Abstract
Sudden cardiac death among top athletes is very rare, however, it is 2–4 times more frequent than in the age-matched control population. In the present study, the electrophysiological consequences of long-term exercise training were investigated on Ca2+ homeostasis and ventricular repolarization, together with the underlying alterations of ion channel expression, in a rat athlete's heart model. 12-week swimming exercise-trained and control Wistar rats were used. Electrophysiological data were obtained by using ECG, patch clamp and fluorescent optical measurements. Protein and mRNA levels were determined by the Western immunoblot and qRT-PCR techniques. Animals in the trained group exhibited significantly lower resting heart rate, higher incidence of extrasystoles and spontaneous Ca2+ release events. The Ca2+ content of the sarcoplasmic reticulum (SR) and the Ca2+ transient amplitude were significantly larger in the trained group. Intensive physical training is associated with elevated SR Ca2+ content, which could be an important part of physiological cardiac adaptation mechanism to training. However, it may also sensitize the heart for the development of spontaneous Ca2+ release and extrasystoles. Training-associated remodeling may promote elevated incidence of life threatening arrhythmias in top athletes.
Subject terms: Cardiology, Medical research, Risk factors
Introduction
Many clinical and epidemiological studies provided evidence that moderate physical exercise markedly improves cardiovascular function, decreases mortality and prevents sudden cardiac death1–3. In contrast, highly increased physical exercise performed regularly by competitive top athletes causes structural remodeling of the left ventricle, including cardiac hypertrophy (structural remodeling)4,5, alterations in the ion channel densities, possibly causing electrical instability (electrical remodeling), bradycardia6 and arrhythmias. These alterations accompanied with a preserved ejection fraction have been classically termed as physiology of the “athlete’s heart”7. Although sudden cardiac death in top athletes is very rare8, it is 2–4 times more frequent than in the age-matched control population9 and is mostly attributed to ventricular fibrillation. Therefore, long-term high intensity endurance exercise may produce increased arrhythmia sensitivity associated with sudden cardiac death2,10. One of the major suspected cause of sudden cardiac death in top athletes is hypertrophic cardiomyopathy which generates high electrical instability in ventricular tissues11.
Several studies investigated the electrophysiological consequences of intensive exercise training and provided evidence that training can distort the normal transmural repolarization heterogeneity primarily by inducing changes in Ito density and ameliorating Ca2+ handling abnormalities12. Furthermore, repolarization attenuation was also reported13,14. In spite of these data, the electrophysiological consequences of cardiac adaptation during intensive exercise training are still controversial. We hypothesized that sudden cardiac death in top athletes could be the consequence of the parallel existence of a trigger mechanism, such as delayed afterdepolarizations, and repolarization inhomogeneity, representing an enhanced arrhythmogenic substrate15.
Our aim was therefore to investigate, in a rat athlete’s heart model, the role of selected components of exercise-induced cardiac adaptation in the increased arrhythmia propensity.
Materials and methods
Animals
All experimental procedures were reviewed and approved by Ethical Committee of Hungary for Animal Experimentation in accordance with the ‘‘Principles of Laboratory Animal Care’’ defined by the National Society for Medical Research (permission number: PEI/001/2374-4/2015) and the Guide for the Care and Use of Laboratory Animals, provided by the Institute of Laboratory Animal Resources and published by the National Institute of Health (NIH Publication No. 85-23, revised 1996.); and to the EU Directive 2010/63/EU guidelines. All animals received human care.
8-week-old Wistar male rats (Toxi-Coop, Dunakeszi, Hungary) (n = 36, m = 240-280 g) were housed in standard rat cages at a constant room temperature (22 ± 2 °C) with 12/12-h light/dark cycles. Rats received standard laboratory diet and water ad libitum. The body weight of animals was controlled regularly (three times a week).
Exercise training protocol
Following acclimatization the rats were randomly divided into control (n = 18) and trained groups (n = 18). Animals of the trained group underwent a 12-week-long swimming training protocol to induce physiological myocardial hypertrophy as described previously16. In brief, swimming training was performed 5 days/week in a divided container filled with tap water (45 cm deep) maintained at 30–32 °C. For adaptation, the duration of swimming was increased by 15 min every second training day from a baseline of 15 min on the first day, until obtaining the maximal 200 min/day. During this 12-week-long period, control animals were accommodated to water 5 min/day, 5 days/week to reduce the possible differences caused by the stress of water contact.
Echocardiography
At the completion of the swimming training program, LV morphological alterations in control (n = 18) and trained (n = 18) rats were observed by echocardiography as described before17, except that rats were anesthetized with isoflurane (5% induction dose, 1–2% maintenance dose). Animals were placed on controlled heating pads, and the core temperature was maintained at 37 °C. After shaving the anterior chest, transthoracic echocardiography was performed in the supine position using a 13 MHz linear transducer (12L-RS, GE Healthcare, Horten, Norway), connected to an echocardiography system (Vivid i, GE Healthcare). Standard two-dimensional and M-mode long- and short axis (at mid-papillary level) images were acquired. Recordings were analyzed off-line using a dedicated software (EchoPac, GE Healthcare). We calculated heart rate (HR) on images recorded by M-mode. On two-dimensional recordings of the short-axis at the mid-papillary level, LV anterior (AWT) and posterior (PWT) wall thickness in diastole (index: d) and systole (index: s) as well as LV end-diastolic (LVEDD) and end-systolic diameter (LVESD) were measured. End-systole was defined as the time point of minimal LV dimensions, while end-diastole as the time point of maximal dimensions. All values were averaged over three consecutive cycles17.
Fractional shortening (FS) was determined from the measurements of LV chamber diameters: FS = [(LVEDD-LVESD)/LVEDD] × 100. LV mass was calculated according to the following formula: LVmass = [(LVEDD + AWTd + PWTd)3 − LVEDD3] × 1.04 × 0.8 + 0.14. To calculate LV mass index, we normalized the LV mass values to the tibial length (TL) of the animal17.
Morphometric assessment
Standard morphometric measurements were obtained including body weight and post-mortem heart weight, as well as tibial length. All animals were weighed before termination. At the end of Langendorff isolated heart measurements, the dry heart weights were measured (n = 12/group). Routinely prepared tibia length was measured after termination. For morphometric analysis, were using a conventional analytical balance and a ruler.
Isolated heart experiments
After 12-week-long swimming training 20-week-old male Wistar rats were used (12 control and 12 trained). ECG and left ventricular pressure of isolated hearts were measured in Langendorff-perfused hearts as described before18. Animals were anaesthetized with Na-pentobarbital (300 mg/kg, i.p.), and were injected with heparin sodium (300 IU) into the portal vein. Hearts were rapidly excised, mounted via the aorta on a Langendorff apparatus and retrogradely perfused with warm (37 °C) modified Krebs–Henseleit bicarbonate buffer (KHB) at a constant pressure (80 Hgmm). The KHB solution contained (in mmol/L): NaHCO3 25; KCl 4.3; NaCl 118.5; MgSO4 1.2; KH2PO4 1.2; glucose 10; CaCl2 1.8, having a pH of 7.4 ± 0.05 when gassed with 95% O2 + 5% CO2. The left ventricular pressure (LVP) was measured by a water-filled latex balloon which was inserted into the left ventricular cavity and inflated to obtain a control state end-diastolic pressure (LVEDP) of 4–8 mmHg18. The constant column pressure was provided by a pump (Masterflex) continuously changing the KHB.
The electrical activity as electrocardiogram (ECG) detected by the three lead self-made electrodes and signal amplifier (Experimetria, Hungary). The LVP and the ECG were simultaneously recorded using the WinWCP software (V4.9.1. Whole Cell Electrophysiology Analysis Program, John Dempster, University of Strathclyde, UK). Ventricular extrasystoles were induced by hypokalemic (2.7 mM K+) KHB solution.
Measurement of ionic currents
Rat ventricular cardiomyocytes were isolated as described in our previous study19. The L-type Ca2+ current, K+ currents, Ca2+ transient measurements were also described earlier20. The estimation of sarcoplasmic reticulum Ca2+ content by caffeine method was applied as previously described21.
Determination of phospho-PKA C, phospho-phospholamban and SERCA2 by western blot
The pan and phosphorylated forms of PKA C, phospholamban (PLN) and SERCA2 were measured in myocardial tissue samples taken from the left ventricle (n = 6/group). Fresh tissue samples were immediately frozen in liquid nitrogen and stored at -80 °C. 30 µg (PKA C, pPKA C), 50 µg (PLN, pPLN) and 20 µg (SERCA2) total protein extracts were resolved using 10% (PKA C, pPKA C), 15% (PLN, pPLN) and 8% (SERCA2) sodium dodecyl sulphate–polyacrylamide gel electrophoresis and transferred onto polyvinylidene fluoride membranes. After blocking in 5% milk-TBS-T, the membranes were immunolabeled with the respective primary antibodies provided by the Calcium Ion Regulation Antibody Sampler Kit (Cell Signalling Technology; Danvers, MA, USA; overnight, at 4 °C; dilutions: anti-PKA C, anti-pPKA C (-α, -β, and -γ when phosphorylated at Thr197): 1:7000, anti-PLN, anti-pPLN (when phosphorylated at Ser16/Thr17): 1:2500, anti-SERCA2: 1:7000). Horseradish peroxidase-conjugated goat anti-rabbit IgG (Southern Biotech, Birmingham, AL, USA; 1 h, RT; 1:8000) was used as a secondary antibody. The membranes were developed with an ECL kit (Advansta; San Jose, CA, USA) and exposed to X-ray film. Equal protein loading was verified by coomassie blue staining, and normalized to total protein. Equal protein loading was verified by coomassie blue staining. Integrated optical density values (sum of each band corrected to the background) was assessed using Image J (FIJI; NIH, Bethesda, MD, USA).
Gene expression analysis by qRT-PCR
All mRNA analyses were carried out as described previously22. Fresh left ventricular tissue samples (n = 6/group) were excised and snap-frozen in liquid nitrogen and stored at − 80 °C. Myocardial samples were homogenized in a lysis buffer (RLT buffer; Qiagen, Hilden, Germany), total RNA was isolated from the tissue using the RNeasy Fibrous Tissue Mini Kit (Qiagen) according to the manufacturer’s instructions and quantified by measuring optical density at 260 nm. 1 µg total RNA was used for reverse transcription [QuantiTect Reverse Transcription Kit (Qiagen)]. Quantitative real-time PCR was performed with the StepOnePlus Real-Time PCR System (Applied Biosystems, Foster City, CA, USA) in triplicates of each sample, in the total volume of 10 µl in each well containing cDNA, TaqMan Universal PCR MasterMix and a TaqMan Gene Expression Assay for the following genes: alpha-1 subunit of a voltage-dependent calcium channel (Cacna1c, assay ID: Rn00709287_m1), alpha-2 and delta subunits of the voltage-dependent calcium channel complex (Cacna2d1, Rn01442580_m1), beta-2 subunit of the voltage-dependent calcium channel complex (CACNB2, Rn00587789_m1), ryanodine receptor 2 (Ryr2, Rn01470303_m1), calsequestrin 2 (CASQ2, Rn00567508_m1), Na+/Ca2+exchanger (NCX) SLC8A1, Rn04338914_m1), sarco/endoplasmic reticulum Ca2+-ATPase (SERCA2) (Atp2a2, Rn00568762_m1) and phospholamban (PLN, Rn01434045_m1) purchased from Applied Biosystems. Data were normalized to glyceraldehyde-3-phosphate dehydrogenase (GAPDH; assay ID: Rn01775763_g1) and expression levels were calculated using the CT comparative method (2-ΔCT). All results are expressed as values normalized to the average values of the control group.
Statistical analysis
All data are presented as mean ± SEM. To compare post-mortem, morphological, hemodynamic parameters and ionic currents of control and trained animals, unpaired Student’s t-test was used. p ≤ 0.05 was considered to be statistically significant.
Results
Echocardiographic results
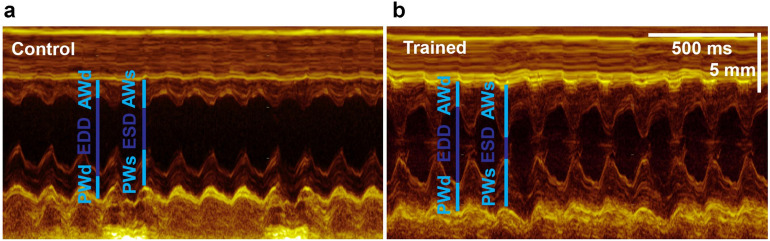
The echocardiographic results are shown in Table 1. The resting heart rate (HR) was significantly decreased in the trained rats, compared to control animals. Echocardiography also revealed significant myocardial hypertrophy, with increased left ventricular (LV) anterior and posterior wall thickness both in systole and diastole, as well as LV mass index. Unchanged LV end-diastolic and decreased end-systolic dimensions resulted in a considerably higher fractional shortening in trained rats, suggesting increased systolic performance (Fig. 1).
Table 1.
Echocardiographic and morphometric data.
| Control (n = 18) | Trained (n = 18) | p | |
|---|---|---|---|
| HR (beat/s) | 371 ± 6 | 314 ± 8 | < 0.05 |
| LVAWTd (mm) | 2.07 ± 0.04 | 2.31 ± 0.05 | < 0.05 |
| LVAWTs (mm) | 3.16 ± 0.07 | 3.67 ± 0.09 | < 0.05 |
| LVPWTd (mm) | 1.98 ± 0.06 | 2.24 ± 0.06 | < 0.05 |
| LVPWTs (mm) | 2.82 ± 0.09 | 3.23 ± 0.09 | < 0.05 |
| LVEDD (mm) | 7.66 ± 0.10 | 7.53 ± 0.13 | 0.415 |
| LVESD (mm) | 4.62 ± 0.10 | 4.17 ± 0.18 | < 0.05 |
| FS (%) | 38.9 ± 1.4 | 45.4 ± 1.8 | < 0.05 |
| LV mass (g) | 1.19 ± 0.04 | 1.41 ± 0.05 | < 0.05 |
| LV mass index (g/kg) | 2.38 ± 0.07 | 3.21 ± 0.10 | < 0.05 |
| Control (n = 12) | Trained (n = 12) | p | |
|---|---|---|---|
| Tibial length (mm) | 45.6 ± 0.8 | 45.1 ± 0.7 | 0.62 |
| Body weight (g) | 501.12 ± 12.2 | 428.85 ± 5.86 | < 0.05 |
| Heart weight (g) | 1.91 ± 0.08 | 2.18 ± 0.08 | < 0.05 |
| Heart weight index (g/kg) | 3.83 ± 0.10 | 5.1 ± 0.20 | < 0.05 |
| Ventricular weight (g) | 1.41 ± 0.06 | 1.63 ± 0.05 | < 0.05 |
| Ventricular weight index (g/kg) | 2.48 ± 0.10 | 3.81 ± 0.12 | < 0.05 |
Figure 1.
Representative left ventricular (LV) M-mode recordings from one control and one trained animal. Exercise training was associated with increased wall thickness values and markedly decreased LV end-systolic diameter.
Morphometric results and Langendorff-perfused experiments
The morphometric data of training induced cardiac hypertrophy, measured at the end of the training program, are shown in Table 1. The unchanged lengths of tibia verified the identical age between control and trained animals. The sedentary rats had significantly larger body weight, whereas physical dimensions of the heart including total weight, weight index, ventricles weight and index were significantly increased in the trained rats.
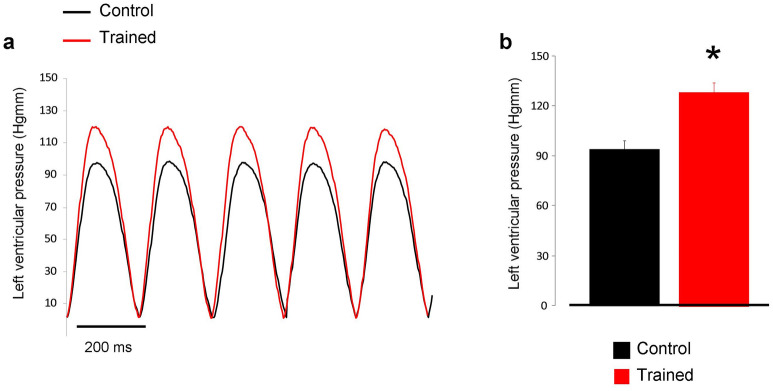
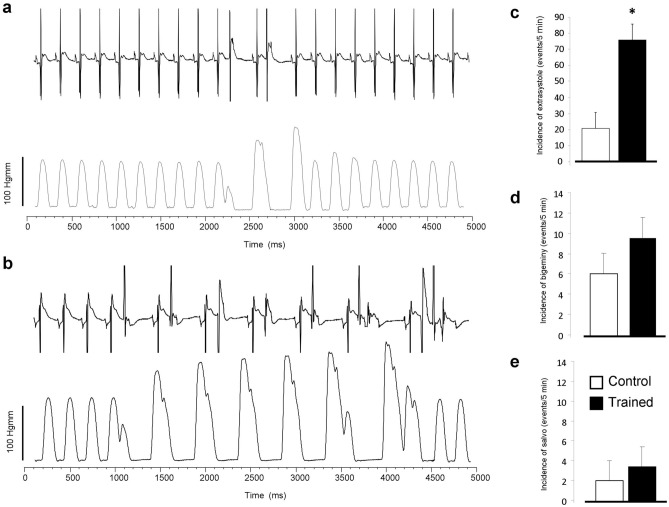
The obtained results of ex-vivo Langendorff experiments are shown in Table 2. ECG recordings showed significantly increased long-term R-R variability in the trained group compared to control hearts, while R-R intervals remained unchanged between the two groups. Similarly, QT intervals were not different, while the long-term QT variability was decreased in trained rats. In line with the echocardiographic data, the LV end-systolic pressure was found to be larger in trained animals (Fig. 2a,b). The arrhythmia analysis revealed that the trained group exerted significantly more ventricular extra beats (21 ± 4 vs 75 ± 21 extra beats, n = 12, p < 0.05). There were no significant difference between groups regarding bigeminy (6 ± 2 vs 10 ± 2 extra beats, n = 12) or salvos (2 ± 1 vs 3 ± 1 salvos, n = 12) (Fig. 3).
Table 2.
ECG and left ventricular pressure parameters measured from isolated, Langendorff perfused rat hearts.
| Control (n = 12) | Trained (n = 12) | p | |
|---|---|---|---|
| RR (ms) | 210.8 ± 5.76 | 214.17 ± 5.36 | 0.670 |
| RRSTV (ms) | 0.77 ± 0.13 | 1.25 ± 0.36 | 0.21 |
| RRLTV (ms) | 0.65 ± 0.06 | 1.57 ± 0.51 | < 0.05 |
| QT (ms) | 87.24 ± 7.46 | 85.43 ± 4.41 | 0.839 |
| QTSTV (ms) | 0.310 ± 0.03 | 0.258 ± 0.06 | 0.44 |
| QTLTV (ms) | 0.506 ± 0.03 | 0.363 ± 0.05 | < 0.05 |
| LVESP (mmHg) | 108.24 ± 6.49 | 133.56 ± 6.53 | < 0.05 |
| LVEDP (mmHg) | 4.69 ± 0.93 | 4.58 ± 0.44 | 0.924 |
| LVDP (mmHg) | 103.55 ± 6.35 | 128.98 ± 6.19 | < 0.05 |
Figure 2.
Panel (a) demonstrates representative left ventricular developed pressure curves in control (black trace) and in exercised rats (red trace) during Langendorff-perfused measurements. As bar graphs in panel (b) illustrate the left ventricular pressure was significantly higher in the case of trained group (red column).
Figure 3.
Arrhythmia incidence between trained and control groups measured by parallel registration of ECG and left ventricular pressure during Langendorff-perfusion. A representative section of ECG and pressure from control group indicate few extrasystoles in panel (a). In the trained group (panel b) the number of extrasystoles significantly increased. Panels (c–e) compares the extrasystole, bigeminy and salvo incidence between control and trained group, respectively.
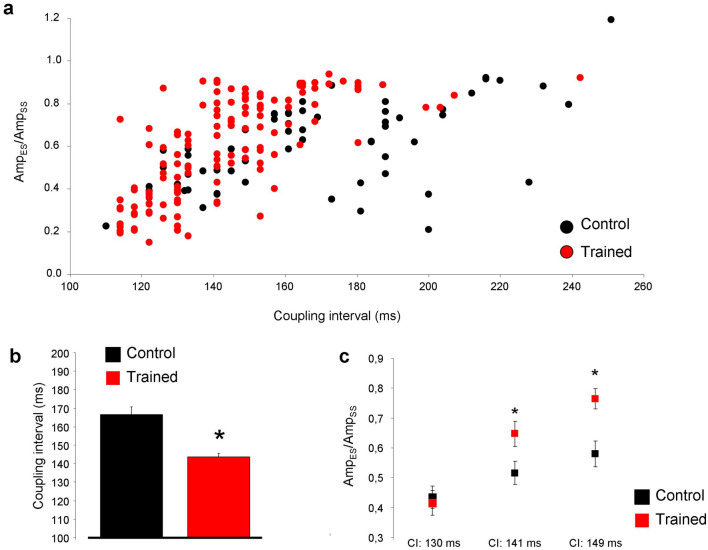
The characteristics of premature beats were further analyzed and the results are demonstrated in Fig. 4. The extra beats were analyzed for a 5-min-long section in all experiments. Only the clearly separated single extra beats were involved in the data analysis, bigeminy and salvo were excluded. Panel a represents the distribution of single extra beats/steady-state beats amplitude ratio in the function of the corresponding coupling interval. The coupling intervals were determined as the time between the initiation of the extra beat and the initiation of the upstroke of the previous steady-state beat. The trained animals (red dots in panel a, and red column in panel b) exerted significantly shorter coupling intervals compared to control (143.7 ± 1.86 ms vs 166.5 ± 4.12 ms; panel b; p < 0.05, n = 135 and 63 respectively, both from 12 animals). The amplitude ratio of premature beats/steady-state beats was compared at 3 discrete coupling intervals, where we could gather sufficient number of data (130, 141 and 149 ms). Since the control group exerted only a few numbers of extra beats at these intervals, we extended the analysis of control group to 10 min. As illustrated in panel c, the ratio of amplitudes were slightly larger in trained animals in 141 ms (0.64 ± 0.04 vs 0.51 ± 0.03, p < 0.05, n = 18 and 12 respectively, both from 12 animals) and 149 ms (0.76 ± 0.03 vs 0.58 ± 0.04, p < 0.05, n = 12 and 13 respectively both from 12 animals) compared to control.
Figure 4.
Analysis of premature beats of Langendorff-perfused hearts. (a,b) show that trained group has shorter coupling interval and larger amplitude of extra beats (c) compared to control.
Measurement of spontaneous Ca2+ releases
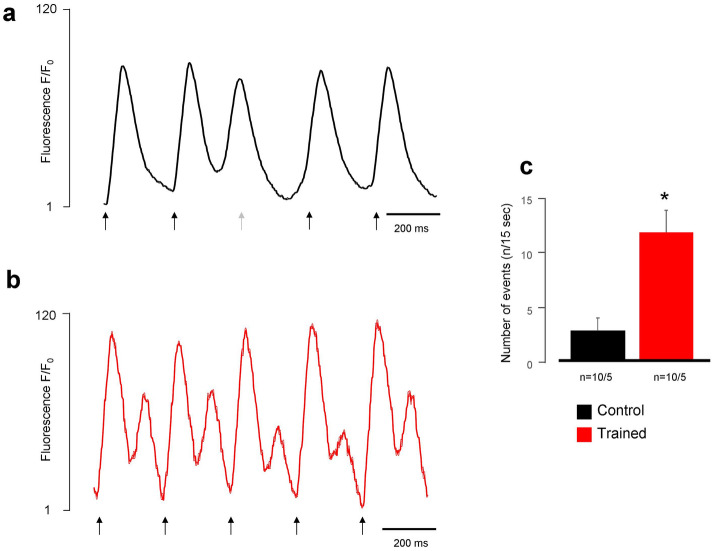
Spontaneous Ca2+ release was measured, in single cardiomyocytes field-stimulated at 4 Hz for 15 s. Although we observed spontaneous Ca2+ release episodes in both groups, the number of spontaneous events was significantly larger in the trained group (11.7 ± 3.9 events/15 s vs 2.7 ± 1.2 events/15 s, n = 10/5 and 10/5 respectively, p < 0.05, Fig. 5).
Figure 5.
Comparison of spontaneous Ca2+ releases under 4 Hz stimulation frequency between control (a) and trained (b) rats. The black arrows indicate the electric stimuli, the grey arrow marks an ineffective stimulus. We found larger number of spontaneous Ca2+ releases in the trained group, compared to control (c).
The ICa,L, SR Ca2+ content and Ca2+ transient measurements
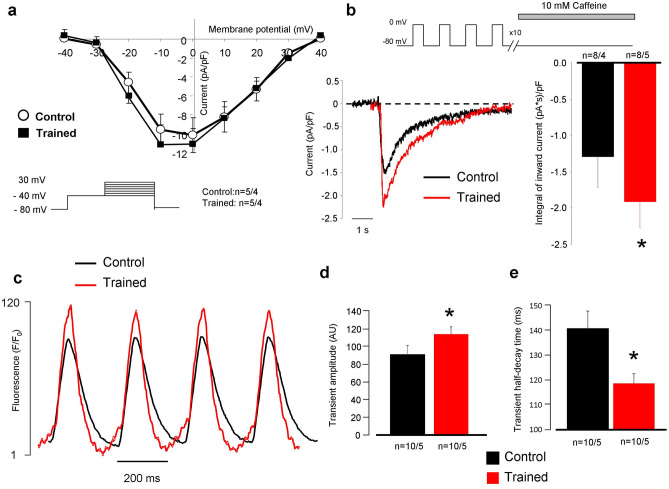
Figure 6a shows the voltage-current relationship of the L-type Ca2+ current (ICa,L) in the presence of buffered intracellular solution. 50 ms-long depolarization pulses from a holding potential of − 80 mV to − 40 mV were applied to inactivate the sodium current, followed by voltage steps to 30 mV to elicit Ca2+ current. As the superimposed plot and the bar graph (at + 10 mV) show, ICa,L density was not different in the trained group at all membrane potentials compared to control (n = 5/4 and n = 5/4). Rapid application of 10 mM caffeine (Fig. 6b) at a holding potential of − 80 mV was used to estimate the SR Ca2+ content. The caffeine flush was preceded by 10 consecutive conditioning pulses from -80 to 0 mV to reach a steady-state SR Ca2+ level. We analyzed the integral of caffeine-induced NCX currents as an indicator of the SR Ca2+ content and found that SR Ca2+ content was significantly increased in the trained group compared to controls (− 1.84 ± 0.4 (pA*s)/pF vs − 1.25 ± 0.5 (pA*s)/pF n = 8/5 and 8/4, respectively, p < 0.05; Fig. 6b). Ca2+ transients were measured at 4 Hz pacing frequency to approximate the physiological heart rate of rats (Fig. 6c). We found that the magnitudes of Ca2+ transients obtained from the trained group were increased compared to control animals (trained: 114.1 ± 8 AU vs control: 91.1 ± 10 AU, n = 10/5 and 10/5, respectively, p < 0.05, Fig. 6d). The half-decay time of the Ca2+ transients, measured at 50% of transient decay, was faster in the case of the trained group (118.7 ± 4 ms vs 140.8 ± 5 ms, n = 10, p < 0.05, Fig. 6e).
Figure 6.
Assessment of Ca2+ handling on isolated cells. Panel (a) shows identical current–voltage relationship of L-type Ca2+ current between groups. Panel (b) illustrates significantly larger inward current as a response of 10 mM caffeine application. Panel (c,d) reports larger Ca2+ transient amplitude in the case of trained rats (red trace) compared to control (black trace). Panel (e) indicates faster transient relaxation kinetics in the case of trained animals.
Repolarizing potassium currents, Ito and IK1
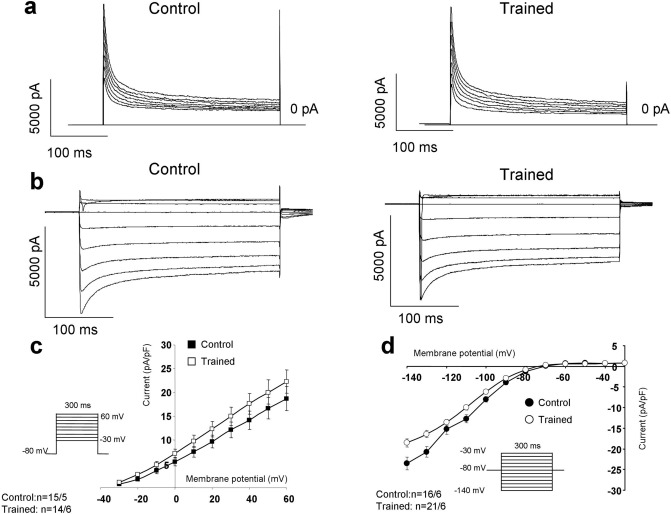
The potential remodeling induced changes in the densities of the Ito and IK1 were examined in the presence of 10 mM EGTA and ICaL inhibition. Ito (Fig. 7a,c) was elicited by 300 ms-long voltage steps to 60 mV from a holding potential of -80 mV. As original current traces (Fig. 7a), as well as current–voltage diagram (Fig. 7c) show, the currents were almost identical between groups. IK1 was measured by using 300 ms-long depolarizing pulses between − 140 and − 30 mV from a holding potential of -80 mV. Similarly to Ito, IK1 did not differ between the control and trained groups (Fig. 7b,d).
Figure 7.
Investigation of the main repolarizing potassium currents on isolated cells. As representative current traces (Panel a,b) and current–voltage diagrams (panel c) illustrate, the currents were found identical between control and trained groups.
Average cell size, as estimated by the whole cell capacitance obtained from our patch clamp experiments was increased significantly, in cardiomyocytes from trained animals compared to control cells (281 ± 12 pF vs 232 ± 15 pF, n = 20, p < 0.05) (Figs. 6a and 7).
Ion channel gene expression levels
The expression levels of genes involved in Ca2+ handling were examined by qRT-PCR. We found that the relative mRNA expression of ryanodine receptor 2 and calsequestrin were significantly higher in the trained group compared to control. The mRNA levels of NCX, SERCA2, LTCC genes and PLN were not different (Fig. 8).
Figure 8.
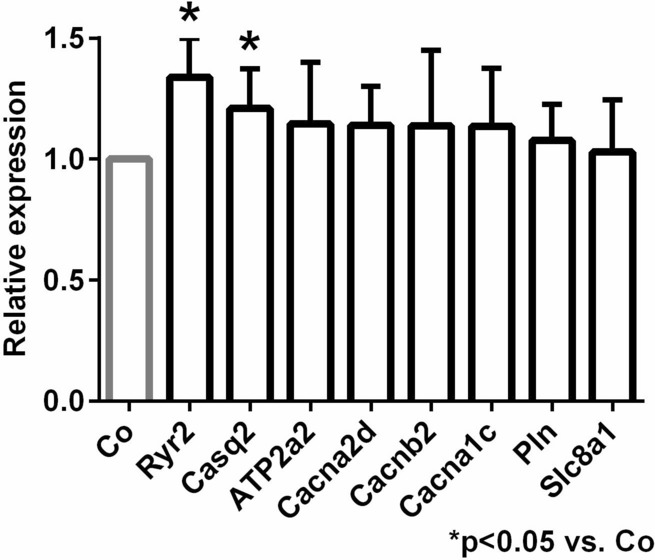
Myocardial gene expression analysis.
Phosphorylation of PKA C, PLN and SERCA2 protein expression
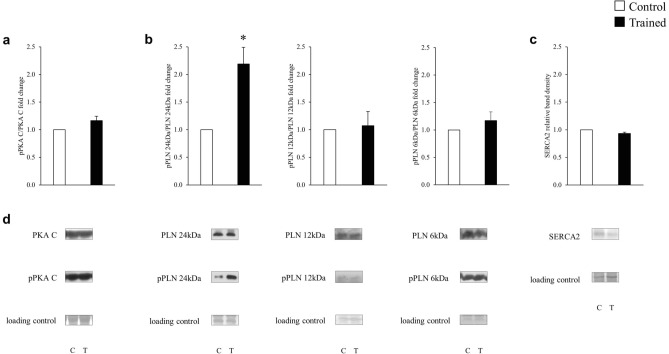
The pan and phosphorylated forms of key proteins involved in the regulation of Ca2+ homeostasis, including PKA C, PLN and SERCA2 protein expression were compared in biopsies from the left ventricles of trained and control rats. Training markedly increased phosphorylation of phospholamban oligomers. There were no significant differences between the groups regarding PKA C phosphorylation, and SERCA expression (Fig. 9).
Figure 9.
The effect of training on the SERCA phosphorylation pathway. The pan and phosphorylated PKA C (a), PLN (b) and the SERCA2 (c) protein expression.
Discussion
In this work, we studied the consequences of structural and electrical remodeling induced by intensive exercise training in rats. The most important findings of the study are: (i) ventricular hypertrophy and increased cardiac output in trained rats is associated with enhanced arrhythmogenic trigger activity; (ii) these arrhythmogenic events are predominantly of ventricular origin, suggesting delayed afterdepolarizations via increased SR Ca2+ content as an underlying mechanism; (iii) the enhanced SR Ca2+ content is maintained by increased expression of the phosphorylated form of phospholamban, and increased level of calsequestrin.
Our results suggest that the physiological adaptation of Ca2+ homeostasis underlying the increased cardiac output demand during exercise, provides a potentially harmful arrhythmia source. In our study, neither key repolarization parameters nor the repolarization inhomogeneity15 differ significantly in trained compared to control rats, which suggests no change in the arrhythmia substrate in the rat model. However, the increased triggering activity observed in the rat model of the athlete’s heart may be coupled with increased transmural dispersion of repolarization in humans (e.g.: as a possible consequence of certain medications and/or dietary supplements, steroid doping agents, or congenital genetic disorders) and therefore can be accounted for the development of life threatening arrhythmias in top athletes. It should be noted that, in contrast to other mammals including human, neither the rapid nor the slow (IKs) delayed rectifier potassium currents (IKr and IKs, respectively) operate in the rat. Therefore, these currents could not be studied in our model, warranting further studies using other species (e.g. rabbits, dogs) to investigate possible changes in repolarization potentially enhancing the arrhythmia substrate during endurance training.
Increased vagal tone may underlie sinus bradycardia
Sinus bradycardia is a hallmark characteristic of the athlete’s heart3,23 as it was the case in several animal training models6,24,25. Sinus bradycardia is generally considered as the consequence of increased vagal tone26, however, D’Souza et al6 demonstrated the down-regulation of the pacemaker (funny) current (If) after the training period of running-trained rats. In our experiments, sinus bradycardia was found in the setting of in-vivo echocardiographic measurements (Table 1), but not in Langendorff-perfused isolated hearts (Table 2). The apparent discrepancy between our study and that of D’Souza6 is may be due to the fundamental differences between the training regime (swimming vs treadmill running). Nevertheless, our present results demonstrate a key role of increased vagal tone leading to sinus bradycardia in the swimming-trained rat model.
Exercise training is associated with improved cardiac output and arrhythmia propensity
Intensive exercise requires increased cardiac output to satisfy the enhanced metabolic demand of the body. This could be associated with remodeling of several components of Ca2+ homeostasis27–29. In line with this, our in vivo and in vitro results unequivocally show increased LV pressure in the trained group (Fig. 2) which was tightly associated with larger incidence of ventricular spontaneous beats during Langendorff perfusion (Fig. 3). ECG analysis revealed the ventricular origin of the extra beats in isolated hearts, suggesting delayed afterdepolarizations mediated by Ca2+-overload, as the underlying mechanism. In this case, the premature beats are governed by facilitated forward NCX activity as a result of larger SR Ca2+ content20,30,31. The shorter coupling intervals of the premature beats in trained animals may support this hypothesis, since a higher SR Ca2+ content due to improved Ca2+ sequestration may lead to earlier spontaneous events (Fig. 4). Accordingly, in field-stimulated Ca2+ transient measurements in isolated cells, a markedly larger spontaneous Ca2+ release activity was found in the trained group that could cause Ca2+-driven extra depolarizations mediated by the NCX (Fig. 5).
Training induced complex remodeling of SR proteins could be responsible to larger Ca2+ content
Experiments with rapid application of caffeine revealed significantly larger Ca2+ content of the SR in trained animals (Fig. 6b). The actual content of the SR is determined by the dynamic balance between the uptake (via SR Ca-ATPase, SERCA) and release through the ryanodine receptors. Phospholamban (PLN), the regulatory protein of SERCA binds to SERCA in its unphosphorylated form reducing its activity. PLN exists in monomeric and pentameric forms. While it is suggested that the monomeric form inhibits SERCA32, the role of pentamers is still unclear. This model is derived from the observation that monomers are better inhibitors of PLN33,34, however, it was also found that a dynamic equilibrium exists between monomeric and pentameric forms. Furthermore, phosphorylation of SERCA or increased cytosolic Ca2+ level increased the proportion of the pentameric pool at the expense of monomeric pool35. Taken together, several independent reports claim that SERCA may interact with oligomeric forms of PLN, and the existence of oligomers appears to offer a functional advantage for the SERCA-PLN interaction36–40. In line with these findings, our results suggest that the increase of PLN pentameric form is associated with improved SERCA kinetics, providing faster decay of the Ca2+ transient, and larger Ca2+ content and possible Ca2+ overload of the sarcoplasmic reticulum (Fig. 6b–e) that may serve as an arrhythmogenic trigger source by spontaneous Ca2+ release. Furthermore, we found enhanced expression level of calsequestrin, an ubiquitous luminal Ca2+ binding protein41 (Fig. 8). It has been proposed that CASQ binds to ryanodine receptor via triadin or junctin, and when free [Ca2+] is low in the SR, ryanodine receptor open probability is reduced. When SR [Ca2+] increases, CASQ dissociates from the ryanodine receptor increasing its open probability42. It has been shown that CASQ intimately determines the magnitude and duration of the Ca2+ release from the SR43 providing a local source of the releasable Ca2+ and it has an important role in ryanodine receptor gating. It was further demonstrated that CASQ modulated ryanodine receptor function via the interaction of two further intraluminal proteins, triadin and junctin44–46. Our results indicate that the increased SR Ca2+ content is maintained by the increased expression level of CASQ, providing the molecular basis for the improved cardiac output via enhancing the storage capacity and the amount of releasable Ca2+ from the SR.
Identical repolarizing currents between trained and control groups
Crucial repolarizing currents, such as Ito and IK1, did not differ in cardiomyocytes isolated form trained and control animals (Fig. 7). In our rat model, exercise induced electrophysiological remodeling is confined to Ca2+ homeostasis and arrhythmias were promoted by increased triggering activity. It is important to note, however, that potassium channel remodeling might occur in large mammals and may critically alter transmural dispersion of potassium currents, providing an enhanced arrhythmogenic substrate. Considering that several potassium channels show Ca2+ dependence to some extent47, the indirect influence of Ca2+ homeostasis alterations on repolarization may also play an important role, and in turn may explain the increased long-term ECG QT variability in the trained group (Table 2).
Conclusion
Our results lead us to conclude that sudden cardiac death associated with training-induced remodeling could possibly arise as the disadvantageous consequence of Ca2+ homeostasis adaptation in the athlete’s heart. The increased Ca2+ content of the SR provides larger available Ca2+ upon its release, which is an adaptive response to meet the enhanced cardiac output demand during exercise. However, the enhanced Ca2+ load of the SR in trained hearts may also serve as a potential arrhythmia trigger source inducing spontaneous Ca2+ release events. The spontaneous Ca2+ release could be amplified the presence of increased sympathetic tone or electrolyte disturbances during training, exposing cardiomyocytes to extra Ca2+ load. The enhanced source of arrhythmogenic triggers, coupled with possible impaired repolarization reserve in human may lead to the development of life threatening arrhythmias such as ‘torsades de pointes’ tachyarrhythmia or ventricular fibrillation in top athletes.
Limitations
(i) Our rat model did not show alterations of the repolarization process, transmural dispersion and therefore changes in the arrhythmia substrate could not be observed. This observation may derive from the species-dependent characteristics of repolarization. Since the rat action potential repolarization relies on Ito and IK1, IKur (Kv1.5), the action potential prolonging effect of a possible IKr and IKs downregulation could not be detected, and should be studied experimentally in other species like rabbit, guinea-pig or dog. (ii) In addition, the Ca2+ removal from the intracellular space in rats depends more on the SERCA activity compared to larger species, including human.
Acknowledgements
We are grateful for helpful suggestions of Prof. Dr. David Eisner (University of Manchester). This work was supported by grants from the National Research Development and Innovation Office (NKFIH PD-125402 (for NN), FK-129117 (for NN), K-119992 and K-120277, the János Bolyai Research Scholarship of the Hungarian Academy of Sciences (for BO and NN), the UNKP-17-4-I-SE-78 New National Excellence Program of the Ministry for Innovation and Technology (for AO), GINOP-2.3.2-15-2016-00006, the Ministry of Human Capacities Hungary (20391-3/2018/FEKUSTRAT and EFOP-3.6.2-16-2017-0006 (LIVE LONGER)), by the Semmelweis University Innovation Found STIA-KF-17 (to AO). University of Szeged Open Access Fund, No.: 5066.
Abbreviations
- AWd
Anterior wall in diastole
- AWs
Anterior wall in systole
- AWT
Anterior wall thickness
- CASQ
Calsequestrin
- EDD
END-diastolic diameter
- ESD
End-systolic diameter
- FS
Fractional shortening
- GAPDH
Glyceraldehyde-3-phospate dehydrogenase
- HR
Heart rate
- ICaL
L-type calcium current
- IgG
Immunoglobulin G
- IK1
Inward rectifier potassium current
- IKr
Rapid component of the delayed rectifier potassium current
- IKs
Slow component of the delayed rectifier potassium current
- Ito
Transient outward potassium current
- LTCC
L-type calcium channel
- LV
Left ventricular
- LVAWTd
Left ventricular anterior wall thickness at diastole
- LVAWTs
Left ventricular anterior wall thickness at systole
- LVDP
Left ventricular developed pressure
- LVEDD
Left ventricular end-diastolic diameter
- LVEDP
Left ventricular end-diastolic pressure
- LVESD
Left ventricular end-systolic diameter
- LVESP
Left ventricular end-systolic pressure
- LVP
Left ventricular pressure
- LVPWTd
Left ventricular posterior wall thickness at diastole
- LVPWTs
Left ventricular posterior wall thickness at systole
- NCX
Sodium/calcium exchanger (Na+/Ca2+ exchanger)
- PLN
Phospholamban
- pPKAC
Phospho-protein kinase A C
- pPLN
Phospho-phospholamban
- PWd
Posterior wall in diastole
- PWs
Posterior wall in systole
- PWT
Posterior wall thickness
- Ryr
Ryanodine receptor
- SERCA2a
Sarcoplasmic reticulum Ca2+-ATPase
- TL
Tibial length
Author contributions
Study conception and design: A.V., J.P., N.N., K.A. Performing experiments: P.G., K.O., V.D.H., B.Ö., J.S., Z.K., A.P., A.B.B., A.O., J.P. Data analysis and visualization: A.O., T.R., J.P., N.N. Writing initial manuscript: N.N. Critical revisions: All authors. Supervision: T.R., B.M., J.G.Y.P., I.B., A.V., J.P. Funding acquisition: T.R., I.B., A.V., N.N.
Competing interests
The authors declare no competing interests.
Footnotes
Publisher's note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
These authors jointly supervised this work: Norbert Nagy and János Prorok.
Change history
6/4/2021
A Correction to this paper has been published: 10.1038/s41598-021-91695-1
References
- 1.Marshall KD, et al. Heart failure with preserved ejection fraction: chronic low-intensity interval exercise training preserves myocardial O2 balance and diastolic function. J. Appl. Physiol. 2013;114:131–147. doi: 10.1152/japplphysiol.01059.2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.O'Keefe JH, et al. Potential adverse cardiovascular effects from excessive endurance exercise. Mayo Clin. Proc. 2012;87:587–595. doi: 10.1016/j.mayocp.2012.04.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Benito B, et al. Cardiac arrhythmogenic remodeling in a rat model of long-term intensive exercise training. Circulation. 2011;123:13–22. doi: 10.1161/CIRCULATIONAHA.110.938282. [DOI] [PubMed] [Google Scholar]
- 4.Olah A, et al. Characterization of the dynamic changes in left ventricular morphology and function induced by exercise training and detraining. Int. J. Cardiol. 2019;277:178–185. doi: 10.1016/j.ijcard.2018.10.092. [DOI] [PubMed] [Google Scholar]
- 5.Olah A, et al. Sex differences in morphological and functional aspects of exercise-induced cardiac hypertrophy in a rat model. Front. Physiol. 2019;10:889. doi: 10.3389/fphys.2019.00889. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.D'Souza A, et al. Exercise training reduces resting heart rate via downregulation of the funny channel HCN4. Nat. Commun. 2014;5:3775. doi: 10.1038/ncomms4775. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Maron BJ, Pelliccia A. The heart of trained athletes: cardiac remodeling and the risks of sports, including sudden death. Circulation. 2006;114:1633–1644. doi: 10.1161/CIRCULATIONAHA.106.613562. [DOI] [PubMed] [Google Scholar]
- 8.Maron BJ. Hypertrophic cardiomyopathy and other causes of sudden cardiac death in young competitive athletes, with considerations for preparticipation screening and criteria for disqualification. Cardiol. Clin. 2007;25:399–414, vi. doi: 10.1016/j.ccl.2007.07.006. [DOI] [PubMed] [Google Scholar]
- 9.Corrado D, Michieli P, Basso C, Schiavon M, Thiene G. How to screen athletes for cardiovascular diseases. Cardiol. Clin. 2007;25(391–397):v–vi. doi: 10.1016/j.ccl.2007.07.008. [DOI] [PubMed] [Google Scholar]
- 10.Aschar-Sobbi R, et al. Increased atrial arrhythmia susceptibility induced by intense endurance exercise in mice requires TNFalpha. Nat. Commun. 2015;6:6018. doi: 10.1038/ncomms7018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Maron BJ, Maron MS. Hypertrophic cardiomyopathy. Lancet. 2013;381:242–255. doi: 10.1016/S0140-6736(12)60397-3. [DOI] [PubMed] [Google Scholar]
- 12.Roman-Campos D, et al. Cardiac structural changes and electrical remodeling in a thiamine-deficiency model in rats. Life Sci. 2009;84:817–824. doi: 10.1016/j.lfs.2009.03.011. [DOI] [PubMed] [Google Scholar]
- 13.Basso C, Corrado D, Thiene G. Arrhythmogenic right ventricular cardiomyopathy in athletes: diagnosis, management, and recommendations for sport activity. Cardiol. Clin. 2007;25:415–422, vi. doi: 10.1016/j.ccl.2007.08.009. [DOI] [PubMed] [Google Scholar]
- 14.Hart G. Exercise-induced cardiac hypertrophy: a substrate for sudden death in athletes? Exp. Physiol. 2003;88:639–644. doi: 10.1113/eph8802619. [DOI] [PubMed] [Google Scholar]
- 15.Varro A, Baczko I. Possible mechanisms of sudden cardiac death in top athletes: a basic cardiac electrophysiological point of view. Pflugers Arch. 2010;460:31–40. doi: 10.1007/s00424-010-0798-0. [DOI] [PubMed] [Google Scholar]
- 16.Radovits T, et al. Rat model of exercise-induced cardiac hypertrophy: hemodynamic characterization using left ventricular pressure-volume analysis. Am. J. Physiol. Heart Circ. Physiol. 2013;305:H124–134. doi: 10.1152/ajpheart.00108.2013. [DOI] [PubMed] [Google Scholar]
- 17.Olah A, et al. Complete reversion of cardiac functional adaptation induced by exercise training. Med. Sci. Sports Exerc. 2017;49:420–429. doi: 10.1249/MSS.0000000000001127. [DOI] [PubMed] [Google Scholar]
- 18.Szepesi J, et al. Comparison of the efficiency of Na+/Ca2+ exchanger or Na+/H+ exchanger inhibition and their combination in reducing coronary reperfusion-induced arrhythmias. J. Physiol. Pharmacol. 2015;66:215–226. [PubMed] [Google Scholar]
- 19.Nagy N, et al. Does small-conductance calcium-activated potassium channel contribute to cardiac repolarization? J. Mol. Cell. Cardiol. 2009;47:656–663. doi: 10.1016/j.yjmcc.2009.07.019. [DOI] [PubMed] [Google Scholar]
- 20.Kohajda Z, et al. The effect of a novel highly selective inhibitor of the sodium/calcium exchanger (NCX) on cardiac arrhythmias in in vitro and in vivo experiments. PLoS ONE. 2016;11:e0166041. doi: 10.1371/journal.pone.0166041. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Oravecz K, et al. Inotropic effect of NCX inhibition depends on the relative activity of the reverse NCX assessed by a novel inhibitor ORM-10962 on canine ventricular myocytes. Eur. J. Pharmacol. 2018;818:278–286. doi: 10.1016/j.ejphar.2017.10.039. [DOI] [PubMed] [Google Scholar]
- 22.Olah A, et al. Physiological and pathological left ventricular hypertrophy of comparable degree is associated with characteristic differences of in vivo hemodynamics. Am. J. Physiol. Heart Circ. Physiol. 2016;310:H587–597. doi: 10.1152/ajpheart.00588.2015. [DOI] [PubMed] [Google Scholar]
- 23.al-Ani M, Munir SM, White M, Townend J, Coote JH. Changes in R-R variability before and after endurance training measured by power spectral analysis and by the effect of isometric muscle contraction. Eur. J. Appl. Physiol. Occup. Physiol. 1996;74:397–403. doi: 10.1007/bf02337719. [DOI] [PubMed] [Google Scholar]
- 24.Northcote RJ, Canning GP, Ballantyne D. Electrocardiographic findings in male veteran endurance athletes. Br. Heart J. 1989;61:155–160. doi: 10.1136/hrt.61.2.155. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Chapman JH. Profound sinus bradycardia in the athletic heart syndrome. J. Sports Med. Phys. Fitness. 1982;22:45–48. [PubMed] [Google Scholar]
- 26.Jensen-Urstad K, Saltin B, Ericson M, Storck N, Jensen-Urstad M. Pronounced resting bradycardia in male elite runners is associated with high heart rate variability. Scand. J. Med. Sci. Sports. 1997;7:274–278. doi: 10.1111/j.1600-0838.1997.tb00152.x. [DOI] [PubMed] [Google Scholar]
- 27.Chen Y, et al. Exercise intensity-dependent reverse and adverse remodeling of voltage-gated Ca(2+) channels in mesenteric arteries from spontaneously hypertensive rats. Hypertens. Res. 2015;38:656–665. doi: 10.1038/hr.2015.56. [DOI] [PubMed] [Google Scholar]
- 28.de Waard MC, et al. Detrimental effect of combined exercise training and eNOS overexpression on cardiac function after myocardial infarction. Am. J. Physiol. Heart Circ. Physiol. 2009;296:H1513–1523. doi: 10.1152/ajpheart.00485.2008. [DOI] [PubMed] [Google Scholar]
- 29.da Costa Rebelo RM, Schreckenberg R, Schluter KD. Adverse cardiac remodelling in spontaneously hypertensive rats: acceleration by high aerobic exercise intensity. J. Physiol. 2012;590:5389–5400. doi: 10.1113/jphysiol.2012.241141. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Diaz ME, Graham HK, O'Neill SC, Trafford AW, Eisner DA. The control of sarcoplasmic reticulum Ca content in cardiac muscle. Cell Calcium. 2005;38:391–396. doi: 10.1016/j.ceca.2005.06.017. [DOI] [PubMed] [Google Scholar]
- 31.Nagy N, et al. Selective Na(+)/Ca(2+) exchanger inhibition prevents Ca(2+) overload-induced triggered arrhythmias. Br. J. Pharmacol. 2014;171:5665–5681. doi: 10.1111/bph.12867. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Stokes DL. Keeping calcium in its place: Ca(2+)-ATPase and phospholamban. Curr. Opin. Struct. Biol. 1997;7:550–556. doi: 10.1016/s0959-440x(97)80121-2. [DOI] [PubMed] [Google Scholar]
- 33.Autry JM, Jones LR. Functional co-expression of the canine cardiac Ca2+ pump and phospholamban in Spodoptera frugiperda (Sf21) cells reveals new insights on ATPase regulation. J. Biol. Chem. 1997;272:15872–15880. doi: 10.1074/jbc.272.25.15872. [DOI] [PubMed] [Google Scholar]
- 34.Kimura Y, Kurzydlowski K, Tada M, MacLennan DH. Phospholamban inhibitory function is activated by depolymerization. J. Biol. Chem. 1997;272:15061–15064. doi: 10.1074/jbc.272.24.15061. [DOI] [PubMed] [Google Scholar]
- 35.Cornea RL, Jones LR, Autry JM, Thomas DD. Mutation and phosphorylation change the oligomeric structure of phospholamban in lipid bilayers. Biochemistry. 1997;36:2960–2967. doi: 10.1021/bi961955q. [DOI] [PubMed] [Google Scholar]
- 36.Colyer J. Control of the calcium pump of cardiac sarcoplasmic reticulum. A specific role for the pentameric structure of phospholamban? Cardiovasc. Res. 1993;27:1766–1771. doi: 10.1093/cvr/27.10.1766. [DOI] [PubMed] [Google Scholar]
- 37.Fujii J, Maruyama K, Tada M, MacLennan DH. Expression and site-specific mutagenesis of phospholamban. Studies of residues involved in phosphorylation and pentamer formation. J. Biol. Chem. 1989;264:12950–12955. doi: 10.1016/S0021-9258(18)51579-9. [DOI] [PubMed] [Google Scholar]
- 38.Oxenoid K, Chou JJ. The structure of phospholamban pentamer reveals a channel-like architecture in membranes. Proc. Natl. Acad. Sci. USA. 2005;102:10870–10875. doi: 10.1073/pnas.0504920102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Reddy LG, Jones LR, Thomas DD. Depolymerization of phospholamban in the presence of calcium pump: a fluorescence energy transfer study. Biochemistry. 1999;38:3954–3962. doi: 10.1021/bi981795d. [DOI] [PubMed] [Google Scholar]
- 40.Stokes DL, Pomfret AJ, Rice WJ, Glaves JP, Young HS. Interactions between Ca2+-ATPase and the pentameric form of phospholamban in two-dimensional co-crystals. Biophys. J. 2006;90:4213–4223. doi: 10.1529/biophysj.105.079640. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Gyorke S, Stevens SC, Terentyev D. Cardiac calsequestrin: quest inside the SR. J. Physiol. 2009;587:3091–3094. doi: 10.1113/jphysiol.2009.172049. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Gyorke I, Hester N, Jones LR, Gyorke S. The role of calsequestrin, triadin, and junctin in conferring cardiac ryanodine receptor responsiveness to luminal calcium. Biophys. J . 2004;86:2121–2128. doi: 10.1016/S0006-3495(04)74271-X. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Terentyev D, et al. Modulation of SR Ca release by luminal Ca and calsequestrin in cardiac myocytes: effects of CASQ2 mutations linked to sudden cardiac death. Biophys. J . 2008;95:2037–2048. doi: 10.1529/biophysj.107.128249. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Gyorke S, Terentyev D. Modulation of ryanodine receptor by luminal calcium and accessory proteins in health and cardiac disease. Cardiovasc. Res. 2008;77:245–255. doi: 10.1093/cvr/cvm038. [DOI] [PubMed] [Google Scholar]
- 45.Gyorke S, et al. Regulation of sarcoplasmic reticulum calcium release by luminal calcium in cardiac muscle. Front. Biosci. 2002;7:d1454–1463. doi: 10.2741/gyorke. [DOI] [PubMed] [Google Scholar]
- 46.Terentyev D, Viatchenko-Karpinski S, Valdivia HH, Escobar AL, Gyorke S. Luminal Ca2+ controls termination and refractory behavior of Ca2+-induced Ca2+ release in cardiac myocytes. Circ. Res. 2002;91:414–420. doi: 10.1161/01.res.0000032490.04207.bd. [DOI] [PubMed] [Google Scholar]
- 47.Nagy N, et al. Role of Ca(2)+-sensitive K+ currents in controlling ventricular repolarization: possible implications for future antiarrhytmic drug therapy. Curr. Med. Chem. 2011;18:3622–3639. doi: 10.2174/092986711796642463. [DOI] [PubMed] [Google Scholar]