Abstract
Background
Hypertension-related disease burden is a major challenge globally, with an estimated 1.56 billion adults expected to be affected by hypertension by 2025. Environmental factors, such as metals, could be risk factors for hypertension, but the relationship between airborne metals and hypertension is rarely studied.
Methods
Census-tract airborne metal concentrations (arsenic, cadmium, chromium, cobalt, lead, manganese, mercury, nickel, selenium, and antimony) from the U.S. Environmental Protection Agency 2005 National Air Toxics Assessment database were linked to enrollment residential addresses of 47,595 women in the Sister Study cohort. Hypertension was defined as high systolic (≥140 mm Hg) or diastolic (≥90 mm Hg) blood pressure measured by trained examiners at enrollment or taking anti-hypertensive medications. Multivariable log binomial regression was used to estimate adjusted prevalence ratios (PRs) and 95% confidence intervals (CIs) for the association between individual metals and hypertension, with and without co-adjustment for other metals. Quantile-based g-computation was used to estimate the joint effect of the overall metal mixture.
Results
Comparing the highest to lowest quartiles, risk of hypertension was higher among women with higher residential exposure to arsenic (PR=1.05, 95%CI=1.02,1.09), lead (PR=1.04, 95%CI=1.01,1.08), chromium (PR=1.03, 95%CI=1.00,1.06), cobalt (PR=1.03, 95%CI=1.00,1.07), and manganese (PR=1.03, 95%CI=1.00,1.06). Selenium was associated with lower risk of hypertension (PR=0.96, 95%CI=0.93,0.99). Results were similar with mutual adjustment for all other metals. The associations varied by race/ethnicity, with greater PRs in other races/ethnicities (Hispanic, black, and other) participants compared to non-Hispanic white participants. The joint effect of a quartile increase in exposure to all the metals was 1.02 (95%CI=0.99,1.04).
Conclusion
We found that living in areas of higher exposure to arsenic, lead, chromium, cobalt, and manganese was related to higher risk of hypertension, whereas living in areas with higher selenium was inversely related to the risk of hypertension.
Keywords: Hypertension, Metals, Mixture
1. Introduction
Hypertension affects nearly one billion people worldwide, and the number of people affected by hypertension is predicted to rise in all regions of the world over the next decade(Poulter et al., 2015). The hypertension-related disease burden is a major global public health challenge, with significant race and sex differences in the prevalence of hypertension(Bennett et al., 2016; Shen et al., 2017). Beyond established risk factors such as lack of physical activity, obesity, and alcohol use, exposure to environmental pollutants, such as metals, may also play an important role in the development and severity of hypertension (Cosselman et al., 2015; Wu et al., 2018a; Wu et al., 2018b). The possible biological mechanism involves oxidative stress and inflammation(Cosselman et al., 2015).
Existing studies concerning the relationship between metals and hypertension indicate possible positive associations between several metals and hypertension, while selenium (Se) seems to be inversely associated with hypertension risk. Most of the studies are based on metal exposure levels measured in biological samples or groundwater. Epidemiological studies demonstrate that blood cadmium (Cd), blood lead (Pb), and groundwater arsenic (As) exposure are related to increased prevalence or risk of hypertension(Abhyankar et al., 2012; Eum et al., 2008; Gambelunghe et al., 2016). A meta-analysis evaluating the relationship between mercury (Hg) exposure and hypertension in epidemiological studies found a significant positive association between Hg concentration in hair or nails and hypertension (Hu et al., 2018). High levels of exposure to essential metals (such as nickel (Ni), cobalt (Co), manganese (Mn), and chromium (Cr)) might be involved in development of hypertension, but the tight regulation of these metals in the body make it challenging to study their roles using biomarkers (Cosselman et al., 2015; Wang et al., 2018a). High serum Se has been associated with reduced cardiovascular disease risk (Flores-Mateo et al., 2006; Zhang et al., 2016), but results of clinical and observational studies of Se and hypertension have been mixed (Kuruppu et al., 2014). Se is an essential trace element for antioxidant enzymes(Kuruppu et al., 2014) and can attenuate the inflammatory process(Duntas, 2009). Therefore, it is hypothesized that Se has a protective effect on hypertension.
The association of airborne metals exposure with hypertension is still unclear. Anthropogenic emissions from coal-fired industrial plants, industry, and transportation could enrich trace metal concentrations in the air (Zhou et al., 2017). Heavy metals have the tendency to attach to particulate matter (PM) (Speak et al., 2012), and one study shows that exposure to PM composed of metals such as zinc (Zn), copper (Cu), Cr, Pb, Ni are associated with prevalence of hypertension(Gangwar et al., 2019). It has been reported that 50 percent of metal-containing particles contain more than one metal(Adachi and Buseck, 2010). Most of the previous research focused on the effects of individual metals on hypertension. In this study, we explored the effect of individual airborne metals on hypertension and evaluated the effect of exposure to multiple metals simultaneously, using mutual adjustment and estimating the joint effect of the overall metal mixture.
The prevalence of hypertension varies by race/ethnicity(Bennett et al., 2016) and region of the U.S.(Centers for Disease Control and Prevention, 2017.). This may reflect differences in underlying susceptibility or exposures. We therefore considered the impact of race/ethnicity and region in stratified analyses. Since obesity(Dludla et al., 2018) and metals(Cosselman et al., 2015) are both associated with oxidative stress, which is important in the pathogenesis of hypertension(Guzik and Touyz, 2017), we also considered effect modification by obesity.
2. Methods
2.1. Study design and study population
The Sister Study is a prospective cohort study of women residing in the US designed to study the relationship between environmental factors and chronic diseases. 50,884 women aged 35-74 were enrolled into the cohort from 2003 to 2009 (Sandler et al., 2017). At the time of enrollment, study participants completed an extensive computer-assisted telephone interview that ascertained demographic characteristics, socioeconomic status, personal anti-hypertensive medication use, and risk factors for chronic diseases such as alcohol consumption and smoking status. During a baseline home visit, height and weight were measured, and blood pressure was measured three consecutive times (after sitting and resting for a few minutes) by trained examiners following standardized protocols (Chan et al., 2015; Sandler et al., 2017). Body mass index (BMI) was calculated as a person’s weight in kilograms divided by the square of the person’s height in meters, and we used BMI=30 as a cut-off value to define obesity (World Health Organization). The data used in this study was from Sister Study Data Release 7.2.
The institutional review board (IRB) of the National Institute of Environmental Health Sciences and the Copernicus Group approved the Sister Study. All participants provided written consent. In this cross-sectional analysis, we included 47,595 participants who had baseline blood pressure measurements, information on anti-hypertensive medication use, ambient air metal concentration and covariates adjusted in the model.
2.2. Exposure assessment
The Environmental Protection Agency (EPA) developed the National Air Toxics Assessment (NATA) database, which includes concentrations of air toxics across the United States(Environmental Protection Agency, 2011). The database includes concentrations (μg/m3) for the metals antimony (Sb), As, Cd, Cr, Co, Pb, Mn, Hg, Ni, and Se at the census-tract level. The NATA assessment involved first compiling the National Emission Inventory (NEI) data on releases from point sources (waste incinerators, factories), non-point sources (dry cleaners, small manufacturers), on-road mobile and non-road mobile sources (cars, trucks, trains), background sources (natural sources, persistence in the environment of past years’ emissions, and transport from distant sources), and secondary formation of toxics.
The estimates from these sources were used as input to three EPA air quality models: Human Exposure Model-3 (HEM-3) (AERMOD version), Assessment System for Population Exposure Nationwide (ASPEN), and Community Multiscale Air Quality (CMAQ) model, to estimate ambient concentrations of emitted air toxics(Environmental Protection Agency, 2011). HEM-3 was used to estimate air toxics concentrations from point, on-road mobile, and non-road mobile sources(Environmental Protection Agency, 2011). For major stationary sources, HEM-3 modeling took in to account stack height and diameter, exit gas temperature and velocity, and the effects of terrain elevation. EPA modeled non-road mobile sources at an initial vertical release height of 2 meters and an initial vertical dispersion of 1 meter and modeled on-road mobile sources at an initial vertical height of 1.44 meters and a vertical dispersion of 1.33 meters. ASPEN, which is a steady-state Gaussian model that considers rate of release, location of release, the height of release, wind speed and directions, was used to estimate air toxics from non-point sources(Environmental Protection Agency, 2011). ASPEN estimates ambient concentrations based on pre-set polar receptor grids. Ambient concentrations from background ambient were derived from monitoring data and emissions data(Environmental Protection Agency, 2011). The CMAQ model was used to estimate air toxics from the secondary formation and decay of some air toxics(Environmental Protection Agency, 2011). NATA estimates a total annual average ambient concentration of each air toxic for each census tract in the United States.
EPA has released NATA data based on inventories representative of air toxic emissions in 1996, 1999, 2002, 2005, 2011, and 2014. We used the 2005 EPA NATA database, because it is in the middle of our enrollment period (2003-2009). The 2005 NATA database also has the advantage that it was updated to include information on industrial sources, lead emissions from airports, and other sources, and used HEM (AERMOD version), a more refined dispersion model, to estimate ambient concentrations compared to previous versions. Use of the 2011 and 2014 estimates would not maintain proper temporality of the exposure-outcome relationship. The NATA data were linked to each study participant’s enrollment residential address at the census-tract level to represent residential exposure to airborne metals.
2.3. Outcome assessment
For both systolic blood pressure (SBP) and diastolic blood pressure (DBP), the average of the second and third measurements was used. When only two or one blood pressure measurements were available, the second measurement or the single value was used (<2%). The definition of hypertension is as follows: 1) either SBP ≥140 mm Hg or DBP ≥90 mm Hg; or 2) whether currently taking antihypertensive medication. We also considered airborne metals in relation to continuous blood pressure (BP) measures. When using continuous BP as the outcome variable, we corrected the BP of those who reported using antihypertensive medication by adding 15 mm Hg to their measured SBP and 10 mm Hg to their measured DBP. Adding a constant to the observed BP allows for inclusion of the entire sample and has been shown to reduce bias (Tobin et al., 2005). We selected 15 mm Hg for SBP and 10 mm Hg for DBP based on a meta-analysis of the blood pressure improvement under different treatment regimens (Baguet et al., 2007).
2.4. Statistical analysis
Air metal toxics were divided into quartile cut-point-based categories, because restricted quadratic splines analyses indicated that quartiles maximized model fit (Howe et al., 2011). Median (min and max) were used to describe the distribution of metal concentration (Table A1). Spearman rank correlation coefficients were calculated among metals (Table A2). We used multivariable log binomial regression to explore the relationship between airborne metals and hypertension, with hypertension considered as a binary outcome and quartiles for each metal as a categorical exposure variable (first quartile as referent). Prevalence of hypertension varies by race/ethnicity and region, and BMI, so prevalence ratios (PRs) and 95% confidence intervals (CIs) were estimated for overall risk and stratified by race/ethnicity, obesity (BMI<30 and BMI≥30), and geographic region. Interaction terms were added into the models to explore potential modification between stratified factors and metals. We fit the median of each quartile group as continuous variables in models for trend tests. We used a general linear model to evaluate the association between airborne metals and continuous BP.
To evaluate the effect of co-exposure to metals on hypertension, we used a log binomial regression model with all air metals simultaneously included. To determine the combined effect of the 10 airborne metals on hypertension, the quantile-based g-computation approach was used to estimate a weighted linear index representing the overall mixture effect and to estimate proportional effects for each metal(Alexander P. Keila et al., 2019; Keil et al., 2020). This is a new approach that estimates the overall mixture effect for multiple correlated components of the mixture. Quantile g-computation does not require all exposures have the same direction of association with the outcome and can yield an unbiased estimate of the overall mixture effect for simultaneously increasing all metals. This is easily interpretable and is directly relevant to understanding the impact of public health interventions that may act to reduce levels of multiple metals. Considering the possible protective role of Se in hypertension(Flores-Mateo et al., 2006; Kuruppu et al., 2014), we repeated the mixture analysis with Se excluded.
A directed acyclic graph (DAG) was used to select confounders (figure A1). We adjusted all models for age, race/ethnicity, participants’ highest level of attained education, participants’ annual household income, census-tract level income, and census-tract level education. The census-tract level income and education data were from 2000 U.S. census data, linked to participants’ baseline address. We used the most frequently reported education level within a census-tract to define the education level for that census tract. For example, if the percentage of people within a census tract with high school or less was larger than the percentages with other education levels, the census-tract was assigned a level of education of high school or less. We conducted the following sensitivity analyses: (1) excluded participants who began living at their current residence after 2005, because air metal levels abstracted from the 2005 NATA database would not accurately represent their residential exposure in 2005; (2) additionally adjusted for smoking status and alcohol consumption; (3) updated the definition of hypertension to be consistent with the recently released classification by the American College of Cardiology and the American Heart Association (Whelton et al., 2018), in which they recommended considering those with SBP≥130 mm Hg or DBP≥80 mm Hg as hypertensive. Statistical significance was defined as p<0.05 (two-tailed). Analysis was done using SAS 9.4 software (SAS Institution, Inc., Cary, NC). Quantile-based g-computation was done using R 3.6.1 package “qgcomp”(Alexander P. Keila, 2019).
3. Results
Among the 47,595 participants included in our study, 15,814 (33%) had hypertension at baseline (table 1). Participants with hypertension were on average older than those without hypertension. Most participants were non-Hispanic white, but those with hypertension were more often other races/ethnicities (Hispanic, Black, and other) (87%) than those without hypertension (81%). Hypertensive participants had lower levels of education and income compared with non-hypertensive participants, and they were more likely to be past smokers, past drinkers, obese, and more likely to live in census tracts with residents having lower levels of income and education.
Table 1.
Baseline characteristics of hypertensive and non-hypertensive participants in the Sister Study (n=47,595) [N (%)]
| Characteristic | Hypertensive (N=15,814) | Non-hypertensive (N=31,781) |
|---|---|---|
| Age (years) | ||
| ≤45 | 893(6) | 6609(21) |
| 46-50 | 1607(10) | 6240(20) |
| 51-55 | 2862(18) | 6738(21) |
| 56-60 | 3426(22) | 5636(18) |
| 61-65 | 3278(21) | 3679(12) |
| >65 | 3748(24) | 2879(9) |
| Race/ethnicity | ||
| Non-Hispanic white | 12773(81) | 27718(87) |
| Other a | 3041(19) | 4063(13) |
| Education | ||
| High school or less | 2986(19) | 4173(13) |
| Some college | 5929(37) | 10183(32) |
| Bachelor’s degree or more | 6899(44) | 17425(55) |
| Annual household income | ||
| ≤$49,999 | 5140(32) | 6654(21) |
| $50,000-$99,999 | 6551(41) | 13044(41) |
| ≥$100,000 | 4123(26) | 12083(38) |
| Smoking status | ||
| Never | 8342(53) | 18198(57) |
| Past | 6165(39) | 10913(34) |
| Current | 1305(8) | 2669(8) |
| Missing | 2(0) | 1(0) |
| Alcohol consumption | ||
| Never | 666(4) | 949(3) |
| Past | 3087(20) | 4037(13) |
| Current (drinks/week) | ||
| ≤1 | 6655(42) | 12444(39) |
| 1-3 | 1843(12) | 5046(16) |
| >3 | 3536(22) | 9269(29) |
| Missing | 27(0) | 36(0) |
| Region | ||
| Northeast | 2419(15) | 5656(18) |
| Midwest | 4443(28) | 8623(27) |
| South | 5709(36) | 10269(32) |
| West | 3243(20) | 7233(23) |
| BMI (kg/m2) | ||
| BMI<30 | 8298(52) | 24904(78) |
| BMI≥30 | 7509(48) | 6868(22) |
| Missing | 7(0) | 9(0) |
| Census-tract income | ||
| ≤$49,999 | 6071(38) | 9590(30) |
| $50,000-99,999 | 8809(56) | 19170(60) |
| ≥$100,000 | 934(6) | 3021(10) |
| Census-tract education | ||
| High school or less | 9501(60) | 16189(51) |
| Some college | 2220(14) | 4561(14) |
| Bachelor’s degree or more | 4093(26) | 11031(35) |
Other includes Hispanic, black and other
Estimated residential airborne metal exposures was higher for As (median=4.36×10−4 μg/m3, Q1=2.56×10−4, Q3=6.90×10−4), Cr (median=5.06×10−4 μg/m3, Q1=3.01×10−4, Q3=8.70×10−4), Pb(median=1.67×10−3 μg/m3, Q1=1.16×10−3, Q3=2.59×10−3), Mn(median=9.35×10−4 μg/m3, Q1=6.83×10−4, Q3=1.46×10−3) and Ni(median=5.73×10−4 μg/m3, Q1=2.66×10−4, Q3=1.12×10−3); whereas exposures to Cd(median=7.54×10−5 μg/m3, Q1=5.11×10−5, Q3=1.22×10−4), Co (median=9.71×10−6 μg/m3, Q1=2.36×10−6, Q3=2.75×10−5), Hg(median=3.11×10−5 μg/m3, Q1=1.25×10−5, Q3=6.45×10−5), Se(median=8.51×10−5 μg/m3, Q1=2.86×10−5, Q3=2.00×10−4) and Sb(median=9.11×10−6 μg/m3, Q1=1.43×10−6, Q3=3.25×10−5) were relatively lower (table A1). Airborne metals were positively correlated, and most correlations were moderate (correlation coefficient 0.4-0.8) (table A2). Exposure variables with moderate or high correlations are suitable for mixture analysis.
As shown in figure 1, comparing the highest quartile of exposure with the lowest, overall hypertension risk was associated with As(PR=1.05, 95%CI=1.02,1.09; p trend=0.002), Pb (PR=1.04, 95%CI=1.01,1.08; p trend=0.02, Cr (PR=1.03, 95%G=1.00,1.06; p trend=0.15), Co (PR=1.03, 95%CI=1.00,1.07; p trend=0.10), and Mn (PR=1.03, 95%CI=1.00,1.06; p trend=0.29), with increasing dose-response trends for some of these. There was a non-monotonic trend observed overall for Cd (PR quartile 3 vs 1=1.05, 95%CI=1.01,1.08; p trend=0.88). We observed a higher overall risk for Hg (PR quartile 2 vs 1=1.04, 95%CI=1.01,1.08; PR quartile 3 vs 1=1.03, 95%CI=1.00,1.06; p trend=0.05) and Sb (PR quartile 2 vs 1=1.04, 95%CI=1.01,1.07; p trend=0.09), but we did not observe a higher PR in the fourth quartile. In all women, hypertension was inversely associated with Se (PR quartile 4 vs 1=0.96, 95%CI= 0.93,0.99; p trend=0.01). The association between Ni exposure and risk of hypertension was not statistically significant.
Figure 1.
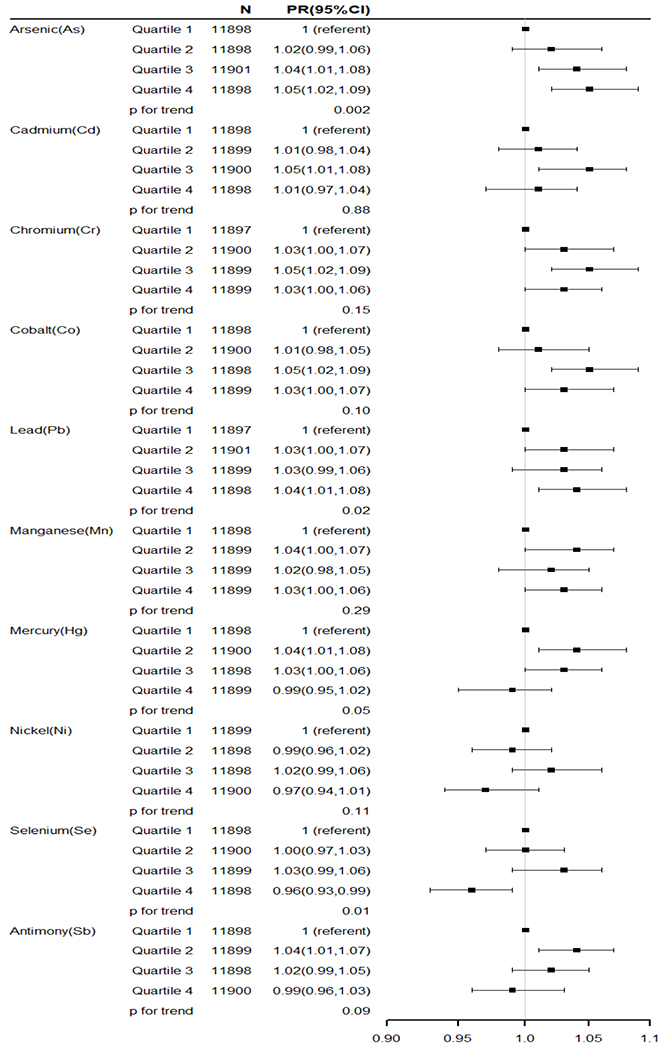
Ambient airborne metals and overall hypertension risk in the Sister Study (N=47,595). The models were adjusted for age, race/ethnicity, education, annually household income, census-tract level income, and census-tract level
Associations differed by race/ethnicity for As (p interaction=0.01), Co (p interaction=0.01), and Pb (p interaction=0.02) with higher PRs for As among participants of other races/ethnicities than non-Hispanic white participants (table 2). There was a significant trend for Co (p trend=0.01) and Pb (p interaction=0.02) among participants of other races/ethnicities but not among non-Hispanic white participants.
Table 2.
Ambient airborne metals and hypertension risk stratified by race/ethnicity in the Sister Study
| Non-Hispanic white (N=40,491) |
Otherb (N=7,104) |
P for interaction | |||
|---|---|---|---|---|---|
| N | PR a (95% CI) | N | PR a (95% CI) | ||
| Arsenic (As) | |||||
| Quartile 1 | 10798 | 1(referent) | 1100 | 1(referent) | |
| Quartile 2 | 10218 | 1.00(0.96,1.04) | 1680 | 1.13(1.04,1.23) | |
| Quartile 3 | 9950 | 1.02(0.98,1.06) | 1951 | 1.14(1.05,1.24) | |
| Quartile 4 | 9525 | 1.03(0.99,1.07) | 2373 | 1.16(1.07,1.25) | |
| p for trend | 0.13 | 0.002 | 0.01 | ||
| Cadmium (Cd) | |||||
| Quartile 1 | 10537 | 1(referent) | 1361 | 1(referent) | |
| Quartile 2 | 10305 | 1.01(0.98,1.05) | 1594 | 1.01(0.94,1.10) | |
| Quartile 3 | 10321 | 1.04(1.00,1.08) | 1579 | 1.11(1.03,1.20) | |
| Quartile 4 | 9328 | 0.99(0.95,1.03) | 2570 | 1.06(0.99,1.13) | 0.15 |
| p for trend | 0.41 | 0.15 | 0.15 | ||
| Chromium (Cr) | |||||
| Quartile 1 | 10733 | 1(referent) | 1164 | 1(referent) | |
| Quartile 2 | 10319 | 1.02(0.98,1.05) | 1581 | 1.08(0.99,1.17) | |
| Quartile 3 | 9857 | 1.05(1.01,1.09) | 2042 | 1.08(1.00,1.16) | |
| Quartile 4 | 9582 | 1.03(0.99,1.07) | 2317 | 1.06(0.98,1.14) | 0.52 |
| p for trend | 0.20 | 0.48 | 0.52 | ||
| Cobalt (Co) | |||||
| Quartile 1 | 10375 | 1(referent) | 1523 | 1(referent) | |
| Quartile 2 | 10091 | 1.03(0.99,1.07) | 1809 | 0.97(0.90,1.04) | |
| Quartile 3 | 10040 | 1.04(1.00,1.07) | 1858 | 1.11(1.03,1.19) | |
| Quartile 4 | 9985 | 1.02(0.99,1.06) | 1914 | 1.08(1.01,1.16) | 0.01 |
| p for trend | 0.56 | 0.01 | 0.01 | ||
| Lead (Pb) | |||||
| Quartile 1 | 10717 | 1(referent) | 1180 | 1(referent) | |
| Quartile 2 | 10208 | 1.03(0.99,1.07) | 1693 | 1.05(0.97,1.14) | |
| Quartile 3 | 9982 | 1.00(0.96,1.04) | 1917 | 1.09(1.01,1.18) | |
| Quartile 4 | 9584 | 1.03(0.99,1.07) | 2314 | 1.09(1.02,1.18) | 0.02 |
| p for trend | 0.24 | 0.02 | 0.02 | ||
| Manganese (Mn) | |||||
| Quartile 1 | 10470 | 1(referent) | 1428 | 1(referent) | |
| Quartile 2 | 10218 | 1.04(1.00,1.07) | 1681 | 1.03(0.95,1.11) | |
| Quartile 3 | 10250 | 1.02(0.98,1.06) | 1649 | 0.99(0.92,1.07) | |
| Quartile 4 | 9553 | 1.03(0.99,1.06) | 2346 | 1.04(0.98,1.12) | 0.91 |
| p for trend | 0.49 | 0.21 | 0.91 | ||
| Mercury (Hg) | |||||
| Quartile 1 | 10623 | 1(referent) | 1275 | 1(referent) | |
| Quartile 2 | 10198 | 1.03(0.99,1.07) | 1702 | 1.10(1.02,1.18) | |
| Quartile 3 | 10016 | 1.01(0.97,1.04) | 1882 | 1.10(1.02,1.18) | |
| Quartile 4 | 9654 | 0.98(0.94,1.02) | 2245 | 1.01(0.94,1.08) | 0.09 |
| p for trend | 0.07 | 0.16 | 0.09 | ||
| Nickel (Ni) | |||||
| Quartile 1 | 10768 | 1(referent) | 1131 | 1(referent) | |
| Quartile 2 | 10352 | 0.98(0.95,1.02) | 1546 | 1.03(0.95,1.12) | |
| Quartile 3 | 9787 | 1.00(0.97,1.04) | 2111 | 1.09(1.02,1.18) | |
| Quartile 4 | 9584 | 0.96(0.93,1.00) | 2316 | 1.01(0.94,1.09) | 0.13 |
| p for trend | 0.08 | 0.59 | 0.13 | ||
| Selenium (Se) | |||||
| Quartile 1 | 10567 | 1(referent) | 1331 | 1(referent) | |
| Quartile 2 | 9984 | 0.99(0.96,1.03) | 1916 | 1.03(0.96,1.10) | |
| Quartile 3 | 9993 | 1.01(0.98,1.05) | 1906 | 1.06(0.99,1.14) | |
| Quartile 4 | 9947 | 0.95(0.92,0.99) | 1951 | 0.97(0.90,1.05) | |
| p for trend | 0.01 | 0.18 | 0.46 | ||
| Antimony (Sb) | |||||
| Quartile 1 | 10483 | 1(referent) | 1415 | 1(referent) | |
| Quartile 2 | 10125 | 1.04(1.00,1.08) | 1774 | 1.05(0.98,1.13) | |
| Quartile 3 | 9800 | 1.01(0.97,1.05) | 2098 | 1.08(1.00,1.16) | |
| Quartile 4 | 10083 | 0.98(0.95,1.02) | 1817 | 1.04(0.97,1.12) | |
| p for trend | 0.05 | 0.76 | 0.39 | ||
Adjusted for age, education, annually household income, census-tract level income, and census-tract level education
Other includes Hispanic, black and other
Most associations did not differ by baseline obesity status (table A3). For Cr (p interaction=0.01), there was a higher risk for those with BMI<30 (PR quartile 3 vs 1=1.07, 95%CI=1.01,1.12; p trend=0.13) and for those with BMI≥30 (PR quartile 2 vs 1=1.04, 95%CI=1.00,1.08; p trend=0.56), but the trend was slightly different. After stratifying by region (table A4), we found that many exposure-response trends were more apparent among participants from the south compared with participants living in other parts of the United States, including risks associated with Cd (p trend=0.01), Cr (p trend=0.02), Co (p trend=0.06) and Mn (p trend=0.02), but we did not observe a significant interaction effect.
When considering multiple metal exposures (table A5), after mutually adjusting for all other metals, risk of hypertension was associated with higher levels of As (PR quartile 4 vs 1=1.06, 95%CI=1.00,1.12; p trend=0.004) and Co (PR quartile 4 vs 1=1.06, 95%CI=1.01,1.10; p trend=0.01). There was also a non-monotonic trend for Cd (PR quartile 3 vs 1=1.05, 95%CI= 1.00,1.09; p trend=0.25). Ni (PR quartile 4 vs 1=0.90, 95%CI=0.86,0.95; p trend=0.001) and Se (PR quartile 4 vs 1=0.92, 95%CI=0.87,0.96; p trend=0.002) were inversely associated with hypertension risk.
Using quantile g-computation, the joint effect of increasing all ten metals by one quartile was 1.02 (95%CI=0.99,1.04) (table 3). Co, As, Cr, Pb, Cd, and Mn were positively weighted in the mixture with risk of hypertension. As (0.27) and Co (0.26) had the greatest proportional positive contribution to the mixture effect. Ni, Se, Hg, and Sb were negatively weighted in the mixture with risk of hypertension. Ni (0.40) and Se (0.39) were the metals with the strongest negative weights. After excluding Se from the mixture, the overall mixture odds ratio (OR) was 1.03 (95%CI=1.00, 1.06), with the greatest positive contribution for As and Co and the greatest negative contribution for Ni and Hg (table A6). When stratified by race/ethnicity, the mixture OR for participants of other races/ethnicities (Hispanic, Black, and other) was 1.07 (95%CI=1.01,1.14) (figure A2).
Table 3.
Quantile-based g-computation of ambient metals and hypertension
| Mixture OR a (95% CI) | Weights | |
|---|---|---|
| Mixture OR | 1.02(0.99,1.04) | |
| Positive direction | ||
| Arsenic (As) | 0.27 | |
| Cobalt (Co) | 0.26 | |
| Chromium (Cr) | 0.15 | |
| Cadmium (Cd) | 0.14 | |
| Lead (Pb) | 0.10 | |
| Manganese (Mn) | 0.08 | |
| Negative direction | ||
| Nickel (Ni) | 0.40 | |
| Selenium (Se) | 0.39 | |
| Mercury (Hg) | 0.12 | |
| Antimony (Sb) | 0.10 |
Adjusted for age, race/ethnicity, education, annually household income, census-tract income, and census-tract education; OR was the effect when increasing all ten metals by one quartile.
In analyses of continuous BP, we found that Pb (SBP: β quartile 4 vs 1=0.54, 95%CI=0.12,0.95; DBP: β quartile 4 vs 1=0.28, 95%CI=0.01,0.56) and Mn (SBP: β quartile 4 vs 1=0.65, 95%CI=0.24,1.05; DBP: β quartile 4 vs 1=0.44, 95%CI=0.17,0.7) were associated with higher blood pressure, and Se (SBP: β quartile 4 vs 1=−0.46, 95%CI=−0.86,−0.05; DBP: β quartile 4 vs 1=−0.65, 95%CI=−0.91,−0.38) was associated with lower blood pressure (table A7).
Results were largely unchanged in sensitivity analysis excluding participants who began living at their current residence after 2005, additionally adjusting for smoking status and alcohol consumption, or using the newer definition of hypertension (table A8).
4. Discussion
In an analysis of individual airborne metals, we found that higher exposure to As, Cr, Co, Pb, and Mn was associated with increased hypertension risk. There was minimal attenuation of associations with mutual adjustment for all the metals. Ambient Se exposure was inversely related to the risk of hypertension. Results varied somewhat by race/ethnicity and obesity and some associations were more pronounced in the southern U.S. region. When considering the combined metal mixture, As and Co had the greatest contribution to hypertension risk. This study provides evidence that some airborne metals might be risk factors for hypertension.
4.1. Airborne metal levels
The levels of exposure in our cohort are lower than some air quality standards and exposures in communities monitored by the EPA. For example, according to National Ambient Air Quality Standards, the concentration of Pb should not exceed 0.15μg/m3 for a 3-month average(United States Environmental Protection Agency, 2008), which is higher than the annual average of Pb level in our study (median=1.67×10−3 μg/m3, Q1=1.16×10−3, Q3=2.59×10−3). Exposure levels in the cohort are also lower than the mean of annual average metal concentrations (As: 0.002 μg/m3, Cr: 0.002 μg/m3, Pb: 0.006 μg/m3, Mn: 0.004 μg/m3, Ni: 0.003 μg/m3) of the 13 sites used by the EPA to monitor air pollution(Chen and Lippmann, 2009; United States Environmental Protection Agency, 2004).
The metals (As, Pb, Cr, Co, and Mn) which were positively associated with risk of hypertension in our study are likely from traffic, combustion, and incineration sources(Adachi and Tainosho, 2004; Sanderson et al., 2014; Tolocka et al., 2004). The correlations for these metals are from 0.5 to 0.7, which indicates that they may come from the same sources. Metals from anthropogenic sources can be transported in the air, deposited on the soil surface, and penetrate into the groundwater(Mazurek et al., 2017). The contribution to human exposure of metals from different environmental media may differ. For Mn, soil and air are the largest contributors to human exposure, with Mn from air contributing twice that from soil to saliva Mn(Butler et al., 2019). Pb mostly come from soil or dust, whereas Cr mostly come from air or dust(Butler et al., 2019). According to the US Agency for Toxic Substances and Disease Registry, while As exposure may come from air, water, and food, food is generally the largest source of exposure for the general population (Agency for Toxic Substances and Disease Registry). Even at low levels, exposure to metals from air is widespread, so exploring the health effects of airborne metals is critical.
4.2. As and Pb
Toxic metals, such as As and Pb, pose potential threats to human health and are the top two chemicals on the Agency for Toxic Substances and Disease Registry 2019 Priority List of Hazardous Substances(Agency for Toxic Substances and Disease Registry, 2019). Some epidemiological studies demonstrated that exposure to As is related to increased risk of hypertension, but there is no consistent evidence of dose-response association(Abhyankar et al., 2012). Both cross-sectional and prospective studies have shown that elevations in BP levels and risk of hypertension are associated with Pb exposure, with a dose-response relationship, although the shape of the dose-response relationship is not completely characterized(Navas-Acien et al., 2007). In our study, risk of hypertension was higher for participants residing in areas with higher levels of exposure to As and Pb, with significantly positive dose-response relationship. Results were similar across race/ethnicity and BMI subgroups and co-adjusting for other metals. In the mixture analysis, airborne As exposure contributed the most to the increase of hypertension risk. The possible mechanisms of Pb and As exposure leading to hypertension is promotion of oxidative stress and decreased ability of the antioxidant defense system(Jomova et al., 2011; Vaziri, 2008).
4.3. Hg and Cd
A plausible mechanism for the toxicity of Hg and Cd might be in promoting lipid peroxidation and inducing oxidative stress(M. Valko, 2005). In addition, both metals can replace Zn and Cu in anti-oxidative enzymes to reduce the effectiveness of metalloenzymes(Houston, 2011; M. Valko, 2005). A meta-analysis of epidemiological studies demonstrated a significant positive association between Hg and prevalent hypertension in populations with high exposure, but not low-to-moderate exposure (hair Hg <2μg/g) (Hu et al., 2018). We only found a slightly higher risk associated with Hg above the lower quartile of exposure, but Hg was found to contribute negatively to the association between the whole metal mixture and hypertension. Our analysis considered only ambient exposure, but fish consumption is a major source of Hg exposure(United States Environmental Protection Agency, 1997).
Several epidemiological studies suggest a positive association between blood Cd and higher blood pressure and an inverse association between urinary Cd and hypertension (Gallagher and Meliker, 2010; Garner and Levallois, 2017). The different results may be because blood and urinary Cd represent short-term and long-term exposure, respectively (Jarup and Akesson, 2009). Besides, Cd is also nephrotoxic and Cd-induced kidney damage will lead to a low Cd concentration in urine, so a lower Cd concentration in urine sometimes represents damage due to Cd exposure (Jarup and Akesson, 2009). Our findings for Cd suggested a potential association with hypertension risk that was stronger among those from the South. Tobacco smoking is an important source of Cd exposure(Jarup and Akesson, 2009). When we additionally adjusted for smoking status, however, the results remained the same.
4.4. Essential metals (Cr, Co, Mn, Ni, and Se)
Cr, Co, Mn, Ni, and Se are essential metals called transition metals. They are absorbed from inhalation and ingestion, then used as cofactors in enzymatic reactions. The deficiency of these metals may lead to reduced enzyme activity, while high levels could generate reactive oxygen species(Rines and Ardehali, 2013). As oxidative stress caused by extra reactive oxygen species is a possible mechanism for hypertension, high exposure to essential metals could be involved in development of hypertension. Two cross-sectional studies demonstrate that risk of hypertension is higher with a higher level of blood Cr, Co, and Mn(Wu et al., 2018a; Wu et al., 2018b), but their participants were patients at a physical examination center or a hospital-based department of cardiology, which may not represent general populations. Another epidemiological study with a relatively small sample size showed in women that hair Cr and Co were related to lower risk of hypertension, whereas Mn was not (Wang et al., 2018a). The heterogenous results may be due to variability between different biological samples and populations. In our study, we found women living in areas with higher airborne levels of Cr, Co, and Mn had a higher prevalence of hypertension.
The association between Se and hypertension is controversial. A meta-analysis of studies of multiple designs and mostly small sample sizes found no conclusive evidence supporting an association between Se levels and hypertension, although there did appear to be a reduced risk of hypertension or reduced blood pressure in some observational studies(Kuruppu et al., 2014; Nawrot et al., 2007). In our study, we found a lower risk of hypertension for participants who live in areas with higher airborne levels of Se, with and without co-adjustment for other metals. In the mixture analysis, Se contributed negatively to risk of hypertension, and the weight was the second largest. When excluding Se, the joint effect of the metal mixture was related to higher risk of hypertension.
The association between Ni exposure and hypertension is also controversial. A study on the association between ambient air pollution and hospital visits shows that higher exposure to Ni in fine particulate matter (PM2.5) constituents is related to higher hospital visits for hypertension(Lu et al., 2019). However, another study with a relatively small sample size focusing on indoor air pollution demonstrates that in women higher hair Ni concentration is related to lower risk of hypertension(Wang et al., 2018a). In our study, individual exposure to Ni was not significantly related to risk of hypertension overall, but it was associated with reduced risk after adjusting for other metals.
4.5. Stratification by race/ethnicity
Multiple studies have demonstrated that the rate of hypertension among Blacks in the US is notably higher than among Whites (Fryar et al., 2017; Lackland, 2014). Air pollution distributions also vary by race/ethnicity, with non-Hispanic blacks and Hispanics living in areas with higher levels of air pollution compared with non-Hispanic whites(Parker et al., 2018). Because of these differences, we carried out analyses stratified by race/ethnicity. In the race/ethnicity-stratified analyses, the direction of associations for each of the metals was similar across the strata. We did however find significant interaction effects of race/ethnicity on the associations between some of the metals (As, Co, and Pb) and hypertension. When exposed to the same level of As, the risk of hypertension was higher in participants of other races/ethnicities compared with non-Hispanic white participants. Trend tests for Co and Pb were significant in participants of other races/ethnicities, but not in non-Hispanic white participants. The mixture analysis shows that exposure to these ten metals as a mixture was associated with higher risk of hypertension in participants of other races/ethnicities. Our results provide evidence that participants of other races/ethnicities (Hispanic, Black, and other) may be more sensitive to adverse effects when exposed to higher levels of airborne metals. Participants of other races/ethnicities in the Sister Study live in areas with relatively higher levels of airborne metals compared with non-Hispanic white participants(White et al., 2019), which may be one of the reasons why the prevalence of hypertension is higher among participants of other races/ethnicities (32% in non-Hispanic white and 43% in other races/ethnicities, table 1).
4.6. Stratification by BMI and geographical regions
Obesity is a risk factor for hypertension and has been reported to be responsible for more than half of hypertension(Whelton et al., 2018). This relationship may involve oxidative stress(Dludla et al., 2018). In humans exposed to metals, metals may interact with one or more tissues. Redox reactions may produce reactive oxygen species which will in turn result in oxidative stress (Cosselman et al., 2015). Several epidemiological studies show that exposure to metals as a mixture is related to obesity(Niehoff et al., 2020; Wang et al., 2018b). Obesity may be a modifier or a mediator of the association between airborne metals exposure and hypertension, which may lead to different associations in obese and non-obese participants. Thus, we stratified the analysis by obesity based on BMI. In the stratified analyses, we found a significant interaction of obesity and Cr in relation to risk of hypertension. The result shows that non-obese participants seem more sensitive to a higher level of Cr exposure. When exposed to higher level of Cr, the risk of hypertension for non-obese participants is higher and the trend is more obvious even though it is not statistically significant.
The prevalence of hypertension in the southern U.S. is higher than elsewhere in the U.S.(Centers for Disease Control and Prevention, 2017.). This difference could be due to population demographics, access to health care, differential risk factor distributions, or other factors which in turn could affect susceptibility to effects of metal exposure. After stratifying by region, we found that participants living in the south may be more sensitive to Cd, Cr, Co and Mn exposure. In addition, more participants of other races/ethnicities were enrolled in the south in the Sister Study(Sandler et al., 2017), which may also contribute to this result.
4.7. Limitations and strengths
The present study was cross-sectional, so the temporal relationship between exposure and outcome was not considered, and we cannot conclude a causal relationship. Another limitation is that our study was based on a volunteer cohort with characteristics that could limit generalizability. Participants were women with enhanced risk for breast cancer which may share risk factors associated with hypertension. History of cardiovascular diseases was not considered in the inclusion criteria and we did not adjust for history of cardiovascular diseases in this analysis. Participants in this cohort also have higher socioeconomic status (Sandler et al., 2017) than the U.S. as a whole. In addition, we used airborne metal levels based on census-tract level exposures rather than participants’ individual exposure. A person could encounter different metal concentrations depending on their behavior and activity pattern, which the estimate of metal exposure based on census-tract level did not consider. We used 2005 NATA data to characterize exposure levels for all participants, some of whom enrolled before or after 2005. This could lead to exposure misclassification, especially for those with greater residential mobility, but we used quartile cut-point-based categories to represent their exposure levels, which may be less subject to misclassification. Furthermore, we only considered metals from air, but metals exposure could come from many sources, including diet. Since NATA estimates a total annual average ambient concentration of each airborne metal, short-term temporal variability was not considered in this study.
A strength of the study is that blood pressure, height, and weight were measured by trained examiners at enrollment instead of self-report, which provides a more accurate assessment of hypertension and obesity than in other studies. Another strength is that we considered both individual and joint effects of the overall metal mixture on hypertension. To our best knowledge, this study is the first large-scale study considering the relationship between airborne metals and hypertension.
5. Conclusions
Our findings suggest that living in areas of higher exposure to As, Cr, Co, Pb, or Mn is related to higher risk of hypertension, whereas exposure to Se is inversely related to the risk of hypertension. Hypertension is a major issue globally, and it is important to identify potentially modifiable environmental risk factors for hypertension beyond the traditional risk factors. Although the magnitude of risks found in our study are small, airborne metal exposures are widespread, and hypertension is common. Thus, the potential association between airborne metals and hypertension may have an important public health impact.
Supplementary Material
Highlights.
This is a large-scale study considering the relationship between airborne metals and hypertension.
Airborne arsenic, lead, chromium, cobalt, and manganese were related to higher risk of hypertension, whereas selenium was inversely related to the risk of hypertension.
The association between airborne metals and risk of hypertension varied somewhat by race, BMI, and geographic region.
The overall metal mixture was associated with higher risk of hypertension among participants of other races/ethnicities, but not among non-Hispanic white participants.
Acknowledgments
Funding sources
This work was supported by the Intramural Research Program of the NIH, NIEHS (Z01ES044005); and the China Scholarship Council (201906210460).
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Human subject study
The institutional review board (IRB) of the National Institute of Environmental Health Sciences and the Copernicus Group approved the Sister Study.
Declaration of interests
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
References
- Abhyankar LN, et al. , 2012. Arsenic exposure and hypertension: a systematic review. Environ Health Perspect. 120, 494–500. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Adachi K, Buseck PR, 2010. Hosted and free-floating metal-bearing atmospheric nanoparticles in Mexico City. Environ Sci Technol. 44, 2299–304. [DOI] [PubMed] [Google Scholar]
- Adachi K, Tainosho Y, 2004. Characterization of heavy metal particles embedded in tire dust. Environ Int. 30, 1009–17. [DOI] [PubMed] [Google Scholar]
- Agency for Toxic Substances and Disease Registry, Arsenic https://www.atsdr.cdc.goV/sites/toxzine/arsenic_toxzine.html#exposure.
- Agency for Toxic Substances and Disease Registry, Priority List of Hazardous Substances https://www.atsdr.cdc.goV/spl/index.html#2019spl, 2019.
- Keila Alexander P., et al. , A quantile-based g-computation approach to addressing the effects of exposure mixtures. . archivePrefix, 2019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Keila, b. Alexander P., Buckleyc Jessie P., O’Brienb Katie M., Fergusonb Kelly K., Zhaod Shanshan White b Alexandra J., A quantile-based g-computation approach to addressing the effects of exposure mixtures. . archivePrefix, 2019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baguet JP, et al. , 2007. Updated meta-analytical approach to the efficacy of antihypertensive drugs in reducing blood pressure. Clin Drug Investig. 27, 735–53. [DOI] [PubMed] [Google Scholar]
- Bennett A, et al. , 2016. Hypertension and ethnicity. Curr Opin Cardiol. 31, 381–6. [DOI] [PubMed] [Google Scholar]
- Butler L, et al. , 2019. Assessing the contributions of metals in environmental media to exposure biomarkers in a region of ferroalloy industry. J Expo Sci Environ Epidemiol. 29, 674–687. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Centers for Disease Control and Prevention, Hypertension Maps and Data Sources. . 2017. [Google Scholar]
- Chan SH, et al. , 2015. Long-Term Air Pollution Exposure and Blood Pressure in the Sister Study. Environ Health Perspect. 123, 951–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen LC, Lippmann M, 2009. Effects of metals within ambient air particulate matter (PM) on human health. Inhal Toxicol. 21, 1–31. [DOI] [PubMed] [Google Scholar]
- Cosselman KE, et al. , 2015. Environmental factors in cardiovascular disease. Nat Rev Cardiol. 12, 627–42. [DOI] [PubMed] [Google Scholar]
- Dludla PV, et al. , 2018. Inflammation and Oxidative Stress in an Obese State and the Protective Effects of Gallic Acid. Nutrients. 11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Duntas LH, 2009. Selenium and inflammation: underlying anti-inflammatory mechanisms. Horm Metab Res. 41, 443–7. [DOI] [PubMed] [Google Scholar]
- Environmental Protection Agency, An Overview of Methods for EPA’s National-Scale Air Toxics Assessment Office of Air Quality Planning and Standards, Research Triangle Park, NC. , 2011. [Google Scholar]
- Eum KD, et al. , 2008. Cadmium in blood and hypertension. Sci Total Environ. 407, 147–53. [DOI] [PubMed] [Google Scholar]
- Flores-Mateo G, et al. , 2006. Selenium and coronary heart disease: a meta-analysis. Am J Clin Nutr. 84, 762–73. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fryar CD, et al. , 2017. Hypertension Prevalence and Control Among Adults: United States, 2015-2016. NCHS Data Brief 1–8. [PubMed] [Google Scholar]
- Gallagher CM, Meliker JR, 2010. Blood and urine cadmium, blood pressure, and hypertension: a systematic review and meta-analysis. Environ Health Perspect. 118, 1676–84. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gambelunghe A, et al. , 2016. Low-level exposure to lead, blood pressure, and hypertension in a population-based cohort. Environ Res. 149, 157–163. [DOI] [PubMed] [Google Scholar]
- Gangwar C, et al. , 2019. Assessment of air pollution caused by illegal e-waste burning to evaluate the human health risk. Environ Int. 125, 191–199. [DOI] [PubMed] [Google Scholar]
- Garner RE, Levallois P, 2017. Associations between cadmium levels in blood and urine, blood pressure and hypertension among Canadian adults. Environ Res. 155, 64–72. [DOI] [PubMed] [Google Scholar]
- Guzik TJ, Touyz RM, 2017. Oxidative Stress, Inflammation, and Vascular Aging in Hypertension. Hypertension 70, 660–667. [DOI] [PubMed] [Google Scholar]
- Houston MC, 2011. Role of mercury toxicity in hypertension, cardiovascular disease, and stroke. J Clin Hypertens (Greenwich) 13, 621–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Howe CJ, et al. , 2011. Splines for trend analysis and continuous confounder control. Epidemiology. 22, 874–5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hu XF, et al. , 2018. Mercury Exposure, Blood Pressure, and Hypertension: A Systematic Review and Dose-response Meta-analysis. Environ Health Perspect. 126, 076002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jarup L, Akesson A, 2009. Current status of cadmium as an environmental health problem. Toxicol Appl Pharmacol. 238, 201–8. [DOI] [PubMed] [Google Scholar]
- Jomova K, et al. , 2011. Arsenic: toxicity, oxidative stress and human disease. J Appl Toxicol. 31, 95–107. [DOI] [PubMed] [Google Scholar]
- Keil AP, et al. , 2020. A Quantile-Based g-Computation Approach to Addressing the Effects of Exposure Mixtures. Environ Health Perspect. 128, 47004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kuruppu D, et al. , 2014. Selenium levels and hypertension: a systematic review of the literature. Public Health Nutr. 17, 1342–52. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lackland DT, 2014. Racial differences in hypertension: implications for high blood pressure management. Am J Med Sci. 348, 135–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lu Y, et al. , 2019. Assessing the association between fine particulate matter (PM2.5) constituents and cardiovascular diseases in a mega-city of Pakistan. Environ Pollut. 252, 1412–1422. [DOI] [PubMed] [Google Scholar]
- Valko M, H. M. a. M. T. D. C., 2005. Metals, Toxicity and Oxidative Stress. Current Medicinal Chemistry. 12, 1161–1208. [DOI] [PubMed] [Google Scholar]
- Mazurek R, et al. , 2017. Assessment of heavy metals contamination in surface layers of Roztocze National Park forest soils (SE Poland) by indices of pollution. Chemosphere. 168, 839–850. [DOI] [PubMed] [Google Scholar]
- Navas-Acien A, et al. , 2007. Lead exposure and cardiovascular disease--a systematic review. Environ Health Perspect. 115, 472–82. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nawrot TS, et al. , 2007. Blood pressure and blood selenium: a cross-sectional and longitudinal population study. Eur Heart J. 28, 628–33. [DOI] [PubMed] [Google Scholar]
- Niehoff NM, et al. , 2020. Metals and trace elements in relation to body mass index in a prospective study of US women. Environ Res. 184, 109396. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Parker JD, et al. , 2018. Particulate Matter Air Pollution Exposure and Heart Disease Mortality Risks by Race and Ethnicity in the United States: 1997 to 2009 National Health Interview Survey With Mortality Follow-Up Through 2011. Circulation. 137, 1688–1697. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Poulter NR, et al. , 2015. Hypertension. Lancet. 386, 801–12. [DOI] [PubMed] [Google Scholar]
- Rines AK, Ardehali H, 2013. Transition metals and mitochondrial metabolism in the heart. J Mol Cell Cardiol. 55, 50–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sanderson P, et al. , 2014. A review of chemical and physical characterisation of atmospheric metallic nanoparticles. Atmospheric Environment. 94, 353–365. [Google Scholar]
- Sandler DP, et al. , 2017. The Sister Study Cohort: Baseline Methods and Participant Characteristics. Environ Health Perspect. 125, 127003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shen W, et al. , 2017. Race and Sex Differences of Long-Term Blood Pressure Profiles From Childhood and Adult Hypertension: The Bogalusa Heart Study. Hypertension. 70, 66–74. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Speak AF, et al. , 2012. Urban particulate pollution reduction by four species of green roof vegetation in a UK city. Atmospheric Environment. 61, 283–293. [Google Scholar]
- Tobin MD, et al. , 2005. Adjusting for treatment effects in studies of quantitative traits: antihypertensive therapy and systolic blood pressure. Stat Med. 24, 2911–35. [DOI] [PubMed] [Google Scholar]
- Tolocka MP, et al. , 2004. Number concentrations of fine and ultrafine particles containing metals. Atmospheric Environment. 38, 3263–3273. [Google Scholar]
- United States Environmental Protection Agency, Mercury Study Report to Congress. Vol. 4, 1997. [Google Scholar]
- United States Environmental Protection Agency, Air Quality Criteria for Particulate Matter. 2004. [Google Scholar]
- United States Environmental Protection Agency, National Ambient Air Quality Standards Table. https://www.epa.gov/criteria-air-pollutants/naaqs-table#1, 2008.
- Vaziri ND, 2008. Mechanisms of lead-induced hypertension and cardiovascular disease. Am J Physiol Heart Circ Physiol. 295, H454–65. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang B, et al. , 2018a. Indoor air pollution affects hypertension risk in rural women in Northern China by interfering with the uptake of metal elements: A preliminary cross-sectional study. Environ Pollut. 240, 267–272. [DOI] [PubMed] [Google Scholar]
- Wang X, et al. , 2018b. Associations of cumulative exposure to heavy metal mixtures with obesity and its comorbidities among U.S. adults in NHANES 2003–2014. Environ Int. 121, 683–694. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Whelton PK, et al. , 2018. 2017 ACC/AHA/AAPA/ABC/ACPM/AGS/APhA/ASH/ASPC/NMA/PCNA Guideline for the Prevention, Detection, Evaluation, and Management of High Blood Pressure in Adults: A Report of the American College of Cardiology/American Heart Association Task Force on Clinical Practice Guidelines. J Am Coll Cardiol. 71, e127–e248. [DOI] [PubMed] [Google Scholar]
- White AJ, et al. , 2019. Metallic Air Pollutants and Breast Cancer Risk in a Nationwide Cohort Study. Epidemiology 30, 20–28. [DOI] [PMC free article] [PubMed] [Google Scholar]
- World Health Organization, Body mass index - BMI http://www.euro.who.int/en/health-topics/disease-prevention/nutrition/a-healthy-lifestyle/body-mass-index-bmi.
- Wu W, et al. , 2018a. Environmental exposure to metals and the risk of hypertension: A cross-sectional study in China. Environ Pollut. 233, 670–678. [DOI] [PubMed] [Google Scholar]
- Wu W, et al. , 2018b. Associations of environmental exposure to metals with the risk of hypertension in China. Sci Total Environ. 622-623, 184–191. [DOI] [PubMed] [Google Scholar]
- Zhang X, et al. , 2016. Selenium status and cardiovascular diseases: meta-analysis of prospective observational studies and randomized controlled trials. Eur J Clin Nutr. 70, 162–9. [DOI] [PubMed] [Google Scholar]
- Zhou X, et al. , 2017. Using Moss to Assess Airborne Heavy Metal Pollution in Taizhou, China. Int J Environ Res Public Health. 14. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


