Abstract
Introduction:
Chronic pain conditions are common among children and engender cascading effects across social, emotional, and behavioral domains for the child and family. Mobile health (mHealth) describes the practice of delivering healthcare via mobile devices and may be an ideal solution to increase access and reach of evidence-based behavioral health interventions.
Areas Covered:
The aim of this narrative review is to present a state-of-the-art overview of evidence-based mHealth efforts within the field of pediatric chronic pain and consider new and promising directions for study. Given the nascent nature of the field, published mHealth interventions in all stages of development are discussed. Literature was identified through a non-systematic search in PubMed and Google Scholar, and a review of reference lists of papers that were identified as particularly relevant or foundational (within and outside of the chronic pain literature).
Expert Opinion:
mHealth is a promising interventional modality with early evidence suggesting it is primed to enhance behavioral health delivery and patient outcomes. There are many exciting future directions to be explored including drawing inspiration from digital health technology to generate new ways of thinking about the optimal treatment of pediatric chronic pain.
Keywords: mHealth, pediatric, chronic pain, self-management
1. Introduction
Up to 25% of children experience chronic pain (i.e., pain lasting 3 or more months) worldwide, with 5–6% of children experiencing moderate to severe levels of chronic pain associated with functional disability [1–6]. In the United States, the impact of pediatric chronic pain translates to $19.5 billion in annual costs to society (e.g. procedural, pharmacological and non-pharmacological treatment, emergency room visits, parent productivity loss) [7]. Pediatric chronic pain engenders cascading effects across social, emotional, and behavioral domains for the child and family [8, 9]. These concerns at patient, family, and societal levels are enduring, as chronic pain in childhood predicts symptom continuity across the lifespan [10].
The current best practice for the management of chronic pain is rooted in biopsychosocial theory-driven frameworks. Biopsychosocial theory is an integrative model highlighting how interacting biological, cognitive, behavioral, and sociocultural influences all contribute to the complex nature of pain and associated functional disability [11, 12]. As informed by biopsychosocial theory, optimal evidence-based treatment espouses a multidisciplinary approach to include behavioral health, physiological, and pharmacological therapies [13, 14]. Behavioral health interventions, namely, Cognitive Behavior Therapy (CBT), are well established in the treatment of chronic pain. CBT harnesses cognitive and behavioral techniques to help patients identify relationships between thoughts, feelings, behaviors and pain. Strategies may include pain psychoeducation, cognitive restructuring, physiological self-regulation (e.g. relaxation training, biofeedback, mindfulness), activity pacing, and graded exposure, among others[15]. There is a rich literature providing support for the utility of behavioral health interventions in ameliorating pain intensity, functional disability, and comorbid psychiatric conditions associated with pediatric chronic pain [16, 17].
Widespread availability of behavioral health for pediatric chronic pain is undermined by a shortage of services outside of urban centers, significant treatment-related costs, and long provider waitlists [18, 19]. Those with the greatest socioeconomic barriers are likely to have most difficulty obtaining services, which serves to exacerbate healthcare disparities. Ongoing access-level barriers underscore the critical need to develop treatment delivery systems that are highly efficient, effective, and yield broad reach. Digitally delivered healthcare may be an ideal solution. Electronic health [eHealth] is an overarching class which refers to “health services and information delivered or enhanced through the Internet and related technologies” (e.g. Internet treatment, virtual sessions with a provider) [20, 21]. Mobile health interventions (mHealth), which falls under the broader category of eHealth, specifically refer to the use of mobile devices and wireless technology to deliver healthcare (e.g. smartphone applications, wearable devices, text messaging) [22].
Although the research base for mHealth for pediatric chronic pain is emerging, there is strong support for Internet-delivered eHealth systems, particularly for adult chronic pain [23–26]. As smartphone technology continues to evolve, the potential for mHealth platforms to build upon the success of eHealth is clear, particularly given that youth are more likely to access the Internet using smartphones as compared to desktop computers [27]. Access to smartphones has become ubiquitous for individuals across developmental groups and socioeconomic backgrounds, rendering mHealth modalities as uniquely suited for the rapid dissemination of interventions across differing healthcare settings with relatively minimal staffing needs [28]. Increasingly sophisticated programming has supported the development of interactive programs that are able to adapt to an individual’s responses and generate tailored content. To encourage patient engagement, smartphone applications can also adopt game-like interactions, known as “gamification,” such as having an avatar and earning points, which may be particularly engaging for pediatric patients [29, 30].
The overarching aim of this narrative review is to present a state-of-the-art overview of evidence-based mHealth efforts within the field of pediatric chronic pain and consider new and promising directions for further study. While mHealth applications are available to support pharmacological management of pain (e.g. medication tracking apps), this review will focus on behavioral health interventions for pediatric chronic pain. To date, there have been several promising apps developed to address disease-specific chronic pain conditions, such as Pain Squad [31] and Pain Buddy [32] for cancer-related pain, and SCD-PROMIS [33] and iManage for sickle cell disease [34]. While these apps show promise in supporting coping to improve chronic pain, they also contain elements specific to the medical condition that may limit the interchangeability of these applications across other pain conditions. This review focuses on the behavioral health apps for pediatric patients with non-disease specific pain conditions. Given the nascent nature of the field, published mHealth interventions in all stages of development are discussed. Literature was identified through a non-systematic search in PubMed and Google Scholar utilizing the following search terms: mHealth, internet, application, child, pediatric, adolescent, chronic pain. No language or other restrictions were applied. We also searched reference lists of papers that were identified as particularly relevant and reviewed studies with foundational or innovative approaches to mHealth that were outside of the chronic pain literature.
2. mHealth for pediatric chronic pain: state of the field
There is an emerging collection of applications developed by healthcare providers and researchers for pediatric chronic pain [35, 36]. Although pediatrics commonly refers to patients ages 0 to 21 [37], extant mHealth for pediatric chronic pain has largely targeted late childhood and adolescence (e.g. ages 10 to 18) [38, 39]. mHealth systems were initially developed as electronic diaries, often referred to as ecological momentary assessment (EMA) [40], and later evolved to include self-management techniques originating from the CBT tradition (e.g. psychoeducation, cognitive restructuring, physiological self-regulation, goal-setting, mindfulness) and dual EMA and self-management. Delivery and assessment are generally accomplished through stand-alone applications, responsive websites with content intended to be accessed via mobile applications, and text messaging. There are also mHealth/eHealth hybrid systems that incorporate some elements of eHealth interventions, such as virtual sessions with a healthcare provider that take place outside of the app. These approaches are generally considered to be mHealth, not eHealth, when intervention content is primarily delivered via mobile application [41].
EMA originated from researchers’ desire to collect multiple data points within ecologically-valid contexts to improve upon the inherent limitations of retrospective self-report. Mobile EMA delivery, as opposed to pencil and paper assessment, advances this endeavor by providing researchers with unbiased time stamps of data report. Mobile delivery has also been found to improve adherence to daily self-report, as compared to pencil and paper approaches [40]. Beyond research utility, mobile EMA and symptom tracking may serve a clinical role. For example, EMA embedded within the Web-based Management of Adolescent Pain (WebMAP) application allows youth to track pain intensity, pain interference, sleep, and mood, and visually see the trajectory of their interacting symptoms over time. Some have proposed that access to this personal data may help patients to better understand their symptoms and to encourage their sharing this information with providers [42]. It is also possible that focused attention on pain symptoms may maintain or exacerbate pain intensity or pain-related anxiety or some other aspect of functional impairment [43]. Additional study is needed to better understand if mobile EMA, particularly regular symptom tracking and self-report, influences the pain experience among participants.
Self-management has been defined by Modi and colleagues as “the interaction of health behaviors and related processes that patients and families engage in to care for a chronic condition” [44]. Common self-management techniques for chronic pain include goal setting, activity pacing, and relaxation techniques, all with the goals of supporting confidence and self-efficacy in managing symptoms. Self-management interventions are commonly introduced and monitored by a health care provider and have been shown to improve pain, functional disability, and psychological symptoms associated with pain among pediatric patients [16]. There is evidence that self-management delivered via mobile apps can support symptom improvement among adult patients with chronic pain [45]. Clinical trials to evaluate the efficacy of app-delivered self-management for pediatric pain patients are ongoing [38, 46]. Schults and colleagues [36] and Hunter and colleagues [47] provide recent reviews of self-management apps for pediatric chronic pain. It is also of relevance to look to the adult chronic pain literature to consider when it may be possible to adapt apps developed for adult patients, or components of these apps, for pediatric patients[48]. This approach may be particularly relevant for older adolescents.
3. mHealth for pediatric chronic pain: innovative directions
In the broader chronic pain and mHealth literatures, there are promising directions and innovations that set the groundwork for the future of digital interventions for pediatric chronic pain. Domains include transforming care based on digital health technology, translating in-person interventions to the digital sphere, novel service delivery models, and innovative research designs and analyses.
3.1. Transforming care based on digital health technology
Technological advancements in the field may help to reimagine optimal treatment for pediatric chronic pain. We will discuss: 1) just-in-time adaptive interventions (JITAI); 2) passive mobile sensing; and 3) wearable sensors that assess and/or treat a target behavior or sensory experience. These modalities are in early stages of development but are all innovative directions that may serve to enhance mHealth for pediatric chronic pain.
Just-in-time Adaptive Interventions (JITAI).
JITAIs are an applied extension of the EMA literature that seek to monitor aspects of a patient’s changing status and contexts with the goal of flexibly providing tailored support, in real time, when the individual needs it [49]. Although this modality is early in development, it has been examined within the context of smoking, obesity, alcohol use, schizophrenia, and physical activity [50–53]. For example, the Addiction–Comprehensive Health Enhancement Support System (A-CHESS) seeks to continue care for individuals transitioning out of residential treatment for alcohol use disorders [53]. A-CHESS was designed to promote communication with peer support groups and addiction experts, monitor symptoms to assess risk of relapse, and provide tailored CBT techniques, among other features. A-CHESS uses global positioning system [GPS] to identify if a participant nears a high-risk location (e.g. predetermined location where a participant has previously used or obtained alcohol]) and sends real time alerts and reminders to encourage adherence to treatment goals. Preliminary work has incorporated aspects of JITAI into mHealth treatment for adult chronic pain [54, 55]. For example, McDonald and colleagues conducted an RCT where they emailed home care nurses of patients with chronic cancer pain with information highlighting six pain-specific clinical recommendations [79]. Nurses randomized into the “augmented” group also received provider prompts, patient education material, and clinical nurse specialist outreach. They found that patients receiving care from nurses in the augmented group reported lower pain intensity as compared to the treatment as usual group. There is significant potential for JITAI approaches to enhance engagement with mHealth interventions for pediatric patients with chronic pain and provide tailored, critical feedback on meeting their treatment goals in real time, when needed.
Passive Mobile Sensing.
There is an array of tools assessing behaviors that have the potential to enhance JITAI and mHealth. Passive mobile sensing refers to all of the data that is collected by smartphones through everyday use, including social interactions, physical activity, app usage logs, social media use, digital media use, among other domains [56, 57]. The Effortless Assessment of Risk States (EARS) is a tool designed to capture mobile sensing data for research use. EARS is able to assess an individual’s social and affective behavior via facial expressions, acoustic vocal quality, natural language use, physical activity, music choice, phone use duration, sleep, and geographical location [58]. Other solutions include ResearchKit, released by Apple in 2015 (https://www.apple.com/ca/researchkit), which supports passive and active data collection, as well as eConsent and electronic patient-reported outcomes (ePROs) [59]. Similarly, Android developed Research Stack (http://researchstack.org) [60].
Wearable sensors.
Sensors are worn by patients to measure or sense a given target behavior/physiological state and communicate this information to an app [61, 62]. Target behaviors/physiological states may include sleep, accelerometers and GPS (activity tracking), heart rate monitors, skin temperature and conductance, blood glucose monitoring, muscle tension, among others [63, 64]. In emerging work by Lopez-Martinez and colleagues, an estimate of nociceptive pain intensity was developed by collecting autonomic activity via a wrist sensor [65].
Beyond research utility, there are some wearable devices that have been designed with the intention of therapeutic benefit. For example, Lewis and colleagues [66] developed a wearable, self-applied, low-intensity therapeutic ultrasound for adult patients with chronic myofascial pain. Results from the pilot study were favorable with regard to preliminary feasibility and patient-reported improvements in pain intensity. There is increasing evidence that virtual reality (VR) is a therapeutic adjunctive that has been found to encourage physical activity for patients with chronic pain [67–69]. There are also mobile versions of VR headsets that connect to smartphones that are also being evaluated [70].
Though advancements in digital health technology have the possibility to significantly contribute to innovations in the assessment and intervention of pediatric chronic pain, it is essential to note that they remain in nascent stages of development and evaluation. One primary challenge relates to establishing equivalency of wearable devices with traditional measurement modalities, including both objective assessment and patient-report. For example, reliability of findings from wearable devices designed to assess sleep is not equivalent with those generated by gold standard in-person laboratory sleep studies (polysomnography) [71]. It is expected that the reliability of wearable devices will improve as technology continues to evolve. At present, objective assessment and patient-reported measures have the stronger evidence-base and continue to be the gold standard for use in healthcare [62, 71–74].
3.2. Translating in-person interventions to the digital sphere
In addition to advancements in digital technology, there are a number of evidence-based in-person interventions for pediatric chronic pain that have not yet been fully explored digitally. We review the potential contributions of the following behavioral health interventions: 1) motivational approaches, 2) exposure-based treatments, and 3) caregiver interventions.
Motivational Approaches.
Following multidisciplinary evaluation, only 50% of pediatric patients with chronic pain referred to behavioral health interventions actually initiate these services [75]. Among patients who initiate treatment, engagement and readiness for change is associated with improved outcomes [76–78]. For many patients, increases in behavior needed to achieve functional improvements leads to short-term elevations in pain and discomfort, which may limit patient willingness to engage in treatment [79]. Therapeutic strategies that enhance motivation for change may be particularly beneficial in encouraging treatment engagement, despite discomfort. Motivational Interviewing (MI) is a person-centered therapeutic approach that seeks to resolve patients’ ambivalence about behavior change by strengthening their intrinsic motivation and commitment to change [80]. MI was originally developed as treatment for substance dependence [e.g. alcohol use, smoking cessation] [81] but since has expanded to successfully promote a range of health behaviors within the context of pediatric chronic illness [e.g. obesity, type 1 diabetes, asthma] [82, 83] and adult chronic pain [84]. MI has been successfully integrated in mHealth systems for other pediatric chronic illnesses [85]. Ongoing research is needed to evaluate the possibility of harnessing MI in mHealth to improve treatment adherence, pain, and function among pediatric patients with chronic pain.
Exposure-based Treatments.
There are a few emerging interventions examining graded in-vivo exposure therapy (GET) for children and adolescents with chronic pain [86–88]. For example, GET interventions specifically target pain-related fear and avoidance by gradually exposing patients to activities they have been avoiding due to fear of pain. Outside of pediatric chronic pain populations, exposure therapy has been developed and delivered via mHealth for youth with anxiety disorders (e.g., Smartphone-enhanced Child Anxiety Treatment, SmartCAT) [89, 90]. SmartCAT was adapted from the in-vivo intervention Coping Cat and addresses barriers to home-based skills practice for children by providing automatic cues to practice skills and providing interactive ways to learn the skills and offering in app learning exercises to increase understanding of skills as well as daily personalized home-based exposures. Within SmartCAT, exposure tasks are tailored to the children’s specific fears, and the child and therapist work together to develop a list of in vivo tasks that the child needs to complete. REACH is another smartphone-based intervention for anxiety in children and adolescents [91]. REACH incorporates multiple evidence-based treatment activities, including behavioral exposures. REACH has not been evaluated for efficacy but has demonstrated adequate usability. Although GET has not yet been digitized for chronic pain, its potential for positive impact on clinical outcomes necessitates further inquiry.
Caregiver Interventions.
Caregivers play a critical role in helping their children manage pain by modelling and reinforcing adaptive cognitions and behaviors, including encouraging the use of adaptive coping strategies, all of which can help the child to maintain treatment-related functional gains. In-person interventions that include parents of youth with chronic pain typically focus on teaching parents the copings skills their child has learned during treatment [e.g., relaxation skills, cognitive restructuring] to support generalization of these skills outside of treatment session. Interventions for caregivers also aim to provide education on the inadvertent reinforcing effects that attending to their child’s pain symptoms can have on a child’s functioning, with training and support on ways to shift attention away from pain complaints and toward increased functioning, thus reinforcing the desired behavior (e.g., coping, functioning) [92–94]. As digital and mobile interventions for this population are developed, these treatment components for caregivers are important to consider and integrate into mHealth interventions. In WebMAP, an 8-week digital intervention for youth with chronic pain, caregivers receive a separate login to access a unique portion of the intervention [23]. The 8 parent-focused modules include pain education, operant training to use praise, attention, and reinforcement to support youth engagement in coping strategies and meet treatment goals, modeling of appropriate responding to pain, and relapse prevention [23].
Aside from teaching caregivers the skills to support their child’s coping and functional gains, it is also important to consider caregivers own psychological distress as it relates to having a child with chronic pain, as this distress has been found to impact child functioning. Research on in-vivo interventions have recently begun to address caregiver mental health in pediatric chronic pain populations, with results demonstrating that psychological interventions focused on reducing caregiver distress were effective, and that addressing this distress impacts child’s functioning [23, 95, 96]. In addition to the treatment components mentioned above, WebMAP also includes modules on aimed at helping caregivers recognize and managing their own distress, with results showing improvement in parent anxiety, depression, and self-blame as it relates to caring for a child with chronic pain [23]. While WebMAP for youth has now evolved into a smartphone application (WebMAP Mobile), the caregiver intervention continues to be delivered via an internet-based platform [38]. Recently, an internet-based Acceptance and Commitment-based intervention for youth with chronic pain (ACTsmart Youth) was developed for mobile use. ACTsmart Youth also contains a caregiver intervention component which focuses on identifying and intervening on parents’ pain-related distress [97]. As mobile technologies continue to develop, the most effective methods for engaging parents in mHealth interventions should continue to be empirically explored.
3.3. Service delivery model: stepped care
Optimal treatment for pediatric chronic pain conditions adopts a multidisciplinary approach to encourage functional improvements. Treatment team members may include psychologists, psychiatrists, physicians, physical and occupational therapists, among others [98, 99]. Depending on degree of patient pain severity and functional disability, treatment may proceed on an outpatient basis with one or more providers; higher levels of care include intensive outpatient and inpatient rehabilitation programs. It is neither feasible nor clinically indicated to provide the highest level of care [e.g. intensive inpatient rehabilitation] for all pediatric patients with chronic pain.
Stepped Care models have been introduced to provide guidance on stratifying patients into appropriate levels of intervention in an effort to deliver cost-effective interventions that match the need of the patient. Originating in the mental health literature, Stepped Care characterizes effective treatments from least resource intensive to most resource intensive. Patient symptom data and response to treatment is examined on an ongoing basis to determine which step of treatment should be delivered, often commencing with the least resource intensive option at the outset [100]. More recently, these models have been adjusted to integrate self-directed eHealth and mHealth interventions at lower levels of need, see Figure 1 [101].
Figure 1:
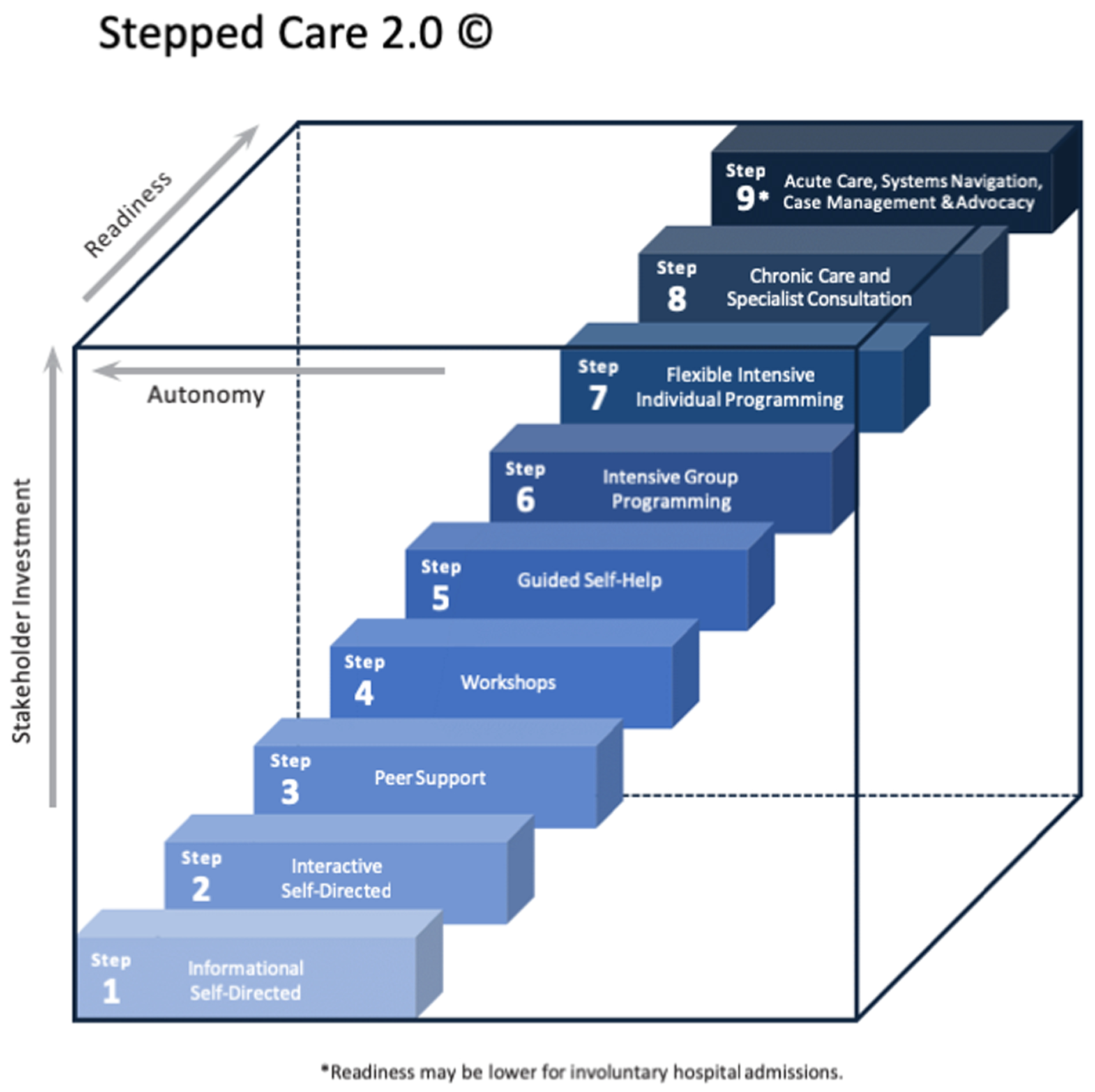
Stepped Care 2.0
Digital health solutions may be particularly important in early steps of the model. For example, the Ottawa Hospital Pain Clinic Stepped Care Approach, based on Stepped Care 2.0, included the following 8 steps: 1] Online reading/self-directed educational modules*; 2] Peer-led self-management programs; 3] Interactive online or in person group-based workshops led by health care professionals*; 4] Online therapist-assisted self-directed therapy*; 5] Online or in-person group therapy*; 6] Interprofessional chronic pain rehab program; 7] 1:1 treatment; and 8] Complex case management [102]. Figure adapted under CC BY 4.0 license from Bell L, Cornish P, Gauthier R, et al. Implementation of The Ottawa Hospital Pain Clinic Stepped Care Program: A Preliminary Report. Canadian Journal of Pain. 2020 [102].
Stepped Care 2.0 has been adapted by the Ottawa Hospital Pain Clinic to better meet the needs of patients with chronic pain. Preliminary findings from the integration of Stepped Care 2.0 into clinical services were that 90% of patients were able to be seen within 1 month of referral and the 6-month waitlist for intensive rehabilitation was eliminated [102]. The success of Stepped Care hinges on developing efficient and effective self-directed interventions that are intended to be widely disseminated as a front-line treatment: digital health solutions. Although Stepped Care has the potential to have a salient impact on within-clinic stratification of patients and wait time for care, it is important to note that a dearth of trained specialty pain providers continues to undermine patient access to optimal chronic pain treatment. Efforts need to continue to increase the number of pain providers and multidisciplinary pain clinics, while better incorporating pain management into health professional training curriculums [103].
3.4. Innovative research designs and analyses
Adaptive theoretical modeling.
As the field of mHealth continues to grow, it is essential that clinical researchers attend to theoretically-grounded development and evaluation frameworks. Assessing the feasibility and efficacy of interventions is essential to support optimal patient care. Traditional models of healthcare development are often stepwise and linear, proceeding from idea generation, to usability, feasibility, effectiveness, and full-scale efficacy trials that are both time and cost intensive. Randomized-controlled efficacy trials, on average, have a duration of approximately 7 years from grant submission to publication of results [104] and it has been estimated that it may take as long as 17 years to translate research findings into practice [105]. This approach is not amenable to mHealth development, as it yields apps using outdated technology and/or irrelevant content before they are even widely accessible [106, 107]. To enhance the development and rapid implementation of mHealth interventions, particularly where there is evidence that targeting the same mechanisms through in-person treatment is effective, adaptive trial designs are needed [108]. Adaptive designs are able to account for rapid advancements in technology and incorporate, in real time, how patients engage with differing features and functions within an app [109]. An emerging model that adopts these principles is the mHealth Agile Development & Evaluation Lifecycle framework, developed by Wilson and colleagues [110, 111] (see Figure 2). This model presents an interactive approach to mHealth intervention research that grants flexibility, rapid evaluation, and changing protocols as technology advances, with the overarching goal of generating flexible evaluations of digital solutions. The model includes four overarching phases: 1) user experience design, development, & alpha testing; 2) beta testing; 3) clinical trial evaluation; and 4) post-market surveillance. All phases of the mHealth Agile Development & Evaluation Lifecycle exist within the context of continuous and iterative feedback to account for advances in clinical knowledge and technology. Important next steps will involve using adaptive trial designs to conduct the following: feasibility trial (may be sufficient to recommend app use if efficacy has already been shown through in-person delivery), non-inferiority trial of mHealth over in-person delivery, safety & tolerability trial, and superiority trial (does mHealth improve upon in-person delivery). These studies will support mHealth as an evidence-based modality of behavioral health intervention.
Figure 2:
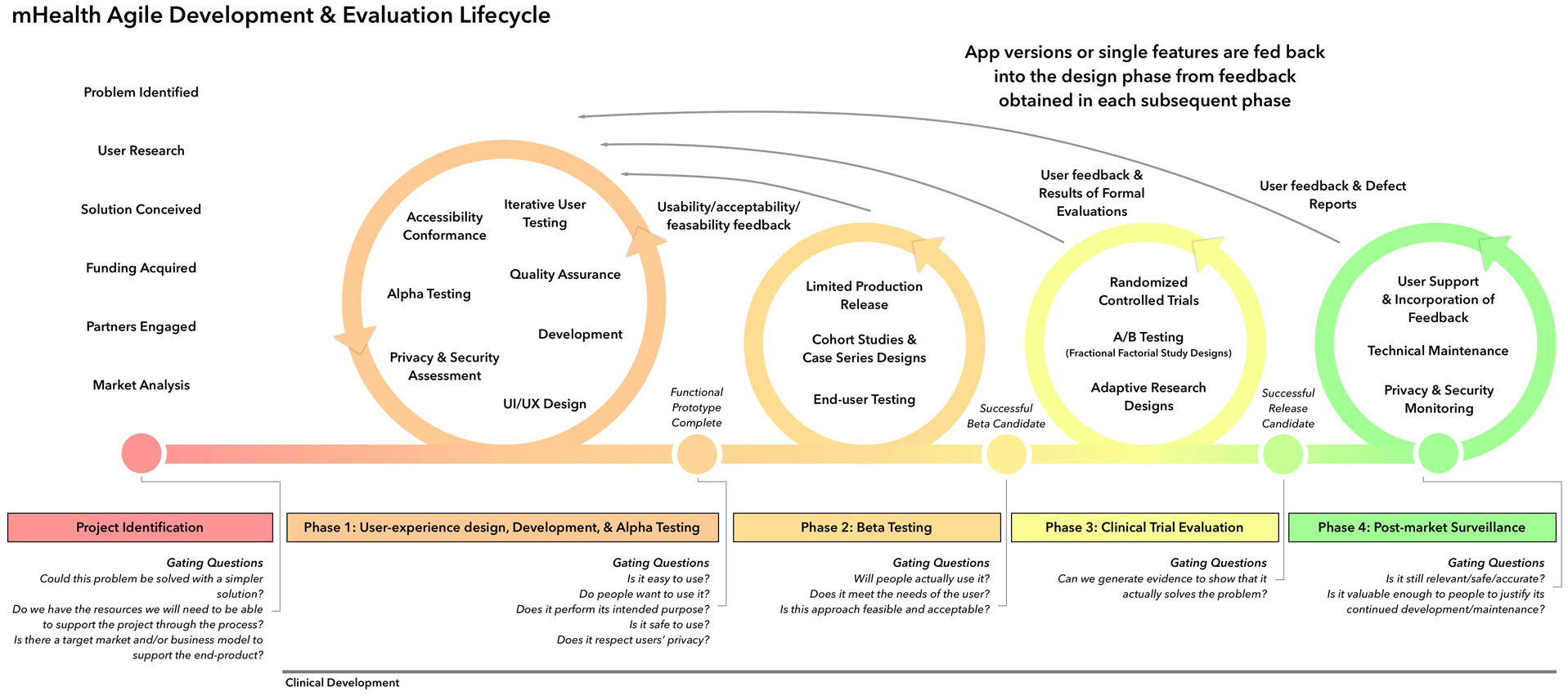
mHealth Agile Development & Evaluation Lifecycle
Figure reproduced under CC BY 4.0 license from Wilson K, Bell C, Wilson L, Witteman H. Agile research to complement agile development: a proposal for an mHealth research lifecycle. NPJ digital medicine. 2018;1:1–6 [110].
Bridging the Gap between Industry and the Scientific Community.
The majority of publicly available health and wellness apps that patients interact with have been developed by lay professionals/industry, and only 2% have supporting empirical publications [e.g. feasibility or efficacy] [112]. Publicly available apps often include foundational theoretical underpinnings of behavioral health and associated interventions, such as mindfulness, biofeedback, relaxation, and yoga, among others. Most that are specific to chronic pain target adults and have been found to include only select components of evidence-based domains known to make self-management effective [e.g. pain education, physiological self-regulation, goal-setting] [113]. Self-management techniques and addressing pain-related difficulties [e.g. sleep, activity, physiological self-regulation] have all been found to improve pain and function in pediatric patients [17]. Thus, there may be reason to believe apps generated by the lay professionals/industry could be beneficial tools for patient care, despite the fact that they have not been empirically evaluated, which implies the need for scientific methods that can be easily integrated in rapid development processes.
Several researchers have sought to provide empirical data on the clinical utility of publicly available apps. In a study conducted by Lalloo and colleagues [113], their team reviewed pain management apps [n = 279 apps identified] across iOS, Android BlackBerry, and Windows operating systems. They rated the content and functionality of each app. Among other indices, functionality of each app was assessed as present/absent across the following criteria: 1] having a pain tracking function; 2] ability to set goals related to improving pain and functioning; 3] provision of pain-related education; 4] provision of skills training related to specific pain self-care strategies; and 5] provision of social support. Smith and colleagues [114] expanded their app search terms to include both chronic pain and pain-related difficulties, but only for iOS (e.g. relaxation, sleep, anxiety; n = 57 apps identified). They rated each app on the following categories, to develop an overall measure “clinical usefulness”: a) included pain-management educational content; b) included empirically supported pain management skills; c) engaging; and d) ease of use. Combining functionality and clinical utility, we summarize proposed criteria for evaluating lay profession/industry apps in Table 1. Publicly available apps may serve an important role in treatment and the above reviews have provided helpful methodology for how researchers may apply an empirical framework to evaluate lay/industry apps. This approach has merit in increasing clinician confidence in utilizing apps for health and wellness generated by lay professionals/community in patient care.
Table 1.
Criteria to Evaluate Lay Professional/Industry Apps
| Functionality | Clinical Utility |
|---|---|
| Pain tracking | Evidence-based content |
| Other symptom tracking (e.g. sleep, mood) | Engaging content |
| Ability to set goals | Ease of use |
| Pain psychoeducation | Developmentally relevant |
| Skills training | Impact on clinical outcomes |
| Integration of wearable sensors/other patient data | Positive user ratings |
| Social support |
Single Case Experimental Design.
In addition to adaptive models and novel methodology to evaluate lay apps, it may also be beneficial to consider analytic designs that can be easily integrated in the rapid development processes and generate individual-level data analyses. Single case experimental design studies (SCED) “are experimental designs in which a single unit (e.g., a client, group of clients, classroom, ward, hospital) is repeatedly observed for a predetermined period of time at various levels of at least one manipulated variable (e.g., the treatment)” [115].
SCED facilitates the generation of meaningful findings in real time that may positively impact app development using a small sample of patients [i.e. n = 1–3]. See Vlaeyen and colleagues for a recent review that outlines the utility of SCED within behavioral science [115].
Digital Health and Big Data.
Data generated by advancements in digital health technologies, in concert with other healthcare data, require sophisticated analyses. Such “Big Data” approaches need to be able to process a multitude of data points on differing scales, including data from wearable sensors, apps, administrative healthcare data, clinical registries, electronic health record, diagnostic imaging, among others [116]. To process Big Data, machine learning algorithms and artificial intelligence (AI) are employed with the goal of predicting, preventing, and optimally treating target health outcomes. In one recent example within the context of chronic pain, a measure of pain volatility (i.e. variability in pain intensity over time) was developed for patients using a pain management app at 1 and 6 months (Manage My Pain app) [117]. A total of 130 demographic, clinical, and application usage variables were collected within the first month of app use. Machine learning algorithms were then employed to analyze the 130 variables to successfully predict pain volatility 6 months later. The above-described study by Rahman and colleagues [117] is a promising example of how mHealth and Big Data can be synthesized to assess and ultimately improve personalized care for patients with chronic pain. The healthcare industry is only beginning to explore the pragmatics of healthcare analytics [118]. A literature base is needed to evaluate if Big Data approaches will be able to improve upon existing assessment and interventional paradigms in the treatment of pediatric chronic pain.
4. Expert opinion
4.1. The digitally-informed future of healthcare.
Emerging trends in healthcare call for a precision medicine approach, which processes patient data (e.g. genetics, disease markers, lifestyle, and psychosocial indices) using healthcare analytics to classify patients into subgroups [119]. Patient subgroups then inform optimal, targeted interventions. The success of precision medicine is dependent on the collection of patient data across the above-described domains as well as the development of efficient and effective targeted treatments. Within the context of precision medicine, mHealth has the potential to integrally contribute to both data collection and intervention. At the core of optimal healthcare, data collected from the child and family (EMA, passive mobile sensing, wearable sensors) are used to inform digital interventions for the family, and are communicated, in real time, to medical providers and the healthcare system via clinician dashboards integrated within the electronic medical record (EMR). Research teams utilize patient generated data, as well as other healthcare data sources (e.g. EMR), to inform provider clinical judgement and support tailored, point of care decisions that result in science-forward, targeted, and effective interventions. We envision this synergistic and dynamic model as the future of evidence-based treatment for pediatric chronic pain, see Figure 3.
Figure 3:
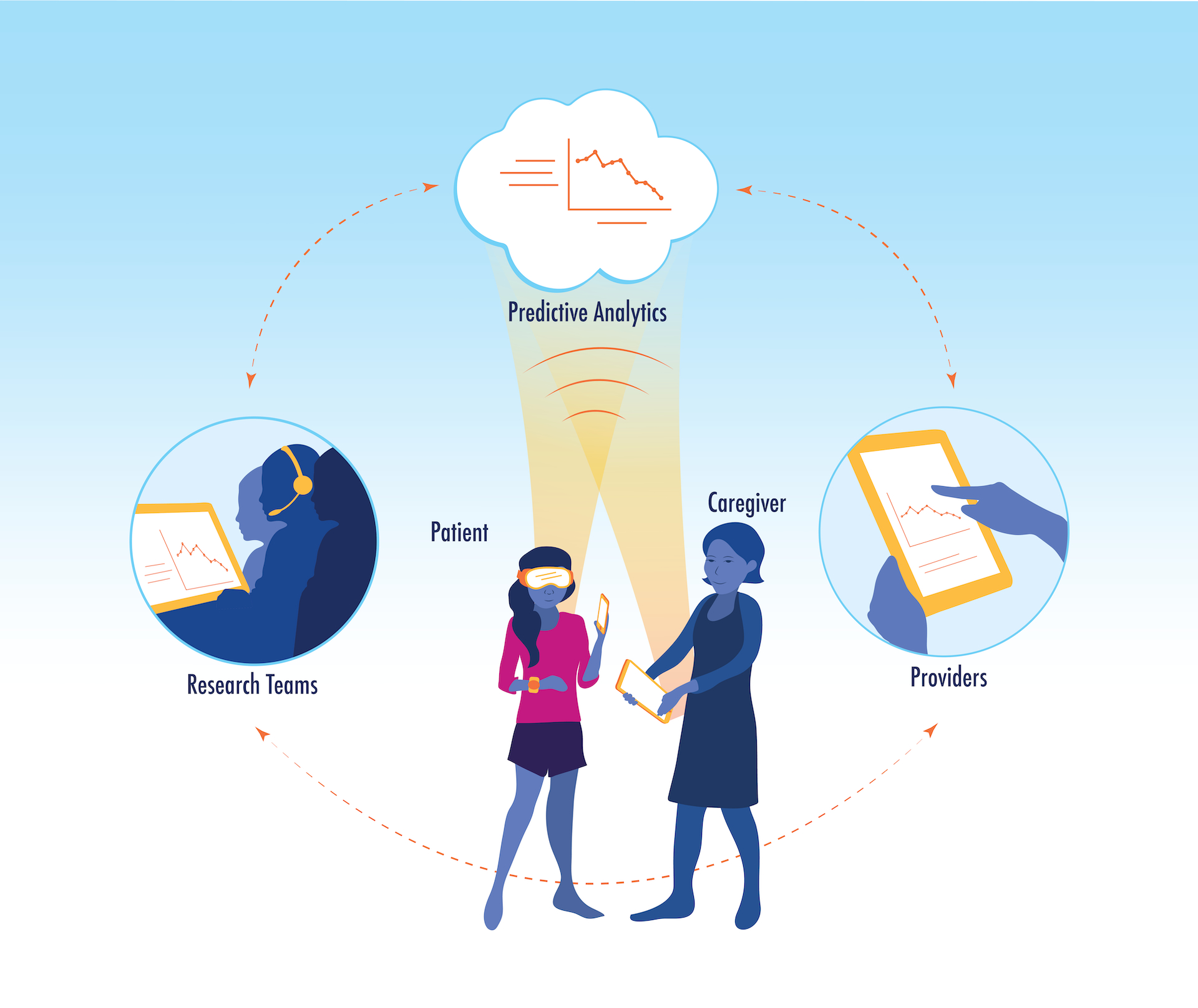
Transforming Healthcare using Digital Solutions
4.2. Key considerations.
As mHealth continues to develop as a viable and promising intervention for pediatric chronic pain, multiple research priorities remain. One priority relates to generating developmentally informed content that is appropriate for differing ages groups. Although not empirically explored, it is expected that digital content would not be equally appropriate across age groups which could, reasonably, have an impact on patient engagement with the material. At present, there are no chronic pain apps for younger children, despite increasing access to smartphones and digital technology. In addition to developmental considerations, cultural adaptations via app have yet to be explored. In some cases, relatively simple, yet highly effective solutions would increase treatment accessibility by offering child and caregiver apps in differing languages.
Although optimal treatment for pediatric chronic pain espouses a multidisciplinary specialty-care approach, primary care providers (PCPs) are often on the frontline of initial pain evaluation and intervention. Very few studies have evaluated the utility of eHealth for pediatric or adult chronic pain patients treated within primary care settings [69, 120]. However, primary care may be a beneficial setting for eHealth and mHealth deployment as it provides a larger catchment area as compared to specialty care pain clinics and improves the possibility of interrupting trajectories of pain symptoms without the need for referral to pain specialists.
There is also a need to better understand how differing mHealth formats can be harnessed. For example, there is varying degree of clinician involvement within the realm of mHealth. Some apps are designed to be self-guided by patients; others incorporate clinician check-ins (digitally or in-person). Beyond health care professional involvement, it will also be beneficial to examine treatment delivery via virtual group interventions (e.g. using videolink meetings, chat forums), as well as studies designed to assess the relative merits of personalized versus standardized healthcare. There is also the need to study the utility of app development across conditions. For many children, it is not well understood why acute pain transitions to chronic pain. EMA could be used to monitor symptom progression, and digital interventions could be applied within that system to provide preventive or early interventions. Implications of differing service delivery formats and transdiagnostic systems on treatment outcomes for pediatric patients with chronic pain are not yet well understood yet reflect important domains for future inquiry.
Finally, it is essential to note that our field has historically struggled with the transition from housing digital interventions in academic systems to widespread public access. As documented by Higgins and colleagues, only 28% of eHealth or mHealth-based tools developed for pediatric pain are publicly disseminated. Given that the average cost per resource was $314,425.31 USD, this has resulted in significant “research waste” [121]. Bridging the gap between mHealth development and widespread access should be a primary goal for the field moving forward. This is a complex issue that hinges on the ability to scale up once built, maintain economic costs, and remain adaptive with regard to technological advancements. At present, there is little structure in place within academic medicine that supports researchers in this endeavor. Strengthening partnerships with industry may be an ideal solution to these challenges as industry has the infrastructure to develop, maintain, and adapt apps.
Digitally-delivered care is rapidly expanding across pediatrics. mHealth is a promising interventional modality with early evidence suggesting it is primed to enhance behavioral health delivery and patient outcomes. There are there are many exciting future directions to be explored including advancing the current evidence base, translating empirically supported practices to the digital realm, and reimagining behavioral health interventions by drawing inspiration from advancements in digital health technology, that may generate new ways of thinking about pediatric chronic pain treatment.
Article highlights.
There is a rich literature providing support for the utility of behavioral health interventions in ameliorating pain intensity, functional disability, and comorbid psychiatric conditions associated with pediatric chronic pain
Widespread availability of behavioral health for pediatric chronic pain is undermined by a shortage of services outside of urban centers, significant treatment-related costs, and long provider waitlists.
Access to smartphones has become ubiquitous for individuals across developmental groups and socioeconomic backgrounds, rendering mobile health interventions (mHealth) as uniquely suited for the rapid dissemination of interventions across differing healthcare settings.
mHealth systems for pediatric chronic pain have been developed as symptom diaries, often referred to as ecological momentary assessment (EMA), and to deploy self-management techniques (e.g. psychoeducation, cognitive restructuring, physiological self-regulation, goal-setting, mindfulness).
In the broader chronic pain and mHealth literatures, there are promising directions and innovations that set the groundwork for the future of digital interventions for pediatric chronic pain, including: transforming care based on digital health technology, translating in-person interventions to the digital sphere, novel service delivery models, and innovative research designs and analyses.
As mHealth continues to develop as a viable and promising intervention for pediatric chronic pain, multiple research priorities remain, including generating developmentally informed content that is appropriate for differing ages groups, considering cultural adaptations, and evaluating differing mHealth formats. (e.g. self-guided, clinician check-ins, group interventions, personalized vs standardized content).
Funding
This investigation was supported by NIAMS/R21 AR072921 awarded to LE Simons.
Footnotes
Declaration of interests
The authors have no other relevant affiliations or financial involvement with any organization or entity with a financial interest in or conflict with the subject matter or materials discussed in this manuscript apart from those disclosed.
Reviewer disclosures
Peer reviewers on this manuscript have no relevant financial or other relationships to disclose.
References
Papers of special note have been highlighted as either of interest (*) or of considerable interest (**) to readers.
- 1.Haraldstad K, Sørum R, Eide H, Natvig GK, Helseth S. Pain in children and adolescents: prevalence, impact on daily life, and parents’ perception, a school survey. Scandinavian journal of caring sciences. 2011;25:27–36 [DOI] [PubMed] [Google Scholar]
- 2.Hoftun GB, Romundstad PR, Rygg M. Factors associated with adolescent chronic non-specific pain, chronic multisite pain, and chronic pain with high disability: the Young–HUNT Study 2008. The Journal of Pain. 2012;13:874–83 [DOI] [PubMed] [Google Scholar]
- 3.Roth‐Isigkeit A, Thyen U, Raspe H, Stöven H, Schmucker P. Reports of pain among German children and adolescents: an epidemiological study. Acta paediatrica. 2004;93:258–63 [PubMed] [Google Scholar]
- 4.Treede R-D, Rief W, Barke A, et al. Chronic pain as a symptom or a disease: the IASP Classification of Chronic Pain for the: International Classification of Diseases:(: ICD-11:). Pain. 2019;160:19–27 [DOI] [PubMed] [Google Scholar]
- 5.Huguet A, Miró J. The severity of chronic pediatric pain: an epidemiological study. The Journal of Pain. 2008;9:226–36 [DOI] [PubMed] [Google Scholar]
- 6.Datz H, Tumin D, Miller R, et al. Pediatric chronic pain and caregiver burden in a national survey. Scandinavian journal of pain. 2019;19:109–16 [DOI] [PubMed] [Google Scholar]
- 7.Groenewald CB, Essner BS, Wright D, Fesinmeyer MD, Palermo TM. The economic costs of chronic pain among a cohort of treatment-seeking adolescents in the United States. The Journal of Pain. 2014;15:925–33 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Palermo TM. Impact of recurrent and chronic pain on child and family daily functioning: a critical review of the literature. Journal of Developmental and Behavioral Pediatrics. 2000 [DOI] [PubMed] [Google Scholar]
- 9.Richardson PA, Birnie KA, Harrison LE, Rajagopalan A, Bhandari RP. Profiling Modifiable Psychosocial Factors Among Children With Chronic Pain: A Person-Centered Methodology. The Journal of Pain. 2019 [DOI] [PubMed] [Google Scholar]
- 10.Hassett AL, Hilliard PE, Goesling J, et al. Reports of chronic pain in childhood and adolescence among patients at a tertiary care pain clinic. The Journal of Pain. 2013;14:1390–7 [DOI] [PubMed] [Google Scholar]
- 11.Melzack R, Wall PD. Pain mechanisms: a new theory. Science. 1965;150:971–9 [DOI] [PubMed] [Google Scholar]
- 12.Gatchel RJ, Peng YB, Peters ML, Fuchs PN, Turk DC. The biopsychosocial approach to chronic pain: scientific advances and future directions. Psychological bulletin. 2007;133:581. [DOI] [PubMed] [Google Scholar]
- 13.Harrison LE, Pate JW, Richardson PA, et al. Best-Evidence for the rehabilitation of chronic pain Part 1: pediatric pain. Journal of clinical medicine. 2019;8:1267. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Ayling Campos A, Amaria K, Campbell F, McGrath PA. Clinical impact and evidence base for physiotherapy in treating childhood chronic pain. Physiotherapy Canada. 2011;63:21–33 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Palermo TM. Cognitive-behavioral therapy for chronic pain in children and adolescents: Oxford University Press; 2012. [Google Scholar]
- 16.Fisher E, Law E, Dudeney J, Eccleston C, Palermo TM. Psychological therapies (remotely delivered) for the management of chronic and recurrent pain in children and adolescents. Cochrane Database of Systematic Reviews. 2019 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Eccleston C, Palermo TM, de C Williams AC, et al. Psychological therapies for the management of chronic and recurrent pain in children and adolescents. Cochrane Database of Systematic Reviews. 2014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Palermo T Remote management of pediatric pain Encyclopedia of Pain 2nd Edition New York: Springer; 20133389–93 [Google Scholar]
- 19.Peng P, Stinson JN, Choiniere M, et al. Dedicated multidisciplinary pain management centres for children in Canada: the current status. Canadian journal of anaesthesia. 2007;54:985. [DOI] [PubMed] [Google Scholar]
- 20.Eysenbach G What is e-health J Med Internet Res 2001; 3 (2): e20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Borrelli B, Ritterband LM. Special issue on eHealth and mHealth: Challenges and future directions for assessment, treatment, and dissemination. Health Psychology. 2015;34:1205. [DOI] [PubMed] [Google Scholar]
- 22.Kay M, Santos J, Takane M. mHealth: New horizons for health through mobile technologies. World Health Organization. 2011;64:66–71 [Google Scholar]
- 23.Palermo TM, Law EF, Fales J, et al. Internet-delivered cognitive-behavioral treatment for adolescents with chronic pain and their parents: a randomized controlled multicenter trial. Pain. 2016;157:174. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Slater H, Jordan JE, Chua J, et al. Young people’s experiences of persistent musculoskeletal pain, needs, gaps and perceptions about the role of digital technologies to support their co-care: a qualitative study. BMJ open. 2016;6:e014007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Du S, Liu W, Cai S, Hu Y, Dong J. The efficacy of e-health in the self-management of chronic low back pain: A meta analysis. International Journal of Nursing Studies. 2019103507. [DOI] [PubMed] [Google Scholar]
- 26.Slattery BW, Haugh S, O’Connor L, et al. An Evaluation of the Effectiveness of the Modalities Used to Deliver Electronic Health Interventions for Chronic Pain: Systematic Review With Network Meta-Analysis. Journal of medical Internet research. 2019;21:e11086. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Anderson M, Jiang J. Teens, social media & technology 2018. Pew Research Center. 2018;31:2018 [Google Scholar]
- 28.Chassiakos YLR, Radesky J, Christakis D, Moreno MA, Cross C. Children and adolescents and digital media. Pediatrics. 2016;138:e20162593. [DOI] [PubMed] [Google Scholar]
- 29.Miller A, Cafazzo J, Seto E, Plan AG. Gamification Design Principles in mHealth Applications for Chronic Disease Management. Health Informatics Journal.22: [DOI] [PubMed] [Google Scholar]
- 30.Ma BD, Ng SL, Schwanen T, et al. Pokémon GO and physical activity in Asia: multilevel study. Journal of medical Internet research. 2018;20:e217. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Stinson JN, Jibb LA, Nguyen C, et al. Construct validity and reliability of a real-time multidimensional smartphone app to assess pain in children and adolescents with cancer. Pain. 2015;156:2607–15 [DOI] [PubMed] [Google Scholar]
- 32.Fortier MA, Chung WW, Martinez A, Gago-Masague S, Sender L. Pain buddy: A novel use of m-health in the management of children’s cancer pain. Computers in biology and medicine. 2016;76:202–14 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Gary K, Rallabhandi P, Quezado Z, Cleary K, editors. A Pain Reporting Platform for Adolescents with Sickle-Cell Disease. Proceedings of the 52nd Hawaii International Conference on System Sciences; 2019 [Google Scholar]
- 34.Crosby LE, Ware RE, Goldstein A, et al. Development and evaluation of iManage: a self‐management app co‐designed by adolescents with sickle cell disease. Pediatric blood & cancer. 2017;64:139–45 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Heathcote LC, Pate JW, Park AL, et al. Pain neuroscience education on YouTube. PeerJ. 2019;7:e6603. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Schults J, Cooke M, Richards J, et al. mHealth Applications for Children and Young People With Persistent Pain: A Scoping Review. Clinical nursing research. 2019;28:779–94 [DOI] [PubMed] [Google Scholar]; *This review details extant mHealth apps for pediatric chronic pain.
- 37.Hardin AP, Hackell JM, Practice Co, Medicine A. Age limit of pediatrics. Pediatrics. 2017;140:e20172151. [DOI] [PubMed] [Google Scholar]
- 38.Palermo TM, de la Vega R, Dudeney J, Murray C, Law E. Mobile health intervention for self-management of adolescent chronic pain (WebMAP mobile): Protocol for a hybrid effectiveness-implementation cluster randomized controlled trial. Contemporary clinical trials. 2018;74:55–60 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Stinson JN, Lalloo C, Harris L, et al. iCanCope with Pain™: User-centred design of a web-and mobile-based self-management program for youth with chronic pain based on identified health care needs. Pain Research and Management. 2014;19: [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Heron KE, Everhart RS, McHale SM, Smyth JM. Using mobile-technology-based ecological momentary assessment (EMA) methods with youth: A systematic review and recommendations. Journal of pediatric psychology. 2017;42:1087–107 [DOI] [PubMed] [Google Scholar]
- 41.Dugas M, Gao G, Agarwal R. Unpacking mHealth interventions: A systematic review of behavior change techniques used in randomized controlled trials assessing mHealth effectiveness. Digital Health. 2020;6:2055207620905411. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Lalloo C, Hundert A, Harris L, et al. Capturing daily disease experiences of adolescents with chronic pain: mHealth-mediated symptom tracking. JMIR mHealth and uHealth. 2019;7:e11838. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Hufford MR, Shields AL, Shiffman S, Paty J, Balabanis M. Reactivity to ecological momentary assessment: An example using undergraduate problem drinkers. Psychology of addictive behaviors. 2002;16:205. [PubMed] [Google Scholar]
- 44.Modi AC, Pai AL, Hommel KA, et al. Pediatric self-management: a framework for research, practice, and policy. Pediatrics. 2012;129:e473–e85 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Devan H, Farmery D, Peebles L, Grainger R. Evaluation of self-management support functions in apps for people with persistent pain: systematic review. JMIR mHealth and uHealth. 2019;7:e13080. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Birnie KA, Campbell F, Nguyen C, et al. iCanCope PostOp: User-Centered Design of a Smartphone-Based App for Self-Management of Postoperative Pain in Children and Adolescents. JMIR formative research. 2019;3:e12028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Hunter JF, Kain ZN, Fortier MA. Pain relief in the palm of your hand: harnessing mobile health to manage pediatric pain. Pediatric Anesthesia. 2019;29:120–4 [DOI] [PubMed] [Google Scholar]; * This review details extant mHealth apps for pediatric chronic pain.
- 48.Sundararaman LV, Edwards RR, Ross EL, Jamison RN. Integration of mobile health technology in the treatment of chronic pain: a critical review. Regional Anesthesia & Pain Medicine. 2017;42:488–98 [DOI] [PubMed] [Google Scholar]
- 49.Nahum-Shani I, Smith SN, Spring BJ, et al. Just-in-time adaptive interventions (JITAIs) in mobile health: key components and design principles for ongoing health behavior support. Annals of Behavioral Medicine. 2018;52:446–62 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Patrick K, Raab F, Adams M, et al. A text message-based intervention for weight loss: randomized controlled trial. Journal of medical Internet research. 2009;11:e1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Riley W, Obermayer J, Jean-Mary J. Internet and mobile phone text messaging intervention for college smokers. Journal of American College Health. 2008;57:245–8 [DOI] [PubMed] [Google Scholar]
- 52.Ben-Zeev D, Kaiser SM, Brenner CJ, et al. Development and usability testing of FOCUS: A smartphone system for self-management of schizophrenia. Psychiatric rehabilitation journal. 2013;36:289. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Gustafson DH, McTavish FM, Chih M-Y, et al. A smartphone application to support recovery from alcoholism: a randomized clinical trial. JAMA psychiatry. 2014;71:566–72 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.McDonald MV, Pezzin LE, Feldman PH, Murtaugh CM, Peng TR. Can just-in-time, evidence-based “reminders” improve pain management among home health care nurses and their patients? Journal of pain and symptom management. 2005;29:474–88 [DOI] [PubMed] [Google Scholar]
- 55.Pelle T, Bevers K, Van Der Palen J, Van Den Hoogen FH, Van Den Ende CH. Development and evaluation of a tailored e-self-management intervention (dr. Bart app) for knee and/or hip osteoarthritis: study protocol. BMC musculoskeletal disorders. 2019;20:398. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Vaid SS, Harari GM. Smartphones in Personal Informatics: A Framework for Self-Tracking Research with Mobile Sensing Digital Phenotyping and Mobile Sensing: Springer; 2019. p. 65–92 [Google Scholar]
- 57.Kumar S, Al’Absi M, Beck J, Ertin E, Scott M. Behavioral monitoring and assessment via mobile sensing technologies. Behavioral Healthcare Technol: Using Science-Based Innovations to Transform Practice. 2014;27:621–4 [Google Scholar]; *This chapter reviews mobile sensing and wearable devices.
- 58.Lind MN, Byrne ML, Wicks G, Smidt AM, Allen NB. The Effortless Assessment of Risk States (EARS) tool: an interpersonal approach to mobile sensing. JMIR mental health. 2018;5:e10334. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.ResearchKit AI. Giving Medical Researchers the Tools to Revolutionize Medical Studies. [Google Scholar]
- 60.Haddad D Introducing: ResearchStack [Press release]. Retrieved from. 2016 [Google Scholar]
- 61.Tully J, Dameff C, Longhurst CA. Wave of Wearables: Clinical Management of Patients and the Future of Connected Medicine. Clinics in Laboratory Medicine. 2020 [DOI] [PubMed] [Google Scholar]
- 62.Iqbal MH, Aydin A, Brunckhorst O, Dasgupta P, Ahmed K. A review of wearable technology in medicine. Journal of the Royal Society of Medicine. 2016;109:372–80 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Smith MT, McCrae CS, Cheung J, et al. Use of actigraphy for the evaluation of sleep disorders and circadian rhythm sleep-wake disorders: an American Academy of Sleep Medicine systematic review, meta-analysis, and GRADE assessment. Journal of Clinical Sleep Medicine. 2018;14:1209–30 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Beck RW, Riddlesworth T, Ruedy K, et al. Effect of continuous glucose monitoring on glycemic control in adults with type 1 diabetes using insulin injections: the DIAMOND randomized clinical trial. Jama. 2017;317:371–8 [DOI] [PubMed] [Google Scholar]
- 65.Martinez DL, Rudovic O, Doughty D, Subramony JA, Picard R. (340) Automatic pain intensity estimation using multi-modal data from wearable sensors. The Journal of Pain. 2017;18:S60 [Google Scholar]
- 66.Lewis GK Jr, Langer MD, Henderson CR Jr, Ortiz R. Design and evaluation of a wearable self-applied therapeutic ultrasound device for chronic myofascial pain. Ultrasound in medicine & biology. 2013;39:1429–39 [DOI] [PubMed] [Google Scholar]
- 67.Won AS, Bailey J, Bailenson J, et al. Immersive virtual reality for pediatric pain. Children. 2017;4:52. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Won AS, Tataru CA, Cojocaru CM, et al. Two virtual reality pilot studies for the treatment of pediatric CRPS. Pain Medicine. 2015;16:1644–7 [DOI] [PubMed] [Google Scholar]
- 69.Ahmadpour N, Randall H, Choksi H, et al. Virtual Reality interventions for acute and chronic pain management. The international journal of biochemistry & cell biology. 2019;114:105568. [DOI] [PubMed] [Google Scholar]
- 70.Wiederhold BK, Miller IT, Wiederhold MD. Using virtual reality to mobilize health care: mobile virtual reality technology for attenuation of anxiety and pain. IEEE Consumer Electronics Magazine. 2017;7:106–9 [Google Scholar]
- 71.Guillodo E, Lemey C, Simonnet M, et al. Clinical Applications of Mobile Health Wearable–Based Sleep Monitoring: Systematic Review. JMIR mHealth and uHealth. 2020;8:e10733. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Dobkin BH, Martinez C. Wearable sensors to monitor, enable feedback, and measure outcomes of activity and practice. Current neurology and neuroscience reports. 2018;18:87. [DOI] [PubMed] [Google Scholar]
- 73.Nag A, Mukhopadhyay SC, Kosel J. Wearable flexible sensors: A review. IEEE Sensors Journal. 2017;17:3949–60 [Google Scholar]
- 74.Coravos A, Goldsack JC, Karlin DR, et al. Digital medicine: a primer on measurement. Digital Biomarkers. 2019;3:31–71 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Simons LE, Logan DE, Chastain L, Cerullo M. Engagement in multidisciplinary interventions for pediatric chronic pain: parental expectations, barriers, and child outcomes. The Clinical journal of pain. 2010;26:291–9 [DOI] [PubMed] [Google Scholar]
- 76.Nicholas MK, Asghari A, Corbett M, et al. Is adherence to pain self‐management strategies associated with improved pain, depression and disability in those with disabling chronic pain? European Journal of Pain. 2012;16:93–104 [DOI] [PubMed] [Google Scholar]
- 77.Guite JW, Logan DE, Simons LE, Blood EA, Kerns RD. Readiness to change in pediatric chronic pain: initial validation of adolescent and parent versions of the Pain Stages of Change Questionnaire. PAIN®. 2011;152:2301–11 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Logan DE, Conroy C, Sieberg CB, Simons LE. Changes in willingness to self-manage pain among children and adolescents and their parents enrolled in an intensive interdisciplinary pediatric pain treatment program. PAIN®. 2012;153:1863–70 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Parker DM, Harrison LE, Simons LE. Chronic pain Adherence and Self-Management in Pediatric Populations: Elsevier; 2020. p. 133–58 [Google Scholar]
- 80.Rollnick S, Miller WR, Butler C. Motivational interviewing in health care: helping patients change behavior: Guilford Press; 2008. [Google Scholar]
- 81.DiClemente CC, Corno CM, Graydon MM, Wiprovnick AE, Knoblach DJ. Motivational interviewing, enhancement, and brief interventions over the last decade: A review of reviews of efficacy and effectiveness. Psychology of Addictive Behaviors. 2017;31:862. [DOI] [PubMed] [Google Scholar]
- 82.Burke BL, Arkowitz H, Menchola M. The efficacy of motivational interviewing: a meta-analysis of controlled clinical trials. Journal of consulting and clinical psychology. 2003;71:843. [DOI] [PubMed] [Google Scholar]
- 83.Cushing CC, Jensen CD, Miller MB, Leffingwell TR. Meta-analysis of motivational interviewing for adolescent health behavior: efficacy beyond substance use. Journal of Consulting and Clinical Psychology. 2014;82:1212. [DOI] [PubMed] [Google Scholar]
- 84.Jensen MP, Nielson WR, Kerns RD. Toward the development of a motivational model of pain self-management. The Journal of Pain. 2003;4:477–92 [DOI] [PubMed] [Google Scholar]
- 85.Bianchi-Hayes J, Schoenfeld E, Cataldo R, et al. Combining activity trackers with motivational interviewing and mutual support to increase physical activity in parent-adolescent dyads: Longitudinal observational feasibility study. JMIR pediatrics and parenting. 2018;1:e3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Dekker C, Goossens ME, Bastiaenen CH, Verbunt JA. Study protocol for a multicentre randomized controlled trial on effectiveness of an outpatient multimodal rehabilitation program for adolescents with chronic musculoskeletal pain (2B Active). BMC musculoskeletal disorders. 2016;17:317. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Simons LE, Vlaeyen JW, Declercq L, et al. Avoid or engage? Outcomes of graded exposure in youth with chronic pain using a sequential replicated single-case randomized design. Pain. 2020;161:520–31 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Simons LE, Harrison LE, O’Brien SF, et al. Graded exposure treatment for adolescents with chronic pain (GET Living): Protocol for a randomized controlled trial enhanced with single case experimental design. Contemporary clinical trials communications. 2019;16:100448. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Pramana G, Parmanto B, Lomas J, et al. Using mobile health gamification to facilitate cognitive behavioral therapy skills practice in child anxiety treatment: open clinical trial. JMIR serious games. 2018;6:e9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Silk JS, Pramana G, Sequeira SL, et al. Using a smartphone app and clinician portal to enhance brief cognitive behavioral therapy for childhood anxiety disorders. Behavior Therapy. 2020;51:69–84 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Stoll RD, Pina AA, Gary K, Amresh A. Usability of a smartphone application to support the prevention and early intervention of anxiety in youth. Cognitive and behavioral practice. 2017;24:393–404 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Kanstrup M, Wicksell RK, Kemani M, et al. A clinical pilot study of individual and group treatment for adolescents with chronic pain and their parents: Effects of acceptance and commitment therapy on functioning. Children. 2016;3:30. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Law EF, Fales JL, Beals-Erickson SE, et al. A single-arm feasibility trial of problem-solving skills training for parents of children with idiopathic chronic pain conditions receiving intensive pain rehabilitation. Journal of pediatric psychology. 2017;42:422–33 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Palermo TM, Law EF, Bromberg M, et al. Problem solving skills training for parents of children with chronic pain: A pilot randomized controlled trial. Pain. 2016;157:1213. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Law E, Fisher E, Eccleston C, Palermo TM. Psychological interventions for parents of children and adolescents with chronic illness. Cochrane Database of Systematic Reviews. 2019 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Simons LE, Smith A, Kaczynski K, Basch M. Living in fear of your child’s pain: the parent fear of pain questionnaire. Pain. 2015;156:694. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Zetterqvist VG C; Rickardsson J; Holmstrom L, & Wicksell RK. Open clinical trial of Acceptance and Commitment Therapy for children and adolescents with chronic pain – outcome and characteristics of responders. 2019 [Google Scholar]
- 98.Clinch J, Eccleston C. Chronic musculoskeletal pain in children: assessment and management. Rheumatology. 2009;48:466–74 [DOI] [PubMed] [Google Scholar]
- 99.Odell S, Logan DE. Pediatric pain management: the multidisciplinary approach. Journal of pain research. 2013;6:785. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Anderson DR, Zlateva I, Coman EN, et al. Improving pain care through implementation of the Stepped Care Model at a multisite community health center. Journal of pain research. 2016;9:1021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Cornish PA, Berry G, Benton S, et al. Meeting the mental health needs of today’s college student: Reinventing services through Stepped Care 2.0. Psychological services. 2017;14:428. [DOI] [PubMed] [Google Scholar]
- 102.Bell L, Cornish P, Gauthier R, et al. Implementation of The Ottawa Hospital Pain Clinic Stepped Care Program: A Preliminary Report. Canadian Journal of Pain. 2020 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Martin SR, Zeltzer LK. Prioritizing pediatric chronic pain and comprehensive pain treatment in the context of the opioid epidemic. Future Medicine; 2018 [DOI] [PubMed] [Google Scholar]
- 104.Riley WT, Glasgow RE, Etheredge L, Abernethy AP. Rapid, responsive, relevant (R3) research: a call for a rapid learning health research enterprise. Clinical and translational medicine. 2013;2:10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Balas EA, Boren SA. Managing clinical knowledge for health care improvement. Yearbook of medical informatics. 2000;9:65–70 [PubMed] [Google Scholar]
- 106.Gentili C, Zetterqvist V, Rickardsson J, et al. ACTsmart–development and feasibility of digital Acceptance and Commitment Therapy for adults with chronic pain. NPJ digital medicine. 2020;3:1–12 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.Baker TB, Gustafson DH, Shah D. How can research keep up with eHealth? Ten strategies for increasing the timeliness and usefulness of eHealth research. Journal of medical Internet research. 2014;16:e36. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108.Pham Q, Wiljer D, Cafazzo JA. Beyond the randomized controlled trial: a review of alternatives in mHealth clinical trial methods. JMIR mHealth and uHealth. 2016;4:e107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109.Pham Q, Graham G, Lalloo C, et al. An analytics platform to evaluate effective engagement with pediatric mobile health apps: design, development, and formative evaluation. JMIR mHealth and uHealth. 2018;6:e11447. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110.Wilson K, Bell C, Wilson L, Witteman H. Agile research to complement agile development: a proposal for an mHealth research lifecycle. NPJ digital medicine. 2018;1:1–6 [DOI] [PMC free article] [PubMed] [Google Scholar]; **This manuscript describes an innovative approach to adaptive trial designs for mHealth.
- 111.Moser R, Abrahamsson P, Pedrycz W, et al. Balancing agility and formalism in software engineering. Berlin, Heidelberg: Springer-Verlag; 2008252–66 [Google Scholar]
- 112.Lau N, O’Daffer A, Colt S, et al. Science or Snake Oil: Systematic Search of iPhone and Android Mobile Apps for Psychosocial Wellness and Stress Management. JMIR mHealth and uHealth. 2020 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113.Lalloo C, Jibb LA, Rivera J, Agarwal A, Stinson JN. There’sa Pain App for that. The Clinical journal of pain. 2015;31:557–63 [DOI] [PubMed] [Google Scholar]
- 114.Smith K, Iversen C, Kossowsky J, et al. Apple apps for the management of pediatric pain and pain-related stress. Clinical Practice in Pediatric Psychology. 2015;3:93 [Google Scholar]
- 115.Vlaeyen J, Wicksell RK, Simons LE, et al. From Boulder to Stockholm in 70 Years: Single Case Experimental Designs in Clinical Research. The Psychological Record: a quarterly journal in theoretical and experimental psychology. 2020 [Google Scholar]
- 116.Palanisamy V, Thirunavukarasu R. Implications of big data analytics in developing healthcare frameworks–A review. Journal of King Saud University-Computer and Information Sciences. 2019;31:415–25 [Google Scholar]
- 117.Rahman QA, Janmohamed T, Pirbaglou M, et al. Defining and predicting pain volatility in users of the manage my pain app: analysis using data mining and machine learning methods. Journal of medical Internet research. 2018;20:e12001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 118.Mehta N, Pandit A. Concurrence of big data analytics and healthcare: A systematic review. International journal of medical informatics. 2018;114:57–65 [DOI] [PubMed] [Google Scholar]
- 119.Ginsburg GS, Phillips KA. Precision medicine: from science to value. Health Affairs. 2018;37:694–701 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 120.Glynn LG, Hayes PS, Casey M, et al. Effectiveness of a smartphone application to promote physical activity in primary care: the SMART MOVE randomised controlled trial. British Journal of General Practice. 2014;64:e384–e91 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 121.Higgins KS, Tutelman PR, Chambers CT, et al. Availability of researcher-led eHealth tools for pain assessment and management: barriers, facilitators, costs, and design. Pain Reports. 2018;3: [DOI] [PMC free article] [PubMed] [Google Scholar]


