Abstract
Myriapods were, together with arachnids, the earliest animals to occupy terrestrial ecosystems, by at least the Silurian. The origin of myriapods and their land colonization have long remained puzzling until euthycarcinoids, an extinct group of aquatic arthropods considered amphibious, were shown to be stem-group myriapods, extending the lineage to the Cambrian and evidencing a marine-to-terrestrial transition. Although possible respiratory structures comparable to the air-breathing tracheal system of myriapods are visible in several euthycarcinoids, little is known about the mechanism by which they respired. Here, we describe a new euthycarcinoid from Upper Devonian alluvio-lagoonal deposits of Belgium. Synchrotron-based elemental X-ray analyses were used to extract all available information from the only known specimen. Sulfur X-ray fluorescence (XRF) mapping and spectroscopy unveil sulfate evaporation stains, spread over the entire slab, suggestive of a very shallow-water to the terrestrial environment prior to burial consistent with an amphibious lifestyle. Trace metal XRF mapping reveals a pair of ventral spherical cavities or chambers on the second post-abdominal segment that do not compare to any known feature in aquatic arthropods, but might well play a part in air-breathing. Our data provide additional support for amphibious lifestyle in euthycarcinoids and show that different respiratory strategies were used during the marine-to-terrestrial transition in the myriapod lineage.
Keywords: terrestrialization, fossil arthropods, air-breathing, synchrotron radiation, X-ray fluorescence imaging, X-ray spectroscopy
1. Introduction
The establishment of complex terrestrial ecosystems (terrestrialization) is a critical event in the history of life, which imposed multiple constraints upon the water-to-land transition of aquatic organisms, such as osmoregulation (water balance to avoid dehydration), respiration and reproduction in air, as well as locomotion without the help of buoyancy, and exposure to ultraviolet radiation (e.g. [1] and references therein). It is therefore not surprising that arthropods were the first animals to take the step, with their exoskeleton comprising a waxy layer that controls water loss and facilitates osmoregulation, and their jointed appendages supporting surface locomotion ([1] and references therein). However, air-breathing is more challenging, as the gill-modified appendages of aquatic arthropods are not rigid and are highly vulnerable to dehydration. In general, respiratory organs invaginate in terrestrial organisms, while they evaginate in aquatic ones (e.g. [2]). Pulmonate arachnids (e.g. scorpions, whip scorpions and spiders) and (semi-)terrestrial crabs have internalized and/or adapted their gills, respectively, into book lungs found inside open ventral abdominal air-filled cavities ([1,3] and references therein), and through gill stiffening and gill chamber enlargement and/or smooth to highly convoluted lining (e.g. [4]). Terrestrial isopods (including woodlice) use their gills, formed by part of their abdominal legs (endopods), for respiration; some groups also modify exopods into invaginations to form lung-like structures ([1,3] and references therein). Myriapods (e.g. centipedes and millipedes), apulmonate arachnids (e.g. harvestmen, mites, ticks and pseudoscorpions) and terrestrial hexapods (bristletails, silverfish and insects) except for some springtails (which exchange gases by diffusion across the cuticle, a capability linked to their small size), independently evolved an entirely new respiratory system, the tracheal system, which consists of small pores (the spiracles) opening on an extensive and elaborate system of air-tubes (the tracheae).
These respiratory organs are already present in the earliest known terrestrial animals, the myriapods and arachnids, which colonized terrestrial ecosystems at least by the Silurian [5,6]. One of the oldest known myriapods, the millipede (Diplopoda) Pneumodesmus newmani (Wilson & Anderson, 2004) from the Early Devonian (ca 414 Ma; [7,8]) of Scotland, very likely possessed a tracheal system as suggested by the presence of slit-like spiracle openings [6]. Book lungs indistinguishable from those in modern Arachnida have been found in trigonotarbids (an extinct group of spider-like arachnids) from the Early Devonian (ca 410 Ma) Rhynie chert hot spring complex in Scotland [9]. Book lungs in the scorpion Pulmonoscorpius kirktonensi (Jeram, 1994) from the Lower Carboniferous of Scotland show similarities both with those in modern scorpions and Limulus book gills suggesting that arachnid book lungs are derived from internalized book gills [10]. Gene expression data and embryology of modern scorpions, however, showed that part of the book lung (the operculum) is derived from the walking legs of abdominal appendages (telopodites), rather than from epipods (the outermost ramus of appendages) as for book gills [11]. The fossil record further yields insights into the origin and marine history of arachnids (Chelicerata). It can be traced as far back as the early Cambrian [12–15], and even documents marine-to-terrestrial adaptations in scorpions [16]. By contrast, the water-to-land transition in the myriapod lineage is mostly a mystery as (i) the oldest known fossils are terrestrial and show evidence for a tracheal system [6] making it impossible to know whether the latter arose during terrestrialization or whether it replaced an earlier respiratory organ [3], and (ii) no aquatic or terrestrial stem group of Myriapoda had been recognized until very recently [17]. The identification of the extinct euthycarcinoids as aquatic stem-group myriapods extends the myriapod lineage to the Cambrian [17], as suggested by fossil-calibrated molecular phylogenies [18,19], and provides the first insights into the water-to-land transition in Myriapoda [17].
Euthycarcinoids form an extinct group of rare (only 18 taxa formally described), and often overlooked, arthropods extending from the Cambrian [20,21] to the Middle Triassic [22–25]. Cambrian taxa were marine [20,21], younger forms inhabited brackish to freshwater environments [26]. They have been alternatively interpreted as related to crustaceans, xiphosuran chelicerates, hexapod-like arthropods or transitional between myriapods and hexapods (see [26] and references therein). A position as stem-group myriapods had recently emerged as the most plausible based on Bayesian phylogenetic analysis [27,28] and is now supported by new, high-resolution details of head structures from the Early Devonian euthycarcinoid Heterocrania rhyniensis (Hirst & Maulik, 1926) from the Rhynie and Windyfield cherts [17]. Euthycarcinoids are considered amphibious and were probably one of the earliest animals to venture onto land, providing unique insights into terrestrial adaptations in the myriapod lineage. Coastal trackways from the Cambrian [21,29–31] suggest that they were indeed capable of at least brief terrestrial excursions [1,26], yet the mechanism by which euthycarcinoids aerially respired remains poorly understood. Several euthycarcinoids display a pair of ventral pores per pre-abdominal somite, located anteriorly to internal tube-like structures [25,32,33] resembling the sternal apodemes of progoneate myriapods. Edgecombe & Morgan [25], however, interpreted such ‘sternal pores’ in Synaustrus brookvalensis (Riek, 1964) as vesicles having a role in minimizing water loss, rather than as spiracles of a tracheal system. Here, we report a new Upper Famennian (Upper Devonian) euthycarcinoid from the very shallow-water alluvio-lagoonal deposits of Belgium that possesses a pair of ventral spherical cavities or chambers on the post-abdomen forming, most likely, a different air-breathing system.
2. Material and methods
2.1. Origin of the fossil material
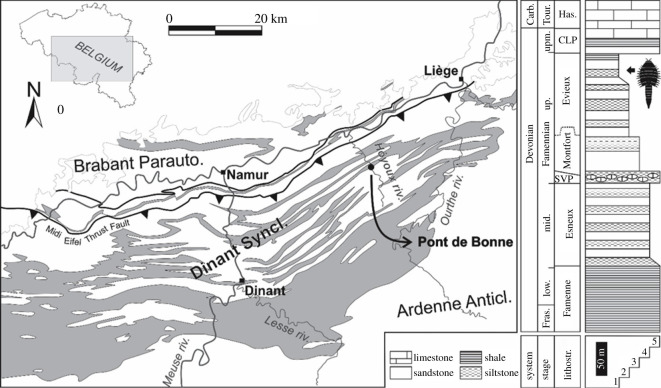
The euthycarcinoid fossil was collected in the 1970s by Prof. Édouard Poty (Liège University) from the Upper Famennian (Evieux Formation) strata at Pont de Bonne, Belgium (figure 1), in an outcrop famous for its fossil plants [36] and eurypterids [37,38] but nowadays partly inaccessible and walled (see [37] for a complete description of the section, and electronic supplementary material, geological background). At Pont de Bonne, the Evieux Formation is interpreted as showing both tidal flat and lagoonal settings [39], locally developed as a slightly dolomitic shale with Racophyton remains. It is commonly acknowledged that this fern grew in brackish water marshes [40]. The euthycarcinoid fossil comes from such a Racophyton dolomitic shale. Contrary to Fraipont's material [37], the specimen is fully articulated indicating very little post-mortem (or post-moulting) transport and presents an uncommon limonitic preservation, similar to that of plant remains coming from the same bed. No counterpart was found, and the specimen is preserved in ventral view. Only the margins and dorsal carinae (preserved as negative reliefs, supporting the interpretation of the fossil as preserved in ventral view) of the pre-abdomen cuticle are visible, indicating that the specimen displays the internal view of the tergites. The post-abdomen and telson cuticle, however, preserves complete surfaces and therefore displays the external view of the sternites.
Figure 1.
Geological and stratigraphical settings of the Pont de Bonne locality, Liège Province, Belgium. Geological map after [34], grey areas represent the Famennian outcrop zones. Famennian lithostratigraphic succession of the Hoyoux valley after [35] with localization of the fossiliferous horizon. Legend: 1, alluvial and alluvio-lagoonal facies; 2, back barrier to lagoonal facies; 3, tidal flat and barrier complex facies; 4, proximal subtidal facies; 5, open-marine subtidal facies. Abbreviations: Anticl., Anticline; Carb., Carboniferous; CLP, Comblain-au-Pont Formation; Fras., Frasnian; Has., Hastière Formation; Lithostr., Lithostratigraphic units; low., lower; mid., middle; Parauto., Parautochton; SVP, Souverain-Pré Formation; Syncl., Syncline; Tour., Tournaisian; up., upper; upm., upermost.
2.2. Synchrotron analyses
The chemical composition of the fossil was investigated using synchrotron rapid scanning X-ray fluorescence (SRS-XRF) mapping at the wiggler beamline 6–2 of the Stanford Synchrotron Radiation Lightsource (SSRL) (figure 2). The diameter of the incoming X-ray beam was reduced to 50 µm using a pinhole (see [41,42] for detailed information about the beamline optics). The fossil was mounted on an x-y scanner stage allowing large movements relative to the X-ray beam with micrometric accuracy. Fluoresced photons were collected using a single element XRF Vortex silicon drift detector placed in the horizontal plane. Integrated intensities in preselected spectral regions corresponding to the emission lines of elements of interest (electronic supplementary material, figure S1) were recorded on the fly in the horizontal direction at a pixel rate corresponding to a scan distance of 50 µm per approximately 4 ms. Full XRF spectra were additionally collected in several points of interest with a 30 s count time (electronic supplementary material, figure S1). Distributions of Ca, Mn, Fe, Ni, Cu, Zn, Ga, As, Br (Kα1) and Pb (Lβ1) XRF lines were mapped using an incident beam energy of 13.5 keV. Note that fitting of the full XRF spectra using the PyMCA data-analysis software [43] indicates that (i) although largely dominated by As, the As distribution also includes signal arising from Pb (Lα1 line, which falls in the same energy domain as the As Kα1 line), (ii) the Pb distribution (Lβ1 line) is actually dominated by pile-up signal from iron, and (iii) no Br was detected (electronic supplementary material, figure S1). Distributions of Al, Si, P, S and Cl Kα1 XRF lines were mapped using an incident beam energy of 3.15 keV while the fossil was enclosed within a custom-built helium-purged chamber to reduce absorption and scattering of the incident and fluoresced low-energy X-rays by air. All elemental distributions presented herein (figure 2, electronic supplementary material, figures S1, S2) are displayed using a linear grey scale going from white (low abundance) to black (high abundance), normalized between the 10th and 90th percentiles of each distribution (except for the S map, slightly saturated to better reveal S associated with the fossil).
Figure 2.
Ericixerxes potii gen. et sp. nov. (a) optical photograph. (b–g) SRS-XRF elemental distributions of As (b–e), Mn (f) and S (only cephalic region and pre-abdomen; (g)) from integrated intensities in their Kα emission energy domains. (c,d) Close-up and interpretative line drawing from the top dotted box area in (b). (e) Close-up from the bottom dotted box area in (b). (h) XANES spectroscopy at the S K-edge from the areas identified with coloured circles in (g), corresponding to the fossil cuticle (orange and blue), and one of the evaporation bubbles (green). Abbreviations: ACT, anterior cephalic tergite; ant., antenna; ?clyp./lab., possible clypeus and/or labrum; ?mand., possible mandibles; PA1–5, post-abdominal somites 1–5; PCT, posterior cephalic tergite; T1–5, pre-abdominal tergites 1–5; sph. ch., spherical chambers. Mapping parameters of the entire maps: scanning step: 50 µm; 901 × 2001 pixels for As and Mn, 801 × 2001 pixels for S; colour scale goes from white (low abundance) to black (high abundance).
X-ray absorption near edge structure (XANES) spectroscopy at the S K-edge was performed to determine sulfur speciation (figure 2h). S XANES spectra were collected in fluorescence mode in the 2450–2550 eV range with energy steps of 1 eV between 2450 and 2460 eV, 0.25 eV between 2460 and 2490 eV, and 1 eV between 2490 and 2550 eV. The count time was set to 0.5 s per energy step. ZnSO4 (prepared as a pellet) was used for energy calibration by setting the position of the main resonance (i.e. sulfate peak) at 2481.75 eV.
3. Results
3.1. Systematic palaeontology
Arthropoda von Siebold, 1848
Mandibulata Snodgrass, 1938
Euthycarcinoidea Gall & Grauvogel, 1964
Euthycarciniformes Starobogatov, 1988
Ericixerxes potii gen. et sp. nov.
3.1.1. Etymology
The genus name is from ‘ericius’ (Latin for hedgehog), referring to the sharp epimera of the pre-abdomen tergites (gender masculine). The species name honours Prof. Édouard Poty who collected the fossil.
3.1.2. Stratigraphic age and distribution
Upper Famennian (Upper Devonian) Evieux Formation, Pont de Bonne, Modave municipality, Liège Province, Belgium (50°27′06″ N, 5°17′05″ E).
3.1.3. Material
Holotype and only known specimen ULg.PA.2016.07.01 (figure 2a), complete individual in ventral view, part only.
3.1.4. Diagnosis
Euthycarciniform arthropod with pre-abdomen tergites bearing sharp epimera and a median carina; fifth post-abdominal somite with long and very sharp epimera; second post-abdominal somite with a pair of large, medial circular cavities.
3.2. Description
The specimen is 75 mm long from the top of the cephalic region to the end of the post-abdomen (figure 2a); maximum width of the pre-abdominal tergites, 19 mm. Semicircular anterior cephalic tergite (ACT) and posterior cephalic tergite (PCT), PCT 1.5 times larger than ACT. Semicircular pre-abdominal tergites (T1–T5) slightly convex posteriorly, with a dorsal median longitudinal carina extending from the middle of the tergite to its posterior margin; at least T2 carina ends in a small spine; T1–T4 of similar dimensions, T5 smaller; T1–T4 bear large and sharp lateral flattened protrusions (epimera), as long as the tergites; T5 with shorter epimera; T1 covering half the PCT; T2–T4 largely covering (ca one third) the preceding tergite; T5 covering both T4 and the first post-abdominal somite. Post-abdomen one-half narrower than the pre-abdomen; subrectangular post-abdominal somites (PA1–PA5); PA1–PA4 bear short, blunt epimera; PA5 tapers into long (twice the length of PA5), extremely sharp epimera. Pyriform telson ending in a sharp spine. Cephalic appendages very poorly preserved; abdominal appendages not preserved.
3.3. Preservation and palaeoenvironment
The specimen preserves cuticular remains as orangish iron oxides, in a greenish dolomitic shale deposited in brackish water marshes (see §2.1 and supplementary material, geological background). Replacement of fossil tissues by iron oxides often results from the oxidation of pyrite (e.g. [44–46]), which forms in large quantities in such organically rich depositional environments, especially considering that sulfate was present (see below). Besides Fe, major-to-trace elemental mapping using SRS-XRF (figure 2, electronic supplementary material, figure S1, S2) shows that the fossil is also enriched in Cu, As (figure 2b,c,e) and to a lesser extent Ca. The fact that the distribution of these elements strictly matches the cuticular remains of the specimen is evidence that it has been controlled (post-mortem) by and reflects original anatomical cuticular structures of the organism. Mn (figure 2f), Ni and to a lesser extent Cu (electronic supplementary material, figure S2) delineate the outer and posterior margins of the tergites and sternites, as well as the median carina and telson, allowing a better distinction of the different trunk segments. This indicates thickening of the cuticle margin, very common in arthropods (e.g. in eurypterids [47]). Zn signal is similar in the fossil and in the sedimentary matrix (electronic supplementary material, figure S2), which mainly consists of Al, Si and Fe.
In addition to transition metals, the fossil contains S (figure 2g, electronic supplementary material, figure S2). XANES spectroscopy at the S K-edge indicates that S is mostly present as sulfates (figure 2h). Traces of organic sulfur, namely cysteine thiol/thioether/heterocyclic S (2472.2–2473.5 eV) and methionine sulfoxide (2475.7 eV), are shown by XANES to be present in several places of the fossil cuticle, most probably representing remnants and/or breakdown products of the chitin-protein complex (figure 2h, orange spectrum). Strikingly, the S map reveals bubble-like subspherical to subcylindrical patches enriched in sulfates at their periphery and depleted at their centre, absolutely invisible with regular light (figure 2g) and closely resembling evaporation stains (‘coffee ring’ effect). These stains are present on the entire surface of the sample (both sediment and specimen) but not enriched within fractures (i.e. not secondary infilling), suggesting very shallow-water to subaerial conditions prior to burial.
3.4. Additional anatomical details revealed by trace metal distributions
The distribution of As (Kα1 emission line: 10.54 keV; probably also including some signal arising from the Pb Lα1 line: 10.55 keV, see above) is particularly interesting as its information depth on the order of a couple of hundred micrometres allows unveiling additional anatomical details buried under surface layers (figure 2b–e). An elongated and annulated structure originating in the ACT and directed forward very likely represents the left antenna (figure 2c,d). Anteriorly to the PCT, a trapezoidal to triangular anteriorly oriented structure, richer in As than the rest of the fossil, stands out as a thicker, or more sclerotized, plate, covering posteriorly two triangular, laterally rounded, processes (figure 2c,d). Racheboeuf et al. [48], in their redescription of Schramixerxes gerem (Schram & Rolfe, 1982) tentatively interpreted a plate with a similar morphology and a similar location on the ventral side of the head as a labrum covering the mouth. This plate also closely resembles the clypeus and/or labrum covering the mandibles in chilopod myriapods (e.g. fig. 4 in [49]), the two triangular laterally rounded processes presumably being the mandibles.
Surprisingly, the As map also uncovers a pair of spherical structures (ca 2 mm large) selectively depleted in As (paler areas) and laying ventrally on PA2 (figure 2b,e), which were not or hardly visible on the specimen. The clear spherical and symmetrical shape of these structures, not seen elsewhere in the specimen or the sedimentary matrix, make them very unlikely to be the result of diagenetic processes. As their concentration in As is closer to that of the sedimentary matrix than the surrounding cuticle area, it indicates that the cuticle is locally thinner, and that they most likely represent cavities or chambers inside the post-abdomen. There could be a second pair on PA3, but these ones look very different, as they are well visible on the fossil and therefore rather represent lacking parts of the cuticle. They are also larger, closer to each other, and more square, rather similar to the square spaces created by the intersection of the medial carina of T4 and the anterior margin of T5. The pair of spherical structures on PA2 also appears on the Cu map (electronic supplementary material, figure S2).
4. Discussion
Ericixerxes potii gen. et sp. nov. is only the second Devonian euthycarcinoid known with H. rhyniensis from the Early Devonian Rhynie and Windyfield cherts. E. potii gen. et sp. nov. and H. rhyniensis share a very similar morphology, but E. potii gen. et sp. nov. clearly differs by its pre-abdomen bearing large and sharp epimera and a median carina, and its post-abdominal somite 5 with extremely sharp epimera. The peculiarity of E. potii gen. et sp. nov. is the pair of quite large, ventral spherical cavities or chambers on the second post-abdominal segment revealed by the distribution of As (figure 2b,e). Ventral abdominal cavities or chambers are not known in extant aquatic arthropods.
No pore or opening structure is visible in E. potii gen. et sp. nov., whereas some other euthycarcinoids exhibit ventral pores located anteriorly to internal tube-like structures [25,32,33] resembling the sternal apodemes that form the tracheal respiratory system within progoneate dignathan myriapods (these pores have alternatively been interpreted as vesicles minimizing water loss [25]). The tracheal system morphologically varies within Myriapoda. Tracheae differ substantially in their positioning, gross morphology and fine structure (e.g. [50]). Scutigeromorph centipedes have unpaired dorsal spiracles opening into a chamber (the atrium) that gives rise to two large bunches of tracheae bathed in haemolymph [51]. Pleurostigmophoran centipedes exhibit a variable number of pleural spiracles, suggesting that tracheal systems evolved more than once within the Chilopoda [50]. Symphylans have a single pair of spiracles on the head. In diplopod millipedes, two pairs of spiracles occur in each segment (at bases of legs) and open into atria and numerous tracheae tubes penetrating organs or ending in the haemolymph (e.g. [3,50] and references therein). A pair of ventral abdominal cavities or chambers as observed in E. potii gen. et sp. nov. does not correspond to any of these morphologies.
Millipedes also possess paired circular gonopores (genital pores), opening on eversible or permanently everted devices, associated with the base of the second pair of walking legs (e.g. [52]). Vaccari et al. [20] recognized ‘a circular structure slightly offset from mid-width of the second post-abdominal segment’ as a possible gonopore. The absence of eversible or permanently everted devices in the fossils is not conclusive as the latter are rarely sclerotized (e.g. [52]), and as such have a weak preservation potential. However, the fact that euthycarcinoids lack appendages on their post-abdomen precludes the identification of the circular cavities seen in E. potii gen. et sp. nov. and Apankura machu (Vaccari et al., 2004) as gonopores homologous to those of diplopods.
The other open abdominal ventral features known in arthropods are the chambers enclosing book lungs in arachnids (e.g. [53,54]) and those enclosing lamellate gills in eurypterids (most probably capable of subaerial breathing via a secondary respiratory structure that comprises the vascularized ventral wall gill-tracts (Kiemenplatten) of the branchial chamber [55]; note that the recent discovery of trabeculae on the dorsal surface of each gill lamella confirms that eurypterids were capable of subaerial breathing [56]). The cavities in E. potii gen. et sp. nov. are highly comparable in shape and position, particularly resembling those enclosing lamellate gills in eurypterids as figured by Laurie [47]. Together with the lack of appendage on these segments, a similar function as chambers enclosing respiratory organs (yet of unknown type and morphology) appears the most likely and unique plausible interpretation for them. The enlarged branchial chambers of some terrestrial crabs, forming cutaneous brachial lungs from vascularization of the chamber wall (e.g. [4]), might be analogues to the cavities in E. potii gen. et sp. nov., as proposed for eurypterid Kiemenplatten [55].
The recognition of air-breathing adaptation in E. potii gen. et sp. nov., from a specimen preserved fully articulated (i.e. most likely with no or limited transport) in very shallow-water to subaerial sediments, provides additional evidence for an amphibious lifestyle for euthycarcinoids. A second independent evolution (as for the tracheal system in apulmonate arachnids, myriapods and terrestrial insects) of a system similar to the book lungs within Arachnida [54], or to the lamellate gills in eurypterids (Chelicerata), cannot be discarded. However, it is very unlikely that euthycarcinoids (stem myriapods, Mandibulata) developed the same organs, but rather different structures, supporting the possibility that the earliest terrestrial animals may have adopted unique respiratory strategies [1]. Our findings indicate that different respiratory strategies have been used during the marine-to-terrestrial transition in the myriapod lineage and suggest that the tracheal systems in myriapods replaced earlier respiratory organs.
Supplementary Material
Acknowledgements
Portions of this research were carried out at the Stanford Synchrotron Radiation Lightsource (CA, USA), a national user facility operated by Stanford University on behalf of the U.S. Department of Energy, Office of Basic Energy Sciences, and we thank the user support staff at SSRL. P.G. thanks G.D. Edgecombe for discussion. U.B. and L.B. acknowledge support from the France – Stanford Center for Interdisciplinary Studies Program, and P.G. from the Institute of Earth Sciences, University of Lausanne. We thank two anonymous referees for their reviews of the manuscript.
Data accessibility
Data are available in the following Dryad Digital Repository at: https://doi.org/10.5061/dryad.cjsxksn2w [57].
Authors' contributions
J.D. rediscovered the fossil in collections and composed the geological background and figure 1. P.G. described the fossil and composed the rest of the paper with input from J.C.L., P.L.M. and L.B. P.G., R.A.W., P.L.M., V.M.E., U.B. and L.B. participated in the synchrotron analyses. P.G. and R.A.W. processed the synchrotron data. All authors interpreted and discussed the results.
Competing interests
The authors have no competing interests.
Funding
Portions of this research were funded by the France – Stanford Center for Interdisciplinary Studies Program and the Institute of Earth Sciences, University of Lausanne, which also supported open access publication costs.
References
- 1.Dunlop JA, Scholtz G, & Selden PA. 2013. Water-to-land transitions. In Arthropod biology and evolution: molecules, development, morphology (eds Minelli A, Boxshall G, Fusco G), pp. 417–439. Heidelberg, Germany: Springer. [Google Scholar]
- 2.Calow P. 1981. Invertebrate biology: a functional approach. New York, NY: John Wiley & Sons. [Google Scholar]
- 3.Hsia CC, Schmitz A, Lambertz M, Perry SF, Maina JN. 2013. Evolution of air breathing: oxygen homeostasis and the transitions from water to land and sky. Compr. Physiol. 3, 849–915. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Burggren WW. 1992. Respiration and circulation in land crabs: novel variations on the marine design. Am. Zool. 32, 417–427. ( 10.1093/icb/32.3.417) [DOI] [Google Scholar]
- 5.Jeram AJ, Selden PA, Edwards D. 1990. Land animals in the Silurian: arachnids and myriapods from Shropshire, England. Science 250, 658–661. ( 10.1126/science.250.4981.658) [DOI] [PubMed] [Google Scholar]
- 6.Wilson HM, Anderson LI. 2004. Morphology and taxonomy of paleozoic millipedes (Diplopoda: Chilognatha: Archipolypoda) from Scotland. J. Paleontol. 78, 169–184. () [DOI] [Google Scholar]
- 7.Suarez SE, Brookfield ME, Catlos EJ, Stöckli DF. 2017. A U-Pb zircon age constraint on the oldest-recorded air-breathing land animal. PLoS ONE 12, e0179262. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Brookfield ME, Catlos EJ, Suarez SE. 2020. Myriapod divergence times differ between molecular clock and fossil evidence: U/Pb zircon ages of the earliest fossil millipede-bearing sediments and their significance. Hist. Biol. ( 10.1080/08912963.2020.1761351) [DOI] [Google Scholar]
- 9.Kamenz C, Dunlop JA, Scholtz G, Kerp H, Hass H. 2008. Microanatomy of Early Devonian book lungs. Biol. Lett. 4, 212–215. ( 10.1098/rsbl.2007.0597) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Jeram AJ. 1990. Book-lungs in a Lower Carboniferous scorpion. Nature 343, 360–361. ( 10.1038/343360a0) [DOI] [Google Scholar]
- 11.Di Z, Edgecombe GD, Sharma PP. 2018. Homeosis in a scorpion supports a telopodal origin of pectines and components of the book lungs. BMC Evol. Biol. 18, 73 ( 10.1186/s12862-018-1188-z) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Legg DA. 2014. Sanctacaris uncata: the oldest chelicerate (Arthropoda). Naturwissenschaften 101, 1065–1073. ( 10.1007/s00114-014-1245-4) [DOI] [PubMed] [Google Scholar]
- 13.Jago JB, García-Bellido DC, Gehling JG. 2016. An early Cambrian chelicerate from the Emu Bay Shale, South Australia. Palaeontology 59, 549–562. ( 10.1111/pala.12243) [DOI] [Google Scholar]
- 14.Aria C, Caron JB. 2017. Mandibulate convergence in an armoured Cambrian stem chelicerate. BMC Evol. Biol. 17, 261 ( 10.1186/s12862-017-1088-7) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Aria C, Caron JB. 2019. A middle Cambrian arthropod with chelicerae and proto-book gills. Nature 573, 586–589. ( 10.1038/s41586-019-1525-4) [DOI] [PubMed] [Google Scholar]
- 16.Waddington J, Rudkin DM, Dunlop JA. 2015. A new mid-Silurian aquatic scorpion—one step closer to land? Biol. Lett. 11, 20140815 ( 10.1098/rsbl.2014.0815) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Edgecombe GD, Strullu-Derrien C, Góral T, Hetherington AJ, Thompson C, Koch M. 2020. Aquatic stem group myriapods close a gap between molecular divergence dates and the terrestrial fossil record. Proc. Natl Acad. Sci. USA 117, 8966–8972. ( 10.1073/pnas.1920733117) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Rota-Stabelli O, Daley AC, Pisani D. 2013. Molecular timetrees reveal a Cambrian colonization of land and a new scenario for ecdysozoan evolution. Curr. Biol. 23, 392–398. ( 10.1016/j.cub.2013.01.026) [DOI] [PubMed] [Google Scholar]
- 19.Lozano-Fernandez J, et al. 2016. A molecular palaeobiological exploration of arthropod terrestrialization. Phil. Trans. R. Soc. B 371, 20150133 ( 10.1098/rstb.2015.0133) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Vaccari NE, Edgecombe GD, Escudero C. 2004. Cambrian origins and affinities of an enigmatic fossil group of arthropods. Nature 430, 554–557. ( 10.1038/nature02705) [DOI] [PubMed] [Google Scholar]
- 21.Collette JH, Hagadorn JW. 2010. Three-dimensionally preserved arthropods from Cambrian Lagerstätten of Quebec and Wisconsin. J. Paleontol. 84, 646–667. ( 10.1666/09-075.1) [DOI] [Google Scholar]
- 22.Handlirsch A. 1914. Eine interessante Crustaceenform aus der Trias der Vogesen. Verhandlungen der Kaiserlich Königlichen Zoologisch-Botanischen in Wien 64, 1–8. [Google Scholar]
- 23.Gall J-C, Grauvogel L. 1964. Un arthropode peu connu le genre Euthycarcinus Handlirsch. Annales de Paléontologie, Invertébrés 50, 1–18. [Google Scholar]
- 24.Riek EF. 1964. Merostomoidea (Arthropoda, Trilobitomorpha) from the Australian Middle Triassic. Records of the Australian Museum 26, 327–332. ( 10.3853/j.0067-1975.26.1965.681) [DOI] [Google Scholar]
- 25.Edgecombe GD, Morgan H. 1999. Synaustrus and the euthycarcinoid puzzle. Alcheringa 23, 193–213. ( 10.1080/03115519908619516) [DOI] [Google Scholar]
- 26.Ortega-Hernández J, Legg DA, Tremewan J, Braddy SJ. 2010. Euthycarcinoids. Geol. Today 26, 195–198. ( 10.1111/j.1365-2451.2010.00770.x) [DOI] [Google Scholar]
- 27.Vannier J, Aria C, Taylor RS, Caron JB. 2018. Waptia fieldensis Walcott, a mandibulate arthropod from the middle Cambrian Burgess Shale. R. Soc. Open Sci. 5, 172206 ( 10.1098/rsos.172206) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Giribet G, Edgecombe GD. 2019. The phylogeny and evolutionary history of arthropods. Curr. Biol. 29, R592–R602. ( 10.1016/j.cub.2019.04.057) [DOI] [PubMed] [Google Scholar]
- 29.MacNaughton RB, Cole JM, Dalrymple RW, Braddy SJ, Briggs DEG, Lukie TD. 2002. First steps on land: arthropod trackways in Cambrian-Ordovician eolian sandstone, southeastern Ontario, Canada. Geology 30, 391–394. () [DOI] [Google Scholar]
- 30.Hagadorn JW, Collette JH, Belt ES. 2011. Eolian-aquatic deposits and faunas of the middle Cambrian Potsdam Group. Palaios 26, 314–334. ( 10.2110/palo.2010.p10-061r) [DOI] [Google Scholar]
- 31.Collette JH, Gass KC, Hagadorn JW. 2012. Protichnites eremita unshelled? Experimental model-based neoichnology and new evidence for a euthycarcinoid affinity for this ichnospecies. J. Paleontol. 86, 442–454. ( 10.1666/11-056.1) [DOI] [Google Scholar]
- 32.Schram FR. 1971. A strange arthropod from the Mazon Creek of Illinois and the trans Permo-Triassic Merostomoidea (Trilobitoidea). Field Museum Nat. Hist. 20, 85–102. [Google Scholar]
- 33.Anderson LI, Trewin NH. 2003. An early Devonian arthropod fauna from the Windyfield cherts, Aberdeenshire, Scotland. Palaeontology 46, 467–509. ( 10.1111/1475-4983.00308) [DOI] [Google Scholar]
- 34.de Béthune P. 1954. Carte géologique de Belgique (échelle 1/500.000). Atlas de Belgique, planche 8 Académie royale de Belgique, Bruxelles.
- 35.Mottequin B, Marion J-M. In press. Carte géologique de Wallonie : Huy – Nandrin 48/3-4, 1/25.000. Namur, Ministère de la Région wallonne, Direction générale des ressources naturelles et de l'environnement.
- 36.Stockmans F. 1948. Végétaux du Dévonien supérieur de la Belgique. Mémoires du Musée royal d'histoire naturelle de Belgique 110, 1–85. [Google Scholar]
- 37.Fraipont J. 1889. Euryptérides nouveaux du Dévonien supérieur de Belgique (Psammites du Condroz). Annales de la Société géologique de Belgique 17, 53–62. [Google Scholar]
- 38.Størmer L, Waterston CD. 1968. Cyrtoctenus gen. nov., a large Late Palaeozoic arthropod with pectinate appendages. Trans. R. Soc. Edinburgh 68, 1–104. ( 10.1017/S0080456800014472) [DOI] [Google Scholar]
- 39.Thorez J, Dreesen R. 1986. A model of a regressive depositional system around the Old Red Continent as exemplified by a field trip in the Upper Famennian ‘Psammites du Condroz’ in Belgium. Annales de la Société géologique de Belgique 109, 285–323. [Google Scholar]
- 40.Scheckler SE. 1986. Geology, floristics and paleoecology of Late Devonian coal swamps from Appalachian Laurentia (U.S.A.). Annales de la Société géologique de Belgique 109, 209–222. [Google Scholar]
- 41.Bergmann U, Morton RW, Manning PL, Sellers WI, Farrar S, Huntley KG, Wogelius RA, Larson P. 2010. Archaeopteryx feathers and bone chemistry fully revealed via synchrotron imaging. Proc. Natl Acad. Sci. USA 107, 9060–9065. ( 10.1073/pnas.1001569107) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Bergmann U, Manning PL, Wogelius RA. 2012. Chemical mapping of paleontological and archeological artifacts with synchrotron X-rays. Ann. Rev. Anal. Chem. 5, 361–389. ( 10.1146/annurev-anchem-062011-143019) [DOI] [PubMed] [Google Scholar]
- 43.Solé V, Papillon E, Cotte M, Walter P, Susini J. 2007. A multiplatform code for the analysis of energy-dispersive X-ray fluorescence spectra. Spectrochim. Acta, Part B 62, 63–68. ( 10.1016/j.sab.2006.12.002) [DOI] [Google Scholar]
- 44.Gabbott SE, Hou X-g, Norry MJ, Siveter DJ. 2004. Preservation of Early Cambrian animals of the Chengjiang biota. Geology 32, 901–904. ( 10.1130/G20640.1) [DOI] [Google Scholar]
- 45.Osés GL, et al. 2016. Deciphering the preservation of fossil insects: A case study from the Crato Member, Early Cretaceous of Brazil. PeerJ 4, e2756 ( 10.7717/peerj.2756) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Saleh F, et al. 2020. Taphonomic pathway of exceptionally preserved fossils in the Lower Ordovician of Morocco. Geobios 60, 99–115. ( 10.1016/j.geobios.2020.04.001) [DOI] [Google Scholar]
- 47.Laurie M. 1893. The anatomy and relations of the Eurypteridae. Trans. R. Soc. Edinburgh, Earth Sci. 37, 509–528. ( 10.1017/S0080456800032713) [DOI] [Google Scholar]
- 48.Racheboeuf PR, Vannier J, Schram FR, Chabard D, Sotty D. 2008. The euthycarcinoid arthropods from Montceau-les-Mines, France: functional morphology and affinities. Earth Environ. Sci. Trans. R. Soc. Edinburgh 99, 11–25. ( 10.1017/S1755691008006130) [DOI] [Google Scholar]
- 49.Edgecombe GD. 2004. Morphological data, extant Myriapoda, and the myriapod stem-group. Contrib. Zool. 73, 207–252. ( 10.1163/18759866-07303002) [DOI] [Google Scholar]
- 50.Hilken G. 1997. Tracheal systems in Chilopoda: a comparison under phylogenetic aspects. Entomologica Scandinavica Supplmentum 51, 49–60. [Google Scholar]
- 51.Edgecombe GD, Giribet G. 2002. Myriapod phylogeny and the relationships of Chilopoda. Biodiversidad, taxonomía y biogeografia de artrópodos de México: hacia una síntesis de su conocimiento 3, 143–168. [Google Scholar]
- 52.Koch M. 2015. Diplopoda – general morphology. In The myriapoda, vol. 2 (ed. Minelli), pp. 7–67. Leiden, The Netherlands: Koninklijke Brill; NV. [Google Scholar]
- 53.Dunlop JA. 1998. The origin of tetrapulmonate book lungs and their significance for chelicerate phylogeny. In Proceedings of the 17th European Colloquium of Arachnology, Edinburgh, 1997 (ed. Selden PA.), pp. 9–16. British Arachnological Society. [Google Scholar]
- 54.Scholtz G, Kamenz C. 2006. The book lungs of Scorpiones and Tetrapulmonata (Chelicerata, Arachnida): evidence for homology and a single terrestrialisation event of a common arachnid ancestor. Zoology 109, 2–13. ( 10.1016/j.zool.2005.06.003) [DOI] [PubMed] [Google Scholar]
- 55.Manning PL, Dunlop JA. 1995. The respiratory organs of eurypterids. Palaeontology 38, 287–298. [Google Scholar]
- 56.Lamsdell JC, McCoy VE, Perron-Feller OA, Hopkins MJ. In press. Air breathing in an exceptionally preserved 340 million year old sea scorpion. Curr. Biol. ( 10.1016/j.cub.2020.08.034) [DOI] [PubMed] [Google Scholar]
- 57.Gueriau P, Lamsdell JC, Wogelius RA, Manning PL, Egerton VM, Bergmann U, Bertrand L, Denayer J.. 2020. Data from: A new Devonian euthycarcinoid reveals the use of different respiratory strategies during the marine-to-terrestrial transition in the myriapod lineage Dryad Digital Repository. ( 10.5061/dryad.cjsxksn2w) [DOI] [PMC free article] [PubMed]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Citations
- Gueriau P, Lamsdell JC, Wogelius RA, Manning PL, Egerton VM, Bergmann U, Bertrand L, Denayer J.. 2020. Data from: A new Devonian euthycarcinoid reveals the use of different respiratory strategies during the marine-to-terrestrial transition in the myriapod lineage Dryad Digital Repository. ( 10.5061/dryad.cjsxksn2w) [DOI] [PMC free article] [PubMed]
Supplementary Materials
Data Availability Statement
Data are available in the following Dryad Digital Repository at: https://doi.org/10.5061/dryad.cjsxksn2w [57].