Abstract
Background
Asian population are at increased risk of bleeding during the warfarin treatment, so the recommended optimal international normalized ratio (INR) level may be lower in Asians than in Westerners. The aim of this prospective multicenter study was to determine the optimal INR level in Thai patients with non-valvular atrial fibrillation (NVAF).
Methods
Patients with NVAF who were on warfarin for stroke prevention were recruited from 27 hospitals in the nationwide COOL-AF registry in Thailand. We collected demographic data, medical history, risk factors for stroke and bleeding, concomitant disease, electrocardiogram and laboratory data including INR and antithrombotic medications. Outcome measurements included ischemic stroke/transient ischemic attack (TIA) and major bleeding. Optimal INR level was assessed by the calculation of incidence density for six INR ranges (< 1.5, 1.5–1.99, 2–2.49, 2.5–2.99, 3–3.49, and ≥ 3.5).
Results
A total of 2, 232 patients were included. The mean age of patients was 68.5 ± 10.6 years. The mean follow-up duration was 25.7 ± 10.6 months. There were 63 ischemic stroke/TIA and 112 major bleeding events. The lowest prevalence of ischemic stroke/TIA and major bleeding events occurred within the INR range of 2.0–2.99 for patients < 70 years and 1.5–2.99 for patients ≥ 70 years.
Conclusions
The INR range associated with the lowest risk of ischemic stroke/TIA and bleeding in the Thai population was 2.0–2.99 for patients < 70 years and 1.5–2.99 for patients ≥ 70 years. The rates of major bleeding and ischemic stroke/TIA were both higher than the rates reported in Western population.
Keywords: Bleeding, Ischemic stroke, Non-valvular atrial fibrillation, Optimal international normalized ratio, Thailand, Warfarin
1. Introduction
The prevalence of non-valvular atrial fibrillation (NVAF) is approximately 1% in the general population.[1, 2] However, that rate increases up to approximately 9% among patients aged older than 80 years.[1] Due to the large size of Asian population and the increasing proportion of elderly population, it is estimated that approximately 49 million men and 23 million women will be affected by NVAF by 2050.[2] It is estimated that the risk of ischemic stroke in patients with NVAF is approximately 5% per year or five times the risk in general population.[1]
Oral anticoagulant is generally recommended for the prevention of ischemic stroke in patients with one or more stroke risk factors.[3] Recent practice guidelines recommend non-vitamin K antagonist anticoagulant (NOAC) over warfarin for stroke prevention in NVAF due to its relative efficacy, safety and convenience compared to warfarin.[3-6] However, in many Asian countries and in many developing countries, warfarin is still the most commonly used oral anticoagulant for stroke prevention in patients with NVAF.[7, 8] The use of these drugs is still comparatively low due to the fear of bleeding and misconception or misunderstanding about this drug class.[7]
In Thailand, NOAC has not been proven to be more cost effective than warfarin because the willingness of to pay level in the Thai population is lower than in Western countries.[9] Also, the Asian population has an increased risk of bleeding, especially intracranial hemorrhage (ICH), compared to Caucasians, and this increased risk could 4-fold higher with warfarin use.[10] The recent NOAC trials also demonstrated an increased risk of bleeding in the Asian population compared to Caucasians among the patients randomized to warfarin treatment.[11] Current guidelines recommend that patients on warfarin should have an international normalized ratio (INR) of 2-3, and that the time in therapeutic range (TTR) should be more than 70%;[3, 12] however, most patients with NVAF in many Asian countries have a TTR of less than 70% - even in controlled settings, such as clinical trials.[13] Due to a fear of bleeding, certain guidelines from Asia recommend a target INR of lower than 2-3, especially amongst elderly patients where a target INR range of 1.6-2.6 is recommended.[14] Results from one study in Chinese population also suggested a lower INR target may be needed.[15]
In this study, we aimed to determine the optimal INR target in elderly and non-elderly Thai population with NVAF in a prospective multicenter registry, the cohort of antithrombotic use and optimal INR level in patients with NVAF in Thailand (COOL-AF) registry.
2. Methods
2.1. Study population
We enrolled patients who were 18 years of age or more with a diagnosis of NVAF. There were 27 university hospitals, regional hospitals, or general hospitals from all five regions of Thailand that participated in this study. The study protocol was approved by the Institutional Review Board (IRB) of each participating hospital. For hospitals that are under the Thailand Ministry of Public Health (MOPH), the study protocol was approved by the IRB of the MOPH. All patients provided written informed consent prior to participation. Patients were excluded if they had one or more of the following conditions: (1) ischemic stroke within three months; (2) prosthetic valve, valve repair, rheumatic valve disease, or significant valve disease; (3) thrombocytopenia (< 100, 000/mm3), myeloproliferative disorders; (4) atrial fibrillation (AF) from transient reversible cause (e.g., during respiratory tract infection); (5) life expectancy less than three years; (6) pregnancy; (7) inability to provide follow-up data; (8) refusal to participate in the study; and/or (9) current hospitalization or hospitalization within one month prior to enrollment. To determine optimal INR levels by incidence density analysis, we included only patients who were on warfarin and who had at least two INR tests.
2.2. Study protocol
After written informed was obtained, data were retrieved from medical records and patient interview by the study team at each hospital. Data were recorded in a case record form (CRF), and then the study team uploaded that data into a web-based system. The CRF for each patient was mailed to the central data management site. Research staff at the central site verified the accuracy and completeness of the data between the data on the CRF and the data entered into the data management system. Any data-related questions by the data management team at the central site were forwarded to the study team at that enrollment site. After data were determined to be accurate and complete, those data were locked. Site monitoring was performed at every study site to ensure compliance with the study protocol and to ensure the collection and recording of consistently accurate and high-quality data.
The study staff at each center was tasked with collecting and recording data every six months from medical records and telephone interview during follow-up until three years. Data relating to cardiovascular events, vital signs, laboratory results, and medications were collected at each follow-up visit. All data collected during the study period was recorded on a CRF with subsequent transfer of that data into the web-based system.
2.3. Data collection and outcome measurement
The following data were collected: demographic data, risk factors for ischemic stroke/transient ischemic attack (TIA) and bleeding as listed in the CHA2DS2-VASc and HAS-BLED score, medical history, types and duration of AF, antithrombotic medications, laboratory (including every INR), and 12-lead electrocardiogram data. Each component of the CHA2DS2-VASc score was scored and recorded, as follows: C = congestive heart failure (1 point); H = hypertension (1 point); A = age > 75 years (2 points); D = diabetes (1 point); S = stroke (2 points); V = vascular disease (1 point); A = age 65-74 (1 point); and Sc = female sex category (1 point). Each component of the HAS-BLED score was scored and recorded, as follows: uncontrolled Hypertension, Abnormal renal, or liver function; history of Stroke; history of Bleeding; Labile INR; Elderly (age above 65 years); and Drugs or alcohol (all 1 point each).
The main outcomes during the follow-up were ischemic stroke or TIA and major bleeding event. We also collected data for minor bleeding. Any main outcome events and their related data were recorded and reported according to the previously described data reporting process. All events were evaluated and verified by the adjudication committee. Any questions or concerns raised by the adjudication committee were forwarded to the study team at that site for explanation or clarification.
INR level was classified into the following six groups for analysis: < 1.5, 1.5-1.99, 2-2.49, 2.5-2.99, 3-3.49, and ≥ 3.5. INR-specific incidence density was calculated as the ratio of the number of thromboembolic or bleeding events that occurred in each INR group to the total amount of time (patient-years) that each patient stayed in each INR group, according to the method described by Rosendaal, et al.[16] in 1993. Assignment of an event to an INR group was made using the first INR during the event or the most recent INR level within seven days prior to the occurrence of the thromboembolic or bleeding event.
The time that each patient stayed in each INR group was calculated by taking the duration between when a patient was in one INR group to when that same patient had an INR score that was in another INR group. That total duration was then divided in half, and half of the time was allocated to the prior INR group and the other half of the time was allocated to the new INR group. For example, if a patient had an INR of 2.1 that became 2.6 twelve weeks later, the time in the 2.0-2.49 INR group was six weeks, and the time in the 2.5-2.99 INR group was six weeks. In order to have been included in this analysis, patients had to have at least two INR tests.
Ischemic stroke was defined as sudden-onset neurologic deficit lasting greater than 24 hours. TIA was similarly defined, except the duration of the neurologic deficit had to have been less than 24 hours. Major bleeding was defined using criteria published by the International Society of Thrombosis and Haemostasis, [17] which includes: (1) fatal bleeding; (2) bleeding in critical area or organs; or (3) bleeding that results in a decrease in hemoglobin level of 20 g/L or more, or that requires a transfusion of two units of red cells or more.
Among patients who died, if the death was related to ischemic stroke, we counted this event as an ischemic stroke. If, however, the death was related to severe bleeding, we counted this a major bleeding event. Deaths that were not related to either ischemic stroke or severe bleeding were not included in either main outcome category. Main outcome events were further subcategorized, as follows: fatal or non-fatal stroke, and fatal and non-fatal major bleeding.
2.4. Statistical analysis
SPSS Statistics version 23.0 (SPSS, Inc., Chicago, IL, USA) was used for all statistical analyses. Categorical data, such as gender and comorbid diseases, are presented as frequency and percentage. Continuous variables, such as age, are expressed as mean ± SD. The optimal INR level was determined by comparing the incidence density among all six INR groups. In this study, for the purpose of comparison with previous data in Asian population, [14, 18] we defined patients older or equal to 70 years as elderly. The incidence density of thromboembolic and bleeding complications was compared using Pearson's chi-square test. The optimal INR level was defined as the lowest incidence density of thromboembolic or hemorrhagic complications. Concerning the comparison of repeated measure INR data, since the number of INR tests during follow-up varied among the study population, repeated measures analysis of variance (ANOVA) was judged not to be suitable for this analysis. Alternatively, linear mixed model (fixed effect) was selected to compare the repeated measurement INR level-related outcome measures at time. A P-value less than 0.05 was considered as being statistically significant for all tests.
3. Results
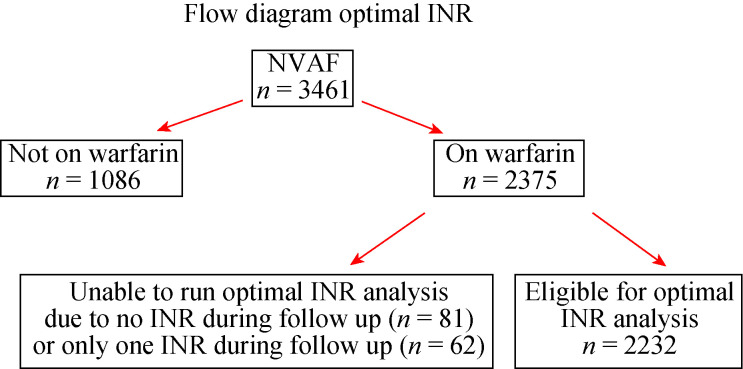
There was a total of 3, 461 patients with NVAF enrolled in the main study. Warfarin was used in 2, 375 patients (68.6%). After excluding 143 patients with less than two available INR tests during the follow-up, 2, 322 patients remained for inclusion into this 'optimal INR' analysis (Figure 1). The average age of patients was 68.5 ± 10.6 years, and 56% were male. Baseline characteristics are shown in Table 1. The average CHA2DS2-VASc and HAS- BLED score was 3.3 ± 1.6 and 1.6 ± 1.0, respectively. According to the requirement of this analysis, all patients were on warfarin. Antiplatelet therapy was used in 277 patients (12.4%), mostly aspirin.
Figure 1.
Flowchart describing the patient recruitment process.
INR: international normalized ratio; NVAF: non-valvular atrial fibrillation.
Table 1.
Baseline characteristics of the study population.
| Characteristics | All (n = 2, 232) |
| Data are presented as means ± SD or n (%). TIA: transient ischemic attack. | |
| Age, yrs | 68.5 ± 10.6 |
| Male gender | 1, 251 (56.0%) |
| Time after diagnosis of atrial fibrillation, yrs | 3.6 ± 4.4 |
| Atrial fibrillation | |
| Paroxysmal | 631 (28.3%) |
| Persistent | 421 (18.9%) |
| Permanent | 1, 180 (52.9%) |
| History of heart failure | 626 (28.0%) |
| History of coronary artery disease | 356 (15.9%) |
| Devices | 216 (9.7%) |
| History of ischemic stroke/TIA | 485 (21.7%) |
| Hypertension | 1, 639 (73.4%) |
| Diabetes mellitus | 609 (27.3%) |
| History of bleeding | 241 (10.8%) |
| CHA2DS2-VASc score | |
| 0 (male), 1 (female) (low risk) | 76 (3.4%) |
| 1 (male), 2 (female) (intermediate risk) | 294 (13.2%) |
| ≥ 2 (male), ≥ 3 (female) (high risk) | 1, 862 (83.4%) |
| HAS-BLED score | |
| 0 | 269 (12.1%) |
| 1-2 | 1, 579 (70.7%) |
| ≥ 3 | 384 (17.2%) |
The average follow-up time was 25.7 ± 10.6 months, and patients were followed-up for 4, 786 patient-years. Table 2 demonstrates the rate of ischemic stroke/TIA and bleeding events, including ICH. There was a total of 63 ischemic stroke/TIA (1.64 per 100 patient-years) in 59 patients, and 112 major bleeding events (2.92 per 100 patient-years) in 104 patients. The overall rate of ischemic stroke/TIA, major bleeding, and ICH was 1.64, 2.92, and 0.76 per 100 person-years, respectively. Although the majority of cases with ischemic stroke/TIA or major bleeding were non-fatal, the fatality rate among patients with ICH was 37.9%.
Table 2.
Ischemic stroke/TIA and bleeding events documented during the follow-up period.
| Events | Number of patients | Number of events | Per 100 person-years |
| ICH: intracranial hemorrhage; TIA: transient ischemic attack. | |||
| Total ischemic stroke/TIA | 59 (2.6%) | 63 (2.8%) | 1.64 (2.41-3.52) |
| Fatal ischemic stroke/TIA | 9 (0.4%) | 9 (0.4%) | 0.23 (0.11-0.45) |
| Non-fatal ischemic stroke/TIA | 50 (2.2%) | 54 (2.4%) | 1.41 (1.06-1.84) |
| Total major bleeding | 104 (4.7%) | 112 (5.0%) | 2.92 (1.26-2.10) |
| Fatal major bleeding | 15 (0.7%) | 15 (0.7%) | 0.39 (0.22-0.65) |
| Non-fatal major bleeding | 89 (4.0%) | 97 (4.3%) | 2.53 (2.05-3.09) |
| Total ICH | 28 (1.3%) | 29 (1.3%) | 0.76 (0.51-1.09) |
| Fatal ICH | 11 (0.5%) | 11 (0.5%) | 0.29 (0.14-0.51) |
| Non-fatal ICH | 17 (0.8%) | 18 (0.8%) | 0.47 (0.28-0.74) |
| Minor bleeding | 330 (14.8%) | 478 (21.4%) | 12.47 (11.38-13.64) |
| Other death | 101 (4.5%) | 101 (4.5%) | 2.64 (2.15-3.20) |
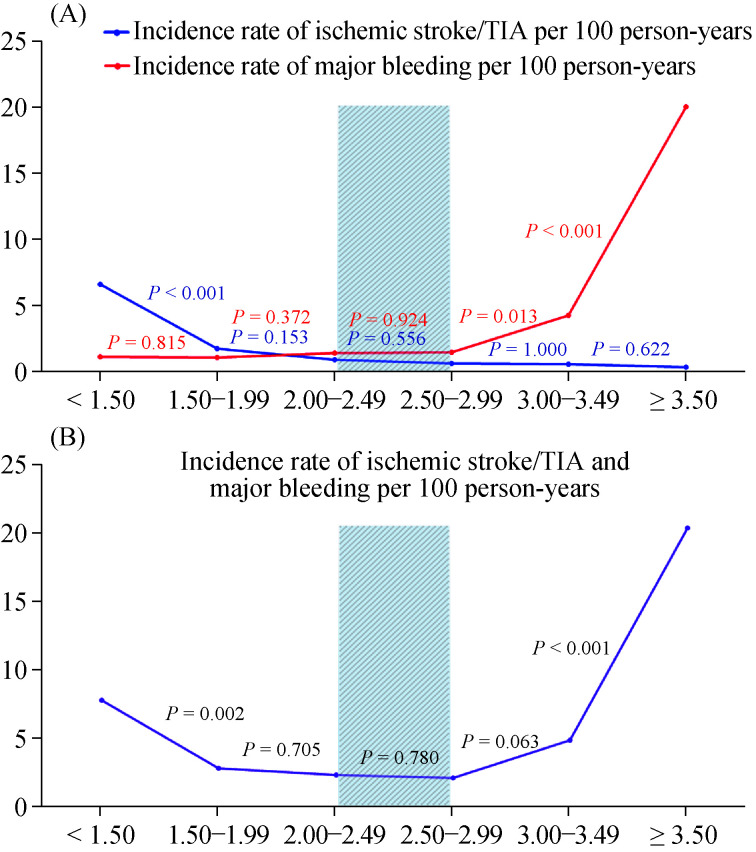
The risk of ischemic stroke/TIA increased as the INR level decreased, and the risk of major bleeding increased as the INR level increased (Table 3 & Figure 2). Figure 2B shows the combined rate of ischemic stroke/TIA and major bleeding to be lowest when the INR was within the range of 2-2.99. There was no significant difference in this combined incidence rate for INR ranges 2-2.49 and 2.5-2.99. The combined event incidence rate was increased when the INR was below 2.0, and when the INR was 3.0 or greater, especially at the high and low extremes of INR level. Ischemic stroke/TIA rate increased twice for INR 1.5-1.99 compared to 2.0-2.49 and major bleeding rate increased almost three times when INR 3-3.49 compared to 2.5-2.99. This is especially true for patients younger than 70 years as shown in Table 3. The incidence rate of ischemic stroke/ TIA markedly increased when the INR less than 1.5, whereas the incidence rate of major bleeding markedly increased when the INR more than 3.0 in patients ≥ 70 years as compared to those < 70 years. For patients < 70 years, the incidence rate of ischemic stroke/TIA increased from 0.49 to 1.64 per 100 person-years when for INR 2.0-2.49 compared to INR 1.5-1.99, whereas the incidence rate of major bleeding was similar (0.66 to 0.82 per 100 person-years). For patients ≥ 70 years, the incidence rate of ischemic stroke/TIA increased from 1.42 to 1.94 per 100 person- years when for INR 2.0-2.49 compared to INR 1.5-1.99, whereas the incidence rate of major bleeding decreased from 2.30 to 1.45 per 100 person-years. The incidence rate of combined ischemic stroke/TIA and major bleeding in patients < 70 years was lowest at INR 2.0-2.99, whereas in patients 70 years, it was lowest at INR 1.5-2.99 indicating a different target INR for these populations.
Table 3.
Rates of ischemic stroke/TIA and bleeding events during follow-up stratified by INR level.
| INR level | Ischemic stroke/TIA and bleeding events (per 100 person-years) | ||||
| Ischemic stroke/TIA | Major bleeding | Minor bleeding | Total bleeding | Ischemic stroke/TIA or major bleeding | |
| INR: international normalized ratio; TIA: transient ischemic attack. | |||||
| All (n = 2, 232) | |||||
| < 1.5 | 6.63 (4.41-9.59) | 1.18 (0.38-2.77) | 11.37 (8.39-15.08) | 12.56 (9.41-16.43) | 7.82 (5.38-10.98) |
| 1.5-2 | 1.78 (1.02-2.88) | 1.11 (0.53-2.04) | 10.88 (8.83-13.26) | 11.99 (9.83-14.47) | 2.89 (1.89-4.23) |
| 2-2.5 | 0.94 (0.47-1.68) | 1.45 (0.84-2.32) | 8.35 (6.78-10.18) | 9.80 (8.09-11.77) | 2.39 (1.59-3.45) |
| 2.5-3 | 0.68 (0.22-1.59) | 1.50 (0.75-2.68) | 10.89 (8.63-13.55) | 12.39 (9.97-15.20) | 2.18 (1.24-3.54) |
| 3-3.5 | 0.61 (0.07-2.21) | 4.28 (2.34-7.18) | 15.91 (11.88-20.85) | 20.19 (15.61-25.68) | 4.90 (2.80-7.95) |
| > 3.5 | 0.36 (0.01-2.03) | 20.03 (15.07-26.03) | 37.14 (30.24-45.03) | 57.17 (48.51-66.75) | 20.39 (15.38-26.44) |
| Total | 1.64 (2.41-3.52) | 2.92 (1.26-2.10) | 12.47 (11.38-13.64) | 15.39 (14.18-16.69) | 4.57 (3.92-5.30) |
| Age ≥ 70 yrs (n = 1, 083) | |||||
| < 1.5 | 10.71 (6.73-16.25) | 1.95 (0.53-5.00) | 12.18 (7.89-18.00) | 14.12 (9.47-20.32) | 12.66 (8.29-18.58) |
| 1.5-2 | 1.94 (0.84-3.82) | 1.45 (0.53-3.16) | 11.37 (8.33-15.13) | 12.82 (9.61-16.79) | 3.39 (1.85-5.69) |
| 2-2.5 | 1.42 (0.61-2.79) | 2.30 (1.23-3.94) | 8.33 (6.12-11.08) | 10.64 (8.12-13.69) | 3.72 (2.30-5.69) |
| 2.5-3 | 0.86 (0.18-2.50) | 2.57 (1.18-4.88) | 13.43 (9.87-17.86) | 16.00 (12.09-20.78) | 3.43 (1.77-5.99) |
| 3-3.5 | 0.65 (0.02-3.59) | 4.52 (1.18-9.31) | 18.06 (12.00-26.11) | 22.58 (15.73-31.40) | 5.16 (2.23-10.17) |
| > 3.5 | 0.71 (0.02-3.92) | 27.54 (19.53-37.55) | 43.08 (32.86-55.18) | 70.62 (57.30-85.65) | 28.25 (20.12-38.36) |
| Total | 2.35 (1.70-3.17) | 4.26 (3.37-5.32) | 13.94 (12.28-15.75) | 18.20 (16.30-20.28) | 6.61 (5.49-7.90) |
| Age < 70 yrs (n = 1, 149) | |||||
| < 1.5 | 2.77 (1.01-6.02) | 0.46 (0.01-2.57) | 10.61 (6.72-15.90) | 11.07 (1.09-16.46) | 3.23 (1.30-6.65) |
| 1.5-2 | 1.64 (0.71-3.23) | 0.82 (0.22-2.10) | 10.46 (7.78-13.74) | 11.28 (8.49-14.67) | 2.46 (1.27-4.30) |
| 2-2.5 | 0.49 (0.10-1.44) | 0.66 (0.18-1.68) | 8.37 (6.24-11.01) | 9.03 (6.80-11.76) | 1.15 (0.46-2.37) |
| 2.5-3 | 0.52 (0.06-1.88) | 0.52 (0.06-1.88) | 8.58 (5.90-12.04) | 9.10 (6.33-12.64) | 1.04 (0.28-2.66) |
| 3-3.5 | 0.58 (0.01-3.24) | 4.07 (1.64-8.39) | 13.97 (8.94-20.76) | 18.04 (12.25-25.58) | 4.66 (2.01-9.16) |
| > 3.5 | 0 | 12.03 (6.88-19.54) | 30.83 (22.12-41.82) | 42.86 (32.46-55.53) | 12.03 (6.88-19.54) |
| Total | 1.00 (0.61-1.54) | 1.70 (1.18-2.37) | 11.13 (9.72-12.69) | 12.83 (11.31-14.50) | 2.70 (2.03-3.52) |
Figure 2.
Incidence rate of ischemic stroke/TIA compared to incidence rate of major bleeding per 100 person-years in each INR group (A) and incidence rate of ischemic stroke/TIA and major bleeding combined per 100 person-years in each INR group (B).
INR 2-3 is indicated by the shade area. INR: international normalized ratio; TIA: transient ischemic attack.
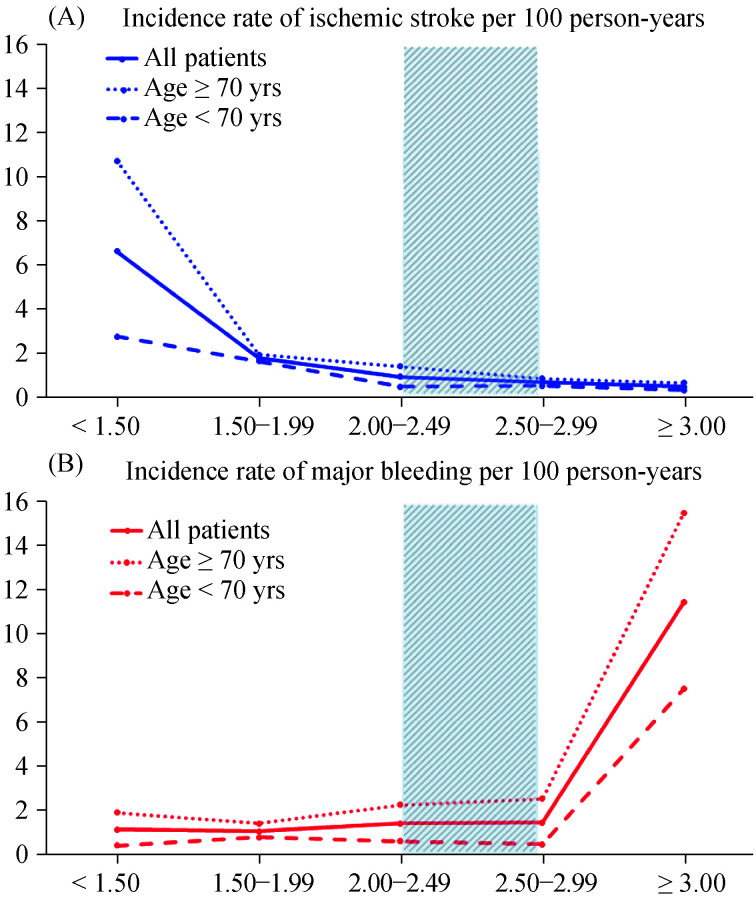
The incidence rate of ischemic stroke/TIA within the INR range of 2-2.99 was 0.79 per 100 person-years, which is 8.4 times lower than incidence rate of ischemic stroke/ TIA when the INR level was < 1.5. The incidence rate of major bleeding within the INR range of 2-2.99 was 1.52 per 100 person-years, which was 12.9 times lower than incidence rate of major bleeding when the INR level was ≥ 3.5. Figure 2 shows a survival graph of ischemic stroke/TIA compared with major bleeding (Figure 2A), and of combination of ischemic stroke/TIA and major bleeding (Figure 2B) - both stratified by INR range/group. Figure 3 demonstrated the incidence rate of ischemic stroke/TIA and major bleeding among patients ≥ 70 years, < 70 years as well as all patients.
Figure 3.
Incidence rate of ischemic stroke/TIA (A) and major bleeding (B) per 100 person-years in each INR group among patients ≥ 70 years, < 70 years as well as all patients.
INR 2-3 is indicated by the shade area. INR: international normalized ratio; TIA: transient ischemic attack.
Linear mixed model analysis was used to determine the difference in INR levels between patients with and without events considering the effect of repeated measure of INR tests. The results, as shown in Table 4, show that the mean INR in patients with ischemic stroke/TIA was significantly lower than the mean INR among patients without ischemic stroke/TIA, and that the mean INR in patients with major or minor bleeding was significantly higher than the mean INR among patients without major or minor bleeding.
Table 4.
The results of linear mixed model analysis to test the difference of INR levels in patients with and without outcomes based on repeated INR data.
| Mean INR | Standard error of the mean | Mean difference (95% CI) | Effect estimate (Beta) | Effect standard error | P-value | |
| A P-value < 0.05 indicates statistical significance. The interaction effect between thromboembolic, bleeding, minor bleeding, and major bleeding event and time was non-significant (P > 0.05). ICH: intracranial hemorrhage; INR: international normalized ratio; TIA: transient ischemic attack. | ||||||
| Ischemic stroke/TIA | 1.79 | 0.17 | -0.60 (-0.93--0.28) | 0.60 | 0.17 | < 0.001 |
| No ischemic stroke/TIA | 2.40 | 0.01 | ||||
| Total bleeding | 3.41 | 0.05 | 1.05 (0.94-1.16) | -1.05 | 0.05 | < 0.001 |
| No bleeding | 2.37 | 0.01 | ||||
| Major bleeding | 5.12 | 0.13 | 2.74 (2.50-2.99) | -2.74 | 0.13 | < 0.001 |
| No major bleeding | 2.38 | 0.01 | ||||
| ICH | 2.94 | 0.25 | 0.55 (0.06-1.03) | -0.55 | 0.25 | 0.028 |
| No ICH | 2.39 | 0.01 | ||||
| Minor bleeding | 3.02 | 0.06 | 0.63 (0.51-0.75) | -0.63 | 0.06 | < 0.001 |
| No minor bleeding | 2.38 | 0.01 | ||||
| Follow-up time point | 2.45 | 0.02 | < 0.001 | |||
4. Discussion
In this large prospective multicenter nationwide registry of patients with NVAF, our principal findings was that the optimal INR level that was associated with the lowest incidence rate of combined efficacy and safety endpoints (ischemic stroke/TIA and major bleeding) was 2-2.99. Ischemic stroke/TIA rate increased twice for INR 1.5-1.99 compared to INR 2.0-2.49 and major bleeding rate increased almost three times when INR 3-3.49 compared to INR 2.5-2.99.
Warfarin is still used in the majority of patients in many Asian countries, especially in low or middle-income countries, due to its comparatively lower drug costs.[9] It is recommended that the TTR be greater than 70% in patients who use warfarin.[3, 12] However, data from one large clinical trial revealed a TTRs within the range of 50%-65%, [13] which was even worse in real-world clinical settings, especially among Asian population.[19, 20] In general, Asian populations have a lower TTR compared to Western countries.[21] For example, the Global Anticoagulant Registry in the Field (GARFIELD) study reported that only 17% of Asian population had a TTR > 65% compared to 49% in Western population.[19] Importantly, efficacy and safety outcomes are related to the quality of INR control.[19, 22]
Previous data indicated that Asian population had an increased risk of ICH, especially while on warfarin therapy. This increased risk of major bleeding and ICH in Asians was also evident in the four large clinical trials that compared warfarin with NOACs. Although baseline patient characteristics compared between our study and other study, such as the GARFIELD registry, [23] are relatively similar, the rate of major bleeding in our study (1.64 per 100 person-years) was much higher than the rate reported in the GARFIELD registry (0.7% per 100 person-years). Of note, the Asian population account for approximately 21% of patients in the GARFIELD registry.[21]
Racial differences in the risk of bleeding may account for differences between and among studies. Also, all patients in our study were on warfarin and all had NVAF, whereas the GARFIELD registry included all types of patients with AF, and many used NOACs, which have been associated with a lower rate of major bleeding compared to warfarin.[21]
Major international guidelines recommend a target INR within the range of 2.0-3.0, and a TTR of greater than 70%.[3, 5, 6] Previous data from a Western population revealed the INR range associated with the lowest event rate to be 2.0-3.0.[24] Ischemic stroke event rates rose steeply at an INR level below 2.0; and even at an INR level of 1.7, the ischemic stroke rate increased two times compared to the rate at INR 2.0.[24]
These data are at odds with some Japanese and Chinese studies. Yamaguchi, et al.[25] compared the clinical outcomes of low-intensity warfarin (target INR 1.5-2.1) with those of the reference group that had a target INR of 2.2-3.5 among 115 Japanese patients with NVAF; the annual rate of ischemic stroke was similar between the two groups, but low-intensity warfarin was safer. However, the target INR in the reference group was higher compared to the standard recommendation of INR 2.0-3.0. Suzuki, et al.[26] studied major bleeding rate in 667 patients with NVAF and a target INR within the range of 1.6-2.6. They reported a major bleeding rate of 2.38%, which is significantly higher than the rates reported in Western populations. They also found an INR of greater than 2.27 to be an independent predictor of major bleeding. Thus, Japanese guidelines for management of patients with NVAF recommends an INR target of 1.6-2.6 in patients older than 70 years of age. This recommendation was based on data from a study by Yasaka, et al., [27] which found the lowest event rates in an elderly population occurred when the INR was within the range of 1.6-2.6. Chen, et al.[15] randomized 786 Chinese patients with NVAF into the following three groups: warfarin target INR 1.6-2.0, warfarin target INR 2.1-2.6, and aspirin 200 mg/day, the annual thromboembolic event rate was 2.6%, 3.1%, and 6.9%, respectively; whereas the major bleeding rate was 2.6%, 2.4%, and 0.4%, respectively. The authors therefore recommended a target INR within the range of 1.6-2.5. The results of our study for patients ≥ 70 years and < 70 years demonstrated a similar trend as Japanese data.[18] We showed that the best INR for the whole group was 2.0-2.99. The results were more obvious for those younger than 70 years. However, for those older than 70 years, the acceptable INR would be 1.5-2.99 since the risk of increased major bleeding was more than the benefit for ischemic stroke/TIA reduction when compared INR 2.0-2.49 and INR 1.5-1.99.
In the present study, among the 2, 232 included patients with NVAF receiving warfarin therapy, the INR range with the lowest combined ischemic stroke/TIA and major bleeding event rate was 2.0-2.99. This finding supports the recommended target INR levels from major practice guidelines.[3, 6] Nevertheless, the major bleeding rate in our study population was much higher than that reported from Western population.[28] Data from the GARFIELD study showed a major bleeding event rate of 0.7 events per 100 person- years, [28] which is substantially lower than the 2.9 events per 100 person-years found in our study using a similar definition. However, the rate of major bleeding in our study was comparable to the rate reported from previous studies in Japanese (2.4 events per 100 person-years) and Chinese (2.4-2.6 events per 100 person-years) populations.[15, 26] The rate of ICH in our study was also higher than that seen in the GARFIELD study, [28] but was similar to the rate from a previous Japanese report.[26] It should also be emphasized that a significant proportion of patients who developed ICH had an INR less than three prior to the ICH event. Lopes, et al.[29] reported that from ARISTOTLE study, 78.5% of patients who developed ICH had a pre-ICH INR of less than three. Asian population was an independent predictor for INR in this study. Data from our study indicated that 62.1% of patients who had ICH had a pre-ICH INR of less than three. Therefore, the INR within target does not guarantee that the patients will be safe from ICH. The ischemic stroke/TIA rate in our study was also greater than the rate reported from the GARFIELD study. Data from the Fushimi AF Registry, which enrolled 3, 878 patients with NVAF in Japan, also found a higher rate of ischemic stroke and major bleeding in Japanese population compared to a Western population.[30]
4.1. Limitations
This study is limited by its registry design, and we report associations rather than causality. Nonetheless, we have recruited subjects from centres nationwide, being representative of the Thailand population and routine clinical practice.
4.2. Conclusions
The optimal INR level in patients with NVAF in this multicenter nationwide study in Thailand was 2.0-2.99 for patients < 70 years and 1.5-2.99 for patients ≥ 70 years. The rates of major bleeding and ischemic stroke/TIA were both higher than the rates reported in Western population.
Acknowledgments
This study was supported by the Health System Research Institute (59-053) and the Heart Association of Thailand under the Royal Patronage of H.M. the King. All authors had no conflicts of interest to disclose. The authors gratefully acknowledge Pontawee Kaewcomdee and Olaree Chaiphet for data management, and all investigators and nurse coordinators of the COOL-AF registry.
References
- 1.Lip GYH, Brechin CM, Lane DA. The global burden of atrial fibrillation and stroke: a systematic review of the epidemiology of atrial fibrillation in regions outside North America and Europe. Chest. 2012;142:1489–1498. doi: 10.1378/chest.11-2888. [DOI] [PubMed] [Google Scholar]
- 2.Tse HF, Wang YJ, Ahmed Ai-Abdullah M, et al. Stroke prevention in atrial fibrillation: an Asian stroke perspective. Heart Rhythm. 2013;10:1082–1088. doi: 10.1016/j.hrthm.2013.03.017. [DOI] [PubMed] [Google Scholar]
- 3.Kirchhof P, Benussi S, Kotecha D, et al. 2016 ESC guidelines for the management of atrial fibrillation developed in collaboration with EACTS. Eur Heart J. 2016;37:2893–2962. doi: 10.1093/eurheartj/ehw210. [DOI] [PubMed] [Google Scholar]
- 4.Chiang CE, Okumura K, Zhang S, et al. 2017 consensus of the Asia Pacific Heart Rhythm Society on stroke prevention in atrial fibrillation. J Arrhythm. 2017;33:345–367. doi: 10.1016/j.joa.2017.05.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Lip GYH, Banerjee A, Boriani G, et al. Antithrombotic therapy for atrial fibrillation: CHEST guideline and expert panel report. Chest. 2018;154:1121–1201. doi: 10.1016/j.chest.2018.07.040. [DOI] [PubMed] [Google Scholar]
- 6.January CT, Wann LS, Calkins H, et al. 2019 AHA/ACC/HRS focused update of the 2014 AHA/ACC/HRS guideline for the management of patients with atrial fibrillation: a report of the American College of Cardiology/American Heart Association Task Force on Clinical Practice Guidelines and the Heart Rhythm Society. J Am Coll Cardiol. 2019;74:104–132. doi: 10.1016/j.jacc.2019.01.011. [DOI] [PubMed] [Google Scholar]
- 7.Yang X, Li Z, Zhao X, et al. Use of warfarin at discharge among acute ischemic stroke patients with non-valvular atrial fibrillation in China. Stroke. 2016;47:464–470. doi: 10.1161/STROKEAHA.115.011833. [DOI] [PubMed] [Google Scholar]
- 8.Krittayaphong R, Winijkul A, Methavigul K, et al. Risk profiles and pattern of antithrombotic use in patients with non-valvular atrial fibrillation in Thailand: a multicenter study. BMC Cardiovasc Disord. 2018;18:174. doi: 10.1186/s12872-018-0911-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Dilokthornsakul P, Nathisuwan S, Krittayaphong R, et al. Cost-effectiveness analysis of non-vitamin K antagonist oral anticoagulants versus warfarin in Thai patients with non-valvular atrial fibrillation. Heart Lung Circ. 2020;29:390–400. doi: 10.1016/j.hlc.2019.02.187. [DOI] [PubMed] [Google Scholar]
- 10.Shen AY, Yao JF, Brar SS, et al. Racial/ethnic differences in the risk of intracranial hemorrhage among patients with atrial fibrillation. J Am Coll Cardiol. 2007;50:309–315. doi: 10.1016/j.jacc.2007.01.098. [DOI] [PubMed] [Google Scholar]
- 11.Chiang CE, Wang KL, Lip GY. Stroke prevention in atrial fibrillation: an Asian perspective. Thromb Haemost. 2014;111:789–797. doi: 10.1160/TH13-11-0948. [DOI] [PubMed] [Google Scholar]
- 12.Lip G, Freedman B, De Caterina R, et al. Stroke prevention in atrial fibrillation: past, present and future. Comparing the guidelines and practical decision-making. Thromb Haemost. 2017;117:1230–1239. doi: 10.1160/TH16-11-0876. [DOI] [PubMed] [Google Scholar]
- 13.Wallentin L, Yusuf S, Ezekowitz MD, et al. Efficacy and safety of dabigatran compared with warfarin at different levels of international normalised ratio control for stroke prevention in atrial fibrillation: an analysis of the RE-LY trial. Lancet. 2010;376:975–983. doi: 10.1016/S0140-6736(10)61194-4. [DOI] [PubMed] [Google Scholar]
- 14.JCS joint working group Guidelines for pharmacotherapy of atrial fibrillation (JCS 2013) Circ J. 2014;78:1997–2021. doi: 10.1253/circj.CJ-66-0092. [DOI] [PubMed] [Google Scholar]
- 15.Chen KP, Huang CX, Huang DJ, et al. Anticoagulation therapy in Chinese patients with non-valvular atrial fibrillation: a prospective, multi-center, randomized, controlled study. Chin Med J (Engl) 2012;125:4355–4360. [PubMed] [Google Scholar]
- 16.Rosendaal FR, Cannegieter SC, van der Meer FJ, et al. A method to determine the optimal intensity of oral anticoagulant therapy. Thromb Haemost. 1993;69:236–239. doi: 10.1055/s-0038-1651587. [DOI] [PubMed] [Google Scholar]
- 17.Schulman S, Kearon C, Subcommittee on Control of Anticoagulation of the Scientific and Standardization Committee of the International Society on Thrombosis and Haemostasis. Definition of major bleeding in clinical investigations of antihemostatic medicinal products in non-surgical patients. J Thromb Haemost. 2005;3:692–694. doi: 10.1111/j.1538-7836.2005.01204.x. [DOI] [PubMed] [Google Scholar]
- 18.Inoue H, Okumura K, Atarashi H, et al. Target international normalized ratio values for preventing thromboembolic and hemorrhagic events in Japanese patients with non-valvular atrial fibrillation: results of the J-RHYTHM registry. Circ J. 2013;77:2264–2270. doi: 10.1253/circj.CJ-13-0290. [DOI] [PubMed] [Google Scholar]
- 19.Haas S, Ten Cate H, Accetta G, et al. Quality of vitamin K antagonist control and 1-year outcomes in patients with atrial fibrillation: a global perspective from the GARFIELD-AF registry. PLoS One. 2016;11:e0164076. doi: 10.1371/journal.pone.0164076. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Poli D, Antonucci E, Dentali F, et al. Recurrence of ICH after resumption of anticoagulation with VK antagonists: CHIRONE study. Neurology. 2014;82:1020–1026. doi: 10.1212/WNL.0000000000000245. [DOI] [PubMed] [Google Scholar]
- 21.Oh S, Goto S, Accetta G, et al. Vitamin K antagonist control in patients with atrial fibrillation in Asia compared with other regions of the world: real-world data from the GARFIELD- AF registry. Int J Cardiol. 2016;223:543–547. doi: 10.1016/j.ijcard.2016.08.236. [DOI] [PubMed] [Google Scholar]
- 22.Proietti M, Senoo K, Lane DA, et al. Major bleeding in patients with non-valvular atrial fibrillation: impact of time in therapeutic range on contemporary bleeding risk scores. Sci Rep. 2016;6:24376. doi: 10.1038/srep24376. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Kakkar AK, Mueller I, Bassand JP, et al. Risk profiles and antithrombotic treatment of patients newly diagnosed with atrial fibrillation at risk of stroke: perspectives from the international, observational, prospective GARFIELD registry. PLoS One. 2013;8:e63479. doi: 10.1371/journal.pone.0063479. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Hylek EM, Skates SJ, Sheehan MA, et al. An analysis of the lowest effective intensity of prophylactic anticoagulation for patients with nonrheumatic atrial fibrillation. N Engl J Med. 1996;335:540–546. doi: 10.1056/NEJM199608223350802. [DOI] [PubMed] [Google Scholar]
- 25.Yamaguchi T. Optimal intensity of warfarin therapy for secondary prevention of stroke in patients with nonvalvular atrial fibrillation: a multicenter, prospective, randomized trial. Japanese Nonvalvular Atrial Fibrillation-Embolism Secondary Prevention Cooperative Study Group. Stroke. 2000;31:817–821. doi: 10.1161/01.STR.31.4.817. [DOI] [PubMed] [Google Scholar]
- 26.Suzuki S, Yamashita T, Kato T, et al. Incidence of major bleeding complication of warfarin therapy in Japanese patients with atrial fibrillation. Circ J. 2007;71:761–765. doi: 10.1253/circj.71.761. [DOI] [PubMed] [Google Scholar]
- 27.Yasaka M, Minematsu K, Yamaguchi T. Optimal intensity of international normalized ratio in warfarin therapy for secondary prevention of stroke in patients with non-valvular atrial fibrillation. Intern Med. 2001;40:1183–1188. doi: 10.2169/internalmedicine.40.1183. [DOI] [PubMed] [Google Scholar]
- 28.Bassand JP, Accetta G, Camm AJ, et al. Two-year outcomes of patients with newly diagnosed atrial fibrillation: results from GARFIELD-AF. Eur Heart J. 2016;37:2882–2889. doi: 10.1093/eurheartj/ehw233. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Lopes RD, Guimarães PO, Kolls BJ, et al. Intracranial hemorrhage in patients with atrial fibrillation receiving anticoagulation therapy. Blood. 2017;129:2980–2987. doi: 10.1182/blood-2016-08-731638. [DOI] [PubMed] [Google Scholar]
- 30.Ogawa H, Hamatani Y, Doi K, et al. Sex-related differences in the clinical events of patients with atrial fibrillation: the Fushimi AF registry. Circ J. 2017;81:1403–1410. doi: 10.1253/circj.CJ-17-0071. [DOI] [PubMed] [Google Scholar]