Abstract
BACKGROUND AND PURPOSE: Long considered to have a role limited largely to motor-related functions, the cerebellum has recently been implicated as being involved in both perceptual and cognitive processes. Our purpose was to determine whether cerebellar activation occurs during cognitive tasks that differentially engage the component processes of word identification in reading.
METHODS: Forty-two neurologically normal adults underwent functional MR imaging of the cerebellum with a gradient-echo echo-planar technique while performing tasks designed to study the cognitive processing used in reading. A standard levels-of-processing paradigm was used. Participants were asked to determine whether pairs of words were written in the same case (orthographic processing), whether pairs of words and non-words rhymed with each other, respectively (phonologic assembly), and whether pairs of words belonged to the same category (semantic processing). Composite maps were generated from a general linear model based on a randomization of statistical parametric maps.
RESULTS: During phonologic assembly, cerebellar activation was observed in the middle and posterior aspects of the posterior superior fissure and adjacent simple lobule and semilunar lobule bilaterally and in posterior aspects of the simple lobule, superior semilunar lobule, and inferior semilunar lobule bilaterally. Semantic processing, however, resulted in activation in the deep nuclear region on the right and in the inferior vermis, in addition to posterior areas active in phonologic assembly, including the simple, superior semilunar, and inferior semilunar lobules.
CONCLUSION: The cerebellum is engaged during reading and differentially activates in response to phonologic and semantic tasks. These results indicate that the cerebellum contributes to the cognitive processes integral to reading.
The cerebellum's importance in planning and implementing movement has been well documented (1−5), but recent evidence points to its having a potentially broader role that includes sensory perception and cognitive processes. In humans, studies of cerebellar lesions show deficits in error detection, language, attentional control, and problem-solving (6). Functional neuroimaging studies of unimpaired participants report cerebellar activation in response to a variety of tasks, including problem-solving, working memory, verb generation, tactile stimulation, olfaction, and attention (7−15). Based on these studies and on the known anatomic pathways between the cerebral cortex and the cerebellum (6, 16, 17), we postulated that the cerebellum might play a part in word identification in reading.
Theories of word identification in reading indicate that at least three component processes are engaged by printed words (18−20). Initially, orthographic processing results in letter (or letter-cluster) identification. Phonologic assembly then maps these orthographic codes onto the phonemic units (sound structures) they represent. Based on the output from these orthographic and phonologic coding processes, lexical-semantic processing is associated with providing access to basic information regarding a target word's meanings in the mental lexicon.
In previous studies, we used a series of hierarchical reading tasks to identify cerebral areas in unimpaired readers that are engaged by each of these component processes integral to word identification in reading (19, 21). In the current study, we used a modification of this paradigm more appropriate to determine whether the cerebellum plays a specific cognitive, as opposed to a perceptual-motor, role in reading. We designed experimental conditions in which perceptual input and motor output (response) were held constant across a series of tasks that systematically increased demands on the component (cognitive) dimensions of word identification described above. We used a standard levels-of-processing paradigm to accomplish this (22). For each of the three basic tasks in this paradigm, participants viewed pairs of words, and for each pair, they signaled a positive response or a negative response with an appropriate key press, keeping perceptual input (stimulus type) and motor output (forced choice button press) constant across the tasks. In one task, however, participants judged whether the words were printed in the same case (orthography); this constitutes a “shallow” level of processing in the standard levels-of-processing paradigm. In the other task, participants judged whether the words rhymed (phonologic judgment), and in the third task, participants judged whether the word pairs were from the same semantic category (semantic judgment). These latter two tasks increase demands on cognitive processing in a progressive manner. In episodic memory studies, which use variants of these tasks, a universally obtained result is a higher probability of recalling items processed in the deep (semantic) as opposed to shallow (orthographic) condition, with phonologic processing recall probabilities intermediate between these two end points (22, 23). This memory continuum is evidence that the deeper processing tasks (phonologic and semantic) engender more extensive cognitive processing (with a greater probability of laying down an episodic memory trace) than the shallower orthographic condition. We hypothesized that if the cerebellum plays a role in phonologic or semantic processing in reading, we would observe systematic increases in cerebellar activation across tasks even though input and output are identical.
Finally, we also included a non-word rhyme task as a contrast with the word rhyme task. The non-word rhyme task, which uses unfamiliar stimuli, should make greater demands on phonologic assembly (orthographic to phonologic decoding routines) than familiar words. We sought to determine whether greater demands on phonologic assembly would be reflected in changes in cerebellar activation. The contrast between non-word rhyme and word rhyme holds task-constant, but because the stimulus familiarity differs, it allows a relatively specific investigation of phonologic assembly effects.
Methods
Participants
Forty-two right-handed participants without neurologic impairment gave written consent and participated voluntarily in the study. There were 23 women (mean age, 25.8 ± 5.9 years) and 19 men (mean age, 24.4 ± 5.3 years). All participants spoke English as their primary language and had completed secondary schooling. Studies were approved by our institutional review board.
Tasks
There were four test tasks (case, word rhyme, category, and non-word rhyme) and one baseline task (line task). For each task, two visual stimuli were presented simultaneously, one above the other, on a projection screen. Participants judged whether the stimuli were the same or different in some aspect (see below) and responded via a button push, so that each of the tasks involved the same decision and motor response. The baseline task, a line-orientation task, was a pure perceptual task that did not include a verbal component. There were two stimuli, each composed of four lines, and participants judged whether the lines had the same pattern of orientation (eg, “/ / \ /” and “/ / \ /” would constitute a yes trial). The case task required orthographic processing of words. Two words of equal length were displayed in either upper case or lower case letters. Participants judged whether the two words were written in the same case (eg, “COW” and “dog” would be a no trial). The word rhyme task required both orthographic and phonologic processing. The participants determined whether two words rhymed (eg, “rice” and “mice” would be a yes trial). The category task required not only orthographic and phonologic processing of the word but also lexical semantic processing. Participants judged whether two words belonged to the same semantic category (eg, “man” [top stimulus] and “boy” [bottom stimulus] would constitute a yes trial). The category task had the same stimuli as the word rhyme task, but participants performed a task that required greater cognitive demand. In the non-word version of the rhyme task, participants determined whether two pseudo-words rhymed (eg, “leat” [top stimulus] and “jete” [bottom stimulus] would be a yes trial). Compared with the word rhyme task, the non-word rhyme task required that the participants perform a similar process (rhyme judgment) but on stimuli that were unfamiliar, which places a greater demand on phonologic assembly. For each of the tasks, half of the displays were yes trials and half were no trials. Stimulus pairs were presented at a rate of one every 4 seconds. The stimuli varied in length (three to six letters), but stimuli within a pair were always matched in length to control for size of the visual display.
Imaging
Functional MR imaging was performed on a 1.5-T GE system (Milwaukee, WI) equipped with resonant gradients (Advanced NMR, Wilmington, MA). Participants were supine in the magnet with their heads immobilized by a neck support, foam wedges, and a restraining band drawn around the forehead. Scout images in the sagittal plane were acquired with parameters of 500/11 (TR/TE), a field of view of 24 cm, an imaging matrix of 256 × 192, and a 5-mm contiguous sections. Seven anatomic images were acquired with parameters of 500/11, a field of view of 40 cm, and an imaging matrix of 256 × 192. In 22 participants, the imaging sections were 9 mm thick, and in 21 participants, the sections were 7 mm thick with a 1-mm gap. All sections were acquired in an oblique coronal plane perpendicular to the intercommissural line, extending from the brain stem to posterior aspect of the cerebellum. Using a single-shot echo-planar, gradient-echo sequence (2685/60/1 [TR/TE, number of excitations]; flip angle, 60°; field of view, 40 × 20 cm; and an imaging matrix, 128 × 64), 146 activation images were collected at the same seven locations and section thickness. For every participant, each of the four tasks (case, word rhyme, non-word rhyme, and category) was presented as one imaging trial, and each trial was run four times. During a trial, the task epoch alternated with the baseline epoch (line task), with each epoch lasting approximately 35 seconds (eight task epochs and eight line epochs in each trial).
Data Analysis
Before conducting statistical analysis, the images from each run were motion-corrected for three translation directions and for the three possible rotations using the SPM-96 program (24). The corrected images were spatially filtered using a gaussian filter with a full-width half-maximum value of 6.5 mm.
For each task (case, word rhyme, non-word rhyme, and category), the t statistic was calculated for each voxel, comparing each task and its own baseline (line task) for each imaging series. A correction for linear drift was built into this calculation (25). Values obtained for each imaging series were then averaged to create a statistical parametric map (SPM), one for each task and each participant. These SPMs and the anatomic images from individual participants were transformed by in-plane transformation and section interpolation into a normalized 3D grid defined by Talairach and Tournoux (26).
The SPMs were not used to compute probabilities of activation because the autocorrelation in the raw time-series data violate assumptions of the t test. Instead, they were used as a derived measure of task-related activity. To generate composite activation maps, we used the SPMs to compute seven standard linear contrast measures (27) as linear combinations of the four basic SPMs, using the coefficients presented in Table. Under the null hypothesis of no effect, the expected value of the mean of this contrast across participants is equal to zero. With an appropriate error term, the observed linear contrast could be used to generate a t statistic and a P value for the significance of an effect. To avoid distribution assumptions, however, we used instead a randomization test to generate a distribution of task-related SPMs (t values) to obtain a P value.
Coefficients of the linear contrasts used to generate composite activation maps
The randomization test creates the population distribution for each voxel by calculating the randomized mean value of the contrast in which a randomly chosen subset of half the contrast measures has a reversed sign. This randomization was performed 1000 times, generating a sampling distribution of the linear contrast measures. The observed linear contrast measure, calculated without sign reversal, was assigned a P value based on its position in this distribution. The proportion of times that the observed linear contrast measure was more extreme than the randomized linear contrast measure represents a P value. It is the proportion of times we would expect to obtain a linear contrast measure as large or larger than the one obtained if the null hypothesis were true. The P value for each voxel was overlaid upon the mean anatomic image for display. The threshold used was P = .005 (uncorrected).
The composite contrast maps were designed to test our hypothesis regarding differences in cerebellar activation in response to changes in cognitive tasks and stimuli. In the non-word rhyme condition (non-word rhyme/line versus case/line), the task was identical to the word rhyme condition (judge whether letter strings rhyme) but the stimuli were non-words as opposed to real words, allowing a more specific investigation of phonologic assembly. The category condition (category/line versus case/line) interrogated cerebellar activity when participants viewed stimuli (real words) identical to those in the word rhyme condition but performed a semantic categorization task that placed greater demands on cognitive processing than did the rhyming judgment in the word task.
Results
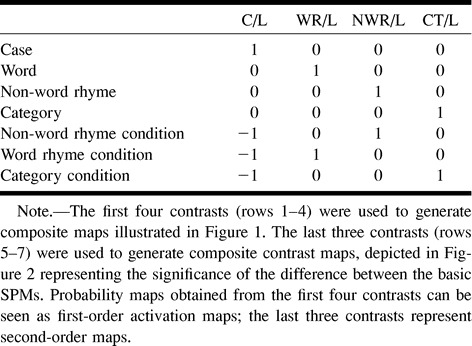
Composite maps of cerebellar activation (P = .005) comparing each of the four reading tasks with the line task are shown in Figure 1. Each reading task, when compared with the line baseline, resulted in activation in the cerebellar hemispheres. This included activation in the middle and lateral aspects of the posterior superior fissure and the adjacent simple lobule and superior semilunar lobule and, more posteriorly, in the posterior and lateral aspects of the horizontal fissure and adjacent superior semilunar lobule and inferior semilunar lobule. In both the non-word rhyme task and category task, however, there was a general increase in area of cerebellar activation in the lateral cerebellar hemispheres (simple lobule, superior semilunar lobule, and inferior semilunar lobule) compared with the word rhyme or case tasks. This increased area of activation was more prominent in the right hemisphere than in the left. Cerebellar regions with more activation in the line task than in the reading tasks are also shown in Figure 1 and include parts of the inferior vermis, the tonsils, the biventer lobule, and the posterior aspect of the superior semilunar and inferior semilunar lobules.
fig 1.
Composite maps (first order, see Table) show regional cerebellar activation during the four reading tasks. Column one compares the case task with the line task (C/L), column two compares the word rhyme task with the line task (WR/L), column three compares the non-word rhyme task with the line task (NWR/L), and column four compares the category task with the line task (CT/L). There is a progressive increase in demand on cognitive processing in going from column one to column four. Numbers indicate cerebellar regions that were more active (P = .005, red-yellow scale) in a reading task compared with the line task; letters indicate areas of the cerebellum that were more active (P = .005, blue-purple scale) in the line task compared with a reading task. 1, anterior aspect of the simple lobule; 2, middle and lateral aspects of the posterior superior fissure and adjacent simple lobule and superior semilunar lobule; 3, middle aspect of the horizontal fissure and adjacent superior semilunar lobule and inferior semilunar lobule; 4, middle aspect of the prepyramidal fissure and adjacent inferior semilunar lobule; 5, posterior and lateral aspects of the horizontal fissure and adjacent superior semilunar lobule and inferior semilunar lobule; 6, posterior and lateral aspects of the posterior superior fissure and adjacent simple lobule and superior semilunar lobule; 7, posterior aspect of inferior semilunar lobule; and 8, posterior and medial aspects of the posterior superior fissure and adjacent simple lobule and superior semilunar lobule. a, inferior vermis; b, postpyramidal fissure and medial aspect of the tonsils; c, biventer lobule; d, medial aspect of the biventer lobule; and e, the middle and posterior aspects of the horizontal fissure and adjacent superior semilunar lobule and inferior semilunar lobule. Section locations in each column from superior to inferior correspond to the following approximate y axis positions of the Talairach atlas: −40, −50, −60, −70, −80, and −90.
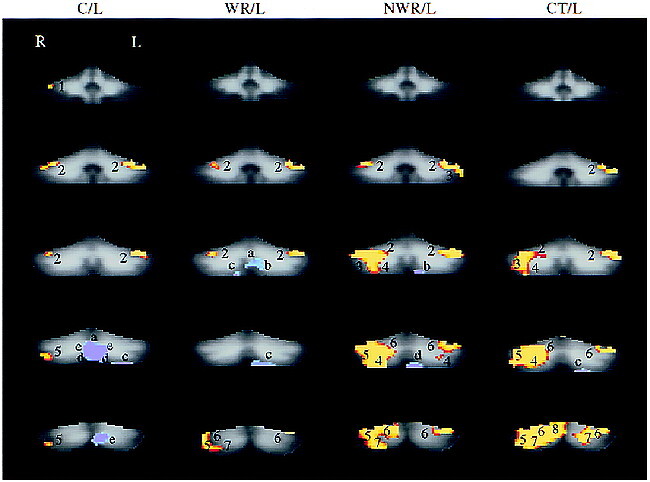
Composite contrast maps of cerebellar activation (P = .005) during reading conditions are shown in Figure 2. There was no significant difference in cerebellar activation when word rhyme condition was contrasted with case condition (Fig 2, column 2). In the non-word rhyme condition (Fig 2, column 1, arrow A), participants performed the same task (judged whether letter strings rhyme) as in the word rhyme condition but on novel stimuli consisting of non-word letter strings. This comparison of activation patterns in columns one and two highlights differences in cerebellar activity when the task is held constant but stimulus familiarity is varied. The non-word rhyme condition revealed activation in the medial aspect of the posterior superior fissure, the adjacent simple lobule and superior semilunar lobule bilaterally, the medial and posterior aspects of the superior semilunar lobule bilaterally, the posterior aspect of the posterior superior fissure and adjacent simple and superior semilunar lobules bilaterally, the posterior and medial aspect of the simple lobule bilaterally, and the posterior and medial aspects of the inferior semilunar lobule bilaterally. In the category condition (Fig 2, column 2, arrow B), participants viewed similar word pairs as in the word-rhyme condition but were required to make a more elaborate semantic analysis, allowing us to investigate cerebellar activation when the stimuli were held constant but the task increased in cognitive demands. This contrast map indicated cerebellar activation in the right deep nuclei, the medial and posterior aspects of the superior semilunar lobule bilaterally, the posterior and medial aspects of the simple lobule bilaterally, and the posterior and medial aspects of the inferior semilunar lobule bilaterally. There were no areas of cerebellum that were more active (P = .005, blue-purple scale) in the case condition compared with the other reading conditions.
fig 2.
Composite maps (second order, see Table) contrast cerebellar activation during different reading conditions. The SPMs from the case/line comparison served as a baseline for comparison with the SPMs from other task comparisons to generate the three composite maps: non-word rhyme (non-word rhyme/line versus case/line, column one), word rhyme (word-rhyme/line versus case/line, column two), and category (category/line versus case/line, column three). Numbers indicate cerebellar regions that were more active (P = .005, red-yellow scale) in either category/line, word rhyme/line, or non-word rhyme/line compared with case/line, respectively. Column two (word rhyme) shows no significant difference in activation between word rhyme/line and case/line. In the non-word rhyme condition (column 1, arrow A) , participants performed the same task (judge whether letter strings rhyme) as in the word rhyme condition but on unfamiliar stimuli (non-word letter strings). Activation in the non-word rhyme condition occurred in the medial aspect of posterior superior fissure and adjacent simple lobule and superior semilunar lobule bilaterally (1), the medial and posterior aspects of the superior semilunar lobule bilaterally (3), the posterior aspect of the posterior superior fissure and adjacent simple and superior semilunar lobules bilaterally (4), the posterior and medial aspect of the simple lobule on the right (6), and the posterior and medial aspects of the inferior semilunar lobule on the left (7). In the category condition (column 3, arrow B), participants viewed similar stimuli (word pairs) as in the word-rhyme condition but were required to make a more elaborate semantic analysis (category judgment versus rhyme judgment). Cerebellar activation in the category condition was observed in the right deep nuclear region (2), the middle and posterior aspects of the horizontal fissure and adjacent superior semilunar lobule and inferior semilunar lobule bilaterally (3), the inferior vermis (5), the posterior and medial aspects of the simple lobule bilaterally (6), and the posterior and medial aspects of the inferior semilunar lobule bilaterally (7). There were no areas of the cerebellum that were more active (P = .005, blue-purple scale) in the case/line condition compared with the other reading conditions. Section locations in each column from superior to inferior correspond to the following y axis positions of the Talairach atlas: −40, −50, −60, −70, −80, and −90.
Discussion
The cerebellum's involvement in language has been relegated historically to control and coordination of the motor output of speech. By combining functional MR imaging with hierarchically organized reading tasks, we showed that the cerebellum activates during reading tasks and that more of the cerebellum is active as cognitive demands increase (Fig 1). Using a standard levels-of-processing paradigm, we identified neuroanatomic foci in the cerebellum that were differentially engaged by phonologic assembly and lexical-semantic processing (Fig 2), indicating that in addition to motor function, the cerebellum is involved in cognitive processes that are integral to word identification in reading.
Phonologic assembly was examined by comparing activation patterns in the non-word rhyme condition (Fig 2, column 1) with the word rhyme condition (Fig 2, column 2). In these two conditions, the higher order operations specific to a rhyming task were held constant as participants judged whether two monosyllabic targets did or did not rhyme. The word rhyme condition, however, used highly familiar stimuli, whereas the non-word rhyme condition required participants to decode a novel letter string to recover its phonologic form before performance of the rhyme judgment. This decoding operation, a process implicated as being deficient in dyslexic readers (28−32), seems to be associated with an increased cerebellar role when compared with the word-rhyme condition with which the use of highly familiar stimuli lessens demands on this operation. During phonologic assembly, activation was seen in the middle and posterior aspects of the posterior superior fissure and adjacent simple lobule and semilunar lobule bilaterally.
Semantic processing was investigated by comparing activation patterns of the category condition (Fig 2, column 3) with those in the word rhyme condition (Fig 2, column 2). The essential difference between the word rhyme condition and the category condition was that the latter is thought to engender more extensive cognitive processing (access to lexicon) than the former. Increased cognitive processing resulted in activation in the inferior vermis and the deep nuclear region on the right that was not seen in phonologic assembly. These findings indicate that there are neuroanatomic correlates for an increased functional role of the cerebellum as higher order lexical operations are engaged in print tasks.
Our results complement a growing body of evidence based on anatomic research, lesion studies, and functional imaging research that the cerebellum is involved in language tasks and other cognitive processes. Anatomically, the cerebellum has more neurons than the cerebrum and has an input-to-output axon ratio of 40:1 (33, 34). The cerebellum is a widely connected region, having physiologic links with all of the major divisions of the CNS (6). These anatomic links, especially those to regions in the frontal, temporal, and parietal lobes that are engaged by reading, may be a reason for part of the cerebellar activity in our study. Bilateral projections to the cerebellum from cortical and brain stem areas that include pathways of subvocal articulation could also account for a portion of the cerebellar activation we report. Differences, however, in the activation pattern across levels of processing at which these subvocal articulatory demands are thought to be similar suggest that subvocal articulation cannot account for all of the cerebellar activation during language tasks. Both the non-word rhyme and category tasks resulted in increased activation relative to word rhyme, yet the precise locations varied, suggesting that the increase in activation is more likely attributed to general language and cognitive factors and not simply to motor control associated with subvocalization. In lesion studies, infarcts of the right cerebellum have resulted in impaired linguistic processing manifested as agrammatism or impaired error detection and learning of a verb generation task (35, 36). Both phonologic assembly and semantic processing activated the right cerebellum in our study, with the right deep nuclear region observed only in semantic processing. Finally, functional imaging studies of verb generation and attention have reported activations near the simple and superior semilunar lobules that are similar in location to activations that we found in the phonology and semantic conditions (7, 8, 10, 11, 13).
A relatively recent concept of cerebellar function supersedes the idea of the cerebellum's being only a motor structure; the cerebellum may be important in predicting internal conditions needed for mental or motor operations and in preparing those conditions for a particular operation (37). This preparatory function is thought to be a general one that facilitates sensory processing and mental and motor performance in response to subsequent sensory events. We have shown that the cerebellum is active during the cognitive operations needed for reading. The cerebellum's role in reading seems to be relevant to both phonologic assembly and lexical-semantic processing. In future studies of disorders known to involve linguistic function (for example, infantile autism and developmental dyslexia), it might be important to investigate both cerebellar and cerebral function.
Conclusion
The cerebellum is active during tasks of word identification and differentially activates during phonologic assembly (posterior superior fissure and adjacent simple and superior semilunar lobules in the middle part of the cerebellum) and semantic processing (the right deep nuclear region and the inferior vermis). These results provide evidence that the cerebellum contributes to the cognitive processes integral to reading.
Acknowledgments
This research was supported by grants from the National Institute of Child Health and Human Development (P01 HD21888 and P50 HD25802).
Footnotes
Address reprint requests to Robert K. Fulbright, MD, Section of Neuroradiology, Department of Radiology, Yale University School of Medicine, Box 208042, New Haven, CT 06520
References
- 1.Ito M, The Cerebellum and Neural Control.. New York: Raven; 1984;354-460
- 2.Thach WT, Goodkin HP, Keating JG, The cerebellum and the adaptive coordination of movement. Ann Rev Neurosci 1992;15:403-442 [DOI] [PubMed] [Google Scholar]
- 3.Horne MK, Butler EG, The role of the cerebello-thalamo-cortical pathway in skilled movement. Prog Neurobiol 1995;46:199-213 [PubMed] [Google Scholar]
- 4.Lisberger SG, The neural basis for learning of simple motor skills. Science 1988;242:728-735 [DOI] [PubMed] [Google Scholar]
- 5.Lisberger SG, Pavelko TA, Bronte-Stewart HM, Stone LS, Neural basis for motor learning in the vestibuloocular reflex of primates: II. changes in the responses of horizontal gaze velocity Purkinje cells in the cerebellar flocculus and ventral paraflocculus. J Neurophysiol 1994;72:954-973 [DOI] [PubMed] [Google Scholar]
- 6.Schmahmann JD, The Cerebellum and Cognition.. San Diego: Academic Press; 199:665
- 7.Petersen SE, Fox PT, Posner MI, Mintun M, Raichle ME, Positron emission tomographic studies of the processing of single words. J Cogn Neurosci 1989;1:153-170 [DOI] [PubMed] [Google Scholar]
- 8.Raichle Me, Fiez JA, Videen TO, et al. Practice-related changes in human brain functional anatomy during nonmotor learning. Cereb Cortex 1994;4:342-353 [DOI] [PubMed] [Google Scholar]
- 9.Kim SG, Ugurbil K, Strick PL, Activation of a cerebellar output nucleus during cognitive processing. Science 1994;265:949-951 [DOI] [PubMed] [Google Scholar]
- 10.Klein D, Milner B, Zatorre RJ, Meyer E, Evans AC, The neural substrates underlying word generation: a bilingual functional-imaging study. Proc Natl Acad Sci U S A 1995;92:2899-2903 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Martin A, Haxby JV, Lalonde FM, Wiggs CL, Ungerleider LG, Discrete cortical regions associated with knowledge of color and knowledge of action. Science 1995;270:102-105 [DOI] [PubMed] [Google Scholar]
- 12.Gao J-H, Parsons LM, Bower JM, Xiong J, Li J, Fox PT, Cerebellum implicated in sensory acquisition and discrimination rather than motor control. Science 1996;272:545-547 [DOI] [PubMed] [Google Scholar]
- 13.Allen G, Buxton RB, Wong EC, Courchesne E, Attentional activation of the cerebellum independent of motor involvement. Science 1997;275:1940-1943 [DOI] [PubMed] [Google Scholar]
- 14.Desmond JE, Gabrieli JDE, Wagner AD, Ginier BL, Glover GH, Lobular patterns of cerebellar activation in verbal working-memory and finger-tapping tasks as revealed by funtional MRI. J Neurosci 1997;17:9675-9685 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Sobel N, Prabhakaran V, Hartley CA, Desmond JE, Zhao Z, Glover GH, Odorant-induced and sniff-induced activation in the cerebellum of the human. J Neurosci 1998;18:8990-9001 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Middleton FA, Strick PL, Anatomical evidence for cerebellar and basal ganglia involvement in higher cognitive function. Science 1994;266:458-461 [DOI] [PubMed] [Google Scholar]
- 17.Schmahmann JD, From movement to thought: anatomic substrates of the cerebellar contribution to cognitive processing. Hum Brain Mapping 4: 1996;174-198 [DOI] [PubMed] [Google Scholar]
- 18.Coltheart M, Curtis B, Atkins P, Haller M, Models of reading aloud: dual-route and parallel-distributed-processing approaches. Psychol Rev 1993;100:589-608 [Google Scholar]
- 19.Pugh KR, Shaywitz BA, Shaywitz SE, et al. Cerebral organization of component processes in reading. Brain 1996;119:1221-1238 [DOI] [PubMed] [Google Scholar]
- 20.Pugh KR, Shaywitz BA, Shaywitz SE, et al. Predicting reading performance from neuroimaging profiles: a relation between phonological effects in printed word identification and cerebral organization of language processes. J Exper Psychol 1997;23:299-318 [DOI] [PubMed] [Google Scholar]
- 21.Shaywitz BA, Shaywitz SE, Pugh KR, et al. Sex differences in the functional organization of the brain for language. Nature 1995;373:607-609 [DOI] [PubMed] [Google Scholar]
- 22.Craik FIM, Lockhart RS, Levels of processing: a framework for memory research. J Verbal Learning Verbal Behav 1972;11:671-694 [Google Scholar]
- 23.Craik FIM, Human memory. Ann Rev Psychol 1979;30:63-102 [Google Scholar]
- 24.Friston KJ, Holmes AP, Worsley KJ, Poline J-B, Frith CD, Frackowiak RSJ, Statistical parametric maps in functional imaging: a general linear approach. Hum Brain Mapping 1995;2:189-210 [Google Scholar]
- 25.Skudlarski P, Constable RT, Gore JC, ROC analysis of statistical methods used in functional MRI: individual subjects. Neuroimage 1999;9:3311-329 [DOI] [PubMed] [Google Scholar]
- 26.Talairach J, Tournoux P, Co-planar Stereotaxic Atlas of the Human Brain.. New York: Thieme; 1988;1-122
- 27.Hays WL, Statistics.. Orlando: Holt, Rinehart & Winston, Inc.; 1988;384-411
- 28.Bradley L, Bryant PE, Categorizing sounds and learning to read: a causal connection. Nature 1983;301:419-421 [Google Scholar]
- 29.Wagner RK, Torgesen JK, The nature of phonological processing and its causal role in the acquisition of reading skills. Psychol Bull 1987;101:192-212 [Google Scholar]
- 30.Stanovich KE, Explaining the differences between the dyslexic and the garden-variety poor reader: the phonological-core-variable-model. J Learn Disabil 1988;21:590-604 [DOI] [PubMed] [Google Scholar]
- 31.Shaywitz SE, Shaywitz BA, Pugh KR, et al. Functional disruption in the organization of the brain for reading in dyslexia. Proc Natl Acad Sci U S A 1998;95:2636-2641 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Shaywitz SE, Dyslexia. N Engl J Med 1998;338:307-312 [DOI] [PubMed] [Google Scholar]
- 33.Williams RW, Herrup K, The control of neuron number. Ann Rev Neurosci 1988;11:423-453 [DOI] [PubMed] [Google Scholar]
- 34.Carpenter MB, Core Text of Neuroanatomy. 4th ed. Baltimore: Williams & Wilkins; 1991 224-249
- 35.Fiez JA, Petersen SE, Cheney MK, Raichle ME, Impaired non-motor learning and error detection associated with cerebellar damage: a single case study. Brain 1992;115:155-178 [DOI] [PubMed] [Google Scholar]
- 36.Silveri MC, Leggio MG, Molinari M, The cerebellum contributes to linguistic production: a case of agrammatic speech following a right cerebellar lesion. Neurology 1994;44:2047-2050 [DOI] [PubMed] [Google Scholar]
- 37.Courchesne E, Allen G, Prediction and preparation, fundamental functions of the cerebellum. Learn Mem 1997;4:1-35 [DOI] [PubMed] [Google Scholar]


