Abstract
Honeybees can be directed to profitable food sources by following waggle dances performed by other bees. Followers can often choose between using this social information or relying on memories about food sources they have visited in the past, so-called private information. While the circumstances that favour the use of either social or private information have received considerable attention, still little is known about the neurophysiological basis of information use. We hypothesized that octopamine and dopamine, two biogenic amines with important functions in reward signalling and learning, affect dance use in honeybees. We orally administered octopamine and dopamine when bees collected food at artificial feeders and tested if this affected interest in dance information about a new food source. We predicted that octopamine reduces interest in dances and strengthens private information use via an increase in the perceived value of the previously exploited resource. Since dopamine has been shown to lower reward perception, we expected it to act in the opposite direction. Octopamine-treated foragers indeed followed 32% fewer dances than control bees and increased the use of private information. Conversely, dopamine-treated bees followed dances 15% longer than control bees, but surprisingly did not use social information more. Overall, our results suggest that biogenic amine signalling affects interactions among dancers and dance followers and, thus, information flow about high-quality food sources.
Keywords: honeybees, waggle dances, social learning, reward system, octopamine, dopamine
1. Introduction
Social learning is a learning that is influenced by other individuals or their products, either through observation or interaction [1,2]. Honeybees, Apis spp., use a unique form of social learning, the waggle dance communication [3–7]. During their waggle dances, dancers attract hivemates and provide them with information about the location and odour of a food source [3,6–10]. Experienced foragers can decide to follow dances and decode this vector information (social information) or to revisit food sources they remember from previous foraging trips (private information) [11–16]. The dance follower's interest in social information can be gauged by the number of waggle runs followed, with bees that decode waggle dances following more waggle runs [11,13,14]. A third strategy, called scouting, is to ignore both social and private information about foraging locations and search for a new food source independently [6,7,17]. Empirical and theoretical studies suggest that the benefits of independent exploration, social information and private information depend strongly on the spatio-temporal distribution of food sources [18–22].
While social information use has been studied extensively from a behavioural ecological perspective [2,17,23–25], less is known about the molecular and neurophysiological basis of the decision to use social versus private information. Previous research suggests that, in honeybee foragers, the perception of rewards is likely to play an important role in the use of social and private information. When foragers experience that their food source is no longer rewarding, they increase their dance following and social information use [13,14], whereas foragers that experienced higher-quality rewards in the past use private information more [26]. Likewise, when foragers exploit more distant (and thus less profitable) food sources, they are more likely to use social dance information [16]. This suggests that neurophysiological mechanisms of reward perception play an important role in the decision to use waggle dance information versus private information. Octopamine (OA) and dopamine (DA) are biogenic amines that function as neuromodulators in the central nervous system of invertebrates, and they play important roles in reward signalling in honeybees [27–31]. They bind to the specific membrane proteins mainly belonging to the family of G-protein-coupled receptors in different parts of the brain [32–35], such as the mushroom bodies and the antennal lobes (i.e. brain areas with important functions in the processing and integration of information [36–38]). OA mediates the reward information during reward learning and, if administered to honeybees, increases the responsiveness of bees to sucrose [29,31,39,40] and to olfactory stimuli [28–30,41]. In addition, oral or topical treatment of foragers with OA increases the motivation to perform waggle dances, probably by increasing the perceived value of rewards [27]. Interestingly, some instances of OA signalling in Drosophila mushroom bodies require DA neurons [42,43]. In honeybees, DA has been found to reduce the response to sucrose rewards and conditioned olfactory stimuli [29–31]. DA has various other effects (e.g. on avoidance learning [44], scouting [45] and locomotion [46]), which could directly or indirectly affect waggle dance communication and the use of private information.
We hypothesized that OA would reduce the use of new social information and strengthen the use of private information by increasing the perceived value of a currently exploited food source. As a result, we expected a decrease in the interest in waggle dances by OA-treated foragers. DA effects are more difficult to predict since DA signalling seems to also complement OA signalling in Drosophila during reward learning [42,43]. But due to the contrasting effects of DA on sucrose responsiveness and extinction in honeybees, we suspected that treatment with DA reduces the use of private information about previous foraging sites and increase interest in waggle dances and thus advertising new food sources. To test these predictions, we trained bees to collect sucrose solution with or without biogenic amines and then exposed these foragers to dances for an alternative, unknown food source. We quantified the interest of trained foragers in these alternative dances and recorded whether they used private information or social information provided by the dance when deciding which feeder to visit.
2. Material and methods
Experiments took place from August to October 2016. We used three colonies (H1–H3) of Apis mellifera carnica housed in glass-walled observation hives in a hut situated on the campus of the University in Mainz, Germany. The colonies consisted of 2000–3000 workers, a queen, brood, pollen and honey reserves.
(a). Experimental procedure
One hive at a time was studied, and two trials per hive were performed (one with OA and one with DA; 6–14 days between the two trials). The order of the trials was randomized for each hive. Each trial lasted 3–4 days and consisted of 1–2 days of training, followed by a treatment day and the test day. We used standard training procedures [7] to simultaneously train two groups of 50–60 foragers to two feeders (unscented 0.8 M sucrose solution—a sugar concentration that induced bees to perform waggle dances) at a distance of 150 m from the hive and 7 m from each other (figure 1). One group was trained to a feeder with a blue underlay (TFa) and the other group to a feeder with a yellow underlay (TFb). Colours were randomly assigned for each trial. The distance of 7 m between the two feeders and the two different colours made sure that trained foragers would visit just one of the two feeders. Afterwards, usually on the same day, we trained a third group of 10–20 foragers to a third feeder (DF, dance feeder) 160 m from the training feeders (TFs) and 150 m from the hive (figure 1). All trained foragers were individually marked with numbered tags of different colours glued to the notum (Opalithplättchen). On the day after training, all feeders provided 0.3 M of identically scented sucrose solution (5 µl essential oil per 100 ml sucrose solution; Primavera Life GmbH, Oy-Mittelberg, Germany). For each hive, we used a different odour: sage for H1, jasmine for H2 and peppermint for H3. On this treatment day, sucrose solution was provided for 60 min, from about 12.00 to 13.00 h. The sugar concentration was lower to prevent the recruitment of more bees, but make sure trained bees returned to their feeder. The duration of 60 min allowed foragers to learn the association among location, reward and scent and to form a long-term memory [47]. The number and time of each visit were noted for all marked bees during the 60 min treatment time.
Figure 1.
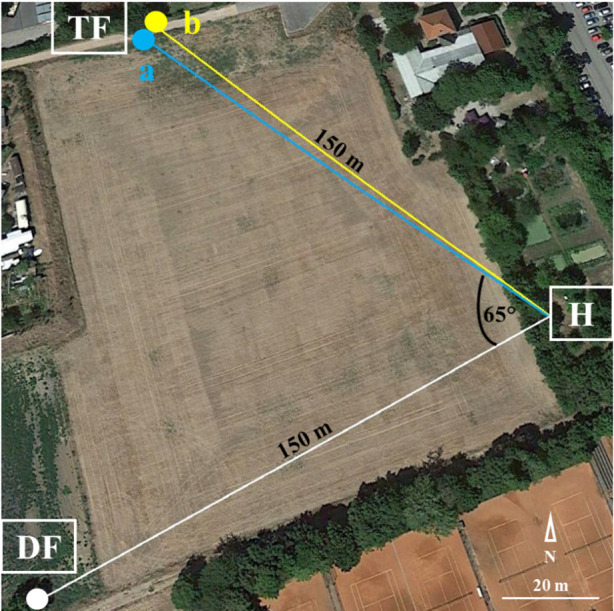
Experimental set-up. Location of the hive (H), dance feeder (DF) and training feeders (TF). The distance between DF and TF was 160 m. Picture taken from Google Earth (49°59′15.63″ N, 8°14′07.20″ E). (Online version in colour.)
In addition, at one TF (either a or b), we added 2 mg ml−1 of biogenic amine (OA or DA hydrochloride, Sigma Aldrich) during the treatment period. This concentration has induced behavioural changes in previous studies [27,44,47]. The other TF served as a control (untreated bees). All solutions (training, treatment and test) also contained 1.75 mg ml−1 ascorbic acid (Sigma Adrich) to reduce oxidation of the biogenic amines [31]. Orally administering biogenic amines has been shown to have similar effects on behaviour as other administration methods, such as topical application [27,40,48,49]. The exact routes of biogenic amines from the crop to the brain remain to be investigated. Gmeinbauer & Crailsheim [50], for example, found that glucose solution consumed by bees after flight quickly appeared in the haemolymph, suggesting a rapid transfer from the crop to the open circulatory system. This would explain why the feeding of biogenic amines leads to rapid changes in biogenic amine titres in the head [51] and in reward perception [40].
While experimentally administered biogenic amines are metabolized and cleared relatively quickly from the brain, probably within a couple of hours [29,51], we expected that our treatment would affect the perception of and learning about food sources during treatment [29,31], which is likely to have long-term effects. Long-term memory can affect foraging decisions in honeybees for several days [47].
On the test day, the day after the treatment, DF foragers were allowed to collect 1.8 M sucrose solution for 60–180 min (approx. 12.00–15.00 h) at the DF, whereas both TFs remained empty. This sucrose concentration made sure DF bees were likely to perform waggle dances advertising the DF location. The sucrose solution at the DF contained the same scent as during training. During this test period, 5–10 DF dancers made repeated foraging trips and performed waggle dances inside the hive. Meanwhile, TF foragers following these dances could decide whether to decode the dances advertising the DF (i.e. use social information or use private information to fly to the TFs). Previous studies have shown that experienced foragers are attracted to dancers carrying a familiar scent, which made it likely that a large proportion of TF foragers interacted with DF dancers [7,14]. The arrival times of all bees at all feeders were noted. At the same time, we filmed the ‘dance floor’ [6] to record DF dances and the dance-following behaviour of TF foragers with high-definition video cameras.
A waggle dance usually consists of many waggle runs (range: 1 to >100) [6,7]. While waggle dances are frequently attended by both social and private information users, bees that attempt to decode dances follow more waggle runs [11,13,14]. We defined dance following as directing the head towards a dancer and being within a distance of one antenna length during the waggle run phase [52,53]. If a bee stopped dancing for at least 5 s, we considered this dance to have ended [52,53]. We analysed the time, the number of dances TF foragers followed and the number of waggle runs they followed.
(b). Statistical analyses
Statistical analyses were performed in R 3.2.3 (https://www.r-project.org/). The data were analysed using generalized linear mixed-effects models (GLMM) for Poisson and binomial distribution. For normally distributed data, we used linear mixed-effects models (LMEs). R fitted these models with the packages ‘lme4’ and ‘nlme’ [54,55]. In the case of zero inflation or overdispersion (estimated with the ‘Dharma’ package), we used GLMMs for zero-inflated data with the ‘glmmADMB’ (Poisson distribution) and the ‘glmmTMB’ (negative binomial distribution, nb) functions [56] (see electronic supplementary material for details on final models). As random effects, we chose ‘hive’ and ‘trial’ to account for any hive or day effects. Occasionally, models failed to converge. In this case, we used only ‘trial’ as a random effect because ‘trial’ effects were stronger. We tested for differences in the number of dances followed, the number of waggle runs followed, the visited test feeder (DF or TF) and the recruitment probability between the two treatments (OA, DA) versus the control. Interactions between two fixed effects were tested by comparing a model with and without the interaction using the likelihood ratio test (LRT) [57]. By means of a survival analysis for a constant hazard with exponential distribution [57] (‘survival’ package), we compared the time of leaving the hive between the three treatment groups.
3. Results
During the six trials (two trials per hive), DF dancers performed 678 dances and a total of 10 789 waggle runs (table 1). Overall, 259 bees were trained to the TF (5.24 ± 3.79 visits during the treatment time), and of those, 84% followed DF dances. Of this latter group, 40% were recruited to the DF by the end of the test period, whereas the remaining 60% exclusively visited the TF (table 1).
Table 1.
Dancing and dance-following behaviour. Data shown are sample size or the mean ± SD.
| hive | trial | dances to DF | waggle runs performed | trained to TF | bees followeda | waggle runs followed | waggle runs/danceb | dances followedc | recruitedd | visits to TFe |
|---|---|---|---|---|---|---|---|---|---|---|
| 1 | OA | 60 | 1040 | 48 | 40 | 626 | 6.7 ± 3.0 | 2.6 ± 1.7 | 15 | 1.7 ± 1.1 |
| 1 | DA | 79 | 1447 | 36 | 31 | 644 | 5.5 ± 2.1 | 3.5 ± 2.8 | 12 | 2.6 ± 2.0 |
| 2 | OA | 182 | 2706 | 42 | 31 | 1673 | 4.8 ± 1.3 | 10.9 ± 8.3 | 13 | 3.0 ± 1.8 |
| 2 | DA | 102 | 979 | 35 | 28 | 578 | 6.8 ± 3.3 | 3.4 ± 2.4 | 17 | 2.7 ± 1.7 |
| 3 | OA | 114 | 2717 | 40 | 34 | 849 | 6.5 ± 3.0 | 3.7 ± 2.2 | 12 | 2.1 ± 1.3 |
| 3 | DA | 141 | 1900 | 58 | 57 | 2440 | 5.4 ± 1.6 | 7.9 ± 4.4 | 15 | 4.1 ± 2.5 |
DF, dance feeder; TF, training feeder.
aNumber of TF foragers that followed DF dances.
bAverage number of DF waggle runs followed per dance by TF foragers.
cNumber of DF dances followed per TF forager.
dNumber of TF foragers recruited to the DF.
eNumber of visits of the TF by TF foragers during testing. Note that the values for ‘TF foragers’ include both treatment and control foragers in a given trial.
(a). Dance-following behaviour
Overall, TF foragers followed 4.7 ± 5.1 dances with an average number of 5.9 ± 2.5 waggle runs per dance (table 1). Bees that were recruited to the DF followed dances approximately 20% longer than bees visiting only the TF feeder (6.70 ± 2.72 versus 5.6 ± 1.78 waggle runs per dance) (LME: t = 2.25, p = 0.026), but there was no difference in the number of dances followed (nb GLMM: z = −1.73, p = 0.08) or the total number of waggle runs followed (LME: t = −1.25, p = 0.21).
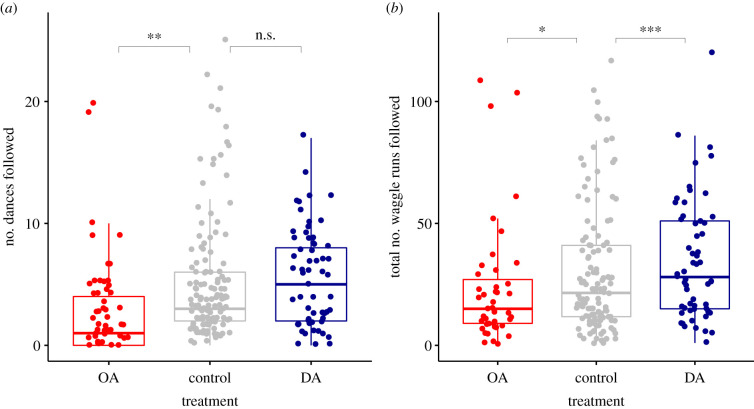
OA-treated foragers followed 3.4 ± 5.7 dances and 27.5 ± 34.3 waggle runs in total, and the control group followed 5.0 ± 5.2 dances and 30.2 ± 26.4 waggle runs. DA-treated foragers followed 5.3 ± 3.8 dances and 34.7 ± 24.4 waggle runs in total (figure 2a,b). OA-treated foragers followed significantly fewer DF dances than control bees (Poisson GLMM: z = −3.1, p = 0.0017). Considering only the bees that followed at least one dance, OA-treated foragers also followed fewer waggle runs (Poisson GLMM: z = −2.4, p = 0.016) compared with the control group. We found no difference in the number of dances followed between DA-treated foragers and control bees (figure 2a; Poisson GLMM: z = 1.42, p = 0.14). However, DA-treated foragers that followed dances followed significantly more waggle runs in total (figure 2b; Poisson GLMM: z = 5.6, p < 0.0001). We found no differences between the treatment groups in the average number of waggle runs followed per dance (LME, OA versus C: t = 0.36; p = 0.72; DA versus C: t = 1.37; p = 0.17).
Figure 2.
Effect of biogenic amine treatment on dance-following behaviour. (a) The number of waggle dances bees followed after oral treatment with octopamine (OA), control solution and dopamine (DA). (b) The effect of OA, control solution and DA on the total number of waggle runs followed by TF bees that followed at least one dance. Boxplots show medians, and interquartile ranges (top line 75% quartile, bottom line 25% quartile) and whiskers show the 5% and 95% percentile). n.s. = p > 0.05, *p < 0.05, **p < 0.001 and ***p < 0.001). Control bees from both trials per colony are combined. Dots represent individual bees. (Online version in colour.)
We also tested whether there was an interaction between the treatment and the number of treatment visits. Indeed, these two factors significantly interacted in their effects on the number of dances followed (Poisson GLMM: LRT = 11.93; p = 0.003) and the total number of waggle runs followed (Poisson GLMM: LRT = 19.4; p < 0.0001). Therefore, we analysed the effect of feeder visits for each treatment group separately. The number of treatment visits had no effect on the number of dances followed in control and DA foragers (Poisson GLMM, control: z = 0.04, p = 0.97; DA: z = −0.97, p = 0.33), but we found a positive relationship between the treatment visit number and the number of dances followed in OA-treated bees (z = 1.98, p = 0.048). Likewise, treatment visits did not affect the total number of waggle runs followed in control and DA-treated bees (Poisson GLMM, control: z = 0.27, p = 0.79; DA: z = −1.24, p = 0.22), but we again found a positive effect of the number of treatment visits in OA-treated bees (nb GLMM: z = 3.1, p = 0.002).
(b). Feeder visitation probability
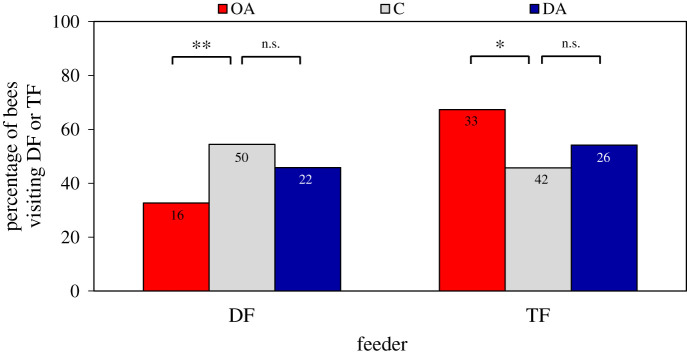
The DF was visited by 33% of OA foragers, 54% of control foragers and 45% of DA foragers (figure 3). Of all bees visiting either feeder, OA-treated bees were significantly less likely to visit the DF than control bees (binomial GLMM: z = −2.6, p = 0.0085), but significantly more likely to visit only the TF (binomial GLMM: z = 2.5, p = 0.011). OA foragers also visited the TF more often than control bees (Poisson GLMM: z = 2.7, p = 0.0080). Conversely, the probability to visit the DF or the TF did not differ between DA foragers and control group foragers (binomial GLMM: DF: z = −0.6, p = 0.54; TF: z = 0.7, p = 0.47). Also, the number of visits of the TF did not differ between these two groups (Poisson GLMM: z = −1.0, p = 0.30).
Figure 3.
Effect of biogenic amine treatment on visitation probability. The percentage of bees that visited the dance feeder at least once, i.e. was recruited (DF, left) or exclusively visited the training feeder, i.e. only used private information (TF, right) after oral treatment with octopamine (OA), control solution (C) and dopamine (DA). Numbers in bars represent the number of bees. (Online version in colour.)
With a survival analysis, we studied the temporal dynamics of the arrival times at the TF during testing. In this analysis, we included all bees that visited a feeder during the 60 min treatment period (including those that did not visit a feeder during the testing). Again, more OA-treated visited the TF than control bees (figure 4) (survival analysis for exponential response: z = −1.6, p < 0.001), and this effect seems especially clear at the beginning of the test period. A larger number of DA bees visited the TF than control bees (survival analysis for exponential response: z = −0.8, p < 0.001). This difference became apparent after approximately 20 min (figure 4).
Figure 4.
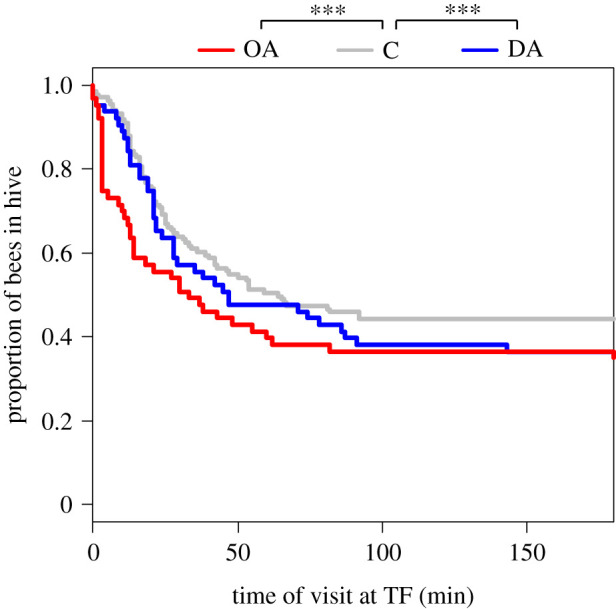
Proportion of bees not yet visiting the training feeder (TF) during the testing period. The first visit of a bee at the TF counted as the beginning (time = 0 min). A survival analysis suggests that there are differences in the temporal dynamics when comparing octopamine-treated foragers (OA, n = 62) versus bees that were fed with a control solution (C, n = 134) and when comparing dopamine-treated bees (DA, n = 63) with control bees. (Online version in colour.)
4. Discussion
We found that the oral treatment of honeybee foragers with OA and DA affected dance-following behaviour and information use. Foragers treated with OA followed fewer waggle dances and, if they followed dances, they followed fewer waggle runs compared with control bees. This is consistent with our prediction that OA-treated bees are less interested in new social information. Despite experiencing that the food source they exploited in the past (TF) was not presently rewarding, these bees mostly relied on their private information and inspected this feeder more often than control bees. Site fidelity is well known in honeybees, even if the visited foraging site does not currently offer rewards [13,14,26]. A possible explanation for an increased use of private information by bees treated with OA is that OA increased the reward perception of bees collecting food at the TF during the treatment period. OA plays a crucial role in reward signalling and has been shown to increase responsiveness to sucrose, learning and retrieval of information in honeybees [27,29,31,40].
OA could also directly reduce the use of social information. Boulay et al. [58] found that OA negatively affects social interactions in ants. Conversely, low levels of OA brain titres are associated with an increased motivation to engage in trophallaxis, which represents an important mechanism of social learning in ants and honeybees [59–61]. Thus, OA treatment might have reduced dance following by reducing the motivation of bees to interact with hivemates. This is consistent with the findings that OA treatment increases scouting (i.e. the search for food without following dances [45]) and that scouts have higher tyramine titres, a precursor of OA, than recruits [62]. Thus, OA might not only strengthen the use of private information by increasing the perceived value of the reward offered at the TF but also reduce social information use by lowering the motivation to engage in social interactions, such as following waggle dances. The negative effects of OA on dance following are also consistent with the observation that older and more experienced foragers appear to rely more on private information and follow dances less [11,63]: OA titres change with age and are higher in older bees [64–66]. Surprisingly, OA-treated foragers showed more interest in dances if they visited the OA feeder more often during the treatment period. It could, thus, be that the OA treatment has a weaker inhibitory effect on foragers that are more motivated to forage, i.e. those that performed more visits during the treatment time. For instance, a larger dose of OA could induce molecular mechanisms that attenuate OA signalling in the brain, thereby reducing signalling when OA titres are very high [67]. More research is needed to better understand the relationship among experience, communication behaviour and biogenic amine signalling.
While DA-treated bees did not follow more dances overall, those bees that did follow dances followed significantly more waggle runs than control bees (figure 2). Interestingly, despite their increased interest in dances, DA-treated bees were not more likely to be recruited to the advertised feeder, suggesting that an increased interest in waggle dances does not necessarily increase the decoding and use of social information. On the contrary, we found evidence that DA increased the use of private information. A survival analysis that included all treated bees found that DA-treated bees were significantly more likely than control bees to visit the training feeder (figure 4). In other words, while DA caused bees to follow dances more thoroughly, it may also have increased their use of private information. These contradictory effects are puzzling but could be explained by the diverse and complex roles that DA plays in the insect brain. Felsenberg et al. [68], for example, demonstrated that there are different subsets of dopaminergic neurons in Drosophila mushroom bodies (see also [69]). One subset neutralizes or extinguishes previously gained memory, whereas the other subset reconsolidates the original memory. Furthermore, DA signalling is involved in both aversive and reward learning in fruit flies and is suspected to signal the nutritive value of a reward, while OA signals sweetness [42,43]. Much less is currently known about the role of DA in reward signalling in honeybees [43]. Distinct functions of DA together with the discrete compartmentalization of dopaminergic neurons in the mushroom bodies [33,68,70] might explain the complex effects on information use we found. Disentangling these effects would require a much more targeted way of treating honeybee foragers (e.g. by injecting DA into specific parts of the brain and the mushroom bodies).
It is possible that there are distinct types of information users: private information users that consistently persist at familiar feeding sites [14,71,72] and social information users that have a high propensity to abandon their food source if it is below a certain threshold and follow dances to find better ones. Scouting bees (i.e. bees that have a high propensity to search for new food sources without following dances) differ substantially in their brain gene expression and learning performance compared to non-scouting bees [45,62]. The probability to follow one of these three strategies seems to be influenced by biogenic amines in complex ways (see also [62]). Currently, we have a limited understanding of how biogenic amines affect the use of different types of information, but social insects are excellent model systems that can help us uncover the role of biogenic amines in individual decision-making and the coordination of foraging activities of colonies.
Supplementary Material
Supplementary Material
Acknowledgements
We thank Prof. Dr Ricarda Scheiner and Dr Markus Thamm for helpful advice.
Data accessibility
The raw data is available as supplementary material.
Authors' contributions
M.L. and C.G. conceived and designed the study. M.L., S.M.G. and T.P. carried out the experiments and analysed the data. M.L. wrote the original draft of the manuscript. C.G., S.M.G. and T.P. reviewed and edited the manuscript.
Competing interests
We declare we have no competing interests.
Funding
S.M.G. was supported by a grant from the ‘Inneruniversitäre Forschungsförderung’ of the University of Mainz. T.P. was supported by a fellowship of the China Scholarship Council (File No. 201606170134).
References
- 1.Heyes CM. 1994. Social learning in animals: categories and mechanisms. Biol. Rev. 69, 207–231. ( 10.1111/j.1469-185X.1994.tb01506.x) [DOI] [PubMed] [Google Scholar]
- 2.Hoppitt W, Laland KN. 2013. Social learning: an introduction to mechanisms, methods, and models. Princeton, NJ: Princeton University Press. [Google Scholar]
- 3.Couvillon MJ. 2012. The dance legacy of Karl von Frisch. Insectes Sociaux 59, 297–306. ( 10.1007/s00040-012-0224-z) [DOI] [Google Scholar]
- 4.Dyer FC. 2002. The biology of the dance language. Annu. Rev. Entomol. 47, 917–949. ( 10.1146/annurev.ento.47.091201.145306) [DOI] [PubMed] [Google Scholar]
- 5.I'Anson Price R, Grüter C. 2015. Why, when and where did honey bee dance communication evolve? Front. Ecol. Evol. 3, 1–7. ( 10.3389/fevo.2015.00040) [DOI] [Google Scholar]
- 6.Seeley TD. 1995. The wisdom of the hive: the social physiology of honey bee colonies. Cambridge, MA: Harvard University Press. [Google Scholar]
- 7.von Frisch K. 1967. The dance language and orientation of bees. Cambridge, MA: Harvard University Press. [Google Scholar]
- 8.Grüter C, Farina WM. 2009. The honeybee waggle dance: can we follow the steps? Trends Ecol. Evol. 24, 242–247. ( 10.1016/j.tree.2008.12.007) [DOI] [PubMed] [Google Scholar]
- 9.Riley JR, Greggers U, Smith AD, Reynolds DR, Menzel R. 2005. The flight paths of honeybees recruited by the waggle dance. Nature 435, 205–207. ( 10.1038/nature03526) [DOI] [PubMed] [Google Scholar]
- 10.Schürch R, Zwirner K, Yambrick BJ, Pirault T, Wilson JM, Couvillon MJ. 2019. Dismantling Babel: creation of a universal calibration for honey bee waggle dance decoding. Anim. Behav. 150, 139–145. ( 10.1016/j.anbehav.2019.01.016) [DOI] [Google Scholar]
- 11.Biesmeijer JC, Seeley TD. 2005. The use of waggle dance information by honey bees throughout their foraging careers. Behav. Ecol. Sociobiol. 59, 133–142. ( 10.1007/s00265-005-0019-6) [DOI] [Google Scholar]
- 12.Grüter C, Balbuena MS, Farina WM. 2008. Informational conflicts created by the waggle dance. Proc. R. Soc. London, Ser. B 275, 1321–1327. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Grüter C, Segers FHID, Ratnieks FLW. 2013. Social learning strategies in honeybee foragers: do the costs of using private information affect the use of social information? Anim. Behav. 85, 1443–1449. ( 10.1016/j.anbehav.2013.03.041) [DOI] [Google Scholar]
- 14.Grüter C, Ratnieks FLW. 2011. Honeybee foragers increase the use of waggle dance information when private information becomes unrewarding. Anim. Behav. 81, 949–954. ( 10.1016/j.anbehav.2011.01.014) [DOI] [Google Scholar]
- 15.Menzel R, et al. 2011. A common frame of reference for learned and communicated vectors in honeybee navigation. Curr. Biol. 21, 645–650. ( 10.1016/j.cub.2011.02.039) [DOI] [PubMed] [Google Scholar]
- 16.Wray MK, Klein BA, Seeley TD. 2012. Honey bees use social information in waggle dances more fully when foraging errors are more costly. Behav. Ecol. 23, 125–131. ( 10.1093/beheco/arr165) [DOI] [Google Scholar]
- 17.Grüter C, Leadbeater E. 2014. Insights from insects about adaptive social information use. Trends Ecol. Evol. 29, 177–184. ( 10.1016/j.tree.2014.01.004) [DOI] [PubMed] [Google Scholar]
- 18.Beekman M, Lew JB. 2008. Foraging in honeybees—when does it pay to dance? Behav. Ecol. 19, 255–262. ( 10.1093/beheco/arm117) [DOI] [Google Scholar]
- 19.Dornhaus A, Klügl F, Oechslein C, Puppe F, Chittka L. 2006. Benefits of recruitment in honey bees: effects of ecology and colony size in an individual-based model. Behav. Ecol. 17, 336–344. ( 10.1093/beheco/arj036) [DOI] [Google Scholar]
- 20.Dornhaus A, Chittka L. 2004. Why do honey bees dance? Behav. Ecol. Sociobiol. 55, 395–401. ( 10.1007/s00265-003-0726-9) [DOI] [Google Scholar]
- 21.I'Anson Price R, Dulex N, Vial N, Vincent C, Grüter C. 2019. Honeybees forage more successfully without the ‘dance language’ in challenging environments. Sci. Adv. 5, eaat0450. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Schürch R, Grüter C. 2014. Dancing bees improve colony foraging success as long-term benefits outweigh short-term costs. PLoS ONE 9, e104660 ( 10.1371/journal.pone.0104660) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Kendal RL, Coolen I, Laland KN. 2009. Adaptive trade-offs in the use of social and personal information. In Cognitive ecology II (eds Dukas R, Ratcliffe JM), pp. 249–271. Chicago, IL: The University of Chicago Press. [Google Scholar]
- 24.Laland KN. 2004. Social learning strategies. Learn. Behav. 32, 4–14. ( 10.3758/BF03196002) [DOI] [PubMed] [Google Scholar]
- 25.Rendell L, et al. 2010. Why copy others? Insights from the social learning strategies tournament. Science 328, 208–213. ( 10.1126/science.1184719) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Al Toufailia HM, Grüter C, Ratnieks FLW. 2013. Persistence to unrewarding feeding locations by honeybee foragers (Apis mellifera): the effects of experience, resource profitability and season. Ethology 119, 1096–1106. ( 10.1111/eth.12170) [DOI] [Google Scholar]
- 27.Barron AB, Maleszka R, Vander Meer RK, Robinson GE. 2007. Octopamine modulates honey bee dance behavior. Proc. Natl. Acad. Sci. USA 104, 1703–1707. ( 10.1073/pnas.0610506104) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Hammer M. 1997. The neural basis of associative reward learning in honeybees. Trends Neurosci. 20, 245–252. ( 10.1016/S0166-2236(96)01019-3) [DOI] [PubMed] [Google Scholar]
- 29.Mercer AR, Menzel R. 1982. The effects of biogenic amines on conditioned and unconditioned responses to olfactory stimuli in the honeybee Apis mellifera. J. Comp. Physiol. A 145, 363–368. ( 10.1007/BF00619340) [DOI] [Google Scholar]
- 30.Perry CJ, Barron AB. 2013. Neural mechanisms of reward in insects. Annu. Rev. Entomol. 58, 543–562. ( 10.1146/annurev-ento-120811-153631) [DOI] [PubMed] [Google Scholar]
- 31.Scheiner R, Plückhahn S, Öney B, Blenau W, Erber J. 2002. Behavioural pharmacology of octopamine, tyramine and dopamine in honey bees. Behav. Brain Res. 136, 545–553. ( 10.1016/S0166-4328(02)00205-X) [DOI] [PubMed] [Google Scholar]
- 32.Beggs KT, Tyndall JDA, Mercer AR. 2011. Honey bee dopamine and octopamine receptors linked to intracellular calcium signaling have a close phylogenetic and pharmacological relationship. PLoS ONE 6, e26809 ( 10.1371/journal.pone.0026809) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.McQuillan HJ, Nakagawa S, Mercer AR. 2012. Mushroom bodies of the honeybee brain show cell population-specific plasticity in expression of amine-receptor genes. Learn. Mem. 19, 151–158. ( 10.1101/lm.025353.111) [DOI] [PubMed] [Google Scholar]
- 34.Mustard JA, Vergoz V, Mesce KA, Klukas KA, Beggs KT, Geddes LH, McQuillan HJ, Mercer AR. 2012. Dopamine signaling in the bee. In Honeybee neurobiology and behavior (eds Galizia CG, Eisenhardt D, Giurfa M), pp. 199–209. Heidelberg, Germany: Springer. [Google Scholar]
- 35.Roeder T, Seifert M, Kähler C, Gewecke M. 2003. Tyramine and octopamine: antagonistic modulators of behavior and metabolism. Arch. Insect. Biochem. Physiol. 54, 1–13. ( 10.1002/arch.10102) [DOI] [PubMed] [Google Scholar]
- 36.Giurfa M. 2007. Behavioral and neural analysis of associative learning in the honeybee: a taste from the magic well. J. Comp. Physiol. A 193, 801–824. ( 10.1007/s00359-007-0235-9) [DOI] [PubMed] [Google Scholar]
- 37.McNeill MS, Kapheim KM, Brockmann A, McGill TA, Robinson GE. 2016. Brain regions and molecular pathways responding to food reward type and value in honey bees. Genes Brain Behav. 15, 305–317. ( 10.1111/gbb.12275) [DOI] [PubMed] [Google Scholar]
- 38.Zars T. 2000. Behavioral functions of the insect mushroom bodies. Curr. Opin Neurobiol. 10, 790–795. ( 10.1016/S0959-4388(00)00147-1) [DOI] [PubMed] [Google Scholar]
- 39.Giray T, Galindo-Cardona A, Oskay D. 2007. Octopamine influences honey bee foraging preference. J. Insect. Physiol. 53, 691–698. ( 10.1016/j.jinsphys.2007.03.016) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Pankiw T, Page RE. 2003. Effect of pheromones, hormones, and handling on sucrose response thresholds of honey bees (Apis mellifera L). J. Comp. Physiol. A 189, 675–684. ( 10.1007/s00359-003-0442-y) [DOI] [PubMed] [Google Scholar]
- 41.Spivak M, Masterman R, Ross R, Mesce KA. 2003. Hygienic behavior in the honey bee (Apis mellifera L.) and the modulatory role of octopamine. Dev. Neurobiol. 55, 341–354. ( 10.1002/neu.10219) [DOI] [PubMed] [Google Scholar]
- 42.Burke CJ, et al. 2012. Layered reward signalling through octopamine and dopamine in Drosophila. Nature 492, 433–439. ( 10.1038/nature11614) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Søvik E, Perry CJ, Barron AB. 2015. Insect reward systems: comparing flies and bees. Adv. Insect Physiol. 48, 189–226. [Google Scholar]
- 44.Agarwal M, Guzmán MG, Morales-Matos C, Díaz RADV, Abramson CI, Giray T. 2011. Dopamine and octopamine influence avoidance learning of honey bees in a place preference assay. PLoS ONE 6, e25371 ( 10.1371/journal.pone.0025371) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Liang ZS, Nguyen T, Mattila HR, Rodriguez-Zas SL, Seeley TD, Robinson GE. 2012. Molecular determinants of scouting behavior in honey bees. Science 335, 1225–1228. ( 10.1126/science.1213962) [DOI] [PubMed] [Google Scholar]
- 46.Mustard JA, Pham PM, Smith BH. 2010. Modulation of motor behavior by dopamine and the D1-like dopamine receptor AmDOP2 in the honey bee. J. Insect. Physiol. 56, 422–430. ( 10.1016/j.jinsphys.2009.11.018) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Menzel R. 1999. Memory dynamics in the honeybee. J. Comp. Physiol. A Neuroethol. Sens. Neural Behav. Physiol. 185, 323–340. ( 10.1007/s003590050392) [DOI] [Google Scholar]
- 48.Schulz DJ, Robinson GE. 2001. Octopamine influences division of labor in honey bee colonies. J. Comp. Physiol. A 187, 53–61. ( 10.1007/s003590000177) [DOI] [PubMed] [Google Scholar]
- 49.Barron A, Schulz D, Robinson G. 2002. Octopamine modulates responsiveness to foraging-related stimuli in honey bees (Apis mellifera). J. Comp. Physiol. A 188, 603–610. ( 10.1007/s00359-002-0335-5) [DOI] [PubMed] [Google Scholar]
- 50.Gmeinbauer R, Crailsheim K. 1993. Glucose utilization during flight of honeybee (Apis mellifera) workers, drones and queens. J. Insect. Physiol. 39, 959–967. ( 10.1016/0022-1910(93)90005-C) [DOI] [Google Scholar]
- 51.Barron AB, Maleszka J, Wander Meer RK, Robinson GE, Maleszka R. 2007. Comparing injection, feeding and topical application methods for treatment of honeybees with octopamine. J. Insect. Physiol. 53, 187–194. ( 10.1016/j.jinsphys.2006.11.009) [DOI] [PubMed] [Google Scholar]
- 52.Al Toufailia HM, Couvillon MJ, Ratnieks FLW, Grüter C. 2013. Honey bee waggle dance communication: signal meaning and signal noise affect dance follower behaviour. Behav. Ecol. Sociobiol. 67, 549–556. ( 10.1007/s00265-012-1474-5) [DOI] [Google Scholar]
- 53.Tanner D, Visscher K. 2009. Does the body orientation of waggle dance followers affect the accuracy of recruitment? Apidologie 40, 55–62. ( 10.1051/apido:2008074) [DOI] [Google Scholar]
- 54.Bates D, Maechler M, Bolker B, Walker S. 2015. Fitting linear mixed-effects models using lme4. J. Stat. Softw. 67, 1–48. ( 10.18637/jss.v067.i01) [DOI] [Google Scholar]
- 55.Pinheiro J, Bates D, DebRoy S, Sarkar D. 2019. nlme: linear and nonlinear mixed effects models. R package version 3.1-140. See https://cran.r-project.org/web/packages/nlme/index.html. [Google Scholar]
- 56.Brooks ME, Kristensen K, van Benthem KJ, Magnusson A, Berg CW, Nielsen A, Skaug HJ, Maechler M, Bolker BM. 2017. glmmTMB balances speed and flexibility among packages for zero-inflated generalized linear mixed modeling. R J. 9, 378–400. ( 10.32614/RJ-2017-066) [DOI] [Google Scholar]
- 57.Crawley MJ. 2007. The R book. Chichester, UK: John Wiley & Sons. [Google Scholar]
- 58.Boulay R, Soroker V, Godzinska EJ, Hefetz A, Lenoir A. 2000. Octopamine reverses the isolation-induced increase in trophallaxis in the carpenter ant Camponotus fellah. J. Exp. Biol. 203, 513–520. [DOI] [PubMed] [Google Scholar]
- 59.Farina WM, Grüter C, Diaz PC. 2005. Social learning of floral odours within the honeybee hive. Proc. R. Soc. London, Ser. B 272, 1923–1928. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Farina WM, Grüter C. 2009. Trophallaxis—a mechanism of information transfer. In Food exploitation by social insects: ecological, behavioral, and theoretical approaches (eds Jarau S, Hrncir M), pp. 173–187. Boca Raton, FL: CRC Press. [Google Scholar]
- 61.Provecho Y, Josens R. 2009. Olfactory memory established during trophallaxis affects food search behaviour in ants. J. Exp. Biol. 212, 3221–3227. ( 10.1242/jeb.033506) [DOI] [PubMed] [Google Scholar]
- 62.Cook CN, Mosqueiro T, Brent CS, Ozturk C, Gadau J, Pinter-Wollman N, Smith BH. 2019. Individual differences in learning and biogenic amine levels influence the behavioural division between foraging honeybee scouts and recruits. J. Anim. Ecol. 88, 236–246. ( 10.1111/1365-2656.12911) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Gil M, Farina WM. 2002. Foraging reactivation in the honeybee Apis mellifera L.: factors affecting the return to known nectar sources. Naturwissenschaften 89, 322–325. ( 10.1007/s00114-002-0323-1) [DOI] [PubMed] [Google Scholar]
- 64.Harris JW, Woodring J. 1992. Effects of stress, age, season, and source colony on levels of octopamine, dopamine and serotonin in the honey bee (Apis mellifera L.) brain. J. Insect. Physiol. 38, 29–35. ( 10.1016/0022-1910(92)90019-A) [DOI] [Google Scholar]
- 65.Schulz DJ, Robinson GE. 1999. Biogenic amines and division of labor in honey bee colonies: behaviorally related changed in the antennal lobes and age-related changes in the mushroom bodies. J. Comp. Physiol. A 184, 481–488. ( 10.1007/s003590050348) [DOI] [PubMed] [Google Scholar]
- 66.Wagener-Hulme C, Kuehn JC, Schulz DJ, Robinson GE. 1999. Biogenic amines and division of labor in honey bee colonies. J. Comp. Physiol. A 184, 471–479. ( 10.1007/s003590050347) [DOI] [PubMed] [Google Scholar]
- 67.Böhm SK, Grady EF, Bunnett NW. 1997. Regulatory mechanisms that modulate signalling by G-protein-coupled receptors. Biochem. J. 322, 1–18. ( 10.1042/bj3220001) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Felsenberg J, Barnstedt O, Cognigni P, Lin S, Waddell S. 2017. Re-evaluation of learned information in Drosophila. Nature 544, 240–244. ( 10.1038/nature21716) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Tedjakumala SR, Rouquette J, Boizeau M-L, Mesce KA, Hotier L, Massou I, Giurfa M. 2017. A tyrosine-hydroxylase characterization of dopaminergic neurons in the honey bee brain. Front. Syst. Neurosci. 11, 47 ( 10.3389/fnsys.2017.00047) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Krashes MJ, DasGupta S, Vreede A, White B, Armstrong JD, Waddell S. 2009. A neural circuit mechanism integrating motivational state with memory expression in Drosophila. Cell 139, 416–427. ( 10.1016/j.cell.2009.08.035) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Biesmeijer JC, de Vries H. 2001. Exploration and exploitation of food sources by social insect colonies: a revision of the scout-recruit concept. Behav. Ecol. Sociobiol. 49, 89–99. ( 10.1007/s002650000289) [DOI] [Google Scholar]
- 72.Wagner AE, van Nest BN, Hobbs CN, Moore D. 2013. Persistence, reticence and the management of multiple time memories by forager honey bees. J. Exp. Biol. 216, 1131–1141. ( 10.1242/jeb.064881) [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
The raw data is available as supplementary material.