Abstract
Introduction:
The antifungal therapy currently available includes three major classes of drugs: polyenes, azoles and echinocandins. However, the clinical use of these compounds faces several challenges: while polyenes are toxic to the host, antifungal resistance to azoles and echinocandins has been reported.
Areas covered:
Fungal sphingolipids (SL) play a pivotal role in growth, morphogenesis and virulence. In addition, fungi possess unique enzymes involved in SL synthesis, leading to the production of lipids which are absent or differ structurally from the mammalian counterparts. In this review, we address the enzymatic reactions involved in the SL synthesis and their relevance to the fungal pathogenesis, highlighting their potential as targets for novel drugs and the inhibitors described so far.
Expert opinion:
The pharmacological inhibition of fungal serine palmitoyltransferase depends on the development of specific drugs, as myriocin also targets the mammalian enzyme. Inhibitors of ceramide synthase might constitute potent antifungals, by depleting the pool of complex SL and leading to the accumulation of the toxic intermediates. Acylhydrazones and aureobasidin A, which inhibit GlcCer and IPC synthesis, are not toxic to the host and effectively treat invasive mycoses, emerging as promising new classes of antifungal drugs.
Keywords: fungi, sphingolipids, antifungal therapy, glucosylceramide, inositol phosphorylceramide, Cryptococcus, Candida, Aspergillus
1. Introduction
Fungal species affect billions of people worldwide, causing superficial infections of the skin or nails or invasive disease [1]. Despite the lack of epidemiological data, it is estimated that at least one and a half million individuals die every year due to invasive fungal infections (IFIs). This high number of deaths is comparable to those caused by tuberculosis [2], one of the top 10 global killers in 2016, according to the World Health Organization.
Cryptococcus, Candida, Aspergillus and Pneumocystis correspond to the most frequently isolated fungi from the clinical practice, accounting for more than 90% of the cases of invasive mycoses [2]. These fungal species are usually found in the environment or as commensal organisms, being cleared out by the immune system of healthy individuals. Immunosuppressive conditions, such as HIV/AIDS, chemotherapy, corticosteroid therapy and hematological malignancies, substantially increase the susceptibility to IFIs.
The main classes of antifungals currently used, and their simplified mechanism of action, are illustrated in Figure 1. The antifungal arsenal explores molecules or synthesis pathways unique to fungi, therefore limiting any potential damage to mammalian cells in host tissues. Ergosterol is the most abundant sterol in fungal membranes, playing a role in their fluidity and permeability [3]. Additionally, ergosterol differs in structure from its mammalian counterpart, cholesterol, making this lipid a suitable target for antifungal drugs. Indeed, two distinct classes of antifungal drugs disrupt the ergosterol pathway through binding to ergosterol (polyenes) or disrupting its synthesis (azoles).
Figure 1.
Schematic representation of the mechanism of action of azoles, polyenes and echinocandins. Both the polyenes and azoles disrupt the major fungal sterol, ergosterol. Whilst the polyene amphotericin B binds to ergosterol and disrupts membrane function, the azoles inhibit 14α-demethylase enzyme, impairing ergosterol synthesis. β−1,3-glucan synthase, a key enzyme for cell wall integrity, is inhibited by the echinocandins.
The polyenes are natural products of Streptomyces nodosus, a soil actinomycete [4], and amphotericin B, its main prototype, has been used for systemic fungal infections since 1959. Despite its clinical use for several decades, amphotericin B is still one of the most potent antifungals, exhibiting a broad spectrum of activity, including against most isolates of Cryptococcus spp, Candida spp and Aspergillus spp. For a long time, it was thought that amphotericin B exerts its fungicidal activity through the formation of pores. However, Gray showed that the antifungal activity of this polyene relies on simply binding to ergosterol, subsequently disrupting membrane function [5]. The membrane permeabilization caused by channel formation constitutes a secondary mechanism which increases amphotericin B potency [6].
Azoles target ergosterol synthesis by inhibiting lanosterol 14α-demethylase, which converts lanesterol to ergosterol [7,8]. This enzyme is involved in a rate-limiting step of the late pathway in ergosterol synthesis [9,10]. The resulting inhibition of 14α-demethylase activates the alternate pathway, leading to the accumulation of the metabolite dienol, which exerts a fungistatic effect [11,12]. The first generation of azoles was introduced in 1969, being mainly used in the following decades as a miconazole parenteral formulation or as ketoconazole oral tablets which showed high gastrointestinal toxicity. The development of second (fluconazole, itraconazole) and third (voriconazole, posaconazole) generation azoles improved spectrum of activity, safety and oral availability [13].
The newest class of antifungals comprise echinocandins, particularly caspofungin, micafungin and anidulafungin, developed in the early 2000s. These drugs target the cell wall, a branched matrix of polysaccharides, present in fungi but not in higher eukaryotes such as mammals [14]. Specifically, echinocandins inhibit the β−1,3-glucan synthase, which leads to cell death in Candida species and a modest, fungistatic effect in Aspergillus [15,16].
The widespread use of antifungal agents promoted the emergence of drug resistance, especially to azoles and echinocandins [17]. Fungal cells can circumvent the inhibitory activity of azoles by upregulating multidrug transporters and therefore increasing the drug efflux [18–21]. A second mechanism of acquired resistance is target modification or overexpression, which limits the deleterious effect of the drug. In Candida, amino acid substitutions in Erg11p have been reported in clinical isolates resistant to fluconazole [22,23]; this resistance phenotype might be explained by a lower affinity of the mutated proteins to azoles [24]. Additionally, an increased number of copies of chromosome 5 and gain-of-function mutations in the transcriptional regulator Upc2 lead to ERG11 overexpression, reducing fungal susceptibility to azoles [25–27]. A. fumigatus resistance to azoles has also been associated with overexpression of the genes encoding 14α-demethylase (cyp51A and cyp51B) or mutations in its sequence [28]. Similarly, mutations in the catalytic subunit of the glucan synthases, called Fks, confer echinocandin resistance in yeasts and molds [29,30]. The development of resistance, along with the therapeutic limitations of the current drugs (e.g. polyenes’ high toxicity, lack of echinocandins’ oral formulations), emphasize the urgent need for new classes of antifungals.
Sphingolipids (SL) are composed of a sphingoid base or long chain base (LCB) amide-linked to a fatty acid, forming the ceramide backbone, which is attached to a polar group via C1-OH (Figure 2A) [31]. SL synthesis starts in the endoplasmic reticulum and, once produced, ceramides are transported to the Golgi, where the synthesis of complex SL occur [32]. In the fungal membrane, SL form microdomains along with sterols named lipid rafts, being crucial to growth, cell polarity establishment, hyphal formation and ultimately, virulence ([33–36], reviewed in [37]). SL are also found in the cell wall or secreted to the extracellular environment in vesicles, which stimulate cytokine production by macrophages [38–41]. More than structural components of membranes, SL act as signaling molecules, regulating cellular processes such as apoptosis and senescence [42]. Fungal SL are not produced by higher eukaryotes or differ structurally from their mammalian counterparts, highlighting the potential use of SL as novel targets for selective antifungal drugs.
Figure 2.
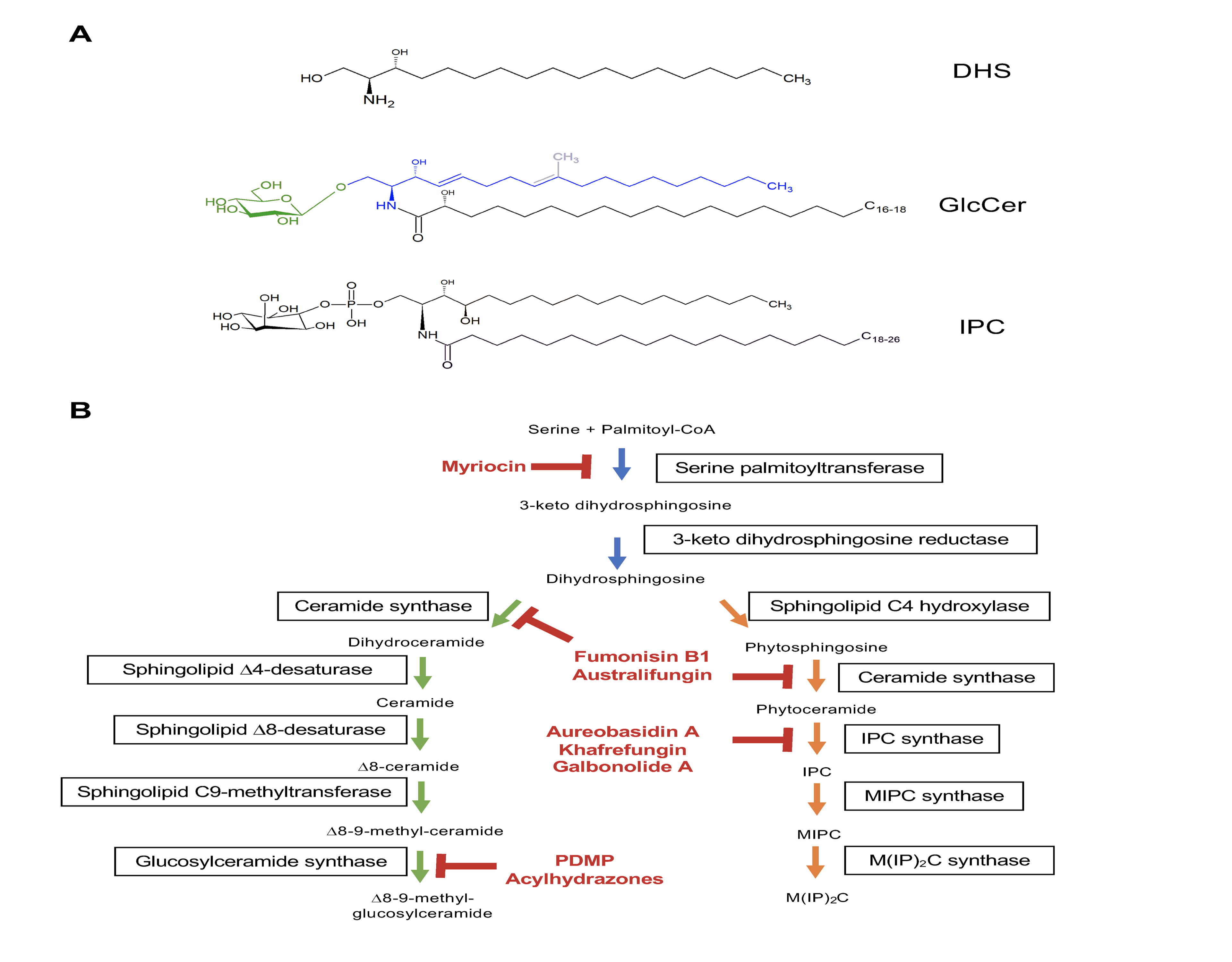
(A) Structure of dihydrosphingosine, glucosylceramide (GlcCer) and inositol phosphorylceramide (IPC). The sugar and the long-chain base in GlcCer are highlighted in green and blue, respectively. The C8-unsaturation and C9-methyl group, present in the fungal but not in the mammalian GlcCer, are shown in gray. (B) The sphingolipid synthesis pathway in fungi. Inhibitors of SL production are highlighted in red and include: myriocin (targets Spt); fumonisin B1 and australifungin (targets ceramide synthases); aureobasidin A, khafrefungin and galbonolide A (targets the Ipc synthase); PDMP analogs and acylhydrazones (targets GlcCer synthase).
The sphingolipid synthesis can be subdivided in three major branches, discussed in the distinct subsections of this review. The shared sphingolipid pathway (first subsection) includes the production of the precursor dihydrosphingosine (DHS), which is further acylated by ceramide synthases to generate dihydroceramide and phytoceramide (Figure 2B, blue arrows). The two pools of ceramides are used as building blocks for the synthesis of neutral (Figure 2B, green arrows) or acidic glycosphingolipids (Figure 2B, orange arrows), addressed in the second and third subsections of this review, respectively.
2. The shared sphingolipid synthesis
The first step of the sphingolipid synthesis consists of the condensation of serine and palmitoyl-CoA, catalyzed by serine palmitoyltransferase (SPT), generating 3-ketodihydrosphingosine. SPT enzyme is a heterodimer encoded by the essential LCB1 and LCB2 genes, conserved from yeast to mammals [43–45]. The Aspergillus homologue of Lcb1, lcbA, is essential for fungal viability, and the conditional repression of lcbA severely impaired polarized growth and germling formation [46]. As the establishment of cell polarity is a crucial hallmark of fungal pathogenesis, these results suggest that pharmacological targeting of SPT might compromise virulence. Myriocin, a metabolite isolated from the insect fungus Isaria sinclairii, irreversibly inhibits SPT [47,48]. In Aspergillus, myriocin prevented spore germination and induced abnormal branching in hyphae, suggesting that the establishment of the cell polarity axis depends on the SL production [46]. Further applications of myriocin in antifungal therapy were limited as this compound is not specific to fungal SPT, showing toxicity towards mammalian and insect cells. In fact, myriocin severely impaired de novo SL synthesis and growth in Chinese hamster ovary (CHO) cells [49]. Additionally, the administration of myriocin to mice reduced the SPT activity in liver cells in a dose-dependent manner [50]. Myriocin also exerts an immunosuppressant effect in the host. Larvae of Galleria mellonella pre-treated with myriocin and infected with C. albicans showed a survival rate significantly lower than those infected with fungi only [51]. Therefore, the use of myriocin derivatives as novel antifungals depends on the synthesis of selective compounds, which do not show host toxicity. The drug repurposing might represent a promising alternative to circumvent the myriocin toxicity in SPT inhibition. Cycloserine, used for the treatment of tuberculosis and neurological disorders, did not show antifungal activity alone but improved the fluconazole activity [52]. Interestingly, heterozygous strains of S. cerevisiae lacking one copy of LCB1 or LCB2 were hypersensitive to cycloserine, suggesting that the compound targets the fungal SPT [52].
Once produced, 3-ketodihydrosphingosine (3-ketosphinganine, 3KS) is converted to dihydrosphingosine (DHS or sphinganine) through 3-keto dihydrosphingosine reductase (Ksr) activity. Ksr1 and KsrA proteins, from C. albicans and A. fumigatus respectively, were shown to reduce 3KS to DHS in vitro [53]. Also, deletion of KSR1 impaired yeast-to-hypha transition, essential for Candida pathogenesis [53,54]. DHS structure is shared by fungi and higher eukaryotes [55]. In fact, FVT1, the mammalian homologue of KSR, can replace yeast KSR in DHS synthesis [56], suggesting a conserved role of the enzyme across species. Although FVT1 and fungal KSR share some sequence homology, these proteins are predicted to differ structurally [57]. Therefore, molecules targeting unique regions of fungal KSR might act as potent inhibitors of fungal differentiation.
The production of DHS represents a branching point in the sphingolipid synthesis [58]. DHS can be acylated by ceramide synthase, forming dihydroceramide, the precursor of the neutral glycosphingolipids (GSLs) as glucosylceramide (GlcCer). Alternatively, DHS can be hydroxylated, generating phytosphingosine (PHS), which will be used in the synthesis of acidic glycosphingolipids as inositolphosphoryl ceramides (IPCs) and mannosyl inositolphosphoryl ceramides (MIPCs). Distinct ceramide synthases use DHS or PHS as substrates; therefore, GlcCer and IPCs differ not only in their polar group (glucose or glycosylphosphoinositol), but also in the ceramide backbone structure (please compare GlcCer and IPC structures in Figure 2A) [55].
DHS conversion to dihydroceramide is catalyzed by Bar1p/BarA and its orthologs Lac1 in C. albicans and Cer1 in C. neoformans. The barA gene was first identified in a screen of Aspergillus mutants resistant to the antifungal compound HSAF [59]. Bar-like ceramide synthases show a preference for dihydroxi sphingoid bases and C16–18 fatty acids [60]. In addition, the disruption of barA or its homologs abolishes GlcCer synthesis, suggesting that dihydroxi ceramides bearing C16–18 fatty acids are the building blocks of GlcCer production [60–63]. Curiously, the role of this class of ceramide synthases diverge in fungal species. For instance, the C. albicans lac1Δ/lac1Δ mutant showed hyphal growth and lipid raft organization in hyphal tips similar to the wild type control [61]. In contrast, the disruption of Aspergillus barA impaired growth and raft formation [59]. Cryptococcus Cer1 was crucial for in vitro growth under acidic and neutral/alkaline conditions, mimicking the environments found in the alveolar macrophages and alveolar space respectively [63]. In agreement with these observations, lack of Cer1 rendered C. neoformans cells avirulent [63] . These results suggest that inhibitors of dihydroceramide synthesis might be more efficacious against Aspergillus and Cryptococcus infections rather than invasive candidiasis.
DHS can also be hydroxylated to phytosphingosine (PHS) by sphingolipid C4 hydroxylase prior to production of phytoceramide [58]. In Aspergillus, the gene encoding the C4 hydroxylase, basA, is essential for fungal viability [64]. This suggests that inhibitors of the C4 hydroxylase might compromise fungal virulence and pathogenesis.
The acylation of PHS with very long-chain fatty acids (VLCFA, C24 or C26) and consequent formation of phytoceramide is catalyzed by a clade of ceramide synthases that differ from those involved in dihydroceramide production [58,60]. LagA is an essential ceramide synthase in Aspergillus, producing the bulk of sphingolipids required for hyphal growth [59]. Similarly, the disruption of Candida LAG1 gene also caused severe defects in polarized growth [61].
Besides the bulk of ceramides produced by de novo pathway as described above, this molecule can also be generated through the salvage pathway. This metabolic route occurs in the acidic lysosomes, where complex SL are broken down to sphingosine [65]. Finally, the sphingosine is re-acylated by ceramide synthases, producing ceramide [65].
The inhibition of ceramide synthesis can also lead to increased levels of DHS and PHS. These sphingoid bases inhibit Aspergillus growth and induce death by apoptosis [66]. Therefore, drugs targeting the ceramide synthases might be potent antifungals, showing a dual mechanism of action: depletion of the sphingolipids content and accumulation of toxic sphingoid bases. So far, two inhibitors of fungal ceramide synthase have been reported. Fumonisin B1 is a mycotoxin produced by Fusarium that competes with the sphingoid base as an enzyme substrate and also affects the binding of the fatty acid chain [67,68]. Although fumonisin B1 inhibits the ceramide synthases, this compound apparently showed poor antifungal activity [68]. In contrast, australifungin, a compound isolated from Sporormiella australis, inhibits the ceramide production and show a broad-spectrum activity against Candida, Aspergillus and Cryptococcus [69]. The use of these mycotoxins in the antifungal therapy has been limited due to their high reactivity and lack of specificity towards the fungal ceramide synthase, also inhibiting the mammalian enzyme [68,69]. In fact, fumonisin B1 is toxic to animals and causes hepato and nephrotoxicity in rodents [70,71]. A fluorescent assay to detect ceramide synthase activity has been reported [72], enabling the screen of chemical libraries for more selective compounds, active against the fungal but not the mammalian enzyme.
3. The GlcCer synthesis
Dihydroceramide is the main substrate used by the sphingolipid Δ4-desaturase enzyme, which catalyzes a C4-reduction in the sphinganine backbone, producing ceramide [73,74]. This reaction occurs in both fungi and mammals [74], and the relevance of (E)-sphing-4-enine for fungal growth and virulence remains to be elucidated.
The unsaturation between C8–C9 and methylation in C9 of the LCB are catalyzed by sphingolipid Δ8-desaturase (Sld) and sphingolipid C9-methyltransferase (Smt) , respectively, generating Δ8–9,Me-Cer. These structural modifications are not observed in mammalian cells, which produce Cer. In Aspergillus, the deletion of sphingolipid Δ8-desaturase encoding gene compromised growth and the formation of sterol-rich rafts in the hyphal tip [75]. Candida cells lacking sphingolipid Δ8-desaturase also exhibited impaired hyphal growth, along with a striking reduction in fungal virulence [76,77]. Curiously, the C9-methylation of LCB had a modest relevance for Candida infection, but was crucial for Cryptococcus pathogenesis [77,78]. In fact, C. neoformans cells deficient in SMT1 gene were not able to replicate in the murine lung, being restricted in granulomas [78]. These observations suggest that potential inhibitors of the sphingolipid C9-methyltransferase might be more effective against Cryptococcus than Candida infections. The pharmacological inhibition of the sphingolipid Δ8-desaturase remains as a promising strategy for novel antifungals, especially for the treatment of invasive candidiasis. The role of the sphingolipid Δ8-desaturase in Cryptococcus and Aspergillus virulence needs to be further investigated, so the use of desaturase inhibitors as panfungal drugs can be considered.
The last step of the GlcCer synthesis is the transfer of a glucose residue from UDP-glucose to the ceramide backbone [79]. This reaction occurs in the Golgi through the activity of glucosylceramide synthase (GCS) [80], which plays a key role in fungal virulence. Indeed, C. neoformans cells lacking GCS1 are avirulent in a murine model of cryptococcosis [81]. As the deletion of GCS1 also impaired Cryptococcus ability to grow at neutral/alkaline pH, it was suggested that GlcCer production is crucial for the colonization of alveolar spaces and the host extracellular environment [81]. The GlcCer synthesis is also important for the growth and virulence of C. albicans and A. nidulans [75,77,82]. Due to the relevance of GlcCer to fungal pathogenesis, GlcCer synthase inhibitors and targeting of GlcCer itself have already been reported. Levery et al. (2002) showed that analogs of the D-threo-PDMP (D-threo-1-phenyl-2-decanoylamino-3-morpholino-1-propanol), known inhibitors of the mammalian GlcCer synthase, also prevented germination and hyphal growth in Aspergillus [83]. The development of new antifungals from D-threo-PDMP relies on the synthesis of fungal-specific compounds which ideally lack toxicity to the host.
In contrast to the PDMP derivatives, the acylhydrazones BHBM and D0 inhibited the GlcCer synthesis in fungi but not in mammalian cells [84]. BHBM and D0 also showed a broad spectrum of antifungal activity, efficiently improving the survival of mice infected with C. neoformans and C. albicans [84]. Derivatives of these acylhydrazones retained the fungicidal activity in vitro and in vivo, while showing suitable pharmacokinetic properties and even lower toxicity towards mammalian cells [85,86]. Therefore, these compounds constitute promising candidates for antifungal therapy.
The immunological targeting of GlcCer might represent an efficacious strategy to prevent mortality caused by fungal infections. In fact, the administration of an anti-GlcCer antibody prior to a lethal Cryptococcus infection improved mice survival by 60% [87]. Vaccination of mice with the GlcCer extracted from the non-pathogenic Candida utilis also protected partially against cryptococcosis, preventing fungal dissemination to the brain [88]. These results highlight the potential of GlcCer synthesis pathway for: i) the development of novel inhibitors that treat invasive infections and ii) the isolation of lipids that differ structurally from the mammalian counterparts and can be used as antigens in fungal vaccines, preventing mortality, especially in the individuals more susceptible to the invasive mycoses.
4. IPC synthesis
IPCs are produced by the transfer of a phosphoinositol from phosphatidylinositol to the C1-hydroxyl group of phytoceramide [89]. This reaction is catalyzed by the product of the IPC1 (or AUR1) gene, which is essential in fungal species [90,91]. The conditional repression of IPC1 in C. neoformans reduced growth under acidic conditions, such as the macrophage intracellular environment, impairing the virulence in rabbits [92]. Similarly, the genetic inactivation of Aspergillus aurA was accompanied by growth defects in spores and germlings, which were unable to establish a normal polarity axis [46]. Since the IPC synthase plays a pivotal role in fungal virulence and is not found in mammalian cells, this enzyme constitutes a potential target for drug development. In fact, the IPC synthase encoding gene was first identified in S. cerevisiae mutants resistant to the compound aureobasidin A [90]. Aureobasidin A, a cyclic peptide isolated from Aureobasidium pullulans, inhibits the IPC synthase at nanomolar concentrations and shows antifungal activity against Candida, Cryptococcus and Aspergillus [93–95]. In addition, aureobasidin A shows low toxicity in mice and prolonged survival in a murine model of candidiasis [93]. The efficacy of aureobasidin A in treating infections caused by Cryptococcus and filamentous/dimorphic fungi remains to be determined. Other potent drugs that inhibit IPC synthase at nanomolar levels include khafrefungin and galbonolide A (rustmicin) [96,97]. Khafrefungin showed fungicidal activity against C. albicans and C. neoformans, promoting the accumulation of ceramides [98]. This suggests that Ipc1 relevance is related to the regulation of phytoceramide levels, which control growth arrest and stress response [99,100], rather than to the IPC production itself. Indeed, the conversion of phytoceramide to IPC enable C. albicans to tolerate fluconazole concentrations above the MIC (minimal inhibitory concentration) [101]. Inhibition of IPC synthase and consequently accumulation of phytoceramides abolish the tolerance phenotype, rendering fluconazole a fungicidal effect [101]. Therefore, the tight regulation of phytoceramide levels seems to play a crucial role in fungal survival and growth, including the slow growth of azole-tolerant subpopulations. Galbonolide A was mainly active against C. neoformans in vitro and reduced the brain fungal burden in a murine model of cryptococcosis [102]. However, this drug showed poor stability in different pHs, quickly degrading into inactive derivatives [102]. SAR (Structure Activity Relationship) studies of galbonolide A derivatives might enable the development of stable compounds showing broad-spectrum activity.
To generate MIPC, the IPC is further mannosylated by MIPC synthase, which is encoded by the MIT1 and mitA genes in Candida and Aspergillus, respectively [103,104]. The deletion of C. albicans MIT1 did not impair growth or morphogenesis, but attenuated virulence in at least 50% [103]. In contrast, A. fumigatus cells lacking MitA were as virulent as the wild-type control [104]. So, inhibitors of the MIPC synthase might reduce the lethality of invasive candidiasis, albeit showing limited spectrum of activity.
The M(IP)2C synthase adds a second inositol phosphate to the MIPC, forming mannosyl diinositol diphosphoceramide, M(IP)2C. In C. albicans, the disruption of the IPT1 gene, encoding for the M(IP)2C synthase, led to the accumulation of MIPC and increased sensitivity to fluconazole and itraconazole [105]. The length of the fatty acid chain composing MIPC and M(IP)2C also affects azole susceptibility. Recently, it was shown that inactivation of FEN1 or FEN12 genes promote fluconazole resistance in C. albicans [106]. FEN1 and FEN12 encode fatty acid elongases, involved in the synthesis of VLCFAs which are transferred to phytosphingosine to form phytoceramide [106]. Indeed, the deletion of C. albicans FEN1 or FEN12 reduced the content of IPCs and MIPCs with 26-carbon fatty acids [106]. In the presence of fluconazole, Δfen1 and Δfen12 mutants produced higher levels of MIPC and DMCDD [14,24-dimethylcholesta-8,24(28)-dien-3β,6α-diol] than the wild-type strain [106]. DMCDD is the toxic sterol accumulated by Erg11 inhibition, which causes membrane permeabilization [107]. Together, these results suggest that the production of complex SL containing very long fatty acids mediates fungal resistance to fluconazole, possibly by reducing DMCDD insertion into the membrane and attenuating the azoles’ effect.
5. The biological level of sphingolipids within fungal cells
GlcCer is the most abundant sphingolipid in C. neoformans, corresponding to 16 – 45 % of total SL content [108]. In contrast, IPCs correspond to 2.4 – 4.4 % of SL production [108]. Similarly, sphingolipidomic analysis suggest that GlcCer is also more abundant than IPCs in A. fumigatus [109]. To the best of our knowledge, the relative levels of GlcCer are not determined in Candida yet. Interestingly, in Candida, the IPC levels were associated with several processes, such as biofilm formation and susceptibly to azoles [110,111]. Variations in the SL level are also observed across fungal species: while IPC levels are higher than MIPC and M(IP)2C in Candida [111], M(IP)2C corresponds to the most abundant SL in S. cerevisiae [112]. Finally, LCB species (DHS, sphingosine and PHS) were found in C. neoformans in very low levels, accounting for 1.2 – 2.6 % of total SLs [108]. Together, these results suggest that SL content is highly regulated, draining the toxic LCBs into complex sphingolipids. Therefore, drugs altering the SL homeostasis might act as potent antifungals.
5. Expert opinion
The development of new drugs targeting sphingolipid synthesis might represent a therapeutic alternative to the antifungals that are currently available, overcoming some of the limitations faced when using azoles, polyenes and echinocandins. The polyene main prototype, amphotericin B, binds to ergosterol [5], utilizing the structural differences found between the fungal and mammalian sterol. Still, amphotericin B shows toxicity towards host tissues, possibly due to unspecific binding to cholesterol [13]. Fungi produce two main classes of sphingolipids, neutral (GlcCer/GalCer) and acidic (IPC, MIPC, M(IP)2C). Like with ergosterol and cholesterol, fungal and mammalian GlcCer also show distinct architectures [79], and one might expect that inhibitors of the fungal GlcCer show some level of toxicity in higher eukaryotes. However, this does not seem to always be true as acylhydrazones were not toxic to mice [84]. Off-target effects caused by inhibiting the synthesis of IPC are also rare, as mammals lack the production of these sphingolipids.
Besides drug selectivity, another challenge in the development of new antifungals is to find molecules that show fungicidal rather than a fungistatic effect. Compounds that impair fungal growth, not leading to cell death, require the host immune system to clear the infection [113]. Many of the reactions involved in the SL synthesis are crucial for the fungal viability, suggesting that inhibitors might have fungicidal activity by inducing a lethal phenotype. Indeed, the inhibition of the IPC synthase by aureobasidin A killed C. albicans cells [93], likely by phytoceramide accumulation. Interestingly, drugs that target non-essential enzymes such as acylhydrazones can also exhibit fungicidal activity [86]. This might be explained by the fact that GlcCer regulates cell cycle progression in C. neoformans [81]. Therefore, the depletion of certain SL which regulate growth arrest, rather than the accumulation of toxic intermediates, might also cause a fungicidal phenotype.
Several questions remain to be elucidated by future studies. Current antifungals exhibit an underlying effect as immune modulators [114]. As GlcCer administration provoked a protective response for Cryptococcus infection in mice [88], it’d be valuable to determine if compounds targeting SL synthesis also induce a direct stimulation of immune cells, facilitate phagocyte activity or “unmask” potential antigens. In addition, GlcCer production has been reported in several species, including dimorphic and filamentous fungi (for review, [115]). If the GlcCer vaccination prevents disseminated infections caused by distinct species, it might constitute a valuable tool for prophylaxis.
Further advances in the use of SL inhibitors for antifungal therapy depend on some advances in this field of research. The structural determination of proteins involved in the SL synthesis pathway would bring new insights about their activity and regulation, enabling the design of novel inhibitors and high-throughput screenings. In addition, SAR studies of inhibitors that already have been reported would elucidate compounds’ groups that drive antifungal activity and toxicity towards mammalian cells. This would allow the development of potent myriocin, fumonisin B1 and aureobasidin A derivatives that do not show unspecific or off-target effects in the host (reviewed by [116]).
SL synthesis inhibitors that emerge as promising candidates for clinical trials include the acylhydrazones and aureobasidin A derivatives. In fact, aureobasidin A derivatives that show enhanced activity against yeasts and filamentous fungi have been developed [13]. Importantly, aureobasidin A constitutes a promising candidate for combination therapy with fluconazole, as improved its antifungal activity [101]. SAR studies also enabled the synthesis of second and third acylhydrazone derivatives, with increased potency and selectivity towards fungal cells [86]. Therefore, acylhydrazones and aureobasidin A derivatives arise as new, promising drugs to be used in antifungal therapy.
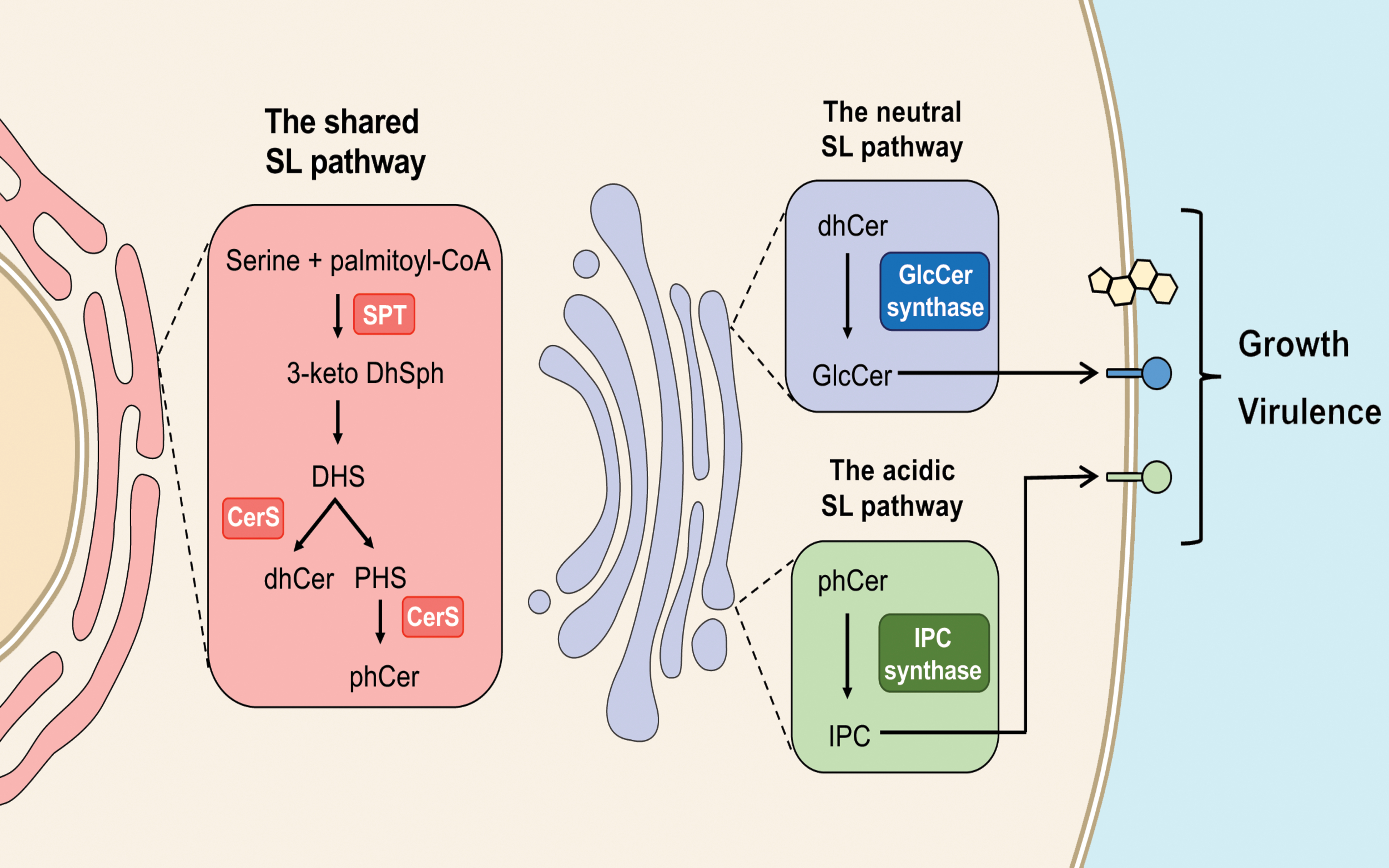
Figure 3.
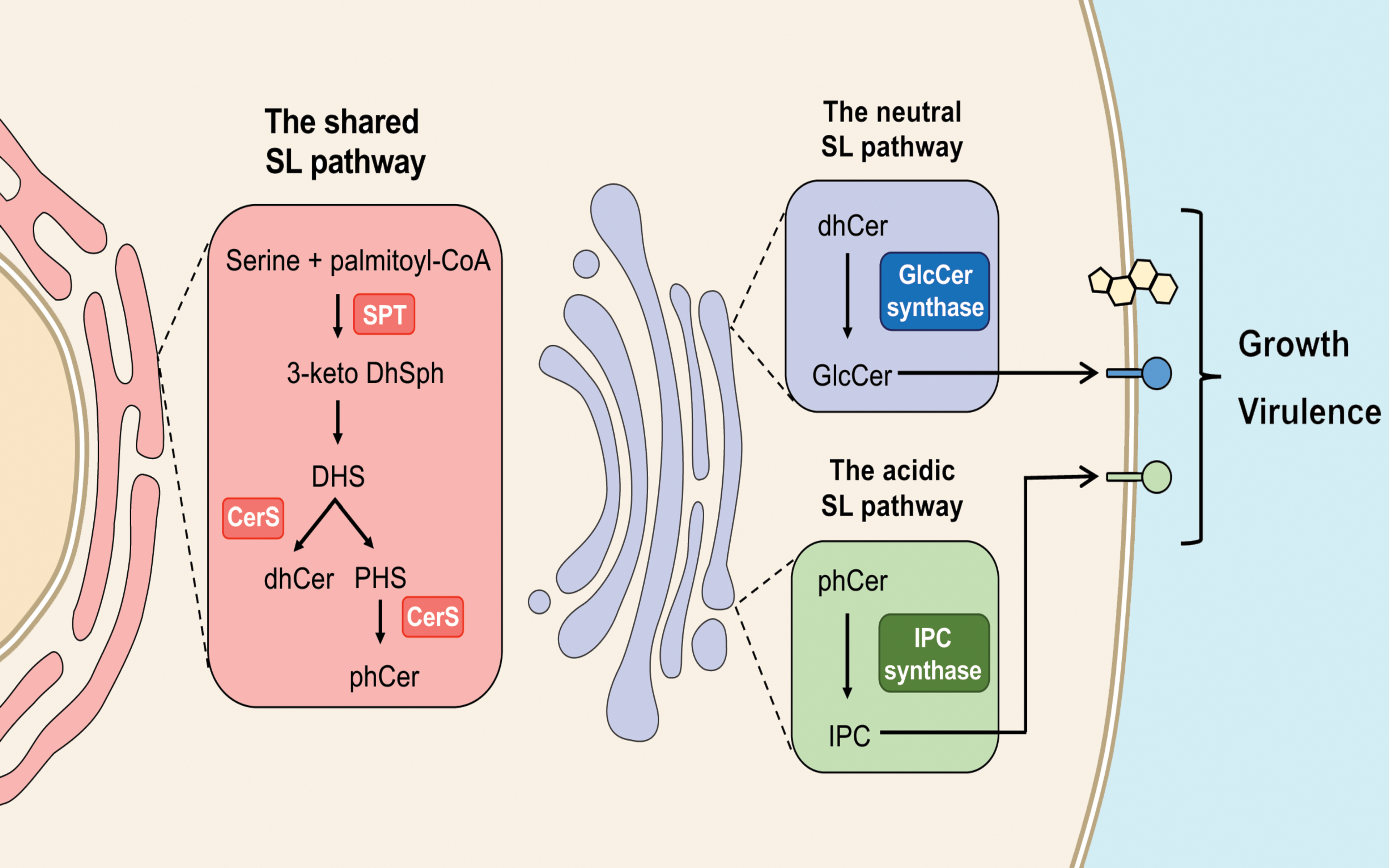
Sphingolipid synthesis starts in the endoplasmic reticulum, with the reaction catalyzed by Spt. Once produced by distinct ceramide synthases (CerS), dihydroceramide (dhCer) and phytoceramide (phCer) are transported to the Golgi for the synthesis of complex sphingolipids (GlcCer and IPC). The conversion of toxic phCer to IPC and the production of GlcCer regulate fungal growth and virulence.
Article highlights.
Fungal sphingolipids play a crucial role in growth and virulence.
The unique structure of fungal sphingolipids makes them promising targets for drug development.
Novel inhibitors of ceramide synthases might act as potent drugs, by depleting the pool of complex sphingolipids and leading to the accumulation of toxic intermediates.
Promising compounds for clinical trials include acylhydrazones and aureobasidin A, which inhibit the production of glucosylceramide and IPC, efficiently treating invasive fungal infections.
Funding
This paper was funded by supported by NIH grants AI116420, AI125770 and AI136934 to M. Del Poeta and by Merit Review grant I01BX002624 from the Veterans Affairs Program to M. Del Poeta
Footnotes
Declaration of interest
M. Del Poeta is a cofounder and Chief Scientific Officer (CSO) of MicroRid Technologies, Inc. The authors have no other relevant affiliations or financial involvement with any organization or entity with a financial interest in or financial conflict with the subject matter or materials discussed in the manuscript.
Reviewer disclosures
Peer reviewers on this manuscript have no relevant financial or other relationships to disclose.
References
Papers of special note have been highlighted as:
* of interest
** of considerable interest
- 1.Brown GD, Denning DW, Levitz SM. Tackling human fungal infections. American Association for the Advancement of Science; 2012. [DOI] [PubMed]
- 2.Brown GD, Denning DW, Gow NA, et al. Hidden killers: human fungal infections. Science translational medicine. 2012;4(165):165rv13–165rv13. [DOI] [PubMed] [Google Scholar]
- 3.Rodrigues ML. The multifunctional fungal ergosterol. MBio. 2018;9(5):e01755–18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Gallis HA, Drew RH, Pickard WW. Amphotericin B: 30 years of clinical experience. Reviews of infectious diseases. 1990;12(2):308–329. [DOI] [PubMed] [Google Scholar]
- 5.Gray KC, Palacios DS, Dailey I, et al. Amphotericin primarily kills yeast by simply binding ergosterol. Proceedings of the National Academy of Sciences. 2012;109(7):2234–2239. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Anderson TM, Clay MC, Cioffi AG, et al. Amphotericin forms an extramembranous and fungicidal sterol sponge. Nature chemical biology. 2014;10(5):400. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Allen D, Wilson D, Drew R, et al. Azole antifungals: 35 years of invasive fungal infection management. Expert review of anti-infective therapy. 2015;13(6):787–798. [DOI] [PubMed] [Google Scholar]
- 8.Kathiravan MK, Salake AB, Chothe AS, et al. The biology and chemistry of antifungal agents: a review. Bioorganic & medicinal chemistry. 2012;20(19):5678–5698. [DOI] [PubMed] [Google Scholar]
- 9.Veen M, Stahl U, Lang C. Combined overexpression of genes of the ergosterol biosynthetic pathway leads to accumulation of sterols in Saccharomyces cerevisiae. FEMS yeast research. 2003;4(1):87–95. [DOI] [PubMed] [Google Scholar]
- 10.Bhattacharya S, Esquivel BD, White TC. Overexpression or deletion of ergosterol biosynthesis genes alters doubling time, response to stress agents, and drug susceptibility in Saccharomyces cerevisiae. MBio. 2018;9(4):e01291–18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Sheehan DJ, Hitchcock CA, Sibley CM. Current and emerging azole antifungal agents. Clinical microbiology reviews. 1999;12(1):40–79. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Kelly S, Lamb D, Kelly D, et al. Resistance to fluconazole and cross‐resistance to amphotericin B in Candida albicans from AIDS patients caused by defective sterol Δ5, 6‐desaturation. FEBS letters. 1997;400(1):80–82. [DOI] [PubMed] [Google Scholar]
- 13.Perfect JR. The antifungal pipeline: a reality check. Nature reviews Drug discovery. 2017;16(9):603. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Gow NA, Latge J-P, Munro CA. The fungal cell wall: structure, biosynthesis, and function. The fungal kingdom. 2017:267–292. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Denning DW. Echinocandin antifungal drugs. The Lancet. 2003;362(9390):1142–1151. [DOI] [PubMed] [Google Scholar]
- 16.Chen SC-A, Slavin MA, Sorrell TC. Echinocandin antifungal drugs in fungal infections. Drugs. 2011;71(1):11–41. [DOI] [PubMed] [Google Scholar]
- 17.Cowen LE. The evolution of fungal drug resistance: modulating the trajectory from genotype to phenotype. Nature Reviews Microbiology. 2008;6(3):187–198. [DOI] [PubMed] [Google Scholar]
- 18.Sanglard D, Odds FC. Resistance of Candida species to antifungal agents: molecular mechanisms and clinical consequences. The Lancet infectious diseases. 2002;2(2):73–85. [DOI] [PubMed] [Google Scholar]
- 19.Coste A, Selmecki A, Forche A, et al. Genotypic evolution of azole resistance mechanisms in sequential Candida albicans isolates. Eukaryotic cell. 2007;6(10):1889–1904. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.da Silva Ferreira ME, Capellaro JL, dos Reis Marques E, et al. In vitro evolution of itraconazole resistance in Aspergillus fumigatus involves multiple mechanisms of resistance. Antimicrobial agents and chemotherapy. 2004;48(11):4405–4413. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Nascimento AM, Goldman GH, Park S, et al. Multiple resistance mechanisms among Aspergillus fumigatus mutants with high-level resistance to itraconazole. Antimicrobial agents and chemotherapy. 2003;47(5):1719–1726. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Flowers SA, Colón B, Whaley SG, et al. Contribution of clinically derived mutations in ERG11 to azole resistance in Candida albicans. Antimicrobial agents and chemotherapy. 2015;59(1):450–460. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Xiang M-J, Liu J-Y, Ni P-H, et al. Erg11 mutations associated with azole resistance in clinical isolates of Candida albicans. FEMS yeast research. 2013;13(4):386–393. [DOI] [PubMed] [Google Scholar]
- 24.Rodero L, Mellado E, Rodriguez AC, et al. G484S amino acid substitution in lanosterol 14-α demethylase (ERG11) is related to fluconazole resistance in a recurrent Cryptococcus neoformans clinical isolate. Antimicrobial agents and chemotherapy. 2003;47(11):3653–3656. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Flowers SA, Barker KS, Berkow EL, et al. Gain-of-function mutations in UPC2 are a frequent cause of ERG11 upregulation in azole-resistant clinical isolates of Candida albicans. Eukaryotic cell. 2012;11(10):1289–1299. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Selmecki A, Forche A, Berman J. Aneuploidy and isochromosome formation in drug-resistant Candida albicans. Science. 2006;313(5785):367–370. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Selmecki A, Gerami‐Nejad M, Paulson C, et al. An isochromosome confers drug resistance in vivo by amplification of two genes, ERG11 and TAC1. Molecular microbiology. 2008;68(3):624–641. [DOI] [PubMed] [Google Scholar]
- 28.Chowdhary A, Sharma C, Hagen F, et al. Exploring azole antifungal drug resistance in Aspergillus fumigatus with special reference to resistance mechanisms. Future microbiology. 2014;9(5):697–711. [DOI] [PubMed] [Google Scholar]
- 29.Park S, Kelly R, Kahn JN, et al. Specific substitutions in the echinocandin target Fks1p account for reduced susceptibility of rare laboratory and clinical Candida sp. isolates. Antimicrobial agents and chemotherapy. 2005;49(8):3264–3273. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Perlin DS. Resistance to echinocandin-class antifungal drugs. Drug Resistance Updates. 2007;10(3):121–130. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Dickson RC, Lester RL. Sphingolipid functions in Saccharomyces cerevisiae. Biochimica et Biophysica Acta (BBA)-Molecular and Cell Biology of Lipids. 2002;1583(1):13–25. [DOI] [PubMed] [Google Scholar]
- 32.Ren J, Hannun Y. Metabolism and roles of sphingolipids in yeast Saccharomyces cerevisiae. Springer International Publishing, Cham, Switzerland. 2017:1–21. [Google Scholar]
- 33.Martin SW, Konopka JB. Lipid raft polarization contributes to hyphal growth in Candida albicans. Eukaryotic cell. 2004;3(3):675–684. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Nichols CB, Fraser JA, Heitman J. PAK kinases Ste20 and Pak1 govern cell polarity at different stages of mating in Cryptococcus neoformans. Molecular biology of the cell. 2004;15(10):4476–4489. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Pearson CL, Xu K, Sharpless KE, et al. MesA, a novel fungal protein required for the stabilization of polarity axes in Aspergillus nidulans. Molecular biology of the cell. 2004;15(8):3658–3672. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Takeshita N, Higashitsuji Y, Konzack S, et al. Apical sterol-rich membranes are essential for localizing cell end markers that determine growth directionality in the filamentous fungus Aspergillus nidulans. Molecular biology of the cell. 2008;19(1):339–351. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Farnoud AM, Toledo AM, Konopka JB, et al. Raft-like membrane domains in pathogenic microorganisms Current topics in membranes. Vol. 75: Elsevier; 2015. p. 233–268. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Rodrigues ML, Travassos LR, Miranda KR, et al. Human antibodies against a purified glucosylceramide from Cryptococcus neoformans inhibit cell budding and fungal growth. Infection and immunity. 2000;68(12):7049–7060. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Rodrigues ML, Nimrichter L, Oliveira DL, et al. Vesicular polysaccharide export in Cryptococcus neoformans is a eukaryotic solution to the problem of fungal trans-cell wall transport. Eukaryotic cell. 2007;6(1):48–59. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Oliveira DL, Freire-de-Lima CG, Nosanchuk JD, et al. Extracellular vesicles from Cryptococcus neoformans modulate macrophage functions. Infection and immunity. 2010;78(4):1601–1609. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Vargas G, Rocha JD, Oliveira DL, et al. Compositional and immunobiological analyses of extracellular vesicles released by C andida albicans. Cellular microbiology. 2015;17(3):389–407. [DOI] [PubMed] [Google Scholar]
- 42.Singh A, Del Poeta M. Lipid signalling in pathogenic fungi. Cellular microbiology. 2011;13(2):177–185. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Dickson RC. Thematic review series: sphingolipids. New insights into sphingolipid metabolism and function in budding yeast. Journal of lipid research. 2008;49(5):909–921. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Nagiec MM, Baltisberger JA, Wells GB, et al. The LCB2 gene of Saccharomyces and the related LCB1 gene encode subunits of serine palmitoyltransferase, the initial enzyme in sphingolipid synthesis. Proceedings of the National Academy of Sciences. 1994;91(17):7899–7902. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Buede R, Rinker-Schaffer C, Pinto W, et al. Cloning and characterization of LCB1, a Saccharomyces gene required for biosynthesis of the long-chain base component of sphingolipids. Journal of bacteriology. 1991;173(14):4325–4332. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Cheng J, Park T-S, Fischl AS, et al. Cell cycle progression and cell polarity require sphingolipid biosynthesis in Aspergillus nidulans. Molecular and Cellular Biology. 2001;21(18):6198–6209. [DOI] [PMC free article] [PubMed] [Google Scholar]; *shows the relevance of SL synthesis for Aspergillus growth.
- 47.Miyake Y, Kozutsumi Y, Nakamura S, et al. Serine palmitoyltransferase is the primary target of a sphingosine-like immunosuppressant, ISP-1/myriocin. Biochemical and biophysical research communications. 1995;211(2):396–403. [DOI] [PubMed] [Google Scholar]
- 48.Wadsworth JM, Clarke DJ, McMahon SA, et al. The chemical basis of serine palmitoyltransferase inhibition by myriocin. Journal of the American Chemical Society. 2013;135(38):14276–14285. [DOI] [PubMed] [Google Scholar]
- 49.Hanada K, Nishijima M, Fujita T, et al. Specificity of inhibitors of serine palmitoyltransferase (SPT), a key enzyme in sphingolipid biosynthesis, in intact cells: a novel evaluation system using an SPT-defective mammalian cell mutant. Biochemical pharmacology. 2000;59(10):1211–1216. [DOI] [PubMed] [Google Scholar]
- 50.He Q, Johnson VJ, Osuchowski MF, et al. Inhibition of serine palmitoyltransferase by myriocin, a natural mycotoxin, causes induction of c-myc in mouse liver. Mycopathologia. 2004;157(3):339–347. [DOI] [PubMed] [Google Scholar]
- 51.de Melo NR, Abdrahman A, Greig C, et al. Myriocin significantly increases the mortality of a non-mammalian model host during Candida pathogenesis. PLoS One. 2013;8(11). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Spitzer M, Griffiths E, Blakely KM, et al. Cross‐species discovery of syncretic drug combinations that potentiate the antifungal fluconazole. Molecular systems biology. 2011;7(1). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Fornarotto M, Xiao L, Hou Y, et al. Sphingolipid biosynthesis in pathogenic fungi: Identification and characterization of the 3-ketosphinganine reductase activity of Candida albicans and Aspergillus fumigatus. Biochimica et Biophysica Acta (BBA)-Molecular and Cell Biology of Lipids. 2006;1761(1):52–63. [DOI] [PubMed] [Google Scholar]
- 54.Gow NA, Brown AJ, Odds FC. Fungal morphogenesis and host invasion. Current opinion in microbiology. 2002;5(4):366–371. [DOI] [PubMed] [Google Scholar]
- 55.Warnecke D, Heinz E. Recently discovered functions of glucosylceramides in plants and fungi. Cellular and Molecular Life Sciences CMLS. 2003;60(5):919–941. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Kihara A, Igarashi Y. FVT-1 is a mammalian 3-ketodihydrosphingosine reductase with an active site that faces the cytosolic side of the endoplasmic reticulum membrane. Journal of Biological Chemistry. 2004;279(47):49243–49250. [DOI] [PubMed] [Google Scholar]
- 57.Gupta SD, Gable K, Han G, et al. Tsc10p and FVT1: topologically distinct short-chain reductases required for long-chain base synthesis in yeast and mammals. Journal of lipid research. 2009;50(8):1630–1640. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Barreto-Bergter E, Pinto MR, Rodrigues ML. Structure and biological functions of fungal cerebrosides. Anais da Academia Brasileira de Ciências. 2004;76(1):67–84. [DOI] [PubMed] [Google Scholar]
- 59.Li S, Du L, Yuen G, et al. Distinct ceramide synthases regulate polarized growth in the filamentous fungus Aspergillus nidulans. Molecular biology of the cell. 2006;17(3):1218–1227. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Ternes P, Wobbe T, Schwarz M, et al. Two pathways of sphingolipid biosynthesis are separated in the yeast Pichia pastoris. Journal of Biological Chemistry. 2011;286(13):11401–11414. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Cheon SA, Bal J, Song Y, et al. Distinct roles of two ceramide synthases, CaLag1p and CaLac1p, in the morphogenesis of Candida albicans. Molecular microbiology. 2012;83(4):728–745. [DOI] [PubMed] [Google Scholar]
- 62.Rittenour WR, Chen M, Cahoon EB, et al. Control of glucosylceramide production and morphogenesis by the Bar1 ceramide synthase in Fusarium graminearum. PLoS One. 2011;6(4). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Munshi MA, Gardin JM, Singh A, et al. The role of ceramide synthases in the pathogenicity of Cryptococcus neoformans. Cell reports. 2018;22(6):1392–1400. [DOI] [PMC free article] [PubMed] [Google Scholar]; **shows the relevance of ceramide synthases for Cryptococcus virulence.
- 64.Li S, Bao D, Yuen G, et al. basA regulates cell wall organization and asexual/sexual sporulation ratio in Aspergillus nidulans. Genetics. 2007;176(1):243–253. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Gault CR, Obeid LM, Hannun YA. An overview of sphingolipid metabolism: from synthesis to breakdown. Sphingolipids as signaling and regulatory molecules: Springer; 2010. p. 1–23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Cheng J, Park T-S, Chio L-C, et al. Induction of apoptosis by sphingoid long-chain bases in Aspergillus nidulans. Molecular and cellular biology. 2003;23(1):163–177. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Humpf H-U, Schmelz E-M, Meredith FI, et al. Acylation of naturally occurring and synthetic 1-deoxysphinganines by ceramide synthase formation of N-palmitoyl-aminopentol produces a toxic metabolite of hydrolyzed fumonisin, AP1, and a new category of ceramide synthase inhibitor. Journal of Biological Chemistry. 1998;273(30):19060–19064. [DOI] [PubMed] [Google Scholar]
- 68.Delgado A, Casas J, Llebaria A, et al. Inhibitors of sphingolipid metabolism enzymes. Biochimica et Biophysica Acta (BBA)-Biomembranes. 2006;1758(12):1957–1977. [DOI] [PubMed] [Google Scholar]
- 69.Mandala SM, Thornton RA, Frommer BR, et al. The discovery of australifungin, a novel inhibitor of sphinganine N-acyltransferase from Sporormiella australis. The Journal of antibiotics. 1995;48(5):349–356. [DOI] [PubMed] [Google Scholar]
- 70.Harrison LR, Colvin BM, Greene JT, et al. Pulmonary edema and hydrothorax in swine produced by fumonisin B1, a toxic metabolite of Fusarium moniliforme. Journal of Veterinary Diagnostic Investigation. 1990;2(3):217–221. [DOI] [PubMed] [Google Scholar]
- 71.Voss KA, Riley RT, Norred W, et al. An overview of rodent toxicities: liver and kidney effects of fumonisins and Fusarium moniliforme. Environmental Health Perspectives. 2001;109(suppl 2):259–266. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Kim HJ, Qiao Q, Toop HD, et al. A fluorescent assay for ceramide synthase activity. Journal of lipid research. 2012;53(8):1701–1707. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Michel C, van Echten-Deckert G, Rother J, et al. Characterization of ceramide synthesis a dihydroceramide desaturase introduces the 4, 5-trans-double bond of sphingosine at the level of dihydroceramide. Journal of Biological Chemistry. 1997;272(36):22432–22437. [DOI] [PubMed] [Google Scholar]
- 74.Ternes P, Franke S, Zähringer U, et al. Identification and characterization of a sphingolipid Δ4-desaturase family. Journal of Biological Chemistry. 2002;277(28):25512–25518. [DOI] [PubMed] [Google Scholar]
- 75.Fernandes C, De Castro P, Singh A, et al. Functional characterization of the A spergillus nidulans glucosylceramide pathway reveals that LCB Δ8‐desaturation and C9‐methylation are relevant to filamentous growth, lipid raft localization and Psd1 defensin activity. Molecular microbiology. 2016;102(3):488–505. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Oura T, Kajiwara S. Disruption of the sphingolipid Δ8-desaturase gene causes a delay in morphological changes in Candida albicans. Microbiology. 2008;154(12):3795–3803. [DOI] [PubMed] [Google Scholar]
- 77.Noble SM, French S, Kohn LA, et al. Systematic screens of a Candida albicans homozygous deletion library decouple morphogenetic switching and pathogenicity. Nature genetics. 2010;42(7):590. [DOI] [PMC free article] [PubMed] [Google Scholar]; **shows the relevance of GlcCer synthesis for Candida virulence.
- 78.Singh A, Wang H, Silva LC, et al. Methylation of glycosylated sphingolipid modulates membrane lipid topography and pathogenicity of Cryptococcus neoformans. Cellular microbiology. 2012;14(4):500–516. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Del Poeta M, Nimrichter L, Rodrigues ML, et al. Synthesis and biological properties of fungal glucosylceramide. PLoS pathogens. 2014;10(1). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Halter D, Neumann S, van Dijk SM, et al. Pre-and post-Golgi translocation of glucosylceramide in glycosphingolipid synthesis. The Journal of cell biology. 2007;179(1):101–115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Rittershaus PC, Kechichian TB, Allegood JC, et al. Glucosylceramide synthase is an essential regulator of pathogenicity of Cryptococcus neoformans. The Journal of clinical investigation. 2006;116(6):1651–1659. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Oura T, Kajiwara S. Candida albicans sphingolipid C9-methyltransferase is involved in hyphal elongation. Microbiology. 2010;156(4):1234–1243. [DOI] [PubMed] [Google Scholar]
- 83.Levery SB, Momany M, Lindsey R, et al. Disruption of the glucosylceramide biosynthetic pathway in Aspergillus nidulans and Aspergillus fumigatus by inhibitors of UDP‐Glc: ceramide glucosyltransferase strongly affects spore germination, cell cycle, and hyphal growth. FEBS letters. 2002;525(1–3):59–64. [DOI] [PubMed] [Google Scholar]
- 84.Mor V, Rella A, Farnoud AM, et al. Identification of a new class of antifungals targeting the synthesis of fungal sphingolipids. MBio. 2015;6(3):e00647–15. [DOI] [PMC free article] [PubMed] [Google Scholar]; **describes the efficacy of acylhydrazones in treating fungal infections.
- 85.Lazzarini C, Haranahalli K, Rieger R, et al. Acylhydrazones as antifungal agents targeting the synthesis of fungal sphingolipids. Antimicrobial agents and chemotherapy. 2018;62(5):e00156–18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Haranahalli K, Lazzarini C, Sun Y, et al. SAR Studies on Aromatic Acylhydrazone-Based Inhibitors of Fungal Sphingolipid Synthesis as Next-Generation Antifungal Agents. Journal of medicinal chemistry. 2019;62(17):8249–8273. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Rodrigues ML, Shi L, Barreto-Bergter E, et al. Monoclonal antibody to fungal glucosylceramide protects mice against lethal Cryptococcus neoformans infection. Clin Vaccine Immunol. 2007;14(10):1372–1376. [DOI] [PMC free article] [PubMed] [Google Scholar]; *shows the potential of GlcCer vaccination.
- 88.Mor V, Farnoud AM, Singh A, et al. Glucosylceramide administration as a vaccination strategy in mouse models of cryptococcosis. PloS one. 2016;11(4). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Heidler SA, Radding JA. Inositol phosphoryl transferases from human pathogenic fungi. Biochimica et Biophysica Acta (BBA)-Molecular Basis of Disease. 2000;1500(1):147–152. [DOI] [PubMed] [Google Scholar]
- 90.Heidler SA, Radding JA. The AUR1 gene in Saccharomyces cerevisiae encodes dominant resistance to the antifungal agent aureobasidin A (LY295337). Antimicrobial agents and chemotherapy. 1995;39(12):2765–2769. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Hashida-Okado T, Ogawa A, Endo M, et al. AUR1, a novel gene conferring aureobasidin resistance onSaccharomyces cerevisiae: a study of defective morphologies in Aur1p-depleted cells. Molecular and General Genetics MGG. 1996;251(2):236–244. [DOI] [PubMed] [Google Scholar]
- 92.Luberto C, Toffaletti DL, Wills EA, et al. Roles for inositol-phosphoryl ceramide synthase 1 (IPC1) in pathogenesis of C. neoformans. Genes & development. 2001;15(2):201–212. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.TAKESAKO K, KURODA H, INOUE T, et al. Biological properties of aureobasidin A, a cyclic depsipeptide antifungal antibiotic. The Journal of antibiotics. 1993;46(9):1414–1420. [DOI] [PubMed] [Google Scholar]; **describes the antifungal activity of aureobasidin A.
- 94.Nagiec MM, Nagiec EE, Baltisberger JA, et al. Sphingolipid synthesis as a target for antifungal drugs complementation of the inositol phosphorylceramide synthase defect in a mutant strain of Saccharomyces cerevisiae by the AUR1 gene. Journal of Biological Chemistry. 1997;272(15):9809–9817. [DOI] [PubMed] [Google Scholar]
- 95.Georgopapadakou NH. Antifungals targeted to sphingolipid synthesis: focus on inositol phosphorylceramide synthase. Expert opinion on investigational drugs. 2000;9(8):1787–1796. [DOI] [PubMed] [Google Scholar]
- 96.Sugimoto Y, Sakoh H, Yamada K. IPC synthase as a useful target for antifungal drugs. Current Drug Targets-Infectious Disorders. 2004;4(4):311–322. [DOI] [PubMed] [Google Scholar]
- 97.Harris GH, Shafiee A, Cabello MA, et al. Inhibition of fungal sphingolipid biosynthesis by rustmicin, galbonolide B and their new 21-hydroxy analogs. The Journal of antibiotics. 1998;51(9):837–844. [DOI] [PubMed] [Google Scholar]
- 98.Mandala SM, Thornton RA, Rosenbach M, et al. Khafrefungin, a novel inhibitor of sphingolipid synthesis. Journal of Biological Chemistry. 1997;272(51):32709–32714. [DOI] [PubMed] [Google Scholar]
- 99.Jenkins GM, Richards A, Wahl T, et al. Involvement of yeast sphingolipids in the heat stress response of Saccharomyces cerevisiae. Journal of Biological Chemistry. 1997;272(51):32566–32572. [DOI] [PubMed] [Google Scholar]
- 100.Chung N, Jenkins G, Hannun YA, et al. Sphingolipids signal heat stress-induced ubiquitin-dependent proteolysis. Journal of Biological Chemistry. 2000;275(23):17229–17232. [DOI] [PubMed] [Google Scholar]
- 101.Rosenberg A, Ene IV, Bibi M, et al. Antifungal tolerance is a subpopulation effect distinct from resistance and is associated with persistent candidemia. Nature communications. 2018;9(1):1–14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Mandala SM, Thornton RA, Milligan J, et al. Rustmicin, a potent antifungal agent, inhibits sphingolipid synthesis at inositol phosphoceramide synthase. Journal of Biological Chemistry. 1998;273(24):14942–14949. [DOI] [PubMed] [Google Scholar]
- 103.Mille C, Janbon G, Delplace F, et al. Inactivation of CaMIT1 inhibits Candida albicans phospholipomannan β-mannosylation, reduces virulence, and alters cell wall protein β-mannosylation. Journal of Biological Chemistry. 2004;279(46):47952–47960. [DOI] [PubMed] [Google Scholar]
- 104.Kotz A, Wagener J, Engel J, et al. The mitA gene of Aspergillus fumigatus is required for mannosylation of inositol-phosphorylceramide, but is dispensable for pathogenicity. Fungal Genetics and Biology. 2010;47(2):169–178. [DOI] [PubMed] [Google Scholar]
- 105.Prasad T, Saini P, Gaur NA, et al. Functional analysis of CaIPT1, a sphingolipid biosynthetic gene involved in multidrug resistance and morphogenesis of Candida albicans. Antimicrobial agents and chemotherapy. 2005;49(8):3442–3452. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.Gao J, Wang H, Li Z, et al. Candida albicans gains azole resistance by altering sphingolipid composition. Nature communications. 2018;9(1):1–14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.Bossche HV, Koymans L, Moereels H. P450 inhibitors of use in medical treatment: focus on mechanisms of action. Pharmacology & therapeutics. 1995;67(1):79–100. [DOI] [PubMed] [Google Scholar]
- 108.Singh A, MacKenzie A, Girnun G, et al. Analysis of sphingolipids, sterols, and phospholipids in human pathogenic Cryptococcus strains. Journal of lipid research. 2017;58(10). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109.Fabri JHTM, Godoy NL, Rocha MC, et al. The AGC kinase YpkA regulates sphingolipids biosynthesis and physically interacts with SakA MAP kinase in Aspergillus fumigatus. Frontiers in microbiology. 2019;9:3347. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110.Lattif AA, Mukherjee PK, Chandra J, et al. Lipidomics of Candida albicans biofilms reveals phase-dependent production of phospholipid molecular classes and role for lipid rafts in biofilm formation. Microbiology. 2011;157(Pt 11):3232. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111.Prasad R, Singh A. Lipids of Candida albicans and their role in multidrug resistance. Current genetics. 2013;59(4):243–250. [DOI] [PubMed] [Google Scholar]
- 112.Ejsing CS, Sampaio JL, Surendranath V, et al. Global analysis of the yeast lipidome by quantitative shotgun mass spectrometry. Proceedings of the National Academy of Sciences. 2009;106(7):2136–2141. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113.Cowen LE, Sanglard D, Howard SJ, et al. Mechanisms of antifungal drug resistance. Cold Spring Harbor perspectives in medicine. 2015;5(7):a019752. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114.Ami RB, Lewis R, Kontoyiannis DP. Immunopharmacology of modern antifungals. Clinical Infectious Diseases. 2008;47(2):226–235. [DOI] [PubMed] [Google Scholar]
- 115.Fernandes CM, Goldman GH, Del Poeta M. Biological roles played by sphingolipids in dimorphic and filamentous fungi. MBio. 2018;9(3):e00642–18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 116.Rollin-Pinheiro R, Singh A, Barreto-Bergter E, et al. Sphingolipids as targets for treatment of fungal infections. Future medicinal chemistry. 2016;8(12):1469–1484. [DOI] [PMC free article] [PubMed] [Google Scholar]


