Abstract
Objective
Vestibular evoked myogenic potentials (VEMPs) are short-latency muscle potentials measured from the neck (cervical VEMP; cVEMP) or under the eyes (ocular VEMP; oVEMP), which provide information regarding function of the saccule and utricle, respectively. VEMPs are reliable when performed in adults; however, reliability of VEMPs in children is unknown. Therefore, the purpose of the study was to determine the test-retest reliability of c- and oVEMP testing in normal control children.
Study Design
Prospective.
Setting
Hospital.
Patients
Ten adults, 14 adolescent children and 13 young children with normal hearing.
Interventions
c- and oVEMP testing were completed across two test sessions in response to air-conduction 500 Hz tone-burst and impulse hammer stimuli. Additionally, oVEMP was completed using eyes-open and eyes-closed conditions.
Main Outcome Measures
Intraclass correlation coefficients were calculated to determine the reliability of c- and oVEMP outcomes.
Results
When using air-conduction stimuli, c- and oVEMP amplitudes are reliable across test sessions in normal control children and adults. With impulse hammer stimuli, cVEMP amplitudes showed high reliability; however, oVEMP amplitudes showed low reliability in both eyes-open and eyes-closed conditions. Comparison between eyes-open and eyes-closed oVEMP conditions revealed shorter latencies and higher peak-to-peak amplitudes in the eyes-open condition.
Conclusions
In this small cohort of normal control children, cVEMPs are reliable using air-conduction and impulse hammer stimuli and oVEMPs are reliable using air-conduction stimuli in the eyes-open condition. oVEMP in eyes-closed conditions were less reliable compared to eyes-open conditions and resulted in a large number of absent responses.
Keywords: Vestibular evoked myogenic potential, pediatrics, reliability, vestibular
INTRODUCTION
Vestibular evoked myogenic potentials (VEMP) are commonly used to assess both portions of the vestibular nerve and otolith organs (1–5). In adults, VEMPs can be reliably elicited with either air- or bone-conducted stimuli (6); however, similar studies assessing the reliability of VEMPs in children have not been published. The VEMP is a muscle potential elicited in response to high intensity stimuli (1). The cervical VEMP (cVEMP) is a short-latency inhibitory response recorded ipsilaterally from the contracted sternocleidomastoid (SCM) muscle and provides information about saccule and inferior vestibular nerve function (1). The ocular VEMP (oVEMP) is an excitatory response recorded contralaterally from the inferior oblique muscle and provides information about utricle and superior vestibular nerve function (7).
VEMP responses are not dependent on auditory sensitivity and can be recorded in individuals with sensorineural hearing loss (8;9). However, conductive hearing loss can reduce or eliminate air-conduction VEMP responses due to attenuation of the stimulus reaching the otoliths (10–12). Therefore, the use of bone-conduction stimuli can assist in evaluating otolith function in populations with middle ear issues. Various methods of bone-conduction, including bone oscillators, mini-shakers and reflex hammers, have been shown to effectively evoke VEMP responses (12–14). Reflex hammer VEMP generally result in shorter latencies and larger amplitudes, likely due to more effective activation of vestibular afferents (12–14).
Studies investigating the reliability of VEMPs in children have not been explored, based on the authors’ literature search. Several factors could impact reliability of VEMP responses in children, including maintaining effective SCM contraction during cVEMP and sustainment of up-gaze during oVEMP testing. We hypothesized children would have more difficulty maintaining adequate SCM contraction, and therefore, demonstrate greater cVEMP variability than adults. In oVEMP, children may not be able to sustain up-gaze due to inattention. One strategy utilized in our laboratory is to mount an iPod at 30-degrees up-gaze and have children watch a video. However, an alternative method is to obtain oVEMP with eyes closed (15;16). oVEMP can be obtained with eyes closed because of Bell’s Phenomenon, the eyes roll upward and inward with closure, allowing for recording from the contracted inferior oblique muscle (17;18). Bell’s Phenomenon is present in 90–97% of individuals (19;20). The reliability of eyes-closed oVEMP has not been explored in adults or children; however, we hypothesized that oVEMP variability may improve in children if completed with eyes closed.
Several other variables can affect VEMP reliability. Variations in electrode placement can impact amplitude and latency (21;22). Ear canal volume can affect stimulus intensity (i.e. higher sound pressure levels with smaller ear canal volumes) and thus affect VEMP amplitude (23;24). Increased neck length and thickness has been correlated with increased cVEMP latency (25). Similarly, we hypothesize that external anatomic landmarks might correlate with oVEMP (i.e., the inter-mastoid distance, which can be used to infer the inter-utricular distance (26).
There is limited information regarding the reliability of vestibular testing in children, including VEMP testing. Therefore, the purpose of the present study was to determine: 1) the reliability and repeatability of c- and oVEMP responses in normal control children, and 2) factors that affect VEMP response characteristics in normal control children.
MATERIALS AND METHODS
Participants
Ten adults (mean age = 25.1 years; range = 21–30; 4 males), 14 adolescents (mean age = 13.3 years; range = 10–19; 4 males) and 13 children (mean age = 6.8 years; range = 4–9; 7 males) with normal hearing participated.
All participants passed a bilateral hearing screening at 25 dB HL at 1000, 2000, and 4000 Hz. Outer and middle ear function was evaluated using otoscopy and tympanometry. Participants were included if they had normal middle ear function bilaterally (compliance ≥ 0.2 mmhos, peak pressure −100 to 30 daPa on tympanometry). By case history, all participants denied neurological disorders, dizziness and balance issues. Aside from VEMPs, described below, no additional vestibular testing was completed.
Informed consent was obtained from all participants for testing approved by the Institutional Review Board at Boys Town National Research Hospital (Protocol # 12–13-XP, PI: KLJ).
VEMP Testing
All participants completed VEMP testing using air- and bone-conduction stimuli. Air-conducted c- and oVEMPs were recorded using the ICS Otometrics Chartr EP 200 system (Taastrup, DK) with ER-3A insert earphones. Stimuli consisted of 500 Hz tone bursts (TBs; condensation, one cycle rise/fall, no plateau, Blackman-gated) presented at 125 dB SPL (94 dB nHL) when equivalent ear canal volumes were > 0.8 ml, and at 120 dB SPL (89 dB nHL) when equivalent ear canal volumes were ≤ 0.8 ml for safe sound exposure during VEMP testing (24). Stimuli were presented at a repetition rate of 5.1/sec, and 75 sweeps were averaged for each test.
Bone-conducted c- and oVEMPs were recorded using the Intelligent Hearing Systems 1.30 Opti-Amp differential amplifier (Miami, FL, USA). Taps were manually presented at Fz (approximately 2 taps per second) through a small gauze pad using a PCB Piezotronics impulse hammer (Model 086C01, PCB Corporation, Depew, New York, USA), which quantified the amount of force delivered for each tap. The epoch began when the hammer force exceeded a 2.0 Newton threshold. Using a miniature accelerometer (Model 166 352A24; PCB Corporation, Depew, New York, USA), an ~1 ms delay was measured, thus all latencies were adjusted accordingly. The data collection software provided real-time feedback regarding individual tap force. Taps between 21 and 40 Newtons (~146 dB FL and 152 dB FL) were included for averaging. Two trials of thirty taps were delivered for each condition. A bandpass filter of 5–500 Hz and amplifier gain of 5k was used for both air- and bone-conduction VEMP testing. Impedances were below 5 kΩ.
Responses from impulse hammer stimuli were saved using a Fireface UCX soundcard (RME, Germany) and custom data collection software running on a Windows 7 PC. VEMP waveforms were imported into Matlab (Mathworks, Natick, MA; Version 2016a) for analysis. Two consecutive runs were obtained in each condition to ensure reproducibility. If replication did not occur, the response was considered absent. Latency and uncorrected amplitude of the responses (p13/n23 for cVEMP; n10/p16 for oVEMP) were recorded.
cVEMP Testing
cVEMP testing was completed with participants in the supine position with their head lifted up, nose upward, to contract the SCM muscles bilaterally. The active electrode was placed on the SCM belly, the reference on the manubrium of the sternum, and the ground electrode under the chin. During air-conduction cVEMP testing, EMG monitoring electrodes were placed directly below the active electrodes, and EMG was monitored throughout the entire trial. Responses were accepted when EMG was between 50 and 300 μV. Subjects watched a video on an iPhone (Apple Inc., Cupertino, CA, USA) for entertainment during testing.
For impulse hammer cVEMP testing, EMG monitoring was integrated into the recording electrodes. An estimation of EMG contraction was calculated using the root mean square (RMS) of the pre-stimulus window from −30 to −10 ms (27;28). EMG was calculated differently for 500 Hz TB and impulse hammer testing due to differences in equipment. For both air- and bone-conducted cVEMP, corrected amplitudes were calculated by dividing the uncorrected cVEMP amplitude by the average EMG.
oVEMP Testing
oVEMP testing was completed with participants seated on an examination table. Two conditions were performed. First, participants closed their eyes throughout testing (eyes-closed condition, EC). Second, participants watched a video on an iPhone positioned at 30 degrees up-gaze (eyes-open condition, EO). The active electrode was placed between the midline of the orbit and the lateral canthus of the eye, the reference on the inner canthus of the right eye and the ground under the chin. This is a modification from the belly tendon electrode montage (21).
Testing Sessions
To assess the reliability and repeatability of VEMP testing, all participants underwent testing twice, each on a separate day (mean time difference = 10 days [range = 1–30]). VEMPs were completed by the same examiner (EF) for Session 1 and 2 for all participants.
Physical Measurements
The following measurements were made during the first session only: 1) the length of the SCM muscle from the mastoid tip perpendicular to the clavicle (25), and 2) the inter-mastoid distance measured posterior to the entrance of the ear canal using a digital caliper. This was inserted into the following equation (0.567 + 0.497(IMD (10−2m)) to estimate the inter-utricular distance (26).
Statistical Analysis
To investigate whether subject cooperation disrupted oVEMP responses in the EC conditions, Chi-square analysis was completed. A paired samples t-test was completed to compare VEMP responses between Session 1 and 2 and EO vs EC-oVEMP responses. Intraclass correlation coefficients (ICCs) were calculated to determine the reliability of all VEMP outcomes. ICC were considered excellent if > 0.75, fair-to-good if between 0.4 and 0.75, and poor if < 0.4. Pearson correlations were calculated to investigate the relationship between VEMP outcomes and physical measurements.
RESULTS
Test-retest Reliability: Cervical and Ocular VEMP
c- and oVEMP response rates for all 74 ears across each VEMP condition are shown in Table 1. cVEMP response rates were excellent for 500 Hz TB and impulse hammer stimuli (96 – 100%). oVEMP response rates were excellent for 500 Hz TB (97–99%); however, response rates decreased with impulse hammer stimuli (86–92%) and further decreased in all conditions with EC (74–81%). Across all conditions 89/888 (10%) responses were absent. The majority of absent responses occurred in EC-oVEMP (67/89, 75%). Among subjects, absent responses occurred sporadically except for 3 subjects that accounted for 23/89 (26%) absent responses. No participant had absent responses in the same ear across all conditions.
Table 1.
Percent of Present VEMP Response Rates for All Ears and VEMP Trials Across Session
| Ocular VEMP | Cervical VEMP | |||||
|---|---|---|---|---|---|---|
| ACS-EO | ACS-EC | IH-EO | IH-EC | ACS | IH | |
| Session 1 | 97% (72/74) | 74% (55/74) | 86% (64/74) | 77% (57/74) | 100% (74/74) | 100% (74/74) |
| Session 2 | 99% (73/74) | 77% (57/74) | 92% (68/74) | 81% (60/74) | 100% (74/74) | 96% (71/74) |
ACS = 500 Hz tone burst, air-conducted stimuli, IH = Impulse hammer, EO = eyes open, EC = eyes closed
In the EC conditions, some adolescents and children had difficulty maintaining eye closure. To investigate whether subject cooperation disrupted EC-oVEMP, oVEMP responses were coded as present or absent, and eye closure disruptions (i.e., opened their eyes or closed eyes tightly) were coded as present or absent. There was no relationship between absent EC-oVEMP and the presence of a disruption for 500 Hz TB (X2 = 0, p = .99) or impulse hammer (X2 = 1.36, p = .24).
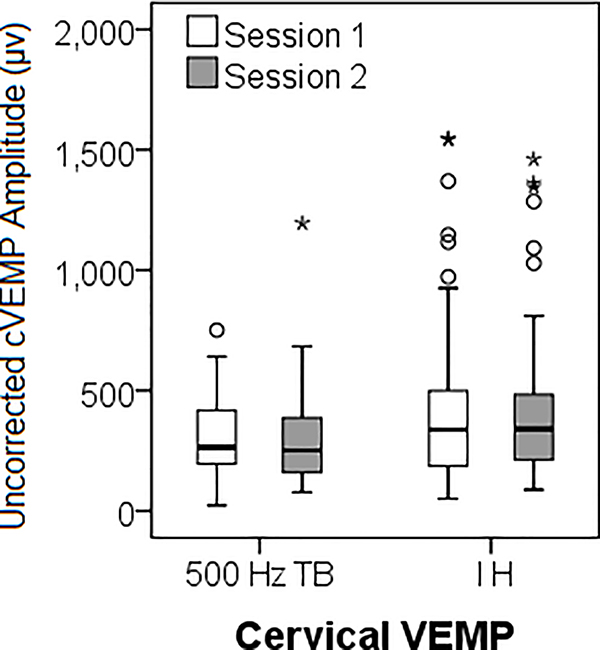
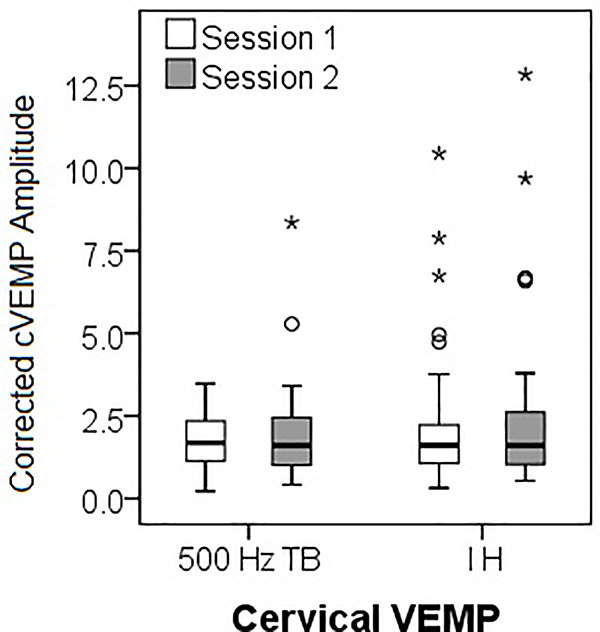
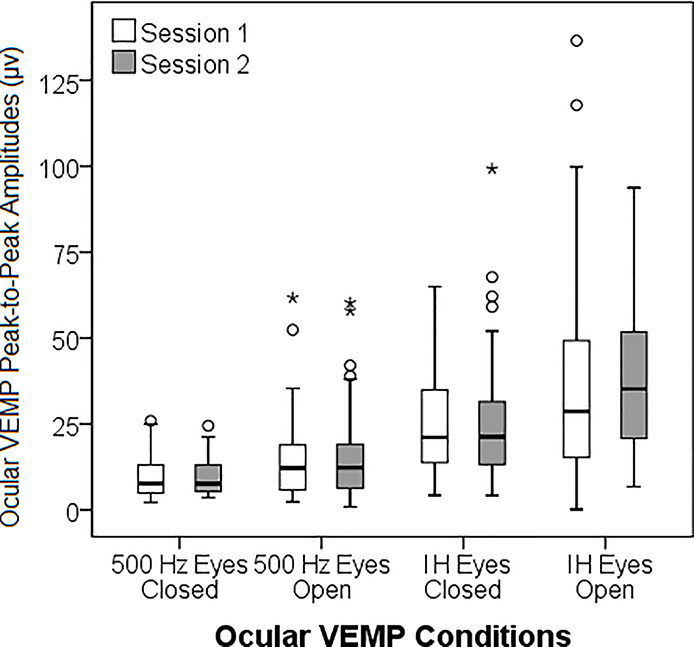
Descriptive data for cVEMP and oVEMP across sessions are shown in Tables 2 and 3, respectively. There were no significant mean differences between Session 1 and 2 for any cVEMP (Table 2, Figure 1A and 1B) or oVEMP (Table 3, Figure 1C) outcomes (p = .12 – .97). Representative tracings from a 17-year-old subject are included as supplemental digital content.
Table 2.
Cervical VEMP Means (SD) and Statistical Results of Test Session Comparison
| P13 (ms) | N23 (ms) | Peak-to-peak Amplitude (μν) | EMG (μν) | Corrected Amplitude | |||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Session | 1 | 2 | p | 1 | 2 | p | 1 | 2 | p | 1 | 2 | p | 1 | 2 | p |
| ACS | 14.6 (1.5) | 14.6 (1.5) | .96 | 21.9 (2.2) | 22.0 (2.2) | .58 | 302.9 (166.8) | 297.4 (186.2) | .76 | 166.6 (36.1) | 174.9 (56.2) | .38 | 1.8 (0.8) | 1.8 (1.2) | .87 |
| IH | 15.0 (1.8) | 15.3 (1.7) | .25 | 21.6 (3.0) | 22.1 (3.2) | .20 | 423.8 (332.7) | 415.9 (316.6) | .80 | 222.5 (89.5) | 219.9 (104.2) | .87 | 2.0 (1.7) | 2.2 (2.1) | .12 |
ACS = 500 Hz tone burst, air-conducted stimuli, IH = impulse hammer, p is the p-value from the paired samples t-test between Session 1 and 2, standard deviation is shown in parentheses
Table 3.
Ocular VEMP Means (SD) and Statistical Results of Test Session Comparison
| n10 (ms) | p16 (ms) | Peak-to-peak Amplitude (μv) | |||||||
|---|---|---|---|---|---|---|---|---|---|
| Session | 1 | 2 | p | 1 | 2 | p | 1 | 2 | p |
| ACS | 9.74 | 9.78 | .62 | 15.14 | 14.96 | .18 | 15.04 | 15.41 | .36 |
| EO | (0.64) | (0.61) | (1.23) | (1.22) | (12.19) | (12.50) | |||
| ACS | 10.48 | 10.62 | .56 | 16.77 | 16.87 | .70 | 9.43 | 9.58 | .77 |
| EC | (1.20) | (1.21) | (1.44) | (1.67) | (5.68) | (5.01) | |||
| IH | 11.36 | 11.27 | .57 | 16.47 | 17.28 | .36 | 35.23 | 39.25 | .27 |
| EO | (0.92) | (0.70) | (1.18) | (6.49) | (27.59) | (22.87) | |||
| IH | 11.95 | 11.94 | .97 | 18.08 | 17.82 | .39 | 24.82 | 25.92 | .71 |
| EC | (1.53) | (1.36) | (1.73) | (1.44) | (14.16) | (18.98) | |||
ACS = 500 Hz tone burst air-conducted stimuli, IH = reflex hammer, EO = eyes open, EC = eyes closed, p is the p-value from the paired samples t-test between Session 1 and 2, standard deviation is shown in parentheses
Figure 1:
Average VEMP amplitudes for each stimulus (500 Hz tone burst (TB) and impulse hammer (IH)) and session (Session 1 and Session 2), for: A) Cervical VEMP uncorrected peak-to-peak amplitude, B) Cervical VEMP corrected amplitude, and C) Ocular VEMP amplitude across test condition.
ICC values were calculated for the entire group (74 ears) and for each age group for both cVEMP and oVEMP (Tables 4 and 5, respectively). Participants with a no response were given an amplitude value of 0 and coded as no response for latency. For 500 Hz TB cVEMP, reliability was significant for the entire group across all response parameters. For the individual groups, contrary to our hypothesis, reliability was highest for the child group. For impulse hammer cVEMP, latency was a less reliable parameter compared to uncorrected peak-to-peak and corrected amplitude, which demonstrated excellent reliability for all groups. For impulse hammer, the corrected amplitude was more reliable than the uncorrected peak-to-peak amplitude.
Table 4.
Cervical VEMP ICC
| ACS | IH | |||||||
|---|---|---|---|---|---|---|---|---|
| p13 | n23 | AMP | cAMP | p13 | n23 | AMP | cAMP | |
| ALL | .789* | .879* | .761* | .724* | .426* | .684* | .809* | .933* |
| Adult | .708* | .84* | .436 | .552* | .517 | .533* | .707* | .944* |
| Adolescent | .71* | .889* | .791* | .827* | .259 | .67* | .892* | .784* |
| Children | .854* | .784* | .918* | .866* | .468* | .489* | .714* | .942* |
ACS = 500 Hz tone burst air-conducted stimuli, IH = Impulse hammer, AMP = peak-to-peak amplitude, cAMP = corrected peak-to-peak amplitude
Statistically significant ICC values are denoted with an asterisk while ICC values with excellent reliability (> 0.75) are denoted with bold font
Table 5.
Ocular VEMP ICC
| ACS EO | ACS EC | IH EO | IH EC | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| n10 | p16 | AMP | n10 | p16 | AMP | n10 | p16 | AMP | n10 | p16 | AMP | |
| ALL | .42* | .75* | .978* | .258 | .441 | .873* | −.215 | −.064 | .474* | .432* | .304 | .396* |
| Adult | .469 | .362 | .923* | .516 | .821* | .983* | .047 | −.045 | .639* | .322 | .256 | .300 |
| Adolescent | .19 | .619* | .906* | .491 | −.008 | .762* | −.176 | .268 | .236 | .495 | .252 | .323 |
| Children | .834* | .665* | .859* | −.151 | .173 | .584* | −.613 | .094 | .267 | .466 | .374 | .438 |
ACS = 500 Hz tone burst air-conducted stimuli, IH = impulse hammer, AMP = peak-to-peak amplitude. Statistically significant ICC values are denoted with an asterisk while ICC values with excellent reliability (> 0.75) are denoted with bold font
For 500 Hz TB oVEMP with EO and EC, latency was a less reliable response parameter; however, the peak-to-peak amplitude demonstrated excellent reliability across all age groups apart from the child group, who demonstrated poor reliability with EC. For impulse hammer oVEMP, reliability was fair-to-good for peak-to-peak amplitude overall and in adults, but poor in both the adolescent and child groups.
Physical Measurements
As expected, both SCM length (r = .696, p < .001) and inter-utricular distance (r = .531, p = .001) were positively correlated with age. There was a significant relationship between 500 Hz TB n23 latency and SCM length (r = .505, p = .001); however, there were no relationships between SCM length and any impulse hammer cVEMP parameters. There was a significant relationship between the inter-utricular distance and 500 Hz TB peak-to-peak oVEMP amplitude for both EO (r = .52, p = .001) and EC (r = .612, p = .001) conditions and the p16 latency for the EO condition (r = −.478, p = .003), suggesting that longer inter-utricular distances are related to higher amplitudes and shorter p16 latencies. There were no significant relationships between the inter-utricular distance and any impulse hammer oVEMP outcomes.
Ocular VEMP: Eyes-Open vs Eyes-Closed Conditions
For 500 Hz TB, the EO condition resulted in significantly shorter n10 latencies (EO: 9.6, EC: 10.54, t = 6.27, p < .001), p16 latencies (EO: 15.09, EC: 16.67, t = 5.77, p < .001), and higher peak-to-peak amplitudes (EO: 16.68, EC: 8.5, t = −6.267, p < .001). For impulse hammer stimuli, the EO condition resulted in significantly shorter p16 latencies (EO: 15.48, EC: 17.05, t = 5.617, p < .001) and higher peak-to-peak amplitudes (EO: 38.76, EC: 25.12, t = −4.033, p < .001). However, there was no significant difference in n10 latency (EO: 10.35, EC: 10.76, t = 1.751, p = .086).
DISCUSSION
Test-Retest Reliability: Cervical and Ocular VEMP
VEMP testing has been reported to be reliable in adults (6;30–33); however, there is limited information on the reliability of VEMP testing in children. Thus, the purpose of the study was to determine the reliability and repeatability of VEMP responses in normal control children and factors that affect VEMP response characteristics. Overall, there were no significant differences in cVEMP responses using either stimulus type (500 Hz TB or impulse hammer) from Session 1 to Session 2 and all cVEMP response parameters (latencies and amplitudes) demonstrated at least fair-to-good reliability (> 0.4). When analyzed by age group, contrary to our hypothesis, children demonstrated higher reliability compared to adults, achieving excellent (or near excellent) ICCs for both uncorrected and corrected cVEMP amplitude regardless of stimuli, suggesting that children can maintain adequate SCM contraction and sustain neck elevation for the duration of the test. Reliability was poorest in our adult group. We attribute this to a large degree of variability in SCM contraction and/or variations in electrode placement. For impulse hammer stimuli, cVEMP corrected amplitudes demonstrated excellent reliability in all age groups suggesting the effectiveness of correcting for amount of muscle tension. However, using either type of stimuli, latency was a less reliable parameter compared to amplitude. Because subjects were seen on separate days, we attribute this to differences in electrode placement.
Similar to cVEMP, there were no significant differences in oVEMP responses using either stimulus type (500 Hz TB or impulse hammer) from Session 1 to Session 2. However, there was a wide degree of variability in reliability across all oVEMP outcomes. oVEMP amplitude had excellent reliability in all age groups using 500 Hz TB stimuli and then reliability decreased with EC and further decreased using the impulse hammer. The high reliability noted for oVEMP amplitude in the EO condition was contrary to our hypothesis in that we hypothesized that children would be the poorest performing group. We attribute the excellent reliability in children to the visual task (iPhone at 30 degrees up-gaze), which motivated children to sustain their gaze upward. In response to 500 Hz TB, oVEMP amplitude in the EC condition indicated excellent reliability for all age groups except the child group, which is unfortunate as this is the population where this paradigm would be most helpful. EC response rates increased at the second session, which could be due to a learned effect.
Interestingly, between the EO and EC conditions using the impulse hammer, reliability was better in the EC conditions in both the adolescent and child groups. We attribute poor reliability in children to the method of delivery. For each trial, the impulse hammer was delivered at Fz from behind or to the side of the participant to avoid being in the line of sight. Although the examiner was not in direct view, the impulse hammer may have been in the peripheral visual field. Participants may have had an anticipatory blink reflex to the onset of each tap, causing artifact, and contributing to poorer EO-oVEMP reliability. Furthermore, most absent responses to impulse hammer stimuli were children. Comparison of all oVEMP response rates showed an improvement from Session 1 to Session 2, indicating a possible learned effect. This observation could warrant a practice trial with children to ensure the task will be completed appropriately or completing oVEMP with an impulse hammer in the EC condition. Compared to 500 Hz TB, poorer reliability was noted in all oVEMP impulse hammer trials (EO and EC). We attribute this to the variability in force that is inherent when using an impulse hammer.
Our results are consistent with others who report both cVEMP and oVEMP amplitude to be reliable in adults (6;30–33). Similarly, cVEMP latencies have been demonstrated by others to be less reliable than cVEMP amplitude (6;31). Factors affecting the reliability of VEMP responses include variability in electrode placement, repositioning of the insert phones and variations in muscle tension for cVEMP (6;32;33). All of which were felt to be factors in the current study. Nguyen et al (2010) found better reliability for oVEMP compared to cVEMP amplitude; this difference was attributed to better precision in electrode placement on the face compared to the neck, less fatigue with up-gaze compared to SCM contraction, less variability in soft tissue on the face compared to the neck, and the excitatory versus inhibitory origin of the oVEMP compared to cVEMP. For air-conduction stimuli, our results follow a similar pattern. In addition to the factors presented by Nguyen et al. (2010), we further speculate this pattern is due to the large degree of variability in SCM muscle tension with cVEMP. This same pattern was not evident using the impulse hammer, particularly for children. As noted above, this is likely due to children being distracted by the impulse hammer.
Overall, our data support the use of both air-conducted and impulse hammer cVEMP as a reliable assessment of saccular function and air-conducted oVEMP as a reliable assessment of utricular function in normal control children. Using impulse hammer stimuli can be especially beneficial in patients with conductive hearing loss, as a traditional air-conducted VEMP will be absent or reduced (10–12). When completing oVEMP with the impulse hammer, our results suggest that an EC condition is more reliable than EO in normal control children, but not for adults.
Eyes-Closed Characteristics
Reduced response rates and reliability were observed in the EC-oVEMP conditions using both stimuli, likely because not all individuals exhibit Bell’s Phenomenon. It is estimated that Bell’s phenomenon occurs in 90–97% of individuals (19;20). While this estimate is higher than the response rates obtained in this study, it is unsure how long Bell’s Phenomenon lasts following eye closure. Takagi (1992) concluded there were larger upward eye movements with longer blink durations; however, the authors did not report on eye movements when eyes were closed for longer than a few seconds (17). Therefore, if Bell’s Phenomenon lasts seconds, it may suggest a short time window for measuring EC-oVEMPs. Because the response is averaged over many samples (75 sweeps for 500 Hz TB; 30 taps for impulse hammer), present oVEMP responses could have been eliminated due to the protocol duration. Bell’s Phenomenon may also be associated with interfering involuntary blinks (16), or eye closure may have to be met with resistance to elicit the response (20;34). As we did not provide resistance to eye closure, the eye may not have moved upward for successful recording of an oVEMP response.
EC-oVEMP responses were lower in amplitude and longer in latency compared to EO conditions. This is consistent with others (16, 35). Because oVEMP amplitudes are influenced by degree of up-gaze (36), the smaller oVEMP amplitudes and high rate of absent responses may be due to variations in gaze angle that cannot be controlled during eye closure (35).
Physical Measurements
Previous studies indicated a relationship between SCM length and p13 and n23 cVEMP latencies. Longer latencies were associated with longer SCM, and thus a longer path length (29;37). This relationship is particularly evident for SCMs < 15.3 cm (37). As such, adult norms are recommended for use when neck length is > 15.3 cm (37). Our study only found a relationship with n23 latency when using air-conducted stimuli. While we measured SCM length using the same protocol as Chang et al. (2007) and Wang et al. (2008), we did not use a cut-off of 15.3 cm; however, only 1 of our subjects had an SCM measuring > 15.3 cm. We did not find a significant relationship between anatomical physical measurements and latencies in response to impulse hammer VEMP. This suggests the type of stimuli may affect latency of the response.
Regarding oVEMP, our results suggest that longer inter-utricular distances were related to higher amplitudes and shorter p16 latencies in response to 500 Hz TB. This was an unexpected finding. We expected latencies would be longer with longer inter-utricular distances, thus accounting for some variability in latencies. Our results are contrary to others who report that oVEMP responses are adult-like by age 3 with no differences in outcome parameters (latency or amplitude) compared to adults (15;38).
Limitations
There were several limitations to the current study. First, while results suggest both air-conducted and impulse hammer cVEMP and air-conducted oVEMPs are reliable assessments in normal control children, 10% of normal control ears demonstrated absent responses. While the majority (75%) of absent responses were in the EC-oVEMP conditions, absent responses were noted in all remaining conditions with the exception of air-conducted cVEMP (16 ears in EO-IH oVEMP, 3 ears in EO-500 Hz TB oVEMP). Additionally, a post hoc power analysis suggests the study is adequately powered for the majority of cVEMP outcomes and air-conducted oVEMP amplitude. This suggests the EC condition is not a reliable means of completing oVEMP and that air-conduction is a preferable stimulus to the impulse hammer. This also suggests that in cases of absent responses, additional trials could be attempted. In the current study, 2 trials were completed for each VEMP type; however, trials could be increased to 3 or 4 to ensure responses are absent. Second, all testing was completed by the same examiner; therefore, reliability calculations do not account for inter-examiner variability. Lastly, our population consisted of a small number of children and young adults with normal hearing; therefore, results do not reflect reliability of patient populations.
CONCLUSION
In conclusion, air-conducted c- and oVEMPs, and impulse hammer cVEMPs are reliable tests of otolith function in normal control children. EC-oVEMP were less reliable compared to EO-oVEMP and resulted in a large number of absent responses; therefore, EC-oVEMP is not considered a reliable condition. However, when a bone-conducted stimulus is needed (i.e., in children with middle ear issues), impulse hammer oVEMPs can be used. When using an impulse hammer in children, EC-oVEMP resulted in better reliability compared to the EO condition; however, this trend was not present for adults. Undiagnosed and untreated vestibular dysfunction can have a significant impact on children’s motor development (39) and academic difficulties, due to the inability to maintain stable gaze (40). The potential risks of vestibular dysfunction warrant the importance of effective testing and diagnosis in children.
Supplementary Material
Source of Funding
Research reported in this publication was supported by the National Institute of General Medical Sciences of the National Institutes of Health under award number P20GM109023 and by the National Institute on Deafness and Other Communication Disorders under award numbers T35 DC 008757, 5T32DC00013–36, R03DC015318 and P30DC004662.
Footnotes
Conflicts of Interest: KLJ is a consultant for Otometrics. There are otherwise no conflicts of interests to disclose.
Reference List
- 1.Colebatch JG, Halmagyi GM, Skuse NF. Myogenic potentials generated by a click-evoked vestibulocollic reflex. J Neurol Neurosurg Psychiatry 1994;57(2):190–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Zhou G, Dargie J, Dornan B, Whittemore K. Clinical uses of cervical vestibular-evoked myogenic potential testing in pediatric patients. Medicine (Baltimore) 2014;93(4):e37. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Weber KP, Rosengren SM, Michels R, Sturm V, Straumann D, Landau K. Single motor unit activity in human extraocular muscles during the vestibulo-ocular reflex. J Physiol 2012;590:3091–101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Rosengren SM, McAngus Todd NP, Colebatch JG. Vestibular-evoked extraocular potentials produced by stimulation with bone-conducted sound. Clin Neurophysiol 2005;116(8):1938–48. [DOI] [PubMed] [Google Scholar]
- 5.Sheykholeslami K, Megerian CA, Arnold JE, Kaga K. Vestibular-evoked myogenic potentials in infancy and early childhood. Laryngoscope 2005;115(8):1440–4. [DOI] [PubMed] [Google Scholar]
- 6.Nguyen KD, Welgampola MS, Carey JP. Test-retest reliability and age-related characteristics of the ocular and cervical vestibular evoked myogenic potential tests. Otol Neurotol 2010;31(5):793–802. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Todd NP, Rosengren SW, Colebatch JG. Ocular vestibular evoked myogenic potentials (oVEMPs) produced by air- and bone-conducted sound. Clin Neurophysiol, 2007;118:381–390. [DOI] [PubMed] [Google Scholar]
- 8.Chihara Y, Iwasaki S, Ushio M, Murofushi T. Vestibular-evoked extraocular potentials by air-conducted sound: another clinical test for vestibular function. Clin Neurophysiol 2007;118(12):2745–51. [DOI] [PubMed] [Google Scholar]
- 9.Tribukait A, Brantberg K, Bergenius J. Function of semicircular canals, utricles and saccules in deaf children. Acta Otolaryngol 2004;124(1):41–8. [DOI] [PubMed] [Google Scholar]
- 10.Yang TL, Young YH. Vestibular-evoked myogenic potentials in patients with otosclerosis using air- and bone-conducted tone-burst stimulation. Otol Neurotol 2007;28(1):1–6. [DOI] [PubMed] [Google Scholar]
- 11.Yang TL, Young YH. Comparison of tone burst and tapping evocation of myogenic potentials in patients with chronic otitis media. Ear Hear 2003;24(3):191–4. [DOI] [PubMed] [Google Scholar]
- 12.Halmagyi GM, Yavor RA, Colebatch JG. Tapping the head activates the vestibular system: a new use for the clinical reflex hammer. Neurology 1995;45(10):1927–9. [DOI] [PubMed] [Google Scholar]
- 13.Rosengren SM, Govender S, Colebatch JG. Ocular and cervical vestibular evoked myogenic potentials produced by air- and bone-conducted stimuli: comparative properties and effects of age. Clin Neurophysiol 2011;122(11):2282–9. [DOI] [PubMed] [Google Scholar]
- 14.Welgampola MS, Rosengren SM, Halmagyi GM, Colebatch JG. Vestibular activation by bone conducted sound. J Neurol Neurosurg Psychiatry 2003;74(6):771–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Wang SJ, Hsieh WS, Young YH. Development of ocular vestibular-evoked myogenic potentials in small children. Laryngoscope 2013;123(2):512–7. [DOI] [PubMed] [Google Scholar]
- 16.Huang YC, Yang TL, Young YH. Feasibility of ocular vestibular-evoked myogenic potentials (oVEMPs) recorded with eyes closed. Clin Neurophysiol 2012;123(2):376–81. [DOI] [PubMed] [Google Scholar]
- 17.Takagi M, Abe H, Hasegawa S, Usui T. Reconsideration of Bell’s phenomenon using a magnetic search coil method. Doc Ophthalmol 1992;80(4):343–52. [DOI] [PubMed] [Google Scholar]
- 18.Bell C On the motion of the eye, in illustration of the uses of teh muscles and nerves of the orbit. Philosophical Transactions of the Royal Society of London 1823;113:166–86. [Google Scholar]
- 19.Snir M, Kremer I, Kuperman A, Merlob P, Yassur Y. Bell’s phenomenon in newborns and premature babies. Br J Ophthalmol 1996;80(6):553–5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Hall AJ. Some observations on the acts of closing and opening the eyes. Br J Ophthalmol 1936;20(5):257–95. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Sandhu JS, George SR, Rea PA. The effect of electrode positioning on the ocular vestibular evoked myogenic potential to air-conducted sound. Clin Neurophysiol 2013;124(6):1232–6. [DOI] [PubMed] [Google Scholar]
- 22.Sheykholeslami K, Murofushi T, Kaga K. The effect of sternocleidomastoid electrode location on vestibular evoked myogenic potential. Auris Nasus Larynx 2001;28(1):41–3. [DOI] [PubMed] [Google Scholar]
- 23.Thomas MLA, Fitzpatrick D, McCreery R, Janky KL. Big Stimulus, Little Ears: Safety in Administering Vestibular-Evoked Myogenic Potentials in Children. J Am Acad Audiol 2017;28(5):395–403. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Rodriguez AI, Thomas MLA, Fitzpatrick D, Janky KL. Effects of High Sound Exposure During Air-Conducted Vestibular Evoked Myogenic Potential Testing in Children and Young Adults. Ear Hear 2018;39(2):269–77. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Chang CH, Yang TL, Wang CT, Young YH. Measuring neck structures in relation to vestibular evoked myogenic potentials. Clin Neurophysiol 2007;118(5):1105–9. [DOI] [PubMed] [Google Scholar]
- 26.Nowe V, Wuyts FL, Hoppenbrouwers M, Van de Heyning PH, De Schepper AM, Parizel PM. The interutricular distance determined from external landmarks. J Vestib Res 2003;13(1):17–23. [PubMed] [Google Scholar]
- 27.McCaslin DL, Fowler A, Jacobson GP. Amplitude normalization reduces cervical vestibular evoked myogenic potential (cVEMP) amplitude asymmetries in normal subjects: proof of concept. J Am Acad Audiol 2014;25(3):268–77. [DOI] [PubMed] [Google Scholar]
- 28.Bogle JM, Zapala DA, Criter R, Burkard R. The effect of muscle contraction level on the cervical vestibular evoked myogenic potential (cVEMP): usefulness of amplitude normalization. J Am Acad Audiol 2013;24(2):77–88. [DOI] [PubMed] [Google Scholar]
- 29.Chang CH, Yang TL, Wang CT et al. Measuring neck structures in relation to vestibular evoked myogenic potentials. Clin Neurophysiol 2007;118:1105–1109. [DOI] [PubMed] [Google Scholar]
- 30.Versino M, Colgaghi S, Callieco R, Cosi V Vestibular evoked myogenic potentials: Test-retest reliability. Funct Neurol 2001, 16(4): 299–309. [PubMed] [Google Scholar]
- 31.Isaradisaikul S, Strong D, Moushey J, Gabbard S, Ackley S, Jenkins H Reliability of vestibular evoked myogenic potentials in healthy subjects. Otol Neurotol 2008,29(4):542–544. [DOI] [PubMed] [Google Scholar]
- 32.Maes L, Vinch BM, De Vel E, D’haenens W, Bockstael A, Keppler H, Philips B, Swinnen F, Dhooge I The vestibular evoked myogenic potential: A test-retest reliability study. Clin Neurophys 2009, 120: 594–600. [DOI] [PubMed] [Google Scholar]
- 33.Leyssens L, Heinze B, Vinck B, Van Ombergen A, Vanspauwen R, Wuyts FL, Maes LK. ‘Standard’ versus ‘nose reference’ electrode placement for measuring oVEMPs with air-conducted sound: Test-retest reliability and preliminary patient results. Clin Neurophys 2017, 128: 312–322. [DOI] [PubMed] [Google Scholar]
- 34.Mustafa TA. The Bell`s phenomenon in newborns. Neurosciences (Riyadh ) 2005;10:41–43. [PubMed] [Google Scholar]
- 35.Todai JK, Congdon SL, Sangi-Haghpeykar H, Cohen HS. Ocular vestibular evoked myogenic potentials in response to three test positions and two frequencies. Laryngoscope 2014, 124(6):E237–E240. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Govender S, Rosengren SM, Colebatch JG. The effect of gaze direction on the ocular vestibular evoked myogenic potential produced by air-conducted sound. Clin Neurophys 2009, 120:1386–1391. [DOI] [PubMed] [Google Scholar]
- 37.Wang SJ, Yeh TH, Chang CH et al. Consistent latencies of vestibular evoked myogenic potentials. Ear Hear 2008;29:923–929. [DOI] [PubMed] [Google Scholar]
- 38.Kuhn JJ, Lavender VH, Hunter LL, McGuire SE, Meinzen-Derr J, Keith RW, Greinwald JH. Ocular Vestibular Evoked Myogenic Potentials: Normative Findings in Children. J Am Acad Audiol 2018, 29:443–450. [DOI] [PubMed] [Google Scholar]
- 39.Rine RM, Cornwall G, Gan K et al. Evidence of progressive delay of motor development in children with sensorineural hearing loss and concurrent vestibular dysfunction. Percept Mot Skills 2000;90:1101–1112. [DOI] [PubMed] [Google Scholar]
- 40.Braswell J, Rine RM. Evidence that vestibular hypofunction affects reading acuity in children. Int J Pediatr Otorhinolaryngol 2006;70:1957–1965. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.