Abstract
As contaminant exposures in aquatic ecosystems continue to increase, the need for streamlining research efforts in environmental toxicology using predictive frameworks also grows. One such framework is the Adverse Outcome Pathway (AOP). An AOP framework organizes and utilizes toxicological information to connect measurable molecular endpoints to an adverse outcome of regulatory relevance via a series of events at different levels of biological organization. Molecular endpoints or biomarkers are essential to develop AOPs and are valuable early warning signs of toxicity of pollutants, including contaminants of emerging concern (CECs). Ecological risk assessment (ERA) approaches using tools such as biomarkers and AOPs benefit from identification of molecular targets conserved across species. Bivalve models are useful in such approaches and are integral to our understanding of ecological and human health risks associated with contaminant exposures. Here we discuss the value of using biomarker approaches in bivalve models to meet the demands of 21st century toxicology.
Keywords: Biomarkers, Adverse outcome pathway, Contaminants of emerging concern, Bivalves
BIOMARKERS, AOPs AND ERA
There is a growing effort in the scientific community to adopt predictive and computational strategies towards ecological risk assessment (ERA) (Vinken et al. 2017). With rapidly expanding consumer markets and industrial manufacturing, new chemicals and materials enter the aquatic environment every year. Therefore, methods for measuring adverse effects as well as regulatory approaches must evolve at a fast pace to identify, evaluate and mitigate the risks associated with contaminants of emerging concern (CECs) in aquatic ecosystems. This is complicated not only by the number of chemicals to be tested, but also, in part, due to species-specific sensitivities and limited understanding of molecular targets and mechanistic pathways in environmental toxicology. Integration of molecular and cellular effects in ERA is both challenging and essential in developing a targeted toxicological approach that is relevant to the growing demands of chemical evaluations. One such framework that connects existing toxicological knowledge to adverse effects of regulatory relevance is the Adverse Outcome Pathway (AOP). An AOP is a representation of a chemical interaction leading to an adverse effect via a series of events based on causal relationships at multiple levels of biological organization. The initial anchor of an AOP is the molecular initiating event (MIE) which is described as the first interaction of the chemical with a biological system, at the molecular level, that triggers a downstream chain of key events (KEs). The directed relationships that identify one KE as an upstream event and the other one as downstream are defined as key event relationships (KERs). The final anchor in an AOP is an adverse outcome (AO) which is usually detected at the organism or population level and is relevant to performing a risk assessment (Ankley et al. 2010). Evaluation of the connections between these two anchors is vital to understanding the nature of the environmental and human health risks and minimizing the use of large-scale, time-, money- and resource- intensive ERA approaches that do not utilize the underlying biological information and predictive power of AOPs. At the lower levels of organization (i.e., molecular, organelle, cellular, and tissue levels), toxicity assessments are typically rapid, relatively inexpensive to conduct, and use fewer resources. However, their applicability in ERA approaches is poor in the absence of the direct effects on apical endpoints (Lam 2009). To close this gap, AOPs have emerged as a promising concept to streamline toxicological research and contribute to effective ERA for pollutants including CECs. AOPs are living documents that evolve over time as new information becomes available and are not specific to a chemical. They consist of reusable components (such as KEs and KERs) that can be used across multiple AOPs in an AOP network (Villeneuve et al. 2014).
Biomarkers (or biological markers) are defined as measurable changes in cellular or biochemical processes, structures or components that are induced by xenobiotics, disease, or other physical agents. They are mostly measured at sub-organismic levels as molecular, cellular, and physiological effects and are often used in mode of action and mechanistic studies in toxicology. Biomarker assessments can contribute to AOP development (Lee et al. 2015) by identifying MIEs and KEs at lower levels of biological organization, and serve as biological-effects monitoring tools for the evaluation of organismal health (Bolognesi and Cirillo 2014; Nicholson and Lam 2005; UNEP/RAMOGE 1999). The application of biomarker tools in understanding effects at higher levels of biological organization is not recent (DeCaprio 1997). Similar to their use in the field of human health and medicine, multi-biomarker approaches offer diagnostic value and detection of early warning signals in environmental health studies (Lee et al. 2015; Ringwood et al. 1999a). Use of environmental assessment tools such as Integrated Biomarker Response (IBR) (Beliaeff and Burgeot 2002) have allowed toxicity evaluations using integration of a suite of biomarkers (Dagnino et al. 2007b; Fossi Tankoua et al. 2013; Parolini et al. 2013). Such integrative approaches and models are instrumental in advancing the applicability of biomarkers in ERA and biomonitoring (Dagnino et al. 2007a; Damiens et al. 2007; Pytharopoulou et al. 2008; Regoli et al. 2014). In this article, we focus on the use of biomarker tools and approaches in development of AOPs using bivalve molluscs as model systems.
BIVALVE MOLLUSCS AS GOOD MODEL SYSTEMS
Models such as bivalve molluscs play an essential role in invertebrate environmental toxicological research. Bivalve biomarker assessments have been successfully utilized for evaluating chemical-induced physiological stress and overall health. Further, it has been suggested that the power of using molluscs as model systems can be exponentially increased via integration of the information obtained from multidisciplinary research efforts ranging from molecular to ecosystem levels (Rittschof and McClellan-Green 2005). Bivalves are widely distributed, particle-feeding molluscs that are easy to collect and maintain in laboratory settings. Many bivalves have commercial importance as a protein-rich seafood group for humans and directly contribute to human health and food safety concerns.
Aquatic sediments act as sinks as well as sources of organic and metal contaminants (Di Toro et al. 1991). The dynamic interface between sediments and overlying water (as well as porewater) is essential in examining the bioavailability and toxicity of contaminants. Benthic fauna, including both epifaunal and infaunal bivalves, are key to understanding how microenvironments and site-specific variables affect chemical or particle behavior (Byrne and O’Halloran 2001). For example, uptake and accumulation of metal contaminants by bivalves is valuable for understanding and defining the role of benthic processes in affecting contaminant bioavailability and the resulting toxicity (Griscom and Fisher 2004). Most bivalves have limited to no mobility and their sedentary lifestyle makes them good candidates for bioaccumulation studies and representatives of habitat quality. Because these shelled molluscs are more resilient to contaminants than many other resident species (O’Connor 2002), they can also provide information about the water column and sediment interface which only continuous long-term monitoring that captures diel and seasonal trends could offer. Therefore, bivalve studies serve a pivotal role as tools to understand habitat quality and in efficient management of stressed ecosystems (Besse et al. 2012). In addition, such approaches can be used to identify hotspots and prioritize conservation efforts, as well as outline critical exposure questions for other species. One example of an extensive national monitoring program is the Mussel Watch Program that started in the US in 1986 (Farrington et al. 2016; Goldberg 1986) and utilizes mussels as a bioindicator species to track chemical contamination trends in aquatic habitats. Other monitoring programs (e.g., MedPol, UNEP Mediterranean Biomonitoring Program; OSPAR Convention, RAMOGE) have also used bivalves to assess spatio-temporal trends in chemical pollution around the world (Andral et al. 2004; Casas and Bacher 2006; Hamza-Chaffai 2014).
Filter-feeding
Most bivalves are filter-feeders and are recognized as bioindicators of aquatic pollution (Bolognesi and Cirillo 2014; Moore 2006; Oehlmann and Schulte-Oehlmann 2003; Ringwood et al. 1999a). They are known to bioconcentrate contaminants (O’Connor 2002) and are sensitive to exposures to inorganic (Hédouin et al. 2011; Waykar and Deshmukh 2012; Yusof et al. 2004) as well as organic contaminants (Freitas et al. 2015; Sericano et al. 1996; Zuloaga et al. 2009). Bivalves have highly developed processes for internalization of nano- to micro-scale particles (Canesi et al. 2012; Moore 2006), making them ideal models for assessing nanomaterial toxicity. Bivalve capture efficiencies for microscale particles are species-specific (Riisgård 1988; Ward and Shumway 2004) and must be considered in particle toxicity assessments. Despite species-specific differences in the particle selection (Baker and Levinton 2003; Kiorboe and Mohlenberg 1981; Winkel and Davids 1982), adverse effects of exposures to nanomaterials have been reported in both freshwater and marine bivalves (Rocha et al. 2015). Further, nanomaterials incorporated into aggregates are shown to be more efficiently captured and ingested by bivalves than freely suspended (100 nm) particles (Ward and Kach 2009). Many nanomaterials undergo aggregation under natural conditions, particularly in marine waters, which could increase their bioavailability to bivalves (Hotze et al. 2010). Agglomerates may disintegrate, after being captured by the gills or entering the stomach, freeing smaller components that may cross cellular barriers. However, scientific evidence supporting this possibility is lacking and detailed investigations regarding the fate of nanomaterials in biological systems are needed.
Gills play critical roles in filtration, feeding and respiratory processes in bivalves. Their large surface area and direct exposure to the microenvironment around the bivalve increase their vulnerability to contaminants. Gill sensitivity and evidence of conserved targets, along with the ease of harvesting gills for biomarker testing, make bivalves good candidates for evaluation of adverse outcomes of CEC exposures. Molecular changes as well as histopathological damage in bivalve gills have been reported, indicating their sensitivity to many contaminant classes (Cappello et al. 2013; Ciacci et al. 2012; Cossu et al. 2000; Gregory et al. 2002; Nogueira et al. 2013). Beating of lateral cilia in the bivalve gills, critical to filter-feeding, is controlled via serotonergic-dopaminergic innervation (Carroll and Catapane 2007). A class of widely prescribed pharmaceutical drugs, selective serotonin re-uptake inhibitors (SSRIs), which includes the antidepressants Fluoxetine (commonly known as Prozac) and Sertraline (commonly known as Zoloft) have been shown to cause adverse effects in bivalves (Estévez-Calvar et al. 2017; Franzellitti et al. 2014; Hazelton et al. 2014). In bivalve physiology, serotonin plays an important role not just in gill ciliary activity, but also in regulation of several reproductive processes, including maturation and release of gametes (Fong and Ford 2014). Widespread occurrence of SSRIs in environmental samples such as wastewater, groundwater, surface water as well as evidence in sediments and biota have raised environmental concerns for possible adverse effects in aquatic ecosystems (Ford and Fong 2016; Silva et al. 2012).
Another class of CECs, β-adrenoceptor antagonists that are used as antihypertensive drugs, has also been shown to adversely affect molecular and physiological processes in marine and freshwater bivalves (Contardo-Jara et al. 2010; Fabbri et al. 2009; Franzellitti et al. 2011; Khan et al. 2018a). Along with their adrenergic antagonistic properties, first-generation antihypertensives have also been documented to possess antagonistic properties towards serotonin-receptors (Huggett et al. 2002). When CECs enter aquatic ecosystems, interactions among multiple prescription drugs at the molecular level may result in physiological effects at the organism level even at low exposure concentrations (Franzellitti et al. 2015). There is also evidence for the occurrence of pharmaceutical compounds in aquatic organisms, including bivalves, which raises concerns around human consumption of bivalves as seafood (Álvarez-Muñoz et al. 2015; McEneff et al. 2014). Transfer of pharmaceuticals to higher trophic levels in aquatic food webs is poorly understood and more research is required to characterize risks of emerging contaminant exposures to humans via dietary intake (Gaw et al. 2014). This growing and overlapping concern relating to human and environmental health is a multi-disciplinary platform for AOP development as well as for identification of conserved pathways, receptors and molecular targets across taxa, and resulting physiological effects. Bivalve research can provide such cross-disciplinary links between human and ecological health. The expanding field of molecular and high-throughput studies with bivalves as models (Dodder et al. 2014; Fabbri et al. 2014; Smital et al. 2004) has provided opportunities towards identification of biological responses that can be extrapolated across taxa.
Detoxification and immune function
The bivalve digestive system and immune processes extend their utility as a model system (Canesi et al. 2008; Canesi et al. 2007). Bivalve digestive tissues are sites for processing as well as detoxification and have been widely used for biomarker assessments in physical, xenobiotic, and biological stress studies (Aguirre-Martínez et al. 2016; Canesi et al. 2010; Khan et al. 2018b). Food particles in the incoming water are trapped by the gill sieve (ctenidia) in the mucous string and are moved towards labial palps in many bivalve species. The trapped food particles eventually travel to the mouth and, subsequently, enter the gut. Digestive cells possess a lysosomal system for intracellular digestion characterized by endo- and phago-cytotic processes. Lysosomes in digestive cells have been shown to accumulate small scale particulate material and are regarded as valuable indicators of contaminant-induced damage in freshwater and marine bivalves (Canesi et al. 2010; Guidi et al. 2010; Ringwood et al. 1999a; Ringwood et al. 1999b).
Bivalve immune cells, referred to as hemocytes, also utilize endo- and phago-cytotic processes for immune functions and defense against invading pathogens (Canesi et al. 2010; Canesi et al. 2002). Hemocytes are structurally and functionally similar to mammalian macrophages and monocytes. These cell types provide cell-mediated immunity through phagocytosis and humoral immunity via the release of lysosomal enzymes and oxyradicals, agglutinins, and antimicrobial peptides (Canesi et al. 2002). Hemocytes are known to respond to pathogen as well as contaminant (chemical and nanomaterial) exposures (Aguirre-Martínez et al. 2016; Canesi et al. 2002; Sauvé et al. 2002). The lysosomal-autophagic reactions are therefore key components of cellular processes associated with exposures to CECs and have been proposed as tools for predicting environmental risk and ecological health (Moore et al. 2006). Interestingly, similar to the mammalian immune cells, bivalve hemocytes also mount responses by induction of signaling pathways involving Mitogen Activated Protein Kinases (MAPKs, p38 and JNKs) and Protein Kinase C (Canesi et al. 2006). While bivalves lack acquired immunity and solely depend on innate components of immune function, the cellular processes that outline innate immune responses have been conserved through evolution (Cooper et al. 2006) and, hence, provide the opportunity to use such responses for the characterization of effects of contaminants on a variety of organisms.
Bivalves have an open circulatory system comprised of a systemic heart pumping hemolymph (containing hemocytes) into the arteries which eventually open into sinuses (Jones 1983). These sinuses carry hemolymph to the body cavity (i.e., the hemocoel) where tissues are bathed in hemolymph. Hemolymph transports respiratory gases, nutrients, metabolic wastes, toxins and contaminants throughout the body. As a health assessment tool, non-invasive sampling of hemolymph in freshwater as well as marine bivalves has been recommended for obtaining valuable information on immune and toxicological responses (Gustafson et al. 2005; Yanick and Heath 2000).
One of the molecular components of bivalve detoxification is the multixenobiotic resistance (MXR) mechanism that is closely related to the mammalian multi-drug resistance (MDR) protein family which is implicated in resistance to chemotherapeutic drugs (Litman et al. 2001). Both MDR and MXR belong to the ATP binding cassette (ABC) superfamily of membrane transporters (such as p-glycoprotein) and have overlapping membrane binding capacities, drug inhibition properties, and antibody cross-reactivities. In bivalve studies, MXR has been shown to be a key biomarker of environmental pollution (Pain and Parant 2007) along with phase I and II detoxification markers, such as, cytochrome P450 and glutathione-s-transferase, respectively (Bonnafé et al. 2015). The similarities between the components of detoxification mechanisms across species, and their connections to human health and responses to environmental pollution together hold promise for use of molecular markers in integrative frameworks such as AOPs for toxicity assessments.
Use of multiple life stages
One key advantage of using bivalve models in environmental toxicology is the ability to utilize all life stages starting from embryos to sexually mature adults (Rittschof and McClellan-Green 2005). Toxicological information from all life stages allows for integration of our knowledge of exposures and effects while highlighting areas of overlap as well as critical data gaps. Such integration could assist in AOP development by incorporation of reproductive outcomes and success of early life-stages together with physiological effects that could impact population-level consequences. Studies have reported effects of CECs on reproductive processes in bivalves (Ciocan et al. 2010; Fabbri et al. 2014). Additionally, linkages between chemical stress, biomarker responses, and reproductive processes have been drawn in bivalves (Edge et al. 2012; Ringwood et al. 2004; Ringwood and Conners 2000).
Most marine bivalves are broadcast spawners and go through a shelled, free swimming larval stage, called a veliger, before they settle to metamorphose to juveniles (Ackerman et al. 1994). Recent advances in our understanding of bivalve genomes (Zhang et al. 2012) reveal a complexity in shell formation and stress responses that suggests studies with veliger larvae can provide insights into the developmental effects of CECs. Indeed, a high-throughput bivalve embryotoxicity assay for screening of CECs has been proposed (Fabbri et al. 2014). Additionally, sensitivity to chemical stress and application of biomarkers towards the goal of biomonitoring have been documented in bivalve developmental stages (Damiens et al. 2004). In addition to the shelled veliger larvae, specialized developmental stages, such as glochidia, found in freshwater mussels of the family Unionoidea have also been shown to be responsive to contaminant exposures (Cope et al. 2008). Adverse developmental effects and larval deformities may compromise the success of wild populations and disrupt community dynamics in aquatic ecosystems. The high sensitivity of early life stages is a useful tool in developing information networks applying biomarker approaches to outline potential AOs at higher levels of biological organization.
BIOMARKER APPROACHES
Application of the wealth of biomarker information from bivalves has been suggested for wide-scale biomonitoring programs using a tiered approach (Viarengo et al. 2007). Tier-1 of this approach utilizes in vitro assessment of a sensitive, rapid and low-cost biomarker (such as lysosomal membrane stability and cell survival rate) as an early warning and scanning system. Based on tier-1 results, tier-2 is employed with a larger suite of biomarkers that considers biological trends using longer term in vivo assessments. Therefore, in vitro tier-1 studies are important for identifying sensitive markers, but they must be verified in longer-term tier-2 in vivo evaluations. In vitro studies provide critical information, especially for potential toxicity of CECs, and serve as screening tools that streamline the two-tiered approach, making it easier and more effective. However, their applicability in developing robust AOPs is only as good as the validity of the selected marker in an in vivo context with downstream physiological effects relevant in a whole-animal exposure system.
One advantage of using bivalve models is the ability to conduct caging studies in coastal, lagoon and estuarine biomonitoring programs to evaluate long-term effects of ecological relevance (Nasci et al. 2002). Additionally, transplantation studies with bivalves are also valuable in ERA and biomonitoring using integrative approaches such as IBR (discussed in the section titled Biomarkers, AOPs and ERA) (Damiens et al. 2007). With an ever-growing list of chemical and material stressors, the most optimal use of biomarker tools is an integrative assessment approach that combines human and environmental health. The concept of finding common endpoints across taxa that can be used at multiple scales of biological organization has been a focal point of risk assessment frameworks globally over the last few decades (Munns et al. 2003). One of the challenges in implementing the recommendations from such frameworks is the identification of sensitive markers in relevant model species and examination of the physiological relevance of biomarker measurements across taxa. Evolutionary conservation of molecular targets, such as receptors that bind pharmaceuticals or enzymes that metabolize xenobiotics, has been utilized by approaches such as the read-across hypothesis in predictive assessment frameworks. This hypothesis states that a drug or chemical will have a measurable effect in a non-target organism if the molecular targets of that drug have been conserved, resulting in a specific pharmacological effect (Rand-Weaver et al. 2013), and holds promise as an alternative to animal- and time-intensive in vivo testing (Stuard and Heinonen 2018). Recent advances in bivalve genomics (Murgarella et al. 2016; Uliano-Silva et al. 2016; Zhang et al. 2012) and other high throughput omic techniques (Campos et al. 2012; Suárez-Ulloa et al. 2013) have opened an exciting path towards evaluation of conserved targets and responses across taxa. Another molecular tool that allows for extrapolation of toxicological information across species is Sequence Alignment to Predict Across Species Susceptibility (SeqAPASS) (LaLone et al. 2016). Using an NCBI (National Center for Biotechnology Information) database, SeqAPASS compares the amino acid sequence and protein structure to evaluate the presence of a potential molecular target in a non-model species. Such comparisons for chemical interactions can identify susceptibility of taxa to chemicals with a known mode of action. This approach maximizes and expands the application of information derived from AOP constructs and from high throughput sequencing. There are examples where existing information is available on the homology and functions of protein families that participate in bivalve stress responses as well in mammalian cancer biology. Heat shock proteins (HSPs) (Margulis et al. 1989) and p53 (Walker et al. 2011) are two examples of how such overlap between taxa can be useful in application of biomarker tools from bivalve models to provide integrative approaches for human and ecological health assessment (Fernández Robledo et al. 2019; Galloway 2006).
In addition to integrating information across taxa, application of new approaches in chemical read-across based on existing tools such as QSAR (Quantitative Structure-Activity Relationship) modeling is growing for AOP development and decision making (Berggren et al. 2015). QSARs are based on the hypothesis that changes in the molecular structure of a chemical reflect changes in biological activity. They have been used in the field of ecotoxicology for decades (Calamari and Vighi 1988; Veith et al. 1983) and their incorporation in AOPs relies on grouping of chemicals based on structural and mechanistic information and quantitative data. Most widely accepted QSAR models are based on mode of action of a chemical. However, QSAR and chemical read-across approaches need to be considered along with the information across taxa that highlights species-specific similarities as well as differences. At lower levels of biological organization, QSAR approaches could help to predict KEs and contribute to AOP development (Hodges et al. 2018). Several online tools such as chemical databases (OECD toolbox (https://qsartoolbox.org/), PubChem (https://pubchem.ncbi.nlm.nih.gov/), Chemspider (http://www.chemspider.com/)) offer information to identify and utilize chemical similarity and biological activity towards the development of different components of AOPs. Other databases, such as DrugBank (https://www.drugbank.ca/), EcoDrug (http://www.ecodrug.org/) ToxCast (https://www.epa.gov/chemical-research/toxcast-chemicals), ChEMBL (https://www.ebi.ac.uk/chembl/), STITCH (http://stitch.embl.de/), are also available to assist with AOP development through identification of chemical initiators for a known MIE (Fay et al. 2017).
Using available toxicological information and the application of chemical- and taxa-read across, AOP development may undergo several phases (Villeneuve et al. 2014) as briefly summarized here. Based on biological plausibility derived from extrapolation of existing data and theoretical relevance, putative AOPs can be assembled using hypothesized KEs and KERs. Such efforts are key to highlighting data gaps and identifying research needs in AOP development. Empirical evidence and weight of evidence for a putative AOP are utilized to qualitatively assemble various components of the AOP describing how KEs can be measured. Such linkages define causal relationships between events at different levels of biological organisation, supported by documented empirical evidence, and contribute to the development of qualitative AOPs based on OECD guidelines (OECD 2013). Further development of quantitative linkages between KEs helps in identifying response relationships that add precision to the performance of risk assessment and regulatory decision-making (Perkins et al. 2019). Development of AOPs and their use at each phase must be guided by a research question and its relevance in predicting an adverse outcome. The predictive power and modular nature of AOPs are linked to their application across taxa and across chemical classes. Therefore, application of existing biomarker data available for multiple species and chemical categories provides opportunities to develop extensive AOP networks. As described in the previous sections, the bivalve literature offers biomarker information related to their filter-feeding mechanisms, conserved components of detoxification and immune function, and effects on multiple life stages. Bivalve biomarker data, when assembled in an AOP framework, can therefore contribute to the screening of chemicals for AOs as well as determining concentration-based relationships.
AOP DEVELOPMENT USING BIVALVES
This case study (Fay et al. 2017) is an example of the bottom-up approach (Villeneuve et al. 2014) using bivalve models and a chemical class that was applied towards the development of an AOP. Antidepressant pharmaceuticals, such as Fluoxetine, are widely prescribed and their low-concentration exposures to aquatic species raise concerns about their adverse effects in non-target organisms. Fluoxetine and many other antidepressant drugs are known to be SSRIs (discussed in the section titled Filter-feeding). The motivation behind this AOP development was to identify AOs in relevant model organisms and KEs that can assist in monitoring of measurable endpoints associated with SSRI exposures (Fay et al. 2017). Limited information is available on the biological activity of SSRIs in non-target organisms. However, using databases such DrugBank (https://www.drugbank.ca/), information on specific therapeutic targets and human protein-drug interactions can be obtained. Using the database, it was found that the therapeutic target of Fluoxetine in humans is the sodium-dependent serotonin transporter, 5-HTT. Given enough evolutionary conservation of 5-HTT across taxa, Fluoxetine was hypothesized to have effects in other species through its interactions with the common human target. To establish biological plausibility, SeqAPASS analysis was applied to confirm substantial conservation of 5-HTT across multiple aquatic taxa. The conservation was identified for the primary amino acid sequence and functional domain of the target and inhibition of this receptor was noted as the MIE. Further, a group of 40 additional drugs with similar inhibitory effects on 5-HTT was identified based on chemical read-across and only four drugs were eventually selected based on pharmacological activity at the therapeutic target and no off-target activity based on DrugBank information (Fay et al. 2017). Inhibitory effects on 5-HTT leads to inhibition of the reuptake of 5-HT (serotonin) resulting in elevated serotonin at neural junctions and subsequent increased serotonergic signaling. Based on information obtained from the USEPA’s database ECOTOX (https://cfpub.epa.gov/ecotox/), aquatic molluscs and crustaceans were identified to be uniquely sensitive to Fluoxetine (Fong and Ford 2014; Hazelton et al. 2014). This sensitivity was hypothesized to be extended to other similar chemical exposures based on whole-organism studies and due to sequence similarities and physiological factors. The development of these AOPs was therefore focused on investigations into the adverse effects of SSRIs on bivalves.
One of the key effects of increased serotonergic signaling in bivalves is release of muscle contraction state leading to opening of the valve (Cunha and Machado 2001; Muneoka and Twarog 1983). Along with depletion of energy, elevated active valve movement can result in water retention in the foot leading to foot detachment and increased susceptibility to predation (Cunha and Machado 2001; Rajagopal et al. 2005). Fluoxetine-exposed marine mussels have been shown to have reduced clearance rates, growth and gonadosomatic index (Peters and Granek 2016) highlighting the role of energy depletion in this AOP. In freshwater mussels, fluoxetine exposures have been linked to increased daytime movement that could result in increased susceptibility to predation due to higher visibility and energy depletion (Hazelton et al. 2014). Based on current literature and biological plausibility associated with the effects of serotonergic signaling on valve movement, retention of water in the foot and its detachment, increased locomotion and energy depletion, AOP 97 (https://aopwiki.org/aops/97) was developed. In this AOP, inhibition of 5-HTT (MIE) was linked to elevated risk to predation (AO) affecting survival and resulting in population-level effects.
Using the same MIE, other AOPs (AOP 195, 203, 204) were also developed and the AOs were identified as altered reproductive success (Fay et al. 2017). AOP 195 (https://aopwiki.org/aops/195) and 204 (https://aopwiki.org/aops/204) show an increase in reproductive success while AOP 203 (https://aopwiki.org/aops/203) reports a decline. Bivalve spawning and egg maturation are affected by serotonergic signaling and effects of 5-HTT inhibition on elevation of release of gametes have been reported in marine bivalves and freshwater dreissenids (Fong and Molnar 2008; Fong 1998). These increases in spawning are linked to serotonin-induced effects such as valve movement and ciliary action that increase water flow and gamete expulsion (Gibbons and Castagna 1984; Hirai et al. 1988; Ram et al. 1996) and could lead to increased reproductive success and population increase (AOP 195). Unlike broadcast spawners, unionid freshwater bivalves possess internal fertilization and parturition of obligate parasitic glochidia larvae. Parturition and detachment of glochidia prior to metamorphosis into the adult stage is documented to be under serotonergic control (Fong et al. 1998; Meechonkit et al. 2012) and is hypothesized to be related to increased valve movement and gill activity due to 5-HTT inhibition. While there are studies that suggest that SSRI exposures induced parturition of viable larvae (Cunha and Machado 2001; Fong et al. 1998) that could result in population increases (AOP 204), there is also evidence of release of immature and nonviable glochidia post parturition in SSRI-exposed mussels (Bringolf et al. 2010) that may be responsible for population decline (AOP 203). Another aspect that induces parturition in unionid bivalves is mantle display that is used as a lure for host fish and this behavior is seen to increase in mussels exposed to SSRIs (Bringolf et al. 2010; Hazelton et al. 2013). Excessive and poorly timed mantle display could lead to negative reproductive results due to inappropriate host selection as well as render the organism susceptible to predation (Hazelton et al. 2014). It must be noted that altered reproductive success is the AO in the AOP associated with induction of spawning and parturition. These population-level effects are important to consider in reference to timing of such reproductive success events from an ERA perspective. SSRI-induced untimely release of viable gametes could result in downstream challenges for population survival related to food availability (Fong and Molnar 2008) and other seasonal parameters. Further, premature release of gametes/larvae in unionids leading to decreased reproductive success and population declines is critical for conservation considerations for native bivalves, especially with the possibility of increased reproductive success of broadcast spawners such as invasive dreissenids (Fay et al. 2017).
The MIE describing inhibition of 5-HTT (https://aopwiki.org/events/619) as well as KE of increased serotonin (5-HT) (https://aopwiki.org/events/626) are common to the four AOPs described above. Further, these events are also in use for another AOP currently under development (https://aopwiki.org/aops/98). The utilization of 5-HTT inhibition as the MIE for AOP 98 is based on existing literature on molluscs (as discussed above), crustaceans and fish (Bossus et al. 2014; McDonald 2017). Such connections emphasize the utility of read-across approaches to develop information networks while maximizing application of existing literature and guiding future investigations to close data gaps.
FUTURE DIRECTIONS AND CHALLENGES
Bivalves are of special interest in human health and ecological risk assessment due to their ecological services, commercial value and far-reaching socio-economic impacts that could arise because of population-level effects of chemical exposures in aquatic ecosystems. At the molecular level, bivalves are valuable for identification and comparison of conserved targets across taxa as well as changes in diagnostic health markers. Further, at the population level, their relevance as model organisms is strengthened due to their contribution towards maintaining water and habitat quality as well as seafood value. Use of bivalve models in ecological and human health risk assessments with predictive frameworks such as AOPs (Figure 1) offers an efficient and cost-effective avenue for toxicological research and environmental management. Integration of information across taxa using evolutionary conserved targets of toxicological relevance to develop AOP networks is still in its early stages (Berggren et al. 2015; Fay et al. 2017; Hodges et al. 2018). Future efforts in predictive toxicology must also include developing quantitative relationships between causal events in the AOP framework and evaluating the magnitude or probability of an AO. Application of quantitative AOP approaches and mathematical modeling that identifies dose-response and/or response-response relationships will contribute towards better hazard predictions (Perkins et al. 2019). For ERA purposes, it is valuable to identify the chemical concentration required to trigger the MIE in an AOP. Such identification of the effective internal dose is dependent on toxicokinetic differences. Linkages that examine relationships between external hazardous exposure concentrations and internal dose therefore enhance AOP applicability in risk characterization of chemical exposures (Perkins et al. 2013; Perkins et al. 2019). Coupling quantitative AOP development with toxicokinetics models to consider chemical absorption and metabolism will allow us to characterize internal dose at the MIE. Further, these developments could also support and strengthen more efficient diagnostic use of biomarkers in future investigations aimed at detection of adverse effects of chemical exposures. It must be noted that extrapolations of chemical and biological information must consider differences in binding efficiency (based on sequence homology) and routes of exposures (waterborne exposures via gills in aquatic organisms versus inhalation via lungs in terrestrial organisms versus dietary exposures via gastrointestinal tract) as they affect dose-response relationships relevant to risk assessment.
Figure 1.
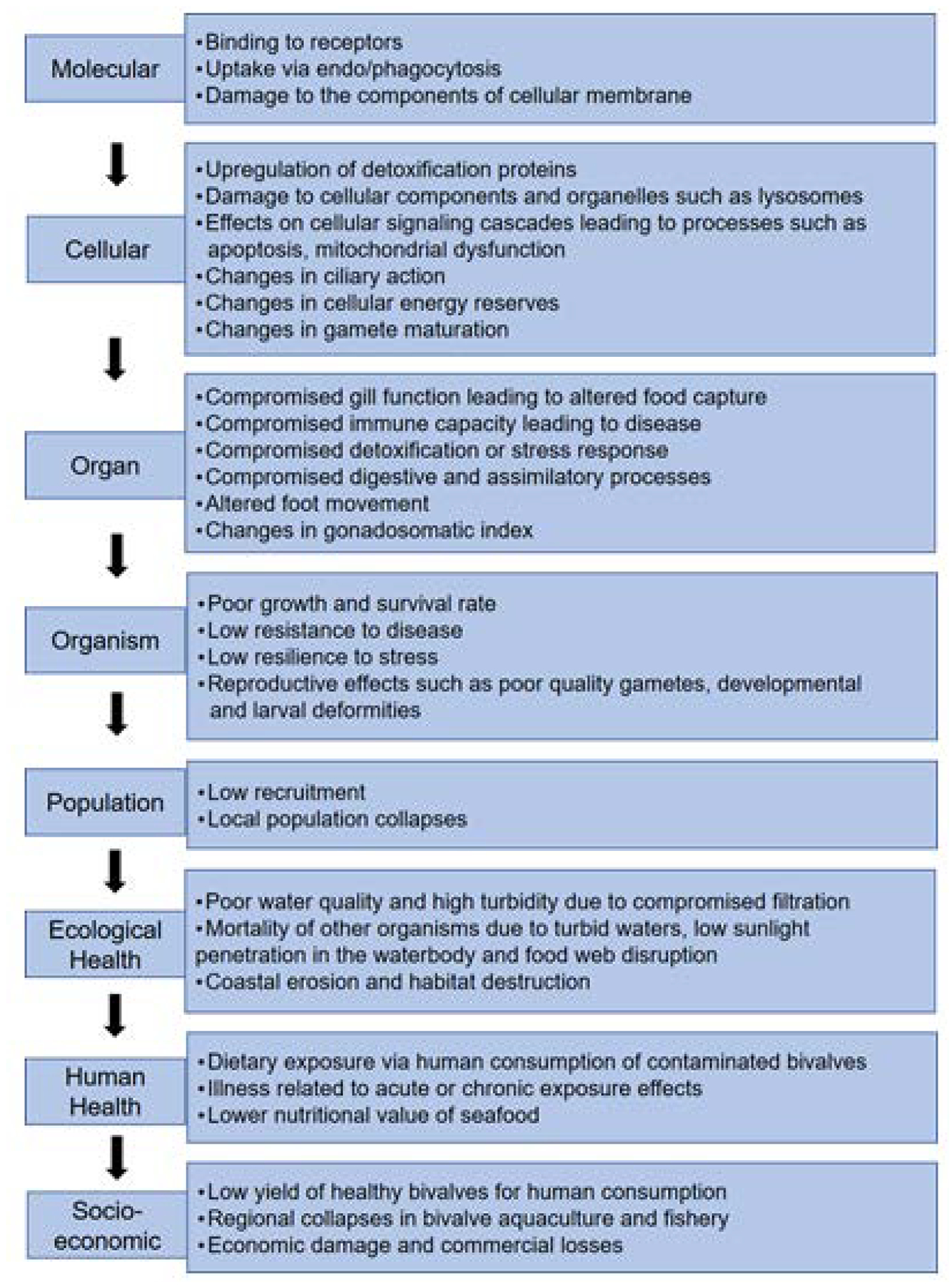
Conceptual diagram showing putative sequential effects of bivalve exposures to Contaminants of Emerging Concern (CECs). Using the Adverse Outcome Pathway (AOP) framework, initiating events at the molecular level can be connected to higher order effects at the organism and population levels which are relevant to risk assessment. Biomarker assessments typically identify molecular and cellular level effects. Higher order effects, at the level of organism and population, can result in ecological and human health impacts which, in turn, result in socio-economic consequences.
One of initial steps in predictive toxicology is extraction and organization of current biomarker literature to focus research efforts and identify information gaps. Existing biomarker data in bivalves could be utilized to identify KEs and postulate potential MIEs. Depending on data availability, a bottom-up approach (MIE to AO) or, in some cases, a top-down (AO to MIE) approach can be employed to construct an AOP. Another valuable approach is the middle-out AOP (Villeneuve et al. 2014) which utilizes intermediate KEs, such as events at the tissue- or organ-level, as anchors linking to an AO and traced back to a MIE. Identification of such relevant pieces of toxicological information from bivalve literature and their quantitative applications are key to cross-species extrapolation for risk assessment.
Use of invertebrate species to obtain information based on similarities in biological pathways is slowly evolving in human and ecological risk assessment (Hodges et al. 2018; Perkins et al. 2013). Therefore, biomarker approaches in bivalve models in an AOP context (Figure 1) using current mathematical models, omics techniques, and chemical databases are valuable tools in cumulative risk assessment, especially as CECs continue to rise in number. However, application of information across taxa must be weighed and developed with physiological and ecological considerations. As toxicological information is assembled across chemical classes and species, limitations and challenges associated with such approaches must be recognized. In many cases, conserved molecular targets may represent one putative mechanism of effect. Non-target organisms that appeared before mammals during evolution, such as bivalves, may possess multiple alternative mechanisms leading to significant, and sometimes unexpected, physiological outcomes. In order to understand toxicological responses for ecological assessments, research must also consider putative mechanisms of contaminant effects in addition to the conserved molecular targets and pathways. Additionally, it is essential to consider the variables that affect responses and are well characterized for human health studies such as overall health, age, metabolism, and reproductive status. It must also be noted that not every biomarker can be integrated into predictive assessments due to limited physiological relevance or applicability across taxa. High-throughput techniques could be instrumental in selection of relevant markers when using the read-across hypothesis. Application of invertebrate models in omics research and AOP approaches has been suggested for identification of pathway-level responses to contaminants across species (Hodges et al. 2018). Further, omics tools offer to maximize the amount of information obtained from one sample, which is important to minimize excessive use of resources in animal testing and to establish the diagnostic value of biomarkers.
Due to selective processes during filter-feeding in bivalves, special attention must be given to particle toxicity evaluations. Bivalve rejection of particles is based on size and numerous surface properties and, as recently demonstrated, uptake of particles doesn’t necessarily reflect ingestion and exposure (Ward et al. 2019). Such considerations are critical in prevention of over-estimation and misrepresentation of particle-based toxicity. Further, association of contaminants (dissolved and particulate) with food and other organic matter in the water column could affect uptake, stability, ingestion, and the toxicological potential of CECs. Another consideration is cellular compartmentalization of the contaminant, its stability upon entry and the effects of cellular processes on the toxicity of CECs. At the tissue level, accumulation of contaminants in different organs at different rates may result in different levels of susceptibility to adverse physiological implications. Dissolved contaminants may accumulate in bivalve gills or, if associated with food particles, can end up in gut and digestive glands leading to adverse physiological outcomes (Faggio et al. 2018; Rajalakshmi and Mohandas 2005). In sexually mature adults, contaminants could also be compartmentalized in lipid-rich gonadal regions and gametes. Such knowledge of tissue-specific contaminant accumulation and molecular assessments are imperative to development of AOPs using bivalve model systems.
One of the challenges in ecotoxicology is assessment of the cross-talk between stress and detoxification pathways and compensatory mechanisms. Integration of such mechanistic strategies by organisms under stress and understanding of their quantitative linkages are critical to predicting risks of chemical exposures. In bivalves, physiological adaptations employed to survive diel and seasonal environmental challenges may affect responses to low-level chemical stress for short periods. To this end, seasonal biomarker ranges and variability among populations must be established to accurately assess chemical effects for bivalve models. Further, species-specific sensitivity is a critical aspect of toxicology (Khan et al. 2018a; Pereira et al. 2011) and differences in responses can arise from baseline level differences in compensatory mechanisms and metabolic rates as well as changes in energy budgets. Bioenergetic endpoints and biomarkers associated with energy depletion and reallocation in bivalves exposed to contaminant stress have been documented in xenobiotic studies (Sokolova et al. 2012). Therefore, integration of models such as the Dynamic Energy Budget (DEB) approach (Kooijman and Kooijman 2000) into the AOP framework can provide linkages between energetic effects and apical/organismal endpoints such as growth, survival, and reproduction in bivalves (Goodchild et al. 2019). Such use of bioenergetic modeling with bivalves (Steeves et al. 2018) in conjunction with toxicity datasets (Jager and Selck 2011) can support development of improved predictive approaches in ecological management and risk assessment. DEB modeling describes how an organism assimilates and utilizes the available energy for processes such as growth, reproduction, maintenance and maturation. Within the DEB framework lies the concept of physiological mode of action which describes how a chemical interferes with energy assimilation and allocation and leads to adverse effects on life history processes (Hodges et al. 2018). It has been suggested that physiological mode of action and its variations across species and chemicals can strengthen the predictive toxicology network and provide linkages to higher levels of biological organization in AOPs (Ashauer and Jager 2018). DEB and AOP frameworks can inform and enhance each other’s predictive powers. Additionally, application of toxicokinetic modeling will provide connections between exposure concentrations and internal doses which must be further linked to organismal level effects as well as to DEB parameters (Murphy et al. 2018).
CONCLUDING REMARKS
It has been widely known and accepted that there is no “silver-bullet” in biomarker research for ERA (Lam 2009). Similarly, based on the current status of literature, there is no “perfect” model system in integrative risk assessment. However, with the goal of minimizing animal-testing and maximizing the efficient application of existing information and predictive toxicology, it is critical to identify model species important to ecological and human health considerations. Bivalves offer a model system that is useful in development of AOPs using integrative biomarker tools (Figure 1) with the help of emerging omics techniques, chemical databases and mathematical models. Bivalve toxicology offers an overlapping and dynamic knowledge base that provides building blocks towards AOP development. However, data gaps remain regarding inter- and intra-species baseline variabilities in biomarker assessments as well as long-term evaluation of trends in biomarkers induced by chemical effects. As more research studies provide toxicological datasets, use of conserved molecular targets in bivalve models would allow us to expand our qualitative and quantitative understanding of the impacts of CECs using the AOP framework. Grouping contaminants into chemical classes and molecular targets across species for development of information networks is a promising approach to keep up with the demands of 21st century toxicology. Despite the overlap in mechanistic findings and risks associated with CECs, currently, there is not adequate dialogue among biomedical researchers, and mammalian and environmental toxicologists. Multidisciplinary research efforts involving the utilization of toxicological evidence across research fields and sentinel model species, such as bivalves, are imperative to the improvement of human and ecological health.
Acknowledgements--
The authors would like to acknowledge L. Mills, D. Nacci and M. Cashman for their contributions towards the review of this manuscript. This manuscript was completed while B. Khan was an ORISE Research Participant at the US EPA, ORD/CEMM, Atlantic Coastal Environmental Sciences Division.
Footnotes
Publisher's Disclaimer: Disclaimer--The views expressed in this article are those of the authors and do not necessarily reflect the views or policies of the US Environmental Protection Agency (EPA). The present manuscript is number ORD-034853 of the Atlantic Coastal Environmental Sciences Division of the US EPA, Office of Research and Development (ORD), Center for Environmental Measurement and Modeling (CEMM).
Conflict of Interest Statement--The authors declare no conflict of interest.
Data availability—Data, associated metadata, and calculation tools are available from the corresponding author (khan.bushra@epa.gov).
REFERENCES
- Ackerman JD, Sim B, Nichols SJ, Claudi R. 1994. A review of the early life history of zebra mussels (dreissena polymorpha): Comparisons with marine bivalves. Canadian Journal of Zoology. 72(7):1169–1179. [Google Scholar]
- Aguirre-Martínez GV, DelValls TA, Martín-Díaz ML. 2016. General stress, detoxification pathways, neurotoxicity and genotoxicity evaluated in ruditapes philippinarum exposed to human pharmaceuticals. Ecotoxicology and Environmental Safety. 124:18–31. [DOI] [PubMed] [Google Scholar]
- Álvarez-Muñoz D, Rodríguez-Mozaz S, Maulvault A, Tediosi A, Fernández-Tejedor M, Van den Heuvel F, Kotterman M, Marques A, Barceló D. 2015. Occurrence of pharmaceuticals and endocrine disrupting compounds in macroalgaes, bivalves, and fish from coastal areas in europe. Environmental Research. 143:56–64. [DOI] [PubMed] [Google Scholar]
- Andral B, Stanisiere JY, Sauzade D, Damier E, Thebault H, Galgani F, Boissery P. 2004. Monitoring chemical contamination levels in the mediterranean based on the use of mussel caging. Marine Pollution Bulletin. 49(9–10):704–712. [DOI] [PubMed] [Google Scholar]
- Ankley GT, Bennett RS, Erickson RJ, Hoff DJ, Hornung MW, Johnson RD, Mount DR, Nichols JW, Russom CL, Schmieder PK. 2010. Adverse outcome pathways: A conceptual framework to support ecotoxicology research and risk assessment. Environmental Toxicology and Chemistry. 29(3):730–741. [DOI] [PubMed] [Google Scholar]
- Ashauer R, Jager T. 2018. Physiological modes of action across species and toxicants: The key to predictive ecotoxicology. Environmental Science: Processes & Impacts. 20(1):48–57. [DOI] [PubMed] [Google Scholar]
- Baker SM, Levinton JS. 2003. Selective feeding by three native north american freshwater mussels implies food competition with zebra mussels. Hydrobiologia. 505(1–3):97–105. [Google Scholar]
- Beliaeff B, Burgeot T. 2002. Integrated biomarker response: A useful tool for ecological risk assessment. Environmental Toxicology and Chemistry. 21(6):1316–1322. [PubMed] [Google Scholar]
- Berggren E, Amcoff P, Benigni R, Blackburn K, Carney E, Cronin M, Deluyker H, Gautier F, Judson RS, Kass GE. 2015. Chemical safety assessment using read-across: Assessing the use of novel testing methods to strengthen the evidence base for decision making. Environmental health perspectives. 123(12):1232–1240. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Besse J-P, Geffard O, Coquery M. 2012. Relevance and applicability of active biomonitoring in continental waters under the water framework directive. TrAC Trends in Analytical Chemistry. 36:113–127. [Google Scholar]
- Bolognesi C, Cirillo S. 2014. Genotoxicity biomarkers in aquatic bioindicators. Current Zoology. 60(2):273–284. [Google Scholar]
- Bonnafé E, Sroda S, Budzinski H, Valière A, Pedelluc J, Marty P, Geret F. 2015. Responses of cytochrome p450, gst, and mxr in the mollusk corbicula fluminea to the exposure to hospital wastewater effluents. Environmental Science and Pollution Research. 22(14):11033–11046. [DOI] [PubMed] [Google Scholar]
- Bossus MC, Guler YZ, Short SJ, Morrison ER, Ford AT. 2014. Behavioural and transcriptional changes in the amphipod echinogammarus marinus exposed to two antidepressants, fluoxetine and sertraline. Aquatic Toxicology. 151:46–56. [DOI] [PubMed] [Google Scholar]
- Bringolf RB, Heltsley RM, Newton TJ, Eads CB, Fraley SJ, Shea D, Cope WG. 2010. Environmental occurrence and reproductive effects of the pharmaceutical fluoxetine in native freshwater mussels. Environmental Toxicology and Chemistry. 29(6):1311–1318. [DOI] [PubMed] [Google Scholar]
- Brockmeier EK, Hodges G, Hutchinson TH, Butler E, Hecker M, Tollefsen KE, Garcia-Reyero N, Kille P, Becker D, Chipman K et al. 2017. The role of omics in the application of adverse outcome pathways for chemical risk assessment. Toxicological Sciences. 158(2):252–262. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Byrne PA, O’Halloran J. 2001. The role of bivalve molluscs as tools in estuarine sediment toxicity testing: A review. Hydrobiologia. 465(1–3):209–217. [Google Scholar]
- Calamari D, Vighi M. 1988. Experiences on qsars and evaluative models in ecotoxicology. Chemosphere. 17(8):1539–1549. [Google Scholar]
- Campos A, Tedesco S, Vasconcelos V, Cristobal S. 2012. Proteomic research in bivalves: Towards the identification of molecular markers of aquatic pollution. Journal of Proteomics. 75(14):4346–4359. [DOI] [PubMed] [Google Scholar]
- Canesi L, Betti M, Ciacci C, Lorusso L, Pruzzo C, Gallo G. 2006. Cell signalling in the immune response of mussel hemocytes. Invertebrate Survival Journal. 3:40–49. [Google Scholar]
- Canesi L, Ciacci C, Betti M, Fabbri R, Canonico B, Fantinati A, Marcomini A, Pojana G. 2008. Immunotoxicity of carbon black nanoparticles to blue mussel hemocytes. Environment International. 34(8):1114–1119. [DOI] [PubMed] [Google Scholar]
- Canesi L, Ciacci C, Fabbri R, Marcomini A, Pojana G, Gallo G. 2012. Bivalve molluscs as a unique target group for nanoparticle toxicity. Marine Environmental Research. 76:16–21. [DOI] [PubMed] [Google Scholar]
- Canesi L, Fabbri R, Gallo G, Vallotto D, Marcomini A, Pojana G. 2010. Biomarkers in mytilus galloprovincialis exposed to suspensions of selected nanoparticles (nano carbon black, c60 fullerene, nano-tio2, nano-sio2). Aquatic Toxicology. 100(2):168–177. [DOI] [PubMed] [Google Scholar]
- Canesi L, Gallo G, Gavioli M, Pruzzo C. 2002. Bacteria-hemocyte interactions and phagocytosis in marine bivalves. Microscopy Research and Technique. 57(6):469–476. [DOI] [PubMed] [Google Scholar]
- Canesi L, Lorusso L, Ciacci C, Betti M, Regoli F, Poiana G, Gallo G, Marcomini A. 2007. Effects of blood lipid lowering pharmaceuticals (bezafibrate and gemfibrozil) on immune and digestive gland functions of the bivalve mollusc, mytilus galloprovincialis. Chemosphere. 69(6):994–1002. [DOI] [PubMed] [Google Scholar]
- Cappello T, Mauceri A, Corsaro C, Maisano M, Parrino V, Lo Paro G, Messina G, Fasulo S. 2013. Impact of environmental pollution on caged mussels mytilus galloprovincialis using nmr-based metabolomics. Marine Pollution Bulletin. 77(1):132–139. [DOI] [PubMed] [Google Scholar]
- Carroll MA, Catapane EJ. 2007. The nervous system control of lateral ciliary activity of the gill of the bivalve mollusc, crassostrea virginica. Comparative Biochemistry and Physiology Part A: Molecular & Integrative Physiology. 148(2):445–450. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Casas S, Bacher C. 2006. Modelling trace metal (hg and pb) bioaccumulation in the mediterranean mussel, mytilus galloprovincialis, applied to environmental monitoring. Journal of Sea Research. 56(2):168–181. [Google Scholar]
- Ciacci C, Barmo C, Gallo G, Maisano M, Cappello T, D’Agata A, Leonzio C, Mauceri A, Fasulo S, Canesi L. 2012. Effects of sublethal, environmentally relevant concentrations of hexavalent chromium in the gills of mytilus galloprovincialis. Aquatic Toxicology. 120–121:109–118. [DOI] [PubMed] [Google Scholar]
- Ciocan CM, Cubero-Leon E, Puinean AM, Hill EM, Minier C, Osada M, Fenlon K, Rotchell JM. 2010. Effects of estrogen exposure in mussels, mytilus edulis, at different stages of gametogenesis. Environmental Pollution. 158(9):2977–2984. [DOI] [PubMed] [Google Scholar]
- Contardo-Jara V, Pflugmacher S, Nützmann G, Kloas W, Wiegand C. 2010. The β-receptor blocker metoprolol alters detoxification processes in the non-target organism dreissena polymorpha. Environmental pollution. 158(6):2059–2066. [DOI] [PubMed] [Google Scholar]
- Cooper EL, Kvell K, Engelmann P, Nemeth P. 2006. Still waiting for the toll? Immunology Letters. 104(1):18–28. [DOI] [PubMed] [Google Scholar]
- Cope WG, Bringolf RB, Buchwalter DB, Newton TJ, Ingersoll CG, Wang N, Augspurger T, Dwyer FJ, Barnhart MC, Neves RJ. 2008. Differential exposure, duration, and sensitivity of unionoidean bivalve life stages to environmental contaminants. Journal of the North American Benthological Society. 27(2):451–462. [Google Scholar]
- Cossu C, Doyotte A, Babut M, Exinger A, Vasseur P. 2000. Antioxidant biomarkers in freshwater bivalves, unio tumidus, in response to different contamination profiles of aquatic sediments. Ecotoxicology and Environmental Safety. 45(2):106–121. [DOI] [PubMed] [Google Scholar]
- Crewe H, Lennard M, Tucker G, Woods F, Haddock R. 1992. The effect of selective serotonin re-uptake inhibitors on cytochrome p4502d6 (cyp2d6) activity in human liver microsomes. British journal of clinical pharmacology. 34(3):262–265. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cunha E, Machado J. 2001. Parturition in anodonta cygnea induced by selective serotonin reuptake inhibitors (ssris). Canadian Journal of Zoology. 79(1):95–100. [Google Scholar]
- Dagnino A, Allen J, Moore M, Broeg K, Canesi L, Viarengo A. 2007a. Development of an expert system for the integration of biomarker responses in mussels into an animal health index. Biomarkers. 12(2):155–172. [DOI] [PubMed] [Google Scholar]
- Dagnino A, Allen JI, Moore MN, Broeg K, Canesi L, Viarengo A. 2007b. Development of an expert system for the integration of biomarker responses in mussels into an animal health index. Biomarkers. 12(2):155–172. [DOI] [PubMed] [Google Scholar]
- Damiens G, Gnassia-Barelli M, Loquès F, Roméo M, Salbert V. 2007. Integrated biomarker response index as a useful tool for environmental assessment evaluated using transplanted mussels. Chemosphere. 66(3):574–583. [DOI] [PubMed] [Google Scholar]
- Damiens G, His E, Gnassia-Barelli M, Quiniou F, Roméo M. 2004. Evaluation of biomarkers in oyster larvae in natural and polluted conditions. Comparative Biochemistry and Physiology Part C: Toxicology & Pharmacology. 138(2):121–128. [DOI] [PubMed] [Google Scholar]
- DeCaprio AP. 1997. Biomarkers: Coming of age for environmental health and risk assessment. Environmental Science & Technology. 31(7):1837–1848. [Google Scholar]
- Di Toro DM, Zarba CS, Hansen DJ, Berry WJ, Swartz RC, Cowan CE, Pavlou SP, Allen HE, Thomas NA, Paquin PR. 1991. Technical basis for establishing sediment quality criteria for nonionic organic chemicals using equilibrium partitioning. Environmental Toxicology and Chemistry: An International Journal. 10(12):1541–1583. [Google Scholar]
- Dodder NG, Maruya KA, Lee Ferguson P, Grace R, Klosterhaus S, La Guardia MJ, Lauenstein GG, Ramirez J. 2014. Occurrence of contaminants of emerging concern in mussels (mytilus spp.) along the california coast and the influence of land use, storm water discharge, and treated wastewater effluent. Marine Pollution Bulletin. 81(2):340–346. [DOI] [PubMed] [Google Scholar]
- Edge KJ, Johnston EL, Roach AC, Ringwood AH. 2012. Indicators of environmental stress: Cellular biomarkers and reproductive responses in the sydney rock oyster (saccostrea glomerata). Ecotoxicology. 21(5):1415–1425. [DOI] [PubMed] [Google Scholar]
- Estévez-Calvar N, Canesi L, Montagna M, Faimali M, Piazza V, Garaventa F. 2017. Adverse effects of the ssri antidepressant sertraline on early life stages of marine invertebrates. Marine Environmental Research. 128:88–97. [DOI] [PubMed] [Google Scholar]
- Fabbri E, Franzellitti S, Capuzzo A. 2009. Effects of the beta-blocker propranolol on health status and gene regulation in the mussel, mytilus galloprovincialis. Comparative Biochemistry and Physiology. 154(1):S13–S22. [Google Scholar]
- Fabbri R, Montagna M, Balbi T, Raffo E, Palumbo F, Canesi L. 2014. Adaptation of the bivalve embryotoxicity assay for the high throughput screening of emerging contaminants in mytilus galloprovincialis. Marine Environmental Research. 99:1–8. [DOI] [PubMed] [Google Scholar]
- Faggio C, Tsarpali V, Dailianis S. 2018. Mussel digestive gland as a model tissue for assessing xenobiotics: An overview. Science of the Total Environment. 636:220–229. [DOI] [PubMed] [Google Scholar]
- Farrington JW, Tripp BW, Tanabe S, Subramanian A, Sericano JL, Wade TL, Knap AH. 2016. Edward d. Goldberg’s proposal of “the mussel watch”: Reflections after 40years. Marine Pollution Bulletin. 110(1):501–510. [DOI] [PubMed] [Google Scholar]
- Fay KA, Villeneuve DL, LaLone CA, Song Y, Tollefsen KE, Ankley GT. 2017. Practical approaches to adverse outcome pathway (aop) development and weight of evidence evaluation as illustrated by ecotoxicological case studies. Environmental Toxicology and Chemistry. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fernández Robledo JA, Yadavalli R, Allam B, Pales Espinosa E, Gerdol M, Greco S, Stevick RJ, Gómez-Chiarri M, Zhang Y, Heil CA et al. 2019. From the raw bar to the bench: Bivalves as models for human health. Developmental & Comparative Immunology. 92:260–282. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fong P, Molnar N. 2008. Norfluoxetine induces spawning and parturition in estuarine and freshwater bivalves. Bulletin of Environmental Contamination and Toxicology. 81(6):535–538. [DOI] [PubMed] [Google Scholar]
- Fong PP. 1998. Zebra mussel spawning is induced in low concentrations of putative serotonin reuptake inhibitors. The biological bulletin. 194(2):143–149. [DOI] [PubMed] [Google Scholar]
- Fong PP, Ford AT. 2014. The biological effects of antidepressants on the molluscs and crustaceans: A review. Aquatic Toxicology. 151:4–13. [DOI] [PubMed] [Google Scholar]
- Fong PP, Huminski PT, D’Urso LM. 1998. Induction and potentiation of parturition in fingernail clams (sphaerium striatinum) by selective serotonin re-uptake inhibitors (ssris). Journal of Experimental Zoology. 280(3):260–264. [DOI] [PubMed] [Google Scholar]
- Ford AT, Fong PP. 2016. The effects of antidepressants appear to be rapid and at environmentally relevant concentrations. Environmental toxicology and chemistry. 35(4):794–798. [DOI] [PubMed] [Google Scholar]
- Fossi Tankoua O, Buffet PE, Amiard JC, Berthet B, Mouneyrac C, Amiard-Triquet C. 2013. Integrated assessment of estuarine sediment quality based on a multi-biomarker approach in the bivalve scrobicularia plana. Ecotoxicology and Environmental Safety. 88:117–125. [DOI] [PubMed] [Google Scholar]
- Franzellitti S, Buratti S, Capolupo M, Du B, Haddad SP, Chambliss CK, Brooks BW, Fabbri E. 2014. An exploratory investigation of various modes of action and potential adverse outcomes of fluoxetine in marine mussels. Aquatic Toxicology. 151:14–26. [DOI] [PubMed] [Google Scholar]
- Franzellitti S, Buratti S, Du B, Haddad SP, Chambliss CK, Brooks BW, Fabbri E. 2015. A multibiomarker approach to explore interactive effects of propranolol and fluoxetine in marine mussels. Environmental Pollution. 205:60–69. [DOI] [PubMed] [Google Scholar]
- Franzellitti S, Buratti S, Valbonesi P, Capuzzo A, Fabbri E. 2011. The β-blocker propranolol affects camp-dependent signaling and induces the stress response in mediterranean mussels, mytilus galloprovincialis. Aquatic Toxicology. 101(2):299–308. [DOI] [PubMed] [Google Scholar]
- Freitas R, Almeida Â, Pires A, Velez C, Calisto V, Schneider RJ, Esteves VI, Wrona FJ, Figueira E, Soares AMVM. 2015. The effects of carbamazepine on macroinvertebrate species: Comparing bivalves and polychaetes biochemical responses. Water Research. 85:137–147. [DOI] [PubMed] [Google Scholar]
- Galloway TS. 2006. Biomarkers in environmental and human health risk assessment. Marine Pollution Bulletin. 53(10):606–613. [DOI] [PubMed] [Google Scholar]
- Gaw S, Thomas KV, Hutchinson TH. 2014. Sources, impacts and trends of pharmaceuticals in the marine and coastal environment. Phil Trans R Soc B. 369(1656):20130572. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gibbons M, Castagna M. 1984. Serotonin as an inducer of spawning in six bivalve species. Aquaculture. 40(2):189–191. [Google Scholar]
- Goldberg ED. 1986. The mussel watch concept. Environmental Monitoring and Assessment. 7(1):91–103. [DOI] [PubMed] [Google Scholar]
- Goodchild CG, Simpson AM, Minghetti M, DuRant SE. 2019. Bioenergetics-adverse outcome pathway: Linking organismal and suborganismal energetic endpoints to adverse outcomes. Environmental Toxicology and Chemistry. 38(1):27–45. [DOI] [PubMed] [Google Scholar]
- Gregory MA, Marshall DJ, George RC, Anandraj A, McClurg TP. 2002. Correlations between metal uptake in the soft tissue of perna perna and gill filament pathology after exposure to mercury. Marine Pollution Bulletin. 45(1):114–125. [DOI] [PubMed] [Google Scholar]
- Griscom SB, Fisher NS. 2004. Bioavailability of sediment-bound metals to marine bivalve molluscs: An overview. Estuaries. 27(5):826–838. [Google Scholar]
- Guidi P, Frenzilli G, Benedetti M, Bernardeschi M, Falleni A, Fattorini D, Regoli F, Scarcelli V, Nigro M. 2010. Antioxidant, genotoxic and lysosomal biomarkers in the freshwater bivalve (unio pictorum) transplanted in a metal polluted river basin. Aquatic Toxicology. 100(1):75–83. [DOI] [PubMed] [Google Scholar]
- Gustafson LL, Stoskopf MK, Bogan AE, Showers W, Kwak TJ, Hanlon S, Levine JF. 2005. Evaluation of a nonlethal technique for hemolymph collection in elliptio complanata, a freshwater bivalve (mollusca: Unionidae). Diseases of aquatic organisms. 65(2):159–165. [DOI] [PubMed] [Google Scholar]
- Hamza-Chaffai A 2014. Usefulness of bioindicators and biomarkers in pollution biomonitoring. International Journal of Biotechnology for Wellness Industries. 3(1):19–26. [Google Scholar]
- Hazelton PD, Cope WG, Mosher S, Pandolfo TJ, Belden JB, Barnhart MC, Bringolf RB. 2013. Fluoxetine alters adult freshwater mussel behavior and larval metamorphosis. Science of the total environment. 445:94–100. [DOI] [PubMed] [Google Scholar]
- Hazelton PD, Du B, Haddad SP, Fritts AK, Chambliss CK, Brooks BW, Bringolf RB. 2014. Chronic fluoxetine exposure alters movement and burrowing in adult freshwater mussels. Aquatic toxicology. 151:27–35. [DOI] [PubMed] [Google Scholar]
- Hédouin L, Pringault O, Bustamante P, Fichez R, Warnau M. 2011. Validation of two tropical marine bivalves as bioindicators of mining contamination in the new caledonia lagoon: Field transplantation experiments. Water research. 45(2):483–496. [DOI] [PubMed] [Google Scholar]
- Hirai S, Kishimoto T, Kadam A, Kanatani H, Koide S. 1988. Induction of spawning and oocyte maturation by 5-hydroxytryptamine in the surf clam. Journal of Experimental Zoology. 245(3):318–321. [Google Scholar]
- Hodges G, Gutsell S, Taylor N, Brockmeier E, Butler E, Rendal C, Colbourne J. 2018. Invertebrate model species in aop development A systems biology approach to advancing adverse outcome pathways for risk assessment. Springer; p. 75–106. [Google Scholar]
- Hotze EM, Phenrat T, Lowry GV. 2010. Nanoparticle aggregation: Challenges to understanding transport and reactivity in the environment. Journal of Environmental Quality. 39(6):1909–1924. [DOI] [PubMed] [Google Scholar]
- Huggett D, Brooks B, Peterson B, Foran C, Schlenk D. 2002. Toxicity of select beta adrenergic receptor-blocking pharmaceuticals (b-blockers) on aquatic organisms. Archives of Environmental Contamination and Toxicology. 43(2):229–235. [DOI] [PubMed] [Google Scholar]
- Jager T, Selck H. 2011. Interpreting toxicity data in a deb framework: A case study for nonylphenol in the marine polychaete capitella teleta. Journal of Sea Research. 66(4):456–462. [Google Scholar]
- Jones H 1983. The circulatory systems of gastropods and bivalves. The Mollusca: Physiology, Part 2 5:189–238. [Google Scholar]
- Khan B, Burgess RM, Fogg SA, Cantwell MG, Katz DR, Ho KT. 2018a. Cellular responses to in vitro exposures to β-blocking pharmaceuticals in hard clams and eastern oysters. Chemosphere. 211:360–370. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Khan B, Clinton SM, Hamp TJ, Oliver JD, Ringwood AH. 2018b. Potential impacts of hypoxia and a warming ocean on oyster microbiomes. Marine Environmental Research. 139:27–34. [DOI] [PubMed] [Google Scholar]
- Kiorboe T, Mohlenberg F. 1981. Particle selection in suspension-feeding bivalves. Mar Ecol Prog Ser. 5(3):291–296. [Google Scholar]
- Kooijman SALM, Kooijman SALM. 2000. Dynamic energy and mass budgets in biological systems. Cambridge university press. [Google Scholar]
- LaLone CA, Villeneuve DL, Lyons D, Helgen HW, Robinson SL, Swintek JA, Saari TW, Ankley GT. 2016. Editor’s highlight: Sequence alignment to predict across species susceptibility (seqapass): A web-based tool for addressing the challenges of cross-species extrapolation of chemical toxicity. Toxicological Sciences. 153(2):228–245. [DOI] [PubMed] [Google Scholar]
- Lam PKS. 2009. Use of biomarkers in environmental monitoring. Ocean & Coastal Management. 52(7):348–354. [Google Scholar]
- Lee JW, Won E-J, Raisuddin S, Lee J-S. 2015. Significance of adverse outcome pathways in biomarker-based environmental risk assessment in aquatic organisms. Journal of Environmental Sciences. 35:115–127. [DOI] [PubMed] [Google Scholar]
- Litman T, Druley TE, Stein WD, Bates SE. 2001. From mdr to mxr: New understanding of multidrug resistance systems, their properties and clinical significance. Cellular and Molecular Life Sciences CMLS. 58(7):931–959. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Margulis BA, Antropova OY, Kharazova AD. 1989. 70 kda heat shock proteins from mollusc and human cells have common structural and functional domains. Comparative Biochemistry and Physiology Part B: Comparative Biochemistry. 94(4):621–623. [DOI] [PubMed] [Google Scholar]
- McDonald MD. 2017. An aop analysis of selective serotonin reuptake inhibitors (ssris) for fish. Comparative Biochemistry and Physiology Part C: Toxicology & Pharmacology. 197:19–31. [DOI] [PubMed] [Google Scholar]
- McEneff G, Barron L, Kelleher B, Paull B, Quinn B. 2014. A year-long study of the spatial occurrence and relative distribution of pharmaceutical residues in sewage effluent, receiving marine waters and marine bivalves. Science of the Total Environment. 476–477:317–326. [DOI] [PubMed] [Google Scholar]
- Meechonkit P, Asuvapongpatana S, Jumromn W, Kovitvadhi U, Weerachatyanukul W. 2012. Sexual differences in serotonin distribution and induction of synchronous larval release by serotonin in the freshwater mussel hyriopsis bialatus. Journal of Molluscan Studies. 78(3):297–303. [Google Scholar]
- Moore M 2006. Do nanoparticles present ecotoxicological risks for the health of the aquatic environment? Environment International. 32(8):967–976. [DOI] [PubMed] [Google Scholar]
- Moore MN, Allen JI, McVeigh A, Shaw J. 2006. Lysosomal and autophagic reactions as predictive indicators of environmental impact in aquatic animals. Autophagy. 2(3):217–220. [DOI] [PubMed] [Google Scholar]
- Muneoka Y, Twarog BM. 1983. Neuromuscular transmission and excitation-contraction coupling in molluscan muscle The mollusca. Elsevier; p. 35–76. [Google Scholar]
- Munns WR, Suter II GW, Damstra T, Kroes R, Reiter LW, Marafante E. 2003. Integrated risk assessment-results from an international workshop. Human and Ecological Risk Assessment. 9(1):379–386. [Google Scholar]
- Murgarella M, Puiu D, Novoa B, Figueras A, Posada D, Canchaya C. 2016. A first insight into the genome of the filter-feeder mussel mytilus galloprovincialis. PLoS One. 11(3):e0151561. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Murphy CA, Nisbet RM, Antczak P, Garcia-Reyero N, Gergs A, Lika K, Mathews T, Muller EB, Nacci D, Peace A et al. 2018. Linking adverse outcome pathways to dynamic energy budgets: A conceptual model In: Garcia-Reyero N, Murphy CA, editors. A systems biology approach to advancing adverse outcome pathways for risk assessment. Cham: Springer International Publishing; p. 281–302. [Google Scholar]
- Nasci C, Nesto N, Monteduro R, Da Ros L. 2002. Field application of biochemical markers and a physiological index in the mussel, mytilus galloprovincialis: Transplantation and biomonitoring studies in the lagoon of venice (ne italy). Marine environmental research. 54(3–5):811–816. [DOI] [PubMed] [Google Scholar]
- Nicholson S, Lam P. 2005. Pollution monitoring in southeast asia using biomarkers in the mytilid mussel perna viridis (mytilidae: Bivalvia). Environment International. 31(1):121–132. [DOI] [PubMed] [Google Scholar]
- Nogueira LS, Wood CM, Gillis PL, Bianchini A. 2013. Isolation and fractionation of gill cells from freshwater (lasmigona costata) and seawater (mesodesma mactroides) bivalves for use in toxicological studies with copper. Cytotechnology. 65(5):773–783. [DOI] [PMC free article] [PubMed] [Google Scholar]
- O’Connor TP. 2002. National distribution of chemical concentrations in mussels and oysters in the USA. Marine Environmental Research. 53(2):117–143. [DOI] [PubMed] [Google Scholar]
- OECD O. 2013. Guidance document on developing and assessing adverse outcome pathways.
- Oehlmann J, Schulte-Oehlmann U. 2003. Trace metals and other contaminants in the environment Molluscs as bioindicators. Elsevier. [Google Scholar]
- Pain S, Parant M. 2007. Identification of multixenobiotic defence mechanism (mxr) background activities in the freshwater bivalve dreissena polymorpha as reference values for its use as biomarker in contaminated ecosystems. Chemosphere. 67(6):1258–1263. [DOI] [PubMed] [Google Scholar]
- Parolini M, Pedriali A, Binelli A. 2013. Application of a biomarker response index for ranking the toxicity of five pharmaceutical and personal care products (ppcps) to the bivalve dreissena polymorpha. Archives of Environmental Contamination and Toxicology. 64(3):439–447. [DOI] [PubMed] [Google Scholar]
- Pereira SM, Fernández-Tajes J, Rábade T, Flórez-Barrós F, Laffon B, Méndez J. 2011. Comparison between two bivalve species as tools for the assessment of pollution levels in an estuarian environment. Journal of Toxicology and Environmental Health, Part A. 74(15–16):1020–1029. [DOI] [PubMed] [Google Scholar]
- Perkins EJ, Ankley GT, Crofton KM, Garcia-Reyero N, LaLone CA, Johnson MS, Tietge JE, Villeneuve DL. 2013. Current perspectives on the use of alternative species in human health and ecological hazard assessments. Environmental health perspectives. 121(9):1002–1010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Perkins EJ, Ashauer R, Burgoon L, Conolly R, Landesmann B, Mackay C, Murphy CA, Pollesch N, Wheeler JR, Zupanic A et al. 2019. Building and applying quantitative adverse outcome pathway models for chemical hazard and risk assessment. Environmental Toxicology and Chemistry. 38(9):1850–1865. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Peters JR, Granek EF. 2016. Long-term exposure to fluoxetine reduces growth and reproductive potential in the dominant rocky intertidal mussel, mytilus californianus. Science of the Total Environment. 545:621–628. [DOI] [PubMed] [Google Scholar]
- Pytharopoulou S, Sazakli E, Grintzalis K, Georgiou CD, Leotsinidis M, Kalpaxis DL. 2008. Translational responses of mytilus galloprovincialis to environmental pollution: Integrating the responses to oxidative stress and other biomarker responses into a general stress index. Aquatic Toxicology. 89(1):18–27. [DOI] [PubMed] [Google Scholar]
- Rajagopal S, Van der Velde G, Van der Gaag M, Jenner H. 2005. Byssal detachment underestimates tolerance of mussels to toxic compounds. Marine Pollution Bulletin. 50(1):20–29. [DOI] [PubMed] [Google Scholar]
- Rajalakshmi S, Mohandas A. 2005. Copper-induced changes in tissue enzyme activity in a freshwater mussel. Ecotoxicology and Environmental Safety. 62(1):140–143. [DOI] [PubMed] [Google Scholar]
- Ram JL, Fong PP, Garton DW. 1996. Physiological aspects of zebra mussel reproduction: Maturation, spawning, and fertilization. American zoologist. 36(3):326–338. [Google Scholar]
- Rand-Weaver M, Margiotta-Casaluci L, Patel A, Panter GH, Owen SF, Sumpter JP. 2013. The read-across hypothesis and environmental risk assessment of pharmaceuticals. Environmental Science & Technology. 47:11384–11395. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Regoli F, Pellegrini D, Cicero AM, Nigro M, Benedetti M, Gorbi S, Fattorini D, D’Errico G, Di Carlo M, Nardi A et al. 2014. A multidisciplinary weight of evidence approach for environmental risk assessment at the costa concordia wreck: Integrative indices from mussel watch. Marine Environmental Research. 96:92–104. [DOI] [PubMed] [Google Scholar]
- Riisgård HU. 1988. Efficiency of particle retention and filtration rate in 6 species of northeast american bivalves. Marine Ecology Progress Series. 45(3):217–223. [Google Scholar]
- Ringwood A, Hameedi M, Lee R, Brouwer M, Peters E, Scott G, Luoma S, Digiulio R. 1999a. Bivalve biomarker workshop: Overview and discussion group summaries. Biomarkers. 4(6):391–399. [DOI] [PubMed] [Google Scholar]
- Ringwood A, Hoguet J, Keppler C, Gielazyn M. 2004. Linkages between cellular biomarker responses and reproductive success in oysters-crassostrea virginica. Marine environmental research. 58(2–5):151–155. [DOI] [PubMed] [Google Scholar]
- Ringwood AH, Conners DE. 2000. The effects of glutathione depletion on reproductive success in oysters, crassostrea virginica. Marine Environmental Research. 50(1):207–211. [DOI] [PubMed] [Google Scholar]
- Ringwood AH, Conners DE, Keppler CJ, Dinovo AA. 1999b. Biomarker studies with juvenile oysters (crassostrea virginica) deployed in-situ. Biomarkers. 4(6):400–414. [DOI] [PubMed] [Google Scholar]
- Rittschof D, McClellan-Green P. 2005. Molluscs as multidisciplinary models in environment toxicology. Marine pollution bulletin. 50(4):369–373. [DOI] [PubMed] [Google Scholar]
- Rocha TL, Gomes T, Sousa VS, Mestre NC, Bebianno MJ. 2015. Ecotoxicological impact of engineered nanomaterials in bivalve molluscs: An overview. Marine Environmental Research. 111:74–88. [DOI] [PubMed] [Google Scholar]
- Sauvé S, Brousseau P, Pellerin J, Morin Y, Senécal L, Goudreau P, Fournier M. 2002. Phagocytic activity of marine and freshwater bivalves: In vitro exposure of hemocytes to metals (ag, cd, hg and zn). Aquatic Toxicology. 58(3):189–200. [DOI] [PubMed] [Google Scholar]
- Sericano JL, Wade TL, Brooks JM. 1996. Accumulation and depuration of organic contaminants by the american oyster (crassostrea virginica). Science of the Total Environment. 179:149–160. [Google Scholar]
- Silva LJG, Lino CM, Meisel LM, Pena A. 2012. Selective serotonin re-uptake inhibitors (ssris) in the aquatic environment: An ecopharmacovigilance approach. Science of The Total Environment. 437:185–195. [DOI] [PubMed] [Google Scholar]
- Smital T, Luckenbach T, Sauerborn R, Hamdoun AM, Vega RL, Epel D. 2004. Emerging contaminants-pesticides, ppcps, microbial degradation products and natural substances as inhibitors of multixenobiotic defense in aquatic organisms. Mutation Research/Fundamental and Molecular Mechanisms of Mutagenesis. 552(1–2):101–117. [DOI] [PubMed] [Google Scholar]
- Sokolova IM, Frederich M, Bagwe R, Lannig G, Sukhotin AA. 2012. Energy homeostasis as an integrative tool for assessing limits of environmental stress tolerance in aquatic invertebrates. Marine environmental research. 79:1–15. [DOI] [PubMed] [Google Scholar]
- Steeves LE, Filgueira R, Guyondet T, Chassé J, Comeau L. 2018. Past, present, and future: Performance of two bivalve species under changing environmental conditions. Frontiers in Marine Science. 5(184). [Google Scholar]
- Stuard SB, Heinonen T. 2018. Relevance and application of read-across - mini review of european consensus platform for alternatives and scandinavian society for cell toxicology 2017 workshop session. Basic & Clinical Pharmacology & Toxicology. 123:37–41. [DOI] [PubMed] [Google Scholar]
- Suárez-Ulloa V, Fernández-Tajes J, Manfrin C, Gerdol M, Venier P, Eirín-López J. 2013. Bivalve omics: State of the art and potential applications for the biomonitoring of harmful marine compounds. Marine drugs. 11(11):4370–4389. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Uliano-Silva M, Americo J, Bastos AS, Furtado C, Rebelo MdF, Prosdocimi F. 2016. Complete mitochondrial genome of the brown mussel perna perna (bivalve, mytilidae). Mitochondrial DNA Part A. 27(6):3955–3956. [DOI] [PubMed] [Google Scholar]
- UNEP/RAMOGE. 1999. Manual on the biomarkers recommended for the med pol biomonitoring programme. Athens, Greece: United Nations Environment Programme [Google Scholar]
- Veith GD, Call DJ, Brooke L. 1983. Structure-toxicity relationships for the fathead minnow, pimephales promelas: Narcotic industrial chemicals. Canadian Journal of Fisheries and Aquatic Sciences. 40(6):743–748. [Google Scholar]
- Viarengo A, Lowe D, Bolognesi C, Fabbri E, Koehler A. 2007. The use of biomarkers in biomonitoring: A 2-tier approach assessing the level of pollutant-induced stress syndrome in sentinel organisms. Comparative Biochemistry and Physiology Part C. 146(3):281–300. [DOI] [PubMed] [Google Scholar]
- Villeneuve DL, Crump D, Garcia-Reyero N, Hecker M, Hutchinson TH, LaLone CA, Landesmann B, Lettieri T, Munn S, Nepelska M et al. 2014. Adverse outcome pathway (aop) development i: Strategies and principles. Toxicological Sciences. 142(2):312–320. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vinken M, Knapen D, Vergauwen L, Hengstler JG, Angrish M, Whelan M. 2017. Adverse outcome pathways: A concise introduction for toxicologists. Archives of Toxicology. 91(11):3697–3707. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Walker CW, Van Beneden RJ, Muttray AF, Böttger SA, Kelley ML, Tucker AE, Thomas WK. 2011. P53 superfamily proteins in marine bivalve cancer and stress biology In: Lesser M, editor. Advances in marine biology. Elsevier; p. 1–36. [DOI] [PubMed] [Google Scholar]
- Ward JE, Kach DJ. 2009. Marine aggregates facilitate ingestion of nanoparticles by suspension-feeding bivalves. Marine Environmental Research. 68(3):137–142. [DOI] [PubMed] [Google Scholar]
- Ward JE, Shumway SE. 2004. Separating the grain from the chaff: Particle selection in suspension-and deposit-feeding bivalves. Journal of Experimental Marine Biology and Ecology. 300(1–2):83–130. [Google Scholar]
- Ward JE, Zhao S, Holohan BA, Mladinich KM, Griffin TW, Wozniak J, Shumway SE. 2019. Selective ingestion and egestion of plastic particles by the blue mussel (mytilus edulis) and eastern oyster (crassostrea virginica): Implications for using bivalves as bioindicators of microplastic pollution. Environmental Science & Technology. [DOI] [PubMed] [Google Scholar]
- Waykar B, Deshmukh G. 2012. Evaluation of bivalves as bioindicators of metal pollution in freshwater. Bulletin of Environmental Contamination and Toxicology. 88(1):48–53. [DOI] [PubMed] [Google Scholar]
- Winkel ET, Davids C. 1982. Food selection by dreissena polymorpha pallas (mollusca: Bivalvia). Freshwater Biology. 12(6):553–558. [Google Scholar]
- Yanick JF, Heath DD. 2000. Survival and growth of mussels subsequent to hemolymph sampling for DNA. Journal of Shellfish Research. 19(2):991. [Google Scholar]
- Yusof A, Yanta N, Wood A. 2004. The use of bivalves as bio-indicators in the assessment of marine pollution along a coastal area. Journal of Radioanalytical and Nuclear Chemistry. 259(1):119–127. [Google Scholar]
- Zhang G, Fang X, Guo X, Li L, Luo R, Xu F, Yang P, Zhang L, Wang X, Qi H. 2012. The oyster genome rreveals stress adaptation and complexity of shell formation. Nature. 490(7418):49–54. [DOI] [PubMed] [Google Scholar]
- Zuloaga O, Prieto A, Usobiaga A, Sarkar SK, Chatterjee M, Bhattacharya BD, Bhattacharya A, Alam MA, Satpathy KK. 2009. Polycyclic aromatic hydrocarbons in intertidal marine bivalves of sunderban mangrove wetland, india: An approach to bioindicator species. Water, Air, and Soil Pollution. 201(1):305. [Google Scholar]


