Abstract
Objective
Myotonia is caused by involuntary firing of skeletal muscle action potentials and causes debilitating stiffness. Current treatments are insufficiently efficacious and associated with side effects. Myotonia can be triggered by voluntary movement (electrically-induced myotonia) or percussion (mechanically-induced myotonia). Whether distinct molecular mechanisms underlie these triggers is unknown. Our goal was to identify ion channels involved in mechanically-induced myotonia and to evaluate block of the channels involved as a novel approach to therapy.
Methods
We developed a novel system to enable study of mechanically-induced myotonia using both genetic and pharmacologic mouse models of myotonia congenita. We extended ex vivo studies of excitability to in vivo studies of muscle stiffness.
Results
As previous work suggests activation of transient receptor potential vanilloid 4 (TRPV4) channels by mechanical stimuli in muscle, we examined the role of this cation channel. Mechanically-induced myotonia was markedly suppressed in TRPV4-null muscles and in muscles treated with TRPV4 small molecule antagonists. The suppression of mechanically-induced myotonia occurred without altering intrinsic muscle excitability such that myotonia triggered by firing of action potentials (electrically-induced myotonia) was unaffected. When injected intraperitoneally, TRPV4 antagonists lessened the severity of myotonia in vivo by approximately 80%.
Interpretation
These data demonstrate for the first time that there are distinct molecular mechanisms triggering electrically- and mechanically-induced myotonia. Our data indicates that activation of TRPV4 during muscle contraction plays an important role in triggering myotonia in vivo. Elimination of mechanically-induced myotonia by TRPV4 inhibition offers a new approach to treating myotonia.
Introduction
Muscle stiffness due to involuntary contraction of muscle (myotonia) is present in a number of dystrophic and non-dystrophic muscle diseases 1–4. The inability of muscle to relax rapidly following cessation of voluntary contraction leads to motor disability, which adversely affects quality of life 2, 4, 5. The mainstay of therapy for myotonia is drugs that block Na+ channels, such as mexiletine 2, 5–8. Block of Na+ channels reduces muscle excitability such that it is harder to trigger action potentials. While this may reduce myotonia, it can also lead to weakness due to impaired action potential generation during voluntary movement 9. In addition, block of Na+ channels can alter cardiac excitability increasing the risk of arrhythmias. Many patients suffer side effects or incomplete symptom resolution, highlighting the need for novel treatments 2, 5–8.
Myotonia can be triggered in two ways: by previous firing of voluntary action potentials or by mechanical stimulation of muscle. Myotonia triggered by voluntary action potentials we term electrically-induced myotonia. Contributors to the generation of electrically-induced myotonia include build-up of K+ in the transverse tubules (t-tubules) and activation of a Na+ persistent inward current (NaPIC) 10–13. Mechanically-induced myotonia can be elicited by tapping muscle with a reflex hammer (percussion myotonia) 1, 2. The contribution of mechanically-induced myotonia to motor dysfunction has never been determined.
To our knowledge, mechanisms underlying mechanically-induced myotonia have not been previously examined. One potential mechanism is the activation of mechanosensitive ion channels. A number of mechanosensitive channels have recently been described and they play central roles in diverse processes such as the sensation of touch, hearing and regulation of blood pressure 14. Skeletal muscle has been shown to express several mechanosensitive channels 15, 16.
TRPV4 is a member of the transient receptor potential (TRP) ion channel family that is activated by mechanical stimuli, heat, hypo-osmolarity and arachidonic acid metabolites 17, 18. In urothelial cells, TRPV4 mediates mechanically-evoked elevations of intracellular Ca2+ and plays a central role in regulation of bladder function 19, 20. TRPV4 is expressed in skeletal muscle 21–23 and has been implicated in modulation of resting Ca2+ influx and muscle fatigue 24, 25. Mechanically-activated currents present in wild type skeletal muscle are absent in TRPV4-null mice, strongly suggesting that TRPV4 contributes to mechanically-activated currents in skeletal muscle 26.
TRPV4 is most permeant to Ca2+ 27 such that its activation would be expected to depolarize muscle. Combining this with the finding that TRPV4 is activated following mechanical stimuli led us to explore the possibility that activation of TRPV4 triggers mechanically-induced myotonia. Our data demonstrate that mechanically-induced activation of TRPV4 is a significant contributor to triggering of myotonia in vivo such that TRPV4 antagonism may offer a new approach to treatment of myotonia.
Methods
Mice
All animal procedures were performed in accordance with the policies of the Animal Care and Use Committee of Wright State University. The genetic mouse model of myotonia congenita used was Clcn1adr-mto/J (ClCadr) mice, which have a null mutation in the Clcn1 gene (Jackson Laboratory Stock #000939). Genotyping was performed as previously described to select heterozygous mice for breeding 28. Otherwise, homozygous myotonic mice were identified by appearance and behavior as previously described 29. To pharmacologically induce myotonia, muscle from asymptomatic littermates was treated with 100 μM 9-anthracenecarboxylic acid (9AC) to block ClC-1 chloride channels 30. Mice were used from 6 weeks to 4 months of age.
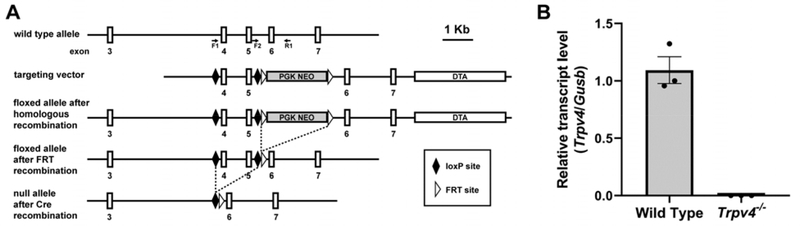
TRPV4-null (Trpv4−/−) mice were generated by homologous recombination using a targeting vector including loxP sites flanking Trpv4 exons 4 and 5, followed by outcrossing to Sox2Cre-expressing mice (Jackson Laboratory Stock #004783; Fig 1A). Quantitative reverse transcription PCR (RT-qPCR) analyses confirm the absence of Trpv4 mRNA expression in these mice (Fig 1B). Trpv4−/− mice and wild type littermates were generated by interbreeding of Trpv4+/− mice. Genotyping was performed by PCR analysis of tail DNA using the following primers: F1, 5′-GACAGCTGTGTCTCCACCAA; F2, 5′-GTGAGTAGCGGTGGAGGCTA; R1, 5′-GGACCAAAATGAGGGTGTGAACTTT (Fig 1A). This multiplex PCR produces a 183-bp amplicon for the wild type allele, a 200-bp amplicon for the null allele, and both amplicons in heterozygous mice. Comparable to previously reported Trpv4 knockout mouse lines 31, Trpv4+/− mice from this newly generated line did not exhibit deficits in weight, survival, or motor function (data not shown).
Figure 1:
Generation and characterization of Trpv4−/− mice. (A) Schematic representation of the targeting strategy. The structure of the endogenous mouse Trpv4 allele is shown at top, including the locations of the three genotyping primers, above the targeting vector in which Trpv4 exons 4 and 5 are flanked by loxP sites. Following excision of the Neo cassette, mice were outcrossed to a Sox2Cre-expressing mouse strain enabling Cre recombinase (Cre)-mediated gene deletion. (B) Trpv4 mRNA levels in extensor digitorum longus muscle of adult WT and Trpv4−/− mice, as assessed by RT-qPCR (n=3 for WT and Trpv4−/−).
Intracellular recording from individual myofibers
Mice were sacrificed using CO2 inhalation followed by cervical dislocation, and both extensor digitorum longus muscles were dissected out tendon-to-tendon. Muscles were maintained at 20–22°C and recorded within 6 hours of sacrifice. The recording chamber was continuously perfused with Ringer solution containing (in mM): NaCl, 118; KCl, 3.5; CaCl2, 1.5; MgSO4, 0.7; NaHCO3, 26.2; NaH2PO4, 1.7; glucose, 5.5 (pH 7.3–7.4), and equilibrated with 95% O2 and 5% CO2.
Intracellular recordings were performed as previously described 13, 29. Briefly, muscles were loaded with 50 μM N-benzyl-p-toluenesulfonamide (BTS) for at least 30 minutes prior to recording to prevent contraction 32. To aid in visualization of fibers during impalement, muscle was stained for 3 minutes with 10 μM 4-(4-diethylaminostyrl)-N-methylpyridinium iodide (4-Di-2-ASP) and imaged with an upright epifluorescence microscope. Micro-electrodes were filled with 3M KCl solution containing 1 mM sulforhodamine 101 to visualize the electrodes with epifluorescence. Resistances were between 15 and 30 MΩ, and capacitance compensation was optimized prior to recording. To perform intracellular recordings of action potentials, individual fibers were impaled with two sharp electrodes, one to pass current and one to record the voltage response. For comparisons of vehicle treatment to drug treatment the bilateral EDL muscles were dissected out. One was treated with vehicle and the other with drug. This allowed for paired recordings from single mice for vehicle versus drug treatment.
Fibers with resting potentials more depolarized than –74 mV were excluded from analysis. Passive properties and properties of single action potentials were measured as previously described 28. Action potential voltage threshold was defined as the voltage at which the dV/dt was 0.03 of its maximum (a value near 10 mV/ms) based on methods described by Sekerli et al 33. Input resistance was calculated by injecting hyperpolarizing current and measuring the steady-state response.
Reverse transcription quantitative PCR (RT-qPCR)
Tissues were homogenized in TRIzol (Thermo Fisher) utilizing Lysing Matrix D 1.4 mm ceramic spheres (MP Biomedicals) and a FastPrep-24 5G Instrument (MP Biomedicals). Total RNA was then isolated and converted to cDNA as described previously 34. RT-qPCR analyses were performed using an ABI 7900HT Real-Time PCR System (Thermo Fisher), with Taqman Universal PCR Master Mix (Thermo Fisher) and the following Taqman Gene Expression Assays: Gusb, Mm00446953_m1 (Thermo Fisher); Trpv4, Mm00499025_m1 (Thermo Fisher). Trpv4 transcript levels were quantified using the delta-delta Ct (ΔΔCt) method with normalization to the reference gene Gusb. Each reaction was performed in triplicate and all data are presented as average normalized Trpv4 transcript levels relative to a single wild type sample, which was assigned the value of 1.
Drugs
HC-067047 was obtained from Hello Bio (Princeton, NJ), GSK2193874 from Tocris (Minneapolis, MN), 9AC and sulforhodamine 101 from Sigma Aldrich (St. Louis, MO), BTS from TCI America (Portland, OR), and 4-Di-2-ASP from Molecular Probes (Eugene, OR).
In vivo force recording
In vivo muscle force recordings were performed as previously described 28. Mice were anesthetized via isoflurane inhalation; then the distal tendon of the triceps surae was attached to a force transduction motor and the sciatic nerve stimulated while isometric muscle force generation was measured. Muscle temperature was monitored with a laser probe and maintained between 28 ˚C and 30 ˚C with a heat lamp. The muscle was kept moist by applying mineral oil. Force recordings in ClCadr mice were performed before and 30 minutes following intraperitoneal (IP) injection. For HC-067047 (20 mg/kg), 0.2 ml of solution containing drug or vehicle was injected (0.015 ml of DMSO and 0.185 ml of saline). GSK2193874 was first dissolved in DMSO at a concentration of 7.5 mg/ml (0.375 mg/50 μl DMSO) using sonication. Distilled water was then added in a 1:1 ratio, followed by another round of sonication. Close to 0.1 mls of this solution was injected IP, with the volume adjusted to administer the correct dose of drug.
Statistical analysis and blinding
For statistical comparisons of intracellular recordings from individual muscle fibers, a repeated measures ANOVA with a random effect for mouse was run using SAS version 9.4 (SAS Institute, Inc., Cary, NC). The “simulate” post-hoc multiple comparison procedure was used for all post-hoc multiple comparisons. Origin software (OriginLab, Northampton, MA) was used to perform paired t-tests for analysis of in vivo force recordings before and after administration of HC-067047 or GSK2193874. All data are presented as means ± SEM, p < 0.05 was considered to be significant. The numbers of mice and muscle fibers used are described in the corresponding figure legends and text. For statistical analysis “n” was the number of mice.
For ex vivo experiments comparing mechanically-induced myotonia in Trpv4+/+ to Trpv4−/− mice, the experimenter was blinded as to the genotype of the mice. Similarly, ex vivo experiments testing efficacy of drugs were performed with the experimenter blinded as to which vial contained drug and which contained vehicle. For in vivo experiments, as the drug solution was opaque, GSK2193874 solutions were taken up in a syringe by a different experimenter and the syringe wrapped in tape to maintain blinding until analysis of data was complete.
Results
We studied muscle isolated from two mouse models of myotonia congenita: i) a pharmacologic model in which ClC-1 chloride channels are blocked with 100 μM 9-anthracenecarboxylic acid (the 9AC model) 30; and ii) a genetic model (Clcn1adr-mto/J, ClCadr mice) in which affected mice are homozygous for a null mutation in the Clcn1 gene 35. In each model of myotonia we studied the response of skeletal muscle to both electrical stimulation and mechanical stimulation, two distinct triggers of myotonia.
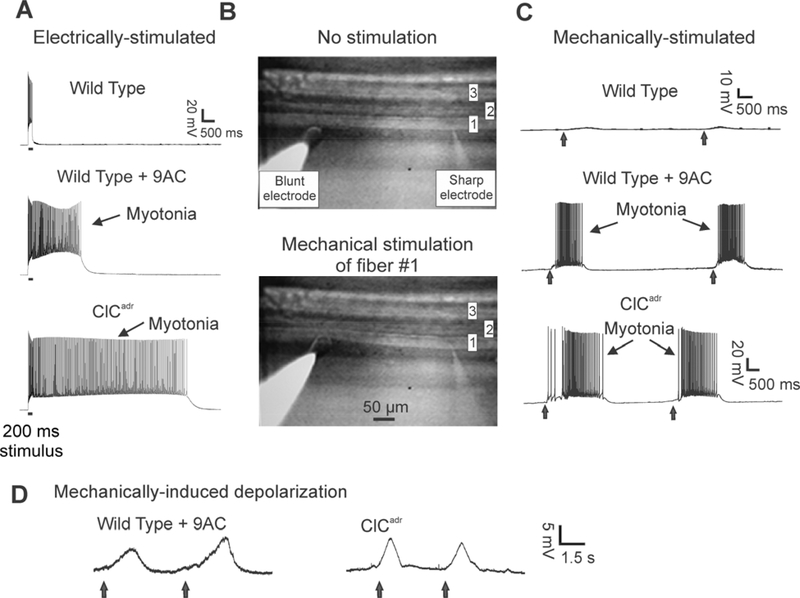
Muscle isolated from wild type mice responded to a 200 ms current injection of depolarizing current by repetitively firing action potentials that immediately ceased when current injection was terminated (Fig 2A). In contrast, muscles from both models of myotonia congenita responded to current injection with firing of action potentials that persisted following cessation of current injection (Fig 2A). We define this as electrically-induced myotonia to distinguish it from mechanically-induced myotonia.
Figure 2:
Electrically- and mechanically-induced myotonia are present in both pharmacologic and genetic mouse models of myotonia congenita. A) Intracellular recording from muscle fibers during stimulation by injection of depolarizing current. In each of the three traces, a 200 ms injection of current (horizontal line under the trace) triggers repeated firing of action potentials during the current injection. In wild type muscle, there is no firing of action potentials following termination of current injection (0/63 fibers from 7 mice). In wild type muscle myofibers in which ClC-1 chloride channels are blocked with 100 μM 9AC and in muscle from ClCadr mice, there is continued firing of action potentials (myotonia) following termination of current injection (58/58 fibers from 8 mice and 72/72 fibers from 9 mice, respectively). B) The arrangement of electrodes used to mechanically stimulate individual muscle fibers. Three muscles fibers are in focus in the image shown. A sharp electrode is impaled into fiber #1 on the right of the image. On the left, a blunt electrode is resting on the bottom of fiber #1, over 200 μm away from the sharp recording electrode. In the top image (No stimulation), the blunt electrode is gently resting on fiber #1. In the lower image the blunt electrode has been manually advanced to mechanically stimulate fiber #1. C) At the times indicated by vertical arrows, the blunt electrode was manually advanced. In wild type muscle, mechanical stimulation did not trigger myotonia (0/56 fibers from 8 mice). In both 9AC-treated and ClCadr muscle, mechanical stimulation triggered myotonia in all fibers tested (42/42 fibers from 6 mice and 56/56 fibers from 8 mice, respectively). D) Examples of mechanically-induced depolarization in muscle when 1 μM TTX was added to the perfusate to block Na+ channels.
In order to study mechanically-induced myotonia, it was necessary to develop a system that allowed for intracellular recording from individual muscle fibers during mechanical displacement of the fiber. For these studies, fibers were impaled with a single, sharp recording electrode. Approximately 200 μm away from the sharp electrode, a blunt 50 μm diameter, polished glass pipette was rested against the impaled fiber. The 200 μm distance was selected to be as close as possible, yet far enough away that pushing on the fiber with the blunt electrode did not cause loss of impalement (Fig 2B). The blunt electrode was manually advanced ~20 μm while recording the myofiber’s response to mechanical stimulation with the intracellular electrode. This distance was found to trigger mechanically-induced myotonia in all myotonic fibers. To avoid bias in manual advancement of the blunt electrode the experimenter was blinded as to genotype or drug treatments in all experiments of mechanically-induced myotonia.
In wild type muscle, mechanical stimulation triggered a depolarization of less than 3 mV that did not trigger myotonia (56 fibers from 8 mice, Fig 2C). In contrast, in myofibers from both models of myotonia congenita, 100% of myofibers exhibited mechanically-induced myotonia (Fig 2C). To directly measure the depolarization triggered by mechanical stimulation, action potentials were blocked with 1 μM tetrodotoxin (TTX; Fig 2D). In myofibers derived from the 9AC model of myotonia congenita, the depolarization averaged 8.6 ± 0.5 mV (35 fibers from 5 mice). In myofibers from the ClCadr model, it averaged 9.5 ± 0.6 mV (21 fibers from 3 mice). To our knowledge, this is the first measurement of mechanically-induced depolarization of skeletal muscle.
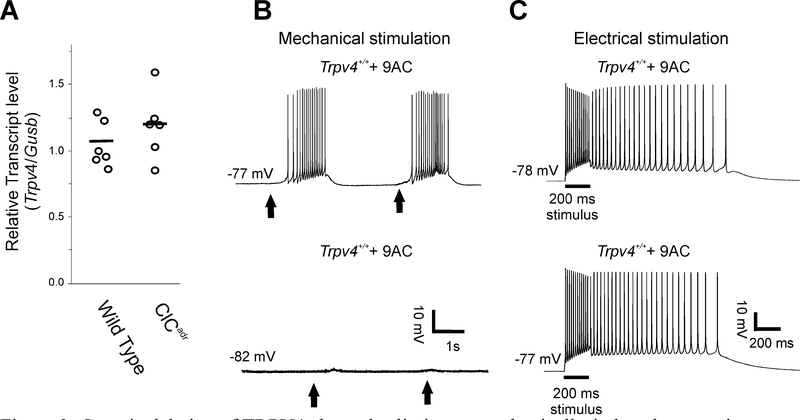
It has been suggested that mechanically-activated currents are absent in muscle lacking TRPV4 ion channels 26. To determine whether TRPV4 might contribute to mechanically-induced myotonia, we first assessed Trpv4 transcript levels in the extensor digitorum longus muscle (EDL), the muscle studied physiologically here. RT-qPCR analyses indicated that Trpv4 transcript was detectable in the EDL, with equivalent expression levels in wild type and ClCadr mice (Fig 3A, 1.05 ± 0.07 wild type vs 1.19 ± 0.10 ClCadr, p = 0.27).
Figure 3:
Genetic deletion of TRPV4 channels eliminates mechanically-induced myotonia without affecting electrically-induced myotonia. A) Scatter plot of the relative Trpv4 transcript levels normalized to the reference gene Gusb for 6 wild type and 6 ClCadr extensor digitorum longus muscles. The horizontal bars represent the mean of each group. B) In 9AC-treated muscle from Trpv4+/+ mice, mechanically-induced myotonia was present in 21/28 fibers from 4 mice whereas in 9AC-treated muscle fibers from Trpv4−/− mice, mechanically-induced myotonia was present in 0/28 fibers from 4 mice. At the times indicated by vertical arrows, a blunt electrode was manually advanced to mechanically stimulate the fiber being studied. C) Electrically-induced myotonia was present in 35/35 9AC-treated Trpv4+/+ fibers from 4 mice and 22/22 Trpv4−/− fibers from 3 mice.
To determine whether activation of TRPV4 plays a role in triggering mechanically-induced myotonia, we recorded from muscle from Trpv4−/− mice. Muscles from wild type siblings and Trpv4−/− mice were treated ex vivo with 9AC to cause myotonia and mechanically stimulated with a blunt electrode. In muscles from Trpv4+/+ mice, mechanically-induced myotonia was present in 21/28 fibers from 4 mice (Fig 3B). In Trpv4−/− mice, mechanically-induced myotonia was present in 0/28 fibers from 4 mice (Fig 3A). The elimination of mechanically-induced myotonia in 9AC-treated Trpv4−/− muscle was not accompanied by changes in membrane properties or properties of single action potentials (Table 1). All 9AC-treated Trpv4+/+ and Trpv4−/− muscle fibers had electrically-induced myotonia (Fig 3C). Following addition of TTX to block action potentials, Trpv4−/− fibers exhibited a close to 50% reduction in mechanically-induced depolarization relative to wild type myofibers (5.6 ± 0.5 mV in Trpv4+/+ vs 2.8 ± 0.5 in Trpv4−/−, p < 0.01, n = 28 fibers from 4 mice for each group). These data demonstrate that loss of TRPV4 selectively eliminates mechanically-induced myotonia by decreasing mechanically-induced depolarization of muscle.
Table 1.
Genetic deletion and pharmacologic inhibition of TRPV4 have no effect on skeletal muscle passive properties and single action potentials
| Resting potential (mV) | Input resistance (MΩ) | Action potential | |||
|---|---|---|---|---|---|
| Threshold (mV) | Maximum rate of rise (mV/ms) | Peak (mV) | |||
| TRPV4+/+ + 9AC | −83.9 ± 0.4 | 1.17 ± 0.09 | −62.7 ± 0.2 | 239.4 ± 7.6 | 32.5 ± 0.9 |
| TRPV4−/− + 9AC | −83.6 ± 0.8 | 1.20 ± 0.07 | −62.9 ± 0.7 | 231.9 ± 2.0 | 29.6 ± 0.6 |
| ClCadr + vehicle | −80.9 ± 0.5 | 0.85 ± 0.03 | −56.7 ± 0.5 | 234.1 ± 30.4 | 30.1 ± 4.7 |
| ClCadr + HC-067047 | −80.9 ± 0.5 | 0.79 ± 0.05 | −56.9 ± 0.6 | 236.6 ± 30.6 | 29.9 ± 3.9 |
| ClCadr + vehicle | −84.5 ± 0.3 | 1.06 ± 0.04 | −60.4 ± 0.4 | 244.9 ± 15.0 | 35.7 ± 1.0 |
| ClCadr + GSK2193874 | −82.8 ± 1.4 | 1.15 ± 0.07 | −59.8 ± 1.0 | 242.8 ± 2.7 | 36.6 ± 0.7 |
For studies of HC-067047, n = 28 fibers from 4 muscles for both groups. For studies of GSK2193874, n = 25 fibers from 3 muscles for vehicle and 19 fibers from 3 muscles for drug. For studies of TRPV4−/− mice, n = 39 fibers from 3 TRPV4+/+ mice, and 22 fibers from 3 TRPV4−/− mice. Data shown are mean ± SEM. None of the differences between vehicle and drug treatment groups and between TRPV4 genotypes were statistically significant.
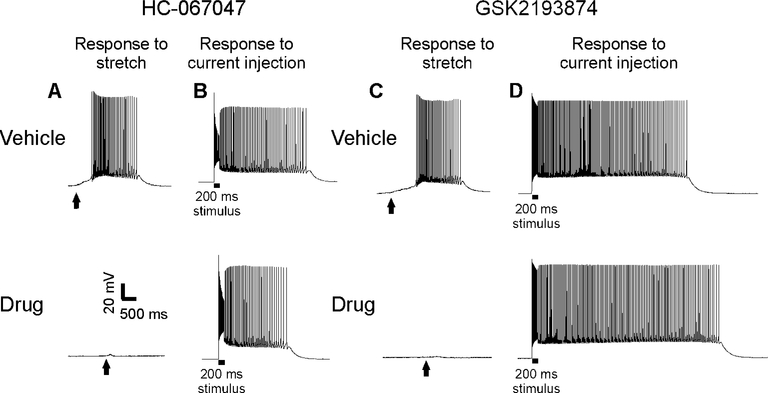
To determine whether small molecule antagonism of TRPV4 might be useful as therapy for myotonia, we examined whether antagonism of TRPV4 could lessen mechanically-induced myotonia ex vivo. HC-067047 at a dose of 1 μM has previously been shown to fully inhibit TRPV4 in urothelial cells 20. Muscle from ClCadr mice was exposed to either 1 μM HC-067047 or DMSO vehicle at a final concentration of 0.1%. The response to mechanical stimulation was dramatically altered by application of HC-067047. After application of vehicle, 100% of fibers exhibited mechanically-induced myotonia (28/28 fibers from 4 mice, Fig 4A), whereas following application of HC-067047, no fibers exhibited mechanically-induced myotonia (0/28 fibers from 4 mice, Fig 4A). To quantitate the effect of HC-067047 on the mechanically-induced depolarization, 1 μM TTX was added to the bath to block generation of action potentials. ClCadr muscle fibers depolarized by 9.5 ± 0.6 mV following mechanical stimulation. Treatment with 1 μM HC-067047 decreased this to 5.5 ± 0.6 mV (p < 0.01 vs vehicle, n = 21 vehicle-treated fibers from 3 mice and 21 HC-067047-treated fibers from 3 mice). Application of HC-067047 had no effect on mechanically-induced depolarization in Trpv4−/− muscle (2.8 ± 0.5 vs 2.7 ± 0.4 mV, n = 28 fibers from 4 mice for each group). These data strongly suggest that HC-067047 abolishes mechanically-induced myotonia by blocking TRPV4 channels.
Figure 4:
Treatment with small molecule inhibitors of TRPV4 greatly reduces mechanically-induced myotonia in ClCadr muscle. A) Mechanically-induced myotonia was present in 28/28 vehicle-treated fibers from 4 mice, and 0/28 fibers treated with 1 μM HC-067047 from 4 mice. At the times indicated by vertical arrows, a blunt electrode was manually advanced to mechanically stimulate the fiber being studied. B) Electrically-induced myotonia was present in 33/33 vehicle-treated fibers from 5 mice and 48/48 HC-067047-treated fibers from 5 mice. C) Following application of vehicle 19/20 fibers from 3 mice had mechanically-induced myotonia. After application of GSK2193874, 3/20 fibers from 3 mice had mechanically-induced myotonia. D) Electrically-induced myotonia was present in 21/21 vehicle-treated fibers from 3 mice and 19/19 fibers treated with 1 μM GSK2193874 from 3 mice.
Application of HC-067047 did not eliminate electrically-induced myotonia (Fig 4B). After treatment with either vehicle or HC-067047, electrically-induced myotonia was present in all fibers (33/33 vehicle-treated fibers from 5 mice and 48/48 HC-067047-treated fibers from 5 mice, Fig 4B). Similar results were obtained using 1 μM GSK2193874 to block TRPV4 channels 36. Application of GSK2193874 greatly reduced the incidence of mechanically-induced myotonia (19/20 fibers with mechanically-induced myotonia in 3 mice treated with vehicle and 3/20 fibers with mechanically-induced myotonia in 3 mice treated GSK2193874, Fig 4C) while having no effect on the incidence of electrically-induced myotonia (21/21 fibers from 3 mice for vehicle and 19/19 fibers from 3 mice for GSK2193874, Fig 4D). These data suggest that drugs that block TRPV4 selectively antagonize mechanically-induced myotonia.
Muscle excitability is determined by the amount of depolarizing current or depolarization of membrane potential required to trigger an action potential. In hyperexcitable myotonic muscle, muscle is too easily depolarized to action potential threshold, while in hypoexcitable muscle it is difficult to reach action potential threshold. To determine whether HC-067047 and GSK2193874 were eliminating mechanically-induced myotonia via a reduction in excitability, we measured passive membrane properties and properties of single action potentials. These properties of muscle determine the difficulty in reaching action potential threshold. Resting potential, input resistance and properties of single action potentials were unchanged by either drug (Table 1), suggesting neither drug altered excitability.
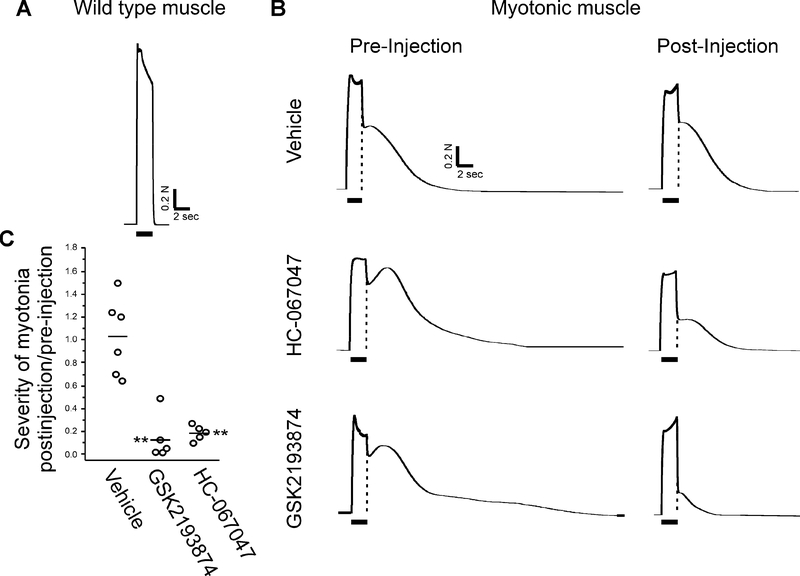
To determine whether antagonism of TRPV4 might be effective as therapy, we measured the severity of myotonia using in vivo contractile force recordings of gastrocnemius muscle as described previously 28. The sciatic nerve was stimulated at 60 Hz while measuring isometric muscle force generation. In wild type muscle, force fused during 60 Hz stimulation and relaxed immediately upon cessation of stimulation (Fig 5A). In ClCadr muscle, fusion of force was normal during stimulation, but relaxation following termination of stimulation was only partial due to myotonia (Fig 5B). Both amplitude and duration of muscle force have been previously used to estimate severity of myotonia 37. To combine these into a single value we took the integral of force with respect to time 9. To eliminate differences in measurement of myotonia due to differences in muscle force between mice, the integral of myotonia was normalized to force generated by a single stimulus before and after administration of drug. We measured severity of myotonia before and 30 minutes after IP injection of 25 mg/kg GSK2193874, 20 mg/kg HC-067047 or vehicle in ClCadr mice. Following injection of vehicle there was no change in severity of myotonia (Fig 5B–C). Following injection of both GSK2193874 and HC-067047 myotonia was reduced by over 80% (Fig 5B–C, p < 0.01 versus vehicle for both drugs, n = 5 mice for each drug treatment group and 6 for vehicle).
Figure 5:
Block of TRPV4 is effective in treating myotonia in vivo. A) The response of wild type muscle to 2 s of 60 Hz stimulation of the sciatic nerve. The horizontal bar under the trace indicates the period of 60 Hz stimulation of the sciatic nerve. Force is fully fused and there is immediate, complete relaxation following termination of stimulation. B) The response of muscle from ClCadr mice to 60 Hz stimulation. The vertical dotted line in each trace represents the rapid relaxation of force that occurs in wild type muscle following termination of nerve stimulation. Pre-injection, force generation during 60 Hz stimulation is fused normally, but relaxation following termination of stimulation is only partial and there is continued contraction for many seconds secondary to myotonia. At 45 minutes post-injection of vehicle, myotonia is minimally changed, but is greatly decreased in mice that received either HC-067047 or GSK2193874. C) Scatter plot of the ratio of severity of myotonia post-injection to severity of myotonia pre-injection in each mouse. The horizontal bars represent the mean of each group. Following injection of vehicle there was no change in severity of myotonia. Following injection of either drug there was a marked reduction. ** indicates p < 0.01 versus vehicle.
Discussion
Current treatment of myotonia is only partially effective and can cause unwanted side effects due, in part, to nonspecific suppression of excitability of both cardiac and skeletal muscle. Identifying currents in skeletal muscle that contribute to triggering of myotonia may lead to development of better targeted therapies that treat muscle stiffness secondary to myotonia without unwanted side effects. We established a novel ex vivo system to study currents responsible for mechanically-induced (percussion) myotonia. Genetic deletion of TRPV4 suppressed generation of mechanically-induced myotonia, while having no effect on myotonia triggered by previous firing of action potentials (electrically-induced myotonia). These data provide the first definitive evidence of distinct molecular mechanisms underlying electrically- and mechanically-induced myotonia. Small molecule inhibitors of TRPV4 reduced the incidence of mechanically-induced myotonia ex vivo and treatment of mice with systemic administration of these inhibitors also significantly reduced myotonia triggered by repetitive stimulation of the sciatic nerve in vivo. Our findings suggest antagonism of TRPV4 may offer a novel therapeutic approach to treating myotonia. A particular advantage of TRPV4 blockade is that it did not affect muscle excitability (the ease of generating action potentials) such that unwanted side effects of weakness and altered cardiac excitability might be avoided.
Two molecularly distinct triggers of myotonia
It has been known for many years that myotonia can be triggered by either voluntary firing of action potentials or percussion of muscle 1, 2. We term these electrically- and mechanically-induced myotonia, respectively. Although it has been presumed that distinct mechanisms are involved, direct proof has been lacking. One reason for the lack of progress in identifying the currents responsible for generation of mechanically-induced myotonia has been the inability to record intracellularly from intact muscle fibers during mechanical stimulation of muscle. We developed a system that allowed for intracellular recording of the depolarization of membrane potential and the subsequent myotonia triggered by mechanical stimulation of individual fibers. This made it possible to determine that genetic deletion of TRPV4 ion channels greatly reduced mechanically-induced myotonia, while having no effect on electrically-induced myotonia.
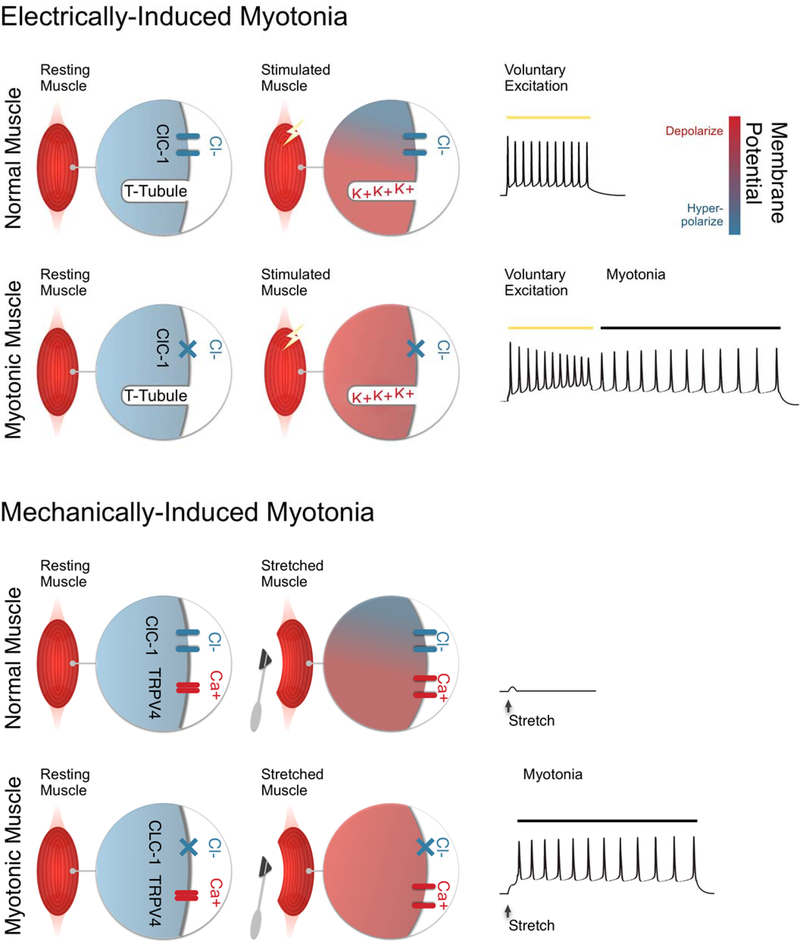
Shown in Figure 6 is a depiction of our current understanding of the distinct mechanisms underlying electrically- and mechanically-induced myotonia in the muscle disease myotonia congenita. In myotonia congenita there is a reduction in the number of functional Cl- channels. ClC-1-mediated Cl- conductance accounts for 70–80% of resting muscle membrane conductance and stabilizes the membrane potential near the resting potential 35, 38–40. In the absence of Cl- current, stimuli that would otherwise cause only modest depolarization, lead to depolarization large enough to trigger myotonia.
Figure 6:
Distinct mechanisms underlying electrically- and mechanically-induced myotonia. Electrically-induced myotonia (top two rows): in wild type muscle Cl- current plays a central role in controlling resting potential both at rest and during firing of action potentials during voluntary movement. Because of the stabilizing influence of Cl- current, K+ build-up in t-tubules does not cause sufficient depolarization to trigger myotonia following voluntary activation of muscle. In myotonia congenita, muscle Cl- current is reduced such that K+ build-up causes depolarization large enough to trigger myotonia. Mechanically-induced myotonia (bottom two rows): mechanical stimulation of wild type muscle activates TRPV4 channels, which depolarizes muscle. This depolarization is opposed by Cl- current such that myotonia is not triggered. Activation of TRPV4 channels during mechanical stimulation of muscle with reduced Cl- current causes depolarization sufficient to trigger myotonia.
In electrically-induced myotonia, an important source of depolarization is exit of K+ from the fiber during repolarization of action potentials. The exit of K+ leads to build-up of K+ in t-tubules, a depolarized shift in the K+ equilibrium potential and depolarization of the membrane potential 10–12, 38. When the Cl- current is small or absent the depolarization caused by K+ build-up triggers myotonia.
In mechanically-induced myotonia, there have been no preceding action potentials so build-up of K+ in t-tubules cannot be a contributor to depolarization. Instead, mechanical distortion of the sarcolemma results in opening of TRPV4 channels, which are nonselectively permeable to monovalent and divalent cations 27, such that their activation causes depolarization and myotonia. A prediction of this model of mechanically-induced myotonia is that agonists of TRPV4 channels would worsen myotonia, though this remains to be tested experimentally.
Ex vivo experiments demonstrated that electrically- and mechanically-induced myotonia could be triggered independently. However, in vivo, block of TRPV4 channels significantly reduced the severity of myotonia triggered by repetitive stimulation of the sciatic nerve (electrically-induced myotonia). The difference is likely due to experimental conditions. During ex vivo studies muscle contraction was eliminated with BTS, a drug that blocks interaction of actin and myosin 32, 41 because contraction of muscle during intracellular recording causes damage-induced loss of membrane potential. During in vivo experiments, muscle contraction was not blocked so that force could be measured. Thus, firing of action potentials in vivo increased tension as muscle contracted, which appears to activate TRPV4 to worsen myotonia. We conclude that build-up of K+ in t-tubules and activation of TRPV4 combine to trigger myotonia in vivo. The lessening of myotonia following block of TRPV4 strongly suggests mechanically-induced activation of TRPV4 is a contributor to generation of myotonia in vivo.
Mechanisms underlying mechanically-induced depolarization of muscle
The simplest explanation for the contribution of TRPV4 to mechanically-induced myotonia is that TRPV4 is activated by mechanical forces exerted on the sarcolemma. Several in vitro studies have provided evidence that TRPV4 is insensitive to membrane stretch 42–45, but can be activated by mechanical stimuli applied at cell-substrate contacts 43 or to cell surface β1-integrins 46. Given the importance of cell-matrix interactions to skeletal muscle physiology 47, cell-substrate contacts may play a key role in sarcolemmal TRPV4 activation in response to mechanical stimulation. However, little is currently known about how TRPV4 is activated by mechanical stimuli in vivo. It also remains to be determined whether the biomechanical properties of skeletal muscle change in the context of myotonia and whether such changes alter the sensitivity of TRPV4 to mechanical stimuli.
In several cell types TRPV4 activation in response to osmomechanical stress occurs with a latency of seconds and is mediated by phospholipase A2 (PLA2)-dependent generation of second messengers, such as arachidonic acid 48–51. In contrast, TRPV4 activation by deflection at cell-substrate interfaces occurs with a rapid latency (<2 ms) and is PLA2-independent, consistent with direct transduction of the mechanical stimulus 43. Similarly, calcium influx through TRPV4 resulting from the application of mechanical force to β1-integrins occurs with a latency of milliseconds 46. The rapid onset of mechanically-induced myotonia observed in both the pharmacologic and genetic models described here (Figure 2) suggests TRPV4 activation in this context may be mediated by mechanisms comparable to those underlying channel responses to cell-substrate deflections or integrin stimulation, rather than via activation by second messengers.
It remains to be determined whether all mechanically-activated currents contributing to triggering of myotonia have been identified. While there is one study suggesting genetic deletion of TRPV4 abrogates mechanically activated currents in muscle 26, we did not observe elimination of mechanically-induced depolarization. The remaining depolarization may be due to a technical issue, such as depolarization by the impaling electrode during mechanical stimuli. Alternatively, a second mechanically activated channel expressed in muscle such as Piezo1, TRPC1, TRPC6 or TRPV2 might also contribute to generation of mechanically-induced myotonia 21, 22, 52–55.
TRPV4 antagonists as a novel approach to therapy of myotonia
Myotonia is present in muscle diseases such as myotonia congenita, myotonic dystrophy, sodium channel myotonia, paramyotonia congenita and hyperkalemic periodic paralysis 1–4. Myotonia causes functionally limiting muscle stiffness, weakness and pain 2, 4, 5. For movement to occur normally, muscle needs to sequentially contract and relax during flexion and extension of a joint. For this to occur, muscle must fire action potentials during contraction, and be electrically silent during relaxation as antagonist muscles contract and the muscle is stretched as it lengthens. If muscle is unable to relax while lengthening, due to mechanically-induced myotonia, this will negatively impact motor performance. The contribution of mechanically-induced myotonia to motor dysfunction in patients with myotonia has never been determined, as it has not been possible to selectively block mechanically-induced myotonia. As described above, our studies suggest mechanically-induced myotonia is a significant contributor to motor dysfunction in vivo such that block of mechanically-induced myotonia may improve motor performance.
The current approach to therapy of myotonia is focused on block of Na+ channels responsible for generation of action potentials 2, 5–8. This reduces excitability and thus reduces myotonia. A problem with this approach is that activation of Na+ channels is required during the action potentials necessary for voluntary movement. Thus, care must be taken to not block too high a percentage of Na+ channels, so as not to cause weakness/paralysis.
A superior approach to therapy would be to block pathologic excitation of muscle without affecting normal excitation 9. Knockout of TRPV4 had no effect on properties of single action potentials that depend on Na+ current, suggesting that excitability is not significantly reduced. Thus, it may be possible to reduce myotonia by blocking TRPV4 without adversely affecting strength during voluntary movement. It has been reported that there are muscle transcriptional changes in Trpv4−/− mice, and that TRPV4 activation modulates resting Ca2+ influx and muscle fatigue 24, 25, such that block of TRPV4 may have side effects related to muscle function. However, despite TRPV4 being expressed in a number of tissues, knockout mice have a surprisingly mild phenotype, suggesting that block of TRPV4 in vivo might not lead to intolerable side effects 31. Administration of selective TRPV4 blockers is well tolerated in both rodents and patients 36, 56. Thus, using block of TRPV4 as therapy for patients with myotonia may become possible in the near future. One approach would be to add block of TRPV4 to current therapy, as there would be little risk of causing muscle hypoexcitability.
The studies reported here focused on myotonia resulting from loss of muscle Cl- current. However, as TRPV4 channels are present in wild type muscle, it is possible that activation of TRPV4 contributes to muscle stiffness in other diseases with myotonia. Thus, block of TRPV4 may be useful in treating myotonia in diseases such as myotonic dystrophy, sodium channel myotonia, paramyotonia congenita and hyperkalemic periodic paralysis.
Acknowledgements
This work was supported by NIH grants AR074985 (M.M.R.), NS087579 (C.J.S) and NS099850 (A.A.V.) and MDA grant 602459 (M.M.R.).
Footnotes
Potential conflicts of interest: Nothing to report.
References
- 1.Trivedi JR, Bundy B, Statland J, et al. Non-dystrophic myotonia: prospective study of objective and patient reported outcomes. Brain. 2013. July;136(Pt 7):2189–200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Cannon SC. Channelopathies of skeletal muscle excitability. Compr Physiol. 2015. April;5(2):761–90. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Thornton CA. Myotonic dystrophy. Neurol Clin. 2014. August;32(3):705–19, viii. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Cannon SC. Sodium Channelopathies of Skeletal Muscle. Handb Exp Pharmacol. 2017. September 23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Statland JM, Bundy BN, Wang Y, et al. Mexiletine for symptoms and signs of myotonia in nondystrophic myotonia: a randomized controlled trial. Jama. 2012. October 3;308(13):1357–65. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Lehmann-Horn F, Jurkat-Rott K, Rudel R. Diagnostics and therapy of muscle channelopathies--Guidelines of the Ulm Muscle Centre. Acta Myol. 2008. December;27:98–113. [PMC free article] [PubMed] [Google Scholar]
- 7.Trivedi JR, Cannon SC, Griggs RC. Nondystrophic myotonia: challenges and future directions. Exp Neurol. 2014. March;253:28–30. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Matthews E, Hanna MG. Repurposing of sodium channel antagonists as potential new anti-myotonic drugs. Exp Neurol. 2014. November;261:812–5. [DOI] [PubMed] [Google Scholar]
- 9.Metzger S, Dupont C, Voss AA, Rich MM. Central Role of Subthreshold Currents in Myotonia. Ann Neurol. 2020. February;87(2):175–83. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Adrian RH, Marshall MW. Action potentials reconstructed in normal and myotonic muscle fibres. J Physiol. 1976. June;258(1):125–43. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Wallinga W, Meijer SL, Alberink MJ, Vliek M, Wienk ED, Ypey DL. Modelling action potentials and membrane currents of mammalian skeletal muscle fibres in coherence with potassium concentration changes in the T-tubular system. Eur Biophys J. 1999;28(4):317–29. [DOI] [PubMed] [Google Scholar]
- 12.Fraser JA, Huang CL, Pedersen TH. Relationships between resting conductances, excitability, and t-system ionic homeostasis in skeletal muscle. J Gen Physiol. 2011. July;138(1):95–116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Hawash AA, Voss AA, Rich MM. Inhibiting persistent inward sodium currents prevents myotonia. Ann Neurol. 2017. September;82(3):385–95. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Ranade SS, Syeda R, Patapoutian A. Mechanically Activated Ion Channels. Neuron. 2015. September 23;87(6):1162–79. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Franco-Obregon A, Lansman JB. Changes in mechanosensitive channel gating following mechanical stimulation in skeletal muscle myotubes from the mdx mouse. J Physiol. 2002. March 1;539(Pt 2):391–407. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Yeung EW, Whitehead NP, Suchyna TM, Gottlieb PA, Sachs F, Allen DG. Effects of stretch-activated channel blockers on [Ca2+]i and muscle damage in the mdx mouse. J Physiol. 2005. January 15;562(Pt 2):367–80. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Darby WG, Grace MS, Baratchi S, McIntyre P. Modulation of TRPV4 by diverse mechanisms. Int J Biochem Cell Biol. 2016. September;78:217–28. [DOI] [PubMed] [Google Scholar]
- 18.Shibasaki K TRPV4 ion channel as important cell sensors. J Anesth. 2016. December;30(6):1014–9. [DOI] [PubMed] [Google Scholar]
- 19.Mochizuki T, Sokabe T, Araki I, et al. The TRPV4 cation channel mediates stretch-evoked Ca2+ influx and ATP release in primary urothelial cell cultures. J Biol Chem. 2009. August 7;284(32):21257–64. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Everaerts W, Zhen X, Ghosh D, et al. Inhibition of the cation channel TRPV4 improves bladder function in mice and rats with cyclophosphamide-induced cystitis. Proc Natl Acad Sci U S A. 2010. November 2;107(44):19084–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Brinkmeier H TRP channels in skeletal muscle: gene expression, function and implications for disease. Adv Exp Med Biol. 2011;704:749–58. [DOI] [PubMed] [Google Scholar]
- 22.Gailly P TRP channels in normal and dystrophic skeletal muscle. Curr Opin Pharmacol. 2012. June;12(3):326–34. [DOI] [PubMed] [Google Scholar]
- 23.Blackburn DM, Lazure F, Corchado AH, Perkins TJ, Najafabadi HS, Soleimani VD. High-resolution genome-wide expression analysis of single myofibers using SMART-Seq. J Biol Chem. 2019. December 27;294(52):20097–108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Kusudo T, Wang Z, Mizuno A, Suzuki M, Yamashita H. TRPV4 deficiency increases skeletal muscle metabolic capacity and resistance against diet-induced obesity. J Appl Physiol (1985). 2012. April;112(7):1223–32. [DOI] [PubMed] [Google Scholar]
- 25.Pritschow BW, Lange T, Kasch J, Kunert-Keil C, Liedtke W, Brinkmeier H. Functional TRPV4 channels are expressed in mouse skeletal muscle and can modulate resting Ca2+ influx and muscle fatigue. Pflugers Arch. 2011. January;461(1):115–22. [DOI] [PubMed] [Google Scholar]
- 26.Ho TC, Horn NA, Huynh T, Kelava L, Lansman JB. Evidence TRPV4 contributes to mechanosensitive ion channels in mouse skeletal muscle fibers. Channels (Austin). 2012. Jul-Aug;6(4):246–54. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Voets T, Prenen J, Vriens J, et al. Molecular determinants of permeation through the cation channel TRPV4. J Biol Chem. 2002. September 13;277(37):33704–10. [DOI] [PubMed] [Google Scholar]
- 28.Dupont C, Denman KS, Hawash AA, Voss AA, Rich MM. Treatment of myotonia congenita with retigabine in mice. Exp Neurol. 2019. May;315:52–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Novak KR, Norman J, Mitchell JR, Pinter MJ, Rich MM. Sodium channel slow inactivation as a therapeutic target for myotonia congenita. Ann Neurol. 2015. February;77(2):320–32. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Palade PT, Barchi RL. On the inhibition of muscle membrane chloride conductance by aromatic carboxylic acids. J Gen Physiol. 1977;69(6):879–96. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Grace MS, Bonvini SJ, Belvisi MG, McIntyre P. Modulation of the TRPV4 ion channel as a therapeutic target for disease. Pharmacol Ther. 2017. September;177:9–22. [DOI] [PubMed] [Google Scholar]
- 32.Macdonald WA, Pedersen TH, Clausen T, Nielsen OB. N-Benzyl-p-toluene sulphonamide allows the recording of trains of intracellular action potentials from nerve-stimulated intact fast-twitch skeletal muscle of the rat. Exp Physiol. 2005. November;90(6):815–25. [DOI] [PubMed] [Google Scholar]
- 33.Sekerli M, Del Negro CA, Lee RH, Butera RJ. Estimating action potential thresholds from neuronal time-series: new metrics and evaluation of methodologies. IEEE Trans Biomed Eng. 2004. September;51(9):1665–72. [DOI] [PubMed] [Google Scholar]
- 34.Ramos DM, d’Ydewalle C, Gabbeta V, et al. Age-dependent SMN expression in disease-relevant tissue and implications for SMA treatment. J Clin Invest. 2019. November 1;129(11):4817–31. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Steinmeyer K, Klocke R, Ortland C, et al. Inactivation of muscle chloride channel by transposon insertion in myotonic mice. Nature. 1991;354(6351):304–8. [DOI] [PubMed] [Google Scholar]
- 36.Thorneloe KS, Cheung M, Bao W, et al. An orally active TRPV4 channel blocker prevents and resolves pulmonary edema induced by heart failure. Sci Transl Med. 2012. November 07;4(159):159ra48. [DOI] [PubMed] [Google Scholar]
- 37.van Lunteren E, Spiegler SE, Moyer M. Fatigue-inducing stimulation resolves myotonia in a drug-induced model. BMC Physiol. 2011;11:5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Adrian RH, Bryant SH. On the repetitive discharge in myotonic muscle fibres. J Physiol. 1974. July;240(2):505–15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Palade PT, Barchi RL. Characteristics of the chloride conductance in muscle fibers of the rat diaphragm. J Gen Physiol. 1977;69(3):325–42. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Steinmeyer K, Ortland C, Jentsch TJ. Primary structure and functional expression of a developmentally regulated skeletal muscle chloride channel. Nature. 1991;354(6351):301–4. [DOI] [PubMed] [Google Scholar]
- 41.Cheung A, Dantzig JA, Hollingworth S, et al. A small-molecule inhibitor of skeletal muscle myosin II. Nat Cell Biol. 2002. January;4(1):83–8. [DOI] [PubMed] [Google Scholar]
- 42.Strotmann R, Harteneck C, Nunnenmacher K, Schultz G, Plant TD. OTRPC4, a nonselective cation channel that confers sensitivity to extracellular osmolarity. Nat Cell Biol. 2000. October;2(10):695–702. [DOI] [PubMed] [Google Scholar]
- 43.Servin-Vences MR, Moroni M, Lewin GR, Poole K. Direct measurement of TRPV4 and PIEZO1 activity reveals multiple mechanotransduction pathways in chondrocytes. Elife. 2017. January 30;6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Nikolaev YA, Cox CD, Ridone P, et al. Mammalian TRP ion channels are insensitive to membrane stretch. J Cell Sci. 2019. December 10;132(23). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Martinac B, Poole K. Mechanically activated ion channels. Int J Biochem Cell Biol. 2018. April;97:104–7. [DOI] [PubMed] [Google Scholar]
- 46.Matthews BD, Thodeti CK, Tytell JD, Mammoto A, Overby DR, Ingber DE. Ultra-rapid activation of TRPV4 ion channels by mechanical forces applied to cell surface beta1 integrins. Integr Biol (Camb). 2010. September;2(9):435–42. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Goody MF, Sher RB, Henry CA. Hanging on for the ride: adhesion to the extracellular matrix mediates cellular responses in skeletal muscle morphogenesis and disease. Dev Biol. 2015. May 1;401(1):75–91. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Vriens J, Watanabe H, Janssens A, Droogmans G, Voets T, Nilius B. Cell swelling, heat, and chemical agonists use distinct pathways for the activation of the cation channel TRPV4. Proc Natl Acad Sci U S A. 2004. January 6;101(1):396–401. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Vriens J, Owsianik G, Fisslthaler B, et al. Modulation of the Ca2 permeable cation channel TRPV4 by cytochrome P450 epoxygenases in vascular endothelium. Circ Res. 2005. October 28;97(9):908–15. [DOI] [PubMed] [Google Scholar]
- 50.Ryskamp DA, Jo AO, Frye AM, et al. Swelling and eicosanoid metabolites differentially gate TRPV4 channels in retinal neurons and glia. J Neurosci. 2014. November 19;34(47):15689–700. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Toft-Bertelsen TL, Yarishkin O, Redmon S, Phuong TTT, Krizaj D, MacAulay N. Volume sensing in the transient receptor potential vanilloid 4 ion channel is cell type-specific and mediated by an N-terminal volume-sensing domain. J Biol Chem. 2019. November 29;294(48):18421–34. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Maroto R, Raso A, Wood TG, Kurosky A, Martinac B, Hamill OP. TRPC1 forms the stretch-activated cation channel in vertebrate cells. Nat Cell Biol. 2005. February;7(2):179–85. [DOI] [PubMed] [Google Scholar]
- 53.Spassova MA, Hewavitharana T, Xu W, Soboloff J, Gill DL. A common mechanism underlies stretch activation and receptor activation of TRPC6 channels. Proc Natl Acad Sci U S A. 2006. October 31;103(44):16586–91. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Alessandri-Haber N, Dina OA, Chen X, Levine JD. TRPC1 and TRPC6 channels cooperate with TRPV4 to mediate mechanical hyperalgesia and nociceptor sensitization. J Neurosci. 2009. May 13;29(19):6217–28. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Zanou N, Mondin L, Fuster C, et al. Osmosensation in TRPV2 dominant negative expressing skeletal muscle fibres. J Physiol. 2015. September 1;593(17):3849–63. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Goyal N, Skrdla P, Schroyer R, et al. Clinical Pharmacokinetics, Safety, and Tolerability of a Novel, First-in-Class TRPV4 Ion Channel Inhibitor, GSK2798745, in Healthy and Heart Failure Subjects. Am J Cardiovasc Drugs. 2019. June;19(3):335–42. [DOI] [PubMed] [Google Scholar]