Abstract
Summary: We herein report the case of a patient who had paradoxical brain embolism owing to a pulmonary arteriovenous fistula (PAVF) who was diagnosed as having a right-to-left shunt by transcranial Doppler (TCD) with saline contrast medium. TCD with saline contrast medium failed to detect any high-intensity transient signals immediately after catheter embolization of the PAVF. Thus, TCD with saline contrast medium was useful for identifying the presence of a right-to-left shunt and for confirming that the shunt had been obliterated after endovascular treatment.
Recently, transcranial Doppler (TCD) with saline contrast medium has been reported to be useful for detecting right-to-left shunts, including patent foramen ovales, atrial septal defects, and pulmonary arteriovenous fistulas (PAVFs) (1). We herein report the case of a patient who had paradoxical brain embolism associated with a PAVF who was successfully treated by radiologic intervention after being diagnosed by TCD with saline contrast medium to have a right-to-left shunt. TCD with saline contrast medium provided a real-time demonstration of the disappearance of a right-to-left shunt immediately after catheter embolization of a PAVF.
Case Report
A 62-year-old right-handed woman was admitted to the National Cardiovascular Center because of the sudden onset of aphasia and right-sided hemiparesis 1 hour after the onset of stroke. The patient had experienced a transient speech disturbance 1 month before admission. She did not have any episodes of hemoptysis or dyspnea, either before or after admission. At the time of her admission, her blood pressure was 104/60 mm Hg, and she had a regular heart rate of 66 beats per minute and a respiratory rate of 16 breaths per minute. Neither pathologic breath sounds nor heart murmurs were auscultated, and no edema was present in her extremities. No cutaneous vascular malformations were observed. Neurologic examinations revealed that the patient had aphasia and right hemiparesis. Laboratory findings showed normal blood cell counts, normal liver and renal functions, and normal serum cholesterol and blood glucose levels. Plasma protein C and antithrombin III levels were within a normal range, but D-dimer was at 2.4 μg/mL and thrombin-antithrombin III complex was at 4.22 μg/L, both of which were above normal levels. Arterial blood gas analysis revealed hypoxemia, with a PaO2 of 75.5 mm Hg and a PaCO2 of 44.2 mm Hg in room air.
At the time of admission, the patient's chest X-ray and ECG readings were normal. Sonography of the cervix with color-coded Doppler failed to show any abnormalities but rather showed normal blood flow velocities in the common carotid and vertebral arteries. In addition, the results of transthoracic ECG were also normal and a CT scan of the brain showed no abnormalities. Two hours after the onset of stroke, intra-arterial digital subtraction angiography revealed an intraluminal filling defect at an ascending branch of the left middle cerebral artery (MCA) (Fig 1A). As a result, 240,000 IU of urokinase was promptly administered locally through a microcatheter, and disappearance of the filling defect was detected angiographically (Fig 1B). We diagnosed the patient as having an embolic infarction, but the source of the infarction could not be determined. The next day, a CT scan of the brain revealed a hypodense area in the striatocapsular region of the left MCA. The IV administration of heparin (15,000 units/day) was begun on day 2. We transtemporally performed TCD using a 2-MHz transducer (Neurogurd, Medasonics) for 30 minutes to detect embolic signals from the left MCA, but no high-intensity transient signals (HITS) were found. The patient's neurologic deficits improved gradually until they disappeared 7 days after the onset of stroke.
fig 1.
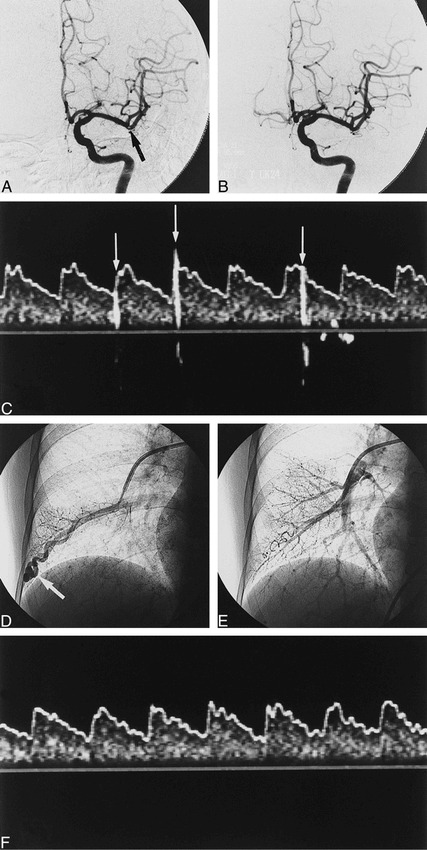
Images from the case of a 62-year-old right-handed woman who experienced the sudden onset of aphasia and right-sided hemiparesis 1 hour after the onset of stroke.
A, Cerebral angiogram shows the intraluminal filling defect (arrow) at an ascending branch of the left MCA 2 hours after the onset of symptoms.
B, After 240,000 IU of urokinase was administered locally through a microcatheter, the filling defect disappeared.
C, Administered before embolization therapy of the PAVF, TCD with saline contrast medium during normal breathing shows many high-intensity transient signals (arrows) from the left MCA 5 days after the onset of stroke.
D, Selective pulmonary angiography shows a PAVF (arrow) in the right lower lobe.
E, After embolization therapy with metallic coil, the feeding vessel to the PAVF is completely occluded.
F, TCD with saline contrast medium failed to reveal any HITS immediately after embolization therapy of the PAVF.
Five days after the onset of stroke, TCD with saline contrast medium was performed to examine the presence of the right-to-left shunt. The TCD with saline contrast medium study was performed using a mixture of saline solution (9 mL) and air (1 mL), agitated between two 10-mL syringes that were connected by a three-way stopcock. The solution was injected into the right antecubital vein within 2 to 3 seconds. The TCD with saline contrast medium showed multiple HITS from the left MCA, not only during a Valsalva maneuver but also during normal breathing (Fig 1C). The results indicated the presence of a continuous right-to-left shunt. On the same day, transesophageal ECG with saline contrast medium showed a significant entry of saline contrast medium into the left atrium through the pulmonary veins, suggesting the presence of a PAVF. Twelve days after the onset of stroke, a contrast-enhanced CT scan of the chest revealed a small nodular lesion connected with pulmonary artery and vein branches in the lower lobe of the right lung. Twenty-two days after the onset of stroke, a ventilation-perfusion lung scintigraphy revealed a perfusion defect in the right lower lung. Venography of the bilateral lower limbs failed to reveal any filling defect in the deep veins. Selective pulmonary angiography performed 32 days after the onset of stroke showed a simple PAVF in the lower right lobe (Fig 1D). We diagnosed the patient at this point as having paradoxical brain embolism associated with an isolated PAVF.
Thirty-six days after the onset of stroke, the PAVF was embolized with Embolization Coil (Cook, Bloomington, IN) while monitoring HITS by TCD (Fig 1E). Although multiple HITS were detected, the TCD with saline contrast medium failed to detect any HITS immediately after the feeding vessels had been occluded (Fig 1F). The results indicated that the right-to-left shunt was successfully embolized.
After embolization therapy, PaO2 (78.1 mm Hg in room air) improved slightly. D-dimer (<0.1 μg/mL), and thrombin-antithrombin III complex (0.68 μg/L) both became within a normal range. On day 43, the patient was free of neurologic deficits and was thus discharged.
Discussion
Hereditary hemorrhagic telangiectasia, or Rendu-Osler-Weber syndrome, is frequently associated with PAVFs. Hereditary hemorrhagic telangiectasia has been found in 36% of patients with isolated PAVFs and in 57% of patients with multiple PAVFs (2). It is characterized by multiple dermal, mucosal, and visceral telangiectasia associated with recurrent bleeding. The patient whose case is reported herein had a simple PAVF without evidence of vascular malformations in other organs.
PAVFs are usually asymptomatic but occasionally cause hemoptysis and dyspnea (3). Because PAVFs are associated with an extracardiac direct communication between the pulmonary artery and vein, they have the potential to cause paradoxical embolism (4, 5). Embolization therapy of PAVFs is reportedly useful in preventing further ischemic events (6). A diagnosis of PAVF can be made by a combination of chest X-ray, CT scan of the chest, pulmonary angiography, and contrast-enhanced ECG. Chest X-rays are not always useful for detecting PAVFs. Remy et al (7) reported that a conventional CT scan of the chest could identify 98% of all PAVFs, whereas pulmonary angiography did so in only 60% of cases. PAVFs smaller than 0.5 mm in diameter cannot be visualized by conventional angiography.
TCD is a reliable and noninvasive technique that can show intracranial hemodynamic changes. Recently, TCD with saline contrast medium was found to be a useful, noninvasive, bedside tool for detecting the presence of a right-to-left shunt in patients with unknown causes of embolic stroke (1). In the present case, TCD with saline contrast medium revealed multiple HITS without the need for provocative steps, such as a Valsalva maneuver or cough. The results in the present case suggested the presence of a PAVF or a large patent foramen ovale with an increase in right atrial pressure owing to pulmonary hypertension.
TCD has been used to monitor flow velocity changes in the MCA during percutaneous transluminal angioplasty of the carotid artery and also in the treatment of arteriovenous malformations and giant aneurysms (8). Moreover, TCD may have the potential to reveal microembolic signals. Recently, HITS have been detected during carotid endarterectomy, and percutaneous transluminal angioplasty and stenting of the carotid artery (9-11). HITS are a marker of an increased risk of intraoperative embolic brain infarction.
In the present patient, HITS detected by TCD with saline contrast medium disappeared immediately after PAVF embolization. Yeung et al (12) reported, in their case study, that TCD with saline contrast medium revealed multiple HITS even after PAVF embolization therapy. If TCD with saline contrast medium shows HITS after embolization of a PAVF, the following issues should be considered: first, if PAVF embolization therapy is not successful and further embolization is needed; and second, other PAVFs may exist, considering that multiple PAVFs occur in 20% of all patients. Therefore, when PAVFs are treated with coil embolization therapy, TCD monitoring is very useful for confirming the disappearance of a right-to-left shunt during PAVF embolization therapy.
Conclusion
In this experience, TCD with saline contrast medium was useful for identifying the presence of a right-to-left shunt as well as for confirming that the shunt had been obliterated after endovascular treatment.
Acknowledgments
This study was supported in part by Research Grants for Cardiovascular Diseases (8C–4, 9A–2, 9A–3, 9A–8) from the Ministry of Health and Welfare of Japan, and by Special Coordinating Funds for Promoting Science and Technology (Strategic Promotion System for Brain Science) from the Science and Technology Agency of Japan.
Footnotes
Address reprint requests to Kazumi Kimura, MD, Cerebrovascular Division, Department of Medicine, National Cardiovascular Center, 5-7-1 Fujishirodai, Suita, Osaka 565-8565, Japan.
References
- 1.Chimowitz MI, Nemec JJ, Marwick TH, Lorig RJ, Furlan AJ, Salcedo EE, Transcranial Doppler ultrasound identifies patients with right-to-left cardiac or pulmonary shunts. Neurology 1991;41:1902-1904 [DOI] [PubMed] [Google Scholar]
- 2.Bosher LH, Jr, Blake DA, Byrd BR, An analysis of the pathologic anatomy of pulmonary arteriovenous aneurysms with particular reference to the applicability of local excision. Surgery 1959;45:91-104 [PubMed] [Google Scholar]
- 3.Blatchford JW, Bolman RM, Hunter DW, Amplatz K, Concomitant pulmonary and cerebral arteriovenous fistulae. Chest 1985;88:782-784 [DOI] [PubMed] [Google Scholar]
- 4.Reguera JM, Colmenero JD, Guerrero M, Pastor M, Martin-Palanca A, Paradoxical cerebral embolism secondary to pulmonary arteriovenous fistula (letter). Stroke 1990;21:504-505 [DOI] [PubMed] [Google Scholar]
- 5.Loscalzo J, Paradoxical embolism: clinical presentation, diagnostic strategies, and therapeutic options. Am Heart J 1986;112:141-145 [DOI] [PubMed] [Google Scholar]
- 6.White RI, Lynch-Nyhan A, Terry P, et al. Pulmonary arteriovenous malformations: technique and long-term outcome of embolotherapy. Radiology 1988;169:663-669 [DOI] [PubMed] [Google Scholar]
- 7.Remy J, Remy-Jardin M, Wattine L, Deffontaines C, Pulmonary arteriovenous malformations: evaluation with CT of the chest before and after treatment. Radiology 1992;182:809-816 [DOI] [PubMed] [Google Scholar]
- 8.Lagalla G, Ceravolo MG, Provinciali L, et al. Transcranial Doppler sonographic monitoring during cerebral aneurysm embolization: a preliminary report. AJNR Am J Neuroradiol 1998;19:1549-1553 [PMC free article] [PubMed] [Google Scholar]
- 9.Spencer MP, Transcranial Doppler monitoring and causes of stroke from carotid endarterectomy. Stroke 1997;28:685-691 [DOI] [PubMed] [Google Scholar]
- 10.Muller M, Behnke S, Walter P, et al. Microembolic signals and intraoperative stroke in carotid endoarterectomy. Acta Neurol Scand 1998;97:110-117 [DOI] [PubMed] [Google Scholar]
- 11.Naylor AR, Bolia A, Abbott RJ, et al. Randomized study of angioplasty and stenting versus carotid endarterectomy: a stopped trial. J Vasc Surg 1998;28:326-334 [DOI] [PubMed] [Google Scholar]
- 12.Yeung M, Khan KA, Antecol DH, Walker DR, Shuaib A, Transcranial Doppler ultrasonography and transesophageal echocardiography in the investigation of pulmonary arteriovenous malformation in a patient with hereditary hemorrhagic telangiectasia presenting with stroke. Stroke 1995;26:1941-1944 [DOI] [PubMed] [Google Scholar]


