Abstract
Objective
Coronavirus disease 2019 (COVID-19) is associated with abnormal inflammatory and coagulation markers, potentially mediating thrombotic events. Our objective was to investigate the incidence, time course, laboratory features, and in-hospital outcomes of COVID-19 patients with suspected venous thromboembolism (VTE).
Methods
A retrospective observational cohort study was conducted of patients hospitalized with COVID-19 who had undergone ultrasound imaging for suspected VTE from March 13 to May 18, 2020. The medical records of the included patients were reviewed for D-dimer, fibrinogen, prothrombin time, partial thromboplastin time, platelet count, C-reactive protein (CRP), and high-sensitivity troponin T at admission and at up to seven time points before and after ultrasound examination. The clinical outcomes included superficial venous thrombosis, deep vein thrombosis, pulmonary embolism, intubation, and death. Mixed effects logistic, linear, and Cox proportional hazards methods were used to evaluate the relationships between the laboratory markers and VTE and other in-hospital outcomes.
Results
Of 138 patients who had undergone imaging studies, 44 (31.9%) had evidence of VTE. On univariable analysis, an elevated admission CRP (odds ratio [OR], 1.05; 95% confidence interval [CI], 1.01-1.09; P = .02; per 10-U increase in CRP), platelet count (OR, 1.48; 95% CI, 1.04-2.12; P = .03; per 1000-U increase in platelet count), and male sex (OR, 2.64; 95% CI, 1.19-5.84; P = .02), were associated with VTE. However only male sex remained significant on multivariable analysis (OR, 2.37; 95% CI, 1.01-5.56; P = .048). The independent predictors of death included older age (hazard ratio [HR], 1.04; 95% CI, 1.00-1.07; P = .04), active malignancy (HR, 4.39; 95% CI, 1.39-13.91; P = .01), elevated admission D-dimer (HR, 1.016; 95% CI, 1.003-1.029; P = .02), and evidence of disseminated intravascular coagulation (HR, 4.81; 95% CI, 1.76-13.10; P = .002).
Conclusions
Male sex, elevated CRP, and elevated platelet count at admission were associated with VTE on univariable analysis. However, only male sex remained significant on multivariable analysis. Older age, active malignancy, disseminated intravascular coagulation, and elevated D-dimer at admission were independently associated with death for patients hospitalized with COVID-19.
Keywords: C-reactive protein, COVID-19, D-dimer, Disseminated intravascular coagulation, Intubation, Venous thromboembolism
Article Highlights.
-
•
Type of Research: A multicenter, retrospective observational study
-
•
Key Findings: Of 138 patients undergoing imaging studies, 44 had evidence of venous thromboembolism despite thromboprophylaxis. The median interval between symptom onset and venous thromboembolism was 13.5 days. Elevated C-reactive protein (odds ratio, 1.05; P = .02) and platelet count (odds ratio, 1.48; P = .03) at admission were independently associated with a greater risk of thrombosis.
-
•
Take Home Message: Admission laboratory values might improve the prognostication, treatment, and resource management for patients hospitalized with coronavirus disease 2019.
Severe acute respiratory syndrome coronavirus-2 (SARS-CoV-2) and the corresponding clinical syndrome of coronavirus disease 2019 (COVID-19) have been primarily noted for pneumonia and acute respiratory distress syndrome, accompanied by respiratory and/or multiorgan system failure.1 , 2 Ongoing research during the global pandemic has continued to uncover a broader array of cardiovascular, neurologic, and renal complications that might, in part, be associated with thrombosis.2, 3, 4, 5
Recent studies have suggested that patients with COVID-19 will have abnormal hematologic and coagulation markers, reflecting a state of heightened inflammation, activated coagulation, and endothelial dysfunction, predisposing to a hypercoagulable state and adverse outcomes.4, 5, 6, 7, 8, 9 Elevations in fibrinogen,10 fibrin degradation products, and D-dimer have been linked with severe disease4 , 11 , 12 and increased odds of death.7 , 8 Other studies have suggested that, although rare overall, disseminated intravascular coagulation (DIC) can also be associated with COVID-19–related death.3 , 7 , 13
Studies specifically investigating symptomatic venous thromboembolic events in COVID-19 patients have reported an overall incidence ranging from 20% to 36%.13, 14, 15, 16, 17, 18 Laboratory test results that can independently predict for thrombotic complications include elevated D-dimer,5,9 prolongation of the prothrombin time (PT), and/or activated partial thromboplastin time (aPTT).14 Although inflammation is a hallmark of severe COVID-19 and elevated inflammatory markers such as C-reactive protein (CRP) have been shown in nonsurvivors,2 , 5 , 19 the trends over time in laboratory markers and the development of venous thromboembolism (VTE) remain unknown. Furthermore, although previous studies of COVID-19 patients have investigated deep vein thrombosis (DVT) and pulmonary embolism (PE), a paucity of data is available on superficial venous thrombosis (SVT) in these patients. Additional information on the trends in laboratory markers and VTE in the progression of COVID-19 could help to refine treatment decisions, anticoagulation strategies, resource management, and overall prognostication for these patients.
We, therefore, investigated the incidence, time course, and relationship of inflammatory and coagulation markers with VTE and in-hospital outcomes for patients admitted with COVID-19.
Methods
Study design and patient selection
Our institutional review board approved the present retrospective two-center cohort study and waived the requirement for written informed consent. We included consecutive patients aged >18 years who had been admitted to one of two large academic hospitals from March 13, 2020 to May 18, 2020 with reverse transcriptase (RT)-polymerase chain reaction (PCR)–confirmed SARS-CoV-2 infection who had undergone ultrasound evaluation for venous thrombosis in the upper and lower extremities during the same admission. The decision to perform ultrasonography or computed tomography pulmonary angiography (CTPA) was determined by clinical suspicion. The main exclusion criterion was pregnancy.
Clinical, laboratory, and imaging features
The patients' electronic medical records were reviewed by two of us (D.M. and V.T.) for demographic data, date of COVID-19 symptom onset, hyperlipidemia, hypertension, diabetes, active tobacco use, coronary artery disease, active malignancy, and thrombosis prophylaxis before the imaging studies. A history of DVT, PE, and/or respiratory diseases such as asthma and chronic obstructive pulmonary disease, was also recorded.
The diagnosis of SARS-CoV2 infection was established using RT-PCR from a nasopharyngeal swab. The admission laboratory values for D-dimer, fibrinogen, PT, aPTT, platelet count, CRP, and high-sensitivity troponin T were recorded within 24 hours of hospitalization. These laboratory tests were also performed and the data recorded for up to seven time points before and after the ultrasound examination, with the date of the ultrasound scan considered the reference point. Patients were evaluated for DIC using the International Society of Thrombosis and Haemostasis criteria, with DIC considered present at a score of ≥5.20
All venous ultrasound studies were performed using the Zonare ZS3 (Mindray, Shenzhen, China), LOGIQ E9, or LOGIQ E10 (GE Medical Systems, Milwaukee, Wis) scanners. DVT was diagnosed if the inability to compress a venous segment under gentle ultrasound probe pressure was present.21 , 22 CTPA was performed using ≥64-slice multidetector computed tomography scanners.
Clinical endpoints and outcomes
The primary outcome of interest was the incidence of VTE, defined as a composite of SVT, DVT, and PE. Other outcomes included a composite of DVT and PE only, intensive care unit (ICU) admission, length of hospitalization, invasive mechanical ventilation, and death during the same hospitalization. Patients were followed up until death, discharge, or August 7, 2020, whichever occurred first.
Statistical analysis
Categorical variables are presented as counts and percentages. Continuous variables were analyzed using the Shapiro-Wilk test and are presented as the median and interquartile range(IQR). Descriptive analyses of the clinical variables and laboratory values were performed using the Wilcoxon rank-sum test or the Fisher exact test, as appropriate. Mixed effects logistic regression was used to assess the association of the clinical and laboratory factors in relation to VTE, with a random patient-level intercept to account for patients with multiple imaging scans. Linear regression was used to analyze the length of stay in relation to the clinical and laboratory factors. Exploratory clinical endpoints of intubation and death were assessed using Cox logistic regression. Patients discharged, deceased, or still hospitalized as of August 7, 2020 were censored at the respective date of occurrence. Factors associated with P < .05 on univariable analysis were included in the multivariable analysis. Trends in laboratory values were assessed using the generalized estimating equations (GEEs) to analyze repeated measures over time. The results are presented as odds ratios (ORs), hazard ratios (HRs), or β-coefficients, as appropriate, with the 95% confidence intervals (CIs). All tests were two-tailed, with an α-level of 0.05 indicating statistical significance. Statistical calculations were performed using R 2019, version 3.6.2 (R Foundation for Statistical Computing, Vienna, Austria) and MATLAB 2019b (Mathworks, Natick, Mass). The lme4 package was used for mixed effects logistic regression, and GEE analysis was performed using the GEEQBOX Statistical Toolbox (MATLAB; Mathworks).23, 24, 25
Results
Patient and scan characteristics
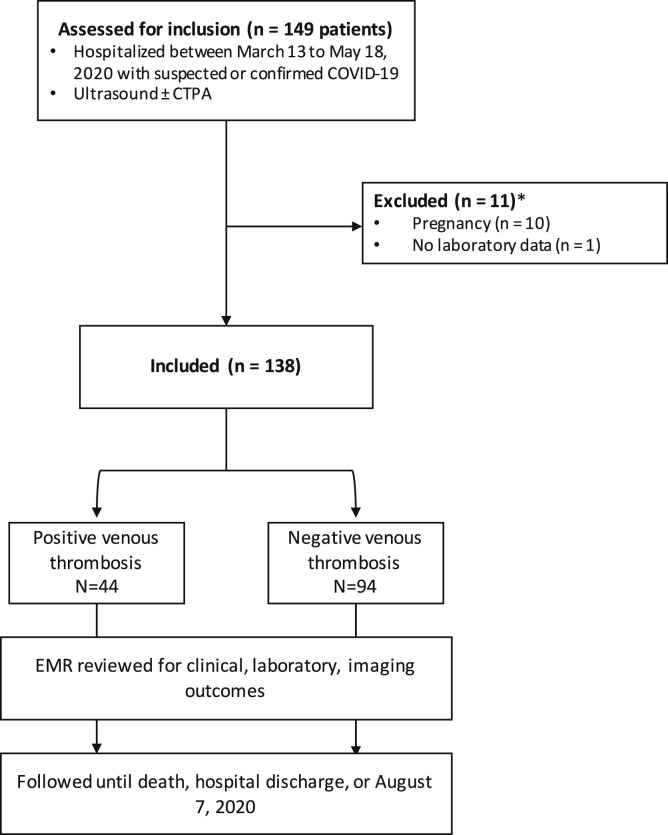
During the study period, 2004 patients had been admitted to our institutions with an RT-PCR–confirmed diagnosis of COVID-19. Of these 2004 patients, 149 (7.4%) had undergone ultrasound scans for suspected VTE and were assessed for study inclusion. Of these 149 patients, 11 were excluded because of pregnancy (n = 10) or no laboratory data available (n = 1), leaving 138 patients (165 ultrasound scans) in the final analysis (Supplementary Fig, online only). Of the 165 scans, 117 (70.9%) were of the lower extremity and 48 (29.1%) were of the upper extremity. The most common indication for obtaining an ultrasound scan was pain and/or swelling in the limb. Of the 138 patients, 126 (91.3%) had been receiving pharmacologic thrombosis prophylaxis before the imaging studies. Of these 129 patients, 83 (60.1%) had received subcutaneous low-molecular-weight heparin, 28 (20.3%) subcutaneous heparin, and 10 (7.2%) pharmacologic prophylaxis, although the specific drug had not been recorded in the medical record. Additionally, five patients (3.6%) had been receiving chronic anticoagulation (three [2.2%], direct oral anticoagulant drugs; and two [1.4%], warfarin) because of a history of VTE, atrial fibrillation, or other indication. Twelve patients (8.7%) had contraindications to pharmacologic thrombosis prophylaxis, including recent or acute gastrointestinal bleeding and thrombocytopenia. The patient characteristics are presented in Table I .
Supplementary Fig (online only).
Study flowchart showing study design, inclusion and exclusion criteria, and outcomes. COVID-19, Coronavirus disease 2019; CTPA, computed tomography pulmonary angiography; EMR, electronic medical record.
Table I.
Patient characteristics and outcomes (N = 138)
| Variable | Value |
|---|---|
| Age, years | 59.2 ± 14.7 |
| Male sex | 83 (60.1) |
| Hypertension | 74 (53.6) |
| Hyperlipidemia | 60 (43.5) |
| Diabetes mellitus | 61 (44.2) |
| Active malignancy | 7 (5.1) |
| Active tobacco use | 22 (15.9) |
| Coronary artery disease | 11 (8.0) |
| Previous DVT or PE | 8 (5.8) |
| Respiratory disease | 23 (16.7) |
| Thrombosis prophylaxis | 126 (91.3) |
| Interval from symptom onset to ultrasound scan, days | |
| Median | 15.0 |
| IQR | 8.0-23.8 |
| Lower extremity ultrasound scan | 117 (70.9) |
| Positive DVT or SVT | 24 (20.5) |
| Upper extremity ultrasound scan | 48 (29.1) |
| Positive DVT or SVT | 27 (56.3) |
| Associated with indwelling catheter | 12 (44.4) |
| Any VTE | 44 (31.9) |
| DVT alone | 27 (61.4) |
| PE alone | 6 (13.6) |
| DVT + PE | 1 (2.3) |
| SVT alone | 10 (22.7) |
| DIC | 8 (5.8) |
| Invasive mechanical ventilation | 92 (66.7) |
| ICU level of care | 95 (68.8) |
| Death | 23 (16.7) |
| Discharged | 114 (82.6) |
| Still inpatient | 2 (1.4) |
| Total length of stay, days | |
| Median | 23.0 |
| IQR | 10.0-38.8 |
DIC, Disseminated intravascular coagulation; DVT, deep venous thrombosis; ICU, intensive care unit; IQR, interquartile range; PE, pulmonary embolism; SVT, superficial venous thrombosis; VTE, venous thromboembolism.
Data presented as mean ± standard deviation or number (%), unless noted otherwise.
Incidence of DVT, PE, intubation, and death
Of the 138 patients, 44 (31.9%) had evidence of VTE, and 40 (29%) had undergone CTPA because of a clinical suspicion of PE. Of the latter 40 patients, 7 (17.9%) were subsequently diagnosed with PE. In addition, 28 patients had DVT (20.2%) and 28 had SVT (20.2%). Some patients had more than one type of VTE. Of the 138 patients, 34 (24.6%) had VTE alone (6 had PE alone, 27 had DVT alone, and 1 had DVT and PE) and 10 had SVT alone (Table I). Of 27 patients with upper extremity venous thrombosis, 12 (44.4%) had had an indwelling catheter (4 with SVT and 8 with DVT). In the patients with VTE, the median time to diagnosis from COVID-19 symptom onset was 13.5 days (IQR, 7-24.8 days).
Overall, 92 patients(66.7%) required intubation, 95 (68.8%) required ICU level of care, and 23 patients (16.7%) died. Eight patients(5.8%) met the criteria for DIC, of whom five had had VTE and four had died. Two patients (1.4%) remained hospitalized as of August 7, 2020. The median follow-up period was 39.5 days (IQR, 15.3-100 days).
VTE, intubation, death, and length of stay related to clinical factors and laboratory markers
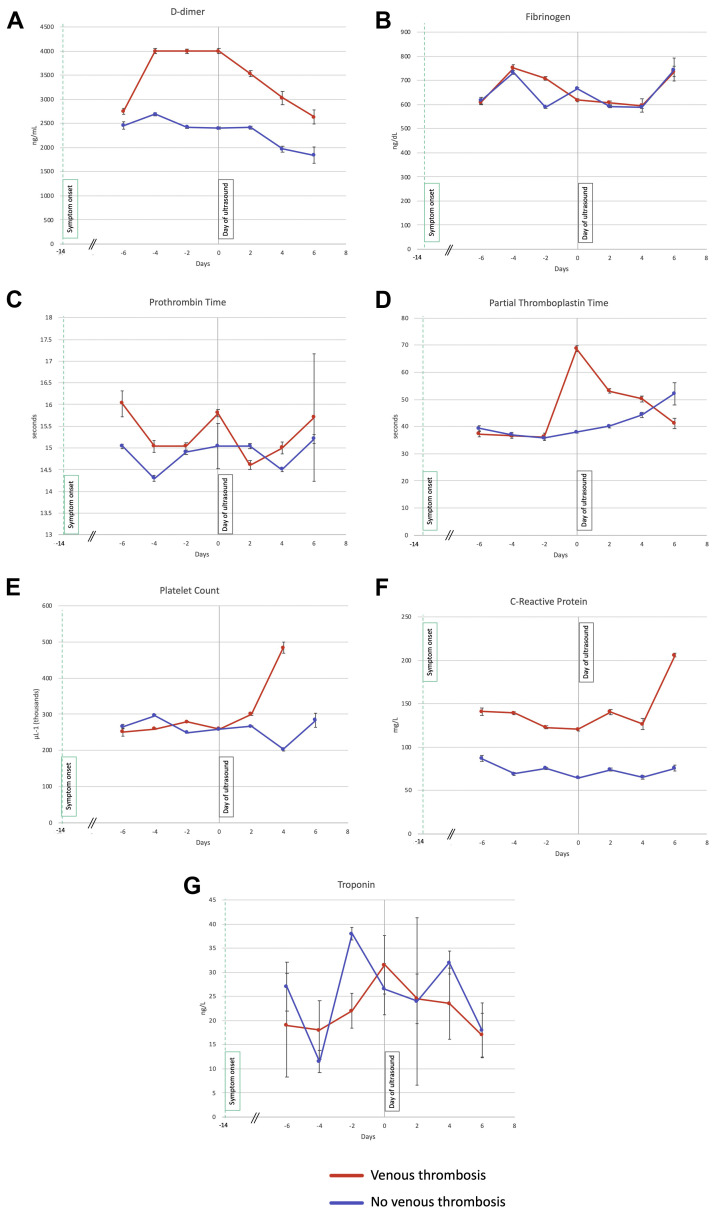
The incidence of male sex, ICU admission, and intubation were significantly greater for patients with VTE (Table II ). An analysis of the overall trends over time in the laboratory test results showed that D-dimer had decreased in both groups (β-coefficient, −107.9; 95% CI, −211.6 to −4.17; P = .04) and the aPTT had increased (β-coefficient, 2.02; 95% CI, 0.002-4.05; P = .05; Supplementary Table I, online only). The PT was lower in patients with VTE (β-coefficient, 0.88; 95% CI, 0.03-0.88; P = .04). However, the D-dimer (β-coefficient, 1108.5; 95% CI, 693.6-1523.4; P < .0001) and CRP (β-coefficient, 69.18; 95% CI, 49.09-89.27; P < .0001) were significantly higher in the patients with VTE. The overall trends in each laboratory test relative to the day of the ultrasound examination are shown in Fig .
Table II.
Patient characteristics stratified by venous thrombosis
| Characteristic | Venous thrombosis |
P value | |
|---|---|---|---|
| Yes | No | ||
| Patients, no. | 44 | 94 | NA |
| Age, years | 59.5 ± 14.3 | 59 ± 15 | .85 |
| Male sex | 33 (75.0) | 50 (53.2) | .02 |
| Hypertension | 22 (50.0) | 52 (55.3) | .59 |
| Hyperlipidemia | 16 (36.4) | 44 (46.8) | .27 |
| Diabetes mellitus | 19 (43.2) | 42 (44.7) | 1.00 |
| Active tobacco use | 1 (2.3) | 6 (6.4) | .43 |
| Coronary artery disease | 9 (20.5) | 13 (13.8) | .33 |
| Previous DVT or PE | 4 (9.1) | 7 (7.4) | .74 |
| Active malignancy | 4 (9.1) | 4 (4.3) | .27 |
| Respiratory disease | 8 (18.2) | 15 (16.0) | .81 |
| Thrombosis prophylaxis | 41 (93.2) | 85 (90.4) | .75 |
| Symptom onset to ultrasound scan, days | .33 | ||
| Median | 16.0 | 13.5 | |
| IQR | 11.0-21.0 | 7.0-24.8 | |
| DIC | 5 (11.4) | 3 (3.2) | .11 |
| ICU | 36 (81.8) | 59 (62.8) | .03 |
| Invasive mechanical ventilation | 35 (79.5) | 57 (60.6) | .03 |
| Death | 10 (22.7) | 13 (13.8) | .22 |
| Discharged | 34 (77.3) | 80 (85.1) | .34 |
| Still inpatient | 0 (0.0) | 2 (2.1) | 1.00 |
| Total length of stay, days | .16 | ||
| Median | 25.0 | 22.5 | |
| IQR | 18.0-40.5 | 8.0-37.8 | |
DIC, Disseminated intravascular coagulation; DVT, deep venous thrombosis; ICU, intensive care unit; IQR, interquartile range; NA, not applicable; PE, pulmonary embolism.
Data presented as mean ± standard deviation or number (%), unless noted otherwise. Boldface P values represent statistical significance.
Fig.
Trends in laboratory test results relative to day-of-ultrasound laboratory markers before and after ultrasound examination (day 0, gray vertical line) in patients with and without venous thrombosis. Owing to the variability in clinical ordering of each laboratory test, the data were grouped into 2-day intervals, and the median was plotted with standard error bars. The green dashed line indicates the median onset of coronavirus disease 2019 (COVID-19) symptoms relative to the day of ultrasonography. D-dimer (A), fibrinogen (B), prothrombin time (C), partial thromboplastin time (D), platelet count (E), C-reactive protein (CRP; F), and high-sensitivity troponin T (G).
Patients with VTE had higher admission, maximum, and day-of-ultrasound CRP levels (Supplementary Tables II and III, online only). On univariable logistic regression, male sex (OR, 2.64; 95% CI, 1.19-5.84; P = .02), elevated CRP (OR, 1.05; 95% CI, 1.01-1.09; P = .02; per 10-U increase in CRP), and platelet count (OR, 1.48; 95% CI, 1.04-2.12; P = .03; per 1000-U increase in platelet count) on admission were associated with VTE during hospitalization. However, only male sex remained significant on multivariable analysis (Table III ).
Table III.
Relation of clinical and laboratory markers for venous thrombosis
| Variable | Univariable |
Multivariable |
||||
|---|---|---|---|---|---|---|
| OR | 95% CI | P value | OR | 95% CI | P value | |
| Age | 1.00 | 0.98-1.03 | .86 | – | – | – |
| Male sex | 2.64 | 1.19-5.84 | .02 | 2.37 | 1.01-5.56 | .048 |
| Hypertension | 0.81 | 0.39-1.65 | .56 | – | – | – |
| Hyperlipidemia | 0.65 | 0.31-1.36 | .25 | – | – | – |
| Diabetes | 0.94 | 0.46-1.94 | .87 | – | – | – |
| Tobacco (active user) | 0.34 | 0.04-2.92 | .33 | – | – | – |
| Coronary artery disease | 1.60 | 0.63-4.09 | .33 | – | – | – |
| Previous DVT or PE | 1.24 | 0.34-4.49 | .74 | – | – | – |
| Respiratory disease | 1.17 | 0.46-3.01 | .74 | – | – | – |
| Active malignancy | 2.25 | 0.54-9.45 | .27 | – | – | – |
| DIC | 3.89 | 0.89-17.08 | .07 | – | – | – |
| Admission laboratory markers | ||||||
| D-dimer, per 100-U increase | 1.006 | 0.992-1.019 | .41 | – | – | – |
| Fibrinogen, per 100-U increase | 1.046 | 0.792-1.382 | .75 | – | – | – |
| PT | 1.084 | 0.970-1.211 | .16 | – | – | – |
| PTT | 1.009 | 0.987-1.032 | .40 | – | – | – |
| Platelet count, per 1000-U increase | 1.482 | 1.035-2.122 | .03 | 1.252 | 0.832-1.882 | .28 |
| CRP, per 10-U increase | 1.049 | 1.009-1.089 | .02 | 1.040 | 0.998-1.082 | .06 |
| hs-Troponin T | 1.000 | 0.998-1.002 | .92 | – | – | – |
CI, Confidence interval; CRP, C-reactive protein; DIC, disseminated intravascular coagulation; DVT, deep vein thrombosis; hs, high sensitivity; OR, odds ratio; PE, pulmonary embolism; PT, prothrombin time; PTT, partial thromboplastin time.
Boldface P values represent statistical significance.
Elevated admission D-dimer and aPTT were associated with an increased hazard for intubation on univariable analysis. However only D-dimer remained significant on multivariable analysis (HR, 1.01; 95% CI, 1.001-1.02; P = .04; Table IV ). Male sex and intubation were independently associated with an increased length of stay (Supplementary Table IV, online only). On multivariable analysis, the independent predictors of death included increasing age (HR, 1.04; 95% CI, 1.00-1.07; P = .04), active malignancy (HR, 4.39; 95% CI, 1.39-13.91; P = .01), DIC (HR, 4.81; 95% CI, 1.76-13.1; P = .002), and elevated admission D-dimer (HR, 1.016; 95% CI, 1.003-1.029; P = .02). An elevated admission CRP showed a trend toward an increased risk of death; however, the difference was not statistically significant (Table V ). VTE was not associated with an increased length of stay or death.
Table IV.
Relation of clinical and admission laboratory markers for invasive mechanical ventilation
| Variable | Univariable |
Multivariable |
||||
|---|---|---|---|---|---|---|
| HR | 95% CI | P value | HR | 95% CI | P value | |
| Age | 1.00 | 0.99-1.02 | .84 | – | – | – |
| Male sex | 0.94 | 0.60-1.47 | .79 | – | – | – |
| Hypertension | 1.05 | 0.69-1.60 | .81 | – | – | – |
| Hyperlipidemia | 0.87 | 0.57-1.32 | .52 | – | – | – |
| Diabetes mellitus | 0.97 | 0.64-1.47 | .88 | – | – | – |
| Active tobacco use | 2.47 | 0.98-6.20 | .054 | 2.06 | 0.72-5.87 | .18 |
| Coronary artery disease | 0.95 | 0.50-1.79 | .87 | – | – | – |
| Previous DVT or PE | 1.33 | 0.53-3.30 | .54 | – | – | – |
| Respiratory disease | 1.28 | 0.68-2.41 | .45 | – | – | – |
| Active malignancy | 1.60 | 0.64-3.97 | .31 | – | – | – |
| DIC | 1.75 | 0.80-3.80 | .16 | – | – | – |
| Admission laboratory markers | ||||||
| D-dimer, per 100-U increase | 1.011 | 1.004-1.017 | .001 | 1.010 | 1.001-1.020 | .04 |
| Fibrinogen, per 100-U increase | 1.151 | 1.000-1.324 | .049 | – | – | – |
| PT | 1.027 | 0.984-1.071 | .23 | – | – | – |
| PTT | 1.007 | 1.001-1.014 | .028 | 1.004 | 0.99-1.02 | .56 |
| Platelet count, per 1000-U increase | 2.951 | 0.245-35.630 | .39 | – | – | – |
| CRP, per 10-U increase | 1.000 | 0.980-1.020 | .90 | – | – | – |
| hs-Troponin T | 1.000 | 0.999-1.000 | .51 | – | – | – |
CI, Confidence interval; CRP, C-reactive protein; DIC, disseminated intravascular coagulation; DVT, deep vein thrombosis; HR, hazard ratio; hs, high sensitivity; PE, pulmonary embolism; PT, prothrombin time; PTT, partial thromboplastin time.
Boldface P values represent statistical significance.
Table V.
Relation of clinical and admission laboratory markers for death
| Variable | Univariable |
Multivariable |
||||
|---|---|---|---|---|---|---|
| HR | 95% CI | P value | HR | 95% CI | P value | |
| Age | 1.04 | 1.01-1.07 | .01 | 1.04 | 1.00-1.07 | .04 |
| Male sex | 0.99 | 0.40-2.45 | .99 | – | – | – |
| Hypertension | 1.64 | 0.71-3.80 | .25 | – | – | – |
| Hyperlipidemia | 1.11 | 0.49-2.54 | .80 | – | – | – |
| Diabetes | 1.17 | 0.51-2.67 | .71 | – | – | – |
| Active tobacco use | 0.00 | 0.00-Inf | 1.00 | – | – | – |
| Coronary artery disease | 1.88 | 0.73-4.81 | .19 | – | – | – |
| Previous DVT or PE | 1.64 | 0.38-7.02 | .51 | – | – | – |
| Respiratory disease | 0.67 | 0.19-2.40 | .54 | – | – | – |
| Active malignancy | 6.46 | 2.37-17.65 | .0003 | 4.39 | 1.39-13.91 | .01 |
| Venous thrombosis | 1.46 | 0.64-3.33 | .37 | – | – | – |
| Invasive mechanical ventilation | 0.40 | 0.12-1.32 | .13 | – | – | – |
| DIC | 4.36 | 1.38-13.74 | .01 | 4.81 | 1.76-13.10 | .002 |
| Admission laboratory markers | ||||||
| D-dimer, per 100-U increase | 1.014 | 1.002-1.025 | .02 | 1.016 | 1.003-1.029 | .02 |
| Fibrinogen, per 100-U increase | 1.185 | 0.756-1.859 | .46 | – | – | – |
| PT | 1.069 | 0.968-1.180 | .19 | – | – | – |
| PTT | 0.991 | 0.970-1.013 | .42 | – | – | – |
| Platelet count, per 1000-U increase | 3.558 | 0.030-4.275 | .60 | – | – | – |
| CRP | 1.030 | 0.998-1.070 | .07 | – | – | – |
| hs-Troponin T | 1.000 | 0.997-1.002 | .85 | – | – | – |
CI, Confidence interval; CRP, C-reactive protein; DIC, disseminated intravascular coagulation; DVT, deep vein thrombosis; HR, hazard ratio; hs, high sensitivity; PE, pulmonary embolism; PT, prothrombin time; PTT, partial thromboplastin time.
Boldface P values represent statistical significance.
Discussion
The primary findings of the present study were as follows. First, the incidence of VTE in COVID-19 patients undergoing ultrasonography or CTPA because of clinical suspicion was 32% despite thrombosis prophylaxis. Second, the median time from COVID-19 symptom onset to the diagnosis of VTE was 13.5 days. Third, male sex and elevated admission CRP and platelet count were associated with VTE on univariable analysis. However, only male sex remained significantly associated on multivariable analysis. Fourth, older age, active malignancy, DIC, and elevated D-dimer at admission were independently associated with death. Finally, VTE was not associated with an increased length of stay or death.
Studies of VTE rates in critically ill patients before the COVID-19 pandemic reported an incidence of 16% on clinically indicated imaging studies26 and from 8% to 37% for prospectively screened patients regardless of symptoms.27, 28, 29 Recent studies have suggested that hospitalized COVID-19 patients have an increased predisposition to thrombotic events, with an incidence ranging from 20% to 36% in the subset undergoing clinically indicated imaging.13, 14, 15, 16, 17, 18 In a more general cohort of COVID-19 patients, the VTE incidence was reported to be 4.8%.9 However, the incidence was reported as high as 85% in those undergoing ultrasound screening examinations regardless of clinical suspicion.30 In an Italian cohort of ICU and general ward patients, 16 of 44 patients (36%) who had undergone clinically indicated imaging studies were found to have VTE.13 Another study of 198 patients demonstrated a 20% incidence of VTE in ICU and general ward patients.15 A third study of 184 patients admitted to the ICU reported a 27% incidence of VTE.14 The number of patients who had undergone imaging studies was not explicitly reported in either of the latter two studies. Finally, an 85% incidence of lower extremity DVT was reported in a study of 48 patients with severe COVID-19 admitted to the ICU who had undergone lower extremity ultrasound screening at least twice, regardless of symptoms.30
We specifically investigated patients who had undergone imaging studies because of clinical suspicion and found an overall incidence of any VTE of 32%. The overall incidence was similar to that from previous studies despite the longer median follow-up (40 days vs 7 days,14 7 days,15 10 days,13 and 12 days17). The added context of our observation that the median time from COVID-19 symptom onset to a positive diagnosis of VTE was 13.5 days raises the possibility that the risk of thrombotic events peaks at ∼2 weeks after symptom onset. The caveat in comparing these studies, however, is that the proportion of patients still hospitalized and remaining at risk of VTE varied considerably: 1.4% in the present study vs 6.7%,13 8%,15 10%,17 and 76%.14 Nevertheless, the results from the present study, and from previous studies, together suggest a lower threshold of clinical suspicion for VTE in these patients might be indicated, especially in the second and third weeks after COVID-19 symptom onset.
The propensity for thromboembolic events in COVID-19 patients is mirrored by the abnormal hematologic and coagulation markers indicating an induced hypercoagulable state. At least one previous study has shown an independent association of an elevated white blood cell count, neutrophil/lymphocyte ratio, and D-dimer with VTE.15 In another study, coagulopathy, defined by the investigators as a prolongation of PT of >3 seconds or aPTT of >5 seconds, was associated with an adjusted HR of 4.1 (95% CI, 1.9-9.1) for thrombotic events.14 In our study, a higher maximum and day-of-ultrasound aPTT were attributed to the initiation of therapeutic anticoagulation after VTE was diagnosed. However, on univariable analysis, we observed an association of elevated admission CRP and platelet count with subsequent VTE, and the D-dimer level was not associated. Although the admission CRP and platelet count did not remain associated on multivariable analysis, suggesting that male sex (which remained significant) affects these laboratory variables, we found a trend toward significance for admission CRP on multivariable analysis (P = .06). Larger studies might be better poised to investigate this signal. Although only eight patients in our study had met the International Society of Thrombosis and Haemostasis criteria for consumptive coagulopathy, elevated admission D-dimer and DIC during hospitalization were independently associated with death, consistent with previous studies.7 , 8 , 13
Severe and fatal manifestations of COVID-19 are characterized by a dysregulated inflammatory response that potentially leads to DIC, acute respiratory distress syndrome, and multiorgan failure.2 , 3 , 6 These overwhelming inflammatory reactions are often accompanied by marked elevations in factors such as the erythrocyte sedimentation rate, interleukin-1β, interleukin-6, tumor necrosis factor-α, and CRP.2 , 31 Elevated admission D-dimer was not associated with venous thrombosis in our study but was independently associated with the need for mechanical ventilation and death. These results suggest that D-dimer expression might be more reflective of the overall disease severity than as a specific risk for venous thrombosis in patients with COVID-19, an observation consistent with previous studies documenting elevated CRP and D-dimer in SARS-CoV-2 infection, with further elevations in those with severe COVID-19.2 , 5 , 19 CRP is also one of several laboratory abnormalities associated with the severity of lung injury32 and lung lesions33 in COVID-19 patients.
The present study had several limitations. First, we used a retrospective design, which contributed to the variable laboratory data as dictated by the standard of care assessment during hospitalization. In the context of attempting to mitigate exposure of healthcare workers, we also acknowledge that shifting clinical thresholds for ordering ultrasound scans could have affected the number and characteristics of the patients who had undergone imaging studies.34 A prospective study design mandating standardized collection intervals would alleviate these limitations. Second, because our primary hypotheses were specifically focused on the features of suspected VTE in the setting of SARS-CoV-2 infection, we did not include COVID-19–positive patients who had not undergone imaging studies. Therefore, we do not know the true incidence of VTE in all COVID-19 patients. Also, whether the findings from the present study are applicable to a more generalized population of COVID-19 patients remains unaddressed. However, to the best of our knowledge, the present study is one of the largest cohorts to date of COVID-19 patients with imaging studies for VTE available. Third, the follow-up period was limited to hospitalization only; therefore, we do not know whether COVID-19 patients will remain at an elevated risk of VTE after discharge. However, in a recent retrospective study evaluating the postdischarge rate of thrombosis in patients with COVID-19, the cumulative incidence of VTE alone at day 30 after discharge was 0.6% (95% CI, 0.1%-4.6%).35 In our study, only 1.4% of patients remained hospitalized at the end of the data collection period. Therefore, those remaining at risk of VTE and other events was very low. Given the acute nature of the COVID-19 pandemic and the need for rapidly improved understanding of the disease, additional analyses of long-term postdischarge events are needed. However, the median length of follow-up in our study of hospitalized patients was considerably longer than that reported by similar previous studies.13, 14, 15
Conclusions
The incidence of VTE in hospitalized COVID-19 patients undergoing imaging studies was 32%, and the median time from symptom onset to the diagnosis of VTE was 13.5 days. Male sex and elevated CRP and platelet count at admission were associated with VTE on univariable analysis. However, only male sex remained significantly associated on multivariable analysis. Elevated D-dimer at admission was independently associated with intubation and death in these patients, and older age, active malignancy, and DIC were also independently associated with death. These results might reflect the hypercoagulable and inflammatory nature of SARS-CoV-2 infection and, if confirmed by larger prospective studies, suggest the potential role of laboratory-based markers in the assessment of adverse outcomes for patients with COVID-19.
Author contributions
Conception and design: VT, DM, MGH, SH
Analysis and interpretation: VT, DM, RR, AD, SM, ML, BG, UH, MGH, SH
Data collection: VT, DM, SH
Writing the article: VT, DM, SH
Critical revision of the article: VT, DM, RR, AD, SM, ML, BG, UH, MGH, SH
Final approval of the article: VT, DM, RR, AD, SM, ML, BG, UH, MGH, SH
Statistical analysis: VT, DM, SH
Obtained funding: Not applicable
Overall responsibility: SH
VT and DM contributed equally to this article and share co-first authorship.
Footnotes
Author conflict of interest: U.H. receives research support on behalf of the institution (unrelated to the present research) from KOWA, MedImmune, HeartFlow, and Duke University, consulting fees (unrelated to the present research) from Duke University and Recor Medical. V.T., D.M., R.R., A.D., S.M., M.T.L., B.G., M.D.G.-H., and S.H. have no conflicts of interest.
Additional material for this article may be found online at www.jvsvenous.org.
The editors and reviewers of this article have no relevant financial relationships to disclose per the Journal policy that requires reviewers to decline review of any manuscript for which they may have a conflict of interest.
Additional material for this article may be found online at www.jvsvenous.org.
Appendix (online only).
Supplementary Table I (online only).
Trends in laboratory values over time
| Variable | Trend over time |
VTE vs no VTE |
||||
|---|---|---|---|---|---|---|
| β-Coefficient | 95% CI | P value | β-Coefficient | 95% CI | P value | |
| D-dimer, per 100-U increase | −107.9 | −211.6 to −4.17 | .04 | 1108.5 | 693.6-1523.4 | <.0001 |
| Fibrinogen, per 100-U increase | 1.27 | −15.31 to 17.85 | .88 | 13.71 | −52.61 to 80.0 | .68 |
| PT | −0.01 | −0.12 to 0.10 | .88 | 0.45 | 0.03-0.88 | .04 |
| PTT | 2.02 | 0.002-4.05 | .05 | 5.20 | −2.90 to 13.30 | .21 |
| Platelet count, per 1000-U increase | 11.45 | −4.68 to 27.58 | .16 | 50.99 | −10.08 to 112.05 | .10 |
| CRP, per 10-U increase | 2.59 | −2.43 to 7.62 | .31 | 69.18 | 49.09-89.27 | <.0001 |
| hs-Troponin T | 0.13 | −1.59 to 1.86 | .88 | −3.07 | −9.97 to 3.82 | .38 |
CI, Confidence interval; CRP, C-reactive protein; hs, high sensitivity; PT, prothrombin time; PTT, partial thromboplastin time, VTE, venous thromboembolism.
Boldface P values represent statistical significance.
Supplementary Table II (online only).
Laboratory values stratified by venous thrombosis
| Laboratory marker | Venous thrombosis |
P value | |
|---|---|---|---|
| Yes (n = 44) | No (n = 94) | ||
| Admission | |||
| D-dimer, ng/mL | 1854 (926-4000) | 1274 (834.5-2787) | .13 |
| Fibrinogen, mg/dL | 674 (495.5-775.75) | 615 (516.5-725.5) | .52 |
| PT, seconds | 14.4 (13.8-15.5) | 14 (13.2-15) | .21 |
| PTT, seconds | 34.1 (30.9-38.6) | 34.3 (32-38.8) | .78 |
| Platelet count, ×103/μL | 225.5 (176-320) | 208.5 (161-254.25) | .052 |
| CRP, mg/L | 158.9 (99.9-245.7) | 132.5 (58.8-161.9) | .03 |
| hs-Troponin T, ng/L | 15 (0-33) | 14 (6-38) | .73 |
| Ultrasound day | |||
| D-dimer, ng/mL | 4000 (2605.5-6546) | 2800 (1276-5017) | .03 |
| Fibrinogen, mg/dL | 618 (512-768) | 645 (541.5-767.5) | .54 |
| PT, seconds | 15.8 (14.8-17.1) | 14.7 (14-16) | .02 |
| PTT, seconds | 74.1 (35.3-135.2) | 37.3 (32.3-43.8) | .01 |
| Platelet count, ×103/μL | 259 (214-387) | 266 (199.25-344.75) | .48 |
| CRP, mg/L | 120.8 (67-170.7) | 64 (26.2-127) | .01 |
| hs-Troponin T, ng/L | 31 (10-47) | 23 (11-40) | .77 |
| Maximum | |||
| D-dimer, ng/mL | 4704 (4000-7970.5) | 3426 (2074.5-6972) | .007 |
| Fibrinogen, mg/dL | 696 (552-891) | 690 (576.25-875.25) | 1.00 |
| PT, seconds | 16.4 (14.9-18) | 15.35 (14.43-17) | .03 |
| PTT, seconds | 82.6 (48.2-150) | 43.4 (36.83-83.23) | .004 |
| Platelet count, ×103/μL | 344 (252.25-505.25) | 332 (225-421) | .13 |
| CRP, mg/L | 212.6 (140.8-285.2) | 144.8 (68.6-238.2) | .01 |
| hs-Troponin T, ng/L | 31 (14-77) | 26 (11-50) | .18 |
| Minimum | |||
| Fibrinogen, mg/dL | 549 (468-672) | 551.5 (429.8-718) | .84 |
| Platelet count, ×103/μL | 216.5 (159.5-328.25) | 202 (149-279) | .42 |
CRP, C-reactive protein; hs, high sensitivity; PT, prothrombin time; PTT, partial thromboplastin time.
Data presented as median (interquartile range). Boldface P values represent statistical significance.
Supplementary Table III (online only).
Relation of laboratory markers for venous thrombosis
| Laboratory marker | OR | 95% CI | P value |
|---|---|---|---|
| Ultrasound scan day | |||
| D-dimer, per 100-U increase | 1.014 | 0.997-1.032 | .11 |
| Fibrinogen, per 100-U increase | 0.914 | 0.694-1.204 | .52 |
| PT | 0.995 | 0.976-1.015 | .63 |
| PTT | 1.022 | 1.008-1.037 | .002 |
| Platelet count, per 1000-U increase | 1.072 | 0.810-1.420 | .63 |
| CRP, per 10-U increase | 1.062 | 1.000-1.12 | .049 |
| hs-Troponin T | 1.000 | 0.997-1.003 | .96 |
| Maximum | |||
| D-dimer, per 100-U increase | 1.010 | 0.998-1.022 | .11 |
| Fibrinogen, per 100-U increase | 0.991 | 0.857-1.147 | .91 |
| PT | 0.997 | 0.985-1.010 | .67 |
| PTT | 1.012 | 1.002-1.022 | .02 |
| Platelet count, per 1000-U increase | 1.193 | 0.936-1.519 | .15 |
| CRP, per 10-U increase | 1.046 | 1.005-1.087 | .03 |
| hs-Troponin T | 1.000 | 0.999-1.002 | .72 |
| Minimum | |||
| Fibrinogen, per 100-U increase | 0.950 | 0.784-1.150 | .60 |
| Platelet count, per 1000-U increase | 1.114 | 0.816-1.520 | .50 |
CI, Confidence interval; CRP, C-reactive protein; hs, high sensitivity; OR, odds ratio; PT, prothrombin time; PTT, partial thromboplastin time.
Boldface P values represent statistical significance.
Supplementary Table IV (online only).
Relation of clinical and admission laboratory markers to length of stay
| Variable | Univariable |
Multivariable |
||||
|---|---|---|---|---|---|---|
| β-Coefficient | 95% CI | P value | β-Coefficient | 95% CI | P value | |
| Age | −0.10 | −0.35 to 0.15 | .42 | – | – | – |
| Male sex | 9.54 | 2.23-16.84 | .01 | 9.92 | 0.32-19.53 | .047 |
| Hypertension | −2.27 | −9.60 to 5.06 | .54 | – | – | – |
| Hyperlipidemia | −2.39 | −9.77 to 5.00 | .53 | – | – | – |
| Diabetes mellitus | 4.81 | −2.53 to 12.14 | .20 | – | – | – |
| Active tobacco use | −3.84 | −20.50 to 12.83 | .65 | – | – | – |
| Coronary artery disease | −0.61 | −10.62 to 9.40 | .90 | – | – | – |
| Previous DVT or PE | −8.61 | −22.05 to 4.83 | .21 | – | – | – |
| Respiratory disease | 2.49 | −7.33 to 12.30 | .62 | – | – | – |
| Active malignancy | −8.33 | −23.93 to 7.28 | .30 | |||
| Venous thrombosis | 3.18 | −4.66 to 11.03 | .43 | – | – | – |
| Invasive mechanical ventilation | 26.82 | 20.50-33.15 | <.0001 | 27.16 | 16.00-38.32 | <.0001 |
| DIC | −1.43 | −17.09 to 14.24 | .86 | – | – | – |
| Admission laboratory marker | ||||||
| D-dimer, per 100-U increase | −0.04 | −0.18 to 0.10 | .58 | – | – | – |
| Fibrinogen, per 100-U increase | 0.48 | −2.39 to 3.35 | .74 | – | – | – |
| PT | −1.07 | −2.26 to 0.13 | .08 | – | – | – |
| PTT | 0.33 | 0.08-0.58 | .01 | 0.43 | 0.20-0.66 | .0005 |
| Platelet count, per 1000-U increase | −2.79 | −6.42 to 0.84 | .13 | – | – | – |
| CRP | 0.36 | −0.08 to 0.75 | .07 | – | – | – |
| hs-Troponin T | 0.00 | −0.02 to 0.03 | .68 | – | – | – |
CI, Confidence interval; CRP, C-reactive protein; DIC, disseminated intravascular coagulation; hs, high sensitivity; PT, prothrombin time; PTT, partial thromboplastin time.
Boldface P values represent statistical significance.
References
- 1.Tay M.Z., Poh C.M., Renia L., MacAry P.A., Ng L.F.P. The trinity of COVID-19: immunity, inflammation and intervention. Nat Rev Immunol. 2020;20:363–374. doi: 10.1038/s41577-020-0311-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Zhang W., Zhao Y., Zhang F., Wang Q., Li T., Liu Z. The use of anti-inflammatory drugs in the treatment of people with severe coronavirus disease 2019 (COVID-19): the perspectives of clinical immunologists from China. Clin Immunol. 2020;214:108393. doi: 10.1016/j.clim.2020.108393. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Menter T., Haslbauer J.D., Nienhold R., Savic S., Hopfer H., Deigendesch N. Post-mortem examination of COVID19 patients reveals diffuse alveolar damage with severe capillary congestion and variegated findings of lungs and other organs suggesting vascular dysfunction. Histopathology. 2020;77:198–209. doi: 10.1111/his.14134. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Wang D., Hu B., Hu C., Zhu F., Liu X., Zhang J. Clinical characteristics of 138 hospitalized patients with 2019 novel coronavirus-infected pneumonia in Wuhan, China. JAMA. 2020;323:1061–1069. doi: 10.1001/jama.2020.1585. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Zhang J.J., Dong X., Cao Y.Y., Yuan Y.D., Yang Y.B., Yan Y.Q. Clinical characteristics of 140 patients infected with SARS-CoV-2 in Wuhan, China. Allergy. 2020;75:1730–1741. doi: 10.1111/all.14238. [DOI] [PubMed] [Google Scholar]
- 6.Qin C., Zhou L., Hu Z., Zhang S., Yang S., Tao Y. Dysregulation of immune response in patients with COVID-19 in Wuhan, China. Clin Infect Dis. 2020;71:762–768. doi: 10.1093/cid/ciaa248. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Tang N., Li D., Wang X., Sun Z. Abnormal coagulation parameters are associated with poor prognosis in patients with novel coronavirus pneumonia. J Thromb Haemost. 2020;18:844–847. doi: 10.1111/jth.14768. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Zhou F., Yu T., Du R., Fan G., Liu Y., Liu Z. Clinical course and risk factors for mortality of adult inpatients with COVID-19 in Wuhan, China: a retrospective cohort study. Lancet. 2020;395:1054–1062. doi: 10.1016/S0140-6736(20)30566-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Al-Samkari H., Leaf R., Dzik W., Carlson J., Fogerty A., Waheed A. COVID and coagulation: bleeding and thrombotic manifestations of SARS-CoV2 infection. Blood. 2020;136:489–500. doi: 10.1182/blood.2020006520. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Di Micco P., Russo V., Carannante N., Imparato M., Rodolfi S., Cardillo G. Clotting factors in COVID-19: epidemiological association and prognostic values in different clinical presentations in an Italian cohort. J Clin Med. 2020;9:1371. doi: 10.3390/jcm9051371. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Guan W.J., Ni Z.Y., Hu Y., Liang W.H., Ou C.Q., He J.X. Clinical characteristics of coronavirus disease 2019 in China. N Engl J Med. 2020;382:1708–1720. doi: 10.1056/NEJMoa2002032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Huang C., Wang Y., Li X., Ren L., Zhao J., Hu Y. Clinical features of patients infected with 2019 novel coronavirus in Wuhan, China. Lancet. 2020;395:497–506. doi: 10.1016/S0140-6736(20)30183-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Lodigiani C., Iapichino G., Carenzo L., Cecconi M., Ferrazzi P., Sebastian T. Venous and arterial thromboembolic complications in COVID-19 patients admitted to an academic hospital in Milan, Italy. Thromb Res. 2020;191:9–14. doi: 10.1016/j.thromres.2020.04.024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Klok F.A., Kruip M., van der Meer N.J.M., Arbous M.S., Gommers D., Kant K.M. Incidence of thrombotic complications in critically ill ICU patients with COVID-19. Thromb Res. 2020;191:145–147. doi: 10.1016/j.thromres.2020.04.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Middeldorp S., Coppens M., van Haaps T.F., Foppen M., Vlaar A.P., Muller M.C.A. Incidence of venous thromboembolism in hospitalized patients with COVID-19. J Thromb Haemost. 2020;18:1995–2002. doi: 10.1111/jth.14888. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Chi G., Lee J.J., Jamil A., Gunnam V., Najafi H., Memar Montazerin S. Venous thromboembolism among hospitalized patients with COVID-19 undergoing thromboprophylaxis: a systematic review and meta-analysis. J Clin Med. 2020;9:2489. doi: 10.3390/jcm9082489. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Trimaille A., Curtiaud A., Marchandot B., Matsushita K., Sato C., Leonard-Lorant I. Venous thromboembolism in non-critically ill patients with COVID-19 infection. Thromb Res. 2020;193:166–169. doi: 10.1016/j.thromres.2020.07.033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Wang Y., Shi L., Yang H., Duan G., Wang Y. Pooled prevalence of deep vein thrombosis among coronavirus disease 2019 patients. Crit Care. 2020;24:466. doi: 10.1186/s13054-020-03181-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Ruan Q., Yang K., Wang W., Jiang L., Song J. Clinical predictors of mortality due to COVID-19 based on an analysis of data of 150 patients from Wuhan, China. Intensive Care Med. 2020;46:846–848. doi: 10.1007/s00134-020-05991-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Wada H., Thachil J., Di Nisio M., Mathew P., Kurosawa S., Gando S. Guidance for diagnosis and treatment of DIC from harmonization of the recommendations from three guidelines. J Thromb Haemost. 2013 Feb 4 doi: 10.1111/jth.12155. [E-pub ahead of print] [DOI] [PubMed] [Google Scholar]
- 21.Bates S.M., Jaeschke R., Stevens S.M., Goodacre S., Wells P.S., Stevenson M.D. Diagnosis of DVT: antithrombotic therapy and prevention of thrombosis, 9th ed: American College of Chest Physicians evidence-based clinical practice guidelines. Chest. 2012;141(Suppl):e351S–e418S. doi: 10.1378/chest.11-2299. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Tapson V.F., Carroll B.A., Davidson B.L., Elliott C.G., Fedullo P.F., Hales C.A. The diagnostic approach to acute venous thromboembolism: clinical practice guideline of the American Thoracic Society. Am J Respir Crit Care Med. 1999;160:1043–1066. doi: 10.1164/ajrccm.160.3.16030. [DOI] [PubMed] [Google Scholar]
- 23.Bates D., Mächler M., Bolker B., Walker S. Fitting linear mixed-effects models using lme4. J Stat Softw. 2015;67:1–48. [Google Scholar]
- 24.R Core Team . 2 ed. Vol. 3.6. R Foundation for Statistical Computing; Vienna, Austria: 2019. (R: A Language and Environment for Statistical Computing). [Google Scholar]
- 25.Ratcliffe S.J., Shults J. GEEQBOX: a MATLAB toolbox for generalized estimating equations and quasi-least squares. J Stat Softw. 2008;25:1–14. [Google Scholar]
- 26.Gibson C.D., Colvin M.O., Park M.J., Lai Q., Lin J., Negassa A. Prevalence and predictors of deep vein thrombosis in critically ill medical patients who underwent diagnostic duplex ultrasonography. J Intensive Care Med. 2020;35:1062–1066. doi: 10.1177/0885066618813300. [DOI] [PubMed] [Google Scholar]
- 27.Zhang C., Zhang Z., Mi J., Wang X., Zou Y., Chen X. The cumulative venous thromboembolism incidence and risk factors in intensive care patients receiving the guideline-recommended thromboprophylaxis. Medicine (Baltimore) 2019;98:e15833. doi: 10.1097/MD.0000000000015833. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Kaplan D., Casper T.C., Elliott C.G., Men S., Pendleton R.C., Kraiss L.W. VTE incidence and risk factors in patients with severe sepsis and septic shock. Chest. 2015;148:1224–1230. doi: 10.1378/chest.15-0287. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Lawall H., Oberacker R., Zemmrich C., Bramlage P., Diehm C., Schellong S.M. Prevalence of deep vein thrombosis in acutely admitted ambulatory non-surgical intensive care unit patients. BMC Res Notes. 2014;7:431. doi: 10.1186/1756-0500-7-431. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Ren B., Yan F., Deng Z., Zhang S., Xiao L., Wu M. Extremely high incidence of lower extremity deep venous thrombosis in 48 patients with severe COVID-19 in Wuhan. Circulation. 2020;142:181–183. doi: 10.1161/CIRCULATIONAHA.120.047407. [DOI] [PubMed] [Google Scholar]
- 31.Liu P.P., Blet A., Smyth D., Li H. The science underlying COVID-19: implications for the cardiovascular system. Circulation. 2020;142:68–78. doi: 10.1161/CIRCULATIONAHA.120.047549. [DOI] [PubMed] [Google Scholar]
- 32.Liu Y., Yang Y., Zhang C., Huang F., Wang F., Yuan J. Clinical and biochemical indexes from 2019-nCoV infected patients linked to viral loads and lung injury. Sci China Life Sci. 2020;63:364–374. doi: 10.1007/s11427-020-1643-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Wang L. C-reactive protein levels in the early stage of COVID-19. Med Mal Infect. 2020;50:332–334. doi: 10.1016/j.medmal.2020.03.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Dua A., Thondapu V., Rosovsky R., Hunt D., Latz C., Waller H.D. DVT protocol optimization to minimize healthcare worker exposure in COVID-19. J Vasc Surg Venous Lymphat Disord. 2020 Aug 11 doi: 10.1016/j.jvsv.2020.08.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Patell R., Bogue T., Koshy A., Bindal P., Merrill M., Aird W. Post-discharge thrombosis and hemorrhage in patients with COVID-19. Blood. 2020;136:1342–1346. doi: 10.1182/blood.2020007938. [DOI] [PMC free article] [PubMed] [Google Scholar]