Abstract
Introduction:
Neurodegenerative diseases, such as Alzheimer’s disease, Parkinson’s disease, Huntington’s disease, amyotrophic lateral sclerosis and prion disease represent important public health concerns. The etiology of neurodegenerative diseases is not completely understood, but genetic factors and environmental factors clearly contribute to their development. As environmental factors, heavy metals such as manganese (Mn) an essential trace element, are required for several physiological processes. In addition, exposures to high levels may culminate in adverse health effects.
Areas covered:
In this critical review, the authors address the role of Mn in the etiology of neurodegenerative diseases and discuss emerging treatments of Mn overload, such as chelation therapy. In addition, the authors discuss natural and synthetic compounds under development as prospective therapeutics. Moreover, bioinformatic approaches to identify new potential targets and therapeutic substances to reverse the neurodegenerative diseases are discussed.
Expert opinion:
Here, the authors highlight the importance of better understanding the molecular mechanisms of toxicity associated with neurodegenerative diseases, and the role of Mn in these diseases. Additional emphasis should be directed to the discovery of new agents to treat Mn-induced diseases, since present day chelator therapies have limited bioavailability. Furthermore, the authors encourage the scientific community to develop research using libraries of compounds to screen those compounds that show efficacy in regulating brain Mn levels. In addition, bioinformatics may provide novel insight for pathways and clinical treatments associated with Mn-induced neurodegeneration, leading to a new direction in Mn toxicological research.
Keywords: Alzheimer’s disease, Huntington’s disease, Parkinson’s disease, amyotrophic lateral sclerosis, manganese, heavy metals
1. Introduction
Neurodegenerative diseases such as amyotrophic lateral sclerosis (ALS), Alzheimer’s disease (AD), Huntington’s disease (HD), Parkinson’s disease (PD) and prion disease are global public health concern. These diseases are characterized by progressive dysfunction and loss of neurons in certain anatomical regions with involvement of different functional systems and a variety of clinical presentations. There are several pathways that contribute to neuronal and/or glial cell damage, and a fundamental phenomenon inherent to most neurodegenerative diseases is the deposition of proteins such as alpha-synuclein, β-amyloid, tau, prion protein in the human brain. These proteins show conformational changes and biochemical modifications with altered physicochemical properties. Moreover, genetic mutations heighten susceptibility to these disorders [1].
Several studies have shown that chronic exposure to pesticides [2,3], particulate matter [4,5], and high levels of heavy metals [6-8] increase the risk of developing different neurodegenerative diseases. Epidemiological studies demonstrated an association between toxic non-essential metals, for instance mercury and AD [9,10], cadmium and AD and PD [11,12]. However, long- term exposure to high levels of essential elements, such as manganese (Mn), may also be toxic to humans and trigger neurodegenerative diseases [13,14].
The Mn is one of the most important metals in mammals and it is required for physiological processes, including brain and skeletal development, blood clotting, reproduction, neuronal function, antioxidant defense, and immune integrity [15]. Mn has also an important function as cofactor of numerous enzymes required for glial and neuronal cells, as well as enzymes involved in synthesis and metabolism of amino acids, proteins, lipids, such as pyruvate carboxylase, glutamine synthetase and isocitrate dehydrogenase [15-17].
Several trace elements, such as iron (Fe), calcium (Ca), zinc (Zn) and cupper (Cu) may interfere with Mn homeostasis [18]. In fact, Mn and other metals may compete for the same transporters, for instance, the divalent metal transporter 1 (DMT-1), which is not specific and able to transport multiple metals and may influence the Mn uptake and tissue distribution, affecting Mn and Fe homeostasis [19]. Moreover, Mn exposure may interfere with Ca 2+ absorption, leading to calcium signaling dysfunction that is associated with neurodegenerative diseases [20].
Mn is found in a large variety of foods, including rice, nuts, whole grains, leafy green vegetables, chocolate, seafood, fruits and seeds [15]. The major, non-occupational source of exposure to Mn is food with high levels of Mn or contaminated water (where rocks with high levels of Mn into the aquifers may increase Mn concentrations in groundwater in certain geologic regions) [21-23]. In addition, the use of total parenteral nutrition in ill infants and upon surgical resections may be an important source of Mn overload, since in parenteral nutrition, 100% of the Mn present in the preparation is bioavailable, while only 3–5% is bioavailable by enteral nutrition [24,25] and inhalation of combusted methylcyclopentadienyl manganese tricarbonyl (MMT), an Mn-containing gasoline additive, also represents an important source for Mn overload [26-28]. Exposure to Mn may also occur in several industrial activities, such as mining, welding, ferroalloy production, smelting and battery manufacturing. Mn-containing fumes and dust in industrial settings represent especially a high risk of increased exposure, where chronic inhalation of high levels of airborne particulate may result in elevated levels of Mn in the brain [29,30]. Chronic occupational exposure has been associated with development of neurotoxicity and neurodegenerative diseases [31]. For instance, in an American welding cohort study it was reported that Mn exposure generated a dose-dependent progression of manganism [32]. Similarly, a dose-dependent dopaminergic dysfunction associated with neurotoxicity in Mn occupational workers was observed as well as Mn exposure positive dose-response to occupational Mn exposure and severity of clinical symptoms of manganism[33].
Regulatory agencies have determined the limit to occupational exposure. The U.S. Federal Occupational Safety and Health Administration (OSHA) set a value of 5000 μg/m3 as an exposure limit, while according to the National Institute for Occupational Safety and Health (NIOSH) this value is 1000 μg/m3 [29]. The first course of action in the event of high Mn exposure is to remove the individual from the source of the exposure [34].
Manganism, a disease associated with exposure to high levels of Mn, has phenotypic features analogous to idiopathic PD such as tremors, cognitive deficits, bradykinesia, and rigidity, reflecting excessive Mn accumulation in the basal ganglia [31,35,36]. However, manganism is void of one of PD’s hallmark, namely the presence of Lewy bodies [36,37]. This is commensurate with the clinical presentations which appear distinct for clinical idiopathic PD vs. manganism.
Overexposure to Mn may be associated with neurological disorders such as AD, PD, HD and ALS [38-41]. In fact, potential mechanisms underlying Mn-induced neurotoxicity and neurodegenerative diseases include changes in proteins aggregation; for instance, β-amyloid, tau, α-synuclein and huntingtin. Indeed, these proteins have been studied as possible new targets to treat Mn poisoning and neurodegenerative disorders [42-44]. Moreover, intracellular signaling pathways such as PI3K/Akt/mTOR, ERK, p53, and transcription factors such as NF-E2-related factor 2 (Nrf2) and NF-κB, may be also involved in neurotoxic effects of Mn and the understanding of how Mn induces changes in these pathways may be used as a strategy to treat or prevent neurodegenerative diseases [45-47].
Although the mechanisms associated with Mn-induced neurotoxicity are not entirely understood, there is abundance of evidence showing that Mn promotes reactive oxygen species (ROS) generation, impairs endoplasmic reticulum (ER) homeostasis, promotes mitochondrial dysfunction, and contributes to apoptosis [13,48].
A growing number of studies has detailed promising neuroprotective strategies to mitigate Mn neurotoxicity in different models (in vitro and in vivo). In this review, we address cellular and molecular mechanisms of Mn-induced neurotoxicity and neurodegenerative diseases, and highlight contemporary neurotherapeutic approaches, such as chelation, and natural and synthetic compounds in mitigating its neurotoxicity.
2. Role of Mn in neurodegenerative diseases
The most common neurodegenerative disease is AD, which is characterized by cognitive impairment with progressive dementia in the elderly [49]. Since Dr. Alois Alzheimer reported the first case in 1907, several studies have identified the pathology, risk factors for the development of the disease, as well as possible pathways involved its etiology [49,50]. The major features of AD are misfolding and aggregation of two proteins, Aβ and tau, forming Aβ plaques and neurofibrillary tangles [51,52].
A study performed by Tong et al. [38] showed an inverse correlation between Mn levels in whole blood with various cognitive and neuropsychological test scores where individuals with lower Mn levels had better score in Mini-Mental State Examination. Interestingly, they also observed that plasma Aβ peptides increase with raised Mn levels, suggesting that Mn in whole blood may be correlated with the progressive cognitive impairment of AD.
Mn homeostasis is perturbed in AD, triggering aggregation of Aβ peptides, the hallmark of AD. Rats exposed to Mn show high concentrations of this metal in several brain regions such as hippocampus and cortex, suggesting that Mn accelerates Aβ deposition and memory dysfunction [53]. Moreover, Mn exposure has been linked to alterations in the expression of amyloid precursor-like protein 1 (APLP1, a member of the amyloid precursor protein family with limited expression in the brain), resulting in diffuse Aβ plaques and degenerating cells, which can potentiate Mn neurotoxic effects [42,54]. Animals exposed to Mn show increased APLP1 protein expression in apoptotic neurons and glial cells, leading to Aβ plaques formation in the frontal cortex, and thereby resulting in cognitive and working memory deficits in these animals [42]. Chronic Mn exposure produces increases in p53 levels (a transcription factor that regulates DNA synthesis and repair, and its activation occurs after injuries leading oxidative stress and genotoxicity) in cortical neurons associated with neurodegeneration [42,55]. Binding of Mn to monomeric Aβ peptide demonstrated weak affinity and transient interactions in vitro when evaluated by spectroscopy and fluorescence methods, suggesting no prolonged effects on plaque formation, even though future studies are required to elucidate the effects of Mn/Aβ peptide interaction in plaque formation [56]. Although AD is a widely studied neurodegenerative disease, how Mn contributes to neurodegenerative process and AD progression are still unclear.
The second most prevalent neurodegenerative disorder is PD, which is characterized by movement disorder, tremor, rigidity, and bradykinesia leading to loss of motility. Other neurological effects, such as dementia, are also observed in PD patients [57]. Although the etiology of PD has yet to be fully understood, both genetic and environmental factors are involved [58]. Exposure to Mn is associated with a parkinsonian-like symptoms that resembles idiopathic PD (referred to as manganism) [59]. This was first described by Couper (1837) among workers exposed to excessive airborne Mn levels. Chronic occupational exposure to high levels of Mn upon welding, smelting, mining, battery manufacturing, ferroalloy and Mn-rich agro-chemicals, is known to cause PD-like manganism [36,60].
Several epidemiological studies evaluated the correlation between Mn exposure and parkinsonism. In a cohort study with 886 American workers, exposed to Mn-containing welding fume was associated with progression of parkinsonism in a dose-dependent manner [32]. Likewise, it was reported that Mn mine workers in South Africa associated cumulative Mn exposure with parkinsonian signs and poorer quality of life [61]. Methcathinone abuse, a psychostimulant that uses potassium permanganate as an oxidant, has also been correlated with extrapyramidal syndrome [62]. However, other authors do not report cognitive deficits and there is a lack of neuropathological studies in methcathinone users to clarify the association between methcathinone users and the PD development [63,64].
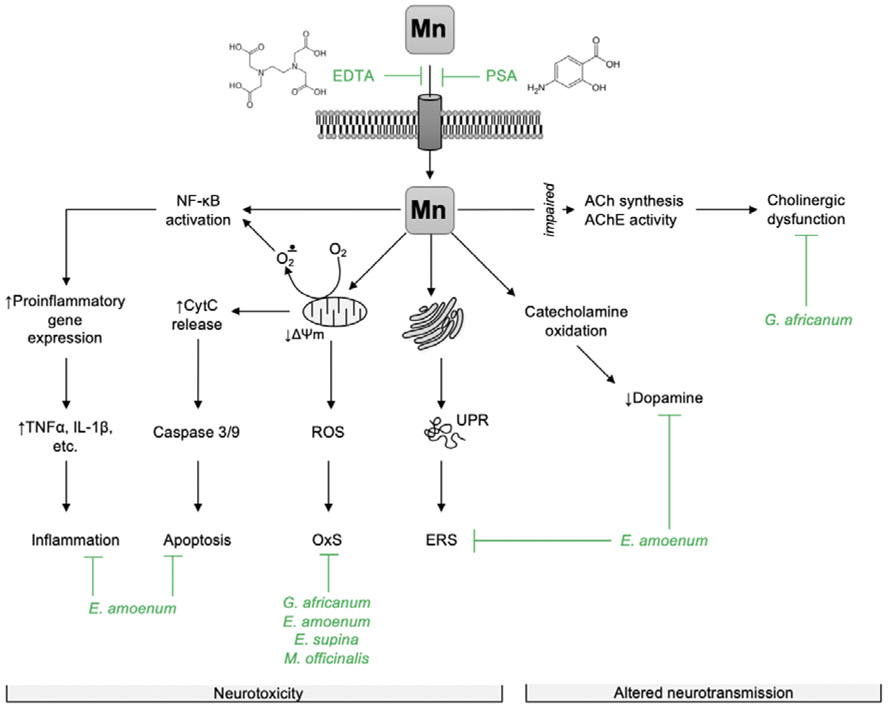
Putative mechanisms associated with its neurotoxicity are shown in Figure 1. An important role has been ascribed to alpha-synuclein (αSyn) [65,66]. αSyn is a chaperon protein which is expressed and localized mainly in presynapitic terminals and regulates synaptic plasticity and dopaminergic neurotransmission [67]. The major aggregated component of Lewy body depositions (a PD’s hallmark) is αSyn. Nonetheless, the role of αSyn in PD is controversial, with a complex role in both neuroprotection and neurodegeneration. Mn exposure alters αSyn expression, aggregation and cytotoxicity [68,69]. In fact, a study using neuronal PC12 cells reported that Mn induces overexpression of αSyn, leading to αSyn aggregation and misfolding, which is associated with cytotoxicity [69]. Interestingly, αSyn acts as neuroprotective chelator in acute Mn exposure by binding to C-terminal sites of this protein [70,71]. However, in chronic Mn overexposure, the natively conformed proteins undergo misfolding, then promoting αSyn aggregation, a critical factor responsible for Mn-induced autophagic degradation and neuronal damage [68]. Indeed, it was reported that Mn exposure could promote cell-to-cell transmission of αSyn, enhancing release of misfolded αSyn that evoke pro-inflammatory responses, potentially contributing to progressive neurodegeneration in dopaminergic neurons [72-74].
Figure 1.
Potential mechanisms of chelators and phytoextracts against Mn-induced neurotoxicity. Briefly, EDTA and PSA prevent Mn2+ from entering the cell, thus reducing its intracellular level and toxic effects. Phytoextracts and particular phytochemicals were shown to counteract mechanisms of Mn2+-induced neurotoxicity including NF-κB activation with subsequent proinflammatory cytokine expression (E. amoenum); mitochondrial dysfunction resulting in ROS overaccumulation and oxidative stress (G. africanum, E. amoenum, E. supina, M. officinalis), as well as apoptotic signaling (E. amoenum); alteration of protein folding, unfolded protein response, and endoplasmic reticulum stress (E. amoenum). Natural extracts were also shown to prevent reduction of catecholamine levels due to autooxidation (E. amoenum) and counteract cholinergic dysfunction (G. africanum). Other plant extracts sharing similar phytochemical spectrum may also possess protective effect. It is also highly expected that these phytoextracts or other phytochemicals may modulate specific mechanisms of Mn2+-induced neurodegeneration, including amyloid-β (Alzheimer’s disease) and α-synuclein (Parkinson’s disease) aggregation, although direct evidence for these effects have yet to be established.
EDTA - ethylenediaminetetraacetate, PSA – para-aminosalicylic acid, ACh - acetylcholin, AChE - acetylcholinesterase, ROS – reactive oxygen species, OxS – oxidative stress, UPR – unfolded protein response, ERS – endoplasmic reticulum stress, CytC – cytochrome c, TNFα – tumor necrosis factor α, IL-1β – interleukin 1β.
There are other factors associated with PD, such as genes of familial form (parkin, pink1 and dj1) that regulate oxidative stress pathways and are implicated in the onset of PD. Using a C. elegans model, Bornhorst et al. [70] showed that worms lacking pdr1 and djr1 have increased Mn-induced oxidative stress. Interestingly, the overexpression of αsyn in these worms was able to rescue this effect, suggesting a complex interplay among αsyn, Mn and pdr1 or djr1 (Figure 1)
HD is an autosomal dominant neurodegenerative disorder, which neuropathological features result from the loss of projections from striatal neurons, and clinically is characterized by psychiatric and cognitive alteration, and progressive motor impairment, ultimately leading to death of patients [75]. Altered Mn homeostasis is likely involved in HD pathology, as suggested by several lines of evidence showing a gene-environment interaction between Mn and the mutant (HTT) gene [76,77]. Williams et al. [77] reported that transgenic mice in a HD mouse model had a selective Mn accumulation in the striatum (the most susceptible brain region in HD) compared to wild-type. In addition, another study demonstrated that the disease-metal interaction changes dendritic morphology characterized by length and branching complexity, and decreases DA levels in the striatum [76].
Building evidence supports a role for Mn deficiency to underlie HD pathology through diminished activity of Mn-dependent processes, including Mn-dependent enzymes such as arginase glutamine synthetase and pyruvate carboxylase, as well as Mn-dependent cell signaling networks and cell processes such as insulin growth factor receptor and autophagy [44,78,79]. Strong evidence in support of this includes the amelioration of arginase metabolic activity deficits by a one-week Mn supplementation in a mouse model of HD [80]. Likewise, supplementation with Mn rescues an HD defect in autophagic cargo loading [81]. The HD pathophysiology is associated with deficits in Mn accumulation in human and mouse neuroprogenitors with pathogenic alleles of Huntingtin. This deficiency induces ATM-p53 activation with enhanced p53 accumulation and phosphorylation in HD patients and mice [82]. Therefore, perturbations in Mn homeostasis can contribute to HD, and future studies to further elucidate the intracellular signaling alterations and mechanisms are warranted.
Another important neurodegenerative disease is ALS that affects more than 12,000 people in the United States, mainly occurring in younger people [44]. This fatal neurodegenerative disease is characterized by loss of motor neurons in the brain and spinal cord, resulting in motor neuron degeneration. This leads to loss of voluntary muscle control, resulting in a progressive paralysis of skeletal muscles and, in late-stages, respiratory failure [83,84]. Although the etiology of ALS is unknown, some patients have a family history of ALS, suggesting a genetic origin factor [85,86]. However, environmental factors play an important role in ALS development. Metal ion dysregulation is a relevant environmental factor, since Mn overexposure and ALS was first reported in German smelters [85,87]. In this regard, Mn has been studied to evaluate the association between Mn levels and ALS disease with conflicting results reported in the literature. The concentration of Mn in cerebrospinal fluid and plasma were higher in ALS patient than control group [88]. Kapaki et al. [89] demonstrated that Mn levels were enhanced in serum of ALS patient but no differences were observed in cerebrospinal fluid. On the other hand, in an Italian cohort of ALS patients, no significant difference was found in serum Mn levels in ALS patients and controls [90], and similar evidence was reported by Peters et al. [91]. Thus, studies to clarify these intriguing findings and studies to develop novel treatments for this illness are necessary.
Prion diseases are progressive neurodegenerative diseases that affect animals, such as goats and sheep (scrapie), cattle (bovine spongiform encephalopathy (BSE)), as well as human s(Creutzfeldt-Jakob disease, Gerstmann-Sträussler-Scheinker disease and kuru) [92]. Prion diseases are associated with neuropathological changes in normal cellular prion protein (PrPC) leading to the formation of an abnormal pathogenic prion protein (PrPSc). The latter triggers a chain-reaction-like process of protein misfolding and progressive accumulation of abnormal isoforms and substantial neuronal degeneration [93]. Prion disorders are associated with metal dyshomeostasis. The prion protein PrPC has a high-binding affinity for divalent metals such as Cu 2+, Zn2+, Fe2+ and Mn2+ [18,94]. Indeed, studies have been reported that Mn could be involved in the misfolding and aggregation of the PrPSC both in vitro and in vivo studies [95,96]. Brown et al. showed that Mn may be incorporated into PrP expressed by astrocytes generating proteinase resistance and loss of function [97]. Likewise, it was reported that Mn can induce stabilization of prion protein, contributing to prion protein misfolding and prion disease pathogenesis [95]. Interestingly, individuals with Creutzfeldt−Jakob Disease, the most common form of human prion disease, have increased Mn levels in the blood and brain, suggesting that Mn blood in prion disease is a highly specific characteristic of the disease [98]. However, neurodegenerative mechanisms are not completely elucidated and need further exploration to better understand the role of Mn in prion diseases.
3. Treatment of Mn induced neurotoxicity
3.1. Calcium disodium ethylene-diamine-tetra-acetic acid (CaNa2EDTA)
There are few clinical treatment options for Mn-induced neurotoxicity. Putative treatment modalities are shown in Figure 1. Chelation therapy is by far the principal treatment modality. This thrapy is based on the infusion of ions or molecules that bind to the metal, removing it through complex formation and urinary excretion [99,100]. Calcium disodium Ethylene-diamine-tetra-acetic acid (CaNa2EDTA) is a synthetic compound that has been used with some success as chelator. Its mode of action is based on the ability of toxic metals to replace calcium in the EDTA core [101]. Both in vitro and in vivo studies have demonstrated the efficacy of CaNa2EDTA to reduce dopaminergic autooxidation upon Mn exposure and the decrease in Mn concentration in both brain and liver of rats exposed to Mn [102,103].
The first study using CaNa2EDTA to treat Mn intoxication evaluated miners [104]. In this study, seven welder/foundry workers affected by Mn-induced Parkinsonism which had deposition of Mn in the basal ganglia received intravenous CaNa2EDTA chelating therapy. Four workers exhibited effective regression of the disease with improved muscle rigidity, while another worker showed a mild improvement of tremor. Also, increased Mn elimination in urine and reduction of Mn in blood were noted [105]. Likewise, two patients with hypermanganesemia that received CaNa2EDTA chelating therapy had decreased Mn blood levels and increased Mn urinary excretion that were associated with improvement of symptoms [106,107]. In addition, hypermanganesemia may occur by mutations in the SLC30A10 gene, which encodes a Mn transporter resulting in Mn accumulation in the brain [108]. In this regard, a report of the 2 year follow-up after chelation therapy in individual carrying SLC30A10 mutations showed reduced clinical disability due to parkinsonian-like movement disorder [109]. Likewise, it was reported a new variant of SLC39A14 gene mutation that may affect Mn accumulation. The patient carrying this mutation underwent chelation therapy with intravenous CaNa2EDTA[110]. Moreover, a strong correlation between chelating treatment and clinical improvement by MRI confirmed by the reduction of Mn deposition in basal ganglia was reported after treatment in workers exposed to Mn [111]. Taken together, these findings suggest that chelation therapy has a therapeutic benefit with positive results. However, some limitations of chelating therapy should be considered, once that positive results were demonstrated in small sample size [34]. For instance, increased Mn excretion in urine and decreased blood Mn levels may not be associated with significant improvement in clinical symptoms [112,113]. In addition, chelating molecules do not readily cross the blood-brain barrier, thus they exhibit low bioavailability and efficacy, which leads to controversial results regarding amelioration provided by CaNa2EDTA [114]. Moreover, other limitation is that chelator substances may not be specific to Mn, since they also decrease other essential metals, potentially increasing possible toxic metals in the body [99]. Additionally, common adverse effects in patients receiving chelation therapy consist of fever, headache, nausea, vomiting, gastrointestinal stress. In some cases, severe adverse effects were reported, such as heart and respiratory failure, breathing difficulty, low blood pressure, convulsions, and low blood calcium [99].
3.2. Para-aminosalicylic acid (PAS)
The substance para-aminosalicylic acid (also known as PAS, 4-amino-2-hydroxybenzoic acid or 4-aminosalicylic acid) is used as antibacterial drug for treatment of tuberculosis [115]. PAS consists of carboxyl, hydroxyl and amine groups, providing favorable chelating properties for metals [101]. This substance was tested for three and a half months in a patient chronically and occupationally exposed to Mn, and authors observed improvement in clinical symptoms such as tremor in hands, handwriting ability, and walking [116]. In fact, another study followed one woman who was exposed for 21 years to airborne Mn and, after treatment with PSA, all clinical symptoms and signs of manganism at the time of treatment were improved, while 17 years after treatment, clinically normal conditions were observed [114].
Yuan et al. demonstrated that Mn exposure disturbs the balance of most divalent metals (particularly in cortex), and that PAS post-treatments can repair these alterations [117]. Impaired spatial learning and memory abilities, which were observed in rats after Mn exposure, returned to levels indistinguishable from controls by PAS treatment [118]. In fact, PAS treatment efficiently reversed the Mn-induced learning and memory deficits associated to decreased Mn levels in the whole blood and brain tissue such as cortex, hippocampus and thalamus [119].
Some antioxidant enzymes and neurotransmitters may be affected by Mn exposed and are related with neurodegenerative disease. Consistent with this observation, decreased glutathione peroxidase and catalase activity were noted in basal ganglia after Mn exposure, which were restored upon PAS treatment [120]. In addition, recent studies have investigated the efficacy of PAS treatment not only as chelating agent to mobilize whole blood Mn, but also in its efficacy to reduce inflammatory cytokines that are related with neurodegenerative diseases, suggesting that PAS may be used to ameliorate and retardate symptoms related with inflammatory responses after Mn exposure [121,122].
3.3. Natural compounds
Plants are frequently able to tolerate extreme concentrations of toxic metals. Such ability has been explored in phytoremediation strategies (e.g., Amaranth (Amaranthus crentus)) since Mn can be oxidized and hyperaccumulated in plants [123,124]. Here, we discuss new perspectives considering natural extracts and phytochemicals reported as prevention or therapy alternatives to Mn intoxication. We considered several outcomes from animal models since natural products are not the first choice for the clinical intervention of Mn poisoning.
Oxidative stress has been implied to mediate Mn toxicity; this is a crucial connection for plant extract usage as a therapy, especially for the ubiquitous antioxidant and chemoprotective properties of phytomedicines. Plant metabolites possess chelating activity against several metals, and generally act by improving the activity of endogenous antioxidants (e.g., superoxide dismutase (SOD), catalase (CAT), glutathione S-transferase (GST)) [125,126]. We discuss here some of the latest outcomes regarding natural compounds or isolated phytomedicines used for Mn-induced toxicity in animal models (Table 1).
Table 1:
Natural extracts used against Mn toxicity.
| Plant | Phytochemical composition | Manganese protective effects | Experimental model |
|---|---|---|---|
|
Echium amoenum Fisch. & CA Mey Popular name: Red feathers Family: Boraginaceae |
Rosmarinic acid, anthocyanin and delphinidin [127,128] | Anti-apoptotic Antioxidant Increased catecholamine levels Behavioral improvements [131] | Rat |
|
Euterpe oleracea Mart. Popular name: Açaí Family: Arecaceae |
Carotenoids, flavonoids, anthocyanins, and other polyphenols [138] [139]. | Antioxidant [138] | Primary cultured astrocytes |
|
Euphorbia supine Raf. Popular name: Prostrate spurge Family: Euphorbiaceae |
Quercetin, gallic acid, protocatechuic acid, nodakenin, quercetin 3-O-hexoside, quercetin 3-O-pentoside, kaempferol 3-O-hexoside, kaempferol 3-O-pentoside, and kaempferol [144] | Reduced the oxidative and endoplasmic reticulum (ER) Reestablished apoptotic markers [141] | Neuroblastoma SKNMC cells and Sprague-Dawley male rat brain |
|
Melissa officinalis L. Popular name: Common balm Family: Lamiaceae |
Rosmarinic acid and flavonoids [147] | Neuroprotection Antioxidant Reestablished superoxide dismutase (SOD) activity [148] | Mice |
|
Gnetum africanum Welw. Popular name: African jointfir Family: Gnetaceae |
Alkaloids [149] | Reestablished acetylcholinesterase (AChE) activity Reduced nitric oxide (NO), and reactive oxygen species (ROS) levels [149] | Drosophila melanogaster |
|
Basella alba L. Popular name: Malabar spinach Family: Basellaceae |
Phenol compounds, carotenoids, ascorbic acid, saponins, coumarins, limonoids [153] | Improved the reproductive dysfunction induced by Mn-containing compound MANEB [153] | Rat |
Echium amoenum (E. amoenum) has been reported as an important source of phenolic compounds, presenting natural antioxidant compounds, such as rosmarinic acid, cyaniding, and delphinidin [127,128]. Phenolic compounds are characterized by their capacity to eliminate high levels of ROS as well as chelating activity for metals [129,130]. The extract of E. amoenum invoked beneficial effects by inhibiting Mn neurotoxicity in the rat hippocampus, which was likely related to decreased oxidative stress, caspase 3 and 9 induction, and apoptosis [131]. The neuroprotective mechanisms of E. amoenum have been attributed to the presence of cyanidin 3-glucoside, the most common anthocyanin in petals of this plant. Cyanidin 3-glucoside can inhibit inflammation by blocking the translocation of c-Jun and NF-κB factors into the nucleus [132]. Mn has been reported both to activate c-Jun and upregulate mRNA expression of NF-κB [133,134]. Moreover, E. amoenum also increased catecholamine levels and improved depression-like behavior in rats [131]. Mn overexposure has been invoked to reduce catecholamine levels, which are frequently associated with behavioral changes in different models. including rat, Drosophila melanogaster (D. melanogaster), and Caenorhabditis elegans (C. elegans) [131,135,136]. Previous data support the concept that Mn can chemically interact with dopamine, leading to autoxidation and decreased dopamine levels [137].
Açaí (Euterpe oleracea Mart.) extract attenuated Mn-induced oxidative stress in primary cultured astrocytes at nutritionally relevant concentrations. The authors also revealed the potential of anthocyanins obtained from açaí to mitigate Mn neurotoxicity [138]. Açaí berry is consumed worldwide in beverages, fruit mix, ice cream and capsules. Besides the palatable taste, açaí berry provides a unique nutritional profile due the high levels of antioxidant compounds, including carotenoids, flavonoids, anthocyanins, and other polyphenols [139]. Açaí is a Mn dietary source. However, it needs to taken into account that a daily consumption of 300 ml açaí pulp exceeds by six-fold (14.6 mg on average) the reference daily intake of Mn for an adult [140], hence at high consumption açaí may have toxic effects despite it antioxidant properties.
Polyphenolic extract of Euphorbia supina (PPEES) has been evaluated against Mn-induced neurotoxicity in both neuroblastoma SKNMC cells and Sprague-Dawley male rat brain. PPEES treatment decreased Mn-induced oxidative stress in both models while reestablished Mn-changed parameters related to endoplasmic reticulum (ER) stress and ER stress-mediated apoptosis markers (GRP78, GADD34, XBP-1, CHOP, Bcl-2, Bax and cleaved caspase-3) [141]. The phenolic features that trigger cellular protection from oxidation have been investigated in the context of several neurodegenerative diseases, including PD and AD [142,143]. In addition to quercetin, eight biologically polyphenols were isolated from Euphorbia supina including gallic acid, protocatechuic acid, nodakenin, quercetin 3-O-hexoside, quercetin 3-O-pentoside, kaempferol 3-O-hexoside, kaempferol 3-O-pentoside, and kaempferol [144].
Similar to E. supina, Melissa officinalis (M. officinalis) presented a neuroprotective role in an AD model [145,146]. M. officinalis extract combines free radical scavenging and antioxidant polyphenolic compounds, including rosmarinic acid, trimeric compounds, and some flavonoids [147]. The neuroprotective effect of M. officinalis was also observed in Mn-induced neurotoxicity in mice, as well as the attenuation in oxidative stress and recoversion of SOD enzyme activity [148].
In several cases, the pharmacological properties have been assigned to their phytomedical compounds. On the other hand, many studies have compared the differences between the extract effects and the isolated phytomedical compounds (Table2). For instance, the efficacy of the alkaloid boldine, component of boldo (Peumus boldus) extract, in preventing/ameliorating the Mn toxic effects in D. melanogaster has been shown. Interestingly, while Boldo extract reduced the mortality rate of flies exposed to Mn, boldine did not show such effectiveness [135]. Boldine partially improved the Mn-induced locomotor dysfunction and decreased TBARS levels, which were fully ameliorated by boldo’s crude extract [135]. The protective properties of an alkaloid extract from African Jointfir (Gnetum africanum) leaf against Mn toxicity was also tested in D. melanogaster as a model. The extract counteracted Mn-induced increase in acetylcholinesterase enzyme (AChE) activity, nitric oxide (NO), and ROS levels [149].
Table 2:
Plant extract effects on Mn intoxication in animal models
| Phytomedicines | Class | Molecular structure | Manganese protective effects |
Experimental model |
|---|---|---|---|---|
| Silymarin (Silybum marianum) | Flavonoid | 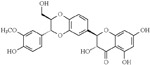 |
Nephroprotection Decreased lipid peroxidation Antioxidant [152] | Rat |
| Boldine (Peumus boldus) | Alkaloid | 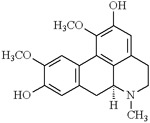 |
Partially improved locomotor dysfunction and TBARS levels [135] | Drosophila melanogaster |
Flavonoids belong to a class of secondary metabolites of plants, having a polyphenolic structure and being widely found in fruits and vegetables [150]. It has been recognized that flavonoids show antioxidant and anti-inflammatory activities, as well as anti-mutagenic and anti-carcinogenic properties, and their effects on human nutrition and health are considerable [150]. Their ability to diminish oxidative stress as an antioxidant function includes the capacity of flavonoids to chelate metal ions [130].
Silymarin, an antioxidant flavonoid complex isolated from the seed of Silybum marinum (milk thistle), has been reported as an Aβ aggregation inhibitor, antioxidant, and neuroprotective compound [151]. Beyond affecting the CNS, Mn-induced toxicity is inherent to other organs, including the liver, lungs, and heart, as well as the reproductive system [101]. Chtourou et al. investigated the Mn-associated nephrotoxicity, as well as the beneficial effects of Silymarin, which reduced the alterations in the renal and urine markers, decreasing lipid peroxidation endpoints, increasing the antioxidant cascade, and decreasing Mn-induced damage [152].
Human exposure to Mn mainly occurs via intake of contaminated food and water, by occupational sources (inhalation of industrial applications, dust, mist, or fumes with Mn), and by environmental sources which include pesticides containing Mn [101]. Maneb (ethylenebis-dithiocarbamate) is a Mn-containing fungicide whose chronic exposure may produce symptoms and signs of central nervous system (CNS) Mn poisoning. Maneb exposure in rats caused a reduction in fertility and testosterone levels. In contrast, its co-administration with Basella alba L. (Basellaceae) extract minimized such changes in reproductive function [153]. B. alba showed efficacy in restoring the antioxidant system in the testicular tissue likely due to the presence of various antioxidant compounds, including phenolic compounds, carotenoids, ascorbic acid, saponins, coumarins and limonoids [154]. Finally, beyond the pharmacological advantages of plants usage, an extensive historical background of their consumption is available, thus reducing toxicological risks and adverse effects when compared to newly synthesized molecules.
4. New approaches to identify targets and molecules to treat Mn neurotoxicity and neurodegeneration
Currently, chelators are widely used to treat conditions of Mn overload. Nonetheless, life-long chelation treatment may induce imbalance in other essential trace element and collateral effects such as nausea, causing vomiting, gastrointestinal intolerance and abdominal discomfort [155]. Therefore, to avoid these adverse effects, searching for new targets and new substances for the treatment of Mn poisoning and its related diseases through new approaches such as bioinformatics, is increasing.
Bioinformatics methods have been used to identify key genes and pathways, predicting the possible molecular mechanisms underlying Mn-induced neurotoxicity and neurodegeneration, as well as screen potential molecules to reverse the neurotoxicity or potential therapeutic agents. For instance, to explore a possible molecular mechanism underlying Mn induced AD, a screening of differentially expressed genes showed 140 upregulated and 267 downregulated genes. Using the connectivity map (CMAP) tool, it was shown that Tyrphostin AG-825, an inhibitor of tyrosine phosphorylation, might prevent and reverse process related with Mn induced neurotoxicity and AD [156]. Thus, bioinformatics methods are important tools to investigate potential targets and new therapeutic substances to reverse neurodegenerative diseases.
Proteomic analyses are also an important tool to identify altered proteins. In this regard, a study explored changes in wild type and HD (YAC128Q) mutant mice exposed to Mn. The results showed changes in proteins involved in the inhibition of glycolysis and energy metabolism, excitotoxicity and dysregulation in cytoskeletal dynamics in the striatal region, suggesting that altered proteins may be used as novel markers of Mn toxicity [157]. Additionally, using a metabolomic approach in HD immortalized striatal neurons of mouse to identify metabolic disruptions after Mn exposure, the authors showed lower metabolite levels of pantothenic acid and glutathione in HD striatal cells compared to control cells. Also, the authors observed impaired induction of isobutyryl carnitine in response to increasing Mn exposure, and induction of metabolites in the pentose shunt pathway, demonstrating that changes in energetic processes underlie the pathobiology of both HD and Mn neurotoxicity [158].
Recently, Fernandes et al. reported metabolomic responses to Mn in SH-SY5Y human neuroblastoma cells, showing that changes in cellular metabolism, such as amino acids, neurotransmitters, energy, and fatty acids metabolism, occur in response to Mn, resulting in subsequent cell death. This suggests that metabolomics analyses represent a valuable approach to investigate and evaluate the toxic molecular responses to Mn toxicity [159].
Kumar et al. performed a high throughput screen of 40,167 small molecules for modifiers of cellular Mn content in a mouse striatal neuron cell line using chemical informatics with stringent validation assays to identify a chemical ‘toolbox’ with 41 small molecules modifiers of neuronal transport [160]. Two of these molecules, VU0063088 and VU0026921, were tested in the Caenorhabditis elegans model, showed potential protective efficacy against Mn-induced DAergic neurodegeneration, consistent with partial recovery in DAergic neuronal morphology and behavior in worms exposed to Mn [161]. Another of these molecules (MESM) was found to be a Mn-selective ionophore that rapidly reduced excessive cellular manganese levels in cultured cells (lowering >90% excess Mn levels in ~15 minutes). It is noteworthy; the MESM molecule was able to decrease even the basal levels of intracellular Mn without altering levels of calcium, iron, cobalt, copper, zinc or molybdenum. This specificity suggests that future studies of MESM as a therapeutic molecule in patients with excess levels of Mn may hold promise [162].
Taken together these studies demonstrate that new technical approaches are essential to better understand the molecular mechanisms underlying Mn-induced neurodegenerative disorders, as well as the identification of new targets for its treatment.
5. Conclusions
Mn exposure is an important health issue, mainly for individuals receiving parenteral nutrition and workers in occupational settings that are exposed to high levels from air. Growing evidence suggests the involvement of Mn in neurodegenerative diseases. Chelation therapy with EDTA and PSA has been widely used in response to Mn poisoning. Several studies have advanced the efficacy of several natural compounds and isolated phytochemicals in treating Mn neurotoxicity, its reprotoxic and nephrotoxic effects, secondary to their antioxidant and chelating properties.
Although, the association between Mn overexposure and neurotoxicity has been well characterized, treatment modalities are scarce. Bioinformatics may provide novel insight for pathways and clinical treatments associated with Mn-induced neurodegeneration. Furthermore, research on new therapeutic targets and new substances that can be used in the treatment of diseases related to Mn are necessary and may recognize a new direction in Mn toxicological research.
6. Expert opinion
Mn is required for proper physiological functions in the brain and other tissues, yet at high exposures it may lead to neurological disorders. To date, a limited number of studies have investigated Mn brain levels in of patients afflicted with the neurological diseases mentioned above. Better understanding the role of Mn in these brain diseases is a timely step. In addition, it will be incumbent upon the scientific community to determine whether patients suffering from the above disorders show elevated levels of brain Mn by means of MRI. While readily feasible, it is prohibitable expensive, hence such efforts have been curtailed to date. Therefore, new modalities should be advanced to cost-effectively monitor brain Mn content. Additional emphasis should be directed at developing Mn transport regulators such as the small molecule noted above, which will be able to pharmacologically control Mn homeostasis. The challenge with those, will be also to develop the means to directly target them to those brain areas which exhibit high or low Mn levels. Thus, it is incumbent upon the scientific community to develop large libraries of compounds to screen those that show efficacy in regulating brain Mn levels. In addition, it is abundantly clear that none of the chelators available to date is even remotely efficacious for reducing bodily Mn levels. Hence, a contemporary need exists for the development of new chelators to treat these Mn-induced diseases.
Article highlights.
Manganese (Mn) is an essential trace element that is required for several physiological process and it has an important function as cofactor of numerous enzymes required for glial and neuronal cells. However, the Mn overload may result in neurotoxicity and development of diseases.
Neurodegenerative diseases, such as Alzheimer’s disease (AD), Huntington’s disease (HD), Parkinson’s disease (PD) and amyotrophic lateral sclerosis (ALS), prion diseases are global public health concern. These diseases are characterized by progressive dysfunction and loss of neurons in certain anatomical regions with a variety of clinical presentations. Environmental factors such as Mn exposure may be involved in the etiology and progression of these diseases.
Chelation therapy using CaNa2EDTA and PAS have been utilized to treatment of symptoms related to Mn over exposure. However, several limitations, such as low bioavailability and efficacy, absence of specificity of chelating molecules, difficulty to cross the blood-brain barrier, and potential common and severe adverse effects in patients receiving chelation therapy hinder effective treatment.
Several natural compounds and isolated phytochemicals have been studied in the treatment of Mn neurotoxicity. Phenolic compounds, such as rosmarinic acid, cyaniding, and delphinidin, and silymarin, an antioxidant flavonoid, have demonstrated promising results in mitigating Mn neurotoxicity.
Bioinformatic analysis to identify key genes and pathways, predicting possible molecular mechanisms underlying Mn-induced neurotoxicity and neurodegeneration and new approaches such as proteomic and metabolomic analyses are essential to better understand the molecular mechanisms underlying Mn-induced neurodegenerative disorders, as well as the identification of new targets for its treatment.
Acknowledgments
Funding
This work was supported by the National Institutes of Health to MA (NIEHS R01ES007331 and R01ES10563) and EL (R01 ES024756).
Footnotes
Declaration of interests
The authors have no other relevant affiliations or financial involvement with any organization or entity with a financial interest in or conflict with the subject matter or materials discussed in this manuscript apart from those disclosed.
Reviewer disclosures
Peer reviewers on this manuscript have no relevant financial or other relationships to disclose.
References
- 1.Kovacs GG. Molecular Pathological Classification of Neurodegenerative Diseases: Turning towards Precision Medicine. Int J Mol Sci. 2016. February 2;17(2). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Bosma H, van Boxtel MP, Ponds RW, et al. Pesticide exposure and risk of mild cognitive dysfunction. Lancet. 2000. September 9;356(9233):912–3. [DOI] [PubMed] [Google Scholar]
- 3.Richardson JR, Roy A, Shalat SL, et al. Elevated serum pesticide levels and risk for Alzheimer disease. JAMA Neurol. 2014. March;71(3):284–90. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Kioumourtzoglou MA, Schwartz JD, Weisskopf MG, et al. Long-term PM2.5 Exposure and Neurological Hospital Admissions in the Northeastern United States. Environ Health Perspect. 2016. January;124(1):23–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Palacios N Air pollution and Parkinson’s disease - evidence and future directions. Rev Environ Health. 2017. December 20;32(4):303–313. [DOI] [PubMed] [Google Scholar]
- 6.Reuben A Childhood Lead Exposure and Adult Neurodegenerative Disease. J Alzheimers Dis. 2018;64(1):17–42. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Li SJ, Ren YD, Li J, et al. The role of iron in Parkinson’s disease monkeys assessed by susceptibility weighted imaging and inductively coupled plasma mass spectrometry. Life Sci. 2020. January 1;240:117091. [DOI] [PubMed] [Google Scholar]
- 8.Wu S, Liu H, Zhao H, et al. Environmental lead exposure aggravates the progression of Alzheimer’s disease in mice by targeting on blood brain barrier. Toxicol Lett. 2020. February 1;319:138–147. [DOI] [PubMed] [Google Scholar]
- 9.Sun YH, Nfor ON, Huang JY, et al. Association between dental amalgam fillings and Alzheimer’s disease: a population-based cross-sectional study in Taiwan. Alzheimers Res Ther. 2015. November 12;7(1):65. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Gerhardsson L, Lundh T, Minthon L, et al. Metal concentrations in plasma and cerebrospinal fluid in patients with Alzheimer’s disease. Dement Geriatr Cogn Disord. 2008;25(6):508–15. [DOI] [PubMed] [Google Scholar]
- 11.Peng Q, Bakulski KM, Nan B, et al. Cadmium and Alzheimer’s disease mortality in U.S. adults: Updated evidence with a urinary biomarker and extended follow-up time. Environ Res. 2017. August;157:44–51. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Komatsu F, Kagawa Y, Kawabata T, et al. A high accumulation of hair minerals in Mongolian people: 2(nd) report; influence of manganese, iron, lead, cadmium and aluminum to oxidative stress, Parkinsonism and arthritis. Curr Aging Sci. 2011. February;4(1):42–56. [DOI] [PubMed] [Google Scholar]
- 13.Martins AC Jr., Morcillo P, Ijomone OM, et al. New Insights on the Role of Manganese in Alzheimer’s Disease and Parkinson’s Disease. Int J Environ Res Public Health. 2019. September 22;16(19). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Balachandran RC, Mukhopadhyay S, McBride D, et al. Brain Manganese and the Balance between Essential Roles and Neurotoxicity. J Biol Chem. 2020. March 18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Aschner JL, Aschner M. Nutritional aspects of manganese homeostasis. Mol Aspects Med. 2005. Aug-Oct;26(4–5):353–62. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Andreini C, Bertini I, Cavallaro G, et al. Metal ions in biological catalysis: from enzyme databases to general principles. J Biol Inorg Chem. 2008. November;13(8):1205–18. [DOI] [PubMed] [Google Scholar]
- 17.Fitsanakis VA, Au C, Erikson KM, et al. The effects of manganese on glutamate, dopamine and gamma-aminobutyric acid regulation. Neurochem Int. 2006. May-Jun;48(6–7):426–33. [DOI] [PubMed] [Google Scholar]
- 18.Kawahara M, Kato-Negishi M, Tanaka K. Cross talk between neurometals and amyloidogenic proteins at the synapse and the pathogenesis of neurodegenerative diseases. Metallomics. 2017. June 21;9(6):619–633. [DOI] [PubMed] [Google Scholar]
- 19.Fitsanakis VA, Zhang N, Garcia S, et al. Manganese (Mn) and iron (Fe): interdependency of transport and regulation. Neurotox Res. 2010. August;18(2):124–31. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Ijomone OM, Aluko OM, Okoh COA, et al. Role for calcium signaling in manganese neurotoxicity. J Trace Elem Med Biol. 2019. December;56:146–155. [DOI] [PubMed] [Google Scholar]
- 21.Da Silva ALC, Urbano MR, Almeida Lopes ACB, et al. Blood manganese levels and associated factors in a population-based study in Southern Brazil. J Toxicol Environ Health A. 2017;80(19–21):1064–1077. [DOI] [PubMed] [Google Scholar]
- 22.Oulhote Y, Mergler D, Barbeau B, et al. Neurobehavioral function in school-age children exposed to manganese in drinking water. Environ Health Perspect. 2014. December;122(12):1343–50. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Khan K, Wasserman GA, Liu X, et al. Manganese exposure from drinking water and children’s academic achievement. Neurotoxicology. 2012. January;33(1):91–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Martins AC, Krum BN, Queiros L, et al. Manganese in the Diet: Bioaccessibility, Adequate Intake, and Neurotoxicological Effects. J Agric Food Chem. 2020. April 29. [DOI] [PubMed] [Google Scholar]
- 25.Aschner JL, Anderson A, Slaughter JC, et al. Neuroimaging identifies increased manganese deposition in infants receiving parenteral nutrition. Am J Clin Nutr. 2015. December;102(6):1482–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Amin H, Shawkat A. Manganese Toxicity Complicating Parenteral Nutrition. Am J Ther. 2019. April 17. [DOI] [PubMed] [Google Scholar]
- 27.Livingstone C Manganese Provision in Parenteral Nutrition: An Update. Nutr Clin Pract. 2018. June;33(3):404–418. [DOI] [PubMed] [Google Scholar]
- 28.Gulson B, Mizon K, Taylor A, et al. Changes in manganese and lead in the environment and young children associated with the introduction of methylcyclopentadienyl manganese tricarbonyl in gasoline--preliminary results. Environ Res. 2006. January;100(1):100–14. [DOI] [PubMed] [Google Scholar]
- 29.Miah MR, Ijomone OM, Okoh COA, et al. The effects of manganese overexposure on brain health. Neurochem Int. 2020. January 20;135:104688. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Sriram K, Lin GX, Jefferson AM, et al. Modifying welding process parameters can reduce the neurotoxic potential of manganese-containing welding fumes. Toxicology. 2015. February 3;328:168–78. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Racette BA, Criswell SR, Lundin JI, et al. Increased risk of parkinsonism associated with welding exposure. Neurotoxicology. 2012. October;33(5):1356–61. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Racette BA, Searles Nielsen S, Criswell SR, et al. Dose-dependent progression of parkinsonism in manganese-exposed welders. Neurology. 2017. January 24;88(4):344–351. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Criswell SR, Warden MN, Searles Nielsen S, et al. Selective D2 receptor PET in manganese-exposed workers. Neurology. 2018. September 11;91(11):e1022–e1030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Erikson KM, Aschner M. Manganese: Its Role in Disease and Health. Met Ions Life Sci. 2019. January 14;19. [DOI] [PubMed] [Google Scholar]
- 35.Lee EY, Flynn MR, Lewis MM, et al. Welding-related brain and functional changes in welders with chronic and low-level exposure. Neurotoxicology. 2018. January;64:50–59. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Cersosimo MG, Koller WC. The diagnosis of manganese-induced parkinsonism. Neurotoxicology. 2006. May;27(3):340–6. [DOI] [PubMed] [Google Scholar]
- 37.Perl DP, Olanow CW. The neuropathology of manganese-induced Parkinsonism. J Neuropathol Exp Neurol. 2007. August;66(8):675–82. [DOI] [PubMed] [Google Scholar]
- 38.Tong Y, Yang H, Tian X, et al. High manganese, a risk for Alzheimer’s disease: high manganese induces amyloid-beta related cognitive impairment. J Alzheimers Dis. 2014;42(3):865–78. [DOI] [PubMed] [Google Scholar]
- 39.Ajsuvakova OP, Tinkov AA, Willkommen D, et al. Assessment of copper, iron, zinc and manganese status and speciation in patients with Parkinson’s disease: A pilot study. J Trace Elem Med Biol. 2020. May;59:126423. [DOI] [PubMed] [Google Scholar]
- 40.Joshi P, Bodnya C, Ilieva I, et al. Huntington’s disease associated resistance to Mn neurotoxicity is neurodevelopmental stage and neuronal lineage dependent. Neurotoxicology. 2019. December;75:148–157. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Roos PM, Vesterberg O, Syversen T, et al. Metal concentrations in cerebrospinal fluid and blood plasma from patients with amyotrophic lateral sclerosis. Biol Trace Elem Res. 2013. February;151(2):159–70. [DOI] [PubMed] [Google Scholar]
- 42.Guilarte TR, Burton NC, Verina T, et al. Increased APLP1 expression and neurodegeneration in the frontal cortex of manganese-exposed non-human primates. J Neurochem. 2008. June;105(5):1948–59. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Verina T, Schneider JS, Guilarte TR. Manganese exposure induces alpha-synuclein aggregation in the frontal cortex of non-human primates. Toxicol Lett. 2013. March 13;217(3):177–83. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Bryan MR, Bowman AB. Manganese and the Insulin-IGF Signaling Network in Huntington’s Disease and Other Neurodegenerative Disorders. Adv Neurobiol. 2017;18:113–142. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Bryan MR, Uhouse MA, Nordham KD, et al. Phosphatidylinositol 3 kinase (PI3K) modulates manganese homeostasis and manganese-induced cell signaling in a murine striatal cell line. Neurotoxicology. 2018. January;64:185–194. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Cheng H, Xia B, Su C, et al. PI3K/Akt signaling pathway and Hsp70 activate in hippocampus of rats with chronic manganese sulfate exposure. J Trace Elem Med Biol. 2018. December;50:332–338. [DOI] [PubMed] [Google Scholar]
- 47.Ma X, Han J, Wu Q, et al. Involvement of dysregulated Wip1 in manganese-induced p53 signaling and neuronal apoptosis. Toxicol Lett. 2015. May 19;235(1):17–27. [DOI] [PubMed] [Google Scholar]
- 48.Wang T, Li X, Yang D, et al. ER stress and ER stress-mediated apoptosis are involved in manganese-induced neurotoxicity in the rat striatum in vivo. Neurotoxicology. 2015. May;48:109–19. [DOI] [PubMed] [Google Scholar]
- 49.Lane CA, Hardy J, Schott JM. Alzheimer’s disease. Eur J Neurol. 2018. January;25(1):59–70. [DOI] [PubMed] [Google Scholar]
- 50.Huat TJ, Camats-Perna J, Newcombe EA, et al. Metal Toxicity Links to Alzheimer’s Disease and Neuroinflammation. J Mol Biol. 2019. April 19;431(9):1843–1868. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Pearson HA, Peers C. Physiological roles for amyloid beta peptides. J Physiol. 2006. August 15;575(Pt 1):5–10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Kane MD, Lipinski WJ, Callahan MJ, et al. Evidence for seeding of beta -amyloid by intracerebral infusion of Alzheimer brain extracts in beta -amyloid precursor protein-transgenic mice. J Neurosci. 2000. May 15;20(10):3606–11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Kaur G, Prakash A. Involvement of the nitric oxide signaling in modulation of naringin against intranasal manganese and intracerbroventricular beta-amyloid induced neurotoxicity in rats. J Nutr Biochem. 2020. February;76:108255. [DOI] [PubMed] [Google Scholar]
- 54.Venkataramani V, Doeppner TR, Willkommen D, et al. Manganese causes neurotoxic iron accumulation via translational repression of amyloid precursor protein and H-Ferritin. J Neurochem. 2018. December;147(6):831–848. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Guilarte TR. APLP1, Alzheimer’s-like pathology and neurodegeneration in the frontal cortex of manganese-exposed non-human primates. Neurotoxicology. 2010. September;31(5):572–4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Wallin C, Kulkarni YS, Abelein A, et al. Characterization of Mn(II) ion binding to the amyloid-beta peptide in Alzheimer’s disease. J Trace Elem Med Biol. 2016. December;38:183–193. [DOI] [PubMed] [Google Scholar]
- 57.Lotharius J, Brundin P. Pathogenesis of Parkinson’s disease: dopamine, vesicles and alpha-synuclein. Nat Rev Neurosci. 2002. December;3(12):932–42. [DOI] [PubMed] [Google Scholar]
- 58.de Lau LM, Breteler MM. Epidemiology of Parkinson’s disease. Lancet Neurol. 2006. June;5(6):525–35. [DOI] [PubMed] [Google Scholar]
- 59.Olanow CW. Manganese-induced parkinsonism and Parkinson’s disease. Ann N Y Acad Sci. 2004. March;1012:209–23. [DOI] [PubMed] [Google Scholar]
- 60.Caudle WM. Occupational Metal Exposure and Parkinsonism. Adv Neurobiol. 2017;18:143–158. [DOI] [PubMed] [Google Scholar]
- 61.Dlamini WW, Nelson G, Nielsen SS, et al. Manganese exposure, parkinsonian signs, and quality of life in South African mine workers. Am J Ind Med. 2020. January;63(1):36–43. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Sikk K, Taba P. Methcathinone “Kitchen Chemistry” and Permanent Neurological Damage. Int Rev Neurobiol. 2015;120:257–71. [DOI] [PubMed] [Google Scholar]
- 63.Chong TT, Bonnelle V, Veromann KR, et al. Dissociation of reward and effort sensitivity in methcathinone-induced Parkinsonism. J Neuropsychol. 2018. June;12(2):291–297. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Ennok M, Sikk K, Haldre S, et al. Cognitive profile of patients with manganese-methcathinone encephalopathy. Neurotoxicology. 2020. January;76:138–143. [DOI] [PubMed] [Google Scholar]
- 65.Benskey MJ, Perez RG, Manfredsson FP. The contribution of alpha synuclein to neuronal survival and function - Implications for Parkinson’s disease. J Neurochem. 2016. May;137(3):331–59. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Harischandra DS, Ghaisas S, Zenitsky G, et al. Manganese-Induced Neurotoxicity: New Insights Into the Triad of Protein Misfolding, Mitochondrial Impairment, and Neuroinflammation. Front Neurosci. 2019;13:654. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Polymeropoulos MH, Lavedan C, Leroy E, et al. Mutation in the alpha-synuclein gene identified in families with Parkinson’s disease. Science. 1997. June 27;276(5321):2045–7. [DOI] [PubMed] [Google Scholar]
- 68.Harischandra DS, Jin H, Anantharam V, et al. alpha-Synuclein protects against manganese neurotoxic insult during the early stages of exposure in a dopaminergic cell model of Parkinson’s disease. Toxicol Sci. 2015. February;143(2):454–68. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Cai T, Yao T, Zheng G, et al. Manganese induces the overexpression of alpha-synuclein in PC12 cells via ERK activation. Brain Res. 2010. November 4;1359:201–7. [DOI] [PubMed] [Google Scholar]
- 70.Bornhorst J, Chakraborty S, Meyer S, et al. The effects of pdr1, djr1.1 and pink1 loss in manganese-induced toxicity and the role of alpha-synuclein in C. elegans. Metallomics. 2014. March;6(3):476–90. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Yan DY, Liu C, Tan X, et al. Mn-Induced Neurocytes Injury and Autophagy Dysfunction in Alpha-Synuclein Wild-Type and Knock-Out Mice: Highlighting the Role of Alpha-Synuclein. Neurotox Res. 2019. July;36(1):66–80. [DOI] [PubMed] [Google Scholar]
- 72.Harischandra DS, Ghaisas S, Rokad D, et al. Environmental neurotoxicant manganese regulates exosome-mediated extracellular miRNAs in cell culture model of Parkinson’s disease: Relevance to alpha-synuclein misfolding in metal neurotoxicity. Neurotoxicology. 2018. January;64:267–277. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Harischandra DS, Rokad D, Neal ML, et al. Manganese promotes the aggregation and prion-like cell-to-cell exosomal transmission of alpha-synuclein. Sci Signal. 2019. March 12;12(572). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Zhang Z, Miah M, Culbreth M, et al. Autophagy in Neurodegenerative Diseases and Metal Neurotoxicity. Neurochem Res. 2016. February;41(1–2):409–22. [DOI] [PubMed] [Google Scholar]
- 75.Ross CA, Tabrizi SJ. Huntington’s disease: from molecular pathogenesis to clinical treatment. Lancet Neurol. 2011. January;10(1):83–98. [DOI] [PubMed] [Google Scholar]
- 76.Madison JL, Wegrzynowicz M, Aschner M, et al. Disease-toxicant interactions in manganese exposed Huntington disease mice: early changes in striatal neuron morphology and dopamine metabolism. PLoS One. 2012;7(2):e31024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Williams BB, Li D, Wegrzynowicz M, et al. Disease-toxicant screen reveals a neuroprotective interaction between Huntington’s disease and manganese exposure. J Neurochem. 2010. January;112(1):227–37. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Horning KJ, Caito SW, Tipps KG, et al. Manganese Is Essential for Neuronal Health. Annu Rev Nutr. 2015;35:71–108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Bryan MR, Nordham KD, Rose DIR, et al. Manganese Acts upon Insulin/IGF Receptors to Phosphorylate AKT and Increase Glucose Uptake in Huntington’s Disease Cells. Mol Neurobiol. 2020. March;57(3):1570–1593. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Bichell TJV, Wegrzynowicz M, Tipps KG, et al. Reduced bioavailable manganese causes striatal urea cycle pathology in Huntington’s disease mouse model. Biochim Biophys Acta Mol Basis Dis. 2017. June;1863(6):1596–1604. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Bryan MR, O’Brien MT, Nordham KD, et al. Acute manganese treatment restores defective autophagic cargo loading in Huntington’s disease cell lines. Hum Mol Genet. 2019. November 15;28(22):3825–3841. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Tidball AM, Bryan MR, Uhouse MA, et al. A novel manganese-dependent ATM-p53 signaling pathway is selectively impaired in patient-based neuroprogenitor and murine striatal models of Huntington’s disease. Hum Mol Genet. 2015. April 1;24(7):1929–44. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Daube JR. Electrodiagnostic studies in amyotrophic lateral sclerosis and other motor neuron disorders. Muscle Nerve. 2000. October;23(10):1488–502. [DOI] [PubMed] [Google Scholar]
- 84.Gonzalez-Fernandez C, Gonzalez P, Rodriguez FJ. New insights into Wnt signaling alterations in amyotrophic lateral sclerosis: a potential therapeutic target? Neural Regen Res. 2020. September;15(9):1580–1589. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Wang MD, Little J, Gomes J, et al. Identification of risk factors associated with onset and progression of amyotrophic lateral sclerosis using systematic review and meta-analysis. Neurotoxicology. 2017. July;61:101–130. [DOI] [PubMed] [Google Scholar]
- 86.Olesen MN, Wuolikainen A, Nilsson AC, et al. Inflammatory profiles relate to survival in subtypes of amyotrophic lateral sclerosis. Neurol Neuroimmunol Neuroinflamm. 2020. May;7(3). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Sutedja NA, Veldink JH, Fischer K, et al. Exposure to chemicals and metals and risk of amyotrophic lateral sclerosis: a systematic review. Amyotroph Lateral Scler. 2009. Oct-Dec;10(5–6):302–9. [DOI] [PubMed] [Google Scholar]
- 88.Roos PM, Lierhagen S, Flaten TP, et al. Manganese in cerebrospinal fluid and blood plasma of patients with amyotrophic lateral sclerosis. Exp Biol Med (Maywood). 2012. July;237(7):803–10. [DOI] [PubMed] [Google Scholar]
- 89.Kapaki E, Zournas C, Kanias G, et al. Essential trace element alterations in amyotrophic lateral sclerosis. J Neurol Sci. 1997. April 15;147(2):171–5. [DOI] [PubMed] [Google Scholar]
- 90.Garzillo EM, Lamberti M, Genovese G, et al. Blood lead, manganese, and aluminum levels in a regional Italian cohort of ALS patients: does aluminum have an influence? J Occup Environ Med. 2014. October;56(10):1062–6. [DOI] [PubMed] [Google Scholar]
- 91.Peters TL, Beard JD, Umbach DM, et al. Blood levels of trace metals and amyotrophic lateral sclerosis. Neurotoxicology. 2016. May;54:119–126. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Prusiner SB. Biology and genetics of prions causing neurodegeneration. Annu Rev Genet. 2013;47:601–23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Kawahara M, Kato-Negishi M, Tanaka KI. Amyloids: Regulators of Metal Homeostasis in the Synapse. Molecules. 2020. March 23;25(6). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Mizuno D, Koyama H, Ohkawara S, et al. Involvement of trace elements in the pathogenesis of prion diseases. Curr Pharm Biotechnol. 2014;15(11):1049–57. [DOI] [PubMed] [Google Scholar]
- 95.Choi CJ, Anantharam V, Martin DP, et al. Manganese upregulates cellular prion protein and contributes to altered stabilization and proteolysis: relevance to role of metals in pathogenesis of prion disease. Toxicol Sci. 2010. June;115(2):535–46. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Pass R, Frudd K, Barnett JP, et al. Prion infection in cells is abolished by a mutated manganese transporter but shows no relation to zinc. Mol Cell Neurosci. 2015. September;68:186–93. [DOI] [PubMed] [Google Scholar]
- 97.Brown DR, Hafiz F, Glasssmith LL, et al. Consequences of manganese replacement of copper for prion protein function and proteinase resistance. EMBO J. 2000. March 15;19(6):1180–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Hesketh S, Sassoon J, Knight R, et al. Elevated manganese levels in blood and CNS in human prion disease. Mol Cell Neurosci. 2008. March;37(3):590–8. [DOI] [PubMed] [Google Scholar]
- 99.Kim JJ, Kim YS, Kumar V. Heavy metal toxicity: An update of chelating therapeutic strategies. J Trace Elem Med Biol. 2019. July;54:226–231. [DOI] [PubMed] [Google Scholar]
- 100.Khandelwal S, Kachru DN, Tandon SK. Chelation in metal intoxication. IX. Influence of amino and thiol chelators on excretion of manganese in poisoned rabbits. Toxicol Lett. 1980. August;6(3):131–5. [DOI] [PubMed] [Google Scholar]
- 101.O’Neal SL, Zheng W. Manganese Toxicity Upon Overexposure: a Decade in Review. Curr Environ Health Rep. 2015. September;2(3):315–28. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Nachtman JP, Delor S, Brennan CE. Manganese neurotoxicity: effects of varying oxygen tension and EDTA on dopamine auto-oxidation. Neurotoxicology. 1987. Summer;8(2):249–53. [PubMed] [Google Scholar]
- 103.Kosal MF, Boyle AJ. Ethylenediaminetetraacetic acid in manganese poisoning of rats; a preliminary study. Ind Med Surg. 1956. January;25(1):1–3. [PubMed] [Google Scholar]
- 104.Penalver R Manganese poisoning. Ind Med Surg. 1956. Apr;25(4):190. [PubMed] [Google Scholar]
- 105.Herrero Hernandez E, Discalzi G, Valentini C, et al. Follow-up of patients affected by manganese-induced Parkinsonism after treatment with CaNa2EDTA. Neurotoxicology. 2006. May;27(3):333–9. [DOI] [PubMed] [Google Scholar]
- 106.Tuschl K, Clayton PT, Gospe SM Jr., et al. Syndrome of hepatic cirrhosis, dystonia, polycythemia, and hypermanganesemia caused by mutations in SLC30A10, a manganese transporter in man. Am J Hum Genet. 2012. March 9;90(3):457–66. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.Quadri M, Federico A, Zhao T, et al. Mutations in SLC30A10 cause parkinsonism and dystonia with hypermanganesemia, polycythemia, and chronic liver disease. Am J Hum Genet. 2012. March 9;90(3):467–77. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108.Stamelou M, Tuschl K, Chong WK, et al. Dystonia with brain manganese accumulation resulting from SLC30A10 mutations: a new treatable disorder. Mov Disord. 2012. September 1;27(10):1317–22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109.Di Toro Mammarella L, Mignarri A, Battisti C, et al. Two-year follow-up after chelating therapy in a patient with adult-onset parkinsonism and hypermanganesaemia due to SLC30A10 mutations. J Neurol. 2014. January;261(1):227–8. [DOI] [PubMed] [Google Scholar]
- 110.Zeglam A, Abugrara A, Kabuka M. Autosomal-recessive iron deficiency anemia, dystonia and hypermanganesemia caused by new variant mutation of the manganese transporter gene SLC39A14. Acta Neurol Belg. 2019. September;119(3):379–384. [DOI] [PubMed] [Google Scholar]
- 111.Discalzi G, Pira E, Herrero Hernandez E, et al. Occupational Mn parkinsonism: magnetic resonance imaging and clinical patterns following CaNa2-EDTA chelation. Neurotoxicology. 2000. October;21(5):863–6. [PubMed] [Google Scholar]
- 112.Ono K, Komai K, Yamada M. Myoclonic involuntary movement associated with chronic manganese poisoning. J Neurol Sci. 2002. July 15;199(1–2):93–6. [DOI] [PubMed] [Google Scholar]
- 113.Walter E, Alsaffar S, Livingstone C, et al. Manganese toxicity in critical care: Case report, literature review and recommendations for practice. J Intensive Care Soc. 2016. August;17(3):252–257. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114.Jiang YM, Mo XA, Du FQ, et al. Effective treatment of manganese-induced occupational Parkinsonism with p-aminosalicylic acid: a case of 17-year follow-up study. J Occup Environ Med. 2006. June;48(6):644–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115.Zheng J, Rubin EJ, Bifani P, et al. para-Aminosalicylic acid is a prodrug targeting dihydrofolate reductase in Mycobacterium tuberculosis. J Biol Chem. 2013. August 9;288(32):23447–56. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 116.Ky SQ, Deng HS, Xie PY, et al. A report of two cases of chronic serious manganese poisoning treated with sodium para-aminosalicylic acid. Br J Ind Med. 1992. January;49(1):66–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 117.Yuan ZX, Chen HB, Li SJ, et al. The influence of manganese treatment on the distribution of metal elements in rats and the protection by sodium para-amino salicylic acid. J Trace Elem Med Biol. 2016. July;36:84–9. [DOI] [PubMed] [Google Scholar]
- 118.Li SJ, Ou CY, He SN, et al. Sodium p-Aminosalicylic Acid Reverses Sub-Chronic Manganese-Induced Impairments of Spatial Learning and Memory Abilities in Rats, but Fails to Restore gamma-Aminobutyric Acid Levels. Int J Environ Res Public Health. 2017. April 10;14(4). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 119.Li SJ, Qin WX, Peng DJ, et al. Sodium P-aminosalicylic acid inhibits sub-chronic manganese-induced neuroinflammation in rats by modulating MAPK and COX-2. Neurotoxicology. 2018. January;64:219–229. [DOI] [PubMed] [Google Scholar]
- 120.Li SJ, Li Y, Chen JW, et al. Sodium Para-aminosalicylic Acid Protected Primary Cultured Basal Ganglia Neurons of Rat from Manganese-Induced Oxidative Impairment and Changes of Amino Acid Neurotransmitters. Biol Trace Elem Res. 2016. April;170(2):357–65. [DOI] [PubMed] [Google Scholar]
- 121.Peng DJ, Zhang YW, Li ZC, et al. Preventive impacts of PAS-Na on the slow growth and activated inflammatory responses in Mn-exposed rats. J Trace Elem Med Biol. 2019. July;54:134–141. [DOI] [PubMed] [Google Scholar]
- 122.Li ZC, Wang F, Li SJ, et al. Sodium Para-aminosalicylic Acid Reverses Changes of Glutamate Turnover in Manganese-Exposed Rats. Biol Trace Elem Res. 2019. December 14. [DOI] [PubMed] [Google Scholar]
- 123.Horiguchi T Mechanism of Manganese Toxicity and Tolerance of Plants .2. Deposition of Oxidized Manganese in Plant-Tissues. Soil Sci Plant Nutr. 1987. December;33(4):595–606. [Google Scholar]
- 124.dos Santos GCG, Rodella AA, de Abreu CA, et al. Vegetable species for phytoextraction of boron, copper, lead, manganese and zinc from contaminated soil. Sci Agr. 2010;67(6):713–719. [Google Scholar]
- 125.Kasote DM, Katyare SS, Hegde MV, et al. Significance of antioxidant potential of plants and its relevance to therapeutic applications. Int J Biol Sci. 2015;11(8):982–91. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 126.Djenidi H, Khennouf S, Bouaziz A. Antioxidant activity and phenolic content of commonly consumed fruits and vegetables in Algeria. Progress in Nutrition 2020;22(1):224–235 [Google Scholar]
- 127.Ranjbar A, Khorami S, Safarabadi M, et al. Antioxidant Activity of Iranian Echium amoenum Fisch & C.A. Mey Flower Decoction in Humans: A cross-sectional Before/After Clinical Trial. Evid Based Complement Alternat Med. 2006. December;3(4):469–73. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 128.Belyaeva EA, Sokolova TV, Emelyanova LV, et al. Mitochondrial electron transport chain in heavy metal-induced neurotoxicity: effects of cadmium, mercury, and copper. ScientificWorldJournal. 2012;2012:136063. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 129.Liu K, Luo M, Wei S. The Bioprotective Effects of Polyphenols on Metabolic Syndrome against Oxidative Stress: Evidences and Perspectives. Oxid Med Cell Longev. 2019;2019:6713194. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 130.Agati G, Azzarello E, Pollastri S, et al. Flavonoids as antioxidants in plants: Location and functional significance. Plant Sci. 2012. November;196:67–76. [DOI] [PubMed] [Google Scholar]
- 131.Sadeghi L, Tanwir F, Yousefi Babadi V. Physiological and Biochemical Effects of Echium Amoenum Extract on Mn(2+)-Imposed Parkinson Like Disorder in Rats. Adv Pharm Bull. 2018. November;8(4):705–713. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 132.Sayyah M, Boostani H, Pakseresht S, et al. Efficacy of aqueous extract of Echium amoenum in treatment of obsessive-compulsive disorder. Prog Neuropsychopharmacol Biol Psychiatry. 2009. November 13;33(8):1513–6. [DOI] [PubMed] [Google Scholar]
- 133.Suzuki T, Tsukamoto I. Manganese-induced apoptosis in hepatocytes after partial hepatectomy. Eur J Pharmacol. 2005. November 21;525(1–3):48–53. [DOI] [PubMed] [Google Scholar]
- 134.Du Y, Zhu Y, Teng X, et al. Toxicological Effect of Manganese on NF-kappaB/iNOS-COX-2 Signaling Pathway in Chicken Testes. Biol Trace Elem Res. 2015. November;168(1):227–34. [DOI] [PubMed] [Google Scholar]
- 135.Bianchini MC, Gularte CO, Escoto DF, et al. Peumus boldus (Boldo) Aqueous Extract Present Better Protective Effect than Boldine Against Manganese-Induced Toxicity in D. melanogaster. Neurochem Res. 2016. October;41(10):2699–2707. [DOI] [PubMed] [Google Scholar]
- 136.Gubert P, Puntel B, Lehmen T, et al. Metabolic effects of manganese in the nematode Caenorhabditis elegans through DAergic pathway and transcription factors activation. Neurotoxicology. 2018. July;67:65–72. [DOI] [PubMed] [Google Scholar]
- 137.Sistrunk SC, Ross MK, Filipov NM. Direct effects of manganese compounds on dopamine and its metabolite Dopac: an in vitro study. Environ Toxicol Pharmacol. 2007. May;23(3):286–96. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 138.Santos VD, Bisen-Hersh E, Yu YC, et al. Anthocyanin-Rich Acai (Euterpe oleracea Mart.) Extract Attenuates Manganese-Induced Oxidative Stress in Rat Primary Astrocyte Cultures. J Toxicol Env Heal A. 2014. April 3;77(7):390–404. [DOI] [PubMed] [Google Scholar]
- 139.Alessandra-Perini J, Rodrigues-Baptista KC, Machado DE, et al. Anticancer potential, molecular mechanisms and toxicity of Euterpe oleracea extract (acai): A systematic review. Plos One. 2018;13(7):e0200101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 140.da Silva Santos V, de Almeida Teixeira GH, Barbosa F Jr. Acai (Euterpe oleracea Mart.): a tropical fruit with high levels of essential minerals-especially manganese-and its contribution as a source of natural mineral supplementation. J Toxicol Environ Health A. 2014;77(1–3):80–9. [DOI] [PubMed] [Google Scholar]
- 141.Bahar E, Lee GH, Bhattarai KR, et al. Polyphenolic Extract of Euphorbia supina Attenuates Manganese-Induced Neurotoxicity by Enhancing Antioxidant Activity through Regulation of ER Stress and ER Stress-Mediated Apoptosis. Int J Mol Sci. 2017. January 30;18(2). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 142.Huyut Z, Beydemir S, Gulcin I. Antioxidant and Antiradical Properties of Selected Flavonoids and Phenolic Compounds. Biochem Res Int. 2017;2017:7616791. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 143.Freyssin A, Page G, Fauconneau B, et al. Natural polyphenols effects on protein aggregates in Alzheimer’s and Parkinson’s prion-like diseases. Neural Regen Res. 2018. June;13(6):955–961. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 144.Song Y, Jeong SW, Lee WS, et al. Determination of Polyphenol Components of Korean Prostrate Spurge (Euphorbia supina) by Using Liquid Chromatography-Tandem Mass Spectrometry: Overall Contribution to Antioxidant Activity. J Anal Methods Chem. 2014;2014:418690. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 145.Bayat M, Azami Tameh A, Hossein Ghahremani M, et al. Neuroprotective properties of Melissa officinalis after hypoxic-ischemic injury both in vitro and in vivo. Daru. 2012. October 3;20(1):42. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 146.Dos Santos-Neto LL, de Vilhena Toledo MA, Medeiros-Souza P, et al. The use of herbal medicine in Alzheimer’s disease-a systematic review. Evid Based Complement Alternat Med. 2006. December;3(4):441–5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 147.Sentkowska A, Biesaga M, Pyrzynska K. Polyphenolic Composition and Antioxidative Properties of Lemon Balm (Melissa officinalis L.) Extract Affected by Different Brewing Processes. Int J Food Prop. 2015;18(9):2009–2014. [Google Scholar]
- 148.Martins EN, Pessano NT, Leal L, et al. Protective effect of Melissa officinalis aqueous extract against Mn-induced oxidative stress in chronically exposed mice. Brain Res Bull. 2012. January 4;87(1):74–9. [DOI] [PubMed] [Google Scholar]
- 149.Oboh G, Ogunsuyi OB, Awonyemi OI, et al. Effect of Alkaloid Extract from African Jointfir (Gnetum africanum) Leaves on Manganese-Induced Toxicity in Drosophila melanogaster. Oxid Med Cell Longev. 2018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 150.Panche AN, Diwan AD, Chandra SR. Flavonoids: an overview. J Nutr Sci. 2016;5:e47. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 151.Singh A, Kumar A, Verma RK, et al. Silymarin encapsulated nanoliquid crystals for improved activity against beta amyloid induced cytotoxicity. Int J Biol Macromol. 2020. February 7;149:1198–1206. [DOI] [PubMed] [Google Scholar]
- 152.Chtourou Y, Garoui el M, Boudawara T, et al. Protective role of silymarin against manganese-induced nephrotoxicity and oxidative stress in rat. Environ Toxicol. 2014. October;29(10):1147–54. [DOI] [PubMed] [Google Scholar]
- 153.Manfo FP, Nantia EA, Dechaud H, et al. Protective effect of Basella alba and Carpolobia alba extracts against maneb-induced male infertility. Pharm Biol. 2014. January;52(1):97–104. [DOI] [PubMed] [Google Scholar]
- 154.Nantia EA, Manfo FPT, Beboy NSE, et al. In vitro antioxidant activity of the methanol extract of Basella alba L (Basellaceae) in rat testicular homogenate. Oxid Antioxid Med Sci. 2013;2(2):131–136. [Google Scholar]
- 155.Marreilha Dos Santos AP, Andrade V, Aschner M. Neuroprotective and Therapeutic Strategies for Manganese-Induced Neurotoxicity. Clin Pharmacol Transl Med. 2017;1(2):54–62. [PMC free article] [PubMed] [Google Scholar]
- 156.Ling J, Yang S, Huang Y, et al. Identifying key genes, pathways and screening therapeutic agents for manganese-induced Alzheimer disease using bioinformatics analysis. Medicine (Baltimore). 2018. June;97(22):e10775. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 157.Wegrzynowicz M, Holt HK, Friedman DB, et al. Changes in the striatal proteome of YAC128Q mice exhibit gene-environment interactions between mutant huntingtin and manganese. J Proteome Res. 2012. February 3;11(2):1118–32. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 158.Kumar KK, Goodwin CR, Uhouse MA, et al. Untargeted metabolic profiling identifies interactions between Huntington’s disease and neuronal manganese status. Metallomics. 2015. February;7(2):363–70. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 159.Fernandes J, Chandler JD, Liu KH, et al. Metabolomic Responses to Manganese Dose in SH-SY5Y Human Neuroblastoma Cells. Toxicol Sci. 2019. May 1;169(1):84–94. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 160.Kumar KK, Lowe EW, Jr., Aboud AA, et al. Cellular manganese content is developmentally regulated in human dopaminergic neurons. Sci Rep. 2014. October 28;4:6801. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 161.Peres TV, Horning KJ, Bornhorst J, et al. Small Molecule Modifiers of In Vitro Manganese Transport Alter Toxicity In Vivo. Biol Trace Elem Res. 2019. March;188(1):127–134. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 162.Horning KJ, Joshi P, Nitin R, et al. Identification of a selective manganese ionophore that enables nonlethal quantification of cellular manganese. J Biol Chem. 2020. March 20;295(12):3875–3890. [DOI] [PMC free article] [PubMed] [Google Scholar]