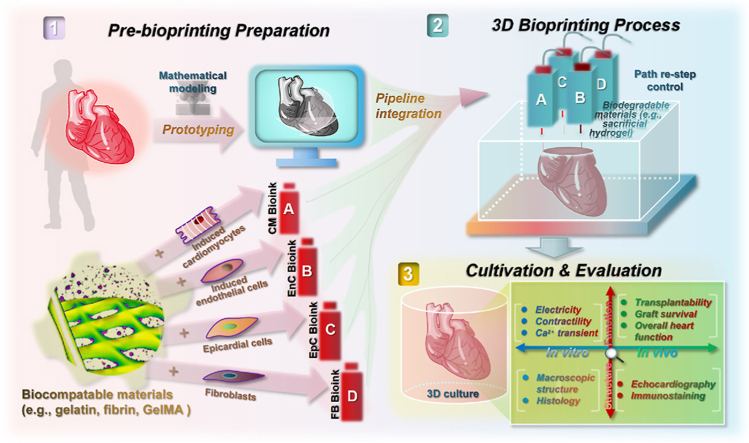
Abstract
Cardiovascular disease is still one of the leading causes of death in the world, and heart transplantation is the current major treatment for end-stage cardiovascular diseases. However, because of the shortage of heart donors, new sources of cardiac regenerative medicine are greatly needed. The prominent development of tissue engineering using bioactive materials has creatively laid a direct promising foundation. Whereas, how to precisely pattern a cardiac structure with complete biological function still requires technological breakthroughs. Recently, the emerging three-dimensional (3D) bioprinting technology for tissue engineering has shown great advantages in generating micro-scale cardiac tissues, which has established its impressive potential as a novel foundation for cardiovascular regeneration. Whether 3D bioprinted hearts can replace traditional heart transplantation as a novel strategy for treating cardiovascular diseases in the future is a frontier issue. In this review article, we emphasize the current knowledge and future perspectives regarding available bioinks, bioprinting strategies and the latest outcome progress in cardiac 3D bioprinting to move this promising medical approach towards potential clinical implementation.
Keywords: 3D bioprinting, Stem cell therapy, Bioink, Heart repair and regeneration
Graphical abstract
Highlights
-
•
The research progress of 3D bioprinting technology integrating biomaterials with heart cells is systematically reviewed.
-
•
The reproduction of both structure and contraction is an indispensable way for perfect heart repair and regeneration.
-
•
For the success of cardiac 3D bioprinting, the process flow of bioink should be standardized in an orderly manner.
-
•
3D bioprinting technology is increasing the personalized utilization of biomaterials in cardiac repair and regeneration.
1. Introduction
Pathological changes caused by the loss of the structure and function of the heart are still the main cause of death among the population [1]. The factors that cause such losses are often diverse, such as congenital heart disease, ischaemic heart disease, trauma, inflammation, etc., which will collectively lead to the progressive impairment of cardiac function [2]. Due to the lack of natural endogenous cardiac stem cells in adult individuals, prolonged heart damage may cause irreversible acute or chronic heart failure, mainly manifested by arrhythmia or a significant reduction in ejection fraction. At present, the corresponding therapeutic methods are still mostly limited to symptomatic treatment, failing to achieve complete in situ cardiac resurrection similar to the repair of skin epidermal tissue. For patients with end-stage heart failure, heart transplantation is a viable treatment. However, because the number of donor lags far behind the number required by patients, coupled with the high probability of immune rejection and surgical complications, the desire for the complete and long-term recovery of heart function is still unfulfilled [3]. Therefore, how to apply cutting-edge technology to artificially rebuild a heart that can match the structure and function of natural myocardial tissue has become a frontier direction in tissue engineering and regenerative medicine [4].
In the past decade, with the development of in vitro induced pluripotent stem cells (iPSCs) [5,6] and subsequent in vitro and in vivo direct cardiac reprogramming [7,8], new explorative cell sources for cardiology studies were soon targeted. These emerging strategies, as we had previously followed [[9], [10], [11]], can produce cell types of certain cardiovascular lineages through the boost of genetic and epigenetic-mediated cell identity transformation, which is a practical solution to the limited number of target cardiac cells caused by cell fate finalization. However, due to the lack of solid mechanical support and directional guidance from the nearby extracellular matrix (ECM), these cells are not morphologically sufficient for higher-dimension organization, which is a common basic for the anatomical shaping and organic integration. For overjoyed, we found that the excavation of biocompatible and biodegradable polymer materials [12], which can partially play the role of ECM in tissue engineering, had shown potential auxiliary effects [13,14]. However, how to bond active cells and biomaterials into a stable complex to faithfully restore the heart microenvironment is still a prominent obstacle.
The era of three-dimensional (3D) bioprinting originates from the extreme needs of biotechnological development. It is a layer-by-layer additive manufacturing technology that can accurately deposit biological materials and active cells in accordance with a certain spatial pattern [15]. In addition, it can produce a highly continuous and stable biological pattern, which can achieve a high-resolution simulation of the key state of the heart and pave the way for the innovative exploration of myocardial tissue repair and regeneration using a next-generation technology [16,17]. However, it is important to realize that 3D bioprinting-based heart regeneration technology is still in the early stages of exploration and therefore there is much that can be achieved through the collaboration of scientists from various areas. For example, the bioink parameters covered by this technology need to be continuously explored at the physical and chemical levels to better characterize the structure and physiological complexity of the myocardium [18,19]. In this review, we focus on the recent innovations regarding using hydrogel materials as bioinks and seed cells to clarify the significant impact of 3D bioprinting on the field of cardiovascular tissue engineering.
2. Anatomy of heart and cardiac diseases
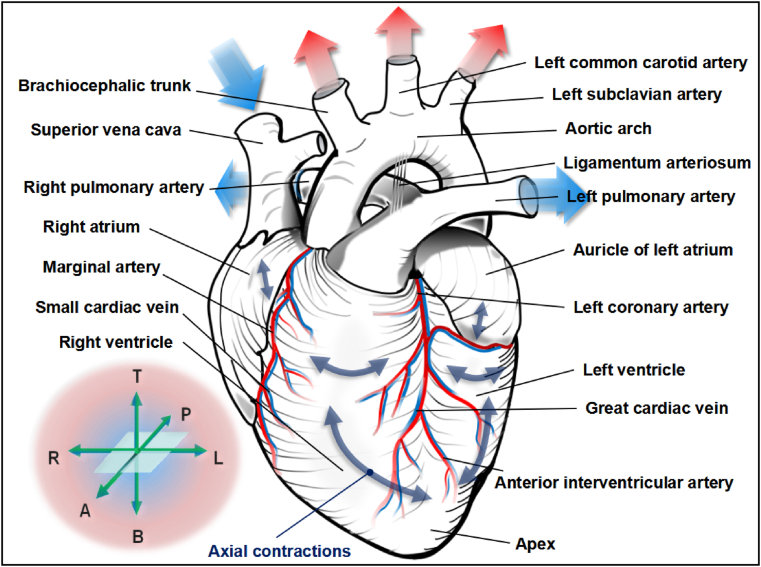
For adult mammals, the heart functioning as the cross-centre between systemic circulation and pulmonary circulation is approximately the size of one's fist and has a stylized four-chambered muscular anatomy. Precisely, the portion near the apex of the heart is divided into the left and right ventricles by the ventricular septum, while the atrial septum above is responsible for maintaining the isolation between the left and right atria (Fig. 1). Based on the pattern of this architecture, the left and right parts of the heart are both equipped with a regular reciprocating electrophysiological programme in order to accurately control the haemodynamics of the body using a series of substantial axial movements [20]. Precisely, oxygen-deficient blood inside the vena cava is pulled into the heart by the diastolic right atrium, and it is then pumped into the lungs for gas exchange by right ventricular muscles. The renewed blood with a high oxygen content is subsequently squeezed into the left heart to efficiently infuse fresh energy into the body (Fig. 1). Therefore, the muscle fibre population of the heart, especially the myocardial tissue that moves longitudinally, laterally, and obliquely along the ventricular wall, is essential for the effective clockwise and counter-clockwise torsional motion during systole and diastole to achieve optimal ejection and filling [21,22].
Fig. 1.
Anatomy and function of human heart. The unique structure of the heart's strong muscle and large/small blood vessel that closely merge together as one is the mechanical force and energy basis for the human body in performing various physiological activities. In particular, the striated muscle fibrous tissue that undergoes autonomic contraction at the same electro-physiological rhythm is a critical feature of coordination between the left heart and the right heart, in order to efficiently handle the repeated remodeling of blood biochemistry. Coordinate icons in the lower left corner are “T” for top, “P” for posterior, “A” for anterior, “R” for right, “L” for left, “B” for bottom.
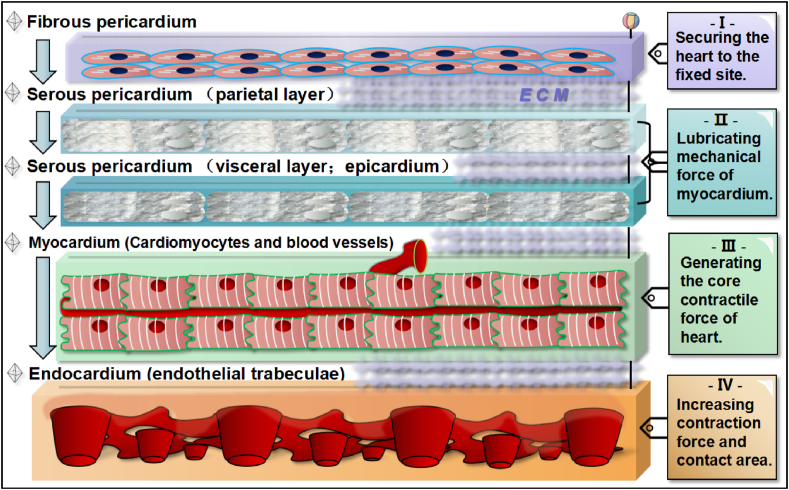
The natural arrangement of the myocardial microstructure at the sub-organ level, as summarized in Fig. 2, is quite delicate and distinct [23,24]. Specifically, the outer myocardial tissue is a layer of fibrous pericardium, and its main function is locating the anatomical position of the entire heart. Downward is the parietal and epicardial layers of the serous pericardium, which together form the pericardial cavity and secrete adequate fluid that lubricates the non-stop moving organic components below. Close to the epicardial layer is the heart's core functional unit, namely, the layer of the active myocardium, containing striated cardiomyocytes (CMs) and nourishing blood vessels (Fig. 2). The innermost layer of the myocardium is the endocardium and endothelial trabeculae, which can significantly enhance the internal contractility and force area of the myocardium. The ECM network containing collagen is distributed among the gaps between the above-mentioned layers and unites the integrity of the heart. Such a model of tacit division and union of labour naturally forges the heart's unique operating mode, and meanwhile it poses a high standard for challenging the artificial reshaping of this active organization.
Fig. 2.
Five cross-sectional layers of myocardial tissue from exterior to interior. The first layer is fibrous pericardium consisting of fibroblasts that can secure the heart's anatomical position. The second and third layer is both serous pericardium, which can lubricate mechanical force of myocardium. The forth layer is the thick myocrdium consisting of active cardiomyocytes and supportive blood vessels. The fifth layer is the robust endocardium that has endothelial trabeculae in order to increase pumping force and area inside. Each layer of myocardial tissue is physically and orderly bond by the components of ECM.
The factors that cause pathological changes in the structure and/or function of the heart are diverse, such as coronary ischaemic heart diseases [25], severe congenital heart diseases [26], and heart dysfunction caused by certain bacterial or viral infections [27]. The pathophysiology that specifically affects one or more layers of myocardial tissue is moreover highly heterogeneous; and if the lesions are treated inappropriately or they are too complicated to cure, this often leads to intractable heart failure [28]. Clinically, heart transplantation is the preferred routine treatment for end-stage heart disease, and other attempts (such as the infusion therapy with mere bioactive cells) are still limited to the laboratory stage. However, due to the scarcity of donors and the inevitability of immune rejection, the long-term survival of patients receiving heart transplantation is difficult to guarantee [29]. Therefore, conducting an in-depth exploration into the clinical transformation of advanced tissue engineering hearts and heart parts, such as using 3D bioprinting technology to form a workable myocardium, is urgently needed [30].
3. 3D bioprinting technology
3.1. General modalities of 3D bioprinting
3D bioprinting stands for an emerging category on a procedural manufacturing technology capable of fabricating 3D constructs in possession of bio-activities or bio-functions, and mostly in a sequential layer-by-layer manner of sophisticated depositions following a specific digital pattern [31,32]. As the designation indicates, the mature “printing” product and the extensive 3D patterning experience are the two engines of 3D “bioprinting” [[31], [32], [33]]. With the development of the additive manufacturing industry, the reconstruction strategies and specific operations dealing with the 3D digital standardization of source materials have been derived into various fixed systems, such as pneumatic extrusion printing, needle-droplet printing and photocuring-based printing [[32], [33], [34]]. For extrusion and droplet bioprinting channels, the materials available per unit time mainly rely on persistent external mechanical force and gravity to draw forth a 3D structure directed by an established route, while laser-assisted bioprinting relies on the sensitive optical guidance as the main workmanship for material sculpture. Typically, the common feature of these modalities lies in the composition of coagulative rheological “inks” required for rapid volumetric prototyping.
While the traditional additive manufacturing procedures simply focus on the ink's shaping ability to forge the target topography, the highlighting characteristics of tissue and organ-targeted 3D bioprinting consist in that the methods (e.g. the normal way, the microfluidic bioprinting, and embedded bioprinting) and inks should have high-level biocompatibility and/or biodegradability, thus requiring live cells or bioactive molecules well merged inside the “bioink” [34]. In general, through a chain of spatiotemporal manufacturing procedures, the established organic objects composed of bioactive materials and functional cells can be vividly reproduced inside the laboratory's culture chamber [[31], [32], [33], [34]]. Notably, one attractive advancement of 3D bioprinting method is the high-fidelity replication of flexible and tough textures de novo, such as the printing of a bunch of functional skeletal muscle [35]. Indeed, by permitting the accurate micro-control of the composition, spatial distribution and exquisite shape of the engineered tissues, such an advanced expertise aimed to achieve 100% bionics clearly surpasses many cruel limitations of the primitive 3D bioprocessing strategy [36]. Moreover, 3D bioprinting technology opens a new methodological portal for tissue engineering and regenerative medicine, especially in perfecting the challenging reconstruction of complex tissues or organs [33,36].
The development of engineering strategies based on 3D bioprinting technology has currently been through different periods. Originally, exploratory researchers mainly employed printable hydrogel materials as biological scaffolds or substrates and coated them with cells or infiltrated active molecules afterwards [37,38]. With the continuous expansion of bio-adaptive materials, using crosslinkable hydrogel as one half of the bioink and functional living cells and/or cofactors as the other is constantly being applied at the frontier of the field [36]. In addition, benefitting from the significant improvement of bioprinting equipment [39], not only can the coordinated extrusion of prepared materials and cells be stably achieved but also the primary single-arm and single-ink method has also been upgraded to advanced versions that can run multi-arms and multi-inks, which greatly improves the systematic accuracy, resolution and diversity for 3D bioprinting supported research [40,41]. Furthermore, in response to the requirement for functional science in the upcoming post-3D era, researchers have also been accelerating the absorption of time as the fourth dimension, that is, exploring the higher-level equations of regenerative medicine based on potential 4D bioprinting [42].
3.2. Standardized bioinks for 3D bioprinting
3.2.1. Integral biomaterials
As one of the core necessary components of the bioink in a 3D bioprinting system, a satisfactory printable biomaterial must take into account the real-time maintenance of the physical shape and cell function of the printed body to obtain reliable and sufficient active transplants for regenerative applications [43]. At present, the bioprinting materials that have been reported and applied mainly include two types, namely, the hydrogels derived from natural polymers and synthetic hydrogel polymers [44,45]. Indeed, each member of these two types of matrix materials can be used individually or can be mixed as a composite hydrogel ink according to bespoke preparation needs or extracellular component patterns.
Natural polymer-derived hydrogels mainly include collagen, gelatine, hyaluronic acid, fibrin, alginate, agarose, chitosan, keratin, Matrigel and decellularized ECM (dECM) [46]. Due to the excellent biological activity and the fact that many members are naturally present in the human body, the natural source group has been regarded as the most popular application component of the bioink skeleton [44,47]. However, their shortcomings are their low mechanical strength, potential to induce immune responses, and batch-to-batch variability [48]. Synthetic hydrogel polymers, on the other hand, mainly include polyacrylic acid derivatives, polyethylene glycol copolymers, polyvinyl alcohol, polyphosphazene and synthetic peptides [45,49]. The biocompatibility of a synthetic hydrogel itself is slightly inferior to that of natural purified hydrogels, but due to its powerful mechanical properties, ease of control, low immunogenicity and no batch shift concerns, it has received much attention in diverse categories of complex tissue engineering such as cardiovascular regeneration [50,51].
3.2.2. Cytological elements
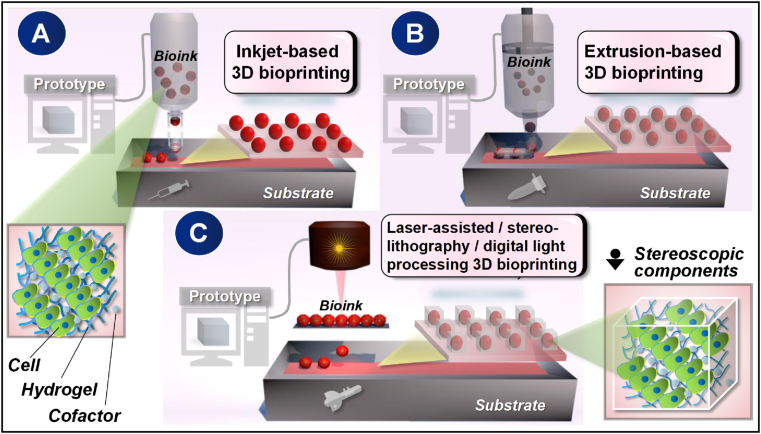
The composition of bioink composed of infiltrated versatile cells and/or cofactors is palpably the pioneering centre for advanced 3D bioprinting to perform its full function in tissue engineering and regeneration [51,52]. In comfortable 3D structures, both cell-cell and cell-hydrogel can form tightly connected and supportive interactions that not only ensure the needs of anatomy or bionics but also obtain the spatio temporal regulation of positive biological behaviours, which excellently promote the development towards targeted organs [53]. Indeed, it is important to answer whether the cells from a conventional 2D culture can be transformed into a biologically active 3D component since the regulation process is theoretically challenging and unknown. Therefore, the cytological part needs to match the microenvironment by means of proper numerical controlled 3D bioprintersin order to execute biological patterning accordingly. At present, according to the bioactive topological trait of a target 3D print object, whether it is simple or complex in mechanical control, the prepared living cells along with cofactors could be effectively patterned through either injection, extrusion, or light-assisted 3D bioprinters (Fig. 3).
Fig. 3.
Major classification of tissue engineering-targeted 3D bioprinting technology. Briefly, a standardized micro-construction as designed by the digital cubic prototype can be achieved either in use of gravity hanging drops controlled by an inkjet-based 3D bioprinter (A), by facilitating mechanical squeezing forces through the regulation of an extrusion-based 3D bioprinter (B), or through the direct-writing function of a laser-guided, stereolithography, or digital light processing 3D bioprinter (C). The above three printing strategies can be used either individually or in combination according to the specific needs of tissue engineering, the purpose of which is to achieve a closer simulation of native tissues and organs.
According to the purpose of the construction and application, functional cells encapsulated by biocompatible and printable hydrogel could include natural stem cells or differentiated lines, iPSCs or derived subgroups, and the improved cells directly transdifferentiated from somatic ones [[54], [55], [56]]. Likewise, in order to guarantee sufficient biological activity of the 3D printed construct, active molecules, such as differentiation-inducing genes, cell growth factors or key signaling proteins, can be reasonably applied according to the cytological and histological characteristics [57,58]. The compatibility between the cell-led active ingredient and the hydrogel before and after mixing should be tested by the researcher during bioink preparation to prevent deplorable cell death and/or poor moulding of the biomaterials.
4. Frontier of 3D bioprinting in cardiac tissue engineering
4.1. The topical tendency of 3D-engineered cardiac tissue
For the past one decade, applications of 3D bioprinting technology in the field of cardiovascular repair and regeneration, particularly those aspiring to generate a holistic engineered heart tissue (HEHT) with long-term contractility for transplantation, have achieved significant progress. Notably, the fabrication of HEHT by a 3D bioprinting system, such as the prevailing extrusion-based 3D bioprinter [59], relies largely on precise depositions of cardiac related cell-laden polymerous bioink in a well-organized manner to strongly form the prototype-established route. The realistic design of this mechanical operation that redraws the anatomy and pump function of a mammalian heart has gradually deepened due to the micro-scale control progress of 3D printing technology itself and because of the cardiac-oriented developments of iPSCs and biomaterials [60]. Nevertheless, the emerging field, born from the interdisciplinarity of these territories, shows impressive vitality and has excellent prospects for the future clinical translation of cardiology [61].
In view of the difficulty of regenerating and repairing damaged or defective hearts, the direction of 3D bioprinting currently focuses on the tight fitting of a sufficient number of functional CMs with a feasible scaffold on a micro scale. The source of the cells being applied during the exploration stage could be selective while its key common criteria revolve around how to ensure the qualified long-term survival and powerful export of the exquisite bionic body both in vivo and in vitro [62]. The biomaterial polymer network, which plays the role of the skeleton and ECM, is generally responsible for maintaining the shape of such cardiac-related organizations, and sometimes it needs to perform the task of the controlled release of the inner bio-active molecules [63]. Significantly, how to establish its muscle-like systematic stability by quantifying and controlling these dynamic factors should be based on the proper mastery of the protein-cell, cell-cell and cell-ECM interactions underlying bottom-up cardiac tissue fabrication [64,65].
In recent studies, as shown in Tables 1 and 3D bioprinting strategies for the fabrication of functional cardiac assemblies with different objectives are primarily focused on the fine-tuning of heart-oriented engineering inspections during pre-, on-, and post-bioprinting, respectively. Most of the reports have been investigating cardiac tissue engineering (CTE) by means of de novo fabrication, which means that the requirement standards for the intrinsic technical details could be stricter than the regular approaches [52,66]. Typically, the exploratory researchers have gradually and commonly formed a nascent system consisting of multiform paradigmatic methodologies that ranges from bioink preparation to the bio-function evaluation of a 3D-engineered HEHT in situ (Table 1). Through the inductive analysis of these typical findings, we can not only sort out the pioneering lines towards the standardized and innovative development of higher-order active cardiac replicas but also provide a good trend reference for the engineering roadmap of 3D bioprinting in other tissues and organs.
Table 1.
List of recent progress of 3D bioprinting for cardiac tissue engineering.
| Bioprinting method | Printer hardware | Bioink hydrogel | Bioink cell | Bioink cofactor | Application condition | Outcome of the effect | Reference |
|---|---|---|---|---|---|---|---|
| Inkjet-based 3D bioprinting | One-head operation | Fbronectin & gelatin | hiPS-CM & fibroblast | dECM | Following the 3D-engineered cardiac muscle fiber unit, co-culture with human cardiac microvascular endothelial cell (HMVEC) was then utilized for tests on functional properties in vitro. | Vascularization and contractility of the fabricated unit can be simultaneously possessed. | [69] |
| Needle-arrayed system | None | Sphere-like cell population composed of human iPSC-derived CM, fibroblast and umbilical vein endothelial cell (HUVEC) | Wnt signaling activator | The direct-write bioprinted cardiac patch was cultured in vitro with shaking for 72 h to obtain synchronized beating function, followed by being implanted to the infarcted area with pull-up omentum as a fixture of nude mice. | Compared with the control group, the infarct area in the myocardial infarction area decreased, while its angiogenesis and cardiac ejection fraction increased. | [73] | |
| Extrusion-based 3D bioprinting | One-headed operation | GelMA, fibronectin, laminin-111, & collagen methacrylate (MeCol) | hiPSC | None | iPSC was first printed and proliferated, and was subsequently differentiated into cardiomyocyte for testing long-term viability and function in vitro. | The heart organoid built sequentially could be endowed with contractility and pump functions. | [90] |
| Two-headed operation | Polyvinyl alcohol (PVA), agarose & alginate | H9c2 & HUVEC | Platelet-rich plasma | Heart-shaped PVA scaffold was first printed, followed by cell-hydrogel 3D bioprinting along the structure, so as to check heart-oriented biological properties in vitro. | Complex functional heart might be well engineered by an appropriate cell-hydrogel ratio. | [68] | |
| Fibrin | iPS-CM | None | The cell ink was firstly printed as a path, which the structural ink closely followed, so as to engineered a 3D cardiac chip for in vitro biological evaluation. | The engineered body showed excellent myocardial tissue-like functions. | [148] | ||
| Gelatin | hiPS-CM & endothelial cell (EC) | Decellularized porcine omentum | A complete, thick vascularized cardiac patch and heart were printed of in one step, and were tested in vitro. | The realistic heart-like active structure with vascularization presented human affinity and could potentially be suitable for future clinical use. | [117] | ||
| Collagen composited freeform reversible embedding of suspended hydrogels (FRESH) | Human CM and EC | Vascular endothelial growth factor (VEGF) | The highly simulated heart model with its own vascularization system was 3D bioprinted, and was tested in vitro. | In addition to the retractable heart, FRESH v2.0 system might engineer many other complex tissue scaffolds. | [102] | ||
| Three-headed operation | Fibrinogen, gelatin, aprotinin, glycerol, hyaluronic acid, sacrificial hydrogel, & polycaprolactone (PCL) | Primary CM from rat ventricular | None | Cell-laden hydrogel, sacrificial hydrogel, and PCL polymer were 3D bioprinted in order to produce an engineered cardiac patch for testing of electrophysiology and biomechanics in vitro, especially the Notch signaling pathway. | Micro-sensing and controlling of the printed cardiomyocyte might be necessary for the creation of functional heart tissues, and Notch inhibitor can improve the maturity level. | [87] | |
| Multi-headed operation | Type-1 atelo-collagen, & polyethylene-vinyl acetate (PEVA) | Cardiomyocyte (CM) of left ventricle of neonatal rat | Porcine left ventricular dECM | Living cells were added after scaffold formation in vitro. | Cardiomyocyte behaved differently in respond to bioink composition and culture conditions. | [70] | |
| GelMA & fibronectin | CM & cardiac fibroblast | None | Mechanical force and biological activity were quantified to clarify how the printing process affected the two main types of heart cells in vitro after the heart cross-sectional shape was printed. | Adding extra molecules, using two-cell bio-ink, and lowering the concentration of GelMA might improve cell survival and network formation. | [89] | ||
| Alginate & PEG-Fibrinogen (PF) | Mice iPS-CM & HUVEC | None | The scaffold and cells are printed one after another, and then the organizations were evaluated both in vitro and in vivo. | The transplanted engineered tissue can merge well with the host's heart by vasculature. | [71] | ||
| Visible lihgt-assisted system | GelMA | Neonatal human cardiac progenitor cell | Cardiac dECM | The myocardial patch was subjected to in vitro biological testing and transplanted into the heart of healthy rats to evaluate the effect in vivo. | The patches attached effectively to rat hearts for 14 d and showed microangiogenesis. | [84] | |
| Fibrin & furfuryl-gelatin | iPS-CM & cardiac fibroblast | None | 3D herringbone construct was printed for identifying the bioink feasibility of combination of cardiomyocyte and cardiac fibroblast in Vitro. | Connexin-43 might be important for the communications between cardiomyocyte and cardiac fibroblast in 3D-engineered tissue. | [119] | ||
| Rapid digital light processing (DLP)-based 3D bioprinting | GelMA | Human iPSC-derived CM (hiPSC-CM) | Porcine left ventricular dECM | After the cells are mixed with the hydrogel, the stripe-like micro-structure is printed and cultured in vitro. | A myocardial microstructure with complex geometry was produced in just a few seconds, and its controllable resolution was as fine as 30 μm. | [85] | |
| UV-assisted 3D bioprinting | One-headed operation | GelMA & methacrylated hyaluronic acid (HAMA) | Valvular interstitial cell | None | The 3D layered structure of heart valve tissue were reproduced for the test of mechanical properties and biomechanics in vitro. | The nature and patient delicate hierarchical structure of heart valve tissues can be engineered. | [88] |
| GelMA | Human embryonic stem cell-derived CM | Green Fluorescent Protein/Calmodulin/M13 Peptide (GCaMP 3) | The block-patterned multicellular cardiac element was rapidly created and long-term cultured for the valuation of calcium transients and mechanical forces in vitro. | Connexin-43 might serve as another critical bio-marker of electrical signal transmission for quality control of bioprinted myocrdium. | [86] | ||
| Two-headed operation | Alginate, MeCol, & arboxyl functionalized carbon nanotubes (CNTs) | Human coronary artery endothelial cell (HCAEC) | None | Alginate (with or without CNTs) was printed as a net-like scaffold, and the MeCol filled with cells was then printed alongside to yield cytological features. | Proliferation, differentiation, and lumen-like organization of HCAEC got short-term improved by the additive CNTs in vitro. | [67] | |
| Sequential | None | Cardiac progenitor derived from hiPSC | Wnt signaling activator | Functional pacemaker-like tissue was created in a hydrogel-free manner, and cardiac gene expression was tested in vitro. | Wnt5b activated a conserved canonical Wnt signaling pathway to guide the differentiation of Nkx2.5+ cardiac progenitors into primary pacemakers. | [72] |
4.2. Critical pre-bioprinting groundwork of cardiac 3D engineering
As the basic unit of a structural and functional bio-simulator, the CM or progenitor suitable for the effective 3D bioprinting of a cardiac system is no doubt of priority importance, and whether or not it can be stably obtained and sustained determines the ultimate availability or unavailability of the construct. Before the official printing operation, the preparation of active cardiac-bioink cells could be divided into three steps, namely, acquisition, cultivation or induction, and encapsulation. Currently, the practicable heart cell source can be human, mouse, or rat, and the first-hand genre covers the primary cells (such as primary rat ventricular CMs and human coronary artery endothelial cells), the cell lines (such as H9C2), or the iPSCs and other progenitor cells derived from iPSCs [[67], [68], [69]]. Because of the cell growth proficiency of the first-hand genre in vitro, the 2D culture systems for cell expansion are generally experienced, while the methods towards of stem cell differentiation and somatic cell trans-differentiation tend to be explorative and optimizable [70,71]. Nevertheless, with the continuous deepening of the related molecular mechanisms, the cell fate induction technology oriented to CM regeneration will be continuously optimized and will also make the cell preparation more personalized and customized. For example, based on the in-depth understanding of the Wnt pathway in cardiac determination, 3D bioprinting qualified induced CMs (iCMs) can be derived from iPSCs by adding a Wnt signaling activator [[72], [73], [74]]. Functional iCMs mainly refer to cardiac cells with striated muscle segments and spontaneous contraction (Fig. 2 Ⅲ); due to its high chemical sensitivity to ischaemia and hypoxia, the in vitro treatment process requires careful attention be given to the suitability of the environment [75]. Interestingly, in the process of cultivating these heart cells to become eligible for mechanical printing, more key genes related to heart development and maturity might in turn be found [76,77]. As is equally important, during the period when the nourished cells are encapsulated as bioink, a high survival rate and a stable biocompatibility of the population itself must be well-guaranteed to constantly deliver a CTE specified module [78,79].
As previously described, the screening of biomaterials suitable for the engineering of a myocardium-like hydrogel scaffold in situ and/or being further transplanted is another key step in the preliminary preparation of most heart-guided 3D bioprinting. To a certain extent, the texture of an ideal 3D bioprinted cardiac construct is mostly determined by the autogeneic hydrogel biomaterials, which could be deemed as an active polymer network harnessing characteristics of simultaneous skin-like softness and muscle-like toughness [80,81]. The hydrogel materials for general tissue engineering mainly includes the chemical cross-linking type and the photo-cross-linking type [82], which are also widely covered in cardiac bioink. Regardless of the differences between each research goal, it seems that in the selection of printing materials, the use of a natural hydrogel as the main bioink backbone for encapsulating CTE cells seems to be more popular. For example, biocompatible methacrylic anhydride gelatine (GelMA) prepared by the reaction of methacrylic anhydride (MA) and gelatine [83] has recently been adopted for the production of functional CTE prints; and the reason is that the appropriate proportion of GelMA, whether in monomer form [[84], [85], [86]] or in composite form [[88], [89], [90]], addresses the basic mechanical requirements. In addition, the hydrogel assembled with a nano-improved biomaterial also shows important abilities in adapting to a 3D bioprinting heart system, which can enhance the interaction between cells and materials to form a stable structure [67,91]. Whereas for the 3D-CTE requiring a relatively simple shape [73] or just a dire-write manner [72,92], the preparation of bioink containing only cells and no hydrogel component added has also been determined to be conditionally feasible. Such attempts may to some extent avoid potential physical and chemical damage to the cells in the bioink using extra additives, but that whether they perform better than biocompatible hydrogels under the same conditions still needs more evidence.
Another 3D-CTE bioink preparation method that can additionally increase the systematic mechanical property and/or bio-activity is the input of a hydrogel dECM [93], which is also a frontier hotspot in this field [94,95]. As a cofactor for the encapsulated living heart cells, a pre-printed cardiac dECM can be either processed solely with the biomaterial as a scaffold source [70] or mixed with the cells [69,85]. Similar to the source of instrumental cells, the preparation of a cardiac dECM reproducing the original physical and mechanical microenvironment of the heart can be different, which is mostly determined by the species and myocardial regions according to the specific requirements of 3D bioprinting applications [96,97]. Interestingly, in addition to providing a supportive microenvironment in tissue engineering, the cardiac dECM also seems to have individual potential for cardiovascular therapeutic effects [98], and its natural components seem to be affected by ageing factors [99]. With the continuous improvement of the production process and formulation, the preparation of the dECM has gradually developed from traditional methods such as lyophilized powder and acid digestion to today's higher fidelity methods such as ultrasonic homogenization, which allows its natural protein structure and function to be better retained [100,101]. More importantly, proteomic explorations of the physiological and pathological dECM of the cardiovascular system will help extract more functional factors such as the VEGF [102] and MMP2 [103], which offer critical support for the generation of a more standardized and opulent preparation of 3D-CTE bioink [104,105].
4.3. Engineering process of cardiac 3D bioprinting
Once the preparation of the bioink is completed, a set of consecutive manufacturing operations guided by integrated computer numerical control machinery (CNCM) is required [106], the goal of which is to establish a biosimulated network of heart cells and bio-materials for evaluation and/or application [107]. Due to the desperation of homeostasis including cell survival and biological effects such as contractility and electrophysiology, the 3D bioprinting process using the layer by layer deposition of cardiac bioink (cbLBLD) tends to be rapidly manufactured under physiologically suitable conditions such as those regarding the temperature and pH [108]. Precisely, important accurate parameters of cbLBLD mainly include the elastic modulus and electrical conductivity [108,109]. The specific design of cbLBLD could be different in each study; however, the evaluation indicators can generally be summarized as aspects including the fundamental operating parameters (FOPs), cross-link moulding (CLM) and the print rheology measurement (PRM), which can be collectively regarded as the basic industry references for HEHT printing operations [31,110,111]. Since the accuracy and available parameters of each bioprinter are distinct, the printing accuracy of the engineered heart body should be optimized according to the single printer used.
To use tissue engineering to generate local heart tissues or complete whole tissues that conform to the mechanical pattern of the prototype design with precise spatial and temporal control, the personalized FOPs of cbLBLD including the printing nozzle's aperture, printing speed, layer thickness, and number of layers need to be well standardized [112,113]. The diameter of the print nozzle for 3D-CTE is generally in the range of tens to hundreds of microns, which can be selected properly according to the viscosity and modulus requirements of the bioink or practical experience [114,115]. The deposition speed of the mechanical device is generally tens to hundreds of millimetres per second so that the production of a single engineering body can be completed as rapidly as possible to avoid a large amount of damage to or the death of heart cells caused by mechanical shear forces [115,116]. The layer thickness is determined by the bioink-related deformability, the deposition volume and the streaming speed; and the continuous printing action can produce a single-layer elongated 3D-CTE structure with a height of hundreds of microns to several millimetres [85,117]. The purpose of setting the number of layers is mainly to consolidate the completeness of the printed body according to heart research or application goals, and the range can be set from several layers to tens or even hundreds of layers, thereby forming a height of several millimetres to several centimetres [102,118]. It is worth noting that in different steps or with the aid of additional biomaterials (such as supportive sacrificial materials) in addition to bioink, the FOP settings can be given different CTE specificities [66,68]. For example, compared with a platform bioprinting system, the suspension printing system exhibited better accuracy and flexibility regarding the precise control of complex HEHTs [118].
The formulation of cell-laden CLMs suitable for 3D-CTE such as photocrosslinking contributes as a finishing-touch step towards the structural stabilization of the ECM scaffold. In the photo-mediated reaction of photo-manufacturing technology, the photosensitive polymer including the LAP [88] and RB [119] can perform cross-linking reactions in situ in the presence of living heart cells and bioactive molecules to greatly promote the rapid prototyping of the printed structure [120], which can also control the density of the hydrogel networks in real time, mimic biological functions, and release biochemical ligands at a micron-level resolution [121]. It is through this fixation procedure that 3D-CTE based on hydrogel assistance can be truly fastened into an elaborate bionic shape with topographical characteristics (Fig. 4), thus providing a partial or complete micro-environmental stent for the corresponding mechanism and application research on the heart. Particularly, for an active heart shaping strategy that uses advanced sacrificial gelatine combined with two-ink printing, the cross-linking condition of gelatinization-and-liquefaction in situ can be that used for a heart cell culture [32,117]. Such ingenious highlights that the unnecessary components other than the prototype heart shape are liquefied for abandonment while the bioink is simultaneously gelled, achieving an engineering outcome with two effects [122]. Moreover, due to the high-level biocompatibility of both the floating supportive gelatine and the pre-coagulated dECM that encapsulates the cells, a highly viable and uniformly distributed state of iCMs can be completely maintained [82,123].
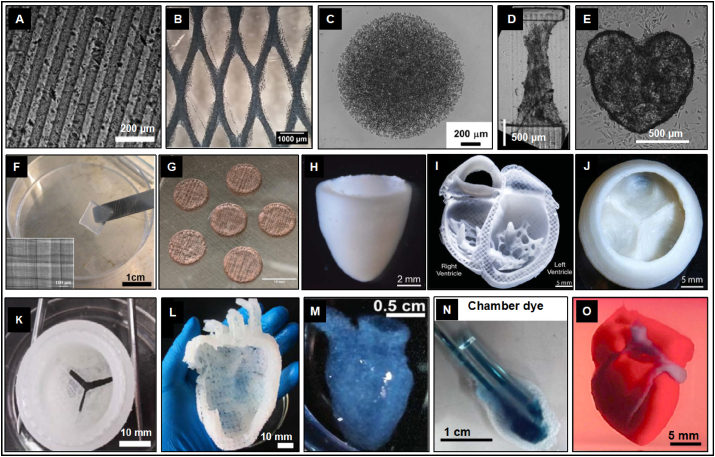
Fig. 4.
Full-fledged and high-resolution cardiac structures engineered by advanced 3D bioprinting. Through accurate printing and cross-linking of cell-loading cardiac bioink, the original structure of heart can be realistically reproduced in construction of: linear columnar (A) [85], grid-like pattern (B) [67], spherical cluster (C) [148], rectangular parallelepiped (D) [86], valentine heart-shaped material (E) [72], cube-shaped block (F) [71], perforated column (G) [84], conical container (H) [102], four-chamber sagittal plane (I) [102], heart valve-like structure (J & K) [68,102], left heart with large vessel-like construction (L) [68], right ventricle-like construction (M & N) [90], and micro vascularized HEHT (O) [117].
For HEHT production that incorporates biomaterials as bioink, in addition to static topological characteristics, the key PRMs of these active bio-elements may still need to be tested during printing operations [124,125]. The reason for this is that the complexity of the structure and function of the cardiovascular system itself is likely to cause both internal and external shear forces on the ontic construct [126], and it is therefore necessary to maintain an appropriate rheological ability of the hydrogel network, especially for the vascularized HEHT that might be transplantable in vivo [127]. Rheology refers to the increase in the viscosity of non-Newtonian fluids such as hydrogels with time under shear stress [128]. The critical 3D-CTE rheological assessment should include the viscous modulus (VM), elastic modulus (EM), yield stress (YS) and shear thinning (ST) [129]. The VM is responsible for maintaining the integrity of the printed structure and is closely related to the nonlinear viscoelasticity (thixotropy/rheology), heart cell density, and overall surface tension. By balancing the speed and pressure of cbLBLD, the bioink with an appropriate viscosity can be positioned within a very small distance from the nozzle of the extruder, thereby eliminating the interference of the previous printing volume [90,130]. Furthermore, setting the EM higher than the VM can ensure the structural stability after the induction of the shrink function, which in turn increases the self-supporting ability, and helps maintain the shape of the bioink by reducing deformation [68,131]. Meanwhile, the YS caused by the non-covalent and electrostatic interactions inside the bioink, that is, the minimum stress required for flow, generates plug flow at the centre of the flow profile to limit the shear force to the narrowness along the extruder wall area, thereby protecting the wrapped cells from shear forces during the printing process [132,133]. Furthermore, the increased shear rate during printing will force the polymer chains to line up in the flow direction, forming the ST (also known as pseudo-plasticity) required for 3D-CTE and decreasing the viscosity of high molecular weight polymers [134]. In addition, the destruction of electrostatic interactions at higher shear rates can also participate in the reduction of the apparent viscosity, which assists in obtaining high printing fidelity and high biological activity. In fact, the HEHT effect of cbLBLD does depend largely on rheological characteristics, such as the pump storage and the response to the blood flow shear stress [135]. These newly emerging feature-function relationships, as well as the mathematical models that may be available in the future, will provide a more targeted method for the rational design of cbLBLD. Undeniably, researchers need to perform cell viability analysis before and after printing to meet the minimum biological requirements for bioink.
4.4. Post-bioprinting fundamental cardiac evaluation
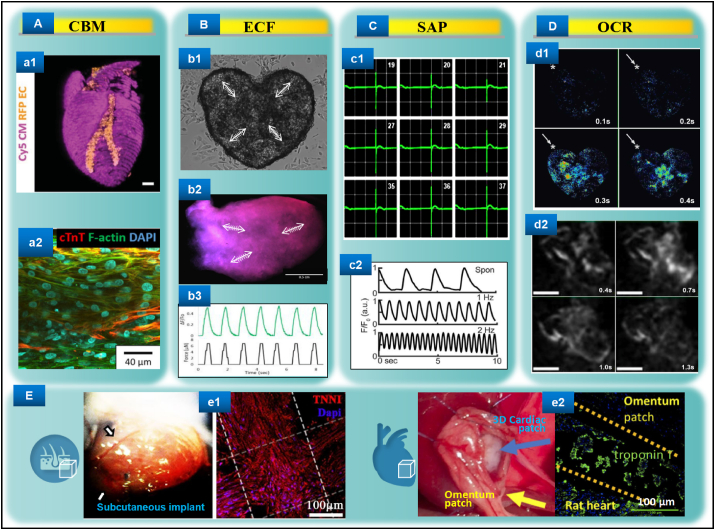
After the 3D bioprinting process is completed, the cardiac biological functions of the cultured engineered structure need to be meticulously implemented both in vitro and in vivo for deep ongoing study. Evaluations that could be carried out in vitro mainly include cardiac biomarker (CBM), efficient contractile force (ECF), spontaneous action potential (SAP), and overall calcium regulation (OCR) assessments, while the repair and regeneration functions could be advanced through in situ engraftment [136,137]. Here, we briefly summarize these aspects as follows (Fig. 5).
Fig. 5.
Evaluations of the representative advanced 3D bioprinted cardiac tissues. A. Immunostaining of cardiac biomarkers may include views of whole (a1) [117] and part (a2) [69]. B. Spontaneous muscle-like contraction after stabilization can be documented by video (b1 and b2) [72,90] and quantification (b3) [86]. C. The micro-engineered cardiac tissues can also excite stable cardiomyocyte-like depolarization-repolarization action potentials (c1 and c2) [102,148]. D. Dynamic calcium transient processes of the advanced 3D bioprinted functional cardiomyocytes may be real-time detected (d1 and d2) [72,90]. E. The 3D bioprinted patch-like tissues composed of iPSC-CMs and blood vessel endothelial cells could be well integrated into mice physiological subcutaneous tissue (e1) [71] and rat myocardial infarcted heart (e2) [73] for in vivo measurements, respectively.
On the basis of maintaining cell viability and structural stability, achieving the stable and continuous expression of the cardiac maturation markers is of great significance for subsequent research on and the application of tissue engineering. In particular, for studies using iCMs as the bioink component, the specific expression of cardiac troponin I and T (cTnI and cTnT) and sarcomeric actinin at the transcription and translation level inside the print body can suggest normal myocardium function [117,138,139]. This CBM expression profile should match the corresponding structural characteristics and have a uniformity tissue distribution to obtain excellent myocardial bionicity and availability [69]. In addition, since perfusion could be critically independent of the culture, the artificial heart prints containing lumen-like microvessels should have a wide and uniform expression of mature smooth muscle markers such as luciferin-labelled α-actin [117,140] so that the gas exchange and cellular metabolism can be maintained at a steady state and further enhance the bionicity and availability [141,142]. It is worth noting that a 3D-fabricated cardiac construction that uses dECM bioink should be provided with a cell-free dECM print as a control to exclude false positive interference from microenvironmental biochemical factors [143]. Indeed, the rational use and development of CBMs is also conducive to the in-depth exploration of unknown regeneration events and mechanisms of bioprinting-related epigenetics [144].
Once the molecule-guided microenvironment is properly established, the mature functional 3D-cultured CM/iCM can be rapidly assembled into one organism, which might trigger a significant programme of ECFs that would probably show skeletal muscle-like systole-diastole movement (not chaotic movement) through microscopic observation [72]. Indeed, one of the main obstacles for using vitro tissue engineering to produce active heart tissue is that the communication among terminally differentiated CMs tends to be incoherent or difficult to maintain, making it difficult to form a continuous thick layer of muscle to fill the gaps in damaged myocardium [145]. As a solution for this issue, a strategy based on using iPSC proliferation to form a sufficient number of precursors followed by in situ structural CM differentiation had shown strong evidence of forming a lifelike cardiac organoid [90]. This organoid could generate synchronized ECFs mimicking the myocardial pump contraction with a thickness of up to half a millimetre. Meanwhile, the cell density could be maintained at the same order of magnitude as that of natural myocardial tissue, which collectively is the representative result closest to HEHT by far [90,146]. In addition, continuous ECFs can also be quantified using specific formulas under specific processing conditions [86], further advancing and enriching the functional modeling system of 3D bioprinting in cardiology. As a matter of course, how to obtain ECFs with a longer duration (such as weeks or even months) and the power to address the physiological ejection fraction level will still be one of the aspects in the field that requires innovative breakthroughs in the following studies.
Another important characteristic that is closely related to the estimation of the contractile functioning of a heart (especially the left and right ventricles) is the electrophysiology mediated by the Na+-K+ exchange pump, namely, the active SAP of 3D-CTE [147]. On the one hand, when pacemaker CMs (or the encapsulated stem cells destined to form pacemaker CMs) were employed in the printing system, even the use of a fine-needle pendant drop printing could excite a typical QRS/T waveform [148]. On the other hand, for the 3D-CTE without typical pacemaker CMs, the regular ventricular-like SAP waveform could also be well triggered or respond to the stimulation of additional electrical signals [102]. Apparently, the stable and regular appearance of SAP had endowed 3D-CTE with a higher dimension of physical characteristics, which is particularly close to the sound regeneration of the native ventricle or “perfect” heart [149]. More importantly, SAP could be imperative for engineered heart tissues in reducing the occurrence of arrhythmias in vivo [150,151], and it should therefore become a routine evaluation for similar studies. From the opposite perspective, the topic with regards to how to improve 3D-CTE maturity by revealing the specific SAP regulation mechanisms underlining de novo constructions of complex HEHT and stem cell therapy will be attractive and of significant value [152,153].
The attractive functionality of a mature heart is additionally manifested in the present of OCR, especially the dynamic changes of the rapid calcium transient capable of coupling ECFs and SAP [154]. For that matter, in order to evaluate the functional integrity of the biomimetic cardiac prints, especially for transplantation or drug screening, the non-destructive capture of the OCR is also needed [155]. For example, in the 3D bioprinting study using CMs/iCMs, the tracing method of calmodulin labelled with green fluorescent protein (GFP), combined with microscopic calcium imaging technology, enables the temporal change of calcium transients to be visually monitored [85]. Such spontaneous calcium transients basically coincide with the mechanical beating forces over time, and their propagation trajectory could also be very similar to the physiological heart electrical sensing path [72,90]. Moreover, the use of additional current to stimulate the printed structure could be measured in response to changes in the calcium flow, providing good support for the discovery of new specific calmodulin genes related to heart regeneration [86]. Seemingly, in addition to being used as a patch or graft, the dynamic monitoring of 3D-CTE-OCR can potentially act in a subsidiary role in the in vitro study of regulatory molecular mechanisms towards cardiac development and disease [156,157].
As the core goal pursued by other tissue engineering and stem cell strategies [158,159], producing functional HEHT suitable for in vivo transplantation to treat heart failure is the high-level expectation of 3D-CTE. Precisely, to preliminarily assess the tissues’ fusion ability in multi-component CM/iCM prints [160], mature in vitro cultured structures were transplanted into the hypodermic position of adaptive immune response-deficient mice [71] so that extra local vascularization and myocardium transformation could be evaluated by molecular biology and immunoimaging methods, respectively [71,136]. Such subcutaneous biocompatible 3D constructions showed micro-vascular mosaicism with angiogenesis, myocardial molecular characterizations, and potential vascular-myocardial interactions [14,71]. In addition, in order to evaluate the orthotopic safety and effectiveness, the 3D-patterned cardiac patch could be transplanted into the infarcted hearts of immune-deficient rats, followed by the tracking of the living heart function and post-sacrificial heart histology [73]. This application had shown a significant therapeutic effect, that is, the infarction area was greatly reduced and the neovascularization was significantly increased, and furthermore the ejection fraction and cardiac output could be statistically improved to a certain extent [73,161]. These successful reports provide an outstanding starting point for the upcoming pre-clinical and clinical exploration of 3D-CTE and could probably contribute as meaningful references for peer studies. All in all, consistent in vitro and in vivo myocardial effects might be achieved by engineering a proper 3D bioprinting design, which could support more functional and/or mechanical studies that could be further conducted.
5. Limitations and prospects
There have been reports on the use of innovative bioinks and strategies such as using the emerging extrusion-based floating 3D printing to engineer eye-catching vascularized contractile myocardial parts or heart-shape constructions, which collectively marks an impressive milestone in the industry [162,163]. However, these artificial prints, compared to the hearts of large mammals or primates, are still naive in their generation of both the input and output mechanical strengths for long-term effects. For example, the physiological simulation effect of 3D bioprinting used in the current field of heart regeneration mostly relies on the introduction of functional cardiomyocytes and endothelial cells [67,75], while cell types like epicardial cells and fibroblasts that can naturally provide mechanical support and conduction are rarely included. The incompleteness of such seed cells in cardiac 3D bioprinting may pose a severe challenge to the stability of the component body once the bioactive materials gradually undergo spontaneous degradation [55,57]. On the other hand, for the diverse production strategies, there hasn't been many comprehensive comparisons between 3D post-bioprinted cardiac cell types and natural heart cells in molecular biology level (e.g. genomics, proteomics and metabolomics), which may generate a certain sort of showcase-application disconnect. Moreover, the current cardiac 3D bioprinting is more focused on top-down production methods, while therapeutic printing strategies for pathological cardiac structure and function loss, especially for the cellular and molecular challenges associated with ischemic injury or myocardial hypertrophy from cellular to organ level, seem to have not been widely approached.
In addition, due to the complexity of heart movement, whether these 3D bioengineered bodies may achieve or get close to 100% cardiac replication physically and chemically remains to be further explored [164]. Currently, there are two different applications of cardiac bioprinting, namely regeneration and in vitro modeling, and they should be equipped with different detailed parameters (e.g. the flexible and conductive bioactive materials for modified modalities and bioinks) for cardiac 3D bioprinting. More importantly, since such craftsmanship of 3D printing and 3D cultures is literally a significant mechanic alteration for living heart cells or stem cells, there are still few reports on the responding epigenetic events, which could be theoretically important for the regulation and extension of cardiac functionality. Undoubtedly, this technology is in its infancy and these limited aspects closely related to cardiac repair and regeneration constrain current-state 3D bioprinting technology in cardiology, which also means that more stringent but reasonable standards may be put forward for upcoming similar studies. For example, the cell types or subtypes (e.g. the atrial, ventricular, and nodal cardiomyocyte subtypes) in the printed body produced by a refined cardiac 3D bioprinting machine should be more comprehensively and meticulously tested with standardized spatiotemporal expression of biomarkers to ensure its biological consistency and functional reliability.
Nonetheless, the high-throughput 3D bioprinting technology is growing, and its combination of stem cell technology is extremely advantageous in the field of tissue engineering and regenerative medicine. With more exploration of the combination of 3D bioprinting technology and cardiology, the issues mentioned above are expected to be effectively solved through more practical updates. Moreover, rather than just being capable of reproducing the smallest functional element, the minimum but not the last goal of 3D bioprinting in cardiac repair and regeneration should be to build an entire living heart for clinical applications to solve the current bottlenecks of donor shortages and immune rejection. To achieve this goal, future research needs to continue to focus on the improvement of sufficient clinical-grade heart stem/progenitor cells, multiple robust myocardium-targeted bioinks, 4D integrated methodology, skillful and comprehensive use of various types of cardiac 3D bioprinters, and multi-dimensional evaluation of maturity for the culture-transplant-monitoring package [75,82,165,166]. It is believed that by conducting continuous chain studies and corresponding epigenetic mechanisms (such as DNA methylations and histone modifications) closely around these issues, 3D-CTE can better serve and be clinically applied for patients sooner [167].
6. Conclusions
Regarding the core topic of loyal reproduction of the cardiac structure and function, a set of detailed cardiac tissue engineering methods and strategies supported by 3D bioprinting technology have been gradually explored, and a considerate degree of interesting integration with cutting-edge biomaterials and stem cell therapy has been obtained. With the development of the constituent elements, the system can be continuously enriched and renewed, thereby producing more in-depth outcomes with cardiac repair and regeneration value. In summary, the reproduction of vascularized myocardium or vivid heart valves, which can integrate the physiological laws of the cardiovascular system and take into account the specific needs of a disease, is one of the most successful representatives of multi-disciplinary integration in the current discipline, showing unique charm and advantages in clinical translation.
CRediT authorship contribution statement
Nanbo Liu: Writing - original draft. Xing Ye: Writing - original draft. Bin Yao: Writing - review & editing. Mingyi Zhao: Investigation. Peng Wu: Investigation. Guihuan Liu: Investigation. Donglin Zhuang: Software. Haodong Jiang: Software. Xiaowei Chen: Data curation. Yinru He: Data curation. Sha Huang: Conceptualization, Supervision, Resources. Ping Zhu: Supervision, Resources, Funding acquisition.
Declaration of competing interest
All the authors agree that no conflict of interest is declared.
Acknowledgement
This research was funded by National Key Research and Development Program of China (2018YFA0108700, 2017YFA0105602, 2017YFC1103300), NSFC Projects of International Cooperation and Exchanges (81720108004), National Natural Science Foundation of China (81974019), The Research Team Project of Natural Science Foundation of Guangdong Province of China (2017A030312007). The key program of guangzhou science research plan (201904020047). The Special Project of Dengfeng Program of Guangdong Provincial People's Hospital (DFJH201812; KJ012019119; KJ012019423).
Footnotes
Peer review under responsibility of KeAi Communications Co., Ltd.
Contributor Information
Sha Huang, Email: stellarahuang@sina.com.
Ping Zhu, Email: tanganqier@163.com.
References
- 1.Virani S.S., Alonso A., Benjamin E.J., Bittencourt M.S., Callaway C.W., Carson A.P. Heart disease and stroke statistics-2020 update: a report from the American heart association. Circulation. 2020;141(9):e139–e596. doi: 10.1161/CIR.0000000000000757. [DOI] [PubMed] [Google Scholar]
- 2.Riggs K.W., Zafar F., Radzi Y., Yu P., Bryant R., Morales D.L.S. Adult congenital heart disease: current early expectations after cardiac transplantation. Ann. Thorac. Surg. 2020;109(2):480–486. doi: 10.1016/j.athoracsur.2019.06.067. [DOI] [PubMed] [Google Scholar]
- 3.Hernandez G.A., Lemor A., Clark D., Blumer V., Burstein D., Byrne R. Heart transplantation and in-hospital outcomes in adult congenital heart disease patients with Fontan: a decade nationwide analysis from 2004 to 2014. J. Card. Surg. 2020;35(3):603–608. doi: 10.1111/jocs.14430. [DOI] [PubMed] [Google Scholar]
- 4.Tomov M.L., Gil C.J., Cetnar A., Theus A.S., Lima B.J., Nish J.E. Engineering functional cardiac tissues for regenerative medicine applications. Curr. Cardiol. Rep. 2019;21(9):105. doi: 10.1007/s11886-019-1178-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Takahashi K., Tanabe K., Ohnuki M., Narita M., Ichisaka T., Tomoda K. Induction of pluripotent stem cells from adult human fibroblasts by defined factors. Cell. 2007;131(5):861–872. doi: 10.1016/j.cell.2007.11.019. [DOI] [PubMed] [Google Scholar]
- 6.Yu J., Vodyanik M.A., Otto K.S., Antosiewicz-Bourget J., Frane J.L., Tian S. Induced pluripotent stem cell lines derived from human somatic cells. Science. 2007;318(5858):1917–1920. doi: 10.1126/science.1151526. [DOI] [PubMed] [Google Scholar]
- 7.Ieda M., Fu J.D., Delgado-Olguin P., Vedantham V., Hayashi Y., Bruneau B.G. Direct reprogramming of fibroblasts into functional cardiomyocytes by defined factors. Cell. 2010;142(3):375–386. doi: 10.1016/j.cell.2010.07.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Mohamed T.M.A., Stone N.R., Berry E.C., Radzinsky E., Huang Y., Pratt K. Chemical enhancement of in vitro and in vivo direct cardiac reprogramming. Circulation. 2017;135(10):978–995. doi: 10.1161/CIRCULATIONAHA.116.024692. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Huang W., Liang J., Feng Y., Jia Z., Jiang L., Cai W. Heterogeneity of adult masseter muscle satellite cells with cardiomyocyte differentiation potential. Exp. Cell Res. 2018;371(1):20–30. doi: 10.1016/j.yexcr.2018.05.028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Chen R., Xie W., Cai B., Qin Y., Wu C., Zhou W. Establishment and identification of a CiPSC lineage reprogrammed from FSP-tdTomato mouse embryonic fibroblasts (MEFs) Stem Cell. Int. 2018:5965727. doi: 10.1155/2018/5965727. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Wang B., Wu L., Li D., Liu Y., Guo J., Li C. Induction of pluripotent stem cells from mouse embryonic fibroblasts by jdp2-jhdm1b-mkk6-glis1-nanog-essrb-sall4. Cell Rep. 2019;27(12):3473–3485. doi: 10.1016/j.celrep.2019.05.068. [DOI] [PubMed] [Google Scholar]
- 12.Asghari F., Samiei M., Adibkia K., Akbarzadeh A., Davaran S. Biodegradable and biocompatible polymers for tissue engineering application: a review, artif. Cells nanomed. Biotechnol. 2017;45(2):185–192. doi: 10.3109/21691401.2016.1146731. [DOI] [PubMed] [Google Scholar]
- 13.Liu N., Chen J., Zhuang J., Zhu P. Fabrication of engineered nanoparticles on biological macromolecular (PEGylated chitosan) composite for bio-active hydrogel system in cardiac repair applications. Int. J. Biol. Macromol. 2018;117:553–558. doi: 10.1016/j.ijbiomac.2018.04.196. [DOI] [PubMed] [Google Scholar]
- 14.Yang W., Zhu P., Huang H., Zheng Y., Liu J., Feng L. Functionalization of novel theranostic hydrogels with kartogenin-grafted USPIO nanoparticles to enhance cartilage regeneration. ACS Appl. Mater. Interfaces. 2019;11(38):34744–34754. doi: 10.1021/acsami.9b12288. [DOI] [PubMed] [Google Scholar]
- 15.Amir T., Nakamura M. Three-dimensional bioprinting: toward the era of manufacturing human organs as spare parts for healthcare and medicine. Tissue Eng. B Rev. 2017;23(3):245–256. doi: 10.1089/ten.TEB.2016.0398. [DOI] [PubMed] [Google Scholar]
- 16.Ong C.S., Nam L., Ong K., Krishnan A., Huang C.Y., Fukunishi T. 3D and 4D bioprinting of the myocardium: current approaches, challenges, and future prospects. BioMed Res. Int. 2018 doi: 10.1155/2018/6497242. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Ma X., Liu J., Zhu W., Tang M., Lawrence N., Yu C. 3D bioprinting of functional tissue models for personalized drug screening and in vitro disease modeling. Adv. Drug Deliv. Rev. 2018;132:235–251. doi: 10.1016/j.addr.2018.06.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Shao L., Gao Q., Xie C., Fu J., Xiang M., He Y. Biofabrication; 2020. Directly Coaxial 3D Bioprinting of Large-Scale Vascularized Tissue Constructs. [DOI] [PubMed] [Google Scholar]
- 19.Pitaktong I., Lui C., Lowenthal J., Mattson G., Jung W.H., Bai Y. Early vascular cells improve microvascularization within 3D cardiac spheroids. Tissue Eng. C Methods. 2020;26(2):80–90. doi: 10.1089/ten.tec.2019.0228. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Enriquez A., Saenz L.C., Rosso R., Silvestry F.E., Callans D., Marchlinski F.E. Use of intracardiac echocardiography in interventional cardiology: working with the anatomy rather than fighting it. Circulation. 2018;137(21):2278–2294. doi: 10.1161/CIRCULATIONAHA.117.031343. [DOI] [PubMed] [Google Scholar]
- 21.Adhyapak S.M., Parachuri V.R. Architecture of the left ventricle: insights for optimal surgical ventricular restoration. Heart Fail. Rev. 2010;15(1):73–83. doi: 10.1007/s10741-009-9151-0. [DOI] [PubMed] [Google Scholar]
- 22.Sanz J., Sánchez-Quintana D., Bossone E., Bogaard H.J., Naeije R. Anatomy, function, and dysfunction of the right ventricle: JACC state-of-the-art review. J. Am. Coll. Cardiol. 2019;73(12):1463–1482. doi: 10.1016/j.jacc.2018.12.076. [DOI] [PubMed] [Google Scholar]
- 23.Sommer G., Schriefl A.J., Andrä M., Sacherer M., Viertler C., Wolinski H., Holzapfel G.A. Biomechanical properties and microstructure of human ventricular myocardium. Acta Biomater. 2015;24:172–192. doi: 10.1016/j.actbio.2015.06.031. [DOI] [PubMed] [Google Scholar]
- 24.Abdeltawab H., Khalifa F., Taher F., Alghamdi N.S., Ghazal M., Beache G. A deep learning-based approach for automatic segmentation and quantification of the left ventricle from cardiac cine MR images. Comput. Med. Imag. Graph. 2020;81:101717. doi: 10.1016/j.compmedimag.2020.101717. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Spertus J.A., Jones P.G., Maron D.J., O'Brien S.M., Reynolds H.R., Rosenberg Y. Health-status outcomes with invasive or conservative care in coronary disease. N. Engl. J. Med. 2020;382(15):1408–1419. doi: 10.1056/NEJMoa1916370. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.de Soysa T.Y., Ranade S.S., Okawa S., Ravichandran S., Huang Y., Salunga H.T. Single-cell analysis of cardiogenesis reveals basis for organ-level developmental defects. Nature. 2019;572(7767):120–124. doi: 10.1038/s41586-019-1414-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Spellberg B., Chambers H.F., Musher D.M., Walsh T.L., Bayer A.S. Evaluation of a paradigm shift from intravenous antibiotics to oral step-down therapy for the treatment of infective endocarditis: a narrative review. JAMA Intern. Med. 2020;180(5):769–777. doi: 10.1001/jamainternmed.2020.0555. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Arrigo M., Jessup M., Mullens W., Reza N., Shah A.M., Sliwa K. Acute heart failure. Nat. Rev. Dis. Primers. 2020;6(1):16. doi: 10.1038/s41572-020-0151-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Shudo Y., Wang H., Lingala B., He H., Kim F.Y., Hiesinger W. Evaluation of risk factors for heart-lung transplant recipient outcome: an analysis of the united network for organ sharing database. Circulation. 2019;140(15):1261–1272. doi: 10.1161/CIRCULATIONAHA.119.040682. [DOI] [PubMed] [Google Scholar]
- 30.Alonzo M., AnilKumar S., Roman B., Tasnim N., Joddar B. 3D Bioprinting of cardiac tissue and cardiac stem cell therapy. Transl. Res. 2019;211:64–83. doi: 10.1016/j.trsl.2019.04.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Kelly B.E., Bhattacharya I., Heidari H., Shusteff M., Spadaccini C.M. Volumetric additive manufacturing via tomographic reconstruction. Science. 2019;63(6431):1075–1079. doi: 10.1126/science.aau7114. [DOI] [PubMed] [Google Scholar]
- 32.McCormack A., Highley C.B., Leslie N.R., Melchels F.P.W. 3D printing in suspension baths: keeping the promises of bioprinting afloat. Trends Biotechnol. 2020;S0167–7799(19) doi: 10.1016/j.tibtech.2019.12.020. 30315-4. [DOI] [PubMed] [Google Scholar]
- 33.Murphy S.V., Atala A. 3D bioprinting of tissues and organs. Nat. Biotechnol. 2014;32(8):773–785. doi: 10.1038/nbt.2958. [DOI] [PubMed] [Google Scholar]
- 34.O'Connell C., Ren J., Pope L., Li Y., Mohandas A., Blanchard R. Characterizing bioinks for extrusion bioprinting: printability and rheology. Methods Mol. Biol. 2020;2140:111–133. doi: 10.1007/978-1-0716-0520-2_7. [DOI] [PubMed] [Google Scholar]
- 35.Ostrovidov S., Salehi S., Costantini M., Suthiwanich K., Ebrahimi M., Sadeghian R.B. 3D bioprinting in skeletal muscle tissue engineering. Small. 2019;15(24) doi: 10.1002/smll.201805530. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Heinrich M.A., Liu W., Jimenez A., Yang J., Akpek A., Liu X. 3D bioprinting: from benches to translational applications. Small. 2019;15(23) doi: 10.1002/smll.201805510. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Mironov V., Visconti R.P., Kasyanov V., Forgacs G., Drake C.J., Markwald R.R. Organ printing: tissue spheroids as building blocks. Biomaterials. 2009;30(12):2164–2174. doi: 10.1016/j.biomaterials.2008.12.084. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Gauvin R., Chen Y.C., Lee J.W., Soman P., Zorlutuna P., Nichol J.W. Microfabrication of complex porous tissue engineering scaffolds using 3D projection stereolithography. Biomaterials. 2012;33(15):3824–3834. doi: 10.1016/j.biomaterials.2012.01.048. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Ahn S.H., Lee J., Park S.A., Kim W.D. Three-dimensional bio-printing equipment technologies for tissue engineering and regenerative medicine. Tissue Eng. Regen. Med. 2016;13(6):663–676. doi: 10.1007/s13770-016-0148-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Moncal K.K., Ozbolat V., Datta P., Heo D.N., Ozbolat I.T. Thermally-controlled extrusion-based bioprinting of collagen. J. Mater. Sci. Mater. Med. 2019;30(5):55. doi: 10.1007/s10856-019-6258-2. [DOI] [PubMed] [Google Scholar]
- 41.Levato R., Jungst T., Scheuring R.G., Blunk T., Groll J., Malda J. From shape to function: the next step in bioprinting. Adv. Mater. 2020;32(12) doi: 10.1002/adma.201906423. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Yang G.H., Yeo M., Koo Y.W., Kim G.H. 4D bioprinting: technological advances in biofabrication. Macromol. Biosci. 2019;19(5) doi: 10.1002/mabi.201800441. [DOI] [PubMed] [Google Scholar]
- 43.Oliveira E.P., Malysz-Cymborska I., Golubczyk D., Kalkowski L., Kwiatkowska J., Reis R.L. Advances in bioinks and in vivo imaging of biomaterials for CNS applications. Acta Biomater. 2019;95:60–72. doi: 10.1016/j.actbio.2019.05.006. [DOI] [PubMed] [Google Scholar]
- 44.Parak A., Pradeep P., du Toit L.C., Kumar P., Choonara Y.E., Pillay V. Functionalizing bioinks for 3D bioprinting applications. Drug Discov. Today. 2019;24(1):198–205. doi: 10.1016/j.drudis.2018.09.012. [DOI] [PubMed] [Google Scholar]
- 45.Morgan F.L.C., Moroni L., Baker M.B. Dynamic bioinks to advance bioprinting. Adv. Healthc. Mater. 2020 doi: 10.1002/adhm.201901798. [DOI] [PubMed] [Google Scholar]
- 46.Bejleri D., Davis M.E. Decellularized extracellular matrix materials for cardiac repair and regeneration. Adv. Healthc. Mater. 2019;8(5) doi: 10.1002/adhm.201801217. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Dobos A., Van Hoorick J., Steiger W., Gruber P., Markovic M., Andriotis O.G. Thiol-Gelatin-norbornene bioink for laser-based high-definition bioprinting. Adv. Healthc. Mater. 2019;26 doi: 10.1002/adhm.201900752. [DOI] [PubMed] [Google Scholar]
- 48.Choudhury D., Tun H.W., Wang T., Naing M.W. Organ-derived decellularized extracellular matrix: a game changer for bioink manufacturing? Trends Biotechnol. 2018;36(8):787–805. doi: 10.1016/j.tibtech.2018.03.003. [DOI] [PubMed] [Google Scholar]
- 49.Konta A.A., García-Piña M., Serrano D.R. Personalised 3D printed medicines: which techniques and polymers are more successful? Bioengineering. 2017;4(4):E79. doi: 10.3390/bioengineering4040079. pii. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Datta L.P., Manchineella S., Govindaraju T. Biomolecules-derived biomaterials. Biomaterials. 2020;230:119633. doi: 10.1016/j.biomaterials.2019.119633. [DOI] [PubMed] [Google Scholar]
- 51.Ji S., Almeida E., Guvendiren M. 3D bioprinting of complex channels within cell-laden hydrogels. Acta Biomater. 2019;95:214–224. doi: 10.1016/j.actbio.2019.02.038. [DOI] [PubMed] [Google Scholar]
- 52.Jeong H.J., Nam H., Jang J., Lee S.J. 3D bioprinting strategies for the regeneration of functional tubular tissues and organs. Bioengineering. 2020;7(2):E32. doi: 10.3390/bioengineering7020032. pii. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Jia H., Schwille P. Bottom-up synthetic biology: reconstitution in space and time. Curr. Opin. Biotechnol. 2019;60:179–187. doi: 10.1016/j.copbio.2019.05.008. [DOI] [PubMed] [Google Scholar]
- 54.Crook J.M., Tomaskovic-Crook E. Bioprinting 3D human induced pluripotent stem cell constructs for multilineage tissue engineering and modeling. Methods Mol. Biol. 2020;2140:251–258. doi: 10.1007/978-1-0716-0520-2_17. [DOI] [PubMed] [Google Scholar]
- 55.Crook J.M. Cell processing for 3D bioprinting: quality requirements for quality assurance in fundamental research and translation. Methods Mol. Biol. 2020;2140:19–26. doi: 10.1007/978-1-0716-0520-2_2. [DOI] [PubMed] [Google Scholar]
- 56.Ngan C., Quigley A., O'Connell C., Kita M., Bourke J., Wallace G.G. 3D bioprinting and differentiation of primary skeletal muscle progenitor cells. Methods Mol. Biol. 2020;2140:229–242. doi: 10.1007/978-1-0716-0520-2_15. [DOI] [PubMed] [Google Scholar]
- 57.Zimmermann R., Hentschel C., Schrön F., Moedder D., Büttner T., Atallah P. High resolution bioprinting of multi-component hydrogels. Biofabrication. 2019;11(4) doi: 10.1088/1758-5090/ab2aa1. [DOI] [PubMed] [Google Scholar]
- 58.Mirani B., Pagan E., Shojaei S., Duchscherer J., Toyota B.D., Ghavami S. A 3D bioprinted hydrogel mesh loaded with all-trans retinoic acid for treatment of glioblastoma. Eur. J. Pharmacol. 2019;854:201–212. doi: 10.1016/j.ejphar.2019.04.007. [DOI] [PubMed] [Google Scholar]
- 59.Frost B.A., Sutliff B.P., Thayer P., Bortner M.J., Foster E.J. Gradient poly(ethylene glycol) diacrylate and cellulose nanocrystals tissue engineering composite scaffolds via extrusion bioprinting. Front. in Bioeng. and Biotechnol. 2019;7:280. doi: 10.3389/fbioe.2019.00280. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Silbernagel N., Körner A., Balitzki J., Jaggy M., Bertels S., Richter B. Shaping the heart: structural and functional maturation of iPSC-cardiomyocytes in 3D-micro-scaffolds. Biomaterials. 2020;227:119551. doi: 10.1016/j.biomaterials.2019.119551. [DOI] [PubMed] [Google Scholar]
- 61.Murphy S.V., De Coppi P., Atala A. Opportunities and challenges of translational 3D bioprinting. Nat. Biomed. Eng. 2019;4(4):370–380. doi: 10.1038/s41551-019-0471-7. [DOI] [PubMed] [Google Scholar]
- 62.Correia C.S., Begum R., Perriman A.W. 3D bioprinting: the emergence of programmable biodesign. Adv. Healthc Mater. 2019 doi: 10.1002/adhm.201900554. [DOI] [PubMed] [Google Scholar]
- 63.Thattaruparambil R.N., Vaquette C., Meinert C., Samuel I.D., Ivanovski S. Optimization of 3D bioprinting of periodontal ligament cells, Dent. Materials. 2019;35(12):1683–1694. doi: 10.1016/j.dental.2019.08.114. [DOI] [PubMed] [Google Scholar]
- 64.Neto M.D., Oliveira M.B., Mano J.F. Microparticles in contact with cells: from carriers to multifunctional tissue modulators. Trends Biotechnol. 2019;37(9):1011–1028. doi: 10.1016/j.tibtech.2019.02.008. [DOI] [PubMed] [Google Scholar]
- 65.Gaspar V.M., Lavrador P., Borges J., Oliveira M.B., Mano J.F. Advanced bottom-up engineering of living architectures. Adv. Mater. 2020;32(6) doi: 10.1002/adma.201903975. [DOI] [PubMed] [Google Scholar]
- 66.Birla R.K., Williams S.K. 3D bioprinting and its potential impact on cardiac failure treatment: an industry perspective. APL Bioeng. 2020;4(1) doi: 10.1063/1.5128371. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Izadifar M., Chapman D., Babyn P., Chen X., Kelly M.E. UV-assisted 3D bioprinting of nanoreinforced hybrid cardiac patch for myocardial tissue engineering. Tissue Eng. C Methods. 2018;24(2):74–88. doi: 10.1089/ten.TEC.2017.0346. [DOI] [PubMed] [Google Scholar]
- 68.Zou Q., Grottkau B.E., He Z., Shu L., Yang L., Ma M. Biofabrication of valentine-shaped heart with a composite hydrogel and sacrificial material. Mater. Sci. Eng. C Mater. Biol. Appl. 2020;108:110205. doi: 10.1016/j.msec.2019.110205. [DOI] [PubMed] [Google Scholar]
- 69.Tsukamoto Y., Akagi T., Akashi M. Vascularized cardiac tissue construction with orientation by layer-by-layer method and 3D printer. Sci. Rep. 2020;10(1):5484. doi: 10.1038/s41598-020-59371-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Das S., Kim S.W., Choi Y.J., Lee S., Lee S.H., Kong J.S. Decellularized extracellular matrix bioinks and the external stimuli to enhance cardiac tissue development in vitro. Acta Biomater. 2019;95:188–200. doi: 10.1016/j.actbio.2019.04.026. [DOI] [PubMed] [Google Scholar]
- 71.Maiullari F., Costantini M., Milan M., Pace V., Chirivì M., Maiullari S. A multi-cellular 3D bioprinting approach for vascularized heart tissue engineering based on HUVECs and iPSC-derived cardiomyocytes. Sci. Rep. 2018;8(1):13532. doi: 10.1038/s41598-018-31848-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Ren J., Han P., Ma X., Farah E.N., Bloomekatz J., Zeng X.I. Canonical Wnt5b signaling directs outlying Nkx2.5+ mesoderm into pacemaker cardiomyocytes. Dev. Cell. 2019;50(6):729–743. doi: 10.1016/j.devcel.2019.07.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Yeung E., Fukunishi T., Bai Y., Bedja D., Pitaktong I., Mattson G. Cardiac regeneration using human-induced pluripotent stem cell-derived biomaterial-free 3D-bioprinted cardiac patch in vivo. J. Tissue Eng. Regen. Med. 2019;13(11):2031–2039. doi: 10.1002/term.2954. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Burns C.G., Burns C.E. Canonical Wnt signaling sets the pace. Dev. Cell. 2019;50(6):675–676. doi: 10.1016/j.devcel.2019.09.002. [DOI] [PubMed] [Google Scholar]
- 75.Karbassi E., Fenix A., Marchiano S., Muraoka N., Nakamura K., Yang X., Murry C.E. Cardiomyocyte maturation: advances in knowledge and implications for regenerative medicine. Nat. Rev. Cardiol. 2020;17(6):341–359. doi: 10.1038/s41569-019-0331-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Christoffels V., Jensen B. Cardiac morphogenesis: specification of the four-chambered heart. Cold Spring Harb. Perspect. Biol. 2020 doi: 10.1101/cshperspect.a037143. pii. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Zhao M.T., Shao N.Y., Garg V. Subtype-specific cardiomyocytes for precision medicine: where are we now? Stem Cell. 2020 doi: 10.1002/stem.3178. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Datta P., Ayan B., Ozbolat I.T. Bioprinting for vascular and vascularized tissue biofabrication. Acta Biomater. 2017;51:1–20. doi: 10.1016/j.actbio.2017.01.035. [DOI] [PubMed] [Google Scholar]
- 79.Datta P., Barui A., Wu Y., Ozbolat V., Moncal K.K., Ozbolat I.T. Essential steps in bioprinting: from pre- to post-bioprinting. Biotechnol. Adv. 2018;36(5):1481–1504. doi: 10.1016/j.biotechadv.2018.06.003. [DOI] [PubMed] [Google Scholar]
- 80.Yan W.C., Davoodi P., Vijayavenkataraman S., Tian Y., Ng W.C., Fuh J.Y.H. 3D bioprinting of skin tissue: from pre-processing to final product evaluation. Adv. Drug Deliv. Rev. 2018;132:270–295. doi: 10.1016/j.addr.2018.07.016. [DOI] [PubMed] [Google Scholar]
- 81.Ostrovidov S., Salehi S., Costantini M., Suthiwanich K., Ebrahimi M., Sadeghian R.B. 3D bioprinting in skeletal muscle tissue engineering. Small. 2019;15(24) doi: 10.1002/smll.201805530. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Lim K.S., Galarraga J.H., Cui X., Lindberg G.C.J., Burdick J.A., Woodfield T.B.F. Fundamentals and applications of photo-cross-linking in bioprinting. Chem. Rev. 2020;120(19):10662–10694. doi: 10.1021/acs.chemrev.9b00812. [DOI] [PubMed] [Google Scholar]
- 83.Yin J., Yan M., Wang Y., Fu J., Suo H. 3D bioprinting of low-concentration cell-laden gelatin methacrylate (GelMA) bioinks with a two-step cross-linking strategy. ACS Appl. Mater. Interfaces. 2018;10(8):6849–6857. doi: 10.1021/acsami.7b16059. [DOI] [PubMed] [Google Scholar]
- 84.Bejleri D., Streeter B.W., Nachlas A.L.Y., Brown M., Gaetani R., Christman K.L., Davis M.E. A bioprinted cardiac patch composed of cardiac-specific extracellular matrix and progenitor cells for heart repair. Adv. Healthc. Mater. 2018;7(23) doi: 10.1002/adhm.201800672. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Yu C., Ma X., Zhu W., Wang P., Miller K.L., Stupin J. Scanningless and continuous 3D bioprinting of human tissues with decellularized extracellular matrix. Biomaterials. 2019;194:1–13. doi: 10.1016/j.biomaterials.2018.12.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Liu J., He J., Liu J., Ma X., Chen Q., Lawrence N. Rapid 3D bioprinting of in vitro cardiac tissue models using human embryonic stem cell-derived cardiomyocytes. Bioprinting. 2019;13 doi: 10.1016/j.bprint.2019.e00040. pii. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Wang Z., Lee S.J., Cheng H.J., Yoo J.J., Atala A. 3D bioprinted functional and contractile cardiac tissue constructs. Acta Biomater. 2018;70:48–56. doi: 10.1016/j.actbio.2018.02.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.van der Valk D.C., van der Ven C.F.T., Blaser M.C., Grolman J.M., Wu P.J., Fenton O.S. Engineering a 3D-bioprinted model of human heart valve disease using nanoindentation-based biomechanics. Nanomaterials. 2018;8(5):E296. doi: 10.3390/nano8050296. pii. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Koti P., Muselimyan N., Mirdamadi E., Asfour H., Sarvazyan N.A. Use of GelMA for 3D printing of cardiac myocytes and fibroblasts. J. 3D Print. Med. 2019;3(1):11–22. doi: 10.2217/3dp-2018-0017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Kupfer M.E., Lin W.H., Ravikumar V., Qiu K., Wang L., Gao L. In situ expansion, differentiation and electromechanical coupling of human cardiac muscle in a 3D bioprinted, chambered organoid. Circ. Res. 2020;127(2):207–224. doi: 10.1161/CIRCRESAHA.119.316155. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Bouguéon G., Kauss T., Dessane B., Barthélémy P., Crauste-Manciet S. Micro- and nano-formulations for bioprinting and additive manufacturing. Drug Discov. Today. 2019;24(1):163–178. doi: 10.1016/j.drudis.2018.10.013. [DOI] [PubMed] [Google Scholar]
- 92.Masaeli E., Marquette C. Direct-write bioprinting approach to construct multilayer cellular tissues. Front. Bioeng. Biotechnol. 2020;7:478. doi: 10.3389/fbioe.2019.00478. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Patel K.H., Dunn A.J., Talovic M., Haas G.J., Marcinczyk M., Elmashhady H. Aligned nanofibers of decellularized muscle ECM support myogenic activity in primary satellite cells in vitro, Biomed. Materials. 2019;14(3) doi: 10.1088/1748-605X/ab0b06. [DOI] [PubMed] [Google Scholar]
- 94.Kabirian F., Mozafari M. Decellularized ECM-derived bioinks: prospects for the future. Methods. 2019;S1046–2023(18) doi: 10.1016/j.ymeth.2019.04.019. 30437-7. [DOI] [PubMed] [Google Scholar]
- 95.Dzobo K., Motaung K.S.C.M., Adesida A. Recent trends in decellularized extracellular matrix bioinks for 3D printing: an updated review. Int. J. Mol. Sci. 2019;20(18):E4628. doi: 10.3390/ijms20184628. pii. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Alexanian R.A., Mahapatra K., Lang D., Vaidyanathan R., Markandeya Y.S., Gill R.K. Induced cardiac progenitor cells repopulate decellularized mouse heart scaffolds and differentiate to generate cardiac tissue. Biochim. Biophys. Acta Mol. Cell Res. 2020;1867(3):118559. doi: 10.1016/j.bbamcr.2019.118559. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Brink M., Zuppinger C. 9th ascona international workshop on cardiomyocyte biology. Biochim. Biophys. Acta Mol. Cell Res. 2020;1867(3):118618. doi: 10.1016/j.bbamcr.2019.118618. [DOI] [PubMed] [Google Scholar]
- 98.Traverse J.H., Henry T.D., Dib N., Patel A.N., Pepine C., Schaer G.L. First-in-Man study of a cardiac extracellular matrix hydrogel in early and late myocardial infarction patients. JACC (J. Am. Coll. Cardiol.): Basic Transl. Sci. 2019;4(6):659–669. doi: 10.1016/j.jacbts.2019.07.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Wang Z., Long D.W., Huang Y., Chen W.C.W., Kim K., Wang Y. Decellularized neonatal cardiac extracellular matrix prevents widespread ventricular remodeling in adult mammals after myocardial infarction. Acta Biomater. 2019;87:140–151. doi: 10.1016/j.actbio.2019.01.062. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Spector M. Decellularized tissues and organs: an historical perspective and prospects for the future. Biomed. Mater. 2020;11(2) doi: 10.1088/1748-6041/11/2/020201. [DOI] [PubMed] [Google Scholar]
- 101.Hussey G.S., Nascari D.G., Saldin L.T., Kolich B., Lee Y.C., Crum R.J. Ultrasonic cavitation to prepare ECM hydrogels. Acta Biomater. 2020;108(20):77–86. doi: 10.1016/j.actbio.2020.03.036. [DOI] [PubMed] [Google Scholar]
- 102.Lee A., Hudson A.R., Shiwarski D.J., Tashman J.W., Hinton T.J., Yerneni S. 3D bioprinting of collagen to rebuild components of the human heart. Science. 2019;365(6452):482–487. doi: 10.1126/science.aav9051. [DOI] [PubMed] [Google Scholar]
- 103.Qian Z., Sharma D., Jia W., Radke D., Kamp T., Zhao F. Engineering stem cell cardiac patch with microvascular features representative of native myocardium. Theranostics. 2019;9(8):2143–2157. doi: 10.7150/thno.29552. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Garcia-Puig A., Mosquera J.L., Jiménez-Delgado S., García-Pastor C., Jorba I., Navajas D. Proteomics analysis of extracellular matrix remodeling during zebrafish heart regeneration. Mol. Cell. Proteomics. 2019;18(9):1745–1755. doi: 10.1074/mcp.RA118.001193. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Sart S., Jeske R., Chen X., Ma T., Li Y. Tissue Eng. Part B Rev.; 2020. Engineering stem cell-derived extracellular matrices: decellularization and biological function. [DOI] [PubMed] [Google Scholar]
- 106.Fay C.D. Computer-aided design and manufacturing (CAD/CAM) for bioprinting. Methods Mol. Biol. 2020;2140:27–41. doi: 10.1007/978-1-0716-0520-2_3. [DOI] [PubMed] [Google Scholar]
- 107.Gorabi A.M., Tafti S.H.A., Soleimani M., Panahi Y., Sahebkar A. Cells, scaffolds and their interactions in myocardial tissue regeneration. J. Cell. Biochem. 2017;118(8):2454–2462. doi: 10.1002/jcb.25912. [DOI] [PubMed] [Google Scholar]
- 108.Lee J., Oh S.J., An S., Kim W.D., Kim S. Biofabrication; 2020. Machine Learning-Based Design Strategy for 3D-Printable Bioink: Elastic Modulus and Yield Stress Determine Printability. [DOI] [PubMed] [Google Scholar]
- 109.Tomaskovic-Crook E., Crook J.M. 3D bioprinting electrically conductive bioink with human neural stem cells for human neural tissues. Methods Mol. Biol. 2020;2140:159–170. doi: 10.1007/978-1-0716-0520-2_10. [DOI] [PubMed] [Google Scholar]
- 110.Cui H., Nowicki M., Fisher J.P., Zhang L.G. 3D bioprinting for organ regeneration. Adv. Healthc. Mater. 2017;6(1) doi: 10.1002/adhm.201601118. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111.Engler A.J., Cooper-White J. Academic vs industry perspectives in 3D bioprinting. APL Bioeng. 2020;4(1) doi: 10.1063/5.0004340. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112.Miri A.K., Mirzaee I., Hassan S., Mesbah Oskui S., Nieto D., Khademhosseini A., Zhang Y.S. Effective bioprinting resolution in tissue model fabrication. Lab Chip. 2019;19(11):2019–2037. doi: 10.1039/c8lc01037d. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113.Matai I., Kaur G., Seyedsalehi A., McClinton A., Laurencin C.T. Progress in 3D bioprinting technology for tissue/organ regenerative engineering. Biomaterials. 2020;226:119536. doi: 10.1016/j.biomaterials.2019.119536. [DOI] [PubMed] [Google Scholar]
- 114.Blaeser A., Duarte Campos D.F., Puster U., Richtering W., Stevens M.M., Fischer H. Controlling shear stress in 3D bioprinting is a key factor to balance printing resolution and stem cell integrity. Adv. Healthc. Mater. 2016;5(3):326–333. doi: 10.1002/adhm.201500677. [DOI] [PubMed] [Google Scholar]
- 115.Sun W., Starly B., Daly A.C., Burdick J.A., Groll J., Skeldon G. The bioprinting roadmap. Biofabrication. 2020;12(2) doi: 10.1088/1758-5090/ab5158. [DOI] [PubMed] [Google Scholar]
- 116.Schwartz R., Malpica M., Thompson G.L., Miri A.K. Cell encapsulation in gelatin bioink impairs 3D bioprinting resolution. J. Mech. Behav. Biomed. Mater. 2020;103:103524. doi: 10.1016/j.jmbbm.2019.103524. [DOI] [PubMed] [Google Scholar]
- 117.Noor N., Shapira A., Edri R., Gal I., Wertheim L., Dvir T. 3D printing of personalized thick and perfusable cardiac patches and hearts. Adv. Sci. 2019;6(11):1900344. doi: 10.1002/advs.201900344. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 118.Corbett D.C., Olszewski E., Stevens K. A FRESH take on resolution in 3D bioprinting. Trends Biotechnol. 2019;37(11):1153–1155. doi: 10.1016/j.tibtech.2019.09.003. [DOI] [PubMed] [Google Scholar]
- 119.Anil Kumar S., Alonzo M., Allen S.C., Abelseth L., Thakur V., Akimoto J. A visible light-cross-linkable, fibrin-gelatin-based bioprinted construct with human cardiomyocytes and fibroblasts. ACS Biomater. Sci. Eng. 2019;5(9):4551–4563. doi: 10.1021/acsbiomaterials.9b00505. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 120.Lee M., Rizzo R., Surman F., Zenobi-Wong M. Guiding lights: tissue bioprinting using photoactivated materials. Chem. Rev. 2020;120(19):10950–11027. doi: 10.1021/acs.chemrev.0c00077. [DOI] [PubMed] [Google Scholar]
- 121.Magalhães L.S.S.M., Santos F.E.P., Elias C.M.V., Afewerki S., Sousa G.F., Furtado A.S.A. Printing 3D hydrogel structures employing low-cost stereolithography technology. J. Funct. Biomater. 2020;11(1) doi: 10.3390/jfb11010012. pii:E12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 122.Zhu K., Chen N., Liu X., Mu X., Zhang W., Wang C., Zhang Y.S. A general strategy for extrusion bioprinting of bio-macromolecular bioinks through alginate-templated dual-stage crosslinking. Macromol. Biosci. 2018;18(9) doi: 10.1002/mabi.201800127. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 123.Valot L., Martinez J., Mehdi A., Subra G. Chemical insights into bioinks for 3D printing. Chem. Soc. Rev. 2019;48(15):4049–4086. doi: 10.1039/c7cs00718c. [DOI] [PubMed] [Google Scholar]
- 124.Paxton N., Smolan W., Böck T., Melchels F., Groll J., Jungst T. Proposal to assess printability of bioinks for extrusion-based bioprinting and evaluation of rheological properties governing bioprintability. Biofabrication. 2017;9(4) doi: 10.1088/1758-5090/aa8dd8. [DOI] [PubMed] [Google Scholar]
- 125.Emmermacher J., Spura D., Cziommer J., Kilian D., Wollborn T., Fritsching U. Engineering considerations on extrusion-based bioprinting: interactions of material behavior, mechanical forces and cells in the printing needle. Biofabrication. 2020;12(2) doi: 10.1088/1758-5090/ab7553. [DOI] [PubMed] [Google Scholar]
- 126.Bianco C.M., Farjo P.D., Ghaffar Y.A., Sengupta P.P. Myocardial mechanics in patients with normal LVEF and diastolic dysfunction. JACC Cardiovasc. Imaging. 2020;13(1 Pt 2):258–271. doi: 10.1016/j.jcmg.2018.12.035. [DOI] [PubMed] [Google Scholar]
- 127.Zhang G., Varkey M., Wang Z., Xie B., Hou R., Atala A. ECM concentration and cell-mediated traction forces play a role in vascular network assembly in 3D bioprinted tissue. Biotechnol. Bioeng. 2020;117(4):1148–1158. doi: 10.1002/bit.27250. [DOI] [PubMed] [Google Scholar]
- 128.Chimene D., Kaunas R., Gaharwar A.K. Hydrogel bioink reinforcement for additive manufacturing: a focused review of emerging strategies. Adv. Mater. 2020;32(1) doi: 10.1002/adma.201902026. [DOI] [PubMed] [Google Scholar]
- 129.Townsend J.M., Beck E.C., Gehrke S.H., Berkland C.J., Detamore M.S. Flow behavior prior to crosslinking: the need for precursor rheology for placement of hydrogels in medical applications and for 3D bioprinting. Prog. Polym. Sci. 2019;91:126–140. doi: 10.1016/j.progpolymsci.2019.01.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 130.Heo D.N., Alioglu M.A., Wu Y., Ozbolat V., Ayan B., Dey M. 3D bioprinting of carbohydrazide-modified gelatin into microparticle-suspended oxidized alginate for the fabrication of complex-shaped tissue constructs. ACS Appl. Mater. Interfaces. 2020;12(18):20295–20306. doi: 10.1021/acsami.0c05096. [DOI] [PubMed] [Google Scholar]
- 131.Yan D., Chang J., Zhang H., Liu J., Song H., Xue Z. Soft three-dimensional network materials with rational bio-mimetic designs. Nat. Commun. 2020;11(1):1180. doi: 10.1038/s41467-020-14996-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 132.Ribeiro A., Blokzijl M.M., Levato R., Visser C.W., Castilho M., Hennink W.E. Assessing bioink shape fidelity to aid material development in 3D bioprinting. Biofabrication. 2017;10(1) doi: 10.1088/1758-5090/aa90e2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 133.Ahlfeld T., Cubo-Mateo N., Cometta S., Guduric V., Vater C., Bernhardt A. A novel plasma-based bioink stimulates cell proliferation and differentiation in bioprinted, mineralized constructs. ACS Appl. Mater. Interfaces. 2020;12(11):12557–12572. doi: 10.1021/acsami.0c00710. [DOI] [PubMed] [Google Scholar]
- 134.Patrício S.G., Sousa L.R., Correia T.R., Gaspar V.M., Pires L.S., Luís J.L. Biofabrication; 2020. Freeform 3D Printing Using a Continuous Viscoelastic Supporting Matrix. [DOI] [PubMed] [Google Scholar]
- 135.Li H., Bao M., Nie Y. Rev.; 2020. Extracellular matrix-based biomaterials for cardiac regeneration and repair, Heart Fail. [DOI] [PubMed] [Google Scholar]
- 136.Qasim M., Haq F., Kang M.H., Kim J.H. 3D printing approaches for cardiac tissue engineering and role of immune modulation in tissue regeneration. Int. J. Nanomed. 2019;14:1311–1333. doi: 10.2147/IJN.S189587. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 137.Kristen M., Ainsworth M.J., Chirico N., van der Ven C.F.T., Doevendans P.A., Sluijter J.P.G. Fiber scaffold patterning for mending hearts: 3D organization bringing the next step. Adv. Healthc. Mater. 2020;9(1) doi: 10.1002/adhm.201900775. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 138.Marques M.A., Parvatiyar M.S., Yang W., de Oliveira G.A.P., Pinto J.R. The missing links within troponin. Arch. Biochem. Biophys. 2019;663:95–100. doi: 10.1016/j.abb.2018.12.026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 139.Johnston J.R., Landim-Vieira M., Marques M.A., de Oliveira G.A.P., Gonzalez-Martinez D., Moraes A.H. The intrinsically disordered C terminus of troponin T binds to troponin C to modulate myocardial force generation. J. Biol. Chem. 2019;294(52):20054–20069. doi: 10.1074/jbc.RA119.011177. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 140.Chen J., Peters A., Papke C.L., Villamizar C., Ringuette L.J., Cao J. Loss of smooth muscle α-actin leads to NF-κB-Dependent increased sensitivity to angiotensin II in smooth muscle cells and aortic enlargement. Circ. Res. 2017;120(12):1903–1915. doi: 10.1161/CIRCRESAHA.117.310563. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 141.Schuerlein S., Schwarz T., Krziminski S., Gätzner S., Hoppensack A., Schwedhelm I. A versatile modular bioreactor platform for Tissue Engineering. Biotechnol. J. 2017;12(2) doi: 10.1002/biot.201600326. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 142.de Bournonville S., Lambrechts T., Vanhulst J., Luyten F.P., Papantoniou I., Geris L. Towards self-regulated bioprocessing: a compact benchtop bioreactor system for monitored and controlled 3D cell and tissue culture. Biotechnol. J. 2019;14(7) doi: 10.1002/biot.201800545. [DOI] [PubMed] [Google Scholar]
- 143.Baroni S., Troiani E., Santonocito C., Moretti G., De Luca C., Antenucci M., Urbani A. A false positive case of high-sensitivity cardiac troponin in a patient with acute chest pain: analytical study of the interference. Clin. Biochem. 2019;66:103–105. doi: 10.1016/j.clinbiochem.2019.02.002. [DOI] [PubMed] [Google Scholar]
- 144.Soler-Botija C., Forcales S.V., Bayés Genís A. Spotlight on epigenetic reprogramming in cardiac regeneration, Semin. Cell Dev. Biol. 2020;97:26–37. doi: 10.1016/j.semcdb.2019.04.009. [DOI] [PubMed] [Google Scholar]
- 145.Sereti K.I., Nguyen N.B., Kamran P., Zhao P., Ranjbarvaziri S., Park S. Analysis of cardiomyocyte clonal expansion during mouse heart development and injury. Nat. Commun. 2018;9(1):754. doi: 10.1038/s41467-018-02891-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 146.Richards D.J., Li Y., Kerr C.M., Yao J., Beeson G.C., Coyle R.C. Human cardiac organoids for the modelling of myocardial infarction and drug cardiotoxicity. Nat. Biomed. Eng. 2020;4(4):446–462. doi: 10.1038/s41551-020-0539-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 147.Jakovljevic D.G. Physical activity and cardiovascular aging: physiological and molecular insights. Exp. Gerontol. 2018;109:67–74. doi: 10.1016/j.exger.2017.05.016. [DOI] [PubMed] [Google Scholar]
- 148.Chikae S., Kubota A., Nakamura H., Oda A., Yamanaka A., Akagi T., Akashi M. Three-dimensional bioprinting human cardiac tissue chips of using a painting needle method, Biotechnol. Bioengineered. 2019;116(11):3136–3142. doi: 10.1002/bit.27126. [DOI] [PubMed] [Google Scholar]
- 149.Palmquist-Gomes P., Pérez-Pomares J.M., Guadix J.A. Cell-based therapies for the treatment of myocardial infarction: lessons from cardiac regeneration and repair mechanisms in non-human vertebrates. Heart Fail. Rev. 2019;24(1):133–142. doi: 10.1007/s10741-018-9750-8. [DOI] [PubMed] [Google Scholar]
- 150.Lemoine M.D., Krause T., Koivumäki J.T., Prondzynski M., Schulze M.L., Girdauskas E. Human induced pluripotent stem cell-derived engineered heart tissue as a sensitive test system for QT prolongation and arrhythmic triggers. Circ. Arrhythm. Electrophysiol. 2018;11(7) doi: 10.1161/CIRCEP.117.006035. [DOI] [PubMed] [Google Scholar]
- 151.Zhao Y., Rafatian N., Feric N.T., Cox B.J., Aschar-Sobbi R., Wang E.Y. A platform for generation of chamber-specific cardiac tissues and disease modeling. Cell. 2019;176(4):913–927. doi: 10.1016/j.cell.2018.11.042. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 152.Sadek H., Olson E.N. Toward the goal of human heart regeneration. Cell Stem Cell. 2020;26(1):7–16. doi: 10.1016/j.stem.2019.12.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 153.Nair N., Gongora E. Stem cell therapy in heart failure: where do we stand today? Biochim. Biophys. Acta (BBA) - Mol. Basis Dis. 2020;1866(4):165489. doi: 10.1016/j.bbadis.2019.06.003. [DOI] [PubMed] [Google Scholar]
- 154.Ladd D., Tilūnaitė A., Roderick H.L., Soeller C., Crampin E.J., Rajagopal V. Assessing cardiomyocyte excitation-contraction coupling site detection from live cell imaging using a structurally-realistic computational model of calcium release. Front. Physiol. 2019;10:1263. doi: 10.3389/fphys.2019.01263. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 155.Scranton K., John S., Escobar A., Goldhaber J.I., Ottolia M. Modulation of the cardiac Na+-Ca2+ exchanger by cytoplasmic protons: molecular mechanisms and physiological implications. Cell Calcium. 2020;87:102140. doi: 10.1016/j.ceca.2019.102140. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 156.Chazin W.J., Johnson C.N. Calmodulin mutations associated with heart arrhythmia: a status report. Int. J. Mol. Sci. 2020;21(4):E1418. doi: 10.3390/ijms21041418. pii. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 157.Moore K., Moore R., Wang C., Norris R.A. Tugging at the heart strings: the septin cytoskeleton in heart development and disease. J. Cardiovasc. Dev. Dis. 2020;7(1) doi: 10.3390/jcdd7010003. pii: E3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 158.Guo R., Morimatsu M., Feng T., Lan F., Chang D., Wan F., Ling Y. Stem cell-derived cell sheet transplantation for heart tissue repair in myocardial infarction. Stem Cell Res. Ther. 2020;11(1):19. doi: 10.1186/s13287-019-1536-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 159.Park J., Anderson C.W., Sewanan L.R., Kural M.H., Huang Y., Luo J. Modular design of a tissue engineered pulsatile conduit using human induced pluripotent stem cell-derived cardiomyocytes. Acta Biomater. 2020;102:220–230. doi: 10.1016/j.actbio.2019.10.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 160.Tang J., Su T., Huang K., Dinh P.U., Wang Z., Vandergriff A. Targeted repair of heart injury by stem cells fused with platelet nanovesicles. Nat. Biomed. Eng. 2018;2:17–26. doi: 10.1038/s41551-017-0182-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 161.Khorramirouz R., Kameli S.M., Fendereski K., Daryabari S.S., Kajbafzadeh A.M. Evaluating the efficacy of tissue-engineered human amniotic membrane in the treatment of myocardial infarction. Regen. Med. 2019;14(2):113–126. doi: 10.2217/rme-2018-0024. [DOI] [PubMed] [Google Scholar]
- 162.Correia Carreira S., Begum R., Perriman A.W. 3D bioprinting: the emergence of programmable biodesign. Adv. Healthc. Mater. 2019 doi: 10.1002/adhm.201900554. [DOI] [PubMed] [Google Scholar]
- 163.Gardin C., Ferroni L., Latremouille C., Chachques J.C., Mitrečić D., Zavan B. Recent applications of three dimensional printing in cardiovascular medicine. Cells. 2020;9(3):E742. doi: 10.3390/cells9030742. pii. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 164.Tang S.W., Tong W.Y., Pang S.W., Voelcker N.H., Lam Y.W. Deconstructing, replicating, and engineering tissue microenvironment for stem cell differentiation. Tissue Eng. B Rev. 2020;2020 doi: 10.1089/ten.TEB.2020.0044. [DOI] [PubMed] [Google Scholar]
- 165.Yu C., Schimelman J., Wang P., Miller K.L., Ma X., You S. Photopolymerizable biomaterials and light-based 3D printing strategies for biomedical application. Chem. Rev. 2020;120(19):10695–10743. doi: 10.1021/acs.chemrev.9b00810. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 166.Miao S., Cui H., Esworthy T., Mahadik B., Lee S.J., Zhou X. 4D self-morphing culture substrate for modulating cell differentiation. Adv. Sci. 2020;7(6):1902403. doi: 10.1002/advs.201902403. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 167.Soler-Botija C., Forcales S.V., Bayés Genís A. Spotlight on epigenetic reprogramming in cardiac regeneration, Semin. Cell Dev. Biol. 2020;97:26–37. doi: 10.1016/j.semcdb.2019.04.009. [DOI] [PubMed] [Google Scholar]