Abstract
BACKGROUND AND PURPOSE: As in adult imaging, FLAIR can be applied to pediatric brain imaging, and this requires an appreciation of the normal pediatric brain appearance by FLAIR imaging. The purpose of this study was to describe the MR appearance of the brain in normal infants and young children as demonstrated by fluid-attenuated inversion-recovery (FLAIR) MR imaging.
METHODS: We retrospectively examined MR brain studies, interpreted as normal by pediatric radiologists, from 29 patients (aged 1 to 42 months) to catalog the appearance of myelination in multiple brain areas.
RESULTS: On T2-weighted images, white matter progressed from hyperintense to hypointense relative to adjacent gray matter over the first 2 years of life. An analogous, although slightly delayed sequence was observed on FLAIR images with the exception of the deep cerebral hemispheric white matter, which followed a triphasic sequence of development. On FLAIR images, the deep cerebral white matter was heterogeneously hypointense relative to gray matter in the young infant, became hyperintense early in the first few months of life, and then reverted to hypointense during the second year of life.
CONCLUSION: The normal appearance and development of brain white matter must be taken into account when interpreting FLAIR images of infants and young children.
Fluid-attenuated inversion-recovery (FLAIR) imaging has become an important part of clinical MR examinations of the adult brain (1, 2). The FLAIR pulse sequence produces heavily T2-weighted images with CSF signal nulled by an appropriately chosen inversion time (TI). Because the CSF signal is suppressed, FLAIR enhances the conspicuity of lesions adjacent to the ventricles and makes the sequence especially useful in the evaluation of cerebral white matter (3).
Not much has been written about FLAIR imaging of infants and young children (4, 5). Although the role of FLAIR in the study of infants and young children is yet to be fully determined, given the lack of published data on the appearance of normal myelination on FLAIR images, we thought it important to document the normal FLAIR appearance of brain white matter in this population. In addition, because any interpretation of MR studies of infants and young children must take into account the changing appearance of white matter caused by the progression of myelination (6–14), we chose to focus this study on the FLAIR appearance of different areas of normal brain as they undergo myelination.
Methods
We retrospectively evaluated brain MR studies from 29 developmentally normal infants and young children, comparing the appearance of the brain on T1-weighted, T2-weighted, and FLAIR MR images.
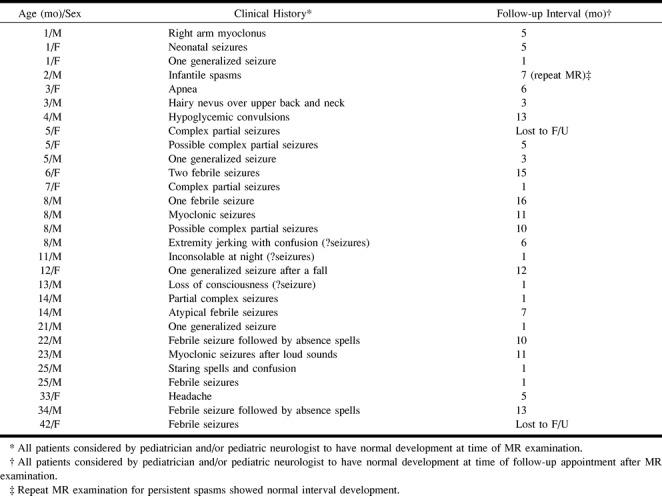
A review of our radiologic records over the last 3 years, since FLAIR imaging became available on our scanner, revealed 36 infants and young children with normal brain MR studies. After review of all the patients' medical records, seven were eliminated from our study because the patients were thought to be developmentally delayed. The remaining 29 patients, born at term and between the ages of 1 and 42 months at the time of the MR examination, made up the study population. All patients were judged to have normal neurologic development at the time of the MR study on the basis of clinical evaluation by the patients' pediatricians and/or pediatric neurologists. The majority of the 29 patients were scanned as part of a work-up for seizures. Table 1 outlines the study population, the reasons for the MR examination, and the follow-up intervals available for all patients. Follow-up clinical data were obtained by chart review and direct communication with the patients' physicians, when necessary. All the brain studies were clinically interpreted as normal. One of the patients has returned for a repeat MR examination.
TABLE 1:
Patient clinical history and follow-up
Patients were scanned on a 1.5-T system (General Electric, version 5.4–5.5) using either an adult bird cage head coil or a pediatric phased-array combination head and spine coil. T2-weighted, T1-weighted, and FLAIR images were obtained in matching axial planes using a field of view of 16 to 20 cm and a section thickness of 4 to 5 mm. T2-weighted images were acquired using a fast spin-echo (FSE) technique with parameters of 3000–5500/80–126 (TR/TEeff) acquired as a dual-echo sequence with an echo train length (ETL) of 8. T1-weighted images were acquired either with a spin-echo (SE) technique with parameters of 400–600/11–16 (TR/TE) or an inversion recovery (IR) technique with parameters of 2000/11–13 (TR/TE) and TI = 800. FLAIR images were acquired with an FSE technique with parameters of 10,002/136–149 (TR/TEeff), TI = 2200–2300, and ETL = 8. The TI was chosen on the basis of the vendor's recommended FLAIR imaging protocol to suppress maximally the signal from CSF. Depending on the clinical circumstance, the patients were either sedated by a radiology nurse using chloral hydrate (50 to 75 mg/kg) orally or by a pediatric anesthesiologist.
Subjective analysis of the intensity of different white matter areas relative to adjacent gray matter was performed by two of the authors after consensual review of representative cases by all three authors to determine appropriate criteria for intensity grading. The grading system was based on a prior report (8). The white matter on T1-weighted, T2-weighted, and FLAIR images was judged to be hypointense, isointense, or hyperintense relative to adjacent gray matter in the following areas: middle cerebellar peduncle, anterior and posterior limbs of the internal capsule, genu and splenium of the corpus callosum, and deep cerebral hemispheric white matter in the occipital, frontal, and temporal lobes. The two authors' independent data sets were compared and scoring discrepancies were resolved by consensus. Since we do not use proton density–weighted imaging for the evaluation of myelination, these images were not compared with the T1-weighted, T2-weighted, and FLAIR images.
The progression of signal changes related to myelination as a function of age was compared among the T1-weighted, T2-weighted, and FLAIR images. For each of the brain areas studied, we determined the approximate age at which the white matter in that area changed in signal intensity relative to its adjacent gray matter. Specifically, we determined the age at which the white matter underwent a transition from being darker than adjacent gray matter to being lighter than adjacent gray matter or vice versa for the three pulse sequences. This age was designated as the point midway between the age at which the signal change was apparent in the first patient and that at which it was observed in all the patients.
Results
On T2-weighted images, the white matter intensity progressed from hyperintense to hypointense relative to adjacent gray matter over the first 2 years of life. With the exception of the deep cerebral white matter, which will be discussed separately, FLAIR images showed the same signal transition slightly later than seen on T2-weighted images (Table 2). On FLAIR images, the white matter changed from hyperintense to hypointense relative to adjacent gray matter first in the middle cerebellar peduncle and the posterior limb of the internal capsule, then in the splenium and genu of the corpus callosum, followed by the anterior limb of the internal capsule (Table 2).
TABLE 2:
Approximate ages at which signal changes related to myelination are visible
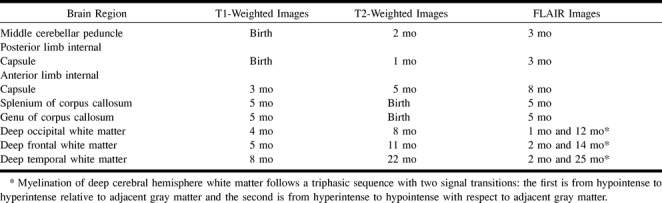
Unlike T1- and T2-weighted images, which show a biphasic sequence of cerebral hemispheric white matter signal, FLAIR images show a triphasic sequence of relative white matter signal change. In our patients, T1-weighted images showed the deep cerebral hemispheric white matter progressing from hypointense to hyperintense relative to adjacent gray matter during the first year of life. T2-weighted images showed the same areas of cerebral hemispheric white matter progressing from hyperintense to hypointense relative to adjacent gray matter between 8 and 14 months of life. FLAIR images initially showed the deep cerebral hemispheric white matter to be heterogeneously hypointense, becoming hyperintense early in the first several months of life, and finally reconverting to hypointense relative to adjacent gray matter during the second year of life (Fig 1). Cerebral deep white matter myelination appears first in the occipital lobes, then in the frontal lobes, and last in the temporal lobes.
fig 1.
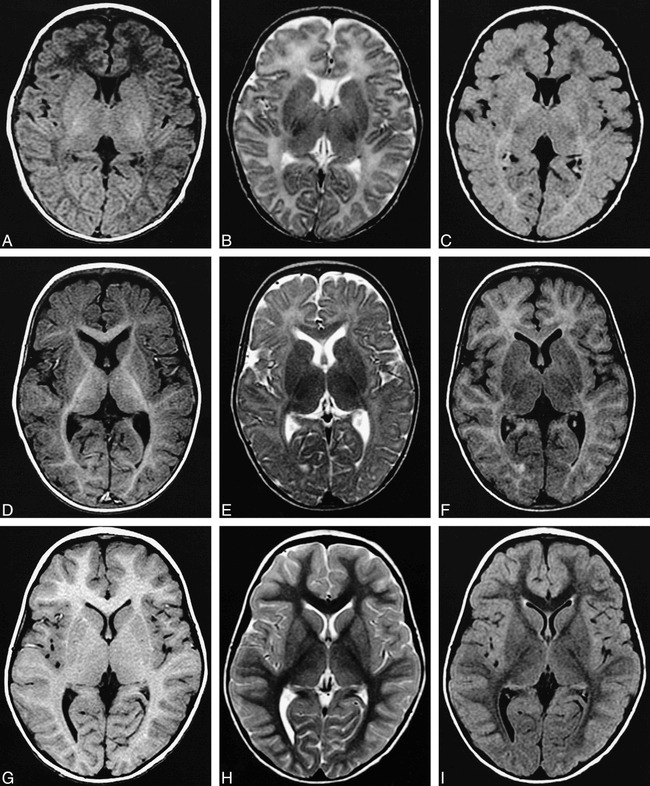
A–I, Matching T1-weighted, T2-weighted, and FLAIR images from three patients ages 5 weeks (A–C), 8 months (D–F), and 3 years (G–I). Note the triphasic signal progression in the deep frontal white matter on the FLAIR images, and that the FLAIR image of the 8 month old (F) looks deceivingly like a T1-weighted image (D, G) except for the internal capsule, which is hypointense rather than hyperintense relative to adjacent gray matter.
A, T1-weighted image, 600/11 (TR/TE); B, T2-weighted image, 4000/119 (TR/TEeff), split echo train with ETL = 8; C, FLAIR image, 10,002/149 (TR/TEeff) TI = 2200, ETL = 8; D, T1-weighted image, 2000/11 (TR/TE), TI = 800; E, T2-weighted image, 3000/119 (TR/TEeff), split echo train with ETL = 8; F, FLAIR image, 10,002/149 (TR/TEeff), TI = 2200, ETL = 8; G, T1-weighted image, 500/11 (TR/TE); H, T2-weighted image, 3000/80 (TR/TEeff), split echo train with ETL = 8; I, FLAIR image, 10,002/149 (TR/TEeff), TI = 2200, ETL = 8.
As with T1- and T2-weighted imaging, FLAIR imaging displays signal changes in the peripheral white matter of the cerebral hemispheres after that seen in the deep white matter. As a result, the peripheral white matter in the temporal lobes is one of the last areas to undergo myelination. It remained hyperintense relative to adjacent gray matter beyond 24 months of age in our series.
A comparison of the scores from the two observers revealed discordant grades in 7% of the measures (50 discrepancies out of 696 scores by each reviewer). The discrepancies were never off by more than one step in the grading system; for example, white matter was graded as hyperintense rather than isointense or as hypointense rather than isointense.
Discussion
The signal changes on T1- and T2-weighted images caused by myelination have been studied by previous investigators (6–14). Because we have recently added FLAIR imaging to our routine clinical pediatric brain MR evaluation, and because FLAIR imaging relies on both T2 and T1 relaxation times for image contrast, we thought it important to document the normal signal changes of myelination as seen on FLAIR images.
We retrospectively examined matching axial T1-weighted, T2-weighted, and FLAIR brain studies interpreted as normal in 29 developmentally normal infants and young children who were scanned primarily because of seizures (Table 1). Although no brain abnormalities were detected in any of our patients, and all the patients were judged clinically to be developmentally normal, some of the patients may have had alterations in brain maturation that could have affected our analysis of myelination. The most likely resultant artifact would be a delay in myelination that would result in an overestimation of the age when signal changes of myelination ought to appear. Arguing against this type of bias in our data is the fact that we saw changes of myelination on our T1-weighted images at ages similar to those reported in prior studies and on our T2-weighted images usually earlier than those reported previously (12, 13). In our sample, the corpus callosum (genu and splenium) was visible as a hypointense structure on T2-weighted images at birth, as compared with prior reports, in which it was not visible until 4 to 8 months of age (12, 13). In addition, in our sample, the anterior limb of the internal capsule and the deep occipital and frontal white matter were visible as hypointense structures on T2-weighted images several months earlier than expected, given prior reports (12, 13).
Older studies used conventional spin-echo techniques to acquire T2-weighted images (6, 7, 9, 11, 12). In contrast, we used FSE technology employing ETLs of 8 and longer TRs and TEeffs. These technical differences most likely explain the earlier visibility of myelination on our T2-weighted images relative to prior reports (12, 13). Myelination has been noted to be visible sooner on FSE-acquired T2-weighted images than on conventional SE T2-weighted images (15). The increased magnetization transfer that occurs on multisection FSE acquisitions can increase the relative conspicuity of myelination (15). In addition, the longer TRs and TEeffs we used in relation to those older studies would also be expected to enhance the conspicuity of myelinated areas because of increased T2 weighting.
FLAIR imaging relies primarily on differences in T2 relaxation for image contrast, because in most FLAIR protocols the TEeff is chosen to be relatively long (136–149 in this study). Because there is an inversion pulse with a long TI (2200 in this study), the signal from tissues with very long T1 relaxation times can be nulled. In adult brain imaging, the only area that normally has a sufficiently long T1 relaxation time for this phenomenon is CSF. In infants, as discussed below, certain brain areas can have sufficiently long T1 relaxation times for them also to appear hypointense.
Except for the deep white matter in the cerebral hemispheres, which will be discussed separately, the white matter on FLAIR images converts from hyperintense to hypointense relative to adjacent gray matter during the first 24 months of life. This finding is not unexpected given that most of the contrast on FLAIR images stems from T2 relaxation effects. Previous authors have attributed this decrease in relative signal of the white matter on T2-weighted images to “tightening of the spiral of myelin around the axon” (7), to “laying down of myelin” (6), or to “maturation” of the myelin (11).
The white matter signal change from hyperintense to hypointense relative to adjacent gray matter on FLAIR images occurs slightly later than the analogous change seen on T2-weighted images. This delay most likely reflects the T1 sensitivity of FLAIR imaging. The inversion pulse before the excitation pulse in the FLAIR experiment imparts a T1 weighting to the images. At the age when the white matter is becoming hypointense relative to gray matter on both T2-weighted and FLAIR images, it is already hyperintense on T1-weighted images. Therefore, the propensity of white matter to become hypointense on FLAIR images because of T2 relaxation effects is partially offset by its propensity to remain hyperintense as a result of T1 relaxation effects. The net effect of these opposing forces on image contrast is likely to be the slight delay in white matter signal progression from hyperintense to hypointense on the FLAIR images relative to the T2-weighted images. Because of the T1 and T2 dependence of FLAIR, it is possible that cerebral lesions could become more or less conspicuous when imaged against the background white matter relative to standard T1- and T2-weighted images, depending on the exact developmental stage of the child and the origin of the lesions. This is beyond the scope of this article and will need to be addressed in future work.
In the deep cerebral hemispheric white matter, FLAIR images show a triphasic sequence of signal change relative to adjacent gray matter not seen on T1- or T2-weighted images (Fig 1). In infants, the deep cerebral hemispheric white matter was hypointense relative to adjacent gray matter on FLAIR images. On matching T1-weighted images, these deep white matter areas were subtly more hypointense than the surrounding white matter. On matching T2-weighted images, these deep white matter areas were subtly more hyperintense than the surrounding white matter. The deep white matter areas are difficult to pick out as distinct areas on T1- and T2-weighted images because they reside within background white matter, which is also hypointense on T1-weighted images and hyperintense on T2-weighted images. These areas are thought to contain a relatively large amount of free water at this time (7, 11). We postulate that the amount of free water increases the T1 value sufficiently to cause the signal to be suppressed and therefore hypointense on FLAIR images in a manner similar to CSF.
The second phase of the triphasic FLAIR sequence occurs between 1 and 2 months of life, when the deep cerebral hemispheric white matter progresses from hypointense to hyperintense with respect to adjacent gray matter. This reflects the decrease in brain free water, with a resultant decrease in T1 relaxation time, that occurs during this time and contributes to the concurrent rise in white matter signal seen with T1-weighted imaging (6, 9, 11). The decreased T1 relaxation time prevents the deep cerebral white matter from being suppressed along with the CSF by the inversion pulse. As the T1 relaxation time decreases, T2 relaxation effects begin to dominate image contrast, resulting in the deep cerebral white matter appearing hyperintense relative to adjacent gray matter on the FLAIR images.
The third phase of the triphasic FLAIR sequence follows during the ensuing 2 years. This final phase of signal change is when the white matter becomes briefly isointense with and then finally hypointense to adjacent gray matter in an analogous though slightly delayed manner relative to the T2-weighted images.
A triphasic sequence of FLAIR signal change was not seen in the other areas of the white matter. An initial hypointense phase was never seen in the middle cerebellar peduncle, the internal capsule, or the corpus callosum. Some of the areas, such as the middle cerebellar peduncle, most likely have matured beyond this point prior to imaging. Alternatively, these areas may never have had a sufficient quantity of free water to cause them to be hypointense with respect to adjacent gray matter. Some of these areas, such as the internal capsule and, to a lesser extent, the corpus callosum, are also small, and subtle decreased signal may be obscured by volume-averaging effects.
Because the temporal lobes are nearly the last areas to undergo myelination, maturing after the frontal lobes (16, 17), the deep white matter in the temporal lobes remained hyperintense on FLAIR images beyond 24 months of age in our series. More peripheral white matter in the temporal lobes can remain hyperintense on FLAIR images beyond 24 months of age. This relatively increased signal in the temporal lobe white matter on FLAIR images is normal in children of this age and should not be confused with a pathologic process.
Thin linear increased signal is almost always seen around the lateral and third ventricles on FLAIR images of adults (5). Its origin is not entirely clear, but it is thought to be related to alterations in axon packing density adjacent to the ependyma (5). This thin line of periventricular increased signal on FLAIR images is not visible in young children until the adjacent deep white matter tracts begin to undergo myelination, around the age of 7 to 8 months. At this time, the deep white matter in FLAIR images is changing from isointense to hypointense relative to adjacent gray matter. It is not clear whether the periventricular bright line appears because of some change in the structure of the periventricular tissues at this time or because the increased signal is not conspicuous enough to appreciate until it is placed between the dark CSF and the darkening periventricular white matter. An analogous periventricular bright line appears around the fourth ventricle at 3 to 4 months of life, when the adjacent middle cerebellar peduncle begins to change on FLAIR images from isointense to hypointense relative to adjacent gray matter.
The utility of FLAIR imaging in children has yet to be proved. At least one author has stated that the FLAIR sequence is not useful in neonates (5). We use the FLAIR sequence in imaging evaluations of infants and children at our institution. Although we have not made a formal study of its utility in this population, it has proved useful to us. Given that FLAIR imaging is in clinical use by us, and most likely by others, we pursued this investigation of the appearance of normal myelination as seen on FLAIR sequences. Only further work will determine whether FLAIR imaging has utility in the study of the different pathologic states encountered in infants and young children.
Conclusion
We have documented the FLAIR appearance of the brain in radiologically normal infants and small children. With the exception of the deep cerebral white matter, myelination follows an orderly progression on FLAIR images, analogous, although slightly delayed, to that seen on T2-weighted images. The FLAIR signal intensity of the deep cerebral hemispheric white matter follows a triphasic sequence of signal change, in which it is initially hypointense, becomes hyperintense in the first few months of life, and then reverts to hypointense in the second year of life. The temporal lobe undergoes myelination last, resulting in normal increased FLAIR signal in the peripheral white matter of the temporal lobe beyond 24 months of age. Interpretation of pediatric FLAIR images should be made with an understanding of the expected signal intensity changes that occur during the first few years of life.
Footnotes
Address reprint requests to James W. Murakami, MD.
References
- 1.Kates R, Atkinson D, Brant-Zawadzki M. Fluid-attenuated inversion recovery (FLAIR): clinical prospectus of current and future applications. . Top Magn Reson Imaging 1996;8:389-396 [PubMed] [Google Scholar]
- 2.Rydberg JN, Hammond CA, Grimm RC,, et al. Initial clinical experience in MR imaging of the brain with a fast fluid-attenuated inversion-recovery pulse sequence. . Radiology 1994;193:173-180 [DOI] [PubMed] [Google Scholar]
- 3.Gawne-Cain ML, Silver NC, Moseley IF, Miller DH. Fast FLAIR of the brain: the range of appearances in normal subjects and its application to quantification of white-matter disease. . Neuroradiology 1997;39:243-249 [DOI] [PubMed] [Google Scholar]
- 4.Sargent MA, Poskitt KJ. Fast fluid-attenuated inversion recovery (FLAIR) magnetic resonance imaging of the brain: a comparison of multi-shot echo-planar and fast spin-echo techniques. . Pediatr Radiol 1997;27:545-549 [DOI] [PubMed] [Google Scholar]
- 5.Barkovich AJ. MR of the normal neonatal brain: assessment of deep structures. . AJNR Am J Neuroradiol 1998;19:1397-1403 [PMC free article] [PubMed] [Google Scholar]
- 6.McArdle CB, Richardson CJ, Nicholas DA,, et al. Developmental features of the neonatal brain: MR imaging, I: gray-white matter differentiation and myelination. . Radiology 1987;162:223-229 [DOI] [PubMed] [Google Scholar]
- 7.Barkovich AJ, Lyon G, Evrard P. Formation, maturation, and disorders of white matter. . AJNR Am J Neuroradiol 1992;13:447-461 [PMC free article] [PubMed] [Google Scholar]
- 8.Dietrich RB, Bradley WG, Zaragoza EJ,, et al. MR evaluation of early myelination patterns in normal and developmentally delayed infants. . AJR Am J Roentgenol 1988;150:889-896 [DOI] [PubMed] [Google Scholar]
- 9.Baierl P, Forster Ch, Fendel H,, et al. Magnetic resonance imaging of normal and pathological white matter maturation. . Pediatr Radiol 1988;18:183-189 [DOI] [PubMed] [Google Scholar]
- 10.Staudt M, Schropp C, Staudt F,, et al. MRI assessment of myelination: an age standardization. . Pediatr Radiol 1994;24:122-127 [DOI] [PubMed] [Google Scholar]
- 11.Girard N, Raybaud C, Du Lac P. MRI study of brain myelination. . J Neuroradiol 1991;18:291-307 [PubMed] [Google Scholar]
- 12.Barkovich AJ, Truwit CL. Practical MRI Atlas of Neonatal Brain Development. . New York: Raven; 1990
- 13.Grodd W. Normal and abnormal patterns of myelin development of the fetal and infantile human brain using magnetic resonance imaging. . Curr Opin Neurol Neurosurg 1993;6:393-397 [PubMed] [Google Scholar]
- 14.Daldrup HE, Schuierer G, Link TM,, et al. Evaluation of myelination and myelination disorders with turbo inversion recovery magnetic resonance imaging. . Eur Radiol 1997;7:1478-1484 [DOI] [PubMed] [Google Scholar]
- 15.Shaw DWW, Weinberger E, Astley SJ, Tsuruda JS. Quantitative comparison of conventional spin echo and fast spin echo during brain myelination. . J Comput Assist Tomogr 1997;21:867-871 [DOI] [PubMed] [Google Scholar]
- 16.Brody BA, Kinney HC, Kloman AS, Gilles FH. Sequence of central nervous system myelination in human infancy, I: an autopsy study of myelination. . J Neuropathol Exp Neurol 1987;46:283-301 [DOI] [PubMed] [Google Scholar]
- 17.Kinney HC, Brody BA, Kloman AS, Gilles FH. Sequence of central nervous system myelination in human infancy, II: patterns of myelination in autopsied infants. . J Neuropathol Exp Neurol 1988;47:217-234 [DOI] [PubMed] [Google Scholar]


