Abstract
Interleukin-2 (IL-2) is a critical regulator of immune homeostasis through its impact on both regulatory T (Treg) and effector T (Teff) cells. However, the precise role of IL-2 in the maintenance and function of Treg cells in the adult peripheral immune system remains unclear. Here, we report that neutralization of IL-2 in mice abrogated all IL-2 receptor signaling in Treg cells, but was well tolerated and only gradually impacted Treg cell function and immune homeostasis. By contrast, despite substantially reduced IL-2 sensitivity, Treg cells maintained selective IL-2 signaling and prevented immune dysregulation following treatment with the inhibitory anti-CD25 antibody PC61. Reduction of Treg cells with a depleting version of the same CD25 antibody permitted CD8+ Teff proliferation before progressing to more widespread immune dysregulation. Thus, despite severely curtailed CD25 expression and function, Treg cells retain selective access to IL-2 that supports their anti-inflammatory functions in vivo. Antibody-mediated targeting of CD25 is being actively pursued for treatment of autoimmune disease and preventing allograft rejection, and our findings help inform therapeutic manipulation and design for optimal patient outcomes.
Introduction
Interleukin-2 (IL-2) is a critical regulator of immune homeostasis through its role in the development, maintenance and function of T regulatory (Treg) cells and its impact on effector cell proliferation and differentiation (1, 2). The IL-2 receptor (IL-2R) can be composed of 2 or 3 subunits: IL-2Rβ (CD122) and the common gamma (γ) chain (CD132) together form the intermediate affinity receptor, and the addition of IL-2Rα (CD25) creates the high affinity receptor. Binding of CD25 to IL-2 induces a conformational change that decreases the energy needed to bind to the rest of the receptor, whereas CD122 and CD132 are the critical signaling chains (3). Treg cells constitutively express CD25, which under homeostatic conditions allows them to outcompete CD25- T effector (Teff) cells and natural killer (NK) cells for limiting amounts of IL-2. This is most important in the secondary lymphoid organs (SLOs), where pro-survival signals downstream of IL-2 signaling maintain Treg cells (4, 5). Notably, Treg cells cannot make their own IL-2 (6, 7), and therefore depend on IL-2 produced mainly from autoreactive CD4+ Teff cells (8, 9). In this way, Teff and Treg cell populations are dynamically linked and reciprocally control each other to maintain immune homeostasis (10).
When the IL-2-dependent balance of Treg and Teff cells is disrupted, autoimmunity and inflammation can occur. Genetic deficiency in CD25, CD122, or IL-2 results in systemic autoimmune disease in mice (11), and single nucleotide polymorphisms (SNPs) in the IL2 and IL2RA genes are associated with multiple autoimmune diseases in both mice and humans (12, 13). Therefore, manipulating the IL-2 signaling pathway therapeutically for treatment of autoimmune disease is an area of immense interest. Low dose IL-2 therapy, which enriches Treg cells, has shown efficacy in murine autoimmune models (14–19), and has also benefitted patients with graft versus host disease (GVHD) (20), Hepatitis C virus-induced vasculitis (21), alopecia areata (22), and lupus (23). However, because IL-2 also acts on effector cells, high dose IL-2 can promote inflammatory responses and this is used for treatment of cancer (24). As such, safety of therapeutic IL-2 remains a concern, and efficacy can vary widely depending on the current disease activity and immune history of the patient. Indeed, in two mouse models of type 1 diabetes, early intervention with IL-2 prevented disease, but initiation of treatment after loss of tolerance (but before overt hyperglycemia) accelerated disease progression (13, 19). The fact that monoclonal antibodies against CD25 are also used as an immunosuppressive to treat organ transplant rejection (25) and demonstrated efficacy against multiple sclerosis (MS) (26) further highlights the complexity of targeting this signaling pathway.
The inhibitory anti-CD25 antibody PC61 has been extensively used to examine the role of CD25 in IL-2 signaling in Treg cells in mice (27, 28), and model the impact of blocking IL-2 signaling in vivo. However, interpretation of results is difficult due to uncertainty of whether the observed in vivo effects are mediated by functional blockade of CD25, Treg cell depletion, or a combination (29–32). Using PC61 derivatives with identical epitope specificity but divergent constant region effector function, a recent study showed that only depletion of CD25hi cells and not blockade of CD25 could disrupt immune homeostasis (33). However, the fact that blockade of CD25 for up to four weeks caused no disturbance in immune homeostasis is surprising, given the central role IL-2 is thought to play in the maintenance of Treg cells in SLOs. For instance, acute blockade of IL-2 using the IL-2 antibody S4B6–1 (S4B6) significantly reduces Treg cells, and when administered early in life causes Treg cell dysfunction sufficient to induce autoimmune gastritis in Balb/c mice (8). However, in addition to blocking IL-2 binding to CD25 (34), this antibody forms superagonistic IL-2 immune complexes that are specifically targeted to CD122hi effector populations such as NK cells and memory T cells (35) and this may have contributed to disease development in these animals. These divergent results may reflect differences in the importance of IL-2 for the induction vs. maintenance of immune tolerance, or may reflect idiosyncrasies in how the reagents used for IL-2 and CD25 blockade actually impact IL-2 availability and signaling in Treg and Teff cells.
In light of this confusion, we comprehensively examined how manipulating the IL-2/CD25 axis by different methods perturbs Treg cell maintenance, phenotype and function in maintaining normal immune homeostasis. We found that neutralization of IL-2 abrogated all STAT5 phosphorylation (pSTAT5) in Treg cells, but did not immediately disrupt Treg cell function or immune homeostasis. However, sustained blockade of IL-2 led to mild dendritic cell (DC) activation and Teff cell proliferation and expansion. By contrast, Treg cells maintained normal IL-2 signaling in the presence of the inhibitory anti-CD25 antibody PC61 in vivo, despite substantially reduced sensitivity to IL-2. Continued IL-2 signaling was dependent on residual CD25 function, and we found that even CD25lo Treg cells that escape depletion after treatment with a strongly depleting IgG2a version of PC61 initially maintain IL-2 responsiveness and functionality in vivo. These findings demonstrate that even with severely curtailed CD25 function, Treg cells retain their selective access to IL-2 in vivo, and this is sufficient to maintain normal Treg cell function and immune homeostasis. These data warrant re-examination of previous studies using the PC61 antibody (31–33), and have important implications for efforts to target the IL-2/CD25 axis therapeutically to dampen inflammation and induce immune tolerance.
Materials and Methods
Mice
C57BL/6 (B6) mice were purchased from The Jackson Laboratory. All mice were maintained at Benaroya Research Institute, and experiments were pre-approved by the Office of Animal Care and Use Committee of Benaroya Research Institute. Mice used in experiments were between 6–12 weeks of age at time of sacrifice.
Flow cytometry
For DC isolations, minced whole spleens were digested in basal RPMI supplemented with 2.5 mg/mL Collagenase D for 20 minutes under agitation at 37°C. Cell suspensions were then passed through 70 μm strainers into RPMI + 10% FBS (RPMI-10). Erythrocytes were lysed in ACK lysis buffer, and the remaining cells were washed in RPMI-10. DCs were enriched using CD11c-microbeads (Miltenyi) according to the manufacturer’s protocol. Cell surface staining for flow cytometry was performed in FACS buffer (PBS-2% BCS) using the following antibody clones: LiveDead, CD4 (GK1.5, RM4–5), CD8 (53–6.7), CD25 (PC61, 7D4), ICOS (C398.4A), CD44 (IM7), CD62L (MEL-14), NK1.1 (PK136), CD122 (5H4), CD132 (TUGm2), CD5 (53–7.3), CD19 (6D5), Gr-1 (RB6–8C5), CD11b (M1/70), CD11c (N418), MHCII (M5/114.15.2), DC marker (33D1), CD80 (16.10A1), CD86 (GL-1), CD40 (3/23), and CD45.2 (104). Cells were incubated in the antibody mixture for 20 min at 4°C and then washed in FACS buffer before collecting events on an LSRII. For intracellular staining, surface antigens were stained before fixation and permeabilization with FixPerm buffer (eBioscience). Cells were washed and stained with antibodies to Foxp3 (FJK-16s), Ki67 (11F6), pSTAT5 (47/pStat5[pY694]), IFN-γ (XMG1.2), and CTLA-4 (UC10–4F10–11). Flow cytometry data was analyzed using FlowJo software.
Ex vivo staining
To assess pSTAT5 levels directly ex vivo, spleens were immediately disrupted between glass slides into eBioscience FixPerm. Cells were incubated for 20 min at room temperature, washed in FACS buffer, resuspended in 500 μL 90% methanol (MeOH), and incubated on ice for at least 30 minutes. Cells were stained with surface and intracellular antigens, including pSTAT5 (pY694) for 45 minutes at room temperature.
Fc.IL-2 proteins
Fc.IL-2 proteins (Fc.WT or Fc.Mut24) were generated and characterized as previously described (36).
In vitro assays
For in vitro CD25 blockade, splenocytes were isolated from untreated B6 mice as described. 5×105 cells were plated per well into a 96-well round bottom plate. Commercially available PC61 (BioXcell) was added to designated wells at 1μg/mL final concentration, and samples were incubated at 37°C for 30 min and then washed. Meanwhile, 1000 U/mL recombinant IL-2 (eBioscience) was incubated with 50 μg/mL S4B6–1 (BioXcell) for 30 minutes at room temperature. rIL-2:S4B6 complexes were then serially diluted 10-fold to achieve all desired concentrations for the experiment. rIL-2 without S4B6 was subject to the same treatment. rIL-2 or rIL-2:S4B6 dilutions were then added to appropriate wells and samples were incubated at 37°C for 30 min. For experiments testing responses to IL-2 muteins, serially diluted Fc.WT or Fc.Mut24 were added to appropriate wells and otherwise experiments were set up in the same manner. Samples were then washed and fixed with FixPerm (eBioscience) for 20 min at room temp, washed and incubated in 500 μL MeOH on ice for at least 30 min, washed and finally stained with antibodies for 45 min at room temp. For in vivo CD25 blockade, animals were injected intraperitoneally as described with 500 μg PC61N297Q or PC612a. Spleens were harvested 24 hours after injection, and in vitro response to IL-2 was measured as described above (without any incubation with commercial PC61).
In vivo antibody treatments
For CD25 blocking or depleting, mice were given 500 μg PC61N297Q or PC612a by intraperitoneal injection every 7 days, or as otherwise specified. For IL-2 blocking experiments, mice were given 150 μg S4B6–1 (BioXcell) only, 150 μg JES6–1A (BioXcell) only, 150 μg S4B6–1 and 150 μg JES6–1A together, or 150 μg S4B6–1 and 500 μg JES6–1A together by intraperitoneal injection every 5 days, or as otherwise specified. For IL-2 complex treatments, 50 μg JES6–1A and 1.5 μg recombinant IL-2 (eBioscience) per mouse were mixed together and incubated at room temperature for 30 minutes. Volume was brought up to 100 uL per mouse with sterile PBS and mice were injected intraperitoneally on day 0 and day 2.
Results
Complete antibody-mediated neutralization of IL-2 in vivo
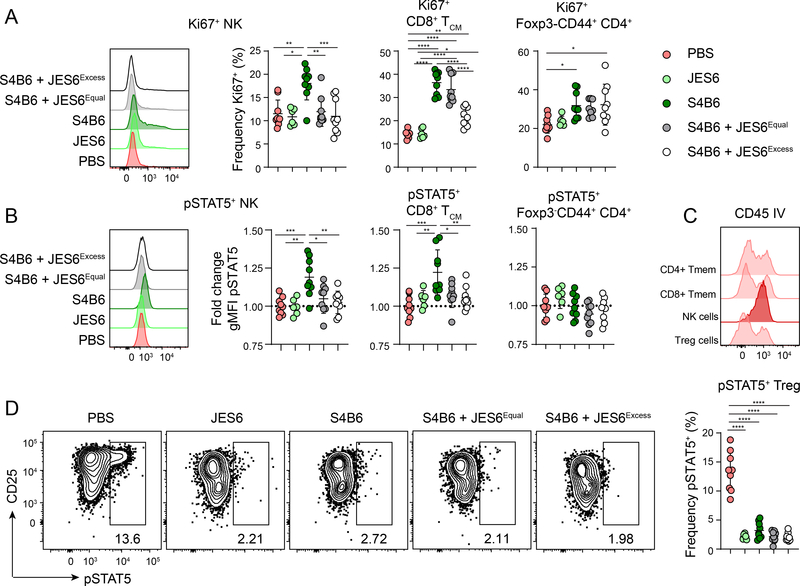
When complexed with recombinant IL-2, the anti-IL-2 monoclonal antibodies S4B6 and JES6–1A12 (JES6) act as super-agonists for different leukocyte populations depending on the antibody used and the IL-2R component expression of the cell. There is evidence that injected antibody can complex with endogenous IL-2 as well (35), and thus, we first wanted to determine if the S4B6 and JES6 antibodies could be used alone or in combination to effectively neutralize IL-2 in vivo. To test this, we treated mice with either JES6 alone, S4B6 alone, equal amounts of S4B6 and JES6, or an excess of JES6 over S4B6, and assessed IL-2 signaling and upregulation of the proliferation marker Ki67 in Treg cells, CD44+CD62L+ CD8+ central memory T cells (CD8+ TCM), CD44+CD62L-Foxp3- CD4+ Teff cells and NK cells after seven days. Due to the qualitative difference in STAT5 phosphorylation response to IL-2 in Treg cells (bimodal) compared to NK and CD8+ TCM cells (a weaker unimodal shift) (Fig. S1A), we reported response to IL-2 as frequency pSTAT5+ of Treg cells and geometric mean fluorescence intensity (gMFI) of the effector populations, respectively.
Injection of JES6, which inhibits IL-2 binding to CD122 and CD132, did not promote proliferation or phosphorylation of STAT5 in NK, CD8+ TCM, or CD4+ Teff cells (Fig. 1 A-B). In contrast, S4B6 blocks IL-2 binding to CD25, and can target IL-2 to cells expressing high levels of CD122. Accordingly, S4B6 treatment induced robust proliferation of NK cells and Teff cell populations. Proliferation was associated with increased STAT5 phosphorylation in NK and CD8+ TCM cells but not CD4+ Teff, which may be responding indirectly to S4B6 treatment (Fig. 1A-B). Addition of JES6 to S4B6 completely blocked proliferation of NK cells, and partially inhibited the proliferation of CD8+ TCM cells. These differential effects of JES6 on the effector populations may be due to their microenvironmental localization in the spleen. Indeed, intravascular labeling experiments showed that the CD4+ and CD8+ Teff reside predominantly in the white pulp (WP) where IL-2 is produced (37, 38), whereas NK cells are almost exclusively located the red pulp (RP) (Fig. 1C). Thus, the relative proximity of the CD8+ TCM cells to sites of IL-2 production may limit the ability of JES6 to block activation of these cells by locally produced S4B6/IL-2 complexes. Finally, consistent with the ability of S4B6 to effectively block IL-2 interaction with CD25, all treatments with S4B6 potently inhibited STAT5 phosphorylation in Treg cells (Fig. 1D). Surprisingly, even though JES6/IL-2 immune complexes generated ex vivo favor activation of CD25hi cells through a triggered exchange mechanism (34), treatment with JES6 alone also completely blocked IL-2 signaling in Treg cells, and did not cause an increase in IL-2 signaling or cell proliferation in any of the cell populations examined (Fig 1A, B, D). Thus we used treatment with the JES6 antibody alone for IL-2 neutralization in all subsequent experiments.
Figure 1.
JES6 effectively neutralizes IL-2 in vivo from all leukocytes
WT B6 mice were treated IP with PBS, 150μg JES6 alone (no S4B6), 150μg S4B6 alone (no JES6), 150μg S4B6 and 150μg JES6, or 500μg JES6 and 150μg S4B6 on day 0 and day 5, and sacrificed on day 7 for analysis. (A) Representative flow cytometry histograms of Ki67 expression in gated NK1.1+ splenic NK cells in each treatment group. Corresponding graphical analysis of frequency of Ki67+ in splenic NK cells, CD8+ TCM cells, and Foxp3-CD44+ CD4+ Teff cells. (B) Representative flow cytometry histograms of pSTAT5 expression in gated NK1.1+ splenic NK cells in each treatment group. Corresponding graphical analysis of fold change gMFI over PBS controls of pSTAT5 in splenic NK cells, CD8+ TCM cells, and Foxp3-CD44+ CD4+ Teff cells. (C) Representative flow cytometry histograms of CD45 IV labeling to assess relative location of splenic leukocyte populations in PBS treated controls. (D) Representative flow cytometry analysis of pSTAT5 and CD25 (clone 7D4) expression by gated splenic Foxp3+ Treg cells. Right, graphical analysis of frequency of pSTAT5+ Treg cells in each treatment group. Data is combined from three independent experiments, 6–9 mice per group total. Significance determined by one-way ANOVA with Tukey post-test for multiple comparisons. *p<0.05, **p<0.01, ***p<0.001, ****p<0.0001.
Differential impacts of targeting CD25 or IL-2 on CD25+ Treg cells
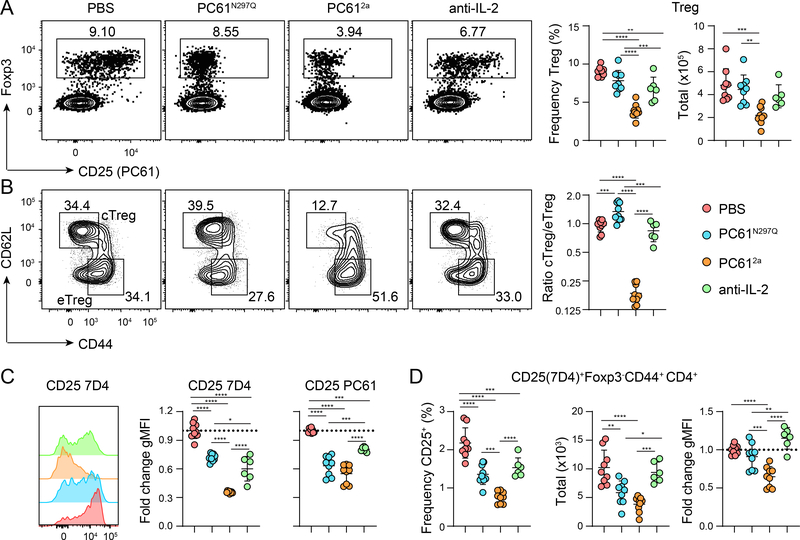
To determine how inhibiting the IL-2/CD25 axis by targeting either CD25 or IL-2 impacts Treg cell abundance and immune homeostasis, C57BL/6 (B6) mice were treated intraperitoneally (IP) with an engineered isoform of PC61 (PC61N297Q) that inhibits CD25 function but does not deplete CD25-expressing cells, an engineered isoform of PC61 (PC612a) that has strong depleting activity, or anti-IL-2 (JES6) as above. In line with previously reported findings (33), seven days after treatment Treg cells were reduced by ~50% in mice treated with PC612a relative to PBS-treated controls (Fig. 2A). Surprisingly, mice treated with anti-IL-2 had only a slightly reduced frequency of Treg cells, and no significant change in the frequency or absolute number of Treg cells was observed in PC61N297Q treated mice. Treg cells can be divided into central (c)Treg and effector (e)Treg cells based on differential expression of CD62L and CD44. In PC612a-treated mice, there was a specific loss of CD62L+CD44lo cTreg cells (Fig. 2B), which express the highest levels of CD25 and are the most dependent on IL-2 for their homeostatic maintenance within the spleen (37). In contrast, there was little change in the ratio of Treg subsets in anti-IL-2 treated mice, and a slight increase in the prevalence of cTreg in PC61N297Q treated mice. Staining isolated cells with a fluorochrome-conjugated PC61 seven days after PC61N297Q and PC612a treatment showed essentially complete coverage of the epitope (Fig. 2A and Fig. 2C), verifying that we used a saturating concentration of injected antibody. To assess CD25 expression in treated animals, we stained cells with the 7D4 anti-CD25 antibody, which recognizes a distinct epitope and does not compete with PC61 for binding. As expected, PC612a treatment effectively depleted CD25hi cells, and the remaining Treg cells in these animals were CD25mid/lo. CD25 expression was also significantly reduced in anti-IL-2-treated mice, which is likely due to the ability of IL-2 signaling and activated STAT5 to promote CD25 expression in a positive feedback loop (39). Interestingly, despite lacking the ability to deplete CD25+ cells, CD25 expression was also significantly decreased on Treg cells from mice that had been treated with PC61N297Q (Fig. 2C), indicating that this antibody may induce surface cleavage or internalization of CD25. Finally, CD4+ and CD8+ Teff cells can transiently express high levels of CD25 upon activation, and thus could also be affected by the treatments administered. While very few CD8+ Teff cells expressed CD25 in any treatment group (not shown), about 2% of Foxp3-CD44+ CD4+ Teff cells were CD25+ (Fig. 2D) and both PC61N297Q and PC612a treatment significantly reduced the frequencies and absolute numbers of this population.
Figure 2.
Impacts of targeting CD25 or IL-2 on Treg cells
WT B6 mice were treated IP with PBS, PC61N297Q, PC612a, or anti-IL-2 (JES6), and sacrificed for analysis after seven days. (A) Representative flow cytometric analysis of Foxp3 and CD25 (clone PC61) expression by gated splenic CD4+ T cells. Foxp3+ Treg cells are gated as indicated. Right, Graphical analysis of frequency and total number of splenic Treg cells in each treatment group. (B) Representative flow cytometry analysis of CD44 and CD62L expression by gated splenic Foxp3+ Treg cells showing gates used to define cTreg and eTreg populations. Right, graphical analysis of the ratio of cTreg cells to eTreg cells in the spleens of each treatment group. (C) Representative flow cytometry histograms of CD25 7D4 staining in Treg cells. Right, graphical analysis of fold change in gMFI over controls of CD25 PC61 and CD25 7D4 staining by Treg cells in each treatment group. (D) Graphical analysis of frequency, total number, and fold change gMFI of CD25+ (7D4) Foxp3-CD44+CD4+ splenic T cells in each treatment group. Data is combined from three independent experiments, 6–9 mice per group total. Significance determined by one-way ANOVA with Tukey post-test for multiple comparisons. *p<0.05, **p<0.01, ***p<0.001, ****p<0.0001.
Short-term targeting of the IL-2/CD25 axis does not disturb immune homeostasis
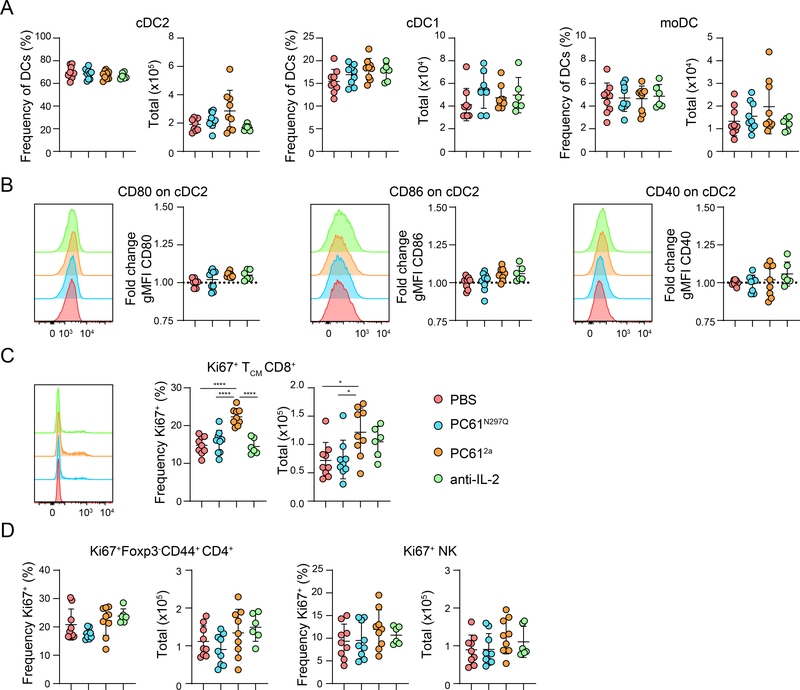
Substantial cross-regulation occurs between Treg cells and DCs (40, 41), and therefore we examined how changes in Treg cell numbers in PC61N297Q, PC612a, and JES6 treated mice impacted the three resident DC populations present in the spleen. No changes in the frequency or number of 33D1-CD11b-CD8+ type-1 conventional DCs (cDC1), 33D1-CD11bhiCD8- monocyte-derived DCs (moDCs), or 33D1+CD11b+ type-2 conventional DCs (cDC2) were observed (Fig. 3A, Fig S1B). Thus, although the frequency and number of Treg cells are reduced in the PC612a and anti-IL-2 treated mice, this was not sufficient to drive expansion of DCs.
Figure 3.
Impacts of targeting the IL-2/CD25 axis on DCs and Teff cells
(A-B) WT B6 mice were treated IP with PBS, PC61N297Q, PC612a, or anti-IL-2 (JES6), and sacrificed after seven days for analysis. (A) Graphical analysis of frequency and total number of splenic MHCII+CD11c+33D1+CD11b+ cDC2, MHCII+CD11c+33D1-CD11b-CD8+ cDC1, and MHCII+CD11c+33D1-CD11b+CD8- moDC. (B) Representative flow cytometry histograms of CD80, CD86 and CD40 expression by gated splenic cDC2. Corresponding graphical analysis of fold change gMFI over PBS controls of CD80, CD86 and CD40 in splenic cDC2s in each treatment group. (C) Representative flow cytometry histograms of Ki67 expression by gated CD44+CD62L+ CD8+ TCM cells. Graphical analysis of frequency and total number of splenic Ki67+ CD8+ TCM cells in each treatment group. (D) Graphical analysis of frequency and total number of splenic Ki67+Foxp3-CD44+CD4+ Teff cells, and Ki67+ NK cells in each treatment group. Data is combined from three independent experiments, 6–9 mice per group total. Significance determined by one-way ANOVA with Tukey post-test for multiple comparisons. *p<0.05, **p<0.01, ***p<0.001, ****p<0.0001.
In addition to regulating their DC abundance, Treg cells also restrain DC activation and prevent excessive T cell priming. Therefore, to assess the functionality of Treg cells in the anti-CD25 and anti-IL-2 treated mice, we examined DC activation in the spleen by measuring expression of CD80 and CD86 (42, 43), and CD40 (40), three important costimulatory molecules that are upregulated in activated DCs. No changes in costimulatory molecule expression were observed on splenic DCs in any treatment group (Fig. 3B and Fig. S1C-D), although there was a trend towards increased expression of CD86 and CD40 in cDC2 of JES6-treated mice. However, expression of costimulatory molecules on cDC2s did increase when IL-2 was blocked by S4B6, even in the presence of JES6 (Fig. S1D). This illustrates that we can detect small changes in activation of DCs in this system, and that activation is truly absent with PC61 or JES6 treatment. Furthermore, it suggests that in contrast to total Treg depletion or deficiency (44), blocking IL-2 signaling in Treg cells is not sufficient, at least at this timepoint, to relieve Treg cell suppression of DCs, and that agonism of effector populations by endogenous IL-2/S4B6 immune complexes potentiates cDC2 activation. In contrast, promoting IL-2 signaling in Treg cells in mice with ex vivo generated IL-2/JES6 complexes robustly expanded Treg cells, leading to increased cDC2 frequency and number, but decreased expression of costimulatory molecules (Fig. S2).
Interestingly, we observed increased proliferation of CD8+ TCM cells only in the PC612a treated mice, whereas there were no significant changes in the proliferation of NK cell or Foxp3-CD44+ CD4+ Teff cell populations with any treatment compared to controls (Fig. 3C, D). As CD8+ TCM are more sensitive to increases in IL-2 than other effector cells (43, 45), proliferation of only this population likely reflects increased bioavailability of IL-2 in PC612a treated mice, where there are fewer CD25hi Treg cells present to consume it.
Treg cells retain selective access to IL-2 in a CD25-dependent manner in the presence of PC61
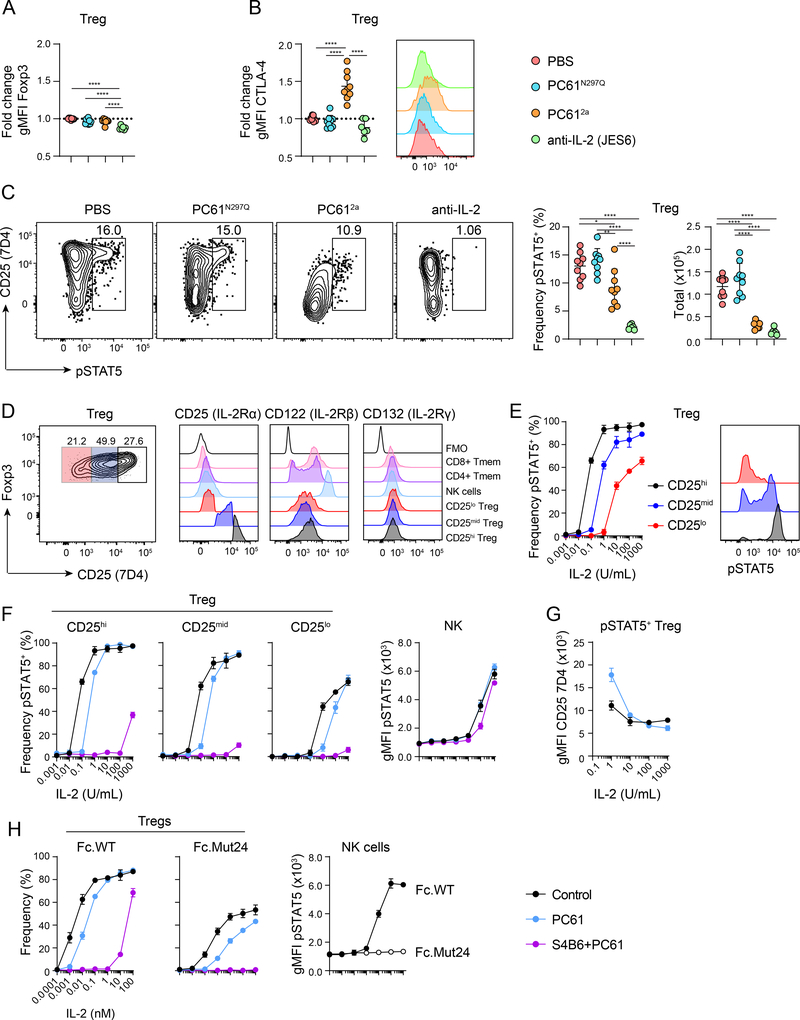
The lack of acute immune dysregulation in mice treated with CD25 or IL-2 antibodies suggests that Treg cells in these animals retain significant functional capacity, in spite of alterations observed in Treg cell number and composition. This contrasts with Treg cell depletion from Foxp3-DTR mice, where loss of Treg cells results in significant expansion of DCs, NK cells, and Teff cells after seven days (40). Therefore, we examined expression of various proteins important for suppressive function in Treg cells from the treatment groups. We found that the gMFI of Foxp3 was significantly reduced in anti-IL-2 treated mice, whereas PC61N297Q and PC612a had only minor impacts on Foxp3 expression (Fig. 4A). Furthermore, there was a trend toward decreased CTLA-4 expression on Treg cells from anti-IL-2 treated mice compared to PBS and PC61N297Q treated mice (Fig. 4B), while agonistic IL-2 immune complexes vastly increased CTLA-4 expression (Fig. S2B). Treg cells from PC612a treated mice had elevated expression of CTLA-4 compared to all other treatment groups, although this increase was at least partly driven by a higher proportion of eTreg cells in this group, which express more CTLA-4 than cTreg cells.
Figure 4.
Treg cells retain selective access to IL-2 in vivo despite severely curtained CD25 function
(A-C) WT B6 mice were treated IP with PBS, PC61N297Q, PC612a, anti-IL-2 (JES6), or anti-IL-2 (S4B6 + JES6Excess) and sacrificed after seven days for analysis. (A) Graphical analysis of fold change gMFI over PBS controls of Foxp3 in gated Foxp3+ Treg cells in the spleens of each treatment group. (B) Graphical analysis of fold change in gMFI over controls of CTLA-4 staining by Treg cells in each treatment group. Right, representative flow cytometric analysis of CTLA-4 by gated splenic Treg cells. (C) Representative flow cytometric analysis of pSTAT5 and CD25 (7D4) by gated splenic Treg cells. Right, graphical analysis of frequency and total number of pSTAT5+ splenic Treg cells in each treatment group. (D) Representative flow cytometric analysis of CD25 (7D4) expression in untreated Treg cells, and gates defining CD25hi, CD25mid and CD25lo cells are shown. Right, representative flow cytometric analysis of CD25, CD122 and CD132 expression by the indicated cell populations and fluorescence minus one (FMO) controls. (E) Graphical analysis of frequency of pSTAT5+ splenic Treg cells within each CD25 expression subset in response to rIL-2. Right, representative flow cytometric analysis of pSTAT5 staining in Treg cells in each CD25 subset in response to 1 U/mL rIL-2. (F) Graphical analysis of frequency of pSTAT5+ Treg cells in response to IL-2 after treatment in vitro with either PC61, or PC61 and S4B6 compared to controls. Right, graphical analysis of gMFI of pSTAT5 in NK cells under the same treatment conditions. (G) Graphical analysis of gMFI of CD25 (7D4) in pSTAT5+ Treg cells in response to IL-2 after treatment in vitro with PC61 compared to controls. (H) Graphical analysis of frequency of pSTAT5+ Treg cells in response to WT.Fc or Mut24.Fc after treatment in vitro with either PC61, or PC61 and S4B6 compared to controls. Right, graphical analysis of gMFI of pSTAT5 in NK cells in response to WT.Fc or Mut24.Fc. (A-C) Data is combined from three independent experiments, 6–9 mice per group total. Significance determined by one-way ANOVA with Tukey post-test for multiple comparisons. *p<0.05, **p<0.01, ***p<0.001, ****p<0.0001. (D-H) Data is from one representative experiment, with three technical replicates per condition. Experiments were repeated independently at least three times.
IL-2 signaling helps maintain Treg cell function by promoting high expression of Foxp3 (46) and other inhibitory molecules like CTLA-4 (47), and we speculated that the different expression levels of these molecules could be due to differences in IL-2 signaling in mice given PC61 compared to JES6. Therefore, we examined pSTAT5 directly ex vivo in different cell populations one week after antibody administration, when PC61 epitopes on CD25 were still completely saturated (Fig. 2A, C). Whereas neutralization of IL-2 blocked all pSTAT5 as expected, Treg cells from animals treated with PC61N297Q maintained normal levels of pSTAT5, and even treatment with PC612a had only a modest impact on the frequency of pSTAT5+ Treg cells (Fig. 4C). The pSTAT5 staining we observed in the treated animals does not simply reflect prolonged IL-2 signaling that occurred prior to treatment initiation, as we have previously shown that injection of IL-2 antibodies as little as 30 minutes prior to sacrifice ameliorates all detectable pSTAT5 in Treg cells (9). We could not detect increased IL-2 signaling in effector populations, as the gMFI of pSTAT5 in both NK cells and CD8+ TCM was not increased by any of the treatments (Fig. S3A). However, we speculate that this is due to the limited sensitivity of our assay, and that treatment with PC612a does result in more IL-2 consumption by CD8+ TCM that drives their enhanced proliferation in these mice.
The ability of Treg cells to maintain IL-2 responsiveness in the presence of the PC61 antibodies led us to two competing hypotheses. Either the CD25 remaining on the cell surface was still functional and mediating IL-2 signaling, or residual IL-2 signaling was CD25-independent. CD25-independent signaling could result from either upregulation of the other IL-2R components on Treg cells, or from increased sensitivity to IL-2 resulting from changes in the IL-2R signaling pathways such as downregulation of the negative regulator protein phosphatase 2A (PP2A) (48). Co-staining with the 7D4 anti-CD25 antibody clearly showed that as in control mice, pSTAT5 was enriched among Treg cells expressing the highest amounts of CD25 in both PC61N297Q- and PC612a-treated mice (Fig. 4C). We therefore compared the expression of the other IL-2R components from untreated splenic Treg cells divided into three subsets based on their expression of CD25 by 7D4 staining. Expression of CD122 and CD132 was similar between all three subsets of Treg cells (Fig. 4D). Furthermore, CD122 expression by Treg cells was much lower than expression by memory T cells or NK cells. Thus, enhanced expression of the intermediate affinity IL-2R does not explain the ability of CD25hi Treg cells to selectively respond to IL-2 in the presence of the PC61 antibodies.
To directly assess the impact of PC61 on CD25 function and the sensitivity of splenic Treg cells to IL-2, we performed in vitro stimulations in the presence of PC61 and the anti-IL-2 clone S4B6, which directly blocks interaction between IL-2 and CD25 but has minimal impact on IL-2 signaling via the intermediate affinity CD122/CD132 complex (34). For analysis, Treg cells were subsetted based on their expression of CD25 by 7D4 staining as in Fig 4D. CD25hi Treg cells achieved maximal pSTAT5 at a relatively low dose of rIL-2 (1 U/mL), while CD25mid Treg cells were approximately 10-fold less sensitive and CD25lo Treg cells were more than 100-fold less sensitive (Fig. 4E). Pre-treatment with PC61 for 30 min prior to IL-2 stimulation reduced IL-2 sensitivity by ~10-fold in all three Treg cell populations (Fig 4F), but all were still able to achieve the maximal level of pSTAT5 observed in untreated cells. However, further addition of S4B6 severely curtailed IL-2 sensitivity in all Treg cells, indicating that they do not efficiently signal through the intermediate-affinity IL-2 receptor. By contrast, in NK cells, which lack CD25 but have high levels of CD122, IL-2 responses were completely unaffected by the addition of PC61 (Fig. 4F, right), and S4B6 had only a small effect on signaling which is due to minor steric inhibition in vitro (34). Thus, we conclude that CD25 retains significant functionality when bound by PC61. Indeed, PC61 does not directly occlude IL-2 binding, but rather inhibits CD25 function by inducing a conformational change in the IL-2 binding pocket (49). Interestingly, at low doses of IL-2 (1 U/mL), treatment with PC61 actually makes Treg cells more CD25 dependent, as evidenced by the increased CD25 gMFI of pSTAT5+ Treg cells treated with PC61 compared to untreated control cells (Fig. 4G).
To further explore residual CD25 function in PC61-treated Treg cells, we stimulated cells with an IL-2 ‘mutein’ (Mut24) that cannot signal in the absence of CD25 due to mutations that reduce its affinity for CD122 (36). Here, we used the IL-2 mutein as a fused Fc-homodimer (Fc.Mut24), and compared it to Fc-fused WT IL-2 (Fc.WT). As before, splenocytes from B6 mice were treated in vitro with PC61 before stimulation with a range of doses of either Fc.WT or Fc.Mut24 IL-2. Treg cells responded to both Fc.WT and Fc.Mut24 in a dose-dependent manner, and addition of PC61 resulted in a ~10-fold decrease in sensitivity to both (Fig. 4H). Furthermore, addition of S4B6 completely blocked signaling in response to Fc.Mut24, demonstrating that Fc.Mut24 stimulation even in the presence of PC61 is CD25 dependent. Treg cells could still respond to high concentrations of Fc.WT in the presence of PC61 and S4B6 through the intermediate affinity receptor. In contrast, NK cells did not respond at all to Fc.Mut24, but responded normally to Fc.WT with little impact of either antibody, consistent their exclusive use of the intermediate-affinity receptor (Fig. 4H, right). Taken together, these data show that even with substantially reduced CD25 function, Treg cells selectively access IL-2 when in competition with effector cells.
To examine how in vivo treatment with either PC61N297Q or PC612a affected IL-2 sensitivity, we performed similar dose-response experiments on cells isolated from mice 24h after in vivo antibody treatment. Even at this early timepoint, PC61 had saturated all detectable epitopes in mice treated with PC61N297Q or PC612a (Fig. S3B), and by 7D4 staining we observed reduced CD25 expression in PC61N297Q-treated mice, and nearly complete depletion of CD25hi Treg cells in PC612a-treated animals (Fig. S3C). IL-2 sensitivity of CD25lo, CD25mid and CD25hi Treg cell populations from treated mice was reduced by about 50-fold (Fig. S3D). However, these Treg cells were still more IL-2 responsive than both CD8+ TCM and NK cells (Fig. S3E). Again, addition of S4B6 to further block IL-2/CD25 interaction severely curtailed IL-2 signaling in treated cells. Together, these data show that although PC61 does substantially reduce the sensitivity of Treg cells to IL-2, sustained CD25 expression and function in PC61N297Q and PC612a treated mice maintains the Treg cell-dominated hierarchy of access to IL-2 in vivo.
Extended treatment with PC612a or JES6 disrupts Treg cell mediated immune homeostasis differently
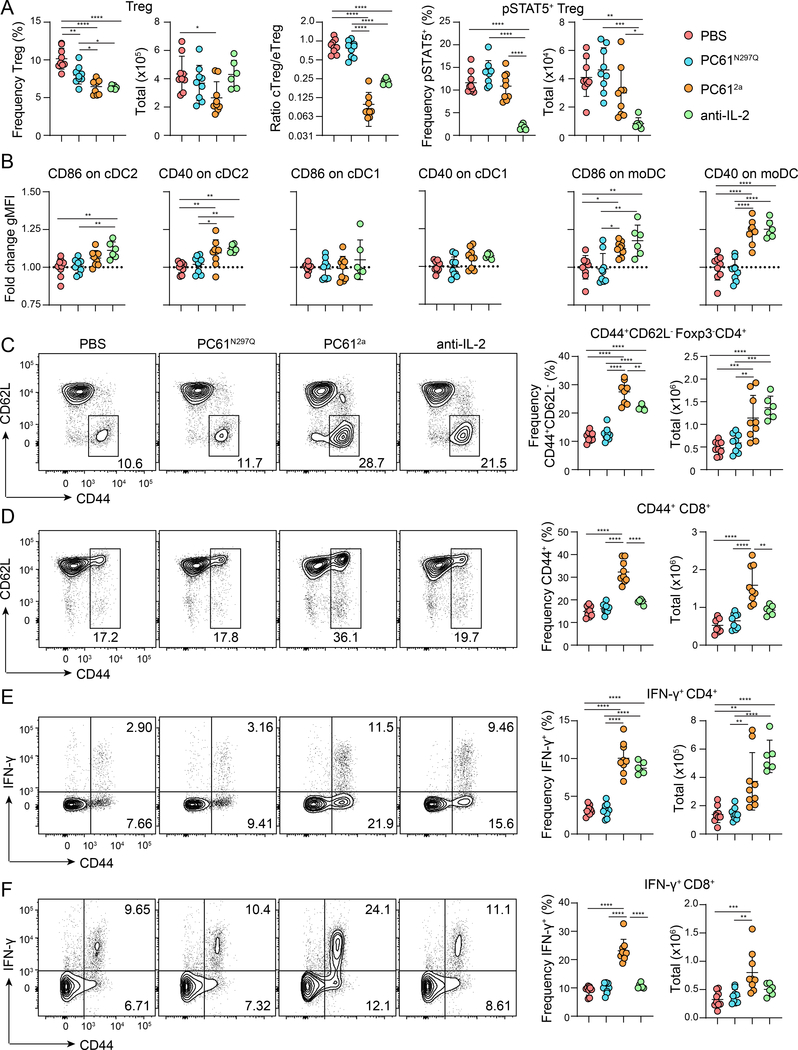
Whereas minor immune dysregulation evidenced by elevated proliferation of CD8+ T cells was only apparent in the PC612a treated mice after one week of treatment, we wondered if long-term inhibition of CD25 with the PC61N297Q or neutralization of IL-2 would ultimately result in compromised Treg cell function. As we observed after one week, the frequency of Treg cells was significantly decreased in mice treated with PC612a for four weeks, and this predominantly impacted CD25hi cTreg cells (Fig. 5A). Interestingly, prolonged treatment also resulted in a small but significant decrease in Treg cell frequency in PC61N297Q treated mice, and a large decrease in the anti-IL-2 treated mice (Fig. 5A). However, absolute numbers of Treg cells in the spleen were diminished only in the PC612a-treated mice. Endogenous STAT5 phosphorylation in Treg cells was strikingly similar at four weeks compared to one week, but we now observed activation by enhanced costimulatory molecule expression of cDC2 and moDC in PC612a and anti-IL-2 treated animals (Fig. 5B). Levels of CD86 expression were highest on DCs from anti-IL-2 treated mice, while levels of CD40 were comparable between this treatment group and PC612a. Accordingly, we detected increased frequencies and numbers of CD44hiCD62Llo CD4+ (Fig. 5C) Teff cells in PC612a and anti-IL-2 treated mice. In contrast, as we observed at 1 week post-treatment, CD44hi CD8+ Teff cells were only expanded in the PC612a treated mice (Fig. 5D). The expanded Teff cell populations were associated with enhanced production of the pro-inflammatory cytokine IFN-γ by both CD4+ and CD8+ T cells (Fig. 5E, F). Therefore, continued IL-2 signaling after Treg cell depletion with PC612a can preserve immune homeostasis only in the short term, while even extended treatment with PC61N297Q does not lead to immune dysregulation. Neutralization of IL-2 by JES6 in the periphery does not acutely disrupt immune homeostasis, but loss of Treg functionality contributes to dysregulation after several weeks.
Figure 5.
Impact of long-term blockade of IL-2 or CD25 on Treg cells and immune homeostasis
WT B6 mice were treated with PBS, PC61N297Q, or PC612a every 7 days or anti-IL-2 every 5 days, and sacrificed after 28 days for analysis. (A) Left, graphical analyses of frequency and total number of splenic Treg cells in each treatment group. Middle, graphical analyses of the ratio of CD62L+ cTreg to CD44+ eTreg in the spleens of each treatment group. Right, graphical analysis of frequency and total number of pSTAT5+ splenic Treg cells in each treatment group. (B) Graphical analysis of fold change gMFI over PBS controls of CD86 and CD40 in splenic cDC2s, cDC1s, and moDCs in each treatment group. Representative analysis of CD44 and CD62L expression by gated splenic CD4+Foxp3- T cells (C) and CD8+ T cells (D) in each treatment group. Right, corresponding graphical analyses of frequency and total number of gated CD4+Foxp3-CD44+CD62L- (C) and CD8+CD44+ (D) T cells. (D) Representative analysis of CD44 and IFN-γ expression in gated CD4+ (E) and CD8+ (F) T cells from each treatment group after stimulation in vitro for 4 hours with PMA, ionomycin and monensin. Right, graphical analysis of frequency and total number of IFN-γ producing CD4+ and CD8+ T cells in each treatment group. Data is combined from three independent experiments, 6–9 mice per group total. Significance determined by one-way ANOVA with Tukey post-test for multiple comparisons. *p<0.05, **p<0.01, ***p<0.001, ****p<0.0001.
Discussion
The importance of CD25 for Treg cell-mediated suppressive function is well established. Although genetic models using conditional ablation of CD25 on Treg cells demonstrate this (43, 50, 51), it is less clear how therapeutically inhibiting CD25 or IL-2 impacts Treg cells. As the IL-2/CD25 axis is central to mediating immune homeostasis, a precise understanding of how it could be targeted to treat human disease is critical. In this study, we define how different aspects of immune regulation are disrupted when the IL-2 signaling pathway is altered by targeting CD25 or IL-2 in various ways. Treg depletion with PC612a treatment or IL-2 blockade led to enhanced DC activation. Similarly, CD4+ T cell activation and expansion relies on a combination of decreased Treg cell numbers and reduced Treg cell function due to blunted or absent IL-2 signaling. By contrast, activation and proliferation of CD8+ T cells does not require loss of IL-2 dependent functions in Treg cells, but seems to occur when Treg cell consumption of IL-2 is impaired, as in the PC612a treatment. Strikingly, although PC61N297Q reduces IL-2 sensitivity ~10–50-fold in Treg cells, this is not sufficient to upset their competitive advantage over Teff cells and NK cells in accessing IL-2, and does not disrupt homeostasis of any of the aforementioned immune parameters, even after prolonged treatment.
Whereas previous studies have struggled to distinguish requirements for IL-2 in earlier developmental stages versus subsequent maintenance in adult peripheral immune tissue, we demonstrate here that continued IL-2 signaling in the periphery is not necessary for maintenance of Treg cell function and immune homeostasis in the short term, but does eventually lead to some immune dysregulation that is far more mild than complete loss of IL-2 or CD25. Previously, we and others have shown that maintenance of eTreg cells in non-lymphoid tissues can be IL-2 independent (37, 52), and the present study shows this can be true in the lymphoid organs as well.
The population sizes and activation states of DCs, Teff cells and Treg cells are dynamically interconnected (40, 41, 44), and work from our lab previously determined that the frequency and function of IL-2 dependent Treg cells in the spleen depends on antigen presentation to autoreactive CD4+ T cells largely by CD80/86-bearing cDC2s (9). Here, we extend these findings to show that when IL-2 is neutralized by JES6 in the short term, immune homeostasis is maintained despite a lack of pSTAT5 and reduction in suppressive molecules in Treg cells. However, S4B6 treatment blocks IL-2 signaling in Treg cells, and simultaneously redirects IL-2 to NK cells and CD8+ TCM, resulting in rapid upregulation of the costimulatory ligands CD86 and CD40 on DCs. This shows that acute blockade of IL-2 signaling is not sufficient to functionally inactivate Treg cells.
It remains unclear why S4B6 can complex with endogenous IL-2 to stimulate CD122hi cells, but JES6 does not do the same with CD25hi cells. S4B6 acts as a structural mimic of CD25 when bound to IL-2 and interacting with the IL-2R (34), and we show here that it can potentiate receptor binding signaling even with limiting amounts of IL-2. In contrast, JES6 has a complicated exchange mechanism with the IL-2R that only allows binding on IL-2 with sufficient CD25 expression on the target cell (34). We speculate that this “hand-off” is dependent on the relative concentrations of IL-2 and JES6 antibody. This could explain why JES6 blocks IL-2 signaling in Treg cells when given in vast excess in vivo, but expands Treg cells when given as ex vivo generated immune complexes with recombinant IL-2 at optimal molar ratios.
In addition to robust expansion, Treg cells displayed high levels of suppressive molecules and reduced levels of costimulatory markers on DCs following treatment with IL-2/JES6 immune complexes (our work and (42)). These data mirror results from Chinen and colleagues (43), in which Treg cells with constitutively active pSTAT5 had an enhanced ability to form conjugates with DCs, resulting in their decreased expression of costimulatory molecules. Treg cells can outcompete naïve T cells to bind to DCs (53), and modulate levels of costimulatory molecules on those DCs through provision of the inhibitory receptor CTLA-4 in vitro (54) and in vivo (42, 55). The trend toward decreased CTLA-4 expression we observed on Treg cells in the anti-IL-2-treated mice likely led to decreased inhibition of DCs with prolonged treatment. IL-2-dependent regulation of other adhesion and inhibitory molecules could also be involved in providing Treg cells’ enhanced ability to interact with and even strip MHCII-peptide from DCs in order to limit priming of conventional T cells and maintain immune homeostasis (56). However, consumption of IL-2 by Treg cells in order to sequester it away from conventional T cells seems to play as critical a role in restraining activation and expansion of Teff, especially in the CD8+ compartment as suggested previously (43).
We further show that Treg cells maintain substantial CD25 function and selective access to IL-2 in a CD25-dependent manner in the presence of PC61, critically clarifying the effects of this commonly used antibody in murine models. Our data strongly support the conclusions of Huss et al. that any effects observed in mice treated with PC61 must be due to active depletion of CD25hi Treg cells (33). However, conclusions made in this study and others based on the ability of PC61 to inhibit CD25 function on Treg cells that are not depleted should be re-evaluated (31, 33). The PC61N297Q antibody was designed expressly for the purpose of assessing effects of IL-2 blockade in Treg cells compared to depletion with the PC612a antibody, and these prior studies assumed that the binding action of the PC61 antibody to CD25 blocks the CD25-dependent IL-2 survival signal in Treg cells. However, we show definitively this is not the case, as Treg cells in the presence of PC61N297Q sustained normal levels of IL-2 signaling and preserved immune homeostasis after treatment for four weeks, while Treg cells treated with anti-IL-2 lost function in the absence of IL-2 signaling and failed to prevent immune activation.
Vargas and colleagues recently demonstrated that tumor-bearing mice treated with a strong depleting CD25 antibody have a significant reduction of intra-tumoral Treg cells and subsequent improved control of tumors, while a non-depleting CD25 antibody has no effect (57). In cancer therapy this result is desirable. In contrast, more subtle inhibition of the IL-2/CD25 axis, such as treatment with PC61N297Q, may be more advantageous for treatment of autoimmunity. For instance, we found that continued IL-2 signaling in splenocytes treated with PC61 was skewed towards the highest CD25 expressing Treg cells compared to the untreated controls. This could be beneficial in an autoimmune setting, particularly in humans where CD25 is expressed on CD56bright NK cells and activated T cells, but at a lower level than on Treg cells (58). The presence of PC61N297Q could therefore allow a further advantage for Treg cells to preferentially access IL-2 over other effector cells, and improve tolerance in a mechanism similar to Treg selective IL-2 ‘muteins’ (36, 59), or even be given in combination IL-2 muteins for an exceedingly selective CD25hi cell response.
In humans, the anti-CD25 antibodies daclizumab and basilixumab have been used as anti-inflammatory agents to treat MS and prevent graft rejection. This is somewhat counterintuitive given the central role of IL-2/CD25 in maintaining immune tolerance, and the mechanisms of action of these drugs is not well understood. Unlike PC61, these antibodies directly bind to and occlude the IL-2 binding site of CD25, and this results in a reduction in Treg cell frequency (although these cells retain function (60, 61)), increased serum levels of IL-2, and an IL-2-dependent increase in CD56bright NK cells (62–65). The increase in CD56bright NK cells correlates positively with therapeutic response in patients with MS. While normally thought to be regulatory and more immature, CD56bright NK cells expanded in daclizumab treated patients display enhanced expression of activation markers and receptors that mediate NK cell activation and cytolytic capacity (66), and in vitro studies indicate the ability of these NK cells to kill activated encephalitogenic T cells (63, 67). Although NK cells appear to have a beneficial therapeutic effect in this setting, it is also possible they are contributing to adverse events in these treated patients, such as dermatitis, malignancies, infections, and encephalitis (26, 68). Identification of antibodies that limit CD25 function but allow Treg cells to maintain their selective access to IL-2 may also be therapeutically beneficial in autoimmunity for limiting Teff cell and NK cell responses while maintaining robust Treg cell function.
Targeting of the IL-2/CD25 axis holds incredible promise for treatment of immune dysfunction. Our study emphasizes the complexity of this pathway, and that changes in the sensitivity of cells to IL-2 can produce strikingly different effects on the immune system. Total blockade of IL-2 gradually progresses to immune dysregulation, whereas residual CD25 function, even in the face of inhibitory or depleting antibodies, can maintain Treg cell preferential access to IL-2 and therefore allow preservation of immune homeostasis to varying degrees. Thus, subtle differences in CD25 antibody specificity and activity could result in a wide range of beneficial outcomes, and could be selectively utilized to maximize therapeutic benefit.
Supplementary Material
Key Points.
Neutralization of IL-2 in vivo does not immediately disrupt Treg cell function.
Treg cells maintain normal IL-2 signaling with the CD25 antibody PC61 in vivo.
Acknowledgments
We thank J. Fontenot and Biogen, Inc. for providing engineered PC61 antibodies. We thank A. Wojno, K. Arumuganathan and T. Nguyen for help with flow cytometry, and members of the Campbell laboratory for helpful discussions.
This work was supported by grants from the NIH to DJC (AI136475, AI124693). ETH was supported by the University of Washington Cell and Molecular Biology Training Grant (5T32GM007270–43).
References
- 1.Malek TR, and Castro I. 2010. Interleukin-2 receptor signaling: at the interface between tolerance and immunity. Immunity 33: 153–165. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Boyman O, and Sprent J. 2012. The role of interleukin-2 during homeostasis and activation of the immune system. Nat Rev Immunol 12: 180–190. [DOI] [PubMed] [Google Scholar]
- 3.Wang X, Rickert M, and Garcia KC. 2005. Structure of the quaternary complex of interleukin-2 with its alpha, beta, and gammac receptors. Science 310: 1159–1163. [DOI] [PubMed] [Google Scholar]
- 4.Malek TR 2003. The main function of IL-2 is to promote the development of T regulatory cells. J Leukoc Biol 74: 961–965. [DOI] [PubMed] [Google Scholar]
- 5.Smigiel KS, Srivastava S, Stolley JM, and Campbell DJ. 2014. Regulatory T-cell homeostasis: steady-state maintenance and modulation during inflammation. Immunol Rev 259: 40–59. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Wu Y, Borde M, Heissmeyer V, Feuerer M, Lapan AD, Stroud JC, Bates DL, Guo L, Han A, Ziegler SF, Mathis D, Benoist C, Chen L, and Rao A. 2006. FOXP3 controls regulatory T cell function through cooperation with NFAT. Cell 126: 375–387. [DOI] [PubMed] [Google Scholar]
- 7.Ono M, Yaguchi H, Ohkura N, Kitabayashi I, Nagamura Y, Nomura T, Miyachi Y, Tsukada T, and Sakaguchi S. 2007. Foxp3 controls regulatory T-cell function by interacting with AML1/Runx1. Nature 446: 685–689. [DOI] [PubMed] [Google Scholar]
- 8.Setoguchi R, Hori S, Takahashi T, and Sakaguchi S. 2005. Homeostatic maintenance of natural Foxp3(+) CD25(+) CD4(+) regulatory T cells by interleukin (IL)-2 and induction of autoimmune disease by IL-2 neutralization. J Exp Med 201: 723–735. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Stolley JM, and Campbell DJ. 2016. A 33D1+ Dendritic Cell/Autoreactive CD4+ T Cell Circuit Maintains IL-2-Dependent Regulatory T Cells in the Spleen. J Immunol 197: 2635–2645. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Amado IF, Berges J, Luther RJ, Mailhe MP, Garcia S, Bandeira A, Weaver C, Liston A, and Freitas AA. 2013. IL-2 coordinates IL-2-producing and regulatory T cell interplay. J Exp Med 210: 2707–2720. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Malek TR 2008. The biology of interleukin-2. Annu Rev Immunol 26: 453–479. [DOI] [PubMed] [Google Scholar]
- 12.Garg G, Tyler JR, Yang JH, Cutler AJ, Downes K, Pekalski M, Bell GL, Nutland S, Peakman M, Todd JA, Wicker LS, and Tree TI. 2012. Type 1 diabetes-associated IL2RA variation lowers IL-2 signaling and contributes to diminished CD4+CD25+ regulatory T cell function. J Immunol 188: 4644–4653. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Tang Q, Adams JY, Penaranda C, Melli K, Piaggio E, Sgouroudis E, Piccirillo CA, Salomon BL, and Bluestone JA. 2008. Central role of defective interleukin-2 production in the triggering of islet autoimmune destruction. Immunity 28: 687–697. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Dinh TN, Kyaw TS, Kanellakis P, To K, Tipping P, Toh BH, Bobik A, and Agrotis A. 2012. Cytokine therapy with interleukin-2/anti-interleukin-2 monoclonal antibody complexes expands CD4+CD25+Foxp3+ regulatory T cells and attenuates development and progression of atherosclerosis. Circulation 126: 1256–1266. [DOI] [PubMed] [Google Scholar]
- 15.Grinberg-Bleyer Y, Baeyens A, You S, Elhage R, Fourcade G, Gregoire S, Cagnard N, Carpentier W, Tang Q, Bluestone J, Chatenoud L, Klatzmann D, Salomon BL, and Piaggio E. 2010. IL-2 reverses established type 1 diabetes in NOD mice by a local effect on pancreatic regulatory T cells. J Exp Med 207: 1871–1878. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Lee SY, Cho ML, Oh HJ, Ryu JG, Park MJ, Jhun JY, Park MK, Stone JC, Ju JH, Hwang SY, Park SH, Surh CD, and Kim HY. 2012. Interleukin-2/anti-interleukin-2 monoclonal antibody immune complex suppresses collagen-induced arthritis in mice by fortifying interleukin-2/STAT5 signalling pathways. Immunology 137: 305–316. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Liu R, Zhou Q, La Cava A, Campagnolo DI, Van Kaer L, and Shi FD. 2010. Expansion of regulatory T cells via IL-2/anti-IL-2 mAb complexes suppresses experimental myasthenia. Eur J Immunol 40: 1577–1589. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Webster KE, Walters S, Kohler RE, Mrkvan T, Boyman O, Surh CD, Grey ST, and Sprent J. 2009. In vivo expansion of T reg cells with IL-2-mAb complexes: induction of resistance to EAE and long-term acceptance of islet allografts without immunosuppression. J Exp Med 206: 751–760. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Wesley JD, Sather BD, Perdue NR, Ziegler SF, and Campbell DJ. 2010. Cellular requirements for diabetes induction in DO11.10xRIPmOVA mice. J Immunol 185: 4760–4768. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Koreth J, Matsuoka K, Kim HT, McDonough SM, Bindra B, Alyea EP 3rd, Armand P, Cutler C, Ho VT, Treister NS, Bienfang DC, Prasad S, Tzachanis D, Joyce RM, Avigan DE, Antin JH, Ritz J, and Soiffer RJ. 2011. Interleukin-2 and regulatory T cells in graft-versus-host disease. N Engl J Med 365: 2055–2066. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Saadoun D, Rosenzwajg M, Joly F, Six A, Carrat F, Thibault V, Sene D, Cacoub P, and Klatzmann D. 2011. Regulatory T-cell responses to low-dose interleukin-2 in HCV-induced vasculitis. N Engl J Med 365: 2067–2077. [DOI] [PubMed] [Google Scholar]
- 22.Castela E, Le Duff F, Butori C, Ticchioni M, Hofman P, Bahadoran P, Lacour JP, and Passeron T. 2014. Effects of low-dose recombinant interleukin 2 to promote T-regulatory cells in alopecia areata. JAMA Dermatol 150: 748–751. [DOI] [PubMed] [Google Scholar]
- 23.Humrich JY, von Spee-Mayer C, Siegert E, Alexander T, Hiepe F, Radbruch A, Burmester GR, and Riemekasten G. 2015. Rapid induction of clinical remission by low-dose interleukin-2 in a patient with refractory SLE. Ann Rheum Dis 74: 791–792. [DOI] [PubMed] [Google Scholar]
- 24.Rosenberg SA 2014. IL-2: the first effective immunotherapy for human cancer. J Immunol 192: 5451–5458. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Chapman TM, and Keating GM. 2003. Basiliximab: a review of its use as induction therapy in renal transplantation. Drugs 63: 2803–2835. [DOI] [PubMed] [Google Scholar]
- 26.Gold R, Giovannoni G, Selmaj K, Havrdova E, Montalban X, Radue EW, Stefoski D, Robinson R, Riester K, Rana J, Elkins J, O’Neill G, and S. s. investigators. 2013. Daclizumab high-yield process in relapsing-remitting multiple sclerosis (SELECT): a randomised, double-blind, placebo-controlled trial. Lancet 381: 2167–2175. [DOI] [PubMed] [Google Scholar]
- 27.Lowenthal JW, Corthesy P, Tougne C, Lees R, MacDonald HR, and Nabholz M. 1985. High and low affinity IL 2 receptors: analysis by IL 2 dissociation rate and reactivity with monoclonal anti-receptor antibody PC61. J Immunol 135: 3988–3994. [PubMed] [Google Scholar]
- 28.Lowenthal JW, Zubler RH, Nabholz M, and MacDonald HR. 1985. Similarities between interleukin-2 receptor number and affinity on activated B and T lymphocytes. Nature 315: 669–672. [DOI] [PubMed] [Google Scholar]
- 29.McHugh RS, and Shevach EM. 2002. Cutting edge: depletion of CD4+CD25+ regulatory T cells is necessary, but not sufficient, for induction of organ-specific autoimmune disease. J Immunol 168: 5979–5983. [DOI] [PubMed] [Google Scholar]
- 30.Setiady YY, Coccia JA, and Park PU. 2010. In vivo depletion of CD4+FOXP3+ Treg cells by the PC61 anti-CD25 monoclonal antibody is mediated by FcgammaRIII+ phagocytes. Eur J Immunol 40: 780–786. [DOI] [PubMed] [Google Scholar]
- 31.Couper KN, Blount DG, de Souza JB, Suffia I, Belkaid Y, and Riley EM. 2007. Incomplete depletion and rapid regeneration of Foxp3+ regulatory T cells following anti-CD25 treatment in malaria-infected mice. J Immunol 178: 4136–4146. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Kohm AP, McMahon JS, Podojil JR, Begolka WS, DeGutes M, Kasprowicz DJ, Ziegler SF, and Miller SD. 2006. Cutting Edge: Anti-CD25 monoclonal antibody injection results in the functional inactivation, not depletion, of CD4+CD25+ T regulatory cells. J Immunol 176: 3301–3305. [DOI] [PubMed] [Google Scholar]
- 33.Huss DJ, Pellerin AF, Collette BP, Kannan AK, Peng L, Datta A, Wipke BT, and Fontenot JD. 2016. Anti-CD25 monoclonal antibody Fc variants differentially impact regulatory T cells and immune homeostasis. Immunology 148: 276–286. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Spangler JB, Tomala J, Luca VC, Jude KM, Dong S, Ring AM, Votavova P, Pepper M, Kovar M, and Garcia KC. 2015. Antibodies to Interleukin-2 Elicit Selective T Cell Subset Potentiation through Distinct Conformational Mechanisms. Immunity 42: 815–825. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Boyman O, Kovar M, Rubinstein MP, Surh CD, and Sprent J. 2006. Selective stimulation of T cell subsets with antibody-cytokine immune complexes. Science 311: 1924–1927. [DOI] [PubMed] [Google Scholar]
- 36.Khoryati L, Pham MN, Sherve M, Kumari S, Cook K, Pearson J, Bogdani M, Campbell DJ, and Gavin MA. 2019. Regulatory T cell expansion by a highly CD25-dependent IL-2 mutein arrests ongoing autoimmunity. bioRxiv. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Smigiel KS, Richards E, Srivastava S, Thomas KR, Dudda JC, Klonowski KD, and Campbell DJ. 2014. CCR7 provides localized access to IL-2 and defines homeostatically distinct regulatory T cell subsets. J Exp Med 211: 121–136. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Liu Z, Gerner MY, Van Panhuys N, Levine AG, Rudensky AY, and Germain RN. 2015. Immune homeostasis enforced by co-localized effector and regulatory T cells. Nature 528: 225–230. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Kim HP, Imbert J, and Leonard WJ. 2006. Both integrated and differential regulation of components of the IL-2/IL-2 receptor system. Cytokine Growth Factor Rev 17: 349–366. [DOI] [PubMed] [Google Scholar]
- 40.Kim JM, Rasmussen JP, and Rudensky AY. 2007. Regulatory T cells prevent catastrophic autoimmunity throughout the lifespan of mice. Nat Immunol 8: 191–197. [DOI] [PubMed] [Google Scholar]
- 41.Darrasse-Jeze G, Deroubaix S, Mouquet H, Victora GD, Eisenreich T, Yao KH, Masilamani RF, Dustin ML, Rudensky A, Liu K, and Nussenzweig MC. 2009. Feedback control of regulatory T cell homeostasis by dendritic cells in vivo. J Exp Med 206: 1853–1862. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Bolton HA, Zhu E, Terry AM, Guy TV, Koh WP, Tan SY, Power CA, Bertolino P, Lahl K, Sparwasser T, Shklovskaya E, and Fazekas de St Groth B. 2015. Selective Treg reconstitution during lymphopenia normalizes DC costimulation and prevents graft-versus-host disease. J Clin Invest 125: 3627–3641. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Chinen T, Kannan AK, Levine AG, Fan X, Klein U, Zheng Y, Gasteiger G, Feng Y, Fontenot JD, and Rudensky AY. 2016. An essential role for the IL-2 receptor in Treg cell function. Nat Immunol 17: 1322–1333. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Bolton HA, Roediger B, and Fazekas de St Groth B. 2015. The effects of IL-2 and Treg cells on dendritic cell homeostasis are mediated indirectly via activation of conventional T cells. Eur J Immunol 45: 1141–1147. [DOI] [PubMed] [Google Scholar]
- 45.Smith GA, Taunton J, and Weiss A. 2017. IL-2Rbeta abundance differentially tunes IL-2 signaling dynamics in CD4(+) and CD8(+) T cells. Sci Signal 10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Rubtsov YP, Niec RE, Josefowicz S, Li L, Darce J, Mathis D, Benoist C, and Rudensky AY. 2010. Stability of the regulatory T cell lineage in vivo. Science 329: 1667–1671. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Barron L, Dooms H, Hoyer KK, Kuswanto W, Hofmann J, O’Gorman WE, and Abbas AK. 2010. Cutting edge: mechanisms of IL-2-dependent maintenance of functional regulatory T cells. J Immunol 185: 6426–6430. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Ding Y, Yu A, Tsokos GC, and Malek TR. 2019. CD25 and Protein Phosphatase 2A Cooperate to Enhance IL-2R Signaling in Human Regulatory T Cells. J Immunol 203: 93–104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Moreau JL, Nabholz M, Diamantstein T, Malek T, Shevach E, and Theze J. 1987. Monoclonal antibodies identify three epitope clusters on the mouse p55 subunit of the interleukin 2 receptor: relationship to the interleukin 2-binding site. Eur J Immunol 17: 929–935. [DOI] [PubMed] [Google Scholar]
- 50.Fan MY, Low JS, Tanimine N, Finn KK, Priyadharshini B, Germana SK, Kaech SM, and Turka LA. 2018. Differential Roles of IL-2 Signaling in Developing versus Mature Tregs. Cell Rep 25: 1204–1213 e1204. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Toomer KH, Lui JB, Altman NH, Ban Y, Chen X, and Malek TR. 2019. Essential and non-overlapping IL-2Ralpha-dependent processes for thymic development and peripheral homeostasis of regulatory T cells. Nat Commun 10: 1037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Gratz IK, Truong HA, Yang SH, Maurano MM, Lee K, Abbas AK, and Rosenblum MD. 2013. Cutting Edge: memory regulatory t cells require IL-7 and not IL-2 for their maintenance in peripheral tissues. J Immunol 190: 4483–4487. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Onishi Y, Fehervari Z, Yamaguchi T, and Sakaguchi S. 2008. Foxp3+ natural regulatory T cells preferentially form aggregates on dendritic cells in vitro and actively inhibit their maturation. Proc Natl Acad Sci U S A 105: 10113–10118. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Oderup C, Cederbom L, Makowska A, Cilio CM, and Ivars F. 2006. Cytotoxic T lymphocyte antigen-4-dependent down-modulation of costimulatory molecules on dendritic cells in CD4+ CD25+ regulatory T-cell-mediated suppression. Immunology 118: 240–249. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Qureshi OS, Zheng Y, Nakamura K, Attridge K, Manzotti C, Schmidt EM, Baker J, Jeffery LE, Kaur S, Briggs Z, Hou TZ, Futter CE, Anderson G, Walker LS, and Sansom DM. 2011. Trans-endocytosis of CD80 and CD86: a molecular basis for the cell-extrinsic function of CTLA-4. Science 332: 600–603. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Akkaya B, Oya Y, Akkaya M, Al Souz J, Holstein AH, Kamenyeva O, Kabat J, Matsumura R, Dorward DW, Glass DD, and Shevach EM. 2019. Regulatory T cells mediate specific suppression by depleting peptide-MHC class II from dendritic cells. Nat Immunol 20: 218–231. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Arce Vargas F, Furness AJS, Solomon I, Joshi K, Mekkaoui L, Lesko MH, Miranda Rota E, Dahan R, Georgiou A, Sledzinska A, Ben Aissa A, Franz D, Werner Sunderland M, Wong YNS, Henry JY, O’Brien T, Nicol D, Challacombe B, Beers SA, Melanoma TC, Renal TC, Lung TC, Turajlic S, Gore M, Larkin J, Swanton C, Chester KA, Pule M, Ravetch JV, Marafioti T, Peggs KS, and Quezada SA. 2017. Fc-Optimized Anti-CD25 Depletes Tumor-Infiltrating Regulatory T Cells and Synergizes with PD-1 Blockade to Eradicate Established Tumors. Immunity 46: 577–586. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Hirakawa M, Matos TR, Liu H, Koreth J, Kim HT, Paul NE, Murase K, Whangbo J, Alho AC, Nikiforow S, Cutler C, Ho VT, Armand P, Alyea EP, Antin JH, Blazar BR, Lacerda JF, Soiffer RJ, and Ritz J. 2016. Low-dose IL-2 selectively activates subsets of CD4(+) Tregs and NK cells. JCI Insight 1: e89278. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Peterson LB, Bell CJM, Howlett SK, Pekalski ML, Brady K, Hinton H, Sauter D, Todd JA, Umana P, Ast O, Waldhauer I, Freimoser-Grundschober A, Moessner E, Klein C, Hosse RJ, and Wicker LS. 2018. A long-lived IL-2 mutein that selectively activates and expands regulatory T cells as a therapy for autoimmune disease. J Autoimmun 95: 1–14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Bluestone JA, Liu W, Yabu JM, Laszik ZG, Putnam A, Belingheri M, Gross DM, Townsend RM, and Vincenti F. 2008. The effect of costimulatory and interleukin 2 receptor blockade on regulatory T cells in renal transplantation. Am J Transplant 8: 2086–2096. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Vlad G, Ho EK, Vasilescu ER, Fan J, Liu Z, Cai JW, Jin Z, Burke E, Deng M, Cadeiras M, Cortesini R, Itescu S, Marboe C, Mancini D, and Suciu-Foca N. 2007. Anti-CD25 treatment and FOXP3-positive regulatory T cells in heart transplantation. Transpl Immunol 18: 13–21. [DOI] [PubMed] [Google Scholar]
- 62.Huss DJ, Mehta DS, Sharma A, You X, Riester KA, Sheridan JP, Amaravadi LS, Elkins JS, and Fontenot JD. 2015. In vivo maintenance of human regulatory T cells during CD25 blockade. J Immunol 194: 84–92. [DOI] [PubMed] [Google Scholar]
- 63.Bielekova B, Catalfamo M, Reichert-Scrivner S, Packer A, Cerna M, Waldmann TA, McFarland H, Henkart PA, and Martin R. 2006. Regulatory CD56(bright) natural killer cells mediate immunomodulatory effects of IL-2Ralpha-targeted therapy (daclizumab) in multiple sclerosis. Proc Natl Acad Sci U S A 103: 5941–5946. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Elkins J, Sheridan J, Amaravadi L, Riester K, Selmaj K, Bielekova B, Parr E, and Giovannoni G. 2015. CD56(bright) natural killer cells and response to daclizumab HYP in relapsing-remitting MS. Neurol Neuroimmunol Neuroinflamm 2: e65. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Cohan SL, Lucassen EB, Romba MC, and Linch SN. 2019. Daclizumab: Mechanisms of Action, Therapeutic Efficacy, Adverse Events and Its Uncovering the Potential Role of Innate Immune System Recruitment as a Treatment Strategy for Relapsing Multiple Sclerosis. Biomedicines 7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Ranganath T, Simpson LJ, Seiler C, Ferreira A-M, Vendrame E, Zhao N, Fontenot JD, Holmes S, and Blish CA. 2019. Characterization of the impact of daclizumab beta on circulating natural killer cells by mass cytometry. bioRxiv [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Jiang W, Chai NR, Maric D, and Bielekova B. 2011. Unexpected role for granzyme K in CD56bright NK cell-mediated immunoregulation of multiple sclerosis. J Immunol 187: 781–790. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Kappos L, Wiendl H, Selmaj K, Arnold DL, Havrdova E, Boyko A, Kaufman M, Rose J, Greenberg S, Sweetser M, Riester K, O’Neill G, and Elkins J. 2015. Daclizumab HYP versus Interferon Beta-1a in Relapsing Multiple Sclerosis. N Engl J Med 373: 1418–1428. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.