Abstract
In shaping how individuals explore their environment and interact with others, personality may mediate both individual and social learning. Yet increasing evidence indicates that personality expression is contingent on social context, suggesting that group personality composition may be key in determining how individuals learn about their environment. Here, we used recovery latency following simulated predator attacks to identify Trinidadian guppies (Poecilia reticulata) that acted in a consistently bold or shy manner. We then employed network-based diffusion analysis to track the spread of a novel foraging behaviour through groups containing different proportions of bold and shy fish. Informed associates promoted learning to a greater extent in bold individuals, but only within groups composed predominately of bold fish. As the proportion of shy fish within groups increased, bold individuals instead emerged as especially effective demonstrators that facilitated learning in others. Individuals were also more likely to learn overall within shy-dominated groups than in bold-dominated ones. We demonstrate that whether and how individuals learn is conditional on group personality composition, indicating that selection may favour traits enabling individuals to better match their behavioural phenotype to their social environment.
Keywords: social learning, social information, animal personality, network-based diffusion analysis, fish, collective behaviour
1. Introduction
Evidence for consistent individual differences in behaviour (referred to as animal personalities) suggests that animals will often show limited flexibility in how they acquire and use ecologically relevant information [1,2]. Bolder (more risk-prone) individuals may be more likely to directly sample the environment [3–6], and thereby act as ‘information producers' within groups [2,3,7]. Furthermore, because boldness often correlates with leadership during collective movements [3,7–9], these individuals may also be more likely to transmit acquired knowledge to naive followers. Conversely, risk-averse tendencies (i.e. shyness) have been linked to increased social information use ([5,10]; though see [11,12]), possibly resulting either from increased attraction [7,13–15] or responsiveness towards group mates [9,16], or because these individuals weight social information more heavily [5,10].
Because reliance on social information enables individuals to avoid costs typically associated with exploration [17]—such as increased predation risk and greater time and energy costs—this body of work suggests that the costs of learning may systematically differ between bold and shy phenotypes. Yet how personality is expressed can be strongly dependent on the social environment [2]. For example, both leadership effectiveness and risk-taking behaviour are contingent not only on an individual's own personality type, but on that of its group mates [3,9,18,19], while collective outcomes may be shaped by the personality traits of key individuals [20]. Accordingly, group personality composition may determine the dominant pathways of information flow by influencing how individuals interact with their environment and with one another.
Here, we evaluate this hypothesis by tracking the spread of a novel foraging behaviour through groups of Trinidadian guppies (Poecilia reticulata) that contained different proportions of bold and shy fish, where boldness was measured as the speed at which individuals resumed activity following simulated predator attacks. We formed groups composed primarily of either bold or shy individuals or that contained an even mix of the two and presented them with a novel foraging task. We then performed a network-based diffusion analysis (NBDA) [21,22] to investigate whether group personality composition influenced the dominant social learning pathways operating within guppy shoals and the relative importance of social versus individual learning for acquiring the foraging task solution.
2. Methods
(a). Study subjects
Trials were conducted September 2015–May 2016 using laboratory-reared descendants of wild-caught fish (Trinidad, 2003). Fry were collected en masse from four 208 l stock tanks, each containing several hundred fish, and haphazardly assigned to 48 rearing tanks (37.9 l). To ensure similar developmental experiences (electronic supplementary material) and to control for sex-based differences in shoaling behaviour and foraging motivation [23,24], only females raised to maturity within the rearing tanks were used in the experiment.
(b). Boldness assay
On day 1, an experimental trial was initiated by collecting 32 non-gravid females from separate rearing tanks and placing them in 500 ml water in separate opaque containers. This permitted individual identification during boldness assays without requiring marking. On day 2, females were exposed individually to a simulated aerial predator attack (design adapted from [25]). Assays were carried out from 8 : 00 to 13 : 00. A female was introduced into the assay tank (electronic supplementary material, figure S1) and allowed 8 min acclimation. Immediately afterwards, a cardboard piece was released, allowing it to travel down a monofilament line above the tank. As it passed beneath a light source, a shadow swept across the tank, eliciting a startle response (erratic dashes prior to freezing) in all guppies tested. Boldness was quantified from video recordings as latency to resume movement, defined as moving at least one body length from where that individual froze. Individuals that did not move for 3 min were given scores of 180 s. Afterwards, individuals were returned to their holding container. Following every other assay, the water within the tank was replaced completely. Individuals were tested again on day 3, enabling repeatability of latency scores to be estimated. Testing order was counterbalanced, such that each individual was tested once as both the first and second fish following a water change.
(c). Experimental procedure
Experimental groups were constructed on day 4. Individuals with a mean latency to resume movement less than 40 s and more than 60 s were labelled as bold and shy, respectively. In addition, no single latency score was more than 50 s for bold individuals, nor less than 50 s for shy ones. Subjects failing to meet these criteria were returned to the stock tanks and not used in the experiment. Our rationale behind these cut-offs was to identify sets of individuals that consistently differed in risk-taking behaviour. An experimental group was then constructed with one of three compositions: bold-dominated groups (n = 12 groups) which contained eight bold and two shy fish; shy-dominated groups (n = 12 groups) which were made up of two bold and eight shy fish and mixed groups (n = 12 groups) which contained five of each type. Individuals were size-matched (≤3 mm standard length) within each group (mean ± s.d.) standard length: 1.79 ± 0.11 cm). Mean (±s.d.) boldness scores were 18.5 ± 8.9 s for bold fish (n = 179 individuals) and 140.1 ± 31.3 s for shy fish (n = 180 individuals). See the electronic supplementary material, figure S2 for distributions of mean latency scores for all individuals tested (n = 853) and for those in the experimental groups.
To permit individual identification, individuals were anaesthetized with buffered MS-222 and injected with two elastomer tags (Northwest Marine Technologies, Inc.). Following this procedure, groups were observed for 1 h. No mortalities occurred during this time. Groups were then introduced into the test arena (electronic supplementary material, figure S3): a black rectangular tank (84 × 51 cm) containing black gravel substrate (water depth: 7 cm). Water temperature was maintained at 26–27°C. Seventeen black plastic partitions (10 × 10 cm) were arranged to break up sight lines within the arena. Trials were recorded from above the arena. Black cloth around the arena minimized external visual disturbance. One hour following introduction, groups were fed flake food ad libitum; uneaten food was removed 1 h later. Groups were then left within the arena overnight.
Trials commenced at 13.00 on day 5. Groups were recorded for 120 min to collect shoaling association data following methods described in [26]. Briefly, focal individuals were randomly selected and observed for 4 min. Every 10 s, we recorded whether that individual was alone (no group mates within four body lengths [27]) or shoaling. If the latter, the identity of its nearest neighbour was recorded (measured from the centre of their heads). Focal individuals' elective group size [27] was also recorded every 60 s, where a group included all individuals within four body lengths of at least one other group member. After 4 min, a new focal was randomly selected until all individuals had been observed in this way. This process was repeated twice more to provide 72 nearest-neighbour and 12 elective group size observations per individual.
After 120 min, a novel foraging device was gently introduced. This was a PVC tube (height: 8.6 cm; diameter: 8.9 cm) with a plastic base and a hole (diameter: 2 cm) near the bottom to permit entry. The cylinder was stocked with 32 freeze-dried blood worms; these floated on the water's surface and were not visible from the outside. Groups were filmed for a further 20 min, during which we recorded individual latencies to enter the device. Most individuals (285 out of 359) entered the device at least once during this period. Following testing, groups were returned to the stock tanks. One bold individual in a bold-dominated group died prior to testing, meaning one group of nine fish was included in the analysis; similar results are obtained when this group is excluded from the analysis (see the electronic supplementary material).
This 5 day procedure was carried out 27 times to generate the 36 experimental groups (it was occasionally possible to construct and test two experimental groups simultaneously).
(d). Statistical analyses
(i). Repeatability of boldness
Repeatability of boldness scores was estimated using rptR in R [28]. A generalized linear mixed-effects model (GLMM) with a Poisson error distribution and log-link function was fitted with boldness score as a dependent variable and individual identity and testing cohort as random effects. In addition, an observation-level random effect was included to account for overdispersion. Repeatability was calculated from variance estimates extracted from the individual identity random effect and random error terms as described in [28]. Parametric bootstrapping (104 simulated datasets) was used to obtain 95% confidence intervals (CIs). Repeatability was estimated across all females that underwent boldness assays, regardless of whether they were used to form the experimental groups.
(ii). Mean elective group size
Individuals’ mean elective group size was included as the response variable in a linear mixed-effects (LMM) model fitted with restricted maximum likelihood using lme4 in R [29]. As one group contained only nine individuals, mean elective group sizes were divided by the maximum elective group size possible. Explanatory variables included group composition (bold-dominated/mixed/shy-dominated), individual personality (bold/shy), the interaction between these terms and body length. Categorical input variables were mean-centred, while body length was centred within each group. Group identity was included as a random intercept term. Inspection of residuals revealed no model specification issues. Statistical significance was assessed via likelihood ratio tests.
(iii). Solving probability and entry rate
We modelled individual probabilities of solving the foraging task using a binomial GLMM fit with lme4 [29]. For those individuals that solved the task, we further modelled the number of times an individual entered the device by fitting a truncated Poisson GLMM with glmmTMB [30], including as an offset the time remaining in the trial once an individual was informed. The same set of explanatory variables and random effects used in the analysis of mean elective group size was considered here. GLMM validation was carried out using DHARMa [31]. Statistical significance was assessed via likelihood ratio tests.
(iv). Network-based diffusion analysis
We used NBDA to evaluate the relative strength of social influences on task solving rate and to identify the typical social learning pathways within the experimental groups [21,22]. NBDA infers social learning if the rate at which naive individuals acquire a behavioural innovation increases with connectedness to informed individuals, taken here as those that entered the foraging device. Specifically, we used a stratified order-of-acquisition analysis [32], taking as data the order in which individuals across all groups solved the foraging task and including all individuals in a single network with all between-group connections set to 0. Treating the data in this way increases the power to detect social learning while simultaneously requiring that fewer assumptions be made about how learning rates change over time (see the electronic supplementary material for more details).
We considered two social network types in the NBDA: proximity networks and group-membership networks. Inclusion of the proximity networks allowed us to evaluate whether social learning rates were predicted by how often individuals were observed as nearest-neighbours of one another. Alternately, support for the group-membership networks (in which all group members are equally connected) would indicate that social learning was not constrained by nearest-neighbour associations. For details of how proximity networks were constructed, see the electronic supplementary material.
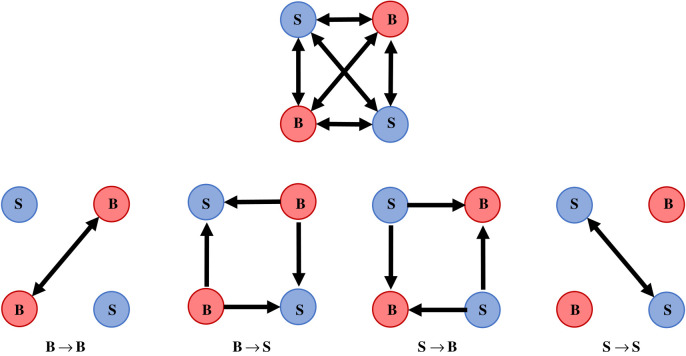
The standard NBDA model takes only a single network as input and so cannot infer whether social learning rates (estimated by the parameter s) vary across different classes of social connection. Here, we used a recently developed multiple-network NBDA [33] that allows for partitioning a social network into multiple pathways of information flow (see the electronic supplementary material for details). Networks for each group were deconstructed into four sub-networks (figure 1), each including only a specific connection type (e.g. connections from bold to shy fish, B → S). We then considered the following ways in which social learning rates may have differed across these pathways: (i) social learning rates, s, did not depend on either demonstrator or observer boldness: sB→B = sB→S = sS→B = sS→S; (ii) demonstrators' boldness influenced how effectively they facilitated learning in others: (sB→B = sB→S) ≠ (sS→B = sS→S); (iii) observers’ boldness influenced their rates of social learning: (sB→B = sS→B) ≠ (sS→S = sB→S); and (iv) social learning rates depended on both demonstrator and observer boldness: sB→B ≠ sS→B ≠ sS→S ≠ sB→S. Following [32], we use demonstrator and observer to refer to informed and naive individuals respectively, without assuming any particular social learning process. Note that although the number of connections varied across sub-networks (e.g. within a bold-dominated group, there are 16 B → S and two S → S connections), NBDA estimates social transmission rates per unit of network connection. For example, in the group-membership networks, s is estimated per informed group member. As such, s can be meaningfully compared across sub-networks, despite mismatches in the number or strength of connections [22].
Figure 1.
Partitioning networks based on boldness. In the complete network (above), all connections are undirected and either reflect shoaling associations (proximity networks) or were set to 1 between all group members (group-membership networks). In order to assess whether the strength of social learning (s) varied across different types of social connection, networks were partitioned into four sub-networks, each of which included only a particular type of social connection (e.g. B → S indicates connections from bold to shy individuals). Though connections were undirected in the complete network, connections in the sub-networks were directed with strength equal to that of the undirected edge. This allowed for teasing apart how boldness impacts the transfer of social information versus its reception. (Online version in colour.)
NBDA also allows for inclusion of individual-level variables (ILVs) that can modify asocial and/or social learning rates [22,32]. We included group composition (bold-dominated/mixed/shy-dominated), individual personality (bold/shy) and body length (continuous; mean-centred within groups) as ILVs that could modify asocial learning rates. We further considered the influence of body length on social learning rates; potential impacts of group composition and individual personality on social learning were captured through constraints on s.
We employed an information-theoretic approach [34], fitting all possible models (electronic supplementary material, table S1) and using Akaike's information criterion corrected for sample size (AICc) to obtain Akaike weights (wi) for each model [34]. The overall support for each network type, social learning pathway and ILV was obtained by summing Akaike weights, Σwi, across all relevant models. Model-averaging [34] was conducted across all models that incorporated the group-membership network, owing to the strong support it received. Profile likelihood techniques were used to obtain CIs for s, as these tend to be highly asymmetric [22,35]. Model-averaged 95% CIs were obtained across those models that included the best-supported network and social learning pathway (electronic supplementary material, table S1). Model-averaged estimates for differences in social transmission rates were also conditional on this model subset. For ILVs, 95% Wald CIs were constructed using unconditional standard errors [34]. The estimated percentage of learning events attributable to social learning were obtained as described in [36]. The NBDA was conducted in R using the NBDA package v.0.9.4 [37].
3. Results
Across all groups, 285 out of 359 individuals solved the task by entering the device. There were 78 solvers in bold-dominated groups (mean solvers group−1 = 6.5; range = 0–10), 96 solvers in mixed groups (mean solvers group−1 = 8; range = 0–10) and 111 solvers in shy-dominated groups (mean solvers group−1 = 9.25; range = 8–10) (figure 2). Bold individuals were not disproportionately likely to be the first to solve the task: among groups that solved the task, the initial solver was bold in 8 out of 11 bold-dominated groups, 7 out of 11 mixed groups and 2 out of 12 shy-dominated groups. The median (interquartile range) gap between consecutive solving events was 15.95 s (5.14–59.56 s; n = 67) in bold-dominated groups, 15.1 s (4.19–53.39 s; n = 85) in mixed groups and 27.44 s (5.69–70.51 s; n = 99) in shy-dominated groups. Feeding strikes were observed for nearly all individuals that entered the device (283 individuals). Of those individuals, 93% began feeding during their first entry (mean ± s.d. latency from initial entry to first feeding strike: bold individuals: 11.1 ± 24.4 s, n = 134; shy individuals: 14.4 ± 37.4 s, n = 149).
Figure 2.
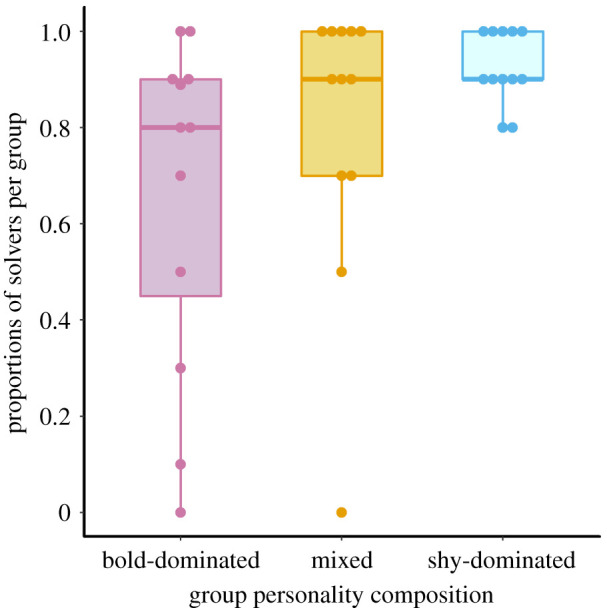
Proportion of individuals that solved the task per group. Owing to a bold-dominated group that contained only nine individuals, the number of solvers was divided by the number of individuals present in each group. The thick line within each box indicates the median, while the boxes indicate the interquartile range. Whiskers extend up to 1.5× the interquartile range. (Online version in colour.)
(a). Repeatability of boldness
Boldness scores were repeatable across consecutive days (n = 853, link-scale: R (95% CI) = 0.33 (0.27, 0.39); original-scale: R (95% CI) = 0.21 (0.17, 0.26); electronic supplementary material, table S2). Furthermore, a pilot study established that scores were repeatable over a 5 day period (electronic supplementary material, table S2), suggesting that individual differences in risk-taking remained consistent during trials. Within the experimental groups, there was evidence of a weak positive relationship between body size and mean boldness score—i.e. smaller fish tended to be bolder (Pearson's r = 0.10, p = 0.06, n = 359).
(b). Mean elective group size
There was little evidence that individuals' mean elective group size depended on group composition (χ22 = 2.38, p = 0.30), body length (, p = 0.41), nor on an interaction between group composition and individual personality (, p = 0.37). There was at best a weak relationship between personality type and mean elective group size (, p = 0.07): shy individuals occupied groups 1.06× larger on average than those occupied by bold fish. See the electronic supplementary material, table S3 for parameter estimates.
(c). Solving probability and entry rate
We did not detect statistically significant effects of body length (, p = 0.58), personality type (, p = 0.18), nor an interaction between group composition and personality (, p = 0.45) on individuals’ overall likelihood of solving the foraging task (whether via individual or social learning). Solving probability varied across different group compositions (, p = 0.03). Individuals were most likely to solve the task within shy-dominated groups and least likely within bold-dominated ones (task solving probability (95% CI): bold-dominated: 0.69 (0.41, 0.88); mixed: 0.90 (0.71, 0.97); shy-dominated: 0.97 (0.88, 0.99)). See the electronic supplementary material, table S4 for parameter estimates.
Informed individuals entered the device 0.62 times min−1 (s.d. = 1.1) on average, although substantial variation was present within and across groups (electronic supplementary material, figure S3). There was little evidence that the number of times an individual entered the device varied as a function of group composition (, p = 0.84) or its interaction with personality (, p = 0.45). However, there was weak evidence linking more frequent entry with increasing body length (, p = 0.06) and a bold personality type (, p = 0.08). See the electronic supplementary material, table S5 for parameter estimates.
(d). Network-based diffusion analysis
The NBDA indicated overwhelming support for knowledgeable group members increasing the rate at which naive individuals solved the foraging task (models with social effects: Σwi > 0.999), providing clear evidence of social learning [32]. Rather than solving events occurring at regularly spaced intervals (as would be expected in the absence of social influences), diffusion curves reveal that group members tended to solve in collective bursts (figure 3). There was little evidence that the spread of the task solution followed the proximity networks (Σwi = 0.001). Rather, there was strong support for the group-membership networks (Σwi = 0.999), in which all group members were connected by ties of equal strength, indicating that social information flow was not constrained by nearest-neighbour preferences.
Figure 3.
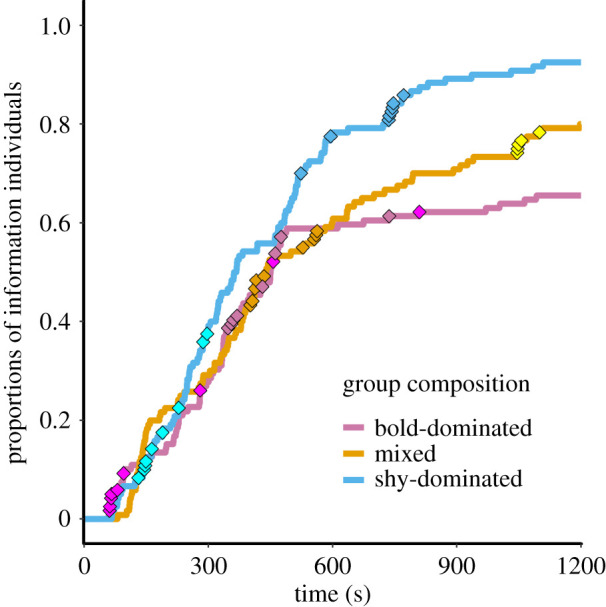
Diffusion curves. Lines indicate the proportion of individuals that had solved the task across all groups of a given personality composition as the trials progressed. Diamonds indicate solving events for a subset of six groups (colours represent different groups).
Social learning pathways were shaped by individual personality, contingent on group composition (table 1). Within bold-dominated groups, the majority of evidence (Σwi = 0.56; table 1) favoured models in which an individual's social learning rate depended on its boldness. Specifically, informed individuals promoted learning in bold group mates to a greater degree than in shy ones (table 2), with the model-averaged difference (sB/S→B – sB/S→S) estimated at 1.36 (95% CI: 0.08, 3.07). Put another way, the addition of a single informed individual meant that a bold fish of average length was expected to solve the task 3.4–3.7× sooner than before, whereas a shy individual was expected to solve the task only 2.3–2.5× sooner (table 2). Conversely, within mixed and shy-dominated groups, models in which social learning rates depended on the risk-taking tendency of informed demonstrators instead received the most support (mixed groups: Σwi = 0.40; shy groups: Σwi = 0.53; table 1). Moreover, the best-supported models constrained social learning rates to be equal across mixed and shy-dominated groups. Together, these findings indicate that the dynamics of information flow were similar within mixed and shy-dominated groups, with bold demonstrators more strongly accelerating the rate at which other group members solved the task than shy demonstrators (model-averaged difference: sB→B/S – sS→B/S = 2.49; 95% CI: 0.29, 5.89). However, models of undifferentiated social learning received nearly as much support within mixed groups as those in which s varied according to demonstrator boldness (table 1).
Table 1.
Total strength of support (Σwi) for alternative social learning pathways operating within the experimental groups, contingent on group personality composition. (Undifferentiated social learning refers to a scenario in which social learning rates (s) do not depend on the boldness of either demonstrator or observer (sB→B = sB→S = sS→B = sS→S). Alternately, s may depend on demonstrator boldness, (sB→B = sB→S) ≠ (sS→B = sS→S); observer boldness, (sB→B = sS→B) ≠ (sS→S = sB→S) or the boldness of both demonstrator and observer, sB→B ≠ sS→B ≠ sS→S ≠ sB→S.)
| social learning pathway | bold-dominated | mixed | shy-dominated |
|---|---|---|---|
| undifferentiated social learning | 0.194 | 0.358 | 0.233 |
| s depends on demonstrator boldness | 0.084 | 0.397 | 0.525 |
| s depends on observer boldness | 0.556 | 0.156 | 0.11 |
| s depends on demonstrator and observer | 0.165 | 0.089 | 0.132 |
Table 2.
Social transmission rates (95% CI) across different group compositions. (The parameter s provides the rate at which an individual acquires information per unit of network connection, relative to the average asocial learning rate (set here as the learning rate for a shy individual of average body length in a bold-dominated group) [22]. Model-averaged 95% CIs were obtained using profile likelihood techniques [35] across all models that included the group-membership networks and best-supported social learning pathway (indicated in bold in the electronic supplementary material, table S1). This pathway constrained certain s parameters and their associated CIs to be equal (e.g. across mixed and shy-dominated groups).)
| social connection | bold-dominated | mixed | shy-dominated |
|---|---|---|---|
| sB→B | 2.35 (1.37, 4.68) | 4.63 (2.88, 9.76) | 5.28 (2.88, 9.76) |
| sB→S | 1.47 (0.35, 2.85) | 4.55 (2.88, 9.76) | 4.79 (2.88, 9.76) |
| sS→S | 1.33 (0.35, 2.85) | 3.48 (1.47, 5.22) | 2.86 (1.47, 5.22) |
| sS→B | 2.70 (1.37, 4.68) | 3.67 (1.47, 5.22) | 3.01 (1.47, 5.22) |
The NBDA did not detect differences in the rate of individual learning across different group compositions (Σwi = 0.189; table 3), nor between bold and shy individuals (Σwi = 0.267; table 3). Although group composition shaped the predominant pathways of social learning, it did not impact the relative importance of individual versus social learning at the group level. Of the 78 solving events in bold-dominated groups, the NBDA estimated that 25.9% (95% CI: 21.4–33.6%) occurred independently of any social influences. Likewise, of the 96 solves in mixed groups and 111 solves in shy-dominated groups, 17.9% (95% CI: 10.3–28.9%) and 19.2% (95% CI: 11.2–30.8%), respectively, were attributed to individual learning alone. In other words, the NBDA predicts that most individuals solved the foraging task at least partly through social learning.
Table 3.
Strength of support and parameter estimates for individual-level variables (ILVs). (ILVs modify either the baseline learning rate (asocial effect) or social learning (social effect). ILV estimates provide the linear effect on the log scale: e.g. bold individuals had an asocial learning rate exp(0.02) = 1.02× that of shy individuals. Unconditional standard errors were used to construct 95% Wald CIs for ILVs [34].)
| individual-level variable | support (Σwi) | model-averaged estimate (95% CI) |
|---|---|---|
| mixed group membership: asocial effect | 0.189 | −0.04 (−0.16, 0.09) |
| shy-dominated group membership: asocial effect | 0.189 | 0.05 (−0.11, 0.22) |
| bold personality: asocial effect | 0.267 | 0.02 (−0.09, 0.13) |
| body length (cm): asocial effect | 0.288 | −0.34 (−3.65, 2.97) |
| body length (cm): social effect | 0.292 | −0.15 (−0.75, 0.45) |
4. Discussion
Variation in personality has previously been linked to differences in how individuals acquire and use information [4,5,10,11], but how personality is expressed frequently depends on the social environment [2,18,19]. Using NBDA, we found that individual variation in boldness impacted how social information spread within guppy shoals, but these effects varied across different group compositions. Specifically, as the proportion of shy group members increased, bold individuals transitioned from being the primary users of social information to those mainly responsible for promoting its spread. In addition, group personality composition influenced the overall probability that individuals would learn how to exploit a novel foraging opportunity. Taken together, these findings suggest that group personality composition may be a key factor in determining whether and how individuals acquire information relevant for adaptive decision-making.
Consistent with previous work in bird flocks and fish shoals [3,7], we found that under certain circumstances, informed bold individuals facilitated learning in others. However, this effect was observed only within mixed and shy-dominated groups, suggesting that a certain number of risk-averse group mates needed to be present before bold individuals emerged as effective demonstrators. Within groups containing relatively few shy individuals, boldness was instead associated with higher social learning rates, potentially reflecting a greater likelihood of interacting with the device once other group mates had begun to do so [11]. These results indicate that the dominant pathways of social learning shifted according to the ratio of risk-prone to risk-averse group members. Indeed, although the top-ranked models suggest that similar social learning pathways operated within mixed and shy-dominated groups, the overall support for personality-based differences in observer learning rates and demonstrator effectiveness in mixed groups was intermediate between bold- and shy-dominated groups (table 1). This may also explain the relatively strong support for undifferentiated social learning in mixed groups (table 1)—i.e. increased effectiveness of bold demonstrators may have been partly offset by bold observers retaining an elevated social learning rate within mixed groups, relative to shy observers (table 2). Social learning is thought to be advantageous in that it allows individuals to forgo the greater costs typically associated with individual exploration [17,32]. Accordingly, by determining the typical pathways of social learning, group personality composition may also govern the costs of acquiring information for different behavioural phenotypes.
The greater effectiveness of bold demonstrators in mixed and shy-dominated groups may have resulted from bold fish generating more opportunities for social learning by interacting with the device more often [4,38]. Although there was no indication that bold fish were more likely to solve the task than shy individuals, they tended to enter the device more frequently once they were informed (though evidence for this latter effect was fairly weak). Alternately, bold individuals may have facilitated information transfer through more effective leadership, potentially enabled by stronger social attractive tendencies in shy individuals [7–9,13–16]. Although there was only a weak relationship at best between shyness and grouping tendencies, group sizes were recorded prior to the device's introduction. If interacting with the novel device was perceived as potentially risky, this may have promoted more cohesive shoaling behaviour [8], particularly in more risk-averse individuals.
Despite previous work linking boldness to increased reliance on individual exploration [3–6], the NBDA did not detect a difference in the rate of individual learning between bold and shy fish, nor did individual learning account for substantially more solving events in bold-dominated groups relative to mixed or shy-dominated ones. Within the group sizes used here, latencies to approach and interact with the device may have been determined more by collective behaviour than individual risk-taking tendencies [6,8]. For example, boldness determines how rapidly sticklebacks (Gasterosteus aculeatus) approach feeding sites when alone, whereas within groups of 10, approach latency is governed by consensus decision-making [8]. Nonetheless, the probability of solving the task (through either individual or social learning) increased with the proportion of risk-averse group mates. If shy individuals were more likely to form larger or more cohesive shoals in response to the device's introduction, such conditions could enable reduced investment in threat-sensitive behaviours (e.g. vigilance and refuging), a concomitant increase in activity and exploration [2], and ultimately increase the likelihood of solving the task. The extent to which variation in personality composition may alter collective responses to environmental challenges is an interesting area for further work.
In principle, our findings might be explained by bolder individuals entering the device first, with apparent differences across group compositions in the pathways of information flow simply reflecting the availability of potential learners. Put another way, rather than bolder individuals being more effective demonstrators in shy-dominated groups, it may be that they simply happened to enter the device first, such that subsequent solving events are largely consistent with social transmission from bold to shy individuals. However, our data do not support this interpretation for several reasons. The NBDA found no evidence that bolder individuals solved the task more rapidly through asocial means, nor were bold individuals disproportionately likely to be the first in a group to solve the task. Furthermore, there was little evidence that the overall likelihood of solving the task differed between bold and shy individuals. Instead, the results of the NBDA are more consistent with personality-based differences in social responses that shifted according to group personality composition.
In summary, both the likelihood of acquiring novel foraging information and the typical social learning pathways operating within guppy shoals depended on the ratio of risk-prone to risk-averse group members. By directing the spread of social information, group personality composition may alter the costs of learning, contingent on individual phenotype, suggesting that individuals may enhance their fitness by associating with group mates whose personalities complement their own [2]. Determining whether such effects may drive evolutionary responses—e.g. in grouping mechanisms, behavioural plasticity or social influence—remains a key next step [39]. Finally, it is important to note that we manipulated group composition solely in terms of risk-taking tendency, whereas in reality, groups vary along multiple behavioural axes [1,40]. By enabling fine-scale, multidimensional characterization of individual- and group-level behavioural variation, advances in high-resolution tracking offer a promising means to better capture this reality and elucidate the functional consequences of group personality composition.
Supplementary Material
Ethics
All experimental procedures and animal care protocols were approved by the University of Louisville's Institutional Animal Care and Use Committee (IACUC no. 13020).
Data accessibility
Raw data and code are available from the Dryad Digital Repository: https://dx.doi.org/10.5061/dryad.8sf7m0ck8 [41].
Authors' contributions
M.J.H. and L.A.D. conceived of and designed the experiment. M.J.H. collected the data. M.J.H. and W.H. analysed the data. M.J.H. drafted the manuscript and all authors contributed to revisions.
Competing interests
We declare we have no competing interests.
Funding
This work was supported by grants from the Animal Behavior Society and the Fisheries Society of the British Isles (awarded to M.J.H.) and by grants from the University of Louisville and the Kentucky Science and Engineering Foundation (awarded to L.A.D.).
References
- 1.Réale D, Reader SM, Sol D, McDougall PT, Dingemanse NJ. 2007. Integrating animal temperament within ecology and evolution. Biol. Rev. 82, 291–318. ( 10.1111/j.1469-185X.2007.00010.x) [DOI] [PubMed] [Google Scholar]
- 2.Webster MM, Ward AJW. 2011. Personality and social context. Biol. Rev. 86, 759–773. ( 10.1111/j.1469-185X.2010.00169.x) [DOI] [PubMed] [Google Scholar]
- 3.Dyer JRG, Croft DP, Morrell LJ, Krause J. 2009. Shoal composition determines foraging success in the guppy. Behav. Ecol. 20, 165–171. ( 10.1093/beheco/arn129) [DOI] [Google Scholar]
- 4.Trompf L, Brown C. 2014. Personality affects learning and trade-offs between private and social information in guppies, Poecilia reticulata. Anim. Behav. 88, 99–106. ( 10.1016/j.anbehav.2013.11.022) [DOI] [Google Scholar]
- 5.Smit JAH, van Oers K. 2019. Personality types vary in their personal and social information use. Anim. Behav. 151, 185–193. ( 10.1016/j.anbehav.2019.02.002) [DOI] [Google Scholar]
- 6.Kurvers RHJM, Adamczyk VMAP, van Wieren SE, Prins HHT. 2011. The effect of boldness on decision-making in barnacle geese is group-size-dependent. Proc. R. Soc. B 278, 2018–2024. ( 10.1098/rspb.2010.2266) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Aplin LM, Farine DR, Mann RP, Sheldon BC. 2014. Individual-level personality influences social foraging and collective behaviour in wild birds. Proc. R. Soc. B 281, 20141016 ( 10.1098/rspb.2014.1016) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.McDonald ND, Rands SA, Hill F, Elder C, Ioannou CC. 2016. Consensus and experience trump leadership, suppressing individual personality during social foraging. Sci. Adv. 2, e1600892 ( 10.1126/sciadv.1600892) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Harcourt JL, Ang TZ, Sweetman G, Johnstone RA, Manica A. 2009. Social feedback and the emergence of leaders and followers. Curr. Biol. 19, 248–252. ( 10.1016/j.cub.2008.12.051) [DOI] [PubMed] [Google Scholar]
- 10.Kurvers RHJM, van Oers K, Nolet BA, Jonker RM, van Wieren SE, Prins HHT, Ydenberg RC. 2010. Personality predicts the use of social information. Ecol. Lett. 13, 829–837. ( 10.1111/j.1461-0248.2010.01473.x) [DOI] [PubMed] [Google Scholar]
- 11.Marchetti C, Drent PJ. 2000. Individual differences in the use of social information in foraging by captive great tits. Anim. Behav. 60, 131–140. ( 10.1006/anbe.2000.1443) [DOI] [PubMed] [Google Scholar]
- 12.Webster MM, Ward AJW, Hart PJB. 2007. Boldness is influenced by social context in threespine sticklebacks (Gasterosteus aculeatus). Behaviour 144, 351–371. ( 10.1163/156853907780425721) [DOI] [Google Scholar]
- 13.Ward AJW, Thomas P, Hart PJB, Krause J. 2004. Correlates of boldness in three-spined sticklebacks (Gasterosteus aculeatus). Behav. Ecol. Sociobiol. 55, 561–568. ( 10.1007/s00265-003-0751-8) [DOI] [Google Scholar]
- 14.Michelena P, Jeanson R, Deneubourg J-L, Sibbald AM. 2010. Personality and collective decision-making in foraging herbivores. Proc. R. Soc. B 277, 1093–1099. ( 10.1098/rspb.2009.1926) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Jolles JW, Fleetwood-Wilson A, Nakayama S, Stumpe MC, Johnstone RA, Manica A. 2015. The role of social attraction and its link with boldness in the collective movements of three-spined sticklebacks. Anim. Behav. 99, 147–153. ( 10.1016/j.anbehav.2014.11.004) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.van Oers K, Klunder M, Drent PJ. 2005. Context dependence of personalities: risk-taking behavior in a social and a non-social situation. Behav. Ecol. 16, 716–723. ( 10.1093/beheco/ari045) [DOI] [Google Scholar]
- 17.Rendell L, Fogarty L, Hoppitt WJE, Morgan TJH, Webster MM, Laland KN. 2010. Cognitive culture: theoretical and empirical insights into social learning strategies. Trends Cogn. Sci. 15, 68–76. ( 10.1016/j.tics.2010.12.002) [DOI] [PubMed] [Google Scholar]
- 18.Magnhagen C, Staffan F. 2005. Is boldness affected by group composition in young-of-the-year perch (Perca fluviatilis)? Behav. Ecol. Sociobiol. 57, 295–303. ( 10.1007/s00265-004-0834-1) [DOI] [Google Scholar]
- 19.Nakayama S, Harcourt JL, Johnstone RA, Manica A. 2016. Who directs group movement? Leader effort versus follower preference in a stickleback fish of different personality. Biol. Lett. 12, 20160207 ( 10.1098/rsbl.2016.0207) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Brown C, Irving E. 2014. Individual personality traits influence group exploration in a feral guppy population. Behav. Ecol. 25, 95–101. ( 10.1093/beheco/art090) [DOI] [Google Scholar]
- 21.Franz M, Nunn CL. 2009. Network-based diffusion analysis: a new method for detecting social learning. Proc. R. Soc. B 276, 1829–1836. ( 10.1098/rspb.2008.1824) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Hoppitt W, Boogert NJ, Laland KN. 2010. Detecting social transmission in networks. J. Theor. Biol. 263, 544–555. ( 10.1016/j.jtbi.2010.01.004) [DOI] [PubMed] [Google Scholar]
- 23.Reader SM, Laland KN. 2000. Diffusion of foraging innovations in the guppy. Anim. Behav. 60, 175–180. ( 10.1006/anbe.2000.1450) [DOI] [PubMed] [Google Scholar]
- 24.Magurran AE. 2005. Evolutionary ecology: the Trinidadian guppy. Oxford, UK: Oxford University Press. [Google Scholar]
- 25.Chapman BB, Morrell LJ, Krause J. 2010. Unpredictability in food supply during early life influences boldness in fish. Behav. Ecol. 21, 501–506. ( 10.1093/beheco/arq003) [DOI] [Google Scholar]
- 26.Wilson ADM, Krause S, James R, Croft DP, Ramnarine IW, Borner KK, Clement RJG, Krause J. 2014. Dynamic social networks in guppies (Poecilia reticulata). Behav. Ecol. Sociobiol. 68, 915–925. ( 10.1007/s00265-014-1704-0) [DOI] [Google Scholar]
- 27.Pitcher TJ, Parrish JK. 1993. Functions of shoaling behaviour in teleosts. In Behaviour of teleost fishes (ed. Pitcher TJ.), pp. 363–439. London, UK: Chapman & Hall. [Google Scholar]
- 28.Stoffel MA, Nakagawa S, Schielzeth H. 2017. rptR: repeatability estimation and variance decomposition by generalized linear mixed-effects models. Methods Ecol. Evol. 8, 1639–1644. ( 10.1111/2041-210X.12797) [DOI] [Google Scholar]
- 29.Bates D, Maechler M, Bolker B, Walker S. 2015. Fitting linear mixed-effects models using lme4. J. Stat. Softw. 67, 1–48. ( 10.18637/jss.v067.i01) [DOI] [Google Scholar]
- 30.Brooks ME, Kristensen K, van Benthem KJ, Magnusson A, Berg CW, Nielsen A, Skaug HJ, Maechler M, Bolker BB. 2017. glmmTMB balances speed and flexibility among packages for zero-inflated generalized linear mixed modelling. R J. 9, 378–400. ( 10.32614/RJ-2017-066) [DOI] [Google Scholar]
- 31.Hartig F. 2019. DHARMa: residual diagnostics for hierarchical (multi-level/mixed) regression models. R package version 0.2.4 See https://CRAN.R-project.org/package=DHARMa.
- 32.Hoppitt W, Laland KN. 2013. Social learning: an introduction to mechanisms, methods, and models. Princeton, NJ: Princeton University Press. [Google Scholar]
- 33.Farine DR, Aplin LM, Sheldon BC, Hoppitt W. 2015. Interspecific social networks promote information transmission in wild songbirds. Proc. R. Soc. B 282, 20142804 ( 10.1098/rspb.2014.2804) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Burnham KP, Anderson DR. 2002. Model selection and multimodel inference: a practical information-theoretic approach. New York, NY: Springer-Verlag. [Google Scholar]
- 35.Morgan BJT. 2009. Applied stochastic modelling, 2nd edn Boca Raton, FL: Chapman & Hall/CRC Press. [Google Scholar]
- 36.Allen J, Weinrich M, Hoppitt W, Rendell L. 2013. Network-based diffusion analysis reveals cultural transmission of lobtail feeding in humpback whales. Science 340, 485–488. ( 10.1126/science.1231976) [DOI] [PubMed] [Google Scholar]
- 37.Hoppitt W, Photopoulou T, Hasenjager M, Leadbeater E. 2019. NBDA: a package for implementing network-based diffusion analysis. R package version 0.9.4. See https://github.com/whoppitt/NBDA/.
- 38.Dugatkin LA, Alfieri MS. 2003. Boldness, behavioral inhibition and learning. Ethol. Ecol. Evol. 15, 43–49. ( 10.1080/08927014.2003.9522689) [DOI] [Google Scholar]
- 39.Farine DR, Montiglio P-O, Spiegel O. 2015. From individuals to groups and back: the evolutionary implications of group phenotypic composition. Trends Ecol. Evol. 30, 609–621. ( 10.1016/j.tree.2015.07.005) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Jolles JW, Boogert NJ, Sridhar VH, Couzin ID, Manica A. 2017. Consistent individual differences drive collective behaviour and group functioning of schooling fish. Curr. Biol. 27, 2862–2868. ( 10.1016/j.cub.2017.08.004) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Hasenjager MJ, Hoppitt W, Dugatkin LA. 2020. Data from: Personality composition determines social learning pathways within shoaling fish Dryad Digital Repository. ( 10.5061/dryad.8sf7m0ck8) [DOI] [PMC free article] [PubMed]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Citations
- Hasenjager MJ, Hoppitt W, Dugatkin LA. 2020. Data from: Personality composition determines social learning pathways within shoaling fish Dryad Digital Repository. ( 10.5061/dryad.8sf7m0ck8) [DOI] [PMC free article] [PubMed]
Supplementary Materials
Data Availability Statement
Raw data and code are available from the Dryad Digital Repository: https://dx.doi.org/10.5061/dryad.8sf7m0ck8 [41].