Abstract
Foragers rely on various cues to assess predation risk. Information theory predicts that high certainty cues should be valued more than low certainty cues. We measured the latency of black-capped chickadees (Poecile atricapillus) to resume feeding during winter in response to cues that conferred different degrees of certainty about current predation risk: a high certainty visual cue (predator mount) and a lower certainty acoustic cue (conspecific mobbing calls), presented either alone or in combination. As predicted, chickadees took longer to resume feeding after the visual than the acoustic cue, and this effect was greatest under conditions of high starvation risk (i.e. low temperatures). Presenting both cues together produced the same foraging delay as the visual cue alone under low starvation risk, but surprisingly, resulted in lower responses under high starvation risk compared to the visual cue alone. We suggest that this may be due to prey using a form of information updating, whereby differences in the timing of perception of acoustic versus visual cues interacts with energetic constraint to shape perceived risk. Although the sequential perception of cues is likely in a range of decision-making contexts, studies manipulating the order in which cues are perceived are needed to test existing models of multimodal cue integration.
Keywords: information theory, cue uncertainty, foraging behaviour, risk-taking behaviour, black-capped chickadees, predation risk
1. Introduction
Animals have to balance the benefits of energy intake against the risk of predation while foraging, and this trade-off is mediated by multiple factors [1–4]. Conditions that place animals under greater energetic stress, such as lower temperature, or shorter day length for diurnal animals, will tend to favour relatively higher investment in foraging [1,4]. By contrast, ecological conditions that increase predation danger, either by increasing the probability of attack (e.g. the number of predators), or increasing the probability that an attack is successful (e.g. presence of concealing cover for predators) will favour higher investment in predator avoidance [1,4]. Numerous empirical studies have shown that foragers adjust their foraging behaviour adaptively in response to changing costs and benefits of foraging [2]. Such observations demonstrate that animals are able to track changes in food and predation risk landscapes. But how do they do this?
Food availability and quality can be assessed directly through encounters. However, foragers must be able to assess predation danger indirectly, because direct encounters with predators would presumably be too costly [2]. Indeed, in their seminal review, Lima and Dill identified understanding how components of predation risk are ‘measured’ by animals as a major gap in our understanding of decision-making under the risk of predation [2]. Since that time, numerous studies have begun to address this knowledge gap and it is clear that prey rely on multiple cue modalities to assess current predation risk (e.g. chemosensory [5], visual [6], and acoustic [7]), and further, that these modalities may differ in both the type and quality of information they provide [8]. For example, observation of a predator, a visual cue, provides complete certainty that a predator is currently present, but does not necessarily reveal the predator's current hunger level [8]. On the other hand, predator odour, a chemical cue, has a lasting presence in the environment in terrestrial systems, and therefore, on its own, does not provide a high degree of certainty that the predator is currently in the area [5], though it may provide accurate information about the predator's current hunger level or recent diet [8]. Many birds produce mobbing calls in response to the presence of a predator which provide social information to congeners about current risk. Although social information can be gathered inexpensively, it is potentially less accurate than personally generated information [9]. For example, mobbing calls can be elicited as false alarms from conspecifics or heterospecifics under stress or by the presence of novel objects or even deceiving alarms to gain access to food [10,11].
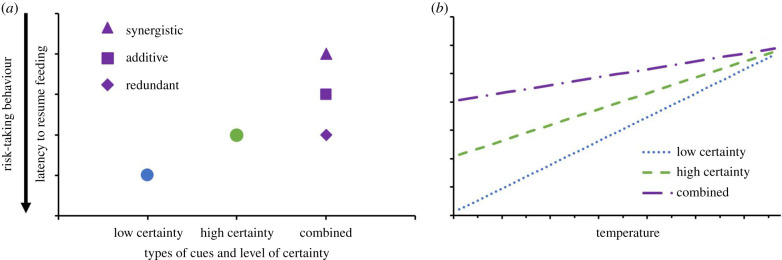
More recently, studies have begun to address how and under what circumstances prey might combine cues from multiple sensory modalities to improve their estimation of current predation risk [8,12]. The decision to respond to a cue or not is expected to depend on the level of reliability of the cue in relation to the level of uncertainty in the environment [8,13–15]. The magnitude of response to a cue is expected to be correlated with the extent to which it reduces uncertainty about the relevant environmental feature. If multiple cues contribute independently to the current assessment of predation risk, providing two complementary cues should reduce uncertainty about current risk levels more compared to either cue on its own [8]. Consequently, two complementary cues would be expected to elicit stronger responses than either cue alone (figure 1a) [8,16]. The extent of increase in response may be additive or synergistic depending on whether the relationship between risk assessment and anti-predator response is linear, as well as how combining cues increases certainty about current risk. Alternatively, if foragers rely only on the higher certainty cue in their assessment of current predation risk, providing a secondary (lower certainty) cue would be expected to elicit the same response as the high certainty cue alone (i.e. redundant effect; figure 1a) [16].
Figure 1.
Graphical representation of alternative hypotheses for how multiple sources of information (cues) affect assessment of current predation risk. Cues that provide greater certainty about current risk (e.g. observation of predator) are predicted to elicit stronger responses (measured as foraging interruption or latency to resume feeding) than cues that provide lower certainty about current risk (e.g. conspecific mobbing calls, predator odour). Panel (a) illustrates three scenarios depicting how multiple cues are used to assess risk. The square illustrates a scenario where multiple cues provide independent certainty about current risk levels, thereby creating an additive effect. The triangle illustrates a scenario where multiple cues provide independent certainty about current risk levels and interact with each other, increasing the magnitude of the response. The diamond illustrates a scenario where cues are redundant and multiple cues elicit a response equal to the higher certainty cue when presented alone. Panel (b) illustrates the predicted interaction between environmental conditions and certainty about current predation danger. Overall, latency is expected to be lower under conditions that increase the probability of mortality due to starvation (i.e. lower temperatures) and higher when the probability of mortality decreases (i.e. higher temperatures). We predict that there should be an interaction between temperature and level of certainty about current risk. With lower certainty about risk, the variation across temperature gradient will vary to a greater degree than when presented with a high certainty cue. For purposes of visualization, we present only the additive hypothesis for the effect of combined cues in interaction with temperature. (Online version in colour.)
In addition, the way that foragers adjust their behaviour in relation to their degree of certainty about current predation risk may be mediated by energetic constraints. Information received through different modalities will act in combination with environmental gradients to shape optimal decision-making [12,16]. Under conditions where foragers have a low risk of mortality due to starvation (e.g. high food availability and warm temperatures), foragers may show strong responses to cues of predation risk (e.g. long latency to resume feeding after detecting the cue) because the cost to mounting a strong response in terms of risk of starvation would be relatively low. However, under conditions of higher energetic constraint (e.g. low food availability and low temperatures), foragers may not only exhibit weaker responses overall but also discriminate more strongly based on the relative certainty associated with a given cue (figure 1b) [8,16].
In this study, we investigated how multiple cues of predation risk interact to shape anti-predator responses in free-living black-capped chickadees (Poecile atricapillus). First, we tested how cues of predation risk, both independently and in combination, affect risk-taking behaviour in chickadees. We used latency to resume feeding as our measure of risk-taking behaviour; longer latencies equated to stronger responses. We focused on two cues of predation risk with different degrees of certainty: a visual presentation of a merlin (Falco columbarius; higher certainty cue of predator presence) and acoustic playbacks of conspecific mobbing calls in response to merlin (lower certainty cue). Additionally, we tested whether greater energetic stress alters the way that chickadees value and integrate different sources of information about predation danger using average daily temperature as a proxy of energetic constraint. During our study, ambient temperatures were always well below the thermoneutral zone of chickadees [17] such that, all else being equal, lower temperatures corresponded to greater energetic constraint. We predicted that this environmental constraint would affect risk-taking behaviour such that the chickadees would resume feeding sooner when presented with low certainty cues as temperature decreases because we expected the relatively high certainty of starvation risk (assessed based on ambient temperature) would outweigh low certainty cues about predation risk when making foraging decisions (figure 1b).
2. Materials and methods
(a). Study area
This study was carried out at the University of Alberta Botanic Garden in Devon (UABG), Alberta, Canada (53°24′27″ N, 113°45′41″ W; electronic supplementary material, figure S1). The UABG is located 22 km SW of Edmonton and 6 km N of Devon within the Devon Dunes natural area. It is a 0.97 km2 property with 0.32 km2 of display gardens and 0.65 km2 of mixed forest. Temperature data used in this study was obtained from the Edmonton International Airport (YEG) weather station, located 10 km SE of the study site (data provided by Alberta Agriculture and Forestry, ACIS: https://agriculture.alberta.ca/acis).
(b). Study species
Black-capped chickadees (hereafter chickadees) are a common non-migratory bird distributed across North America. In winter, they form stable flocks that forage together and are communally vigilant for predators [18]. Flocks display a social hierarchy based on sex and age (males and older individuals hold higher ranks) [18]. Despite seasonal acclimation to low temperatures [19,20], chickadees face a high mortality risk during winter due to limited food availability and reduction in cover for protection from predators [18]. Chickadees use a complex system of vocalizations to communicate within the flock about predation danger [21]. They are also common visitors to anthropogenically provided food sources [18].
A marked population of chickadees was established at the UABG beginning in autumn 2017. Birds were caught using mistnets at feeders located throughout the study area (electronic supplementary material, figure S1). We did not use mobbing call playbacks during catching, as is commonly done with chickadees, to avoid influencing the types of birds captured based on their responsiveness to playbacks as this may have biased our results regarding chickadee responses to experimental playbacks. To avoid carry-over effects from capturing and handling, we ended catching one week prior to the start of data collection, and no catching occurred during the experiments.
Upon capture, all birds that were not already marked were fitted with Canadian Wildlife Service aluminium bands. Immediately after, two short standardized behavioural assays were carried out as part of another study (total duration <4 min). Basic morphometric data was collected (e.g. tarsus, bill, and wing length), body mass was recorded, and a small blood sample was taken from the brachial vein for molecular sexing [22]. All birds were fitted with a unique combination of colour bands to allow for visual recognition.
In total, 334 chickadees were captured prior to the experiment. As part of another study aimed to assess the effects of passive integrated transponder (PIT) tags and methods of PIT tag application, birds were randomly assigned to receive no PIT tag (N = 112), a PIT tag attached to a colour band (N = 141), or a PIT tag implanted subcutaneously (N = 81). The experiment described henceforth relied on PIT tags to automatically register visits by birds to feeders. Preliminary tests revealed that PIT tags embedded in leg bands were read with 100% reliability using our feeder set-up (353 radio frequency identification (RFID) registrations out of 353 video-recorded visits), but implanted PIT tags were not read (0 RFID registrations out of 204 video-recorded visits) (JDA-T 2018, unpublished data). The band-embedded PIT tags were 10 mm × 2 mm, while the implanted PIT tags were 8 mm × 2 mm (EM4102 frequency, Eccel Technology Ltd, UK). The lack of readings from implanted PIT tags was the result of the implanted PIT tags having a smaller detection radius due to their smaller size. Therefore, results presented below are only for birds with PIT tags embedded in leg bands.
(c). Experiment set-up and data collection
Experiments were performed between late November 2018 and early March 2019. We placed eight feeders baited with black-oil sunflower seeds throughout the study area with at least 270 m distance between adjacent feeders (electronic supplementary material, figure S1). Birds could only access seeds via the feeder opening, as discarded seeds became inaccessible (see electronic supplementary material, figure S2). At the beginning of October 2018, we placed antennas connected to RFID readers with an internal clock in the feeders at the point of access to the seeds. This set-up allowed us to register the time of visits and identity of all PIT-tagged birds that were using the feeders (5 cm detection radius, circuit boards and RFID antennas from Priority 1 Design, Australia; electronic supplementary material, figure S2). We visited the feeders every 4 days to replace batteries and to collect the data that had been registered to memory cards in the feeder circuit boards. Battery and SD card exchanges were always carried out on non-experimental days to avoid creating disturbances associated with these visits that might affect our measured responses to the experimental treatments. Details of the protocol for data collection from SD cards and visits to the feeders during experimental days are provided in electronic supplementary material, appendix S1.
To determine the effect of different cues of perceived predation danger, we carried out 1-h treatments using a 2 × 2 factorial design of two variables—visual cue present (yes/no) and acoustic cue present (yes/no)—that resulted in four levels of treatments: control (no visual and no acoustic cues of predation), acoustic only (acoustic), visual only (visual), and acoustic plus visual (acoustic + visual). The four different treatments were designed to simulate the presence of a predator in the natural setting as closely as possible. For the visual treatment, we used six different taxidermic mounts of juvenile merlins, each mounted on a base of wood that was attached to the top of a pole located 3 m in front of the feeder (referred to as ‘experimental pole’; electronic supplementary material, figure S3). Because the areas surrounding feeders were a mosaic of vegetation types, these mounts were visible at distances ranging from around 20 to 50 m (JDA-T 2018, personal observation). We used merlins as the predator species in this experiment because they are present in the study area throughout the winter (based on records in the eBird digital repository (https://ebird.org/species/merlin) and JDA-T 2018, personal observations) and are known to specialize on small birds, including chickadees [21]. The top of the pole was at the same level as the feeder and was facing the feeder opening (electronic supplementary material, figure S2). For the acoustic treatment, we used chickadee mobbing calls instead of predator calls because merlin do not vocalize when mounting attacks. The chickadee mobbing calls used were recorded in another population (around 40 km from this study population) in response to the same merlin mounts used in the present study. From these recordings, we created eight unique, 1-h files which were made up of alternating sequences of mobbing calls (ranging from 5 to 20 s in length and comprised of the mobbing calls of between 1 and 4 chickadees, repeated over 1-min periods) and bouts of silence (ranging from 60 to 180 s). Each of the eight unique files included the same range of flock sizes in the mobbing bouts (1–4). The sequence files were played back using portable speakers (Shockwave, Foxpro, Lewistown, PA; electronic supplementary material, figure S3) that were placed on the experimental poles during treatments (electronic supplementary material, figure S2) and could be heard at distances of approximately 80 m (JDA-T 2018, personal observation). Further details about the recordings are in electronic supplementary material, appendix S2. Because previous work in chickadees has already shown that they can distinguish different predators and respond to the syntax in mobbing calls (e.g. number of ‘dee’ notes) [21], we consider it unlikely that our treatments were perceived as novel objects/sounds. Therefore, our control treatments provided controls for only the non-biological components of our experimental treatments. The visual cue during the control treatment consisted of the experimental pole. The control treatment for the acoustic treatment consisted of the presence of the speaker. The dates of each treatment for each feeder (including the order of the merlin mounts and sound files used) are provided in electronic supplementary material, table S1.
We used a stratified random design to assign treatments to feeders so that (i) each experimental day a maximum of one experimental treatment was carried out at any given feeder and (ii) each experimental day, each of the four treatment levels was carried out (i.e. one control, one acoustic, one visual, and one combined). A complete replicate consisted of one set of all four treatments carried out at each of the eight feeders, which required a total of eight experimental days to complete. Treatment start times were approximately '09:30, 11:00, 12:30, and 14:00. Within each experimental day, the order of the treatments was randomized to avoid confounding variables related to the time of day. To minimize potential carry-over and/or habituation effects of our treatments, we only conducted treatments (experimental days) every second day during any given replicate, with at least 7 days break between successive replicates. This meant that within a given replicate, three 1-h long predator presentations (one visual, one acoustic, and one visual + acoustic) occurred at a single feeder over the course of 16 days. We carried out four complete replicates of the experiment at each of the eight feeders. A schematic representation of a replicate is provided in electronic supplementary material, figure S4.
(d). Data selection and processing
Following the approach used by Mathot et al. [23], we selected only individuals that were present at the feeders in the hour immediately preceding the start of any treatment. This was done to ensure that birds included in our analyses had been present in the vicinity of the feeder immediately prior to the 1-h treatment period and, therefore, likely to have been exposed to the experimental treatment. However, we acknowledge that as this was a study conducted in the field, we could not control which birds were present when a treatment was initiated. As such, stochastic differences in the timing of perception of cues within-individuals may contribute noise to the results, making our findings more conservative. Nonetheless, we confirmed that the choice to restrict analyses to individuals we deemed likely to have experienced the treatment (i.e. birds using the feeder in the 1 h before the treatment commenced) did not unduly influence our results by comparing results from this subset of birds against results using all birds, regardless of whether they were present in the hour immediately preceding the treatment. In all cases, the results were both qualitatively and quantitatively similar (see electronic supplementary material, table S2).
We calculated the response variable for risk-taking behaviour as latency to resume feeding (latency). Latency was defined as the time (in seconds) from the start of any treatment until the first return visit to the feeder on the same day. For the birds that did not return on the same day, we assigned a maximum latency score equivalent to if they had returned at civil twilight (the end of the foraging period). For further details on data processing and calculation of latency to resume feeding, see electronic supplementary material, appendix S3.
(e). Statistical analysis
We used R v. 3.6.0 for all statistical analyses [24]. We analysed the log-transformed latency to resume feeding in seconds as a function of sex, temperature, treatment, and the interaction of treatment and temperature as fixed effects. Treatment was coded with four levels: Control, Acoustic, Visual, and Acoustic + Visual. This was done to facilitate the interpretation of the models' outputs of treatments and their interactions with temperature, and to allow direct comparisons between all pairwise combinations of treatments. The average daily temperature was centred and standardized so that model estimates (other than temperature) reflect estimated effects at the average temperature during our study (−11.15°C). By standardizing average daily temperature, the estimated effect of temperature reflects the effect of 1 s.d. change in temperature on our response variable, facilitating comparison with other fixed effects in our models. Bird identity (79 levels) and feeder number (eight levels) were included as random effects to account for non-independence at these levels. We also included replicate as a random effect to account for potential habituation. If there was habituation to our stimuli across replicates, we would observe the significant among-replicate variance in latency to resume feeding. The random effects were crossed, as the same individual could be detected at more than one feeder, and across multiple replicates, and each replicate was carried out across all eight feeders. We constructed a linear mixed-effects model (LMM) with a Gaussian error distribution using the R package lme4 [25]. Effect sizes were calculated based on 1000 simulations to obtain values of a posterior distribution of the model. We used Markov chain Monte Carlo to obtain an estimate of the effect size (β) using kernel density estimation and 95% confidence intervals (CIs) with the R package MCMCglmm [26]. We considered our results to show strong support for an effect when the 95% CI was not overlapping between estimates. In comparison to a frequentist perspective, when the 95% CI is not overlapping zero or overlapping between estimates, it is equivalent to a p-value < 0.05. Estimates that were biased away from zero but whose 95% CI showed up to 15% overlap with zero were interpreted as showing ‘moderate support’ for an effect because this corresponds to estimates providing at least five times greater support for the interpretation of an effect compared to the interpretation of no effect. For these cases, we provide Bayesian p-values (i.e. the proportion of estimates that overlap). Estimates that were centred on zero were interpreted as showing no support for an effect, or strong support for lack of an effect. This form of interpretation allows us to avoid a dichotomous interpretation of the results that can show a continuous range of support for a given interpretation.
We ran three different models for latency to resume feeding. The first model included only the observations from birds that were present before the experiment and returned at any point within the same day (N = 951 observations of 79 individuals). The second included all observations of individuals that were present before the start of the experiment regardless of whether they returned to the feeder after the treatment occurred (N = 1009 observations of 79 individuals), and the third model included all birds that were recorded at the feeder post-treatment, regardless of whether they were present in the hour immediately preceding the treatment or not (N = 1197 observations of 79 individuals). These models were all qualitatively and quantitatively similar (electronic supplementary material, table S2), indicating that our results were not contingent on our data selection criteria. We present only the results for the model including birds that were present prior to the treatment and that also returned within the same day in the main text. We did not include merlin mount identities or mobbing call sequence numbers in the models as they were assigned randomly for all the trials through the experiment; we assumed that they did not affect the estimates of the other variables and we were not interested in measuring the effect of these variables in this study. Finally, we calculated adjusted repeatability following Nakagawa and Schielzeth [27].
To aid in post hoc interpretation of the latency to resume feeding results, we also evaluated the effect of treatment, temperature, and their interaction on feeding rates once chickadees resumed feeding. Because we were interested in the short-term effect of perceived predation risk on feeding rates, we used the number of visits to the feeder in the first 20 min after a bird returned to the feeder following a treatment to estimate feeding rate (visits/hour = count of visits over 20 min period × 3). The models had the same structure as the model described above for latency to resume feeding but included the additional fixed effect ‘latency to resume feeding’ (in seconds, centred, and scaled). This was done to allow us to disentangle the effects of current energetic needs which would increase with decreasing temperature and with increasing latency to resume feeding from the effect of current perceived risk.
3. Results
A total of 79 individuals (47 males and 32 females) were registered at the feeders before the experimental trials, with each bird present for an average of 13 experimental trials (range 1–23.) Note, that while there were a total of 16 trials per feeder, some birds were recorded at multiple feeders, hence the possibility to be observed >16 times. Birds with few observations were retained in the analyses because they increase power to estimate fixed effects, even if they do not contribute to the estimation of random effects [28]. The number of birds present before (B) and after (A) treatments did not vary as a function of treatment type (control: N = 76 (B), N = 75 (A); acoustic: N = 75 (B), N = 75 (A); visual: N = 75 (B), N = 74 (A); acoustic + visual: (N = 76 (B), N = 74 (A)).
There was substantial variation in the latency to resume feeding, and these differed as a function of treatment (Control: mean = 21.6 min, range = 0.7–249.5 min; Acoustic: mean = 37.0 min, range = 0.5–370.7 min; Visual: mean = 52.6, range = 0.4–291.5 min; Acoustic + Visual: mean = 48.0 min, range = 0.5–206.1 min). Analyses of latency to resume feeding as a function of our four treatment levels showed that chickadees responded differently to different cues, and that these relationships interacted with ambient temperature (table 1 and figure 2). Latency to resume feeding was shorter at colder temperatures for all treatments except the visual treatment (table 1 and figure 2). Latency to resume feeding following the visual treatment was longer than all other treatments and did not vary as a function of temperature (table 1 and figure 2). Across all temperatures, the latency to resume feeding was shortest for the control, followed by acoustic treatment (table 1 and figure 2). At low temperatures, the latency to resume feeding following the acoustic + visual treatment was shorter than following the visual treatment alone, but at high temperatures, the effect sizes were nearly identical (table 1 and figure 2). There was no support for important among-feeder variance, suggesting that flocks responded similarly to the treatments. There was also no support for important among-replicate variance, consistent with the notion that there was not significant habituation to the experimental treatments. However, there were large, repeatable, among-individual differences in the latency to resume feeding (r = 0.21 95% CI = 0.14, 0.30; table 1). The observed differences in latency to resume feeding between the fastest returning individuals and the slowest returning individuals was approximately 80 min, which represents >15% of the foraging day for chickadees at this latitude during winter, a biologically important difference.
Table 1.
LMM model results for latency to resume feeding (LRF) and foraging rates (FR). The models included only those birds that returned to the feeder on the same day of the treatment.
| LMM Log (LRF seconds) | FR (visits h−1) | |
|---|---|---|
| fixed effects | β (95% CI) | β (95% CI) |
| sexa | 0.01 (−0.27, 0.33) | −3.38 (−5.82, −1.14) |
| treatment | ||
| control | 6.49 (6.01, 6.84) | 19.61 (17.51, 22.16) |
| acoustic | 6.64 (6.23, 7.04) | 16.39 (14.12, 18.77) |
| visual | 7.55 (7.15, 7.96) | 17.11 (14.67, 19.31) |
| both | 7.43 (7.02, 7.85) | 17.11 (14.91, 19.70) |
| temperatureb | ||
| temperature by control | 0.22 (0.06, 0.34) | 0.14 (−1.05, 1.28) |
| temperature by acoustic | 0.23 (0.08, 0.36) | −1.49 (−2.85, −0.42) |
| temperature by visual | 0.09 (−0.08, 0.21) | −0.86 (−2.14, 0.28) |
| temperature by acoustic + visual | 0.18 (0.06, 0.37) | −0.08 (−1.46, 1.18) |
| LRFc | NA | 1.96 (1.32, 2.63) |
| random effects | σ (95% CI) | σ (95% CI) |
| individual N = 79 | 0.33 (0.19, 0.50) | 17.09 (9.36, 25.12) |
| feeders N = 8 | 0.12 (0.03, 0.57) | 0.09 (0.00, 0.24) |
| replicate N = 4 | 0.00 (0.00, 0.07) | 3.78 (0.00, 13.66) |
| residual N = 951 | 1.23 (1.14, 1.38) | 87.0 (78.14, 95.01) |
| repeatabilityd | r (95% CI) | r (95% CI) |
| individual | 0.21 (0.14, 0.30) | 0.16 (0.10, 0.24) |
aSex was coded as males = −0.5 and females = 0.5 so that effect estimate is the difference between males and females, and all other estimates are for ‘average sex’ (i.e. the mid-point between males and females).
bMean daily temperature, grand mean centred and divided by 2 s.d.
cLRF, grand mean centred and divided by 2 s.d.
dAdjusted repeatability estimated after taking into account fixed effects.
Figure 2.
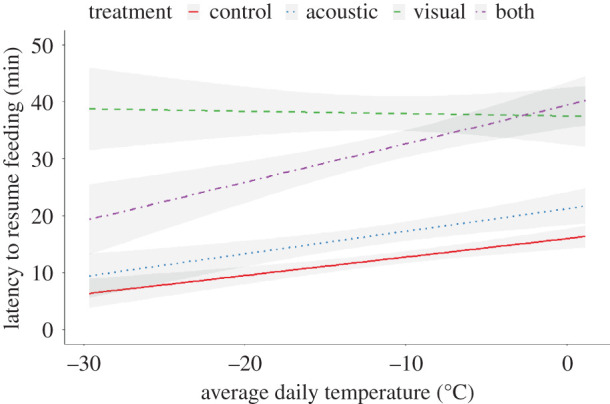
Prediction from the model presented in table 1 for latency to resume feeding measured in minutes in response to the average daily temperature under different treatments. The lines represent the regression of different treatments of cues about predator presence as a function of temperature. The grey areas represent 95% CIs. (Online version in colour.)
We also analysed foraging rates in the 20 min after birds resumed feeding (i.e. the short-term feeding rate response to perceived predation). Latency to resume feeding was positively correlated with feeding rate, when birds took longer to resume feeding after a treatment, they fed at significantly higher rates once they returned (table 1 and figure 3), suggesting that greater latency to resume feeding came at the cost of increased probability of mortality due to starvation. Feeding rates varied across treatments and temperatures such that at milder temperatures, feeding rates were highest following the control treatment, with progressively lower feeding rates for the combined, visual, and acoustic treatments (table 1 and figure 3). At more extreme cold temperatures, feeding rates were relatively similar across all treatments following the return to the feeder (table 1 and figure 3). Again, there was no support for important among-feeder variance, suggesting that flocks responded similarly to the treatments. Additionally, the among-replicate variance was low, consistent with the notion that there was not significant habituation to the experimental treatments. However, there were large, repeatable, among-individual differences in feeding rates (r = 0.16, 95% CI = 0.10, 0.24; table 1). The observed differences in feeding rates (controlling for latency to resume feeding) were again large, with the chickadees with the lowest feeding rates having an average of 2.14 visits h−1, compared with 31.5 visits h−1 for the chickadees with the highest feeding rates, an approximately 15-fold difference.
Figure 3.
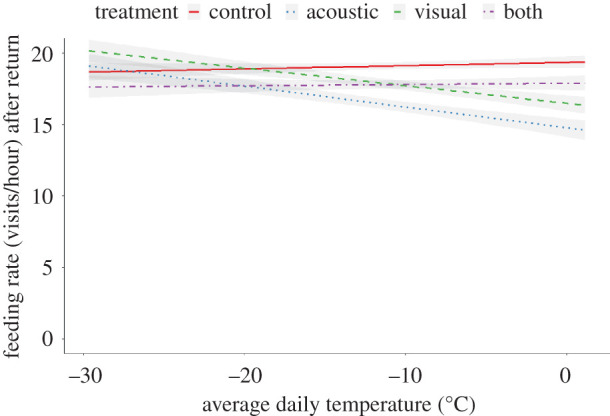
Prediction from the model presented in table 1 for feeding rate (visits h−1) in response to the average daily temperature under different treatments, controlling for latency to resume feeding. The lines represent the regression of different treatments of cues about predator presence as a function of temperature. The grey areas represent 95% CIs. (Online version in colour.)
4. Discussion
Prey can use a variety of cues to evaluate current predation risk, and recently attention has shifted towards understanding how and under what circumstances they should integrate cues across different sensory modalities [8,12]. We studied how chickadees respond to two types of cues of predation danger (visual and acoustic) that vary in the degree of certainty, and how combining these cues shapes foraging decisions. Our study showed that chickadees responded to cues of predation danger by delaying foraging compared with a control disturbance in the vicinity of the feeder, and that they responded more strongly to visual cues compared with acoustic cues across all temperatures recorded in this study. We also found support for cue redundancy, but only under low energetic constraint (i.e. warmer temperatures). Under high energetic constraints, multimodal cues produced intermediate responses compared to either cue type presented alone. Below, we discuss these results in the context of how these cues may have altered perceived predation and perceived competition, as well as the potential importance of differences in the timing of cue perception.
We were interested in understanding how combining cues that provide different degrees of certainty about current predation risk influence decision-making in chickadees. We used predator mounts (merlin) and playbacks of conspecific mobbing calls with the a priori expectation that the visual cue would provide greater certainty that a predator was currently in the area compared with mobbing calls, which can be given as false alarms as shown in other closely related species [10]. Two lines of evidence support this assumption. First, chickadees exhibited stronger responses to the visual cue compared to the acoustic cue across all temperatures recorded in this study (figure 2). Second, we observed that the response to the visual cue was similar across all average daily temperatures recorded during the study, indicating that even under high energetic constraints (i.e. low temperatures), the visual cue elicited strong predator avoidance behaviour. By contrast, at low temperatures, the acoustic cue elicited a foraging delay that was approximately half of that which it elicited under more benign conditions (figure 2). We interpret this as evidence that the acoustic cue was perceived as a lower certainty cue, and thus, reliance on it was devalued under conditions of high starvation risk.
Given that the response to the visual cue was strong and was not modulated by energetic constraints, we predicted to observe a redundant effect when the acoustic cue was presented together with the visual cue. However, this was only observed at relatively mild temperatures. At very low temperatures, chickadees presented with both acoustic and visual cues showed responses that were intermediate to when they were presented with either cue type alone. These results suggest that the integration of multimodal cues is context-dependent, which is consistent with predictions from a recent model of multimodal cue integration [12] and several earlier empirical studies. For example, grey squirrels (Sciurus corlinensis) show multimodal enhancement (i.e. synergism) in response to combined visual and vocal alarm signals from conspecifics, but only in populations with low anthropogenic noise levels [29]. Similarly, reproductive female round gobies (Neogobius melanostomus) exhibit synergistic responses to combined chemical and acoustic cues from reproductive males, but non-reproductive females show little response to these cues when they are presented either alone or in combination [30]. However, the specific form of integration observed in our study at low temperatures was unexpected. Rather than relying on a single cue under high energetic constraint, chickadees showed a response that was intermediate to their response when either cue was presented alone. Below, we discuss three alternative explanations for these results.
First, the acoustic cue may have simultaneously manipulated two perceived dimensions of risk: predation and competition, with the relative importance of competition being greater under high energetic constraint. Previous studies have demonstrated that chickadees can recognize other individuals and flocks by specific components of the calls [31]. Thus, by presenting an acoustic signal from another flock, it is possible that the chickadees perceived a greater number of birds in the surrounding area, which may have increased perception of local resource competition [32]. Under high energetic constraints (i.e. low temperatures), avoiding starvation should become relatively more important. Thus, the presence of the acoustic cue, either alone or in combination with the visual cue, would be expected to favour relatively shorter latencies to resume feeding, but also, higher feeding rates relative to the control. Our analyses of feeding rates post-treatment do not support this idea. At the lowest temperatures in our study, feeding rates were relatively similar across all treatment types after controlling for treatment-related differences in latency to resume feeding (figure 3), and there was no support for the interpretation that feeding rates were elevated when chickadees received acoustic cues under the conditions of our experiment.
An alternative explanation for the intermediate response to the combined cue in terms of latency to resume feeding is that the presence of acoustic cues manipulated perceived dilution of risk benefits via the increased perceived group size [33]. The many-eyes hypothesis posits that group-level vigilance increases with group size. Therefore, by perceiving additional individuals in the acoustic treatment in the present study, the perceived risk should have decreased, and the feeding rates in the absence of acoustic cues should have actually been lower relative to feeding rates in the presence of acoustic cues. However, this was not the response we found (figure 3). Our playbacks included a relatively small number of chickadees vocalizing (range 1–4 within any given bout). Using playbacks with a larger range of conspecifics vocalizing would provide a more comprehensive test of the potential role of competition and/or dilution in buffering the response to the multimodal cue under high energetic constraints.
Finally, we suggest that differences in the timing of cue perception may account for the intermediate response to multimodal cues observed under conditions of high energetic stress. If prey use Bayesian updating of risk assessment, the response to information provided by the second cue may be contingent on the information provided by the first cue [e.g. 12]. For example, perceiving the visual cue of predation first may convey high certainty information to the focal chickadee that the predator is present, and the absence of alarm calls initially may convey information that the threat is not currently being attended to. This may elicit a stronger response compared to the reverse scenario, where a chickadee first hears mobbing calls—identifying both the presence of a threat (with lower certainty compared to the visual cue) and high certainty that if the threat is real, it is being attended to. Subsequently, observing the visual cue would reaffirm this information, while increasing certainty that the threat is real. The two studies described above which found synergistic effects of multimodal cues both employed experimental designs that allowed both cues to be perceived simultaneously by the focal individuals [29,30]. In our study, although the two cues were presented at the same time, the acoustic cue could likely have been perceived sooner given that it could be detected at relatively longer distances (around 80 m, JDA-T 2018, personal observation) compared to the visual cue (20–50 m, JDA-T 2018, personal observation). We are only aware of one other study in which a combined multimodal cue elicited an intermediate response compared to each cue presented alone. In that study, yellow-bellied marmots (Marmota flaviventer) were presented with olfactory (coyote, Canis latrans, urine) and acoustic (coyote vocalizations) cues of predation risk alone and in combination [12]. Similar to our study, the acoustic cue would likely have been detectable at larger distances compared to the olfactory cue, and the authors similarly found that the multimodal foraging response was intermediate to the chemical (urine) or acoustic (coyote vocalizations) cues in isolation [12]. Together, these results are consistent with Bayesian updating model for cue integration, though follow-up experiments that manipulate the order of presentation of cues would be insightful.
Although not the focus of the current study, we also observed large among-individual differences in both the latency to resume feeding and subsequent feeding rates (table 1). The extent of repeatable among-individual variation observed in this study was relatively high compared with two previous studies in great tits, Parus major (r ≈ 0.06 [23,34]). The relatively high degree of repeatable among-individual differences in risk-taking observed in our experiment may be due to the fact that chickadees have strong and stable social hierarchies compared with great tits [35], and social rank is known to affect foraging behaviour, including risk-taking, in a range of passerines [36,37]. However, assessing whether this contributed to the high repeatability observed in the present study would require data on the social rank of individuals, which is not currently available in this population of chickadees.
5. Conclusion
Our results are consistent with the notion that cue certainty influences foraging decisions in chickadees, and that multimodal cue integration is context-dependent [12,29,30]. At mild temperatures, observed latencies to resume feeding were consistent with cue redundancy. However, at low temperatures, chickadees responded to the multimodal cue with a response that was intermediate to the response observed to either cue in isolation. We suggest that this response may result from Bayesian updating of sequentially perceived cues [12], but further studies explicitly testing the effect of cue order are needed. Understanding how organisms combine multiple sources of information has application for a range of decision-making contexts [12]. For example, mate choice decisions can be based on a combination of visual (e.g. plumage and mating displays) and acoustic (e.g. song) cues, and multiple types of cues are often considered either simultaneously or in sequence [38–40]. Understanding the types of conditions that lead to redundant, intermediate, additive, or synergistic effects, including the timing and sequence of cue perception, will allow for a more holistic understanding of uncertainty management in animal decision-making.
Supplementary Material
Acknowledgements
We thank Colleen St. Clair for the insights on the design and interpretation of results of this study. Thank you to Erin Bayne and Catrionna Leven for loaning the recording equipment used to generate our playback files, Jenna Congdon for helping record mobbing calls from wild chickadees, Sheeraja Sridharan for molecular sexing of the chickadees, Megan Westervelt for offering editorial comments, and Lee Foote for facilitating our work at the University of Alberta Botanic Garden. Two anonymous referees provided comments and suggestions that greatly improved the manuscript.
Ethics
These experiments were carried out under permits to K.J.M. and J.J.W. for catching and banding of chickadees from the Bird Banding Office in Canada (banding permit 10277 AK and 10277 AL), permits for experiments from the University of Alberta Biosciences Animal Care and Use Committee (ACUC) (permit AUP00002542), an Environment Canada Canadian Wildlife Service Scientific permit (#13-ABSC004) and an Alberta Fish and Wildlife Capture and Research permits (#56066, #56065, 19-056).
Data accessibility
The datasets supporting this article are in the electronic supplementary material. All data required for the analyses presented in this paper, including R code, are available from the Dryad Digital Repository: https://doi.org/10.5061/dryad.ksn02v72h [41].
Authors' contributions
J.D.A-T. conceived the study. J.D.A-T. and J.J.W. carried out the field work. J.D.A-T. was responsible for data processing and data analysis. J.D.A-T. and K.J.M. wrote the manuscript. All co-authors contributed to study design and manuscript revisions.
Competing interests
We declare we have no competing interests.
Funding
This research was supported by the Alberta Conservation Association (ACA) Grants in Biodiversity to J.D.A-T., a Natural Sciences and Engineering Research Council (NSERC) Discovery Grant to K.J.M. [grant no. RGPIN-2018-04358], the University of Alberta Start-up Funds to K.J.M., and a CRC Research Grant to K.J.M.
References
- 1.Bednekoff PA. 2007. Foraging in the face of danger. In Foraging: behavior and ecology (eds Stephens DW, Brown JS, Ydenberg RC), p. 305 Chicago, IL: University of Chicago Press. [Google Scholar]
- 2.Lima SL, Dill LM. 1990. Behavioral decisions made under the risk of predation: a review and prospectus. Can. J. Zool. 68, 619–640. ( 10.1139/z90-092) [DOI] [Google Scholar]
- 3.Houston AI, McNamara JM, Hutchinson JMC. 1993. General results concerning the trade-off between gaining energy and avoiding predation. Phil. Trans. R. Soc. B 341, 375–397. ( 10.1098/rstb.1993.0123) [DOI] [Google Scholar]
- 4.McNamara JM, Houston AI, Lima SL. 1994. Foraging routines of small birds in winter: a theoretical investigation. J. Avian Biol. 25, 287–302. ( 10.2307/3677276) [DOI] [Google Scholar]
- 5.Kats LB, Dill LM. 1998. The scent of death: chemosensory assessment of predation risk by prey animals. Ecoscience 5, 361–394. ( 10.1080/11956860.1998.11682468) [DOI] [Google Scholar]
- 6.Caro T. 2005. Antipredator defenses in birds and mammals, p. 591 Chicago: University of Chicago Press. [Google Scholar]
- 7.Hettena AM, Munoz N, Blumstein DT. 2014. Prey responses to predator's sounds: a review and empirical study. Ethology 120, 427–452. ( 10.1111/eth.12219) [DOI] [Google Scholar]
- 8.Munoz NE, Blumstein DT. 2012. Multisensory perception in uncertain environments. Behav. Ecol. 23, 457–462. ( 10.1093/beheco/arr220) [DOI] [Google Scholar]
- 9.Webster MM, Laland KN. 2008. Social learning strategies and predation risk: minnows copy only when using private information would be costly. Proc. R. Soc. B 275, 2869–2876. ( 10.1098/rspb.2008.0817) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Møller AP. 2010. False alarm calls as a means of resource usurpation in the Great Tit Parus major. Ethology 79, 25–30. ( 10.1111/j.1439-0310.1988.tb00697.x) [DOI] [Google Scholar]
- 11.Munn CA. 1986. Birds that ‘cry wolf’. Nature 319, 143–145. ( 10.1038/319143a0) [DOI] [Google Scholar]
- 12.Munoz NE, Blumstein DT. 2020. Optimal multisensory integration. Behav. Ecol. 31, 184–193. ( 10.1093/beheco/arz175) [DOI] [Google Scholar]
- 13.McLinn CM, Stephens DW. 2006. What makes information valuable: signal reliability and environmental uncertainty. Anim. Behav. 71, 1119–1129. ( 10.1016/j.anbehav.2005.09.006) [DOI] [Google Scholar]
- 14.Shannon CE. 1948. A mathematical theory of communication. Bell Syst. Tech. J. 27, 379–423. [Google Scholar]
- 15.Stephens DW, Dunlap AS. 2009. Why do animals make better choices in patch-leaving problems? Behav. Processes 80, 252–260. ( 10.1016/j.beproc.2008.11.014) [DOI] [PubMed] [Google Scholar]
- 16.Weissburg M, Smee DL, Ferner MC. 2014. The sensory ecology of nonconsumptive predator effects. Am. Nat. 184, 141–157. ( 10.1086/676644) [DOI] [PubMed] [Google Scholar]
- 17.Chaplin SB. 1976. Physiology of hypothermia in black-capped chickadee, Parus atricapillus. J. Comp. Physiol. 112, 335–344. ( 10.1007/bf00692303) [DOI] [Google Scholar]
- 18.Smith SM. 1992. The black-capped chickadee: behavioral ecology and natural history. Ithaca, NY: Cornell University Press. [Google Scholar]
- 19.Chaplin SB. 1974. Daily energetics of the black-capped chickadee, Parus atricapillus, in winter. J. Comp. Psychol. 89, 321–330. ( 10.1007/bf00695350) [DOI] [Google Scholar]
- 20.Cooper SJ, Swanson DL. 1994. Seasonal acclimatization of thermoregulation in the black-capped chickadee. The Condor 96, 638–646. ( 10.2307/1369467) [DOI] [Google Scholar]
- 21.Templeton CN, Greene E, Davis K. 2005. Allometry of alarm calls: black-capped chickadees encode information about predator size. Science 308, 1934–1937. ( 10.1126/science.1108841) [DOI] [PubMed] [Google Scholar]
- 22.Griffiths R, Double MC, Orr K, Dawson RJ. 1998. A DNA test to sex most birds. Mol. Ecol. 7, 1071–1075. ( 10.1046/j.1365-294x.1998.00389.x) [DOI] [PubMed] [Google Scholar]
- 23.Mathot KJ, Nicolaus M, Araya-Ajoy YG, Dingemanse NJ, Kempenaers B, Grémillet D. 2015. Does metabolic rate predict risk-taking behaviour? A field experiment in a wild passerine bird. Funct. Ecol. 29, 239–249. ( 10.1111/1365-2435.12318) [DOI] [Google Scholar]
- 24.R Core Team. 2019. R: A language and environment for statistical computing. Vienna, Austria: R Foundation for Statistical Computing. [Google Scholar]
- 25.Bates D, Mächler M, Bolker B, Walker S.. 2015. Fitting linear mixed-effects models using lme4. J. Stat. Softw. 67, 1–48. ( 10.18637/jss.v067.i01). [DOI] [Google Scholar]
- 26.Hadfield JD. 2010. MCMC methods for multi-response generalized linear mixed models: the MCMCglmm R package. J. Stat. Softw. 33, 1–22.20808728 [Google Scholar]
- 27.Nakagawa S, Schielzeth H. 2010. Repeatability for Gaussian and non-Gaussian data: a practical guide for biologists. Biol. Rev. Camb. Philos. Soc. 85, 935–956. ( 10.1111/j.1469-185X.2010.00141.x) [DOI] [PubMed] [Google Scholar]
- 28.Martin JGA, Nussey DH, Wilson AJ, Réale D. 2011. Measuring individual differences in reaction norms in field and experimental studies: a power analysis of random regression models. Methods Ecol. Evol. 2, 362–374. ( 10.1111/j.2041-210x.2010.00084.x) [DOI] [Google Scholar]
- 29.Partan SR, Fulmer AG, Gounard MAM, Redmond JE. 2010. Multimodal alarm behavior in urban and rural gray squirrels studied by means of observation and a mechanical robot. Cur. Zool. 56, 313–326. ( 10.1093/czoolo/56.3.313) [DOI] [Google Scholar]
- 30.Kasurak AV, Zielinski BS, Higgs DM. 2012. Reproductive status influences multisensory integration responses in female round gobies, Neogobius melanostomus. Anim. Behav. 83, 1179–1185. ( 10.1016/j.anbehav.2012.02.008) [DOI] [Google Scholar]
- 31.Nowicki S. 1983. Flock-specific recognition of chickadee calls. Behav. Ecol. Sociobiol. 12, 317–320. ( 10.1007/bf00302899) [DOI] [Google Scholar]
- 32.Lima SL. 1990. The influence of models on the interpretation of vigilance. In Interpretation and explanation in the study of animal behavior (eds Bekoff M, Jamieson D), pp. 246–267. Boulder, CO: Westview Press. [Google Scholar]
- 33.Lima SL. 1995. Back to the basics of anti-predator vigilance: the group-size effect. Anim. Behav. 49, 11–20. [Google Scholar]
- 34.Quinn JL, Cole EF, Bates J, Payne RW, Cresswell W. 2012. Personality predicts individual responsiveness to the risks of starvation and predation. Proc. R. Soc. B 279, 1919–1926. ( 10.1098/rspb.2011.2227) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Otter KA. 2007. Ecology and behavior of chickadees and titmice: an integrated approach. New York, NY: Oxford University Press. [Google Scholar]
- 36.Hogstad O. 1989. Social organization and dominance behavior in some Parus species. Wilson Bull. 101, 254–262. ( 10.2307/4162728) [DOI] [Google Scholar]
- 37.Koivula K, Lahti K, Rytkönen S, Orell M. 1994. Do subordinates expose themselves to predation? Field experiments on feeding site selection by willow tits. J. Avian Biol. 25, 178–183. ( 10.2307/3677073) [DOI] [Google Scholar]
- 38.Balsby TJS, Dabelsteen T. 2002. Female behaviour affects male courtship in whitethroats, Sylvia communis: an interactive experiment using visual and acoustic cues. Anim. Behav. 63, 251–257. ( 10.1006/anbe.2001.1920) [DOI] [Google Scholar]
- 39.Gonzalez-Voyer A, den Tex RJ, Castello A, Leonard JA. 2013. Evolution of acoustic and visual signals in Asian barbets. J. Evol. Biol. 26, 647–659. ( 10.1111/jeb.12084) [DOI] [PubMed] [Google Scholar]
- 40.Jennions MD, Petrie M. 1997. Variation in mate choice and mating preferences: a review of causes and consequences. Biol. Rev. Camb. Philos. Soc. 72, 283–327. ( 10.1017/s0006323196005014) [DOI] [PubMed] [Google Scholar]
- 41.Arteaga-Torres JD, Wijmenga JJ, Mathot KJ. 2020. Supplementary Data of “Visual cues of predation risk outweigh acoustic cues: a field experiment in black-capped chickadees” Dryad Digital Repository. ( 10.5061/dryad.ksn02v72h) [DOI] [PMC free article] [PubMed]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Citations
- Arteaga-Torres JD, Wijmenga JJ, Mathot KJ. 2020. Supplementary Data of “Visual cues of predation risk outweigh acoustic cues: a field experiment in black-capped chickadees” Dryad Digital Repository. ( 10.5061/dryad.ksn02v72h) [DOI] [PMC free article] [PubMed]
Supplementary Materials
Data Availability Statement
The datasets supporting this article are in the electronic supplementary material. All data required for the analyses presented in this paper, including R code, are available from the Dryad Digital Repository: https://doi.org/10.5061/dryad.ksn02v72h [41].