Abstract
BACKGROUND AND PURPOSE: Ion implantation is a surface-modification technology that creates a borderless surface on protein-coated platinum; this change in physical and chemical properties on the surface of Guglielmi detachable coils (GDCs) appears to enhance cell proliferation and adhesion. Our purpose was to evaluate the effect of ion implantation on GDCs in an experimental aneurysm model.
METHODS: GDCs were coated with either type I collagen, fibronectin, vitronectin, laminin, or fibrinogen. Using He+ or Ne+ 1 × 1014–15 ions/cm2, ion implantation was performed on these protein-coated GDCs (GDC-Is). A total of 56 experimental aneurysms were constructed microsurgically in the common carotid arteries of 28 swine. These experimental aneurysms were embolized with standard GDCs (n = 23), collagen GDC-Is (n = 11), vitronectin GDC-Is (n = 6), laminin GDC-Is (n = 4), fibrinogen GDC-Is (n = 6), and fibronectin GDC-Is (n = 6). The animals were sacrificed at day 14 after coil embolization. The physical properties of the new coils (friction on delivery, deployment into aneurysms, trackability, etc) and the development of tissue scarring and neoendothelium across the aneurysm's orifice were evaluated macroscopically and microscopically.
RESULTS: No evidence of increased coil friction/stiffness was observed during delivery of GDC-Is through microcatheters in this aneurysm model. A more intense scar formation and neoendothelium at the neck of aneurysms were observed macroscopically when treated with GDC-Is. Significant differences in the proportion of neck coverage between standard GDCs (48.3% ± 20.5%) and all GDC-I groups were observed (collagen GDC-I—89.4% ± 14.9%, P < .01; vitronectin GDC-I—71.5% ± 7.0%, P < .05; laminin GDC-I—76.5% ± 11.0%, P < .05; fibrinogen GDC-I—74.8% ± 13.9%, P < .05; fibronectin GDC-I—87.5% ± 15.0%, P < .01). Light microscopy showed a well-organized fibrous tissue bridging the aneurysm's neck when using GDC-Is, whereas only a fibrin-like thin layer covered the standard GDC surfaces.
CONCLUSION: GDC-Is indicated a more intense inflammatory response in the aneurysm body and dome and faster re-endothelial coverage of the neck of the aneurysm. This accelerated histologic response may decrease the chances of coil compaction and aneurysm recanalization. This technology may improve anatomic and clinical outcomes in patients harboring intracranial aneurysms.
The clinical use of Guglielmi detachable coils (GDCs) has been accepted as a valuable alternative to the surgical treatment of cerebral aneurysms (1–5). Recent clinical experience reported in the literature indicates that complete occlusion can be achieved in more than 80% of small-sized/small-necked aneurysms.
A small neck remnant, however, is seen frequently in wide-necked, large, or giant aneurysms. The natural history of such neck remnants is not well documented. A limited number of long-term angiographic studies has shown that one third of treated aneurysms with small neck remnants developed coil compaction and recanalization. Another third of these aneurysms demonstrated further thrombosis of the neck remnant, and the remaining one third remained anatomically unchanged (4).
GDC coils are biologically inert. They produce a limited cellular response in the aneurysm clot and delayed development of neoendothelial coverage across the neck of the aneurysm. Neoendothelium is histologic evidence that the aneurysm has been totally isolated from the circulation. The relatively slow development of neoendothelial coverage across the neck of the aneurysm allows coil compaction with concomitant aneurysm recanalization and regrowth. Therefore, the development of a more biologically active GDC can improve present anatomic and clinical outcomes by accelerating scar formation and neoendotheilal coverage across the neck of the aneurysm.
Several modifications of the GDC surface have been reported, including the use of extracellular matrix (ECM) proteins, polymer coating, or the addition of cytokines such as growth factors (6–11). These types of GDC modifications have been shown to improve biological activity in vitro and in limited in vivo evaluation in the animal laboratory. They have not been used yet in clinical practice.
A limited number of investigators have studied the surface interaction between blood cells and embolic materials (8, 9, 12–15). Because GDC coils are exposed to high blood flow shear stress at the neck of an aneurysm, strong cell-coil surface adhesion is necessary to achieve strong and accelerated re-endothelialization across the aneurysm's neck. This strong cellular adhesion would limit untoward thromboembolic complications from emboli arising from the coil surface exposed to fibrin and platelets.
Ion implantation is a surface-modification technology that creates a borderless surface on protein-coated platinum (13). This change in physical and chemical properties on the surface of the GDC coils appears to enhance cell proliferation and adhesion. This technology can be used in association with several protein coatings on the surface of GDCs.
We studied this methodology in the animal laboratory, analyzing its impact on cellular proliferation and adhesion as mechanisms of wound healing at the neck of aneurysms. We compared results with standard and ion-implanted GDC coils placed under similar biological conditions.
Methods
Ion Implantation
Ion implantation is a physico-chemical surface-modification process resulting from the impingement of a high-energy ion beam (16). When ion implantation is performed on a protein-coated GDC surface, the coated protein is embedded and mixed into the GDC surface (protein-coated GDC-I). The major difference between conventional coating and ion-implantation technology is in the quality of the interfaces. Any standard coating process has an interface (or “border”) between coating materials (such as proteins) and the base materials (such as platinum). Regular GDC coating may be stripped away by mechanical stress while depositing the coil through long, small microcatheters. Ion implantation creates a very thin film of protein adherent that is embedded into the coil surface, producing neither significant changes in the diameter of the coil nor increased coil rigidity (13).
Animal Preparation
All animal experiments were conducted in accordance with policies set by the UCLA Chancellor's Animal Research Committee and National Institutes of Health guidelines. Twenty-eight Red Duroc swine were used in this study. The animals were 3 to 4 months old, weighed 30 to 40 kg, were of either sex, and were maintained on a standard laboratory diet. After an overnight fast, each swine was premedicated with intramuscular 20 mg/kg ketamine and 2 mg/kg xylazine. General anesthesia was maintained with mechanical ventilation and inhalation of 0.5% to 1.5% halothane after endotracheal intubation.
Coil Preparation (Fig 1)
GDC coils of sizes 8 mm × 40 cm (coil diameter × length), 8 mm × 20 cm, 6 mm × 20 cm, 5 mm × 15 cm and GDC-18 (ie, 0.038-cm) thickness and size 4 mm × 10 cm and GDC-10 (ie, 0.025-cm) thickness were coated with fibronectin (human plasma fibronectin, 0.5 mg/mL; Koken, Tokyo, Japan), type I collagen (bovine dermis collagen, 0.3%; Koken, Tokyo, Japan), vitronectin (bovine plasma vitronectin, 0.1 mg/mL; Koken, Tokyo, Japan), laminin (mouse laminin, 1.0 mg/mL; Koken, Tokyo, Japan), and fibrinogen (human fibrinogen, 20 mg/mL; Midorijuji, Osaka, Japan). These coils underwent Ne+ or He+ (collagen) ion implantation at a dose of 1 × 1014 or 1 × 1015 ions/cm2 at an energy of 150 keV.
Aneurysm Construction
Fifty-six experimental lateral-wall aneurysms were constructed microsurgically in both common carotid arteries of 28 swine. The details of aneurysm construction have been reported previously (13). The aneurysm sacs (8–10 mm) and necks (7 mm) were created of equal size in all vessels (Fig 2A–B).
fig 2.
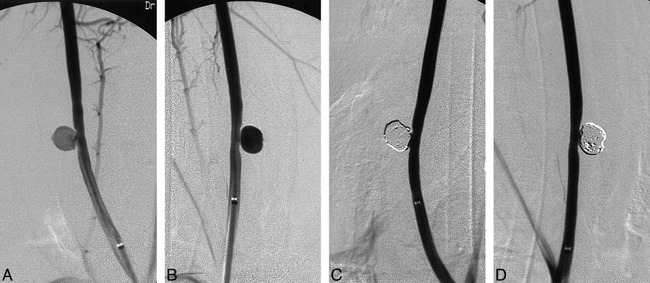
Angiograms of experimental aneurysms created on right and left common carotid arteries (A and B) and occluded aneurysms after standard GDC treatment (C, left) and protein coated GDC-I (D, right). Note tight packing of both aneurysms.
Aneurysm Embolization
Endovascular treatments of aneurysms were performed immediately after their construction. The goal of this study was to compare the wound-healing process (quickness and intensity of scar formation and neoendothelial coverage in the body and neck of the aneurysm) between standard GDC coils and protein-coated GDC coils treated with the ion-implantation technique (GDC-I). It was assumed that spontaneous aneurysm growth/thrombosis would be similar in aneurysms treated with standard GDCs or GDC-Is, as all aneurysms had similar morphology and density of coil deployment.
A total of 56 experimental aneurysms were embolized with either standard GDCs (n = 23), collagen GDC-Is (n = 11), vitronectin GDC-Is (n = 6), laminin GDC-Is (n = 4), fibrinogen GDC-Is (n = 6), and fibronectin GDC-Is (n = 6).
A 6F sheath was placed in the right femoral artery following standard Seldinger puncture and catheterization. Selective common carotid arteriograms were performed using a 6F Fastguide catheter (Target Therapeutics, Freemont, CA), and the aneurysms were shown in multiple projections. An intravenous bolus of 3000 U of heparin was injected to prevent thromboembolic complications. A Tracker-18 microcatheter and Seeker-14 microguidewire combination (Target Therapeutics) was advanced through the guiding catheter and the tip of the microcatheter was positioned in the center of the sac of the aneurysm.
All aneurysms were tightly and densely packed with coils. (Fig 2C–D). This technique eliminated potential hemodynamic differences at the neck of the aneurysm that could have affected the proliferation, migration, and adhesion of endothelial cells in that area. The physical performances of the conventional protein-coated GDC-Is were evaluated during the endovascular procedures (trackability, pushability, smoothness, etc).
A 14-day follow-up angiogram was performed in all cases and the animals then were euthanized using standard approved procedures. This time sequence was based on results of a previously reported study (13).
Particular attention was paid to the extent and density of a fibrous membrane covering the neck of the aneurysm. The development of this membrane was considered as definitive histologic proof of aneurysm isolation from the blood circulation. After resection, the parent arteries were cut along the longitudinal axis, and the necks of the aneurysms were analyzed macroscopically. The largest dimension of the orifice (OF) and the white fibrous membrane (FM) that covered the orifice was the FM to OF proportion (and recorded as the FM/OF × 100%). The details of this measurement were described previously (13). Statistical analysis of the FM to OF proportion was performed using one-way analysis of variance (ANOVA) and the Tukey multiple comparison test. Results were considered significant at P < .05 and reported as the mean ± SD. The harvested aneurysms were fixed with 2% formaldehyde and embedded in plastic resin or fixed with 2% glutaraldehyde. Histologic sections (20 μm to 30 μm thickness) through the midline of the aneurysmal orifice (between each stay suture) were made using a diamond knife. Most of the histologic specimens were stained with hematoxylin and eosin. Some specimens were not usable for light microscopic evaluation because of technical failure of cutting and staining. Only good specimens were used for histopathologic evaluation. Four specimens (one laminin GDC-I, one collagen GDC-I, two standard GDCs) were immersed immediately into saline and then fixed with 2.0% glutaraldehyde. These specimens then were dehydrated in graded ethanol, coated with gold, and observed at 200 to 2000 magnifications in a scanning electron microscope (SEM) at an accelerating voltage of 15 kV.
Results
Angiographic Findings
Fourteen days after embolization, angiographic follow-up in both standard GDC and GDC-I groups demonstrated total occlusion of the aneurysms. None of the aneurysms showed angiographic evidence of coil compaction or recanalization.
Macroscopic Findings
Macroscopic examinations of the aneurysms showed significant differences between the standard GDC and the GDC-I groups. Embolized aneurysms with standard GDC coils demonstrated a larger exposure of the GDC coils to arterial flow (average neck coverage, 48.3% ± 20.5%). The surface of the standard GDCs was covered with a thin fibrin-like material (Fig 3A).
fig 3.
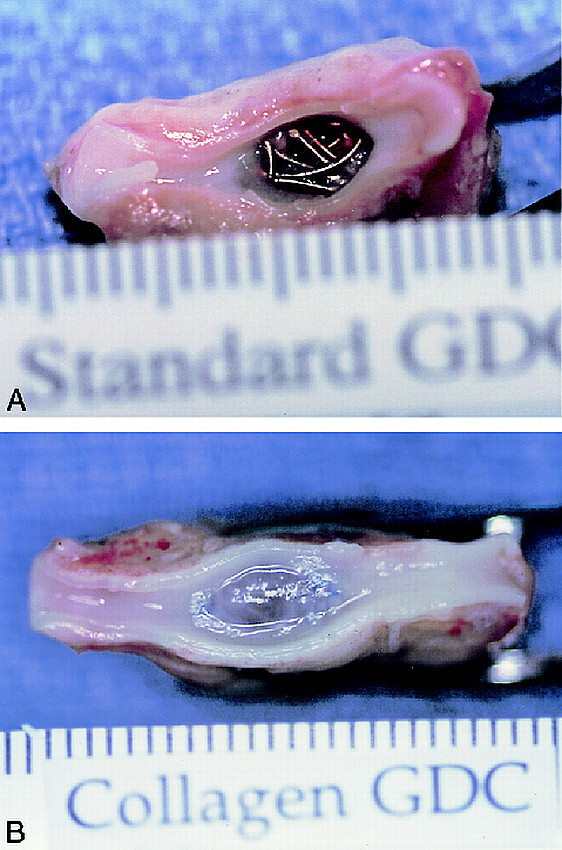
Macroscopic appearances of an aneurysmal orifice 14 days after treatment, with standard GDC (A) and collagen GDC-I (B). Surfaces of standard GDCs were covered with fibrin-like materials. On the other hand, the orifice of collagen GDC-I treated aneurysm was covered completely with thick fibrous tissue.
A denser and thicker fibrous tissue response was observed at the neck of aneurysms embolized with GDC-I (Fig 3B). The predominantly fibrous scarring was covered with neoendothelium arising from the edges of the neck of the aneurysm. One-way ANOVA (P < .001) and the Tukey multiple comparison test showed statistically significant differences in the proportion of neck coverage (FM /OF proportion) between standard GDCs and all GDC-I groups: collagen GDC-I—89.4% ± 14.9%, P < .01; vitronectin GDC-I—71.5% ± 7.0%, P < .05; laminin GDC-I—76.5% ± 11.0%, P < .05; fibrinogen GDC-I—74.8% ± 13.9%, P < .05; fibronectin GDC-I—87.5% ± 15.0%, P < .01) (Fig 4).
fig 4.
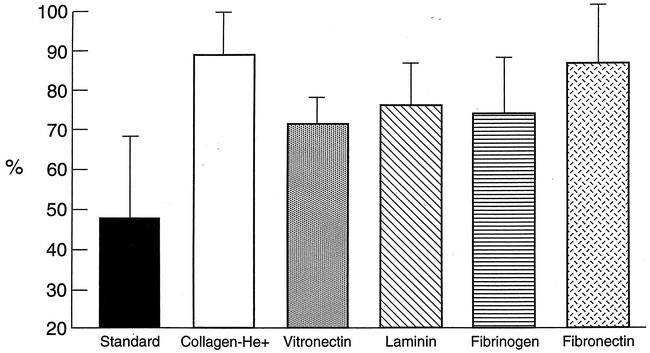
Neck coverage ratios for treated aneurysms at day 14. Largest dimensions of the orifice (OF) of the aneurysm and the fibrous membrane (FM) that covers the orifice were recorded and calculated as FM/OF proportion (fibrous membrane/ length of orifice × 100%). Standard GDC (n = 23), 48.3% ± 20.5%; collagen GDC-I (n = 11), 89.4% ± 14.9%; vitronectin GDC-I (n = 6), 71.5% ± 7.0%; laminin GDC-I (n = 4), 76.5% ± 11.0%; fibrinogen GDC-I, 74.8% ± 13.9% (n = 6); fibronectin GDC-I (n = 6), 87.5% ± 15.0%.
There were no statistically significant differences between each protein-coated GDC-I group; however, collagen-coated GDC-I specimens showed the highest obliteration rate of the aneurysm's neck, with five of 11 specimens showing 100% neck coverage.
Light and Scanning Electron Microscopic Findings (Figs 5 and 6)
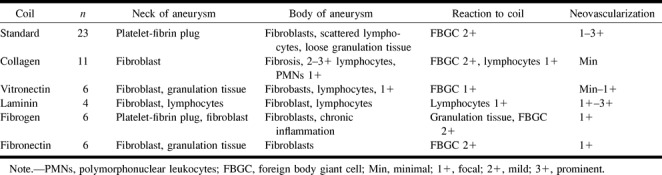
Light microscopic findings on the histologic specimens are detailed in Table 1. Low-magnification light microscopy showed well-organized fibrous tissue bridging the necks of the aneurysms embolized with GDC-Is. A thin fibrin-platelet complex and partial endothelialization were seen in those aneurysms embolized with standard GDCs.
Histologic findings of treated aneurysms
High-magnification light microscopy showed mild and organized fibrous tissue reaction as well as fibroblasts surrounding standard GDCs near the necks of the aneurysms. Interestingly, microscopic neovascularization was seen in the aneurysm's dome.
Collagen GDC-I demonstrated more collagen-rich fibrous tissue and a mild inflammatory cell response. The dominant cells were fibroblasts and macrophages. A few foreign-body giant cells were found also. Little neovascular formation in the body and dome of the aneurysm was seen compared with aneurysms treated with standard GDCs.
Vitronectin, fibronectin, and fibrinogen GDC-Is showed well-organized fibrous tissue response and fibroblasts. Neovascular formation was rare. Laminin GDC-I, however, demonstrated well-organized fibrous repair of the aneurysm neck and neovascularization was seen frequently.
Low-magnification SEM showed coil exposure at the neck of the aneurysm with standard GDCs. High-magnification views showed fibrin deposition on the surface of standard GDCs. GDC-Is demonstrated an almost complete or complete coverage of the neck of the aneurysm with an intact endothelial cell layer.
Discussion
Current Limitations of GDC Techonology
Despite the present popularity of the GDC technology for the treatment of intracranial aneurysms, one can identify important anatomic limitations associated with this endovascular therapeutic procedure.
GDC coils appear less effective when treating aneurysms with wide necks or ones that are large-to-giant in size (4, 5, 18). The mechanism of aneurysm occlusion with the standard GDC system is mechanical in nature. Increasing clinical experience shows that a complete and permanent aneurysm occlusion occurs only in tightly packed aneurysms. The presence of a residual neck in the aneurysm inflow zone precludes complete aneurysm occlusion in many cases. When an aneurysm is embolized incompletely with GDCs, there is a potential risk of aneurysm recanalization. Residual arterial flow in the aneurysm prevents endothelial cell proliferation across the neck of the aneurysm and anatomic isolation of the aneurysm from the blood circulation. No histologic reports in humans have shown organized fibrous tissue or neoendothelial proliferation across the neck of the aneurysm with GDC treatment (19–22).
Platinum is inert biologically and does not elicit a significant biological response on its surface. Tamatani et al (15) demonstrated no endothelial cell proliferation on the platinum coil surface in vitro. Biologically active GDCs may improve the situation by anchoring the GDC coils in the aneurysm and might accelerate intra-aneurysmal and neck inflammatory response with concomitant neoendothelial coverage.
Modification of the GDC
Several investigators have used ECM proteins, growth factors, or polymers to modify coil thrombogenicity and inflammatory cellular response related to their intravascular implantation (6–9, 11, 15). Polyurethane coating increases coil thrombogenicity but also changes its physical characteristics such as memory, shape, softness, trackability, and smoothness (6). Dawson et al (7) reported a collagen-coated platinum coil study with favorable anatomic results. Nonetheless, this coating process resulted in significant increase in coil thickness. This type of coating also can be stripped away easily during coil delivery through a microcatheter and become a potential source of embolic complication. Szikora and colleagues (11) published a study regarding collagen-filled platinum coils. This type of coil modification did not show anatomic improvement angiographically. Only a local effect was seen surrounding the coils. This modification also increased coil friction and stiffness. These results also suggested that this kind of coating may be removed from the platinum surface when it is exposed to high-flow shear stress at the neck of the aneurysm.
Ion-Implantation Technology and Protein Coatings
Recent advances in surface-modification technology allow the development of biologically compatible surfaces. These methods include carbon deposition, plasma deposition, ultraviolet irradiation, and ion implantation (17, 23–30). Ion implantation uses the highest energy delivery to produce surface modifications (16, 17, 30). When ion implantation is performed on a protein-coated surface, proteins are embedded and mixed into the platinum surface like a “tattoo.” The newly created surface does not lose its surface characteristics by mechanical stress. Analysis by Raman spectroscopy has shown that ion implantation produces amorphous carbon and disordered graphitic carbon, which is independent of the ion species and polymers used (17, 30).
Our laboratory results confirmed that there were no untoward mechanical changes in protein-coated GDC-I. Histologic findings proved the advantage of this modification process on the GDCs. A faster and stronger neoendothelial proliferation was observed at the neck of the GDC-I treated aneurysms. This accelerated histologic “cure” also appears to accelerate a neoendothelialization across the neck of the aneurysm. This early anatomic isolation of the aneurysm may decrease coil compaction and aneurysm recanalization.
In this study, we used different proteins and collagen in combination with ion-implantation technology. These proteins play an important role in cell proliferation, attachment, and the process of wound healing (31–46).
ECM proteins also may play a significant role in the development or repair of cerebral aneurysms (47–49). Some studies have examined the effect of various proteins on growth of endothelial cells derived from a rat aorta or canine carotid artery (15, 50). They found that proliferation of endothelial cells was greater on type I collagen than on other ECM proteins such as fibronectin, laminin, or gelatin. Our study did not show statistical significance between the protein-coated GDC-I groups. Aneurysms treated with collagen GDC-I, however, showed more intense neck coverage than did the other aneurysms. Histologic findings also demonstrated a more favorable inflammatory cellular response.
A limited neovascular proliferation was depicted in the dome of some aneurysms embolized with standard GDC and laminin GDC-I. It is not known whether this phenomenon occurs in human aneurysms. Laminin is a basement-membrane protein that accelerates endothelial proliferation and migration (32, 34, 46). The other ECM protein–coated GDC-Is did not show such prominent neovascularization. Although laminin GDC-I showed excellent neoendothelialization, the thickness of the intimal layer was relatively thin compared with the other modified GDCs.
Animal Model
We used a surgically created lateral side-wall aneurysm in the swine carotid artery. This experimental aneurysm model is highly reproducible in its size, shape, and hemodynamics. The swine model that we used has a strong tendency for spontaneous wound healing but the lateral venous-pouch model is sufficient to analyze different anatomic and histologic changes observed in aneurysms when embolized with standard GDCs versus GDC-Is. Fourteen-day postembolization angiographic and histologic evaluation is appropriate to analyze differences in “wound healing” at the neck of the experimental aneurysms (13). It needs to be emphasized that the endothelial growth rate has been related to its embryologic origin, artery size, sex, age, and disease state (51). Further evaluation using different models of flow dynamics in aneurysms is now under investigation.
Conclusion
Protein-coated GDC-Is share similar physical characteristics with standard GDCs. Experimental aneurysms treated with GDC-Is show a faster biological response in the aneurysm body and dome and faster neoendothelial proliferation and migration at the neck of the aneurysm. This accelerated biological response may decrease the chances of coil compaction and recanalization in human cerebral aneurysms treated by GDCs.
fig 1.
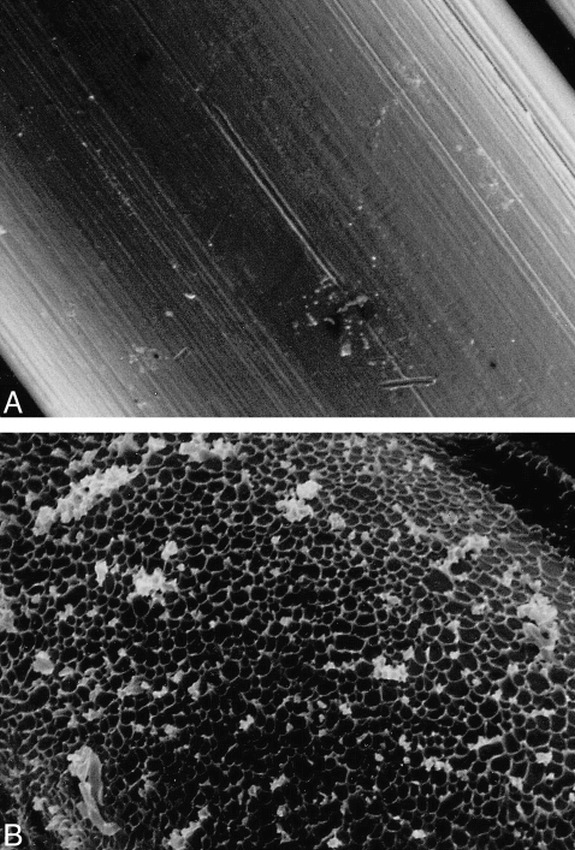
Scanning electron-microscopic appearances of surface of a standard coil (A) and a protein-coated ion-implanted coil (B) (original magnification, ×1500). Note crater-like appearance of ion-beam bombardment on protein-coated coil surface.
fig 5.
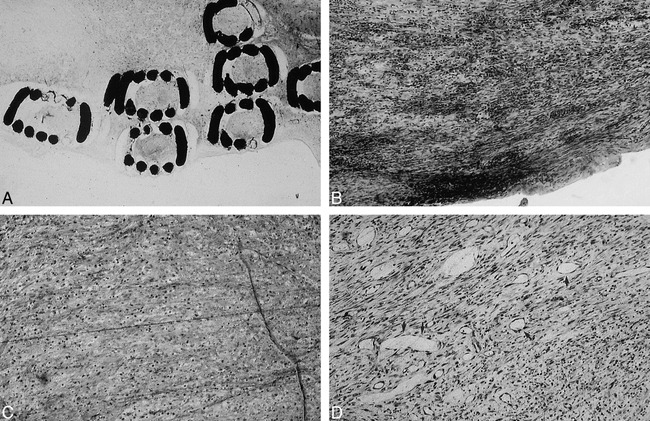
Light microscopic findings in region of aneurysm neck at day 14 after treatment, with standard GDCs (A, original magnification) or vitronectin GDC-Is (B, ×10). Note intense fibroblast response with vitronectin GDC-I. Only a thin fibrous layer was observed with standard GDCs. Light microscopic findings in region of the aneurysm sac. Sac of aneurysm treated with collagen GDC-I was filled with fibroblasts and monocytes. No neovascularization was seen with collagen GDC-Is (C, ×10), whereas microscopic neovascularization was seen in aneurysmal dome with standard GDCs (arrow, D, ×10).
fig 6.
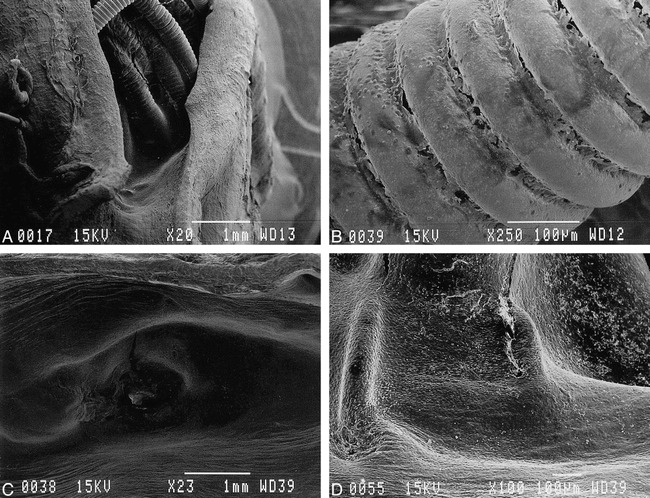
Scanning electron microscopic appearance of orifice of embolized aneurysms.
A and B, Lower (×20) and higher (×250) magnification of orifice treated with standard GDCs. Surface of coils were exposed to the arterial lumen and only fibrin/leukocyte complex was seen on surface.
C and D, Lower (×23) and higher (×100) magnification of orifice treated with laminin GDC-Is. Orifice was covered completely with neoendothelial cell layer.
Acknowledgments
We gratefully acknowledge the technical assistance of John Robert, Shawn McGill, and Fernando Viñuela, Jr of the Leo G. Rigler Radiological Research Center at the University of California, Los Angeles. We also thank James Sayre, PhD, for assistance with the statistical analysis.
Footnotes
Address reprint requests to Yuichi Murayama, MD, Division of Interventional Neuroradiology, Department of Radiological Sciences, UCLA School of Medicine and Medical Center, 10833 Le Conte Avenue, Los Angeles, CA 90024.
References
- 1.Guglielmi G, Viñuela F, Dion J, Duckwiler G, Electrothrombosis of saccular aneurysms via endovascular approach: Part 2-preliminary clinical experience. J Neurosurg 1991;75:8-14 [DOI] [PubMed] [Google Scholar]
- 2.Guglielmi G, Viñuela F, Duckwiler G, et al. Endovascular treatment of posterior circulation aneurysms by electrothrombosis using electrically detachable coils. J Neurosurg 1992;77:515-524 [DOI] [PubMed] [Google Scholar]
- 3.Murayama Y, Malisch T, Guglielmi G, et al. Incidence of cerebral vasospasm after endovascular treatment of acutely ruptured aneurysms: report on 69 cases. J Neurosurg 1997;87:830-835 [DOI] [PubMed] [Google Scholar]
- 4.Murayama Y, Viñuela F, Duckwiler GR, Gobin YP, Guglielmi G, Embolization of incidental cerebral aneurysms by using the Guglielmi detachable coil system. J Neurosurg 1999;90:207-214 [DOI] [PubMed] [Google Scholar]
- 5.Viñuela F, Duckwiler G, Mawad M, Guglielmi detachable coil embolization of acute intracranial aneurysm: perioperative anatomical and clinical outcome in 403 patients. J Neurosurg 1997;86:475-482 [DOI] [PubMed] [Google Scholar]
- 6.Ahuja AA, Hergenrother RW, Strother CM, Rappe AA, Cooper SL, Graves VB, Platinum coil coatings to increase thrombogenicity: a preliminary study in rabbits. AJNR Am J Neuroradiol 1993;14:794-798 [PMC free article] [PubMed] [Google Scholar]
- 7.Dawson RC, Krisht AF, Barrow DL, Joseph GJ, Shengelaia GG, Bonner G, Treatment of experimental aneurysms using collagen-coated microcoils. Neurosurgery 1995;36:133-140 [DOI] [PubMed] [Google Scholar]
- 8.Kalmes DF, Borland MK, Cloft HJ, et al. In vitro proliferation and adhesion of basic fibroblast growth factor-producing fibroblasts on platinum coils. Radiology 1998;206:237-243 [DOI] [PubMed] [Google Scholar]
- 9.Kalmes DF, Williams AD, Cloft HJ, Lopes M-BS, Hankins GR, Helm GA, Platinum coil-mediated implantation of growth factor-secreting endovascular tissue grafts: an in vivo study. Radiology 1998;207:519-523 [DOI] [PubMed] [Google Scholar]
- 10.Kwan ESK, Heilman CB, Roth PA, Endovascular packing of carotid bifurcation aneurysms with polyester fiber-coated platinum coils in a rabbit model. AJNR Am J Neuroradiol 1993;14:323-333 [PMC free article] [PubMed] [Google Scholar]
- 11.Szikora I, Wakhloo AK, Guterman LR, et al. Initial experience with collagen-filled Guglielmi detachable coils for endovascular treatment of experimental aneurysms. AJNR Am J Neuroradiol 1997;18:667-670 [PMC free article] [PubMed] [Google Scholar]
- 12.Mawad ME, Mawad JK, Cartwright J, Jr, Gokaslan Z, Long-term histopathologic changes in canine aneurysms embolized with Guglielmi detachable coils. AJNR Am J Neuroradiol 1995;16:7-13 [PMC free article] [PubMed] [Google Scholar]
- 13.Murayama Y, Suzuki Y, Viñuela F, et al. Ion implantation and protein coating of detachable coils for endovascular treatment of cerebral aneurysms: concepts and preliminary results in swine models. Neurosurgery 1997;40:1233-1244 [DOI] [PubMed] [Google Scholar]
- 14.Spetzger U, Reul J, Weis J, Bertalanffy H, Thron A, Gilsbach JM, Microsurgically produced bifurcation aneurysms in a rabbit model for endovasclar embolization. J Neurosurg 1996;85:488-495 [DOI] [PubMed] [Google Scholar]
- 15.Tamatani S, Ogawa T, Minakawa T, Takeuchi S, Koike T, Tanaka R, Histological interaction of cultured endothelial cells and endovascular embolic materials coated with extracellular matrix. J Neurosurg 1997;86:109-112 [DOI] [PubMed] [Google Scholar]
- 16.Iwaki M, Formation of metal surface layers with high performance by ion implantation. Nucl Instr and Meth B 1989;37/38:661-666 [Google Scholar]
- 17.Suzuki Y, Kusakabe M, Kaibara M, Iwaki M, Sasabe H, Nishisaka T, Cell adhesion control by ion implantation into extra-cellular matrix. Nucl Instr and Meth B 1994;91:588-592 [Google Scholar]
- 18.Zubillaga AF, Guglielmi G, Viñuela F, Duckwiler GR, Endovascular occlusion of intracranial aneurysms with electrically detachable coils: Correlation of aneurysm neck size and treatment results. AJNR Am J Neuroradiol 1994;15:815-820 [PMC free article] [PubMed] [Google Scholar]
- 19.Horowitz MB, Purdy PD, Burns D, Bellotto D, Scanning electron microscopic findings in a basilar tip aneurysm embolized with Guglielmi detachable coils. AJNR Am J Neuroradiol 1997;18:688-690 [PMC free article] [PubMed] [Google Scholar]
- 20.Mizoi K, Yoshimoto T, Takahashi A, Nagamine Y, A pitfall in the surgery of a recurrent aneurysm after coil embolization and its histological observation: technical case report. Neurosurgery 1996;39:65-69 [DOI] [PubMed] [Google Scholar]
- 21.Molyneux AJ, Ellison DW, Morris J, Byrne JV, Histological findings in giant aneurysms treated with Guglielmi detachable coils. Report of two cases with autopsy correlation. J Neurosurg 1995;83:129-132 [DOI] [PubMed] [Google Scholar]
- 22.Ozawa T, Koike T, Takeuchi S, Tanaka R, Scanning electron microscopic study of the migrated platinum coil after endovascular embolization of a giant cerebral aneurysm (Letters). AJNR Am J Neuroradiol 1998;19:594-595 [PMC free article] [PubMed] [Google Scholar]
- 23.Bambauer R, Mestres P, Schiel R, Klinkmann J, Sioshansi P, Surface-treated catheters with ion beam-based process evaluation in rats. Artif Organs 1997;21:1039-1041 [DOI] [PubMed] [Google Scholar]
- 24.Ignatius MJ, Sawheney N, Gupta A, Thibadeau BM, Monteiro OR, Brown IG, Bioactive surface coating for nanoscale instruments: effect on CNS neurons. J Biomed Mater Res 1998;40:264-274 [DOI] [PubMed] [Google Scholar]
- 25.Kaibara M, Iwata H, Wada H, Kawamoto Y, Iwaki M, Suzuki Y, Promotion and control of selective adhesion and proliferation of endothelial cells on polymer surface by carbon deposition. J Biomed Mater Res 1996;31:429-435 [DOI] [PubMed] [Google Scholar]
- 26.Kawamoto Y, Nakao A, Ito Y, Wada N, Kaibara M, Endothelial cells on plasma-treated segmented polyurethane. J Mater Sci Mater Med 1997;8:551-557 [DOI] [PubMed] [Google Scholar]
- 27.Sioshansi P, Medical application of ion beam processes. Nucl Intr and Meth B 1987;19/20:204-208 [Google Scholar]
- 28.Sioshansi P, Tobin EJ, Surface treatment of biomaterials by ion-beam processes. Med Plast Biomater 1995;3:50-59 [Google Scholar]
- 29.Sugawara T, Matsuda T, Photochemical surface derivatization of a peptide containing Arg-Gly-Asp (RGD). J Biomed Mater Res 1995;29:1047-1052 [DOI] [PubMed] [Google Scholar]
- 30.Suzuki Y, Kusakabe M, Lee JS, Kaibara M, Iwaki M, Sasabe H, Endothelial cell adhesion to ion implanted polymers. Nucl Instr and Meth B 1992;65:142-147 [Google Scholar]
- 31.Brotchie H, Wakefield D, Fibronectin: Structure, function and significance in wound healing. Australas J Dermatol 1990;31:47-56 [DOI] [PubMed] [Google Scholar]
- 32.Campbell JH, Terranova VP, Laminin: molecular organization and biological function. J Oral Pathol 1988;17:309-323 [DOI] [PubMed] [Google Scholar]
- 33.Felding-Habermann B, Cheresh DA, Vitronectin and its receptors. Curr Opin Cell Biol 1993;5:864-868 [DOI] [PubMed] [Google Scholar]
- 34.Foidart JM, Bere EW, Jr, Yaar M, et al. Distribution and immunoelectron microscopic localization of laminin, a noncollagenous basement membrane glycoprotein. Lab Invest 1980;42:336-342 [PubMed] [Google Scholar]
- 35.Gray AJ, Bishop JE, Reeves JT, Lauent G, AB chains of fibrinogen stimulate proliferation of human fibroblasts. J Cell Sci 1993;104:409-413 [DOI] [PubMed] [Google Scholar]
- 36.Grinnell F, Feld M, Minter D, Fibroblast adhesion to fibrinogen and fibrin substrata: requirement for cold-insoluble globulin (plasma fibronectin). Cell 1980;19:517-525 [DOI] [PubMed] [Google Scholar]
- 37.Hayman EG, Pierschbacher MD, Suzuki S, Ruoslahti E, Vitronectin-A major cell attachment-promoting protein in fetal bovine serum. Exp Cell Res 1985;160:245-258 [DOI] [PubMed] [Google Scholar]
- 38.Hedin U, Bottger BA, Forsberg E, Johansson S, Thyberg J, Diverse effects of fibronectin and laminin on phenotypic properties of cultured arterial smooth muscle cells. J Cell Biol 1988;107:307-319 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Liesi P, Extracellular matrix and neuronal movement. Experientia 1990;46:900-907 [DOI] [PubMed] [Google Scholar]
- 40.Mosher DF, Fibronectin. Prog Hemostasis Thrombosis 1980;5:111-151 [PubMed] [Google Scholar]
- 41.Preissner KT, Structure and biological role of vitronectin. Annu Rev Cell Biol 1991;7:275-310 [DOI] [PubMed] [Google Scholar]
- 42.Ross R, The fibroblast and wound repair. Biol Rev 1968;43:51-96 [DOI] [PubMed] [Google Scholar]
- 43.Ross R, Wound healing. Sci Amer 1969;220:40-50 [DOI] [PubMed] [Google Scholar]
- 44.Scheel G, Rahfoth B, Franke J, Grau P, Acceleration of wound healing by local application of fibronectin. Arch Oethop Trauma Surg 1991;110:284-287 [DOI] [PubMed] [Google Scholar]
- 45.Tomasini BR, Mosher DF, Vitronectin. Prog Hemostasis Thrombosis 1991;10:269-305 [PubMed] [Google Scholar]
- 46.Tryggvason K, The laminin family. Current Opinion in Cell Biol 1993;5:877-882 [DOI] [PubMed] [Google Scholar]
- 47.Austin G, Fisher S, Dickson D, Anderson D, Richardson S, The significance of the extracellular matrix in intracranial aneurysms. Ann Clin Lab Sci 1993;23:97-105 [PubMed] [Google Scholar]
- 48.Futami K, Yamashita J, Tachibana O, Higashi S, Ikeda K, Yamashita T, Immunohistochemical alterations of fibronectin during the formation and proliferative repair of experimental cerebral aneurysms in rats. Stroke 1995;26:1659-1664 [DOI] [PubMed] [Google Scholar]
- 49.Skirgaudas M, Awad IA, Kim J, Rothbart D, Criscuolo G, Expression of angiogenesis factors and selected vascular wall matrix proteins in intracranial saccular aneurysms. Neurosurgery 1996;39:537-547 [DOI] [PubMed] [Google Scholar]
- 50.McGuire PG, Orkin RW, Isolation of rat aortic endothelial cells by primary explant techniques and their phenotypic modulation by defined substrata. Lab Invest 1987;57:94-105 [PubMed] [Google Scholar]
- 51.Thorin E, Shatos MA, Shreeve M, Walters CL, Bevan JA, Human vascular endothelium heterogeneity. A comparative study of cerebral and peripheral cultured vascular endothelial cells. Stroke 1997;28:375-381 [DOI] [PubMed] [Google Scholar]


