Abstract
Genetic matching between transplant donor and recipient pairs has traditionally focused on the HLA regions of the genome, but recent studies suggest that matching for non-HLA regions may be important as well. We assess four genetic matching scores for use in association analyses of transplant outcomes. These scores describe genetic ancestry distance using IBS, or genetic incompatibility or mismatch of the two genomes and therefore may reflect different underlying biological mechanisms for donor and recipient genes to influence transplant outcomes. Our simulation studies show that jointly testing these scores with the recipient genotype is a powerful method for preliminary screening and discovery of transplant outcome related SNPs and gene regions. Following these joint tests with marginal testing of the recipient genotype and matching score separately can lead to further understanding of the biological mechanisms behind transplant outcomes. In addition, we present results of a liver transplant data analysis that show joint testing can detect SNPs significantly associated with acute rejection in liver transplant.
Keywords: joint testing, genetic matching scores, transplant genetics
Introduction
In the field of transplantation, a major focus is matching potential donors to recipients to have a high probability of successful transplant outcome. Currently, there are a wide range of clinical features that are considered when matching a transplant recipient to a donor. These range from donor aspects, such as age, sex, and whether the donor is living or deceased, to recipient aspects, such as age and sex (Reddy, Varghese, Venkataraman, & Rela, 2013). Genetic matching for transplantation, when the number of similar alleles in donor and recipient genotypes that modify transplant outcome is maximized, has also been performed for some organ types but has mainly focused on the HLA regions of the genome since this region is responsible for immune responses that can lead to transplant rejection (Lim, Wong, Heidt, and Class, 2018; Sypek, Kausman, Holt, and Hughes, 2018). Some studies have shown that there are gene regions outside of the major histocompatibility complex that are genetic modifiers for transplant outcomes (Steers et al., 2019, McCarroll et al., 2009, Grafft et al., 2009, Yang, & Sarwal, 2017; Almoguera, Shaked, & Keating, 2014).
Genetic association studies for transplant outcomes tend to look at the recipient genome only (Dorr et al., 2018). Individual recipient SNPs have been found to be associated with acute rejection outcomes for kidney, liver, and heart transplants (Green et al., 2017; Morris et al., 2015; Almoguera, Shaked, & Keating, 2014). Recipient’s genes have also been found to affect pharmacogenetic outcomes, such as immunosuppressant concentration in transplant recipients (Dorr et al., 2018; Oetting et al., 2016; Almoguera, Shaked, & Keating, 2014). Multiple studies have examined how differences in transplant outcomes that seem to be based on self-reported race may be because of underlying genetic differences between different ethnic groups (Green et al., 2017; Morris et al., 2015). Fewer studies have been carried out looking at the association between the donor genome and transplant outcomes, but polymorphisms in donor APOL1 and TLR4 genes have been found to be significantly associated with negative transplant outcomes in the kidney and liver respectively (Julian et al., 2017; Oetting et al., 2012; Dorr et al., 2018). The biological mechanisms behind genetic matching in transplant studies remain elusive. One common hypothesis is that the recipient mounts an immune response after recognizing the donor organ as non-self. There is also the potential for regulatory pathways to become disrupted if SNPs located in non-coding gene regions differ between donors and recipients. Genetic differences have also been known to affect metabolism of immunosuppressant drugs (Yang, & Sarwal, 2017). In addition, many studies have found discrepancies between transplant outcomes for recipients of different races, especially if donor and recipient are of different races (Morris et al., 2015; Pang et al., 2009; Nair and Thuluvath, 2001; Nair, Eustace, and Thuluvath, 2002; Zhang, 2017; Saxena et al., 2012). It has been conjectured that these discrepancies may have been caused by genetic differences between donor and recipient (Julian et al., 2017). To work towards understanding biological mechanisms behind transplantation success and failure, it may be useful to quantify the genetic differences between transplant donors and recipients beyond one of their genomes.
More recently, researchers have started to examine the joint effect of donor and recipient genomes by looking at genetic mismatch between the two. Using an Allogenomics Mismatch Score (AMS) method that generates a difference score between transplant donor and recipient genomic markers, exonic SNPs coding for transmembrane proteins were found to be associated with long term kidney graft function (Mesnard et al., 2016). Donor/recipient (D/R) pairs with a higher number of non-HLA mismatched variants prior to kidney transplant were found to have an increased risk of antibody mediated rejection after transplant (Pineda et al., 2017). A method similar to the AMS, which we refer to as the binary mismatch score, was used to examine genome-wide mismatches in non-synonymous SNPs (nsSNPs) found in transmembrane and secreted proteins (Reindl-Schwaighofer et al., 2019). This study found that the number of nsSNP mismatches was independently associated with graft loss in a multivariate model that adjusted for HLA eplet mismatch (Reindl-Schwaighofer et al., 2019). Under a genomic-collision model, defined as a scenario in which a recipient who is homozygous for a deletion-tagging allele was paired with a non-homozygous donor (Steers et al., 2019), a single recipient SNP in the LIMS1 locus was confirmed to have association with kidney graft rejection. These works suggest that paired analyses of donor-recipient genomes may shed new light on biological mechanisms underlying transplant outcomes. To date, however, there has not been systematic assessment on the power of the AMS, and binary mismatch scores, and it is of interest to understand the relationship among these scores. The AMS and binary mismatch scores focus on one specific biological mechanism where the recipient would create donor-specific antibodies that would initiate an immune response towards the graft. It is of interest to gain insight into alternative biological mechanisms through the use of alternative paired scores.
In this work, we assess and compare the three methods discussed above and propose a new method based on the degree of identity-by-state (IBS) mismatch between the donor and recipient genomes. The remainder of the paper is formatted as follows. First, we will present the four scoring methods and discuss how these scores are related. This will be followed by results of extensive simulation studies comparing the performance of the four scores. Then we will show an analysis of liver transplant genetic data using these methods. Finally, we will discuss the benefits and potential limitations of these methods.
Methods
Four different methods were utilized to calculate difference scores between transplant donors and recipients, which were then used in analysis to determine if these scores were associated with differential transplant outcomes.
Notation
We define and as the number of minor alleles (0, 1, 2) present at SNP l for the donor and the recipient respectively. For our models, we consider a general regression setting. Depending on the phenotype variables available for analysis, models can either be in the form of generalized linear models for continuous or categorical outcomes, Yi, or Cox proportional hazards models, for a time to event outcome. Our generalized linear model is of the form
where g(.) is the link function, μ = E(Y), and Wi = (Wi1,…,Wip) are covariate values for recipient i with regression coefficients α = (α1,…,αp). We let Xi correspond to the recipient genotype with coefficient β and Zi corresponds to one of the matching scores that will be introduced below with coefficient γ. We let i = {1,…,n}, where n corresponds to the total number of donor/recipient pairs in the study.
For time to event outcomes, we use the Cox proportional hazards model
to assess whether our distance measures are associated with the hazard of transplant outcome. In the above formula, λ(t|W,X,Z) refers to the hazard function at time point t, λ0(t) is the baseline hazard function, and all other notation is the same as was discussed for the generalized linear model. We wish to test the null hypothesis of null genetic matching effect:
or jointly test the global null hypotheses of null genetic effect:
IBS Mismatch Score
The proposed method, the IBS mismatch score, is based on the degree of identity-by-state (IBS) between pairs of individuals. Identity-by-state refers to when two individuals share the same alleles at a genomic locus. This sharing may be because of a recent common ancestor between the two individuals, in which case the region would be considered identical-by-descent, or because of the alleles matching by chance. In either case, the individuals have some number of alleles in common. The IBS mismatch score is calculated as
where and are defined above. The IBS() function is a measure of the dissimilarity between the donor and recipient genomes at a particular SNP, and is defined as
assuming SNPs are diallelic. A version of this measure was used as a kernel function in a least-squares kernel machine approach to test multiple genetic markers in association with quantitative traits (Kwee et al., 2008). Their kernel was used to produce a scalar measure of similarity between multiple SNPs in a genetic region, whereas our score focuses on a single SNP (Kwee et al., 2008).
Incompatibility Score
The IBS score quantifies similarity between the donor and recipient genomes, but it is possible that a difference that exists between the two genomes may matter more than the degree of the difference. This concept motivates a second method that we call “the incompatibility score”. This score is defined as
where the incompatibility function is defined as
A score similar to this has been considered for maternal and fetal genotypes in relation to the risk of pre-eclampsia (PE; Parimi et al, 2008). While none of the SNPs tested were significantly associated with PE after correcting for multiple testing, the authors were able to find SNPs from three candidate genes such that their incompatibility effect was nominally associated with PE. Interestingly, the incompatibility of one of these SNPs showed a protective effect, suggesting that incompatibility between genomes does not necessarily lead to a worse outcome (Parimi et al, 2008). This score has also been utilized in kidney transplant, where both single SNP mismatches and genome-wide mismatch were found to be associated with antibody-mediated rejection and T-cell mediated rejection (Pineda et al., 2017).
The Allogenomics Mismatch Score (AMS) and Binary Mismatch Score
The Allogenomics Mismatch Score (AMS) uses genomic markers to generate a difference score between transplant donors and recipients (Mesnard et al., 2016). It is based on the allogenomics concept, the hypothesis that looking at the difference between transplant donor and recipient alleles in the coding regions of the genome can give insight into which amino acids coded by the donor genome would present as non-self to the recipient immune system. The AMS was defined as
where Grl refers to the genotype of recipient r at genomic site l, Gdl refers to the genotype of donor d at site l, and δl is defined as
where a denotes alleles of a genotype. Essentially, the δl function is equal to 0 if both the donor genotype alleles are in the recipient genotype, is equal to 1 if the donor genotype contains an allele not present in the recipient genotype, and is equal to 2 if the donor genotype contains two alleles not present in the recipient genotype. To compare this method to our scores, we will apply the δl function over both alleles at a SNP to obtain a mismatch score
The binary mismatch score was defined similarly to the AMS,
Instead of summing across the alleles in the genotype, however, this score assigned a 1 to instances when the donor genotype had any alleles not present in the recipient genotype and a 0 otherwise (Reindl-Schwaighofer et al., 2019).
Comparison of Scores
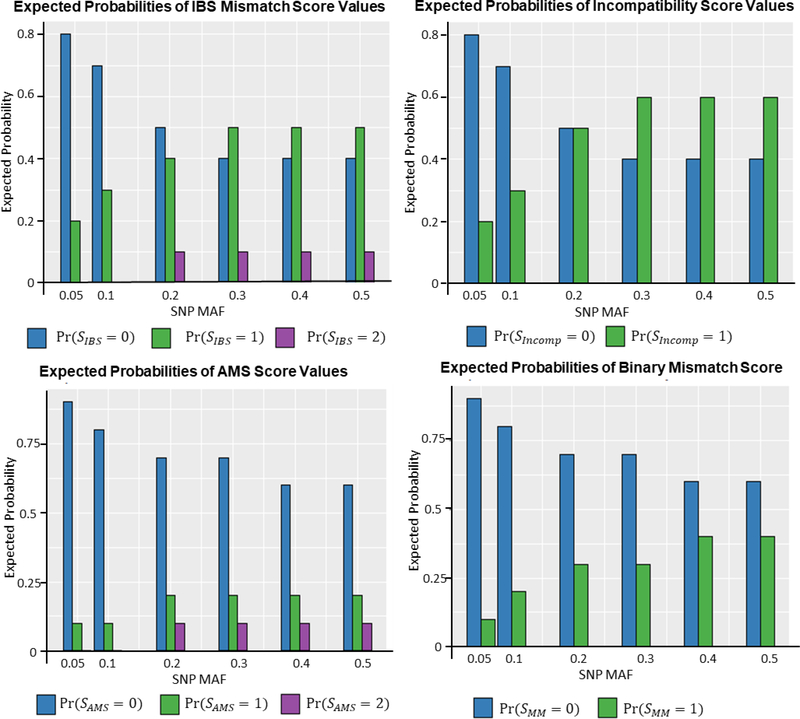
The IBS mismatch score, incompatibility score, the AMS, and the binary mismatch score are related to one another. This relationship can be seen clearly in Table 1, which gives the possible score values for each method and the donor/recipient genotype pairs that result in each score. The incompatibility score acts as a simplified version of the IBS mismatch score, collapsing the IBS scores of one and two into a single score category. The AMS equals the IBS mismatch score when the recipient genotype is homozygous but assigns a score of 0 when the recipient genotype is heterozygous. The binary mismatch score resembles a simplified AMS score and equals the incompatibility score when the recipient genotype is homozygous. Figure 1 shows how the population frequency of each score changes across a range of minor allele frequencies, assuming unrelated donor/recipient pairs and Hardy-Weinberg equilibrium (HWE). For the IBS mismatch score, lower minor allele frequencies (MAFs) result in higher P(SIBS = 0). As MAF increases, P(SIBS = 1) and P(SIBS = 2) increase while P(SIBS = 0) decreases. The incompatibility score and AMS follow the same trend, but P(SMM = 1),P(SAMS = 1) and P(SAMS = 2) always remain relatively small across the range of MAFs, which may lead to low statistical power for association testing, unless the effect size is very large.
Table 1:
The possible score values for the IBS mismatch score (SIBS), incompatibility score (SIncomp), the Allogenomics Mismatch Score (SAMS) and the binary mismatch score (SMM) and the D/R genotype pairs that result in these score values Gd is donor genotype, Gr is recipient genotype.
| Possible Score Values | (Gd,Gr) Pairs | |
|---|---|---|
| SIBS | 0 | {(aa,aa), (Aa,Aa), (AA,AA)} |
| 1 | {(Aa,aa), (aa,Aa), (AA,Aa), (Aa,AA)} | |
| 2 | {(AA,aa), (aa,AA)} | |
| SIncomp | 0 | {(aa,aa), (Aa,Aa), (AA,AA)} |
| 1 | {(Aa,aa), (aa,Aa), (AA,Aa), (Aa,AA), (AA,aa),(aa,AA)} |
|
| SAMS | 0 | {(aa,aa), (aa,Aa), (Aa,Aa), (AA,Aa), (AA,AA)} |
| 1 | {(Aa,aa), (Aa,AA)} | |
| 2 | {(AA,aa), (aa,AA)} | |
| SMM | 0 | {(aa,aa), (aa,Aa), (Aa,Aa), (AA,Aa), (AA,AA)} |
| 1 | {(Aa,aa), (AA,aa), (aa,AA), (Aa,AA)} | |
Figure 1:
Expected probabilities of different score values for the IBS Mismatch Score (SIBS), the incompatibility Score (SIncomp), the Allogenomics Mismatch Score (SAMS) and the binary mismatch score (SMM) under different minor allele frequencies.
Joint Testing of β and γ
In the presence of donor-recipient matching effects, a standard association test between the outcome and recipient genotype can be misleading. We will use an example below to demonstrate how such tests can produce biased results. We therefore recommend a joint test of recipient genotype and matching score as the first step to account for any donor-recipient matching and recipient genetic effects.
Consider a scenario where recipient and donor genotypes are related to a binary phenotype, Y, through the incompatibility score SIncomp:
where . In this model, the donor-recipient matching, not just the recipient genotype, is the risk factor for the outcome. For a standard association test with recipient genotype, like in a recipient-only GWAS, a model as below would be considered:
where for simplicity, we adopt the dominant coding for Grl (0 for aa and 1 for Aa or AA). If we assume that HWE holds for donors and recipients and that the phenotype is rare, the fitted odds ratio, ORFit = exp(βFit), can be expressed as a function of the true odds ratio, ORTrue = exp(βTrue), and the minor allele frequency.
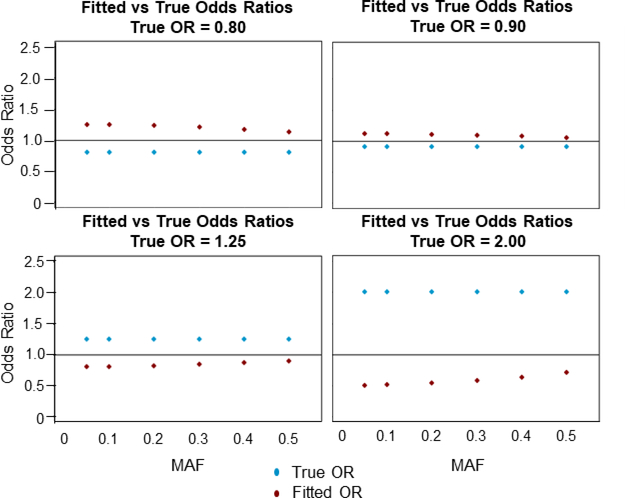
Figure 2 reveals a few insights into the relationship between fitted ORs and true ORs, with SNP MAF ranging from 0.05 to 0.5. First, for all true ORs used, the fitted OR has the opposite effect size. When the true odds ratio is less than 1 indicating a protective effect of incompatibility, for example, the fitted odds ratio is always greater than 1 indicating an increased risk for recipient genotype. Second, for the smallest MAFs, 0.05 and 0.1, the fitted odds ratio tends to be the inverse of the true odds ratio. This is because MAF2 ≈ 0 in these instances, which simplifies the above equation into . Finally, the closer the true odds ratio is to 1, the smaller the difference between the true and fitted OR.
Figure 2:
Minor allele frequency (MAF) versus true and fitted odds ratios (OR) calculated using the empirical equation . The horizontal line corresponds to an OR of 1.00. The blue points correspond to the true OR and the red points correspond to the fitted OR.
If the IBS mismatch or the AMS score were considered instead of the incompatibility score, a similar relationship between the fitted and true ORs would be found but the result would be slightly more complicated because these scores are not binary. Similarly for the binary mismatch score, which acts as a less restrictive incompatibility score by focusing only on alleles present in the donor genotype that are not present in the recipient genotype.
On the other hand, we can consider an opposite situation in which the outcome is associated only with recipient genotype but we test for the matching score. Similar derivation will lead to the same relationship (bias) between the fitted and true ORs. These results signal that caution must be used when performing association analyses when a donor-recipient matching effect is possible. Wrongly specified models may lead to misleading association results, even giving an opposite direction of effect. This misspecification cannot be easily avoided, however, since it is not possible to know whether the recipient genotype or one of the matching scores is truly associated with outcome phenotype before association testing is done. In light of these results, it is of interest to first jointly test the association of both score and recipient genotype as a screening test for association. The joint tests with significant results could then be followed by testing the univariate models of score or genotype to determine which of the two is truly driving the association. A similar two-stage method has been used in gene-environment interaction testing where this method led to improved power for finding relevant associated genes (e.g., Dai et al., 2012).
Simulations
Simulation studies were conducted to address three questions. First, we assess the type I error rates for the different scores. Second, we compare the power of joint testing significance of recipient genotype and score and univariate testing of only the score or genotype. Third, we compare the univariate power between the different scores.
Generation of the Simulated Datasets
Separate datasets were created for 22 immune related genes previously found to be associated with transplant outcomes (Table S5). Phase 2 HapMap CEU genotypes, downloaded from the PLINK website (Purcell et al., 2007), were subset into gene regions based on starting and ending locations. Haplotype frequency data and SNP information was then extracted using Haploview 4.2 (Barrett et al., 2004) (Example: Table S4). This data was then read into R (v 3.6.1; R Core Team, 2018) and two haplotypes were sampled based on haplotype frequency under Hardy-Weinberg Equilibrium and paired up to generate genotype data. Genes ranged from having 2 SNPs (HMOX1, IFNL3) to 68 SNPs (CXCL12) with a median of 14 SNPs per gene. In total, 447 SNPs were tested across the 22 genes. We considered a small, 500 donor/recipient (D/R) pairs, and a large, 1000 D/R pairs, sample size.
The incidence of acute rejection for liver transplant has been reported to be between 10–40%. We therefore chose to fix incidence rates at 15 and 30% for type I error and power analyses (Dogan et al., 2018; Choudhary et al., 2017). Phenotypes were generated using the model
where Yi denotes the outcome phenotype of recipient i in a sample of N total donor/recipient pairs and all other variables are defined in the Methods section. We note that the model is simplified slightly, where Xi in this case can correspond to either a single recipient genotype SNP or one of the single SNP score values.
To determine the power of joint testing, we consider a true model where recipient and donor genotypes are related to binary phenotype only through one of the scores
Of interest is whether jointly testing the significance of the recipient genotype SNP and the score will tend to decrease the power to find the true association. We fit three separate models where we included the single SNP scores only, the recipient genotype SNP only, or both the score and the recipient genotype at the same SNP,
We then tested the power of rejecting the null hypotheses βScore = 0 or βRGeno = 0, for the first two models as well as jointly testing the power of the hypothesis βScore = βRGeno = 0 for the third model. For these analyses, Grl was coded according to the additive model of inheritance (0 for aa, 1 for Aa, 2 for AA). A similar analysis was also conducted where the true model was taken to be
and all three models were once again fit and tested for power as was described above.
For type I error analysis, we used an effect size of β = 0 to generate data under the null model. For power analyses, a range of OR parameters, exp(β),β = ±0.37,±0.30,±0.22, and ± 0.14, were used. In both cases β0 was calculated to ensure the incidence of outcome was either 15 or 30%. Logistic regression models were fit for each SNP of the forms described in the Methods section. A total of 5000 simulations were conducted for each sample size.
Simulation Results
Type I Error Analysis
Type I error analysis gave no indication of inflated false positive rates for any of the simulation models we specified. More details can be seen in the appendix.
Joint Power Analysis
Figure 3 shows the results of power analysis for both the joint modeling and univariate modeling under the true models of IBS mismatch score or recipient genotype SNP being associated with outcome. Both analyses show that jointly testing the significance of the recipient genotype and the score variables has only slightly lower power than the univariate testing of the correct model for all SNP MAFs. Importantly, this relationship occurs despite the fact that the incorrect univariate model has low power to detect an association across all MAFs.
Figure 3:
Plots of estimated statistical power versus SNP minor allele frequency for joint modeling both the recipient genotype and the IBS mismatch score. Results are shown for a true odds ratio of 1.45 and an outcome prevalence of 15%. From left to right, the true model is that IBS mismatch score is associated with the outcome and that recipient genotype is associated with the outcome.
This trend can be seen for all four scores (Figure 4). In general, MAF does not appear to have an impact on power. Comparing the power levels for the scores, it appears that the AMS has the highest power overall. The binary mismatch score has the lowest power overall, although its power is comparable to the incompatibility score. The IBS mismatch score has slightly higher power than the incompatibility score and has similar or slightly lower power compared to the AMS. In all cases, the relatively high power levels do not seem to be because of inflated type I error rates (Figure S1). Overall, these simulations confirm that joint testing is almost as powerful as univariate testing for all SNP MAFs and for all scores.
Figure 4:
Plots of estimated statistical power versus SNP minor allele frequency for joint modeling both the recipient genotype and each of the four scores. Results are shown for a true odds ratio of 1.45 and an incidence of outcome of 15%. From top to bottom, and left to right, the true models are that IBS mismatch score is associated with the outcome, that incompatibility score is associated with the outcome, that the Allogenomics Mismatch Score (AMS) is associated with the outcome, and that binary mismatch score is associated with outcome.
Marginal Power Analysis
Figure 5 shows the results for univariate power analyses, comparing the four scores and the recipient genotype. We see that the IBS mismatch score, the recipient genotype, and the AMS have comparable power across all SNP MAFs. The incompatibility score and binary mismatch score have lower power than the other scores for all MAFs. When the IBS mismatch score is the true model, the incompatibility score has comparable power to the true model and vice versa. This is not surprising as a result of the relationship between these two scores. By comparing the average estimated ORs for each fitted model, we are better able to quantify the bias of the misspecified models (Table S1). When the incompatibility score is used to create the phenotypes, for example, the true model results in estimated ORs around 1.45 (1.43–1.46), while the misspecified models looking at association of the IBS mismatch score with outcome results in estimated ORs ranging from 1.28 to 1.41, the misspecified models where AMS is assumed to be associated with outcome estimate ORs ranging from 1.14 to 1.34, and the misspecified models using the binary mismatch score result in OR estimates ranging from 1.23 to 1.37. In all cases, fitting an incorrect score tends to result in loss of power, as expected, and the loss can be severe. Therefore, if there is no prior hypothesis of which score is best to fit it may be beneficial to fit all four scores although this would lead to a higher amount of multiple testing.
Figure 5:
Estimated power level versus SNP MAF for power analyses. Results shown are for an incidence of outcome of 15% and a true odds ratio value of 1.45. For each plot, a different single SNP covariate value was used to generate the true phenotype values. From top to bottom, and left to right, the covariate used to generate phenotypes is recipient genotype, IBS mismatch score, incompatibility score, Allogenomics Mismatch Score (AMS), and binary mismatch score.
Real Data Analysis
Sample Information
We utilized samples from four liver transplant studies which were conducted on behalf of the international genetics & translational research in transplantation network (iGeneTRAiN). Most of the donors were deceased at the time of transplant, and most donor and recipient pairs were related. All transplants were conducted between 2000 and 2014. The average age of recipient was around 55 and all four studies had around 25% female recipients. All studies also had a majority of Caucasian recipients (73 to 92%). The incidence of acute rejection (AR) outcome ranged from 10–40% in the four studies. In total the data contained information on 1966 donor/recipient pairs of varying ethnicities. Analyses were restricted to a subset of Caucasian only recipients, 1418 pairs, in order to reduce the chance of false-positive findings related to differences in genetic ethnicity. All data sets were genotyped using the Axiom Tx SNP GWAS array, a genotyping chip of approximately 782,000 SNPs that has been specially curated to include variants of importance to transplant, including variants involved in immune response. All the data was imputed using the 1000 Genomes v3 as reference (Keating et al., 2015; Li et al., 2015). After imputation, SNPs were removed from analysis if they had MAF smaller than 0.05, did not follow Hardy-Weinberg equilibrium, or had more than 1% missingness.
The analysis of the whole genome is ongoing. To demonstrate our methods, we gained access to data for 167 SNPs located in 20 immune related genes that had previously been found associated with liver transplant outcomes in the literature (Almoguera, Shaked, & Keating, 2014). For each of the gene regions, SNPs were pulled from PLINK binary files using PLINK 1.7 (Purcell et al., 2007). Gene regions were subset based on the starting location and ending location of each gene from the Genome Reference Consortium Human Build 37 (hg19).
Statistical Analyses
The IBS mismatch score, incompatibility score, the AMS and the binary mismatch score were calculated for each SNP in each gene region using the methods described earlier.
Separate Cox proportional hazards models were created for each scoring method to assess association between each SNP with time to acute rejection after liver transplant. Models were adjusted for the covariates of recipient age, recipient gender, study, categorized year of transplant and the first 3 principal components from the recipient genotype data. If a SNP was found to be significantly associated with AR during joint analysis, then separate univariate models were created that included recipient genotype and score alone. All analyses were conducted using R (v 3.6.1; R Core Team, 2018). Significance was determined based on an alpha of 0.05, and Bonferroni correction was also utilized to account for multiple testing.
Results
Table 2 shows the hazard ratios and uncorrected p-values for the top associated SNP in each gene along with the score that was significantly associated with outcome for the joint model and the results of univariate analyses. For seven genes, joint analyses were able to find SNPs significantly associated with outcome. Univariate analyses were then able to determine whether the recipient genotype or the score was driving the association. When recipient genotype was associated with outcome, such as for SNPs rs4363 (ACE), rs2640539 (AGTR1), rs2856758 (CCR5), and rs800323 (CXCL12), joint analyses of multiple scores tended to produce significant associations. In some instances, both the score and recipient genotype remained associated in univariate analyses, for example both the recipient genotype and the binary mismatch score were found to be associated with AR for SNP rs4363 (ACE). In cases when the score was significantly associated both in joint and univariate analysis, there is little change between the joint and univariate hazard ratio and confidence interval, as seen for both rs3024613 (IL4R) and rs11777928 (TNFRSF10A, but at times the univariate p-values are smaller than the joint testing p-values.
Table 2:
Hazard ratios and 95% confidence intervals for the top SNP in each gene region that was significantly associated with transplant outcome for at least 1 score. Results are based on Caucasian recipients only. Multivariate hazard ratios and joint p-values are shown, as well as univariate hazard ratios and p-values for score or genotype that remained significant in the univariate model. Bold results are significant using alpha of 0.05
| RSID (Gene) | Score used in Joint Model | Joint Analysis Score Hazard Ratio (95% CI) | Joint Analysis R Genotype Hazard Ratio (95% CI) | Joint P-Value | Genotype/Score in Univariate Model | Univar. Analysis Hazard Ratio (95% CI) | Univariate P-Value |
|---|---|---|---|---|---|---|---|
| rs4363 (ACE) | IBS Mismatch Score | 1.00 | 1.29 | 0.03 | IBS Mismatch Score | 0.97 | 0.76 |
| (0.81, 1.23) | (1.07, 1.57) | (0.79, 1.19) | |||||
| Incompatibility Score | 0.94 | 1.29 | 0.03 | Incompatibility Score | 0.92 | 0.55 | |
| (0.72, 1.23) | (1.06, 1.56) | (0.71, 1.20) | |||||
| Allogenomics Mismatch Score | 0.89 | 1.28 | 0.02 | Allogenomics Mismatch Score | 0.86 | 0.17 | |
| (0.72, 1.10) | (1.05, 1.56) | (0.70, 1.07) | |||||
| Binary Mismatch Score | 0.72 | 0.03 | |||||
| Binary Mismatch Score | 0.75 | 1.29 | 4.74E-03† | (0.54, 0.97) | |||
| (0.55, 1.00) | (1.05, 1.58) | Recipient Genotype | 1.29 | 0.01 | |||
| (1.07, 1.56) | |||||||
| rs2640539 (AGTR1) | IBS Mismatch Score | 1.40 | 1.44 | 0.01 | IBS Mismatch Score | 1.21 | 0.10 |
| (1.10, 1.80) | (1.10, 1.88) | (0.97, 1.51) | |||||
| Allogenomics Mismatch Score | 1.33 | 0.01 | |||||
| Allogenomics Mismatch Score | 1.36 | 1.25 | 7.63E-03 | (1.07, 1.66) | |||
| (1.09, 1.71) | (0.99, 1.59) | Recipient Genotype | 1.24 | 0.09 | |||
| (0.97, 1.59) | |||||||
| rs2856758 (CCR5) | IBS Mismatch Score | 0.84 | 1.24 | 0.04 | IBS Mismatch Score | 0.76 | 0.03 |
| (0.63, 1.12) | (0.89, 1.74) | (0.59, 0.98) | |||||
| Incompatibility Score | 0.82 | 1.25 | 0.04 | Incompatibility Score | 0.74 | 0.03 | |
| (0.60, 1.12) | (0.90, 1.74) | (0.56, 0.98) | |||||
| Allogenomics Mismatch Score | 0.77 | 1.40 | 0.02 | Allogenomics Mismatch Score | 0.79 | 0.11 | |
| (0.57, 1.04) | (1.04, 1.90) | (0.58, 1.06) | |||||
| Binary Mismatch Score | 0.75 | 0.11 | |||||
| Binary Mismatch Score | 0.71 | 1.44 | 0.01 | (0.53, 1.07) | |||
| (0.50, 1.02) | (1.06, 1.94) | Recipient Genotype | 1.38 | 0.03 | |||
| (1.02, 1.85) | |||||||
| rs1436926 (CXCL12) | IBS Mismatch Score | 1.47 | 1.53 | 2.56E-03 | IBS Mismatch Score | 1.23 | 0.10 |
| (1.12, 1.92) | (1.16, 2.03) | (0.96, 1.58) | |||||
| Incompatibility Score | 1.45 | 1.49 | 0.01 | Incompatibility Score | 1.24 | 0.13 | |
| (1.08, 1.95) | (1.12, 1.97) | (0.94, 1.63) | |||||
| Allogenomics Mismatch Score | 1.45 | 1.30 | 2.39E-03 | Allogenomics Mismatch Score | 1.43 | 3.86E-03 | |
| (1.13, 1.87) | (1.01, 1.67) | (1.12, 1.83) | |||||
| Binary Mismatch Score | 1.59 | 2.76E-03 | |||||
| Binary Mismatch Score | 1.53 | 1.25 | 3.27E-03 | (1.17, 2.15) | |||
| (1.13, 2.08) | (0.97, 1.61) | Recipient Genotype | 1.31 | 0.04 | |||
| (1.01, 1.71) | |||||||
| rs3024613 (IL4R) | IBS Mismatch Score | 1.38 | 0.98 | 0.01 | IBS Mismatch Score | 1.38 | 2.57E-03 |
| (1.12, 1.70) | (0.81, 1.19) | (1.12, 1.71) | |||||
| Incompatibility Score | 1.49 | 0.98 | 0.02 | Incompatibility Score | 1.49 | 0.01 | |
| (1.11, 2.01) | (0.80, 1.19) | (1.11, 2.01) | |||||
| Allogenomics Mismatch Score | 1.29 | 0.01 | |||||
| Allogenomics Mismatch Score | 1.29 | 0.98 | 0.04 | (1.07, 1.56) | |||
| (1.07, 1.56) | (0.81, 1.18) | Recipient Genotype | 0. 96 | 0.68 | |||
| (0.78, 1.17) | |||||||
| rs12443062 (TNFAIP8L3) | Incompatibility Score | 0.80 | 1.20 | 0.03 | Incompatibility Score | 0.74 | 0.03 |
| (0.57, 0.97) | |||||||
| (0.60, 1.06) | (0.93, 1.56) | Recipient Genotype | 1.29 | 0.04 | |||
| (1.01, 1.65) | |||||||
| rs11777928 (TNFRSF10A) | IBS Mismatch Score | 1.37 | 1.05 | 0.02 | IBS Mismatch Score | 1.34 | 4.49E-03† |
| (1.10, 1.70) | (0.85, 1.29) | (1.10, 1.65) | |||||
| Allogenomics Mismatch Score | 1.35 | 0.99 | 0.01 | Allogenomics Mismatch Score | 1.35 | 1.90E-03† | |
| (1.11, 1.63) | (0.82, 1.20) | (1.12, 1.63) | |||||
| Binary Mismatch Score | 1.49 | 4.65E-03† | |||||
| Binary Mismatch Score | 1.49 | 0.95 | 0.02 | (1.13, 1.96) | |||
| (1.13, 1.96) | (0.79, 1.14) | Recipient Genotype | 0.95 | 0.61 | |||
| (0.78, 1.16) | |||||||
Results reach significance after Bonferroni correction.
Discussion
We studied four methods for use in association analysis of transplant outcomes. These methods, the IBS mismatch score, the incompatibility score, the AMS score, and the binary mismatch score, are genetic matching score methods designed specifically for use with transplant data. We showed that while these four scores are related, they have different probability distributions and it is also likely that they are measuring different aspects of donor/recipient genotype matching. We demonstrated that model misspecification can be extremely detrimental to analysis results. In all cases, regardless of MAF or true OR value, the fitted OR was always of an opposite effect direction as compared to the true OR. These results emphasize the need to be mindful of potential model misspecification and led us to consider joint testing of both score and recipient genotype as the method of choice for screening individual SNPs.
Our simulation studies showed that jointly testing a model containing recipient genotype SNP and matching score was almost equally as powerful as marginally testing the true model. Similar results have been demonstrated before in the context of gene-environment interaction studies. Kraft et al. (2007) showed that a joint two degree of freedom test had comparable power to marginal and interaction-based tests. By jointly testing, we can maintain good power levels even under a misspecified model whereas a misspecified marginal model loses power considerably. After joint testing is complete, those SNPs that were found to be significant using this method can be tested using univariate models in order to determine if the recipient genotype or the score or both are driving the association with outcome.
Our real data analysis of liver transplant data led to identification of seven genes having at least one SNP significantly associated with time to acute rejection. Marginal testing of these top SNPs revealed whether the score or recipient genotype was driving the associations. In cases where recipient genotype SNP was associated with acute rejection outcome, multiple scores showed significance in joint testing and often the confidence intervals for the score hazard ratios crossed 1. When score was significantly associated with outcome, the hazard ratio and confidence interval remain relatively constant between joint and univariate testing. Donor genotype was also considered in our univariate analyses, but the top SNPs were not significantly associated with outcome. Significant SNPs are noted in the supplementary tables (Tables S2 and S3). We note that when a genetic matching score was marginally associated with the probability of acute rejection, but the recipient or donor genotype SNP was not, the hazard ratios of the recipient genotype model and the donor genotype model were not close to being significant. This could mean that the scores are picking up on the difference between the donor and recipient genotypes and are not simply acting as a stand-in for one of these values. In addition, LD analysis conducted using HaploReg (v4.1, Ward and Kellis, 2011) showed that in some cases when multiple SNPs in a gene region were marginally associated with acute rejection probability, there were multiple independent associations.
These associated SNPs should be validated in independent studies. Evidence from previous transplant association studies suggests that the genes ACE, IL4R, and AGTR1 were implicated in acute rejection, and SNPs from ACE, including our top SNP rs4363, were found to be associated with sudden cardiac death in kidney transplant (Moe et al. 2019). Additionally, gene expression studies found that expression levels of AGTR1 tended to be increased for heart transplant recipients with recurrent rejection episodes, and expression levels of IL4R were increased in natural killer cells after kidney transplant (Yamani et al. 2006, Zhu et al. 2018). While these genes were not directly implicated in liver transplant outcomes, the fact that they were found to be significant in other transplant scenarios is encouraging.
Earlier we discussed how these matching scores could be used to help suggest SNPs of interest that may modify transplant outcome. In the case of our real data analyses, we specifically looked at time to first acute rejection, which occurs when the recipient mounts an immune response against the donor organ. Based on our results, some hypotheses of acute rejection mechanisms are possible. A significant SNP was present in the gene CCR5, which is involved in sending various immune cells toward sites of inflammation (Hütter, 2011; Ajuebor, Carey, & Swain, 2006). Results showed that an increase in mismatch score for both the incompatibility score and the IBS mismatch score were associated with decreased odds of acute rejection. It may be the case that mismatch in this gene causes less transcription of the CCR5 protein and in turn leads to less receptors available for binding and instructing other immune cells which could lead to less inflammation and a less acute immune response in the donated liver.
Our methods do have limitations. For real data analyses, we were only able to test our methods on liver transplant data. These methods can be applied to any organ transplant, but it would have been helpful to be able to demonstrate their usefulness for at least one other organ type. In addition, we focused on immune related genes for our initial testing, but it would be interesting to test other genetic regions as well. For our simulation studies, we considered unrelated pairs of donors and recipients as we sampled existing haplotypes to preserve LD among SNPs. These results are also applicable to the related donor and recipient pairs. Future work will consider the degree of relatedness of the matched pairs in association with successful transplant outcomes.
As of now, these scores are only defined in a single SNP setting. Work is ongoing to develop a multi-marker analysis method for these scores to decrease the multiple testing burden and potentially increase power to discover gene regions associated with transplant outcome.
In summary, these four genetic matching scores are powerful tools for future analysis of transplant genetic data. Use of these scores in future association analyses may lead to further understanding of the biology behind acute rejection, and other transplant outcomes, which could lead to the prevention of these negative outcomes for future transplants.
Supplementary Material
Acknowledgements
The research reported in this publication was supported by the National Institutes of Health grants R01-HL138306 (JC, VLA), R21-ES020811 (JC) and R01-ES016626 (JC).
Funding: NIH grants R01-HL138306, R21-ES020811 and R01-ES016626.
Footnotes
Conflict of Interests
The authors declare that there are no conflicts of interest.
Data Availability Statement
The liver transplant data provided by iGeneTRAiN will not be made publicly available. The simulated data that support the findings of this study are available from the corresponding author upon request.
References
- Ajuebor MN, Carey JA, & Swain MG (2006). CCR5 in T cell-mediated liver diseases: what’s going on?. The Journal of Immunology, 177(4), 2039–2045. 10.4049/jimmunol.177.4.2039 [DOI] [PubMed] [Google Scholar]
- Almoguera B, Shaked A, & Keating BJ (2014). Transplantation genetics: current status and prospects. American Journal of Transplantation, 14(4), 764–778. 10.1111/ajt.12653 [DOI] [PubMed] [Google Scholar]
- Barrett JC, Fry B, Maller J, & Daly MJ (2004). Haploview: analysis and visualization of LD and haplotype maps. Bioinformatics, 21(2), 263–265. 10.1093/bioinformatics/bth457 [DOI] [PubMed] [Google Scholar]
- Choudhary NS, Saigal S, Bansal RK, Saraf N, Gautam D, & Soin AS (2017). Acute and chronic rejection after liver transplantation: what a clinician needs to know. Journal of Clinical and Experimental Hepatology, 7(4), 358–366. 10.1016/j.jceh.2017.10.003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dai JY, Kooperberg C, Leblanc M, & Prentice RL (2012). Two-stage testing procedures with independent filtering for genome-wide gene-environment interaction. Biometrika, 99(4), 929–944. 10.1093/biomet/ass044 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dogan N, Hüsing-Kabar A, Schmidt HH, Cicinnati VR, Beckebaum S, & Kabar I (2018). Acute allograft rejection in liver transplant recipients: Incidence, risk factors, treatment success, and impact on graft failure. Journal of International Medical Research, 46(9), 3979–3990. 10.1177/0300060518785543 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dorr CR, Oetting WS, Jacobson PA, & Israni AK (2018). Genetics of Acute Rejection After Kidney Transplantation. Transplant International, 31(3), 263–277. 10.1111/tri.13084 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grafft CA, Cornell LD, Gloor JM, Cosio FG, Gandhi MJ, Dean PG, Stegall MD, & Amer H (2009). Antibody-mediated rejection following transplantation from an HLA-identical sibling. Nephrology Dialysis Transplantation, 25(1), 307–310. 10.1093/ndt/gfp526 [DOI] [PubMed] [Google Scholar]
- Green DJ, Brooks MM, Burckart GJ, Chinnock RE, Canter C, Addonizio LJ, Bernstein D, Kirklin JK, Naftel DC, Girnita DM, Zeevi A, & Webber SA (2017). The Influence of Race and Common Genetic Variations on Outcomes After Pediatric Heart Transplantation. American Journal of Transplantation, 17(6), 1525–1539. 10.1111/ajt.14153 [DOI] [PubMed] [Google Scholar]
- Hütter G (2011). The Impact of CCR5 Polymorphism on the Clinical Outcome of Allogeneic Stem Cell Transplantation. Journal of Transplantation Technologies & Research, 1, 2161–0991. 10.4172/2161-0991.S1-004 [DOI] [Google Scholar]
- Julian BA, Gaston RS, Brown WM, Reeves‐Daniel AM, Israni AK, Schladt DP, Pastan SO, Mohan S, Freedman BI, & Divers J (2017). Effect of Replacing Race With Apolipoprotein L1 Genotype in Calculation of Kidney Donor Risk Index. American Journal of Transplantation, 17(6), 1540–1548. 10.1111/ajt.14113 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Keating BJ, Van Setten J, Jacobson PA, Holmes MV, Verma SS, Chandrupatla HR, Nair N, Gao H, Li YR, Chang B, Wong C, Phillips R, Cole BS, Mukhtar E, Zhang W, Cao H, Mohebnasab M, Hou C, Lee T, Steel L, … & Wong C (2015). Design and Implementation of the International Genetics and Translational Research in Transplantation Network. Transplantation, 99(11), 2401–2412. 10.1097/TP.0000000000000913 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kraft P, Yen YC, Stram DO, Morrison J, & Gauderman WJ (2007). Exploiting Gene-Environment Interaction to Detect Genetic Associations. Human Heredity, 63(2), 111–119. 10.1159/000099183 [DOI] [PubMed] [Google Scholar]
- Kwee LC, Liu D, Lin X, Ghosh D, & Epstein MP (2008). A Powerful and Flexible Multilocus Association Test for Quantitative Traits. The American Journal of Human Genetics, 82(2), 386–397. 10.1016/j.ajhg.2007.10.010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li YR, van Setten J, Verma SS, Lu Y, Holmes MV, Gao H, Lek M, Nair N, Chandrupatla H, Chang B, Karczewski KJ, Wong C, Mohebnasab M, Mukhtar E, Phillips R, Tragante V, Hou C, Steel L, Lee T, Garifallou J, … & Keating BJ (2015). Concept and design of a genome-wide association genotyping array tailored for transplantation-specific studies. Genome medicine, 7(1), 90 10.1186/s13073-015-0211-x [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lim WH, Wong G, Heidt S, & Claas FHJ (2018). Novel Aspects of Epitope Matching and Practical Application in Kidney Transplantation. Kidney International, 93(2), 314–324. 10.1016/j.kint.2017.08.008 [DOI] [PubMed] [Google Scholar]
- McCarroll SA, Bradner JE, Turpeinen H, Volin L, Martin PJ, Chilewski SD, Antin JH, Lee SJ, Ruutu T, Storer B, Warren EH, Zhang B, Zhao LP, Ginsburg D, Soiffer RJ, Partanen J, Hansen JA, Ritz J, Palotie A, & Altshuler D (2009). Donor-recipient Mismatch for Common Gene Deletion Polymorphisms in Graft-Versus-Host Disease. Nature Genetics, 41(12), 1341–4. 10.1038/ng.490 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mesnard L, Muthukumar T, Burbach M, Li C, Shang H, Dadhania D, Lee JR, Sharma VK, Xiang J, Suberbielle C, Carmagnat M, Ouali N, Rondeau E, Friedewald JJ, Abecassis MM, Suthanthiran M, & Campagne F (2016). Exome Sequencing and Prediction of Long-Term Kidney Allograft Function. PLoS Computational Biology, 12(9), e1005088 10.1371/journal.pcbi.1005088 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moe SM, Long J, Schwantes-An THL, Decker BS, Wetherill L, Edenberg HJ, Xuei X, Vatta M, Foroud TM, & Chertow GM (2018). Angiotensin-related Genetic Determinants of Cardiovascular Disease in Patients Undergoing Hemodialysis. Nephrology Dialysis Transplantation, 34(11), 1924–1931. 10.1093/ndt/gfy191 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Morris AA, Kalogeropoulos AP, Zhao L, Owen M, Laskar SR, Vega JD, Smith A, & Butler J (2015). Race and Ethnic Differences in the Epidemiology and Risk Factors for Graft Failure After Heart Transplantation. The Journal of Heart and Lung Transplantation, 34(6), 825–831. 10.1016/j.healun.2014.12.012 [DOI] [PubMed] [Google Scholar]
- Nair S, Eustace J, & Thuluvath PJ (2002). Effect of Race on Outcome of Orthotopic Liver Transplantation: A Cohort Study. The Lancet, 359(9303), 287–293. 10.1016/S0140-6736(02)07494-9 [DOI] [PubMed] [Google Scholar]
- Nair S, & Thuluvath PJ (2001). Does Race-Matched Liver Transplantation Offer Any Graft Survival Benefit?. Transplantation Proceedings 33(1–2), 1523–4. 10.1016/s0041-1345(00)02581-1 [DOI] [PubMed] [Google Scholar]
- Oetting WS, Guan W, Schladt DP, Leduc RE, Jacobson PA, Matas AJ, Chinnakotla S, Schröppel B, Murphy BT, & Israni AK (2012). Donor Polymorphisms of Toll-Like Receptor 4 Associated With Graft Failure in Liver Transplant Recipients. Liver Transplantation, 18(12), 1399–1405. 10.1002/lt.23549 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Oetting WS, Schladt DP, Guan W, Miller MB, Remmel RP, Dorr C, Sanghavi K, Mannon RB, Herrera B, Matas AJ, Salomon DR, Kwok P‐Y, Keating BJ, Israni AK, & Jacobson PA for the DeKAF Investigators (2016). Genomewide Association Study of Tacrolimus Concentrations in African American Kidney Transplant Recipients Identifies Multiple CYP3A5 Alleles. American Journal of Transplantation, 16(2), 574–582. 10.1111/ajt.13495 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pang PS, Kamal A, & Glenn JS (2009). The Effect of Donor Race on the Survival of Black Americans Undergoing Liver Transplantation for Chronic Hepatitis C. Liver Transplantation, 15(9), 1126–1132. 10.1002/lt.21835 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Parimi N, Tromp G, Kuivaniemi H, Nien JK, Gomez R, Romero R, & Goddard KA (2008). Analytical approaches to detect maternal/fetal genotype incompatibilities that increase risk of pre-eclampsia. BMC Medical Genetics, 9(1), 60 10.1186/1471-2350-9-60 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pineda S, Sigdel TK, Chen J, Jackson AM, Sirota M, & Sarwal MM (2018). Novel Non-Histocompatibility Antigen Mismatched Variants Improve the Ability to Predict Antibody-Mediated Rejection Risk in Kidney Transplant. Frontiers in Immunology, 8, 1687 10.3389/fimmu.2017.01687 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Purcell S, Neale B, Todd-Brown K, Thomas L, Ferreira MAR, Bender D, Maller J, Sklar P, de Bakker PIW, Daly MJ, & Sham PC (2007). PLINK: A Tool Set for Whole-Genome Association and Population-Based Linkage Analyses. The American Journal of Human Genetics, 81(3), 559–575. 10.1086/519795 [DOI] [PMC free article] [PubMed] [Google Scholar]
- R Core Team (2018). R: A language and environment for statistical computing. R Foundation for Statistical Computing, Vienna, Austria: URL: https://www.R-project.org/. [Google Scholar]
- Reddy MS, Varghese J, Venkataraman J, & Rela M (2013). Matching donor to recipient in liver transplantation: Relevance in clinical practice. World Journal of Hepatology, 5(11), 603–611. 10.4254/wjh.v5.i11.603 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reindl-Schwaighofer R, Heinzel A, Kainz A, van Setten J, Jelencsics K, Hu K, Loza B, Kammer M, Heinze G, Hruba P, Koňaříková A, Viklicky O, Boehmig GA, Eskandary F, Fischer G, Claas F, Tan JC, Albert TJ, Patel J, Keating B, & Oberbauer R (2019). Contribution of non-HLA incompatibility between donor and recipient to kidney allograft survival: genome-wide analysis in a prospective cohort. The Lancet, 393(10174), 910–917. 10.1016/S0140-6736(18)32473-5 [DOI] [PubMed] [Google Scholar]
- Saxena V, Lai JC, O’Leary JG, Verna EC, Brown RS Jr, Stravitz RT, Trotter JF, Krishnan K, Terrault NA, & Consortium to Study Health Outcomes in HCV Liver Transplant Recipients. (2012). Recipient-donor Race Mismatch for African American Liver Transplant Patients With Chronic Hepatitis C. Liver Transplantation, 18(5), 524–531. 10.1002/lt.22461 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Steers NJ, Li Y, Drace Z, D’Addario JA, Fischman C, Liu L, Xu K, Na Y, Neugut YD, Zhang JY, Sterken R, Balderes O, Bradbury D, Ozturk N, Ozay F, Goswami S, Mehl K, Wold J, Jelloul FZ, … & Kiryluk K (2019). Genomic Mismatch at LIMS1 Locus and Kidney Allograft Rejection. New England Journal of Medicine, 380 (20), 1918–1928. 10.1056/NEJMoa1803731 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sypek M, Kausman J, Holt S, & Hughes P (2018). HLA Epitope Matching in Kidney Transplantation: An Overview for the General Nephrologist. American Journal of Kidney Diseases, 71(5), 720–731. 10.1053/j.ajkd.2017.09.021 [DOI] [PubMed] [Google Scholar]
- Ward LD, & Kellis M (2011). HaploReg: A Resource for Exploring Chromatin States, Conservation, and Regulatory Motif Alterations Within Sets of Genetically Linked Variants. Nucleic Acids Research, 40(D1), D930–D934. 10.1093/nar/gkr917 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yamani MH, Cook DJ, Rodriguez ER, Thomas DM, Gupta S, Alster J, Taylor DO, Hobbs R, Young JB, Smedira N, & Starling RC (2006). Increased Expression of Angiotensin II Type 1 Receptor (AGTR1) in Heart Transplant Recipients With Recurrent Rejection. The Journal of Heart and Lung Transplantation, 25(11), 1283–1289. 10.1016/j.healun.2006.09.012 [DOI] [PubMed] [Google Scholar]
- Yang JYC, & Sarwal MM (2017). Transplant Genetics and Genomics. Nature Reviews Genetics, 18(5), 309 10.1038/nrg.2017.12 [DOI] [PubMed] [Google Scholar]
- Zhang Y (2017). Impact of Donor Recipient Gender and Race Mismatch on Graft Outcomes in Patients With End-Stage Liver Disease Undergoing Liver Transplantation. Progress in Transplantation, 27(1), 39–47. 10.1177/1526924816679839 [DOI] [PubMed] [Google Scholar]
- Zhu L, Aly M, Wang H, Karakizlis H, Weimer R, Morath C, Kuon RJ, Toth B, Ekpoom N, Opelza G, & Daniel V (2018). Changes of NK cell subsets with time post-transplant in peripheral blood of renal transplant recipients. Transplant Immunology, 49, 59–71. 10.1016/j.trim.2018.04.005 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.