Abstract
Introduction:
Despite maximal surgical resection and chemoradiation, glioblastoma (GBM) continues to be associated with significant morbidity and mortality. Novel therapeutic strategies are urgently needed. Given success in treating multiple other forms of cancer, checkpoint inhibitor immunotherapy remains foremost amongst novel therapeutic strategies that are currently under investigation.
Areas covered:
Through a systematic review of both published literature and the latest preliminary data available from ongoing clinical studies, we provide an up-to-date discussion on the immune system in the CNS, a detailed mechanistic evaluation of checkpoint biology in the CNS along with evidence for disruption of these pathways in GBM, and a summary of available preclinical and clinical data for checkpoint blockade in GBM. We also include a discussion of novel, emerging targets for checkpoint blockade which may play an important role in GBM immunotherapy.
Expert opinion:
Evidence indicates that while clinical success of checkpoint blockade for the treatment of GBM has been limited to date, through improved preclinical models, optimization in the context of standard of care therapies, assay standardization and harmonization, and combinatorial approaches which may include novel targets for checkpoint blockade, checkpoint inhibitor immunotherapy may yield a safe and effective therapeutic option for the treatment of GBM.
Keywords: cancer, glioma, immunotherapy, antibodies, checkpoint inhibitors, clinical trials
1. Background
Although representative of only ~2% of all adult cancers, the World Health Organization (WHO) grade IV tumor, glioblastoma (GBM) is one of the deadliest and its treatment remains a significant challenge [1,2]. GBM is the most common primary malignant brain tumor in adults [3,4] and has recently been distinguished from IDH mutant tumors as these IDH-wild type diffuse astrocytic gliomas have histological and genetic features predictive of highly aggressive clinical behavior [5]. The median survival of patients with GBM is 15 months with standard of care treatment [6–8]. Standard of care therapy continues to be centered on maximal surgical resection as a less than gross total resection portends a poorer prognosis [9]. Concurrent temozolomide (TMZ) and radiotherapy is usually initiated four weeks post-surgery to allow for wound healing, followed by adjuvant TMZ [10]. Other Food and Drug Administration (FDA) – approved GBM therapies include alternating electric fields, bevacizumab and intracavitary wafers. Unfortunately, radiotherapy is often ineffective against tumor cells in hypoxic environments such as those found in larger tumors with necrotic cores [11,12]. Of note, recent evidence has emerged to suggest that radiation may in fact drive progression of GBM tumors to more malignant phenotypes [13]. Despite its ability to cross the BBB, only about 20% of TMZ contained within plasma penetrates the cerebrospinal fluid (CSF) due to its low solubility and short half-life (i.e. 1.8 hours) [14]. As such, high systemic doses are typically required to reach therapeutic levels within the CNS [14–16]. This can result in off target toxicity that requires cessation of therapy. Moreover, treatment resistance and subsequent failure is common with continuous and prolonged treatment and can occur through a myriad of mechanisms [17]. For example, O(6)-methylguanine-DNA-methyltransferase (MGMT) directly repairs damaged DNA caused by alkylating agents such as TMZ and is more active in patients with prior exposure to TMZ thus contributing to treatment resistance [18,19]. MGMT methylation status has also been linked to survival [20,21]. Finally, it is prudent to note that GBM is a genetically and functionally heterogeneous tumor, with a subset of cancer stem cells (CSCs) that exhibit self-renewal capacity and resistance to standard of care therapy [22].
Immunotherapy has emerged as a promising alternative treatment strategy for a number of tumors. Critically, the dogma that the brain is an immune privileged environment has been eroded with a litany of evidence now having demonstrated fully functional innate and adaptative immunity within the brain [23,24]. Immunotherapeutic strategies in development for GBM encompass several different approaches which, for example, in addition to checkpoint inhibitors including the delivery of oncolytic viruses [25], bispecific antibodies [26–28], adoptive transfer of chimeric antigen receptors (CAR) T cells [29–32], cytokine therapies [33], tumor or peptide vaccines [34–36] and dendritic cell therapies [37,38]. Given the success of immune checkpoint inhibitor immunotherapy in many other forms of cancer, however, checkpoint inhibitors remain an attractive choice currently under clinical investigation for the treatment of GBM. Indeed, immune checkpoint blockade has shown significant preclinical promise [39–43]. Recent advanced-phase clinical trials, however, have failed to yield improved patient outcomes, suggesting that additional preclinical and clinical studies are necessary to improve our understanding of this immunomodulatory therapeutic platform such that it may achieve meaningful results in the treatment of GBM. Here, we focus on data published over the previous decade in the National Library of Medicine journal citation database (MEDLINE) and the latest preliminary data available from ongoing clinical studies to provide an up-to-date discussion on the immune system in the CNS, a detailed mechanistic evaluation of checkpoint biology in the CNS along with evidence for disruption of these pathways in GBM, and a summary of available preclinical and clinical data for checkpoint blockade in GBM. We also include a discussion of novel, emerging targets for checkpoint blockade which may play an important role in GBM immunotherapy.
2. Tumor Immune Response in GBM
The tumor immune response is a complex interplay between cancer cells and the immune system, consisting of recognition of tumor antigens via the presentation of these antigens by antigen-presenting cells (APCs) to antigen-specific naïve T-cells (Figure 1). Although fewer tumor-associated antigens have been identified from gliomas than from other tumors, viable immunologic targets have been described, such as EGFRvIII, SART3, MAGE1, gp100, IL13Ra2, HER2, EphrinA2, amongst others [44]. To mount an anti-glioma response, the immune system must be able to access these antigens and present them to antigen-specific naïve T-cells. For CNS antigens, this initial activation of T-cells occurs in the periphery [45], as the brain lacks secondary lymphoid organs, and under physiologic conditions the brain’s resident myeloid cell population, microglia, express low levels of the accessory molecules necessary for efficient antigen presentation [46,47]. The CNS possesses a unique system of lymphatics that provide an anatomic pathway for brain antigens to reach lymphoid organs for presentation, with cerebrospinal fluid functioning as lymph. This was first described with the discovery that antigen transferred into the brain collects in the cervical lymph nodes [48], and later the discovery that injection of antigen can lead to an antibody response [49]. The interstitial fluid of the CNS drains via perivascular channels into the CSF, across the cribriform plate to nasal mucosa and collects in the deep cervical lymph nodes (DCLN), where they are taken up by dendritic cells (DCs) and presented to naïve antigen-specific T-cells [23,46]. Several groups have shown that intracerebrally injected DCs migrate to the DCLN chains and activate tumor-specific T-cells that migrate to the brain [50], while others have shown that intracerebrally injected antigen can activate T-cells within DCLN [51]. In addition to DCs in the DCLN, other cell types that may play a role in immune surveillance include choroid plexus epithelial cells, and meningeal, perivascular and ventricular macrophages, all of which are external to the parenchyma, but can sample antigen via access to the CSF [46,47].
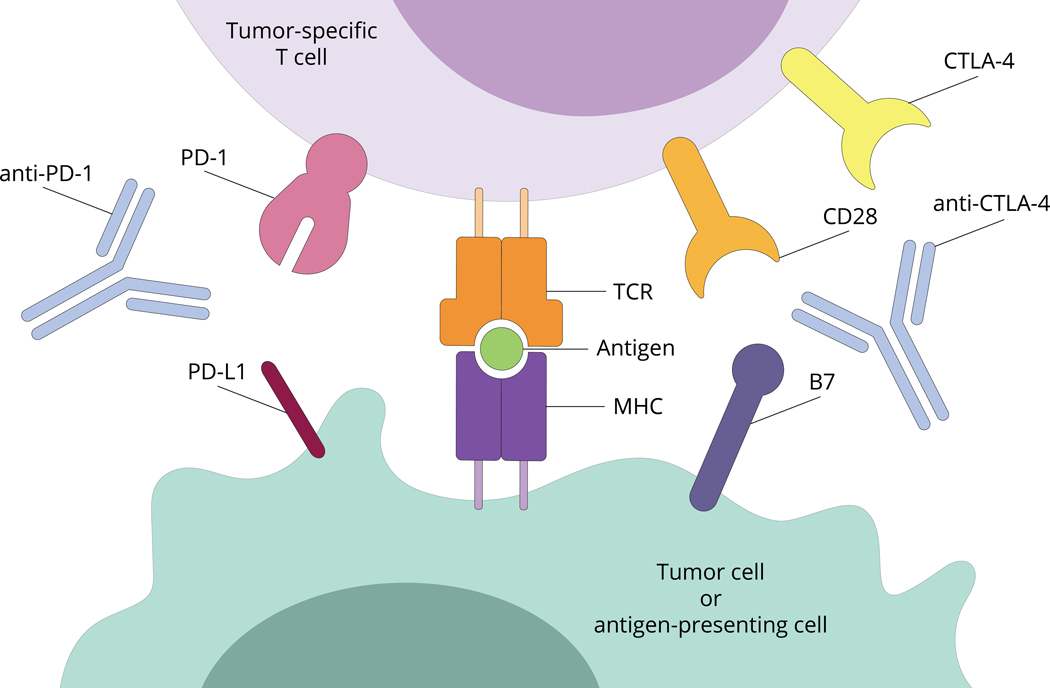
Figure 1 – Schematic overview of T cell engagement with tumor cells or antigen presenting cells.
Tumor cells and antigen presenting cells (APCs) express a transmembrane major histocompatibility complex (MHC) on their surface. T cell receptors (TCRs) on the surface of T cells are capable of recognize MHC bound to target peptide antigen leading to subsequent T cell activation. Secondary signals via PD-L1 to PD-1 binding and B7 to CD28 or B7 to CTLA-4 serve to modulate the T cell response to APCs or tumor cells expressing target antigen. Checkpoint inhibitors including anti-PD-1 and anti-CTLA-4 have been designed to augment the immune response. Please see accompanying text for further details.
Within the lymph nodes, tumor antigen is presented to antigen-specific naïve T-lymphocytes, which then become activated and travel from the blood to the brain under the control of chemokines which guide their pathotropic “homing” to the site of tumor [52,53]. Here, these tumor-infiltrating lymphocytes (TILs) exert their cytotoxic function with increased numbers of TILs having been correlated with greater overall survival in a number of different cancers [54–56]. In glioma, a study of 519 patients, increased CD8+ T cell infiltrate was found to correlate with long-term survival [57], highlighting the role of cytotoxic lymphocytes in the anti-tumor response. Others have likewise shown that increased CD3+ and CD8+ immune cell infiltration correlates with improved survival [58]. Still others have demonstrated that patients with tumors that have a higher density of (Forkhead box P3) Foxp3+ TILs have a shorter progression free survival and overall survival, while a higher density of CD8+ TILs provided no significant difference in survival [59]. Some have concluded that methodological issues including low sample size and limited study designs have precluded more definitive conclusions [60].
Several of these steps in the immune response are subject to regulation by activating or inhibitory signals known as checkpoints, which modify the duration and intensity of the response. Checkpoint signaling can occur during the priming or effector phase. The priming phase, described above, occurs in secondary lymphoid organs with the presentation of antigen by APCs to naïve T-cells, and is subject to inhibitory checkpoints via receptor-ligand interactions between naïve lymphocytes and the APCs (e.g., cytotoxic T-lymphocyte-associated protein 4 (CTLA-4)/B7) [61,62]. The activated cells that make it past the first checkpoint then undergo upregulation of inhibitory receptors such as programmed cell death protein 1 (PD-1) [63]or T-cell immunoglobulin and mucin-domain containing-3 (TIM-3) [64], which can interact with their ligands programmed death-ligand 1 (PD-L1) [65] or galectin (GAL)-9 [66], respectively, in the tumor and/or tumor stroma. In a physiologic state, these checkpoints are critical for downregulating immune responses and thus avoiding potentially devastating neurologic sequela that could occur from an unrestrained response. These checkpoints, however, are often usurped by tumors as a way to evade the tumor immune response (reviewed in [67]); therefore, a firm understanding of the physiologic and pathobiologic function of these checkpoints are critical in an effort to harness them as effective therapies for GBM.
3. An Overview of Checkpoint Biology
3.1. Inhibitors and costimulators
In a simplified sense, the immune synapse between a T-cell and APC or target cell consists of a primary and secondary signal; the former occurs via interactions between the T-cell receptor (TCR) and antigen loaded on a major histocompatibility complex (MHC) while the latter is either co-stimulatory or co-inhibitory, which ultimately determines the trajectory of the T-cell response that unfolds. Importantly, much of what has been described about mechanisms underlying the function of either co-stimulatory (e.g., CD28, CD80, CD86, CD137, OX40) or co-inhibitory (e.g., CTLA-4, PD-1, lymphocyte-activation gene 3 (LAG-3), TIM3) checkpoint proteins has been derived from solid tumor models of melanoma and the underlying immunobiology in GBM is therefore less well-described.
3.2. CTLA-4: activation
CTLA-4 serves as a regulatory checkpoint at the priming stage by interfering with one of the signals required for T-cell activation (Figure 2). As per the above mentioned, priming of naïve T-cells in secondary lymphoid organs requires an immune synapse to form that consists of two signals, 1) peptide antigen presented via the MHC, which engages the TCR, and 2) a costimulatory signal from one of several molecules; for activation this is typically B7 on the APC binding with CD28 on the T-cell surface. However, CD28 shares its ligand, B7, with the receptor CTLA-4, which also localizes to the T-cell surface in response to antigen recognition [68,69]. CTLA-4 possesses a higher affinity for B7 than does CD28 [68], leading to CTLA-4 outcompeting and blocking the costimulatory interaction between CD28 and B7, inhibiting activation and leading to anergy [70]. Ligand-bound CTLA-4 also inhibits intracellular signaling involved in T-cell activation [71,72]. The critical role for CTLA-4 in modulating immune response is demonstrated in CTLA-4-deficient mice, who demonstrate lethal immune system hyperactivation [61]. CTLA-4-mediated immune regulation also occurs via regulatory T-cells (Treg) at the effector site. Treg are characterized by expression of Foxp3 transcription factor, which controls transcription of the Ctla4 gene such that Treg constitutively express CTLA-4 [73]. CTLA-4 is critical to the immunosuppressive function of Treg by binding B7 and leading to its downregulation on APCs [74]. This function of CTLA-4 becomes particularly significant in tumors, which contain increased fractions of Treg cells [75].
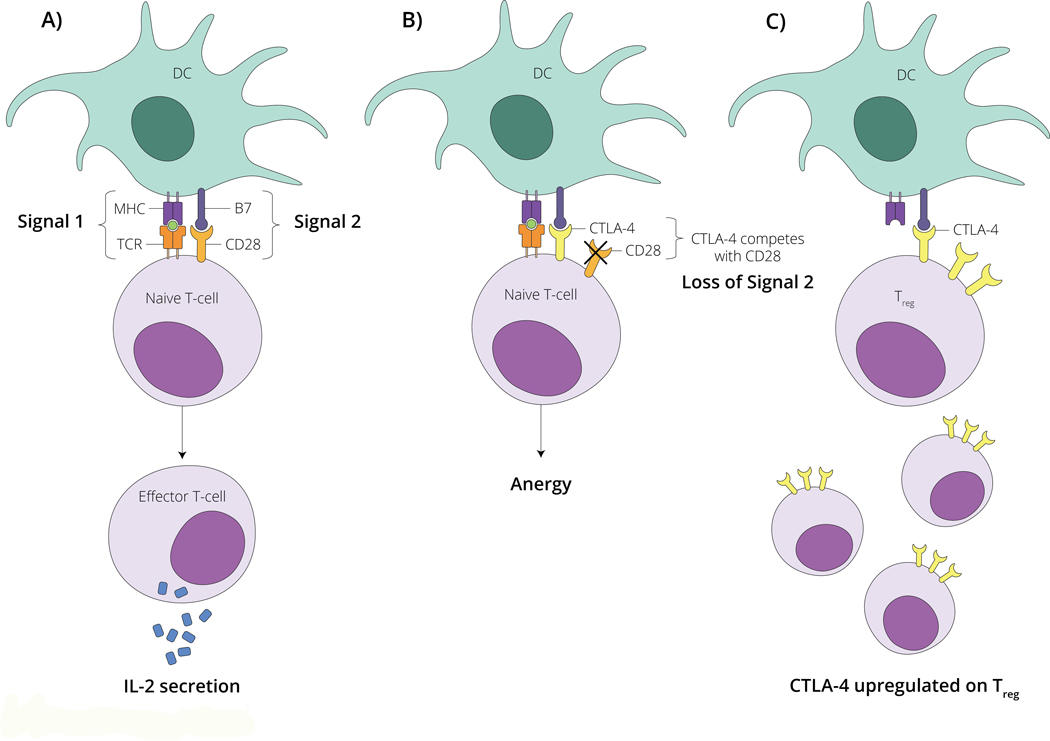
Figure 2 – Schematic overview of CTLA-4 modulated T cell function.
A) T cell are activated by dendritic cells (DC) via signal 1 (MHC-antigen-TCR binding) and signal 2 (B7-CD28 binding) leading to a naïve t cell becoming a functional effector T cell capable of secreting interleukin-2 (IL-2) which serves to further propagate the immune response. B) CTLA-4 on the surface of T cells competes with CD28 for B7 binding. When CTLA-4 binds to B7 (loss of signal 2), a signaling cascade is induced that leads to T cell anergy. C) CTLA-4 is upregulated on the surface of regulatory T cells (Treg). This leads to a predominance of B7-CTLA-4 binding among this T cell subset. Modulating CTLA-4 with therapeutic antibodies can lead to effective anti-tumor immune responses. Please see accompanying text for further details.
3.3. PD-1: effector phase
While CTLA-4 exerts its regulatory activity mainly during the priming of naïve T-cells, PD-1 acts as a checkpoint during the effector stage of immune response (Figure 3). PD-1 is upregulated on the surface of activated T-cells, and its signaling leads to reduced proliferation, cytokine production and impaired cytolytic function [76]. Prolonged antigen exposure, such as that which occurs with chronic viral infection or cancer, leads to persistently high levels of PD-1 expression and can lead to a state of T-cell exhaustion [77]. PD-1’s two ligands, PD-L1 (B7-H1) and PD-L2 (B7-DC) differ. While PD-L2 expression is limited to professional APCs, including DCs and some macrophages, PD-L1 is expressed on peripheral epithelial cells, activated hematopoietic cells, and tumors [76] and is upregulated in response to interferon (IFN)-gamma production by activated T-cells [78]. PD-1/PD-L1 interaction plays an important role in peripheral tolerance [79]. PD-L1 can also interact with B7, (the ligand from CD28 and CTLA-4) to deliver inhibitory signals [80], adding another layer of complexity to this immunoregulatory pathway. PD-L2-PD-1 interaction likewise inhibits TCR-mediated cytokine production and proliferation [81]. APCs from PD-L2-dificient mice have been shown to be more potent in inducing T cell activation compared to wild-type controls [82]. The control of expression of the two ligands differs, however, as PD-L1 expression is primarily upregulated by IFN-gamma, while both IFN-gamma and IFN-beta upregulate PD-L2 expression [83]. PD-L1 and PD-L2 also differ in their interaction with PD-1, with PD-L2 binding to PD-1 with an approximately 3-fold stronger affinity compared to PD-L1 [84–87].
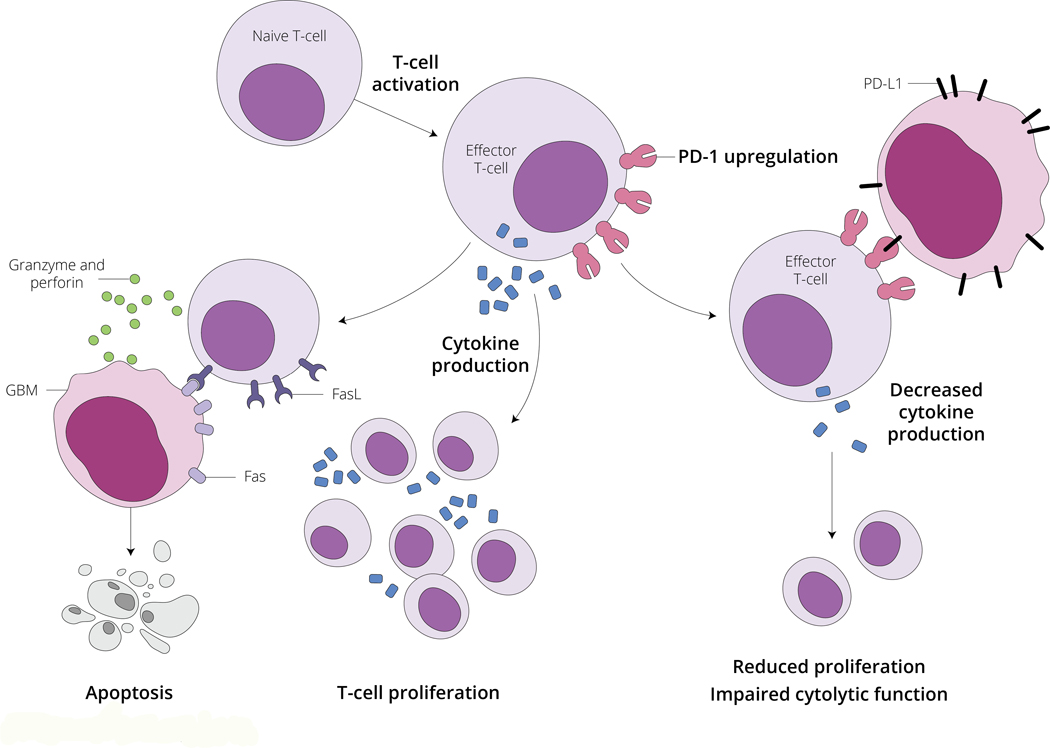
Figure 3 – Schematic overview of PD-1 function and T cell mediated tumor cell death.
Activated T cells produce cytokines, including interleukin-2 (IL-2) which via autocrine and paracrine signaling lead to T cell proliferation. Activated T cells are capable of inducing T cell apoptosis via the Fas-Fas Ligand (FasL) system. Fas-FasL binding results in localized secretion of granzyme and perforin. This locally released complex is capable of inducing tumor cell apoptosis. PD-1 is also upregulated on the surface of activated T cells. PD-L1 is expressed on the surface of tumor cells, and when engaged with its receptor on the surface of T cells, PD-1, results in decreased T cell cytokine secretion, decreased activated T cell proliferation, and impaired T cell cytolytic function. Modulating PD-1 or PD-L1 with therapeutic antibodies can lead to effective anti-tumor immune responses. Please see accompanying text for further details.
4. Immune Dysregulation in GBM
GBM exerts its potent immunosuppression via a variety of mechanisms, including production of immunosuppressive cytokines, impaired antigen presentation by APCs [88], recruitment/proliferation of Treg cells [75], and T-cell exhaustion [89]. One important mechanism of immunosuppression is dysregulation of the checkpoint signaling described above.
The PD-1/PD-L1 axis is a known mediator of immunosuppression in GBM. Interestingly PD-1 expression is increased on peripheral T-cells in patients with grade II through IV astrocytomas as compared to healthy controls, and expression correlates with increasing tumor grade [90]. PD-L1, rarely expressed on the cell surface in normal human tissues (albeit with a few exceptions such as tonsil and placenta, and some cells from the macrophage lineage [91]), is also significantly increased on glioma cells, tumor associated macrophages, and circulating monocytes found in GBM patients [92]. In a sample of 135 human GBM specimens, over 70% of cells expressed PD-L1, and the percentage was even higher in newly diagnosed GBM [93]. This increase in PD-L1 expression likely occurs both through tumor specific mutations and through adaptive response to inflammation. Loss of the tumor suppressor phosphate and tensin homology (PTEN) and activation of the phosphatidylinositol-3-OH kinase (PI(3)K) pathway have been shown to increase expression of PD-L1, and tumors expressing the mutated PTEN show resistance to tumor-specific T-cell lysis as compared to glioma cells expressing wild-type PTEN [94]. PD-L1 expression also increases in response to inflammatory cytokines such as IFN-gamma, which are produced by TILs as a part of the anti-tumor response [91,95]. This upregulation of PD-L1 in response to inflammation has been termed “adaptive resistance” [96]. PD-L1 expression on tumor cells facilitates apoptosis of activated T-cells, T-cell anergy, and T-cell exhaustion [97]. Accordingly, it is perhaps unsurprising that expression of PD-L1 on tumor cells is associated with poor prognosis and decreased overall survival in several types of cancer [98–100], including GBM [101]. Interestingly, expression of PD-L1 on surrounding neurons may actually correlate with a better prognosis as these PD-L1 expressing neurons induce a caspase-dependent apoptosis of glioma cell [101]. Further work will be necessary to fully elucidate the relationship between PD-L1 expression and GBM prognosis, but current evidence suggests an unfavorable cumulative effect on prognosis [102–107].
Engagement of CTLA-4 on naïve T-cells also results in an impaired anti-glioma immune response, demonstrated most saliently by the fact that CTLA-4 polymorphisms which alter gene expression and increase downregulation of T-cell activity yield increased risk for developing gliomas [108]. Because of its inhibitory role in antigen priming, the effects of CTLA-4 pose particular challenges to immunotherapies designed to induce anti-tumor immune responses, such as DC vaccines [109]. CTLA-4 also plays a role in glioma-induced immunosuppression through its role with Treg, which are increased both systemically and within the tumor microenvironment in GBM patients [75]. Finally it is important to reiterate that CTLA-4 is constitutively expressed on Treg, where it augments immunosuppressive functions and prevents T-cell responses to tumor antigen(s) [110].
It is also important to consider the impact of standard of care therapies on the immune response to GBM. Temozolomide chemotherapy and radiation, proven to provide a survival benefit for patients with GBM, carry a major side effect of lymphopenia. Multiple immunotherapeutic approaches have proven efficacious in the context of lymphopenia [111–113], however, likely a result of lymphopenia induced homeostatic cytokines that reduce the activation threshold and induce the proliferation of T-cells [114]. Dexamethasone is also frequently used for the treatment of GBM-induced cerebral edema as it decreases the permeability of the blood-brain barrier [115]. It is the glucocorticoid of choice given its long half-life, high potency, and minimal mineralocorticoid activity [116]. A recent study by Giles et al. evaluated the effect of dexamethasone during checkpoint blockade. The authors found that dexamethasone upregulates CTLA-4 expression in CD4- and CD8-positive T cells and diminishes CD28-mediated T cell differentiation and proliferation. CTLA-4 blockade, however, was found to partially abrogate the immune inhibitory effects of dexamethasone, leading to increased proliferation and IFN-gamma expression in human T cells exposed to dexamethasone and increased IFN-gamma expressing TILs and extended survival in dexamethasone treated preclinical murine glioma models [117].
5. Checkpoint Inhibitor Immunotherapy
As discussed above, inhibitory checkpoints are physiologic mechanisms designed to protect the body from an unrestrained immune response; unfortunately, many cancers, including GBM, usurp these protective pathways and upregulate checkpoints proteins which results in dampening of the host immune response. Checkpoint blockade is an FDA-approved strategy for treating multiple solid tumors, most notably melanoma and non-small-cell lung carcinoma (NSCLC) [118]. The overall approach has been facilitated via development of humanized monoclonal antibodies that selectively block signaling through these inhibitory pathways [119]. Given the observed tumor regression and improved survival in other solid tumors, this approach is being actively explored in GBM in both the preclinical and clinical settings.
5.1. Preclinical Studies
An early preclinical study in this space by Fecci et al. demonstrated that anti-CTLA-4 monotherapy employed against a murine glioma model resulted in ~80% long term survival [40]. The authors showed that anti-CTLA-4 monotherapy reversed the dramatic reductions in CD4+ T-cell counts induced by glioma and conferred resistance to Treg mediated immunosuppression. They also noted that anti-CTLA-4 therapy conferred its therapeutic benefits without inducing experimental encephalomyelitis, allaying fears that checkpoint blockade might unleash an autoimmune response within the brain. A subsequent study by Reardon et al. showed that treatment of glioma-bearing mice with single agent anti-PD-1, anti-PD-L1 and anti-CTLA-4 produced long-term tumor-free survival in 50%, 20% and 15% of animals respectively. Furthermore, combination therapy with anti-CTLA-4 plus anti-PD-1 cured 75% of animals, and even prevented tumor growth upon intracranial tumor rechallenge [39]. Still, accurately predicting human responses to immune checkpoint inhibitor immunotherapy in preclinical models of glioblastoma remains a challenge. To better predict clinical responses, a novel GBM model, SB28, has recently been developed to recapitulate key human GBM characteristics including low mutational load and low MHC-I expression; unlike the commonly used, highly immunogenic GL261 tumor model, this novel tumor model induces modest CD8+ T-cell infiltration and is resistant to immune checkpoint blockade [120]. Such a model better recapitulates human GBM and is useful for ongoing efforts to study checkpoint immunotherapy.
5.2. Clinical Studies
There are many ongoing clinical trials investigating checkpoint blockade in newly diagnosed or recurrent GBM. Trials investigating blockade of PD-1 with the monoclonal antibody, nivolumab are ongoing. Unfortunately the initial results from CheckMate 143 (ClinicalTrials.gov NCT02017717), the first large randomized clinical trial of program death pathway inhibition in the setting of GBM, have failed to demonstrate a prolongation in overall survival when comparing nivolumab to bevacizumab in the treatment of recurrent GBM [121]. Despite this setback the efficacy of nivolumab is currently being investigated in multiple phase 2 trials in newly diagnosed GBM patients. One of these trials is pursuing the addition of nivolumab to standard of care (temozolomide plus radiation) (NCT02667587, CheckMate-548), while another is comparing nivolumab to temozolomide, each combined with radiation therapy (NCT02617589, CheckMate-498). Preliminary results from the CheckMate-498 study, a randomized Phase 3, multi-center study evaluating nivolumab and radiation versus temozolomide and radiation in patients with newly diagnosed MGMT-unmethylated GBM after surgery, demonstrate that the primary endpoint of overall survival was not met at final analysis [122]. Full evaluation of the data is ongoing and peer-reviewed publication of the results is pending.
The results of a recent randomized, multi-institution clinical trial designed to evaluate immune responses and survival following neoadjuvant and/or adjuvant therapy with pembrolizumab (anti-PD-1) in patients with recurrent, surgically resectable GBM have also been published [123]. Via this important work Cloughesy et al. demonstrate that patients who were randomized to receive neoadjuvant pembrolizumab, with continued adjuvant therapy following surgery, had significantly extended overall survival as compared to patients that were randomized to receive adjuvant, post-surgical PD-1 blockade [123]. Of note, neoadjuvant PD-1 blockade was also associated with upregulation of T cell– and IFN-γ-related gene expression, and downregulation of cell-cycle-related gene expression within the tumor, which was not seen in patients that received adjuvant therapy alone [123]. Finally, focal induction of PD-L1 in the tumor microenvironment, enhanced clonal expansion of T-cells, decreased PD-1 expression on peripheral blood T-cells and a decreasing monocytic population was observed more frequently in the neoadjuvant group [123]. A second prospective study conducted by Schalper et al. appears to support the validity of neoadjuvant centered checkpoint strategies [124]. They again demonstrated that neoadjuvant delivery of an anti-PD-1 monoclonal antibody (i.e. nivolumab) resulted in enhanced expression of chemokine transcripts, higher immune cell infiltration and most interestingly appeared to augmented TCR clonal diversity among TILs [124]. These findings are critical and suggest that the neoadjuvant administration of PD-1 blockade enhances both the local and systemic anti-tumor immune response and may represent a more efficacious approach to the treatment of this uniformly lethal brain tumor thereby suggesting that the temporality of checkpoint therapy must be considered in future trials.
Many other trails are currently underway investigating combinations of immunotherapeutic strategies, including dual checkpoint blockade. For example, in patients with recurrent GBM, blockade of LAG-3 or stimulation of CD137 (4–1BB) alone or in combination with anti-PD-1 is being examined (NCT02658981). In patients with newly diagnosed GBM, a phase 1 trial has begun to assess ipilimumab (anti-CTLA-4) and nivolumab, individually and in combination (NCT02311920). Others are combining tumor vaccines with checkpoint blockade (NCT02529071, NCT03018288, NCT03014804).
6. Emerging Targets for Checkpoint Blockade in GBM
While the majority of current clinical trials are investigating the classical immune checkpoints (i.e. PD-1 and CTLA-4), there are newly recognized alternative checkpoints that provide additional opportunities for study and treatment. Characterizations of GBM and TILs within the tumor microenvironment have demonstrated expression of additional markers, including TIM-3, LAG-3, T-cell immunoreceptor with Ig and ITIM domains (TIGIT) and CD39, among others [125–127]. Of note, within GBM, there is significantly lower functionality of TILs that are triple positive for PD-1, TIM-3, and LAG-3 [89], suggesting a correlation between mounting immune checkpoint expression and degree of T-cell exhaustion. These results highlight the importance of exploring alternative checkpoints.
TIM-3 is increasingly recognized as a negative regulator of T-cell responses. The binding of TIM-3 to GAL-9, one of its ligands, induces T-cell death through intracellular calcium influx [66]. Studies have demonstrated increased TIM-3 expression on exhausted CD8+ T-cells [128] and in mouse models of many solid tumors it is often co-expressed with PD-1, representing a severe state of exhaustion [129]. Given its potential for dampening the response of T-cells to tumor antigens, TIM-3 represents an attractive target for therapy. Preclinical study of combination therapy targeting PD-1 and TIM-3 along with focal radiation demonstrated efficacy in murine glioma [130]. While not yet under investigation in human GBM, several groups are exploring anti-TIM-3 monotherapy and combination checkpoint blockade with anti-PD-1 and anti-TIM-3 in phase I and II trials (NCT02817633 [Tesaro, Inc.], NCT03099109 [Eli Lilly], NCT02608268 [Novartis]) for advanced solid tumors.
LAG-3 is an MHC class II ligand expressed on activated T-cells [131]. With similar homology to CD4, LAG-3 competes for MHC class II and when bound is able to negatively regulate antigen-dependent responses [131,132]. Additionally, LAG-3 is expressed on Treg cells and enhances their immunosuppressive capacity [133]. In an alternative manner of immunosuppression, Treg are able to gain expression of MHC class II through the process of trogocytosis [134] and subsequently bind LAG-3 on effector cells, dampening their activity [133]. Given its upregulation on exhausted T-cells, namely TILs in the setting of cancer, the blockade of LAG-3 is being targeted in the clinical setting for GBM and other malignancies. A phase I clinical trial investigating anti-LAG-3 alone or in combination with nivolumab (anti-PD-1) in patients with recurrent GBM has recently begun recruitment (NCT02658981).
Another alternative checkpoint gaining recognition for its role in interfering with anti-tumor immunity is CD39, an ectonucleotidase. When tissues are disrupted in pathologic conditions such as inflammation, hypoxia, and/or malignancy, the extracellular level of ATP rises [135]. Anchored in the cell membrane of B-cells, monocytes, and T-cells, CD39 catalyzes the hydrolysis of ATP and ADP into AMP, which is then converted to adenosine [136]. While its effects vary by receptor binding, in the tumor setting adenosine modulates immune cells, creating an immunosuppressed state with enhanced Treg function and dampened T effector and NK cell function [136]. With preliminary investigations demonstrating increased CD39 expression on Treg in GBM and correlation with decreased survival [137], CD39 may represent a future target for checkpoint blockade.
7. Expert Commentary & Five-Year View
Despite the success of immune checkpoint blockade strategies in many solid tumors, efficacy of immune checkpoint blockade in GBM is still wanting. The failure of the CheckMate-143 trial has highlighted some of the challenges associated with bringing immune checkpoint blockade to bear in GBM. Multiple predictors of patient response to immune checkpoint blockade have been demonstrated in other solid tumors, including tumor PD-L1 expression, high mutational burden and high T-cell infiltration yet their relevance to GBM remains unclear.
Immune checkpoint blockade likely perpetuates the anti-tumor activity of T-cell responses against neo-antigens (T-cell epitopes newly formed by tumor-specific mutations [138]). Therefore, the mutational status of the tumor has been shown to predict responses to immune checkpoint blockade, with tumors that have higher mutational loads predicting sensitivity to immune-checkpoint blockade [139–141]. GBM, in contrast, has a very low mutational burden as highlighted by Hodges et al.; this work demonstrated that only a minority of GBM patients (~3.5%) have a high tumor mutational load thereby suggesting that GBM patients would be unlikely to benefit from immune checkpoint inhibition monotherapy [142]. Interestingly, a recent retrospective study of individuals with recurrent GBM by Zhao et al. has revealed via genomic/transcriptomic analyses a role for PTEN mutations and MAPK pathway alterations in anti-PD-1 therapy in non-responders and responders respectively [143]. Unfortunately, this work also highlighted treatment failure in those patients that were initial responders; their analysis of a small subset of responders and non-responders revealed that neoantigens were lost over the course of treatment and that genes sets related to immunosuppression became enriched post-therapy [143].
Additionally, while the immunosuppressive effects of chemotherapy present a challenge for designing any effective cancer immunotherapy strategies, differences do appear to exist between systemic chemotherapy and local chemotherapy. In line with such thinking Mathios et al. showed that local chemotherapy combined with anti–PD-1 facilitates an anti-tumor immune response and improves survival in GBM [144]. Local chemotherapy treated mice displayed increased infiltration of tumor-associated DCs and clonal expansion of antigen-specific T effector cells whilst in comparison, those animals that received systemic chemotherapy displayed systemic and intratumoral lymphodepletion, with decreased immune memory in long-term survivors [144]. Interestingly evidence has also emerged to suggest that while alkylating chemotherapeutic agents such as TMZ induce tumoral mutations (i.e. thereby creating neoantigens) these are unlikely to be helpful for improving responses to immune checkpoint blockade given their subclonal nature [145].
Despite compelling anti-tumor activity of antibodies targeting PD-1, resistance to these therapies has been observed in cancers previously known to be sensitive. To further elucidate potential mechanisms of adaptive resistance, Koyama et al. analyses the tumor immune microenvironment in the context of anti-PD-1 therapy in two fully immunocompetent mouse models of lung adenocarcinoma [146]. In tumors progressing following response to anti-PD-1 therapy, they observed up-regulation of alternative immune checkpoints, notably TIM-3 [146]. Given that GBM has been noted to induced/upregulate multiple immune checkpoints, monotherapy may not be sufficient [89].
Additionally, bone marrow T-cell sequestration is a newly characterized phenomenon occurring in the setting of intracranial tumors. T-cells become sequestered in the bone marrow following loss of the S1P1 receptor from the T-cell surface [147]. This contributes to the long-described lymphopenia seen in patients with GBM. Sequestration likely also contributes to the failure of checkpoint blockade in GBM, as the effector arm of the tumor immune response is unable to travel and to exert its anti-tumor function(s). Studies in mice showed that reversing sequestration in a “knock-in” model preventing receptor internalization allowed T-cells to travel to the brain tumor and licensed T-cell activating therapies/checkpoint blockade thereby improving survival [147]. Clearly, the development of pharmacologic strategies to prevent sequestration and/or deliver increased numbers of T-cells to the intracranial tumor (e.g. via the provision of oncolytic viruses [148]) will likely improve efficacy of checkpoint blockade in patients with GBM.
Taken together, while checkpoint inhibitors hold immense promise for the treatment of GBM, better outcomes may be realized through improved preclinical models, strategies to enhance checkpoint immunotherapy in the context of other standard of care therapies, combinatorial approaches utilizing alternative emerging targets for checkpoint immunotherapy, and novel strategies to reverse T cell sequestration. Significant progress has been made in the field of cancer immunotherapy and today, following decades of investigation, the field of cancer immunotherapy has come to fruition. There are still, however, significant hurdles to overcome prior to realization of clinically proven effective immunotherapy for brain tumors.
Numerous modalities are being explored relating to checkpoint inhibitors as detailed in this review. In bring these advanced immunotherapies to the clinic it will be prudent to design clinical studies that allow for the collection of tissue before and after the provision of immunotherapies such as checkpoint blockade [149], thereby allowing for a precise molecular and cellular characterization of changes post-treatment (i.e. be they suggestive of responsive or resistant to therapy). Standardization and harmonization among assays used to evaluate responses will also be critical. Laboratory to laboratory variation in assays methodology yield results that are difficult to interpret and at times lead to erroneous conclusions, further hampering progress. Through standardization and harmonization, however, we would better be able to define predictive markers for successful clinical outcomes, thus enabling rational selection of study groups most likely to benefit. We and others have pursued assay standardization and harmonization throughout collaborative networks and believe that such efforts will be critical to advancement of the field.
Furthermore, as additional molecular targets and the mechanism by which they modulate immune responses continue to be elucidated, it is likely that pre-clinical studies and clinical trials will explore additional strategies for immune checkpoints modulation that have yet to be discovered. As clinical data becomes more robust, it is also likely that checkpoint therapy will be custom tailored to a given patients specific immune phenotype. Further studies are needed to inform clinicians as to which patients will respond to a given therapy and which therapies to apply to different subsets of patients. As new targets continue to emerge and our understanding of existing targets continues to be refined, we move quickly to such a reality. Taken together, the studies and scientific progress detailed in this review suggest that five-year outlook for brain tumor immunotherapy in general and checkpoint inhibitor therapy in particular holds promise.
8. Key Issues.
Glioblastoma continues to portend a poor prognosis with standards of care that are centered on gross total resection and chemoradiation
Contrary to previous belief, the immune system plays and active role in the CNS and further understanding of CNS immunology will allow for more effective anti-cancer therapeutics.
New strategies centered on immune engagement and anti-tumoral immune responses will be critical to treatment of GBM. Expediated pre-clinical evaluation and advancement to clinical trials will avail new, potentially life-saving therapeutics for patients.
While checkpoint inhibitor immunotherapy remains promising, there are still challenges to overcome including low mutational burden, loss of neoantigens, resistance, and lymphopenia secondary to bone marrow T cell sequestration.
Better clinical outcomes may be realized through additional studies utilizing improved preclinical models, strategies to optimize timing of checkpoint inhibitor immunotherapy in the context of other standard of care therapies, and utilization of combinatorial approaches which may include novel targets for checkpoint immunotherapy.
Through assay standardization and harmonization, more robust and reliable predictive markers of positive clinical outcomes may be determined. This may lead to additional clinical studies with therapeutic regimens tailored to patient populations most likely to benefit.
Further preclinical and clinical studies are necessary to gain an increased understanding of the safety and efficacy profile of CNS acting immunotherapeutic checkpoint inhibitors, allowing for new and improved therapeutics for the treatment of GBM.
9. Acknowledgements
Figures 1, 2, and 3 were illustrated specifically for this manuscript by Megan Llewellyn, MSMI. Copyright Duke University. Printed here with permission under a CC-BY 4.0 license.
Financial support: This work was supported by funding from the National Institutes of Health: F30CA196199 (P.C. Gedeon), R01NS085412 (J.H. Sampson), U01NS090284 (J.H. Sampson), R01CA177476 (J.H. Sampson), R01NS086943 (J.H. Sampson), and R01NS099463 (J.H. Sampson). The funders had no role in study design, data collection and analysis, decision to publish, or preparation of the manuscript.
Footnotes
Declaration of interest
P.C. Gedeon has declared being a co-inventor of patents which belong to Duke University. J.D. Bernstock has declared positions/equity in CITC Ltd and Avidea Technologies and being on the scientific board of advisors for POCKiT Diagnostics. B.D. Choi has declared being a co-inventor of patents which belong to Duke University and General Hospital Corp. He has also received commercial research grants from ACEA Biosciences. J.H. Sampson has declared being a co-inventor of patents which belong to Duke University; receiving commercial research grants from Annias and Istari; holding ownership interest in Annias, Neuronium, Duke University, and Istari; and is on the advisory board of Bristol Myers Squibb, Medicenna, Insera Health and Annais. The authors have no other relevant affiliations or financial involvement with any organization or entity with a financial interest in or financial conflict with the subject matter or materials discussed in the manuscript apart from those disclosed.
11. References
Papers of special note have been highlighted as either of interest (*) or of considerable interest (**) to readers.
- 1.Ellor SV, Pagano-Young TA, Avgeropoulos NG. Glioblastoma: background, standard treatment paradigms, and supportive care considerations. J Law Med Ethics, 42(2), 171–182 (2014). [DOI] [PubMed] [Google Scholar]
- 2.Chandana SR, Movva S, Arora M, Singh T. Primary brain tumors in adults. Am Fam Physician, 77(10), 1423–1430 (2008). [PubMed] [Google Scholar]
- 3.Ohgaki H, Kleihues P. Genetic pathways to primary and secondary glioblastoma. Am J Pathol, 170(5), 1445–1453 (2007). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Urbanska K, Sokolowska J, Szmidt M, Sysa P. Glioblastoma multiforme - an overview. Contemp Oncol (Pozn), 18(5), 307–312 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Brat DJ, Aldape K, Colman H et al. cIMPACT-NOW update 5: recommended grading criteria and terminologies for IDH-mutant astrocytomas. Acta neuropathologica, 139(3), 603–608 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Stupp R, Hegi ME, Mason WP et al. Effects of radiotherapy with concomitant and adjuvant temozolomide versus radiotherapy alone on survival in glioblastoma in a randomised phase III study: 5-year analysis of the EORTC-NCIC trial. Lancet Oncol, 10(5), 459–466 (2009). [DOI] [PubMed] [Google Scholar]
- 7.Lu J, Cowperthwaite MC, Burnett MG, Shpak M. Molecular Predictors of Long-Term Survival in Glioblastoma Multiforme Patients. PLoS One, 11(4), e0154313 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Das P, Puri T, Jha P et al. A clinicopathological and molecular analysis of glioblastoma multiforme with long-term survival. J Clin Neurosci, 18(1), 66–70 (2011). [DOI] [PubMed] [Google Scholar]
- 9.Khan MB, Chakraborty S, Boockvar JA. Gross Total Resection of Glioblastoma Improves Overall Survival and Progression-Free Survival Compared to Subtotal Resection or Biopsy Alone. Neurosurgery, 79(6), N12–N13 (2016). [DOI] [PubMed] [Google Scholar]
- 10.Pirzkall A, McGue C, Saraswathy S et al. Tumor regrowth between surgery and initiation of adjuvant therapy in patients with newly diagnosed glioblastoma. Neuro Oncol, 11(6), 842–852 (2009). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Sheehan JP, Shaffrey ME, Gupta B, Larner J, Rich JN, Park DM. Improving the radiosensitivity of radioresistant and hypoxic glioblastoma. Future Oncol, 6(10), 1591–1601 (2010). [DOI] [PubMed] [Google Scholar]
- 12.Weller M, Cloughesy T, Perry JR, Wick W. Standards of care for treatment of recurrent glioblastoma--are we there yet? Neuro Oncol, 15(1), 4–27 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Minata M, Audia A, Shi J et al. Phenotypic Plasticity of Invasive Edge Glioma Stem-like Cells in Response to Ionizing Radiation. Cell reports, 26(7), 1893–1905 e1897 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Ostermann S, Csajka C, Buclin T et al. Plasma and cerebrospinal fluid population pharmacokinetics of temozolomide in malignant glioma patients. Clin Cancer Res, 10(11), 3728–3736 (2004). [DOI] [PubMed] [Google Scholar]
- 15.Newlands ES, Blackledge GR, Slack JA et al. Phase I trial of temozolomide (CCRG 81045: M&B 39831: NSC 362856). Br J Cancer, 65(2), 287–291 (1992). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Kumari S, Ahsan SM, Kumar JM, Kondapi AK, Rao NM. Overcoming blood brain barrier with a dual purpose Temozolomide loaded Lactoferrin nanoparticles for combating glioma (SERP-17–12433). Sci Rep, 7(1), 6602 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Miranda A, Blanco-Prieto MJ, Sousa J, Pais A, Vitorino C. Breaching barriers in glioblastoma. Part II: Targeted drug delivery and lipid nanoparticles. Int J Pharm, 531(1), 389–410 (2017). [DOI] [PubMed] [Google Scholar]
- 18.Zhang J, Stevens MF, Bradshaw TD. Temozolomide: mechanisms of action, repair and resistance. Curr Mol Pharmacol, 5(1), 102–114 (2012). [DOI] [PubMed] [Google Scholar]
- 19.Wiewrodt D, Nagel G, Dreimuller N, Hundsberger T, Perneczky A, Kaina B. MGMT in primary and recurrent human glioblastomas after radiation and chemotherapy and comparison with p53 status and clinical outcome. Int J Cancer, 122(6), 1391–1399 (2008). [DOI] [PubMed] [Google Scholar]
- 20.Gessler F, Bernstock JD, Braczynski A et al. Surgery for Glioblastoma in Light of Molecular Markers: Impact of Resection and MGMT Promoter Methylation in Newly Diagnosed IDH-1 Wild-Type Glioblastomas. Neurosurgery, 84(1), 190–197 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Hegi ME, Diserens AC, Gorlia T et al. MGMT gene silencing and benefit from temozolomide in glioblastoma. The New England journal of medicine, 352(10), 997–1003 (2005). [DOI] [PubMed] [Google Scholar]
- 22.Dirks PB. Cancer: stem cells and brain tumours. Nature, 444(7120), 687–688 (2006). [DOI] [PubMed] [Google Scholar]
- 23.Louveau A, Smirnov I, Keyes TJ et al. Structural and functional features of central nervous system lymphatic vessels. Nature, 523(7560), 337–341 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Schlager C, Korner H, Krueger M et al. Effector T-cell trafficking between the leptomeninges and the cerebrospinal fluid. Nature, 530(7590), 349–353 (2016).* [DOI] [PubMed] [Google Scholar]
- 25.Desjardins A, Gromeier M, Herndon JE 2nd et al. Recurrent Glioblastoma Treated with Recombinant Poliovirus. The New England journal of medicine, 379(2), 150–161 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Gedeon PC, Schaller TH, Chitneni SK et al. A Rationally Designed Fully Human EGFRvIII:CD3-Targeted Bispecific Antibody Redirects Human T Cells to Treat Patient-derived Intracerebral Malignant Glioma. Clin Cancer Res, 24(15), 3611–3631 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Schaller TH, Snyder DJ, Spasojevic I, Gedeon PC, Sanchez-Perez L, Sampson JH. First in human dose calculation of a single-chain bispecific antibody targeting glioma using the MABEL approach. J Immunother Cancer, 8(1) (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Gedeon PC, Streicker MA, Schaller TH, Archer GE, Jokinen MP, Sampson JH. GLP toxicology study of a fully-human T cell redirecting CD3:EGFRvIII binding immunotherapeutic bispecific antibody. PloS one, 15(7), e0236374 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Suryadevara CM, Desai R, Abel ML et al. Temozolomide lymphodepletion enhances CAR abundance and correlates with antitumor efficacy against established glioblastoma. Oncoimmunology, 7(6), e1434464 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Suryadevara CM, Desai R, Farber SH et al. Preventing Lck Activation in CAR T Cells Confers Treg Resistance but Requires 4–1BB Signaling for Them to Persist and Treat Solid Tumors in Nonlymphodepleted Hosts. Clin Cancer Res, 25(1), 358–368 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Johnson LA, Scholler J, Ohkuri T et al. Rational development and characterization of humanized anti-EGFR variant III chimeric antigen receptor T cells for glioblastoma. Science translational medicine, 7(275), 275ra222 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.O’Rourke DM, Nasrallah MP, Desai A et al. A single dose of peripherally infused EGFRvIII-directed CAR T cells mediates antigen loss and induces adaptive resistance in patients with recurrent glioblastoma. Science translational medicine, 9(399) (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Batich KA, Reap EA, Archer GE et al. Long-term Survival in Glioblastoma with Cytomegalovirus pp65-Targeted Vaccination. Clin Cancer Res, 23(8), 1898–1909 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Gedeon PC, Choi BD, Sampson JH, Bigner DD. RINDOPEPIMUT Anti-EGFRvIII Peptide Vaccine Oncolytic. Drug Future, 38(3), 147–155 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Reardon DA, Desjardins A, Vredenburgh JJ et al. Rindopepimut with Bevacizumab for Patients with Relapsed EGFRvIII-Expressing Glioblastoma (ReACT): Results of a Double-Blind Randomized Phase II Trial. Clin Cancer Res, 26(7), 1586–1594 (2020). [DOI] [PubMed] [Google Scholar]
- 36.Weller M, Butowski N, Tran DD et al. Rindopepimut with temozolomide for patients with newly diagnosed, EGFRvIII-expressing glioblastoma (ACT IV): a randomised, double-blind, international phase 3 trial. Lancet Oncol, 18(10), 1373–1385 (2017). [DOI] [PubMed] [Google Scholar]
- 37.Reap EA, Suryadevara CM, Batich KA et al. Dendritic Cells Enhance Polyfunctionality of Adoptively Transferred T Cells That Target Cytomegalovirus in Glioblastoma. Cancer Res, 78(1), 256–264 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Mitchell DA, Batich KA, Gunn MD et al. Tetanus toxoid and CCL3 improve dendritic cell vaccines in mice and glioblastoma patients. Nature, 519(7543), 366–369 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Reardon DA, Gokhale PC, Klein SR et al. Glioblastoma Eradication Following Immune Checkpoint Blockade in an Orthotopic, Immunocompetent Model. Cancer Immunol Res, 4(2), 124–135 (2016).* [DOI] [PubMed] [Google Scholar]
- 40.Fecci PE, Ochiai H, Mitchell DA et al. Systemic CTLA-4 blockade ameliorates glioma-induced changes to the CD4+ T cell compartment without affecting regulatory T-cell function. Clin Cancer Res, 13(7), 2158–2167 (2007). [DOI] [PubMed] [Google Scholar]
- 41.Reardon DA, Gokhale PC, Hodi FS et al. Immune checkpoint blockade for glioblastoma: Preclinical activity of single agent and combinatorial therapy. Journal of Clinical Oncology, 32(15_suppl), 2084–2084 (2014). [Google Scholar]
- 42.Belcaid Z, Phallen JA, Zeng J et al. Focal radiation therapy combined with 4–1BB activation and CTLA-4 blockade yields long-term survival and a protective antigen-specific memory response in a murine glioma model. PloS one, 9(7), e101764 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Zeng J, See AP, Phallen J et al. Anti-PD-1 blockade and stereotactic radiation produce long-term survival in mice with intracranial gliomas. International journal of radiation oncology, biology, physics, 86(2), 343–349 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Dunn GP, Dunn IF, Curry WT. Focus on TILs: Prognostic significance of tumor infiltrating lymphocytes in human glioma. Cancer Immun, 7, 12 (2007). [PMC free article] [PubMed] [Google Scholar]
- 45.Korn T, Kallies A. T cell responses in the central nervous system. Nature reviews. Immunology, 17(3), 179–194 (2017). [DOI] [PubMed] [Google Scholar]
- 46.Ransohoff RM, Engelhardt B. The anatomical and cellular basis of immune surveillance in the central nervous system. Nat. Rev. Immunol, 12(9), 623–635 (2012). [DOI] [PubMed] [Google Scholar]
- 47.Schetters STT, Gomez-Nicola D, Garcia-Vallejo JJ, Van Kooyk Y. Neuroinflammation: Microglia and T Cells Get Ready to Tango. Frontiers in immunology, 8, 1905 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Bradbury MW, Cserr HF, Westrop RJ. Drainage of cerebral interstitial fluid into deep cervical lymph of the rabbit. Am. J. Physiol, 240(4), F329–336 (1981). [DOI] [PubMed] [Google Scholar]
- 49.Walter BA, Valera VA, Takahashi S, Matsuno K, Ushiki T. Evidence of antibody production in the rat cervical lymph nodes after antigen administration into the cerebrospinal fluid. Arch. Histol. Cytol, 69(1), 37–47 (2006). [DOI] [PubMed] [Google Scholar]
- 50.Karman J, Ling C, Sandor M, Fabry Z. Initiation of immune responses in brain is promoted by local dendritic cells. J. Immunol, 173(4), 2353–2361 (2004). [DOI] [PubMed] [Google Scholar]
- 51.Walter L, Albert ML. Cutting edge: cross-presented intracranial antigen primes CD8+ T cells. J. Immunol, 178(10), 6038–6042 (2007). [DOI] [PubMed] [Google Scholar]
- 52.Calzascia T, Masson F, Di Berardino-Besson W et al. Homing phenotypes of tumor-specific CD8 T cells are predetermined at the tumor site by crosspresenting APCs. Immunity, 22(2), 175–184 (2005). [DOI] [PubMed] [Google Scholar]
- 53.Masson F, Calzascia T, Di Berardino-Besson W, de Tribolet N, Dietrich PY, Walker PR. Brain microenvironment promotes the final functional maturation of tumor-specific effector CD8+ T cells. Journal of immunology, 179(2), 845–853 (2007). [DOI] [PubMed] [Google Scholar]
- 54.Clemente CG, Mihm MC Jr., Bufalino R, Zurrida S, Collini P, Cascinelli N. Prognostic value of tumor infiltrating lymphocytes in the vertical growth phase of primary cutaneous melanoma. Cancer, 77(7), 1303–1310 (1996). [DOI] [PubMed] [Google Scholar]
- 55.Naito Y, Saito K, Shiiba K et al. CD8+ T cells infiltrated within cancer cell nests as a prognostic factor in human colorectal cancer. Cancer Res, 58(16), 3491–3494 (1998). [PubMed] [Google Scholar]
- 56.Zhang L, Conejo-Garcia JR, Katsaros D et al. Intratumoral T cells, recurrence, and survival in epithelial ovarian cancer. N. Engl. J. Med, 348(3), 203–213 (2003). [DOI] [PubMed] [Google Scholar]
- 57.Yang I, Tihan T, Han SJ et al. CD8+ T-cell infiltrate in newly diagnosed glioblastoma is associated with long-term survival. J Clin Neurosci, 17(11), 1381–1385 (2010). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Kmiecik J, Poli A, Brons NH et al. Elevated CD3+ and CD8+ tumor-infiltrating immune cells correlate with prolonged survival in glioblastoma patients despite integrated immunosuppressive mechanisms in the tumor microenvironment and at the systemic level. Journal of neuroimmunology, 264(1–2), 71–83 (2013). [DOI] [PubMed] [Google Scholar]
- 59.Yue Q, Zhang X, Ye HX et al. The prognostic value of Foxp3+ tumor-infiltrating lymphocytes in patients with glioblastoma. Journal of neuro-oncology, 116(2), 251–259 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Bienkowski M, Preusser M. Prognostic role of tumour-infiltrating inflammatory cells in brain tumours: literature review. Curr Opin Neurol, 28(6), 647–658 (2015). [DOI] [PubMed] [Google Scholar]
- 61.Tivol EA, Borriello F, Schweitzer AN, Lynch WP, Bluestone JA, Sharpe AH. Loss of CTLA-4 leads to massive lymphoproliferation and fatal multiorgan tissue destruction, revealing a critical negative regulatory role of CTLA-4. Immunity, 3(5), 541–547 (1995). [DOI] [PubMed] [Google Scholar]
- 62.Waterhouse P, Penninger JM, Timms E et al. Lymphoproliferative disorders with early lethality in mice deficient in Ctla-4. Science, 270(5238), 985–988 (1995). [DOI] [PubMed] [Google Scholar]
- 63.Agata Y, Kawasaki A, Nishimura H et al. Expression of the PD-1 antigen on the surface of stimulated mouse T and B lymphocytes. International immunology, 8(5), 765–772 (1996). [DOI] [PubMed] [Google Scholar]
- 64.Monney L, Sabatos CA, Gaglia JL et al. Th1-specific cell surface protein Tim-3 regulates macrophage activation and severity of an autoimmune disease. Nature, 415(6871), 536–541 (2002). [DOI] [PubMed] [Google Scholar]
- 65.Barber DL, Wherry EJ, Masopust D et al. Restoring function in exhausted CD8 T cells during chronic viral infection. Nature, 439(7077), 682–687 (2006). [DOI] [PubMed] [Google Scholar]
- 66.Zhu C, Anderson AC, Schubart A et al. The Tim-3 ligand galectin-9 negatively regulates T helper type 1 immunity. Nat Immunol, 6(12), 1245–1252 (2005). [DOI] [PubMed] [Google Scholar]
- 67.Syn NL, Teng MWL, Mok TSK, Soo RA. De-novo and acquired resistance to immune checkpoint targeting. Lancet Oncol, 18(12), e731–e741 (2017). [DOI] [PubMed] [Google Scholar]
- 68.Rudd CE, Taylor A, Schneider H. CD28 and CTLA-4 coreceptor expression and signal transduction. Immunol. Rev, 229(1), 12–26 (2009). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Azuma M, Ito D, Yagita H et al. B70 antigen is a second ligand for CTLA-4 and CD28. Nature, 366(6450), 76–79 (1993). [DOI] [PubMed] [Google Scholar]
- 70.Woroniecka KI, Rhodin KE, Chongsathidkiet P, Keith KA, Fecci PE. T-cell Dysfunction in Glioblastoma: Applying a New Framework. Clin Cancer Res, 24(16), 3792–3802 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Chuang E, Lee KM, Robbins MD et al. Regulation of cytotoxic T lymphocyte-associated molecule-4 by Src kinases. J. Immunol, 162(3), 1270–1277 (1999). [PubMed] [Google Scholar]
- 72.Parry RV, Chemnitz JM, Frauwirth KA et al. CTLA-4 and PD-1 receptors inhibit T-cell activation by distinct mechanisms. Mol. Cell. Biol, 25(21), 9543–9553 (2005). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Hill JA, Feuerer M, Tash K et al. Foxp3 transcription-factor-dependent and -independent regulation of the regulatory T cell transcriptional signature. Immunity, 27(5), 786–800 (2007). [DOI] [PubMed] [Google Scholar]
- 74.Wing K, Onishi Y, Prieto-Martin P et al. CTLA-4 control over Foxp3+ regulatory T cell function. Science, 322(5899), 271–275 (2008). [DOI] [PubMed] [Google Scholar]
- 75.Fecci PE, Mitchell DA, Whitesides JF et al. Increased regulatory T-cell fraction amidst a diminished CD4 compartment explains cellular immune defects in patients with malignant glioma. Cancer Res, 66(6), 3294–3302 (2006). [DOI] [PubMed] [Google Scholar]
- 76.Riley JL. PD-1 signaling in primary T cells. Immunol. Rev, 229(1), 114–125 (2009). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Wherry EJ, Ha SJ, Kaech SM et al. Molecular signature of CD8+ T cell exhaustion during chronic viral infection. Immunity, 27(4), 670–684 (2007). [DOI] [PubMed] [Google Scholar]
- 78.Lee SK, Seo SH, Kim BS et al. IFN-gamma regulates the expression of B7-H1 in dermal fibroblast cells. J. Dermatol. Sci, 40(2), 95–103 (2005). [DOI] [PubMed] [Google Scholar]
- 79.Fife BT, Pauken KE, Eagar TN et al. Interactions between PD-1 and PD-L1 promote tolerance by blocking the TCR-induced stop signal. Nat Immunol, 10(11), 1185–1192 (2009). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Butte MJ, Keir ME, Phamduy TB, Sharpe AH, Freeman GJ. Programmed death-1 ligand 1 interacts specifically with the B7–1 costimulatory molecule to inhibit T cell responses. Immunity, 27(1), 111–122 (2007). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Latchman Y, Wood CR, Chernova T et al. PD-L2 is a second ligand for PD-1 and inhibits T cell activation. Nat Immunol, 2(3), 261–268 (2001). [DOI] [PubMed] [Google Scholar]
- 82.Zhang Y, Chung Y, Bishop C et al. Regulation of T cell activation and tolerance by PDL2. Proc Natl Acad Sci U S A, 103(31), 11695–11700 (2006). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Garcia-Diaz A, Shin DS, Moreno BH et al. Interferon Receptor Signaling Pathways Regulating PD-L1 and PD-L2 Expression. Cell Rep, 19(6), 1189–1201 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Cheng X, Veverka V, Radhakrishnan A et al. Structure and interactions of the human programmed cell death 1 receptor. The Journal of biological chemistry, 288(17), 11771–11785 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Lazar-Molnar E, Yan Q, Cao E, Ramagopal U, Nathenson SG, Almo SC. Crystal structure of the complex between programmed death-1 (PD-1) and its ligand PD-L2. Proc Natl Acad Sci U S A, 105(30), 10483–10488 (2008). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Philips EA, Garcia-Espana A, Tocheva AS et al. The structural features that distinguish PD-L2 from PD-L1 emerged in placental mammals. The Journal of biological chemistry, 295(14), 4372–4380 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Viricel C, Ahmed M, Barakat K. Human PD-1 binds differently to its human ligands: a comprehensive modeling study. J Mol Graph Model, 57, 131–142 (2015). [DOI] [PubMed] [Google Scholar]
- 88.Petersen TR, Dickgreber N, Hermans IF. Tumor antigen presentation by dendritic cells. Crit. Rev. Immunol, 30(4), 345–386 (2010). [DOI] [PubMed] [Google Scholar]
- 89.Woroniecka K, Chongsathidkiet P, Rhodin KE et al. T Cell Exhaustion Signatures Vary with Tumor Type and are Severe in Glioblastoma. Clinical Cancer Research, 24(17), 4175–4186 (2018).* [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Wei B, Wang L, Zhao X, Du C, Guo Y, Sun Z. The upregulation of programmed death 1 on peripheral blood T cells of glioma is correlated with disease progression. Tumour biology : the journal of the International Society for Oncodevelopmental Biology and Medicine, 35(4), 2923–2929 (2013). [DOI] [PubMed] [Google Scholar]
- 91.Dong H, Strome SE, Salomao DR et al. Tumor-associated B7-H1 promotes T-cell apoptosis: a potential mechanism of immune evasion. Nat. Med, 8(8), 793–800 (2002). [DOI] [PubMed] [Google Scholar]
- 92.Bloch O, Crane CA, Kaur R, Safaee M, Rutkowski MJ, Parsa AT. Gliomas promote immunosuppression through induction of B7-H1 expression in tumor-associated macrophages. Clin. Cancer Res, 19(12), 3165–3175 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Berghoff AS, Kiesel B, Widhalm G et al. Programmed death ligand 1 expression and tumor-infiltrating lymphocytes in glioblastoma. Neuro Oncol, 17(8), 1064–1075 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Parsa AT, Waldron JS, Panner A et al. Loss of tumor suppressor PTEN function increases B7-H1 expression and immunoresistance in glioma. Nat. Med, 13(1), 84–88 (2007). [DOI] [PubMed] [Google Scholar]
- 95.Wintterle S, Schreiner B, Mitsdoerffer M et al. Expression of the B7-related molecule B7-H1 by glioma cells: a potential mechanism of immune paralysis. Cancer Res, 63(21), 7462–7467 (2003). [PubMed] [Google Scholar]
- 96.Chen L, Han X. Anti-PD-1/PD-L1 therapy of human cancer: past, present, and future. J. Clin. Invest, 125(9), 3384–3391 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Tsushima F, Yao S, Shin T et al. Interaction between B7-H1 and PD-1 determines initiation and reversal of T-cell anergy. Blood, 110(1), 180–185 (2007). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Thompson RH, Gillett MD, Cheville JC et al. Costimulatory B7-H1 in renal cell carcinoma patients: Indicator of tumor aggressiveness and potential therapeutic target. Proc. Natl. Acad. Sci. U. S. A, 101(49), 17174–17179 (2004). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Ohigashi Y, Sho M, Yamada Y et al. Clinical significance of programmed death-1 ligand-1 and programmed death-1 ligand-2 expression in human esophageal cancer. Clin. Cancer Res, 11(8), 2947–2953 (2005). [DOI] [PubMed] [Google Scholar]
- 100.Hamanishi J, Mandai M, Iwasaki M et al. Programmed cell death 1 ligand 1 and tumor-infiltrating CD8+ T lymphocytes are prognostic factors of human ovarian cancer. Proc. Natl. Acad. Sci. U. S. A, 104(9), 3360–3365 (2007). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Liu Y, Carlsson R, Ambjorn M et al. PD-L1 expression by neurons nearby tumors indicates better prognosis in glioblastoma patients. The Journal of neuroscience : the official journal of the Society for Neuroscience, 33(35), 14231–14245 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Nduom EK, Wei J, Yaghi NK et al. PD-L1 expression and prognostic impact in glioblastoma. Neuro-oncology, 18(2), 195–205 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Zeng J, Zhang XK, Chen HD, Zhong ZH, Wu QL, Lin SX. Expression of programmed cell death-ligand 1 and its correlation with clinical outcomes in gliomas. Oncotarget, 7(8), 8944–8955 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Han J, Hong Y, Lee YS. PD-L1 Expression and Combined Status of PD-L1/PD-1-Positive Tumor Infiltrating Mononuclear Cell Density Predict Prognosis in Glioblastoma Patients. J Pathol Transl Med, 51(1), 40–48 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Miyazaki T, Ishikawa E, Matsuda M et al. Assessment of PD-1 positive cells on initial and secondary resected tumor specimens of newly diagnosed glioblastoma and its implications on patient outcome. Journal of neuro-oncology, 133(2), 277–285 (2017). [DOI] [PubMed] [Google Scholar]
- 106.Lee KS, Lee K, Yun S et al. Prognostic relevance of programmed cell death ligand 1 expression in glioblastoma. Journal of neuro-oncology, 136(3), 453–461 (2018). [DOI] [PubMed] [Google Scholar]
- 107.Hao C, Chen G, Zhao H et al. PD-L1 Expression in Glioblastoma, the Clinical and Prognostic Significance: A Systematic Literature Review and Meta-Analysis. Front Oncol, 10, 1015 (2020).* [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108.Wu Q, Zhan X, Dou T et al. CTLA4 A49G polymorphism shows significant association with glioma risk in a Chinese population. Biochem. Genet, 49(3–4), 190–201 (2011). [DOI] [PubMed] [Google Scholar]
- 109.Fong B, Jin R, Wang X et al. Monitoring of regulatory T cell frequencies and expression of CTLA-4 on T cells, before and after DC vaccination, can predict survival in GBM patients. PloS one, 7(4), e32614 (2012). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110.Walker LS, Sansom DM. The emerging role of CTLA4 as a cell-extrinsic regulator of T cell responses. Nat. Rev. Immunol, 11(12), 852–863 (2011). [DOI] [PubMed] [Google Scholar]
- 111.Nistico P, Capone I, Palermo B et al. Chemotherapy enhances vaccine-induced antitumor immunity in melanoma patients. Int J Cancer, 124(1), 130–139 (2009). [DOI] [PubMed] [Google Scholar]
- 112.Palermo B, Del Bello D, Sottini A et al. Dacarbazine treatment before peptide vaccination enlarges T-cell repertoire diversity of melan-a-specific, tumor-reactive CTL in melanoma patients. Cancer Res, 70(18), 7084–7092 (2010). [DOI] [PubMed] [Google Scholar]
- 113.Sampson JH, Aldape KD, Archer GE et al. Greater chemotherapy-induced lymphopenia enhances tumor-specific immune responses that eliminate EGFRvIII-expressing tumor cells in patients with glioblastoma. Neuro-oncology, 13(3), 324–333 (2011). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114.Klebanoff CA, Khong HT, Antony PA, Palmer DC, Restifo NP. Sinks, suppressors and antigen presenters: how lymphodepletion enhances T cell-mediated tumor immunotherapy. Trends in immunology, 26(2), 111–117 (2005). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115.Salvador E, Shityakov S, Forster C. Glucocorticoids and endothelial cell barrier function. Cell Tissue Res, 355(3), 597–605 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 116.Kostaras X, Cusano F, Kline GA, Roa W, Easaw J. Use of dexamethasone in patients with high-grade glioma: a clinical practice guideline. Curr Oncol, 21(3), e493–503 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 117.Giles AJ, Hutchinson MND, Sonnemann HM et al. Dexamethasone-induced immunosuppression: mechanisms and implications for immunotherapy. J Immunother Cancer, 6(1), 51 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 118.Ott PA, Hodi FS, Robert C. CTLA-4 and PD-1/PD-L1 Blockade: New Immunotherapeutic Modalities with Durable Clinical Benefit in Melanoma Patients. Clinical Cancer Research, 19(19), 5300 (2013). [DOI] [PubMed] [Google Scholar]
- 119.Chowdhury PS, Chamoto K, Honjo T. Combination therapy strategies for improving PD-1 blockade efficacy: a new era in cancer immunotherapy. J Intern Med, 283(2), 110–120 (2018). [DOI] [PubMed] [Google Scholar]
- 120.Genoud V, Marinari E, Nikolaev SI et al. Responsiveness to anti-PD-1 and anti-CTLA-4 immune checkpoint blockade in SB28 and GL261 mouse glioma models. Oncoimmunology, 7(12), e1501137 (2018).* [DOI] [PMC free article] [PubMed] [Google Scholar]
- 121.Reardon DA, Brandes AA, Omuro A et al. Effect of Nivolumab vs Bevacizumab in Patients With Recurrent Glioblastoma: The CheckMate 143 Phase 3 Randomized Clinical Trial. JAMA Oncol, 6(7), 1–8 (2020).** [DOI] [PMC free article] [PubMed] [Google Scholar]
- 122.BMS Newsroom. Bristol-Myers Squibb Announces Phase 3 CheckMate −498 Study Did Not Meet Primary Endpoint of Overall Survival with Opdivo (nivolumab) Plus Radiation in Patients with Newly Diagnosed MGMT-Unmethylated Glioblastoma Multiforme. https://news.bms.com/press-release/corporatefinancial-news/bristol-myers-squibb-announces-phase-3-checkmate-498-study-did. Published May 9, 2019. Accessed on: July 1, 2020.**
- 123.Cloughesy TF, Mochizuki AY, Orpilla JR et al. Neoadjuvant anti-PD-1 immunotherapy promotes a survival benefit with intratumoral and systemic immune responses in recurrent glioblastoma. Nature medicine, 25(3), 477–486 (2019).** [DOI] [PMC free article] [PubMed] [Google Scholar]
- 124.Schalper KA, Rodriguez-Ruiz ME, Diez-Valle R et al. Neoadjuvant nivolumab modifies the tumor immune microenvironment in resectable glioblastoma. Nature medicine, 25(3), 470–476 (2019).** [DOI] [PubMed] [Google Scholar]
- 125.Lucca LE, Lerner BA, Park C et al. Differential expression of the T-cell inhibitor TIGIT in glioblastoma and MS. Neurol Neuroimmunol Neuroinflamm, 7(3) (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 126.Mohme M, Schliffke S, Maire CL et al. Immunophenotyping of Newly Diagnosed and Recurrent Glioblastoma Defines Distinct Immune Exhaustion Profiles in Peripheral and Tumor-infiltrating Lymphocytes. Clin Cancer Res, 24(17), 4187–4200 (2018). [DOI] [PubMed] [Google Scholar]
- 127.Woroniecka K, Chongsathidkiet P, Rhodin K et al. T-Cell Exhaustion Signatures Vary with Tumor Type and Are Severe in Glioblastoma. Clin Cancer Res, 24(17), 4175–4186 (2018).* [DOI] [PMC free article] [PubMed] [Google Scholar]
- 128.Jones RB, Ndhlovu LC, Barbour JD et al. Tim-3 expression defines a novel population of dysfunctional T cells with highly elevated frequencies in progressive HIV-1 infection. The Journal of Experimental Medicine, 205(12), 2763–2779 (2008). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 129.Sakuishi K, Apetoh L, Sullivan JM, Blazar BR, Kuchroo VK, Anderson AC. Targeting Tim-3 and PD-1 pathways to reverse T cell exhaustion and restore anti-tumor immunity. J Exp Med, 207(10), 2187–2194 (2010). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 130.Kim JE, Patel MA, Mangraviti A et al. Combination Therapy with Anti-PD-1, Anti-TIM-3, and Focal Radiation Results in Regression of Murine Gliomas. Clinical cancer research, 23(1), 124–136 (2017).* [DOI] [PMC free article] [PubMed] [Google Scholar]
- 131.Huard B, Gaulard P, Faure F, Hercend T, Triebel F. Cellular expression and tissue distribution of the human LAG-3-encoded protein, an MHC class II ligand. Immunogenetics, 39(3), 213–217 (1994). [DOI] [PubMed] [Google Scholar]
- 132.Huard B, Tournier M, Hercend T, Triebel F, Faure F. Lymphocyte-activation gene 3/major histocompatibility complex class II interaction modulates the antigenic response of CD4+ T lymphocytes. Eur J Immunol, 24(12), 3216–3221 (1994). [DOI] [PubMed] [Google Scholar]
- 133.Nguyen LT, Ohashi PS. Clinical blockade of PD1 and LAG3 — potential mechanisms of action. Nature Reviews Immunology, 15, 45 (2014). [DOI] [PubMed] [Google Scholar]
- 134.Davis DM. Intercellular transfer of cell-surface proteins is common and can affect many stages of an immune response. Nature reviews. Immunology, 7(3), 238–243 (2007). [DOI] [PubMed] [Google Scholar]
- 135.Di Virgilio F, Adinolfi E. Extracellular purines, purinergic receptors and tumor growth. Oncogene, 36(3), 293–303 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 136.Allard B, Longhi MS, Robson SC, Stagg J. The ectonucleotidases CD39 and CD73: Novel checkpoint inhibitor targets. Immunological reviews, 276(1), 121–144 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 137.Mostafa H, Pala A, Högel J et al. Immune phenotypes predict survival in patients with glioblastoma multiforme. Journal of Hematology & Oncology, 9(1), 77 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 138.Kelderman S, Schumacher TN, Kvistborg P. Mismatch Repair-Deficient Cancers Are Targets for Anti-PD-1 Therapy. Cancer Cell, 28(1), 11–13 (2015). [DOI] [PubMed] [Google Scholar]
- 139.Rizvi NA, Hellmann MD, Snyder A et al. Cancer immunology. Mutational landscape determines sensitivity to PD-1 blockade in non-small cell lung cancer. Science (New York, N.Y.), 348(6230), 124–128 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 140.Schumacher TN, Kesmir C, van Buuren MM. Biomarkers in cancer immunotherapy. Cancer Cell, 27(1), 12–14 (2015). [DOI] [PubMed] [Google Scholar]
- 141.Woller N, Gurlevik E, Fleischmann-Mundt B et al. Viral Infection of Tumors Overcomes Resistance to PD-1-immunotherapy by Broadening Neoantigenome-directed T-cell Responses. Mol Ther, 23(10), 1630–1640 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 142.Hodges TR, Ott M, Xiu J et al. Mutational burden, immune checkpoint expression, and mismatch repair in glioma: implications for immune checkpoint immunotherapy. Neuro Oncol, 19(8), 1047–1057 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 143.Zhao J, Chen AX, Gartrell RD et al. Immune and genomic correlates of response to anti-PD-1 immunotherapy in glioblastoma. Nature medicine, 25(6), 1022 (2019). [DOI] [PubMed] [Google Scholar]
- 144.Mathios D, Kim JE, Mangraviti A et al. Anti-PD-1 antitumor immunity is enhanced by local and abrogated by systemic chemotherapy in GBM. Science translational medicine, 8(370), 370ra180 (2016).* [DOI] [PMC free article] [PubMed] [Google Scholar]
- 145.McGranahan N, Furness AJ, Rosenthal R et al. Clonal neoantigens elicit T cell immunoreactivity and sensitivity to immune checkpoint blockade. Science, 351(6280), 1463–1469 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 146.Koyama S, Akbay EA, Li YY et al. Adaptive resistance to therapeutic PD-1 blockade is associated with upregulation of alternative immune checkpoints. Nature communications, 7, 10501 (2016).* [DOI] [PMC free article] [PubMed] [Google Scholar]
- 147.Chongsathidkiet P, Jackson C, Koyama S et al. Sequestration of T cells in bone marrow in the setting of glioblastoma and other intracranial tumors. Nat. Med, 24, 1459–1468 (2018).* [DOI] [PMC free article] [PubMed] [Google Scholar]
- 148.Totsch SK, Schlappi C, Kang KD et al. Oncolytic herpes simplex virus immunotherapy for brain tumors: current pitfalls and emerging strategies to overcome therapeutic resistance. Oncogene, 38:6159–6171 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 149.Ito H, Nakashima H, Chiocca EA. Molecular responses to immune checkpoint blockade in glioblastoma. Nature medicine, 25(3), 359–361 (2019).** [DOI] [PMC free article] [PubMed] [Google Scholar]