Abstract
Summary: Dural arteriovenous fistulas (DAVFs) of the anterior condylar vein are an uncommon but important subset of fistulas occurring at the skull base that can be confused with DAVFs of the marginal sinus on angiography. MR angiography source images can document the intraosseous extent and the relationship to the hypoglossal canal of this type of fistula, which can have significant clinical implications. We present the imaging features of angiography, CT, and MR angiography of three cases of DAVFs localized to the anterior condylar vein and within the hypoglossal canal, which were confirmed by source images from MR angiography. Transvenous coil embolization was curative in two of three cases and would seem to be the treatment of choice when venous access is available.
Intracranial dural arteriovenous fistulas (DAVFs) are abnormal communications between meningeal arteries and dural venous sinuses, or veins, or both. Fifty percent of DAVFs occur in the posterior fossa and usually involve the transverse or sigmoid sinus. Previous articles have described less common fistulas occurring at the skull base in the region of the foramen magnum (1, 2). This report focuses on a previously unreported subset of DAVFs occurring at the skull base involving the anterior condylar vein.
Case Reports
Case 1
A 74-year-old woman presented with an audible pulse-synchronous bruit. Diagnostic angiography showed a DAVF medial to the jugular bulb with unrestricted venous drainage via antegrade flow in the adjacent jugular vein (Fig 1A). Using polyvinyl alcohol, the patient was treated with intra-arterial embolization that partially relieved symptoms. Two years after initial embolization, the patient's bruit suddenly disappeared, followed by concurrent development of ipsilateral chemosis and proptosis. Angiography revealed a dramatic change in venous drainage, with retrograde filling of the inferior petrosal sinus and cavernous sinus to the superior ophthalmic vein, as well as extensive pial drainage. The exact location of the fistula on angiography was revealed most accurately by contralateral common carotid angiography (Fig 1B). MR angiography source images showed abnormal flow medial to the jugular bulb in the region of the hypoglossal canal, with a significant intraosseous component (Fig 1C-D). Complete transvenous occlusion of the fistula was performed using a coaxial system of a 7F Berenstein catheter and a 3F infusion catheter with pushable, fibered, and Guglielmi detachable coils (Fig1E). Postembolization CT showed coils extending from the hypoglossal canal into the bone along the medial aspect of the jugular bulb (Fig 1F-G).
fig 1.
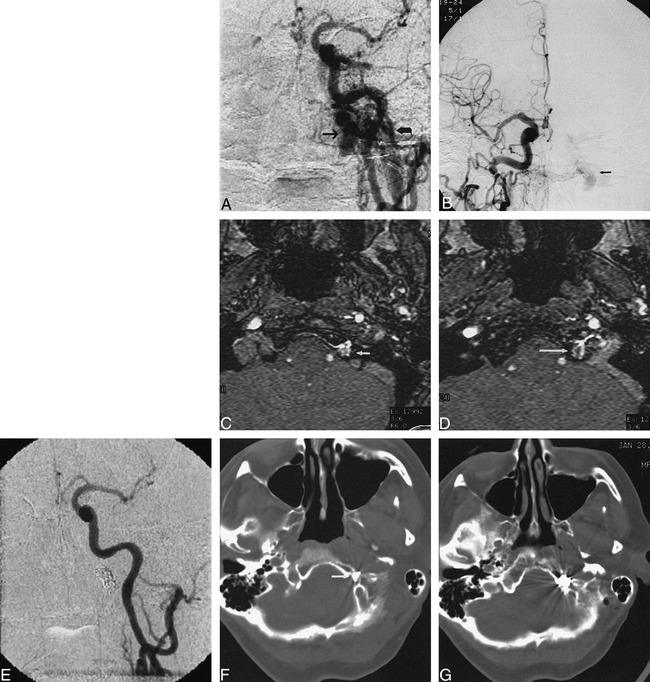
Case 1.
A, Anteroposterior view of left common carotid artery demonstrates a DAVF at skull base. Fistula was located in superior aspect of dilated anterior condylar vein (arrow), medial to jugular bulb (curved arrow).
B, Anteroposterior view of right common carotid artery demonstrates fistula (arrow) medial to jugular bulb. This view was used to direct transvenous embolization to exact site of the fistula.
C, MR angiography source image from three-dimensional time-of-flight sequence (54/9/1 [TR/TE/excitations]) at level of hypoglossal canal shows abnormal flow signal in left hypoglossal canal (arrow).
D, MR angiography source image from three-dimensional time-of-flight sequence (54/9/1) at level of junction of sigmoid sinus and jugular bulb demonstrates abnormal intraosseous flow (arrow) medial to jugular bulb.
E, Anteroposterior view of right common carotid artery following transvenous coil embolization confirms occlusion of fistula.
F, CT scan following transvenous embolization demonstrates portion of coil mass in hypoglossal canal (arrow).
G, CT scan after embolization shows coil mass to be partially intraosseous and located medial to jugular bulb.
Case 2
A 74-year-old woman presented with headache and severe pulse-synchronous bruit. Diagnostic angiography revealed a DAVF medial to the right jugular bulb (Fig 2A). Similar to case 1, the exact site of the fistula was demonstrated best on the contralateral carotid artery angiogram (Fig 2B). Venous drainage flowed antegrade into the jugular vein and retrograde into the sigmoid and transverse sinuses. External carotid intra-arterial embolization was performed initially with polyvinyl alcohol, and the right vertebral artery was occluded. This was followed by stereotactic radiation that resulted in only partial relief of symptoms. MR angiography source images revealed abnormal flow medial to the jugular bulb adjacent to the hypoglossal canal. A significant amount of abnormal flow signal appeared intraosseously (Fig 2C-D). After 1 year with persistent symptoms, the patient underwent transvenous embolization that was performed with pushable, fibered, and Guglielmi detachable coils, resulting in complete occlusion of the fistula. A triaxial catheter system used an 8F outer-guide catheter, a 4F inner-guide catheter, and a 3F infusion catheter. The patient has remained asymptomatic 2 years postembolization.
fig 2.
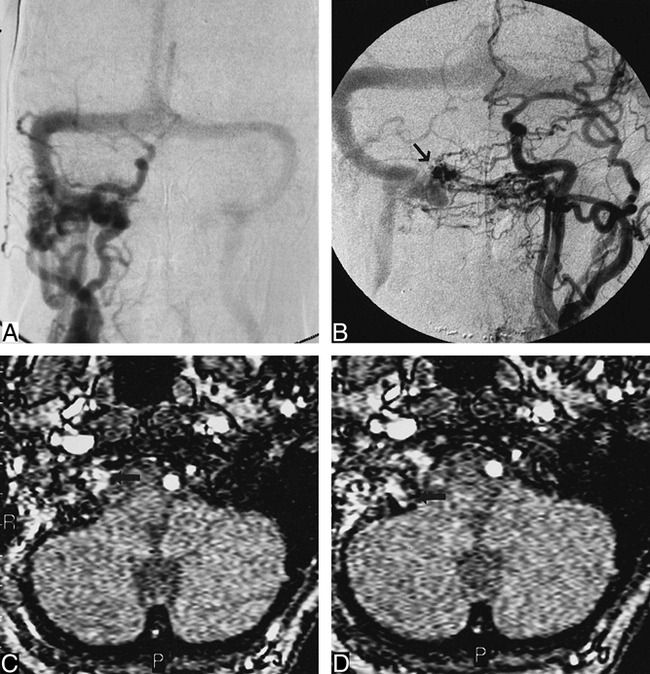
Case 2.
A, Anteroposterior view of right common carotid artery shows dural fistula in region of right jugular bulb. Note poor opacification of intracranial circulation owing to steal. Retrograde filling of the right transverse, sigmoid, straight, and superior sagital sinuses is visible.
B, Anteroposterior view of left common carotid artery demonstrates skull base DAVF located medial to jugular bulb. Fistula is located in superior aspect of dilated anterior condylar vein (arrow).
C, MR angiography source image from three-dimensional time-of-flight (42/9/1) sequence shows abnormal flow in hypoglossal canal (arrow) compatible with DAVF of anterior condylar vein.
D, MR angiography source image at level of jugular bulb shows abnormal intraosseous flow (arrow) medial to jugular bulb.
Case 3
A 57-year-old man presented with headache and mild pulse-synchronous bruit. MR angiography revealed a large DAVF medial to the right jugular bulb. MR angiography source images showed that flow signal was partially intraosseous and within the hypoglossal canal, similar in location to cases 1 and 2. On angiography, the fistula was supplied primarily by the ipsilateral ascending pharyngeal and occipital arteries. Venous drainage was antegrade to the jugular bulb and internal jugular vein. The exact fistula site was shown most accurately by contralateral external carotid artery injection. Treatment consisted of transvenous coil embolization and transarterial polyvinyl alcohol embolization of the ascending pharyngeal artery. Approximately 90% of the fistula was occluded. The patient's tinnitus and bruit resolved with embolization.
Discussion
The venous plexus within the hypoglossal canal is referred to as the anterior condylar vein. This venous plexus originates at the junction of the jugular bulb and inferior petrosal sinus and extends into the hypoglossal canal. The anterior condylar vein traverses the hypoglossal canal (anterior condylar canal) joining the inferomedial aspect of the inferior petrosal sinus to the suboccipital venous plexus, vertebral or paravertebral veins, marginal sinus, or the internal vertebral venous plexus. The hypoglossal canal contains the hypoglossal nerve, the neuromeningeal branch of the ascending pharyngeal artery, and the surrounding venous plexus (Fig 3). An emissary vein, the anterior condylar vein extends through the calvarium to join the intradural sinuses and the extracranial veins (3–6).
fig 3.
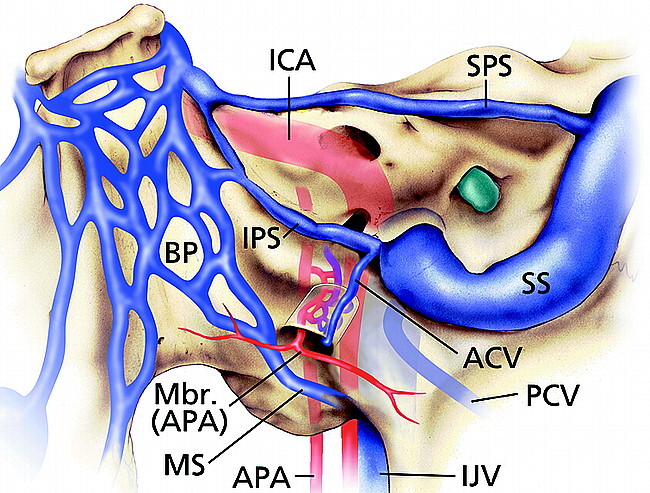
Illustration of normal veins and venous sinuses of skull base demonstrates anterior condylar vein originating at junction of inferior petrosal sinus and jugular bulb and then coursing through hypoglossal (anterior condylar) canal. ICA, internal carotid artery; APA, ascending pharyngeal artery; Mbr (APA), meningeal branch of the ascending pharyngeal artery; ACV, anterior condylar vein; PCV, posterior condylar vein; BP, basilar plexus; SPS, superior petrosal sinus; IPS, inferior petrosal sinus; MS, marginal sinus; SS, sigmoid sinus; IJV, internal jugular vein. (Printed with permission from Mayfield Clinic.)
The majority of DAVFs that occur in the posterior fossa involve the transverse or sigmoid sinuses. Several recent articles, however, have described less common fistulas occurring at the skull base in the region of the foramen magnum. Pierot et al (1) reported six cases of DAVFs involving the skull base located along the margin of the foramen magnum, four of which had venous drainage into a pial vein with retrograde flow. Four of these cases involved intracranial hemorrhage. Three of the patients in these cases were treated with surgery, and all were cured. One patient was treated partially with embolization; however, the patient had a subarachnoid hemorrhage 3 months later and died.
McDougall et al (2) described 14 DAVFs involving the marginal sinus (the sinus located between the layers of dura at the rim of the foramen magnum). The marginal sinus encircles the foramen magnum, linking the basal venous plexus of the clivus and the occipital sinus posteriorly. The marginal sinus drains to the sigmoid sinus or jugular bulb. These DAVFs were separate from the jugular bulb and sigmoid sinus, medial to these structures. Six of their 14 cases had unrestricted venous drainage. Three cases had restricted venous drainage. Three cases had retrograde pial venous drainage. All nine patients with venous access to the fistula were treated and cured via the transvenous route, with adjunctive transarterial embolization performed in seven patients. In the three patients with retrograde pial venous drainage and no access for transvenous embolization, transarterial liquid adhesives were used for definitive treatment, and all were cured. On angiography, these cases of skull base/marginal sinus DAVFs are indistinguishable from our three cases of fistulas of the anterior condylar vein. None of these previous reports emphasized the MR angiography findings and may have included cases similar to ours, related to the anterior condylar vein.
We present three cases of DAVFs localized to the anterior condylar vein. On angiography, these fistulas are medial to the confluens of the inferior petrosal sinus and jugular bulb and are similar in appearance to fistulas involving the marginal sinus on angiography. Their arterial supply is from all directions, giving an erroneous impression of a wide area of fistulous involvement, which is why the contralateral external carotid angiogram was most useful in identifying the location of the fistula on angiography. In case 1, venous drainage was via the jugular vein initially. After development of chemosis and proptosis, there was a dramatic change in venous drainage with retrograde flow in the inferior petrosal sinus and cavernous sinus to the superior ophthalmic vein as well as extensive pial drainage. Case 2 showed retrograde flow in the sigmoid and transverse sinuses. Case 3 demonstrated antegrade flow in the jugular bulb and posterior condylar vein. All cases showed antegrade flow in the jugular bulb and jugular vein. There was no direct venous flow to the extracranial suboccipital venous plexus or paravertebral venous system. We have identified two additional such fistulas by angiography and MR angiography that have yet to be treated and we suspect a number of other similar cases exist after review of prior arteriograms that were not documented by MR angiography. These unproved cases suggest the fistula of the anterior condylar vein may not be as rare as previously thought.
Because fistulas of the anterior condylar vein may be confused with marginal sinus DAVFs on angiography, source images from MR angiography are essential in localizing the fistula to the hypoglossal canal. MR angiography source images in case 1 demonstrated abnormal intraosseous flow signal medial to the jugular bulb and within the hypoglossal canal, in the location of the anterior condylar vein. The location of coil placement as documented by CT in case 1 confirms the significant intraosseous component and its relationship to the hypoglossal canal. MR angiography source images of cases 2 and 3 also showed similar abnormal flow signal localized to the hypoglossal canal and medial to the jugular bulb with a significant intraosseous component.
Intraosseous DAVFs have been described previously. Malik et al reported a surgical series of two patients with intraosseous DAVFs located at the foramen magnum. He hypothesized the intraosseous nature was related to the large number of emissary veins in this region. Our imaging observations confirm his operative experience that considerable hemorrhage could occur during bone removal, and if no DAVF is identified in the region of the dural sinuses, exploration of the adjacent bone should be performed until the entire fistula is exposed (7).
Classically, DAVFs are related directly to the venous sinuses. Nonetheless, extrasinusal DAVFs involving emissary veins have been reported. Piske and Lasjaunias reported three cases in an extrasinusal location: intraorbital; middle cranial fossa; and superior orbital fissure. They hypothesized that DAVFs could develop wherever veins had a transosseous course (emissary veins) (8).
A recent article by Blomquist et al described a case of a skull base DAVF presenting as a unilateral hypoglossal palsy. The DAVF was described as being located in the right paramedian skull base (9). Based on symptoms and location, we suspect this case is similar to our cases of DAVFs of the anterior condylar vein.
Initial treatment of cases 1 and 2 was performed with transarterial embolization of polyvinyl alcohol particles for palliative purposes, which partially relieved symptoms. Right vertebral occlusion and stereotactic radiosurgery in patient 2 had little effect. Definitive treatment was felt to be not necessary initially because the venous flow was antegrade in the jugular vein in case 1 and antegrade in the jugular vein and retrograde in the sigmoid sinus without retrograde cortical venous drainage in case 2.
Two years after initial embolization in case 1, the patient developed the sudden onset of chemosis and proptosis with subsequent diminished visual acuity. The change in venous drainage included pial drainage of the posterior fossa and retrograde flow via the inferior petrosal sinus to the cavernous sinus and then to the superior ophthalmic vein. The patient's symptoms and development of pial venous drainage required definitive therapy. Transvenous embolization of the fistula was performed with coils to complete occlusion. The patient remains asymptomatic.
One year after initial embolization in case 2, the patient's symptoms of headache and bruit slowly worsened. Transvenous embolization was performed with coils until complete occlusion of the fistula. She has remained asymptomatic for 2 years.
Transvenous embolization was performed in case 3 with nearly complete occlusion except for a small medial pocket. This was supplemented by transarterial embolization with polyvinyl alcohol. After embolization, there was slow flow in the remaining fistula. The patient's symptoms of headache and tinnitus resolved.
As in McDougall's series, the treatment of choice is transvenous coil embolization (2, 10). Three similar cases of transvenous embolization of the inferior petrosal sinus/anterior condylar vein complex in patients undergoing skull base surgery involving the jugular bulb were reported recently by Carrier et al (11).
The exact location of the fistula draining into the anterior condylar vein complex needs to be localized on angiography prior to transvenous embolization so that the coil mass can be directed specifically to this region. The fistula location was delineated best on the contralateral carotid arteriogram in all cases. Transvenous embolization of these DAVFs was directed at the dilated venous pouch that was medial and superior to the jugular bulb (Fig 2B). Microcatheter access was directed medially from the inferior aspect of the inferior petrosal sinus at the expected origin of the anterior condylar vein. No immediate complications were apparent. The patient in case 3 developed delayed lower extremity venous thrombosis corresponding to the same side that venous access was obtained.
Hypoglossal nerve palsy is a potential complication in the treatment of DAVFs related to the hypoglossal canal. Transvenous coil embolization could create mass effect on the hypoglossal nerve within the hypoglossal canal. Transarterial embolization of the ascending pharyngeal artery can cause ischemic injury to cranial nerves IX–XII (12). Because the anterior condylar vein is an emissary vein, and if the inferior petrosal sinus is not compromised, transvenous occlusion of the fistula should not cause localized venous hypertension or direct venous flow toward the brain. If transvenous embolization is incomplete, venous flow could be diverted to the marginal sinus or internal vertebral veins.
Alternative therapies include transarterial embolization with liquid adhesives and surgery. These therapies probably should be reserved for similar cases requiring treatment without transvenous access. The major risks of transarterial embolization of the ascending pharyngeal artery with liquid adhesives include cranial nerve IX-XII palsy and unrecognized communication with the vertebral artery. Surgical accessibility is an important consideration. The intraosseous location of the fistula, as demonstrated in our three cases, can make the fistula difficult to identify at surgery. The intraosseous location can result in significant blood loss during bone removal, and condylar resection may lead to craniocervical instability. MR angiography source images are essential for identifying the exact location of the fistula and may be helpful for determining which therapy (transarterial embolization vs surgery) should be attempted.
In summary, DAVFs of the anterior condylar vein are an uncommon skull base fistula, which can be confused with DAVFs of the marginal sinus on angiography. MR angiography source images can document the intraosseous extent and the relationship to the hypoglossal canal, which can have significant clinical implications. Transvenous coil embolization was curative in two of three cases and would seem to be the treatment of choice when venous access is available.
Footnotes
Address reprint requests to Robert Ernst, MD, ℅ Editorial Office, University of Cincinnati Department of Neurosurgery, 231 Bethesda Avenue, Cincinnati, OH 45267-0515.
References
- 1.Pierot L, Chiras J, Meder J, et al. Dural arteriovenous fistulas of the posterior fossa draining into subarachnoid veins. AJNR Am J Neuroradiol 1992;13:315-323 [PMC free article] [PubMed] [Google Scholar]
- 2.McDougall C, Halbach V, Dowd C, et al. Dural arteriovenous fistula of the marginal sinus. AJNR Am J Neuroradiol 1997;18:1565-1572 [PMC free article] [PubMed] [Google Scholar]
- 3.Theron J, Djindjian R, Cervicovertebral phlebography using catheterization. Radiology 1973;108:325-331 [DOI] [PubMed] [Google Scholar]
- 4.Okudera T, Huang Y, Ohta T, et al. Development of posterior fossa dural sinuses, emissary veins, and jugular bulb: morphological and radiologic study. AJNR Am J Neuroradiol 1994;15:1871-1883 [PMC free article] [PubMed] [Google Scholar]
- 5.Katsuta T, Rhoton A, Jr, Matsushima T, The jugular foramen: microsurgical anatomy and operative approaches. Neurosurgery 1997;41:149-202 [DOI] [PubMed] [Google Scholar]
- 6.Wen H, Rhoton A, Jr, Katsuta T, et al. Microsurgical anatomy of the transcondylar, supracondylar, and paracondylar extensions of the far-lateral approach. J Neurosurg 1997;87:555-585 [DOI] [PubMed] [Google Scholar]
- 7.Malik G, Mahmood A, Mehta B, Dural arteriovenous malformation of the skull base with intraosseous vascular nidus. J Neurosurg 1994;81:620-623 [DOI] [PubMed] [Google Scholar]
- 8.Piske R, Lasjaunias P, Extrasinusal dural arteriovenous malformations. Neuroradiology 1988;30:426-432 [DOI] [PubMed] [Google Scholar]
- 9.Blomquist M, Barr J, Hurst R, Isolated unilateral hypoglossal neuropathy caused by dural arteriovenous fistula. AJNR Am J Neuroradiol 1998;19:951-953 [PMC free article] [PubMed] [Google Scholar]
- 10.Halbach V, Higashida R, Heishima G, et al. Transvenous embolization of dural fistulas involving the transverse and sigmoid sinuses. AJNR Am J Neuroradiol 1989;10:385-392 [PMC free article] [PubMed] [Google Scholar]
- 11.Carrier D, Arriaga M, Gorum M, et al. Preoperative embolization of anastamoses of the jugular bulb: an adjuvant in jugular foramen surgery. AJNR Am J Neuroradiol 1997;18:1252-1256 [PMC free article] [PubMed] [Google Scholar]
- 12.Lasjaunias P, edCraniofacial and Upper Cervical Arteries. Baltimore/London: Williams & Wilkins, 1981: 105-118


