Abstract
BACKGROUND AND PURPOSE: Although the central processing of somatic pain has been dealt with in numerous brain imaging studies, the neural correlates of visceral pain have received much more limited attention. Our goal was to assess the feasibility of detecting brain activation patterns induced by rectal pain by means of functional MR imaging. We hypothesized that the cerebral processing of rectal pain would exhibit strong similarities with the central processing of somatic pain.
METHODS: Functional MR imaging data were obtained from eight healthy subjects. A block paradigm was applied. Rectal pain was induced by inflating a latex balloon catheter that had been inserted into the rectum. Functional responses were established by means of cross-correlation analysis.
RESULTS: Activation was detected within the anterior cingulate gyrus, the prefrontal cortex, the insular cortex, the sensory-motor cortex, the inferior parietal lobule, the posterior cingulate gyrus, and the visual cortex.
CONCLUSION: Functional MR imaging of visceral pain is feasible in healthy subjects. The activation patterns observed in this study support the hypothesis that the cerebral processing of visceral pain involves multiple components, similar to the central processing of somatic pain. Our results constitute a first step toward the identification of possible aberrations in the activation patterns of patients suffering from visceral hypersensitivity.
The central processing of somatic pain in humans has been dealt with in numerous studies using positron-emission tomography (PET) (1–7) and, more recently, functional MR imaging (8–10). Multiple components seem to be generally involved in somatic pain processing, including sensory-discriminative components (processed within the primary sensory and insular cortices), affective components (processed within the anterior cingulate gyrus), and cognitive components (processed within the prefrontal cortex).
The central processing of visceral pain has received much more limited attention. To our knowledge, only a few PET studies of visceral pain have been reported (11–14). The regional cerebral activity was explored in normal and abnormal perception of visceral pain induced by rectal pressure (13), during processing of painful and nonpainful esophageal sensations (14), and in response to myocardial ischemia (11, 12).
Functional MR imaging has by now become the method of choice for functional brain mapping. While it shares with PET an intrinsic sensitivity to regional cerebral blood flow increases, it detects these changes by using deoxyhemoglobin as an endogenous paramagnetic tracer (15) rather than H215O as an exogenous radioactive tracer. Furthermore, functional MR imaging presents much better spatial and temporal resolutions, and the matching of functional and anatomic MR imaging data is straightforward.
In this study in healthy subjects, we assessed the feasibility of mapping the neural correlates of rectal pain by means of functional MR imaging. Given the extensive viscerosomatic convergence occurring at the spinal level (14), we hypothesized that the cerebral processing of rectal pain would exhibit strong similarities with the central processing of somatic pain.
Methods
Subjects
Eight healthy volunteers (three women and five men; average age, 29 years) were examined. All subjects were right-handed, as assessed by means of the Edinburgh inventory test (16). None of the subjects had a history of chronic pain or of gastrointestinal symptoms, and all had normal findings at physical examination. Informed consent was obtained in all cases, and the experimental protocol was approved by the local ethics committee.
Paradigm
A block paradigm was applied that was composed of three periods, each comprising a “control” followed by a “stimulation” epoch. During the latter epochs, visceral pain was induced by inflation of a latex balloon catheter that had been inserted into the rectum. This balloon was inflated until a volume inducing a discomfort level of about 80, as measured on a visual analog scale in which 100 was the maximum tolerable volume (MTV), was reached. The MTV had been determined during a separate session, a few days before the functional MR imaging examination. During the control epochs, the balloon was simply deflated. Stimulation and control epochs each lasted 1 minute. Subjects kept their eyes closed during the functional scan, and they wore earplugs.
MR Acquisition
Subjects were examined at 1.5 T on a clinical MR unit (Philips ACS II). The body coil was used for excitation, and the regular quadrature bird cage head coil was used for detection. The volume of interest was composed of seven adjacent, axial sections, each 7-mm thick. This volume was oriented parallel to the bicommissural plane and centered on the commissures. Positioning of the volume was performed on scout images acquired in the sagittal plane. The volume was measured five times during each epoch (total measurement time, about 10 minutes). Between epochs, MR acquisition was interrupted to allow for balloon inflation and deflation. The functional images were obtained using a conventional gradient-recalled echo (GRE) MR sequence. The major parameters of this sequence were as follows: TR/TE, 64/40; acquisition matrix, 45 × 64; field of view, 154 × 220 mm2; digital pixel size, 3.4 × 3.4 × 7 mm3; flip angle, 30°; bandwidth per pixel, 14 Hz; acquisition time per image, 2.9 seconds per slice; reconstruction matrix, 128 × 128. Subsequent to the functional MR imaging, a high-resolution 3D phase-contrast (PC) (17) MR sequence was applied. The brain volume covered by this image was identical to the volume of interest covered by the functional images. The PC MR sequence provided anatomic (T1-weighted) as well as vascular information about the volume of interest.
Data Processing
MR images were analyzed off line by means of a software package developed under the IDL (Research Systems Inc, Boulder, CO) programming language. Within each epoch, the first measurement of the volume was discarded to prevent sensitivity to an initial drift of the system parameters. Following in-plane motion correction of the successively measured volumes (18) and pixelwise baseline correction (by removing the lowest Fourier components of the temporal series), functional responses were detected by means of cross-correlation analysis (19). A three-step approach was followed. In the first step, pixels were identified that presented a cross-correlation coefficient with the paradigm (modeled as a boxcar) above a particular positive threshold. The latter was adjusted so that a vast majority of the individual pixels retained corresponded to gray matter tissue. In practice, these adjustments led to cross-correlation thresholds varying slightly among subjects, around 0.40. In the second step, isolated pixels were rejected by retaining only those pixels that were members of clusters containing a minimum number of adjacent activated pixels (typically four). In the third step, the cross-correlation coefficient of the average signal intensity within each cluster thus obtained was determined. Only those clusters for which the value of the average cross-correlation coefficient was beyond a second threshold (typically 0.55) were ultimately retained. Functional 2D maps were obtained by superimposing the activated pixel clusters onto the anatomic images derived from the 3D PC MR imaging. A vascular mask was applied to reject possible macrovascular responses (20). This mask was equally derived from the 3D PC MR sequence. The functional areas were identified in each subject by comparing the sulci and gyri within the anatomic images with those of the corresponding sections of the Talairach atlas (21). Hemispheric dominance of the functional responses within cortical areas was assessed by means of analysis of variance.
For the sake of presentation, the functional 2D maps (corresponding to individual sections) were also combined into a single functional projection map. This projection map was obtained by superimposing the maximum-intensity projections of the activation clusters onto averaged intensity projections of the corresponding anatomic images. The projections were applied perpendicular to the individual image planes (projection maps thus were also oriented transversely). Following radiologic practice, the left and right hemispheres are presented on the right and left, respectively.
Results
Despite the in-plane motion correction applied, the data sets from two of the eight subjects were unusable, owing to excessive motion artifacts. Those artifacts resulted presumably from excessive out-of-plane (interimage) motion by the subjects and/or from excessive (intraimage) motion while acquiring the k-space data from particular sections.
Typical functional maps of rectal pain obtained from one of the subjects are shown in Figure 1. The maps located to the left of the color scale display the functional responses obtained within four adjacent individual sections, 7-mm thick each. The map located to the right of the color scale is the functional projection map corresponding to these four sections (the thickness of the projected volume is 28 mm). The functional responses within the insulae, within the anterior cingulate gyrus, and within the prefrontal cortex are delineated in red, dark blue, and light blue, respectively. The color scale represents the cross-correlation between the temporal evolution of the pixels and a reference waveform representing the block paradigm applied. The data processing parameters applied were the following: cross-correlation threshold on individual pixels of 0.4, minimum cluster size of four pixels, and cross-correlation threshold on the averaged cluster intensities of 0.55.
fig 1.
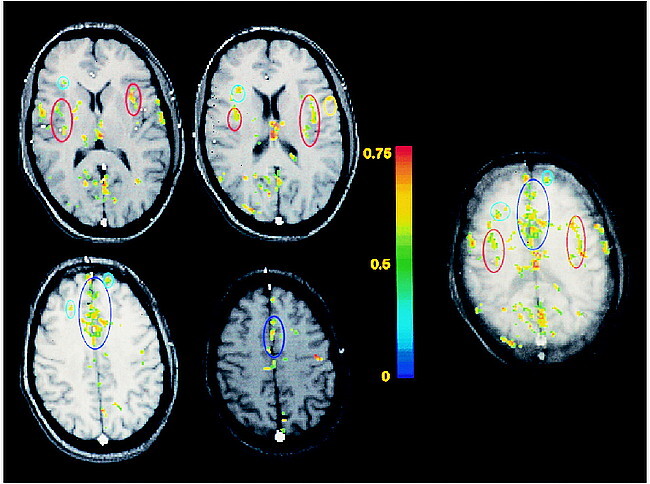
Typical functional maps of rectal pain. The maps located to the left of the color scale display the functional responses obtained within four adjacent individual sections, each 7-mm thick. The map located to the right of the color scale is the functional projection map corresponding to these four sections (thickness of the projected volume is 28 mm). The functional responses within the insulae, anterior cingulate gyrus, and prefrontal cortex are delineated in red, dark blue, and light blue, respectively. The color scale represents the cross-correlation between the temporal evolution of the pixels and a reference waveform representing the block paradigm applied.
Typical functional responses obtained for rectal pain within the anterior cingulate gyrus and within the left and right insulae are shown in Figure 2. The responses displayed here pertain to the subject considered in Figure 1.
fig 2.
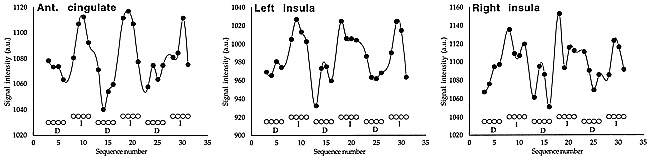
Typical functional responses obtained for rectal pain within the anterior cingulate gyrus and within the left and right insulae. The responses displayed pertain to the subject considered in figure 1. Open circles represent the paradigm applied (D indicates deflation; I, inflation of the rectal balloon).
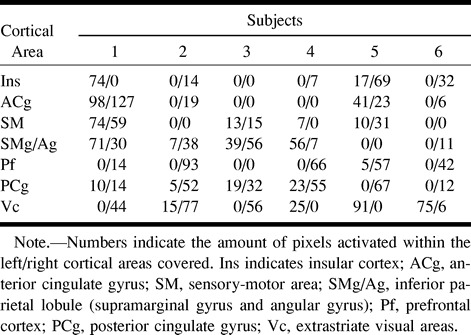
The Table summarizes the activation patterns from the six subjects whose data were retained. Overall, functional responses to rectal pain were detected in the insula (five subjects), the anterior cingulate gyrus (Brodmann areas 24 and 32; four subjects), the inferior parietal lobule (angular gyrus and supramarginal gyrus, Brodmann areas 39 and 40; five subjects), the primary somatosensory and motor cortices (Brodmann areas 3, 2, 1, and 4; four subjects), the dorsolateral prefrontal cortex (Brodmann areas 9, 10, and 46; five subjects), the posterior cingulate gyrus (Brodmann areas 30 and 31; six subjects), and the extrastriate visual cortex (Brodmann areas 18 and 19; six subjects). Analysis of variance on the number of pixels activated in these seven areas, with hemisphere and area as factors, revealed an overall predominance of responses within the right hemisphere, F(1,5) = 7.69; P < .04. Contrast analyses on the hemisphere by area interaction revealed significant right-hemispheric predominance within the prefrontal cortex, F(1,30) = 8.00; P < .009, and within the insular cortex, F(1,30) = 6.05; P < .02.
Discussion
The activation patterns obtained in response to rectal pain appear to be strongly distributed, similar to what has generally been observed in brain-mapping studies of somatic pain in healthy subjects. Furthermore, as with somatic pain, responses are detected within the anterior cingulate gyrus, the prefrontal cortex, and the primary sensory and insular cortices.
The anterior cingulate gyrus forms part of the medial pain system, together with the medial group of thalamic nuclei (22). In the present study, the anterior cingulate gyrus was activated in four of the six subjects. Activation of the anterior cingulate gyrus is generally considered to be related to the affective aspects of pain, such as perceived unpleasantness. Convincing direct experimental evidence supporting this hypothesis in the case of somatic pain has recently been provided by a PET study (3) in which hypnotic suggestions were used to alter selectively the unpleasantness of noxious (heat) stimuli, without affecting the perceived intensity. In that study, activity in the anterior cingulate cortex was found to correlate with the perceived unpleasantness of the painful stimuli, while activity in other pain-related cortical areas did not. In a functional MR imaging study (8), activation of the anterior cingulate gyrus was obtained for moderate or intense pain induced by painful electrical nerve stimulation, and was undetected for mild pain. Furthermore, in a PET study on visceral pain (13), the anterior cingulate was found activated even when a painful stimulus was simply simulated.
In the present study, the level of inflation of the balloon during noxious stimulation was determined by the subjects themselves. Therefore, it may not be excluded that the lack of activation observed within the anterior cingulate gyrus in two of the six subjects reflected subnoxious inflation of the balloon. The strong responses in the prefrontal cortex most likely reflected cognitive processing of the affective aspects of pain. Similarly, the activation detected within the extrastriate visual cortices may have been induced by visual imagery (23) during the painful stimulation epochs.
The primary sensory and insular cortices form part of the lateral pain system, together with the lateral group of thalamic nuclei (22). Those areas are generally considered to be primarily involved in the sensory-discriminative aspects and spatial location of the painful stimuli. In four of the five subjects, the activation within the insular area exhibits right hemispheric predominance. This predominance is consistent with observations made in a recent PET study on esophageal sensation (14), and has been interpreted in terms of the involvement of the right hemisphere in emotional arousal. The lack of activation observed in two of the six subjects in the primary sensory-motor area most likely indicates that the sensory-discriminative and spatial location aspects of the rectal stimulation were similar during both the control and painful stimulation epochs.
In the absence of equipment allowing us to apply instantaneous MR acquisition techniques, such as echo-planar imaging (EPI), we used conventional GRE MR sequences for the functional MR images. Those sequences are known to be suboptimal for functional MR imaging examinations, owing to their high sensitivity to subject motion and to physiologic fluctuations. It may therefore be anticipated that the use of a state-of-the art MR imager (ie, equipped with EPI-like techniques and allowing a large number of images to be acquired within a single scan) will significantly improve the robustness of the functional responses induced by visceral pain.
Demonstrating the feasibility of functional MR imaging mapping of rectal pain and assessing the activation patterns in healthy subjects constitutes our first step toward identifying possible aberrations in the activation patterns of patients with irritable bowel syndrome (IBS). Several observations have suggested that the perception of visceral pain is altered in patients with IBS (24, 25). It has been shown, in particular, that the level of visceral stimulation needed to induce pain appears significantly lower in IBS patients than in healthy subjects (24). It is presently unclear to what extent this visceral hypersensitivity reflects increased sensitivity of the primary afferent neurons and/or impaired processing of the afferent signals within the spinal tract or within the thalamic or cortical projections. Numerous arguments, including the observation that psychiatric disorders (26), stress, and anxiety (27) also affect visceral hypersensitivity, and the observation of aberrant activation patterns in a recent PET study with IBS patients (13), strongly suggest a modulating or an etiologic role of the CNS in the physiopathology of functional digestive disorders. Therefore, functional brain mapping of patients with visceral hypersensitivity is of considerable interest.
Conclusion
This functional MR imaging study of healthy subjects focused on the cerebral representation of visceral pain. Overall, the activation patterns observed support the hypothesis that cerebral processing of visceral pain in healthy humans exhibits strong similarities to the central processing of somatic pain.
Cortical areas involved in rectal pain
Footnotes
Supported by the European Union (BIOMED 2 PL 950870).
Presented at the Fourth International Conference on Functional Mapping of the Human Brain, Montreal, 1998.
Address reprint requests to Christoph Segebarth, PhD, INSERM U438, Centre Hospitalier Universitaire, Pavillon B, BP 217, 38043 Grenoble Cedex 9, France.
References
- 1.Jones AKP, Friston KJ, Frackowiak RSJ, Cerebral localisation of responses to pain in man using positron emission tomography. Science 1992;255:215-216 [DOI] [PubMed] [Google Scholar]
- 2.Talbot JD, Marrett S, Evans AC, Meyer E, Bushnell MC, Duncan GH, Multiple representations of pain in human cerebral cortex. Science 1991;251:1355-1358 [DOI] [PubMed] [Google Scholar]
- 3.Rainville P, Duncan GH, Price DD, Carrier B, Bushnell C, Pain affect encoded in human anterior cingulate but not somatosensory cortex. . Science 1997;277:968-971 [DOI] [PubMed] [Google Scholar]
- 4.Casey KL, Minoshima S, Morrow TJ, Koeppe RA, Comparison of human cerebral activation patterns during cutaneous warmth, heatpain and deep cold pain. . J Neurophysiol 1996;76:571-581 [DOI] [PubMed] [Google Scholar]
- 5.Vogt BA, Derbyshire S, Jones AKP, Pain processing in four regions of human cingulate cortex localized with co-registered PET and MR imaging. . Eur J Neurosci 1996;8:1461-1473 [DOI] [PubMed] [Google Scholar]
- 6.Craig AD, Reiman EM, Evans A, Bushnell MC, Functional imaging of an illusion of pain. . Nature 1996;384:258-260 [DOI] [PubMed] [Google Scholar]
- 7.Coghill RC, Talbot JD, Evans AC, et al. Distributed processing of pain and vibration by the human brain. . J Neurosci 1994;14:4095-4108 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Davis KD, Wood ML, Crawley AP, Mikulis DJ, fMRI of human somatosensory and cingulate cortex during painful electrical nerve stimulation. . Neuroreport 1995;7:321-325 [PubMed] [Google Scholar]
- 9.Oshiro Y, Fuijita N, Tanake H, Hirabuki N, Nakamura H, Yoshiya I, Functional mapping of pain-related activation with echo-planar MRI: significance of the SII-insular region. . Neuroreport 1998;9:2285-2289 [DOI] [PubMed] [Google Scholar]
- 10.Davis KD, Kwan CL, Crawley AP, Mikulis DJ, Event-related fMRI of pain: entering a new era in imaging pain. . Neuroreport 1998;9:3019-3023 [DOI] [PubMed] [Google Scholar]
- 11.Rosen SD, Paulesu E, Frith CD, Frackowiak RSJ, Davies GJ, Jones T, Central nervous pathways mediating angina pectoris. . Lancet 1994;334:147-150 [DOI] [PubMed] [Google Scholar]
- 12.Rosen SD, Paulesu E, Nihoyannopoulos P, et al. Silent ischemia as a central problem: regional brain activation compared in silent and painful myocardial ischemia. . Ann Intern Med 1996;124:939-949 [DOI] [PubMed] [Google Scholar]
- 13.Silverman DHS, Munakata JA, Ennes H, Mandelkern MA, Hoh CK, Mayer EA, Regional cerebral activity in normal and pathological perception of visceral pain. . Gastroenterology 1997;112:64-72 [DOI] [PubMed] [Google Scholar]
- 14.Aziz Q, Andersson JLR, Valind S, et al. Identification of human brain loci processing esophageal sensation using positron emission tomography. . Gastroenterology 1997;113:50-59 [DOI] [PubMed] [Google Scholar]
- 15.Ogawa S, Tank DW, Menon R, et al. Intrinsic signal changes accompanying sensory stimulation: functional brain mapping with magnetic resonance imaging. . Proc Natl Acad Sci U S A 1992;89:5951-5955 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Oldfield RC, The assessment and analysis of handedness: the Edinburgh inventory. . Neuropsychologia 1971;9:97-113 [DOI] [PubMed] [Google Scholar]
- 17.Dumoulin CL, Souza SP, Walker MF, Wagle W, Three dimensional phase contrast angiography. . Magn Reson Med 1989;9:139-149 [DOI] [PubMed] [Google Scholar]
- 18.Maas LC, Frederick BDB, Renshaw PF, Decoupled automated rotational and translational registration for functional MRI time series data: the DART registration algorithm. . Magn Reson Med 1997;37:131-139 [DOI] [PubMed] [Google Scholar]
- 19.Bandettini PA, Jesmanowicz A, Wong EC, Hyde JS, Processing strategies for time-course data sets in functional MRI of the human brain. . Magn Reson Med 1993;30:161-173 [DOI] [PubMed] [Google Scholar]
- 20.Segebarth C, Belle V, Delon C, et al. Functional MRI of the human brain: predominance of signals from extracerebral veins. . Neuroreport 1994;5:813-816 [DOI] [PubMed] [Google Scholar]
- 21.Talairach J, Tournoux P, Co-planar Stereotaxic Atlas of the Human Brain.. New York: Thieme; 1988;
- 22.Vogt BA, Sikes RW, Vogt LJ, Anterior cingulate cortex and the medial pain system. In: Vogt B, Gabriel M, eds.Neurobiology of Cingulate Cortex and Limbic Thalamus: A Comprehensive Handbook. Boston: Birkhauser; 1993;313-344
- 23.Kosslyn SM, Thompson WL, Alpert NM, Neural systems shared by visual imagery and visual perception: a positron emission tomography study. . Neuroimage 1997;6:320-334 [DOI] [PubMed] [Google Scholar]
- 24.Mayer EA, Gebhardt GF, Basic and clinical aspects of visceral hyperalgesia. . Gastroenterology 1994;107:271-293 [DOI] [PubMed] [Google Scholar]
- 25.Cook IJ, Van Eeden A, Collins SM, Patients with irritable bowel syndrome have greater pain tolerance than normal subjects. . Gastroenterology 1987;93:727-733 [DOI] [PubMed] [Google Scholar]
- 26.Barsky AJ, Investigating the psychological aspects of irritable bowel syndrome. Gastroenterology 1987;93:902-904 [DOI] [PubMed] [Google Scholar]
- 27.Enck P, Wienbeck M, Epidemiology and psychological factors of the irritable bowel syndrome. Eur J Gastroenterol Hepatol 1993;5:979-989 [Google Scholar]


