Abstract
The sustainable production of solvents from above ground carbon is highly desired. Several clostridia naturally produce solvents and use a variety of renewable and waste-derived substrates such as lignocellulosic biomass and gas mixtures containing H2/CO2 or CO. To enable economically viable production of solvents and biofuels such as ethanol and butanol, the high productivity of continuous bioprocesses is needed. While the first industrial-scale gas fermentation facility operates continuously, the acetone–butanol–ethanol (ABE) fermentation is traditionally operated in batch mode. This review highlights the benefits of continuous bioprocessing for solvent production and underlines the progress made towards its establishment. Based on metabolic capabilities of solvent producing clostridia, we discuss recent advances in systems-level understanding and genome engineering. On the process side, we focus on innovative fermentation methods and integrated product recovery to overcome the limitations of the classical one-stage chemostat and give an overview of the current industrial bioproduction of solvents.
Keywords: Cell retention and immobilization, Systems biology and genome-scale metabolic models, Complex and renewable feedstocks, Gas fermentation, Integrated product recovery
Introduction
The Paris Agreement adopted in 2016 displays an international effort to reduce carbon emissions and promotes the development of new sustainable processes for fuel and chemical production using “above ground” carbon as feedstocks [149]. To implement sustainable and economically viable processes towards the establishment of a circular bioeconomy, the use of cheap and abundant carbon sources such as municipal solid waste, lignocellulosic biomass and steel mill exhaust gas must be favored over expensive and edible carbon sources like starch [149, 292]. Solventogenic clostridia can grow on a variety of hexose and pentose sugars and produce relevant solvents such as ethanol, butanol and acetone. The Weizmann process was implemented more than a hundred years ago [255], making solventogenic clostridia long-known production hosts of the industrial biotechnology. Acetogens can grow on mixtures of CO, CO2 and H2 which can be obtained directly from furnaces of steel mills or through the gasification of various carbon-rich waste streams and lignocellulosic biomass [170]. The product spectrum strongly depends on the acetogenic strain and includes the commodity chemicals acetate, ethanol and butanol [19, 59]. To enable the commercialization of bioprocess for the production of bulk chemicals like solvents, an estimated product titer of 50 g L−1, the productivity of 3 g L−1 h−1 and yield not less than 80% of the theoretical yield have to be reached [302]. Continuous bioprocessing offers a mean to reach the demanded high productivity [21, 208, 315].
Biofuels such as butanol and ethanol are needed in high quantities and their market shows a steady growth [164, 261, 270, 275]. While ethanol is already used worldwide for biofuel applications, a 50% higher energy density, lower vapor pressure, lower water absorption, lower corrosivity, better blending abilities and the possible use in unmodified combustion engines and existing infrastructure make butanol a promising alternative [41, 58, 161, 211]. To penetrate the biofuel market, butanol production has to compete with the performance of ethanol-producing bioprocesses [95]. Continuous ethanol production reached productivities of ~ 10 g L−1 h−1, yields of up to 0.46 g ethanol per g of pentose or hexose and concentrations of ~ 100 g L−1 [229]. The first commercial scale gas fermenting facility for ethanol production started operation in 2018 and runs in a fully continuous manner with a comparable productivity [149, 284]. The acetone–butanol–ethanol (ABE) fermentation, however, is classically operated in batch mode and the switch to a continuous bioprocess proves challenging [95, 164, 219]. Continuous high cell density cultivations of solventogenic clostridia have already reached butanol productivities of about 10 g L−1 h−1 [125, 187]. Due to the high toxicity of butanol, titers are typically limited to values below 20 g L−1 [79, 133]. Integrated product recovery methods display a meaningful way to compensate for the low product titers and to alleviate product toxicity [82].
In this review, we show the progress made towards the continuous production of solvents with solventogenic and acetogenic clostridia. With the objective of a holistic process design, the first part of the review focuses on the production hosts where we highlight metabolic capabilities and relevant phenotypical properties of clostridia. The recent developments in systems biology and genetic engineering tools increase microbial understanding and enable better strain design. Regarding the implementation of a sustainable and economical process, we give a short overview of the most promising alternative feedstocks. By building the bridge to the current advances of fermentation methods and add-ons used for solvent production, we discuss the challenges and opportunities of continuous fermentation and outline the current situation of industrial bioprocessing for solvent production. Finally, we tie up the threads for the successful industrial implementation of continuous solvent production by emphasizing the importance to combine strain engineering with innovative fermentation methods along with the need for further improvement of monitoring and control strategies for these processes.
Solventogenic and acetogenic clostridia
Solventogenic clostridia have been a part of industrial biotechnology as production hosts of solvents for more than a century [255]. While the research focused on Clostridium acetobutylicum, the model organism of the ABE fermentation, further clostridia including C. beijerinckii, C. saccharoperbutylacetonicum and C. saccharobutylicum were investigated for their high butanol production activity [58, 143, 166]. With the isolation of C. ljungdahlii, acetogenic bacteria (acetogens) have also become interesting hosts for industrial solvent production. This organism was first studied for its ability to form ethanol from gasified coal and is today one of the model acetogens [258, 315]. Acetogens are more relevant than ever as they can utilize the greenhouse gases CO and CO2 as inorganic carbon sources, making them applicable for carbon capture and valorization technologies [61, 149]. Acetogens form a metabolically, ecologically, and phylogenetically diverse group [256]. Several acetogenic clostridia such as C. ljungdahlii, C. autoethanogenum and C. carboxidivorans are investigated for solvent production [19, 59]. Non-clostridial acetogens such as Acetobacterium woodii and Eubacterium limosum are also investigated and modified for the production of bulk chemicals [108, 128, 276]. The most common solventogenic and acetogenic clostridia investigated for industrial application are summarized in Table 1.
Table 1.
Overview of industrially relevant solventogenic and acetogenic clostridia
| Strain | Growth on sole carbon source: | Native fermentation products | References | ||||
|---|---|---|---|---|---|---|---|
| H2/CO2 | CO/CO2 | C6 sugars | C5 sugars | Glycerol | |||
| C. acetobutylicum | – | – | X | X | (X) | Acetate, acetone, butanol, butyrate, ethanol, H2, CO2 | [143, 157] |
| C. beijerinckii | – | – | X | X | (X) | Acetate, acetone, butanol, butyrate, ethanol isopropanola, H2, CO2 | [143, 197, 279] |
| C. saccharoperbutylacetonicum | – | – | X | X | – | Acetate, acetone, butanol, butyrate, ethanol, H2, CO2 | [143] |
| C. saccharobutylicum | – | – | X | X | – | Acetate, acetone, butanol, butyrate, ethanol, H2, CO2 | [143] |
| C. pasteurianum | – | – | X | – | X | Acetate, Butanol, butyrate, ethanol, lactate, 1,3-propanediol, H2, CO2 | [223] |
| C. ljungdahlii | X | X | X | X | – | Acetate, 2,3-butanediol, ethanol, lactate, CO2 | [148, 286] |
| C. carboxidivorans | X | X | X | X | X | Acetate, ethanol, butanol, butyrate, hexanoate, hexanol, CO2 | [80, 172] |
| C. ragsdalei | X | X | X | X | – | Acetate, ethanol, 2,3-butanediol, lactate, CO2 | [115, 148] |
| C. drakei | X | X | X | X | X | Acetate, ethanol, butanol, butyrate, CO2 | [172] |
| C. autoethanogenum | X | X | X | X | – | Acetate, ethanol, 2,3-butanediol, lactate, CO2 | [3, 148, 192] |
| M. thermoacetica | X | X | X | X | – | Acetate, CO2 | [56] |
Metabolic modules
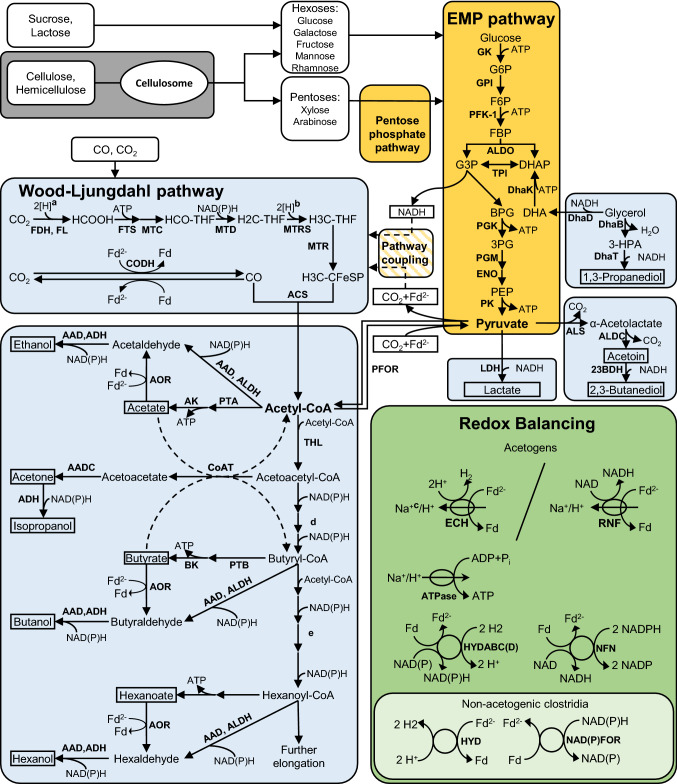
The metabolism of solventogenic and acetogenic clostridia can be subdivided into metabolic modules (see Fig. 1). Oxidative metabolic modules break down heterotrophic carbon sources to the intermediates acetyl-CoA and pyruvate and generate reduction equivalents. Additional reduction equivalents can be obtained from CO and H2 oxidation. Acetogens possess the Wood–Ljungdahl pathway, a reductive metabolic module that uses reduction equivalents to fixate CO2 and to form additional acetyl-CoA [259]. Further reductive modules use reduction equivalents to convert intermediates to products such as butanol, ethanol, acetone and 2,3-butanediol (2,3-BDO) [69, 148, 245]. Balancing modules match the generated and consumed reduction equivalents.
Fig. 1.
Schematic of the metabolism of acetogenic and solventogenic clostridia. Oxidative metabolic modules for the generation of reduction equivalents and intermediates are depicted in yellow. Reductive metabolic modules consuming reduction equivalents and synthesizing products are displayed in light blue. Redox balancing modules for the balancing of formed and consumed reduction equivalents are marked in green. Products of reductive metabolic modules are framed by black boxes. a Reduction of CO2 to formate can use H2, Fd2−, NADPH or even 0.5 Fd + 0.5 NADPH; b NADH is used for the reduction of H2C-THF to H3C-THF in the non-clostridial acetogen Acetobacterium woodii. In C. autoethanogenum, 2 NADH are most likely used to reduce Fd and H2C-THF in an electron bifurcating reaction [300]. c The translocation of Na+ by Ech in some species is likely but experimental evidence is missing [258]. d Subsequent steps for the reduction of acetoacetyl-CoA to butyryl-CoA are catalyzed by 3-hydroxyacyl-CoA dehydrogenase, crotonase and acyl-CoA dehydrogenase. e Subsequent steps for the reduction of butyryl-CoA to hexanoyl-CoA are catalyzed by thiolase, 3-hydroxyacyl-CoA dehydrogenase, crotonase and acyl-CoA dehydrogenase. 23BDH 2,3-butanediol dehydrogenase; 3-HPA 3-hydroxypropionaldehyde; 3PG glycerate 3-phosphate; AAD alcohol/aldehyde dehydrogenase; AADC acetoacetate decarboxylase; ACS acetyl-CoA synthase; ADH alcohol dehydrogenase; AK acetate kinase; ALDC acetolactate decarboxylase; ALDH aldehyde dehydrogenase; ALDO fructose biphosphate aldolase; ALS acetolactate synthase; BK butyrate kinase; BPG 1,3-bisphosphoglycerate; CoAT CoA transferase; CFeSP corrinoid iron–sulfur protein; DHA dihydroxyacetone; DhaB glycerol dehydratase; DhaD glycerol dehydratase; DhaK DHA kinase; DHAP dihydroxyacetone phosphate; DhaT 1,3-propanediol oxidoreductase; ECH energy-converting hydrogenase complex; ENO enolase; F6P fructose 6-phosphate; FBP fructose 1,6-bisphosphate; Fd ferredoxin; FDH formate dehydrogenase; FL formate-H2 lyase; FTS formyl-THF synthase; G3P glyceraldehyde 3-phosphate; G6P glucose 6-phosphate, GAPDH glyceraldehyde phosphate dehydrogenase; GK hexokinase; GPI phosphoglucose isomerase; HYD hydrogenase; HYDABC(D) electron-bifurcating hydrogenase; LDH lactate dehydrogenase; MTC methenyl-THF cyclohydrolase; MTD methylene-THF dehydrogenase; MTR methyl transferase; MTRS methylene-THF reductase; NAD(P)FOR NAD(P)H:Ferredoxin oxidoreductase; NFN electron-bifurcating transhydrogenase; PFK-1 phosphofructokinase; PFOR pyruvate:ferredoxin oxidoreductase; PGK phosphoglycerate kinase; PGM phosphoglycerate mutase; PEP phosphoenolpyruvate; PK pyruvate kinase; PTA phosphotransacetylase; PTB phosphotransbutyrylase; RNF Rnf complex; TPI triosephosphate isomerase
Carbohydrates display a valuable carbon source for clostridia. Complex feedstocks such as lignocellulose may be used directly when the organisms are able to degrade it to fermentable sugars. Cellulolytic clostridia like C. thermocellum produce enzymatic complexes called cellulosomes for this task and are reviewed in detail elsewhere [194, 321]. Released or directly fed carbohydrates are degraded for energy and reduction equivalent generation. The interlinked Embden–Meyerhof–Parnas (EMP) and pentose phosphate pathways (PPP) are the oxidative metabolic modules responsible for the degradation of hexoses and pentoses, respectively [219]. Finally, pyruvate is formed and may be used for acetyl-CoA formation releasing CO2 and generating additional reduction equivalents.
Acetogens can generate acetyl-CoA via the Wood–Ljungdahl pathway (WLP). There are several reviews recommended for further reading [20, 57, 258, 259]. The WLP is a reductive module that can use reduction equivalents generated from oxidative modules (EMP and PPP) or from the oxidation of CO or H2 [20, 259]. CO2 is stepwise reduced to a methyl-group in the Eastern branch of the WLP. The Western branch serves to provide a carbonyl group either directly from CO or from the reduction of CO2. Finally, the methyl-group and a carbonyl-group are combined with coenzyme A (HS-CoA) to form acetyl-CoA [57].
The growth of acetogenic and solventogenic clostridia in batch cultivations can be divided into two phases (‘biphasic’ fermentation): First, produced coenzyme A-bound acids (acetyl-CoA, butyryl-CoA, hexanoyl-CoA) can be released enabling ATP generation and fast growth, leading to the overall production of acids. This growth phase is referred to as acidogenesis [124]. In a second growth phase, the accumulated acids are taken up and converted to alcohols by reductive modules. Due to the accumulation of solvents, this growth phase is called solventogenesis [247]. In solventogenic clostridia, coenzyme A bound acids are reduced to their respective aldehyde by alcohol/aldehyde dehydrogenases (AADs) or aldehyde dehydrogenases (ADH) [38, 317]. Several acetogenic clostridia harbor aldehyde oxidoreductases (AORs) for the direct conversion of carboxylic acids to aldehydes without prior activation [48, 80, 120, 247]. AORs were shown to guide the ethanol formation during autotrophic growth of Clostridium autoethanogenum [169]. However, the direct reduction of acetic acid to acetaldehyde is thermodynamically unfavorable under standard conditions (1 M concentration of acetic acid and acetaldehyde at pH 7) and is facilitated by a low intracellular pH value [198].
Stoichiometric imbalances of reduction equivalents are resolved by redox balance modules: acetogens possess a membrane-bound trans-hydrogenase (Ech or Rnf complex) that transfers electrons from electron carriers with low redox potential (Fd2−) to electron carriers with a higher redox potential (NAD/NADH, H2) and couples the transfer with the translocation of Na+ or H+ out of the cell [258]. The generated chemiosmotic gradient can be used for energy generation by a membrane-bound ATPase. Electron bifurcating hydrogenases like HydABCD are essential for the supply of reduced ferredoxin during growth on mixtures of H2 and CO2 and may also serve for redox balancing during heterotrophic growth [258, 316]. During acidogenic growth, solventogenic clostridia like C. acetobutylicum balance surplus NADH by forming H2 [218].
Parameters and conditions promoting the solvent formation
Overall, the pH value, the acid concentration, and the degree of reduction of the substrate influence the metabolism and the formed products of acetogens and clostridia. These parameters can be used to steer the cultivation towards solventogenesis. During a continuous, phosphate-limited cultivation of C. acetobutylicum ATCC824, a change from acidogenic to solventogenic metabolism could be directed by solely changing the external pH from 5.7 to 4.5 [97, 126]. A two-stage continuous cultivation of C. ljungdahlii also allowed to control acidogenesis and solventogenesis using the pH setpoint [247]. Similarly, solventogenesis was induced during batch cultivation of the acetogen C. aceticum by shifting the pH-value from 8.0 to 6.9 [10]. Interestingly, the pH value was also suggested to favor alcohol formation reactions and to hamper the formation of longer fatty acids like hexanoic acid during the cultivation of acetogenic clostridia [41]. Supplementing a batch culture of C. beijerinckii NCIMB 8052 with acetate, butyrate or both led to an earlier onset of solventogenesis and to higher final butanol titers, highlighting the role of acid concentration in switching to solventogenesis [109, 313]. The supply of reduction equivalents during heterotrophic cultivation can be increased by changing the carbon source. Replacing glucose with glycerol for the cultivation of C. pasteurianum shifted the product spectrum from acids to solvents [46]. During continuous cultivation of the acetogen C. autoethanogenum, increasing the ratio of H2 to inorganic carbon in the feed gas led to an increased ratio of ethanol to acetate, showing that the amount of reduction equivalents supplied from the substrate influences solvent formation [299].
An interesting feature of some acetogens is the ability to use gaseous substrates and organic carbon sources like carbohydrates simultaneously. This ability is referred to as anaerobic, non-photosynthetic (ANP) mixotrophy [134]. By providing additional reduction equivalents via CO or H2 oxidation, the theoretical butanol yield on glucose is increased from 0.97 mol mol−1 to 1.33 mol mol−1 [76]. Advantages and applications of ANP mixotrophy are further discussed elsewhere [61, 76, 78, 192].
Strain stability and changes in strain performance
Aside from parameters that support solventogenic growth behavior, influences on the cellular performance and viability have to be considered: solventogenic clostridia may partially or completely lose their ability to produce solvents from acids during continuous cultivation or repeated batch cultivation [141]. This phenotypical phenomenon called strain degeneration has various causes. In C. acetobutylicum ATCC 824, degeneration is caused by the loss of the mega plasmid pSol carrying the genes for solvent formation [45]. In case of the degenerated strain C. beijerinckii DG 8052, the ability to form solvents was lost without a genetic change and could be restored by addition of CaCO3 [131]. Even phage infection caused strain degeneration during the industrial cultivation of C. madisonii [132]. Interestingly, a degeneration-resistant strain of C. beijerinckii NCIMB 8052 was isolated as early as 1993 [142]. Degeneration has, to the best of our knowledge, not been observed for an acetogen yet.
During the so-called acid crash, the fast accumulation of acids causes the cultivation to end before switching to the solventogenic phase [16, 80]. The acid crash in C. acetobutylicum was shown to be caused by formic acid accumulation to concentrations of ~ 1 mM [311]. In case of the acetogen C. carboxidivorans P7, an acid crash was caused by the fast accumulation of acetic acid at high cultivation temperatures (37 °C) [245].
The solvents produced are toxic to the culture: the growth of the C. acetobutylicum ATCC 824 wild type was inhibited by 50% when butanol, ethanol and acetone were added in concentrations of 7–13 g L−1, 40 g L−1 and 40 g L−1, respectively. 20 g L−1 butanol inhibited growth completely [133]. Growth of C. carboxidivorans cultures with CO as the sole carbon source was inhibited to 50% or even completely by 14.5 and 20 g L−1 butanol, respectively. Tolerance against ethanol was significantly higher: 35 g L−1 ethanol inhibited growth to 50% [79].
The onset of solventogenesis is seen as a survival strategy for dealing with the rising acid concentration during batch cultivation. Sporulation is a second survival strategy of clostridia [326]. Both sporulation and the metabolic switch from acidogenesis to solventogenesis are coordinated by the master regulator Spo0A in C. beijerinckii NCIMB 8052 [246]. However, the coordination of both events seems to differ between clostridial strains [219] and is not completely resolved to date [166].
While problems with sporulation have been reported for the acid-producing strain C. kluyveri [89], sporulation so far has not been identified as a problem regarding acetogenic clostridia because C. ljungdahlii and C. autoethanogenum were found to rarely sporulate [3, 286].
Systems biology and genetic engineering
The characterization of the metabolism of clostridial species and its regulation are the basis of metabolic engineering approaches on the way to high-performance strains for highly efficient industrial solvent production [333]. By applying omics technologies and metabolic modelling, our understanding of production hosts on the systems level is improved and can guide the rational strain design [40, 326, 333].
Genome-scale metabolic (GSM) models allow to describe the metabolic capabilities of different species [51]. GSM models have been developed for solventogenic clostridia such as C. acetobutylicum ATCC 824 and C. beijerinckii NCIMB 8052, cellulolytic clostridia such as C. cellulolyticum and C. thermocellum and several acetogenic clostridia such as C. ljungdahlii, M. thermoacetica, C. autoethanogenum and C. drakei (see Table 2).
Table 2.
Summary of genome-scale metabolic models for Clostridium spp.
| Organism | Acetogen | Metabolic model | References | ||
|---|---|---|---|---|---|
| Genes | Reaction | Metabolites | |||
| C. acetobutylicum ATCC 824 | N | 432 | 502 | 479 | iJL432 [157] |
| C. acetobutylicum ATCC 824 | N | 473 | 522 | 422 | [262] |
| C. acetobutylicum ATCC 824 | N | 700 | 709 | 679 | iFS700 [252] |
| C. acetobutylicum ATCC 824 | N | 490 | 794 | 707 | iCac490 [196] |
| C. acetobutylicum DSM 792 | N | N/A | 592 | 444 | [309] |
| C. acetobutylicum ATCC 824 | N | 802 | 1462 | 1137 | iCac802 [50] |
| C. acetobutylicum ATCC 824 | N | 967 | 1231 | 1058 | iCac967 [332] |
| C. beijerinckii NCIMB 8052 | N | 925 | 938 | 881 | iCM925 [197] |
| C. butyricum IBUN 13A | N | 641 | 891 | 701 | iCbu641 [263] |
| C. cellulolyticum H10 | N | 431 | 621 | 603 | iFS431 [253] |
| C. kluyveri | N | 708 | 994 | 804 | iCKL708 [342] |
| C. thermocellum ATCC 27405 | N | 432 | 577 | 525 | iSR432 [250] |
| C. thermocellum DSM 1313 | N | 601 | 872 | 904 | iAT601 [290] |
| C. thermocellum ATCC 27405 | N | 446 | 637 | 598 | iCth446 [49] |
| C. autoethanogenum | Y | 805 | 1002 | 1075 | [189] |
| C. autoethanogenum | Y | 786 | 1109 | 1097 | iCLAU786 [300] |
| C. autoethanogenum | Y | 699 | 755 | 772 | MetaCLAU [216] |
| C. drakei | Y | 771 | 922 | 854 | iSL771 [271] |
| C. ljungdahlii | Y | 637 | 785 | 698 | iHN637 [206] |
| C. ljungdahlii | Y | 680 | 809 | 718 | iJL680a [177] |
| M. thermoacetica | Y | 558 | 705 | 698 | iAI558 [119] |
aiJL680 is the GSM model that serves as the basis for the ME-model iJL965-ME. iJL965-ME extends iJL680 by adding 196 protein-coding open reading frames (ORFs), 89 RNA genes, 576 transcription units, 19 types of rRNA modifications, 17 types of tRNA modifications, 735 protein complexes with updated stoichiometry, 219 modified protein complexes and 134 translocated proteins
Grouping the metabolism as metabolic modules allows to compare the abilities of different organisms. Interestingly, fragments of modules might also be included in strains that cannot express an entire module functionally. As an example, several GSM models of clostridia contain the carbon monoxide dehydrogenase (CODH) reaction but only acetogens such as C. ljungdahlii harbor the full WLP. A common clostridial ancestor potentially had a functional WLP [51]. The modularity of the metabolism is an impetus for researchers to transfer useful abilities from one strain to another:
Clostridium acetobutylicum ATCC 824 has been equipped with genes from C. thermocellum for the formation of active mini-cellulosomes [151, 318]. Strains equipped with both a functional cellulosome and enzymes for the formation of butanol would allow solvent formation directly from lignocellulosic biomass and enable the use of such a host in a consolidated bioprocess. The establishment of a functional WLP in C. acetobutylicum ATCC 824 was investigated as well [33, 77]. Activity could be demonstrated for both the Eastern and Western branch of the WLP. There was, however, a lack of carbon flux from the WLP to acetyl-CoA that was hypothesized to be caused by a low level of the enzyme acetyl-CoA synthase [77]. Integrating the WLP into solventogenic clostridia would allow to recapture the H2 and CO2 released during metabolization of carbohydrates and to increase the overall carbon yield.
Extended genome scale metabolic models
A recent development was the integration of GSM models of the acetogens C. ljungdahlii and C. autoethanogenum into spatiotemporal models of large-scale (30–125 m3) bubble column reactors for gas fermentation [39, 40, 167]. These models enable prediction of cellular performance considering spatially resolved gradients of solved substrate gases (H2, CO and CO2) in the reactor environment. An integrated GSM model was used to investigate targets for gene knockouts that improve cellular performance in industrial scale [40]. There also exist other models of large-scale bubble column fermentation where the biology was modeled with a fundamental set of reactions [269] or with a biothermodynamics approach [7]. Considering the industrial importance of bubble column reactors for gas fermentation [39, 275, 284], the rise of these models supports further scale-up and industrialization of gas fermentation.
Another exciting advancement is the development of a metabolism and macromolecular synthesis model (ME-model) including protein and RNA synthesis in a GSM model [177]. The obtained model is the first of its kind for gram-positive bacteria and shows an improved prediction of growth rate, acetate formation rate and production of reduced compounds such as ethanol and glycerol compared to the underlying GSM model. It also allows to model the influence of cofactor (Ni+) availability suggesting new applications like media optimization.
Omics approaches
While GSM models can describe the general metabolic capabilities of an organism, gene expression varies depending on environmental conditions. The current metabolic phenotype can be accessed on proteome, transcriptome, and metabolome level with single- and multiomics approaches [285, 333]. Additionally, fluxomics approaches can use GSM models to calculate and estimate metabolite fluxes.
Single- and multiomics approaches have been applied to monitor the transition from solventogenesis to acidogenesis in solventogenic clostridia. The onset of solventogenesis and sporulation superimpose each other during batch cultivation. Continuous cultivation of C. acetobutylicum in a phosphate-limited chemostat allowed the culture to switch from acidogenesis to solventogenesis without triggering sporulation [97]. The possibility to investigate different metabolic states separately and reproducibly makes continuous cultivation a valuable tool for systems biology studies [97, 332, 333].
Naturally, studies aimed to understand phenomena that impair the industrial application and continuous cultivation of solventogenic and acetogenic clostridia. Regarding solventogenic clostridia, such phenomena include strain degeneration [131], solvent tolerance [110] and the response to inhibitors found in hydrolyzed lignocellulose [160, 176, 337]. A review about systems biology studies of C. acetobutylicum has been published recently and is highly recommended to the reader [333].
Several well-known acetogens such as C. ljungdahlii, C. autoethanogenum, C. ragsdalei and C. coskatii can produce acetate and ethanol simultaneously [19]. Ethanol is a desired product and formed acetate leaving the process is considered a “carbon loss” [301]. Several omics studies hence investigated the influence of the pH-value and substrate limitation [249] or the composition of the feed gas [106, 299, 300, 340] on ethanol formation. One interesting finding of proteome studies is that an increase in ethanol production seems not to be linked to key enzyme abundance in both C. ljungdahlii [249] and C. autoethanogenum [299], suggesting that regulation might be thermodynamically or on a posttranslational level rather than on a transcriptional level.
Systems biology approaches are also applied to investigate the function of key enzymes in metabolic pathways. Biochemical studies of relevant oxidoreductases in C. autoethanogenum cell extract in combination with transcriptome analysis allowed to determine the activity and the electron donor and acceptor specificity of key enzymes of the WLP and ethanol formation [198]. However, the activity of the methylenetetrahydrofolate reductase could only be demonstrated with the artificial electron acceptor benzyl viologen. Metabolic modelling employing a GSM model suggested that this enzyme is ferredoxin reducing, potentially filling this gap [300]. Recently, a GSM model of C. drakei coupled with transcriptome analysis and 13C metabolic tracing experiments was used to prove a functional cooperation of the glycine synthase-reductase pathway (GSRP) and the WLP [271]. The subsequent successful expression of the GSRP into E. limosum with a plasmid-based system underlines once again the modularity of metabolism.
Strain engineering and design
The ever-improving understanding of the metabolism of acetogens and clostridia driven by systems biology promotes rational strain design. In addition to studies directly benefiting from mathematical and integrative system support [333], there are plenty of strain engineering studies with straight-forward approaches. Targeted properties include inhibitor tolerance for growth on complex feedstocks [160], increased productivity [124, 276, 335] product selectivity [158, 169, 310] and the expansion of the product spectrum [47, 108, 149]. Advances in metabolically engineered solventogenic clostridia and acetogens have been reviewed recently [41, 116, 149, 166, 208].
Important phenotypical properties for a robust solventogenic producer strain are abolished sporulation and increased solvent tolerance [166, 292]. These traits are especially important for continuous cultivation: sporulation associated with a halt of cell growth would lead to the cells washing out. The culture broth constantly contains increased solvent levels which cause cell stress. Even though clostridial butanol tolerance and its mechanisms are not completely understood to date [331], rational approaches have already been described to increase solvent tolerance [188, 323]. While rationally engineered strains showed a more rapid adaptation to butanol or performed better than the wild type when challenged with butanol, performance above the critical level of 2% (v/v) butanol were not tested [188] or could not be overcome [323]. Rational design of asporogenous C. acetobutylicum strains focused on inactivating the sporulation regulators σF, σE, σG and SpollE [292]. Both deletion of SpollE and σG resulted in asporogenous strains that formed solvents in an inoculum independent manner [22, 293]. However, inactivation of SpollE led to lower final solvent titers as compared to the wild type [22].
Random strain engineering strategies have been a valuable alternative to rational approaches. The generation of a strain library via random mutagenesis and subsequent screening for better producers proved useful to isolate an improved strain: C. acetobutylicum ATCC 55025 is asporogenous and produces high concentrations of butanol and total solvents [122]. This strain was further evolved to the strain JB-200. C. acetobutylicum JB-200 is asporogenous, butanol tolerant and hyper-producing [324], showing that these properties are compatible in clostridia. Comparative genomic analysis of the C. acetobutylicum strains ATCC 55,025, JB-200 and ATCC 824 identified the orphan histidine kinase cac3319 as a knockout target for increased butanol production and tolerance [324]. Butanol stress has also been a major subject of multiomics studies [110, 333]. The improving knowledge on butanol tolerance and asporogenous strains paves the way for future rational strain design.
Genome engineering
Clostridia are challenging hosts for genome engineering. Common challenges are their low transformation and recombination efficiency [135, 149]. Clostridia lack non-homologous end-joining (NHEJ) and show a low activity of homology-directed repair (HDR) [135], both cellular repair mechanism for DNA double-strand breaks. The low activity of repair mechanisms can be used to screen for homologous recombination events with donor DNA. The genomic integration site can be targeted with high sequence specificity using a CRISPR/Cas system. Integration of the donor DNA removes the sequence that is targeted by CRISPR/Cas and protects the cell with the modified genome from the introduction of a lethal double-strand break [195]. To exploit HDR itself for the genomic integration of donor DNA, better understanding of homologous recombination mechanisms in clostridia and acetogens is needed [37]. Despite the challenges, genome engineering has been a focus of recent research and significant progress has been made. The latest published genome engineering tools for clostridia are summarized in Table 3.
Table 3.
Tools for genome engineering of clostridia
| Purpose | Tool | Description | Application | References |
|---|---|---|---|---|
| Genomic integration of whole pathways | Phage serine integrase system for dual integrase casette exchange (DICE) | Allows integrase-mediated site-specific integration into the genome without integration of unwanted DNA-like plasmid backbones | The whole butyric acid production pathway was integrated into the C. ljungdahlii genome | [112] |
| Genomic integration of whole pathways | Genomic integration system based on the Himar1 transposase | The Himar1 transposase is used to integrate the target DNA casette randomly at any AT-site in the genome | The acetone production pathway and an ermC selectable marker were integrated into the C. ljungdahlii genome | [220] |
| Deletion of single genes | CRISPR nickase based system for deletion | The truncated Cas9 protein (trCas9) lacking the RuvCl nucleolytic domain can be used for deletions even when expressed strong and constitutively | Two ermB genes and pyrE were deleted from the Clostridioides difficile genome | [118] |
| Deletion and integration of pathways | Targetron-recombinase system for large-scale genome engineering | Targetrons are used to position markerless lox66 and lox71 sites in the genome. Cre recombinase deletes the DNA in between the lox66 and lox71 site via homologous recombination | A 50-gene prophage island was deleted from the C. phytofermentans genome | [35] |
| Complementation after deletion | CRISPR/Cas9-based complementation strategy employing 24 nt bookmark sequences | A 24 nt bookmark sequence is introduced at the place of a gene that has been deleted. For future complementation studies, the 24 nt bookmark sequence is selected against to integrate the wildtype gene at its original location | The pyrE gene in C. ljungdahlii was replaced with 9 consecutive bookmark sequences. All 9 bookmark sequences allowed complementation with the pyrE wildtype gene | [264] |
| Editing of single nucleotides in genome | CRISPR-targeted base editing via deamination | A combination of nuclease deactivated Cas9 with activation-induced cytidine deaminase is applied for cytosine to thymine substitution without DNA cleavage | Premature stop codons were introduced into genes related to the formation of acetate (pta) and ethanol (adhE1, adhE2, aor1, aor2) in C. ljungdahlii | [320] |
| Editing of single nucleotides in genome | CRISPR nickase assisted base editing via deamination | A fusion of cytidine deaminase, CRISPR-Cas9D10A nickase and uracil DNA glycosylase inhibitor (UGI) is used for base-pair substitutions of C∙G to A∙T | Mutations were introduced into the pyrE, xylR, Spo0A and araR gene of C. beijerinckii | [163] |
Large-scale genome engineering tools such as the deletion of whole prophage islands or the integration of whole metabolic pathways have been developed for clostridial systems [35, 112, 220]. These tools may be used for new applications like the generation of a library of genome reduced strains and to improve the fast engineering of stable producer strains. CRISPR-targeted base editing tools allow genome engineering while avoiding the need for homologous recombination events, the introduction of donor DNA and DNA double-strand breaks [163, 320]. A useful application for base editing tools is the introduction of premature stop codons into genes to disrupt the gene function.
The number of available tools for genome engineering and metabolic engineering of clostridia increased significantly over the past decade. Further tools including plasmid systems for gene overexpression, dCas9 and RNA systems for gene down-regulation and gene deletion and insertion tools are reviewed elsewhere [37, 135, 166, 195]. An impressive testimony for the importance and applicability of genetic engineering of anaerobic microorganisms is the custom-made ‘Clostridia Biofoundry’ for fully automated, high throughput strain engineering used by the commercial syngas fermenting company LanzaTech [106].
Alternative feedstocks
Solventogenic and acetogenic clostridia offer the possibility to use a broad substrate range for fermentation processes. The choice of feedstock has a big impact on the economic viability of the solvent production process and the price of the final product [68, 232]. Since the main solvents butanol and ethanol are bulk chemicals, the feedstock should be cheap and available in large quantities [21]. While basic research mainly relies on costly glucose [14, 81, 204, 222], glycerol and crude glycerol [9, 23, 86, 187], the historical ABE fermentation process mainly utilizes sugar- and starch-rich first-generation feedstocks such as sugarcane, molasses and maize [92, 103, 212]. Alternative feedstocks offering a high potential are food and agricultural waste [2, 75, 236, 237], lignocellulosic biomass [54, 117, 137, 203], and liquid waste streams, for instance of the pulp and paper industry [102] (see Table 4). Waste streams and lignocellulosic feedstocks are abundant, cheap and not in competition with food production [201]. The use of alternative feedstocks is more sustainable and offers a lower carbon footprint by saving the waste streams from incineration and thereby decreasing the greenhouse gas emissions [32, 95]. The European Commission estimated around 88 million tons of food waste produced in Europe which equals 3.3 Gt of CO2 per year [2, 25, 274]. Food waste is defined as a waste of restaurants, canteens, and the food processing industry [2, 85]. Food waste mainly contains sugar and starch but also a large portion of fibers [2, 85]. Agricultural residues and plant-based biomass are also called second-generation feedstocks as they mainly contain lignocellulose [209]. Lignocellulosic biomass is woody and fibrous material composed of a complex structure of cellulose, hemicellulose and lignin [32, 92, 117].
Table 4.
Overview of industrially relevant alternative feedstocks for solventogenic and acetogenic clostridia
| Carbon source | Feedstock | Pretreatment | Organism | References |
|---|---|---|---|---|
| Lignocellulosic biomass | ||||
| C6 and C5 sugars | Apple pomace ultra-filtration sludge | Dilute sulfuric acid pretreatment and detoxification method | C. beijerinckii NRRL B-466 | [186] |
| Barley straw | Acid hydrolysis and overliming | C. beijerinckii P260 | [236] | |
| Cassava bagasse | Mechanically milling, enzymatic hydrolysis | C. acetobutylicum JB200 | [182] | |
| Corn stover | Hot-water with wheat straw hydrolysate and overliming | C. beijerinckii P260 | [237] | |
| Enzymatic hydrolysis | C. saccharobutylicum DSM 13864 | [213] | ||
| Dilute sulfuric acid pretreatment | C. beijerinckii BA101 | [67] | ||
| Domestic organic waste | Steam explosion and enzymatic hydrolysis | C. beijerinckii B-592, C. acetobutylicum DSM 1731 | [42] | |
| Extruded, enzymatic hydrolysis | C. acetobutylicum ATCC 824 | [180] | ||
| Market refused vegetables | Shredded | C. acetobutylicum DSM 792 | [280] | |
| Municipal solid waste | Dilute acid or hot water treatment and enzymatic hydrolysis | C. acetobutylicum NRRL B-591 | [75] | |
| Pine and elm woods | Enzymatic hydrolysis and organosolv pretreatment | C. acetobutylicum NRRL B-591 | [8] | |
| Pineapple peel | Grounded, dried, saccharification, detoxification method | C. acetobutylicum B 527 | [144] | |
| Rice straw | Enzymatic hydrolysis, alkaline and concentrated phosphoric acid pretreatments | C. acetobutylicum NRRL B-591 | [202] | |
| Starch industry wastewater | Dilute sulfuric acid pretreatment and detoxification method | C. beijerinckii NRRL B-466 | [186] | |
| Suspended brewery liquid waste | Dilute sulfuric acid pretreatment and detoxification method | C. beijerinckii NRRL B-466 | [186] | |
| Switchgrass | Dilute sulfuric acid pretreatment, enzymatic hydrolysis | C. beijerinckii P260 | [237] | |
| Alkali-pretreatment | C. saccharobutylicum DSM 13864 | [87] | ||
| Wheat straw | Grounded, hot dilute sulfuric acid hydrolysis | C. beijerinckii P260 | [235, 238, 239] | |
| Wood pulping hydrolysate | Detoxification: ion exchange resins, overliming and activated charcoal adsorption | C. beijerinckii CC101 | [181] | |
| Starch waste streams | ||||
| Starch | Food waste | Shredding | Clostridium sp. BOH3 | [334] |
| Blending and drying | Clostridium sp. strain HN4 | [224] | ||
| Pulverization | C. beijerinckii P260 | [113] | ||
| Sago | Enzymatic hydrolysis | C. saccharobutylicum DSM 13864 | [171] | |
| Starch-based waste packing peanuts | – | C. beijerinckii BA101 | [129] | |
| Potato waste starch | – | C. acetobutylicum NRRL B-591 | [145] | |
| Defibered-sweet-potato slurry | – | C. acetobutylicum P262 | [12] | |
| Inedible dough | – | C. beijerinckii NCIMB 8053 | [297] | |
| Sugar waste streams | ||||
| Sucrose, fructose, raffinose, stachyose, verbascose | Soy molasses | – | C. beijerinckii BA101 | [232] |
| Glucose, mannose | Konjac waste | Enzymatic hydrolysis, simultaneous saccharification and fermentation | C. acetobutylicum ATCC 824 | [265] |
| Cellobiose, glucose | Waste cotton fibers | Phosphoric acid-acetone process and enzymatic hydrolysis | C. acetobutylicum NRRL B-591 | [261] |
| Lactose | Milk dust powder | – | C. acetobutylicum ATCC 824, C. beijerinckii NCIMB 8052 | [296] |
| Cheese whey | – | C. acetobutylicum P262 | [62, 63] | |
| Gaseous feedstocks | ||||
| CO:CO2:H2:N2 (16.5:15.5:5:56) | Gasified Switchgrass | Ash removal by cyclone, scrubbers with 90% water, 10% acetone | C. carboxidivorans | [6] |
| CO:CO2:H2:N2 (42:20:2:36) | Steel mill waste gas | – | C. autoethanogenum | [198] |
| CO:CO2:H2:N2 (44:22:2:32) | – | C. autoethanogenum, C. ljungdahlii, C. ragsdalei | [148] | |
| CO:H2:CO2 (40:30:30) | Syngas | – | Clostridium ragsdalei PTA-7826 | [277] |
| CO:CO2:H2 (10:60:30) | Electrolysis of CO2 and H2O to form CO and H2 | C. autoethanogenum, C. kluyveri | [104] | |
While sugar substrates can be directly used in fermentation processes, feedstocks containing starch are primarily saccharified to glucose by glucoamylase [75, 289]. However, there are clostridia which can directly utilize starch, such as C. acetobutylicum NRRL B-591 and Clostridium beijerinckii BA101 [68, 71, 145, 184]. Therefore, food wastes are easily accessible and do not require expensive pretreatment [2, 113]. Conversely, feedstocks with a high lignocellulosic fraction such as wheat straw, corn stover, rice straw and cassava bagasse (see Table 4) require a pretreatment to release the sugars for conversion [32, 203]. Likewise, hydrolysis and/or saccharification can be integrated into the fermentation [32, 137]. For detailed information about pretreatment and integrated methods, the reader is referred to recent reviews on this topic [21, 32, 92, 117]. According to Ibrahim et al. [117], pretreatment and integrated methods increase capital and operational costs as well as time and energy requirements. Cao and Sheng [32] additionally underlined the negative effect of degradation and loss of carbohydrates. Sugar degradation not only decreases the proportion of convertible sugars but also leads to the formation of toxic compounds (e.g. furfural and 5-hydroxymethylfurfural), which may inhibit cell growth and lower the productivity of the process [32]. To decrease toxicity, hydrolysates can be treated to remove inhibitors prior to fermentation [32]. Liquid waste streams such as soy molasses [232], cheese whey [242] and Kraft paper mill sludge [102] are advantageous as they are already rich in free sugars and do not require hydrolysis. However, some liquid waste streams like paper mill sludge require detoxification to reduce growth-inhibiting components [95].
Saccharified lignocellulose and waste streams of the pulp and paper industry, contain a sugar mixture of hexoses (e.g. glucose, galactose, fructose) and pentoses (e.g. xylose, arabinose) [289]. For high productivity and an economic-efficient production process total sugar utilization is essential [168]. Unlike most natural yeast strains, solventogenic clostridia are particularly well suited to ferment pentose sugars like xylose [338, 343]. Despite the ability to convert a broad spectrum of sugars, the well-known problem of carbon catabolite repression (CCR) in sugar mixtures is a remaining issue. Therefore, recent studies have focused on the efficient conversion of sugar mixtures [289, 292]. Current research wants to go further by focusing on strains naturally capable to degrade cellulose and the genetic modification of the metabolic pathways. The major goal is the direct conversion of the complex structured lignocellulosic biomass to avoid expensive pretreatment steps [123]. For more information about genetic modification of metabolism, the reader is referred to the section “Systems biology and genetic engineering” and Jang et al. [123].
Since acetogens came to the center of attention, there are far more possibilities using alternative feedstocks: acetogenic clostridia can not only grow heterotrophically on a range of carbon sources but also autotrophically on gaseous substrates [192]. Gas mixtures of CO, H2 and CO2 are suitable substrates for gas fermentation of acetogens. These gas mixtures referred to as synthesis gas or syngas can be sustainably produced by the gasification of lignocellulosic biomass and municipal solid waste (MSW) [170]. Gasification yields accessible carbon even from the complex lignin fraction that accounts for up to 40% of the plant biomass [278]. Other sources of syngas include industrial waste streams such as exhaust gas of the steel and oil industry [270] and even gas mixtures obtained electrochemically from CO2 and H2O [104, 275].
While no fixed ratio of H2/CO is needed for syngas fermentation [11], the overall gas composition does influence the bioprocess. A higher ratio of H2 to CO may reduce the loss of carbon as CO2 and influence the product spectrum [170, 299]. The composition of the gas mixture depends on its origin. Syngas obtained from biomass gasification as well as furnace gas from steel mills may contain several detrimental impurities including ammonia (NH3), nitrogen oxide (NOx) and other enzyme inhibitory compounds such as acetylene (C2H2), ethylene (C2H4), ethane (C2H6) and oxygen (O2) [107]. The presence of the inhibitor hydrogen cyanide (HCN) in the feed gas even forced a temporal shutdown of a semi-commercial plant for ethanol production from gasified biomass and MSW [303]. Some impurities may also influence process parameters such as the pH-value, the osmolarity or the oxidation-reduction potential (ORP) [322]. Cleanup methods for removal of different impurities are available but costly and should be reduced to the minimum [48].
CO and H2 are poorly soluble in water (83 and 71% of the solubility of oxygen at 37 °C, respectively [221]) and must be continuously transferred from the gaseous to the liquid phase during gas fermentations. A high mass transfer of gases into the liquid is desired to enable high production rates and near-complete conversion of the feed gas. Unconverted gas leaving the bioreactor means both loss of valuable substrate and emission of greenhouse gas (GHG) [303].
ANP mixotrophy is a common feature of acetogens allowing them to utilize gaseous substrates and organic carbon sources simultaneously [134]. An increase in carbon yield from carbohydrates through ANP mixotrophy has been demonstrated for several acetogens [134] [27, 192] and a patent for the mixotrophic production of butanol, butyrate, isopropanol, acetone and ethanol has been issued [294]. A next step towards industrial application would be to demonstrate the benefits of mixotrophy during growth on complex feedstocks like hydrolyzed lignocellulose.
In conclusion, the use of alternative feedstocks with solventogenic clostridia has been much better researched compared to acetogenic clostridia. With the ability to co-utilize gaseous and organic substrates, however, acetogens seem an attractive option to develop carbon efficient bioprocesses with superior product yields from cheap carbon and energy sources. After focusing on the organism in combination with a cheap and sustainable feedstock for high efficiency of solvent production, the next step is the technical side of process optimization.
Continuous fermentation methods
In this section, we pay special attention to the different operation strategies for continuous fermentations, their properties and potential as a powerful tool to develop solvent production towards industrial implementation. For the design of a new economical process, the choice of the reactor type and the operation strategy are the two major criteria, mostly affecting the formation and activity of biocatalyst, conversion rate, volumetric productivity and downstream processing [179, 275, 314].
Batch and fed-batch
The batch process is easy to operate and requires minimum control. For that reason, it was conventionally used for the first laboratory studies and industrial ABE processes in Europe [21, 133, 179, 343]. Compared to fed-batch and continuous mode, batch mode reached the highest solvent yield for ABE fermentation [165]. However, changing conditions in batch over time (e.g. product concentration) can lead to an uncontrolled switch between the acidogenic and solventogenic phase, inhibited growth or cell death [105, 133, 179, 200]. Major drawbacks for industrial use of the batch mode are downtime periods for reactor preparation and prolonged lag phases leading to an overall low productivity [43, 161, 179].
When referring to batch mode during gas fermentations, the liquid volume remains unchanged, while the gaseous substrate is typically supplied either at the beginning (batch mode) or as continuous flow (fed-batch) [98, 276, 340]. While bioreactors offer control and monitoring possibilities [140], serum bottles represent the only “real” batch cultivations in gas fermentation, delimiting gas exchange and stripping of (intermediate) products.
Feeding strategies in fed-batch mode give the possibility to maintain a certain growth rate and low substrate concentration which offers the use of substrates toxic to the cells in large amounts and to obtain higher biomass and product concentrations than in batch cultivations [70, 92, 179, 226, 227]. Accumulation of products (like butanol) to toxic levels in the fed-batch process can inhibit the growth and product formation. A significantly improved solvent productivity was achieved by the integration of product recovery [70].
Nevertheless, the downtime in a fed-batch is comparable to a batch process and likewise there is no continuous substrate conversion and product formation. Multiple studies have investigated the use of fed-batch in comparison to continuous processes [124, 174, 182, 239, 288, 340]. Li et al. [165] tested batch, fed-batch and continuous process modes for ABE fermentation and recommended continuous fermentations to obtain bioprocesses with superior productivities.
Continuous processes
In contrast to batch and fed-batch cultivations, continuous cultivations are more demanding in terms of process control but offer significantly higher productivity and advanced capabilities for process design. Increased efficiency in industrial scale is offered, due to minimal initial lag phase, possible continuous feeding of permanently accumulating waste streams, steady downstream processing and thereby reduced downtime [16, 72, 92, 95, 107, 164, 179, 343]. Compared to short batch cultivations, continuous processes require increased attention to maintain strictly anaerobic conditions and to avoid microbial contaminations [161, 247, 343]. Table 5 gives a quick overview of the advantages and disadvantages of the continuous fermentation methods and operational strategies for solvent production described in the following sections.
Table 5.
Comparison of the most advantageous continuous fermentation methods and configurations for solvent production with solventogenic and acetogenic clostridia
| Method/configuration | Advantages | Disadvantages | References |
|---|---|---|---|
| One-stage chemostat |
Maintain growth rate at defined value Supports growth-related products Stable gas fermentation with acetogens |
Maximum growth rate limited by dilution rate Low biomass during solvent formation Strain degeneration and difficulty to reach steady state conditions with solventogenic clostridia |
[18, 193, 319] |
| Multi-stage systems |
Tool to stabilize biphasic fermentations Variation of temperature, pH or nutrient supply between the stages |
Higher costs for multiple reactors Complex control |
[18, 205, 247, 275, 279] |
| Cell retention |
Uncouples dilution rate of specific growth rate High volumetric productivity Full control of biocatalyst concentration Increased conversion rates (Toxic) solvents can easily be recovered of cell-free permeate Circulation of effluent possible Biomass reuse lowers propagation costs |
Difficult long-term operation Costly membrane Membrane fouling At high-level biomass concentration operational problems (high viscosity, heavy gas formation and foaming) Higher contamination risk of external separation Nonselective retention (dead, non-viable cells, and substrate particles) Requirement of cell viability monitoring |
[21, 62, 161, 164, 185, 204, 222, 287, 308, 314] |
| Cell immobilization and biofilm reactors |
Prevents washout of cells Allows higher dilution rates Increases reaction rates and productivity Enhanced genetic stability Improved inhibitor resistance of cells Protects cells against shear forces |
Uncontrolled cell growth can lead to blocking or Membrane fouling Maintenance of cell viability and physiology Diffusion limitation of mass-transfer Varying microenvironment Leaking of cells of support Inactive or dead biomass Reduced productivity during longer-term operation Challenging scale-up |
[11, 138, 150, 168, 179, 205, 225, 231, 275, 284, 314, 341] |
| Integrated product recovery |
Energy-efficient at low solvent concentrations Integration of downstream step for solvent recovery into cultivation Operable in continuous mode Lowers the concentration of toxic products in the broth Decreased product inhibition Improved substrate conversion rates, solvent yields, and productivities In situ product recovery: culture broth does not leave the reactor |
In-line method in separate recovery loop affecting the cells In situ product recovery: limited optimization opportunities Disadvantages dependent on product recovery method |
[21, 58, 82, 161, 164, 306, 325] |
One-stage chemostats
Stable continuous fermentation in chemostat was successfully maintained in several publications [13, 23, 43, 127, 307]. A commonly referred strain in stable chemostat runs is Clostridium acetobutylicum ATCC 824 [9, 90, 126, 127, 272]. For instance, more than 70 days of stable chemostat cultivation of C. acetobutylicum ATCC 824 was achieved at pH 6 and a dilution rate of 0.05 h−1 with a substrate-mixture of glucose and low-grade glycerol [9]. Butanol was the major solvent, produced with a yield of 0.34 mol mol−1 and a productivity of 0.42 g L−1 h−1, one of the highest reported productivity values for chemostat cultivations with C. acetobutylicum [9].
Basic lab-scale approaches for gas fermentation were mainly applied in continuous cultivation [84, 106, 146, 199, 275, 299, 300]. The continuous gas fermentation leads to a steady value of dissolved gases in the liquid medium which allows a precise calculation of the substrate consumption rate by monitoring the off-gas composition. The continuous stirred-tank reactor (CSTR) offers extensive mixing capabilities by the steady distribution of gaseous and liquid substrates [28, 107, 275]. The resulting high mass transfer rate is the reason why CSTRs are the first choice for gas fermentation investigations [4, 11, 106, 107, 170, 199, 217, 275, 298, 299]. For industrial-scale gas fermentations, the energy demand for sufficient mixing is significantly increased in CSTRs. As an alternative bubble columns, gas lift and loop reactors showed to be simple and cost-efficient, with the possibility for an energy-efficient scale-up [96, 275, 284]. However, for solvent production from organic substrates with suspended cells, the CSTR is still the dominating reactor type in industrial scale.
Continuous bioprocessing with solventogenic clostridia is challenging due to strain degeneration and because steady-state conditions can be difficult to establish [16, 18, 131, 141, 319, 343]. In the past, the degeneration of different Clostridium acetobutylicum strains (ATCC 824, DSM I73, NCIB 8052 and P262) in chemostat cultivations was investigated in multiple studies [9, 272, 319]. It has been shown that degenerated and solventogenic clostridia are transiently in co-culture but with increased cultivation time the fast-growing degenerated cells outgrow the slow-growing solvent-producing cells [45, 70, 95, 319]. One possible explanation is the strong selection pressure acting on the cells in a long-term cultivation. The increased number of generations, compared to a batch process, is not only detrimental for the genetic stability of genetically engineered organisms but also increases the chance for natural and induced mutations [179, 193].
One-stage chemostats for ABE fermentation often failed to reach steady-state conditions and are marked by the oscillation of biomass, product, and substrate concentration [18, 86, 204]. So far, the influence of culture pH, extracellular addition of butyric acid or acetic acid as co-substrate and phosphate (P) or nitrogen (N) limitations on culture stability has been investigated [13, 46, 105, 130, 200, 343]. Although nutrient limitations can efficiently stabilize cultures, this stability can only be achieved at the expense of incomplete carbon substrate utilization. In contrast to solventogenic clostridia, acetogenic microorganisms easily reached the steady-state in one-stage chemostats and strain degeneration has never been reported. A conclusion on ABE fermentation may be drawn by comparing solventogenic clostridia and acetogens on the systems biological level.
During chemostat cultivation, the close link of the volumetric productivity to the liquid dilution rate and thus, the specific growth rate, offers higher process control. However, the maximum growth rate of the cells limits the dilution rate. While a chemostat process is advantageous for growth-related products, growth inhibition by toxic products and low growth rates during solventogenesis result in a limitation of the dilution rate [26, 161, 165, 166, 179]. Low biomass concentrations were also reported for gas fermentations with acetogens [37]. Low cell concentrations in combination with low dilution rates eventually limit the volumetric solvent productivity in the chemostat. Optimization of processes with solventogenic and acetogenic clostridia, therefore, requires additional modifications of the basic one-stage chemostat, described in the following sections.
Multi-stage systems
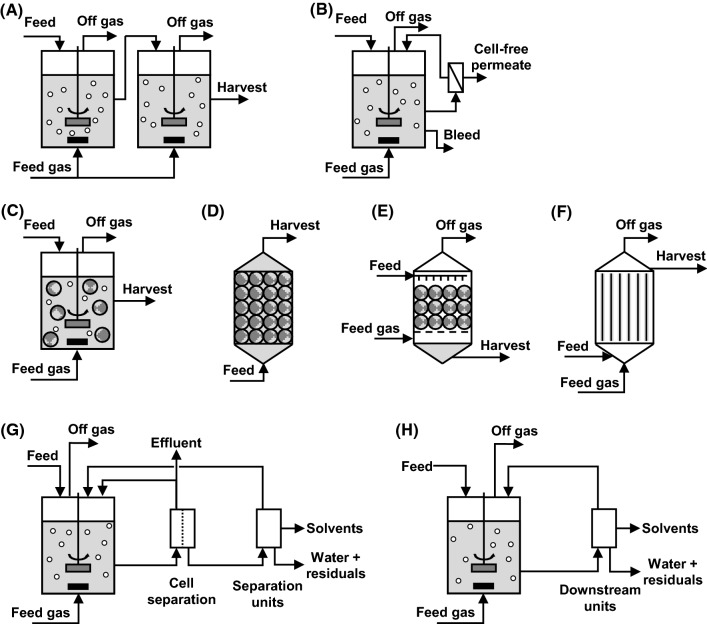
A technical solution for stabilization of the continuous production are multi-stage systems where multiple reactors connected serially form a “reactor cascade” (see Fig. 2a). For example, the process can be split into a nutrient-limited phase (e.g. phosphor or nitrogen) and a solvent forming phase by variation of temperature, pH or nutrient supply between the stages (see Table 6) [18, 88, 154, 205, 279]. Two-staged reactor systems were proven to enhance the stability of the cell physiology and product formation of solventogenic fermentations, either with heterogenic or gaseous substrates [18, 205, 247, 275]. The use of continuous two-stage chemostats for solventogenic clostridia was first discussed by Bahl et al. [14] and has subsequently been investigated as a tool to stabilize biphasic fermentations [18, 91, 154, 205].
Fig. 2.
Overview on the most advantageous fermentation methods and configurations for continuous solvent production with solventogenic and acetogenic clostridia. a Multi-stage process with two chemostat stages; high cell density cultivation in a b continuous cell retention system and with c–f immobilized systems and biofilm reactors: c chemostat with free-flowing immobilized cell particles, d packed-bed reactor (PBR), e trickle bed reactor (TBR), f hollow fiber membrane reactor (HFMBR). TBR (e) and HFMBR (f) are mainly used for gas fermentation. Integrated product recovery methods: g in-line recovery and h in situ recovery. (Modified from [82, 267, 275, 314, 341]
Table 6.
Application of the multi-stage process for continuous solvent production with solventogenic and acetogenic clostridia
| Strain | Continuous system | 1st stage | 2nd stage | Substrate | Product | Titer, productivity, yield | References |
|---|---|---|---|---|---|---|---|
| C. acetobutylicum DSM 1731 | Two-stage chemostat | D 0.125 h−1, P-limited | D 0.04 h−1, P-limited | Glucose | Butanol | 130 mM | [14] |
| Acetone | 74 mM | ||||||
| C. acetobutylicum IFP 918 | Two-stage chemostat | D 0.16 h−1, pH 6.1, T 36 °C | D 0.045 h−1, pH 5.5, T 33 °C, N-limited | Glucose | ABE solvents | 0.28 g g−1 | [18] |
| C. acetobutylicum | Two-stage fermentation | D 0.08 h−1, pH 4.5 | D 0.04 h−1, pH 4.5–5.0 | Glucose | ABE solvents | 21 g L−1, 0.36 g g−1 | [91] |
| C. acetobutylicum ATCC 824 | Two-stage chemostat | D 0.075 h−1, pH 6.0, T 37 °C, N-limited, acid formation | D 0.06 h−1, pH 4.5, T 33 °C, solvent production | Glucose | ABE solvents | 9.11 g L−1, 0.6 g L−1 h−1 | [154] |
| Butanol | 5.93 g L−1, 0.4 g L−1 h−1 | ||||||
| C. acetobutylicum ATCC 824 |
Two-stage turbidostat/ chemostat |
pH 4.62, acid formation | pH 4.37, solvent production and in situ product recovery | Glucose | ABE solvents | 18.0 g L−1, 0.13 g L−1 h−1, 0.30 g g−1 | [305] |
| C. acetobutylicum B 5313 | Two-stage chemostat and cell immob., in situ product recovery | D 0.6 h−1, 37 °C | D 0.3 h−1, 37 °C | Glucose | ABE solvents | 25.32 g L−1, 2.5 g L−1 h−1, 0.35 g g−1 | [17] |
| C. beijerinckii NRRL B592 | Two-stage cascade and cell immobilization | D 0.5–0.6 h−1, pH 4.6–4.7, T 36 °C | D 0.15–0.20 h−1, pH 4.7–4.8, T 36 °C | Glucose | ABE solvents | 9.27 g L−1, 1.24 g L−1 h−1 | [88] |
| Butanol | 5.57 g L−1 | ||||||
| C. beijerinckii NRRL B592 | Two-stage cascade | Turbidostat, D 0.12 h−1, pH 4.7, 34 °C, acid formation | Chemostat, D 0.022 h−1, pH 4.7, 34 °C, solvent production | Glucose | ABE solvents | 15 g L−1, 0.27 g L−1 h−1 | [205] |
| Butanol | 9.1 g L−1 | ||||||
|
C. beijerinckii DSM 6423 |
Two-stage chemostat | D 112 h−1, pH 4.8 | D 0.054 h−1, pH 5.1 | Glucose | ABE solvents | 10.56 g L−1, 0.39 g L−1 h−1 | [279] |
| C. ragsdalei ATCC PTA-7826 | Two-stage cascade with cell recycle and nutrient limitation | D 0.28 mL min−1, acid formation | D 0.28 mL min−1, ethanol production | Syngas: 30% H2, 30% CO2, 40% CO | Ethanol | (14.74 g g−1 cells) | [153] |
|
C. ljungdahlii ERI-2 (ATCC 55380) |
Two-stage cascade | CSTR, pH 5.5–5.7, growth stage | Bubble column, pH 4.4–4.8, with cell and gas recycle ethanol production | Syngas: 60% CO, 35% H2, 5% CO2 | Ethanol | 450 mM, 0.37 g L−1 h−1 | [247] |
| C. ljungdahlii PETC | Two-stage chemostat | CSTR D 0.96 day−1, pH 5.5 | Bubble column with cell recycle, D 0.48 day−1, pH 4.5 | Syngas: 60% CO, 35% H2, 5% CO2 | Ethanol | 19 g L−1, 0.30 g L−1 h−1 | [191] |
| C. ljungdahlii PETC | Two-stage chemostat | Acid formation | Ethanol production | Syngas: 60% CO, 35% H2, 5% CO2 | Ethanol | 188.2 mM | [249] |
The table gives an overview of the settings of the most investigated two-stage process, in detail: dilution rate (D), pH, temperature (T), nutrient limitation (P: phosphate, N: nitrogen) and purpose of the respective stage
In 1998, ButylFuel LLC (Columbus, USA) patented a two-stage fermentation process separating acidogenesis and solventogenesis in two distinct process steps. In the first stage, C. tyrobutyricum converts glucose to butyric acid which is transferred to the second stage and converted to butanol by C. acetobutylicum [243].
Multi-staged processes are the method of choice in semi-continuous industrial ABE fermentation in Russia and China [95]. While ABE processes in Europe were merely focused on batch cultivation in the past, China and Russia continually focused on continuous bioprocessing to produce acetone, butanol, and ethanol [211, 343].
Recently, Richter et al. [247], and Martin et al. [191] applied a two-stage cultivation system for syngas fermentation, separating the process in a growth stage and an ethanol producing stage (see Table 6). In 2016, a continuous multi-stage cultivation in circulated loop reactors for gas fermentation was patented by LanzaTech [295], emphasizing the feasibility and suitability of multi-stage processes for industrial use.
Recent investigations of solventogenic clostridia demonstrate the continuous two-stage cultivations with integrated product recovery in the second stage [17, 304, 305]. As shown in Table 6, there are several possibilities to combine the reactor cascade with other technologies, such as cell recycling [5, 247] and cell immobilization [17, 88]. Cell recycling and immobilization can consolidate the idea of a growth and solvent forming process phase, as described for gas fermentation [247].
High cell density cultivation
To solve the problem of insufficient biomass in continuous cultivations, growth needs to be uncoupled from the liquid feed flow rate. Uncoupling can be done by regulating the cell concentration in a continuous culture equipped with a cell retention technique or by immobilization of the cells [86, 114, 210, 222, 338]. The topics of cell retention and immobilization are described in the following sections.
Cell retention
The introduction of a cell retention or cell recycling unit uncouples the dilution rate from the specific growth rate and therefore allows to accumulate higher biocatalyst concentrations [161, 185, 314]. That way, a ‘retentostat’ offers the possibility of a fully controlled high cell density fermentation by increasing conversion rates for complete substrate uptake and efficient conversion into the target product. Cell retention has been reported to be advantageous for solventogenic clostridia and enables high volumetric productivity during gas fermentation with acetogens [37, 108, 134, 247, 248].
Cell retention with submerged cells can industrially be achieved by centrifugation and filtration, while membrane filtration is primarily used in lab-scale experiments [179, 314]. Using membrane filtration, biomass is increased by holding back the cells by a hollow-fiber membrane module (see Fig. 2b) [62, 222, 308]. The growth rate in the retentostat can be controlled by the value of bleed flow [185]. Of the obtained cell-free permeate, toxic solvents can easily be recovered, while the leftover substrate can be returned to the reactor for an increased conversion [62].
Systematic reuse of biomass can lower the costs of cell propagation [314]. On the other hand, the process may be more complex and difficult to operate in the long-term [62]. The requirement of a membrane for the cross-flow filtration increases the process costs and implies the risk of membrane fouling over time [21, 62, 287]. Cell recycling can be combined with different reactor types such as bubble columns and process modifications such as cell immobilization, latter reduces problems with membrane fouling [21, 173]. The use of an external separation method constitutes a higher risk for contamination compared to a conventional chemostat process. Rapid pumping of the cell broth through the separation device can cause cellular shear stress [204]. The use of a separation unit in industrial gas fermentations can lead to a deficit in gas supply due to longer residence times.
Cell retention has already been demonstrated in the past to increase the productivity in ABE fermentation of glucose by Clostridium acetobutylicum [5, 81, 204, 222, 257]. When research in ABE got back into the focus between 2005–2010, the topic of cell retention was rediscovered. Tashiro et al. [287] maintained a high cell density culture of C. saccharoperbutylacetonicum N1-4 in a membrane cell-recycling reactor, feeding glucose and showed an ABE productivity of 7.55 g L−1 h−1 and concentration of 8.58 g L−1 for more than 200 h without cell degeneration. More than 710 h of stable cell recycling application and conversion of glycerol to a high butanol productivity was shown with the hyper producing Clostridium pasteurianum MBEL_GLY2 [187]. Jang et al. [125] and Nguyen et al. [210] showed some of the highest achieved butanol productivities with 21.1 and 14 g L−1 h−1, respectively (see Table 7). Successful implementation of cell retention for the utilization of C5 sugars like xylose was shown by Zheng et al. [338] and Survase et al. [283].
Table 7.
Application of cell retention for continuous solvent production with solventogenic and acetogenic clostridia
| Strain | Continuous system | Dilution rate | Bleed rate | CDW | Substrate | Product | Titer/productivity /yield | References |
|---|---|---|---|---|---|---|---|---|
| C. acetobutylicum ATCC 824 | Membrane cell-recycle reactor | 0.5 h−1 | 0.025 h−1 | 20 g L−1 | Glucose | ABE solvents | 13 g L−1, 6.5 g L−1 h−1 | [222] |
| C. acetobutylicum ATCC 824 | Cell-recycle reactor | 0.35 h−1 | Total cell retention | 125 g L−1 | Glucose | ABE solvents | 4.5 g L−1 h−1, 0.31 g g−1 | [81] |
| Butanol | 3.7 g L−1 h−1 | |||||||
| C. acetobutylicum ATCC 824 | Spin filter perfusion bioreactor | 0.089 h−1 | Total cell retention | 49 g L−l | Glucose | ABE solvents | 1.14 g L−1 h−1 | [204] |
| C. acetobutylicum AvapClo™ (ATCC 824 with ADH gene) | Membrane cell-recycle reactor with product recovery of the permeate and effluent recirculation | 0.77 h–1 | Bleed for constant CDW | Max 50 g L−1 | C6 lignocellulosic sugars from pine wood | Butanol, acetone, isopropanol and ethanol mix | 10 g L−1 h−1, 0.33 g g−1 sugars | [283] |
| C. acetobutylicum BKM19 | Membrane cell-recycle reactor | 0.86 h−1 | 0.04 h−1 | (OD600nm 335) | Glucose | ABE solvents | 23.5 g L−1, 21.1 g L−1 h−1, 0.34 g g−1 | [125] |
| Butanol | 11.9 g L−1, 10.7 g L−1 h−1, 0.17 g g−1 | |||||||
| C. acetobutylicum CAB1060 | Cell-recycle reactor with in situ extraction and P-limitation | 0.076 h–1 | 0.05 h−1 | 28–30 g L−1 | Glucose | Butanol | 550 g L−1, 14 g L−1 h−1, 0.35 g g−1 | [210] |
| C. acetobutylicum P262 | Membrane cell-recycle reactor | 0.41 h−1 | 0.02 h−1 | 20 g L−1 | Cheese whey permeate | ABE solvents | 0.31 g g−1 | [62] |
| C. acetobutylicum | Cell-recycle reactor with P-limitation | 0.40 h−1 | 13.1 g L−1 | Glucose | Butanol | 4.1 g L−1 h−1 | [257] | |
| C. saccharoperbutyl-acetonicum N1-4 | Membrane cell-recycle reactor | 0.71 h−1 | 0.16 h−1 | 16.3 g L−1 | Glucose | ABE solvents | 8.66 g L−1, 7.54 g L−1 h−1 | [287] |
| C. saccharoperbutyl-acetonicum N1-4 | Membrane cell-recycle reactor | 0.78 h−1 | 17.4 g L−1 | Xylose | Butanol | 4.26 g L−1, 3.32 g L−1 h−1 | [338] | |
| C. autoethanogenum DSM10061 | Membrane cell-recycle reactor | 4.9 day−1 | 0.5 day−1 | 1.83 g L−1 | 65% H2, 23% CO2, 9%N2 | Ethanol | 6.3 g L−1, 0.14 M | [198] |
| C. ljungdahlii ∆SADH (pTCtA) | Cell-recycle reactor | 3–6 mL min−1 | Bleed for constant CDW | 10–18 g L−1 | Fructose | Acetone | 10.8 g L−1 | [134] |
| Co-culture: C. ljungdahlii PETC and C. kluyveri DSM555 | Membrane cell-recycle reactor | 40–80 mL h−1 | 10–20 mL h−1 | (OD600nm 5–10) | Syngas: 60% CO, 35%H2, 5% CO2 | Ethanol | 65.5 mmol CL−1 day−1 | [248] |
| Butanol | 39.2 mmol CL−1 day−1 | |||||||
| Hexanol | 31.7 mmol CL−1 day−1 | |||||||
| C. pasteurianum MBEL_GLY2 | Cell-recycle reactor | 0.90 h−1 | Total cell retention | (OD600nm 407.6) | Glycerol | Total solvents | 9.2 g L−1, 8.3 g L−1 h−1 | [187] |
| Butanol | 8.6 g L−1, 7.8 g L−1 h−1 |
The table focuses on cell recycling systems and shows the essential settings of dilution rate and bleed rate, deployed to achieve high cell dry weight (CDW)
The next step in research with solventogenic clostridia will be the optimized bioprocessing of alternative feedstocks. Liquid waste streams and pretreated substrates like lignocellulose hydrolysates comprise of a mixture of sugars but furthermore can contain a high solid particle concentration and inhibiting substances, leading to decreased cell growth. While cell retention is essential for efficient conversion of this kind of substrates, there may be an upcoming problem: the retention system is not selective for active biomass. Therefore, inactive cells and even substrate particles accumulate equally in the reactor. Consequently, an increase in biomass concentration does not necessarily lead to a proportional increase in productivity [308]. A major approach is the viability monitoring of the cell population and differentiation between cells and background particles via rapid at-line tools such as flow cytometry [291, 308].
Multiple studies showed the implementation of cell retention in (syn)gas fermentation with acetogens [36, 108, 178, 247, 248] (see Table 7). Additionally, Jones et al. [134] successfully showed mixotrophic growth of an engineered Clostridium ljungdahlii strain on syngas and fructose in a cell retention system. Regarding industrial production at scale, there are several patents for gas fermentation equipped with cell retention [84, 260].
Cell immobilization
Another option for continuous high cell density cultivation is the use of cell immobilization and biofilm reactors to prevent cell washout [168, 275, 314]. To that end, immobilization allows operation at higher dilution rates which in turn increases reaction rates and productivities [179, 225]. Advantages of immobilization include enhanced genetic stability, improved resistance of cells to inhibitory substrates or products and protection against shear forces [150, 341].
Immobilization is commonly achieved by entrapment of cells or by binding of cells to a carrier [341]. Cells can be entrapped inside a semipermeable membrane or encapsulated inside a polymeric matrix, for example inside beads of alginate or polyacrylamide (see Fig. 2c) [12, 145, 168, 179, 341]. Binding of the cells to the surface of a solid material is implemented by physical adsorption, ionic bonds, covalent bonds, or a mixture [55, 225, 341]. Entrapment and covalent bond formation require expensive and cell propagation limiting chemicals [179, 225]. In contrast, adsorption on a carrier is more natural, forms stronger bonds and can easily be performed in place [225]. A trend in research of immobilized solvent production is the use of cheap, renewable materials as adsorption carrier such as wood pulp [15], sugarcane bagasse [17], coconut fiber [281], corn stover [86] or clay bricks [240]. During adsorption, cell growth occurs in biofilms [179, 225, 231, 234].
Typical bioreactors for the bioprocessing with floating immobilized cells are CSTR (see Fig. 2c), fluidized bed bioreactors and air-lift reactors [341]. Packed bed reactors (PBR) differ from bioreactors with fully suspended culture as they are tightly packed with a carrier material to support biofilm formation (see Fig. 2d) [16, 225]. For gas fermentation, two special types of immobilized reactors have recently been described: the trickle bed reactor (TBR, see Fig. 2e) and the hollow fiber membrane reactor (HFMBR, see Fig. 2f) [11, 275, 295]. TBR are similar packed as PBR but the bed is sprinkled with liquid nutrient medium from above and flushed with the substrate gas from below to obtain high gas–liquid transport rates with low energy consumption [275]. A microporous membrane is used in a HFMBR for gas distribution and at the same time as carrier surface, providing cell growth at the gas–liquid interface with high mass transfer rate [267, 275]. Uncontrolled cell growth can lead to blocking of the PBR and TBR column, which was reported as a major problem in the first scale-up of the PBR process with solventogenic clostridia [225, 231, 275]. Moreover, membrane fouling of the cost-intensive membranes of HFMBR is a problem which causes the loss of membrane functionality [138, 275]. Immobilization leads to varying microenvironmental conditions and diffusion limitation of substrates and products, either by the thickness of the biofilm, pore size or surface area of the material [138, 179, 341]. The impeded mass transfer leads to inactive or dead biomass and a reduction of the volumetric productivity during Longer operation periods [204].
Several investigations with different cell immobilization techniques were performed over the years with solventogenic clostridia and in recent years with acetogenic gas fermentation (listed in Table 8). Gallazzi et al. [86] used a continuous immobilized packed-bed reactor filled with corn stover pieces for biofilm adsorption of C. pasteurianum DSM 525. During steady-state with 0.44 h−1 dilution rate, they reached a butanol titer of 10.4 g−1 L−1, productivity of 4.2 g−1 L−1 h−1 and 33% butanol to liquid by-products ratio. For syngas fermentation (38% CO, 28.5% CO2, 28.5% H2 and 5% N2, flow: 4.6 mL min−1) with Clostridium ragsdalei, a semi-continuous trickle bed reactor, consisting of a borosilicate glass column filled with 6 mm soda lime glass beads, reached an ethanol titer of 5.7 g L−1 [52].
Table 8.
Application of cell immobilization for continuous solvent production with solventogenic and acetogenic clostridia
| Strain | Immobilized system | Immobilization type and carrier | Substrate | Product | Max. productivity | References |
|---|---|---|---|---|---|---|
| C. acetobutylicum ATCC 55025 | Continuous biofilm reactor | Adsorption, fibrous bed | Glucose and co-substrate butyrate | Butanol | 4.6 g L−1 h−1 | [114] |
| C. acetobutylicum B 5313 | Two-stage chemostat with integrated solvent recovery | Adsorption, sugarcane bagasse | Glucose | ABE solvents | 2.5 g L−1 h−1, 0.35 g g−1 | [17] |
| C. acetobutylicum DSM 792 | Continuous packet bed reactor | Adsorption, Tygon rings | Lactose and yeast extract (cheese whey imitate) | Butanol | 4.4 g L−1 h−1 | [207] |
| C. acetobutylicum DSM 792 | Continuous biofilm reactor | Adsorption, coconut fibers and wood pulp | Sugar mix, synth. (lignocellulose hydrolysate) | ABE solvents | 12.14 g L−1 h−1 | [281] |
| C. acetobutylicum DSM792-ADH | Continuous packed bed reactor | Adsorption, wood pulp fibers | Lignocellulosic biomass hydrolysate | Isopropanol-butanol-ethanol mix | 1.67 g L−1 h−1 | [15] |
| C. acetobutylicum P-262 | Continuous 4-stage biofilm reactor system | Adsorption, ceramic D-21 beads | Defibered-sweet-potato-slurry | ABE solvents | 1.0 g L−1 h−1 | [12] |
| C. beijerinckii BA101 | Continuous packed bed reactor | Adsorption, clay bricks | Glucose | ABE solvents | 15.8 g L−1 h−1 | [240] |
| C. beijerinckii BA101 | Continuous plug-flow biofilm reactor, in-site product recovery and effluent recycling | Adsorption, clay brick | Glucose | Butanol | 16.2 g L−1 h−1 | [168] |
| C. beijerinckii BA101 | Continuous packed bed reactor, Scale-up | Adsorption, brick pieces | Glucose | Butanol | 34.76 g L−1 h−1 | [231] |
| C. beijerinckii DSM 6423 | Continuous packed bed reactor | Adsorption, wood pulp | Sugar mix, synth. (lignocellulose hydrolysate) | ABE solvents | 5.58 g L−1 h−1 | [282] |
| C. beijerinckii NCIMB 8052 | Chemostat with immobilized cells | Encapsulation, porous polyvinyl alcohol media | Glucose and co-substrate butyrate | Butanol | 0.40 g L−1 h−1 | [159] |
| C. beijerinckii ATCC 6014 and C. tyrobutyricum ATCC 25755 | Continuous biofilm reactor | Adsorption, fibrous bed | Cassava bagasse hydrolysate | Isopropanol-butanol mix | 0.44 g L−1 h−1 | [336] |
| C. carboxidivorans P7 | Hollow fiber membrane biofilm reactor | Adsorption, membrane | Syngas: 20% CO, 5% H2, 15% CO2, 60% N2 | Ethanol | 23.93 g L−1, 0.24 mol C mol C−1 | [267] |
| C. carboxidivorans P7 | Biofilm reactor | Adsorption, cordierite-based ceramic monolith cylinder | Syngas: 20% CO, 5% H2, 15% CO2, 60% N2 | Ethanol | 4.89 g L−1, 2.35 g L−1 day−1 | [266] |
| C. pasteurianum DSM 525 | Continuous packed bed reactor | Adsorption, corn stover pieces | Glycerol | Butanol | 4.2 g L−1 h−1 | [86] |
| C. ragsdalei ATCC-PTA-7826 | Trickle bed reactor semi-continuous | Adsorption, 6 mm soda lime glass beads | Syngas: 38% CO, 28.5% CO2, 28.5% H2, 5% N2 | Ethanol | 5.7 g L−1, 0.80 mmol L−1 h−1 | [52] |
| Clostridium ragsdalei ATCC-PTA-7826 | Trickle bed reactor semi-continuous | Adsorption, 6 mm soda lime glass beads | Syngas: 38% CO, 5% N2, 28.5% CO2, 28.5% H2 | Ethanol | 45 mg L−1 h−1 | [53] |
The overview is focused on the immobilized system, type and carrier
Regarding industrial use, there are patents for cell immobilization methods of solventogenic clostridia [44] and for gas fermentation with the acetogen C. ljungdahlii ERI2 ATCC 55380 for a 144-L trickle bed reactor [83]. For an overview of patents for biofilm reactors in gas fermentation, the reader is referred to Stoll et al. [275]. Nevertheless, maintenance of cell viability and physiology in an immobilized system is complicated [179]. Long-term biofilm stability is difficult to maintain and cell leakage from the support material requires an additional separation step [21, 225, 284]. Therefore, the scale-up of an immobilized system for industrial use is challenging and requires additional engineering studies [205, 231, 341]. In contrast, industrial gas fermentations using cell retention have already been demonstrated at scale. Due to the advantages of cell retention for process intensification, the number of applications for continuous high cell density fermentations is expected to increase significantly in the future.
Integrated product recovery
Once the continuous cultivation for solvent production is established, the focus shifts to product toxicity of e.g. butanol or ethanol [133, 152]. One way to address product toxicity is to engineer solvent tolerant strains (see section “Strain engineering and design”).
A second approach is to reduce the concentration of toxic products in the fermentation broth by integrating solvent recovery into the upstream process by in situ or in-line methods [58, 82, 161]. The in-line method is maintained in a separate loop, circling the alcohol-depleted effluent back into the reactor, whereas the culture broth does not leave the reactor during in situ product recovery (see Fig. 2g, h) [82, 306]. The spatial separation of in-line recovery methods from the fermentation process allows independent optimization [82]. The constant recovery of toxic products lowers the actual concentration in the culture broth [21]. That way, product inhibition is decreased, leading to increased solvent yields and productivities and improved substrate conversion rates [164, 273, 325, 328].
The traditional method for product recovery is by distillation using multi-column procedures, particularly in the industrial production of fuel ethanol [21, 306, 327]. The growing interest of the biofuel industry to use lignocellulosic and waste stream feedstocks leads to lowered alcohol concentrations in the fermentation broth [306]. Distillation is a robust and popular method for ethanol recovery but is less suitable for low solvent concentrations due to the high energy requirement [21, 306, 327]. The required energy for the distillation procedure increases exponentially for butanol levels below 10 g L−1 [190] or ethanol concentrations below 40 g L−1 [306]. The boiling point of butanol is higher than that of water. These azeotropic properties hinder the butanol recovery via distillation [117].
Requirements concerning the degree of recovery differ between integrated methods and methods for the final separation at the end of the bioprocess. While the effluent during integrated recovery is circulated by feeding back to the reactor, the product remaining in the effluent after the recovery with final separation technologies like distillation is lost. Consequently, final separation technologies require a higher degree of alcohol recovery [82, 161, 306].
Therefore, alternative methods are employed for integrated product recovery, including gas stripping, liquid–liquid extraction, adsorption, pervaporation and perstraction [65, 82, 166]. Below, we give a short introduction to recovery techniques. For further information, the reader is pointed out to Friedl [82], Vane [306], Bharathiraja et al. [21] and Kujawska et al. [152].
Gas stripping is a simple, physical method for economic in situ solvent recovery [55, 82]. For separation, the cell broth in the reactor is flushed with N2 or CO2, stripping the volatile solvents from the solution [21]. Afterwards, the stripped solvents and entrained water is recovered by condensation from the escaping gas stream [21, 55, 74]. The gas stream can be recycled for several cycles [66]. To lower processing costs, there is also the possibility to directly use the fermentation gas (containing CO2 or H2) as stripping gas [82]. Gas stripping is a quite flexible separation method and can be used in combination with different process types (e.g. batch, fed-batch, continuous, multi-stage processes, fluidized bed reactors) and with other separation techniques [21, 73, 82]. It is claimed as the most studied technique for solvent recovery and as one of the most energy efficient and economic methods [101, 166, 228]. Ezeji et al. [69, 70] showed that gas-stripping efficiently lowers the solvent concentration in the reactor, leading to a 200% improved solvent productivity and 118% improved yield. Friedl [82] suggested the in-line recovery for gas stripping in an external loop as it offers easier optimization of the recovery rate compared to in situ recovery.
Liquid–liquid extraction recovery is realized using an extracting solvent showing a miscibility gap with water and high affinity to the product [82]. The advantages of this method are high capacity and selectivity. However, the design of the extraction process can be complex and expensive to perform [100, 161]. Implementation of in situ liquid–liquid extraction requires a non-toxic extraction solvent [58]. The most recommended non-toxic extraction solvent for in situ recovery in an ABE fermentation is oleyl alcohol [17, 60, 233, 283, 339]. The currently known extraction solvents are applicable, but not ideal in performance, making the choice of the extraction solvent a challenging and ongoing research topic [82].
Of the membrane techniques for solvent recovery, perstraction and pervaporation are the two most promising ones. Perstraction is an expansion of liquid–liquid extraction. The separation of cell broth and extracting solvent via a suitable membrane eliminates the problem of extraction of solvent toxicity and emulsion development [58, 82].
Pervaporation or so called “membrane distillation”, is claimed to be commercially competitive and the best-developed method for in situ solvent removal [21, 82, 121, 226, 305]. Hydrophobic polymeric membranes allow solvents to selectively permeate from the liquid fermentation broth on one membrane site into the gas phase on the other membrane site [305]. The membranes possess a higher affinity to organic solvents, leading to high fluxes and a fast sorption of the organic compounds [305]. The driving force of pervaporation is the difference of vapor pressure between the feed and permeate side [21, 82, 305]. The difference is typically introduced by the application of a vacuum or sweep gas on the permeate side of the membrane [305]. PDMS (polydimethylsiloxane [121, 305], and POMS (polyoctylmethylsilixane [156], are typical used polymers for the pervaporation membranes. For more information on the membrane material, the reader is pointed to Huang et al. [111].
For the in situ implementation of membrane techniques, the membranes need to be mounted inside the reactor. While successfully implemented in lab-scale, the design and scale-up are quite complicated [82]. Common problems of membranes such as fouling and clogging can lead to operational problems since there is no possibility for cleaning when used in situ [58, 82]. The disadvantages of membrane techniques such as high price, limitation of diffusion and fouling problems constitute an obstacle for the implementation of perstraction and pervaporation on an industrial scale [58, 166].
Adsorption is an effective, energy-efficient, and easy to operate separation technique [64, 82, 230]. It has been investigated in several process mode combinations and showed to reduce the inhibiting product concentration [99, 175, 214, 215, 254, 329, 330]. The solvent recovery by adsorption of the fermentation broth can be operated continuously and is carried out in two steps: First, the alcohol is taken up by the adsorbent until maximum loading is obtained. Subsequently, the adsorbent is regenerated to obtain a concentrated butanol solution [306, 325]. Regeneration is accomplished by temperature increase or by reduction of the pressure [82]. For continuous mode, more than one column with adsorption material is needed [82]. Depending on the material, adsorption offers the possibility for selective removal of solvents in a gaseous, vapor or liquid mixture and can also be used with other separation methods to reduce the water content of the concentrated product [82]. Typical adsorption materials are hydrophobic activated carbon, zeolites and polymeric (ion-exchange) resins [82, 111, 325]. According to Abdehagh et al. [1], activated carbon F-400 is the best butanol adsorbent with the highest adsorption capacity, while Friedl [82] pointed out that zeolites are already successfully used in industrial plants for ethanol dehydration. A disadvantage of adsorption for the integration into a fermentation process is the problem of nutrient fouling, which requires the pre-separation with micro- or ultrafiltration before recovering the solvents by adsorption [82]. Depending on the material, adsorption suffers from low selectivity, high resin prices and physical instability [58, 166]. Therefore, the performance of adsorption needs to be evaluated on an industrial scale [82].
Each integrated product recovery method has its benefits and drawbacks [166]. The main target for implementation in the solvent producing industry is the energy-efficiency of the separation method. To minimize the costs and increase the productivity, the recovery step needs to be operable in continuous mode without interferences.
While especially required in fed-batch processes where product inhibition is limiting the productivity, these integrated product recovery strategies have also been applied to continuous processes and systems using cell immobilization [30, 70, 73, 100, 182, 325, 330].
Most frequently used recovery methods in lab-scale are gas stripping and pervaporation, implemented in several investigations [31, 73, 74, 162, 182, 226, 248, 251, 268, 305]. For example, Lienhardt et al. [168] used a continuous biofilm reactor with Clostridium beijerinckii BA101 cells adsorbed onto clay bricks fed with glucose as substrate. The reactor effluent was recycled after the removal of butanol by pervaporation, lowering butanol toxicity while retaining the intermediate acids in the effluent. At a dilution rate of 2.0 h−1, Lienhardt et al. [168] obtained complete sugar utilization with a productivity of 10.2 g−1 L−1 h−1. In industry, combinations of two recovery methods are also applicable, e.g. the combination of liquid–liquid extraction of butanol with oleyl alcohol coupled with gas stripping (patented by Butamax Advanced Biofuels LLC [93]).
One example for successful application of in situ product recovery to increase titer, rate and yield metrics in a continuous fermentation process relied on pervaporation and showed a significant increase of the substrate consumption rate, solvent productivity, and yield by 58% (2.02 g L−1 h−1), 81% (0.75 g L−1 h−1) and 15% (0.38 g g−1), respectively [162]. Using cassava-derived glucose with Clostridium acetobutylicum DP217, final ABE and butanol titers of 574.3 g L−1 and 501.1 g L−1, respectively, were obtained [162]. In a recent study, systems biology tools enabled the engineering of C. acetobutylicum and achieved a stable, highly selective, and high yield butanol production of 0.35 g g−1, which corresponds to 84% of the theoretical maximum [210]. Using the strain in a continuous high cell density cultivation combined with in situ product recovery, a butanol titer of 550 g L−1 was achieved in the recovered product stream, comparable to solvent levels in traditional ethanol plants [210]. The implementation of an integrated product recovery method into an optimized continuous process has therefore been shown to increase the final titer and consequently the economic competitiveness for industrial production.
Industrial application
A look in the past shows the development of industrial applications of solvent production with clostridia. In the 1970s the oil crisis led to a revival of the historical Weizmann process, which was initially established in 1915 during the First World War but with rising substrate prices of molasses, maize, or wheat the ABE process was no longer economically viable [58, 312]. In 2006, DuPont and British Petroleum (BP) announced their cooperation for the reinstallation of new industrial ABE plants, once again leading to an increased research interest in the topic of ABE processing [312]. At the same time, gas fermentation technology using acetogens received increasing attention and has been developed towards industrial implementation. Since then, many plants and projects launched the production of butanol, acetone, and ethanol with solventogenic and acetogenic clostridia from various feedstocks but several needed modernizations or were closed due to economic pressure [211, 326]. In the following, an overview of the recent companies in the field of ABE fermentation and gas fermentation is shown.
Traditional ABE fermentation
Some of the major companies for the industrial ABE process with solventogenic clostridia are Butamax Advanced Biofuels, ButylFuel LLC, Celtic Renewables Ltd and Cathay Industrial Biotech. Companies working on the process development for biobutanol production are Tetravitae Bioscience and METabolic EXplorer [2].
Butamax Advanced Biofuels (US), a joint venture of BP and DuPont, currently operates a biobutanol plant in Lamberton, Minnesota with a capacity to produce 30,000 tons butanol annually from lignocellulosic feedstocks. In addition, Butamax operates a demonstration facility in Hull (UK) and a small-scale unit in Delaware (US), which is using corn and sugar as feedstock. Since no details of the process have been released, it was assumed that Butamax relies on a traditional process using C. beijerinckii.[2, 29, 58, 211]
ButylFuel LLC (US) created a patented two-stage fermentation process with C. tyrobutyricum and C. acetobutylicum (see section “Multi-stage systems”) and uses forest residues, temperate grasses and crop residuals as feedstock for butanol production [58, 244].
Celtic Renewables Ltd (UK) was formed in 2012. In 2017, the company announced the construction of a commercial demonstration plant in Grangemouth, UK for over 500,000 L of biofuel annually. Originally developed and established in 2007 at the Edinburgh Napier University, the ABE process of Celtic Renewables uses a Clostridium sp. able to convert xylose, arabinose and glucose into butanol, ethanol, and acetone. As feedstocks, the whisky by-products pot ale and draff are used, pretreated by thermal hydrolysis. Additionally, solid residues and cell biomass generated during the ABE process is sold as high-grade animal feed [34].
Two former biobutanol companies are Green Biologics Ltd. (UK) and Cobalt Technologies Biofuels (USA). Cobalt Technologies has been operating a pilot plant production for 20,000 L of butanol annually until bankruptcy in 2015 [2, 155]. Green Biologics Ltd. developed a new fermentation process with genetically modified strains and started a commercial butanol production in 2017, aiming to produce from sugar and agricultural waste. Unfortunately, they ran out of money in October 2019 and now offer their knowledge under the name ‘Biocleave Limited’ [24, 94].
Gas fermentation
Three companies are known for their gas fermentation technology and pilot plants: INEOS Bio, Coskata Inc. and LanzaTech. However, only LanzaTech prevailed and has now implemented its technology in a commercial plant at scale [275].
Coskata Inc. was originally founded in 2006 in cooperation with the University of Oklahoma. For the process, methane was reformed into syngas and fermented to ethanol, presumably using HFMBR technology [107, 275]. Coskata was operating a gas fermentation plant for ethanol production from 2009 to 2011 (capacity of 118 t a–1) in Pennsylvania, which was shut down due to financial insolvency in 2015. The Coskata technology was acquired by Synata Bio in 2016 [107, 275].
INEOS Bio was established in 2008, because of the takeover by Bioengineering Resources Inc., which was founded by the gas fermentation pioneer James L. Gaddy [11, 170, 275]. INEOS Bio was one of the first companies, implementing gas fermentation at an industrial scale [149, 275]. In 2012 they started operating a semi-commercial biorefinery plant in Florida, aiming a bioethanol production of 8 million gallons per year (Mgy), produced from syngas, generated from lignocellulosic biomass and municipal waste [241, 275]. The presence of the inhibitor hydrogen cyanide in the feed gas led to severe problems with the syngas fermentation process in 2013. In consequence, the plant was shut down in December 2014 [107, 275]. In 2017 the plant was sold to Alliance Bio-Products Inc. During the same time, INEOS Bio was purchased by Jupeng Bio, Inc. (Texas, US). Jupeng Bio claims to be the first company worldwide introducing large scale cellulosic bioethanol in 2013. For their syngas fermentation they gasify mainly biomass material and waste material [136].
LanzaTech based in Illinois (US) was founded by the gas fermentation pioneers Sean D. Simpson and Richard Forster in 2005 [275]. As one of the first, LanzaTech managed to establish a profitable, stable, and continuous gas fermentation process using syngas for selective ethanol production [107, 108, 147, 149, 275]. Their process was initially extensively tested in a pilot plant in Glenbrook, New Zealand, using steel mill exhaust gases for ethanol production with a proprietary C. autoethanogenum strain [48, 108]. LanzaTech set up two gas fermentation demonstration plants in cooperation with the large Chinese steel manufacturers BaoSteel and Shougang (capacity 300 Mt a–1 ethanol) and now aims to construct numerous commercial plants worldwide [107, 275]. In cooperation with Aemetis, LanzaTech build a plant for biogenic syngas in California, targeting the gasification of non-recyclable MSW, agricultural and forestry waste [139, 149, 275]. With their strong international network, LanzaTech continues to advance gas fermentation technology and to expand to additional products [275]. In addition, LanzaTech has a broad patent portfolio to secure its intellectual property in process technology and strain development and is ready to implement production of chemicals such as 2,3-butanediol, butanol, butadiene and acetone in commercial plants [149, 275].
Prospects
In this review, we show that the intelligent connection of bioprocess technology and strain engineering tools complemented by newly gained knowledge from systems biology studies is the ideal way towards highly efficient fermentation processes for industrial solvent production, competitive to non-sustainable fuel and solvent industry [210]. Nevertheless, the industrial implementation of the continuous fermentation process is limited to one example, the LanzaTech process using CO from steel mill off the gas to produce ethanol with C. autoethanogenum.
The development of solutions providing high productivities will be an impetus to establish continuous bioprocesses for economic solvents and bulk chemical production [183]. Moreover, the utilization of alternative low-cost feedstocks such as lignocellulosic and gaseous substrates will increase economic viability. The progress in the understanding and design of strains as well as fermentation strategies with high cell density cultivation using cell retention techniques and the integration of in situ product recovery methods shows an enormous potential for continuous fermentations.
To exploit this potential, a detailed understanding of strain physiology and metabolism under “production conditions” is required. Using systems level analyses and metabolic modeling, targets for strain improvement can be identified, and emerging genome engineering tools allow to rapidly establish phenotype-genotype relationships.
Additionally, the transfer of promising bioprocessing concepts into larger scales requires the development and implementation of process analytical technology (PAT) concepts to obtain suitable monitoring and control strategies. Here, methods like flow cytometry emerge as promising tools to monitor active biomass in “dirty” substrates, e.g. lignocellulose-based hydrolysates containing particles, thus allowing to obtain more solid data in terms of process performance. Furthermore, flow cytometry can increase knowledge on sporulation and cell viability under different conditions. Other process analyzers include spectroscopy methods, but also omics tools to assess culture response to inhibitors and varying feedstock compositions. Finally, the demand for enhanced control promotes the development of models, ranging from simple software sensors and black box models to fully integrated spatiotemporal models.
Following this integrated approach shall ultimately allow to successfully scale-up and implement novel continuous bioprocessing solutions for solvent production or altogether new products using solventogenic and acetogenic clostridia.
Acknowledgements
Open access funding provided by TU Wien (TUW). We would like to thank Christoph Herwig from TU Wien for his helpful input and critical review of the manuscript.
Author contributions
CV: Conceptualization, Writing—original draft, Visualization. CN: Conceptualization, Writing—original draft, Visualization. SP: Conceptualization, Writing—review and editing, Supervision, Project administration, Funding acquisition. All authors have read and approved the final manuscript.
Funding
Open access funding provided by TU Wien (TUW). CN and SP received funding from the Austrian Research Promotion Agency [Grant number # 874503].
Compliance with ethical standards
Conflict of interest
The authors declare that they have no conflict of interest.
Ethical approval
This article does not contain any studies with human participants or animals performed by any of the authors.
Footnotes
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Charlotte Anne Vees and Christian Simon Neuendorf equal contribution.
Contributor Information
Charlotte Anne Vees, Email: charlotte.vees@tuwien.ac.at.
Christian Simon Neuendorf, Email: christian.neuendorf@tuwien.ac.at.
Stefan Pflügl, Email: stefan.pfluegl@tuwien.ac.at.
References
- 1.Abdehagh N, Tezel FH, Thibault J. Adsorbent screening for biobutanol separation by adsorption: kinetics, isotherms and competitive effect of other compounds. Adsorption. 2013;19:1263–1272. doi: 10.1007/s10450-013-9566-8. [DOI] [Google Scholar]
- 2.Abo BO, Gao M, Wu C, Zhu W, Wang Q. A review on characteristics of food waste and their use in butanol production. Rev Environ Health. 2019;34:447–457. doi: 10.1515/reveh-2019-0037. [DOI] [PubMed] [Google Scholar]
- 3.Abrini J, Naveau H, Nyns E-J. Clostridium autoethanogenum, sp. nov., an anaerobic bacterium that produces ethanol from carbon monoxide. Arch Microbiol. 1994;161:345–351. doi: 10.1007/BF00303591. [DOI] [Google Scholar]
- 4.Abubackar HN, Veiga MC, Kennes C. Biological conversion of carbon monoxide: rich syngas or waste gases to bioethanol. Biofuel Bioprod Biorefin. 2011;5:93–114. doi: 10.1002/bbb.256. [DOI] [Google Scholar]
- 5.Afschar AS, Biebl H, Schaller K, Schügerl K. Production of acetone and butanol by Clostridium acetobutylicum in continuous culture with cell recycle. Appl Microbiol Biotechnol. 1985;22:394–398. doi: 10.1007/BF00252779. [DOI] [Google Scholar]
- 6.Ahmed A, Cateni BG, Huhnke RL, Lewis RS. Effects of biomass-generated producer gas constituents on cell growth, product distribution and hydrogenase activity of Clostridium carboxidivorans P7T. Biomass Bioenergy. 2006;30:665–672. doi: 10.1016/j.biombioe.2006.01.007. [DOI] [Google Scholar]
- 7.Almeida Benalcázar E, Noorman H, Maciel Filho R, Posada JA. Modeling ethanol production through gas fermentation: a biothermodynamics and mass transfer-based hybrid model for microbial growth in a large-scale bubble column bioreactor. Biotechnol Biofuels. 2020;13:59. doi: 10.1186/s13068-020-01695-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Amiri H, Karimi K. Improvement of acetone, butanol, and ethanol production from woody biomass using organosolv pretreatment. Bioprocess Biosyst Eng. 2015;38:1959–1972. doi: 10.1007/s00449-015-1437-0. [DOI] [PubMed] [Google Scholar]
- 9.Andrade JC, Vasconcelos I. Continuous cultures of Clostridium acetobutylicum: culture stability and low-grade glycerol utilisation. Biotechnol Lett. 2003;25:121–125. doi: 10.1023/a:1021911217270. [DOI] [PubMed] [Google Scholar]
- 10.Arslan K, Bayar B, Nalakath Abubackar H, Veiga MC, Kennes C. Solventogenesis in Clostridium aceticum producing high concentrations of ethanol from syngas. Bioresour Technol. 2019;292:121941. doi: 10.1016/j.biortech.2019.121941. [DOI] [PubMed] [Google Scholar]
- 11.Asimakopoulos K, Gavala HN, Skiadas IV. Reactor systems for syngas fermentation processes: a review. Chem Eng J. 2018;348:732–744. doi: 10.1016/j.cej.2018.05.003. [DOI] [Google Scholar]
- 12.Badr HR, Toledo R, Hamdy MK. Continuous acetone–ethanol–butanol fermentation by immobilized cells of Clostridium acetobutylicum. Biomass Bioenergy. 2001;20:119–132. doi: 10.1016/s0961-9534(00)00068-4. [DOI] [Google Scholar]
- 13.Bahl H, Andersch W, Braun K, Gottschalk G. Effect of pH and butyrate concentration on the production of acetone and butanol by Clostridium acetobutylicum grown in continuous culture. Appl Microbiol Biotechnol. 1982;14:17–20. doi: 10.1007/BF00507998. [DOI] [Google Scholar]
- 14.Bahl H, Andersch W, Gottschalk G. Continuous production of acetone and butanol by Clostridium acetobutylicum in a two-stage phosphate limited chemostat. Appl Microbiol Biotechnol. 1982;15:201–205. doi: 10.1007/BF00499955. [DOI] [Google Scholar]
- 15.Bankar SB, Jurgens G, Survase SA, Ojamo H, Granström T. Enhanced isopropanol–butanol–ethanol (IBE) production in immobilized column reactor using modified Clostridium acetobutylicum DSM792. Fuel. 2014;136:226–232. doi: 10.1016/j.fuel.2014.07.061. [DOI] [Google Scholar]
- 16.Bankar SB, Survase SA, Ojamo H, Granström T. Biobutanol: the outlook of an academic and industrialist. RSC Adv. 2013;3:24734. doi: 10.1039/c3ra43011a. [DOI] [Google Scholar]
- 17.Bankar SB, Survase SA, Singhal RS, Granström T. Continuous two stage acetone–butanol–ethanol fermentation with integrated solvent removal using Clostridium acetobutylicum B 5313. Bioresour Technol. 2012;106:110–116. doi: 10.1016/j.biortech.2011.12.005. [DOI] [PubMed] [Google Scholar]
- 18.Barbeau JY, Marchal R, Vandecasteele JP. Conditions promoting stability of solventogenesis or culture degeneration in continuous fermentations of Clostridium acetobutylicum. Appl Microbiol Biotechnol. 1988;29:447–455. doi: 10.1007/BF00269067. [DOI] [Google Scholar]
- 19.Bengelsdorf FR, Poehlein A, Linder S, Erz C, Hummel T, Hoffmeister S, Daniel R, Dürre P. Industrial acetogenic biocatalysts: a comparative metabolic and genomic analysis. Front Microbiol. 2016;7:1036. doi: 10.3389/fmicb.2016.01036. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Bertsch J, Müller V. Bioenergetic constraints for conversion of syngas to biofuels in acetogenic bacteria. Biotechnol Biofuels. 2015;8:210. doi: 10.1186/s13068-015-0393-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Bharathiraja B, Jayamuthunagai J, Sudharsanaa T, Bharghavi A, Praveenkumar R, Chakravarthy M, Yuvaraj D. Biobutanol—an impending biofuel for future: a review on upstream and downstream processing tecniques. Renew Sust Energ Rev. 2017;68:788–807. doi: 10.1016/j.rser.2016.10.017. [DOI] [Google Scholar]
- 22.Bi C, Jones SW, Hess DR, Tracy BP, Papoutsakis ET. SpoIIE is necessary for asymmetric division, sporulation, and expression of σF, σE, and σG but does not control solvent production in Clostridium acetobutylicum ATCC 824. J Bacteriol. 2011;193:5130–5137. doi: 10.1128/JB.05474-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Biebl H. Fermentation of glycerol by Clostridium pasteurianum—batch and continuous culture studies. J Ind Microbiol Biotechnol. 2001;27:18–26. doi: 10.1038/sj.jim.7000155. [DOI] [PubMed] [Google Scholar]
- 24.Biocleave (2019) Biocleave Limited. https://biocleave.com
- 25.Bousquet P, Ciais P, Miller JB, Dlugokencky EJ, Hauglustaine DA, Prigent C, Van Der Werf GR, Peylin P, Brunke EG, Carouge C, Langenfelds RL, Lathière J, Papa F, Ramonet M, Schmidt M, Steele LP, Tyler SC, White J. Contribution of anthropogenic and natural sources to atmospheric methane variability. Nature. 2006;443:439–443. doi: 10.1038/nature05132. [DOI] [PubMed] [Google Scholar]
- 26.Branska B, Pechacova Z, Kolek J, Vasylkivska M, Patakova P. Flow cytometry analysis of Clostridium beijerinckii NRRL B-598 populations exhibiting different phenotypes induced by changes in cultivation conditions. Biotechnol Biofuels. 2018;11:99. doi: 10.1186/s13068-018-1096-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Braun K, Gottschalk G. Effect of molecular hydrogen and carbon dioxide on chemo-organotrophic growth of Acetobacterium woodii and Clostridium aceticum. Arch Microbiol. 1981;128:294–298. doi: 10.1007/BF00422533. [DOI] [PubMed] [Google Scholar]
- 28.Bredwell MD, Srivastava P, Worden RM. Reactor design issues for synthesis-gas fermentations. Biotechnol Prog. 1999;15:834–844. doi: 10.1021/bp990108m. [DOI] [PubMed] [Google Scholar]
- 29.Butamax (2020) Butamax® Advanced Biofuels LLC. https://www.butamax.com/
- 30.Cai D, Chen H, Chen C, Hu S, Wang Y, Chang Z, Miao Q, Qin P, Wang Z, Wang J, Tan T. Gas stripping–pervaporation hybrid process for energy-saving product recovery from acetone–butanol–ethanol (ABE) fermentation broth. Chem Eng J. 2016;287:1–10. doi: 10.1016/j.cej.2015.11.024. [DOI] [Google Scholar]
- 31.Cai D, Hu S, Miao Q, Chen C, Chen H, Zhang C, Li P, Qin P, Tan T. Two-stage pervaporation process for effective in situ removal acetone–butanol–ethanol from fermentation broth. Bioresour Technol. 2016;224:380–388. doi: 10.1016/j.biortech.2016.11.010. [DOI] [PubMed] [Google Scholar]
- 32.Cao G, Sheng Y. Biobutanol production from lignocellulosic biomass: prospective and challenges. J Bioremediat Biodegrad. 2016;7:363. doi: 10.4172/2155-6199.1000363. [DOI] [Google Scholar]
- 33.Carlson ED, Papoutsakis ET. Heterologous expression of the Clostridium carboxidivorans CO dehydrogenase alone or together with the acetyl coenzyme A synthase enables both reduction of CO2 and oxidation of CO by Clostridium acetobutylicum. Appl Environ Microbiol. 2017;83:e00829–e01817. doi: 10.1128/AEM.00829-17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Celtic (2020) Celtic renewables. https://www.celtic-renewables.com
- 35.Cerisy T, Rostain W, Chhun A, Boutard M, Salanoubat M, Tolonen AC. A targetron-recombinase system for large-scale genome engineering of clostridia. mSphere. 2019;4:e00710–e00719. doi: 10.1128/mSphere.00710-19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Chang IS, Kim BH, Lovitt RW, Bang JS. Effect of CO partial pressure on cell-recycled continuous CO fermentation by Eubacterium limosum KIST612. Process Biochem. 2001;37:411–421. doi: 10.1016/s0032-9592(01)00227-8. [DOI] [Google Scholar]
- 37.Charubin K, Bennett RK, Fast AG, Papoutsakis ET. Engineering Clostridium organisms as microbial cell-factories: challenges and opportunities. Metab Eng. 2018;50:173–191. doi: 10.1016/j.ymben.2018.07.012. [DOI] [PubMed] [Google Scholar]
- 38.Chen J-S. Alcohol dehydrogenase: multiplicity and relatedness in the solvent-producing clostridia. FEMS Microbiol Rev. 1995;17:263–273. doi: 10.1111/j.1574-6976.1995.tb00210.x. [DOI] [PubMed] [Google Scholar]
- 39.Chen J, Gomez JA, Höffner K, Phalak P, Barton PI, Henson MA. Spatiotemporal modeling of microbial metabolism. BMC Syst Biol. 2016;10:21. doi: 10.1186/s12918-016-0259-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Chen J, Henson MA. In silico metabolic engineering of Clostridium ljungdahlii for synthesis gas fermentation. Metab Eng. 2016;38:389–400. doi: 10.1016/j.ymben.2016.10.002. [DOI] [PubMed] [Google Scholar]
- 41.Cheng C, Bao T, Yang S-T. Engineering Clostridium for improved solvent production: recent progress and perspective. Appl Microbiol Biotechnol. 2019;103:5549–5566. doi: 10.1007/s00253-019-09916-7. [DOI] [PubMed] [Google Scholar]
- 42.Claassen P, Budde M, López-Contreras A. Acetone, butanol and ethanol production from domestic organic waste by solventogenic clostridia. J Mol Microbiol Biotechnol. 2000;2:39–44. [PubMed] [Google Scholar]
- 43.Clarke K, Hansford G. Production of acetone and butanol by Clostridium acetobutylicum in a product–limited chemostat. Chem Eng Commun. 1986;45:75–81. doi: 10.1080/00986448608911373. [DOI] [Google Scholar]
- 44.Contag PR, Burns-Guydish SM, Meerman HJ, Walther DC, Chen JC, Maddox I, Nienow AW (2009) Enhanced ABE fermentation with high yielding butanol tolerant Clostridium strains. GB Patent No. GB2459756A
- 45.Cornillot E, Nair RV, Papoutsakis ET, Soucaille P. The genes for butanol and acetone formation in Clostridium acetobutylicum ATCC 824 reside on a large plasmid whose loss leads to degeneration of the strain. J Bacteriol. 1997;179:5442–5447. doi: 10.1128/jb.179.17.5442-5447.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Dabrock B, Bahl H, Gottschalk G. Parameters affecting solvent production by Clostridium pasteurianum. Appl Environ Microbiol. 1992;58:1233–1239. doi: 10.1128/AEM.58.4.1233-1239.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Dai Z, Dong H, Zhu Y, Zhang Y, Li Y, Ma Y. Introducing a single secondary alcohol dehydrogenase into butanol-tolerant Clostridium acetobutylicum Rh8 switches ABE fermentation to high level IBE fermentation. Biotechnol Biofuels. 2012;5:44. doi: 10.1186/1754-6834-5-44. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Daniell J, Köpke M, Simpson S. Commercial biomass syngas fermentation. Energies. 2012;5:5372–5417. doi: 10.3390/en5125372. [DOI] [Google Scholar]
- 49.Dash S, Khodayari A, Zhou J, Holwerda EK, Olson DG, Lynd LR, Maranas CD. Development of a core Clostridium thermocellum kinetic metabolic model consistent with multiple genetic perturbations. Biotechnol Biofuels. 2017;10:108. doi: 10.1186/s13068-017-0792-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Dash S, Mueller TJ, Venkataramanan KP, Papoutsakis ET, Maranas CD. Capturing the response of Clostridium acetobutylicum to chemical stressors using a regulated genome-scale metabolic model. Biotechnol Biofuels. 2014;7:144. doi: 10.1186/s13068-014-0144-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Dash S, Ng CY, Maranas CD. Metabolic modeling of clostridia: current developments and applications. FEMS Microbiol Lett. 2016;363:fnw004. doi: 10.1093/femsle/fnw004. [DOI] [PubMed] [Google Scholar]
- 52.Devarapalli M, Atiyeh HK, Phillips JR, Lewis RS, Huhnke RL. Ethanol production during semi-continuous syngas fermentation in a trickle bed reactor using Clostridium ragsdalei. Bioresour Technol. 2016;209:56–65. doi: 10.1016/j.biortech.2016.02.086. [DOI] [PubMed] [Google Scholar]
- 53.Devarapalli M, Lewis R, Atiyeh H. Continuous ethanol production from synthesis gas by Clostridium ragsdalei in a trickle-bed reactor. Fermentation. 2017;3:23. doi: 10.3390/fermentation3020023. [DOI] [PubMed] [Google Scholar]
- 54.Díaz VHG, Tost GO. Butanol production from lignocellulose by simultaneous fermentation, saccharification, and pervaporation or vacuum evaporation. Bioresour Technol. 2016;218:174–182. doi: 10.1016/j.biortech.2016.06.091. [DOI] [PubMed] [Google Scholar]
- 55.Dos Santos Vieira CF, Maugeri Filho F, Maciel Filho R, Pinto Mariano A. Acetone-free biobutanol production: past and recent advances in the isopropanol–butanol–ethanol (IBE) fermentation. Bioresour Technol. 2019;287:121425. doi: 10.1016/j.biortech.2019.121425. [DOI] [PubMed] [Google Scholar]
- 56.Drake HL, Daniel SL. Physiology of the thermophilic acetogen Moorella thermoacetica. Res Microbiol. 2004;155:869–883. doi: 10.1016/j.resmic.2004.10.002. [DOI] [PubMed] [Google Scholar]
- 57.Drake HL, Gößner AS, Daniel SL. Old acetogens, new light. Ann NY Acad Sci. 2008;1125:100–128. doi: 10.1196/annals.1419.016. [DOI] [PubMed] [Google Scholar]
- 58.Dürre P. Biobutanol: an attractive biofuel. Biotechnol J. 2007;2:1525–1534. doi: 10.1002/biot.200700168. [DOI] [PubMed] [Google Scholar]
- 59.Dürre P. Butanol formation from gaseous substrates. FEMS Microbiol Lett. 2016;363:fnw040. doi: 10.1093/femsle/fnw040. [DOI] [PubMed] [Google Scholar]
- 60.Dürre P, Bahl H. Microbial production of acetone/butanol/isopropanol. In: Rehm HJ, Reed G, editors. Biotechnology. 2. Weinheim: VCH Verlagsgesellschaft; 1996. pp. 229–268. [Google Scholar]
- 61.Emerson DF, Stephanopoulos G. Limitations in converting waste gases to fuels and chemicals. Curr Opin Biotechnol. 2019;59:39–45. doi: 10.1016/j.copbio.2019.02.004. [DOI] [PubMed] [Google Scholar]
- 62.Ennis BM, Maddox IS. Production of solvents (ABE fermentation) from whey permeate by continuous fermentation in a membrane bioreactor. Bioprocess Eng. 1989;4:27–34. doi: 10.1007/bf00612667. [DOI] [Google Scholar]
- 63.Ennis BM, Marshall CT, Maddox IS, Paterson AHJ. Continuous product recovery by in-situ gas stripping/condensation during solvent production from whey permeate using Clostridium acetobutylicum. Biotechnol Lett. 1986;8:725–730. doi: 10.1007/BF01032571. [DOI] [Google Scholar]
- 64.Eom M-H, Kim W, Lee J, Cho J-H, Seung D, Park S, Lee JH. Modeling of a biobutanol adsorption process for designing an extractive fermentor. Ind Eng Chem Res. 2013;52:603–611. doi: 10.1021/ie301249z. [DOI] [Google Scholar]
- 65.Ezeji T, Li Y. Advanced product recovery technologies. In: Vertes AA, editor. Biomass to biofuels: strategies for global industries. Wiltshire: Wiley; 2010. pp. 331–345. [Google Scholar]
- 66.Ezeji T, Qureshi N, Blaschek H (2005) Process for continuous solvent production. US Patent No. US20050089979A1
- 67.Ezeji T, Qureshi N, Blaschek HP. Butanol production from agricultural residues: impact of degradation products on Clostridium beijerinckii growth and butanol fermentation. Biotechnol Bioeng. 2007;97:1460–1469. doi: 10.1002/bit.21373. [DOI] [PubMed] [Google Scholar]
- 68.Ezeji TC, Groberg M, Qureshi N, Blaschek HP. Continuous production of butanol from starch-based packing peanuts. Appl Biochem Biotechnol. 2003;106:375–382. doi: 10.1385/abab:106:1-3:375. [DOI] [PubMed] [Google Scholar]
- 69.Ezeji TC, Qureshi N, Blaschek HP. Production of acetone, butanol and ethanol by Clostridium beijerinckii BA101 and in situ recovery by gas stripping. World J Microbiol Biotechnol. 2003;19:595–603. doi: 10.1023/a:1025103011923. [DOI] [Google Scholar]
- 70.Ezeji TC, Qureshi N, Blaschek HP. Acetone butanol ethanol (ABE) production from concentrated substrate: reduction in substrate inhibition by fed-batch technique and product inhibition by gas stripping. Appl Microbiol Biotechnol. 2004;63:653–658. doi: 10.1007/s00253-003-1400-x. [DOI] [PubMed] [Google Scholar]
- 71.Ezeji TC, Qureshi N, Blaschek HP. Continuous butanol fermentation and feed starch retrogradation: butanol fermentation sustainability using Clostridium beijerinckii BA101. J Biotechnol. 2005;115:179–187. doi: 10.1016/j.jbiotec.2004.08.010. [DOI] [PubMed] [Google Scholar]
- 72.Ezeji TC, Qureshi N, Blaschek HP. Bioproduction of butanol from biomass: from genes to bioreactors. Curr Opin Biotechnol. 2007;18:220–227. doi: 10.1016/j.copbio.2007.04.002. [DOI] [PubMed] [Google Scholar]
- 73.Ezeji TC, Qureshi N, Blaschek HP. Production of acetone butanol (AB) from liquefied corn starch, a commercial substrate, using Clostridium beijerinckii coupled with product recovery by gas stripping. J Ind Microbiol Biotechnol. 2007;34:771–777. doi: 10.1007/s10295-007-0253-1. [DOI] [PubMed] [Google Scholar]
- 74.Ezeji TC, Qureshi N, Blaschek HP. Microbial production of a biofuel (acetone–butanol–ethanol) in a continuous bioreactor: impact of bleed and simultaneous product removal. Bioprocess Biosyst Eng. 2013;36:109–116. doi: 10.1007/s00449-012-0766-5. [DOI] [PubMed] [Google Scholar]
- 75.Farmanbordar S, Karimi K, Amiri H. Municipal solid waste as a suitable substrate for butanol production as an advanced biofuel. Energy Convers Manag. 2018;157:396–408. doi: 10.1016/j.enconman.2017.12.020. [DOI] [Google Scholar]
- 76.Fast AG, Papoutsakis ET. Stoichiometric and energetic analyses of non-photosynthetic CO2-fixation pathways to support synthetic biology strategies for production of fuels and chemicals. Curr Opin Chem Eng. 2012;1:380–395. doi: 10.1016/j.coche.2012.07.005. [DOI] [Google Scholar]
- 77.Fast AG, Papoutsakis ET. Functional expression of the Clostridium ljungdahlii acetyl-coenzyme A synthase in Clostridium acetobutylicum as demonstrated by a novel in vivo CO exchange activity en route to heterologous installation of a functional Wood–Ljungdahl pathway. Appl Environ Microbiol. 2018;84:e02307–e02317. doi: 10.1128/AEM.02307-17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Fast AG, Schmidt ED, Jones SW, Tracy BP. Acetogenic mixotrophy: novel options for yield improvement in biofuels and biochemicals production. Curr Opin Biotechnol. 2015;33:60–72. doi: 10.1016/j.copbio.2014.11.014. [DOI] [PubMed] [Google Scholar]
- 79.Fernández-Naveira Á, Abubackar HN, Veiga MC, Kennes C. Carbon monoxide bioconversion to butanol-ethanol by Clostridium carboxidivorans: kinetics and toxicity of alcohols. Appl Microbiol Biotechnol. 2016;100:4231–4240. doi: 10.1007/s00253-016-7389-8. [DOI] [PubMed] [Google Scholar]
- 80.Fernández-Naveira Á, Veiga MC, Kennes C. H–B–E (hexanol–butanol–ethanol) fermentation for the production of higher alcohols from syngas/waste gas. J Chem Technol Biotechnol. 2017;92:712–731. doi: 10.1002/jctb.5194. [DOI] [Google Scholar]
- 81.Ferras E, Minier M, Goma G. Acetonobutylic fermentation: Improvement of performances by coupling continuous fermentation and ultrafiltration. Biotechnol Bioeng. 1986;28:523–533. doi: 10.1002/bit.260280408. [DOI] [PubMed] [Google Scholar]
- 82.Friedl A. Downstream process options for the ABE fermentation. FEMS Microbiol Lett. 2016;363:fnw073. doi: 10.1093/femsle/fnw073. [DOI] [PubMed] [Google Scholar]
- 83.Gaddy JL (2000) Biological production of ethanol from waste gases with Clostridium ljungdahlii. US Patent No. US6136577A
- 84.Gaddy JL, Arora DK, Ko C-W, Phillips JR, Basu R, Wilkstrom CV, Clausen EC (2014) Methods for increasing the production of ethanol from microbial fermentation. US Patent No. US8642302B2
- 85.Galanakis C. Food waste valorization opportunities for different food industries. In: Galanakis C, editor. The interaction of food industry and environment. New York: Academic Press; 2020. pp. 341–422. [Google Scholar]
- 86.Gallazzi A, Branska B, Marinelli F, Patakova P. Continuous production of n-butanol by Clostridium pasteurianum DSM 525 using suspended and surface-immobilized cells. J Biotechnol. 2015;216:29–35. doi: 10.1016/j.jbiotec.2015.10.008. [DOI] [PubMed] [Google Scholar]
- 87.Gao K, Boiano S, Marzocchella A, Rehmann L. Cellulosic butanol production from alkali-pretreated switchgrass (Panicum virgatum) and phragmites (Phragmites australis) Bioresour Technol. 2014;174:176–181. doi: 10.1016/j.biortech.2014.09.152. [DOI] [PubMed] [Google Scholar]
- 88.Gapes JR, Nimcevic D, Friedl A. Long-term continuous cultivation of Clostridium beijerinckii in a two-stage chemostat with on-line solvent removal. Appl Environ Microbiol. 1996;62:3210–3219. doi: 10.1128/aem.62.9.3210-3219.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Gildemyn S, Molitor B, Usack JG, Nguyen M, Rabaey K, Angenent LT. Upgrading syngas fermentation effluent using Clostridium kluyveri in a continuous fermentation. Biotechnol Biofuels. 2017;10:83. doi: 10.1186/s13068-017-0764-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Girbal L. How neutral red modified carbon and electron flow in Clostridium acetobutylicum grown in chemostat culture at neutral pH. FEMS Microbiol Rev. 1995;16:151–162. doi: 10.1016/0168-6445(94)00056-5. [DOI] [Google Scholar]
- 91.Godin C, Engasser JM. Two-stage continuous fermentation of Clostridium acetobutylicum: effects of pH and dilution rate. Appl Microbiol Biotechnol. 1990;33:269–273. doi: 10.1007/BF00164520. [DOI] [Google Scholar]
- 92.Gottumukkala LD, Haigh K, Görgens J. Trends and advances in conversion of lignocellulosic biomass to biobutanol: microbes, bioprocesses and industrial viability. Renew Sustain Energ Rev. 2017;76:963–973. doi: 10.1016/j.rser.2017.03.030. [DOI] [Google Scholar]
- 93.Grady MC, Jahic M, Patnaik R (2009) A method for producing butanol using two-phase extractive fermentation. Patent No. WO2009149270A3
- 94.Green (2020) Green Biologics Limited. https://greenbiologics.com
- 95.Green EM. Fermentative production of butanol—the industrial perspective. Curr Opin Biotechnol. 2011;22:337–343. doi: 10.1016/j.copbio.2011.02.004. [DOI] [PubMed] [Google Scholar]
- 96.Grethlein AJ, Jain MK. Bioprocessing of coal-derived synthesis gases by anaerobic bacteria. Trends Biotechnol. 1992;10:418–423. doi: 10.1016/0167-7799(92)90290-c. [DOI] [Google Scholar]
- 97.Grimmler C, Janssen H, Krauβe D, Fischer R-J, Bahl H, Dürre P, Liebl W, Ehrenreich A. Genome-wide gene expression analysis of the switch between acidogenesis and solventogenesis in continuous cultures of Clostridium acetobutylicum. J Mol Microbiol Biotechnol. 2011;20:1–15. doi: 10.1159/000320973. [DOI] [PubMed] [Google Scholar]
- 98.Groher A, Weuster-Botz D. Comparative reaction engineering analysis of different acetogenic bacteria for gas fermentation. J Biotechnol. 2016;228:82–94. doi: 10.1016/j.jbiotec.2016.04.032. [DOI] [PubMed] [Google Scholar]
- 99.Groot WJ, Luyben KCAM. In situ product recovery by adsorption in the butanol/isopropanol batch fermentation. Appl Microbiol Biotechnol. 1986;25:29–31. doi: 10.1007/BF00252508. [DOI] [Google Scholar]
- 100.Groot WJ, Soedjak HS, Donck PB, Lans RGJM, Luyben KCAM, Timmer JMK. Butanol recovery from fermentations by liquid-liquid extraction and membrane solvent extraction. Bioprocess Eng. 1990;5:203–216. doi: 10.1007/bf00376227. [DOI] [Google Scholar]
- 101.Groot WJ, Van Der Lans RGJM, Luyben KCAM. Technologies for butanol recovery integrated with fermentations. Process Biochem. 1992;27:61–75. doi: 10.1016/0032-9592(92)80012-r. [DOI] [Google Scholar]
- 102.Guan W, Shi S, Tu M, Lee YY. Acetone–butanol–ethanol production from Kraft paper mill sludge by simultaneous saccharification and fermentation. Bioresour Technol. 2016;200:713–721. doi: 10.1016/j.biortech.2015.10.102. [DOI] [PubMed] [Google Scholar]
- 103.Guo T, Sun B, Jiang M, Wu H, Du T, Tang Y, Wei P, Ouyang P. Enhancement of butanol production and reducing power using a two-stage controlled-pH strategy in batch culture of Clostridium acetobutylicum XY16. World J Microbiol Biotechnol. 2012;28:2551–2558. doi: 10.1007/s11274-012-1063-9. [DOI] [PubMed] [Google Scholar]
- 104.Haas T, Krause R, Weber R, Demler M, Schmid G. Technical photosynthesis involving CO2 electrolysis and fermentation. Nat Catal. 2018;1:32–39. doi: 10.1038/s41929-017-0005-1. [DOI] [Google Scholar]
- 105.Hartmanis MG, Gatenbeck S. Intermediary metabolism in Clostridium acetobutylicum: levels of enzymes involved in the formation of acetate and butyrate. Appl Environ Microbiol. 1984;47:1277–1283. doi: 10.1128/AEM.47.6.1277-1283.1984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.Heffernan JK, Valgepea K, de Souza Pinto Lemgruber R, Casini I, Plan M, Tappel R, Simpson SD, Köpke M, Nielsen LK, Marcellin E. Enhancing CO2-valorization using Clostridium autoethanogenum for sustainable fuel and chemicals production. Front Bioeng Biotechnol. 2020;8:204. doi: 10.3389/fbioe.2020.00204. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.Heijstra BD, Leang C, Juminaga A. Gas fermentation: cellular engineering possibilities and scale up. Microb Cell Fact. 2017;16:60. doi: 10.1186/s12934-017-0676-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108.Hoffmeister S, Gerdom M, Bengelsdorf FR, Linder S, Flüchter S, Öztürk H, Blümke W, May A, Fischer R-J, Bahl H, Dürre P. Acetone production with metabolically engineered strains of Acetobacterium woodii. Metab Eng. 2016;36:37–47. doi: 10.1016/j.ymben.2016.03.001. [DOI] [PubMed] [Google Scholar]
- 109.Holt RA, Stephens GM, Morris JG. Production of solvents by Clostridium acetobutylicum cultures maintained at neutral pH. Appl Environ Microbiol. 1984;48:1166–1170. doi: 10.1128/AEM.48.6.1166-1170.1984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110.Horinouchi T, Maeda T, Furusawa C. Understanding and engineering alcohol-tolerant bacteria using OMICS technology. World J Microbiol Biotechnol. 2018;34:157. doi: 10.1007/s11274-018-2542-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111.Huang H-J, Ramaswamy S, Liu Y. Separation and purification of biobutanol during bioconversion of biomass. Sep Purif Technol. 2014;132:513–540. doi: 10.1016/j.seppur.2014.06.013. [DOI] [Google Scholar]
- 112.Huang H, Chai C, Yang S, Jiang W, Gu Y. Phage serine integrase-mediated genome engineering for efficient expression of chemical biosynthetic pathway in gas-fermenting Clostridium ljungdahlii. Metab Eng. 2019;52:293–302. doi: 10.1016/j.ymben.2019.01.005. [DOI] [PubMed] [Google Scholar]
- 113.Huang H, Singh V, Qureshi N. Butanol production from food waste: a novel process for producing sustainable energy and reducing environmental pollution. Biotechnol Biofuels. 2015;8:147. doi: 10.1186/s13068-015-0332-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114.Huang W-C, Ramey DE, Yang S-T (2004) Continuous Production of Butanol by Clostridium acetobutylicum Immobilized in a Fibrous Bed Bioreactor. Paper presented at the biotechnology for fuels and chemicals held May 4–7, 2003, Breckenridge, CO [DOI] [PubMed]
- 115.Huhnke RL, Lewis RS, Tanner RS (2006) Isolation and characterization of novel clostridial species. US Patent No. US7704723B2
- 116.Humphreys CM, Minton NP. Advances in metabolic engineering in the microbial production of fuels and chemicals from C1 gas. Curr Opin Biotechnol. 2018;50:174–181. doi: 10.1016/j.copbio.2017.12.023. [DOI] [PubMed] [Google Scholar]
- 117.Ibrahim MF, Ramli N, Kamal Bahrin E, Abd-Aziz S. Cellulosic biobutanol by clostridia: challenges and improvements. Renew Sustain Energy Rev. 2017;79:1241–1254. doi: 10.1016/j.rser.2017.05.184. [DOI] [Google Scholar]
- 118.Ingle P, Groothuis D, Rowe P, Huang H, Cockayne A, Kuehne SA, Jiang W, Gu Y, Humphreys CM, Minton NP. Generation of a fully erythromycin-sensitive strain of Clostridioides difficile using a novel CRISPR-Cas9 genome editing system. Sci Rep. 2019;9:8123. doi: 10.1038/s41598-019-44458-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 119.Islam MA, Zengler K, Edwards EA, Mahadevan R, Stephanopoulos G. Investigating Moorella thermoacetica metabolism with a genome-scale constraint-based metabolic model. Integr Biol. 2015;7:869–882. doi: 10.1039/C5IB00095E. [DOI] [PubMed] [Google Scholar]
- 120.Isom CE, Nanny MA, Tanner RS. Improved conversion efficiencies for n-fatty acid reduction to primary alcohols by the solventogenic acetogen “Clostridium ragsdalei”. J Ind Microbiol Biotechnol. 2015;42:29–38. doi: 10.1007/s10295-014-1543-z. [DOI] [PubMed] [Google Scholar]
- 121.Izák P, Schwarz K, Ruth W, Bahl H, Kragl U. Increased productivity of Clostridium acetobutylicum fermentation of acetone, butanol, and ethanol by pervaporation through supported ionic liquid membrane. Appl Microbiol Biotechnol. 2008;78:597–602. doi: 10.1007/s00253-008-1354-0. [DOI] [PubMed] [Google Scholar]
- 122.Jain MK, Beacom D, Datta R (1993) Mutant strain of C. acetobutylicum and process for making butanol. US Patent No. US5192673A
- 123.Jang Y-S, Lee J, Malaviya A, Seung DY, Cho JH, Lee SY. Butanol production from renewable biomass: rediscovery of metabolic pathways and metabolic engineering. Biotechnol J. 2012;7:186–198. doi: 10.1002/biot.201100059. [DOI] [PubMed] [Google Scholar]
- 124.Jang Y-S, Lee JY, Lee J, Park JH, Im JA, Eom M-H, Lee J, Lee S-H, Song H, Cho J-H, Seung DY, Lee SY. Enhanced butanol production obtained by reinforcing the direct butanol-forming route in Clostridium acetobutylicum. mBio. 2012;3:e00314-2. doi: 10.1128/mbio.00314-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 125.Jang Y-S, Malaviya A, Lee SY. Acetone–butanol–ethanol production with high productivity using Clostridium acetobutylicum BKM19. Biotechnol Bioeng. 2013;110:1646–1653. doi: 10.1002/bit.24843. [DOI] [PubMed] [Google Scholar]
- 126.Janssen H, Döring C, Ehrenreich A, Voigt B, Hecker M, Bahl H, Fischer R-J. A proteomic and transcriptional view of acidogenic and solventogenic steady-state cells of Clostridium acetobutylicum in a chemostat culture. Appl Microbiol Biotechnol. 2010;87:2209–2226. doi: 10.1007/s00253-010-2741-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 127.Janssen H, Grimmler C, Ehrenreich A, Bahl H, Fischer R-J. A transcriptional study of acidogenic chemostat cells of Clostridium acetobutylicum—Solvent stress caused by a transient n-butanol pulse. J Biotechnol. 2012;161:354–365. doi: 10.1016/j.jbiotec.2012.03.027. [DOI] [PubMed] [Google Scholar]
- 128.Jeong J, Bertsch J, Hess V, Choi S, Choi I-G, Chang IS, Müller V. Energy conservation model based on genomic and experimental analyses of a carbon monoxide-utilizing, butyrate-forming acetogen, Eubacterium limosum KIST612. Appl Environ Microbiol. 2015;81:4782–4790. doi: 10.1128/AEM.00675-15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 129.Jesse TW, Ezeji TC, Qureshi N, Blaschek HP. Production of butanol from starch-based waste packing peanuts and agricultural waste. J Ind Microbiol Biotechnol. 2002;29:117–123. doi: 10.1038/sj.jim.7000285. [DOI] [PubMed] [Google Scholar]
- 130.Jiang M, Chen J-N, He A-Y, Wu H, Kong X-P, Liu J-L, Yin C-Y, Chen W-F, Chen P. Enhanced acetone/butanol/ethanol production by Clostridium beijerinckii IB4 using pH control strategy. Process Biochem. 2014;49:1238–1244. doi: 10.1016/j.procbio.2014.04.017. [DOI] [Google Scholar]
- 131.Jiao S, Zhang Y, Wan C, Lv J, Du R, Zhang R, Han B. Transcriptional analysis of degenerate strain Clostridium beijerinckii DG-8052 reveals a pleiotropic response to CaCO3-associated recovery of solvent production. Sci Rep. 2016;6:38818. doi: 10.1038/srep38818. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 132.Jones DT, Shirley M, Wu X, Keis S. Bacteriophage infections in the industrial acetone butanol (AB) fermentation process. J Mol Microbiol Biotechnol. 2000;2:21–26. [PubMed] [Google Scholar]
- 133.Jones DT, Woods DR. Acetone–butanol fermentation revisited. Microbiol Rev. 1986;50:484–524. doi: 10.1128/MMBR.50.4.484-524.1986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 134.Jones SW, Fast AG, Carlson ED, Wiedel CA, Au J, Antoniewicz MR, Papoutsakis ET, Tracy BP. CO2 fixation by anaerobic non-photosynthetic mixotrophy for improved carbon conversion. Nat Commun. 2016;7:12800. doi: 10.1038/ncomms12800. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 135.Joseph RC, Kim NM, Sandoval NR. Recent developments of the synthetic biology toolkit for Clostridium. Front Microbiol. 2018;9:154. doi: 10.3389/fmicb.2018.00154. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 136.Jupeng (2017) Jupeng Bio Inc. https://www.jupengbio.com
- 137.Jurgens G, Survase S, Berezina O, Sklavounos E, Linnekoski J, Kurkijärvi A, Väkevä M, Van Heiningen A, Granström T. Butanol production from lignocellulosics. Biotechnol Lett. 2012;34:1415–1434. doi: 10.1007/s10529-012-0926-3. [DOI] [PubMed] [Google Scholar]
- 138.Kantzow C, Mayer A, Weuster-Botz D. Continuous gas fermentation by Acetobacterium woodii in a submerged membrane reactor with full cell retention. J Biotechnol Biomater. 2015;212:11–18. doi: 10.1016/j.jbiotec.2015.07.020. [DOI] [PubMed] [Google Scholar]
- 139.Karlson B, Bellavitis C, France N. Commercializing LanzaTech, from waste to fuel: an effectuation case. JMO. 2018 doi: 10.1017/jmo.2017.83. [DOI] [Google Scholar]
- 140.Karmann S, Follonier S, Egger D, Hebel D, Panke S, Zinn M. Tailor-made PAT platform for safe syngas fermentations in batch, fed-batch and chemostat mode with Rhodospirillum rubrum. Microb Biotechnol. 2017;10:1365–1375. doi: 10.1111/1751-7915.12727. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 141.Kashket ER, Cao Z-Y. Clostridial strain degeneration. FEMS Microbiol Rev. 1995;17:307–315. doi: 10.1111/j.1574-6976.1995.tb00214.x. [DOI] [Google Scholar]
- 142.Kashket ER, Cao ZY. Isolation of a degeneration-resistant mutant of Clostridium acetobutylicum NCIMB 8052. Appl Environ Microbiol. 1993;59:4198–4202. doi: 10.1128/AEM.59.12.4198-4202.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 143.Keis S, Shaheen R, Jones DT. Emended descriptions of Clostridium acetobutylicum and Clostridium beijerinckii, and descriptions of Clostridium saccharoperbutylacetonicum sp. nov. and Clostridium saccharobutylicum sp. nov. Int J Syst Evol Microbiol. 2001;51:2095–2103. doi: 10.1099/00207713-51-6-2095. [DOI] [PubMed] [Google Scholar]
- 144.Khedkar MA, Nimbalkar PR, Gaikwad SG, Chavan PV, Bankar SB. Sustainable biobutanol production from pineapple waste by using Clostridium acetobutylicum B 527: drying kinetics study. Bioresour Technol. 2017;225:359–366. doi: 10.1016/j.biortech.2016.11.058. [DOI] [PubMed] [Google Scholar]
- 145.Kheyrandish M, Asadollahi MA, Jeihanipour A, Doostmohammadi M, Rismani-Yazdi H, Karimi K. Direct production of acetone–butanol–ethanol from waste starch by free and immobilized Clostridium acetobutylicum. Fuel. 2015;142:129–133. doi: 10.1016/j.fuel.2014.11.017. [DOI] [Google Scholar]
- 146.Klask C-M, Kliem-Kuster N, Molitor B, Angenent LT. An open-source multiple-bioreactor system for replicable gas-fermentation experiments: Nitrate feed results in stochastic inhibition events, but improves ethanol production of Clostridium ljungdahlii with CO2 and H2. Cold Spring Harb Lab. 2019 doi: 10.1101/2019.12.15.877050. [DOI] [Google Scholar]
- 147.Köpke M, Mihalcea C, Bromley JC, Simpson SD. Fermentative production of ethanol from carbon monoxide. Curr Opin Biotechnol. 2011;22:320–325. doi: 10.1016/j.copbio.2011.01.005. [DOI] [PubMed] [Google Scholar]
- 148.Köpke M, Mihalcea C, Liew F, Tizard JH, Ali MS, Conolly JJ, Al-Sinawi B, Simpson SD. 2,3-butanediol production by acetogenic bacteria, an alternative route to chemical synthesis, using industrial waste gas. Appl Environ Microbiol. 2011;77:5467–5475. doi: 10.1128/AEM.00355-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 149.Köpke M, Simpson SD. Pollution to products: recycling of ‘above ground’ carbon by gas fermentation. Curr Opin Biotechnol. 2020;65:180–189. doi: 10.1016/j.copbio.2020.02.017. [DOI] [PubMed] [Google Scholar]
- 150.Kourkoutas Y, Bekatorou A, Banat IM, Marchant R, Koutinas AA. Immobilization technologies and support materials suitable in alcohol beverages production: a review. Food Microbiol. 2004;21:377–397. doi: 10.1016/j.fm.2003.10.005. [DOI] [Google Scholar]
- 151.Kovács K, Willson BJ, Schwarz K, Heap JT, Jackson A, Bolam DN, Winzer K, Minton NP. Secretion and assembly of functional mini-cellulosomes from synthetic chromosomal operons in Clostridium acetobutylicum ATCC 824. Biotechnol Biofuels. 2013;6:117. doi: 10.1186/1754-6834-6-117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 152.Kujawska A, Kujawski J, Bryjak M, Kujawski W. ABE fermentation products recovery methods—a review. Renew Sustain Energ Rev. 2015;48:648–661. doi: 10.1016/j.rser.2015.04.028. [DOI] [Google Scholar]
- 153.Kundiyana DK, Huhnke RL, Wilkins MR. Effect of nutrient limitation and two-stage continuous fermentor design on productivities during “Clostridium ragsdalei” syngas fermentation. Bioresour Technol. 2011;102:6058–6064. doi: 10.1016/j.biortech.2011.03.020. [DOI] [PubMed] [Google Scholar]
- 154.Lai M-C, Traxler RW. A coupled two-stage continuous fermentation for solvent production by Clostridium acetobutylicum. Enzyme and Microb Technol. 1994;16:1021–1025. doi: 10.1016/0141-0229(94)90136-8. [DOI] [Google Scholar]
- 155.Lane J (2015 ) RIP cobalt technologies or…How Commercializing Butanol Technology is like riding the Tour de France. Biofuels Digest. https://www.biofuelsdigest.com/bdigest/2015/06/24/rip-cobalt-technologies-orhow-commercializing-butanol-technology-is-like-riding-the-tour-de-france/. Accessed 18 May 2020
- 156.Lazarova M, Bösch P, Friedl A. POMS membrane for selective separation of ethanol from dilute alcohol-aqueous solutions by pervaporation. Sep Sci Technol. 2012;47:1709–1714. doi: 10.1080/01496395.2012.658943. [DOI] [Google Scholar]
- 157.Lee J, Yun H, Feist AM, Palsson BØ, Lee SY. Genome-scale reconstruction and in silico analysis of the Clostridium acetobutylicum ATCC 824 metabolic network. Appl Microbiol Biotechnol. 2008;80:849–862. doi: 10.1007/s00253-008-1654-4. [DOI] [PubMed] [Google Scholar]
- 158.Lee JY, Jang Y-S, Lee J, Papoutsakis ET, Lee SY. Metabolic engineering of Clostridium acetobutylicum M5 for highly selective butanol production. Biotechnol J. 2009;4:1432–1440. doi: 10.1002/biot.200900142. [DOI] [PubMed] [Google Scholar]
- 159.Lee S-M, Cho MO, Park CH, Chung Y-C, Kim JH, Sang B-I, Um Y. Continuous butanol production using suspended and immobilized Clostridium beijerinckii NCIMB 8052 with supplementary butyrate. Energy Fuels. 2008;22:3459–3464. doi: 10.1021/ef800076j. [DOI] [Google Scholar]
- 160.Lee S, Lee JH, Mitchell RJ. Analysis of Clostridium beijerinckii NCIMB 8052’s transcriptional response to ferulic acid and its application to enhance the strain tolerance. Biotechnol Biofuels. 2015;8:68. doi: 10.1186/s13068-015-0252-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 161.Lee SY, Park JH, Jang SH, Nielsen LK, Kim J, Jung KS. Fermentative butanol production by clostridia. Biotechnol Bioeng. 2008;101:209–228. doi: 10.1002/bit.22003. [DOI] [PubMed] [Google Scholar]
- 162.Li J, Chen X, Qi B, Luo J, Zhang Y, Su Y, Wan Y. Efficient production of acetone–butanol–ethanol (ABE) from cassava by a fermentation–pervaporation coupled process. Bioresour Technol. 2014;169:251–257. doi: 10.1016/j.biortech.2014.06.102. [DOI] [PubMed] [Google Scholar]
- 163.Li Q, Seys FM, Minton NP, Yang J, Jiang Y, Jiang W, Yang S. CRISPR–Cas9 D10A nickase-assisted base editing in the solvent producer Clostridium beijerinckii. Biotechnol Bioeng. 2019;116:1475–1483. doi: 10.1002/bit.26949. [DOI] [PubMed] [Google Scholar]
- 164.Li S-Y, Chiang C-J, Tseng IT, He C-R, Chao Y-P. Bioreactors and in situ product recovery techniques for acetone–butanol–ethanol fermentation. FEMS Microbiol Lett. 2016;363:107. doi: 10.1093/femsle/fnw107. [DOI] [PubMed] [Google Scholar]
- 165.Li S-Y, Srivastava R, Suib SL, Li Y, Parnas RS. Performance of batch, fed-batch, and continuous A–B–E fermentation with pH-control. Bioresour Technol. 2011;102:4241–4250. doi: 10.1016/j.biortech.2010.12.078. [DOI] [PubMed] [Google Scholar]
- 166.Li S, Huang L, Ke C, Pang Z, Liu L. Pathway dissection, regulation, engineering and application: lessons learned from biobutanol production by solventogenic clostridia. Biotechnol Biofuels. 2020;13:39. doi: 10.1186/s13068-020-01674-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 167.Li X, Griffin D, Li X, Henson MA. Incorporating hydrodynamics into spatiotemporal metabolic models of bubble column gas fermentation. Biotechnol Bioeng. 2019;116:28–40. doi: 10.1002/bit.26848. [DOI] [PubMed] [Google Scholar]
- 168.Lienhardt J, Schripsema J, Qureshi N, Blaschek HP. Butanol production by Clostridium beijerinckii BA101 in an immobilized cell biofilm reactor. Appl Biochem Biotechnol. 2002;98–100:591–598. doi: 10.1385/abab:98-100:1-9:591. [DOI] [PubMed] [Google Scholar]
- 169.Liew F, Henstra AM, Kӧpke M, Winzer K, Simpson SD, Minton NP. Metabolic engineering of Clostridium autoethanogenum for selective alcohol production. Metab Eng. 2017;40:104–114. doi: 10.1016/j.ymben.2017.01.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 170.Liew FM, Köpke M, Simpson SD. Gas fermentation for commercial biofuels production. In: Fang Z, editor. Liquid, gaseous and solid biofuels—conversion techniques. Rijeka: InTech; 2013. pp. 125–173. [Google Scholar]
- 171.Liew ST, Arbakariya A, Rosfarizan M, Raha AR. Production of solvent (acetone–butanol–ethanol) in continuous fermentation by Clostridium saccharobutylicum DSM 13864 using gelatinised sago starch as a carbon source. Malays J Microbiol. 2006;2:42–50. doi: 10.21161/mjm.220608. [DOI] [Google Scholar]
- 172.Liou JSC. Clostridium carboxidivorans sp. nov., a solvent-producing clostridium isolated from an agricultural settling lagoon, and reclassification of the acetogen Clostridium scatologenes strain SL1 as Clostridium drakei sp. nov. Int J Syst Evol Microbiol. 2005;55:2085–2091. doi: 10.1099/ijs.0.63482-0. [DOI] [PubMed] [Google Scholar]
- 173.Lipnizki F, Hausmanns S, Laufenberg G, Field R, Kunz B. Use of pervaporation-bioreactor hybrid processes in biotechnology. Chem Eng Technol. 2000;23:569–577. doi: 10.1002/1521-4125(200007)23:7<569::Aid-ceat569>3.0.Co;2-1. [DOI] [Google Scholar]
- 174.Lipovsky J, Patakova P, Paulova L, Pokorny T, Rychtera M, Melzoch K. Butanol production by Clostridium pasteurianum NRRL B-598 in continuous culture compared to batch and fed-batch systems. Fuel Process Technol. 2016;144:139–144. doi: 10.1016/j.fuproc.2015.12.020. [DOI] [Google Scholar]
- 175.Liu D, Chen Y, Ding F-Y, Zhao T, Wu J-L, Guo T, Ren H-F, Li B-B, Niu H-Q, Cao Z, Lin X-Q, Xie J-J, He X-J, Ying H-J. Biobutanol production in a Clostridium acetobutylicum biofilm reactor integrated with simultaneous product recovery by adsorption. Biotechnol Biofuels. 2014;7:5. doi: 10.1186/1754-6834-7-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 176.Liu H, Zhang J, Yuan J, Jiang X, Jiang L, Zhao G, Huang D, Liu B. Omics-based analyses revealed metabolic responses of Clostridium acetobutylicum to lignocellulose-derived inhibitors furfural, formic acid and phenol stress for butanol fermentation. Biotechnol Biofuels. 2019;12:101. doi: 10.1186/s13068-019-1440-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 177.Liu JK, Lloyd C, Al-Bassam MM, Ebrahim A, Kim J-N, Olson C, Aksenov A, Dorrestein P, Zengler K. Predicting proteome allocation, overflow metabolism, and metal requirements in a model acetogen. PLoS Comput Biol. 2019;15:e1006848. doi: 10.1371/journal.pcbi.1006848. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 178.Liu K, Atiyeh HK, Stevenson BS, Tanner RS, Wilkins MR, Huhnke RL. Continuous syngas fermentation for the production of ethanol, n-propanol and n-butanol. Bioresour Technol. 2014;151:69–77. doi: 10.1016/j.biortech.2013.10.059. [DOI] [PubMed] [Google Scholar]
- 179.Liu S. Cell cultivation. In: Liu S, editor. Bioprocess engineering. 2. Amsterdam: Elsevier; 2017. pp. 699–782. [Google Scholar]
- 180.López-Contreras AM, Claassen PAM, Mooibroek H, De Vos WM. Utilisation of saccharides in extruded domestic organic waste by Clostridium acetobutylicum ATCC 824 for production of acetone, butanol and ethanol. Appl Microbiol Biotechnol. 2000;54:162–167. doi: 10.1007/s002530000374. [DOI] [PubMed] [Google Scholar]
- 181.Lu C, Dong J, Yang S-T. Butanol production from wood pulping hydrolysate in an integrated fermentation–gas stripping process. Bioresour Technol. 2013;143:467–475. doi: 10.1016/j.biortech.2013.06.012. [DOI] [PubMed] [Google Scholar]
- 182.Lu C, Zhao J, Yang S-T, Wei D. Fed-batch fermentation for n-butanol production from cassava bagasse hydrolysate in a fibrous bed bioreactor with continuous gas stripping. Bioresour Technol. 2012;104:380–387. doi: 10.1016/j.biortech.2011.10.089. [DOI] [PubMed] [Google Scholar]
- 183.Luttmann R, Bracewell DG, Cornelissen G, Gernaey KV, Glassey J, Hass VC, Kaiser C, Preusse C, Striedner G, Mandenius C-F. Soft sensors in bioprocessing: a status report and recommendations. Biotechnol J. 2012;7:1040–1048. doi: 10.1002/biot.201100506. [DOI] [PubMed] [Google Scholar]
- 184.Madihah MS, Ariff AB, Sahaid KM, Suraini AA, Karim MIA. Direct fermentation of gelatinized sago starch to acetone–butanol–ethanol by Clostridium acetobutylicum. World J Microbiol Biotechnol. 2001;17:567–576. doi: 10.1023/a:1012351112351. [DOI] [PubMed] [Google Scholar]
- 185.Mainka T, Mahler N, Herwig C, Pflügl S. Soft sensor-based monitoring and efficient control strategies of biomass concentration for continuous cultures of Haloferax mediterranei and their application to an industrial production chain. Microorganisms. 2019;7:648. doi: 10.3390/microorganisms7120648. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 186.Maiti S, Sarma SJ, Brar SK, Le Bihan Y, Drogui P, Buelna G, Verma M. Agro-industrial wastes as feedstock for sustainable bio-production of butanol by Clostridium beijerinckii. Food Bioprod Process. 2016;98:217–226. doi: 10.1016/j.fbp.2016.01.002. [DOI] [Google Scholar]
- 187.Malaviya A, Jang Y-S, Lee SY. Continuous butanol production with reduced byproducts formation from glycerol by a hyper producing mutant of Clostridium pasteurianum. Appl Microbiol Biotechnol. 2012;93:1485–1494. doi: 10.1007/s00253-011-3629-0. [DOI] [PubMed] [Google Scholar]
- 188.Mann MS, Dragovic Z, Schirrmacher G, Lütke-Eversloh T. Over-expression of stress protein-encoding genes helps Clostridium acetobutylicum to rapidly adapt to butanol stress. Biotechnol Lett. 2012;34:1643–1649. doi: 10.1007/s10529-012-0951-2. [DOI] [PubMed] [Google Scholar]
- 189.Marcellin E, Behrendorff JB, Nagaraju S, DeTissera S, Segovia S, Palfreyman RW, Daniell J, Licona-Cassani C, Quek L-E, Speight R, Hodson MP, Simpson SD, Mitchell WP, Köpke M, Nielsen LK. Low carbon fuels and commodity chemicals from waste gases—systematic approach to understand energy metabolism in a model acetogen. Green Chem. 2016;18:3020–3028. doi: 10.1039/C5GC02708J. [DOI] [Google Scholar]
- 190.Mariano AP, Filho RM. Improvements in biobutanol fermentation and their impacts on distillation energy consumption and wastewater generation. BioEnergy Res. 2012;5:504–514. doi: 10.1007/s12155-011-9172-0. [DOI] [Google Scholar]
- 191.Martin ME, Richter H, Saha S, Angenent LT. Traits of selected Clostridium strains for syngas fermentation to ethanol. Biotechnol Bioeng. 2016;113:531–539. doi: 10.1002/bit.25827. [DOI] [PubMed] [Google Scholar]
- 192.Maru BT, Munasinghe PC, Gilary H, Jones SW, Tracy BP. Fixation of CO2 and CO on a diverse range of carbohydrates using anaerobic, non-photosynthetic mixotrophy. FEMS Microbiol Lett. 2018;365:fny039. doi: 10.1093/femsle/fny039. [DOI] [PubMed] [Google Scholar]
- 193.Maxon WD. Continuous fermentation. In: Umbreit WW, editor. Advances in applied microbiology. New York: Academic Press; 1960. pp. 335–355. [DOI] [PubMed] [Google Scholar]
- 194.Mbaneme-Smith V, Chinn MS. Consolidated bioprocessing for biofuel production: recent advances. Energy Emiss Control Technol. 2015;3:23–44. doi: 10.2147/EECT.S63000. [DOI] [Google Scholar]
- 195.McAllister KN, Sorg JA. CRISPR genome editing systems in the genus Clostridium: a timely advancement. J Bacteriol. 2019;201:e00219–00219. doi: 10.1128/JB.00219-19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 196.McAnulty MJ, Yen JY, Freedman BG, Senger RS. Genome-scale modeling using flux ratio constraints to enable metabolic engineering of clostridial metabolism in silico. BMC Syst Biol. 2012;6:42. doi: 10.1186/1752-0509-6-42. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 197.Milne CB, Eddy JA, Raju R, Ardekani S, Kim P-J, Senger RS, Jin Y-S, Blaschek HP, Price ND. Metabolic network reconstruction and genome-scale model of butanol-producing strain Clostridium beijerinckii NCIMB 8052. BMC Syst Biol. 2011;5:130. doi: 10.1186/1752-0509-5-130. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 198.Mock J, Zheng Y, Mueller AP, Ly S, Tran L, Segovia S, Nagaraju S, Köpke M, Dürre P, Thauer RK. Energy conservation associated with ethanol formation from H2 and CO2 in Clostridium autoethanogenum Involving Electron Bifurcation. J Bacteriol. 2015;197:2965–2980. doi: 10.1128/jb.00399-15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 199.Mohammadi M, Younesi H, Najafpour G, Mohamed AR. Sustainable ethanol fermentation from synthesis gas by Clostridium ljungdahlii in a continuous stirred tank bioreactor. J Chem Technol Biotechnol. 2012;87:837–843. doi: 10.1002/jctb.3712. [DOI] [Google Scholar]
- 200.Monot F, Engasser J-M, Petitdemange H. Influence of pH and undissociated butyric acid on the production of acetone and butanol in batch cultures of Clostridium acetobutylicum. Appl Microbiol Biotechnol. 1984;19:422–426. doi: 10.1007/BF00454381. [DOI] [Google Scholar]
- 201.Moon HG, Jang Y-S, Cho C, Lee J, Binkley R, Lee SY. One hundred years of clostridial butanol fermentation. FEMS Microbiol Lett. 2016;363:fnw001. doi: 10.1093/femsle/fnw001. [DOI] [PubMed] [Google Scholar]
- 202.Moradi F, Amiri H, Soleimanian-Zad S, Ehsani MR, Karimi K. Improvement of acetone, butanol and ethanol production from rice straw by acid and alkaline pretreatments. Fuel. 2013;112:8–13. doi: 10.1016/j.fuel.2013.05.011. [DOI] [Google Scholar]
- 203.Morone A, Pandey RA. Lignocellulosic biobutanol production: gridlocks and potential remedies. Renew Sustain Energy Rev. 2014;37:21–35. doi: 10.1016/j.rser.2014.05.009. [DOI] [Google Scholar]
- 204.Mulchandani A, Volesky B. Production of acetone–butanol–ethanol by Clostridium acetobutylicum using a spin filter perfusion bioreactor. J Biotechnol. 1994;34:51–60. doi: 10.1016/0168-1656(94)90165-1. [DOI] [Google Scholar]
- 205.Mutschlechner O, Swoboda H, Gapes JR. Continuous two-stage ABE-fermentation using Clostridium beijerinckii NRRL B592 operating with a growth rate in the first stage vessel close to its maximal value. J Mol Microbiol Biotechnol. 2000;2:101–105. [PubMed] [Google Scholar]
- 206.Nagarajan H, Sahin M, Nogales J, Latif H, Lovley DR, Ebrahim A, Zengler K. Characterizing acetogenic metabolism using a genome-scale metabolic reconstruction of Clostridium ljungdahlii. Microb Cell Fact. 2013;12:118. doi: 10.1186/1475-2859-12-118. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 207.Napoli F, Olivieri G, Russo ME, Marzocchella A, Salatino P. Butanol production by Clostridium acetobutylicum in a continuous packed bed reactor. J Ind Microbiol Biotechnol. 2010;37:603–608. doi: 10.1007/s10295-010-0707-8. [DOI] [PubMed] [Google Scholar]
- 208.Nawab S, Wang N, Ma X, Huo Y-X. Genetic engineering of non-native hosts for 1-butanol production and its challenges: a review. Microb Cell Fact. 2020;19:79. doi: 10.1186/s12934-020-01337-w. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 209.Ndaba B, Chiyanzu I, Marx S. n-Butanol derived from biochemical and chemical routes: a review. Biotechnol Rep. 2015;8:1–9. doi: 10.1016/j.btre.2015.08.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 210.Nguyen N-P-T, Raynaud C, Meynial-Salles I, Soucaille P. Reviving the Weizmann process for commercial n-butanol production. Nat Commun. 2018 doi: 10.1038/s41467-018-05661-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 211.Ni Y, Sun Z. Recent progress on industrial fermentative production of acetone–butanol–ethanol by Clostridium acetobutylicum in China. Appl Microbiol Biotechnol. 2009;83:415–423. doi: 10.1007/s00253-009-2003-y. [DOI] [PubMed] [Google Scholar]
- 212.Ni Y, Wang Y, Sun Z. Butanol production from cane Molasses by Clostridium saccharobutylicum DSM 13864: batch and semicontinuous fermentation. Appl Biochem Biotechnol. 2012;166:1896–1907. doi: 10.1007/s12010-012-9614-y. [DOI] [PubMed] [Google Scholar]
- 213.Ni Y, Xia Z, Wang Y, Sun Z. Continuous butanol fermentation from inexpensive sugar-based feedstocks by Clostridium saccharobutylicum DSM 13864. Bioresour Technol. 2013;129:680–685. doi: 10.1016/j.biortech.2012.11.142. [DOI] [PubMed] [Google Scholar]
- 214.Nielsen DR, Prather KJ. In situ product recovery of n-butanol using polymeric resins. Biotechnol Bioeng. 2009;102:811–821. doi: 10.1002/bit.22109. [DOI] [PubMed] [Google Scholar]
- 215.Nielsen L, Larsson M, Holst O, Mattiasson B. Adsorbents for extractive bioconversion applied to the acetone–butanol fermentation. Appl Microbiol Biotechnol. 1988;28:335–339. doi: 10.1007/BF00268191. [DOI] [Google Scholar]
- 216.Norman ROJ, Millat T, Schatschneider S, Henstra AM, Breitkopf R, Pander B, Annan FJ, Piatek P, Hartman HB, Poolman MG, Fell DA, Winzer K, Minton NP, Hodgman C. Genome-scale model of C. autoethanogenum reveals optimal bioprocess conditions for high-value chemical production from carbon monoxide. Eng Biol. 2019;3:32–40. doi: 10.1049/enb.2018.5003. [DOI] [Google Scholar]
- 217.Orgill JJ, Atiyeh HK, Devarapalli M, Phillips JR, Lewis RS, Huhnke RL. A comparison of mass transfer coefficients between trickle-bed, hollow fiber membrane and stirred tank reactors. Bioresour Technol. 2013;133:340–346. doi: 10.1016/j.biortech.2013.01.124. [DOI] [PubMed] [Google Scholar]
- 218.Papoutsakis ET. Equations and calculations for fermentations of butyric acid bacteria. Biotechnol Bioeng. 1984;26:174–187. doi: 10.1002/bit.260260210. [DOI] [PubMed] [Google Scholar]
- 219.Patakova P, Linhova M, Rychtera M, Paulova L, Melzoch K. Novel and neglected issues of acetone–butanol–ethanol (ABE) fermentation by clostridia: Clostridium metabolic diversity, tools for process mapping and continuous fermentation systems. Biotechnol Adv. 2013;31:58–67. doi: 10.1016/j.biotechadv.2012.01.010. [DOI] [PubMed] [Google Scholar]
- 220.Philipps G, de Vries S, Jennewein S. Development of a metabolic pathway transfer and genomic integration system for the syngas-fermenting bacterium Clostridium ljungdahlii. Biotechnol Biofuels. 2019;12:112. doi: 10.1186/s13068-019-1448-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 221.Phillips JR, Huhnke RL, Atiyeh HK. Syngas fermentation: a microbial conversion process of gaseous substrates to various products. Fermentation. 2017;3:28. doi: 10.3390/fermentation3020028. [DOI] [Google Scholar]
- 222.Pierrot P, Fick M, Engasser JM. Continuous acetone–butanol fermentation with high productivity by cell ultrafiltration and recycling. Biotechnol Lett. 1986;8:253–256. doi: 10.1007/BF01030507. [DOI] [Google Scholar]
- 223.Pyne ME, Liu X, Moo-Young M, Chung DA, Chou CP. Genome-directed analysis of prophage excision, host defence systems, and central fermentative metabolism in Clostridium pasteurianum. Sci Rep. 2016;6:26228. doi: 10.1038/srep26228. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 224.Qin Z, Duns GJ, Pan T, Xin F. Consolidated processing of biobutanol production from food wastes by solventogenic Clostridium sp. strain HN4. Bioresour Technol. 2018;264:148–153. doi: 10.1016/j.biortech.2018.05.076. [DOI] [PubMed] [Google Scholar]
- 225.Qureshi N, Annous BA, Ezeji TC, Karcher P, Maddox IS. Biofilm reactors for industrial bioconversion processes: employing potential of enhanced reaction rates. Microb Cell Fact. 2005;4:24. doi: 10.1186/1475-2859-4-24. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 226.Qureshi N, Blaschek HP. Butanol Production Using Clostridium beijerinckii BA101 hyper-butanol producing mutant strain and recovery by pervaporation. Appl Biochem Biotechnol. 2000;84–86:225–236. doi: 10.1385/abab:84-86:1-9:225. [DOI] [PubMed] [Google Scholar]
- 227.Qureshi N, Blaschek HP. Recovery of butanol from fermentation broth by gas stripping. Renew Energy. 2001;22:557–564. doi: 10.1016/s0960-1481(00)00108-7. [DOI] [Google Scholar]
- 228.Qureshi N, Blaschek HP. Recovery of butanol from fermentation broth by gas stripping. AIDIC. 2016;49:13–18. [Google Scholar]
- 229.Qureshi N, Hodge D, Vertes A. Biorefineries. Integrated biochemical processes for liquid biofuels. 1. Netherlands: Elsevier; 2014. [Google Scholar]
- 230.Qureshi N, Hughes S, Maddox IS, Cotta MA. Energy-efficient recovery of butanol from model solutions and fermentation broth by adsorption. Bioprocess Biosyst Eng. 2005;27:215–222. doi: 10.1007/s00449-005-0402-8. [DOI] [PubMed] [Google Scholar]
- 231.Qureshi N, Lai LL, Blaschek HP. Scale-up of a high productivity continuous biofilm reactor to produce butanol by adsorbed cells of Clostridium beijerinckii. Food Bioprod Process. 2004;82:164–173. doi: 10.1205/0960308041614891. [DOI] [Google Scholar]
- 232.Qureshi N, Lolas A, Blaschek HP. Soy molasses as fermentation substrate for production of butanol using Clostridium beijerinckii BA101. J Ind Microbiol Biotechnol. 2001;26:290–295. doi: 10.1038/sj.jim.7000131. [DOI] [PubMed] [Google Scholar]
- 233.Qureshi N, Maddox IS. Continuous production of acetone–butanol–ethanol using immobilized cells of Clostridium acetobutylicum and integration with product removal by liquid-liquid extraction. J Ferment Bioeng. 1995;80:185–189. doi: 10.1016/0922-338x(95)93217-8. [DOI] [Google Scholar]
- 234.Qureshi N, Paterson AHJ, Maddox IS. Model for continuous production of solvents from whey permeate in a packed bed reactor using cells of Clostridium acetobutylicum immobilized by adsorption onto bonechar. Appl Microbiol Biotechnol. 1988;29:323–328. doi: 10.1007/BF00265814. [DOI] [Google Scholar]
- 235.Qureshi N, Saha BC, Cotta MA. Butanol production from wheat straw hydrolysate using Clostridium beijerinckii. Bioprocess Biosyst Eng. 2007;30:419–427. doi: 10.1007/s00449-007-0137-9. [DOI] [PubMed] [Google Scholar]
- 236.Qureshi N, Saha BC, Dien B, Hector RE, Cotta MA. Production of butanol (a biofuel) from agricultural residues: Part I—use of barley straw hydrolysate. Biomass Bioenergy. 2010;34:559–565. doi: 10.1016/j.biombioe.2009.12.024. [DOI] [Google Scholar]
- 237.Qureshi N, Saha BC, Hector RE, Dien B, Hughes S, Liu S, Iten L, Bowman MJ, Sarath G, Cotta MA. Production of butanol (a biofuel) from agricultural residues: Part II—use of corn stover and switchgrass hydrolysates. Biomass Bioenergy. 2010;34:566–571. doi: 10.1016/j.biombioe.2009.12.023. [DOI] [Google Scholar]
- 238.Qureshi N, Saha BC, Hector RE, Hughes SR, Cotta MA. Butanol production from wheat straw by simultaneous saccharification and fermentation using Clostridium beijerinckii: Part I—batch fermentation. Biomass Bioenergy. 2008;32:168–175. doi: 10.1016/j.biombioe.2007.07.004. [DOI] [Google Scholar]
- 239.Qureshi N, Saha BC, Hector RE, Hughes SR, Cotta MA. Butanol production from wheat straw by simultaneous saccharification and fermentation using Clostridium beijerinckii: Part II—fed-batch fermentation. Biomass Bioenergy. 2008;32:176–183. doi: 10.1016/j.biombioe.2007.07.005. [DOI] [Google Scholar]
- 240.Qureshi N, Schripsema J, Lienhardt J, Blaschek HP. Continuous solvent production by Clostridium beijerinckii BA101 immobilized by adsorption onto brick. World J Microbiol Biotechnol. 2000;16:377–382. doi: 10.1023/A:1008984509404. [DOI] [Google Scholar]
- 241.Rabacal M, Ferreira AF, Silva CAM, Costa M. Targeting energy, high value products and waste valorisation. New York: Biorefineries Springer International Publishing; 2017. [Google Scholar]
- 242.Raganati F, Olivieri G, Procentese A, Russo ME, Salatino P, Marzocchella A. Butanol production by bioconversion of cheese whey in a continuous packed bed reactor. Bioresour Technol. 2013;138:259–265. doi: 10.1016/j.biortech.2013.03.180. [DOI] [PubMed] [Google Scholar]
- 243.Ramey DE (1998) Continuous two stage, dual path anaerobic fermentation of butanol and other organic solvents using two different strains of bacteria. US Patent No. US5753474A
- 244.Ramey S, David R (2005). https://www.butanol.com/
- 245.Ramió-Pujol S, Ganigué R, Bañeras L, Colprim J. Incubation at 25 °C prevents acid crash and enhances alcohol production in Clostridium carboxidivorans P7. Bioresour Technol. 2015;192:296–303. doi: 10.1016/j.biortech.2015.05.077. [DOI] [PubMed] [Google Scholar]
- 246.Ravagnani A, Jennert KCB, Steiner E, Grunberg R, Jefferies JR, Wilkinson SR, Young DI, Tidswell EC, Brown DP, Youngman P, Morris JG, Young M. Spo0A directly controls the switch from acid to solvent production in solvent-forming clostridia. Mol Microbiol. 2000;37:1172–1185. doi: 10.1046/j.1365-2958.2000.02071.x. [DOI] [PubMed] [Google Scholar]
- 247.Richter H, Martin M, Angenent L. A two-stage continuous fermentation system for conversion of syngas into ethanol. Energies. 2013;6:3987–4000. doi: 10.3390/en6083987. [DOI] [Google Scholar]
- 248.Richter H, Molitor B, Diender M, Sousa DZ, Angenent LT. A narrow pH range supports butanol, hexanol, and octanol production from syngas in a continuous co-culture of Clostridium ljungdahlii and Clostridium kluyveri with in-line product extraction. Front Microbiol. 2016;7:1773. doi: 10.3389/fmicb.2016.01773. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 249.Richter H, Molitor B, Wei H, Chen W, Aristilde L, Angenent LT. Ethanol production in syngas-fermenting Clostridium ljungdahlii is controlled by thermodynamics rather than by enzyme expression. Energy Environ Sci. 2016;9:2392–2399. doi: 10.1039/c6ee01108j. [DOI] [Google Scholar]
- 250.Roberts SB, Gowen CM, Brooks JP, Fong SS. Genome-scale metabolic analysis of Clostridium thermocellum for bioethanol production. BMC Syst Biol. 2010;4:31. doi: 10.1186/1752-0509-4-31. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 251.Rochón E, Ferrari MD, Lareo C. Integrated ABE fermentation-gas stripping process for enhanced butanol production from sugarcane-sweet sorghum juices. Biomass Bioenergy. 2017;98:153–160. doi: 10.1016/j.biombioe.2017.01.011. [DOI] [Google Scholar]
- 252.Salimi F, Mandal R, Wishart D, Mahadevan R. Understanding Clostridium acetobutylicum ATCC 824 metabolism using genome-scale thermodynamics and metabolomics-based modeling. IFAC Proc. 2010;43:126–131. doi: 10.3182/20100707-3-BE-2012.0022. [DOI] [Google Scholar]
- 253.Salimi F, Zhuang K, Mahadevan R. Genome-scale metabolic modeling of a clostridial co-culture for consolidated bioprocessing. Biotechnol J. 2010;5:726–738. doi: 10.1002/biot.201000159. [DOI] [PubMed] [Google Scholar]
- 254.Saravanan V, Waijers DA, Ziari M, Noordermeer MA. Recovery of 1-butanol from aqueous solutions using zeolite ZSM-5 with a high Si/Al ratio; suitability of a column process for industrial applications. Biochem Eng J. 2010;49:33–39. doi: 10.1016/j.bej.2009.11.008. [DOI] [Google Scholar]
- 255.Sauer M. Industrial production of acetone and butanol by fermentation—100 years later. FEMS Microbiol Lett. 2016;363:fnw134. doi: 10.1093/femsle/fnw134. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 256.Schiel-Bengelsdorf B, Dürre P. Pathway engineering and synthetic biology using acetogens. FEBS Lett. 2012;586:2191–2198. doi: 10.1016/j.febslet.2012.04.043. [DOI] [PubMed] [Google Scholar]
- 257.Schlote D, Gottschalk G. Effect of cell recycle on continuous butanol-acetone fermentation with Clostridium acetobutylicum under phosphate limitation. Appl Microbiol Biotechnol. 1986;24:1–5. doi: 10.1007/BF00266276. [DOI] [Google Scholar]
- 258.Schuchmann K, Müller V. Autotrophy at the thermodynamic limit of life: a model for energy conservation in acetogenic bacteria. Nat Rev Microbiol. 2014;12:809–821. doi: 10.1038/nrmicro3365. [DOI] [PubMed] [Google Scholar]
- 259.Schuchmann K, Müller V. Energetics and application of heterotrophy in acetogenic bacteria. Appl Environ Microbiol. 2016;82:4056–4069. doi: 10.1128/aem.00882-16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 260.Schultz MA, Raiser TE, Brenc RJ (2017) Product management in biological conversion processes. US Patent No. US20170226538A1
- 261.Seifollahi M, Amiri H. Phosphoric acid-acetone process for cleaner production of acetone, butanol, and ethanol from waste cotton fibers. J Clean Prod. 2018;193:459–470. doi: 10.1016/j.jclepro.2018.05.093. [DOI] [Google Scholar]
- 262.Senger RS, Papoutsakis ET. Genome-scale model for Clostridium acetobutylicum: Part I. Metabolic network resolution and analysis. Biotechnol Bioeng. 2008;101:1036–1052. doi: 10.1002/bit.22010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 263.Serrano-Bermúdez LM, González Barrios AF, Maranas CD, Montoya D. Clostridium butyricum maximizes growth while minimizing enzyme usage and ATP production: metabolic flux distribution of a strain cultured in glycerol. BMC Syst Biol. 2017;11:58. doi: 10.1186/s12918-017-0434-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 264.Seys FM, Rowe P, Bolt EL, Humphreys CM, Minton NP. A gold standard, CRISPR/Cas9-based complementation strategy reliant on 24 nucleotide bookmark sequences. Genes. 2020;11:458. doi: 10.3390/genes11040458. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 265.Shao M, Chen H. Feasibility of acetone–butanol–ethanol (ABE) fermentation from Amorphophallus konjac waste by Clostridium acetobutylicum ATCC 824. Process Biochem. 2015;50:1301–1307. doi: 10.1016/j.procbio.2015.05.009. [DOI] [Google Scholar]
- 266.Shen Y, Brown R, Wen Z. Enhancing mass transfer and ethanol production in syngas fermentation of Clostridium carboxidivorans P7 through a monolithic biofilm reactor. Appl Energy. 2014;136:68–76. doi: 10.1016/j.apenergy.2014.08.117. [DOI] [Google Scholar]
- 267.Shen Y, Brown R, Wen Z. Syngas fermentation of Clostridium carboxidivoran P7 in a hollow fiber membrane biofilm reactor: evaluating the mass transfer coefficient and ethanol production performance. Biochem Eng J. 2014;85:21–29. doi: 10.1016/j.bej.2014.01.010. [DOI] [Google Scholar]
- 268.Shin C, Baer ZC, Chen XC, Ozcam AE, Clark DS, Balsara NP. Block copolymer pervaporation membrane for in situ product removal during acetone–butanol–ethanol fermentation. J Membr Sci. 2015;484:57–63. doi: 10.1016/j.memsci.2015.03.005. [DOI] [Google Scholar]
- 269.Siebler F, Lapin A, Hermann M, Takors R. The impact of CO gradients on C. ljungdahlii in a 125 m3 bubble column: Mass transfer, circulation time and lifeline analysis. Chem Eng Sci. 2019;207:410–423. doi: 10.1016/j.ces.2019.06.018. [DOI] [Google Scholar]
- 270.Smart KF, Mueller AP, James M, Mawdsley H, Mihalcea CD (2017) Fermentation process. US Patent No. US9771603B2
- 271.Song Y, Lee JS, Shin J, Lee GM, Jin S, Kang S, Lee J-K, Kim DR, Lee EY, Kim SC, Cho S, Kim D, Cho B-K. Functional cooperation of the glycine synthase-reductase and Wood–Ljungdahl pathways for autotrophic growth of Clostridium drakei. PNAS. 2020;117:7516–7523. doi: 10.1073/pnas.1912289117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 272.Soni BK, Soucaille P, Goma G. Continuous acetone–butanol fermentation: a global approach for the improvement in the solvent productivity in synthetic medium. Appl Microbiol Biotechnol. 1987;25:317–321. doi: 10.1007/BF00252540. [DOI] [Google Scholar]
- 273.Staggs KW, Nielsen DR. Improving n-butanol production in batch and semi-continuous processes through integrated product recovery. Process Biochem. 2015;50:1487–1498. doi: 10.1016/j.procbio.2015.06.009. [DOI] [Google Scholar]
- 274.Stenmarck Å, Jensen C, Quested T, Moates G (2016) Estimates of European food waste levels. 10.13140/RG.2.1.4658.4721. https://www.eu-fusions.org/phocadownload/Publications/Estimates%20of%20European%20food%20waste%20levels.pdf. Accessed 27 May 2020
- 275.Stoll IK, Boukis N, Sauer J. Syngas fermentation to alcohols: reactor technology and application perspective. Chem Ing Tech. 2019 doi: 10.1002/cite.201900118. [DOI] [Google Scholar]
- 276.Straub M, Demler M, Weuster-Botz D, Dürre P. Selective enhancement of autotrophic acetate production with genetically modified Acetobacterium woodii. J Biotechnol. 2014;178:67–72. doi: 10.1016/j.jbiotec.2014.03.005. [DOI] [PubMed] [Google Scholar]
- 277.Sun X, Atiyeh HK, Zhang H, Tanner RS, Huhnke RL. Enhanced ethanol production from syngas by Clostridium ragsdalei in continuous stirred tank reactor using medium with poultry litter biochar. Appl Energy. 2019;236:1269–1279. doi: 10.1016/j.apenergy.2018.12.010. [DOI] [Google Scholar]
- 278.Sun Y, Cheng J. Hydrolysis of lignocellulosic materials for ethanol production: a review. Bioresour Technol. 2002;83:1–11. doi: 10.1016/s0960-8524(01)00212-7. [DOI] [PubMed] [Google Scholar]
- 279.Survase SA, Jurgens G, Van Heiningen A, Granström T. Continuous production of isopropanol and butanol using Clostridium beijerinckii DSM 6423. Appl Microbiol Biotechnol. 2011;91:1305–1313. doi: 10.1007/s00253-011-3322-3. [DOI] [PubMed] [Google Scholar]
- 280.Survase SA, Sklavounos E, Van Heiningen A, Granström T. Market refused vegetables as a supplement for improved acetone–butanol–ethanol production by Clostridium acetobutylicum DSM 792. Ind Crops Prod. 2013;45:349–354. doi: 10.1016/j.indcrop.2012.12.049. [DOI] [Google Scholar]
- 281.Survase SA, Van Heiningen A, Granström T. Continuous bio-catalytic conversion of sugar mixture to acetone–butanol–ethanol by immobilized Clostridium acetobutylicum DSM 792. Appl Microbiol Biotechnol. 2012;93:2309–2316. doi: 10.1007/s00253-011-3761-x. [DOI] [PubMed] [Google Scholar]
- 282.Survase SA, Van Heiningen A, Granström T. Wood pulp as an immobilization matrix for the continuous production of isopropanol and butanol. J Ind Microbiol Biotechnol. 2013;40:209–215. doi: 10.1007/s10295-012-1219-5. [DOI] [PubMed] [Google Scholar]
- 283.Survase SA, Zebroski R, Bayuadri C, Wang Z, Adamos G, Nagy G, Pylkkanen V. Membrane assisted continuous production of solvents with integrated solvent removal using liquid-liquid extraction. Bioresour Technol. 2019;280:378–386. doi: 10.1016/j.biortech.2019.02.024. [DOI] [PubMed] [Google Scholar]
- 284.Takors R, Kopf M, Mampel J, Bluemke W, Blombach B, Eikmanns B, Bengelsdorf FR, Weuster-Botz D, Dürre P. Using gas mixtures of CO, CO2 and H2 as microbial substrates: the do's and don'ts of successful technology transfer from laboratory to production scale. Microb Biotechnol. 2018;11:606–625. doi: 10.1111/1751-7915.13270. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 285.Tang J. Microbial metabolomics. Curr Genomics. 2011;12:391–403. doi: 10.2174/138920211797248619. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 286.Tanner RS, Miller LM, Yang D. Clostridium ljungdahlii sp. nov., an acetogenic species in clostridial rRNA homology group I. Int J Syst Bacteriol. 1993;43:232–236. doi: 10.1099/00207713-43-2-232. [DOI] [PubMed] [Google Scholar]
- 287.Tashiro Y, Takeda K, Kobayashi G, Sonomoto K. High production of acetone–butanol–ethanol with high cell density culture by cell-recycling and bleeding. J Biotechnol. 2005;120:197–206. doi: 10.1016/j.jbiotec.2005.05.031. [DOI] [PubMed] [Google Scholar]
- 288.Tashiro Y, Takeda K, Kobayashi G, Sonomoto K, Ishizaki A, Yoshino S. High butanol production by Clostridium saccharoperbutylacetonicum N1–4 in fed-batch culture with pH-Stat continuous butyric acid and glucose feeding method. J Biosci Bioeng. 2004;98:263–268. doi: 10.1016/s1389-1723(04)00279-8. [DOI] [PubMed] [Google Scholar]
- 289.Tashiro Y, Yoshida T, Noguchi T, Sonomoto K. Recent advances and future prospects for increased butanol production by acetone–butanol–ethanol fermentation. Eng Life Sci. 2013;13:432–445. doi: 10.1002/elsc.201200128. [DOI] [Google Scholar]
- 290.Thompson RA, Dahal S, Garcia S, Nookaew I, Trinh CT. Exploring complex cellular phenotypes and model-guided strain design with a novel genome-scale metabolic model of Clostridium thermocellum DSM 1313 implementing an adjustable cellulosome. Biotechnol Biofuels. 2016;9:194. doi: 10.1186/s13068-016-0607-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 291.Tracy BP, Gaida SM, Papoutsakis ET. Development and application of flow-cytometric techniques for analyzing and sorting endospore-forming Clostridia. Appl Environ Microbiol. 2008;74:7497–7506. doi: 10.1128/aem.01626-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 292.Tracy BP, Jones SW, Fast AG, Indurthi DC, Papoutsakis ET. Clostridia: the importance of their exceptional substrate and metabolite diversity for biofuel and biorefinery applications. Curr Opin Biotechnol. 2012;23:364–381. doi: 10.1016/j.copbio.2011.10.008. [DOI] [PubMed] [Google Scholar]
- 293.Tracy BP, Jones SW, Papoutsakis ET. Inactivation of σE and σG in Clostridium acetobutylicum illuminates their roles in clostridial-cell-form biogenesis, granulose synthesis, solventogenesis, and spore morphogenesis. J Bacteriol. 2011;193:1414–1426. doi: 10.1128/JB.01380-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 294.Tracy BP, Somekh SR, Phillips JR, Eyal AM (2019) Integrated mixotrophic fermentation method. US Patent No. US 20190144892 A1, May 16, 2019
- 295.Trevethick SR, Bromley JC, Waters GW, Koepke M, Tran LP, Overgaard RJ (2016) Multi-stage bioreactor process. US Patent No. US20160115505A1
- 296.Ujor V, Bharathidasan A, Cornish K, Ezeji T. Evaluation of industrial dairy waste (milk dust powder) for acetone–butanol–ethanol production by solventogenic Clostridium species. Springerplus. 2014;3:387. doi: 10.1186/2193-1801-3-387. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 297.Ujor V, Bharathidasan AK, Cornish K, Ezeji TC. Feasibility of producing butanol from industrial starchy food wastes. Appl Energy. 2014;136:590–598. doi: 10.1016/j.apenergy.2014.09.040. [DOI] [Google Scholar]
- 298.Ungerman AJ, Heindel TJ. Carbon monoxide mass transfer for syngas fermentation in a stirred tank reactor with dual impeller configurations. Biotechnol Prog. 2008;23:613–620. doi: 10.1021/bp060311z. [DOI] [PubMed] [Google Scholar]
- 299.Valgepea K, de Souza Pinto Lemgruber R, Abdalla T, Binos S, Takemori N, Takemori A, Tanaka Y, Tappel R, Köpke M, Simpson SD, Nielsen LK, Marcellin E. H2 drives metabolic rearrangements in gas-fermenting Clostridium autoethanogenum. Biotechnol Biofuels. 2018;11:55. doi: 10.1186/s13068-018-1052-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 300.Valgepea K, de Souza Pinto Lemgruber R, Meaghan K, Palfreyman RW, Abdalla T, Heijstra BD, Behrendorff JB, Tappel R, Köpke M, Simpson SD, Nielsen LK, Marcellin E. Maintenance of ATP homeostasis triggers metabolic shifts in gas-fermenting acetogens. Cell Syst. 2017;4:505–515. doi: 10.1016/j.cels.2017.04.008. [DOI] [PubMed] [Google Scholar]
- 301.Valgepea K, Loi KQ, Behrendorff JB, Lemgruber RdSP, Plan M, Hodson MP, Köpke M, Nielsen LK, Marcellin E. Arginine deiminase pathway provides ATP and boosts growth of the gas-fermenting acetogen Clostridium autoethanogenum. Metab Eng. 2017;41:202–211. doi: 10.1016/j.ymben.2017.04.007. [DOI] [PubMed] [Google Scholar]
- 302.Van Dien S. From the first drop to the first truckload: commercialization of microbial processes for renewable chemicals. Curr Opin Biotechnol. 2013;24:1061–1068. doi: 10.1016/j.copbio.2013.03.002. [DOI] [PubMed] [Google Scholar]
- 303.Van Hecke W, Bockrath R, De Wever H. Effects of moderately elevated pressure on gas fermentation processes. Bioresour Technol. 2019;293:122129. doi: 10.1016/j.biortech.2019.122129. [DOI] [PubMed] [Google Scholar]
- 304.Van Hecke W, Hofmann T, De Wever H. Pervaporative recovery of ABE during continuous cultivation: enhancement of performance. Biotechnol Prog. 2013;129:421–429. doi: 10.1016/j.biortech.2012.11.072. [DOI] [PubMed] [Google Scholar]
- 305.Van Hecke W, Vandezande P, Claes S, Vangeel S, Beckers H, Diels L, De Wever H. Integrated bioprocess for long-term continuous cultivation of Clostridium acetobutylicum coupled to pervaporation with PDMS composite membranes. Bioresour Technol. 2012;111:368–377. doi: 10.1016/j.biortech.2012.02.043. [DOI] [PubMed] [Google Scholar]
- 306.Vane LM. Separation technologies for the recovery and dehydration of alcohols from fermentation broths. Biofuel Bioprod Biorefin. 2008;2:553–588. doi: 10.1002/bbb.108. [DOI] [Google Scholar]
- 307.Vasconcelos I, Girbal L, Soucaille P. Regulation of carbon and electron flow in Clostridium acetobutylicum grown in chemostat culture at neutral pH on mixtures of glucose and glycerol. J Bacteriol. 1994;176:1443–1450. doi: 10.1128/jb.176.5.1443-1450.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 308.Vees CA, Veiter L, Sax F, Herwig C, Pflügl S. A robust flow cytometry-based biomass monitoring tool enables rapid at-line characterization of S. cerevisiae physiology during continuous bioprocessing of spent sulfite liquor. Anal Bioanal Chem. 2020;412:2137–2149. doi: 10.1007/s00216-020-02423-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 309.Wallenius J, Viikilä M, Survase S, Ojamo H, Eerikäinen T. Constraint-based genome-scale metabolic modeling of Clostridium acetobutylicum behavior in an immobilized column. Bioresour Technol. 2013;142:603–610. doi: 10.1016/j.biortech.2013.05.085. [DOI] [PubMed] [Google Scholar]
- 310.Wang S, Dong S, Wang Y. Enhancement of solvent production by overexpressing key genes of the acetone–butanol–ethanol fermentation pathway in Clostridium saccharoperbutylacetonicum N1–4. Bioresour Technol. 2017;245:426–433. doi: 10.1016/j.biortech.2017.09.024. [DOI] [PubMed] [Google Scholar]
- 311.Wang S, Zhang Y, Dong H, Mao S, Zhu Y, Wang R, Luan G, Li Y. Formic acid triggers the “Acid Crash” of Acetone–butanol–ethanol fermentation by Clostridium acetobutylicum. Appl Environ Microbiol. 2011;77:1674–1680. doi: 10.1128/AEM.01835-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 312.Wang Y, Janssen H, Blaschek HP. Fermentative biobutanol production: an old topic with remarkable recent advances. In: Bisaria VS, Kondo A, editors. Bioprocessing of renewable resources to commodity bioproducts. New York: Wiley; 2014. pp. 227–260. [Google Scholar]
- 313.Wang Y, Li X, Blaschek HP. Effects of supplementary butyrate on butanol production and the metabolic switch in Clostridium beijerinckii NCIMB 8052: genome-wide transcriptional analysis with RNA-Seq. Biotechnol Biofuels. 2013;6:138. doi: 10.1186/1754-6834-6-138. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 314.Westman JO, Franzén CJ. Current progress in high cell density yeast bioprocesses for bioethanol production. Biotechnol J. 2015;10:1185–1195. doi: 10.1002/biot.201400581. [DOI] [PubMed] [Google Scholar]
- 315.Whitham JM, Pawlak JJ, Grunden AM. Clostridium ljungdahlii: a review of the development of an industrial biocatalyst. Curr Biotechnol. 2016;5:54–70. doi: 10.2174/2211550105666151208211335. [DOI] [Google Scholar]
- 316.Wiechmann A, Ciurus S, Oswald F, Seiler VN, Müller V. It does not always take two to tango: “Syntrophy” via hydrogen cycling in one bacterial cell. ISME J. 2020;14:1561–1570. doi: 10.1038/s41396-020-0627-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 317.Wiesenborn DP, Rudolph FB, Papoutsakis ET. Coenzyme A transferase from Clostridium acetobutylicum ATCC 824 and its role in the uptake of acids. Appl Environ Microbiol. 1989;55:323–329. doi: 10.1128/AEM.55.2.323-329.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 318.Willson BJ, Kovács K, Wilding-Steele T, Markus R, Winzer K, Minton NP. Production of a functional cell wall-anchored minicellulosome by recombinant Clostridium acetobutylicum ATCC 824. Biotechnol Biofuels. 2016;9:109. doi: 10.1186/s13068-016-0526-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 319.Woolley RC, Morris JG. Stability of solvent production by Clostridium acetobutylicum in continuous culture: strain differences. J Appl Bacteriol. 1990;69:718–728. doi: 10.1111/j.1365-2672.1990.tb01569.x. [DOI] [Google Scholar]
- 320.Xia P-F, Casini I, Schulz S, Klask C-M, Angenent LT, Molitor B. Reprogramming acetogenic bacteria with CRISPR-targeted base editing via deamination. Synth Biol. 2020 doi: 10.1101/2020.04.20.047845. [DOI] [PubMed] [Google Scholar]
- 321.Xin F, Dong W, Zhang W, Ma J, Jiang M. Biobutanol production from crystalline cellulose through consolidated bioprocessing. Trends Biotechnol. 2019;37:167–180. doi: 10.1016/j.tibtech.2018.08.007. [DOI] [PubMed] [Google Scholar]
- 322.Xu D, Tree DR, Lewis RS. The effects of syngas impurities on syngas fermentation to liquid fuels. Biomass Bioenergy. 2011;35:2690–2696. doi: 10.1016/j.biombioe.2011.03.005. [DOI] [Google Scholar]
- 323.Xu M, Zhao J, Yu L, Tang I-C, Xue C, Yang S-T. Engineering Clostridium acetobutylicum with a histidine kinase knockout for enhanced n-butanol tolerance and production. Appl Microbiol Biotechnol. 2015;99:1011–1022. doi: 10.1007/s00253-014-6249-7. [DOI] [PubMed] [Google Scholar]
- 324.Xu M, Zhao J, Yu L, Yang S-T. Comparative genomic analysis of Clostridium acetobutylicum for understanding the mutations contributing to enhanced butanol tolerance and production. J Biotechnol. 2017;263:36–44. doi: 10.1016/j.jbiotec.2017.10.010. [DOI] [PubMed] [Google Scholar]
- 325.Xue C, Liu F, Xu M, Tang IC, Zhao J, Bai F, Yang S-T. Butanol production in acetone–butanol–ethanol fermentation with in situ product recovery by adsorption. Bioresour Technol. 2016;219:158–168. doi: 10.1016/j.biortech.2016.07.111. [DOI] [PubMed] [Google Scholar]
- 326.Xue C, Zhao J, Chen L, Yang S-T, Bai F. Recent advances and state-of-the-art strategies in strain and process engineering for biobutanol production by Clostridium acetobutylicum. Biotechnol Adv. 2017;35:310–322. doi: 10.1016/j.biotechadv.2017.01.007. [DOI] [PubMed] [Google Scholar]
- 327.Yakovlev AV, Shalygin MG, Matson SM, Khotimskiy VS, Teplyakov VV. Separation of diluted butanol–water solutions via vapor phase by organophilic membranes based on high permeable polyacetylenes. J Membr Sci. 2013;434:99–105. doi: 10.1016/j.memsci.2013.01.061. [DOI] [Google Scholar]
- 328.Yang ST, Lu C. Extraction-fermentation hybrid (extractive fermentation) In: Ramaswamy S, Ramarao BV, Huang H, editors. Separation and purification technologies in biorefineries. Chichester: Wiley; 2013. pp. 409–437. [Google Scholar]
- 329.Yang X, Tsai G-J, Tsao GT. Enhancement of in situ adsorption on the acetone–butanol fermentation by Clostridium acetobutylicum. Sep Technol. 1994;4:81–92. doi: 10.1016/0956-9618(94)80009-x. [DOI] [Google Scholar]
- 330.Yang X, Tsao GT. Enhanced acetone–butanol fermentation using repeated fed-batch operation coupled with cell recycle by membrane and simultaneous removal of inhibitory products by adsorption. Biotechnol Bioeng. 1995;47:444–450. doi: 10.1002/bit.260470405. [DOI] [PubMed] [Google Scholar]
- 331.Yang Y, Lang N, Zhang L, Wu H, Jiang W, Gu Y. A novel regulatory pathway consisting of a two-component system and an ABC-type transporter contributes to butanol tolerance in Clostridium acetobutylicum. Appl Microbiol Biotechnol. 2020;104:5011–5023. doi: 10.1007/s00253-020-10555-6. [DOI] [PubMed] [Google Scholar]
- 332.Yoo M, Bestel-Corre G, Croux C, Riviere A, Meynial-Salles I, Soucaille P. A quantitative system-scale characterization of the metabolism of Clostridium acetobutylicum. mBio. 2015;6:e01808–e01815. doi: 10.1128/mbio.01808-15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 333.Yoo M, Nguyen N-P-T, Soucaille P. Trends in systems biology for the analysis and engineering of Clostridium acetobutylicum Metabolism. Trends Microbiol. 2020;28:118–140. doi: 10.1016/j.tim.2019.09.003. [DOI] [PubMed] [Google Scholar]
- 334.Zhang C, Li T, Su G, He J. Enhanced direct fermentation from food waste to butanol and hydrogen by an amylolytic Clostridium. Renew Energy. 2020;153:522–529. doi: 10.1016/j.renene.2020.01.151. [DOI] [Google Scholar]
- 335.Zhang J, Zong W, Hong W, Zhang Z-T, Wang Y. Exploiting endogenous CRISPR-Cas system for multiplex genome editing in Clostridium tyrobutyricum and engineer the strain for high-level butanol production. Metab Eng. 2018;47:49–59. doi: 10.1016/j.ymben.2018.03.007. [DOI] [PubMed] [Google Scholar]
- 336.Zhang S, Qu C, Huang X, Suo Y, Liao Z, Wang J. Enhanced isopropanol and n-butanol production by supplying exogenous acetic acid via co-culturing two Clostridium strains from cassava bagasse hydrolysate. J Ind Microbiol Biotechnol. 2016;43:915–925. doi: 10.1007/s10295-016-1775-1. [DOI] [PubMed] [Google Scholar]
- 337.Zhao X, Condruz S, Chen J, Jolicoeur M. A quantitative metabolomics study of high sodium response in Clostridium acetobutylicum ATCC 824 acetone–butanol–ethanol (ABE) fermentation. Sci Rep. 2016;6:28307. doi: 10.1038/srep28307. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 338.Zheng J, Tashiro Y, Yoshida T, Gao M, Wang Q, Sonomoto K. Continuous butanol fermentation from xylose with high cell density by cell recycling system. Bioresour Technol. 2013;129:360–365. doi: 10.1016/j.biortech.2012.11.066. [DOI] [PubMed] [Google Scholar]
- 339.Zheng Y-N, Li L-Z, Xian M, Ma Y-J, Yang J-M, Xu X, He D-Z. Problems with the microbial production of butanol. J Ind Microbiol Biotechnol. 2009;36:1127–1138. doi: 10.1007/s10295-009-0609-9. [DOI] [PubMed] [Google Scholar]
- 340.Zhu H-F, Liu Z-Y, Zhou X, Yi J-H, Lun Z-M, Wang S-N, Tang W-Z, Li F-L. Energy conservation and carbon flux distribution during fermentation of CO or H2/CO2 by Clostridium ljungdahlii. Front Microbiol. 2020;11:416. doi: 10.3389/fmicb.2020.00416. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 341.Zhu Y. Immobilized cell fermentation for production of chemicals and fuels. In: Yang S-T, editor. Bioprocessing for value-added products from renewable resources. New technologies and applications. Amsterdam: Elsevier; 2007. pp. 373–396. [Google Scholar]
- 342.Zou W, Ye G, Zhang J, Zhao C, Zhao X, Zhang K. Genome-scale metabolic reconstruction and analysis for Clostridium kluyveri. Genome. 2018;61:605–613. doi: 10.1139/gen-2017-0177. [DOI] [PubMed] [Google Scholar]
- 343.Zverlov VV, Berezina O, Velikodvorskaya GA, Schwarz WH. Bacterial acetone and butanol production by industrial fermentation in the Soviet Union: use of hydrolyzed agricultural waste for biorefinery. Appl Microbiol Biotechnol. 2006;71:587–597. doi: 10.1007/s00253-006-0445-z. [DOI] [PubMed] [Google Scholar]