Abstract
Purpose
Upgrade rates of conventional ADH are reported at 10–30%; however, rates for ADH bordering on DCIS (ADH-BD) are largely unknown. We examined the upgrade rate of ADH-BD and core needle biopsy (CNB) features associated with upgrade. Surgical management in patients with concurrent ipsilateral breast cancer (BC) was also examined.
Methods
From 2000–2018, women with CNB diagnosis of ADH-BD were prospectively identified. Women with pure ADH-BD and concurrent ipsilateral ADH-BD/BC were analyzed separately, and upgrade rates were calculated. CNB features associated with upgrade and type of surgery were examined in women with pure ADH-BD; CNB features and concurrent pathology associated with upgrade were examined in women with ipsilateral BC.
Results
108/236 (46%) patients with pure ADH-BD on CNB had DCIS (40%) or invasive carcinoma (6%) on surgical excision. DCIS or invasive carcinoma was more frequently found on excision of a mass that yielded ADH-BD on biopsy than excision of calcifications (65% vs. 38%; p<0.001). The breast conservation success rate was high (80%) in patients who upgraded, despite a high re-excision rate of 46%. The upgrade rate of ADH-BD in women with concurrent ipsilateral BC was 41%. Most women (94%) with ADH-BD in the same quadrant as the BC were candidates for breast conserving surgery, with a success rate of 89%.
Conclusion
The upgrade rate for pure ADH-BD is significantly higher than that reported for women with conventional ADH, especially in women with a mass on imaging. The upgrade rate of concurrent ipsilateral ADH-BD and BC is similarly high. Excision with a margin of normal tissue and specimen inking should be routine to minimize the need for re-excision.
Keywords: atypical ductal hyperplasia, ductal carcinoma in situ, markedly atypical ductal hyperplasia, borderline lesions, breast surgery, ipsilateral breast cancer
Introduction
Atypical ductal hyperplasia (ADH) and low-grade ductal carcinoma in situ (DCIS) are morphologically similar entities with very different clinical significance. ADH is considered a high-risk lesion, and excision is recommended to rule out an underlying malignancy. In the absence of an underlying malignancy, patients are offered endocrine therapy for risk reduction and no additional local therapy. In contrast, DCIS is treated as a cancer with excision to negative margins, endocrine therapy when hormone receptor positive, and adjuvant radiation therapy in many cases. Pathologists distinguish between ADH and low-grade DCIS using quantitative criteria. If the area of atypia is greater than 2 mm in linear extent or involves 2 or more basement membrane-bound spaces, it is considered DCIS.1–3
Borderline epithelial lesions, also known as atypical ductal hyperplasia bordering on DCIS (ADH-BD), are ductal proliferations that elude precise categorization into either ADH or DCIS.4 For example, a patient may have atypical ductal cells similar to that of DCIS, but the lesion span is < 2 mm, leading to interobserver variability in the diagnosis.5 Definitive categorization of these borderline lesions at the time of core biopsy can be challenging given the small sample size and tissue fragmentation. Excision of these borderline lesions is important, as the additional tissue sampled may allow for definitive classification as either ADH or DCIS.
While the surgical excision upgrade rate for conventional ADH ranges from 10–30% 1,6–10, the upgrade rate for ADH-BD is less well described. A single-institution series of 74 patients with “markedly” atypical ductal hyperplasia found a 45% upgrade rate to DCIS and 4% upgrade rate to DCIS and invasive carcinoma 11. Increased utilization of bilateral breast MRI for high-risk surveillance, as well as its increased use as an additional imaging modality for breast cancer patients, has increased the number of high-risk atypical epithelial lesions identified compared to imaging with digital mammography alone 12. In this study, we examined a large contemporary series of patients with ADH-BD on core biopsy, to determine rate of upgrade to DCIS or invasive carcinoma. In addition, we examined the impact on surgical management of ipsilateral ADH-BD in patients with concurrent DCIS and/or invasive carcinoma.
Materials and Methods
Following approval from the Memorial Sloan Kettering Cancer Center Institutional Review Board, an institutional database was queried to identify women with a breast core needle biopsy (CNB) diagnosis of ADH-BD from 2000 through 2018. Search keywords included “markedly atypical ductal hyperplasia”, “markedly atypical ductal hyperplasia bordering on DCIS”, and “markedly atypical ductal hyperplasia focally reaching low-grade DCIS”, all of which were classified as ADH-BD. All patients underwent CNB at Memorial Sloan Kettering Cancer Center or had pathology slides submitted for internal review prior to surgical treatment. Patients without available surgical excision pathology due to treatment at an outside institution following CNB and those with prior history of ipsilateral breast cancer were excluded. Women with synchronous ipsilateral DCIS and/or invasive breast carcinoma (BC) were included, but analyzed as a separate cohort from those without an ipsilateral carcinoma. Upgrade was defined as diagnosis of DCIS or invasive carcinoma on surgical excision. In the synchronous ipsilateral BC/ADH-BD cohort, upgrade rate was determined in patients with ADH-BD in a separate quadrant of the breast who underwent either 2 or more simultaneous lumpectomies or mastectomy. Upgrade rate in patients with synchronous ipsilateral/ADH-BD undergoing single lumpectomy could not be ascertained.
Patient demographics, indication for CNB, biopsy features, and pathologic data were obtained from the medical record and pathology database. Clinical characteristics were compared between groups using Fisher’s exact test for categorical variables and the Wilcoxon rank-sum test for continuous variables. All tests were evaluated for statistical significance at alpha level 0.05. Statistical analysis was performed using SAS version 9.4 (SAS Institute Inc., Cary, NC).
Results
Pure Atypical Ductal Hyperplasia Bordering on Ductal Carcinoma In Situ
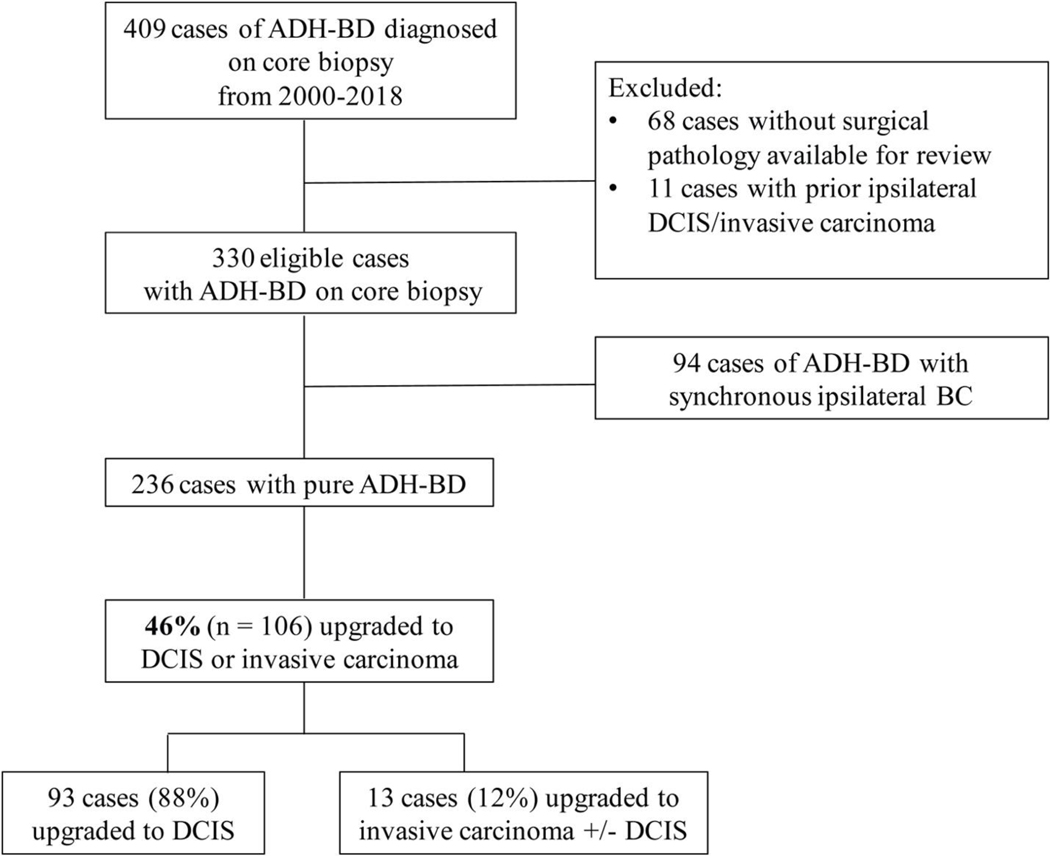
In total, 236 patients with pure ADH-BD on core biopsy were identified between 2000 and 2018 (Figure 1). Median patient age was 53 years (range 29–88 years), and 139 patients (59%) were postmenopausal at diagnosis. The most common radiographic indications for CNB were indeterminate calcifications on screening mammography in 171 patients (72%), a mass on imaging in 52 patients (22%), and suspicious enhancement on screening MRI in 10 patients (4%) (Table 1). Of the 52 patients who presented with a mass on imaging, 18 (35%) were palpable according to documentation by the treating surgeon. 155 patients (66%) underwent stereotactic biopsy, 48 patients (20%) underwent ultrasound-guided biopsy, 10 patients (4%) underwent MRI-guided biopsy, and 23 patients (10%) underwent biopsies with an unknown type of image guidance.
Figure 1.
Rate of upgrade of ADH-BD on core biopsy to in situ or invasive disease
ADH-BD atypical ductal hyperplasia bordering on ductal carcinoma in situ, DCIS ductal carcinoma in situ, BC breast carcinoma
Table 1.
Rate of upgrade in patients with pure ADH-BD by core biopsy factors Frequency (percent) reported unless otherwise noted.
| Overall (n = 236) | Upgraded (n = 108) | Not upgraded (n = 128) | p value* | |
|---|---|---|---|---|
| Core needle biopsy diagnosis | 0.39 | |||
| “Reaching/approaching DCIS” | 29 | 11 (10.2) | 18 (14.1) | |
| “Bordering on DCIS” | 104 | 50 (46.3) | 54 (42.2) | |
| “Suspicious for DCIS” | 16 | 10 (9.3) | 6 (4.7) | |
| “Markedly atypical ductal hyperplasia” | 87 | 37 (34.3) | 50 (39.1) | |
| Indication for biopsy | < 0.001 | |||
| Indeterminate calcifications | 171 | 65 (60.2) | 106 (82.8) | |
| Mass | 52 | 34 (31.5) | 18 (14.1) | |
| Suspicious MRI enhancement | 10 | 6 (5.6) | 4 (3.1) | |
| Unknown | 3 | 3 (2.8) | 0 (0) | |
| Type of core biopsy | 0.001 | |||
| MR-guided | 10 | 6 (5.6) | 4 (3.1) | |
| Stereotactic | 155 | 57 (52.8) | 98 (76.6) | |
| Ultrasound-guided | 48 | 32 (29.6) | 16 (12.5) | |
| Unknown | 23 | 13 (12.0) | 10 (7.8) | |
| Number of core specimens Median (range), n† | 8 (1, 31), 74 | 6 (1, 27), 37 | 8 (1, 31), 37 | 0.04 |
| Needle gauge Median (range), n‡ | 11 (6, 22), 89 | 11 (8, 14), 46 | 11 (6, 22), 43 | 0.27 |
Results from Fisher’s exact test for categorical variables, and the Wilcoxon rank-sum test for continuous variables.
Number of core specimens is missing for 67% of the observations.
Needle gauge is missing for 62% of the observations.
ADH-BD atypical ductal hyperplasia bordering on ductal carcinoma in situ, DCIS ductal carcinoma in situ, MR magnetic resonance
Overall, 108 of 236 patients (46%) with ADH-BD on CNB had DCIS or invasive carcinoma at surgical excision (Table 2). Pure DCIS was present in 40% of cases, and invasive carcinoma was present with or without associated DCIS in 6% of cases. In patients with pure DCIS at surgical excision, 30% of cases (28/93) were low grade, 58% (54/93) were intermediate grade, and 12% (11/93) were high grade. Of the 13 patients who upgraded to invasive carcinoma, 11 patients (85%) had invasive ductal, 1 patient (8%) had invasive lobular, and 1 patient (8%) had invasive tubular; median invasive cancer size was 10 mm (range 1–27 mm). Ten of the 13 patients underwent axillary staging with sentinel lymph node biopsy, and 1 was found to have axillary nodal metastases.
Table 2.
Final pathology at time of surgical excision in patients with pure ADH-BD diagnosed on core biopsy (n = 236)
| Final pathology | n (%) |
|---|---|
| DCIS/invasive carcinoma | 108 (45.8) |
| DCIS | 95 (40.3) |
| Invasive carcinoma | 13 (5.5) |
| High-risk lesion | 82 (34.7) |
| ADH-BD | 10 (4.2) |
| ADH | 33 (14.0) |
| ADH and lobular neoplasia | 18 (7.6) |
| Lobular neoplasia | 21 (8.9) |
| Benign pathology | 46 (19.5) |
CNB core needle biopsy, ADH-BD atypical ductal hyperplasia bordering on ductal carcinoma in situ, ADH atypical ductal hyperplasia
The upgrade rate in patients presenting with a mass was 65% (34/52), compared to 38% (65/171) in those with indeterminate calcifications. In total, 62% of patients (8/13) who upgraded to invasive carcinoma and 56% (52/93) who upgraded to DCIS presented with a mass. Ultrasound-guided biopsy was associated with the highest risk of upgrade (67% [32/48], p < 0.001) compared to stereotactic and MRI-guided biopsies. Patients who had an upgrade at the time of surgery had a lower number of core biopsy specimens compared to patients who did not have an upgrade (6 vs. 8 specimens, p = 0.04).
Most patients with pure ADH-BD (92%, 216/236) underwent wide local excision (WLE) via needle or seed-localization. Upfront mastectomy was performed in 18 patients (8%) due to patient preference or at the recommendation of the treating surgeon due to the presence of extensive residual calcifications following CNB. Ninety-seven patients (44%) who underwent WLE upgraded to DCIS or invasive carcinoma, 57 (59%) had positive or close (≤2 mm) margins, and 45 (46%) underwent at least one re-excision; 19 patients (20%) ultimately underwent mastectomy following WLE.
Of the 128 patients who did not have DCIS or invasive cancer at surgical excision, 82 (64%) had high-risk lesions and 46 (36%) had benign pathology (Table 2). High-risk lesions included conventional ADH (40%), conventional ADH with LCIS (22%), pure LCIS (26%), and persistent ADH-BD (12%). All 10 patients with persistent ADH-BD were managed with lumpectomy alone and were offered chemoprevention. No patients required re-excision for close or positive margins. One patient was referred to radiation oncology for evaluation, but radiation was not ultimately recommended. Nine of 10 patients with available follow-up information had no evidence of disease at a median follow-up of 10 years. Chemoprevention uptake was 15% (12/82) among patients with persistent high-risk lesions at surgical excision.
Synchronous Ipsilateral ADH-BD and DCIS or Invasive Carcinoma
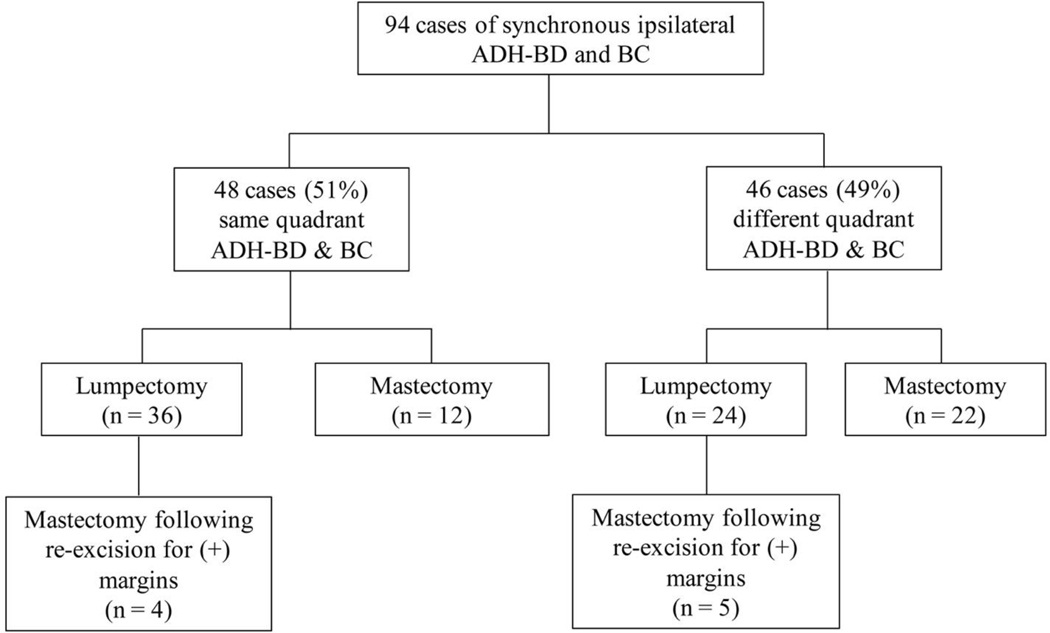
There were 94 patients with concurrent ipsilateral ADH-BD and DCIS or invasive carcinoma (BC). Median age was 56 years (range 20–80 years); 56 patients (60%) were postmenopausal at the time of ADH-BD diagnosis. 47 patients (50%) had a concurrent diagnosis of DCIS and 47 patients (50%) had a concurrent diagnosis of invasive carcinoma. Most patients with invasive carcinoma had invasive ductal (72%); other subtypes included invasive lobular carcinoma (n = 7), invasive mammary carcinoma (n = 3), or papillary carcinoma (n = 3). Approximately half of patients (n = 46) had ADH-BD and BC in the same quadrant of the breast. The remaining 48 patients had ADH-BD and BC in different quadrants. Sixty patients elected to undergo breast-conservation therapy, and, of these, only 9 ultimately required mastectomy.
Of the 48 patients with synchronous ADH-BD and BC in different quadrants, 12 had two separate lumpectomies and 22 underwent mastectomy (Figure 2), and the risk of upgrade for ADH-BD was assessed for these patients. The rate of upgrade for ADH-BD in this setting was 41% (14/34). The presence of synchronous DCIS compared to invasive carcinoma was associated with a higher risk of upgrade of the ADH-BD site (59% vs. 29%, p = 0.01). The majority of patients with synchronous ADH-BD and ipsilateral DCIS or invasive carcinoma in separate quadrants ultimately required mastectomy (59%; 27/46).
Figure 2.
Surgical management following diagnosis of synchronous ipsilateral ADH-BD and DCIS and/or invasive carcinoma
ADH-BD atypical ductal hyperplasia bordering on ductal carcinoma in situ, BC breast carcinoma
Upgrade rates could not be calculated for patients with same-quadrant disease or in patients with one excision specimen, due to imprecise estimation of boundaries between the two lesions. Of the 48 patients with ADH-BD in the same quadrant as the BC, 94% (45 patients) were deemed by the treating surgeon to be candidates for breast conservation. Three patients had extensive residual calcifications following CNB and were recommended to undergo mastectomies. 80% of patients (36/45) who were deemed good candidates underwent lumpectomies: 8 patients (22%) required re-excision for positive or close margins at the ADH-BD site, of whom 4 ultimately required mastectomies. The success rate of breast conservation in women with ADH-BD in the same quadrant as the BC was 89% (32/36), including women who had one successful re-excision for involved margins. 12 patients underwent upfront mastectomy for synchronous ADH-BD and BC in the same quadrant, 8 of whom were candidates for breast conservation, according to the treating surgeon’s documentation.
Discussion
In this study, pure ADH-BD was associated with an upgrade rate of 46%, and synchronous ADH-BD and BC was associated with an upgrade rate of 41%. The risk of upgrade to cancer in the presence of CNB-diagnosed ADH-BD is substantially higher than the 10–30% risk associated with conventional ADH. In spite of the higher risk of upgrade, the majority of women with pure ADH-BD (80%) and with ADH-BD and ipsilateral BC (85%) were able to successfully undergo breast-conservation therapy without conversion to mastectomy.
Upgrade rates of borderline lesions have previously been reported in studies examining outcomes of conventional ADH. Deshaies et al 7 reported an upgrade rate of 59% in 88 patients with CNB-diagnosed “severe ADH.” The adjusted odds ratio for upgrade of severe ADH compared to absence of severe ADH on multivariate analysis was 4.53 (95% confidence interval [CI] 2.69–7.62). An association between “severe ADH” and upgrade at surgery was also reported in two small series 13,14. Of two cohorts of 62 and 26 patients, respectively, “severe” or “marked” ADH was identified in 9 and 6 core specimens, with upgrade rates of 44% and 83%, respectively. To our knowledge, we report the largest series of patients with borderline lesions on CNB and the associated risk of underestimating cancer on CNB.
We found that ADH-BD found on core biopsy of a mass on imaging was significantly associated with upgrade as compared to that found after biopsy of calcifications. Ultrasound-guided biopsies are typically performed for masses rather than calcifications on imaging, and therefore it was not surprising that they were associated with a higher rate of upgrade than mammographic or MRI-guided biopsies. Retrospective studies evaluating upgrade rates of ADH without marked atypia have similarly found a correlation between the presence of a mass, especially if palpable, with increased risk of cancer on surgical excision 8,15–17. However, this was not observed in Vandenbussche’s series of CNB-diagnosed borderline cases, likely due to a small sample size.
More extensive sampling with a higher number of core biopsies was associated with a lower risk of upgrade. These likely allows for better sampling of the lesion, which improves sensitivity of the core biopsy. We hypothesized that larger core needle gauge would be associated with lower risk of upgrade; however, this was not substantiated on univariate analysis. This is likely explained by the relatively high number of missing observations, due to a large proportion of patients who underwent biopsies at outside institutions and submitted slides for institutional review prior to surgical treatment. Nevertheless, the literature supports use of larger needle gauge to reduce underestimation of cancer at surgical excision in the setting of conventional ADH 18,19.
The increased frequency of upgrade for borderline lesions has important clinical implications with regards to preoperative counseling and surgical planning. Complete excision of ADH in the absence of DCIS is not required. Conversely, consensus guidelines for DCIS endorse 2 mm margins in conjunction with whole-breast irradiation (WBRT) to ensure minimal risk of ipsilateral breast tumor recurrence 20. Although no guidelines for margin width exist for ADH-BD, our findings warrant preoperative consideration of excising margins according to DCIS guidelines, with specimen inking, given the significantly higher risk of upgrade. Additionally, we identified a higher re-excision rate for close or positive margins following excision of pure ADH-BD in patients who upgraded (46%). The rate of re-excision for pure ADH-BD is higher than reported rates from our institution for DCIS in patients undergoing whole breast irradiation (38% prior to adoption of SSO-ASTRO guidelines for DCIS and 29% in the postguideline era).21 Among those with close margins in the study by Mamtani et al, the re-excision rate was 65% in the preguideline era versus 63% in the postguideline era, further emphasizing consideration of margins in the setting of pure ADH-BD. The re-excision rate for ADH-BD with concurrent ipsilateral BC (22%) more closely reflects the pooled prevalence of re-excisions for invasive disease reported in a meta-analysis (22% vs 14% after SSO-ASTRO guideline publication)22 and rates reported by authors from our institution (21.4 vs 15.1%).23 Although the re-excision rate was lower in this cohort, specimen inking may still be considered to minimize the need for re-operation in these patients.
The diagnosis of ADH-BD concurrently with ipsilateral breast cancer was influential in surgical decision making from a patient perspective. When found at distinct sites in the same quadrant of the breast, the majority of patients remain candidates for and choose breast-conserving surgery. Interestingly, 75% of patients who had mastectomies for disease in the same quadrant would have been candidates for breast-conserving surgery based on opinion of the treating surgeon. This finding raises the concern that a diagnosis of ADH-BD in the setting of breast cancer may influence patients to pursue more-aggressive surgery without additional oncologic benefit. When diagnosed in separate quadrants of the breast, breast-conserving surgery remains an option in selected patients for whom the cosmetic outcomes are acceptable with multiple excisions or with one large excision. Patient-reported cosmetic outcome data following BCT with adjuvant radiation in patients with multiple ipsilateral BC was recently published as a planned secondary endpoint of the Alliance Z11102 trial.24 No difference was observed in cosmesis scores or overall satisfaction when stratifying by number of lumpectomies or size of the largest area of disease. Good to excellent cosmetic outcomes were reported up to 5 years following treatment. Consequently, patients with ADH-BD and concurrent ipsilateral BC should be counseled on favorable cosmesis in the setting of multiple or one large excision followed by radiation. Although the majority of patients in our series with different-quadrant disease required mastectomy, our findings should inform shared decision-making with patients regarding surgical management.
Long-term follow-up studies of women with borderline lesions identified at surgical excision have previously been published by authors from this institution. Choi et al 25 reported that rates of ipsilateral breast events (IBEs) in patients with borderline lesions were similar to rates for DCIS at a median follow-up of 5 years (7.7 vs. 7.2%, p = 0.80), and nearly identical to that of low-grade DCIS not treated with radiation (p = 0.95) Furthermore, no difference was seen in the 5-year invasive IBE rate between borderline lesions and DCIS (6.5 vs. 2.5%, p = 0.25). Coopey et al 26 reported a 6.8% rate of invasive disease in patients with severe ADH bordering on DCIS at a median follow-up of 5.7 years. These data should be highlighted in discussions of recurrence risk and inform the discussion of chemoprevention with patients diagnosed with ADH-BD following excision.
There are strengths and limitations to our study. Limitations include its retrospective nature as well as the inherent variability in interpretation and reporting of borderline lesions. Additionally, missing data on the number of CNB specimens and needle gauge, due to biopsies being performed at outside institutions, limits the interpretability of our data with regard to technical features of CNB associated with upgrade. Strengths include its large size and pathology diagnoses rendered by experienced subspecialized breast pathologists.
Conclusion
We report a 46% upgrade rate of CNB-diagnosed pure ADH-BD with no increase in upgrade above this rate in women with synchronous ipsilateral DCIS and/or invasive carcinoma. The presence of a mass and biopsy under ultrasound-guidance were associated with an increased risk of upgrade, consistent with literature describing upgrade rates of conventional ADH without marked atypia. Finally, we identified a high rate of re-excisions following upgrade, suggesting that excision of tissue with adequate margins should be considered in the setting of core biopsy-diagnosed ADH-BD.
Acknowledgments
This study has not previously been presented.
Funding: The preparation of this study was supported in part by NIH/NCI Cancer Center Support Grant No. P30 CA008748 to Memorial Sloan Kettering Cancer Center.
Footnotes
Disclosures and Compliance with Ethical Standards
Compliance with Ethical Standards
Ethical approval: All procedures performed in studies involving human participants were in accordance with the ethical standards of the institutional and/or national research committee and with the 1964 Helsinki declaration and its later amendments or comparable ethical standards. This article does not contain any studies with animals performed by any of the authors.
Informed consent: Informed consent was obtained from all individual participants included in the study.
Supporting Data Statement: Supporting data for this study will be made available upon reasonable request.
Conflict of interest: First author Dr. Kate Pawloski declares that she has no conflict of interest. Second author Dr. Nicole Christian declares that she has no conflict of interest. Third author Andrea Knezevic declares that she has no conflict of interest. Fourth author Dr. Hannah Y. Wen declares that she has no conflict of interest. Fifth author Dr. Kimberly J. Van Zee declares that she has no conflict of interest. Sixth author Dr. Monica Morrow declares the receipt of speaking honoraria from Genomic Health. Seventh author Dr. Audree B. Tadros declares that she has no conflict of interest
Publisher's Disclaimer: This Author Accepted Manuscript is a PDF file of an unedited peer-reviewed manuscript that has been accepted for publication but has not been copyedited or corrected. The official version of record that is published in the journal is kept up to date and so may therefore differ from this version.
References:
- 1.Racz JM, Carter JM, Degnim AC. Lobular Neoplasia and Atypical Ductal Hyperplasia on Core Biopsy: Current Surgical Management Recommendations. Ann Surg Oncol. 2017;24(10):2848–2854. [DOI] [PubMed] [Google Scholar]
- 2.Page DL, Dupont WD, Rogers LW, Rados MS. Atypical hyperplastic lesions of the female breast. A long-term follow-up study. Cancer. 1985;55(11):2698–2708. [DOI] [PubMed] [Google Scholar]
- 3.Tavassoli FA, Norris HJ. A comparison of the results of long-term follow-up for atypical intraductal hyperplasia and intraductal hyperplasia of the breast. Cancer. 1990;65(3):518–529. [DOI] [PubMed] [Google Scholar]
- 4.Rosai J. Borderline epithelial lesions of the breast. Am J Surg Pathol. 1991;15(3):209–221. [DOI] [PubMed] [Google Scholar]
- 5.Tozbikian G, Brogi E, Vallejo CE, et al. Atypical Ductal Hyperplasia Bordering on Ductal Carcinoma In Situ. Int J Surg Pathol. 2017;25(2):100–107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Burak WE Jr., Owens KE, Tighe MB, et al. Vacuum-assisted stereotactic breast biopsy: histologic underestimation of malignant lesions. Arch Surg. 2000;135(6):700–703. [DOI] [PubMed] [Google Scholar]
- 7.Deshaies I, Provencher L, Jacob S, et al. Factors associated with upgrading to malignancy at surgery of atypical ductal hyperplasia diagnosed on core biopsy. Breast. 2011;20(1):50–55. [DOI] [PubMed] [Google Scholar]
- 8.Jackman RJ, Birdwell RL, Ikeda DM. Atypical ductal hyperplasia: can some lesions be defined as probably benign after stereotactic 11-gauge vacuum-assisted biopsy, eliminating the recommendation for surgical excision? Radiology. 2002;224(2):548–554. [DOI] [PubMed] [Google Scholar]
- 9.Sohn V, Arthurs Z, Herbert G, et al. Atypical ductal hyperplasia: improved accuracy with the 11-gauge vacuum-assisted versus the 14-gauge core biopsy needle. Ann Surg Oncol. 2007;14(9):2497–2501. [DOI] [PubMed] [Google Scholar]
- 10.Winchester DJ, Bernstein JR, Jeske JM, et al. Upstaging of atypical ductal hyperplasia after vacuum-assisted 11-gauge stereotactic core needle biopsy. Arch Surg. 2003;138(6):619–622; discussion 622–613. [DOI] [PubMed] [Google Scholar]
- 11.Vandenbussche CJ, Khouri N, Sbaity E, et al. Borderline atypical ductal hyperplasia/low-grade ductal carcinoma in situ on breast needle core biopsy should be managed conservatively. Am J Surg Pathol. 2013;37(6):913–923. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Kuhl CK, Keulers A, Strobel K, Schneider H, Gaisa N, Schrading S. Not all false positive diagnoses are equal: On the prognostic implications of false-positive diagnoses made in breast MRI versus in mammography / digital tomosynthesis screening. Breast Cancer Res. 2018;20(1):13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Adrales G, Turk P, Wallace T, Bird R, Norton HJ, Greene F. Is surgical excision necessary for atypical ductal hyperplasia of the breast diagnosed by Mammotome? Am J Surg. 2000;180(4):313–315. [DOI] [PubMed] [Google Scholar]
- 14.Philpotts LE, Lee CH, Horvath LJ, Lange RC, Carter D, Tocino I. Underestimation of breast cancer with II-gauge vacuum suction biopsy. AJR Am J Roentgenol. 2000;175(4):1047–1050. [DOI] [PubMed] [Google Scholar]
- 15.Co M, Kwong A, Shek T. Factors affecting the under-diagnosis of atypical ductal hyperplasia diagnosed by core needle biopsies - A 10-year retrospective study and review of the literature. Int J Surg. 2018;49:27–31. [DOI] [PubMed] [Google Scholar]
- 16.Khoury T, Chen X, Wang D, et al. Nomogram to predict the likelihood of upgrade of atypical ductal hyperplasia diagnosed on a core needle biopsy in mammographically detected lesions. Histopathology. 2015;67(1):106–120. [DOI] [PubMed] [Google Scholar]
- 17.Ko E, Han W, Lee JW, et al. Scoring system for predicting malignancy in patients diagnosed with atypical ductal hyperplasia at ultrasound-guided core needle biopsy. Breast Cancer Res Treat. 2008;112(1):189–195. [DOI] [PubMed] [Google Scholar]
- 18.Jang M, Cho N, Moon WK, Park JS, Seong MH, Park IA. Underestimation of atypical ductal hyperplasia at sonographically guided core biopsy of the breast. AJR Am J Roentgenol. 2008;191(5):1347–1351. [DOI] [PubMed] [Google Scholar]
- 19.Mesurolle B, Perez JC, Azzumea F, et al. Atypical ductal hyperplasia diagnosed at sonographically guided core needle biopsy: frequency, final surgical outcome, and factors associated with underestimation. AJR Am J Roentgenol. 2014;202(6):1389–1394. [DOI] [PubMed] [Google Scholar]
- 20.Morrow M, Van Zee KJ, Solin LJ, et al. Society of Surgical Oncology-American Society for Radiation Oncology-American Society of Clinical Oncology Consensus Guideline on Margins for Breast-Conserving Surgery with Whole-Breast Irradiation in Ductal Carcinoma In Situ. Ann Surg Oncol. 2016;23(12):3801–3810. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Mamtani A, Romanoff A, Baser R, Vincent A, Morrow M, Gemignani ML. Adoption of SSO-ASTRO Margin Guidelines for Ductal Carcinoma in Situ: What Is the Impact on Use of Additional Surgery? Ann Surg Oncol. 2020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Havel L, Naik H, Ramirez L, Morrow M, Landercasper J. Impact of the SSO-ASTRO Margin Guideline on Rates of Re-excision After Lumpectomy for Breast Cancer: A Meta-analysis. Annals of Surgical Oncology. 2019;26(5):1238–1244. [DOI] [PubMed] [Google Scholar]
- 23.Rosenberger LH, Mamtani A, Fuzesi S, et al. Early Adoption of the SSO-ASTRO Consensus Guidelines on Margins for Breast-Conserving Surgery with Whole-Breast Irradiation in Stage I and II Invasive Breast Cancer: Initial Experience from Memorial Sloan Kettering Cancer Center. Annals of Surgical Oncology. 2016;23(10):3239–3246. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Rosenkranz KM, Ballman K, McCall L, et al. Cosmetic Outcomes Following Breast-Conservation Surgery and Radiation for Multiple Ipsilateral Breast Cancer: Data from the Alliance Z11102 Study. Ann Surg Oncol. 2020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Choi DX, Eaton AA, Olcese C, Patil S, Morrow M, Van Zee KJ. Blurry boundaries: do epithelial borderline lesions of the breast and ductal carcinoma in situ have similar rates of subsequent invasive cancer? Ann Surg Oncol. 2013;20(4):1302–1310. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Coopey SB, Mazzola E, Buckley JM, et al. The role of chemoprevention in modifying the risk of breast cancer in women with atypical breast lesions. Breast Cancer Res Treat. 2012;136(3):627–633. [DOI] [PubMed] [Google Scholar]