Abstract
Background
Implementing the current guidelines for leisure-time physical activity (LTPA) provides significant health benefits, especially for middle-aged adults, but it is unclear whether LTPA also translates into cardiovascular health benefits among elderly people. Therefore, we aimed to assess the association of LTPA with the risks of cardiovascular disease (CVD), including coronary heart disease (CHD) and stroke, and all-cause mortality in an elderly population.
Methods
In this prospective cohort study, 32, 942 participants aged 60 years or older who participated in a health check-up programme in China between 2010 and 2018 were included. We evaluated the morbidity and mortality risks through the Cox regression model, competing risk model and restricted cubic spline model.
Results
During a median of 6.84 years of follow-up, there were 6, 857 elderly people with incident CVD; a total of 6, 324 deaths occurred due to all causes and 2, 060 deaths occurred due to CVD. Compared with the inactive group, reductions in CVD morbidity and mortality were observed, with hazard ratios (HRs) of 0.89 (95% CI: 0.83-0.96) and 0.81 (95% CI: 0.71-0.92) in the insufficiently active group, 0.86 (95% CI: 0.80-0.92) and 0.79 (95% CI: 0.69-0.90) in the sufficiently active group, and 0.79 (95% CI: 0.70-0.89) and 0.58 (95% CI: 0.45-0.76) in the highly active group, respectively; but no significant reductions were observed in the very highly active group, with HRs of 0.87 (95% CI: 0.71-1.06) and 0.99 (95% CI: 0.70-1.40), respectively. Compared with the inactive group, reductions in all-cause mortality were also observed, with a HR of 0.90 (95% CI: 0.84-0.97) in the insufficiently active group, 0.82 (95% CI: 0.77-0.89) in the sufficiently active group, 0.77 (95% CI: 0.67- 0.87) in the highly active group, and 0.80 (95% CI: 0.64-0.98) in the very highly active group. A restricted cubic spline diagram showed that there was an L-shaped association between LTPA and the risk of all-cause mortality but a U-shaped or reverse J-shaped relationship between LTPA and the risk of CVD morbidity and mortality, especially stroke. In addition, a subgroup analysis showed that elderly population who consistently performed LTPA for ten years or more had a lower risk of morbidity and mortality.
Conclusions
In an elderly population, even insufficient activity is associated with a decreased risk of all-cause mortality and CVD, and moderate levels of LTPA may be optimal for CVD prevention. In addition, elderly people who consistently perform LTPA over several years may experience greater health benefits.
Keywords: Cardiovascular disease, Chronic coronary disease, Leisure-time physical activity, Stroke
1. Introduction
Cardiovascular disease (CVD) is one of the leading causes of death worldwide, causing a huge economic and health burden, especially among elderly people.[1-3] At present, there are 250 million people over the age of 60 years in China, and the number is increasing by more than 80 million every year. There are approximately 290 million patients with CVD in China, and morbidity and mortality are still increasing.[4] The prevention of CVD is particularly important.
Leisure-time physical activity (LTPA) refers to all of the physical activity that people engage in their free time.[5] Hence, there is a distinction between LTPA and physical activity which is engaged in as part of gainful employment or in the context of daily life. As specific forms of leisure-time behavior that consciously aim at improving physical fitness, sport and exercise constitute the core types of LTPA. Previous studies have revealed that LTPA reduces all-cause mortality in adults.[6, 7] The World Health Organization and the United States government have recently released evidence-based Physical Activity Guidelines, recommending at least 150 min of moderate-intensity or 75 min of vigorous-intensity aerobic activity per week, or an equivalent combination of both.[8] However, less evidence of the effects of LTPA on all-cause mortality and CVD is available in elderly people. It is less well established whether LTPA that does not meet the recommended level could result in health benefits and whether very high LTPA is more conducive to the prevention of CVD among elderly people in China.
Therefore, we used data from a representative cohort of elderly people in central China to explore the relationship between LTPA and morbidity and mortality of CVD, including coronary heart disease (CHD) and stroke, to understand the optimal level of LTPA for CVD prevention in the elderly population.
2. Methods
This was a prospective population-based cohort study approved by the Ethics Committee of Zhengzhou University in China, and all participants provided written informed consent. Study procedures were performed in accordance with the Declaration of Helsinki ethical principles for medical research involving human subjects.
2.1. Cohort description and follow-up
To monitor risk factors for CVD and other noncommunicable diseases in elderly people and improve the health of urban and rural residents, an annual health check-up project has been carried out for the city's elderly population aged 60 years and above since 2010 in Xinzheng City, Henan Province. According to the basic public health service standard, all elderly people in the city were given a free annual health check-up that included questionnaire interviews, physical examinations and laboratory tests, and an electronic health check-up database was formed for this population, which could be integrated with the hospital information system and the cause of death registration system. The health records were updated according to the results of health check-ups or medical records.
2.2. Study population
We used data from the electronic health check-up database of the elderly population in Xinzheng City, Henan Province, Central China from January 2010 to December 2018. The research data can be accessed by contacting the School of Public Health, Zhengzhou University. In total, 36, 998 participants were eligible for the study in 2010. We excluded participants who had any of the following conditions: (1) a history of CVD at the time of entry (n = 2, 441); (2) missing information on LTPA (n = 321); (3) missing information on covariates (n = 566); and (4) data from only one screening examination without repeated measures (n = 728). The final sample size included in the analysis was 32, 942 participants.
2.3. Assessment of LTPA
The primary study exposure was LTPA defined by frequency and duration. Using a combination of frequency (times/week) and duration of LTPA (minutes/time), we defined LTPA in minutes/week.[9] Moderate- and vigorous-intensity physical activities were defined in terms of a coding scheme based on the physical activity compendium.[10] Moderate activities consisted of 3.0 to 5.9 metabolic equivalents (METs), for example: brisk walking (2.4- 4 miles/h), biking (5-9 miles/h), ballroom dancing, active yoga, and recreational swimming; vigorous activities consisted of 6.0 METs or more, for example: jogging/running, biking (≥ 10 miles/h), singles tennis, and swimming. Occupational and routine domestic activities were not included in the present analysis. Physical activity guidelines defined one minute of vigorous-intensity activity as equivalent to two minute of moderate-intensity activity; [11, 12] therefore, we calculated the total LTPA level (minutes/week) for each study participant by converting vigorous-intensity LTPA into the equivalent moderate-intensity LTPA. We used minutes/week instead of METs to understand for our findings more easily.
LTPA was categorized into five groups: inactive (0 min/ week), insufficiently active (1-149 min/week), sufficiently active (150-299 min/week), highly active (300-599 min/ week), and very highly active (≥ 600 min/week).[9, 13, 14]
In addition, we surveyed the number of years that participants had adhered to LTPA. Two subgroups were defined according to the number of years of LTPA, one group performed LTPA for less than ten years, and the other group performed LTPA for ten years or more.
2.4. Covariates
The study covariates included study participants' demographic characteristics, lifestyle behaviors and clinical data. Demographic variables included age, sex (men/women) and medical history of type 2 diabetes mellitus (T2DM). For the assessment of the current severity of smoking, we used a smoking index (SI), which is calculated by multiplying the "number of cigarettes smoked per day" by the "duration of smoking in years".[15] For the assessment of current drinking habits, we used a standard drink, which is defined as a specialized cup for each type of alcohol such as wine, beer, or hard liquor. Each cup has a different volume but contains a similar amount of alcohol (14 g).[16, 17] Lifestyle behaviours included smoking status [(never, former, current light-to- moderate smoker (SI < 400) and current heavy smoker (SI ≥ 400)) and alcohol consumption status (lifetime abstainer, former drinker, current light-to-moderate drinker (men: < 15 drinks/week; women: < 8 drinks/week) and current heavy drinker (men: ≥ 15 drinks/week; women: ≥ 8 drinks/week). The clinical data included anthropometric measurements and laboratory investigations. The measurements including body height, weight, resting heart rate (RHR), systolic blood pressure (SBP), and diastolic blood pressure (DBP) were measured by trained personnel. Body mass index (BMI) was calculated as the weight in kilograms divided by the square of the height in meters. After an overnight fast, peripheral venous blood samples were collected and analyzed with an automatic biochemical analyzer (DIRUI CS380, Changchun, China). Laboratory information, including fasting plasma glucose (FPG), high-density lipoprotein cholesterol (HDL-C), total cholesterol (TC) and triglyceride (TG), was used in this study.
2.5. Outcome definition
The study cohort was followed until the end of 2018. The outcomes of interest in this study were CVD morbidity and mortality, consisting of CHD and stroke morbidity and mortality, and all-cause death. These CVDs were identified from the data of the annual standard health check-up record with digital linkage to the hospital dataset for admissions. Mortality data were obtained from the National Causes of Death Register. Diseases were identified according to the International Classification of Disease (ICD)-10 codes. CHD included ICD-10 codes I20-I25, stroke included ICD-10 codes I60-I69, and CVD was defined as either CHD or stroke.
2.6. Statistical analysis
Data were analyzed in 2019. Participants' baseline characteristics based on the five LTPA categories were presented. Descriptive data were presented as a percentage and frequency for categorical variables, as the mean ± standard deviation (SD) for continuous variables with a normal distribution, and as the median ± interquartile range (IQR) for continuous variables with a non-normal distribution. To compare baseline characteristics, categorical data were assessed using the Pearson's chi-squared or Fisher's exact test. Continuous data were compared using one-way ANOVA or Kruskal-Wallis H test.
Cox proportional hazards regression models were used to estimate the association between physical activity patterns and the risks of CVD, CHD and stroke morbidity and mortality, and all-cause mortality. We examined the proportional hazards assumption by performing statistical testing based on the scaled Schoenfeld residuals, although no appreciable violations were noted. For the present analysis, calendar time (years) was the timescale. Cox proportional hazards regression models were adjusted for age and sex (Model 1) and further adjusted for smoking, alcohol consumption, BMI, medical history of T2DM, SBP, DBP, RHR, FPG, TC, TG, and HDL-C (Model 2). In the survival analysis of CVD morbidity, we assumed that individuals who died of causes other than CVD were still at risk of developing CVD. To address this biologically untenable assumption, we calculated the cumulative incidence of CVD using a competing-risks survival regression and calculated the LTPA for CVD, accounting for the competing risk of nonevent death (Model 3).[18] Competing-risks survival regression also was used for the survival analysis of CHD and stroke morbidity (Model 3). Harrell's C-index, a goodness of fit measure for models, was obtained by use of the STATA stcox postestimation command "estat concordance", and the 95% confidence interval (CI) was obtained by use of the "somersd" package in STATA version 16.0.[19] In addition, the association of the study outcomes with two subgroups according to the number of years of physical activity (< 10 years and ≥ 10 years) was also evaluated.
To evaluate the relationship of LTPA (as a continuous variable) with morbidity and mortality risk, the potentially nonlinear relationship was explored using a restricted cubic spline model. Considering that more than half of elderly people were not participating in LTPA, three knots were placed at the median, 75th and 95th percentiles of total LTPA. A P-value for nonlinearity was calculated by testing the null hypothesis that the coefficient of the second spline was equal to zero.[20] The output included a graphic representation of the fitted splines, with the hazard ratio (HR) for morbidity and mortality on the y-axis and LTPA on the x-axis. Sensitivity analyses were performed according to subgroups (< 10 years and ≥ 10 years).
All statistical analyses were performed using SAS version 9.4 (SAS Institute, Inc., Cary, North Carolina, USA) and STATA version 16.0 (Stata Corporation, College Station, Texas, USA). A two-sided P-value < 0.05 was considered to be statistically significant.
3. Results
A total of 32, 942 survey respondents (mean age: 69.49 ± 7.45 years, 46.89% male, 53.11% female) were included in the present study. Individuals were observed for a median of 6.84 years of follow-up (IQR: 5.15-7.10). Table 1 shows the participants' characteristics at baseline by the five categories of LTPA. Of the participants, 20, 832 participants (63.24%) were classified as inactive at baseline, 4, 422 participants (13.42%) as insufficiently active, 5, 328 participants (16.17%) as sufficiently active, 1, 773 participants (5.38%) as highly active, and 587 participants (1.78%) as very highly active. Individuals with higher LTPA tended to be younger and male. Similarly, higher LTPA was also associated with a lower prevalence of T2DM, blood pressure, RHR and BMI, as well as more favorable blood biochemistry profiles.
Table 1.
Baseline characteristics of the included participants according to the level of LTPA.
| Variables | Inactive (n = 20, 832) | Insufficiently active (n = 4, 422) | Sufficiently active (n = 5, 328) | Highly active (n = 1, 773) | Very highly active (n = 587) | P-value |
| Data are presented as means ± SD or n (%). *Presented as median (interquartile range). BMI: body mass index; DBP: diastolic blood pressure; FPG: fasting plasma glucose; HDL-C: high-density lipoprotein cholesterol; LTPA: leisure-time physical activity; RHR: resting heart rate; SBP: systolic blood pressure; TC: total cholesterol; TG: triglyceride; T2DM: type 2 diabetes mellitus. | ||||||
| Age, yrs | 69.85 ± 7.85 | 69.59 ± 7.04 | 68.74 ± 6.52 | 67.66 ± 6.00 | 67.93 ± 5.94 | < 0.001 |
| Male | 9, 801 (47.05%) | 1, 976 (44.69%) | 2, 410 (45.23%) | 929 (52.40%) | 329 (56.05%) | < 0.001 |
| Smoking | ||||||
| Never | 15, 740 (75.56%) | 3, 373 (76.28%) | 4, 176 (78.38%) | 1, 365 (76.99%) | 448 (76.32%) | < 0.001 |
| Former | 1, 527 (7.33%) | 328 (7.42%) | 340 (6.38%) | 132 (7.45%) | 50 (8.52%) | |
| Light-to-moderate | 1, 482 (7.11%) | 302 (6.83%) | 356 (6.68%) | 144 (8.12%) | 42 (7.16%) | |
| Heavy | 2, 083 (10.00%) | 419 (9.48%) | 456 (8.56%) | 132 (7.45%) | 47 (8.01%) | |
| Alcohol consumption | ||||||
| Lifetime abstainer | 17, 142 (82.29%) | 3, 600 (81.41%) | 4, 402 (82.62%) | 1, 400 (78.96%) | 447 (76.15%) | < 0.001 |
| Former drinker | 426 (2.04%) | 134 (3.03%) | 139 (2.61%) | 75 (4.23%) | 37 (6.30%) | |
| Light-to-moderate | 2, 789 (13.39%) | 593 (13.41%) | 681 (12.78%) | 266 (15.00%) | 92 (15.67%) | |
| Heavy | 475 (2.28%) | 95 (2.15%) | 106 (1.99%) | 32 (1.80%) | 11 (1.87%) | |
| T2DM, % | 2, 763 (13.26%) | 574 (12.98%) | 636 (11.94%) | 185 (10.43%) | 68 (11.58%) | 0.002 |
| BMI, kg/m2 | 24.00 ± 3.27 | 23.98 ± 3.09 | 23.87 ± 3.20 | 23.79 ± 3.10 | 23.61 ± 3.25 | 0.001 |
| SBP, mmHg | 133.61 ± 20.94 | 132.93 ± 19.72 | 132.22 ± 19.80 | 132.45 ± 20.29 | 132.29 ± 18.51 | < 0.001 |
| DBP, mmHg | 79.83 ± 10.10 | 79.72 ± 10.08 | 79.25 ± 10.18 | 79.11 ± 10.56 | 79.05 ± 9.43 | < 0.001 |
| RHR, beats per minute | 74.61 ± 7.20 | 74.03 ± 7.80 | 73.62 ± 7.51 | 73.04 ± 7.40 | 73.44 ± 7.45 | < 0.001 |
| FPG, mmol/L | 5.14 (4.64-5.70)* | 5.12 (4.37-5.80)* | 5.00 (4.30-5.74)* | 5.10 (4.32-5.87)* | 4.90 (4.46-5.75)* | < 0.001 |
| TC, mmol/L | 4.86 (4.32-5.30)* | 4.82 (4.31-5.30)* | 4.65 (4.25-5.27)* | 4.69 (4.23-5.20)* | 4.65 (4.33-5.02)* | < 0.001 |
| TG, mmol/L | 1.45 (1.13-1.71)* | 1.38 (1.04-1.71)* | 1.29 (1.03-1.55)* | 1.28 (1.02-1.54)* | 1.28 (1.05-1.54)* | < 0.001 |
| HDL-C, mmol/L | 1.39 (1.19-1.60)* | 1.41 (1.20-1.62)* | 1.41 (1.19-1.63)* | 1.42 (1.19-1.65)* | 1.42 (1.20-1.65)* | < 0.001 |
From baseline, during a median of 6.84 years of follow-up, there were 6, 857 elderly people with incident CVD (4, 937 elderly people with incident CHD and 2, 240 elderly people with incident stroke). Elderly individuals over 60 years of age had an absolute risk of 37.83 per 1000 per year for CVD morbidity (26.88 per 1000 per year for CHD morbidity and 11.73 per 1000 per year for stroke morbidity).
Table 2 shows the multivariate association between LTPA and the risk of CVD morbidity. Compared with the inactive participants, in competing-risks Model 3, the fully adjusted HR for CVD morbidity was 0.89 (95% CI: 0.83- 0.96) in the insufficiently active participants, 0.86 (95% CI: 0.80-0.92) in the sufficiently active participants, 0.79 (95% CI: 0.70-0.89) in the highly active participants, and 0.87 (95% CI: 0.71-1.06) in the very highly active participants. Harrell's C-index for this model indicated high goodness of fit (C-index = 0.802, 95% CI: 0.787-0.818; supplemental material, Table 1S). Similar effects on CHD and stroke morbidity were also observed across different physical activity categories. The HRs for CHD morbidity for insufficiently active, sufficiently active, highly active, and very highly active were 0.90 (95% CI: 0.83-0.98), 0.89 (95% CI: 0.82-0.96), 0.87 (95% CI: 0.76-0.96) and 0.84 (95% CI: 0.66-1.06), respectively. The HRs for stroke morbidity for insufficiently active, sufficiently active, highly active, and very highly active were 0.88 (95% CI: 0.78-0.99), 0.87 (95% CI: 0.77-0.98), 0.70 (95% CI: 0.56-0.88) and 1.08 (95% CI: 0.79-1.48), respectively.
Table 2.
Association between LTPA level and risk of CVD morbidity.
| Variables | Inactive | Insufficiently active | Sufficiently active | Highly active | Very highly active |
| Model 1: adjusted for sex and age. Model 2: adjusted for variables in Model 1 plus current smoking, alcohol consumption, BMI, medical history of T2DM, SBP, DBP, RHR, FPG, TC, TG and HDL-C. Model 3: competing-risks survival regression adjusted covariates were the same as Model 2. *Presented as HR (95% CI). BMI: body mass index; CHD: coronary heart disease; CI: confidence interval; CVD: cardiovascular disease; DBP: diastolic blood pressure; FPG: fasting plasma glucose; HDL-C: high-density lipoprotein cholesterol; HR: hazards ratio; LTPA: leisure-time physical activity; RHR: resting heart rate; SBP: systolic blood pressure; TC: total cholesterol; TG: triglyceride; T2DM: type 2 diabetes mellitus. | |||||
| CVD | |||||
| Events | 4, 569 | 902 | 1, 002 | 281 | 103 |
| Person-years | 111, 206 | 25, 202 | 31, 089 | 10, 323 | 3, 426 |
| Incidence, events per 1, 000 person-years | 41.09 | 35.79 | 32.23 | 27.22 | 30.07 |
| Model 1 | Reference | 0.84 (0.78-0.90)* | 0.77 (0.72-0.82)* | 0.66 (0.59-0.75)* | 0.72 (0.59-0.88)* |
| Model 2 | Reference | 0.88 (0.82-0.94)* | 0.84 (0.78-0.90)* | 0.78 (0.69-0.88)* | 0.87 (0.71-1.06)* |
| Model 3 | Reference | 0.89 (0.83-0.96)* | 0.86 (0.80-0.92)* | 0.79 (0.70-0.89)* | 0.87 (0.71-1.06)* |
| CHD | |||||
| Events | 3, 253 | 649 | 742 | 224 | 69 |
| Person-years | 112, 700 | 25, 578 | 31, 491 | 10, 441 | 3, 469 |
| Incidence, events per 1, 000 person-years | 28.86 | 25.37 | 23.56 | 21.45 | 19.89 |
| Model 1 | Reference | 0.85 (0.78-0.92)* | 0.79 (0.73-0.86)* | 0.75 (0.65-0.85)* | 0.68 (0.54-0.87)* |
| Model 2 | Reference | 0.88 (0.81-0.96)* | 0.86 (0.80-0.94)* | 0.85 (0.74-0.94)* | 0.83 (0.65-1.05)* |
| Model 3 | Reference | 0.90 (0.83-0.98)* | 0.89 (0.82-0.96)* | 0.87 (0.76-0.96)* | 0.84 (0.66-1.06)* |
| Stroke | |||||
| Events | 1, 502 | 289 | 324 | 82 | 43 |
| Person-years | 117, 468 | 26, 565 | 32, 600 | 10, 784 | 3, 571 |
| Incidence, events per 1, 000 person-years | 12.79 | 10.88 | 9.94 | 7.60 | 12.04 |
| Model 1 | Reference | 0.83 (0.73-0.94)* | 0.77 (0.69-0.87)* | 0.60 (0.48-0.75)* | 0.94 (0.69-1.27)* |
| Model 2 | Reference | 0.86 (0.76-0.97)* | 0.84 (0.74-0.95)* | 0.69 (0.55-0.89)* | 1.08 (0.80-1.46)* |
| Model 3 | Reference | 0.88 (0.78-0.99)* | 0.87 (0.77-0.98)* | 0.70 (0.56-0.88)* | 1.08 (0.79-1.48)* |
Our study depict the stratified analysis on the association of LTPA categories and risk of CVD morbidity according to subgroups of the number of years of LTPA (< 10 years and ≥ 10 years) (supplemental material, Table 2S). We found the same direction of association, and the association was more significant in the group of individuals performing LTPA for ten years or more than in the group with less than ten years of LTPA.
There were a total of 6, 324 deaths due to all causes and 2, 060 deaths due to CVD (981 deaths due to CHD and 1, 082 deaths due to stroke) during 193, 878 person-years of follow-up. Table 3 shows the multivariate associations between LTPA level and risk of all-cause and CVD mortality. Compared with the inactive participants, the fully adjusted HR for all-cause mortality was 0.90 (95% CI: 0.84-0.97) for the insufficiently active participants, 0.82 (95% CI: 0.77-0.89) for the sufficiently active participants, 0.77 (95% CI: 0.67-0.87) for the highly active participants, and 0.80 (95% CI: 0.64-0.98) for the very highly active participants. Compared with the inactive participants, the fully adjusted HR for CVD mortality was 0.81 (95% CI: 0.71-0.92) for the insufficiently active participants, 0.79 (95% CI: 0.69- 0.90) for the sufficiently active participants, 0.58 (95% CI: 0.45-0.76) for the highly active participants, and 0.99 (95% CI: 0.70-1.40) for the very highly active participants. Similar results were observed with the group of individuals with an LTPA history of ten years or more and the group with less than ten years of LTPA (supplemental material, Table 3S).
Table 3.
Association between LTPA level and risk of all-cause and CVD mortality.
| Variables | Inactive | Insufficiently active | Sufficiently active | Highly active | Very highly active |
| Model 1: adjusted for sex and age. Model 2: adjusted for variables in Model 1 plus current smoking, alcohol consumption, BMI, medical history of T2DM, SBP, DBP, RHR, FPG, TC, TG and HDL-C. *Presented as HR (95% CI). BMI: body mass index; CHD: coronary heart disease; CI: confidence interval; CVD: cardiovascular disease; DBP: diastolic blood pressure; FPG: fasting plasma glucose; HDL-C: high-density lipoprotein cholesterol; HR: hazards ratio; LTPA: leisure-time physical activity; RHR: resting heart rate; SBP: systolic blood pressure; TC: total cholesterol; TG: triglyceride; T2DM: type 2 diabetes mellitus. | |||||
| All-cause | |||||
| Deaths | 4, 249 | 862 | 881 | 244 | 88 |
| Model 1 | Reference | 0.89 (0.83-0.96)* | 0.81 (0.76-0.87)* | 0.76 (0.66-0.86)* | 0.77 (0.62-0.95)* |
| Model 2 | Reference | 0.90 (0.84-0.97)* | 0.82 (0.77-0.89)* | 0.77 (0.67-0.87)* | 0.80 (0.64-0.98)* |
| CVD | |||||
| Deaths | 1, 434 | 258 | 276 | 59 | 33 |
| Model 1 | Reference | 0.79 (0.69-0.90)* | 0.74 (0.65-0.85)* | 0.53 (0.41-0.69)* | 0.86 (0.61-1.22)* |
| Model 2 | Reference | 0.81 (0.71-0.92)* | 0.79 (0.69-0.90)* | 0.58 (0.45-0.76)* | 0.99 (0.70-1.40)* |
| CHD | |||||
| Deaths | 688 | 119 | 131 | 30 | 13 |
| Model 1 | Reference | 0.77 (0.63-0.94)* | 0.76 (0.63-0.92)* | 0.59 (0.41-0.85)* | 0.75 (0.43-1.30)* |
| Model 2 | Reference | 0.78 (0.64-0.95)* | 0.81 (0.67-0.97)* | 0.66 (0.46-0.96)* | 0.90 (0.52-1.56)* |
| Stroke | |||||
| Deaths | 749 | 139 | 145 | 29 | 20 |
| Model 1 | Reference | 0.81 (0.67-0.97)* | 0.73 (0.61-0.87)* | 0.47 (0.33-0.69)* | 0.96 (0.61-1.49)* |
| Model 2 | Reference | 0.82 (0.69-0.99)* | 0.77 (0.64-0.92)* | 0.51 (0.35-0.74)* | 1.06 (0.67-1.65)* |
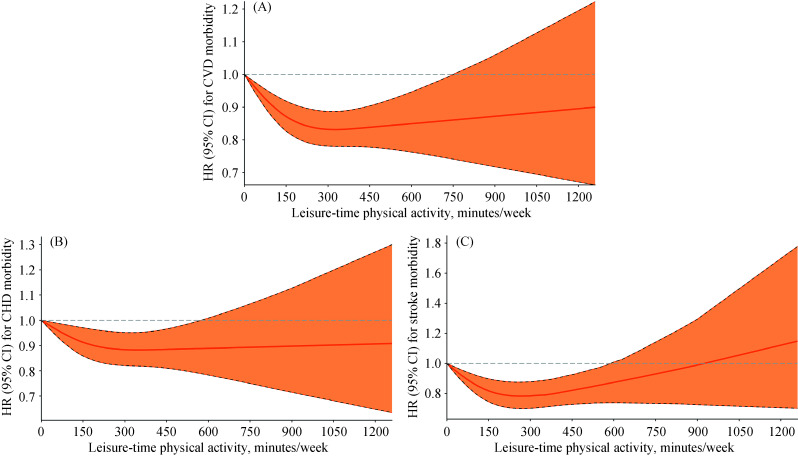
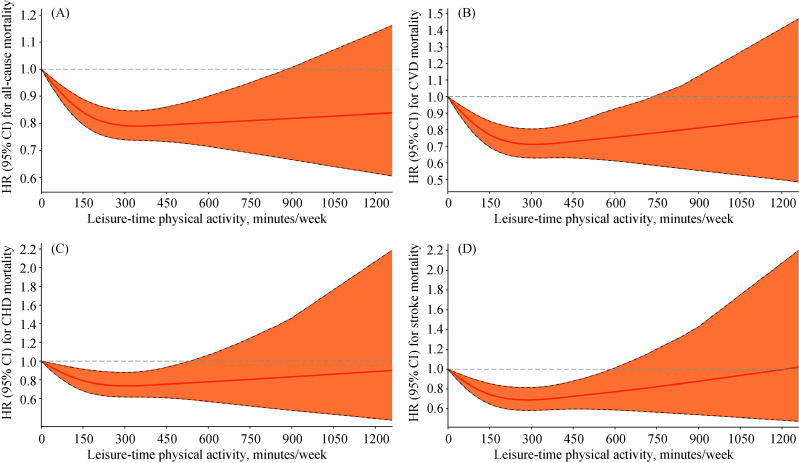
Restricted cubic splines showed that there were curvilinear relationships between LTPA (as a continuous variable) and the risk of CVD morbidity (P-value for nonlinearity < 0.001, Figure 1A), stroke morbidity (P-value for nonlinearity < 0.001, Figure 1C), CVD mortality (P-value for nonlinearity < 0.001, Figure 2B), CHD mortality (P-value for nonlinearity = 0.034, Figure 2C) and stroke mortality (P-value for nonlinearity = 0.002, Figure 2D); but the risks of CHD morbidity (P-value for nonlinearity = 0.068, Figure 1B) and all-cause mortality (P-value for nonlinearity = 0.078, Figure 2A) did not differ significantly from a curvilinear relationship. Our study also shows the tests of non- linearity of LTPA restricted cubic splines (supplemental material, Table 4S). The overall tests of association (likelihood ratio tests) indicated significant associations between LTPA and CVD morbidity and mortality and all-cause mortality of all participants and subgroups (supplemental material, Table 5S).
Figure 1.
HRs for CVD morbidity according to LTPA in the elderly population.
(A): A association between LTPA (as a continuous variable, min/week) and risk of CVD morbidity; (B): a association between LTPA (as a continuous variable, min/week) and risk of CHD morbidity; and (C): a association between LTPA (as a continuous variable, min/week) and risk of stroke morbidity. CHD: coronary heart disease; CI: confidence interval; CVD: cardiovascular disease; HR: hazards ratio; LTPA: leisure-time physical activity.
Figure 2.
HRs for all-cause and CVD mortality according to LTPA in the elderly population.
(A): A association between LTPA (as a continuous variable, min/week) and risk of all-cause mortality; (B): a association between LTPA (as a continuous variable, min/week) and risk of CVD mortality; (C): a association between LTPA (as a continuous variable, min/week) and risk of CHD mortality; and (D): a association between LTPA (as a continuous variable, min/week) and risk of stroke mortality. CHD: coronary heart disease; CI: confidence interval; CVD: cardiovascular disease; HR: hazards ratio; LTPA: leisure-time physical activity.
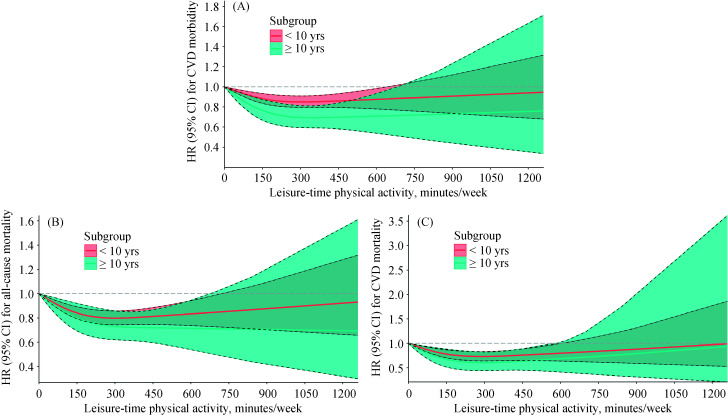
An L-shaped association was seen between LTPA and the risks of CHD morbidity and all-cause mortality. Compared with inactivity, the protective effect of LTPA on CHD morbidity and all-cause mortality started at a low dose and became stronger with an increasing dose until remaining steady at the highest recommended level of LTPA. However, a U-shaped or reverse J-shaped relationship was seen between LTPA and the risk of CVD morbidity and mortality (especially stroke); as the LTPA level increased, the protective effect gradually increased, and when the maximum recommended dose was reached, the protective effect began to gradually decrease and even appeared harmful. Regarding the risk of CVD morbidity and mortality, there was no significant protective effect when physical activity exceeded approximately 750 min/week. We examined the association between LTPA and the risk of all-cause mortality and CVD morbidity and mortality stratified by the number of years of LTPA and found these results were similar to those obtained when analyzing all data (Figure 3). It is worth noting that the elderly people who performed LTPA consistently for ten years or more had a lower risk of all-cause mortality and CVD morbidity and mortality.
Figure 3.
HRs for all-cause mortality and CVD according to LTPA in subgroups.
(A): A association between LTPA (as a continuous variable, min/week) and risk of CVD morbidity in subgroups; (B): a association between LTPA (as a continuous variable, min/week) and risk of all-cause mortality in subgroups; and (C): a association between LTPA (as a continuous variable, min/week) and risk of CVD mortality in subgroups. CI: confidence interval; CVD: cardiovascular disease; HR: hazards ratio; LTPA: leisure-time physical activity.
4. Discussion
In this prospective population-based study of people aged 60 years or older in China, we compared the relationship between LTPA and CVD (including CHD and stroke) morbidity and mortality and all-cause mortality. We found that even insufficient activity was associated with a decreased risk of morbidity and mortality. The risk of CVD morbidity and mortality was lowest among the elderly people who performed approximately twice the recommended minimum level of physical activity. However, very high activity (more than 4-6 times the minimum recommendation) may not significantly reduce the risks of CVD morbidity or mortality compared to the rates associated with inactivity.
Many previous studies of adults or middle-aged adults have shown that physical activity at less than the recommended levels could bring health benefits. For example, a Taiwanese study involving adults over the age of 20 showed that individuals who performed a daily average of 15 minutes of moderate-intensity exercise experienced significant health benefits when compared with individuals who were inactive.[13] A study of middle-aged and elderly Chinese men and women found that even a small amount of moderate-intensity LTPA was associated with a decreased risk of death due to all causes and CVD.[21] A study of 88, 140 American adults aged 40-85 years suggested that individuals who performed 10-59 min/week of light-to-moderate physical activity experienced significant health benefits compared with physically inactive adults.[9] We observed similar effects in our population of elderly people, even insufficient activity was associated with a decreased risk of CVD morbidity and mortality and all-cause mortality. However, approximately two-thirds of the elderly people were inactive in our study. Therefore, it is essential to increase the activity level in this population, even if it does not reach the minimum level recommended by the World Health Organization.
Recent studies have reported that very high levels of LTPA could still have health effects. For all-cause mortality, we also found that very high levels of LTPA were still beneficial compared to inactivity. However, there are some conflicting findings about the relationship between very high levels of LTPA and the risk of CVD morbidity and mortality. Regarding the risk of CVD in elderly people, we did not find a significant health effect. Although a study of middle-age and older adults in the United States suggested that very high levels of LTPA were beneficial, a significant U-shaped curve was found between LTPA and CVD deaths, with a minimum risk of CVD mortality observed at 400 min/week.[9] However, in elderly people, we did not find significant health effects of very high levels of LTPA on CVD mortality, and we also found a similar U-shaped curve, with the lowest risk of CVD mortality observed at approximately 300 min/week. A study involving 17 countries with different income levels also found a U-shaped relationship between recreational physical activity and CVD mortality.[22] The Japan Public Health Center-Based Prospective (JPHC) study found that the associations between physical activity level and stroke risk showed a U-shaped or even J-shaped.[23] The results of these studies are consistent with our conclusions.
A previous meta-analysis including studies mostly from Western countries reported that compared with inactivity, moderately intense physical activity was sufficient to achieve the maximum risk reduction for stroke.[24] A prospective study of 1.1 million women from the United Kingdom showed the lowest CVD risks among women who performed moderate amounts of activity.[25] A large study of 416, 175 adults found that physical activity with > 100 minutes/day of vigorous-intensity activity gave no additional health benefit.[13] Therefore, very high levels of LTPA may not to be encouraged, because of the potential for increased injury and CVD risks.
The mechanisms of LTPA's health effects are complex and require further study. Appropriate physical activity can reduce the risk of CVD by improving factors such as hypertension, obesity, insulin resistance, lipid and lipoprotein profiles, endothelial function, the inflammatory response, plasma viscosity, and platelet aggregation.[26-28] Recent studies have proposed that excessive endurance sports may potentially induce adverse cardiovascular effects, such as arrhythmias and myocardial damage.[13] Very high levels of physical activity may lead to pathological remodelling of the heart and major arteries, elevated levels of cardiac biomarkers, myocardial fibrosis, sudden and transient increases in blood pressure, sudden death, and cerebral hemorrhage.[29, 30]
A study of American male physicians suggested that habitual vigorous exercise diminishes the risk of sudden death during vigorous exertion.[31] We found that elderly people who performed LTPA consistently for ten years or more had a lower risk of all-cause mortality and CVD morbidity and mortality. Performing LTPA consistently for more than ten years may reduce the harm associated with very high levels of LTPA. Therefore, a progressive transitional phase (for example, starting with walking) may be useful to reduce risk, and we encourage regular physical activity from middle age. Increasing physical activity is a simple, widely applicable, low-cost global strategy that could reduce deaths and CVD in middle-aged and elderly people.
4.1. Limitations
One strength of this study is the long follow-up, with yearly health screenings to identify the onset of each type of CVD. Another strength of this study is not only did we study the risk of LTPA and CVD (CHD and stroke) morbidity but also the risk of mortality, as well as the impact of the duration of physical activity on health.
Some limitations of our study need to be addressed. Firstly, LTPA was measured by self-report, so there may be recall bias and further objective measurement methods are needed, such as the implementation of pedometers or accelerometers.[32, 33] Secondly, we studied only LTPA, but the effects of other activities (occupation, transportation, household) and interactions were not considered. Thirdly, the sample size of the very high LTPA group may be relatively small in our study. Next, we need a larger sample size population for verification. Last but not least, we had no data on sedentary behaviors that may modify the association of LTPA with morbidity and mortality risk.[34]
4.2. Conclusions
Our study shows that there is an L-shaped association between LTPA and the risk of all-cause mortality, but a U-shaped or reverse J-shaped relationship between LTPA and the risk of CVD morbidity and mortality (especially stroke). Therefore, for an elderly population, even insufficient activity is associated with a decreased risk of all-cause mortality and CVD, and moderate LTPA may be optimal for CVD prevention. In addition, the elderly people who performed LTPA consistently for ten years or more had a lower risk of all-cause mortality and CVD. In short, we encourage adherence to regular and moderate LTPA from middle-aged adults.
Acknowledgments
This study was supported by National Key Research and Development Programme of China (2017YFC1307705 & 2016YFC0106907) and the Science and Technology Development Programme of Henan (No.201403007). All authors had no conflicts of interest to disclose.
SUPPLEMENTARY DATA
Supplementary data to this article can be found online.
Contributor Information
Xiao-Yan ZHAO, Email: zhaoxy15@lzu.edu.cn.
Song-He SHI, Email: zzussh@126.com.
References
- 1.Timmis A, Townsend N, Gale C, et al. European Society of Cardiology: cardiovascular disease statistics 2017. Eur Heart J. 2018;39:508–579. doi: 10.1093/eurheartj/ehx628. [DOI] [PubMed] [Google Scholar]
- 2.Lewington S, Lacey B, Clarke R, et al. The burden of hypertension and associated risk for cardiovascular mortality in China. JAMA Intern Med. 2016;176:524–532. doi: 10.1001/jamainternmed.2016.0190. [DOI] [PubMed] [Google Scholar]
- 3.Vos T, Abajobir AA, Abate KH, et al. Global, regional, and national incidence, prevalence, and years lived with disability for 328 diseases and injuries for 195 countries, 1990-2016: a systematic analysis for the Global Burden of Disease Study 2016. Lancet. 2017;390:1211–1259. doi: 10.1016/S0140-6736(17)32154-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Chen WW, Gao RL, Liu LS, et al. China cardiovascular diseases report 2015: a summary. J Geriatr Cardiol. 2017;14:1–10. doi: 10.11909/j.issn.1671-5411.2017.01.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Jörgensen S, Martin Ginis KA, Lexell J. Leisure time physical activity among older adults with long-term spinal cord injury. Spinal Cord. 2017;55:848–856. doi: 10.1038/sc.2017.26. [DOI] [PubMed] [Google Scholar]
- 6.Päivärinne V, Kautiainen H, Heinonen A, et al. Relationships of leisure-time physical activity and work ability between different occupational physical demands in adult working men. Int Arch Occup Environ Health. 2019;92:739–746. doi: 10.1007/s00420-019-01410-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Tsenkova VK. Leisure-time, occupational, household physical activity and insulin resistance (HOMAIR) in the Midlife in the United States (MIDUS) national study of adults. Prev Med Rep. 2017;5:224–227. doi: 10.1016/j.pmedr.2016.12.025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Piercy KL, Troiano RP, Ballard RM, et al. The physical activity guidelines for Americans. JAMA. 2018;320:2020–2028. doi: 10.1001/jama.2018.14854. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Zhao M, Veeranki SP, Li S, et al. Beneficial associations of low and large doses of leisure time physical activity with all-cause, cardiovascular disease and cancer mortality: a national cohort study of 88, 140 US adults. Br J Sports Med. 2019;53:1405–1411. doi: 10.1136/bjsports-2018-099254. [DOI] [PubMed] [Google Scholar]
- 10.Ainsworth BE, Haskell WL, Herrmann SD, et al. 2011 compendium of physical activities: a second update of codes and MET values. Med Sci Sports Exerc. 2011;43:1575–1581. doi: 10.1249/MSS.0b013e31821ece12. [DOI] [PubMed] [Google Scholar]
- 11.O'Donovan G, Lee IM, Hamer M, et al. Association of "weekend warrior" and other leisure time physical activity patterns with risks for all-cause, cardiovascular disease, and cancer mortality. JAMA Intern Med. 2017;177:335–342. doi: 10.1001/jamainternmed.2016.8014. [DOI] [PubMed] [Google Scholar]
- 12.Ferrario MM, Roncaioli M, Veronesi G, et al. Differing associations for sport versus occupational physical activity and cardiovascular risk. Heart. 2018;104:1165–1172. doi: 10.1136/heartjnl-2017-312594. [DOI] [PubMed] [Google Scholar]
- 13.Wen CP, Wai JP, Tsai MK, et al. Minimum amount of physical activity for reduced mortality and extended life expectancy: a prospective cohort study. Lancet. 2011;378:1244–1253. doi: 10.1016/S0140-6736(11)60749-6. [DOI] [PubMed] [Google Scholar]
- 14.Hamer M, O'Donovan G, Stamatakis E. Association between physical activity and sub-types of cardiovascular disease death causes in a general population cohort. Eur J Epidemiol. 2019;34:483–487. doi: 10.1007/s10654-018-0460-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Inamura K, Yokouchi Y, Kobayashi M, et al. Tumor B7-H3 (CD276) expression and smoking history in relation to lung adenocarcinoma prognosis. Lung Cancer. 2017;103:44–51. doi: 10.1016/j.lungcan.2016.11.013. [DOI] [PubMed] [Google Scholar]
- 16.Goncalves A, Claggett B, Jhund PS, et al. Alcohol consumption and risk of heart failure: the Atherosclerosis Risk in Communities Study. Eur Heart J. 2015;36:939–945. doi: 10.1093/eurheartj/ehu514. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Van Horn L, Carson JA, Appel LJ, et al. Recommended dietary pattern to achieve adherence to the American Heart Association/American College of Cardiology (AHA/ACC) guidelines: a scientific statement from the American Heart Association. Circulation. 2016;134:e505–e529. doi: 10.1161/CIR.0000000000000462. [DOI] [PubMed] [Google Scholar]
- 18.Austin PC, Fine JP. Practical recommendations for reporting Fine-Gray model analyses for competing risk data. Stat Med. 2017;36:4391–4400. doi: 10.1002/sim.7501. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Harrell FE Jr, Califf RM, Pryor DB, et al. Evaluating the yield of medical tests. JAMA. 1982;247:2543–2546. doi: 10.1001/jama.1982.03320430047030. [DOI] [PubMed] [Google Scholar]
- 20.Orsini N, Li R, Wolk A, et al. Meta-analysis for linear and nonlinear dose-response relations: examples, an evaluation of approximations, and software. Am J Epidemiol. 2012;175:66–73. doi: 10.1093/aje/kwr265. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Liu Y, Wen W, Gao YT, et al. Level of moderate-intensity leisure-time physical activity and reduced mortality in middle-aged and elderly Chinese. J Epidemiol Community Health. 2018;72:13–20. doi: 10.1136/jech-2017-209903. [DOI] [PubMed] [Google Scholar]
- 22.Lear SA, Hu W, Rangarajan S, et al. The effect of physical activity on mortality and cardiovascular disease in 130 000 people from 17 high-income, middle-income, and low-income countries: the PURE study. Lancet. 2017;390:2643–2654. doi: 10.1016/S0140-6736(17)31634-3. [DOI] [PubMed] [Google Scholar]
- 23.Kubota Y, Iso H, Yamagishi K, et al. Daily total physical activity and incident stroke: the Japan public health center-based prospective study. Stroke. 2017;48:1730–1736. doi: 10.1161/STROKEAHA.117.017560. [DOI] [PubMed] [Google Scholar]
- 24.Wendel-Vos GC, Schuit AJ, Feskens EJ, et al. Physical activity and stroke. A meta-analysis of observational data. Int J Epidemiol. 2004;33:787–798. doi: 10.1093/ije/dyh168. [DOI] [PubMed] [Google Scholar]
- 25.Armstrong ME, Green J, Reeves GK, et al. Frequent physical activity may not reduce vascular disease risk as much as moderate activity: large prospective study of women in the United Kingdom. Circulation. 2015;131:721–729. doi: 10.1161/CIRCULATIONAHA.114.010296. [DOI] [PubMed] [Google Scholar]
- 26.Soares-Miranda L, Siscovick DS, Psaty BM, et al. Physical activity and risk of coronary heart disease and stroke in older adults: the Cardiovascular Health Study. Circulation. 2016;133:147–155. doi: 10.1161/CIRCULATIONAHA.115.018323. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Barengo NC, Antikainen R, Borodulin K, et al. Leisure-time physical activity reduces total and cardiovascular mortality and cardiovascular disease incidence in older adults. J Am Geriatr Soc. 2017;65:504–510. doi: 10.1111/jgs.14694. [DOI] [PubMed] [Google Scholar]
- 28.Shortreed SM, Peeters A, Forbes AB. Estimating the effect of long-term physical activity on cardiovascular disease and mortality: evidence from the Framingham Heart Study. Heart. 2013;99:649–654. doi: 10.1136/heartjnl-2012-303461. [DOI] [PubMed] [Google Scholar]
- 29.Weiss SA, Blumenthal RS, Sharrett AR, et al. Exercise blood pressure and future cardiovascular death in asymptomatic individuals. Circulation. 2010;121:2109–2116. doi: 10.1161/CIRCULATIONAHA.109.895292. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Chomistek AK, Chiuve SE, Jensen MK, et al. Vigorous physical activity, mediating biomarkers, and risk of myocardial infarction. Med Sci Sports Exerc. 2011;43:1884–1890. doi: 10.1249/MSS.0b013e31821b4d0a. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Albert CM, Mittleman MA, Chae CU, et al. Triggering of sudden death from cardiac causes by vigorous exertion. N Engl J Med. 2000;343:1355–1361. doi: 10.1056/NEJM200011093431902. [DOI] [PubMed] [Google Scholar]
- 32.Kieffer SK, Croci I, Wisløff U, et al. Temporal changes in a novel metric of physical activity tracking (personal activity intelligence) and mortality: the HUNT Study, Norway. Prog Cardiovasc Dis. 2019;62:186–192. doi: 10.1016/j.pcad.2018.09.002. [DOI] [PubMed] [Google Scholar]
- 33.Matthews CE, Keadle SK, Troiano RP, et al. Accelerometer- measured dose-response for physical activity, sedentary time, and mortality in US adults. Am J Clin Nutr. 2016;104:1424–1432. doi: 10.3945/ajcn.116.135129. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Knaeps S, Bourgois JG, Charlier R, et al. Ten-year change in sedentary behaviour, moderate-to-vigorous physical activity, cardiorespiratory fitness and cardiometabolic risk: independent associations and mediation analysis. Br J Sports Med. 2018;52:1063–1068. doi: 10.1136/bjsports-2016-096083. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplementary data to this article can be found online.