Abstract
The occurrence of harmful algal blooms dominated by toxic cyanobacteria has induced continuous loadings of algal organic matter (AOM) and toxins in drinking water treatment plants. However, the impact of AOM on the active biofilms and microbial community structures of biologically-active filtration (BAF), which directly affects the contaminant removal, is not well understood. In this study, we systematically examined the effects of AOM on BAF performance and bacterial biofilm formation over 240 days, tracing the removal of specific AOM components, a cyanotoxin [microcystin-LR (MC-LR)], and microbial community responses. The component analysis (excitation and emission matrix analysis) results for AOM revealed that terrestrial humic-like substances showed the highest removal among all the identified components and were strongly correlated to MC-LR removal. In addition, reduced empty bed contact time and deactivation of biofilms significantly decreased BAF performances for both AOM and MC-LR. The active biofilm, bacterial community structure, and mlrA gene (involved in microcystin degradation) abundance demonstrated that bacterial biofilm composition responded to AOM and MC-LR, in which Rhodocyclaceae, Saprospiraceae, and Comamonadaceae were dominant. In addition, MC-LR biodegradation appeared to be more active at the top than at the bottom layer in BAF. Overall, this study provides deeper insights into the role of biofilms and filter operation on the fate of AOM and MC-LR in BAF.
Keywords: Algal organic matter (AOM), Microcystin, Biologically-active filtration, Biofilm, Excitation-emission matrix (EEM), Microbial community structure
1. Introduction
Harmful algal blooms (HABs) dominated by toxic cyanobacteria have been increasingly detected in water bodies worldwide. During such blooms, cyanobacterial cells may produce and release undesirable algal metabolites such as cyanotoxins and taste and odor causing compounds, which can severely impair water quality (Chen et al., 2017; Fang et al., 2010a; Li et al., 2012b). Among the cyanotoxins produced by different cyanobacteria, the most prevalent in freshwater systems are microcystins (MCs). Microcystin-LR (MC-LR), one of the most toxic and frequently detected among microcystin congeners, is a hepatotoxin that can be lethal (Gutiérrez-Praena et al., 2012). In addition, cyanobacterial cells release algal organic matter (AOM) containing a wide spectrum of components such as amino acids, peptides, proteins, and polysaccharides (Guo et al., 2017). These are known to serve as precursors for disinfection byproducts (DBPs) (Hong et al., 2009). Hence, to produce safe drinking water, it is critical that drinking water treatment plants (DWTPs) are prepared to control AOM and MCs.
Most DWTPs rely on conventional treatment methods consisting of coagulation/flocculation (C/F), sedimentation, granular media filtration (GMF), and disinfection. During the C/F process, AOM inhibits the C/F effectiveness by increasing the negative particle charge. AOM proteins are likely to form complexes with coagulants, resulting in the reduction of available coagulants for particle destabilization (Pivokonsky et al., 2006; Sano et al., 2011). The C/F process has also shown to be ineffective in removing extracellular MCs (Sun et al., 2013). Consequently, these conditions may lead to continuous loads of AOM with MC-LR in GMF systems.
Recently, biologically-active filtration (BAF) with granular activated carbon (GAC) has been widely employed in place of conventional GMF to reduce biodegradable organic matter, DBP precursors, and taste and odor causing compounds. The biological degradation of MC-LR was also demonstrated by the indigenous microbial biofilm in BAF (Ho et al., 2006). For instance, Morganella morganii isolated from a filtration system showed the biodegradation of MC-LR with and without other carbon sources (Eleuterio and Batista, 2010). However, few studies have reported the performance of GAC-based GMF in the biodegradation of MC-LR. For example, lab-scale filtration with the combination of adsorption and biodegradation showed complete removal of pure MC-LR (5 μg/L) for 6 months of operation using filter effluent from a DWTP (Wang et al., 2007). On the other hand, for full-scale GMF systems with GAC, even less than 5 μg/L of MC-LR in raw water was not completely eliminated (Lahti et al., 2001; Lambert et al., 1996). It should be noted that previous lab-scale tests mostly evaluated the performance of GAC-based GMF at longer filtration times (>15 min), whereas many full-scale GMF systems are operated under rapid filtration conditions (≤10 min) (Brown et al., 2016). Furthermore, in all previous studies, the impacts of AOM on biofilm formation and MC-LR removal have not been evaluated even though AOM and MC-LR co-exist in source water. Results from these previous studies indicate that there are great research needs to better understand and optimize BAF to treat AOM and MC-LR.
The biodegradation performance of BAF heavily relies on microbial community composition and activity within biofilms (Kim et al., 2014; Richter et al., 2008). Additionally, operational variability (e.g. filtration rate and type of filter media) and filter influent water quality (e.g. organic matter) can significantly affect microbial biofilm stability (i.e., their structure and function) (Prest et al., 2016). The large portion of labile organic compounds in AOM and its continuous load may significantly alter biofilm community structure, impacting BAF performance (Bittar et al., 2015; Hammes et al., 2007). Several studies have reported the changes of bacterial community structure associated with HABs and the decomposition of AOM in freshwater systems (Louati et al., 2015; Shi et al., 2017; Woodhouse et al., 2016). However, no studies have focused on understanding the impact of AOM components on microbial community dynamics in BAF even though biofilm community structure not only plays an important role in various organic matter removal at DWTPs, but also shapes the microbial community composition in drinking water distribution systems (DWDSs) (El-Chakhtoura et al., 2015).
To address the aforementioned knowledge gaps, GAC/sand columns simulating BAF in DWTPs were operated to (1) understand the role of biofilms and different operational conditions (slow and rapid filtration) for the removal of AOM and MC-LR, (2) elucidate the treatability of spectrally characterized AOM components and their correlations with MC-LR removal, and (3) investigate the impact of AOM on the activity and community structure of bacterial biofilms in BAF.
2. Materials and methods
2.1. Materials and reagents
MC-LR standards were purchased from Cayman Inc. (purity ≥ 95%). Cyanobacteria-laden water samples (mostly Microcystis species) were obtained from the western basin of Lake Erie during the summer of 2017. AOM from Microcystis sp. contains a mixture of compounds such as amino acids, peptides, polysaccharides, and proteins (Guo et al., 2017). Cyanobacterial cells were separated from the collected water samples by membrane filtration using a 0.45 μm (pore size) filter. The separated cells were mixed with DI water and underwent three freeze-thaw cycles at −80 °C. Then, AOM was obtained by filtering the water sample using a 0.45 μm membrane filter (Zhu et al., 2015) and was stored for column operation.
2.2. Column set-up and operations
Figure S1 shows a schematic diagram of the experimental setup. Five identical glass columns (length: 25 cm, internal diameter: 2.5 cm) were prepared in parallel. Filter media of the columns consisted of GAC (10 cm) and sand (4 cm) layers simulating BAF with GAC in a full-scale DWTP (Toledo, Ohio, USA). Filter influent for the column operation was obtained from the DWTP after alum coagulation and sedimentation, but prior to GMF (Table 1). Selected fresh GAC with an effective particle size of 1.0–1.2 mm was obtained from the Calgon Carbon Corporation. GAC was rinsed with deionized water, autoclaved at 121 °C, and oven-dried prior to use. Filter sand media with an effective size of 0.8–1.0mmwas obtained from the DWTP and prepared following the same procedures for GAC.
Table 1.
Water quality and biological parameters of filter influent.
| TOC (mg/L) | TN (mg/L) | Turbidity (NTU) | pH | HPC (CFU/mL) | |
|---|---|---|---|---|---|
| Day 0–159 | 2.05 ± 0.48 | 2.25 ± 1.32 | 0.96 ± 1.74 | 8.90 ± 0.32 | 1.33 ± 0.95 (× 105) |
| Day 160–242 | 2.38 ± 0.54 | 0.32 ± 0.14 | 0.75 ± 0.46 | 8.71 ± 0.31 | 2.49 ± 0.99 (× 105) |
Operation conditions of the columns are summarized in Table 2. Collected biomass during backwashing of filters at the DWTP was inoculated into columns 1 and 2 before operating the columns to emulate filtration systems with indigenous biofilm formation (Bai et al., 2016). Columns 3, 4, and 5 were operated without biomass inoculation to obtain naturally developed biofilms on filter media by feed solution. In order to determine the abiotic removal of AOM and MC-LR in BAF, sodium azide (a biocide) was tested but significantly oxidized MC-LR. Thus, autoclaving was conducted for column 5 once a week (10 min at 121 °C) to monitor filter performance with reduced biofilm formation (Wang et al., 2007). For 160 days, all five columns were operated at 20 min EBCT (representing slow filtration condition) by feeding the collected filter influent without AOM and MC-LR. Column 4 (without initial biomass inoculation) was sacrificed to analyze active biomass in biofilm and microbial community composition before AOM was introduced. From day 161 on, columns 2, 3, and 5 were operated with filter influent fortified with AOM, while column 1 was continuously operated only with filter influent. AOM was diluted 10,000 times by filter influent and AOM contribution to total organic carbon (TOC) and total nitrogen (TN) was ~0.5 mg L−1 and ~0.1 mg L−1, respectively. AOM concentration was determined based on previous studies, which monitored AOM releases by a pre-oxidation step (Qi et al., 2016; Xie et al., 2013), and our own preliminary laboratory tests to estimate the impact of water treatment on AOM releases at the DWTP seasonally impacted by HABs. MC-LR was not detected in AOM fortified filter influent.
Table 2.
Operations of GAC/sand columns with AOM and MC-LR.
| EBCT(min) | Column 1a | Column 2a | Column 3 | Column 4 | Column 5 b | |
|---|---|---|---|---|---|---|
| Day 1–159 | 20 | FI | FI | FI | FI | FI |
| Day 160–178 | 20 | FI | FI/AOM | FI/AOM | Dismantled c | FI/AOM |
| Day 179–208 | 20 | FI | FI/AOM/MC-LR | FI/AOM/MC-LR | x | FI/AOM/MC-LR |
| Day 209–242 | 10 | FI | FI/AOM/MC-LR | FI/AOM/MC-LR | x | FI/AOM/MC-LR |
FI: Filter influent for the column operation was obtained from a DWTP.
Filter biomass was inoculated before operation to facilitate the formation of biofilm.
Column 5 was regularly sterilized by autoclaving (10 min at 121 °C).
Column 4 was dismantled at day 159 and filter media samples were collected.
After day 179, pure MC-LR (5 μg/L) with AOM was injected into columns 2, 3, and 5 at 20 min EBCT. The observed average MC-LR concentration in the influent of the columns was 5.07 ± 0.11 μg/L. From day 209 on, the columns were operated at 10 min EBCT (simulating a rapid filtration condition) to examine the influence of EBCT on the removal of MC-LR and AOM. All columns were operated under room temperature (23 ± 2 °C). A single column was prepared for each condition due to high operational costs associated with utilizing pure MC-LR for the study.
Surface cleaning for columns was conducted every two weeks during the first phase and once a week after the addition of AOM and MC-LR to minimize the re-stratification of filter media. Influent and effluent samples (0.25 L for each in a sterilized glass flask) were collected from the columns twice per week for water quality analyses. At the end of the column operation, filter media samples in each column were also collected in sterilized tubes from the top, middle, and bottom of 10 cm GAC layers and a 4 cm sand layer for active biomass in biofilm and microbial community structure analyses. In addition, collected filter influent (2 L) from the DWTP was filtered using 0.2 μm membrane filter (Millipore, USA). The filter media samples and membranes were stored at −80 °C until further processing for microbial community analyses.
2.3. Water quality analysis and quantification of biomass
TOC and TN of collected filter influents and effluents were determined using a TOC analyzer (TOC-VCSH, Shimadzu, Japan). The pH and turbidity were also analyzed. Adenosine triphosphate (ATP) analysis was used to regularly monitor active biomass of collected column influent and effluent samples. ATP of collected filter media samples was also analyzed to determine active biomass in columns using an ATP kit (LuminUltra, Canada) developed for biofilms. A standard heterotrophic plate count (HPC) was performed for filter influent samples according to the previously described method (Fujioka et al., 2019). Bacterial counts using the HPC method were expressed with the colony-forming unit (CFU).
2.4. Excitation-emission matrix (EEM) measurements and PARAFAC modeling
To trace removal of dissolved organic matters (DOM) including AOM components in BAF, the EEMs of collected influents and effluents were measured using a spectrofluorometer (RF-6000, Shimadzu, Japan). Details describing the measurements and corrections can be found in Text S1. A total of ninety-six EEM samples (349 EEM spectra) obtained from the study were utilized to identify individual components using parallel factor analysis (PARAFAC) modeling (Murphy et al., 2013). The Fmax value represents the maximum fluorescence intensity of each component. Fmax in influents and effluents was used to calculate the removal efficiency of organic matter in BAF.
2.5. Microcystin-LR analysis
Collected samples in a 250 mL Erlenmeyer flask were treated using the solid phase extraction (SPE) method (available in Text S2) (Kaloudis et al., 2013). High performance liquid chromatography (HPLC) coupled with a photo diode array (PDA) detector (LC-20AD, Shimadzu, Japan) and a C18 column (Phenomenex Kinetex, USA) with a guard column was used to quantify MC-LR. The analysis was performed by isocratic elution (flow rate 0.5 mL/min) using a solution composed of 4:6 (volume) acetonitrile (Sigma Aldrich, USA) to HPLC water with 0.05% trifluoracetic acid (Sigma Aldrich, USA). The PDA detector was set at the wavelength range of 195–300 nm, and 238 nm was selected to determine MC-LR (the detection limit was 0.05 μg/L).
2.6. DNA extraction, quantitative polymerase chain reaction (qPCR), and high-throughput amplicon sequencing analysis
DNA was extracted from collected filter media samples (0.5 g) and membrane filters for filter influent using a commercial DNA isolation Kit (Mo-Bio Laboratories, USA). The concentration and quality of DNA in the extracts were examined using Qubit 3.0 (Life Technologies, USA). DNA extracts were used as templates to detect the mlrA gene, which encodes a microcystinase enzyme involved in MC-LR biodegradation (Hoefel et al., 2009a), and to generate 16S rRNA gene sequencing libraries. Barcoded 16S rRNA gene targeting primers (i.e., 515F and 806R) (Caporaso et al., 2011) were used and the targeted products (i.e., 291 bp) were sequenced in both directions using an Illumina MiSeq with a PE250 sequencing kit as previously described (Inkinen et al., 2016). The obtained 16S rRNA gene sequence data were processed and analyzed using QIIME 1.9.1 software (Caporaso et al., 2010). Detailed procedures for qPCR and the sequencing data analysis are in Text S3.
2.7. Statistical analysis
The R software (version 3.4.4) was used for statistical analyses of the data. Principal coordinate analysis (PCoA) based on unweighted Unifrac distance was performed to visualize the distribution of community structures in filter media using rarefied sequencing data at the library size of 12,900. Permutational Multivariate ANOVA (PERMANOVA) based on Bray-Curtis dissimilarities and similarity percentage (SIMPER) analysis were conducted to monitor the differences among the columns and at different filter depths. Further statistical analysis information about the effect of AOM on filter performances is provided in Text S4.
3. Results and discussions
3.1. Removal of MC-LR by BAF under the presence of AOM
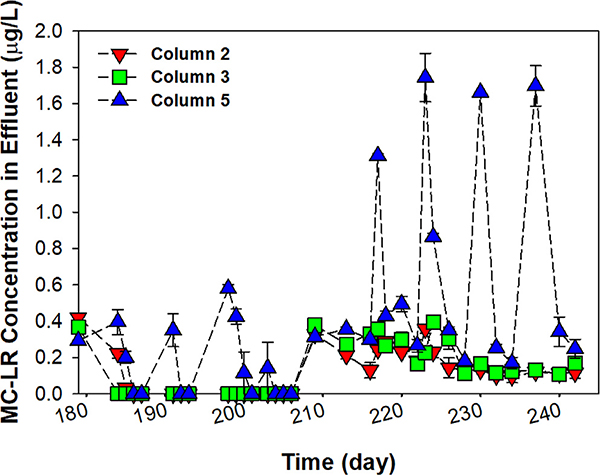
At 20 min EBCT, MC-LR was detected in the effluents of all three columns for the first few days (Fig. 1), but columns 2 and 3 showed the complete removal of MC-LR after 7 days of acclimation period. For column 5 (the autoclaved column), MC-LR breakthrough occurred repeatedly right after each sterilization. The MC-LR removal results for 20 min EBCT indicate that GAC with active biofilms can efficiently eliminate 5 μg/L of MC-LR even under the presence of AOM. However, when 10 min EBCT was applied, MC-LR was found in all effluent samples. Averages of MC-LR detected in the effluents of columns 2, 3, and 5 were 0.17 ± 0.07 μg/L, 0.22 ± 0.09 μg/L, and 0.65 ± 0.56 μg/L, respectively. For column 5 at 10 min EBCT, the breakthrough patterns became increasingly distinct (>1 μg/L of MC-LR). If adsorption was the only removal mechanism for MC-LR in column 5 right after sterilization, the results suggest that 3.39 ± 0.17 μg/L of MC-LR was abiotically removed. After this, its removal was rapidly recovered as much as those of columns 2 and 3. It is worth noting that the sterilization significantly lowered the removal efficiency of MC-LR in column 5 (P < 0.05). Generally, the matrix structures of active biofilms are known to influence the transport of waterborne substances from the bulk fluid to substratum (Stewart, 2003). Previous studies evidenced that biofilms on GAC inhibit the liquid bulk flow but introduce a laminar liquid film around GAC, which effectively allows waterborne substances to diffuse into the adsorption sites in GAC (Stoodley and Lewandowski, 1994). It is also noteworthy that the top layer of column 5 had a higher ATP value than in columns 1 and 4 (columns without AOM and MC-LR) even though column 5 was sterilized two days before collecting filter media samples (Fig. S2). This shows that activity of bacterial biofilms was partially recovered in column 5 between the regular sterilizations. Therefore, we surmise that the recovery of MC-LR removal in column 5 was closely associated with the formation of active biofilms on GAC. However, further studies are required to enhance our understanding on the removal mechanisms (GAC adsorption, biosorption, and biodegradation) of MC-LR and how biofilm characteristics (e.g. thickness, surface coverage, and porosity) affect mass transfer of MC-LR onto GAC surfaces in BAF.
Fig. 1.
Removal of MC-LR through the BAF systems: effluent from column 2 (red, biomass seeded), column 3 (green, no biomass seeded), and column 5 (autoclaved control, blue); influent MC-LR concentration was 5.07 ± 0.11 μg/L. Samples were collected at the EBCT of 20 min (day 179–208) and 10 min (day 209–242) after the addition of AOM/MC-LR. (For interpretation of the references to colour in this figure legend, the reader is referred to the Web version of this article.)
EBCT is a crucial operational parameter for BAF and shorter EBCT is known to decrease BAF performance for organic carbon removal (Terry and Summers, 2018). In this study, after reducing the EBCT from 20 min to 10 min, a significant drop of TOC removal in all columns was observed (P < 0.05) (Fig. S3). At 10 min EBCT, the average TOC removals were 15 ± 7.7%, 20 ± 7.8%, 23 ± 8.2%, and 12 ± 11.9%, for columns 1, 2, 3, and 5, respectively. Likewise, we found that the MC-LR removal also significantly decreased with the reduced EBCT (P < 0.05). BAF or GMF systems in most DWTPs with large treatment capacities are operated at an EBCT less than or equal to 10 min (Brown et al., 2016). Thus, our results suggest that the performance of GMF for MC-LR removal under the rapid filtration (≤10 min) needs to be carefully monitored, especially when a high concentration of MC-LR is present in filter influent.
Previous field studies with sand and anthracite media showed that more than 1 μg/L of MC-LR remained in finished water when raw water contained around 5 μg/L of MC-LR, and even 2.1 μg/L of MC-LR in filter influent was not completely removed (>0.3 μg/L in filter effluent) (Miller et al., 2017; Zamyadi et al., 2012). Even considering the differences in the water quality, operating conditions, and filter media type, our results indicate that BAF with GAC also has limitations in completely removing MC-LR at the short EBCT under the presence of AOM. This suggests that DWTPs need to consider additional treatment barriers (e.g. powdered activated carbon addition to C/F for adsorbing dissolved MC-LR or increased disinfectant dose to oxidize the Adda group of MC-LR for BAF effluent, resulting in a less toxic form) along with increasing EBCT (if feasible) for MC-LR during HABs. More detailed information of the impacts of EBCT and injected AOM on the performance of BAF is available in Text S5.
3.2. Tracking organic matter removal in BAF using EEM-PARAFAC
The PARAFAC model analysis results (Table S1) identified four independent components associated with different dissolved organic matrices in all samples (Fig. S4). Component 2 (C2) contained two maxima at ex/em 270 (370)/466 nm. Components 1, 3, and 4 (C1, C3, and C4) had a single maximum at ex/em 320/394, 280/322, and 250/308 nm, respectively. C1 is associated with marine humic-like substances, C2 relates to terrestrial humic-like substances, and C3 and C4 represent protein-like substances (Table S2). The peak related to C3 contains tryptophan-like substances and autochthonous protein-like substances which are susceptible to microbial degradation (Villacorte et al., 2015). For C4, the peak is similar to tyrosine-like substances in an aromatic protein-like region (Chen et al., 2003).
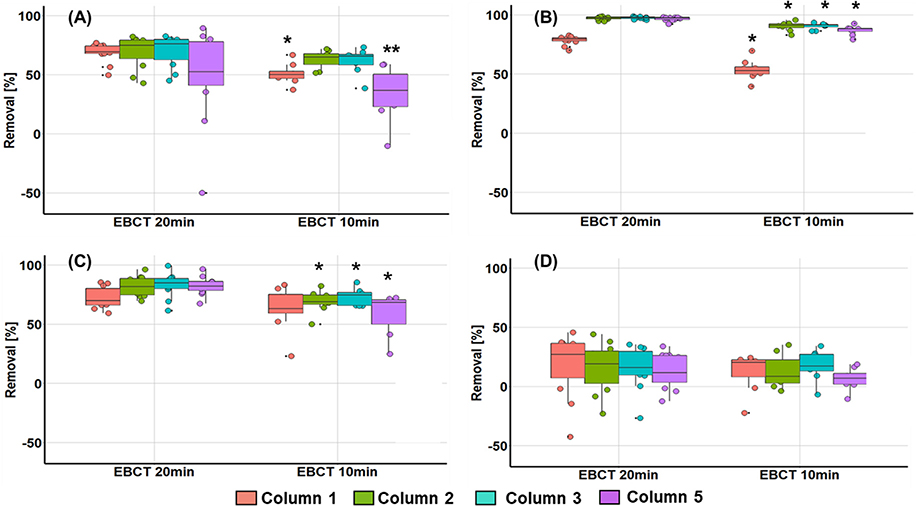
After the characterization, component composition and the % removal efficiency of each component through filtration were obtained using Fmax (Fig. 2). Statistical analyses were also performed to monitor the effects of the EBCT and biofilm sterilization on organic matter removal. We found that the addition of AOM in the filter influent increased components C2 (terrestrial humic-like) and C3 (protein-like) (Fig. S5), which was in line with previous findings (Hong et al., 2008). Most of all, all columns showed higher removal efficiency for C2 than other components at 20 min EBCT (78.2 ± 3.6%, 96.9 ± 1.3%, 97.2 ± 0.9%, and 96.5 ± 1.6% for columns 1, 2, 3, and 5, respectively), confirming that humic-like substances with longer emission wavelengths (394 nm for C1 and 466 nm for C2) are more likely to have a strong affinity to GAC (Sgroi et al., 2018). There was also significant removal of C3 in the columns (71.9 ± 8.7%, 81.9 ± 8.0%, 82.9 ± 9.7%, and 82.1 ± 7.3% for columns 1, 2, 3, 5, respectively). This can be explained by the fact that protein-like materials such as peptides and amino acids with low molecular weight (MW) were effectively removed by GAC through adsorption, and those adsorbed molecules containing high organic nitrogen content could be easily biodegraded in BAF (Chen et al., 2011).
Fig. 2.
Box-and-whisker plots showing percent reduction of maximum fluorescence intensities (Fmax) of PARAFAC components through the BAF systems; column 1 (filter biomass inoculated and operated w/o AOM), column 2 (filter biomass inoculated and operated w/AOM), column 3 (no inoculation and operated w/AOM), and column 5 (no inoculation and operated w/regular autoclaving and AOM); The whiskers indicate the max and min. The box stretches from the lower hinge (25th percentile) to the upper hinge (75th percentile). The median is shown as a line across the box; (A): Component 1 (marine humic-like substances), (B): Component 2 (terrestrial humic-like substances), (C): Component 3 (protein-like substances with tryptophan-like substances), (D): Component 4 (protein-like substances with tyrosine-like substances); * indicates P < 0.05 based on Welch’s t-test between EBCT 20 min and 10 min, ** indicates P < 0.05 based on Welch’s t-test between biological (columns 2 and 3) and sterilized (column 5) columns.
When the EBCT was reduced to 10 min, all component removal decreased significantly except C4 (P < 0.05), consistent with a previous study (Chen et al., 2016). For example, in column 1, C1 and C2 removals dropped by 17% and 24%, respectively. Columns 2 and 3 showed a similar decreasing trend for C2 (6.7% and 7.0%) and C3 (13.1% and 10.1%). Column 5 exhibited 25%, 9%, and 24% reduction for C1, C2, and C3, respectively. Interestingly, lower removal of C1 (marine humic-like substances) was observed in column 5, compared to columns 2 and 3 (P < 0.05) (Fig. 2A). Generally, BAF with GAC provides substantial removal of humic-like substances through adsorption and biodegradation (Pramanik et al., 2014). Notably, the removal of high MW substances was more attributed to the adsorption of those molecules on biofilms rather than GAC because of size exclusion effect on GAC (Huang et al., 2011), which suggests that biofilm plays a more important role in removing marine humic-like substances in the system. For C4 (tyrosine-like substances), low average removal (even negative removal) was observed in all columns (12.7 ± 15.1%) at both EBCTs, which might be due to bacterial exudates produced by biofilms in columns (Shen et al., 2016).
3.3. Correlations between AOM components and MC-LR removal
The EEM-PARAFAC method has been successfully employed to investigate correlations between fluorescence intensities of various components and general water quality parameters (Yang et al., 2015). A recent study also reported a relationship between specific cyanobacteria-derived AOM components and carbonaceous DBP (C-DBP) and nitrogenous DBP (N-DBP) formation, confirming that AOM associated with protein-like substances, corresponding to C3, are strongly correlated with trihalomethanes and more toxic N-DBP yields (Ma et al., 2018). However, no previous studies have explored the applicability of the EEM-PARAFAC method for monitoring MC-LR removal in BAF. It is worth mentioning that the ex/em loadings of MC-LR and C4 were similar to each other as the EEM spectra of pure MC-LR overlapped with the position where C4 was identified (Fig. S6). Consequently, this infers that direct tracking of MC-LR removal using the EEM-PARAFAC method is not feasible under the presence of protein-like substances. To further elucidate any potential correlations between MC-LR removal and the identified AOM components, the Spearman’s rank correlation analysis was conducted, and the results revealed a strong positive correlation between C2 and MC-LR removals in all columns (Table 3). When the component ratios were also evaluated, C1:C2 presented a significant correlation for MC-LR removal in columns 2 (0.55) and 3 (0.75), indicating that the removal of MC-LR was more related to the removal of humic-like substances (C2) than protein-like substances (C3) in BAF. Little is known about the role of C2 in BAF, except that it is related to aromatic carbon compounds with both high (>100 kDa) and low MW portions (<1 kDa) (Qu et al., 2012). There is no available information regarding its impacts on BAF. In the following section, the role of AOM components and their influences on bacterial community structure will be discussed.
Table 3.
Summary of Spearman’s rank correlation coefficients (ρ) between PARAFAC component removal and MC-LR (* indicates significances, P < 0.05).
| MC-LR removal |
|||
|---|---|---|---|
| Column 2 | Column 3 | Column 5 | |
| Component 1 removal | 0.19 | 0.24 | 0.41 |
| Component 2 removal | 0.54* | 0.60* | 0.66* |
| Component 3 removal | 0.47 | 0.26 | 0.14 |
| Component 4 removal | 0.18 | 0.22 | 0.29 |
| Component 1/Component 2 | 0.55* | 0.75* | 0.65* |
| Component 3/Component 2 | 0.34 | 0.44 | 0.37 |
| Component 4/Component 2 | 0.27 | 0.31 | 0.18 |
3.4. AOM impacts on the communities of bacterial biofilms in BAF
Previous studies reported the dynamic changes of fresh water bacterial community structures in response to a wide range of cyanobacteria species and their potential impacts on water quality, followed by AOM release (Li et al., 2012a; Wilhelm et al., 2014). However, there is no study that monitors the impacts of AOM on the bacterial community structures in BAF. To our knowledge, this is the first study that provides insights into the roles of AOM on the highly variable bacterial community composition in BAF.
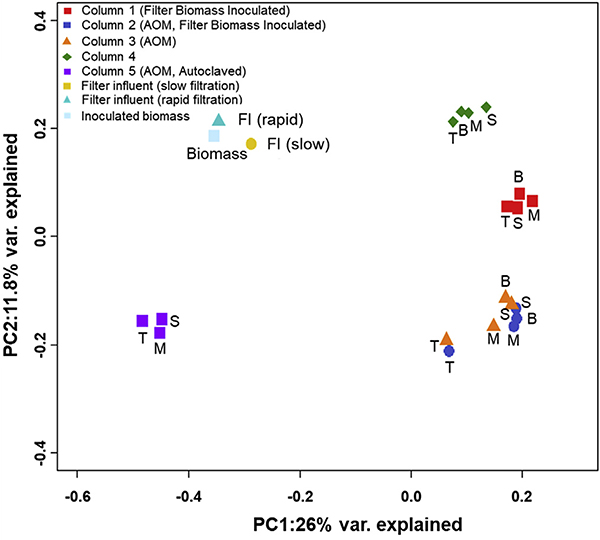
A total of 439,429 sequences were retrieved from the filter media samples using 16S rRNA sequencing analysis and 1646 OTUs were obtained. Samples from column 5 contained the lowest OTUs. The top of the GAC layers in columns 2 and 3 showed lower richness (Chao1) and diversity (Simpson) compared to column 1, which may indicate that the top layer was significantly affected by AOM (Table S3). To visualize the dissimilarity of community structures among samples, the PCoA was performed on unweighted Unifrac distance, and 37.8% variance of the OTU dataset was included in the first two principal coordinate axes (Fig. 3). Samples from columns 1, 4, and 5 formed three distinct groups far separated from the inoculum. Samples from columns 2 and 3 were also clustered together but the top layers were distant from the cluster. The PERMANOVA test on OTU tables confirmed that column 1 was significantly different from columns 2, 3, and 5 (P < 0.05). This indicates that AOM affected the community structures in column 2 although columns 1 and 2 were seeded with the same biomass from the DWTP and appeared very similar with each other at the phylum levels. Moreover, SIMPER results (Table S4) revealed high dissimilarities at top layers among the columns, which also supports that injected AOM influenced the top layers more than bottom layers of filters.
Fig. 3.
Plot of principal coordinate analysis (PCoA) of the microbial communities based on the unweighted Unifrac distance in filter media and inoculum samples. Abbreviations: T: top GAC samples; M: middle GAC samples; B: bottom GAC samples; S: sand samples; FI: filter influent. Column 1 was operated w/o AOM/MC-LR and column 4 was disassembled at day 159 before the addition of AOM/MC-LR. Columns 2, 3, and 5 were exposed to AOM/MC-LR.
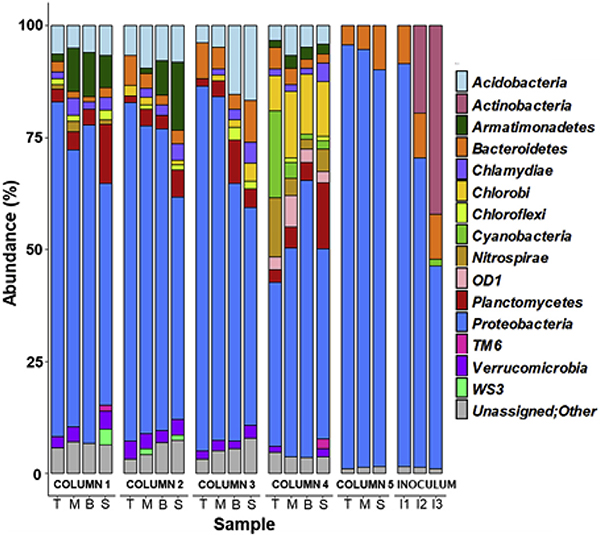
Of all identified OTUs, the major phyla for each sample are shown in Fig. 4. The recovered taxa from this study are composed of fifteen bacterial phyla commonly found in BAF, including Acidobacteria, Actinobacteria, Nitorspirae, Proteobacteria, and Chlorobi (Oh et al., 2018), with Proteobacteria being the most dominant across the samples. Proteobacteria in columns 1, 2, and 3 constituted 77.3 ± 2% at top, 69.1 ± 5% at middle, 65.2 ± 4% at bottom GAC layers, and 49.2 ± 0.3% at sand layers. In column 1, Alphaproteobacteria accounted for 33–50% of the overall abundance. Betaproteobacteria overwhelmingly dominated columns 2 (63–83%), 3 (45–87%), and 5 (60–68%). This implies that AOM as substrate strongly influenced the bacterial communities in the columns, resulting in the dominance of the Betaproteobacteria group (in which members are known to be highly competitive in nutrient-rich environments) (Niemi et al., 2009).
Fig. 4.
Relative abundance of bacterial community composition in filter media samples and inoculum at phylum level. The rare species with relative abundance <1% were included as others. Abbreviations: T: top GAC samples; M: middle GAC samples; B: bottom GAC samples; S: sand samples; I1: Influent (during slow filtration); I2: Influent (during rapid filtration); I3: Inoculated biomass. Column 1 was operated w/o AOM/MC-LR and column 4 was disassembled at day 159 before the addition of AOM/MC-LR. Columns 2, 3, and 5 were exposed to AOM/MC-LR.
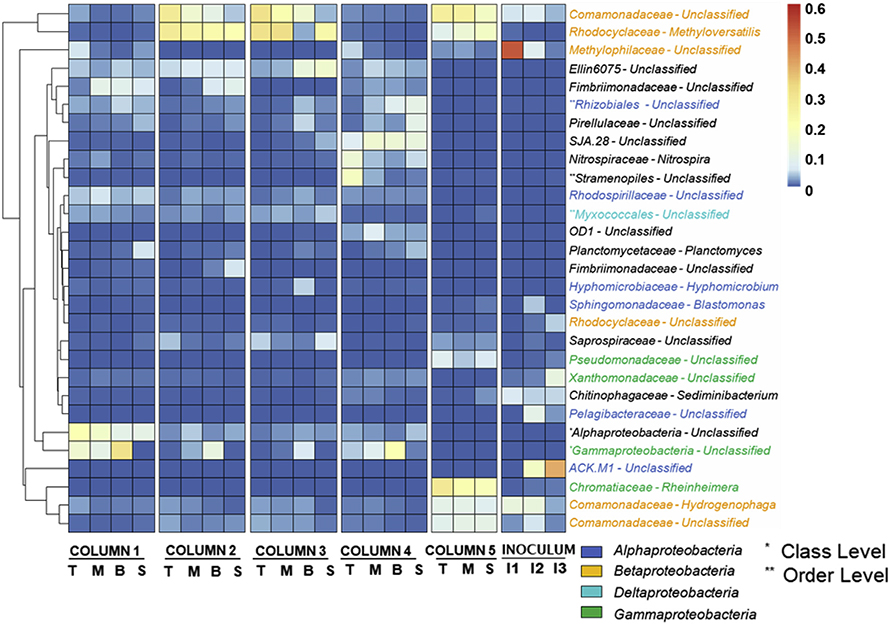
Further characterization at family and genus levels revealed that in columns 2 and 3, genus Methyloversatilis (Rhodocyclaceae family) and unclassified Comamonadaceae accounted for 22.9 ± 8% and 19.4 ± 10%, respectively (Fig. 5). In column 5, the two groups constituted the average of 12.7 ± 3% and 22.4 ± 4%. Members of the genus Methyloversatilis are facultative methylotrophs, which are able to utilize organic acids, aromatic compounds, and methylated amines (Smalley et al., 2015). As we observed increased TN removals in columns 2, 3, and 5 under the presence of AOM (Fig. S3), their predominance may be significantly related to nitrogen contents of AOM such as unidentified organic nitrogen and aliphatic amines (Fang et al., 2010b). For the Comamonadaceae, some of the group members are known to degrade aromatic carbon compounds and produce large amounts of extracellular polymeric substances (EPS) (Feng et al., 2012; Te et al., 2017). A previous study reported that carbon-hydrogen aromatic compounds were originated from humic-like substances of AOM (Villacorte et al., 2015), which were shown to be successfully removed by filtration in our EEM-PARAFAC results (Fig. 2). In our previous study, the family Comamonadaceae was dominant in biofilm impacted by AOM, showing a strong correlation with total EPS contents (Li et al., 2019). Thus, it is inferred that the adsorbed aromatic carbon compounds in C2 helped in the enrichment of Comamonadaceae. In addition, bacteria closely related to members of the family Saprospiraceae were enriched in the top layer of columns 2, 3, and 5. Saprospiraceae as protein hydrolyzers (McIlroy and Nielsen, 2014; Xia et al., 2007) generally coexist with Flavobacteriales, which rely on intermediates hydrolyzed from protein by Saprospiraceae (Kim et al., 2016). In the study, the proliferation of the family Flavobacteriales was also observed in columns 2 and 3, indicating that Saprospiraceae dominated by utilizing protein-like substances of AOM.
Fig. 5.
The heatmap of genera with relative abundances >4%. Abbreviations: T: top GAC samples; M: middle GAC samples; B: bottom GAC samples; S: sand samples; I1: Influent (during slow filtration); I2: Influent (during rapid filtration); I3: Inoculated biomass. Column 1 was operated w/o AOM/MC-LR and column 4 was disassembled at day 159 before the addition of AOM/MC-LR. Columns 2, 3, and 5 were exposed to AOM/MC-LR.
Overall, the bacterial community analysis showed that the addition of AOM significantly decreased the diversity of the top layer while the bacterial community was shifting in the favor of Betaproteobacteria in BAF. Notably, the abundance of Rhodocyclaceae, Saprospiraceae, and Comamonadaceae at the family level was distinct in the presence of AOM.
3.5. Abundances of mlrA gene and potential MC-degrading bacteria
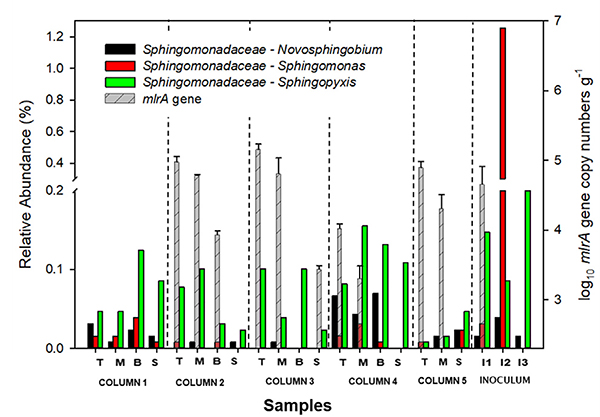
Until now, over 40 MC-degrading bacterial strains have been identified from diverse ecosystems. The characterized degraders mainly belong to Proteobacteria and the most frequently reported genera are Novosphingobium, Sphingomonas and Sphingopyxis, belonging to Alphaproteobacteria (Li et al., 2017). These genera were detected in the sequencing libraries (Fig. 6). For example, Sphingopyxis was well distributed through all columns compared to Novosphingobium and Sphingomonas. This genus was relatively abundant at the top layers in columns 2 and 3, while it was dominant at bottom layers in columns 1, 4, and 5. Hoefel et al. (2009a) demonstrated that the abundance of MC-degraders is positively connected with the abundance of the mlrA gene, which encodes enzymes hydrolytically cleaving the cyclic structure of MC-LR. The authors also reported a strong correlation between the abundance of mlrA gene and MC-LR biodegradation in sand filters (Hoefel et al., 2009b). Our qPCR results revealed that mlrA genes were also present in all columns except column 1 (Fig. 6) and the genes were more abundant at top layers, which implies potential biodegradation of MC-LR linked to some of these bacterial groups. However, further studies are needed to unravel the distribution and activity of MC-LR degraders in BAF under different operational conditions and seasonal changes (with and without HAB), providing absolute resolution at genus/species level and gene expressions associated with MC-LR biodegradation.
Fig. 6.
Relative abundance of potential MC-degrading bacteria and abundance of mlrA gene copies within filter media biofilm (Error bars represent standard deviations of the results for duplicate analyses): Abbreviations: T: top GAC samples; M: middle GAC samples; B: bottom GAC samples; S: sand samples; I1: Influent (during slow filtration); I2: Influent (during rapid filtration); I3: Inoculated biomass. Column 1 was operated w/o AOM/MC-LR and column 4 was disassembled at day 159 before the addition of AOM/MC-LR. Columns 2, 3, and 5 were exposed to AOM/MC-LR.
3.6. Engineering implications
This study highlights the impact of AOM on MC-LR removal and active biomass and community structures of biofilms in BAF. Under the presence of AOM, complete MC-LR removal by both GAC adsorption and biodegradation could be achieved after a seven-day acclimation period at high EBCT, but not at low EBCT, although MC-LR concentration in BAF effluent was lower than 1 μg/L (WHO’s guideline for MC-LR). There were also limitations in eliminating all AOM components at both EBCTs. Protein-like substances were less removed than terrestrial humic-like substances, which may increase concerns over finished water quality. Specifically, since the BAF receiving AOM showed the incomplete removal of protein-like substances, the substances can facilitate toxic DBP formation upon disinfection and biofilm formation in DWDS. Furthermore, detached biofilm clusters and EPS released from AOM-impacted biofilm in BAF can lead to additional DBP and biofilm formation in both DWTPs and DWDSs (Li et al. 2019, 2020). Thus, water utilities may need to consider additional physical and chemical treatments that can supplement BAF for AOM removal during HABs.
In addition, more careful monitoring and operation of BAF and associated processes are required to ensure finished water quality. Most BAF processes are still operated as “black boxes” without any practical guidelines for operation and performance monitoring, especially for AOM. Thus, understanding the fate of AOM is critical to gain deeper insight into upstream treatment process control and BAF optimization. Our EEM-PARAFAC analysis results showed that EEM-PARAFAC can be a useful tool to monitor removal performance for AOM-associated components while predicting MC-LR removal in BAF. Consequently, this can help DWTP operators make informed decisions to optimize DWTP processes, offering a comprehensive understanding of AOM and MC-LR removal during HABs.
Lastly, our community analysis results clearly demonstrated that AOM and MC-LR could impact the community structure of biofilm, increasing the relative abundance of bacteria that potentially utilize AOM components and MC-LR. Our finding displays the future potential to harness biofilm activity and functions in BAF for enhanced removal of cyanobacteria-derived contaminants using feasible strategies such as biostimulation and/or bioaugmentation (Horemans et al., 2017; Mikkelson et al., 2015).
4. Conclusions
Under the presence of AOM, BAF with GAC completely removed 5 μg/L of MC-LR at 20 min EBCT but not at 10 min EBCT.
Deactivation of biofilm with autoclaving showed that C1 (marine humic-like substances) removal was more related to active biofilm formation and up to 68% (~3.4 μg/L) of injected MC-LR could be abiotically removed in BAF.
BAF showed higher removal efficiency for terrestrial humic-like substances than protein-like substances at both EBCTs and there was a strong correlation between the removal of terrestrial humic-like substances and the removal of MC-LR.
The study demonstrated that EBCT and the formation of biofilms play an important role in the removal of AOM and MC-LR; however, further studies are needed to better understand how biofilm formation characteristics are associated with the AOM removal mechanisms in BAF.
Substantial shifts in bacterial community structures in response to AOM led to the predominance of Rhodocyclaceae, Saprospiraceae, and Comamonadaceae. Moreover, the distribution of mlrA gene abundance and MC-degrading bacteria showed potential MC-LR biodegradation in BAF.
Supplementary Material
Acknowledgments
This researchwas supported by the National Science Foundation (GOALI 1605185), the Ohio Water Development Authority (7174), and the Ohio Department of Higher Education (R/SDW-2-BOR). We appreciate Mr. Jacob Goetz at the Toledo DWTP for providing water samples and Ms. Faith Seo and Mr. Brady Spitulsk for proofreading. In addition, this work has been subjected to the U.S. Environmental Protection Agency (EPA)’s administrative review and has been approved for external publication. Any opinions expressed do not reflect the views of the agency; therefore, no official endorsement should be inferred. Any mention of trade names or commercial products does not constitute endorsement or recommendation for use.
Footnotes
Declaration of competing interest
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
Appendix A. Supplementary data
Supplementary data to this article can be found online at https://doi.org/10.1016/j.watres.2020.116120.
References
- Bai Y, Chang Y, Liang J, Chen C, Qu J, 2016. Treatment of groundwater containing Mn (II), Fe (II), as (III) and Sb (III) by bioaugmented quartz-sand filters. Water Res. 106, 126–134. [DOI] [PubMed] [Google Scholar]
- Bittar TB, Vieira AA, Stubbins A, Mopper K, 2015. Competition between photochemical and biological degradation of dissolved organic matter from the cyanobacteria Microcystis aeruginosa. Limnol. Oceanogr 60 (4), 1172–1194. [Google Scholar]
- Brown J, Upadhyaya G, Carter J, Brown T, Lauderdale C, 2016. North American Biofiltration Knowledge Base. Water Research Foundation, Colorado. [Google Scholar]
- Caporaso JG, Kuczynski J, Stombaugh J, Bittinger K, Bushman FD, Costello EK, Fierer N, Pena AG, Goodrich JK, Gordon JI, 2010. QIIME allows analysis of high-throughput community sequencing data. Nat. Methods 7 (5), 335. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Caporaso JG, Lauber CL, Walters WA, Berg-Lyons D, Lozupone CA, Turnbaugh PJ, Fierer N, Knight R, 2011. Global patterns of 16S rRNA diversity at a depth of millions of sequences per sample. Proc. Natl. Acad. Sci. Unit. States Am 108 (Suppl. 1), 4516–4522. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen B, Kim Y, Westerhoff P, 2011. Occurrence and treatment of wastewater-derived organic nitrogen. Water Res. 45 (15), 4641–4650. [DOI] [PubMed] [Google Scholar]
- Chen F, Peldszus S, Elhadidy AM, Legge RL, Van Dyke MI, Huck PM, 2016. Kinetics of natural organic matter (NOM) removal during drinking water biofiltration using different NOM characterization approaches. Water Res. 104, 361–370. [DOI] [PubMed] [Google Scholar]
- Chen J, Gao N, Li L, Zhu M, Yang J, Lu X, Zhang Y, 2017. Disinfection byproduct formation during chlor (am) ination of algal organic matters (AOM) extracted from Microcystis aeruginosa: effect of growth phases, AOM and bromide concentration. Environ. Sci. Pollut. Control Ser 24 (9), 8469–8478. [DOI] [PubMed] [Google Scholar]
- Chen W, Westerhoff P, Leenheer JA, Booksh K, 2003. Fluorescence excitation–emission matrix regional integration to quantify spectra for dissolved organic matter. Environ. Sci. Technol 37 (24), 5701–5710. [DOI] [PubMed] [Google Scholar]
- El-Chakhtoura J, Prest E, Saikaly P, van Loosdrecht M, Hammes F, Vrouwenvelder H, 2015. Dynamics of bacterial communities before and after distribution in a full-scale drinking water network. Water Res. 74, 180–190. [DOI] [PubMed] [Google Scholar]
- Eleuterio L, Batista JR, 2010. Biodegradation studies and sequencing of microcystin-LR degrading bacteria isolated from a drinking water biofilter and a fresh water lake. Toxicon 55 (8), 1434–1442. [DOI] [PubMed] [Google Scholar]
- Fang J, Ma J, Yang X, Shang C, 2010a. Formation of carbonaceous and nitrogenous disinfection by-products from the chlorination of Microcystis aeruginosa. Water Res. 44 (6), 1934–1940. [DOI] [PubMed] [Google Scholar]
- Fang J, Yang X, Ma J, Shang C, Zhao Q, 2010b. Characterization of algal organic matter and formation of DBPs from chlor (am) ination. Water Res. 44 (20), 5897–5906. [DOI] [PubMed] [Google Scholar]
- Feng S, Xie S, Zhang X, Yang Z, Ding W, Liao X, Liu Y, Chen C, 2012. Ammonium removal pathways and microbial community in GAC-sand dual media filter in drinking water treatment. J. Environ. Sci 24 (9), 1587–1593. [DOI] [PubMed] [Google Scholar]
- Fujioka T, Ueyama T, Mingliang F, Leddy M, 2019. Online assessment of sand filter performance for bacterial removal in a full-scale drinking water treatment plant. Chemosphere 229, 509–514. [DOI] [PubMed] [Google Scholar]
- Guo T, Yang Y, Liu R, Li X, 2017. Enhanced removal of intracellular organic matters (IOM) from Microcystic aeruginosa by aluminum coagulation. Separ. Purif. Technol 189, 279–287. [Google Scholar]
- Gutiérrez-Praena D, Pichardo S, Jos Á, Moreno FJ, Cameán AM, 2012. Biochemical and pathological toxic effects induced by the cyanotoxin Cylindrospermopsin on the human cell line Caco-2. Water Res. 46 (5), 1566–1575. [DOI] [PubMed] [Google Scholar]
- Hammes F, Meylan S, Salhi E, Köster O, Egli T, Von Gunten U, 2007. Formation of assimilable organic carbon (AOC) and specific natural organic matter (NOM) fractions during ozonation of phytoplankton. Water Res. 41 (7), 1447–1454. [DOI] [PubMed] [Google Scholar]
- Ho L, Meyn T, Keegan A, Hoefel D, Brookes J, Saint CP, Newcombe G, 2006. Bacterial degradation of microcystin toxins within a biologically active sand filter. Water Res. 40 (4), 768–774. [DOI] [PubMed] [Google Scholar]
- Hoefel D, Adriansen CM, Bouyssou MA, Saint CP, Newcombe G, Ho L, 2009a. Development of an mlrA gene-directed TaqMan PCR assay for quantitative assessment of microcystin-degrading bacteria within water treatment plant sand filter biofilms. Appl. Environ. Microbiol 75 (15), 5167–5169. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hoefel D, Adriansen CM, Bouyssou MA, Saint CP, Newcombe G, Ho L, 2009b. Development of an mlrA gene-directed TaqMan PCR assay for quantitative assessment of microcystin-degrading bacteria within water treatment plant sand filter biofilms. Appl. Environ. Microbiol. 75 (15), 5167–5169. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hong H, Wong M, Liang Y, 2009. Amino acids as precursors of trihalomethane and haloacetic acid formation during chlorination. Arch. Environ. Contam. Toxicol 56 (4), 638–645. [DOI] [PubMed] [Google Scholar]
- Hong HC, Mazumder A,Wong MH, Liang Y, 2008. Yield of trihalomethanes and haloacetic acids upon chlorinating algal cells, and its prediction via algal cellular biochemical composition. Water Res. 42 (20), 4941–4948. [DOI] [PubMed] [Google Scholar]
- Horemans B, Raes B, Vandermaesen J, Simanjuntak Y, Brocatus H, T’Syen J, Degryse J, Boonen J, Wittebol J, Lapanje A, 2017. Biocarriers improve bioaugmentation efficiency of a rapid sand filter for the treatment of 2, 6-dichlorobenzamide-contaminated drinking water. Environ. Sci. Technol 51 (3), 1616–1625. [DOI] [PubMed] [Google Scholar]
- Huang G, Meng F, Zheng X, Wang Y, Wang Z, Liu H, Jekel M, 2011. Biodegradation behavior of natural organic matter (NOM) in a biological aerated filter (BAF) as a pretreatment for ultrafiltration (UF) of river water. Appl. Microbiol. Biotechnol 90 (5), 1795–1803. [DOI] [PubMed] [Google Scholar]
- Inkinen J, Jayaprakash B, Santo Domingo J, Keinänen-Toivola M, Ryu H, Pitkänen T, 2016. Diversity of ribosomal 16S DNA-and RNA-based bacterial community in an office building drinking water system. J. Appl. Microbiol 120 (6), 1723–1738. [DOI] [PubMed] [Google Scholar]
- Kaloudis T, Zervou S-K, Tsimeli K, Triantis TM, Fotiou T, Hiskia A, 2013. Determination of microcystins and nodularin (cyanobacterial toxins) in water by LC–MS/MS. Monitoring of Lake Marathonas, a water reservoir of Athens, Greece. J. Hazard Mater 263, 105–115. [DOI] [PubMed] [Google Scholar]
- Kim N-K, Oh S, Liu W-T, 2016. Enrichment and characterization of microbial consortia degrading soluble microbial products discharged from anaerobic methanogenic bioreactors. Water Res. 90, 395–404. [DOI] [PubMed] [Google Scholar]
- Kim TG, Yun J, Hong S-H, Cho K-S, 2014. Effects of water temperature and backwashing on bacterial population and community in a biological activated carbon process at a water treatment plant. Appl. Microbiol. Biotechnol 98 (3), 1417–1427. [DOI] [PubMed] [Google Scholar]
- Lahti K, Rapala J, Kivimäki A, Kukkonen J, Niemelä M, Sivonen K, 2001. Occurrence of microcystins in raw water sources and treated drinking water of Finnish waterworks. Water Sci. Technol 43 (12), 225–228. [PubMed] [Google Scholar]
- Lambert TW, Holmes CF, Hrudey SE, 1996. Adsorption of microcystin-LR by activated carbon and removal in full scale water treatment. Water Res. 30 (6), 1411–1422. [Google Scholar]
- Li H, Xing P, Wu QL, 2012a. The high resilience of the bacterioplankton community in the face of a catastrophic disturbance by a heavy Microcystis bloom. FEMS Microbiol. Ecol 82 (1), 192–201. [DOI] [PubMed] [Google Scholar]
- Li J, Li R, Li J, 2017. Current research scenario for microcystins biodegradation–a review on fundamental knowledge, application prospects and challenges. Sci. Total Environ. 595, 615–632. [DOI] [PubMed] [Google Scholar]
- Li L, Gao N, Deng Y, Yao J, Zhang K, 2012b. Characterization of intracellular & extracellular algae organic matters (AOM) of Microcystic aeruginosa and formation of AOM-associated disinfection byproducts and odor & taste compounds. Water Res. 46 (4), 1233–1240. [DOI] [PubMed] [Google Scholar]
- Li L, Jeon Y, Lee S-H, Ryu H, Santo Domingo JW, Seo Y, 2019. Dynamics of the physiochemical and community structures of biofilms under the influence of algal organic matter and humic substances. Water Res. 158, 136–145. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li L, Jeon Y, Ryu H, Santo Domingo JW, Seo Y, 2020. Assessing the chemical compositions and disinfection byproduct formation of biofilms: application of fluorescence excitation-emission spectroscopy coupled with parallel factor analysis. Chemosphere 246, 125745. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Louati I, Pascault N, Debroas D, Bernard C, Humbert J-F, Leloup J, 2015. Structural diversity of bacterial communities associated with bloom-forming freshwater cyanobacteria differs according to the cyanobacterial genus. PloS One 10 (11), e0140614. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ma C, Xu H, Zhang L, Pei H, Jin Y, 2018. Use of fluorescence excitation–emission matrices coupled with parallel factor analysis to monitor C-and N-DBPs formation in drinking water recovered from cyanobacteria-laden sludge dewatering. Sci. Total Environ 640, 609–618. [DOI] [PubMed] [Google Scholar]
- McIlroy SJ, Nielsen PH, 2014. The Prokaryotes. Springer, pp. 863–889. [Google Scholar]
- Mikkelson KM, Homme CL, Li D, Sharp JO, 2015. Propane biostimulation in biologically activated carbon (BAC) selects for bacterial clades adept at degrading persistent water pollutants. Environ. Sci. J. Integr. Environ. Res.: Processes & Impacts 17 (8), 1405–1414. [DOI] [PubMed] [Google Scholar]
- Miller T, Beversdorf L, Weirich C, Bartlett S, 2017. Cyanobacterial toxins of the Laurentian Great Lakes, their toxicological effects, and numerical limits in drinking water. Mar. Drugs 15 (6), 160. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Murphy KR, Stedmon CA, Graeber D, Bro R, 2013. Fluorescence spectroscopy and multi-way techniques. PARAFAC. Anal. Methods 5 (23), 6557–6566. [Google Scholar]
- Niemi RM, Heiskanen I, Heine R, Rapala J, 2009. Previously uncultured β-Proteobacteria dominate in biologically active granular activated carbon (BAC) filters. Water Res. 43 (20), 5075–5086. [DOI] [PubMed] [Google Scholar]
- Oh S, Hammes F, Liu W-T, 2018. Metagenomic characterization of biofilter microbial communities in a full-scale drinking water treatment plant. Water Res. 128, 278–285. [DOI] [PubMed] [Google Scholar]
- Pivokonsky M, Kloucek O, Pivokonska L, 2006. Evaluation of the production, composition and aluminum and iron complexation of algogenic organic matter. Water Res. 40 (16), 3045–3052. [DOI] [PubMed] [Google Scholar]
- Pramanik BK, Roddick FA, Fan L, 2014. Effect of biological activated carbon pretreatment to control organic fouling in the microfiltration of biologically treated secondary effluent. Water Res. 63, 147–157. [DOI] [PubMed] [Google Scholar]
- Prest EI, Hammes F, van Loosdrecht M, Vrouwenvelder JS, 2016. Biological stability of drinking water: controlling factors, methods, and challenges. Front. Microbiol 7, 45. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Qi J, Lan H, Liu R, Miao S, Liu H, Qu J, 2016. Prechlorination of algae-laden water: the effects of transportation time on cell integrity, algal organic matter release, and chlorinated disinfection byproduct formation. Water Res. 102, 221–228. [DOI] [PubMed] [Google Scholar]
- Qu F, Liang H, Wang Z, Wang H, Yu H, Li G, 2012. Ultrafiltration membrane fouling by extracellular organic matters (EOM) of Microcystis aeruginosa in stationary phase: influences of interfacial characteristics of foulants and fouling mechanisms. Water Res. 46 (5), 1490–1500. [DOI] [PubMed] [Google Scholar]
- Richter D, Massmann G, Dünnbier U, 2008. Behaviour and biodegradation of sulfonamides (p-TSA, o-TSA, BSA) during drinking water treatment. Chemosphere 71 (8), 1574–1581. [DOI] [PubMed] [Google Scholar]
- Sano D, Ishifuji S, Sato Y, Imae Y, Takaara T, Masago Y, Omura T, 2011. Identification and characterization of coagulation inhibitor proteins derived from cyanobacterium Microcystis aeruginosa. Chemosphere 82 (8), 1096–1102. [DOI] [PubMed] [Google Scholar]
- Sgroi M, Anumol T, Roccaro P, Vagliasindi FG, Snyder SA, 2018. Modeling emerging contaminants breakthrough in packed bed adsorption columns by UV absorbance and fluorescing components of dissolved organic matter. Water Res. 145, 667–677. [DOI] [PubMed] [Google Scholar]
- Shen H, Chen X, Zhang D, Chen H. b., 2016. Generation of soluble microbial products by bio-activated carbon filter during drinking water advanced treatment and its influence on spectral characteristics. Sci. Total Environ 569, 1289–1298. [DOI] [PubMed] [Google Scholar]
- Shi L, Huang Y, Zhang M, Yu Y, Lu Y, Kong F, 2017. Bacterial community dynamics and functional variation during the long-term decomposition of cyanobacterial blooms in-vitro. Sci. Total Environ 598, 77–86. [DOI] [PubMed] [Google Scholar]
- Smalley NE, Taipale S, De Marco P, Doronina NV, Kyrpides N, Shapiro N, Woyke T, Kalyuzhnaya MG, 2015. Functional and genomic diversity of methylotrophic Rhodocyclaceae: description of Methyloversatilis discipulorum sp. nov. Int. J. Syst. Evol. Microbiol 65 (7), 2227–2233. [DOI] [PubMed] [Google Scholar]
- Stewart PS, 2003. Diffusion in biofilms. J. Bacteriol 185 (5), 1485–1491. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stoodley P, Lewandowski Z, 1994. Liquid flow in biofilm systems. Appl. Environ. Microbiol 60 (8), 2711–2716. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sun F, Pei H-Y, Hu W-R, Li X-Q, Ma C-X, Pei R-T, 2013. The cell damage of Microcystis aeruginosa in PACl coagulation and floc storage processes. Separ. Purif. Technol 115, 123–128. [Google Scholar]
- Te SH, Tan BF, Thompson JR, Gin KY-H, 2017. Relationship of microbiota and cyanobacterial secondary metabolites in planktothricoides-dominated bloom. Environ. Sci. Technol 51 (8), 4199–4209. [DOI] [PubMed] [Google Scholar]
- Terry LG, Summers RS, 2018. Biodegradable organic matter and rapid-rate biofilter performance: a review. Water Res. 128, 234–245. [DOI] [PubMed] [Google Scholar]
- Villacorte LO, Ekowati Y, Neu TR, Kleijn JM, Winters H, Amy G, Schippers JC, Kennedy MD, 2015. Characterisation of algal organic matter produced by bloom-forming marine and freshwater algae. Water Res. 73, 216–230. [DOI] [PubMed] [Google Scholar]
- Wang H, Ho L, Lewis DM, Brookes JD, Newcombe G, 2007. Discriminating and assessing adsorption and biodegradation removal mechanisms during granular activated carbon filtration of microcystin toxins.Water Res. 41 (18), 4262–4270. [DOI] [PubMed] [Google Scholar]
- Wilhelm SW, LeCleir GR, Bullerjahn GS, McKay RM, Saxton MA, Twiss MR, Bourbonniere RA, 2014. Seasonal changes in microbial community structure and activity imply winter production is linked to summer hypoxia in a large lake. FEMS Microbiol. Ecol 87 (2), 475–485. [DOI] [PubMed] [Google Scholar]
- Woodhouse JN, Kinsela AS, Collins RN, Bowling LC, Honeyman GL, Holliday JK, Neilan BA, 2016. Microbial communities reflect temporal changes in cyanobacterial composition in a shallow ephemeral freshwater lake. ISME J. 10 (6), 1337. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xia Y, Kong Y, Nielsen PH, 2007. In situ detection of protein-hydrolysing microorganisms in activated sludge. FEMS Microbiol. Ecol 60 (1), 156–165. [DOI] [PubMed] [Google Scholar]
- Xie P, Ma J, Fang J, Guan Y, Yue S, Li X, Chen L, 2013. Comparison of permanganate preoxidation and preozonation on algae containing water: cell integrity, characteristics, and chlorinated disinfection byproduct formation. Environ. Sci. Technol 47 (24), 14051–14061. [DOI] [PubMed] [Google Scholar]
- Yang L, Hur J, Zhuang W, 2015. Occurrence and behaviors of fluorescence EEM-PARAFAC components in drinking water and wastewater treatment systems and their applications: a review. Environ. Sci. Pollut. Control Ser 22 (9), 6500–6510. [DOI] [PubMed] [Google Scholar]
- Zamyadi A, MacLeod SL, Fan Y, McQuaid N, Dorner S, Sauvé S, Prévost M, 2012. Toxic cyanobacterial breakthrough and accumulation in a drinking water plant: a monitoring and treatment challenge. Water Res. 46 (5), 1511–1523. [DOI] [PubMed] [Google Scholar]
- Zhu M, Gao N, Chu W, Zhou S, Zhang Z, Xu Y, Dai Q, 2015. Impact of preozonation on disinfection by-product formation and speciation from chlor (am) ination of algal organic matter of Microcystis aeruginosa. Ecotoxicol. Environ. Saf 120 256–262. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.