Abstract
The Kölliker-Fuse nucleus (KF) is a functionally distinct component of the parabrachial complex, located in the dorsolateral pons of mammals. The KF has a major role in respiration and upper airway control. A comprehensive understanding of the KF and its contributions to respiratory function and dysfunction requires an appreciation for its neurochemical characteristics. The goal of this review is to summarize the diverse neurochemical composition of the KF, focusing on the neurotransmitters, neuromodulators and neuropeptides present. We also include a description of the receptors expressed on KF neurons and transporters involved in each system, as well as their putative roles in respiratory physiology. Finally, we provide a short section reviewing the literature regarding neurochemical changes in the KF in the context of respiratory dysfunction observed in SIDS and Rett syndrome. By overviewing the current literature on the neurochemical composition of the KF, this review will serve to aid a wide range of topics in the future research into the neural control of respiration in health and disease.
Keywords: respiration, SIDS, Rett syndrome, GABA, glutamate, opioids
Graphical Abstract
The Kölliker-Fuse nucleus (KF) plays an important role in respiration and upper airway control. The purpose of this review is to summarize the neurochemical composition of the KF, discussing the neurotransmitters, neuromodulators and neuropeptides present. We also describe the receptors and transporters involved in each system, and provide a summary of their roles in the control of breathing. In addition, we briefly discuss the neurochemical changes in the KF in SIDS and Rett syndrome. This review will serve to support a wide range of topics in the future research into the neural control of breathing in health and disease.
Introduction
The parabrachial complex is a collection of multiple nuclei in the dorsolateral pons surrounding the superior cerebellar peduncle. The three major subdivisions of the parabrachial complex, the lateral and medial parabrachial nuclei and the Kölliker-Fuse nucleus (KF; Figure 1), can be found in animals (Dutschmann and Dick 2012) and humans (Lavezzi et al. 2004b). Each of the pontine nuclei have a different neurochemical composition, as well as anatomical connections, in accordance with distinct functional roles. Due to its important role in respiratory physiology, we highlight the KF and its neurochemical identity in this review.
Figure 1.
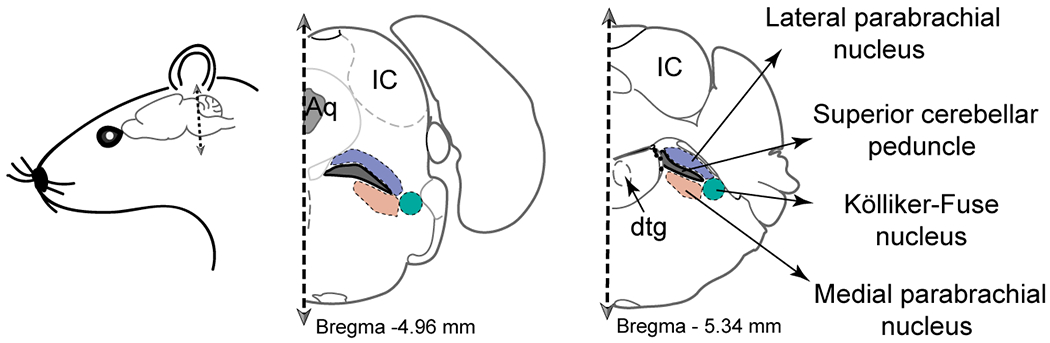
Location of the KF in the adult mouse brain. In the schematics of coronal hemi-sections, the green shaded area indicates the location of the KF in the proximity of the lateral parabrachial nucleus (in purple) and the medial parabrachial nucleus (in orange) surrounding the superior cerebellar peduncle (in gray). Other landmarks included in the diagram: Aq – Aqueduct; IC – inferior colliculus; dtg – dorsal tegmental nucleus.
Respiration is generated and controlled by the coordinated efforts of respiratory nuclei in the pons and medulla. Rhythm generators in the ventrolateral medulla, consisting of the pre-Bötzinger complex, the post-inspiratory complex, and the lateral parafacial nucleus, are thought to generate inspiration, post-inspiration, and expiration respectively (Smith et al. 1991; Anderson et al. 2016; Huckstepp et al. 2015). The Bötzinger complex is a collection of mostly inhibitory neurons that fire during expiration, providing a major source of inhibition for the network (Ezure et al. 2003a; Schreihofer et al. 1999). Neural output from rhythm generators is relayed to premotor neurons in the ventral respiratory group. Medullary respiratory neurons receive modulatory input from pontine structures, including the KF, which is required for normal breathing (Fung and St. John 1995; Dutschmann and Herbert 2006; Dutschmann and Dick 2012; Smith et al. 2007). Together, these nuclei (with others not mentioned here) form a highly interconnected network that produces a three-phase respiratory pattern (inspiration, post-inspiration, and expiration) that constitute a normal breath (see more information on respiratory rhythm in the following reviews: Smith et al., 2013; Del Negro et al., 2018; Ramirez & Baertsch, 2018a, 2018b). The precise control of breathing phases relies on the continuous balance of excitatory and inhibitory neurotransmission in respiratory neural populations. Thus, the neurochemical identity of respiratory nuclei provides valuable information about their functional roles in respiration and respiratory related behaviors, such as swallowing and cough.
Lumsden (1923) described the KF as the “inspiratory off-switch,” important for the transition of inspiration to expiration. Since then, it has been shown that the KF is required for normal breathing and upper airway patency (Fung and St. John 1995; Dutschmann and Herbert 2006; Dutschmann and Dick 2012; Smith et al. 2007). The KF is composed of heterogeneous populations of neurons, most of which have a respiratory function (Cohen and Wang 1959; Dick et al. 1994; Dutschmann and Herbert 2006; Ezure and Tanaka 2006). Excitatory KF neurons project to the majority of the core respiratory nuclei in the brainstem, including the pre-Bötzinger complex, lateral parafacial nucleus, Bötzinger complex, and rostral ventral respiratory group (Geerling et al. 2017; Song et al. 2012). Through these projections, the KF directly participates in the control of respiration, by modulating respiratory rate and pattern (Lumsden 1923; St-John et al. 1971; Cohen 1971; Cohen and Shaw 2004; Fung and St. John 1995; Jodkowski et al. 1994; Gautier and Bertrand 1975; Smith et al. 2007). As demonstrated by Fung and St John (1995), chemical lesion or pharmacological inhibition of the KF changes rate and pattern, by eliminating the post-inspiratory phase and prolonging inspiration. Conversely, pharmacological excitation of the KF prolongs the duration of the post-inspiratory phase (Dutschmann and Herbert 2006). These studies, in conjunction with others, support the critical role of the KF in the neural control of breathing.
KF neurons also project to a range of cranial nerves, for instance the vagus and hypoglossal nerves (Browaldh et al. 2016; Geerling et al. 2017). These projections are important in the coordinated control of the upper airways with breathing during orofacial behaviors involving similar anatomical pathways, such as swallowing, cough and vocalizations (Browaldh et al. 2016; Bautista et al. 2014; Jakus et al. 2008). Additionally, through connections with regions mediating the arousal component of hypoxic/hypercapnic chemoreflexes, the KF may also play a role in the state-dependent adjustments of respiratory rate and pattern (Kaur and Saper 2019). For instance, the KF receives chemosensory information from the lateral hypothalamus, retrotrapezoid nucleus (RTN), medullary raphe, the nucleus of the solitary tract (NTS) and the locus coeruleus (LC) (Guyenet and Bayliss 2015; Herbert et al. 1990; Hancock and Fougerousse 1976; Kaur and Saper 2019; Peyron et al. 1998; Robertson et al. 2013; Yokota et al. 2016).
With this in mind, the purpose of this review is to illustrate the unique neurochemical makeup of the KF, mainly in the context of respiration. We will begin by emphasizing the various neurotransmitters, neuromodulators, neuropeptides, receptors, and transporters expressed in the KF. To gain an understanding of the neurochemical composition of the KF, we drew primarily from autoradiography, immunohistochemistry, in situ hybridization and anatomical tracing studies. In some of these studies the authors were not focused on KF, but included the KF in images and analysis as part of the parabrachial complex more generally. For these studies, we report findings about the parabrachial complex and extract, when possible, information pertaining to KF. Additionally, the described approaches have their pitfalls (e.g. antibody and probe selectivity, background or artifactual staining). Therefore, we also reference physiological studies to functionally confirm or dispute the conclusions made by histological methods. In the later portions of this review, we address neurochemical changes in the KF that lead to breathing disorders. Importantly, we do not intend for this to be an exhaustive review of the KF’s function, since many reviews exist on the physiological role of the pons in breathing (Browaldh et al. 2016; Dutschmann and Dick 2012; Smith et al. 2007; Smith et al. 2013; Mörschel and Dutschmann 2009).
We hope that this review will be a valuable resource in understanding the complex neurochemical makeup of the KF, and by identifying gaps in our knowledge, it will aid future research on the neural control of breathing in health and disease.
Glutamate
Glutamate receptor expression in the KF
Glutamate is the key excitatory neurotransmitter in most brainstem respiratory neurons (reviewed in (Bonham 1995)). Two major classes of glutamate receptors are expressed in the mammalian brain: ionotropic and metabotropic. Ionotropic AMPA, NMDA and kainite receptors are ligand-gated ion-channels, whereas metabotropic glutamate receptors (mGluR1–8) are G protein-coupled receptors. Expression of these glutamate receptors in the respiratory network changes during development (reviewed in Wong-Riley & Liu, 2005).
Glutamate injections into the KF elicit a characteristic post-inspiratory apnea and/or tachypnea, which is a useful step in functionally localizing the KF (Chamberlin and Saper 1994; Dutschmann and Herbert 2006; Saunders and Levitt 2020; Barnett et al. 2018). Multiple studies provide functional evidence for the presence of glutamate receptors in the KF (Dutschmann and Herbert 1998; Lara et al. 2002; Bautista and Dutschmann 2014a; Kuna and Remmers 1999; Dutschmann and Herbert 2006).
AMPA receptors are expressed in a wide range of variants due to multiple forms and combinations of subunits (GluA1-4). Most AMPA receptors contain the subunit GluA2, which makes them impermeable to Ca2+ (Brusa et al. 1995; Jonas and Spruston 1994; Sommer et al. 1991). Detected by immunohistochemistry, the KF of rats contains a moderate amount of GluA1 and GluA2/3, and a low amount of GluA4 (Chamberlin and Saper 1995). In addition to the variety in AMPA subunits, flip or flop splice forms of those subunits also have a substantial impact on the channel’s kinetic and gating properties (Monyer et al. 1991; Lee et al. 1998). More than half of the analyzed KF neurons showed strong mRNA expressions of the GluA2 (formerly GluRB) flip and GluA3 (formerly GluRC) flip subunits. Similarly, the majority of KF neurons expressed GluA1 (formerly GluRA) flop and GluA3 (formerly GluRC) flop mRNAs (Guthmann and Herbert 1999a). Such subunit assemblies likely result in AMPA receptors that have increased glutamate sensitivity, longer desensitization times and higher levels of steady state currents (Mosbacher et al. 1994; Guthmann and Herbert 1999a).
NMDA receptors are heterogeneous due to the diversity of the splice forms and subunits that can form various assemblies and result in distinct NMDA receptors (Feldmeyer and Cull-Candy 1996). This heterogeneity in structure leads to differences in channel properties. The structural differences can affect, for instance, the receptor’s affinity to glutamate, the strength of the Mg2+ block, or deactivation kinetics, leading to more global physiological consequences. The KF expresses an abundance of NR1, NR2B and NR2D subunits confirmed by immunohistochemistry against the proteins, as well as in situ hybridization for mRNA detection in rat brains (Guthmann and Herbert 1999b; Dutschmann et al. 1998; Kron et al. 2008). During development, NR2B expression decreases and NR2D expression increases in the KF (Kron et al. 2008). The NR2D isoform has longer deactivation times, lower affinity for glutamate and a weaker Mg2+ block (Feldmeyer and Cull-Candy 1996). Consistent with a switch from NR2B to NR2D expression, NMDA-mediated synaptic currents in the KF were smaller and decayed slower in brain slices from rats at P14-21 than P1-5 (Kron et al. 2008).
Because noxious nasal stimulation increases the expression of immediate early gene cFos in NR1-labeled KF neurons, these neurons have been described as potential relay sites for the nasotrigeminal reflex circuit, at least in rats (Dutschmann et al. 1998). Furthermore, NR1-labeled KF neurons project to the Bötzinger complex and are implicated in control of post-inspiration (Song et al. 2015).
Currently eight different mGluRs have been identified, termed mGluR1-8 (Hollmann 1994). Based on their composition and pharmacology, the eight receptor subtypes have been classified into three groups. Group I contains mGluR1 and mGluR5 and couples to Gq proteins. Group II contains mGluR2 and mGluR3, and couples to Gi/o proteins. Group III contains mGluR4 and mGluR6-8, and couples to Gi/o proteins. Immunohistochemistry against mGluR1 and mGluR5 (group I receptors) and mGluR2/3 (group II) in rats resulted in strong to moderate staining for all tested receptor subtypes in the KF (Guthmann and Herbert 1999c). The strongest immunoreactivity was observed against mGluR1, while staining for mGluR5 and mGluR2/3 was also present, but it was less prominent. This study also revealed that group I receptors are postsynaptic, with mGluR1 located on KF neuron dendrites and mGluR5 located on the soma. Group II receptors on the other hand, are presumably on presynaptic terminals or reside on glial cells. Studies into the different contributions of mGluRs to KF function and respiratory network function are very important, yet lacking.
Glutamate transporters in the KF
Glutamate transporters can be divided into two main classes, excitatory amino acid transporters (EAATs) and vesicular glutamate transporters (VGLUTs). Currently there are five known EAATs (EAAT1-5). EAAT1-4 are expressed in the brain, however EAAT 1 and 2 are mostly present in glia and EAAT5 has only been found in the retina (for review see (Zhou and Danbolt 2013). The presence of EAAT2 (also known as GLT-1) in the KF has not been specifically investigated. However, low levels of immunocytochemical labeling for different variants of GLT-1 can be detected in the KF and rest of the parabrachial complex in mouse brains (images of KF coronal sections obtained from the open access online microscopic image repository associated with the data set described by (Holmseth et al. 2009); http://www.rbwb.org/). EAAT3 can be found in neurons throughout the entire brain, and is solely localized to the soma and dendrites (Shashidharan et al. 1997). EAAT4 is most highly expressed in cerebellar Purkinje cells in mouse, rat and human brains, but is also found throughout the rodent forebrain (Massie et al. 2008). Neither EAAT3 nor EAAT4 can be detected in the brainstem of adult rodents (Furuta et al. 1997; Nieoullon et al. 2006).
The second family contains three known types of VGLUTs: VGLUT1-3. VGLUTs have a much lower affinity for glutamate than EAATs (Shigeri et al. 2004). VGLUT1 is predominantly expressed in the cortex, cerebellum, and hippocampus, while VGLUT2 is more restricted to the thalamus, deep cerebellar nuclei and the brainstem (Fremeau et al. 2001). Most neurons located in the rostral parts of the KF are glutamatergic and express VGLUT2, however VGLUT2 expressing KF neurons are more sparse in the caudal regions of the nucleus, as shown in mice (Geerling et al. 2017; Kaur et al. 2013; Niu et al. 2010). VGLUT3 can be detected in the parabrachial complex, however its presence in the KF has not been examined yet (Guo et al. 2005).
Sources of glutamate in the KF
The majority of KF (and parabrachial complex) neurons are glutamatergic, however the density of glutamatergic cells decreases progressively towards the caudal portions of the KF (Geerling et al. 2017). These glutamatergic KF neurons send extensive axonal projections to caudal targets in the rodent brain, such as the NTS, medullary reticular formation, Bötzinger complex, pre-Bӧtzinger complex, and rostral ventral respiratory group (Ezure and Tanaka 2006; Yokota et al. 2007; Herbert et al. 1990; Geerling et al. 2017). A large proportion of parabrachial complex neurons express the transcription factor, Forkhead box protein 2 (FoxP2), however KF neurons only show minor expression for this marker. Most FoxP2 expressing neurons are glutamatergic and express VGLUT2 mRNA in the entire parabrachial complex, including the sparse FoxP2+ KF neurons (Geerling et al. 2011; Geerling et al. 2017). The developmental origin of the densely packed, rostral KF glutamatergic neurons is unknown.
KF neurons also receive glutamatergic modulation from a wide range of brainstem respiratory nuclei (Figure 2). For instance, excitatory, somatostatin (SST)-expressing pre-Bӧtzinger complex neurons project to the KF (Yang and Feldman 2018). These excitatory projections between the pre-Bӧtzinger complex and KF are hypothesized to be important in the control of respiratory phase-transitions in the context of breathing, as well as behaviors strictly coordinated with breathing (sniffing, whisking, swallowing, vocalizations, etc.).
Figure 2.
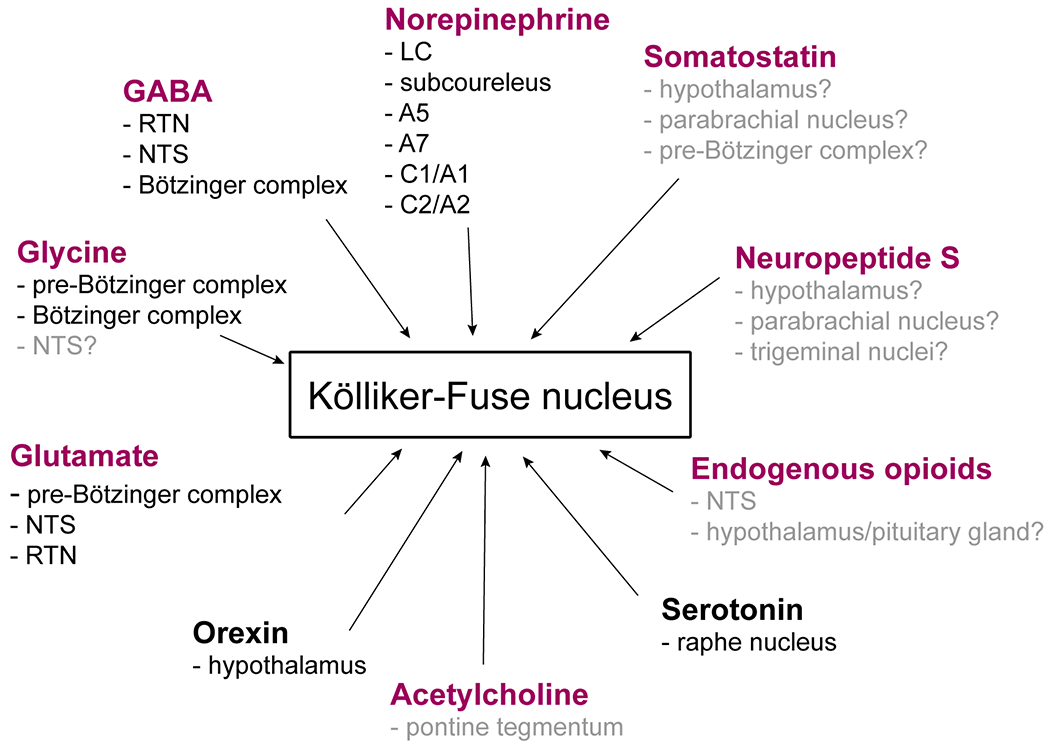
Neurochemical identity and origin of afferent projections to KF. Purple font indicates neurotransmitters and neuropeptides produced locally in the KF. Origins listed in gray font indicate potential neuropeptide and neurotransmitter release from regions with known anatomical projections to the KF. The specific neurochemical identity of these projections has not functionally addressed.
Glutamatergic projections to the KF also originate from the marginal layer of the ventral surface of the medulla (the area corresponding to the retrotrapezoid nucleus [RTN]), detected by a combination of retrograde labeling and in situ hybridization for VGLUT2 mRNA in rats (Weston et al. 2004). While this study did not specifically look at projections to the KF, the example injection sites and summary figures indicate the inclusion of the KF. In a more recent study, a majority of retrograde labeled varicosities from the RTN to the KF were positive for VGLUT2 confirming glutamatergic input from RTN (Silva et al. 2016). Additionally, arousal inducing chemosensory information may arrive indirectly to the KF from the carotid bodies, through the nucleus of the solitary tract (NTS; Song et al., 2011; Kaur et al., 2013). Labeling and antidromic activation experiments performed in rats indicate that hypoxia-activated neurons in the NTS send excitatory projections to the KF that may be glutamatergic (Gozal et al. 1999; Song et al. 2011).
Functional role of glutamate in the KF
Representing the most prevalent type of neurotransmitter in the KF, glutamatergic modulation of KF neurons has widespread functional implications. One of the most important and most heavily studied functions of the KF is in the control of respiratory rate and pattern. NMDA receptor activation in the KF/parabrachial complex stimulates respiratory rate in normal, as well as hypoxic conditions, and influences sleep-wake states (Boon and Milsom 2008; Bonis et al. 2010a; Chamberlin and Saper 1994). Sequential microinjections of both AMPA- and NMDA-antagonists into the KF and parabrachial complex of decerebrate rabbits results in substantially decreased respiratory rate (Navarrete-Opazo et al. 2020). In rats, blockade of NMDA receptors in the KF abolishes the post-inspiratory phase of breathing in baseline and post-hypoxia conditions (Song et al. 2015), and reduces nasotrigeminal reflex induced respiratory depression and bradycardia (Dutschmann and Herbert 1998). Conditional deletion of Vglut2 from specific portions of the parabrachial complex, including the KF, results in delayed or lack of arousal responses to increased CO2, indicating a potential role in the chemosensory control of respiration in mice (Kaur et al. 2013).
In the arterially perfused working heart-brainstem preparation of the rat, glutamate microinjections into the KF prolong the post-inspiratory phase of respiration (which has direct effects on respiratory rate by prolonging expiration potentially through modulation of the rhythm generator), and causes glottis closure in the upper airways (Dutschmann and Herbert 2006). Direct application of glutamate receptor agonists or antagonists into the KF has been shown to modulate the activity of multiple cranial nerves, many of them with important roles in upper airway control, including the recurrent laryngeal, trigeminal and hypoglossal nerves (for a review see: (Browaldh et al. 2016)). For instance, unilateral kainic acid, NMDA or AMPA microinjections in the KF lead to the tonic excitation of the hypoglossal nerve (Kuna and Remmers 1999). These experiments provide further support for the KF’s pivotal role in upper airway patency.
GABA
GABA receptor expression in the KF
The main inhibitory neurotransmitter in the nervous system, gamma-aminobutyric acid (GABA), binds to two types of receptors. The first group, GABA-A receptors are ionotropic, composed of five homologous or heterologous subunits, forming 19 different isoforms of a ligand-gated Cl− channel that is the functional receptor (Olsen and Sieghart 2008). The second group, GABA-B receptors are metabotropic G-protein coupled receptors encoded by three GABA-B receptor genes (Kaupmann et al. 1998).
A detailed study on GABA-A receptor expression in the parabrachial complex, and specifically addressing the KF, was conducted by Guthmann et al. (1998). In this study, immunolabeling of GABA-A receptor subunits revealed the presence of γ2-subunit on the cell bodies and dendritic profiles of KF neurons in rats. The α1 and β2/3 subunits were mainly present on KF neuron dendrites, while they were also detectable on parabrachial neuron somas. Moderate staining was present on KF somas for α2 and α3 subunits, however, these subunits were not evident on dendritic processes in the KF.
Such detailed specific description of the expression of GABA-B receptor variants in the KF is not available. Nevertheless, strong immunohistological labeling of GABA-B-R1 variant was shown in general in the ‘pontine nuclei’ (Margeta-Mitrovic et al. 1999), while a different study found that the GABA-B-R1b variant (the more prevalent version of GABA-B-R1) was seldom expressed in the pons (Fritschy 1999). While the exact variant of GABA-B receptors in the KF is currently not known, acute KF slice experiments conducted in our laboratory provides functional evidence for the presence of GABA-B receptors on KF neurons. Application of the GABA-B agonist baclofen leads to the activation of G-protein coupled inwardly rectifying K+ (GIRK) conductance in a large population (~60%) of KF neurons in both rats and mice (Levitt et al. 2015; Levitt and Williams 2018; Varga et al. 2020). Furthermore, the GABA-B receptor positive neurons are also sensitive to opioids (Varga et al. 2020; Levitt and Williams 2018).
GABA transporters in the KF
Out of the currently known GABA transporter genes, the ones prevalently present in the central nervous system are GAT1 and GAT3 (for a review on GABA transporters see (Zhou and Danbolt 2013)). In the rat KF, an in situ hybridization study detected the presence of both GAT1 and GAT3 mRNA (Durkin et al. 1995). Meanwhile, also performed in rats, immunohistochemical characterization of the KF region revealed contrasting results, with prominent GAT3 immunoreactivity, but no GAT1 labeling at all (Guthmann et al. 1998). Immunolabeling for GAT3 was exclusive to glia and neuronal processes, but not cell bodies, which is in agreement with previous reports on GAT3 expression (Ribak et al. 1996; Minelli et al. 1996; Minelli et al. 1995; Guthmann et al. 1998). Arguably, the presence of GAT1 mRNA detectable by in situ hybridization does not necessitate the expression of the GAT1 protein (detectable by immunohistochemistry), nevertheless further supportive evidence is required to reconcile this discrepancy. Additionally, the GABA vesicular transporter (Vgat) has been shown to co-localize with FoxP2 in the caudal portions of the KF (Geerling et al. 2017).
Sources of GABA in the KF
In the mammalian brain, GABA is synthesized via decarboxylation of glutamate into GABA and CO2, by the catalytic enzyme glutamic acid decarboxylase (GAD). GAD is encoded by two genes in two isoforms, GAD65 and GAD67 (Erlander et al. 1991). While both isoforms are present throughout the neuron, GAD65 is more prominent in axon terminals (Kaufman et al. 1991). GAD immunolabeling has been shown in KF somas as well as axon terminals (Ford et al. 1995; Guthmann et al. 1998). The regional distribution of GAD65 and GAD67 mRNA and/or protein is densely clustered and overlaps in the caudal portions of the KF and the lateral parabrachial nucleus. Both classes of GAD show overlapping, but scattered expression patterns in the rest of the parabrachial complex in rodent brains (Guthmann et al. 1998; Geerling et al. 2017). Thus far two subpopulations of GAD67-expressing neurons have been identified in the caudal KF, with one group co-expressing the transcription factor FoxP2, while the other group lacking FoxP2 (Gray 2008; Miller et al. 2012; Geerling et al. 2011; Geerling et al. 2017). The KF also receives GABAergic inhibitory input from the RTN, NTS, and the Bötzinger complex (Figure 2; Ezure et al., 2003; Silva et al., 2016).
Functional role of GABA in the KF
Although the majority of KF neurons are glutamatergic, the more caudal KF contains GABAergic clusters of neurons that also have extensive projections throughout the brainstem (Geerling et al. 2017). The most prominent GABAergic projections originating from the KF target the sensory trigeminal nuclei, showing dense labeling at the level of pars interpolaris and extending with sparse projections all the way to the medullary reticular formation (Geerling et al. 2017). Most of these projections contact ipsilateral target sites. Geerling and colleagues (2017) also found GABAergic projections from KF to the hypoglossal and facial motor nuclei, the NTS and the ventrolateral medulla (including the Bötzinger and pre-Bötzinger complexes). Rostral GABAergic projections from the KF extend to the periaqueductal gray, midbrain reticular formation and a subset of hypothalamic nuclei (Geerling et al. 2017).
A range of pharmacological studies have provided clues about the role of GABAergic signaling in the KF (to review, see (Browaldh et al. 2016)). Direct administration of GABA-A receptor agonists into the KF has a strong effect on respiratory related functions. For instance, locally applied muscimol decreases respiratory rate, increases the length of inspiration and expiration, increases resting diaphragm EMG amplitude, decreases diaphragm EMG frequency, and decreases genioglossus EMG amplitude in anesthetized, vagotomized rats (Silva et al. 2016). In a different study, using the in situ preparation of the rat, GABA-A agonist isoguvacine abolished post-inspiration and eupneic hypoglossal activity, while also disrupting the breathing pattern (Bautista and Dutschmann 2014a). Isoguvacine injected into the KF also leads to apneusis, abolishes laryngeal adductor activity (Dutschmann and Herbert 2006), and causes drastic changes in the baseline respiratory pattern that have been implicated in the coordination of swallows (Bautista and Dutschmann 2014b; Bautista et al. 2014).
Disinhibition of the KF by bicuculline, a GABA-A receptor antagonist, disrupts the swallowing reflex and leads to delayed swallowing in the in situ preparation of the rat (Bautista and Dutschmann 2014b). Such failure to properly coordinate swallowing and breathing could lead to aspiration. Injection of bicuculline into the KF exacerbates respiratory depression and bradycardia evoked by ethmoidal nerve (sensory branch of the trigeminal nerve) stimulation (Dutschmann and Herbert 1998). These data combined with the anatomical data showing dense KF projections to the trigeminal nuclei, support the KF’s potential role in coupling respiratory and trigeminal inputs, perhaps to coordinate orofacial/whisking behaviors with breathing, or to initiate the diving reflex (Dutschmann and Herbert 1996; Geerling et al. 2017; Dutschmann and Herbert 1998).
Based on the wide spectrum of results, it is clear that the KF is an important mediator of many respiratory related behaviors and GABAergic neurotransmission plays a crucial role in the coordination of the sequential steps involved in these behaviors. As discussed in the “Neurochemical alterations in the context of respiratory dysfunction” section, alterations in KF GABAergic neurotransmission have serious consequences in the pathophysiology of respiratory dysfunction.
Glycine
Glycine Receptor Expression
The amino acid glycine serves as one of the main inhibitory neurotransmitters in the brainstem and spinal cord. The glycine receptor (GlyR) is an ionotropic heteropentamer, located primarily in the brainstem and spinal cord (Rajendra et al. 1997). GlyRs are ligand-gated chloride channels allowing for rapid postsynaptic inhibition. Glycinergic inhibition has been implicated in the modulation of respiratory frequency and pattern (Schmid et al. 1991; Janczewski et al. 2013). A functional GlyR consists of three α subunits and two β subunits, with each α subunit containing a glycine-binding site, while the β subunits are thought to anchor GlyR at synaptic sites (Herbert et al. 2000; Grudzinska et al. 2005).
The expression of mRNA for the α1, α2, and β subunits of the glycine receptor in the parabrachial complex and specifically in the KF has been confirmed by in situ hybridization (Fujita et al. 1991; Sato et al. 1991; Sato et al. 1992). GlyRα1 subunit expression in the KF has also been documented by immunohistochemistry, with differences in both staining intensity and pattern noted between the rostral and caudal portions. GlyRα1 immunoreactivity in the rostral KF is strong, granular and diffusely distributed throughout the fibers, whereas weaker staining of cell bodies and dendrites can be observed in the caudal KF of rats (Herbert et al. 2000).
Glycine Transporter Expression
Glycine transporters consist of 12 transmembrane domains and rely on the co-transport of Na+/Cl− into the cell with glycine (Zafra and Giménez 2008). There are two gene products for the glycine transporter, GlyT1 and GlyT2. GlyT1 is expressed mainly in glial cells, including in astrocytes, where it influences excitatory neurotransmission through the regulation of glycine levels at glutamatergic synapses containing the NMDA receptor, at which glycine acts as a co-agonist (Gabernet et al. 2005). Under this framework, reduced GlyT1 expression corresponds with a potentiation of NMDA receptor function. In the KF of rats, GlyT1 immunoreactivity is relatively weak compared to the surrounding parabrachial nuclei, despite strong expression of mRNA for the NR1 subunit of the NMDA receptor (Herbert et al. 2000).
The GlyT2 transporter is located on the axons and other presynaptic elements of glycinergic neurons, and mediates the uptake of glycine from the extracellular space before it is packaged into vesicles (Rousseau et al. 2008). The uptake of glycine by GlyT2 controls the intracellular concentrations of glycine and therefore determines the vesicular content of glycine (Rousseau et al. 2008). In the parabrachial complex, including the KF, GlyT2 expression is limited to areas that express either glycine or GlyRα1 immunoreactivity (Herbert et al. 2000).
Sources of Glycine in the KF
Glycine is made from serine using the enzymes serine hydroxyl-methyl-transferase and glycine decarboxylase (Hernandes and Troncone 2009). However, the concentrations of glycine available at terminals is significantly higher than can be synthesized locally, indicating that uptake by GlyT2 or other forms of local recapture are needed to maintain presynaptic glycine supplies (Rousseau et al. 2008). Glycine immunoreactivity in the KF takes the form of punctate labeling along axonal fibers and at the terminals of presumed afferent axons. Of these glycine-immunoreative puncta, some are located adjacent to unlabeled cell bodies, suggesting that the KF contains axosomatic glycinergic contacts (Herbert et al., 2000) . Additionally, the presence of scattered cell bodies outlined by glycine immunoreactivity has also been consistently documented in the rat KF (Rampon et al., 1996; Herbert et al., 2000).
Using transgenic mice and Cre-dependent viral labeling, Yang & Feldman (2018) documented projections from GlyT2-positive glycinergic neurons in the pre-Bötzinger complex to many brainstem regions, including the KF (Figure 2). These glycinergic projections parallel projections from glutamatergic somatostatin-positive pre-Bötzinger neurons to many respiratory regions, but their function has not yet been identified.
While no other glycinergic projections to the KF have been directly confirmed, several regions that project to the KF are known to contain glycinergic neurons. Some pump cells of the NTS have been shown to express GlyT2 mRNA, and are thought to co-release GABA and glycine (Ezure and Tanaka 2004). NTS pump cells are known to project to the KF, and may therefore represent a source of glycinergic input (Ezure and Tanaka 2006). Afferent projections also extend to the KF from the inhibitory augmenting expiratory neurons of the Bötzinger complex (Ezure et al. 2003b), some of which co-release glycine and GABA (Schmid et al. 1996), have also been found.
Functional role of Glycine in the KF
While both GABAergic and glycinergic synaptic currents are present in KF neurons, the glycinergic component becomes more predominant later in development, at least in the rat brain (Kron et al. 2007b). Despite the role of the KF as a relay site in trigeminally-evoked bradycardia and respiratory suppression, it appears that transmission through glycine receptors in the KF is not essential for modulating nasotrigeminal reflex responses in rats (Dutschmann and Herbert 1998). While unilateral injections of the GABA-A antagonist bicuculline in the KF enhance trigeminally-evoked bradycardia and respiratory suppression, injections of the GlyR antagonist strychnine does not significantly alter these autonomic responses (Dutschmann and Herbert 1998).
Acetylcholine
Acetylcholine receptor expression in the KF
Acetylcholine (ACh) is a neurotransmitter that activates two flavors of receptor: the ionotropic nicotinic and metabotropic muscarinic acetylcholine receptor (nAChR and mAChR respectively). Both receptors are expressed throughout the central nervous system (Wamsley et al. 1984; Tribollet et al. 2004; Levey et al. 1991) and modulate the excitability and firing rate of neurons (Papke 2014; Felder 1995; Wess 1996). The nAChR is a ligand-gated pentameric cation channel that is expressed pre- and/or post-synaptically and formed by various compositions of α (α 2-10) and β (β 2-4) subunits. Activation by ACh or nicotine causes depolarization and increases neuronal excitability (Papke 2014). Conversely, the mAChR, activated by ACh or muscarine, is coupled to G-proteins and expressed in five different receptor subtypes (M1-M5). M1, M3, and M5 receptor subtypes are excitatory and couple to Gq, while M2 and M4 are inhibitory and couple to Gi/o (Wess 1996; Felder 1995).
Autoradiography studies performed in the lateral parabrachial nucleus identified the expression of α7 nAChR in the vicinity of the KF in rats (Tribollet et al. 2004). In human studies, an increase in α7 nAChR expression was observed in the KF of fetuses and early newborns with smoking mothers (Lavezzi 2018). α2-containing nAChRs are also expressed in the parabrachial complex of rats and mice (Ishii et al. 2005).
There are few histological studies regarding the expression of mAChRs in the KF. Using a nonselective mAChR antagonist probe, mAChR expression was shown in the parabrachial complex by autoradiography (Wamsley et al. 1984), however, receptor subtype expression was not assessed. Examination of the Allen Brain Atlas suggests mAChR mRNA expression in the KF. Further support for mAChR expression specifically in the parabrachial complex and specifically in the KF comes from physiological studies detailed below in the “Functional Implications of Acetylcholine” section.
Sources of Acetylcholine in the KF
Acetylcholine is synthesized in cholinergic neurons by choline acetyltransferase (ChAT). ChAT is expressed in two pontine tegmental nuclei that are nearby the parabrachial complex: 1) the pedunculopontine tegmental nucleus, which is rostral to the parabrachial complex, and 2) the laterodorsal tegmental nucleus, which is medial to the parabrachial complex (Armstrong et al. 1983). Representing the main source of acetylcholine for most forebrain and hindbrain structures, local projections from the pontine tegmental nuclei likely contribute to KF cholinergic signaling (Figure 2; Woolf & Butcher, 1989). In addition, immunohistochemistry identified ChAT expression locally, in the parabrachial complex of cats (Jones and Beaudet 1987). Recently taking advantage of ChATCre transgenic mice, lateral parabrachial and KF neurons have been shown to co-express cholinergic and glutamatergic markers transiently during development, which may be important for maturation of excitatory synapses (Nasirova et al. 2020).
Acetylcholine transporters in the KF
Acetylcholine is packaged into synaptic vesicles by vesicular ACh transporter (VAChT; (Parsons et al. 1993). Coexpression of ChAT and VAChT in a neuron indicates functional cholinergic neurotransmission (Schäfer et al. 1994). In situ hybridization identified VAChT mRNA in the parabrachial complex of adult rats (Schäfer et al. 1994). Furthermore, this finding is observed in the Allen Brain Atlas, which shows VAChT expression in the KF and parabrachial nuclei. In the synaptic cleft, Ach is rapidly hydrolyzed to acetate and choline by acetylcholinesterase (AChE). Choline uptake and recycling is then performed by the choline transporter 1 (CHT1), which is distributed in low densities on fibers in the pontine nuclei (Misawa et al. 2001).
Functional Implications of Acetylcholine
Although few histological studies have confirmed the expression of mAChR in the KF, functional studies provide further support for local expression of this receptor subtype. Egan and North (1986) found that muscarine (mAChR agonist) hyperpolarized parabrachial complex neurons in rat intracellular recordings by acting through the M2 mAChR subtype. Additionally, microdialysis of atropine, a mAChR antagonist, into the KF of goats has been shown to decrease pulmonary ventilation and breathing frequency at night (Bonis et al. 2010b). In a later study using the arterially perfused working heart-brainstem preparation, carbachol, a nonselective AChR agonist, was microinjected into the pons of rats (Brandes et al. 2011). While the study did not focus on the effects of AChR agonist on the KF, in a couple of animals carbachol unilaterally injected in the KF led to increases in respiratory rate, indicating the presence of mAChRs (Brandes et al. 2011). These functional studies, considered in conjunction with the above histological evidence, support mAChR expression in the parabrachial complex and more specifically the KF. Nevertheless, future research, with particular attention given to understanding the specific expression of mAChR subtypes (M1-M5) should provide important details regarding the cholinergic neurochemistry of the KF and its impact on breathing.
Norepinephrine
Norepinephrine receptor expression in the KF
Norepinephrine (NE), or noradrenaline, is a catecholamine synthesized by noradrenergic neurons in the brainstem. NE has differential effects on target neurons depending on the adrenergic receptor subtype(s) present. Adrenergic receptors are GPCRs that can be broadly classified as α1, α2, or β receptors, each of which has three subtypes (α1A-C, α2A-C, and β1-3) (for review, see (Lefkowitz 1990; Strosberg 1993)). α1 receptors are excitatory Gq-coupled, while α2 receptors are inhibitory Gi/o-coupled. All three β receptor subtypes are Gs-coupled.
A wide range of histological methods, including autoradiography and in situ hybridization, indicate the presence of α2 receptors in the pontine nuclei, including the parabrachial nuclei and the KF (Scheinin et al. 1994; Unnerstall et al. 1985; Herbert and Flügge 1995; Andrade et al. 2014; Davern 2014). Specifically, moderate to intense labeling for α2A and α2B-receptor subtypes has been documented in the KF of multiple species (Rosin et al. 1993; Wang et al. 1996; Tavares et al. 1996; Strazielle et al. 1999). To our knowledge, there are no reports directly addressing the presence of β adrenergic receptors in the KF. Since the pons and medulla have been shown to only express very low amounts of β-receptors, the KF likely only expresses a limited amount (if any) of this adrenergic receptor subtype (Palacios and Kuhar 1982).
Norepinephrine transporter in the KF
The norepinephrine transporter (NET) plays a key role in regulating noradrenergic signaling in the brain by maintaining intracellular NE stores and via NE reuptake from the synapse into the presynaptic terminals (Torres et al. 2003). NET mRNA labeling has been described in locations consistent with the presence of catecholaminergic cell groups A4, A5, A6 (or locus coeruleus), A7, A1/C1 and A2/C2 (Lorang et al. 1994). Additionally, the presence of NET correlates with the level of noradrenergic innervation, with autoradiography demonstrating moderate levels of the transporter in the KF region of the mouse brain (Strazielle et al. 1999).
Sources of norepinephrine in the KF
The primary source of NE throughout the brain is the locus coeruleus (LC), however other noradrenergic areas also contribute (Robertson et al. 2013; Swanson and Hartman 1975; Schwarz and Luo 2015; Jones and Moore 1977). Indeed, noradrenergic neurons form an almost continuous column of NE producing nuclei in the hindbrain (Robertson et al. 2013). The cellular makeup and developmental origin of the nuclei is somewhat heterogeneous, with each neuron subtype derived from one of six distinct rhombomere-based genetic lineages (r1-r6, (Robertson et al. 2013)). The parabrachial complex, including the KF, receives axonal projections from r1-derived neurons that make up the majority of the LC, as well as neurons of r3 , r4 and r5 origin, which are present in the subcoeruleus area, A7, A5, and the adrenergic/noradrenergic C1/A1 and C2/A2 areas in the mouse medulla (Figure 2; Robertson et al., 2013). Projections from the LC and subcoeruleus to KF have also been demonstrated by retrograde tracing studies in cats and monkeys (Hancock and Fougerousse 1976). Additionally, a range of anatomical studies performed on cat, rat and mouse brains, indicate the presence of noradrenergic cells in the KF, many of which refer to the region as KF/A7, thus combining the KF with the noradrenergic A7 nucleus (Hermanson and Blomqvist 1995; Stevens et al. 1982; Reddy et al. 1991; Strazielle et al. 1999). Some of these studies imply that the noradrenergic neurons located in the KF functionally belong to the A7 nucleus, but cannot be properly distinguished due to lack of anatomical borders. However, a subset of experiments (done on the same species) indicate that the KF and A7 are independent pontine nuclei, both of which contain NE producing neurons that differentially express a range of other neuropeptides (Figure 2; Holstege & Kuypers, 1982; Clark & Proudfit, 1991; Reddy et al., 1991).
Functional role of norepinephrine in the KF
Most results on the role of noradrenergic KF neurons come from anatomical tracing studies and provide consistent results from a range of species. However, direct functional and physiological evidence is still lacking. The majority of NE producing KF neurons project to the superficial laminae of the dorsal horn and the thoracic sympathetic cell column, and these projections have an inhibitory effect on spinal motoneurons (Reddy et al. 1991; Clark and Proudfit 1991; Holstege and Kuypers 1982; Stevens et al. 1982). The specific role or roles of noradrenergic KF neurons in modulating spinal control of behavior remains unknown.
Serotonin
Serotonin receptor expression in the KF
There are seven general classes of serotonin receptors that include 14 known receptor subtypes (Hoyer et al. 2002; Barnes and Sharp 1999). Serotonin receptors are GPCRs with the exception of 5-HT3 receptors that are ligand-gated ion channels (Bockaert et al. 2006; Maricq et al. 1991; Van-Hooft and Yakel 2003). Of all serotonin receptor types, the 5-HT1A, 5-HT2A, 5-HT2B, 5-HT2C, 5-HT4, and 5-HT7 receptors have been implicated in the involvement of the mammalian respiratory network and therefore speculated to be present in the KF (Barrett et al. 2012; Erickson et al. 2007; Yamauchi et al. 2008; Richter et al. 2003; Dhingra et al. 2016). Current studies have explicitly shown the expression of Gi/o-coupled 5-HT1A, and Gq-coupled 5-HT2A and 5-HT2B receptors in the rodent KF (Dhingra et al. 2016; Niebert et al. 2011).
Sources of serotonin in the KF
In the central nervous system, serotonin is primarily produced in the raphe nuclei found in the midbrain and brainstem (Törk 1990). Among these, the caudal raphe projects extensively to the pontine respiratory group, which includes the KF (Figure 2; Steinbusch, 1981; Steinbusch et al., 1981; Hilaire et al., 2010). Afferent projections from the KF to the nucleus raphe magnus of the medulla have also been shown, thus completing reciprocal connections between the raphe and the KF (Gang et al. 1991; Gang et al. 1990; Gang et al. 1993).
Functional role of serotonin in the KF
Although the activation of 5-HT receptors has been implicated in a wide variety of neurological processes, its effects on respiration provide the most insight into the role of serotonin in the KF (for a review on 5-HT’s role in respiration see Hodges & Richerson, 2008).
Intracerebroventricular (ICV) administration of a 5-HT1A receptor antagonist, WAY100635, increases respiratory variability and silences upper airway muscle respiratory activity in rats (Besnard et al. 2012). In contrast, ICV administration of a 5-HT1A receptor agonist, 8-OH-DPAT, increases upper airway muscle activity (Besnard et al. 2012). Given the importance of the KF in coordination of the upper airways, these effects could be mediated in part by the KF. 5-HT1A receptor activation leads to a substantial increase in the immediate early gene Fos expression in many respiratory nuclei, including the KF (Besnard et al. 2012). Administration of 8-OH-DPAT, a 5-HT1A receptor agonist, directly into the KF improves respiratory regularity in a mouse model of Rett syndrome, providing further evidence for functional 5-HT1A receptors in the KF (Abdala et al. 2010). The importance of KF 5-HT1A receptors on variability was also confirmed in a later study where microinjection of the antagonist WAY100635 into the KF led to a significant increase in respiratory pattern variability, characterized by spontaneous central apneas in mice (Dhingra et al. 2016). Together these data provide evidence for the KF’s involvement in the serotonergic modulation of the upper airways and respiratory pattern control.
Opioids
Opioid receptor expression in the KF
Opioid receptors are Gi/o protein-coupled receptors that are widely distributed throughout the mammalian brain and have been implicated in a multitude of physiological processes depending on their location, ranging from analgesia to reward and addiction, stress resilience and homeostatic control (Toubia and Khalife 2019; Adams et al. 1986). The three major types of opioid receptors are delta (DOR), kappa (KOR) and mu opioid receptors (MOR; for reviews on opioid receptors see Waldhoer et al., 2004; Williams et al., 2013; Manglik, 2019). MORs are abundantly expressed in the KF based on immunohistochemistry and in situ hybridization in rats and analysis of genetic knock-in mice with mCherry-tagged MOR (Chamberlin et al. 1999; Ding et al. 1996; George et al. 1994; Erbs et al. 2014). Moderate intensity of DOR expression was documented by both immunohistochemistry and in situ hybridization in the parabrachial complex (Cahill et al. 2001). Similarly, DOR positive fibers and varicosities, as well as DOR immunoreactive cell bodies were detected in the parabrachial complex and A7 area (Arvidsson et al. 1995). KOR expression has not been documented in the KF.
Physiological studies in rodents also support the expression of MORs in the KF (Levitt et al. 2015; Varga et al. 2020) and nearby parabrachial nuclei (Prkic et al. 2012; Miller et al. 2017). In single-cell recording of KF neurons in brain slice, about 60% of KF neurons express somatodendritic MORs (Levitt et al. 2015). There is no functional evidence of somatodendritic DORs, since the effects of the MOR/DOR agonist [Met5]-enkephalin are blocked by the selective MOR antagonist CTAP (Levitt et al. 2015) and are absent in neurons with MORs deleted (Varga et al. 2020).
Sources of endogenous opioids in the KF
Endogenous opioid peptides have important roles in a wide range of physiological functions, including analgesia, stress relief, feeding behaviors, and importantly, cardiorespiratory control (Adams et al. 1986; Toubia and Khalife 2019). Endogenous opioids are produced via enzymatic splicing of pre-pro-peptides and the functional products of splicing are categorized as: β-endorphin that binds to MOR, enkephalins that bind to DOR and MOR, and dynorphins that bind to KOR and MOR (Corder et al. 2018; Waldhoer et al. 2004). β-endorphin is cleaved from pro-opioimelanocortin in the hypothalamus/pituitary and NTS (Figure 2; Lee & Wardlaw, 2007; Cerritelli et al., 2016). Similarly, endogenous enkephalins are cleaved from the precursor protein pre-pro-enkephalin. Functional enkephalin neuropeptides, Met-and Leu-enkephalin, have high affinity for DOR and also bind to MOR (Udenfriend and Kilpatrick 1983; Corder et al. 2018; Adams et al. 1986). Endogenous opioids are released in response to pain, stress and/or inflammation, and even in response to the administration of exogenous opioids (Schlen and Bentley 1980; Holaday et al. 1977; Adams et al. 1986).
Enkephalin is produced locally in the KF (Figure 2; Blomqvist et al., 1994; Hermanson & Blomqvist, 1995). In situ hybridization in rats revealed a large population of parabrachial complex neurons, including KF neurons, that express pre-pro-enkephalin mRNA (Hermanson and Blomqvist 1997; Engström et al. 2001). KF neurons containing pre-pro-enkephalin send projections to the paragigantocellular nucleus of the ventral medulla, as well as the spinal cord, and participate in analgesia (Blomqvist et al. 1994). Similarly, β-endorphin can be detected with immunolabeling in moderate to high levels in the parabrachial complex of rats, however this study did not specifically investigate peptide expression in the KF (Palkovits and Eskay 1987). On the other hand, endomorphin-1 and endomorphin-2 immunoreactive fibers are distributed differentially in the KF, with endomorphin-2 showing dense labeling and endomorphin-1 only present on a few fibers in rats and guinea pigs (Martin-Schild et al. 1999). Endomorphin-2 labeling is more intense in the KF of mice compared to rats (Martin-Schild et al. 1999).
Functional role of opioids in the KF
While not much is known about the exact function of endogenous opioids in the KF, there are multiple studies focusing on the effects of exogenous opioids. Exogenous MOR agonists have been shown to play a substantial role in opioid-induced respiratory rate depression and breathing pattern degradation in multiple species (Prkic et al. 2012; Miller et al. 2017; Lalley et al. 2014; Levitt et al. 2015; Saunders and Levitt 2020; Varga et al. 2020). Opioid agonists directly hyperpolarize a population of KF neurons by activating GIRK conductance (Levitt et al. 2015). Further details on the KF’s role in opioid-induced respiratory depression are out of the scope of this review.
Neuropeptide S
Neuropeptide S receptor expression in the KF
Neuropeptide S (NPS) is a regulatory neuropeptide expressed in the brainstem. The NPS system has been associated with modulation of many different central functions, including stress/anxiety, arousal, feeding and digestion, addiction, memory, and pain (for review see Guerrini et al., 2010). Expression of mRNA for the NPS precursor peptide in rodents appears to be limited to a few pontine areas, including the parabrachial complex, and specifically the KF. However, expression of the NPS receptor, NPSR, is more widespread, with mRNA distributed throughout areas associated with sleep, anxiety, and sensory processing, including parts of the hippocampus, hypothalamus, and the amygdaloid cortex, as well as in the piriform cortex (Xu et al. 2007). NPSR is a Gq and Gs-coupled receptor, producing increases in intracellular Ca2+ and cAMP after NPS administration (Reinscheid et al. 2005; Xu et al. 2004). Compared to cortical and thalamic areas, low expression of NPSR mRNA has been found in the brainstem (Reinscheid and Xu 2005). In the lateral parabrachial nucleus and KF of mice, only a few scattered NPSR-expressing fibers were found with immunohistochemistry, while in situ hybridization for NPSR mRNA revealed moderate expression in the lateral parabrachial nucleus (Clark et al. 2011).
Sources of Neuropeptide S in the KF
Using in situ hybridization for NPS precursor mRNA, Clark et al. (2011) demonstrated that in the mouse brain, NPS-producing neurons were isolated to the KF and the pericoerulear area (Figure 2). In contrast, NPS-producing neurons in the rat were limited to the area of the principle sensory trigeminal nuclei, the pericoerulear area (LC and nearby catecholaminergic clusters), and the lateral parabrachial nucleus, with a few scattered fibers in the dorsomedial hypothalamus and amygdala. In rats, the number of NPS-producing cells is greater in the LC cluster than the lateral parabrachial cluster, while in mice, the number of NPS-producing cells in the parabrachial cluster was greater than in the LC cluster (Liu et al. 2011). Additionally, the lateral pararachial cluster is considered to align with the KF in mice, but not in rats. However, the parabrachial cluster in both species was found to co-express corticotropin-releasing factor, suggesting they may be functionally similar, despite slight differences in spatial location. In humans, expression of NPS precursor mRNA has been documented in the parabrachial nuclei and KF (Adori et al. 2015).
Functional Role of Neuropeptide S in the KF
In other brain regions, such as the pericoerulear cluster, NPS neurons have been shown to be glutamatergic, suggesting a close association between NPS and excitatory signaling. In the rat parabrachial complex, NPS-expressing neurons have been shown to co-express corticotropin-releasing factor, implicating NPS signaling in arousal and anxiety (Xu et al. 2007). Short term stress, induced via a 10-minute swim test or a 20-minute prolonged restraint test, caused expression of cFos protein in NPS-producing neurons, though co-localization was lesser than in the LC cluster of NPS-producing neurons (Liu et al. 2011). This implies that both the KF and LC clusters of NPS-producing neurons are activated by stress, suggesting they may play a role in anxiety, arousal and other stress-mediated behaviors. Additionally, it is thought that the NPS-producing neurons of the KF region may receive synaptic inputs from Orexin A/hypocretin-positive cells, suggesting a potential role for the KF-NPS system in the arousal-mediated modulation of respiration in mice (Liu et al. 2011). Arousal signaling in the parabrachial complex is particularly important in respiratory control, with the KF and parabrachial nuclei shown to play a role in state-dependent, high CO2-induced arousal (or hypercapnic arousal) - a staple of sleep apnea (Kaur et al. 2013; Kaur and Saper 2019; Kaur et al. 2017).
The NPS peptide is predominantly produced in areas implicated in the control of respiration (Xu et al. 2007). The NPS/NPSR system has also been demonstrated to modify respiratory parameters in vivo. ICV administration of NPS in wild-type and NPSR1-deficient mice resulted in receptor-dependent increases in respiratory frequency and decreases in tidal volume, demonstrating that administration of NPS can directly alter respiratory function through actions initiated in the central nervous system (Zhu et al. 2011).
Orexin
Orexin receptor expression in the KF
Orexins (ORX) or hypocretins are excitatory neuropeptides expressed in two splice variants (ORX-A and ORX-B) in the central nervous system (reviewed in Wang et al., 2018). Orexin receptors (OXR) are GPCRs with two identified isoforms, OX1R and OX2R (Sakurai et al. 1998). ORX-A binds to both OX1R and OX2R with equal affinity, while ORX-B binds mainly to OX2R (Langmead et al. 2004). Marcus and colleagues (2001) have found that OX1R and OX2R mRNA are differentially expressed in the rat brain, as identified by in situ hybridization, with both OX1R and OX2R mRNA only weakly expressed in the KF and rest of the parabrachial complex (Marcus et al. 2001). In contrast, using immunohistochemistry, Yokota et al. (2016) identified a substantial amount of OX2R-expressing neurons in the rat KF. Tracing methods indicate that the projection targets of these OX2R-positive KF neurons are mainly the rostral ventral respiratory group, the phrenic nucleus in the spinal cord, and the hypoglossal nucleus (Yokota et al. 2016). Further supportive data for the existence of OX2Rs in the KF and the projection targets of these neurons came from physiological experiments also conducted in rats (Dutschmann et al. 2007). In these experiments ORX-B microinjected into the KF lead to an increase in both hypoglossal and phrenic nerve output in the in situ working heart-brainstem preparation, likely resulting from ORX-B binding to the OX2R on KF neurons.
Sources of orexin in the KF
Orexins are exclusively produced by hypothalamic orexinergic neurons, located primarily in the tuberal part of the hypothalamus (Peyron et al. 1998; Nambu et al. 1999; Modirrousta et al. 2005; De Lecea et al. 1998; Sakurai et al. 1998). Among many other roles, it has been hypothesized that orexin is important in modulating respiration via multiple respiratory projection targets (Williams and Burdakov 2008; Deng et al. 2007; Zhang et al. 2005). Indeed, anatomical tracing studies showed that orexinergic neurons send axonal projections to the KF and other parabrachial nuclei (Figure 2; Peyron et al., 1998). Yokota and colleagues (2016) demonstrated through retrograde cholera toxin B subunit injections in the KF that up to ~23% of labeled neurons originating in the hypothalamus are immunoreactive for orexin. These neurons are predominantly distributed in the medial and perifornical regions of the hypothalamus, and additional labeling can be detected in the lateral region of the hypothalamic orexin region (Figure 2). The orexin-neuron axon terminals make asymmetrical synapses with somata and dendrites of KF neurons (Yokota et al. 2016).
Functional role of orexin in the KF
ORX serves an important role in regulating energy homeostasis and food intake (Sakurai 2002; Gregor Sutcliffe and De Lecea 2000; Willie et al. 2001), reward and addiction (Harris and Aston-Jones 2006), autonomic function (Kuwaki 2008; Williams and Burdakov 2008; Willie et al. 2001) and sleep-wake states (Sakurai 2007). Most relevant to this review, it has been shown that lack of orexin may lead to sleep disorders, such as narcolepsy (Chemelli et al. 1999; Willie et al. 2001) and sleep apnea (Nakamura et al. 2007). The wide range of functions served by the orexin system are controlled by two subpopulations of ORX neurons in the hypothalamus, with neurons located in the lateral hypothalamus being involved in food intake, reward and addiction, and neurons in the medial and perifornical hypothalamic regions modulating wakefulness and arousal states (Harris and Aston-Jones 2006). Based on the results of Yokota et al. 2016, the majority of hypothalamic neurons projecting to the KF originate in the medial and perifornical areas, hinting at a potential contribution of ORX-modulated KF neurons to the control of sleep-wake state and arousal. This hypothesis is in line with studies demonstrating increased activity of parabrachial complex, including KF area neurons during wakefulness (Sieck and Harper 1980; Lydic and Orem 1979). Additionally, Dutschmann et al. (2007) found that ORX-B microinjected into the KF of rats leads to transient increases in respiratory rate. This increase in breathing also coincides with an increase in hypoglossal motor output, linking orexinergic modulation of KF neural output to increased upper airway patency. This function has clear implications not only in the state-dependent control of respiration, but also in the reduced upper airway resistance, characteristic of obstructive sleep apnea (Dutschmann et al. 2007).
Somatostatin
Somatostatin receptor expression in the KF
Somatostatin (SST; also called growth hormone release-inhibiting hormone and somatotropin release-inhibiting factor) is a neuropeptide that is expressed in three prosomatostatin-derived peptide isoforms (SST-14, SST-28 and SST-28(1-12)) throughout the mammalian central nervous system (Bruno et al. 1992; O’Carroll et al. 1992; Günther et al. 2018). SST achieves its inhibitory effects via Gi/o protein-coupled SST receptors. Six different receptor subtypes have been described for SST to date, SST1-5, with SST2 receptors present in two forms, SST2a and SST2b (Vanetti et al. 1992; Vanetti et al. 1994; Yasuda et al. 1992; Bruno et al. 1992; O’Carroll et al. 1992). In human brains high levels of SST receptor expression has been identified by autoradiographic studies in the parabrachial complex that is highest during the early stages of fetal development and goes through an abrupt decrease during the perinatal period (Carpentier et al. 1996; Carpentier et al. 1997). Importantly, the location of binding sites generally overlaps with SST-positive fibers in infants (Chigr et al. 1989; Carpentier et al. 1997).
Sources of somatostatin in the KF
SST is initially secreted as pre-pro-somatostatin, an inactive precursor protein, which is then cleaved into pro-somatostatin. Pro-somatostatin is further processed into the above described active peptide isoforms (Kumar and Grant 2010). The active peptide SST is widely produced throughout the body, including in the gastrointestinal mucosa, the pancreas, and importantly throughout the central nervous system (Arimura et al. 1975; Polak et al. 1975; Patel and Reichlin 1978; Finley et al. 1981; Johansson et al. 1984). The main source of SST in the brain is the hypothalamus, however other sites, such as areas in the cortex, brainstem and spinal cord, also contribute to the distribution of the neuropeptide (Barnett 2003; Johansson et al. 1984; Finley et al. 1981). For instance, in the mouse brain, SST-expressing excitatory pre-Bӧtzinger complex neurons project to the KF (Figure 2; Yang & Feldman, 2018). Immunocytochemistry on cat brains has revealed a dense distribution of SST-28 (1-12) immunoreactive fibers as well as cell bodies in the KF area, however the pons in general had less immunoreactive structures compared to the medulla (De León et al. 1992). In addition, the parabrachial complex has been shown to have moderate to high densities of immunoreactive neurons for SST labeling (Figure 2; Johansson et al., 1984). To our knowledge, there is no direct evidence for the KF’s involvement in SST production. However, the results of retrograde tracing experiments combined with pre-pro-somatostatin mRNA labeling indicate that the KF represents one of the major sources of SSTergic projections to the rostral ventrolateral medulla and thus may be directly involved in the distribution of SST throughout the adult rat brain (Bou Farah et al. 2016).
Functional role of somatostatin in the KF
Taking into consideration the wide range of projections from the KF to most brainstem respiratory nuclei and the high density of SST receptor expression in most of these regions, KF SSTergic neurons likely play a key role in cardiorespiratory control (Bou Farah et al. 2016; Barnett 2003; Günther et al. 2018; Dutschmann and Dick 2012). However, the direct effect of SST on KF neurons and respiratory and/or cardiovascular control remains unknown.
Neurochemical alterations in the context of respiratory dysfunction
Sudden Infant Death Syndrome (SIDS)
SIDS is defined as the sudden unexpected death of a seemingly healthy infant during sleep, the cause of which remains unknown even after a complete autopsy (Krous et al. 2004). The underlying cause of SIDS is not clear, however the most compelling hypothesis (“triple risk hypothesis”) is supported by both anatomical and functional evidence (Filiano and Kinney 1994). According to this hypothesis, the main culprit in SIDS is the abnormal development of autonomic areas of the brain, which leaves the infants vulnerable to external stressors, such as hypercapnia or hypoxia.
The KF’s crucial role in coordinating breathing in not only normoxia, but also high CO2 and/or low O2 conditions, makes it a prime target for elucidating the underlying abnormalities in SIDS (Damasceno et al. 2014; Lavezzi 2015). Indeed, evidence provided by human histological studies suggests an array of KF abnormalities in fetal, newborn and infant brains (Lavezzi et al. 2004a; Lavezzi et al. 2014; Lavezzi 2015; Lavezzi et al. 2016). A major contributor to late fetal (but not newborn or infant) unexplained deaths is an underdeveloped KF (Lavezzi et al. 2004a; Matturri and Lavezzi 2011). Beyond morphology, neurochemical alterations of the KF are also linked to SIDS. This includes differences in orexinergic innervation and receptor expression in the KF of control and SIDS subjects (Lavezzi et al. 2016). Specifically, in control brains, KF neurons receive extensive orexinergic projections measured by immunohistochemical labeling of OX1R, while such varicosities are minimal or absent in SIDS brains. OX2R labeling is weak or absent in control and SIDS brains, suggesting that there may not be a functional abnormality linked to OX2R in SIDS.
Another important characteristic of KF neurons in SIDS and sudden intrauterine unexplained death syndrome (SIUDS) is the decreased amount of brain-derived neurotrophic factor (BDNF) expression associated with morphological immaturity (Lavezzi et al. 2014; Lavezzi 2015). BDNF is required for the healthy development of brainstem respiratory nuclei and the projections connecting them (Caravagna et al. 2013; Lu 2003; Katz et al. 2009; Balkowiec and Katz 1998). Specifically, BDNF plays an important role in the maturation of GABAergic and glutamatergic fast synaptic transmission in the KF, as supported by data from rats (Kron et al. 2007b; Kron et al. 2007a; Kron et al. 2008). Thus, alterations in BDNF expression pathways may be the causative agents of KF morphological hypoplasia and potentially alterations in KF GABAergic and glutamatergic dysfunction in SIDS and SIUDS (Lavezzi et al. 2014; Lavezzi 2015).
One of the major risk factors of SIDS is smoking, so not surprisingly the majority of the specimens examined in the above described studies were exposed to harmful chemicals, including nicotine, present in cigarettes during pregnancy and/or following birth (Lavezzi et al. 2014; Lavezzi et al. 2016). Interestingly, deficiencies in both OX1R and BDNF expression highly correlate with maternal smoking (Lavezzi et al. 2016; Lavezzi et al. 2014). Future studies should address the potential causal role of nicotine or other harmful cigarette chemicals in abnormal orexin receptor and BDNF expression in the KF.
Rett syndrome (RTT)
Alterations in KF BDNF expression levels and consequent GABAergic dysfunction are also relevant in Rett syndrome (RTT; Vermehren-Schmaedick et al., 2012; Lavezzi et al., 2014, 2016; Lavezzi, 2015; Abdala et al., 2016). RTT results from mutation in a X-linked gene affecting the expression of the nuclear protein methyl-CpG-binding protein 2 (MeCP2), where developmental and functional abnormalities accumulate across the brain and involving multiple neurotransmitter systems (Amir et al., 1999). Among other symptoms, RTT is characterized by irregular respiratory patterns, such as periodic breathing and frequent central and/or obstructive apneas, which indicate the heavy involvement of brainstem expiratory/post-inspiratory respiratory nuclei (Bissonnette et al. 2014; Bissonnette and Knopp 2008; Abdala et al. 2010; Carotenuto et al. 2013; Lugaresi et al. 1985; Stettner et al. 2007). Indeed, anatomical investigation of the KF in a mouse model of RTT showed a substantial decrease in the number of local GABAergic processes and perisomatic bouton-like puncta compared to control mice (Abdala et al. 2016). Additionally, GABA receptor antagonists microinjected into the KF of healthy rats induces RTT-like respiratory patterns (Abdala et al. 2016). Finally, local application of GABA reuptake blockers in the KF rescue the respiratory phenotype in RTT and restores breathing patterns in RTT mice, indicating a major role for the KF in RTT respiratory symptomology (Abdala et al. 2010).
The serotoninergic system also has potential in correcting breathing irregularity in RTT. Sarizotan, a mixed serotonin 5-HT1A receptor agonist and dopamine D2 receptor partial agonist, and F15599, a selective serotonin 5-HT1A agonist, are both effective at improving respiratory dysfunction in mouse models of RTT (Abdala et al. 2014b; Levitt et al. 2013). The KF may be a site of action because administration of the serotonin 5-HT1A receptor agonist 8-OH-DPAT into the KF of MeCP2 deficient mice improved breathing irregularity (Abdala et al. 2010; Abdala et al. 2014a).
Summary
We summarized the neurochemical composition of the KF, including neurotransmitters, neuromodulators, and hormones present. We also addressed the receptors and transporters involved in each system and discussed their potential physiological function. Finally, we provide a brief summary of literature regarding neurochemical changes in the KF in the context of respiratory dysfunction. We predict that future research into the neurophysiology of respiration will benefit from this review.
Acknowledgements
This work was supported by National Institutes of Health Grants R00 DA038069 and R01 DA047978, and International Rett Syndrome Foundation Basic Research Grant #3608 to E.S.L.A.G.V. was funded by the UF Breathing Research and Therapeutics Training Program (T32 HL134621) and the Center for Respiratory Research and Rehabilitation.
Abbreviations:
- 5-HT
5-hydroxytryptamine, serotonin
- ACh
acetylcholine
- AChE
acetylcholinesterase
- AMPA
α-amino-3-hydroxy-5-methyl-4-isoxazolepropionic acid
- BDNF
brain-derived neurotrophic factor
- cAMP
cyclic adenosine-monophosphate
- ChAT
choline acetyl transferase
- CHT1
choline transporter 1
- CSF
cerebrospinal fluid
- DOR
delta opioid receptor
- EAAT
excitatory amino acid transporter
- EMG
electromyography
- FoxP2
Forkhead box protein 2
- GABA
γ-aminobutyric acid
- GAD
glutamic acid decarboxylase
- GAT
GABA transporter
- GIRK
G protein-coupled inwardly-rectifying potassium channel
- GluA
subunits of the glutamate AMPA receptor
- GluN
subunits of the glutamate NMDA receptor
- GlyR
glycine receptor
- GlyT
glycine transporter
- GPCR
G-protein-coupled receptor
- ICV
intracerebroventricular
- KF
Kölliker-Fuse nucleus
- KOR
kappa opioid receptor
- LC
locus coeruleus
- mAChR
muscarinic acetylcholine receptor
- MeCP2
methyl-CpG-binding protein 2
- mGluR
metabotropic glutamate receptor
- MOR
mu opioid receptor
- nAChR
nicotinic acetylcholine receptor
- NE
norepinephrine
- NET
norepinephrine transporter
- NMDA
N-methyl-d-aspartate
- NPS
neuropeptide S
- NPSR
neuropeptide S receptor
- NTS
nucleus of the solitari tract
- ORX
orexin
- RTT
Rett syndrome
- SST
somatostatin
- SIDS
sudden infant death syndrome
- SIUDS
sudden intrauterine unexplained death syndrome
- VAChT
vesicular acetylcholine transporter
- VGLUT
vesicular glutamate transporter
- VGAT
vesicular GABA transporter
Footnotes
Conflict of Interest
The authors declare no competing financial interests.
References
- Abdala AP, Bissonnette JM, Newman-Tancredi A (2014a) Pinpointing brainstem mechanisms responsible for autonomic dysfunction in Rett syndrome: Therapeutic perspectives for 5-HT1A agonists. Front. Physiol 5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Abdala APL, Dutschmann M, Bissonnette JM, Paton JFR (2010) Correction of respiratory disorders in a mouse model of Rett syndrome. Proc. Natl. Acad. Sci. U. S. A 107, 18208–18213. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Abdala AP, Lioy DT, Garg SK, Knopp SJ, Paton JFR, Bissonnette JM (2014b) Effect of sarizotan, a 5-HT1a and D2-like receptor agonist, on respiration in three mouse models of rett syndrome. Am. J. Respir. Cell Mol. Biol 50, 1031–1039. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Abdala AP, Toward MA, Dutschmann M, Bissonnette JM, Paton JFR (2016) Deficiency of GABAergic synaptic inhibition in the Kölliker-Fuse area underlies respiratory dysrhythmia in a mouse model of Rett syndrome. J. Physiol 594, 223–237. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Adams ML, Brase DA, Welch SP, Dewey WL (1986) The role of endogenous peptides in the action of opioid analgesics. Ann. Emerg. Med 15, 1030–1035. [DOI] [PubMed] [Google Scholar]
- Adori C, Barde S, Bogdanovic N, Uhlén M, Reinscheid RR, Kovacs GG, Hökfelt T (2015) Neuropeptide S-and Neuropeptide S receptor-expressing neuron populations in the human pons. Front. Neuroanat 9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Anderson TM, Garcia AJ, Baertsch NA, Pollak J, Bloom JC, Wei AD, Rai KG, Ramirez JM (2016) A novel excitatory network for the control of breathing. Nature 536, 76–80. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Andrade CAF, Andrade-Franzé GMF, Paula P. M. De, Luca L. A. De, Menani JV. (2014) Role of α2-adrenoceptors in the lateral parabrachial nucleus in the control of body fluid homeostasis. Brazilian J. Med. Biol. Res 47, 11–18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Arimura A, Sato H, Dupont A, Nishi N, Schally AV (1975) Somatostatin: Abundance of immunoreactive hormone in rat stomach and pancreas. Science (80-. ). 189, 1007–1009. [DOI] [PubMed] [Google Scholar]
- Armstrong DM, Saper CB, Levey AI, Wainer BH, Terry RD (1983) Distribution of cholinergic neurons in rat brain: Demonstrated by the immunocytochemical localization of choline acetyltransferase. J. Comp. Neurol 216, 53–68. [DOI] [PubMed] [Google Scholar]
- Arvidsson U, Dado RJ, Riedl M, Lee JH, Law PY, Loh HH, Elde R, Wessendorf MW (1995) δ-Opioid receptor immunoreactivity: Distribution in brainstem and spinal cord, and relationship to biogenic amines and enkephalin. J. Neurosci 15, 1215–1235. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Balkowiec A, Katz DM (1998) Brain-derived neurotrophic factor is required for normal development of the central respiratory rhythm in mice. J. Physiol 510, 527–533. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barnes NM, Sharp T (1999) A review of central 5-HT receptors and their function. Neuropharmacology 38, 1083–1152. [DOI] [PubMed] [Google Scholar]
- Barnett P (2003) Somatostatin and somatostatin receptor physiology. Endocrine 20, 255–264. [DOI] [PubMed] [Google Scholar]
- Barnett WH, Jenkin SEM, Milsom WK, Paton JFR, Abdala AP, Molkov YI, Zoccal DB (2018) The kölliker-fuse nucleus orchestrates the timing of expiratory abdominal nerve bursting. J. Neurophysiol 119, 401–412. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barrett KT, Kinney HC, Li A, Daubenspeck JA, Leiter JC, Nattie EE (2012) Subtle alterations in breathing and heart rate control in the 5-HT1A receptor knockout mouse in early postnatal development. J. Appl. Physiol 113, 1585–1593. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bautista TG, Dutschmann M (2014a) Inhibition of the pontine Kölliker-Fuse nucleus abolishes eupneic inspiratory hypoglossal motor discharge in rat. Neuroscience 267, 22–29. [DOI] [PubMed] [Google Scholar]
- Bautista TG, Dutschmann M (2014b) Ponto-medullary nuclei involved in the generation of sequential pharyngeal swallowing and concomitant protective laryngeal adduction in situ. J. Physiol 592, 2605–2623. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bautista TG, Fong AY, Dutschmann M (2014) Spontaneous swallowing occurs during autoresuscitation in the in situ brainstem preparation of rat. Respir. Physiol. Neurobiol 202, 35–43. [DOI] [PubMed] [Google Scholar]
- Besnard S, Khemiri H, Masse F, Denise P, Verdaguer M, Gestreau C (2012) Differential respiratory control of the upper airway and diaphragm muscles induced by 5-HT1A receptor ligands. Sleep Breath. 16, 135–147. [DOI] [PubMed] [Google Scholar]
- Bissonnette JM, Knopp SJ (2008) Effect of inspired oxygen on periodic breathing in methy-CpG-binding protein 2 (Mecp2) deficient mice. J. Appl. Physiol 104, 198–204. [DOI] [PubMed] [Google Scholar]
- Bissonnette JM, Schaevitz LR, Knopp SJ, Zhou Z (2014) Respiratory phenotypes are distinctly affected in mice with common Rett syndrome mutations MECP2 T158A and R168X. Neuroscience 267, 166–176. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Blomqvist A, Hermanson O, Ericson H, Larhammar D (1994) Activation of a bulbospinal opioidergic projection by pain stimuli in the awake rat. Neuroreport 5, 461–464. [DOI] [PubMed] [Google Scholar]
- Bockaert J, Claeysen S, Bécamel C, Dumuis A, Marin P (2006) Neuronal 5-HT metabotropic receptors: Fine-tuning of their structure, signaling, and roles in synaptic modulation. Cell Tissue Res. 326, 553–572. [DOI] [PubMed] [Google Scholar]
- Bonham AC (1995) Neurotransmitters in the CNS control of breathing. Respir. Physiol 101, 219–230. [DOI] [PubMed] [Google Scholar]
- Bonis JM, Neumueller SE, Krause KL, Kiner T, Smith A, Marshall BD, Qian B, Pan LG, Forster HV (2010a) Site-specific effects on respiratory rhythm and pattern of ibotenic acid injections in the pontine respiratory group of goats. J. Appl. Physiol 109, 171–188. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bonis JM, Neumueller SE, Krause KL, Kiner A Smith T, Marshall BD, Qian B, Pan LG, Forster HV (2010b) A role for the Kölliker-Fuse nucleus in cholinergic modulation of breathing at night during wakefulness and NREM sleep. J. Appl. Physiol 109, 159–170. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Boon JA, Milsom WK (2008) NMDA receptor-mediated processes in the Parabrachial/Kölliker fuse complex influence respiratory responses directly and indirectly via changes in cortical activation state. Respir. Physiol. Neurobiol 162, 63–72. [DOI] [PubMed] [Google Scholar]
- Bou Farah L, Bowman BR, Bokiniec P, Karim S, Le S, Goodchild AK, Mcmullan S (2016) Somatostatin in the rat rostral ventrolateral medulla: Origins and mechanism of action. J. Comp. Neurol 524, 323–342. [DOI] [PubMed] [Google Scholar]
- Brandes IF, Stettner GM, Mörschel M, Kubin L, Dutschmann M (2011) REM sleep-like episodes of motoneuronal depression and respiratory rate increase are triggered by pontine carbachol microinjections in in situ perfused rat brainstem preparation. Exp. Physiol 96, 548–555. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Browaldh N, Bautista TG, Dutschmann M, Berkowitz RG (2016) The Kölliker-Fuse nucleus: a review of animal studies and the implications for cranial nerve function in humans. Eur. Arch. Oto-Rhino-Laryngology 273, 3505–3510. [DOI] [PubMed] [Google Scholar]
- Bruno JF, Xu Y, Song J, Berelowitz M (1992) Molecular cloning and functional expression of a brain-specific somatostatin receptor. Proc. Natl. Acad. Sci. U. S. A 89, 11151–11155. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brusa R, Zimmermann F, Koh DS, Feldmeyer D, Gass P, Seeburg PH, Sprengel R, Sakmann B (1995) Early-onset epilepsy and postnatal lethality associated with an editing-deficient GluR-B allele in mice. Science (80-. ). 270, 1677–1680. [DOI] [PubMed] [Google Scholar]
- Cahill CM, McClellan KA, Morinville A, Hoffert C, Hubatsch D, O’Donnell D, Beaudet A (2001) Immunohistochemical distribution of delta opioid receptors in the rat central nervous system: Evidence for somatodendritic labeling and antigen-specific cellular compartmentalization. J. Comp. Neurol 440, 65–84. [DOI] [PubMed] [Google Scholar]
- Caravagna C, Soliz J, Seaborn T (2013) Brain-derived neurotrophic factor interacts with astrocytes and neurons to control respiration. Eur. J. Neurosci 38, 3261–3269. [DOI] [PubMed] [Google Scholar]
- Carotenuto M, Esposito M, D’Aniello A, Rippa CD, Precenzano F, Pascotto A, Bravaccio C, Elia M (2013) Polysomnographic findings in Rett syndrome: A case-control study. Sleep Breath. 17, 93–98. [DOI] [PubMed] [Google Scholar]
- Carpentier V, Vaudry H, Mallet E, Laquerrière A, Tayot J, Leroux P (1996) Anatomical distribution of somatostatin receptors in the brainstem of the human fetus. Neuroscience 73, 865–879. [DOI] [PubMed] [Google Scholar]
- Carpentier V, Vaudry H, Mallet E, Tayot J, Laquerrière A, Leroux P (1997) Ontogeny of somatostatin binding sites in respiratory nuclei of the human brainstem. J. Comp. Neurol 381, 461–472. [DOI] [PubMed] [Google Scholar]
- Cerritelli S, Hirschberg S, Hill R, Balthasar N, Pickering AE (2016) Activation of brainstem proopiomelanocortin neurons produces opioidergic analgesia, bradycardia and bradypnoea. PLoS One 11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chamberlin NL, Mansour A, Watson SJ, Saper CB (1999) Localization of mu-opioid receptors on amygdaloid projection neurons in the parabrachial nucleus of the rat. Brain Res. 827, 198–204. [DOI] [PubMed] [Google Scholar]
- Chamberlin NL, Saper CB (1994) Topographic organization of respiratory responses to glutamate microstimulation of the parabrachial nucleus in the rat. J. Neurosci 14, 6500–6510. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chamberlin NL, Saper CB (1995) Differential distribution of AMPA-selective glutamate receptor subunits in the parabrachial nucleus of the rat. Neuroscience 68, 435–443. [DOI] [PubMed] [Google Scholar]
- Chemelli RM, Willie JT, Sinton CM, Elmquist JK, Scammell T, Lee C, Richardson JA, et al. (1999) Narcolepsy in orexin knockout mice: Molecular genetics of sleep regulation. Cell 98, 437–451. [DOI] [PubMed] [Google Scholar]
- Chigr F, Najimi M, Leduque P, Charnay Y, Jordan D, Chayvialle JA, Tohyama M, Kopp N (1989) Anatomical distribution of somatostatin immunoreactivity in the infant brainstem. Neuroscience 29, 615–628. [DOI] [PubMed] [Google Scholar]
- Clark FM, Proudfit HK (1991) The projection of noradrenergic neurons in the A7 catecholamine cell group to the spinal cord in the rat demonstrated by anterograde tracing combined with immunocytochemistry. Brain Res. 547, 279–288. [DOI] [PubMed] [Google Scholar]
- Clark SD, Duangdao DM, Schulz S, Zhang L, Liu X, Xu YL, Reinscheid RK (2011) Anatomical characterization of the neuropeptide S system in the mouse brain by in situ hybridization and immunohistochemistry. J. Comp. Neurol 519, 1867–1893. [DOI] [PubMed] [Google Scholar]
- Cohen MI (1971) Switching of the respiratory phases and evoked phrenic responses produced by rostral pontine electrical stimulation. J. Physiol 217, 133–158. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cohen MI, Shaw CF (2004) Role in the inspiratory off-switch of vagal inputs to rostral pontine inspiratory-modulated neurons. Respir. Physiol. Neurobiol 143, 127–140. [DOI] [PubMed] [Google Scholar]
- Cohen MI, Wang SC (1959) Respiratory neuronal activity in pons of cat. J. Neurophysiol 22, 33–50. [DOI] [PubMed] [Google Scholar]
- Corder G, Castro DC, Bruchas MR, Scherrer G (2018) Endogenous and Exogenous Opioids in Pain. Annu. Rev. Neurosci 41, 453–473. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Damasceno RS, Takakura AC, Moreira TS (2014) Regulation of the chemosensory control of breathing by Kölliker-Fuse neurons. Am. J. Physiol. - Regul. Integr. Comp. Physiol 307. [DOI] [PubMed] [Google Scholar]
- Davern PJ (2014) A role for the lateral parabrachial nucleus in cardiovascular function and fluid homeostasis. Front. Physiol 5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Del Negro CA, Funk GD, Feldman JL (2018) Breathing matters. Nat. Rev. Neurosci 19, 351–367. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Deng BS, Nakamura A, Zhang W, Yanagisawa M, Fukuda Y, Kuwaki T (2007) Contribution of orexin in hypercapnic chemoreflex: Evidence from genetic and pharmacological disruption and supplementation studies in mice. J. Appl. Physiol 103, 1772–1779. [DOI] [PubMed] [Google Scholar]
- Dhingra RR, Dutschmann M, Dick TE (2016) Blockade of dorsolateral pontine 5HT1A receptors destabilizes the respiratory rhythm in C57BL6/J wild-type mice. Respir. Physiol. Neurobiol 226, 110–114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dick TE, Bellingham MC, Richter DW (1994) Pontine respiratory neurons in anesthetized cats. Brain Res. 636, 259–269. [DOI] [PubMed] [Google Scholar]
- Ding YQ, Kaneko T, Nomura S, Mizuno N (1996) Immunohistochemical localization of μ-opioid receptors in the central nervous system of the rat. J. Comp. Neurol 367, 375–402. [DOI] [PubMed] [Google Scholar]
- Durkin MM, Smith KE, Borden LA, Weinshank RL, Branchek TA, Gustafson EL (1995) Localization of messenger RNAs encoding three GABA transporters in rat brain: an in situ hybridization study. Mol. Brain Res 33, 7–21. [DOI] [PubMed] [Google Scholar]
- Dutschmann M, Dick TE (2012) Pontine mechanisms of respiratory control. Compr. Physiol 2, 2443–2469. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dutschmann M, Guthmann A, Herbert H (1998) NMDA receptor subunit NR1-immunoreactivity in the rat pons and brainstem and colocalization with Fos induced by nasal stimulation. Brain Res. 809, 221–230. [DOI] [PubMed] [Google Scholar]
- Dutschmann M, Herbert H (1996) The Kolliker-Fuse nucleus mediates the trigeminally induced apnoea in the rat. Neuroreport 7, 1432–1436. [DOI] [PubMed] [Google Scholar]
- Dutschmann M, Herbert H (1998) NMDA and GABA(A) receptors in the rat Kolliker-Fuse area control cardiorespiratory responses evoked by trigeminal ethmoidal nerve stimulation. J. Physiol 510, 793–804. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dutschmann M, Herbert H (2006) The Kölliker-Fuse nucleus gates the postinspiratory phase of the respiratory cycle to control inspiratory off-switch and upper airway resistance in rat. Eur. J. Neurosci 24, 1071–1084. [DOI] [PubMed] [Google Scholar]
- Dutschmann M, Kron M, Mörschel M, Gestreau C (2007) Activation of Orexin B receptors in the pontine Kölliker-Fuse nucleus modulates pre-inspiratory hypoglossal motor activity in rat. Respir. Physiol. Neurobiol 159, 232–235. [DOI] [PubMed] [Google Scholar]
- Egan TM, North RA (1986) Acetylcholine hyperpolarizes central neurones by acting on an M2 muscarinic receptor. Nature 319, 405–407. [DOI] [PubMed] [Google Scholar]
- Engström L, Engblom D, Örtegren U, Mackerlova L, Paues J, Blomqvist A (2001) Preproenkephalin mRNA expression in rat parabrachial neurons: Relation to cells activated by systemic immune challenge. Neurosci. Lett 316, 165–168. [DOI] [PubMed] [Google Scholar]
- Erbs E, Faget L, Veinante P, Kieffer BL, Massotte D (2014) In vivo neuronal co-expression of mu and delta opioid receptors uncovers new therapeutic perspectives. Recept. Clin. Investig [DOI] [PMC free article] [PubMed] [Google Scholar]
- Erickson JT, Shafer G, Rossetti MD, Wilson CG, Deneris ES (2007) Arrest of 5HT neuron differentiation delays respiratory maturation and impairs neonatal homeostatic responses to environmental challenges. Respir. Physiol. Neurobiol 159, 85–101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Erlander MG, Tillakaratne NJK, Feldblum S, Patel N, Tobin AJ (1991) Two genes encode distinct glutamate decarboxylases. Neuron 7, 91–100. [DOI] [PubMed] [Google Scholar]
- Ezure K, Tanaka I (2004) GABA, in some cases together with glycine, is used as the inhibitory transmitter by pump cells in the Hering-Breuer reflex pathway of the rat. Neuroscience 127, 409–417. [DOI] [PubMed] [Google Scholar]
- Ezure K, Tanaka I (2006) Distribution and medullary projection of respiratory neurons in the dorsolateral pons of the rat. Neuroscience 141, 1011–1023. [DOI] [PubMed] [Google Scholar]
- Ezure K, Tanaka I, Saito Y (2003a) Brainstem and spinal projections of augmenting expiratory neurons in the rat. Neurosci. Res 45, 41–51. [DOI] [PubMed] [Google Scholar]
- Ezure K, Tanaka I, Saito Y (2003b) Brainstem and spinal projections of augmenting expiratory neurons in the rat. Neurosci. Res 45, 41–51. [DOI] [PubMed] [Google Scholar]
- Felder CC (1995) Muscarinic acetylcholine receptors: Signal transduction through multiple effectors. FASEB J. 9, 619–625. [PubMed] [Google Scholar]
- Feldmeyer D, Cull-Candy S (1996) Functional consequences of changes in NMDA receptor subunit expression during development. J. Neurocytol 25, 857–867. [DOI] [PubMed] [Google Scholar]
- Filiano JJ, Kinney HC (1994) A perspective on neuropathologic findings in victims of the sudden infant death syndrome: The triple-risk model. Neonatology 65, 194–197. [DOI] [PubMed] [Google Scholar]
- Finley JCW, Maderdrut JL, Roger LJ, Petrusz P (1981) The immunocytochemical localization of somatostatin-containing neurons in the rat central nervous system. Neuroscience 6, 2173–2192. [DOI] [PubMed] [Google Scholar]
- Ford B, Holmes CJ, Mainville L, Jones BE (1995) GABAergic neurons in the rat pontomesencephalic tegmentum: Codistribution with cholinergic and other tegmental neurons projecting to the posterior lateral hypothalamus. J. Comp. Neurol 363, 177–196. [DOI] [PubMed] [Google Scholar]
- Fremeau RT, Troyer MD, Pahner I, Nygaard GO, Tran CH, Reimer RJ, Bellocchio EE, Fortin D, Storm-Mathisen J, Edwards RH (2001) The expression of vesicular glutamate transporters defines two classes of excitatory synapse. Neuron 31, 247–260. [DOI] [PubMed] [Google Scholar]
- Fritschy JM (1999) GABAB-receptor splice variants GB1a and GB1b in rat brain: Developmental regulation, cellular distribution and extrasynaptic localization. Eur. J. Neurosci 11, 761–768. [DOI] [PubMed] [Google Scholar]
- Fujita M, Sato K, Sato M, Inoue T, Kozuka T, Tohyama M (1991) Regional distribution of the cells expressing glycine receptor β subunit mRNA in the rat brain. Brain Res. 560, 23–37. [DOI] [PubMed] [Google Scholar]
- Fung ML, John WM St. (1995) The functional expression of a pontine pneumotaxic centre in neonatal rats. J. Physiol 489, 579–591. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Furuta A, Rothstein JD, Martin JL (1997) Glutamate transporter protein subtypes are expressed differentially during rat cns development. J. Neurosci 17, 8363–8375. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gabernet L, Pauly-Evers M, Schwerdel C, Lentz M, Bluethmann H, Vogt K, Alberati D, Mohler H, Boison D (2005) Enhancement of the NMDA receptor function by reduction of glycine transporter-1 expression. Neurosci. Lett 373, 79–84. [DOI] [PubMed] [Google Scholar]
- Gang S, Mizuguchi A, Aoki M (1991) Axonal projections from the pontine pneumotaxic region to the nucleus raphe magnus in cats. Respir. Physiol 85, 329–339. [DOI] [PubMed] [Google Scholar]
- Gang S, Mizuguchi A, Kobayashi N, Aoki M (1990) Descending axonal projections from the medial parabrachial and Kölliker-Fuse nuclear complex to the nucleus raphe magnus in cats. Neurosci. Lett 118, 273–275. [DOI] [PubMed] [Google Scholar]
- Gang S, Nakazono Y, Aoki N (1993) Differential projections to the raphe nuclei from the medial parabrachial-Kölliker-Fuse (NPBM-KF) nuclear complex and the retrofacial nucleus in cats: retrograde WGA-HRP tracing. J. Auton. Nerv. Syst 45, 241–244. [DOI] [PubMed] [Google Scholar]
- Gautier H, Bertrand F (1975) Respiratory effects of pneumotaxic center lesions and subsequent vagotomy in chronic cats. Respir. Physiol 23, 71–85. [DOI] [PubMed] [Google Scholar]
- Geerling JC, Stein MK, Miller RL, Shin JW, Gray PA, Loewy AD (2011) FoxP2 expression defines dorsolateral pontine neurons activated by sodium deprivation. Brain Res. 1375, 19–27. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Geerling JC, Yokota S, Rukhadze I, Roe D, Chamberlin NL (2017) Kölliker–Fuse GABAergic and glutamatergic neurons project to distinct targets. J. Comp. Neurol 525, 1844–1860. [DOI] [PMC free article] [PubMed] [Google Scholar]
- George SR, Zastawny RL, Briones-Urbina R, Cheng R, Nguyen T, Heiber M, Kouvelas A, Chan AS, O′Dowd BF. (1994) Distinct distributions of MU, delta and kappa opioid receptor mRNA in rat brain. Biochem. Biophys. Res. Commun 205, 1438–1444. [DOI] [PubMed] [Google Scholar]
- Gozal D, Xue YD, Simakajornboon N (1999) Hypoxia induces c-Fos protein expression in NMDA but not AMPA glutamate receptor labeled neurons within the nucleus tractus solitarii of the conscious rat. Neurosci. Lett 262, 93–96. [DOI] [PubMed] [Google Scholar]
- Gray PA (2008) Transcription factors and the genetic organization of brain stem respiratory neurons. J. Appl. Physiol 104, 1513–1521. [DOI] [PubMed] [Google Scholar]
- Gregor Sutcliffe J, Lecea L. De (2000) The hypocretins: Excitatory neuromodulatory peptides for multiple homeostatic systems, including sleep and feeding. J. Neurosci. Res 62, 161–168. [DOI] [PubMed] [Google Scholar]
- Grudzinska J, Schemm R, Haeger S, Nicke A, Schmalzing G, Betz H, Laube B (2005) The β subunit determines the ligand binding properties of synaptic glycine receptors. Neuron 45, 727–739. [DOI] [PubMed] [Google Scholar]
- Guerrini R, Salvadori S, Rizzi A, Regoli D, Calo’ G (2010) Neurobiology, pharmacology, and medicinal chemistry of neuropeptide S and its receptor. Med. Res. Rev 30, 751–777. [DOI] [PubMed] [Google Scholar]
- Günther T, Tulipano G, Dournaud P, Bousquet C, Csaba Z, Kreienkamp HJ, Lupp A, et al. (2018) International union of basic and clinical pharmacology. CV. somatostatin receptors: Structure, function, ligands, and new nomenclature. Pharmacol. Rev 70, 763–835. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guo ZL, Moazzami AR, Longhurst JC (2005) Stimulation of cardiac sympathetic afferents activates glutamatergic neurons in the parabrachial nucleus: Relation to neurons containing nNOS. Brain Res 1053, 97–107. [DOI] [PubMed] [Google Scholar]
- Guthmann A, Fritschy JM, Ottersen OP, Torp R, Herbert H (1998) GABA, GABA transporters, GABA(A) receptor subunits, and GAD mRNAS in the rat parabrachial and Kolliker-fuse nuclei. J. Comp. Neurol 400, 229–243. [PubMed] [Google Scholar]
- Guthmann A, Herbert H (1999a) In situ hybridization analysis of flip/flop splice variants of AMPA-type glutamate receptor subunits in the rat parabrachial and Kolliker-Fuse nuclei. Mol. Brain Res 74, 145–157. [DOI] [PubMed] [Google Scholar]
- Guthmann A, Herbert H (1999b) Expression of N-methyl-D-aspartate receptor subunits in the rat parabrachial and Kolliker-Fuse nuclei and in selected pontomedullary brainstem nuclei. J. Comp. Neurol 415, 501–517. [PubMed] [Google Scholar]
- Guthmann A, Herbert H (1999c) Distribution of metabotropic glutamate receptors in the parabrachial and Kolliker-Fuse nuclei of the rat. Neuroscience 89, 873–881. [DOI] [PubMed] [Google Scholar]
- Guyenet PG, Bayliss DA (2015) Neural Control of Breathing and CO2 Homeostasis. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hancock MB, Fougerousse CL (1976) Spinal projections from the nucleus locus coeruleus and nucleus subcoeruleus in the cat and monkey as demonstrated by the retrograde transport of horseradish peroxidase. Brain Res. Bull 1, 229–234. [DOI] [PubMed] [Google Scholar]
- Harris GC, Aston-Jones G (2006) Arousal and reward: a dichotomy in orexin function. Trends Neurosci. 29, 571–577. [DOI] [PubMed] [Google Scholar]
- Herbert H, Flügge G (1995) Distribution of alpha2-adrenergic binding sites in the parabrachial complex of the rat. Anat. Embryol. (Berl) 192, 507–516. [DOI] [PubMed] [Google Scholar]
- Herbert H, Guthmann A, Zafra F, Ottersen OP (2000) Glycine, glycine receptor subunit and glycine transporters in the rat parabrachial and Kolliker-Fuse nuclei. Anat. Embryol. (Berl) 201, 259–272. [DOI] [PubMed] [Google Scholar]
- Herbert H, Moga MM, Saper CB (1990) Connections of the parabrachial nucleus with the nucleus of the solitary tract and the medullary reticular formation in the rat. J. Comp. Neurol 293, 540–580. [DOI] [PubMed] [Google Scholar]
- Hermanson O, Blomqvist A (1995) Enkephalinergic and catecholaminergic neurons constitute separate populations in the rat Kölliker-Fuse/A7 region. Neurosci. Lett 190, 57–60. [DOI] [PubMed] [Google Scholar]
- Hermanson O, Blomqvist A (1997) Preproenkephalin messenger RNA-expressing neurons in the rat parabrachial nucleus: Subnuclear organization and projections to the intralaminar thalamus. Neuroscience 81, 803–812. [DOI] [PubMed] [Google Scholar]
- Hernandes MS, Troncone LRP (2009) Glycine as a neurotransmitter in the forebrain: A short review. J. Neural Transm 116, 1551–1560. [DOI] [PubMed] [Google Scholar]
- Hilaire G, Voituron N, Menuet C, Ichiyama RM, Subramanian HH, Dutschmann M (2010) The role of serotonin in respiratory function and dysfunction. Respir. Physiol. Neurobiol 174, 76–88. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hodges MR, Richerson GB (2008) Contributions of 5-HT neurons to respiratory control: Neuromodulatory and trophic effects. Respir. Physiol. Neurobiol 164, 222–232. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Holaday JW, Law PY, Tseng LF, Loh HH, Li CH (1977) β-Endorphin: pituitary and adrenal glands modulate its action. Proc. Natl. Acad. Sci. U. S. A 74, 4628–4632. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hollmann M (1994) Cloned Glutamate Receptors. Annu. Rev. Neurosci 17, 31–108. [DOI] [PubMed] [Google Scholar]
- Holmseth S, Scott HA, Real K, Lehre KP, Leergaard TB, Bjaalie JG, Danbolt NC (2009) The concentrations and distributions of three C-terminal variants of the GLT1 (EAAT2; slc1a2) glutamate transporter protein in rat brain tissue suggest differential regulation. Neuroscience 162, 1055–1071. [DOI] [PubMed] [Google Scholar]
- Holstege G, Kuypers HGJM (1982) The Anatomy of Brain Stem Pathways to the Spinal Cord in Cat. A Labeled Amino Acid Tracing Study. Prog. Brain Res 57, 145–175. [DOI] [PubMed] [Google Scholar]
- Hoyer D, Hannon JP, Martin GR (2002) Molecular, pharmacological and functional diversity of 5-HT receptors. Pharmacol. Biochem. Behav 71, 533–554. [DOI] [PubMed] [Google Scholar]
- Huckstepp RTR, Cardoza KP, Henderson LE, Feldman JL (2015) Role of parafacial nuclei in control of breathing in adult rats. J. Neurosci 35, 1052–1067. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ishii K, Wong JK, Sumikawa K (2005) Comparison of α2 nicotinic acetylcholine receptor subunit mRNA expression in the central nervous system of rats and mice. J. Comp. Neurol 493, 241–260. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jakus J, Poliacek I, Halasova E, Murin P, Knocikova J, Tomori Z, Bolser DC (2008) Brainstem circuitry of tracheal-bronchial cough: c-fos study in anesthetized cats. Respir. Physiol. Neurobiol 160, 289–300. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Janczewski WA, Tashima A, Hsu P, Cui Y, Feldman JL (2013) Role of inhibition in respiratory pattern generation. J. Neurosci 33, 5454–5465. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jodkowski JS, Coles SK, Dick TE (1994) A “pneumotaxic centre” in rats. Neurosci. Lett 172, 67–72. [DOI] [PubMed] [Google Scholar]
- Johansson O, Hökfelt T, Elde RP (1984) Immunohistochemical distribution of somatostatin-like immunoreactivity in the central nervous system of the adult rat. Neuroscience 13. [DOI] [PubMed] [Google Scholar]
- Jonas P, Spruston N (1994) Mechanisms shaping glutamate-mediated excitatory postsynaptic currents in the CNS. Curr. Opin. Neurobiol 4, 366–372. [DOI] [PubMed] [Google Scholar]
- Jones BE, Beaudet A (1987) Distribution of acetylcholine and catecholamine neurons in the cat brainstem: A choline acetyltransferase and tyrosine hydroxylase immunohistochemical study. J. Comp. Neurol 261, 15–32. [DOI] [PubMed] [Google Scholar]
- Jones BE, Moore RY (1977) Ascending projections of the locus coeruleus in the rat. II. Autoradiographic study. Brain Res. 127, 23–53. [PubMed] [Google Scholar]
- Katz DM, Dutschmann M, Ramirez JM, Hilaire G (2009) Breathing disorders in Rett syndrome: Progressive neurochemical dysfunction in the respiratory network after birth. Respir. Physiol. Neurobiol 168, 101–108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kaufman DL, Houser CR, Tobin AJ (1991) Two Forms of the γ‐Aminobutyric Acid Synthetic Enzyme Glutamate Decarboxylase Have Distinct Intraneuronal Distributions and Cofactor Interactions. J. Neurochem 56, 720–723. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kaupmann K, Malitschek B, Schuler V, Heid J, Froestl W, Beck P, Mosbacher J, et al. (1998) GABA(B)-receptor subtypes assemble into functional heteromeric complexes. Nature 396, 683–687. [DOI] [PubMed] [Google Scholar]
- Kaur S, Pedersen NP, Yokota S, Hur EE, Fuller PM, Lazarus M, Chamberlin NL, Saper CB (2013) Glutamatergic signaling from the parabrachial nucleus plays a critical role in hypercapnic arousal. J. Neurosci 33, 7627–7640. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kaur S, Saper CB (2019) Neural circuitry underlying waking up to hypercapnia. Front. Neurosci 13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kaur S, Wang JL, Ferrari L, Thankachan S, Kroeger D, Venner A, Lazarus M, et al. (2017) A Genetically Defined Circuit for Arousal from Sleep during Hypercapnia. Neuron 96, 1153–1167.e5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kron M, Mörschel M, Reuter J, Zhang W, Dutschmann M (2007a) Developmental changes in brain-derived neurotrophic factor-mediated modulations of synaptic activities in the pontine Kölliker-Fuse nucleus of the rat. J. Physiol 583, 315–327. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kron M, Reuter J, Gerhardt E, Manzke T, Zhang W, Dutschmann M (2008) Emergence of brain-derived neurotrophic factor-induced postsynaptic potentiation of NMDA currents during the postnatal maturation of the Kölliker-Fuse nucleus of rat. J. Physiol 586, 2331–2343. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kron M, Zhang W, Dutschmann M (2007b) Developmental changes in the BDNF-induced modulation of inhibitory synaptic transmission in the Kölliker-Fuse nucleus of rat. Eur. J. Neurosci 26, 3449–3457. [DOI] [PubMed] [Google Scholar]
- Krous HF, Beckwith JB, Byard RW, Rognum TO, Bajanowski T, Corey T, Cutz E, Hanzlick R, Keens TG, Mitchell EA (2004) Sudden infant death syndrome and unclassified sudden infant deaths: A definitional and diagnostic approach. Pediatrics 114, 234–238. [DOI] [PubMed] [Google Scholar]
- Kumar U, Grant M (2010) Somatostatin and somatostatin receptors. Results Probl. Cell Differ 50, 137–184. [DOI] [PubMed] [Google Scholar]
- Kuna ST, Remmers JE (1999) Premotor input to hypoglossal motoneurons from Kolliker-Fuse neurons in decerebrate cats. Respir. Physiol 117, 85–95. [DOI] [PubMed] [Google Scholar]
- Kuwaki T (2008) Orexinergic modulation of breathing across vigilance states. Respir. Physiol. Neurobiol 164, 204–212. [DOI] [PubMed] [Google Scholar]
- Lalley PM, Pilowsky PM, Forster HV, Zuperku EJ (2014) CrossTalk opposing view: The pre-Bötzinger complex is not essential for respiratory depression following systemic administration of opioid analgesics. J. Physiol 592, 1163–1166. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Langmead CJ, Jerman JC, Brough SJ, Scott C, Porter RA, Herdon HJ (2004) Characterisation of the binding of [ 3H]-SB-674042, a novel nonpeptide antagonist, to the human orexin-1 receptor. Br. J. Pharmacol 141, 340–346. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lara JP, Dawid-Milner MS, López MV, Montes C, Spyer KM, González-Barón S (2002) Laryngeal effects of stimulation of rostral and ventral pons in the anaesthetized rat. Brain Res. 934, 97–106. [DOI] [PubMed] [Google Scholar]
- Lavezzi AM (2015) A new theory to explain the underlying pathogenetic mechanism of sudden infant death syndrome. Front. Neurol 6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lavezzi AM (2018) Toxic effect of cigarette smoke on brainstem nicotinic receptor expression: Primary cause of sudden unexplained perinatal death. Toxics 6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lavezzi AM, Corna MF, Matturri L (2014) Disruption of the Brain-Derived neurotrophic factor (BDNF) immunoreactivity in the human Kölliker-Fuse nucleus in victims of unexplained fetal and infant death. Front. Hum. Neurosci 8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lavezzi AM, Ferrero S, Roncati L, Matturri L, Pusiol T (2016) Impaired orexin receptor expression in the Kölliker–Fuse nucleus in sudden infant death syndrome: possible involvement of this nucleus in arousal pathophysiology. Neurol. Res 38, 706–716. [DOI] [PubMed] [Google Scholar]
- Lavezzi AM, Ottaviani G, Ballabio G, Rossi L, Matturri L (2004a) Preliminary study on the cytoarchitecture of the human parabrachial/Kö lliker-Fuse complex, with reference to sudden infant death syndrome and sudden intrauterine unexplained death. Pediatr. Dev. Pathol 7, 171–179. [DOI] [PubMed] [Google Scholar]
- Lavezzi AM, Ottaviani G, Rossi L, Matturri L (2004b) Cytoarchitectural organization of the parabrachial/Kölliker-Fuse complex in man. Brain Dev. 26, 316–320. [DOI] [PubMed] [Google Scholar]
- Lecea L. De, Kilduff TS, Peyron C, Gao XB, Foye PE, Danielson PE, Fukuhara C, et al. (1998) The hypocretins: Hypothalamus-specific peptides with neuroexcitatory activity, in Proc. Natl. Acad. Sci. U. S. A, Vol. 95, pp. 322–327. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee JC, Greig A, Ravindranathan A, Parks TN, Rao MS (1998) Molecular analysis of AMPA-specific receptors: Subunit composition, editing, and calcium influx determination in small amounts of tissue. Brain Res. Protoc 3, 142–154. [DOI] [PubMed] [Google Scholar]
- Lee M, Wardlaw SL (2007) Beta-Endorphin, in Encycl. Stress, pp. 332–335.17853060 [Google Scholar]
- Lefkowitz RJ (1990) The adrenergic receptors: structure/function and regulation, in Eur. J. Pharmacol, Vol. 183, p. 53. [Google Scholar]
- De León M., Coveñas R, Narváez JA, Tramu G, Aguirre JA, González-Barón S (1992) Distribution of somatostatin-28 (1–12) in the cat brainstem: an immunocytochemical study. Neuropeptides 21, 1–11. [DOI] [PubMed] [Google Scholar]
- Levey AI, Kitt CA, Simonds WF, Price DL, Brann MR (1991) Identification and localization of muscarinic acetylcholine receptor proteins in brain with subtype-specific antibodies. J. Neurosci 11, 3218–3226. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Levitt ES, Abdala AP, Paton JFR, Bissonnette JM, Williams JT (2015) μ opioid receptor activation hyperpolarizes respiratory-controlling Kölliker-Fuse neurons and suppresses post-inspiratory drive. J. Physiol 593, 4453–4469. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Levitt ES, Hunnicutt BJ, Knopp SJ, Williams JT, Bissonnette JM (2013) A selective 5-HT1a receptor agonist improves respiration in a mouse model of Rett syndrome. J. Appl. Physiol 115, 1626–1633. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Levitt ES, Williams JT (2018) Desensitization and tolerance of Mu opioid receptors on pontine kölliker-fuse neurons. Mol. Pharmacol 93, 8–13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu X, Zeng J, Zhou A, Theodorsson E, Fahrenkrug J, Reinscheid RK (2011) Molecular fingerprint of neuropeptide s-producing neurons in the mouse brain. J. Comp. Neurol 519, 1847–1866. [DOI] [PubMed] [Google Scholar]
- Lorang D, Amara SG, Simerly RB (1994) Cell-type-specific expression of catecholamine transporters in the rat brain. J. Neurosci 14, 4903–4914. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lu B (2003) BDNF and activity-dependent synaptic modulation. Learn. Mem 10, 86–98. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lugaresi E, Cirignotta F, Montagna P (1985) Abnormal breathing in the Rett syndrome. Brain Dev. 7, 329–333. [DOI] [PubMed] [Google Scholar]
- Lumsden T (1923) Observations on the respiratory centres in the cat. J. Physiol 57, 153–160. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lydic R, Orem J (1979) Respiratory neurons of the pneumotaxic center during sleep and wakefulness. Neurosci. Lett 15, 187–192. [DOI] [PubMed] [Google Scholar]
- Manglik A (2019) Molecular Basis of Opioid Action: From Structures to New Leads. Biol. Psychiatry 87, 6–14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marcus JN, Aschkenasi CJ, Lee CE, Chemelli RM, Saper CB, Yanagisawa M, Elmquist JK (2001) Differential expression of Orexin receptors 1 and 2 in the rat brain. J. Comp. Neurol 435, 6–25. [DOI] [PubMed] [Google Scholar]
- Margeta-Mitrovic M, Mitrovic I, Riley RC, Jan LY, Basbaum AI (1999) Immunohistochemical localization of GABA(B) receptors in the rat central nervous system. J. Comp. Neurol 405, 299–321. [DOI] [PubMed] [Google Scholar]
- Maricq AV, Peterson AS, Brake AJ, Myers RM, Julius D (1991) Primary structure and functional expression of the 5HT3receptor, a serotonin-gated ion channel. Science (80-. ). 254, 432–437. [DOI] [PubMed] [Google Scholar]
- Martin-Schild S, Gerall AA, Kastin AJ, Zadina JE (1999) Differential distribution of endomorphin 1- and endomorphin 2-like immunoreactivities in the CNS of the rodent. J. Comp. Neurol 405, 450–471. [PubMed] [Google Scholar]
- Massie A, Cnops L, Smolders I, McCullumsmith R, Kooijman R, Kwak S, Arckens L, Michotte Y (2008) High-affinity Na+/K+-dependent glutamate transporter EAAT4 is expressed throughout the rat fore- and midbrain. J. Comp. Neurol 511, 155–172. [DOI] [PubMed] [Google Scholar]
- Matturri L, Lavezzi AM (2011) Unexplained stillbirth versus SIDS: Common congenital diseases of the autonomic nervous system-pathology and nosology. Early Hum. Dev 87, 209–215. [DOI] [PubMed] [Google Scholar]
- Miller JR, Zuperku EJ, Stuth EAE, Banerjee A, Hopp FA, Stucke AG (2017) A Subregion of the Parabrachial Nucleus Partially Mediates Respiratory Rate Depression from Intravenous Remifentanil in Young and Adult Rabbits. Anesthesiology 127, 502–514. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miller RL, Knuepfer MM, Wang MH, Denny GO, Gray PA, Loewy AD (2012) Fos-activation of FoxP2 and Lmx1b neurons in the parabrachial nucleus evoked by hypotension and hypertension in conscious rats. Neuroscience 218, 110–125. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Minelli A, Brecha NC, Karschin C, DeBiasi S, Conti F (1995) GAT-1, a high-affinity GABA plasma membrane transporter, is localized to neurons and astroglia in the cerebral cortex. J. Neurosci 15, 7734–7746. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Minelli A, DeBiasi S, Brecha NC, Zuccarello LV, Conti F (1996) GAT-3, a high-affinity GABA plasma membrane transporter, is localized to astrocytic processes, and it is not confined to the vicinity of GABAergic synapses in the cerebral cortex. J. Neurosci 16, 6255–6264. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Misawa H, Nakata K, Matsuura J, Nagao M, Okuda T, Haga T (2001) Distribution of the high-affinity choline transporter in the central nervous system of the rat. Neuroscience 105, 87–98. [DOI] [PubMed] [Google Scholar]
- Modirrousta M, Mainville L, Jones BE (2005) Orexin and MCH neurons express c-Fos differently after sleep deprivation vs. recovery and bear different adrenergic receptors. Eur. J. Neurosci 21, 2807–2816. [DOI] [PubMed] [Google Scholar]
- Monyer H, Seeburg PH, Wisden W (1991) Glutamate-operated channels: Developmentally early and mature forms arise by alternative splicing. Neuron 6, 799–810. [DOI] [PubMed] [Google Scholar]
- Mörschel M, Dutschmann M (2009) Pontine respiratory activity involved in inspiratory/expiratory phase transition. Philos. Trans. R. Soc. B Biol. Sci 364, 2517–2526. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mosbacher J, Schoepfer R, Monyer H, Burnashev N, Seeburg PH, Ruppersberg JP (1994) A molecular determinant for submillisecond desensitization in glutamate receptors. Science (80-. ). 266, 1059–1062. [DOI] [PubMed] [Google Scholar]
- Nakamura A, Zhang W, Yanagisawa M, Fukuda Y, Kuwaki T (2007) Vigilance state-dependent attenuation of hypercapnic chemoreflex and exaggerated sleep apnea in orexin knockout mice. J. Appl. Physiol 102, 241–248. [DOI] [PubMed] [Google Scholar]
- Nambu T, Sakurai T, Mizukami K, Hosoya Y, Yanagisawa M, Goto K (1999) Distribution of orexin neurons in the adult rat brain. Brain Res. 827, 243–260. [DOI] [PubMed] [Google Scholar]
- Nasirova N, Quina LA, Agosto-Marlin IM, Ramirez JM, Lambe EK, Turner EE (2020) Dual recombinase fate mapping reveals a transient cholinergic phenotype in multiple populations of developing glutamatergic neurons. J. Comp. Neurol 528, 283–307. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Navarrete-Opazo A, Cook-Snyder D, Miller J, Callison J, McCarthy N, Palkovic B, Stuth E, Zuperku E, Stucke A (2020) Endogenous glutamatergic inputs to the Parabrachial Nucleus/ Kölliker-Fuse Complex determine respiratory rate. Respir. Physiol. Neurobiol, In press. Accepted Jan. 28. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Niebert M, Vogelgesang S, Koch UR, Bischoff AM, Kron M, Bock N, Manzke T (2011) Expression and function of serotonin 2A and 2B receptors in the mammalian respiratory network. PLoS One 6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nieoullon A, Canolle B, Masmejean F, Guillet B, Pisano P, Lortet S (2006) The neuronal excitatory amino acid transporter EAAC1/EAAT3: Does it represent a major actor at the brain excitatory synapse? J. Neurochem 98, 1007–1018. [DOI] [PubMed] [Google Scholar]
- Niu JG, Yokota S, Tsumori T, Qin Y, Yasui Y (2010) Glutamatergic lateral parabrachial neurons innervate orexin-containing hypothalamic neurons in the rat. Brain Res. 1358, 110–122. [DOI] [PubMed] [Google Scholar]
- O’Carroll AM, Lolait SJ, Konig M, Mahan LC (1992) Molecular cloning and expression of a pituitary somatostatin receptor with preferential affinity for somatostatin-28. Mol. Pharmacol 42, 939–946. [PubMed] [Google Scholar]
- Olsen RW, Sieghart W (2008) International Union of Pharmacology. LXX. Subtypes of γ-aminobutyric acidA receptors: Classification on the basis of subunit composition, pharmacology, and function. Update. Pharmacol. Rev 60, 243–260. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Palacios JM, Kuhar MJ (1982) Beta adrenergic receptor localization in rat brain by light microscopic autoradiography. Neurochem. Int 4, 473–490. [DOI] [PubMed] [Google Scholar]
- Palkovits M, Eskay RL (1987) Distribution and possible origin of β-endorphin and ACTH in discrete brainstem nuclei of rats. Neuropeptides 9, 123–137. [DOI] [PubMed] [Google Scholar]
- Papke RL (2014) Merging old and new perspectives on nicotinic acetylcholine receptors. Biochem. Pharmacol 89, 1–11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Parsons SM, Prior C, Marshall IG (1993) Acetylcholine transport, storage, and release. Int. Rev. Neurobiol 35, 279–390. [DOI] [PubMed] [Google Scholar]
- Patel YC, Reichlin S (1978) Somatostatin in hypothalamus, extrahypothalamic brain, and peripheral tissues of the rat. Endocrinology 102, 523–530. [DOI] [PubMed] [Google Scholar]
- Peyron C, Tighe DK, Pol AN Van Den, Lecea L. De, Heller HC, Sutcliffe JG, Kilduff TS. (1998) Neurons containing hypocretin (orexin) project to multiple neuronal systems. J. Neurosci 18, 9996–10015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Polak JM, Grimelius L, Pearse AGE, Bloom SR, Arimura A (1975) Growth-hormone release-inhibiting hormone in gastrointestinal and pancreatic D cells. Lancet 305, 1220–1222. [DOI] [PubMed] [Google Scholar]
- Prkic I, Mustapic S, Radocaj T, Stucke AG, Stuth EAE, Hopp FA, Dean C, Zuperku EJ (2012) Pontine μ-opioid receptors mediate bradypnea caused by intravenous remifentanil infusions at clinically relevant concentrations in dogs. J. Neurophysiol 108, 2430–2441. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rajendra S, Lynch JW, Schofield PR (1997) The glycine receptor. Pharmacol. Ther 73, 121–146. [DOI] [PubMed] [Google Scholar]
- Ramirez J-M, Baertsch NA (2018a) The Dynamic Basis of Respiratory Rhythm Generation: One Breath at a Time. Annu. Rev. Neurosci 41, 475–499. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ramirez JM, Baertsch N (2018b) Defining the rhythmogenic elements of mammalian breathing. Physiology 33, 302–316. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rampon C, Luppi PH, Fort P, Peyron C, Jouvet M (1996) Distribution of glycine-immunoreactive cell bodies and fibers in the rat brain. Neuroscience 75, 737–755. [DOI] [PubMed] [Google Scholar]
- Reddy VK, Fung SJ, Zhuo H, Barnes CD (1991) Pontospinal transmitters and their distribution, in Prog. Brain Res, Vol. 88, pp. 103–121. [DOI] [PubMed] [Google Scholar]
- Reinscheid RK, Xu Y-L (2005) Neuropeptide S as a novel arousal promoting peptide transmitter. FEBS J. 272, 5689–5693. [DOI] [PubMed] [Google Scholar]
- Reinscheid RK, Xu YL, Okamura N, Zeng J, Chung S, Pai R, Wang Z, Civelli O (2005) Pharmacological characterization of human and murine neuropeptide S receptor variants. J. Pharmacol. Exp. Ther 315, 1338–1345. [DOI] [PubMed] [Google Scholar]
- Ribak CE, Tong WMY, Brecha NC (1996) GABA plasma membrane transporters, GAT-1 and GAT-3, display different distributions in the rat hippocampus. J. Comp. Neurol 367, 595–606. [DOI] [PubMed] [Google Scholar]
- Richter DW, Manzke T, Wilken B, Ponimaskin E (2003) Serotonin receptors: Guardians of stable breathing. Trends Mol. Med 9, 542–548. [DOI] [PubMed] [Google Scholar]
- Robertson SD, Plummer NW, Marchena J De, Jensen P. (2013) Developmental origins of central norepinephrine neuron diversity. Nat. Neurosci 16, 1016–1023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rosin DL, Zeng D, Stornetta RL, Norton FR, Riley T, Okusa MD, Guyenet PG, Lynch KR (1993) Immunohistochemical localization of α2a-adrenergic receptors in catecholaminergic and other brainstem neurons in the rat. Neuroscience 56, 139–155. [DOI] [PubMed] [Google Scholar]
- Rousseau F, Aubrey KR, Supplisson S (2008) The glycine transporter Glyt2 controls the dynamics of synaptic vesicle refilling in inhibitory spinal cord neurons. J. Neurosci 28, 9755–9768. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sakurai T (2002) Roles of orexins in regulation of feeding and wakefulness. Neuroreport 13, 987–995. [DOI] [PubMed] [Google Scholar]
- Sakurai T (2007) The neural circuit of orexin (hypocretin): Maintaining sleep and wakefulness. Nat. Rev. Neurosci 8, 171–181. [DOI] [PubMed] [Google Scholar]
- Sakurai T, Amemiya A, Ishii M, Matsuzaki I, Chemelli RM, Tanaka H, Williams SC, et al. (1998) Orexins and orexin receptors: A family of hypothalamic neuropeptides and G protein-coupled receptors that regulate feeding behavior. Cell 92, 573–585. [DOI] [PubMed] [Google Scholar]
- Sato K, Kiyama H, Tohyama M (1992) Regional distribution of cells expressing glycine receptor α2 subunit mRNA in the rat brain. Brain Res. 590, 95–108. [DOI] [PubMed] [Google Scholar]
- Sato K, Zhang JH, Saika T, Sato M, Tada K, Tohyama M (1991) Localization of glycine receptor α1 subunit mRNA-containing neurons in the rat brain: An analysis using in situ hybridization histochemistry. Neuroscience 43, 381–395. [DOI] [PubMed] [Google Scholar]
- Saunders SE, Levitt ES (2020) Kölliker-Fuse/Parabrachial complex mu opioid receptors contribute to fentanyl-induced apnea and respiratory rate depression. Respir. Physiol. Neurobiol 275, 1–12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schäfer MKH, Weihe E, Varoqui H, Eiden LE, Erickson JD (1994) Distribution of the vesicular acetylcholine transporter (VAChT) in the central and peripheral nervous systems of the rat. J. Mol. Neurosci 5, 1–26. [DOI] [PubMed] [Google Scholar]
- Scheinin M, Lomasney JW, Hayden-Hixson DM, Schambra UB, Caron MG, Lefkowitz RJ, Fremeau RT (1994) Distribution of α2-adrenergic receptor subtype gene expression in rat brain. Mol. Brain Res 21, 133–149. [DOI] [PubMed] [Google Scholar]
- Schlen H, Bentley GA (1980) The possibility that a component of morphine-induced analgesia is contributed indirectly via the release of endogenous opioids. Pain 9, 73–84. [DOI] [PubMed] [Google Scholar]
- Schmid K, Böhmer G, Gebauer K (1991) Glycine receptor-mediated fast synaptic inhibition in the brainstem respiratory system. Respir. Physiol 84, 351–361. [DOI] [PubMed] [Google Scholar]
- Schmid K, Foutz AS, Denavit-Saubié M (1996) Inhibitions mediated by glycine and GABAA receptors shape the discharge pattern of bulbar respiratory neurons. Brain Res. 710, 150–160. [DOI] [PubMed] [Google Scholar]
- Schreihofer AM, Stornetta RL, Guyenet PG (1999) Evidence for glycinergic respiratory neurons: Botzinger neurons express mRNA for glycinergic transporter 2. J. Comp. Neurol 407, 583–597. [DOI] [PubMed] [Google Scholar]
- Schwarz LA, Luo L (2015) Organization of the locus coeruleus-norepinephrine system. Curr. Biol 25, R1051–R1056. [DOI] [PubMed] [Google Scholar]
- Shashidharan P, Huntley GW, Murray JM, Buku A, Moran T, Walsh MJ, Morrison JH, Plaitakis A (1997) Immunohistochemical localization of the neuron-specific glutamate transporter EAAC1 (EAAT3) in rat brain and spinal cord revealed by a novel monoclonal antibody. Brain Res. 773, 139–148. [DOI] [PubMed] [Google Scholar]
- Shigeri Y, Seal RP, Shimamoto K (2004) Molecular pharmacology of glutamate transporters, EAATs and VGLUTs. Brain Res. Rev 45, 250–265. [DOI] [PubMed] [Google Scholar]
- Sieck GC, Harper RM (1980) Pneumotaxic area neuronal discharge during sleep-waking states in the cat. Exp. Neurol 67, 79–102. [DOI] [PubMed] [Google Scholar]
- Silva JN, Lucena EV, Silva TM, Damasceno RS, Takakura AC, Moreira TS (2016) Inhibition of the pontine Kölliker-Fuse nucleus reduces genioglossal activity elicited by stimulation of the retrotrapezoid chemoreceptor neurons. Neuroscience 328, 9–21. [DOI] [PubMed] [Google Scholar]
- Smith JC, Abdala APL, Borgmann A, Rybak IA, Paton JFR (2013) Brainstem respiratory networks: Building blocks and microcircuits. Trends Neurosci. 36, 152–162. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smith JC, Abdala APL, Koizumi H, Rybak IA, Paton JFR (2007) Spatial and functional architecture of the mammalian brain stem respiratory network: A hierarchy of three oscillatory mechanisms. J. Neurophysiol 98, 3370–3387. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smith JC, Ellenberger HH, Ballanyi K, Richter DW, Feldman JL (1991) Pre-Bötzinger complex: A brainstem region that may generate respiratory rhythm in mammals. Science (80-. ). 254, 726–729. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sommer B, Köhler M, Sprengel R, Seeburg PH (1991) RNA editing in brain controls a determinant of ion flow in glutamate-gated channels. Cell 67, 11–19. [DOI] [PubMed] [Google Scholar]
- Song G, Tin C, Poon CS (2015) Multiscale fingerprinting of neuronal functional connectivity. Brain Struct. Funct 220, 2967–2982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Song G, Wang H, Xu H, Poon CS (2012) Kölliker-Fuse neurons send collateral projections to multiple hypoxia-activated and nonactivated structures in rat brainstem and spinal cord. Brain Struct. Funct 217, 835–858. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Song G, Xu H, Wang H, MacDonald SM, Poon CS (2011) Hypoxia-excited neurons in NTS send axonal projections to Kölliker-Fuse/parabrachial complex in dorsolateral pons. Neuroscience 175, 145–153. [DOI] [PMC free article] [PubMed] [Google Scholar]
- St-John WM, Glasser RL, Richard AK (1971) Apneustic breathing after vagotomy in cats with chronic pneumotaxic center lesions. Respir. Physiol 12, 239–250. [DOI] [PubMed] [Google Scholar]
- Steinbusch HWM (1981) Distribution of serotonin-immunoreactivity in the central nervous system of the rat-Cell bodies and terminals. Neuroscience 6, 557–618. [DOI] [PubMed] [Google Scholar]
- Steinbusch HW, Verhofstad AA, Penke B, Varga J, Joosten HW (1981) Immunohistochemical characterization of monoamine-containing neurons in the central nervous system by antibodies to serotonin and noradrenalin. A study in the rat and the lamprey (Lampetra fluviatilis). Acta Histochem. Suppl 24, 107–122. [PubMed] [Google Scholar]
- Stettner GM, Huppke P, Brendel C, Richter DW, Gärtner J, Dutschmann M (2007) Breathing dysfunctions associated with impaired control of postinspiratory activity in Mecp2-/y knockout mice. J. Physiol 579, 863–876. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stevens RT, Hodge CJ, Apkarian AV (1982) Kölliker-Fuse nucleus: the principal source of pontine catecholaminergic cells projecting to the lumbar spinal cord of cat. Brain Res. 239, 589–594. [DOI] [PubMed] [Google Scholar]
- Strazielle C, Lalonde R, Hébert C, Reader TA (1999) Regional brain distribution of noradrenaline uptake sites, and of α1-, α2- and β-adrenergic receptors in PCD mutant mice: A quantitative autoradiographic study. Neuroscience 94, 287–304. [DOI] [PubMed] [Google Scholar]
- Strosberg AD (1993) Structure, function, and regulation of adrenergic receptors. Protein Sci. 2, 1198–1209. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Swanson LW, Hartman BK (1975) The central adrenergic system. An immunofluorescence study of the location of cell bodies and their efferent connections in the rat utilizing dopamine‐B‐hydroxylase as a marker. J. Comp. Neurol 163, 467–505. [DOI] [PubMed] [Google Scholar]
- Tavares A, Handy DE, Bogdanova NN, Rosene DL, Gavras H (1996) Localization of α2A and α2B-Adrenergic Receptor Subtypes in Brain. Hypertension 27, 449–455. [DOI] [PubMed] [Google Scholar]
- Törk I (1990) Anatomy of the Serotonergic System. Ann. N. Y. Acad. Sci 600, 9–34. [DOI] [PubMed] [Google Scholar]
- Torres GE, Gainetdinov RR, Caron MG (2003) Plasma membrane monoamine transporters: Structure, regulation and function. Nat. Rev. Neurosci 4, 13–25. [DOI] [PubMed] [Google Scholar]
- Toubia T, Khalife T (2019) The Endogenous Opioid System: Role and Dysfunction Caused by Opioid Therapy. Clin. Obstet. Gynecol 62, 3–10. [DOI] [PubMed] [Google Scholar]
- Tribollet E, Bertrand D, Marguerat A, Raggenbass M (2004) Comparative distribution of nicotinic receptor subtypes during development, adulthood and aging: An autoradiographic study in the rat brain. Neuroscience 124, 405–420. [DOI] [PubMed] [Google Scholar]
- Udenfriend S, Kilpatrick DL (1983) Biochemistry of the enkephalins and enkephalin-containing peptides. Arch. Biochem. Biophys 221, 309–323. [DOI] [PubMed] [Google Scholar]
- Unnerstall JR, Fernandez I, Orensanz LM (1985) The alpha-adrenergic receptor: Radiohistochemical analysis of functional characteristics and biochemical differences. Pharmacol. Biochem. Behav 22, 859–874. [DOI] [PubMed] [Google Scholar]
- Van-Hooft JA, Yakel JL (2003) 5-HT3 receptors in the CNS: 3B or not 3B? Trends Pharmacol. Sci 24, 157–160. [DOI] [PubMed] [Google Scholar]
- Vanetti M, Kouba M, Wang X, Vogt G, Höllt V (1992) Cloning and expression of a novel mouse somatostatin receptor (SSTR2B). FEBS Lett. 311, 290–294. [DOI] [PubMed] [Google Scholar]
- Vanetti M, Ziólkowska B, Wang X, Horn G, Höllt V (1994) mRNA distribution of two isoforms of somatostatin receptor 2 (mSSTR2A and mSSTR2B) in mouse brain. Mol. Brain Res 27, 45–50. [DOI] [PubMed] [Google Scholar]
- Varga AG, Reid BT, Kieffer BL, Levitt ES (2020) Differential impact of two critical respiratory centers in opioid‐induced respiratory depression in awake mice. J. Physiol 598, 189–205. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vermehren-Schmaedick A, Jenkins VK, Knopp SJ, Balkowiec A, Bissonnette JM (2012) Acute intermittent hypoxia-induced expression of brain-derived neurotrophic factor is disrupted in the brainstem of methyl-CpG-binding protein 2 null mice. Neuroscience 206, 1–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Waldhoer M, Bartlett SE, Whistler JL (2004) Opioid Receptors. Annu. Rev. Biochem 73, 953–990. [DOI] [PubMed] [Google Scholar]
- Wamsley JK, Zarbin MA, Kuhar MJ (1984) Distribution of muscarinic cholinergic high and low affinity agonist binding sites: A light microscopic autoradiographic study. Brain Res. Bull 12, 233–243. [DOI] [PubMed] [Google Scholar]
- Wang C, Wang Q, Ji B, Pan Y, Xu C, Cheng B, Bai B, Chen J (2018) The Orexin/Receptor System: Molecular Mechanism and Therapeutic Potential for Neurological Diseases. Front. Mol. Neurosci 11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang R, Macmillan LB, Fremeau RT, Magnuson MA, Lindner J, Limbird LE (1996) Expression of α2-adrenergic receptor subtypes in the mouse brain: Evaluation of spatial and temporal information imparted by 3 kb of 5′ regulatory sequence for the α2A AR-receptor gene in transgenic animals. Neuroscience 74, 199–218. [DOI] [PubMed] [Google Scholar]
- Wess J (1996) Molecular biology of muscarinic acetylcholine receptors. Crit. Rev. Neurobiol 10, 69–99. [DOI] [PubMed] [Google Scholar]
- Weston MC, Stornetta RL, Guyenet PG (2004) Glutamatergic Neuronal Projections from the Marginal Layer of the Rostral Ventral Medulla to the Respiratory Centers in Rats. J. Comp. Neurol 473, 73–85. [DOI] [PubMed] [Google Scholar]
- Williams JT, Ingram SL, Henderson G, Chavkin C, Zastrow M von, Schulz S, Koch T., Evans CJ, Christie MJ. (2013) Regulation of μ-opioid receptors: Desensitization, phosphorylation, internalization, and tolerance. Pharmacol. Rev 65, 223–254. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Williams RH, Burdakov D (2008) Hypothalamic orexins/hypocretins as regulators of breathing. Expert Rev. Mol. Med 10, 1–10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Willie JT, Chemelli RM, Sinton CM, Yanagisawa M (2001) To Eat or to Sleep? Orexin in the Regulation of Feeding and Wakefulness. Annu. Rev. Neurosci 24, 429–458. [DOI] [PubMed] [Google Scholar]
- Wong-Riley MTT, Liu Q (2005) Neurochemical development of brain stem nuclei involved in the control of respiration. Respir. Physiol. Neurobiol 149, 83–98. [DOI] [PubMed] [Google Scholar]
- Woolf NJ, Butcher LL (1989) Cholinergic systems in the rat brain: IV. descending projections of the pontomesencephalic tegmentum. Brain Res. Bull 23, 519–540. [DOI] [PubMed] [Google Scholar]
- Xu Y-L, Gall CM, Jackson VR, Civelli O, Reinscheid RK (2007) Distribution of neuropeptide S receptor mRNA and neurochemical characteristics of neuropeptide S-expressing neurons in the rat brain. J. Comp. Neurol 500, 84–102. [DOI] [PubMed] [Google Scholar]
- Xu YL, Reinscheid RK, Huitron-Resendiz S, Clark SD, Wang Z, Lin SH, Brucher FA, et al. (2004) Neuropeptide S: A neuropeptide promoting arousal and anxiolytic-like effects. Neuron 43, 487–497. [DOI] [PubMed] [Google Scholar]
- Yamauchi M, Ocak H, Dostal J, Jacono FJ, Loparo KA, Strohl KP (2008) Post-sigh breathing behavior and spontaneous pauses in the C57BL/6J (B6) mouse. Respir. Physiol. Neurobiol 162, 117–125. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yang CF, Feldman JL (2018) Efferent projections of excitatory and inhibitory preBötzinger Complex neurons. J. Comp. Neurol 526, 1389–1402. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yasuda K, Rens-Domiano S, Breder CD, Law SF, Saper CB, Reisine T, Bell GI (1992) Cloning of a novel somatostatin receptor, SSTR3, coupled to adenylylcyclase. J. Biol. Chem 267, 20422–20428. [PubMed] [Google Scholar]
- Yokota S, Oka T, Asano H, Yasui Y (2016) Orexinergic fibers are in contact with Kölliker-Fuse nucleus neurons projecting to the respiration-related nuclei in the medulla oblongata and spinal cord of the rat. Brain Res. 1648, 512–523. [DOI] [PubMed] [Google Scholar]
- Yokota S, Oka T, Tsumori T, Nakamura S, Yasui Y (2007) Glutamatergic neurons in the Kölliker-Fuse nucleus project to the rostral ventral respiratory group and phrenic nucleus: A combined retrograde tracing and in situ hybridization study in the rat. Neurosci. Res 59, 341–346. [DOI] [PubMed] [Google Scholar]
- Zafra F, Giménez C (2008) Glycine transporters and synaptic function. IUBMB Life 60, 810–817. [DOI] [PubMed] [Google Scholar]
- Zhang W, Fukuda Y, Kuwaki T (2005) Respiratory and cardiovascular actions of orexin-A in mice. Neurosci. Lett 385, 131–136. [DOI] [PubMed] [Google Scholar]
- Zhou Y, Danbolt NC (2013) GABA and glutamate transporters in brain. Front. Endocrinol. (Lausanne) 4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhu H, Perkins C, Mingler MK, Finkelman FD, Rothenberg ME (2011) The role of neuropeptide S and neuropeptide S receptor 1 in regulation of respiratory function in mice. Peptides 32, 818–825. [DOI] [PMC free article] [PubMed] [Google Scholar]


