Abstract
CD4 T cells play a major role to orchestrate the immune response. Upon activation, CD4 T cells differentiate into effector T cell (Teff) or regulatory T cell (Treg) subsets that promote or suppress the immune response, respectively. Along with these unique immunological roles, CD4 T cell subsets have specific metabolic requirements and programs that can influence the immune response. We therefore examined the metabolite levels of Teff and Treg in detail. Surprisingly, the metabolite showing the largest difference between Teff and Treg was serotonin (5-HT), revealing a potentially distinct role for serotonin in CD4 T cell function. 5-HT is well known as a neurotransmitter and recently has been recognized to play a role in the immune response; however, little is known about the immune cell type-specific expression of the serotonergic machinery and receptors. We therefore examined the serotonergic-related machinery in Teff and Treg and found differential expression of the serotonin transporter SERT and 5-HT1a and 5-HT2 receptors. We also found that Treg express tryptophan hydroxylase, which converts tryptophan to serotonin, suggesting for the first time that Treg synthesize serotonin. Our results in this study expand the potential immunomodulatory role of serotonin in CD4 T cell biology and could ultimately aid the development of novel immunomodulatory targets for treatment of autoimmune and neuropsychiatric disorders.
Keywords: Serotonin, 5-HT, lymphocytes, T cells, regulatory T cells, Treg
1. Introduction
Serotonin (5-hydroxytryptamine, 5-HT) is a neurotransmitter commonly known for its role in brain function and mood; however, brain-derived or central serotonin only accounts for 5% of the body’s total serotonin (1–3). A role for serotonin in immunity is becoming increasingly recognized, as the use of serotonin modulating drugs such as serotonin reuptake inhibitors (SSRIs) and monoamine oxidase inhibitors have been shown to be immunomodulatory and influence autoimmune and inflammatory processes (4–8). SSRIs bind to the serotonin transporter SERT (also called 5-HTT), an integral membrane protein that mediates the transport of serotonin and other ions into the cell (9, 10). SSRIs have even been described as a novel class of immunosuppressants, as they have been shown in animal models to suppress immune reactions involved in autoimmune disease and in vitro to suppress lymphocyte proliferation, cytokine secretion, and viability (7, 8, 11). Serotonin itself can be transported into the cell via SERT and stored in vesicles mediated by the vesicular monoamine transporter (VMAT), a process which has been largely characterized in neurons but is thought to occur in immune cells as well (12–14). Extracellularly, serotonin also binds to a large family of 5-HT receptors, the downstream effects of which are cell-type specific and not well understood for immune cells (6, 15, 16).
Serotonin is derived from the amino acid tryptophan. Tryptophan is involved in protein synthesis and the production of cellular metabolites, including kynurenine and 5-HT (17). The enzyme indolamine-2,3-dioxygenase (IDO) converts tryptophan to kynurenine in most tissues. The IDO/kynurenine pathway is an important metabolic pathway that generates nicotinamide adenine dinucleotide (NAD), an essential metabolic cofactor. In addition, the IDO pathway has been implicated in immunity, as IDO1 expression can be induced by inflammatory cytokines and has been found to play a role in immune tolerance and the maternal-fetal interface (18–20). Kynurenine and the other metabolites along the IDO pathway have also been shown to play an immunoregulatory role through binding to the aryl hydrocarbon receptor (Ahr) in various immune cell types (21, 22). Tryptophan conversion to 5-HT is mainly controlled by the enzyme tryptophan hydroxylase (TPH) (23). Because both tryptophan degradation pathways have been implicated to influence the immune response, a greater understanding of the role of tryptophan metabolism and the metabolites kynurenine and 5-HT in immunity is critical.
As CD4 T cells are the major orchestrators of the adaptive immune system, the regulation of the T cell response by 5-HT is of great interest. Naïve CD4 T cells are activated by cytokines in the environment and differentiate into T effector cells (Teff), including pro-inflammatory T helper 1 (Th1) and T helper 17 (Th17) cells which direct and coordinate the immune response (24). Another subset of T cells, regulatory T cells (Treg), are responsible for suppressing and moderating the inflammatory response (25, 26). An appropriate balance between Teff and Treg is important, as imbalance can lead to either excessive inflammation or immune suppression. While previous studies have examined the presence of serotoninergic machinery in T cells, these studies have been performed in T cell lines or total T cells (27–29). As Teff and Treg play distinct roles in the immune response, an understanding of the expression of serotonin-processing cellular machinery and levels of metabolites in different CD4 T cell populations (naïve, Teff, Treg) will provide a greater understanding of the role of serotonin in T cell biology. Here, we examine the expression of serotoninergic machinery, including serotonin transport, receptors and intracellular storage in the CD4 T cell subsets.
2. Materials and Methods
2.1. Mice
Six to eight week old C57BL/6J mice from Jackson Laboratory were used for all experiments. All procedures were performed under Duke University Medical Center IACUC-approved protocols.
2.2. T cell isolation and differentiation
Naïve CD4+CD25− T cells were isolated ex vivo from the spleens of the mice described above by magnetic microbead negative selection with >98% purity (Miltenyi #130-104-454). The isolated CD4+CD25− T cells were then cultured on irradiated splenic feeder cells (300 Gy) at a ratio of 1:5 (200,000 T cells:1 million irradiated splenocytes in a 24-well plate) in RPMI supplemented with 10% FBS, sodium pyruvate, penicillin/streptomycin, HEPES and beta-mercaptoethanol and 2.5μg/mL of soluble anti-CD3 antibody (eBioscience). The naïve T cells were then differentiated in vitro by adding the following cytokines to each generate each subset: Th1, 10ng/mL IL-12 (R&D Systems), 10μg/mL anti-IL-4 (eBioscience, clone 11B11), 1μg/mL anti-IFNγ (eBioscience); Th2, 1000U/mL recombinant IL-4 (R&D Systems), 10μg/mL anti-IL-12 (eBioscience), 10μg/mL anti-IFNγ; Th17, 20ng/mL IL-6 (R&D Systems), 2.5ng/mL TGFβ (R&D Systems), 10μg/mL anti-IFNγ; Treg, 3ng/mL TGFβ. On day 3 post stimulation, cells were split 1:2 and expanded with IL-2 for an additional 2 days (30, 31). Cells were then harvested, washed 1x with PBS, pelleted and snap frozen.
2.3. Semi-quantitative real time PCR
RNA was extracted from cell pellets with the RNeasy RNA purification minikit (Qiagen). Reverse-transcription PCR was performed with iScript cDNA synthesis kit (Biorad). Real-time PCR was performed with iQSYBR Green detection chemistry (Biorad) using an iCycler (BioRad). Relative mRNA levels were normalized to actin RNA content. A CT value < 36 was reported as positive for RNA expression. Primer sequences are as follows:
| Name | Sequence | Use |
|---|---|---|
| TPH1FW | GAA CAA AGA CCA TTC CTC CGA AAG AG | qPCR |
| TPH1 REV | GTG AGC TGA TCG GGC GAG TCC A | qPCR |
| HTR2A FW | CAG CCC TCC CTC CTC GTT TTG | qPCR |
| HTR2A REV | GCC GGAAGT TGT AGC AGA TGAAGT | qPCR |
| HTR1A FW | ACC CCA ACG AGT GCA CCA TCA G | qPCR |
| HTR1A REV | GCA GGC GGG GAC ATA GGA G | qPCR |
| HTR1B FW | CGA TGC GGT GGA GTA TTC TGC | qPCR |
| HTR1B REV | TAG CGG CCA TGA GTT TCT TCT TTT | qPCR |
| Slc6a4 FW | ACA ACA TCA CCT GGA CAC TCC ATT C | qPCR |
| Slc6a4 REV | CCG CAT ATG TGA TGA AAA GGA GGC T | qPCR |
| IDO1 FW | AAGGGCTTCTTCCTCGTCTC | qPCR |
| IDO1 REV | AAAAACGTGTCTGGGTCCAC | qPCR |
| VMAT2 FW | TGTGAAGTCTGGTGTGTTTAAGG | qPCR |
| VMAT2 REV | CATCACATCACAAGGCATCC | qPCR |
2.4. Metabolomics
Nontargeted metabolomic analyses were performed as described previously (32). Briefly, CD4 T cells were isolated and differentiated as described above and then subjected to both GC/MS and LC/MS/MS platforms (by Metabolon Inc.) for determination of cellular metabolites. Data were then grouped by unsupervised clustering using MetaboAnalyst software. The samples were normalized using Bradford protein concentration and rescaled to set the median to 1.
2.5. Statistical analysis
Statistical analyses were primarily performed with Prism software (GraphPad) using 2-tailed Student’s t-tests. Statistically significant results are indicated (* = p < 0.05). For the metabolomics data, pair-wise comparisons were performed with Welch’s t-tests and/or Wilcoxon’s rank sum tests using the program “R”.
3. Results
3.1. Metabolomic profiling of CD4+ subsets reveals differences in tryptophan metabolic pathway
The balance of effector CD4 T cells (Teff; e.g. Th1 and Th17) and regulatory T cells (Treg) is critical in a variety of settings, particularly in the context of autoimmunity and inflammatory disease (25, 33–35). An understanding of the metabolic differences between Teff and Treg may lead to new strategies for treating inflammatory diseases by modulating the Teff:Treg balance through metabolic inhibition. While much of the previous work examining the T cell metabolic profiles has focused on glucose and lipid metabolism, there has been an increasing focus on differences in amino acid metabolism in the T cell subsets (36–42). We therefore examined metabolite levels of Teff and Treg in detail, using unsupervised clustering of approximately 400 metabolites measured by high-resolution nontargeted Q exactive–mass spectrometry (QE-MS) metabolomics (32). Surprisingly, the metabolite showing the largest difference between Teff and Treg was serotonin (5-HT). While the levels of the serotonin precursor tryptophan were relatively similar between naïve T cells, Teff (Th1 and Th17) and Treg, 5-HT levels were markedly higher in Treg compared to naïve T cells and Teff (Figure 1). We also examined the other tryptophan metabolite kynurenine and found a trend toward higher levels in Teff, suggesting that Teff and Treg may utilize distinct pathways to metabolize tryptophan.
Figure 1. Metabolomic profiling of CD4+ T cell subsets reveals differences in tryptophan metabolic pathway.
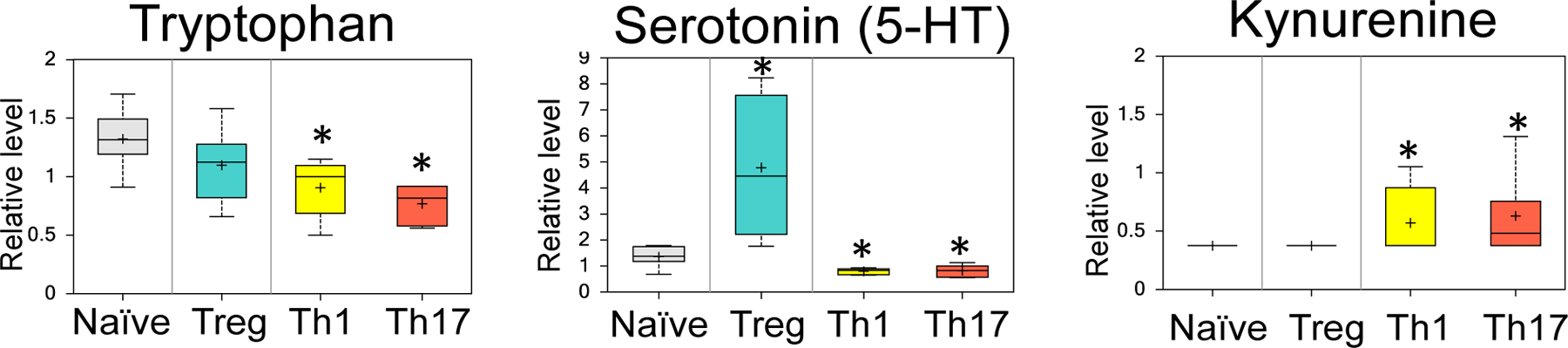
Naive CD4+CD25− T cells were collected or polarized in vitro for 3 days, split 1:2, and cultured with IL-2 alone for an additional 2 days to generate Th1, Th17, or Treg. T cell subset lysates from 3 biological replicate samples were extracted and analyzed using high-resolution LC-QE-MS for determination of cellular metabolites. Relative levels of tryptophan, serotonin (5-HT) and kynurenine are shown. Samples are shown as box plots representing 6 biological replicates per sample. * = p < .05 compared with naïve.
To assess whether Treg have the ability to synthesize serotonin from tryptophan, we performed qPCR analysis and found that Treg express high levels of the enzyme that converts tryptophan to 5-HT, tryptophan hydroxylase 1 (TPH1) (Figure 2). Teff also expressed TPH1 but to a much lesser extent than Treg (20–40 fold compared to 100 fold higher than naïve T cells). The expression of the other TPH isoform, TPH2, was not detected. Therefore, our data shows that Treg have the ability to synthesize 5-HT and contain high levels of intracellular 5-HT. Interestingly, while Teff did not contain significant levels of 5-HT, Th1 and Th17 cells showed higher levels of IDO, the enzyme that converts tryptophan to kynurenine. Th17, in particular, expressed significantly higher levels of IDO1.
Figure 2. CD4+ T cell subsets have differential expression of enzymes in tryptophan metabolic pathway.
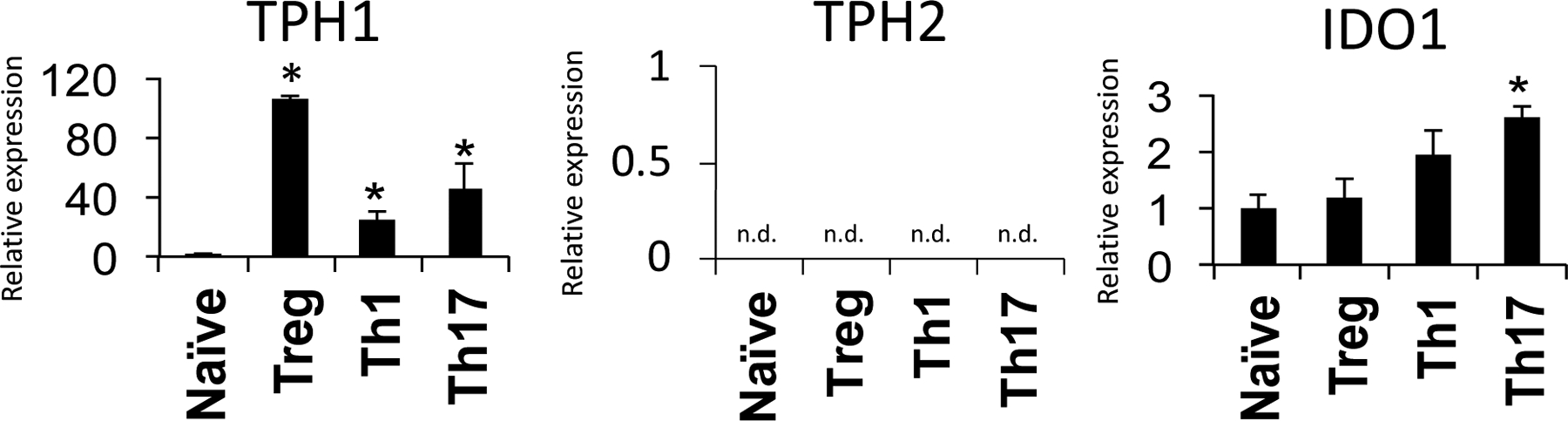
Naive CD4+CD25− T cells were collected or polarized in vitro for 3 days, split 1:2, and cultured with IL-2 alone for an additional 2 days to generate Th1, Th17, or Treg. T cell subset lysates from 3 biological replicate samples were extracted and subjected to qPCR to assess gene expression. The gene expression of tryptophan hydroxylase 1 (TPH1), tryptophan hydroxylase 2 (TPH2) and Indoleamine-pyrrole 2,3-dioxygenase (IDO1) are shown. Samples are normalized to naive T cells. Data are shown as mean ± SD and is representative of 3 independent experiments. n.d. = not detected. * = p < 0.05.
3.2. Treg contains the machinery to synthesize, transport and store intracellular serotonin
Given that Treg contained high levels of 5-HT, we next examined if Teff and Treg have differences in other 5-HT-related machinery that might explain increased intracellular 5-HT levels in Treg. The serotonin transporter, SERT (slc6a4, also called 5-HTT) is expressed by neurons and cells of the GI tract but has more recently been reported in human blood lymphocytes (9, 43). We found that Slc6a4 was expressed by naïve T cells, Teff and Treg with no significance differences between the subtypes (Figure 3A), suggesting that all of the examined CD4 T cell subtypes have a similar capacity to transport extracellular 5-HT into the cell. We next examined the expression of vesicular monoamine transporter 2 (VMAT2 or slc18a2), which is best known for its ability to transport monoamines, including serotonin, from the intracellular space into synaptic vesicles in the brain. Previous studies have suggested that human peripheral blood lymphocytes express VMAT2 but it has only been examined in the context of dopamine in bulk lymphocytes (13). Interestingly, Treg showed significantly higher expression of VMAT2 compared to naïve T cells and Teff (Figure 3A). Altogether, these data suggest that Treg have the capacity to generate 5-HT from tryptophan and store 5-HT in intracellular vesicles via VMAT2.
Figure 3. CD4 T cells differentially express serotonin-related genes.
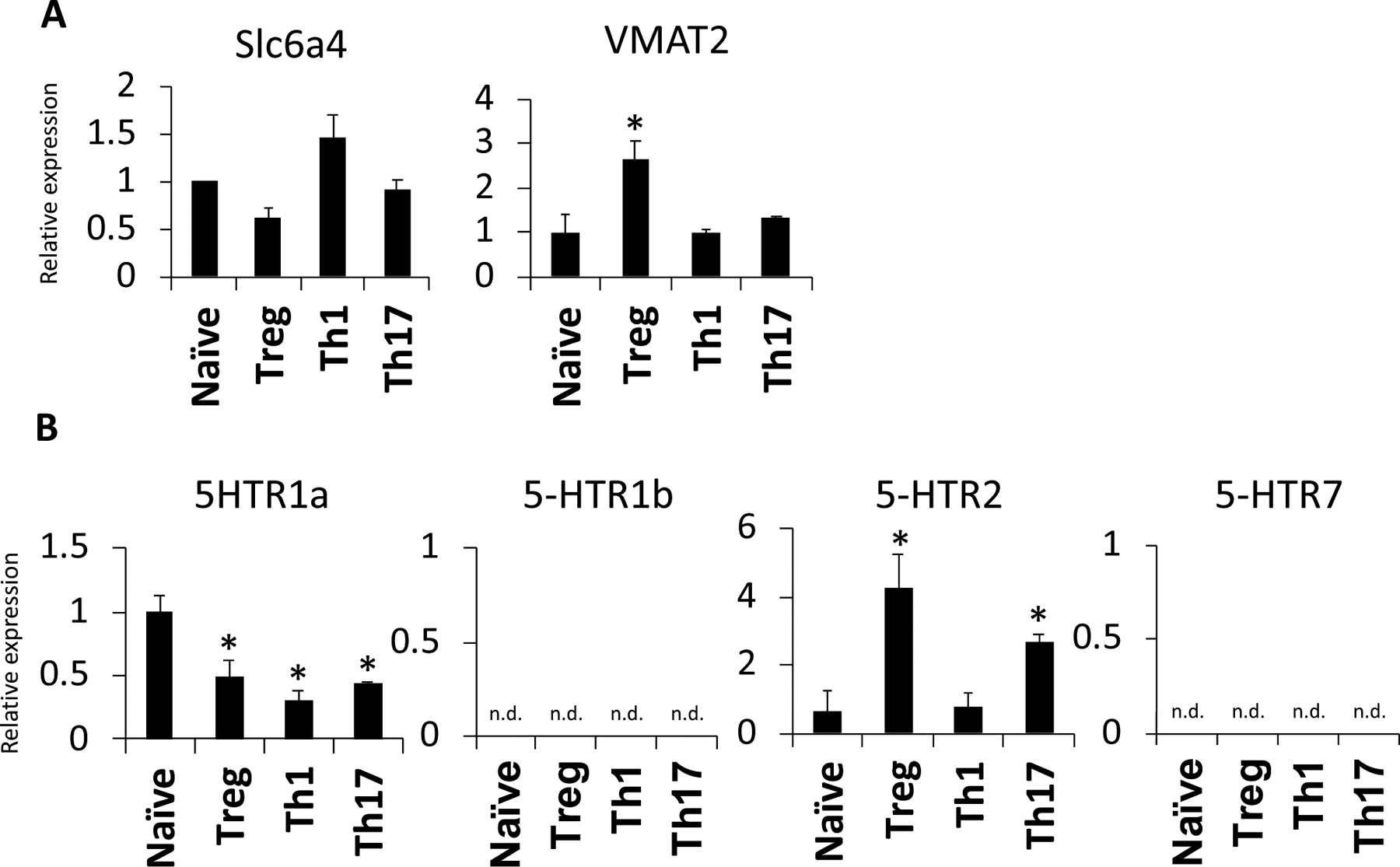
Naive CD4+CD25− T cells were collected or polarized in vitro for 3 days, split 1:2, and cultured with IL-2 alone for an additional 2 days to generate Th1, Th17, or Treg. T cell subset lysates from 3 biological replicate samples were subjected to qPCR to assess gene expression. (A) Relative expression levels of Slc6a4 VMAT2 are shown. (B) Relative expression levels of 5-HTR1a, 5-HTR1b, 5-HTR2 and 5-HTR7 are shown. Samples are normalized to naive T cells and data are shown as mean ± SD and are representative of 3 independent experiments, n.d. = not detected. * = p < .05
3.3. Teff and Treg have different expression patterns of 5-HT receptors
The finding that CD4+ T cells, particularly Treg, express 5-HT related machinery led us to hypothesize that serotonin effects on T cells via the 5-HT receptors may modulate T cell function. Indeed, previous studies have shown that lymphocyte cell lines and human T cells express several of the 5-HT receptors, including the 5-HT1, 5-HT2 and 5-HT7 receptors, although the downstream effects of these receptors in T cells are still the subject of investigation (44–47). Importantly, none of these studies explored differences between the CD4 T cell subsets. Therefore, we performed qPCR to examine if Teff and Treg have differential expression patterns of the 5-HT receptors. While the expression of the 5-HT1a, 5-HT1b, 5-HT2 and 5-HT7 receptors were examined, only 5-HT1aR and 5-HT2R were expressed by the CD4 T cell subsets (Figure 3B). 5-HTR1a was expressed at a higher level in naïve T cells compared to Teff and Treg, while 5-HTR2 had significantly higher expression in Treg. Together, these data suggest that changes in serotonin receptor expression may play a role in Teff versus Treg differentiation and/or function.
4. Discussion
Understanding the mechanisms that control T cell function and differentiation is crucial to develop new strategies to modulate immune function and prevent autoimmune and inflammatory disease. One such emerging area of research is the role of serotonin in immunity (6, 48, 49). While earlier studies have explored the presence of serotonin-related machinery, as well as the expression of serotonin receptors, in T cells, these studies were primarily done in bulk lymphocytes, total T cells or T cell lines (6, 27, 28, 44, 46, 50). To our knowledge, no study had specifically examined the CD4 T cell subsets (Teff and Treg). Total lymphocytes or T cells were shown to express the 5-HT1a, 5-HT1b, 5-HT2, 5-HT3 and 5-HT7 receptors (27, 28, 44, 46, 47). Our results with the differentiated CD4 T cell subsets suggest that naïve CD4 T cells as well as Th1, Th17 and Treg express the mRNA for 5-HT1a and 5-HT2 receptors but not 5-HT1b, or 5-HT7. The effect of serotonin signaling on CD4 T cell fate and function will be important to explore in future studies. Previous work reported an increased expression of the 5-HT1a receptor in the peripheral blood lymphocytes of systemic lupus erythematosus patients, suggesting a possible role for serotonin signaling in autoimmunity (51). The role of 5-HT2 in T cells is less clear; one study showed that treatment of total T cells with a 5-HT2 receptor agonist increased the expression of inflammatory cytokines; however, another used a different agonist and found a reduction in splenic CD8 T cells (52, 53). Therefore, more research is needed to understand the role of 5-HT as a stimulator or a suppressor of T cell function, and in what subtypes and contexts.
Additionally, more research is needed to determine whether serotonin, tryptophan or the balance between the IDO and TPH metabolic pathways influences immune and inflammatory processes (19, 54). Tryptophan is an essential amino acid that can be degraded into either serotonin or kynurenine (17). The expression of IDO in dendritic cells has been shown to promote tolerance and inhibit T cell proliferation (18, 20). Several theories have been proposed, including that the metabolism of tryptophan removes the essential amino acid from the microenvironment, effectively inhibiting T cell proliferation (54, 55). Other data has linked the IDO pathway to the generation of Treg, as tryptophan starvation combined with kynurenine supplementation induced a regulatory phenotype in naïve T cells (56). There is also evidence that kynurenine interacts with the aryl hydrocarbon receptor to generate Treg and that serotonin upregulates Treg and inhibits Teff (57–59). In addition, our data suggests that Treg selectively express TPH-1. Previous work has shown that total CD3 T cells increase their expression of TPH-1 upon activation with concanavalin A as well as activated CD4 T cells stimulated with PMA and ionomycin (27–29). Whether the TPH-1 detected in these previous studies was a result of Treg expression will be important to explore in future studies. In addition, we found that the CD4 T cell subsets express VMAT2, while VMAT1 was previously shown to be expressed in total T cells (29). Therefore, much more work is needed to understand the expression of the various serotonergic-related genes in the different T cell compartments, including CD8 T cells, memory T cells and follicular T helper cells, and confirm the expression of these proteins. Our data suggests that there may be a more direct role for serotonin in CD4 T cell biology, as Teff and Treg express distinct levels of the serotonin receptors and machinery. The high levels of serotonin and expression of TPH1 in Treg compared to Teff also points to a more direct role for serotonin or the removal of tryptophan in the CD4 T cell subsets.
It is becoming increasingly clear that the neurotransmitter serotonin plays a role in immune function. The presence and distinct expression of serotonin pathway components in Teff and Treg suggest a unique role for serotonin in these cell populations. Moving forward, it will be important to confirm these findings in human T cells and further examine the role of serotonin in specific T cell subsets and whether drugs that alter 5-HT signaling have differential effects on the balance of specific T cell subtypes in different disease settings.
5. Conclusions
These data suggest that tryptophan metabolism is differentially regulated in Teff and Treg and that serotonin may be selectively important for Treg. The results of this study expand the potential immunomodulatory role of serotonin in CD4 T cell biology and may ultimately support the development of new immunomodulatory targets for the treatment of autoimmune and neuropsychiatric disorders.
Acknowledgments
We would like to acknowledge Rigel Kishton as well as members of the Rathmell and MacIver labs for support and helpful discussions. This work was supported by the National Institutes of Health (R01-DK106090 and R01-DK105550) and a CNUCOM mini-grant award (VAG).
Abbreviations:
- Teff
effector T cell
- Treg
regulatory T cell
- 5-HT
serotonin, 5-hydroxytryptamine
- SSRIs
serotonin reuptake inhibitors
- SERT
serotonin transporter
- slc6a4
5-HTT
- IDO
indolamine-2,3-dioxygenase
- VMAT
vesicular monoamine transporter
- NAD
nicotinamide adenine dinucleotide
- Ahr
aryl hydrocarbon receptor
- TPH
tryptophan hydroxylase
- Th1
T helper 1 cell
- Th17
T helper 17 cell
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Disclosure
The authors of this manuscript have no conflicts of interest to disclose.
References
- 1.Yadav VK, Oury F, Suda N, Liu ZW, Gao XB, Confavreux C, Klemenhagen KC, Tanaka KF, Gingrich JA, Guo XE, Tecott LH, Mann JJ, Hen R, Horvath TL, Karsenty G. A serotonin-dependent mechanism explains the leptin regulation of bone mass, appetite, and energy expenditure. Cell. 2009;138(5):976–989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Gershon MD, Drakontides AB, Ross LL. Serotonin: Synthesis and Release from the Myenteric Plexus of the Mouse Intestine. Science. 1965;149(3680):197–199. [DOI] [PubMed] [Google Scholar]
- 3.Gershon MD. Serotonin: its role and receptors in enteric neurotransmission. Adv Exp Med Biol. 1991;294:221–230. [DOI] [PubMed] [Google Scholar]
- 4.Zhang Y, Zhen H, Yao W, Bian F, Mao X, Yang X, Jin S. Antidepressant drug, desipramine, alleviates allergic rhinitis by regulating Treg and Th17 cells. Int J Immunopathol Pharmacol. 2013;26(1):107–115. [DOI] [PubMed] [Google Scholar]
- 5.Yuan XQ, Qiu G, Liu XJ, Liu S, Wu Y, Wang X, Lu T. Fluoxetine promotes remission in acute experimental autoimmune encephalomyelitis in rats. Neuroimmunomodulation. 2012;19(4):201–208. [DOI] [PubMed] [Google Scholar]
- 6.Wu H, Denna TH, Storkersen JN, Gerriets VA. Beyond a neurotransmitter: The role of serotonin in inflammation and immunity. Pharmacol Res. 2019;140:100–114. [DOI] [PubMed] [Google Scholar]
- 7.Vollmar P, Nessler S, Kalluri SR, Hartung HP, Hemmer B. The antidepressant venlafaxine ameliorates murine experimental autoimmune encephalomyelitis by suppression of pro-inflammatory cytokines. Int J Neuropsychopharmacol. 2009;12(4):525–536. [DOI] [PubMed] [Google Scholar]
- 8.Taler M, Gil-Ad I, Korob I, Weizman A. The immunomodulatory effect of the antidepressant sertraline in an experimental autoimmune encephalomyelitis mouse model of multiple sclerosis. Neuroimmunomodulation. 2011;18(2):117–122. [DOI] [PubMed] [Google Scholar]
- 9.Faraj BA, Olkowski ZL, Jackson RT. Expression of a high-affinity serotonin transporter in human lymphocytes. Int J Immunopharmacol. 1994;16(7):561–567. [DOI] [PubMed] [Google Scholar]
- 10.Rudnick G. Serotonin transporters--structure and function. J Membr Biol. 2006;213(2):101–110. [DOI] [PubMed] [Google Scholar]
- 11.Gobin V, Van Steendam K, Denys D, Deforce D. Selective serotonin reuptake inhibitors as a novel class of immunosuppressants. Int Immunopharmacol. 2014;20(1):148–156. [DOI] [PubMed] [Google Scholar]
- 12.Anlauf M, Schafer MK, Depboylu C, Hartschuh W, Eiden LE, Kloppel G, Weihe E. The vesicular monoamine transporter 2 (VMAT2) is expressed by normal and tumor cutaneous mast cells and Langerhans cells of the skin but is absent from Langerhans cell histiocytosis. J Histochem Cytochem. 2004;52(6):779–788. [DOI] [PubMed] [Google Scholar]
- 13.Amenta F, Bronzetti E, Cantalamessa F, El-Assouad D, Felici L, Ricci A, Tayebati SK. Identification of dopamine plasma membrane and vesicular transporters in human peripheral blood lymphocytes. J Neuroimmunol. 2001;117(1–2):133–142. [DOI] [PubMed] [Google Scholar]
- 14.Arreola R, Becerril-Villanueva E, Cruz-Fuentes C, Velasco-Velazquez MA, Garces-Alvarez ME, Hurtado-Alvarado G, Quintero-Fabian S, Pavon L. Immunomodulatory effects mediated by serotonin. J Immunol Res. 2015;2015:354957. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Pithadia AB, Jain SM. 5-Hydroxytryptamine Receptor Subtypes and their Modulators with Therapeutic Potentials. J Clin Med Res. 2009;1(2):72–80. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Ahern GP. 5-HT and the immune system. Curr Opin Pharmacol. 2011;11(1):29–33. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Le Floc’h N, Otten W, Merlot E. Tryptophan metabolism, from nutrition to potential therapeutic applications. Amino Acids. 2011;41(5):1195–1205. [DOI] [PubMed] [Google Scholar]
- 18.Munn DH, Sharma MD, Lee JR, Jhaver KG, Johnson TS, Keskin DB, Marshall B, Chandler P, Antonia SJ, Burgess R, Slingluff CL Jr., Mellor AL. Potential regulatory function of human dendritic cells expressing indoleamine 2,3-dioxygenase. Science. 2002;297(5588):1867–1870. [DOI] [PubMed] [Google Scholar]
- 19.Grohmann U, Fallarino F, Puccetti P. Tolerance, DCs and tryptophan: much ado about IDO. Trends Immunol. 2003;24(5):242–248. [DOI] [PubMed] [Google Scholar]
- 20.Fallarino F, Vacca C, Orabona C, Belladonna ML, Bianchi R, Marshall B, Keskin DB, Mellor AL, Fioretti MC, Grohmann U, Puccetti P. Functional expression of indoleamine 2,3-dioxygenase by murine CD8 alpha(+) dendritic cells. Int Immunol. 2002;14(1):65–68. [DOI] [PubMed] [Google Scholar]
- 21.Nguyen NT, Kimura A, Nakahama T, Chinen I, Masuda K, Nohara K, Fujii-Kuriyama Y, Kishimoto T. Aryl hydrocarbon receptor negatively regulates dendritic cell immunogenicity via a kynurenine-dependent mechanism. Proc Natl Acad Sci U S A. 2010;107(46):19961–19966. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Bessede A, Gargaro M, Pallotta MT, Matino D, Servillo G, Brunacci C, Bicciato S, Mazza EM, Macchiarulo A, Vacca C, Iannitti R, Tissi L, Volpi C, Belladonna ML, Orabona C, Bianchi R, Lanz TV, Platten M, Della Fazia MA, Piobbico D, Zelante T, Funakoshi H, Nakamura T, Gilot D, Denison MS, Guillemin GJ, DuHadaway JB, Prendergast GC, Metz R, Geffard M, Boon L, Pirro M, Iorio A, Veyret B, Romani L, Grohmann U, Fallarino F, Puccetti P. Aryl hydrocarbon receptor control of a disease tolerance defence pathway. Nature. 2014;511(7508):184–190. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Stone TW, Darlington LG. Endogenous kynurenines as targets for drug discovery and development. Nat Rev Drug Discov. 2002;1(8):609–620. [DOI] [PubMed] [Google Scholar]
- 24.Zhu J, Yamane H, Paul WE. Differentiation of effector CD4 T cell populations (*). Annu Rev Immunol. 2010;28:445–489. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Sakaguchi S, Yamaguchi T, Nomura T, Ono M. Regulatory T cells and immune tolerance. Cell. 2008;133(5):775–787. [DOI] [PubMed] [Google Scholar]
- 26.Brunkow ME, Jeffery EW, Hjerrild KA, Paeper B, Clark LB, Yasayko SA, Wilkinson JE, Galas D, Ziegler SF, Ramsdell F. Disruption of a new forkhead/winged-helix protein, scurfin, results in the fatal lymphoproliferative disorder of the scurfy mouse. Nat Genet. 2001;27(1):68–73. [DOI] [PubMed] [Google Scholar]
- 27.Leon-Ponte M, Ahern GP, O’Connell PJ. Serotonin provides an accessory signal to enhance T-cell activation by signaling through the 5-HT7 receptor. Blood. 2007;109(8):3139–3146. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.O’Connell PJ, Wang X, Leon-Ponte M, Griffiths C, Pingle SC, Ahern GP. A novel form of immune signaling revealed by transmission of the inflammatory mediator serotonin between dendritic cells and T cells. Blood. 2006;107(3):1010–1017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Chen Y, Leon-Ponte M, Pingle SC, O’Connell PJ, Ahern GP. T lymphocytes possess the machinery for 5-HT synthesis, storage, degradation and release. Acta Physiol (Oxf). 2015;213(4):860–867. [DOI] [PubMed] [Google Scholar]
- 30.Michalek RD, Gerriets VA, Jacobs SR, Macintyre AN, MacIver NJ, Mason EF, Sullivan SA, Nichols AG, Rathmell JC. Cutting edge: distinct glycolytic and lipid oxidative metabolic programs are essential for effector and regulatory CD4+ T cell subsets. J Immunol. 2011;186(6):3299–3303. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Harrington LE, Hatton RD, Mangan PR, Turner H, Murphy TL, Murphy KM, Weaver CT. Interleukin 17-producing CD4+ effector T cells develop via a lineage distinct from the T helper type 1 and 2 lineages. Nat Immunol. 2005;6(11):1123–1132. [DOI] [PubMed] [Google Scholar]
- 32.Gerriets VA, Kishton RJ, Nichols AG, Macintyre AN, Inoue M, Ilkayeva O, Winter PS, Liu X, Priyadharshini B, Slawinska ME, Haeberli L, Huck C, Turka LA, Wood KC, Hale LP, Smith PA, Schneider MA, MacIver NJ, Locasale JW, Newgard CB, Shinohara ML, Rathmell JC. Metabolic programming and PDHK1 control CD4+ T cell subsets and inflammation. J Clin Invest. 2015;125(1):194–207. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Powrie F, Leach MW, Mauze S, Menon S, Caddle LB, Coffman RL. Inhibition of Th1 responses prevents inflammatory bowel disease in scid mice reconstituted with CD45RBhi CD4+ T cells. Immunity. 1994;1(7):553–562. [DOI] [PubMed] [Google Scholar]
- 34.Josefowicz SZ, Lu LF, Rudensky AY. Regulatory T cells: mechanisms of differentiation and function. Annu Rev Immunol. 2012;30:531–564. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Fontenot JD, Gavin MA, Rudensky AY. Foxp3 programs the development and function of CD4+CD25+ regulatory T cells. Nat Immunol. 2003;4(4):330–336. [DOI] [PubMed] [Google Scholar]
- 36.Sinclair LV, Rolf J, Emslie E, Shi YB, Taylor PM, Cantrell DA. Control of amino-acid transport by antigen receptors coordinates the metabolic reprogramming essential for T cell differentiation. Nat Immunol. 2013;14(5):500–508. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Gerriets VA, Kishton RJ, Johnson MO, Cohen S, Siska PJ, Nichols AG, Warmoes MO, de Cubas AA, MacIver NJ, Locasale JW, Turka LA, Wells AD, Rathmell JC. Foxp3 and Toll-like receptor signaling balance Treg cell anabolic metabolism for suppression. Nat Immunol. 2016;17(12):1459–1466. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Gerriets VA, Rathmell JC. Metabolic pathways in T cell fate and function. Trends Immunol. 2012;33(4):168–173. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Johnson MO, Wolf MM, Madden MZ, Andrejeva G, Sugiura A, Contreras DC, Maseda D, Liberti MV, Paz K, Kishton RJ, Johnson ME, de Cubas AA, Wu P, Li G, Zhang Y, Newcomb DC, Wells AD, Restifo NP, Rathmell WK, Locasale JW, Davila ML, Blazar BR, Rathmell JC. Distinct Regulation of Th17 and Th1 Cell Differentiation by Glutaminase-Dependent Metabolism. Cell. 2018;175(7):1780–1795 e1719. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Macintyre AN, Gerriets VA, Nichols AG, Michalek RD, Rudolph MC, Deoliveira D, Anderson SM, Abel ED, Chen BJ, Hale LP, Rathmell JC. The glucose transporter Glut1 is selectively essential for CD4 T cell activation and effector function. Cell Metab. 2014;20(1):61–72. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Nakaya M, Xiao Y, Zhou X, Chang JH, Chang M, Cheng X, Blonska M, Lin X, Sun SC. Inflammatory T cell responses rely on amino acid transporter ASCT2 facilitation of glutamine uptake and mTORC1 kinase activation. Immunity. 2014;40(5):692–705. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Klysz D, Tai X, Robert PA, Craveiro M, Cretenet G, Oburoglu L, Mongellaz C, Floess S, Fritz V, Matias MI, Yong C, Surh N, Marie JC, Huehn J, Zimmermann V, Kinet S, Dardalhon V, Taylor N. Glutamine-dependent alpha-ketoglutarate production regulates the balance between T helper 1 cell and regulatory T cell generation. Sci Signal. 2015;8(396):ra97. [DOI] [PubMed] [Google Scholar]
- 43.Lima L, Urbina M. Serotonin transporter modulation in blood lymphocytes from patients with major depression. Cell Mol Neurobiol. 2002;22(5–6):797–804. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Aune TM, McGrath KM, Sarr T, Bombara MP, Kelley KA. Expression of 5HT1a receptors on activated human T cells. Regulation of cyclic AMP levels and T cell proliferation by 5-hydroxytryptamine. J Immunol. 1993;151(3):1175–1183. [PubMed] [Google Scholar]
- 45.Khan NA, Poisson JP. 5-HT3 receptor-channels coupled with Na+ influx in human T cells: role in T cell activation. J Neuroimmunol. 1999;99(1):53–60. [DOI] [PubMed] [Google Scholar]
- 46.Urbina M, Arroyo R, Lima L. 5-HT7 receptors and tryptophan hydroxylase in lymphocytes of rats: mitogen activation, physical restraint or treatment with reserpine. Neuroimmunomodulation. 2014;21(5):240–249. [DOI] [PubMed] [Google Scholar]
- 47.Yin J, Albert RH, Tretiakova AP, Jameson BA. 5-HT(1B) receptors play a prominent role in the proliferation of T-lymphocytes. J Neuroimmunol. 2006;181(1–2):68–81. [DOI] [PubMed] [Google Scholar]
- 48.El Aidy S, Dinan TG, Cryan JF. Immune modulation of the brain-gut-microbe axis. Front Microbiol. 2014;5:146. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Franco R, Pacheco R, Lluis C, Ahern GP, O’Connell PJ. The emergence of neurotransmitters as immune modulators. Trends Immunol. 2007;28(9):400–407. [DOI] [PubMed] [Google Scholar]
- 50.Aune TM, Golden HW, McGrath KM. Inhibitors of serotonin synthesis and antagonists of serotonin 1A receptors inhibit T lymphocyte function in vitro and cell-mediated immunity in vivo. J Immunol. 1994;153(2):489–498. [PubMed] [Google Scholar]
- 51.Xu J, Zhang G, Cheng Y, Chen B, Dong Y, Li L, Xu L, Xu X, Lu Z, Wen J. Hypomethylation of the HTR1A promoter region and high expression of HTR1A in the peripheral blood lymphocytes of patients with systemic lupus erythematosus. Lupus. 2011;20(7):678–689. [DOI] [PubMed] [Google Scholar]
- 52.Davydova SM, Cheido MA, Gevorgyan MM, Idova GV. Effects of 5-HT2A receptor stimulation and blocking on immune response. Bull Exp Biol Med. 2010;150(2):219–221. [DOI] [PubMed] [Google Scholar]
- 53.Inoue M, Okazaki T, Kitazono T, Mizushima M, Omata M, Ozaki S. Regulation of antigen-specific CTL and Th1 cell activation through 5-Hydroxytryptamine 2A receptor. Int Immunopharmacol. 2011;11(1):67–73. [DOI] [PubMed] [Google Scholar]
- 54.Mellor AL, Munn DH. IDO expression by dendritic cells: tolerance and tryptophan catabolism. Nat Rev Immunol. 2004;4(10):762–774. [DOI] [PubMed] [Google Scholar]
- 55.Nowak EC, de Vries VC, Wasiuk A, Ahonen C, Bennett KA, Le Mercier I, Ha DG, Noelle RJ. Tryptophan hydroxylase-1 regulates immune tolerance and inflammation. J Exp Med. 2012;209(11):2127–2135. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Fallarino F, Grohmann U, You S, McGrath BC, Cavener DR, Vacca C, Orabona C, Bianchi R, Belladonna ML, Volpi C, Santamaria P, Fioretti MC, Puccetti P. The combined effects of tryptophan starvation and tryptophan catabolites down-regulate T cell receptor zeta-chain and induce a regulatory phenotype in naive T cells. J Immunol. 2006;176(11):6752–6761. [DOI] [PubMed] [Google Scholar]
- 57.Mezrich JD, Fechner JH, Zhang X, Johnson BP, Burlingham WJ, Bradfield CA. An interaction between kynurenine and the aryl hydrocarbon receptor can generate regulatory T cells. J Immunol. 2010;185(6):3190–3198. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Sacramento PM, Monteiro C, Dias ASO, Kasahara TM, Ferreira TB, Hygino J, Wing AC, Andrade RM, Rueda F, Sales MC, Vasconcelos CC, Bento CAM. Serotonin decreases the production of Th1/Th17 cytokines and elevates the frequency of regulatory CD4(+) T-cell subsets in multiple sclerosis patients. Eur J Immunol. 2018;48(8):1376–1388. [DOI] [PubMed] [Google Scholar]
- 59.Chabbi-Achengli Y, Coman T, Collet C, Callebert J, Corcelli M, Lin H, Rignault R, Dy M, de Vernejoul MC, Cote F. Serotonin Is Involved in Autoimmune Arthritis through Th17 Immunity and Bone Resorption. Am J Pathol. 2016;186(4):927–937. [DOI] [PubMed] [Google Scholar]


