Abstract
Background:
Tumor growth patterns have important implications for surveillance intervals, prognostication, and treatment decisions but have not been well described for hepatocellular carcinoma (HCC). The aim of our study was to characterize HCC doubling time and identify correlates for indolent and rapid growth patterns.
Methods:
We performed a systematic literature review of Medline and EMBASE databases from inception to December 2019 and national meeting abstracts from 2010 to 2018. We identified studies reporting HCC tumor growth or tumor volume doubling time (TVDT), without intervening treatment, and abstracted data to calculate TVDT and correlates of growth patterns (rapid defined as TVDT <3 months and indolent as TVDT >9 months). Pooled TVDT was calculated using a random effects model.
Results:
We identified 20 studies, including 1374 HCC lesions in 1334 patients. The pooled TVDT was 4.6 months (95%CI 3.9 – 5.3 months I2=94%), with 35% classified as rapid, 27.4% intermediate, and 37.6% indolent growth. In subgroup analysis, studies from Asia reported shorter TVDT than studies elsewhere (4.1 vs. 5.8 months). The most consistent correlates of rapid tumor growth included hepatitis B etiology, smaller tumor size (continuous), AFP doubling time, and poor tumor differentiation. Studies were limited by small sample sizes, measurement bias, and selection bias.
Conclusion:
Tumor volume doubling time of HCC is approximately 4–5 months; however, there is heterogeneity in tumor growth patterns, including more aggressive patterns in Asian hepatitis B-predominant populations. Identifying correlates of tumor growth patterns is important to better individualize HCC prognostication and treatment decisions.
Keywords: liver cancer, HCC, tumor growth rate, tumor biology, overdiagnosis
INTRODUCTION
Hepatocellular carcinoma (HCC) is the 4th leading cause of cancer-related mortality worldwide, contributing to approximately 800,000 deaths annually.1 It is one of the few cancers whose incidence and mortality is increasing in the United States and Europe over recent years, related to an increased burden of chronic liver disease including hepatitis C (HCV) and non-alcoholic steatohepatitis (NASH).2 Further, despite improvements in treatment, HCC survival remains dismal with 5-year survival below 20%.3
In light of these data, HCC has traditionally been considered an aggressive tumor with presumed rapid growth. However, recent multi-center data from the US and Europe have questioned this dogma, demonstrating heterogeneity in tumor growth patterns with over one-third of tumors demonstrating indolent growth patterns.4 These data are important to confirm given the multi-faceted importance of tumor growth patterns. For example, the current recommendation to perform HCC surveillance every 6 months is largely based on early literature suggesting (tumor volume doubling time) TVDT of approximately 70–120 days5. However, semi-annual surveillance is prone to missed lesions in patients with rapidly growing tumors and overdiagnosis in those with indolent tumors6. Similarly, tumor growth patterns likely affect treatment response and are important to understand for accurate prognostication. Data regarding tumor growth patterns in other cancer types, such as prostate7, have highlighted the potential for overdiagnosis and overtreatment. In light of the competing risk of liver-related mortality in most patients with HCC, select patients with indolent tumors may opt for close surveillance, rather than be exposed to potential harms of treatment. Accurate assessment of tumor growth patterns could facilitate individualized treatment decisions, such as transplant eligibility for larger tumors, the need for bridging therapy while awaiting liver transplantation, and potential use of adjuvant therapy after ablation or resection.
Therefore, the aims of our systematic review were to 1) characterize HCC tumor volume doubling time and tumor growth patterns and 2) identify correlates for rapid, intermediate, and indolent growth patterns.
METHODS
Data Sources and Searches
We conducted a computer-assisted search of Medline and EMBASE databases from inception to December 1, 2019 with the following keyword combinations: 1) doubl$ or natural history or lead time AND 2) hepatocellular ca$ or hcc or hepatoma or liver ca$. The search was restricted to human studies and articles published in English. Manual searches of reference lists from applicable studies were performed to identify any studies that may have been missed by the electronic search. Additional searches of national meetings including Digestive Disease Week, American Association for the Study of Liver Diseases, American College of Gastroenterology from 2010–2018 were performed. Finally, consultation with expert hepatologists was performed to identify any references that may have been missed. The study was conducted in accordance with PRISMA guidelines8.
Study Selection
We reviewed all titles and abstracts to identify potentially relevant articles. Two investigators (PN and AS) reviewed all potentially relevant full texts for inclusion, with disagreements resolved through discussion and consensus. We included cohort studies (retrospective or prospective) that reported HCC TVDT, growth patterns, and/or correlates of tumor growth patterns without any intervening treatment as the primary outcomes of interest. Studies reporting correlates of tumor growth patterns but not point estimates for TVDT or tumor growth patterns were still included for the correlates outcome analysis. We excluded studies with non-human data or lack of original data. Although we did not restrict studies based on methodology of assessing tumor size, we performed a pre-planned subgroup analysis of studies exclusively using cross-sectional imaging (multi-phase CT or contrast-enhanced MRI). If publications used overlapping cohorts of patients, we used data from the study with more granular data regarding tumor growth patterns.
Data Extraction and Quality Assessment
We used standardized data extraction forms to collect the following items: geographic region and years of the study, size and characteristics of the patient cohort, inclusion/exclusion criteria, method for assessing tumor size, and relevant data characterizing TVDT or tumor growth patterns. Most included studies reported summary measures for TVDT (with standard deviation) or tumor growth patterns, although granular data on tumor sizes at different times, permitting calculation of TVDT, were collected if available. Calculation of TVDT, when needed, was performed using Schwarz’s formula: TVDT = [(T – T0) ln2] / [ln (V/V0)], where V and V0 are tumor volumes at time point T and T0, respectively.9 We also collected any reported correlates of TVDT or tumor growth patterns, typically identified through linear or logistic regression analyses.
We assessed the risk of bias for each study using a modified Newcastle-Ottawa scale10, which assesses selection of the patient cohort, comparability of study groups, and adequacy of assessing the outcome of interest. Specifically, quality assessment was based on potential selection bias, sample size, representativeness of patient cohort, validity of imaging modality, number of tumor diameters assessed, bias in the assessment of outcome, and appropriateness of statistical analysis.
Data Synthesis and Analysis
Our primary outcome of interest was TVDT, defined in days, and tumor growth patterns. For any studies in which tumor growth patterns were not pre-specified, we a priori defined rapid as TVDT <3 months, intermediate as TVDT 3–9 months, and indolent as TVDT >9 months. These definitions were in part based on guideline recommendations for semi-annual surveillance for HCC early detection11,12. A pooled TVDT estimate was calculated by pooling study-specific estimates, using a random effects model. Heterogeneity was assessed graphically by examination of forest plots and then statistically using the inconsistency index (I2), with an I2 of >75% indicating significant heterogeneity.13 Pre-planned subgroup analyses were performed by region (USA and Europe versus Asia), imaging modality (ultrasound versus CT/MRI), and study year (prior to the year 2000 vs after 2000). We evaluated subgroup analyses by region given potential differences in tumor biology by liver disease etiology, imaging modality given likely differences in accuracy of assessing tumor size, and study year given evolution of HCC characterization and advances in imaging technology over time. A post-hoc subgroup analysis was performed by study sample size given results of funnel plot analysis, which was performed to graphically assess for publication bias. In addition to between-study analyses, we also recorded within-study correlates of tumor growth patterns. All data analyses were conducted using Stata version 14.2 (College Station, TX).
RESULTS
Study Selection and Characteristics
The electronic search returned 7675 total results, which was narrowed to 169 studies after review of study titles and abstracts. After full text review, we identified 20 studies with sufficient data for estimation of TVDT and tumor growth patterns. An additional 5 studies had incomplete data for TVDT but had data regarding correlates of tumor growth patterns. Reasons for exclusion at time of full-text review are detailed in Figure 1. On the basis of funnel plot analysis (Supplemental Figure 1), we could not exclude the possibility of publication bias. Smaller studies reported longer TVDT than those with larger sample sizes, and there was a paucity of small studies reporting short TVDT.
Figure 1.
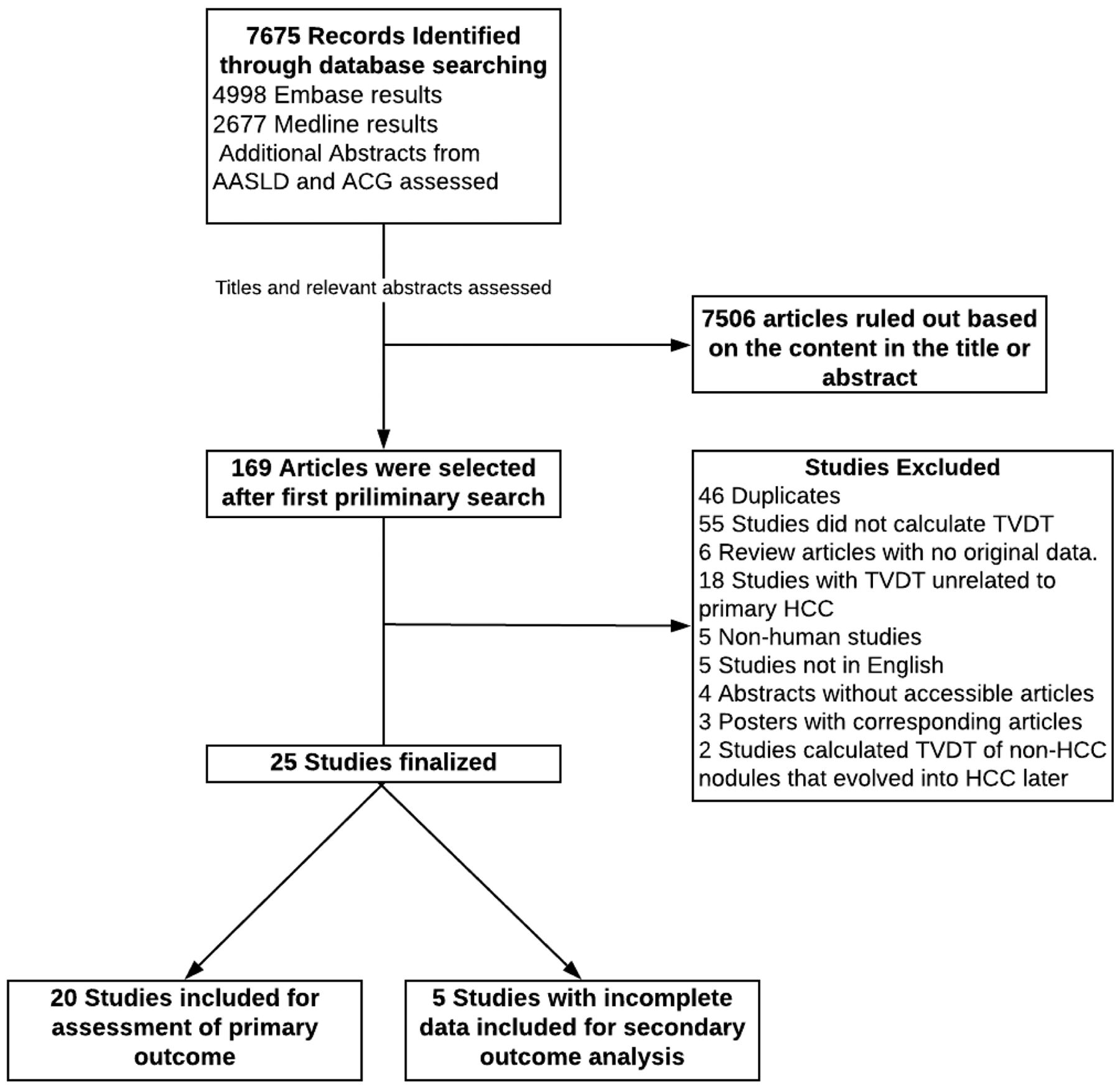
Search and selection process
Characteristics of the included studies are detailed in Table 1. Overall, there were 1572 patients (n=1621 HCC), including 1334 patients (n=1374 HCC) in the 20 studies reporting point estimates for TVDT or tumor growth patterns. Among included studies, the majority were small including less than 50 patients, with a median sample size of 28 patients, and the two largest studies (each including >200 patients) being published in 2017 or later.14, 4 Most studies had a retrospective design (n=16) and were conducted in Asia (n=15). Most studies published prior to 2000 used ultrasound as the modality to assess tumor sizes, whereas those published after 2000 exclusively used cross-sectional imaging.
Table 1.
Study Characteristics
| Source | Study Site | Study design | Imaging modality | # patients | Primary HCC etiology | Tumor volume doubling time (months) |
|---|---|---|---|---|---|---|
| Ebara 198629 | Japan | Prospective | US | 22 | 59 % EtOH 14% HBV |
Mean 6.5 ± 5.73.9123 Median: 5.6 |
| Sheu 198519 | China | Prospective | US | 28 | 86% HBV | Mean: 4.5 ± 3.1 Median: 3.7 |
| Okazaki 198925 | Japan | Prospective | US, CT | 15 | 13% HBV | Mean 3.4 ± 2.5 Median: 2.3 |
| Yoshino 198317 | Japan | Prospective | US, CT | 13 | Mean: 3.9 ± 3.2 | |
| Barbara 199226 | Italy | Retrospective | US | 39 | 28% HBV 28% EtOH |
Mean: 6.7± 4.3 Median: 5.6 |
| Matsuhashi 199632 | Japan | Prospective | US | 21 | 100% HCV 100% EtOH |
Mean: 2.6 ± 1.5 Median: 1.9 |
| Matsuhashi 199632 | Japan | Prospective | US | 14 | 100% HCV | Mean: 4.7 ± 2.0 |
| Trere 199547 | Italy | Prospective | US | 24 | Mean: 11.1 ± 2.2 Median: 8.0 |
|
| Saitoh 199530 | Japan | Retrospective | CT | 15 | 93% HCV | Mean: 10.2 ± 5.0 Median: 9.8 |
| Sadek 199548 | USA | Prospective | MRI | 5 | 40 % HBV 20% HCV |
Mean:2.2 ± 1.2 Median: 2.0 |
| Saito 199833 | Japan | Retrospective | US | 21 | 91% HCV | Mean: 6.8 ± 5.3 Median 4.7 |
| Kubota 200349 | Japan | Retrospective | CT | 22 | 69% HCV | Mean: 3.8 ± 3.4 Median: 2.7 |
| Nakajima 200231 | Japan | Retrospective | US, CT, MRI | 34 | 94% HCV | Mean: 3.0 ± 2.2 Median: 2.5 |
| Taouli 200520 | USA | Retrospective | CT, MRI | 11 | 27% HBV 27% Hep C |
Mean: 6.2 ± 5.3 Median: 4.4 |
| Cucchetti 200522 | Italy | Retrospective | US, CT, MRI | 62 | 65% HCV | |
| Kudo 200850 | Japan | Prospective | US | 52 | ||
| Woo 201051 | Korea | Retrospective | CT | 5 | 100% HBV | Mean: 3.3 ± 1.0 Median: 3.6 |
| Furlan 201224 | USA, Italy, Rome | Retrospective | CT, MRI | 48 | 79% HCV | |
| Mochizuki 201228 | Japan | Prospective | CT | 19 | Mean: 3.6 | |
| Shingaki 201327 | Japan | Retrospective | CT | 53 | 77% HCV | Mean: 3.0 ± 3.1 |
| Rowe 201423 | UK | Retrospective | 57 | >50% HCV | Mean: 5.0 | |
| Villa 201621 | Italy | Prospective | CT | 78 | 56% HCV | Mean: 3.5 ± 3.0 Median: 2.7 |
| Jha 201418 | USA | Retrospective | MRI | 52 | 63% HCV | Mean: 5.3 ± 5.4 |
| An 201515 | Korea | Retrospective | CT, MRI | 175 | 66% HBV | Mean: 4.2 ± 4.2 Median: 2.8 |
| Kim 201714 | Korea | Retrospective | CT, MRI | 269 | 73% HBV | Median: 2.4 |
| Rich 2019 primary cohort4 | USA | Retrospective | CT, MRI | 242 | 68% HCV | Median: 7.5 |
| Rich 2019 validation cohort4 | USA, UK | Retrospective | CT, MRI | 176 | 53% HCV | Median: 4.5 |
CT – computed tomography; EtOH – alcohol-related liver disease; HCC – hepatocellular carcinoma; HCV – hepatitis C virus; MRI – magnetic resonance imaging; NASH – nonalcoholic steatohepatitis
Tumor Volume Doubling time of HCC
Across 20 studies with available data, study-level mean TVDT ranged from 2.2 months to 11.3 months. The pooled mean TVDT was 4.6 (95%CI: 3.9 – 5.4) months, although there was significant heterogeneity both on visual inspection of forest plots and analytically (I2=94%) (Figure 2). Sensitivity analysis, in which one study is removed at a time, failed to demonstrate a change in TVDT exceeding 10 days. In subgroup analyses, we did not observe notable differences in pooled TVDT by imaging modality (4.7 months [95% CI 3.7–5.7 months, I2= 86%] for studies using ultrasound vs. 4.6 months [95% CI 3.5–5.5 months, I2= 94%] for studies using CT or MRI), although there appeared to be differences by study year (5.2 months [95% CI 4.0–6.5 months, I2= 90%] for studies published prior to 2000 vs. 4.1 months [95% CI 3.2–5.1 months, I2= 95%] for those published after 2000) and by location (4.1 months [95% CI 3.4–4.8 months, I2= 91%] for studies conducted in Asia vs. 5.4 months [95% CI 4.0–6.9 months, I2= 94%] for those in the US and Europe. (Supplemental Figure 2). Post-hoc subgroup analysis revealed potential differences by study sample size (4.8 months [95% CI 3.9–5.7 months, I2 = 88%] for studies with <50 patients vs. 4.3 months [95% CI 3.0–5.6 months, I2 = 94%] for studies with >50 patients).
Figure 2.
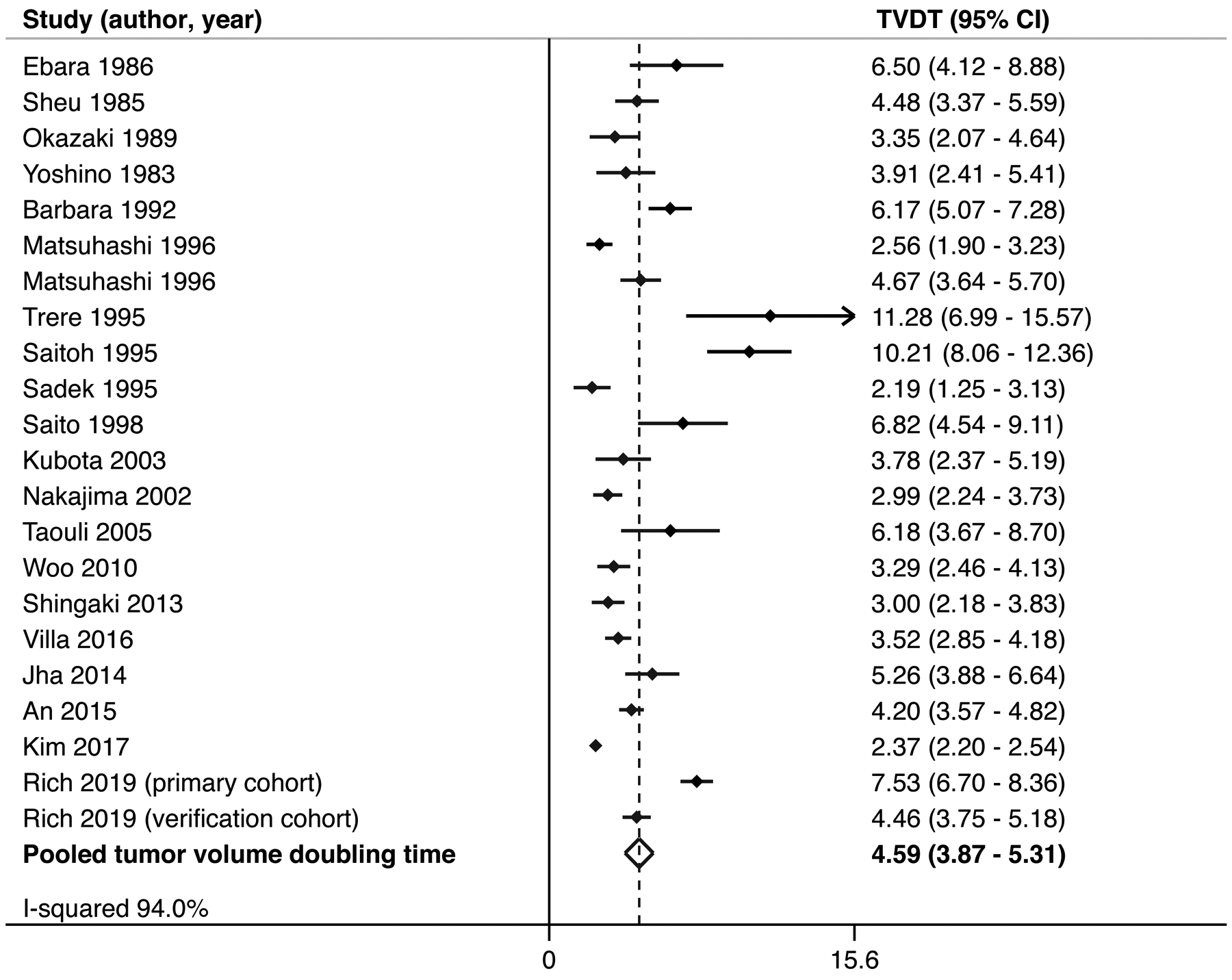
Pooled tumor volume doubling time for hepatocellular carcinoma
Tumor growth patterns were reported in 25 studies. As presented in Table 3, 35% of HCC had rapid growth patterns, 27.4% intermediate growth, and 37.6% had an indolent growth pattern. There appeared to be differences in growth patterns by study location, with a higher proportion of patients with aggressive tumors among studies conducted in Asia (43.8% vs. 25.5%, respectively, p<0.001).
Table 3.
Hepatocellular carcinoma tumor growth patterns
| Study | Rapid growth n (%) | Intermediate growth n (%) | Indolent growth n (%) |
|---|---|---|---|
| Ebara 198629 | 9 (40.9) | 5 (22.7) | 8 (36.4) |
| Sheu 198519 | 10 (32.3) | 17 (54.8) | 4 (12.9) |
| Okazaki 198925 | 10 (66.7) | 4 (26.7) | 1 (6.7) |
| Yoshino 198317 | 7 (43.8) | 7 (43.8) | 2 (12.5) |
| Barbara 199226 | 8 (20.5) | 20 (51.3) | 11 (28.2) |
| Matsuhashi 199632 | 14 (66.7) | 7 (33.3) | 0 |
| Matsuhashi 199632 | 2 (14.3) | 12 (85.7) | 0 |
| Trere 199547 | 2 (10.0) | 7 (35.0) | 11 (55.0) |
| Saitoh 199530 | 1 (4.8) | 4 (19.0) | 16 (76.2) |
| Sadek 199548 | 4 (66.7) | 2 (33.3) | 0 |
| Saito 199833 | 1 (4.8) | 14 (66.7) | 6 (2.9) |
| Kubota 200349 | 12 (54.5) | 9 (40.9) | 1 (4.5) |
| Nakajima 200231 | 21 (61.8) | 12 (35.3) | 1 (2.9) |
| Taouli 200520 | 4 (25.0) | 8 (50.0) | 4 (25.0) |
| Cucchetti 200522 | 34 (54.8) | 25 (42.4) | 3 (4.8) |
| Woo 201051 | 1 (20.0) | 4 (80.0) | 0 |
| Villa 201421* | 19 (24.4) | N/A | 59 (75.6) |
| Rowe 201423* | 39 (59.1) | N/A | 27 (40.9) |
| Kim 201714* | 110 (40.9) | N/A | 159 (59.1) |
| Rich 2019 primary cohort4 | 61 (25.2) | 75 (31.0) | 106 (43.8) |
| Rich 2019 validation cohort4* | 49 (27.8) | 96 (54.6) | 31 (17.6) |
| Total | 418 (35.0) | 328 (27.4) | 450 (37.6) |
Study-specific definition for tumor growth patterns were used. Indolent vs. rapid defined using cut-off of 53 days for Villa 2014 and cut-off of 2 months for Kim 2017. For Rowe 2014, rapid vs. indolent defined using specific growth rate at cut-off of 1%. For Rich 2019 validation cohort, rapid, intermediate and indolent were defined as <90 days, 90–365 days, and >365 days respectively.
Correlates of Tumor Growth Patterns within Studies
Supplemental Table 1 describes factors correlated with HCC growth patterns as reported within individual studies. None of the studies found an association between growth patterns and patient demographics, including age and sex, and most failed to find an association with degree of liver dysfunction, e.g. Child Pugh score. Although there were limited data examining any association between liver disease etiology and TVDT, three studies reported shorter TVDT among patients with chronic HBV infection as compared to other etiologies.15–17 Similarly, Rich et al observed that HCC in the setting of HCV or HBV-related cirrhosis had shorter TVDT than those with non-viral etiologies.4 In contrast, Kim et al14 and An el al15 failed to find an association between liver disease etiology and TVDT. Several studies14,15,18–20 have reported a linear association between smaller tumor diameter and shorter TVDT (i.e. more rapid growth), with tumor diameter evaluated continuously. Recently, Rich et al4 noted a similar association between TVDT and tumor diameter, analyzed using categories of 1–2 cm. 2–5 cm, and >5 cm.
Several studies found an association between higher alpha fetoprotein (AFP) levels and rapid HCC growth,4,15,21,22; however, this was inconsistent with other studies failing to find an association.14,19,21,23–26. In contrast, the association between AFP doubling times and HCC growth patterns appeared to be consistently observed across studies17,19,27–28. In these studies, AFP doubling time was typically calculated using a modification of the Schwartz equation using to calculate TVDT and authors reported high degrees of correlation (r = 0.70 – 0.97). There were few data evaluating the association between other novel biomarkers and tumor growth patterns. Although data on radiomics predicting tumor growth patterns were limited, there were not any baseline imaging features consistently associated with tumor growth patterns. Saitoh and colleagues found tumors with increased arterial blood supply and hypervascularity had more rapid tumor growth,30 although this was not evaluated by others.24 Jha and colleagues evaluated imaging findings in small HCC and found increased signal intensity on T2-weighted imaging was associated with rapid growth whereas increased intensity on T1-weighted imaging was associated with more indolent growth.18
Several studies found an association between degree of differentiation and tumor growth patterns,21,22,27,29–31 although this was not significant in others.4,23,32,33 Other studies reported a positive association between rapid tumor growth and higher proliferation indices25,31,33 and microvascular invasion.21,22 A recent prospective study with 132 patients (78 training set and 54 validation set) found a 5-gene transcriptomic signature with angiopoietin-2, delta-like ligand 4, neuropilin/tolloid-like2, endothelial cell-specific molecule-1, and nuclear receptor subfamily 4 group A, member 1 (NR4A1) was associated with rapid growth and worse survival.21
Study Quality
Results of the quality assessment are detailed in Table 2. In brief, several limitations in study design were prevalent. First, all studies were limited by an inherent selection bias given the inclusion of untreated patients who underwent repeat imaging, who are likely different than those who undergo treatment without interval imaging. Most notably, patients with aggressive tumor biology are less likely to remain sufficiently stable to allow repeated imaging than those with indolent tumors. Second, several older studies further restricted patient cohorts by only including those with biopsy-proven HCC or excluding patients with prolonged stability in tumor volume. Third, most studies had small sample sizes, with less than 100 patients each, resulting in imprecise point estimates. Fourth, many studies had potential measurement bias, given nearly half used ultrasound imaging to measure tumor volumes and others used different imaging modalities to compare tumor sizes at different time points. Further, most studies measured tumors in only one dimension, which falsely assumes that tumors are perfectly spherical, and were dependent upon the measurements of a single radiologist, despite recognized inter-observer variability in tumor measurements. Finally, all analyses assume TVDT is constant, however tumor biology and TVDT may be dynamic, with changes over time.
Table 2.
Quality Assessment of Eligible Studies via Newcastle-Ottawa scale
| Study | Selection bias | Sample Size | Patient inclusion criteria | Imaging test | Tumor volume measurement | Interpretation by >1 radiologist | Statistical analysis |
|---|---|---|---|---|---|---|---|
| Ebara 198629 | ✓ | ||||||
| Sheu 198519 | ✓ | ||||||
| Okazaki 198925 | ✓ | ✓ | |||||
| Yoshino 198317 | ✓ | ✓ | |||||
| Barbara 199226 | ✓ | ✓ | ✓ | ||||
| Matsuhashi 199632 | ✓ | ✓ | |||||
| Trere 199547 | ✓ | ✓ | |||||
| Saitoh 199530 | ✓ | ||||||
| Sadek 199548 | ✓ | ||||||
| Saito 199833 | ✓ | ✓ | ✓ | ||||
| Kubota 200349 | ✓ | ✓ | ✓ | ||||
| Nakajima 200231 | ✓ | ✓ | ✓ | ✓ | |||
| Taouli 200520 | ✓ | ✓ | ✓ | ||||
| Woo 201051 | ✓ | ✓ | ✓ | ||||
| Shingaki 201327 | ✓ | ✓ | ✓ | ✓ | |||
| Villa 201621 | ✓ | ✓ | ✓ | ✓ | ✓ | ||
| Jha 201418 | ✓ | ✓ | ✓ | ✓ | ✓ | ||
| An 201515 | ✓ | ✓ | ✓ | ✓ | |||
| Kim 201714 | ✓ | ✓ | ✓ | ✓ | |||
| Rich 20194 | ✓ | ✓ | ✓ | ✓ | ✓ | ✓ |
Checkmarks denote high-quality metrics and empty cells denote low-quality metrics
DISCUSSION
An accurate understanding of tumor growth patterns is critical for many aspects of cancer care, most notably prognostication and treatment decisions. We found a pooled TVDT for HCC of approximately 4.6 months, although there was significant variation across studies ranging from 2.2 to 11.3 months. Studies also reported variation in tumor growth patterns, with over one-third of HCC described as having rapid growth, one-fourth as intermediate growth, and over one-third as having indolent growth. There are limited data describing correlates of tumor growth patterns, although some studies report more rapid growth for smaller tumors, poorly differentiated tumors, and HCC in patients with viral liver disease. Overall, available literature highlights the variation in HCC growth patterns and the need for further studies to better differentiate patients with rapid versus indolent tumors.
In addition to clear implications for prognostication and treatment decisions, expected tumor growth patterns also impact the potential benefit of HCC surveillance. A cancer screening program is most effective when tumors have intermediate and dependable growth. Rapidly growing tumors are unlikely to be detected by screening and often present symptomatically, whereas indolent tumors are more likely to be detected but prone to overdiagnosis. Overdiagnosis in such situations leads to overtreatment, economic harms, and detriment in quality of life without any benefit in prognosis or mortality.34 Given the lack of level I data evaluating HCC surveillance in patients with cirrhosis, we rely on cohort studies using statistical methods to adjust for potential lead-time and length-time bias based on TVDT assumptions5,35. However, most studies used TVDT of 70–90 days, which likely led to inaccurate assumptions for lead-time and length time biases. Longer TVDT estimates, as suggested by our study, may increase potential lead and length time biases, thereby abating observed benefits of HCC surveillance in prior studies. Further, patient-level predictors of tumor biology could help inform personalized surveillance strategies among at-risk patients.
Although there were inconsistent correlates for TVDT across studies, a few observations were relatively consistent. First, we observed variation in TVDT based on geographic location, likely based on differences in liver disease etiology. Subgroup analyses found a higher proportion of rapidly growing tumors among studies conducted in Asia, and recent studies with diverse liver disease etiologies reported more indolent growth among patients with non-viral liver disease. If confirmed, these differences could be related to variation in molecular pathways of HCC pathogenesis and growth between liver disease etiologies36. These data are particularly important in the Western world, where HCC is increasingly related to non-viral etiologies such as NASH and alcohol-related cirrhosis37,38. Second, several studies suggest HCC may exhibit logarithmic growth, with rapid early growth followed by more indolent growth as the tumor becomes larger.4,14–16,18 It is unclear if this growth pattern would be related to changes in mutational burden and tumor biology, changes in blood supply related to tumor burden (i.e. outgrowing its blood supply), or an artifact of measurement bias and small differences in tumor diameter making a larger difference when tumors are smaller in size. Finally, several studies have reported more rapid growth in patients with poorly differentiated tumors.21,22,27,29–31 Tumor growth patterns and better understanding of the tumor biology of early-stage patients are relevant for several treatment decisions such as need for bridging therapy while awaiting liver transplantation or optimal surveillance interval (versus early ablation) for UNOS stage T1 lesions39. It may also help predict response to HCC treatments such as TACE or systemic therapy.40 These data can particularly affect relative weights placed on HCC-related mortality versus liver-related complications when making treatment decisions in patients with significant portal hypertension or decompensated cirrhosis.
In light of heterogeneity in tumor growth patterns, a biomarker for tumor biology would be of great clinical utility. Several studies have shown that AFP can be prognostic, with high levels associated with poorer response to therapy, including higher risk of post-resection and post-transplant recurrence41,42. However, in our analysis, studies were discordant in finding an association between baseline AFP levels and tumor growth patterns. It is unclear if the lack of association in these studies is simply related to tumor heterogeneity with AFP only being elevated in approximately half of all tumors43–45. However, studies found a consistent association between AFP doubling time and TVDT, which could be a readily available marker for identifying aggressive tumors. Future studies should continue to evaluate longitudinal changes in biomarkers, other novel biomarkers (including biomarker panels), and radiomic features, such as degree of enhancement or timing of washout, as potential indicators of tumor biology. Although the rare use of biopsy for HCC histologic confirmation likely limits the routine use of tissue-based biomarkers, it is possible that continued refinement of liquid biopsy techniques may help identify a prognostic biomarker and surrogate of TVDT46.
Despite the recognized importance of characterizing tumor growth patterns, we noted the current literature evaluating TVDT has several limitations. First, most studies were conducted in Asia, with a predominant HBV-infected patient population, with fewer data from the Western countries with more diverse disease etiologies. It is possible that tumor growth patterns may vary by liver disease etiology so it is unknown if the available data would apply to contemporary cohorts with NASH, alcohol-related cirrhosis, or patients with hepatitis C after sustained viral response. Second, funnel plot analysis suggested the possibility of publication bias, with a paucity of small studies reporting short TVDT. Third and most importantly, most included studies had notable limitations including small sample sizes, potential measurement bias, and an assumption that TVDT is constant over time. Notably, studies conducted prior to 2005 preceded noninvasive diagnostic criteria of HCC and relied on ultrasound for HCC tumor measurement, resulting in increased selection and measurement biases. To evaluate these potential biases, we performed subgroup analyses by geographic location (Asia vs. Europe and US), imaging modality (ultrasound vs. CT/MRI), study year (pre- and post-2000), and study sample size (<50 vs. >50). We found consistent results in most subgroup analyses, although shorter TVDT in studies from Asia compared to Europe and the US. Further subgroup analyses by liver disease etiology were unfortunately not possible without patient-level data. Fourth, there was significant between-study heterogeneity observed in our meta-analysis with an I2 >90%, increasing the degree of uncertainty around the point estimate for pooled TVDT. We evaluated this heterogeneity through sensitivity and subgroup analyses, although heterogeneity persisted, perhaps reflecting the inherent heterogeneity in tumor growth patterns described in our results. Lastly, there is a selection bias inherent to all studies of HCC natural history; however, this may be unavoidable due to ethical concerns regarding design of a prospective study to observe tumor biology in the absence of treatment for all patients with HCC. We believe understanding these limitations of prior studies can inform design of high quality studies evaluating this important topic in the future.
Conclusion
In this systematic review and meta-analysis, we found wide variation in tumor growth patterns, with over one-third of HCC exhibiting rapid growth and over one-third having indolent growth. Correlates of rapid tumor growth include hepatitis B etiology, smaller tumor diameter, AFP doubling time, and poor tumor differentiation. However, current studies have notable limitations, highlighting the need for high quality studies in this area as well a need for novel prognostic biomarkers that correlate with tumor biology.
Supplementary Material
What is already known about this subject?
Hepatocellular carcinoma is traditionally considered an aggressive tumor
Understanding HCC growth patterns has implications for prognostication as well as treatment decisions.
What are the new findings?
HCC has notable variation in tumor growth patterns, with over one-third of HCC being categorized as having indolent growth and over one-third as rapid growth
Correlates of rapid tumor growth include viral liver disease etiology, small tumor size, and poor tumor differentiation.
How might it impact on clinical practice in the foreseeable future
Our data highlight a need for studies to identify prognostic biomarkers that can differentiate tumor growth patterns.
Identification of a subgroup of indolent HCC highlight the potential for overtreatment in some patients.
Financial support:
This work was conducted with support from NIH R01 MD012565 R01 CA222900 and R01 CA212008. The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Institutes of Health.
Abbreviations:
- AFP
alpha fetoprotein
- CT
computed tomography
- HCC
hepatocellular carcinoma
- HCV
hepatitis C virus
- MRI
magnetic resonance imaging
- NASH
nonalcoholic steatohepatitis
- TVDT
tumor volume doubling time
Footnotes
Conflicts of Interest: Neehar Parikh serves as a consultant to Exelixis, Eli Lilly and Bristol-Myers Squibb. He has served on advisory boards for Eisai, Wako Diagnostics and Bayer and has received institutional research funding from Bayer and Exact Sciences. Amit Singal has served on advisory boards or as a consultant for Gilead, Abbvie, Bayer, Eisai, Exelixis, Bristol Meyers Squibb, Wako Diagnostics, Exact Sciences, Roche, and Glycotest. None of the other authors have any relevant conflicts of interest.
REFERENCES
- 1.Organization WH. Cancer https://www-who-int.foyer.swmed.edu/news-room/fact-sheets/detail/cancer: World Health Organization; 2019, September 12 [2019. [Google Scholar]
- 2.Mohammadian M, Soroush A, Mohammadian-Hafshejani A, et al. The incidence and mortality of liver cancer and its relationship with development in Asia. Asian Pacific Journal of Cancer Prevention 2016;17(4):2041–47. [DOI] [PubMed] [Google Scholar]
- 3.Momin BR, Pinheiro PS, Carreira H, et al. Liver cancer survival in the United States by race and stage (2001–2009): Findings from the CONCORD-2 study. Cancer 2017;123 Suppl 24(Suppl 24):5059–78. doi: 10.1002/cncr.30820 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Rich NE, John BV, Parikh ND, et al. Hepatocellular carcinoma demonstrates heterogeneous growth patterns in a multi-center cohort of patients with cirrhosis. Hepatology 2020 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Singal AG, Pillai A, Tiro J. Early detection, curative treatment, and survival rates for hepatocellular carcinoma surveillance in patients with cirrhosis: a meta-analysis. PLoS medicine 2014;11(4) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Overdiagnosis: an understudied issue in hepatocellular carcinoma surveillance Seminars in liver disease; 2017. Thieme Medical Publishers. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Schmid HP, McNeal JE, Stamey TA. Observations on the doubling time of prostate cancer. The use of serial prostate-specific antigen in patients with untreated disease as a measure of increasing cancer volume. Cancer 1993;71(6):2031–40. [DOI] [PubMed] [Google Scholar]
- 8.Stewart LA, Clarke M, Rovers M, et al. Preferred reporting items for a systematic review and meta-analysis of individual participant data: the PRISMA-IPD statement. Jama 2015;313(16):1657–65. [DOI] [PubMed] [Google Scholar]
- 9.Schwartz M A biomathematical approach to clinical tumor growth. Cancer 1961;14(6):1272–94. [DOI] [PubMed] [Google Scholar]
- 10.Peterson J, Welch V, Losos M, et al. The Newcastle-Ottawa scale (NOS) for assessing the quality of nonrandomised studies in meta-analyses. Ottawa: Ottawa Hospital Research Institute; 2011 [Google Scholar]
- 11.Liver EAFTSOT. EASL clinical practice guidelines: management of hepatocellular carcinoma. Journal of hepatology 2018;69(1):182–236. [DOI] [PubMed] [Google Scholar]
- 12.Marrero JA, Kulik LM, Sirlin CB, et al. Diagnosis, staging, and management of hepatocellular carcinoma: 2018 practice guidance by the American Association for the Study of Liver Diseases. Hepatology 2018;68(2):723–50. [DOI] [PubMed] [Google Scholar]
- 13.Higgins JP, Thompson SG. Quantifying heterogeneity in a meta-analysis. Statistics in medicine 2002;21(11):1539–58. [DOI] [PubMed] [Google Scholar]
- 14.Kim JK, Kim H-D, Jun M-J, et al. Tumor volume doubling time as a dynamic prognostic marker for patients with hepatocellular carcinoma. Digestive diseases and sciences 2017;62(10):2923–31. [DOI] [PubMed] [Google Scholar]
- 15.An C, Choi YA, Choi D, et al. Growth rate of early-stage hepatocellular carcinoma in patients with chronic liver disease. Clinical and molecular hepatology 2015;21(3):279. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Park M-S. P1317 EARLY STAGE HEPATOCELLULAR CARCINOMA IN KOREANS WITH CHRONIC LIVER DISEASE: TUMOR GROWTH RATE AND 5-YEAR SURVIVAL. Journal of Hepatology 2014;60(1):S534. [Google Scholar]
- 17.YOSHINO M Growth Kinetics of Hepatocellular Carcinoma. Japanese Journal of Clinical Oncology 1983;13(1):45–52. doi: 10.1093/oxfordjournals.jjco.a038864 [DOI] [PubMed] [Google Scholar]
- 18.Jha RC, Zanello PA, Nguyen XM, et al. Small hepatocellular carcinoma: MRI findings for predicting tumor growth rates. Academic radiology 2014;21(11):1455–64. [DOI] [PubMed] [Google Scholar]
- 19.Sheu J-C, Sung J-L, Chen D-S, et al. Growth rate of asymptomatic hepatocellular carcinoma and its clinical implications. Gastroenterology 1985;89(2):259–66. [DOI] [PubMed] [Google Scholar]
- 20.Taouli B, Goh JS, Lu Y, et al. Growth rate of hepatocellular carcinoma: evaluation with serial computed tomography or magnetic resonance imaging. Journal of computer assisted tomography 2005;29(4):425–29. [DOI] [PubMed] [Google Scholar]
- 21.Villa E, Critelli R, Lei B, et al. Neoangiogenesis-related genes are hallmarks of fast-growing hepatocellular carcinomas and worst survival. Results from a prospective study. Gut 2016;65(5):861–69. [DOI] [PubMed] [Google Scholar]
- 22.Cucchetti A, Vivarelli M, Piscaglia F, et al. Tumor doubling time predicts recurrence after surgery and describes the histological pattern of hepatocellular carcinoma on cirrhosis. Journal of hepatology 2005;43(2):310–16. [DOI] [PubMed] [Google Scholar]
- 23.Rowe I, Haldar D, Houlihan D, et al. Calculating the growth rate of small hepatocellular carcinoma: implications for surveillance and treatment. American Association for the Study of Liver Disease, 2014:858. [Google Scholar]
- 24.Furlan A, Marin D, Agnello F, et al. Hepatocellular carcinoma presenting at contrast-enhanced multi–detector-row computed tomography or gadolinium-enhanced magnetic resonance imaging as a small (≤ 2 cm), indeterminate nodule: growth rate and optimal interval time for imaging follow-up. Journal of computer assisted tomography 2012;36(1):20–25. [DOI] [PubMed] [Google Scholar]
- 25.Okazaki N, Yoshino M, Yoshida T, et al. Evaluation of the prognosis for small hepatocellular carcinoma based on tumor volume doubling time. A preliminary report. Cancer 1989;63(11):2207–10. [DOI] [PubMed] [Google Scholar]
- 26.Barbara L, Benzi G, Gaiani S, et al. Natural history of small untreated hepatocellular carcinoma in cirrhosis: a multivariate analysis of prognostic factors of tumor growth rate and patient survival. Hepatology 1992;16(1):132–37. [DOI] [PubMed] [Google Scholar]
- 27.Shingaki N, Tamai H, Mori Y, et al. Serological and histological indices of hepatocellular carcinoma and tumor volume doubling time. Molecular and clinical oncology 2013;1(6):977–81. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Hitoshi Mochizuki YT, Kenji Hosoda, Yoji Suzuki, Yuji Hoshino, Yuichirou Kojima, Yuichi Hirose, Masao Omata. Validation of Serum Tumor Doubling Time (DT) by Conventional CT (Computed Tomography) DT in Hepatocellular Carcinoma. Asian Pacific Association for the Study of the Liver: Hepatol Int, 2012:212. [Google Scholar]
- 29.Ebara M, Ohto M, Shinagawa T, et al. Natural history of minute hepatocellular carcinoma smaller than three centimeters complicating cirrhosis: a study in 22 patients. Gastroenterology 1986;90(2):289–98. [DOI] [PubMed] [Google Scholar]
- 30.Saitoh S, Ikeda K, Koida I, et al. Serial hemodynamic measurements in well-differentiated hepatocellular carcinomas. Hepatology 1995;21(6):1530–34. [PubMed] [Google Scholar]
- 31.Nakajima T, Moriguchi M, Mitsumoto Y, et al. Simple tumor profile chart based on cell kinetic parameters and histologic grade is useful for estimating the natural growth rate of hepatocellular carcinoma. Human pathology 2002;33(1):92–99. [DOI] [PubMed] [Google Scholar]
- 32.Matsuhashi T, Yamada N, Shinzawa H, et al. Effect of alcohol on tumor growth of hepatocellular carcinoma with type C cirrhosis. Internal medicine 1996;35(6):443–48. [DOI] [PubMed] [Google Scholar]
- 33.Saito Y, Matsuzaki Y, Doi M, et al. Multiple regression analysis for assessing the growth of small hepatocellular carcinoma: the MIB-1 labeling index is the most effective parameter. Journal of gastroenterology 1998;33(2):229–35. [DOI] [PubMed] [Google Scholar]
- 34.Petrasek J, Singal AG, Rich NE. Harms of Hepatocellular Carcinoma Surveillance. Current Hepatology Reports 2019:1–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Choi DT, Kum H-C, Park S, et al. Hepatocellular carcinoma screening is associated with increased survival of patients with cirrhosis. Clinical Gastroenterology and Hepatology 2019;17(5):976–87. e4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Rebouissou S, Nault J-C. Advances in molecular classification and precision oncology in hepatocellular carcinoma. Journal of Hepatology 2020;72(2):215–29. [DOI] [PubMed] [Google Scholar]
- 37.Parikh ND, Marrero WJ, Wang J, et al. Projected increase in obesity and non-alcoholic-steatohepatitis–related liver transplantation waitlist additions in the United States. Hepatology 2019;70(2):487–95. [DOI] [PubMed] [Google Scholar]
- 38.Estes C, Razavi H, Loomba R, et al. Modeling the epidemic of nonalcoholic fatty liver disease demonstrates an exponential increase in burden of disease. Hepatology 2018;67(1):123–33. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Mehta N, Sarkar M, Dodge JL, et al. Intention to treat outcome of T1 hepatocellular carcinoma with the “wait and not ablate” approach until meeting T2 criteria for liver transplant listing. Liver Transplantation 2016;22(2):178–87. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Purcell Y, Sartoris R, Paradis V, et al. Influence of pretreatment tumor growth rate on objective response of hepatocellular carcinoma treated with transarterial chemoembolization. Journal of Gastroenterology and Hepatology 2020;35(2):305–13. [DOI] [PubMed] [Google Scholar]
- 41.Toyoda H, Kumada T, Kaneoka Y, et al. Prognostic value of pretreatment levels of tumor markers for hepatocellular carcinoma on survival after curative treatment of patients with HCC. Journal of hepatology 2008;49(2):223–32. [DOI] [PubMed] [Google Scholar]
- 42.Duvoux C, Roudot–Thoraval F, Decaens T, et al. Liver transplantation for hepatocellular carcinoma: a model including α-fetoprotein improves the performance of Milan criteria. Gastroenterology 2012;143(4):986–94. e3. [DOI] [PubMed] [Google Scholar]
- 43.Marrero JA, Feng Z, Wang Y, et al. α-fetoprotein, des-γ carboxyprothrombin, and lectin-bound α-fetoprotein in early hepatocellular carcinoma. Gastroenterology 2009;137(1):110–18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Gopal P, Yopp AC, Waljee AK, et al. Factors that affect accuracy of α-fetoprotein test in detection of hepatocellular carcinoma in patients with cirrhosis. Clinical Gastroenterology and Hepatology 2014;12(5):870–77. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Tzartzeva K, Obi J, Rich NE, et al. Surveillance imaging and alpha fetoprotein for early detection of hepatocellular carcinoma in patients with cirrhosis: a meta-analysis. Gastroenterology 2018;154(6):1706–18. e1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Mann J, Reeves HL, Feldstein AE. Liquid biopsy for liver diseases. Gut 2018;67(12):2204–12. [DOI] [PubMed] [Google Scholar]
- 47.Trerè D, Gramantieri L, Siringo S, et al. In hepatocellular carcinoma AgNOR protein expression correlates with tumour mass doubling time. Journal of hepatology 1996;24(1):60–65. [DOI] [PubMed] [Google Scholar]
- 48.Sadek AG, Mitchell DG, Siegelman ES, et al. Early hepatocellular carcinoma that develops within macroregenerative nodules: growth rate depicted at serial MR imaging. Radiology 1995;195(3):753–56. [DOI] [PubMed] [Google Scholar]
- 49.Kubota K, Ina H, Okada Y, et al. Growth rate of primary single hepatocellular carcinoma: determining optimal screening interval with contrast enhanced computed tomography. Digestive diseases and sciences 2003;48(3):581–86. [DOI] [PubMed] [Google Scholar]
- 50.Kudo M, Tochio H. Intranodular blood supply correlates well with biological malignancy grade determined by tumor growth rate in pathologically proven hepatocellular carcinoma. Oncology 2008;75(Suppl. 1):55–64. [DOI] [PubMed] [Google Scholar]
- 51.Woo HY, Jang JW, Choi JY, et al. Tumor doubling time after initial response to transarterial chemoembolization in patients with hepatocellular carcinoma. Scandinavian journal of gastroenterology 2010;45(3):332–39. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


