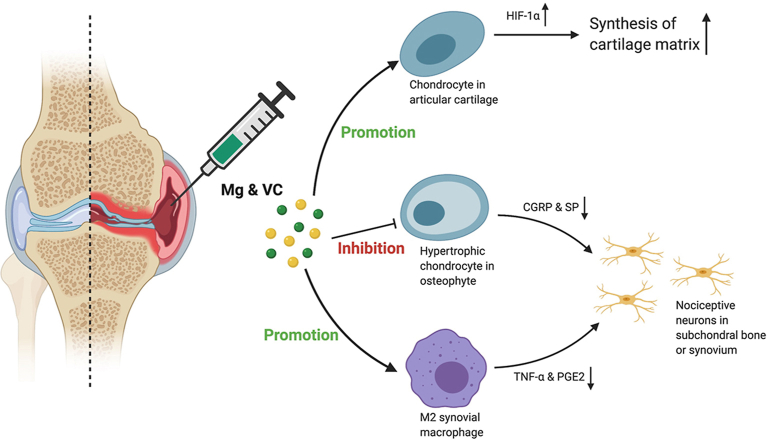
Abstract
Introduction
We previously demonstrated that magnesium ions (Mg2+) was a novel therapeutic alternative for osteoarthritis (OA) through promoting the hypoxia inducible factor-1α (HIF-1α)-mediated cartilage matrix synthesis. However, oxidative stress can inhibit the expression of HIF-1α, amplify the inflammation that potentially impairs the therapeutic efficacy of Mg2+ in OA. Vitamin (VC), a potent antioxidant, may enhance the efficacy of Mg2+ in OA treatment. This study aims to investigate the efficacy of combination of Mg2+ and VC on alleviating joint destruction and pain in OA.
Material and methods
Anterior cruciate ligament transection with partial medial meniscectomy induced mice OA model were randomly received intra-articular injection of either saline, MgCl2 (0.5 mol/L), VC (3 mg/ml) or MgCl2 (0.5 mol/L) plus VC (3 mg/ml) at week 2 post-operation, twice weekly, for 2 weeks. Joint pain and pathological changes were assessed by gait analysis, histology, western blotting and micro-CT.
Results
Mg2+ and VC showed additive effects to significantly alleviate the joint destruction and pain. The efficacy of this combined therapy could sustain for 3 months after the last injection. We demonstrated that VC enhanced the promotive effect of Mg2+ on HIF-1α expression in cartilage. Additionally, combination of Mg2+ and VC markedly promoted the M2 polarization of macrophages in synovium. Furthermore, combination of Mg2+ and VC inhibited osteophyte formation and expressions of pain-related neuropeptides.
Conclusions
Intra-articular administration of Mg2+ and VC additively alleviates joint destruction and pain in OA. Our current formulation may be a cost-effective alternative treatment for OA.
Keywords: Osteoarthritis, Magnesium, Vitamin C, Cartilage, Inflammation, Osteophyte
Graphical abstract
Highlights
-
•
Magnesium ions (Mg2+) and vitamin C (VC) efficaciously alleviates joint pathology and pain in OA.
-
•
Mg2+ + VC markedly increase expression of HIF-1a, a key anabolic factor in cartilage.
-
•
Mg2+ + VC significantly promote M2 polarization of synovial macrophage, may subsequently reduce inflammatory pain.
-
•
Mg2+ + VC also inhibit osteophyte formation and productions of pain-related neuropeptides.
1. Introduction
Osteoarthritis (OA) is the most common degenerative joint disorder that will affect around 78 million people worldwide by 2040 [1]. It remains a grand challenge to treat OA [2,3]. Efforts for developing new approaches have been made. For example, platelet-rich plasma (PRP) or mesenchymal stem cells (MSCs) therapies showed some potentials on cartilage repair [4,5]. However, the cost, potential risks, lack of standardization in preparations of PRP and MSCs are concerns for further clinical applications [2,3]. Nutritional supplements have been developed as non-pharmacological treatment. For example, supplementation of olive tree compound and vitamin D has shown protective effect on articular cartilage [6,7]. Recently, magnesium (Mg)-based biomedical devices have shown great translational potential in orthopaedics [8]. Mg-based alloys exhibit good tissue compatibility, pro-osteogenic property after implantation [9]. Proteome analysis has also indicated that Mg-based biomaterials is beneficial for cartilage regeneration [10]. Moreover, our previous study has demonstrated that magnesium ions (Mg2+) alleviates cartilage degeneration in OA by regulating hypoxia inducible factor-1α (HIF-1α)-mediated cartilage matrix synthesis [11]. With regards to the cost-effective and safety properties, Mg2+ shows a great potential for clinical application. However, this benefit of Mg2+ alone is diminished in a relatively short period after discontinuing the therapy, likely attributed to the fact that OA is a multifactorial disease. Therefore, reagents which can eliminate detrimental factors may help clear the obstacles limiting the efficacy of Mg2+ in OA treatment.
Articular cartilage is an avascular tissue favoring a hypoxic environment. HIF-1α is a key mediator that promotes the chondrocyte adaptation in the hypoxic environment and exerts anabolic and anti-catabolic effects in the chondrocytes of OA [[12], [13], [14], [15]], suggesting that manipulating HIF-1α may have potential therapeutic benefits in OA. We previously reported that Mg2+ could enhance the cartilage matrix synthesis through promoting the expression of HIF-1α [11]. However, previous study indicated that oxidative stress negatively affected oligodendroglial development through inhibiting the expression of HIF-1α [16]. Given the fact that overactivation of oxidative stress has critical role in initiating and potentiating the progression of OA [17]. As a result, oxidative stress may impair the promotive effect of Mg2+ on the expression of HIF-1α in cartilage. Vitamin C (VC) is not only a potent antioxidant, but also a key factor participates in post-translational modification of collagen synthesis and the stabilization of collagen network [[18], [19], [20], [21], [22]]. Whether combining with VC could enhance the promotive effect of Mg2+ on the HIF-1α-mediated cartilage matrix synthesis is worthy to be investigated.
Synovitis is a recognized contributing factor to joint pathology and pain. Released inflammatory cytokines from synoviocytes (for example, macrophage and fibroblast-like synoviocyte) promote cartilage degradation, also induce hyperalgesia by sensitizing nociceptive nerve in the synovium of OA [[23], [24], [25]]. Therefore, alleviating synoviocyte dysfunction is crucial for OA treatment [26,27]. Moreover, overactivated oxidative stress can amplify the inflammation and further aggravate the pathological changes and pain in OA [28,29]. Therefore, VC supplementation is essential for controlling inflammation combined with Mg2+ [11,30]. On the other hand, clinical data showed that osteophyte formation is also in relation to joint pain in OA [[31], [32], [33]]. However, it remains to be investigated how osteophyte induces pain in OA, and whether Mg2+ plus VC could ameliorate osteophyte formation in OA.
In this study, we evaluated the efficacy of intra-articular injection of Mg2+ and VC on alleviating joint destruction and pain in surgical induced OA model in mice. We investigated whether VC could enhance the promotive effect of Mg2+ on the expression of HIF-1α in cartilage. We further clarified whether combination of Mg+ and VC could ameliorate pain in OA through the possible underlying mechanisms of inhibiting the inflammation, productions of pain-related neuropeptides and osteophyte formation.
2. Material and methods
2.1. Establishment of OA model, grouping and treatments
Male C57/BL6 mice (3-month-old) were purchased for this study. The animal experiment was approved by the Animal Ethics and Experimentation Committee of the Chinese University of Hong Kong (Reference No:18/071/MIS-5-C) and conducted following the ARRIVE guidelines. OA was induced by performing anterior cruciate ligament transection with partial medial meniscectomy (ACLT + PMM) surgery in mice [11,34]. From day 1 to day 3 post-operation, the mice received temgesic (0.3 mg/kg of body weight) once per day. The mice were allowed free to food, water and cage activities after the operation. At week 2 post-operation (relative early stage of OA), the mice were randomly assigned to four different groups (n = 6 per group per time point) and received 10 μl intra-articular injection of either saline (control group), 3 mg/ml VC (A5960, Sigma Aldrich, US) (VC group), 0.5 mol/L MgCl2 (M8266, Sigma Aldrich, US) (Mg group) or 0.5 mol/L MgCl2 + 3 mg/ml VC (Mg + VC group) twice per week for two consecutive weeks. The optimal doses of MgCl2 and VC were selected according to our previous studies [11,35]. The grouping of the mice in this study was summarized in Supplementary Table 1.
2.2. Pain assessment by gait analysis
At day one pre-treatment, day 3–84 post-treatment, gait analysis was performed to evaluate the pain-related behaviors of mice by using Catwalk XT 9.0 system (Noldus Information Technology, Wageningen, the Netherlands) [36,37]. Each mouse was placed individually onto a glass walkway (width, 5 cm) and allowed to walk voluntarily back and forth. The footprint was recorded by a high-speed video camera that was positioned (34 cm from the glass walkway) under the glass walkway. Gait parameters (Duty cycle, Intensity, Print area, Single stance, Stand, Swing, Swing speed) of all mice were recorded as baseline one day before treatments [[37], [38], [39]]. At day 3–84 post-treatment, these gait parameters of the mice in all groups were further recorded. The ratio of left hind to right hind (LH/RH) of the parameters was calculated. Limb Idleness Index (LII) was also calculated according to our previous description [37]. Finally, ΔLH/RH (ΔLH/RH = LH/RH post-treatment - LH/RH at baseline) and ΔLII (ΔLII = LII post-treatment - LII pre-treatment) were used for statistical analysis.
2.3. Histomorphometric analysis
At week 6 and 12 post-treatment, knee samples were prepared for fixation in 10% buffered formalin solution and decalcified in 9% formic acid. Paraffin sections were prepared for Safranin O/Fast green or Hematoxylin and Eosin (H&E) staining. The severity of cartilage degeneration and synovitis was evaluated using OARSI scoring system and Krenn scoring system [40,41]. Subchondral bone damages and osteophytes thickness were also evaluated accordingly to previous descriptions [40,42].
2.4. Immunohistochemical (IHC) and immunofluorescence (IF) staining
Primary antibodies were applied and incubated overnight at 4 °C. Horseradish peroxidase (HRP) conjugated secondary antibodies were applied and incubated for 1 h at room temperature. For IHC staining, the signal was developed with DAB kit (TA-060-QHDX, Thermo Fisher scientific) and counterstained with Hematoxylin. For IF staining, DAPI (D1306, Life Technology) was used to stain the nuclei. The antibodies used were listed in the Supplementary Table 2.
2.5. Western blot
At week one post-treatment, four mice from each group were sacrificed for dissecting cartilage tissues from the tibial plateau and divided into two separate samples. The samples were smashed into powder and lysed with RIPA buffer (Cat#89901, Thermo Fisher Scientific). After electrophoresis (protein ladder, BIO-RAD, 1610374), the proteins were electroblotted onto polyvinylidene fluoride membranes. The membranes were blocked and incubated with primary antibodies (HIF-1α, MW 120 kDa; COL2A1, MW 142 kDa; SOX-9, MW 56 kDa; GAPDH, MW 37 kDa) at 4 °C overnight. Subsequently, the membranes were incubated with HRP conjugated secondary antibodies for 1 h at room temperature. Protein bands were detected using an enhanced chemiluminescence kit (Cat#1705062, BIO-RAD). The antibodies were listed in the Supplementary Table 2.
2.6. μCT scanning
At week 12 post-treatment, the fixed knee samples were scanned by μCT (μCT 40, SCANCO MEDICAL, Brttisellen, Switzerland, Voltage = 70 kV, Current = 113 μA; Voxel size = 15 μm; Threshold = 200). Twenty consecutive sagittal images from medial compartment of proximal tibial epiphysis were used to perform 3-D morphometric analyses. Bone volume/total tissue volume (BV/TV), trabecular thickness (Tb.Th), trabecular separation (Tb.Sp), trabecular number (Tb.N) were used to evaluate the subchondral bone changes [43].
2.7. Statistical analysis
Sample size was estimated by power calculation based on our pilot study. Six mice per group was sufficient to provide a 30% difference in the OARSI score (average total score of medial tibial plateau and femoral condyle, α = 0.05, power > 0.85) at week 6 post-treatment. Two mice were excluded from our experiments due to anesthetic accidents. Histological semi-quantitative analysis was performed by two colleagues blindly based on three sections from different regions in each sample. In cases of discrepancies, opinions from a third party was sought. Differences between each treatment and control were analyzed using two-way ANOVA and Turkey's multiple comparisons test using the GraphPad Prism software (Version 8.2) [44]. The significance was defined as P < 0.05.
3. Results
3.1. Intra-articular injection of Mg2+ and VC alleviated structural degeneration in OA
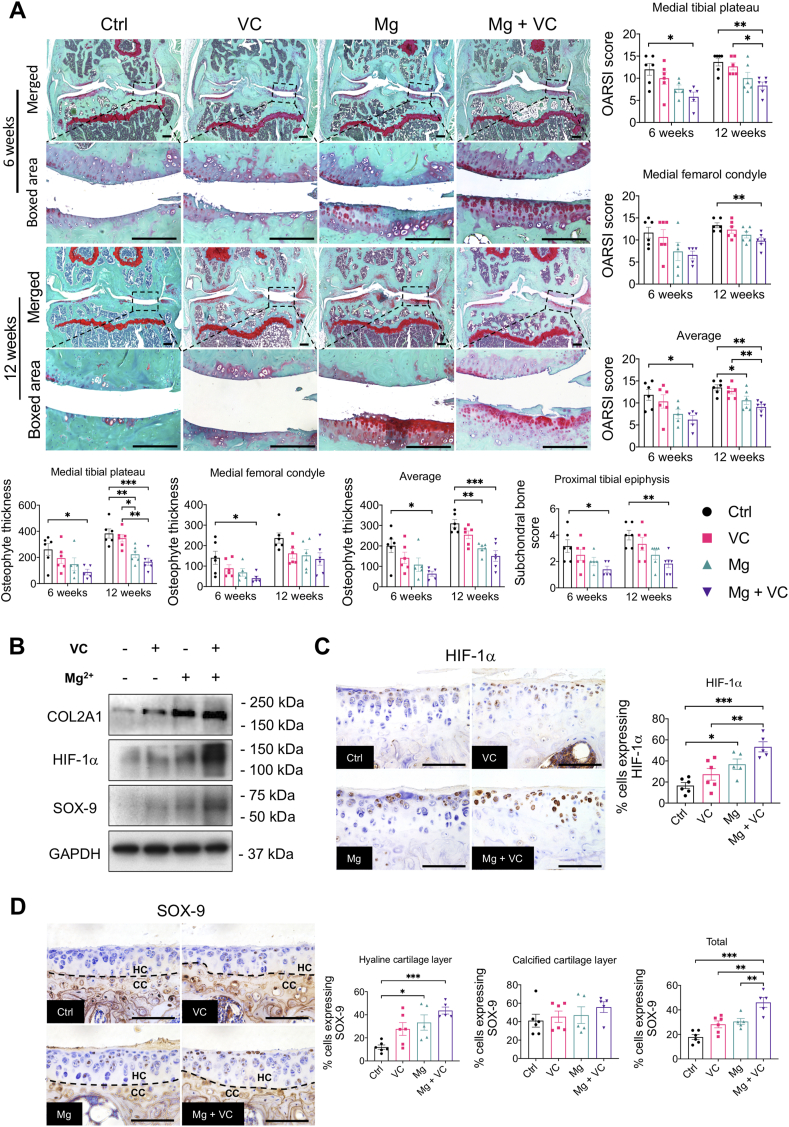
At week 6 post-treatment, in the control group, obvious abrasion on the cartilage surface and focal loss of full-thickness cartilage resulted in an increased OA score. VC only provided a moderate alleviation on cartilage degeneration. Mg2+ alone and Mg combined with VC largely alleviated the cartilage degeneration. Moreover, the mice from Mg + VC group showed the lowest OA score (tibial plateau, P = 0.011; femoral condyle, P = 0.184, average score of tibial plateau and femoral condyle, P = 0.004) compare to the control (Fig. 1A). At week 12 post-treatment, we found severe diffuse cartilage degeneration characterized by absence of entire cartilage at both medial tibial plateau and femoral condyle and resulted in higher OA scores in control and VC groups. Mg2+ slightly ameliorated the cartilage degeneration at this time point. Intriguingly, combination of Mg2+ and VC could still protect the integrity of the cartilage. The OA score was significantly decreased in Mg + VC group (tibial plateau, P = 0.005; femoral condyle, P = 0.010; average score of tibial plateau and femoral condyle, P = 0.001) when comparing to that in the control group (Fig. 1A).
Figure 1.
The structural degeneration of OA was significantly attenuated by intra-articular injection of Mg2+and VC. (A) Left, Safranin O/Fast green staining showed the changes on cartilage in each group at week 6 and 12 post-treatment. Scale bar, 250 μm. Right and bottom, cartilage degeneration, subchondral bone changes and osteophyte development were quantified by OARSI scoring system at the indicated time points. *P < 0.05, **P < 0.01, ***P < 0.001, n = 5–6 animals per group. (B) Western Blotting showed the expressions of COL2A1, HIF-1α and SOX-9 in dissected cartilage tissue at week 1 post-treatment. (C) Left, IHC staining showed the expressions of HIF-1α in articular cartilage at week 6 post-treatment. Scale bar, 100 μm. Right, quantification of HIF-1α positive cells in cartilage at week 6 post-treatment. *P < 0.05, **P < 0.01, ***P < 0.001. n = 5–6 animals per group. (D) Left, IHC staining showed the expression of SOX-9 in hyaline cartilage (HC) and calcified cartilage (CC) layer at week 6 post-treatment. Right, quantification of SOX-9 positive cells in either HC or CC layer. Scale bar, 100 μm. Dash line, tide mark. *P < 0.05, **P < 0.01, ***P < 0.001. n = 5–6 animals per group.
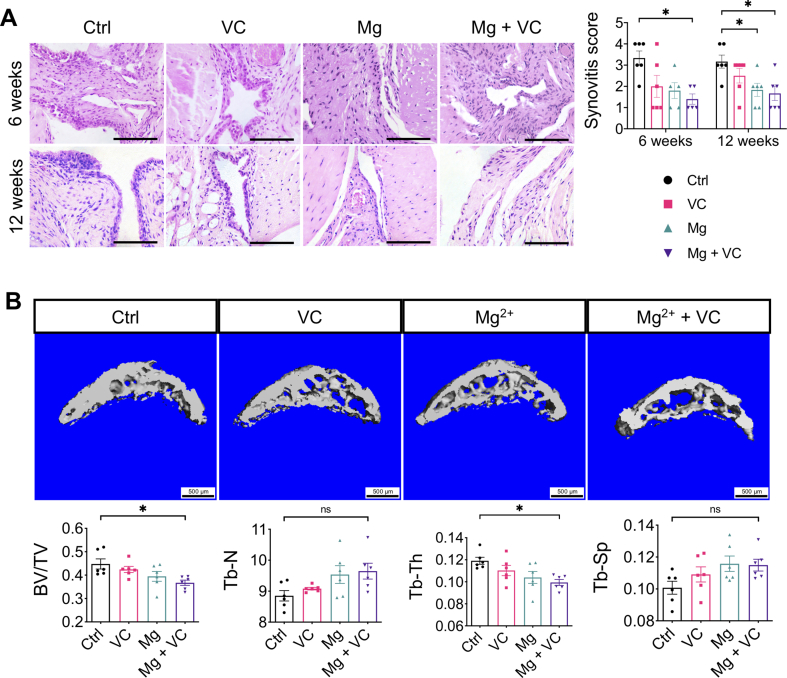
At week 6 and 12 post-treatment, we found mid-grade inflammatory response in synovium characterized by increased lining cell layer and focal inflammatory cells infiltration in the control group. The synovitis was less severe in all treatment groups, while combination of Mg2+ and VC provided better efficacy on alleviating the synovitis with the lowest synovitis score over other treatments when comparing to the control (week 6, P = 0.014; week 12, P = 0.018) (Supplementary Fig. 1A). In addition, we found a progressively increased thickness of osteophyte in both medial tibial plateau and femoral condyle of the mice in the control group, either Mg2+ or VC could partially inhibit the osteophyte formation, combination of Mg2+ and VC could significantly reduce the thickness of osteophyte compared to the control at week 6 (tibial plateau, P = 0.039; femoral condyle, P = 0.025; average score of tibial plateau and femoral condyle, P = 0.016) and 12 (tibial plateau, P < 0.001; femoral condyle, P = 0.061; average score of tibial plateau and femoral condyle, P < 0.001) (Fig. 1A). Moreover, the mice in the control group showed an increased thickening of subchondral bone in proximal tibia, resulting in increased subchondral bone score (based on OARSI scoring system) at week 6 post-treatment. VC and Mg2+ alone only partially alleviated the subchondral bone changes. Whereas, combination of Mg and VC could significantly alleviate the subchondral bone changes compared to the control (P = 0.026) (Fig. 1A). At week 12 post-treatment, apart from the progressively increased thickening of subchondral bone, we also found that the articular cartilage had collapsed into the epiphysis in both the control and VC groups. Mg2+ provided limit potential on alleviating these changes at this time point. However, the results still showed significantly decreased subchondral bone score in Mg + VC group when comparing to that in the control group (P = 0.004) (Fig. 1A). Consistent to the histological findings, at week 12 post-treatment, the results of μCT scanning showed that Mg2+ + VC significantly alleviated the subchondral bone changes with decreased BV/TV (P = 0.019) and Tb.Th (P = 0.015), increased Tb.N (P = 0.065) and Tb.Sp (P = 0.132) compared to the control (Supplementary Fig. 1B).
3.2. Mg2+ and VC prevented the cartilage degeneration via regulating HIF-1α
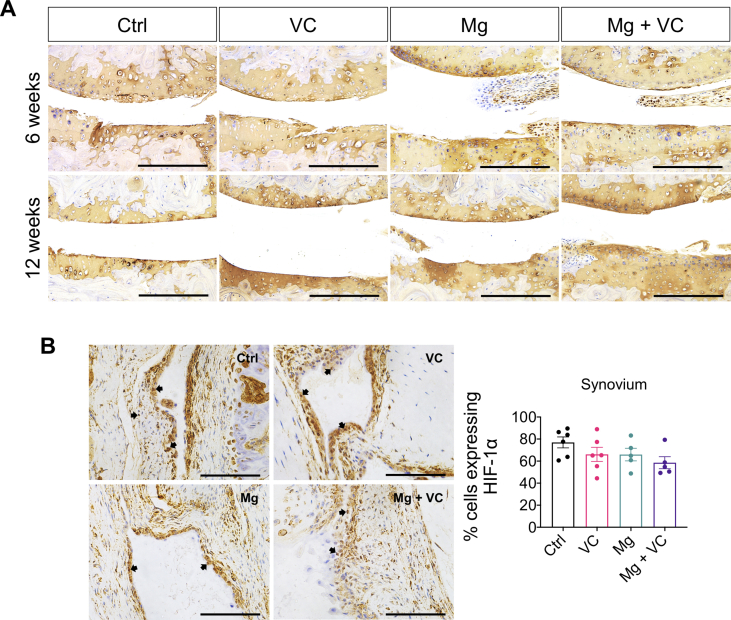
To investigate the efficacy of Mg2+ + VC on prevention of the cartilage matrix degeneration, we evaluated the content of collagen type 2A1 (COL2A1) in cartilage using IHC staining. The results showed no marked difference on COL2A1 between groups. However, the integrity of cartilage in Mg + VC group was well protected compared to that in the control group at week 6 and 12 post-treatment (Supplementary Fig. 2A). We next dissected the cartilage tissue from the mice at week 1 post-treatment, Western blot showed that the expression of COL2A1 in cartilage tissue of Mg + VC group was significantly higher than that of the control group (Fig. 1B). Furthermore, we found that the expression levels of HIF-1α and its downstream marker, transcription factor sex determining region Y-box 9 (SOX-9), were markedly elevated in Mg + VC group with respected to that in the control group (Fig. 1B). We next confirmed the expressions of HIF-1α and SOX-9 in cartilage at week 6 post-treatment using IHC staining. Similarly, we found that Mg2+ and VC had an additive effect to significantly promote the expressions of HIF-1α in articular cartilage (Ctrl vs Mg + VC, P < 0.001, interaction effect, P = 0.544). The expression of SOX-9 in hyaline cartilage layer was also markedly increased after injection of Mg2+ and VC (Ctrl vs Mg + VC, P < 0.001) (Fig. 1D). However, there was no significant difference on the expression of HIF-1α in synovium between the groups (Supplementary Fig. 2B).
3.3. Mg2+ and VC suppressed the expressions of OA markers
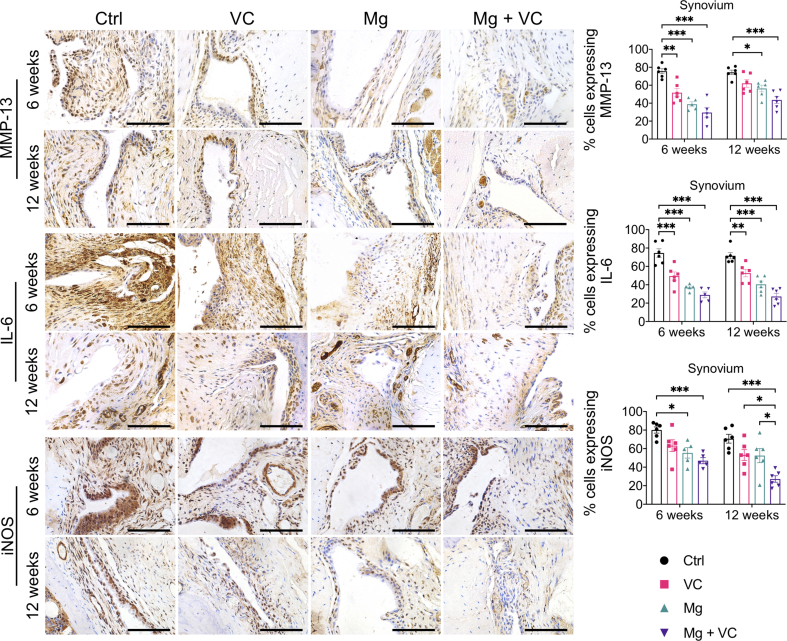
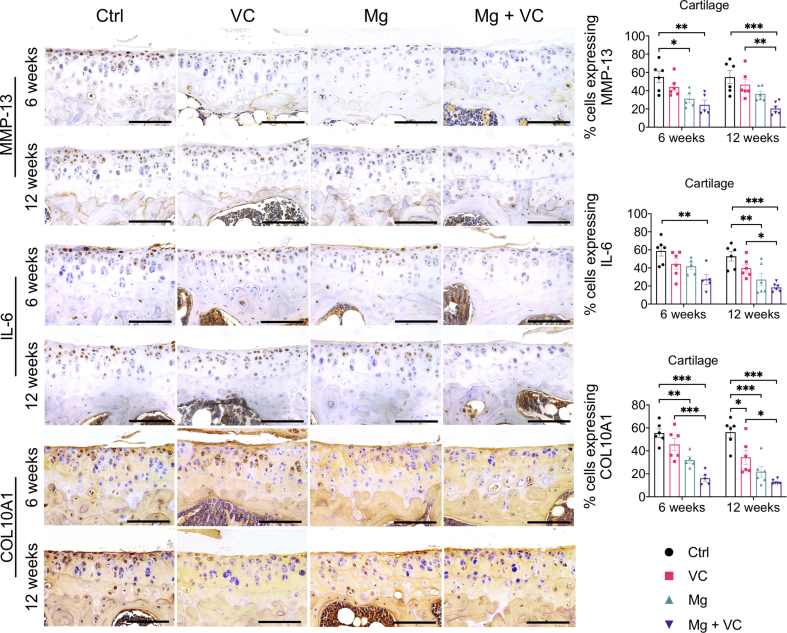
The IHC staining showed increased expression levels of matrix metalloproteinase-13 (MMP-13), interleukin-6 (IL-6) and inducible nitric oxide synthases (iNOS) in both lining cell layer and resident cells in synovium of control group at week 6 and 12 post-treatment (Supplementary Fig. 3). Both VC and Mg2+ reduced the percentage of cells expressing MMP-13, IL-6 and iNOS in the synovium, respectively. The MMP-13, IL-6 and iNOS positive cells in synovium was significantly reduced in Mg2+ + VC group (MMP-13, week 6, P < 0.001; week 12, P < 0.001. IL-6: week 6, P < 0.001; week 12, P < 0.001; iNOS, week 6, P < 0.001; week 12, P < 0.001) compared to that of the control group (Supplementary Fig. 3). We also found similar results in cartilage, the percentage of either MMP-13 or IL-6 positive chondrocytes slightly decreased in VC or Mg groups while significantly reduced in Mg + VC group (MMP-13, week 6, P = 0.003; week 12, P < 0.001. IL-6: week 6, P = 0.003; week 12, P < 0.001) with respected to the control group (Supplementary Fig. 4). The percentage of chondrocytes expressing collagen type 10A1 (COL10A1) was profoundly reduced in Mg + VC group at week 6 (P < 0.001) and week 12 (P < 0.001) post-treatment when comparing to the control group (Supplementary Fig. 4).
3.4. Mg2+ and VC alleviated pain-related animal behaviors
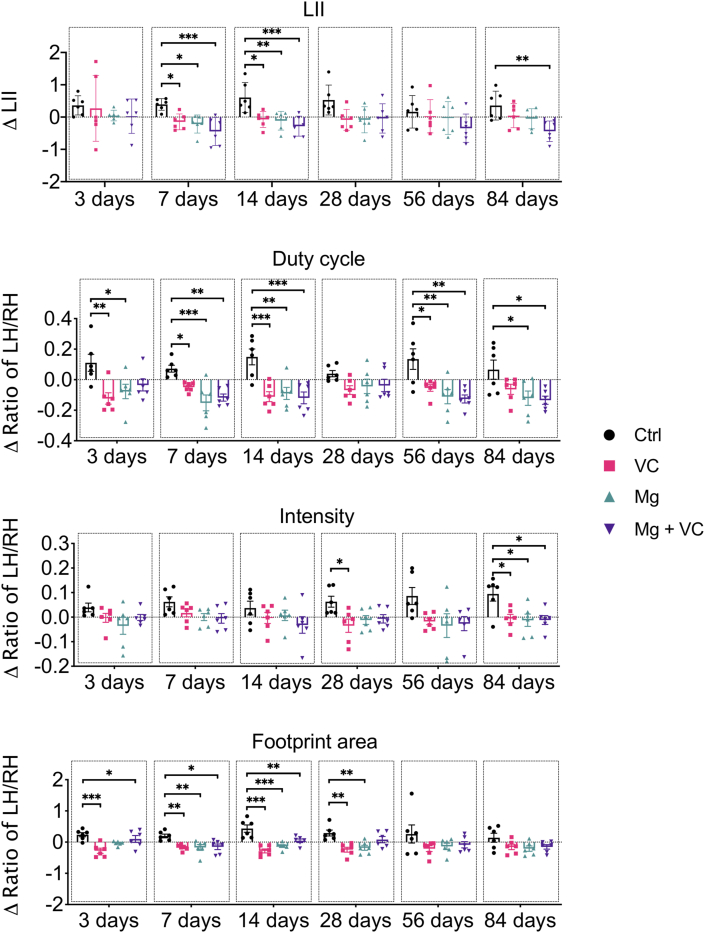
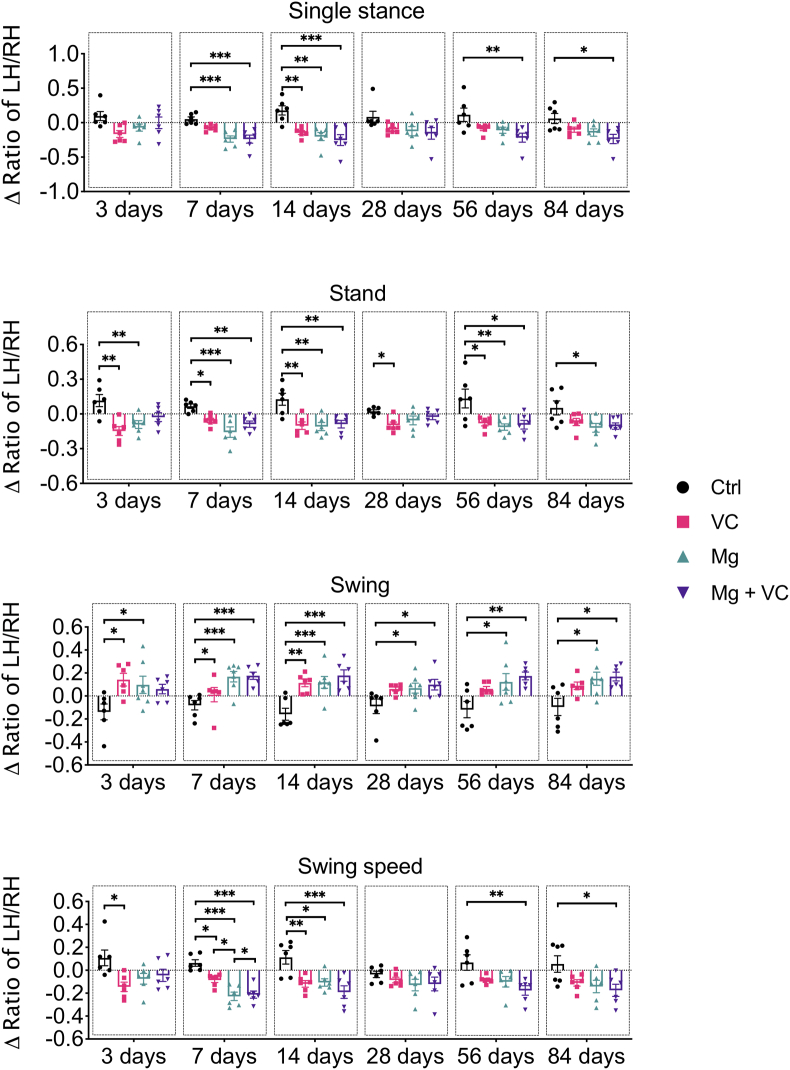
Gait analysis (Catwalk) was used to evaluate the pain-related behaviors of mice. Both VC and Mg2+ reduced OA pain, respectively, at the relatively early time point post-treatment. Intriguingly, only Mg2+ + VC still provided a significant amelioration on the pain-related behaviors over other treatments at the later time points post-treatment (Figure 2, Figure 3). According to the time-course analysis of gait analysis, the ΔLII and Δratios of LH/RH on duty cycle, stand, single stance, intensity and swing speed were deceased, while the Δratio of LH/RH on swing was increased after injection of Mg2+ + VC from day 3–84 post-treatment compared to the control. These data suggested that intra-articular injection of Mg2+ + VC efficaciously alleviated pain-related behaviors (Figure 2, Figure 3).
Figure 2.
Time-course analysis on gait parameters of LII, Duty cycle, Intensity, Footprint area showed the alterations of pain related animal behaviors from day 3–84 after intra-articular injection of Mg2+ + VC. *P < 0.05, **P < 0.01, ***P < 0.001, n = 6 animals per group.
Figure 3.
Time-course analysis on gait parameters of Single stance, Stand, Swing, Swing speed showed the alterations of pain related animal behaviors from day 3–84 after intra-articular injection of Mg2+ + VC. *P < 0.05, **P < 0.01, ***P < 0.001, n = 6 animals per group.
3.5. Mg2+ and VC might ameliorate inflammation-induced pain via regulating synovial macrophage polarization in OA
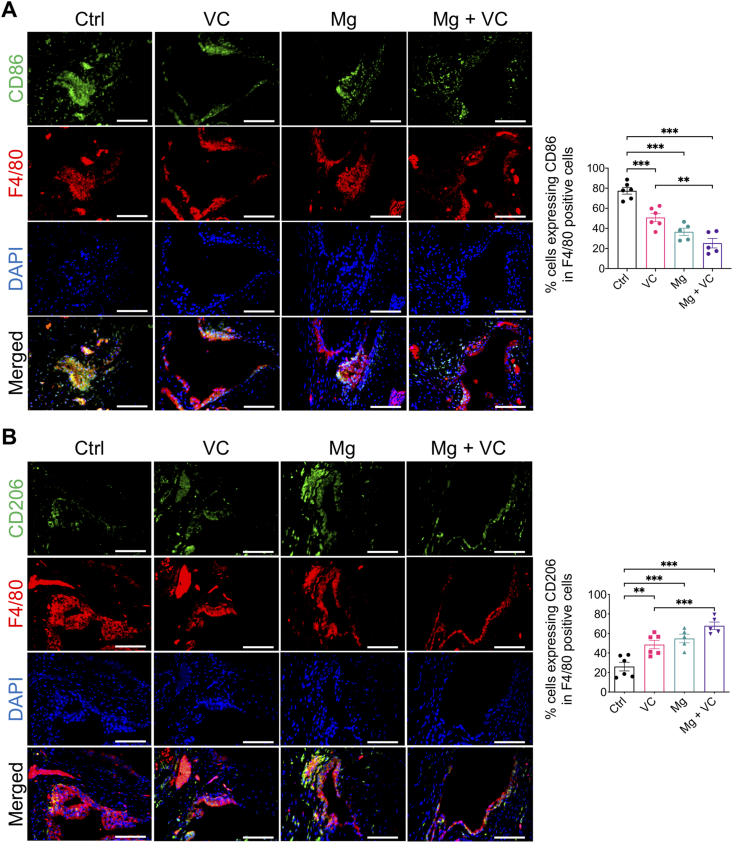
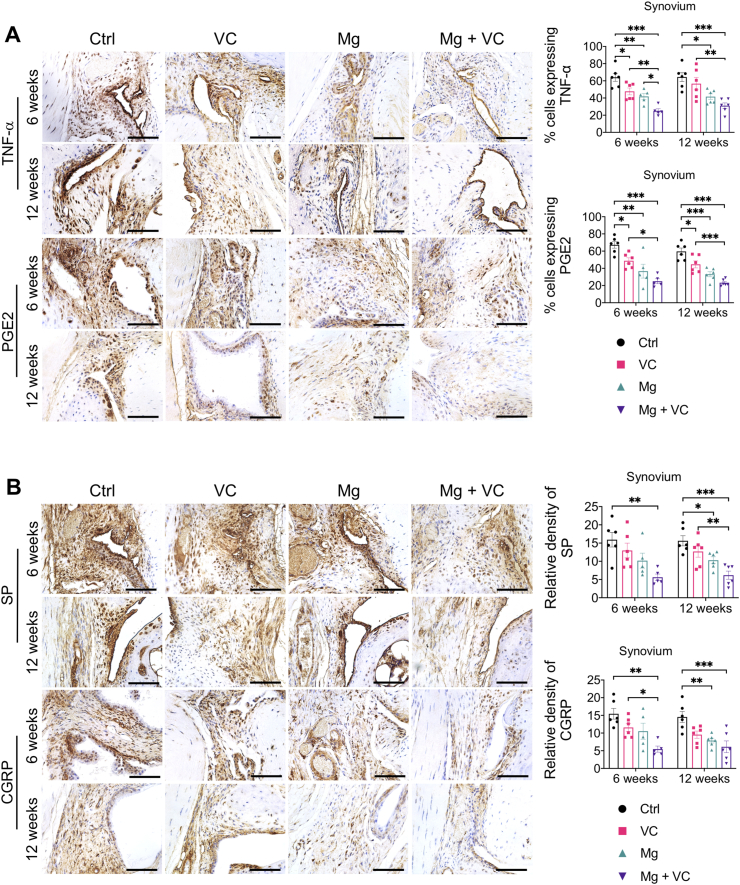
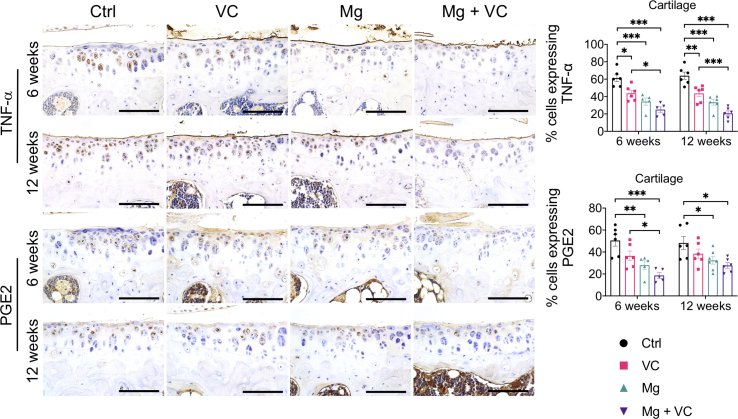
Macrophage infiltration in synovium plays an essential role in triggering the inflammation in OA [24]. Released inflammatory cytokines could sensitize nociceptive nerve terminals in synovium and induce inflammatory OA pain [25]. To investigate the regulatory effects of Mg2+ and VC on synovial macrophage in OA, we identified the phenotypic characterization of macrophage in synovium at week 6 post-treatment. The results of immunofluorescent staining showed a marked elevation of CD86 (M1-like macrophage marker) in F4/80 (macrophage marker) positive cells in synovial tissue of the control group, the percentage of CD86 expressing cells in F4/80 positive cells was decreased in VC (P < 0.001), Mg (P < 0.001) and Mg + VC (P < 0.001) groups compared to the control group (Fig. 4A). Meanwhile, the proportion of cells positive for CD206 (M2-like macrophage marker) in F4/80 positive cells was increased in groups of VC (P = 0.006), Mg (P < 0.001) and Mg + VC (P < 0.001) compared to that of the control group (Fig. 4B). Semi-quantitative analysis indicated that Mg2+ and VC had an additive effect to significantly promote the M2 polarization of macrophage in synovium at weeks 6 post-treatment (CD86, interaction effect, P = 0.070; CD206, interaction effect, P = 0.285). Meanwhile, as expected, Mg2+ and VC also additively suppress the expressions of inflammatory cytokines over other treatments. The results of IHC staining showed that the percentages of tumor necrosis factor-α (TNF-α) and prostaglandin E2 (PGE2) positive cells were significantly decreased in synovial tissues of Mg + VC group compared to that of the control group at week 6 (TNF-α, P < 0.001; PGE2, P < 0.001) and 12 (TNF-α, P < 0.001; PGE2, P < 0.001) post-treatment (Fig. 5A). Additionally, we also found similar results that the expressions of TNF-α and PGE2 were suppressed in cartilage after injection of Mg2+ and VC (Supplementary Fig. 5). To determine the nerve sensitization in synovium after treatments, we further assessed the expressions of substance P (SP) and calcitonin gene-related peptide (CGRP) in synovium. The IHC staining showed that either VC or Mg2+ could reduce the expressions of SP and CGRP. Combination of Mg2+ and VC had larger effect on suppressing the expressions of SP and CGRP than other treatments compared to the control at week 6 (SP, P = 0.005; CGRP, P = 0.001) and 12 (SP, P < 0.001; CGRP, P < 0.001) post-treatment (Fig. 5B). These results indicated that the nerve sensitization in synovium might be reduced [25].
Figure 4.
Intra-articular injection of Mg2+combined with VC promoted the M2 synovial macrophage polarization. (A) Left, IF staining showed the expressions of CD86 (M1 macrophage marker) and F4/80 (macrophage marker) in synovium at week 6 post-treatment. Scale bar, 100 μm. Right, quantification of CD86 in F4/80 positive cells in synovium at week 6 post-treatment. P < 0.01, ***P < 0.001. n = 5–6 animals per group. (B) Lift, IF staining showed the expressions of CD206 (M2 macrophage marker) and F4/80 in synovium at week 6 post-treatment. Scale bar, 100 μm. Right, quantification of CD206 in F4/80 positive cells in synovium at week 6 post-treatment. **P < 0.01, ***P < 0.001. n = 5–6 animals per group.
Figure 5.
Intra-articular injection of Mg2+combined with VC reduced the productions of inflammatory cytokines (TNF-α and PGE2) as well as the pain related neuropeptides (SP and CGRP) in the synovium. (A) Left, IHC staining showed the expressions of TNF-α and PGE2 in synovium at week 6 and 12 post-treatment. Scale bar, 100 μm. Right, quantification of TNF-α and PGE2 positive cells in synovium at the indicated time points. *P < 0.05, **P < 0.01, ***P < 0.001, n = 5–6 animals per group. (B) Left, IHC showed the expressions of SP and CGRP in synovium at week 6 and 12 post-treatment. Scale bar, 100 μm. Right, quantification of SP and CGRP positive cells in synovium at the indicated time points. *P < 0.05, **P < 0.01, ***P < 0.001, n = 5–6 animals per group.
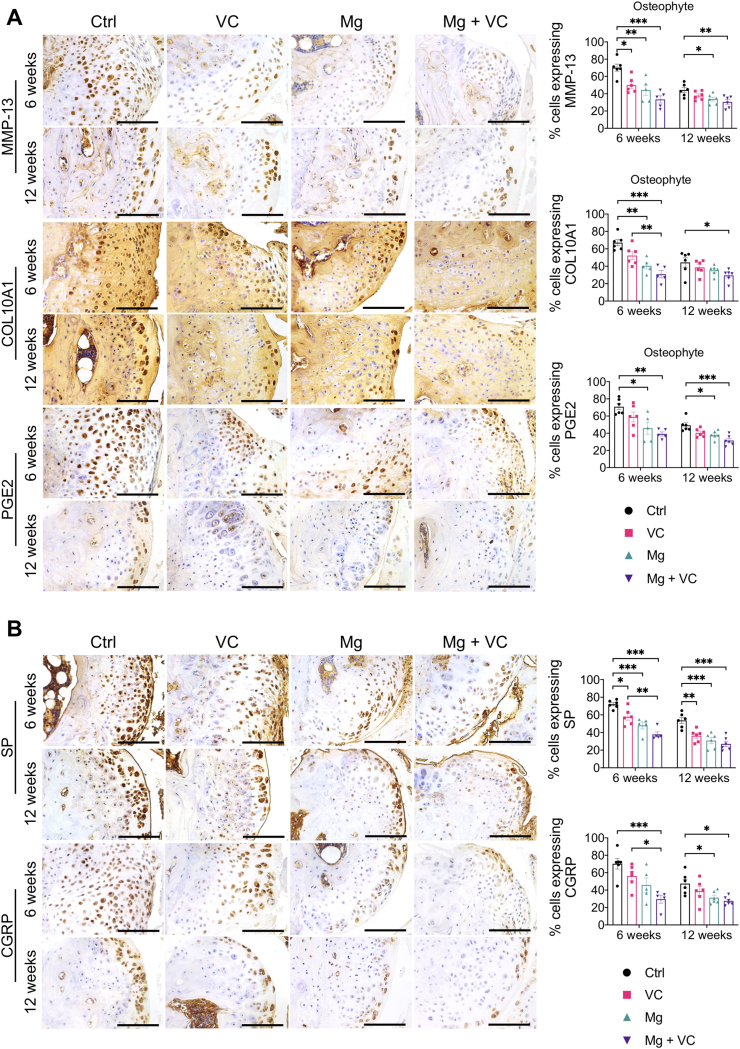
3.6. Mg2+ and VC might alleviate pain through inhibiting osteophytes formation in OA
Our histomorphometric analysis showed that intra-articular injection of Mg + VC limited the thickness of osteophytes (Fig. 1A). It is known that osteophyte formation also occurred through the process of endochondral ossification [45]. In this study, we determined the expressions of MMP-13, COL10A1 and PGE2 in osteophytes using IHC staining. We found significant elevations of MMP-13, COL10A1 or PGE2 positive hypertrophic chondrocytes in osteophytes of the control group at week 6 post-treatment (Fig. 6A). In contrast, combination of Mg2+ and VC significantly reduced the proportion of MMP-13 (P < 0.001), COL10A1 (P < 0.001) and PGE2 (P = 0.002) expressing cells in osteophytes (Fig. 6A). At week 12 post-treatment, along with the osteophyte development, the hypertrophic chondrocytes were replaced by bony tissue thus resulted in a reduced proportion of MMP-13, COL10A1 or PGE2 positive cells in osteophytes of the control group (Fig. 6A). However, combination of Mg2+ and VC could still suppress the expressions of MMP-13 (P = 0.006), COL10A1 (P = 0.043) and PGE2 (P < 0.001) in osteophytes with respected to the control at this time point (Fig. 6A). Previous studies demonstrated that hypertrophic chondrocytes in fracture healing callus could produce neuropeptides including SP [46]. The results from this study showed high expressions of SP and CGRP in hypertrophic chondrocyte in osteophyte of the control group at week 6 and 12 post-treatment, whereas the percentages of SP and CGRP expressing cells (SP, week 6, P < 0.001, week 12, P < 0.001; CGRP, week 6, P = 0.001, week 12, P = 0.012) in osteophyte were significantly reduced in Mg + VC group when comparing to that of the control group (Fig. 6B).
Fig. 6.
Intra-articular injection of Mg2+combined with VC reduced the expressions of hypertrophic markers (MMP-13, COL10A1 and PGE2) and subsequent productions of the pain related neuropeptides (SP and CGRP) in osteophyte. (A) Left, IHC staining showed the expressions of MMP-13, COL10A1 and PGE2 in osteophyte at week 6 and 12 post-treatment. Scale bar, 100 μm. Right, quantification of MMP-13, COL10A1 and PGE2 positive cells in osteophyte at the indicated time points. *P < 0.05, **P < 0.01, ***P < 0.001, n = 5–6 animals per group. (B) Left, IHC staining showed the expressions of SP and CGRP in osteophyte at week 6 and 12 post-treatment. Scale bar, 100 μm. Right, quantification of SP and CGRP positive cells in osteophyte at the indicated time points. *P < 0.05, **P < 0.01, ***P < 0.001, n = 5–6 animals per group.
4. Discussion
In this study, we demonstrated that intra-articular injection of Mg2+ and VC could efficaciously alleviate the joint pathology and pain-related behaviors in ACLT + PMM induced mice OA model. The efficacy of such combination therapy could sustain for 3 months after the last treatment. We demonstrated that VC significantly enhanced the promotive effect of Mg2+ on the expression of HIF-1α in cartilage. In addition, intra-articular injection of Mg2+ and VC markedly promoted the M2 polarization of synovial macrophages. Moreover, combination of Mg2+ and VC also inhibited the osteophyte formation and the productions of pain-related neuropeptides (SP and CGRP).
HIF-1α serves as a key factor for maintenance of hyaline chondrocyte phenotype by promoting the synthesis of type II collagen and aggrecan [[47], [48], [49], [50], [51]]. Depletion of HIF-1α can induce chondrocyte apoptosis and aggravate cartilage degeneration in OA [[52], [53], [54]]. Yet, clinical study has shown that the increased expression of HIF-1α in cartilage is closely associated with severity of OA and indicated HIF-1α may be a harmful factor in OA progression [55]. In fact, HIF-1α plays essential roles in promoting chondrocyte adaptation to survival [56]. In OA, catabolic-stress (for example the inflammatory cytokines, IL-1β) stimulates the expression of HIF-1α in chondrocyte to mediate the anti-catabolic responses [48,53]. Our previous findings also have shown that Mg2+ can further stimulate the HIF-1α expression in human cartilage tissue explants after IL-1β induction in vitro [11]. The Mg2+ induced HIF-1α expression in chondrocyte may contribute to the enhanced matrix synthesis of cartilage and the alleviation of OA progression [11]. Nevertheless, the enhanced oxidative stress in OA may counteract the promotive effect of Mg2+ on the expression of HIF-1α [16]. Apart from confirming our previous finding that Mg2+ promotes the expression of HIF-1α [11], here we further found that intra-articular injection of Mg2+ and VC additively enhanced the expression of HIF-1α in cartilage. Additional supplementation of VC may eliminate the negative effect of oxidative stress thus significantly enhanced the promotive effect of Mg2+ on the expression of HIF-1α in cartilage [16]. Meanwhile, this combination therapy markedly elevates the expressions of SOX-9 and COL2A1 in cartilage. Therefore, the results collectively indicate that VC can enhance the promotive effect of Mg2+ on the HIF-1α-mediated cartilage matrix synthesis, in turn, attenuate the cartilage degeneration in OA. In the future study, experiments are still needed to block the HIF-1α pathway in presence of Mg2+ and VC to consolidate our findings.
As known that pain runs throughout the whole course of OA and is the main complaint of the patients. Multiple factors are involved in causing OA pain. Except for inflammation that plays essential role in pain development of OA [24,25]. Several clinical data show a closed relationship between osteophyte formation and pain in OA [[31], [32], [33]]. In this study, we assessed the efficacy of intra-articular administration of Mg2+ and VC on ameliorating pain in the OA model and further elucidated the possible underlying mechanisms.
According to previous studies on OA pain, Philip G et al. [25] has suggested a concept of inflammatory OA pain which describes a bidirectional crosstalk between immune and nervous system regulates OA pain. Specifically, in the inflamed synovial tissue in OA, activated immune cells (for example, macrophage) secrete number of cytokines include TNF-α and PGE2 which can be recognized by nociceptive nerve terminals in synovium [57,58]. The sensitized nerve terminals, in turn, can produce SP and CGRP which further promote nerve sensitization, vasodilation and extravasation of immune cells [59]. Among the immune cells, macrophage is reported as one of the main cell types triggering the inflammation in OA [24]. Previous studies have indicated that both Mg2+ and VC may have possible roles on regulating the polarization of macrophage [[60], [61], [62]]. In this study, we have found that either Mg2+ or VC has an independent effect to promote the M2 polarization while inhibit M1 polarization of macrophage in synovium. More importantly, Mg2+ and VC acts additively to promote the M2 polarization of synovial macrophage. Consequently, the expression levels of TNF-α and PGE2 are significantly reduced. As aforementioned, these released inflammatory cytokines can enhance the sensitization of nerve terminals in synovium and induce hyperalgesia with increased expressions of neuropeptides [25]. Our results have further showed that Mg2+ and VC can decrease the expressions of CGRP and SP in synovium additively. These results indicate that the nerve sensitization may be reduced after the treatment. Previous studies have shown that Mg2+ deficiency induces raised levels of neuropeptides (CGRP and SP) in peripheral blood [63,64]. We have also reported that Mg2+ can promote the production of CGRP in dorsal root ganglia (DRG) [65]. However, whether local supplementation of Mg2+ could directly influence the expression of CGRP in the nerve terminals of synovium in OA is unclear. In the present study, Mg2+ may inhibit the releases of several inflammatory cytokines through promoting the M2 polarization of synovial macrophage, in turn, ameliorates the inflammatory cytokines induced nerve sensitization by decreasing expressions of CGRP and SP. Meanwhile, supplementation of VC may also exert similar functions with Mg2+. Previous study has indicated that the elevated expressions of CGRP and SP in sciatic nerve after fracture is attributed to oxidative stress [66]. Treatment with VC can inhibit oxidative stress to down-regulate the productions of CGRP and SP, subsequently alleviates the nociceptive features post-fracture [66]. Therefore, VC may enhance the efficacy of Mg2+ on the alleviation of OA pain.
Previous clinical data has indicated that osteophyte formation may be another source of pain in OA [[31], [32], [33]]. However, the mechanism of osteophyte on triggering the OA pain is not fully understood. In this study, we have found marked elevated proportion of hypertrophic chondrocytes with expressions of MMP-13, COL10A1 and PGE2 in osteophyte of the OA model in the control group which is consistent to previous reports [45]. Whereas combination of Mg2+ and VC can significantly inhibit the osteophyte formation with a decreased osteophyte size and reduced hypertrophic markers. It is worth bearing in mind that hypertrophic chondrocytes in fracture healing callus could produce SP and CGRP which facilitated osteogenesis and fracture healing reported by previous studies [46,67]. In our present study, for the first time, we demonstrate that hypertrophic chondrocytes in osteophyte of OA also produce SP and CGRP. These neuropeptides (SP and CGRP) can promote the nerve sensitization and cause the hyperalgesia in OA [[68], [69], [70]]. This evidence provides us a concept that the released SP and CGRP from osteophyte can be recognized by nociceptive nerve terminals distributed in the synovium or even subchondral bone thus promote the OA pain. The results in this study indicate another possible mechanism provided by combination of Mg2+ and VC on pain control in OA.
Our current intra-articular injectable treatment still has limitation. As known that repeated administrations are still needed in this study. In the future study, we will develop a delivery system with controlled release of Mg2+ and VC which can help to minimize the administration frequency. Other relevant assessments (for example, detection of serum concentration of Mg2+ and VC at multiple time points after the treatment) will be also conducted to evaluate the efficiency of drug delivery system on releasing of Mg2+ and VC.
5. Conclusions
In the current study, we demonstrated that combined intra-articular injection of Mg2+ and VC can efficaciously attenuate the joint destruction and pain in a surgical induced OA model in mice. Mg2+ and VC acts additively on promoting the HIF-1α-mediated cartilage matrix synthesis. In addition, Mg2+ and VC have additive effect to significantly promote the M2 polarization of macrophage in synovium, may subsequently alleviate inflammatory OA pain. Furthermore, combination of Mg2+ and VC also inhibits the osteophyte formation and productions of CGRP and SP. The multiple actions of Mg2+ combined with VC injection thus explain the long-term beneficial outcomes in OA. Additionally, the safe and cost-effective properties of this current formulation shed light on the great translational potential. Our study provides essential preclinical evidence for coming clinical trials and bedside applications.
CRediT authorship contribution statement
Hao Yao: Conceptualization, Methodology, Formal analysis, Investigation, Data curation, Writing - original draft. Jiankun Xu: Conceptualization, Methodology, Data curation, Validation, Writing - review & editing. Jiali Wang: Conceptualization, Methodology, Validation. Yifeng Zhang: Conceptualization, Methodology, Validation. Nianye Zheng: Methodology, Investigation. Jiang Yue: Methodology, Investigation. Jie Mi: Methodology, Investigation. Lizhen Zheng: Methodology, Investigation. Bingyang Dai: Methodology, Investigation. Wenhan Huang: Methodology, Investigation. Shuhang Yung: Methodology, Resources. Peijie Hu: Methodology. Yechun Ruan: Methodology. Qingyun Xue: Methodology. Kiwai Ho: Supervision, Writing - review & editing. Ling Qin: Supervision, Writing - review & editing, Project administration, Funding acquisition.
Declaration of competing interest
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
Acknowledgements
We acknowledge Li Ka-Shing Institute of Health Sciences (LiHS) for providing a harmonious working environment and the partial support from Hong Kong RGC Theme-based Research Scheme (T13-402/17-N), National Natural Science Foundation of China (81802152), Collaborative Research Fund (C4026-17WF), and Health and Medical Research Fund (17180671).
Footnotes
Peer review under responsibility of KeAi Communications Co., Ltd.
Supplementary data to this article can be found online at https://doi.org/10.1016/j.bioactmat.2020.10.016.
Contributor Information
Kiwai Ho, Email: kevinho@cuhk.edu.hk.
Ling Qin, Email: lingqin@cuhk.edu.hk.
Appendix A. Supplementary data
The following are the Supplementary data to this article:
Supplementary Fig. 1.
Intra-articular injection of Mg2+combined with VC alleviated the synovitis and subchondral bone sclerosis. (A) Left, H&E staining showed the synovitis development at weeks 6 and 12 post-treatment. Scale bar, 100 μm. Right, severity of synovitis was quantified by synovitis scoring at the indicated time points. *P < 0.05, **P < 0.01. n = 5–6 animals per group. (B) μCT scanning and quantitative analysis on the proximal tibial epiphysis at the medial site. *P < 0.05, n = 6 animals per group.
Supplementary Fig. 2.
(A) IHC staining showed the expression of COLA1 in cartilage at the indicated time points. Scale bar, 250 μm. (B) Left, IHC staining showed the expression of HIF-1α (arrow) in synovium at week 6 post-treatment. Scale bar, 100 μm. Right, quantification of HIF-1α positive cells in synovium at week 6 post-treatment. n = 5–6 animals per group.
Supplementary Fig. 3.
Left, IHC staining showed the expressions of MMP-13, IL-6 and iNOS in synovium at week 6 and 12 post-treatment. Scale bar, 100 μm. Right, quantitation of cells expressing MMP-13, IL-6 and iNOS in synovium at the indicated time points. *P < 0.05, **P < 0.01, ***P < 0.001, n = 5–6 animals per group.
Supplementary Fig. 4.
Left, IHC staining showed the expressions of MMP-13, IL-6 and Col-10 in articular cartilage at week 6 and 12 post-treatment. Scale bar, 100 μm. Right, quantification of cells expressing MMP-13, IL-6 and COL10A1 in cartilage at the indicated time points. *P < 0.05, **P < 0.01, ***P < 0.001, n = 5–6 animals per group.
Supplementary Fig. 5.
Left, IHC staining showed the expressions of TNF-α and PGE2 in cartilage at week 6 and 12 post-treatment. Scale bar, 100 μm. Right, quantification of cells expressing TNF-α and PGE2 in cartilage at the indicated time points. *P < 0.05, **P < 0.01, ***P < 0.001, n = 5–6 animals per group.
References
- 1.Hootman J.M., Helmick C.G., Barbour K.E., Theis K.A., Boring M.A. Updated projected prevalence of self-reported doctor-diagnosed arthritis and arthritis-attributable Activity limitation among US adults, 2015-2040. Arthritis Rheum. 2016;68(7):1582–1587. doi: 10.1002/art.39692. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Bannuru R.R., Osani M.C., Vaysbrot E.E., Arden N.K., Bennell K., Bierma-Zeinstra S.M.A., Kraus V.B., Lohmander L.S., Abbott J.H., Bhandari M., Blanco F.J., Espinosa R., Haugen I.K., Lin J., Mandl L.A., Moilanen E., Nakamura N., Snyder-Mackler L., Trojian T., Underwood M., McAlindon T.E. OARSI guidelines for the non-surgical management of knee, hip, and polyarticular osteoarthritis. Osteoarthritis Cartilage. 2019;27(11):1578–1589. doi: 10.1016/j.joca.2019.06.011. [DOI] [PubMed] [Google Scholar]
- 3.Kolasinski S.L., Neogi T., Hochberg M.C., Oatis C., Guyatt G., Block J., Callahan L., Copenhaver C., Dodge C., Felson D., Gellar K., Harvey W.F., Hawker G., Herzig E., Kwoh C.K., Nelson A.E., Samuels J., Scanzello C., White D., Wise B., Altman R.D., DiRenzo D., Fontanarosa J., Giradi G., Ishimori M., Misra D., Shah A.A., Shmagel A.K., Thoma L.M., Turgunbaev M., Turner A.S., Reston J. 2019 American college of rheumatology/arthritis foundation guideline for the management of osteoarthritis of the hand, hip, and knee. Arthritis Rheum. 2020;72(2):220–233. doi: 10.1002/art.41142. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Campbell K.A., Saltzman B.M., Mascarenhas R., Khair M.M., Verma N.N., Bach B.R., Jr., Cole B.J. Does intra-articular platelet-rich plasma injection provide clinically superior outcomes compared with other therapies in the treatment of knee osteoarthritis? A systematic review of overlapping meta-analyses. Arthroscopy. 2015;31(11):2213–2221. doi: 10.1016/j.arthro.2015.03.041. [DOI] [PubMed] [Google Scholar]
- 5.Kong L., Zheng L.Z., Qin L., Ho K.K.W. Role of mesenchymal stem cells in osteoarthritis treatment. J Orthop Translat. 2017;9:89–103. doi: 10.1016/j.jot.2017.03.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Szychlinska M.A., Imbesi R., Castrogiovanni P., Guglielmino C., Ravalli S., Di Rosa M., Musumeci G. Assessment of vitamin D supplementation on articular cartilage morphology in a young healthy sedentary rat model. Nutrients. 2019;11(6) doi: 10.3390/nu11061260. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Szychlinska M.A., Castrogiovanni P., Trovato F.M., Nsir H., Zarrouk M., Lo Furno D., Di Rosa M., Imbesi R., Musumeci G. Physical activity and Mediterranean diet based on olive tree phenolic compounds from two different geographical areas have protective effects on early osteoarthritis, muscle atrophy and hepatic steatosis. Eur. J. Nutr. 2019;58(2):565–581. doi: 10.1007/s00394-018-1632-2. [DOI] [PubMed] [Google Scholar]
- 8.Sun Y., Wu H., Wang W., Zan R., Peng H., Zhang S., Zhang X. Translational status of biomedical Mg devices in China. Bioact Mater. 2019;4:358–365. doi: 10.1016/j.bioactmat.2019.11.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Chen K., Xie X., Tang H., Sun H., Qin L., Zheng Y., Gu X., Fan Y. In vitro and in vivo degradation behavior of Mg-2Sr-Ca and Mg-2Sr-Zn alloys. Bioact Mater. 2020;5(2):275–285. doi: 10.1016/j.bioactmat.2020.02.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Martinez Sanchez A.H., Omidi M., Wurlitzer M., Fuh M.M., Feyerabend F., Schluter H., Willumeit-Romer R., Luthringer B.J.C. Proteome analysis of human mesenchymal stem cells undergoing chondrogenesis when exposed to the products of various magnesium-based materials degradation. Bioact Mater. 2019;4:168–188. doi: 10.1016/j.bioactmat.2019.04.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Yao H., Xu J.K., Zheng N.Y., Wang J.L., Mok S.W., Lee Y.W., Shi L., Wang J.Y., Yue J., Yung S.H., Hu P.J., Ruan Y.C., Zhang Y.F., Ho K.W., Qin L. Intra-articular injection of magnesium chloride attenuates osteoarthritis progression in rats. Osteoarthritis Cartilage. 2019;27(12):1811–1821. doi: 10.1016/j.joca.2019.08.007. [DOI] [PubMed] [Google Scholar]
- 12.Thoms B.L., Dudek K.A., Lafont J.E., Murphy C.L. Hypoxia promotes the production and inhibits the destruction of human articular cartilage. Arthritis Rheum. 2013;65(5):1302–1312. doi: 10.1002/art.37867. [DOI] [PubMed] [Google Scholar]
- 13.Duval E., Leclercq S., Elissalde J.M., Demoor M., Galera P., Boumediene K. Hypoxia-inducible factor 1alpha inhibits the fibroblast-like markers type I and type III collagen during hypoxia-induced chondrocyte redifferentiation: hypoxia not only induces type II collagen and aggrecan, but it also inhibits type I and type III collagen in the hypoxia-inducible factor 1alpha-dependent redifferentiation of chondrocytes. Arthritis Rheum. 2009;60(10):3038–3048. doi: 10.1002/art.24851. [DOI] [PubMed] [Google Scholar]
- 14.Lafont J.E., Talma S., Hopfgarten C., Murphy C.L. Hypoxia promotes the differentiated human articular chondrocyte phenotype through SOX9-dependent and -independent pathways. J. Biol. Chem. 2008;283(8):4778–4786. doi: 10.1074/jbc.M707729200. [DOI] [PubMed] [Google Scholar]
- 15.Strobel S., Loparic M., Wendt D., Schenk A.D., Candrian C., Lindberg R.L., Moldovan F., Barbero A., Martin I. Anabolic and catabolic responses of human articular chondrocytes to varying oxygen percentages. Arthritis Res. Ther. 2010;12(2):R34. doi: 10.1186/ar2942. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Brill C., Scheuer T., Buhrer C., Endesfelder S., Schmitz T. Oxygen impairs oligodendroglial development via oxidative stress and reduced expression of HIF-1alpha. Sci. Rep. 2017;7:43000. doi: 10.1038/srep43000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Mobasheri A., Rayman M.P., Gualillo O., Sellam J., van der Kraan P., Fearon U. The role of metabolism in the pathogenesis of osteoarthritis. Nat. Rev. Rheumatol. 2017;13:302. doi: 10.1038/nrrheum.2017.50. [DOI] [PubMed] [Google Scholar]
- 18.Aghajanian P., Hall S., Wongworawat M.D., Mohan S. The roles and mechanisms of actions of vitamin C in bone: new developments. J. Bone Miner. Res. 2015;30(11):1945–1955. doi: 10.1002/jbmr.2709. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Sharma G., Saxena R.K., Mishra P. Regeneration of static-load-degenerated articular cartilage extracellular matrix by vitamin C supplementation. Cell Tissue Res. 2008;334(1):111–120. doi: 10.1007/s00441-008-0666-9. [DOI] [PubMed] [Google Scholar]
- 20.Grimmer C., Balbus N., Lang U., Aigner T., Cramer T., Muller L., Swoboda B., Pfander D. Regulation of type II collagen synthesis during osteoarthritis by prolyl-4-hydroxylases: possible influence of low oxygen levels. Am. J. Pathol. 2006;169(2):491–502. doi: 10.2353/ajpath.2006.050738. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Arrigoni O., De Tullio M.C. Ascorbic acid: much more than just an antioxidant. Biochim. Biophys. Acta. 2002;1569(1–3):1–9. doi: 10.1016/s0304-4165(01)00235-5. [DOI] [PubMed] [Google Scholar]
- 22.Shi R., Hu W., Zhang Y., Gao S., Smith A.H., Ye J., Cai L., Graham L.M., Li C. Ascorbate inducible N259 glycans on prolyl 4-hydroxylase subunit alpha1 promote hydroxylation and secretion of type I collagen. Cell. Mol. Life Sci. 2019;76:3449–3464. doi: 10.1007/s00018-019-03081-w. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Kapoor M., Martel-Pelletier J., Lajeunesse D., Pelletier J.P., Fahmi H. Role of proinflammatory cytokines in the pathophysiology of osteoarthritis. Nat. Rev. Rheumatol. 2011;7(1):33–42. doi: 10.1038/nrrheum.2010.196. [DOI] [PubMed] [Google Scholar]
- 24.Sellam J., Berenbaum F. The role of synovitis in pathophysiology and clinical symptoms of osteoarthritis. Nat. Rev. Rheumatol. 2010;6(11):625–635. doi: 10.1038/nrrheum.2010.159. [DOI] [PubMed] [Google Scholar]
- 25.Conaghan P.G., Cook A.D., Hamilton J.A., Tak P.P. Therapeutic options for targeting inflammatory osteoarthritis pain. Nat. Rev. Rheumatol. 2019;15(6):355–363. doi: 10.1038/s41584-019-0221-y. [DOI] [PubMed] [Google Scholar]
- 26.Castrogiovanni P., Di Rosa M., Ravalli S., Castorina A., Guglielmino C., Imbesi R., Vecchio M., Drago F., Szychlinska M.A., Musumeci G. Moderate physical activity as a prevention method for knee osteoarthritis and the role of synoviocytes as biological key. Int. J. Mol. Sci. 2019;20(3) doi: 10.3390/ijms20030511. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Di Rosa M., Castrogiovanni P., Musumeci G. The synovium theory: can exercise prevent knee osteoarthritis? The role of “mechanokines”, A possible biological key. J. Functional Morphol. Kinesiol. 2019;4(11) doi: 10.3390/jfmk4010011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Mobasheri A., Rayman M.P., Gualillo O., Sellam J., van der Kraan P., Fearon U. The role of metabolism in the pathogenesis of osteoarthritis. Nat. Rev. Rheumatol. 2017;13(5):302–311. doi: 10.1038/nrrheum.2017.50. [DOI] [PubMed] [Google Scholar]
- 29.Henrotin Y., Kurz B., Aigner T. Oxygen and reactive oxygen species in cartilage degradation: friends or foes? Osteoarthritis Cartilage. 2005;13(8):643–654. doi: 10.1016/j.joca.2005.04.002. [DOI] [PubMed] [Google Scholar]
- 30.Chiu P.R., Hu Y.C., Huang T.C., Hsieh B.S., Yeh J.P., Cheng H.L., Huang L.W., Chang K.L. Vitamin C protects chondrocytes against monosodium iodoacetate-induced osteoarthritis by multiple pathways. Int. J. Mol. Sci. 2016;18(1) doi: 10.3390/ijms18010038. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Kroon F.P.B., van Beest S., Ermurat S., Kortekaas M.C., Bloem J.L., Reijnierse M., Rosendaal F.R., Kloppenburg M. In thumb base osteoarthritis structural damage is more strongly associated with pain than synovitis. Osteoarthritis Cartilage. 2018;26(9):1196–1202. doi: 10.1016/j.joca.2018.04.009. [DOI] [PubMed] [Google Scholar]
- 32.Zhu Z., Laslett L.L., Jin X., Han W., Antony B., Wang X., Lu M., Cicuttini F., Jones G., Ding C. Association between MRI-detected osteophytes and changes in knee structures and pain in older adults: a cohort study. Osteoarthritis Cartilage. 2017;25(7):1084–1092. doi: 10.1016/j.joca.2017.01.007. [DOI] [PubMed] [Google Scholar]
- 33.Sowers M., Karvonen-Gutierrez C.A., Jacobson J.A., Jiang Y., Yosef M. Associations of anatomical measures from MRI with radiographically defined knee osteoarthritis score, pain, and physical functioning. J Bone Joint Surg Am. 2011;93(3):241–251. doi: 10.2106/JBJS.I.00667. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Fang H., Beier F. Mouse models of osteoarthritis: modelling risk factors and assessing outcomes. Nat. Rev. Rheumatol. 2014;10(7):413–421. doi: 10.1038/nrrheum.2014.46. [DOI] [PubMed] [Google Scholar]
- 35.Fu S.C., Cheng W.H., Cheuk Y.C., Mok T.Y., Rolf C., Yung S.H., Chan K.M. Development of vitamin C irrigation saline to promote graft healing in anterior cruciate ligament reconstruction. J. Orthopaedic Translation. 2013;1:67–77. [Google Scholar]
- 36.Cheuk Y.C., Fu S.C., Mok S.W., Ho K.K., Hung L.K., Chan K.M. Intra-articular injection of an antioxidant formulation did not improve structural degeneration in a rat model of post-traumatic osteoarthritis. J Orthop Translat. 2017;8:25–31. doi: 10.1016/j.jot.2016.08.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Fu S.C., Cheuk Y.C., Hung L.K., Chan K.M. Limb Idleness Index (LII): a novel measurement of pain in a rat model of osteoarthritis. Osteoarthritis Cartilage. 2012;20(11):1409–1416. doi: 10.1016/j.joca.2012.08.006. [DOI] [PubMed] [Google Scholar]
- 38.Zhu S., Zhu J., Zhen G., Hu Y., An S., Li Y., Zheng Q., Chen Z., Yang Y., Wan M., Skolasky R.L., Cao Y., Wu T., Gao B., Yang M., Gao M., Kuliwaba J., Ni S., Wang L., Wu C., Findlay D., Eltzschig H.K., Ouyang H.W., Crane J., Zhou F.Q., Guan Y., Dong X., Cao X. Subchondral bone osteoclasts induce sensory innervation and osteoarthritis pain. J. Clin. Invest. 2019;129(3):1076–1093. doi: 10.1172/JCI121561. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Lakes E.H., Allen K.D. Gait analysis methods for rodent models of arthritic disorders: reviews and recommendations. Osteoarthritis Cartilage. 2016;24(11):1837–1849. doi: 10.1016/j.joca.2016.03.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Gerwin N., Bendele A.M., Glasson S., Carlson C.S. The OARSI histopathology initiative - recommendations for histological assessments of osteoarthritis in the rat. Osteoarthritis Cartilage. 2010;18(Suppl 3):S24–S34. doi: 10.1016/j.joca.2010.05.030. [DOI] [PubMed] [Google Scholar]
- 41.Krenn V., Morawietz L., Burmester G.R., Kinne R.W., Mueller-Ladner U., Muller B., Haupl T. Synovitis score: discrimination between chronic low-grade and high-grade synovitis. Histopathology. 2006;49(4):358–364. doi: 10.1111/j.1365-2559.2006.02508.x. [DOI] [PubMed] [Google Scholar]
- 42.Jeon O.H., Kim C., Laberge R.M., Demaria M., Rathod S., Vasserot A.P., Chung J.W., Kim D.H., Poon Y., David N., Baker D.J., van Deursen J.M., Campisi J., Elisseeff J.H. Local clearance of senescent cells attenuates the development of post-traumatic osteoarthritis and creates a pro-regenerative environment. Nat. Med. 2017;23(6):775–781. doi: 10.1038/nm.4324. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Tong W., Zeng Y., Chow D.H.K., Yeung W., Xu J., Deng Y., Chen S., Zhao H., Zhang X., Ho K.K., Qin L., Mak K.K. Wnt16 attenuates osteoarthritis progression through a PCP/JNK-mTORC1-PTHrP cascade. Ann. Rheum. Dis. 2019;78(4):551–561. doi: 10.1136/annrheumdis-2018-214200. [DOI] [PubMed] [Google Scholar]
- 44.Slinker B.K. The statistics of synergism. J. Mol. Cell. Cardiol. 1998;30(4):723–731. doi: 10.1006/jmcc.1998.0655. [DOI] [PubMed] [Google Scholar]
- 45.Moskowitz R.W., Goldberg V.M. Studies of osteophyte pathogenesis in experimentally induced osteoarthritis. J. Rheumatol. 1987;14(2):311–320. [PubMed] [Google Scholar]
- 46.Niedermair T., Kuhn V., Doranehgard F., Stange R., Wieskotter B., Beckmann J., Salmen P., Springorum H.R., Straub R.H., Zimmer A., Grifka J., Grassel S. Absence of substance P and the sympathetic nervous system impact on bone structure and chondrocyte differentiation in an adult model of endochondral ossification. Matrix Biol. 2014;38:22–35. doi: 10.1016/j.matbio.2014.06.007. [DOI] [PubMed] [Google Scholar]
- 47.Stegen S., Laperre K., Eelen G., Rinaldi G., Fraisl P., Torrekens S., Van Looveren R., Loopmans S., Bultynck G., Vinckier S., Meersman F., Maxwell P.H., Rai J., Weis M., Eyre D.R., Ghesquiere B., Fendt S.M., Carmeliet P., Carmeliet G. HIF-1alpha metabolically controls collagen synthesis and modification in chondrocytes. Nature. 2019;565(7740):511–515. doi: 10.1038/s41586-019-0874-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Zhang F.J., Luo W., Lei G.H. Role of HIF-1alpha and HIF-2alpha in osteoarthritis. Joint Bone Spine. 2015;82(3):144–147. doi: 10.1016/j.jbspin.2014.10.003. [DOI] [PubMed] [Google Scholar]
- 49.Husa M., Liu-Bryan R., Terkeltaub R. Shifting HIFs in osteoarthritis. Nat. Med. 2010;16:641. doi: 10.1038/nm0610-641. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Taheem D.K., Jell G., Gentleman E. Hypoxia inducible factor-1alpha in osteochondral tissue engineering. Tissue Eng. B Rev. 2020;26(2):105–115. doi: 10.1089/ten.teb.2019.0283. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Vinatier C., Merceron C., Guicheux J. Osteoarthritis: from pathogenic mechanisms and recent clinical developments to novel prospective therapeutic options. Drug Discov. Today. 2016;21(12):1932–1937. doi: 10.1016/j.drudis.2016.08.011. [DOI] [PubMed] [Google Scholar]
- 52.Schipani E., Ryan H.E., Didrickson S., Kobayashi T., Knight M., Johnson R.S. Hypoxia in cartilage: HIF-1alpha is essential for chondrocyte growth arrest and survival. Genes Dev. 2001;15(21):2865–2876. doi: 10.1101/gad.934301. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Yudoh K., Nakamura H., Masuko-Hongo K., Kato T., Nishioka K. Catabolic stress induces expression of hypoxia-inducible factor (HIF)-1 alpha in articular chondrocytes: involvement of HIF-1 alpha in the pathogenesis of osteoarthritis. Arthritis Res. Ther. 2005;7(4):R904–R914. doi: 10.1186/ar1765. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Bouaziz W., Sigaux J., Modrowski D., Devignes C.S., Funck-Brentano T., Richette P., Ea H.K., Provot S., Cohen-Solal M., Hay E. Interaction of HIF1alpha and beta-catenin inhibits matrix metalloproteinase 13 expression and prevents cartilage damage in mice. Proc. Natl. Acad. Sci. U. S. A. 2016;113(19):5453–5458. doi: 10.1073/pnas.1514854113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Qing L., Lei P., Liu H., Xie J., Wang L., Wen T., Hu Y. Expression of hypoxia-inducible factor-1alpha in synovial fluid and articular cartilage is associated with disease severity in knee osteoarthritis. Exp Ther Med. 2017;13(1):63–68. doi: 10.3892/etm.2016.3940. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Coimbra I.B., Jimenez S.A., Hawkins D.F., Piera-Velazquez S., Stokes D.G. Hypoxia inducible factor-1 alpha expression in human normal and osteoarthritic chondrocytes. Osteoarthritis Cartilage. 2004;12(4):336–345. doi: 10.1016/j.joca.2003.12.005. [DOI] [PubMed] [Google Scholar]
- 57.Cook A.D., Christensen A.D., Tewari D., McMahon S.B., Hamilton J.A. Immune cytokines and their receptors in inflammatory pain. Trends Immunol. 2018;39(3):240–255. doi: 10.1016/j.it.2017.12.003. [DOI] [PubMed] [Google Scholar]
- 58.Schaible H.G. Nociceptive neurons detect cytokines in arthritis. Arthritis Res. Ther. 2014;16(5):470. doi: 10.1186/s13075-014-0470-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Ji R.R., Chamessian A., Zhang Y.Q. Pain regulation by non-neuronal cells and inflammation. Science. 2016;354(6312):572–577. doi: 10.1126/science.aaf8924. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Li L., Li Y., Fan Z., Wang X., Li Z., Wen J., Deng J., Tan D., Pan M., Hu X., Zhang H., Lai M., Guo J. Ascorbic acid facilitates neural regeneration after sciatic nerve crush injury. Front. Cell. Neurosci. 2019;13:108. doi: 10.3389/fncel.2019.00108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Costantino M.D., Schuster A., Helmholz H., Meyer-Rachner A., Willumeit-Romer R., Luthringer-Feyerabend B.J.C. Inflammatory response to magnesium-based biodegradable implant materials. Acta Biomater. 2019;101:598–608. doi: 10.1016/j.actbio.2019.10.014. [DOI] [PubMed] [Google Scholar]
- 62.Hu T., Xu H., Wang C., Qin H., An Z. Magnesium enhances the chondrogenic differentiation of mesenchymal stem cells by inhibiting activated macrophage-induced inflammation. Sci. Rep. 2018;8(1):3406. doi: 10.1038/s41598-018-21783-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Weglicki W.B., Dickens B.F., Wagner T.L., Chmielinska J.J., Phillips T.M. Immunoregulation by neuropeptides in magnesium deficiency: ex vivo effect of enhanced substance P production on circulating T lymphocytes from magnesium-deficient mice. Magnes. Res. 1996;9(1):3–11. [PubMed] [Google Scholar]
- 64.Weglicki W.B., Phillips T.M. Pathobiology of magnesium deficiency: a cytokine/neurogenic inflammation hypothesis. Am. J. Physiol. 1992;263(3 Pt 2):R734–R737. doi: 10.1152/ajpregu.1992.263.3.R734. [DOI] [PubMed] [Google Scholar]
- 65.Zhang Y., Xu J., Ruan Y.C., Yu M.K., O'Laughlin M., Wise H., Chen D., Tian L., Shi D., Wang J., Chen S., Feng J.Q., Chow D.H., Xie X., Zheng L., Huang L., Huang S., Leung K., Lu N., Zhao L., Li H., Zhao D., Guo X., Chan K., Witte F., Chan H.C., Zheng Y., Qin L. Implant-derived magnesium induces local neuronal production of CGRP to improve bone-fracture healing in rats. Nat. Med. 2016;22(10):1160–1169. doi: 10.1038/nm.4162. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Guo T.Z., Wei T., Huang T.T., Kingery W.S., Clark J.D. Oxidative stress contributes to fracture/cast-induced inflammation and pain in a rat model of complex regional pain syndrome. J. Pain. 2018;19(10):1147–1156. doi: 10.1016/j.jpain.2018.04.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Opolka A., Straub R.H., Pasoldt A., Grifka J., Grassel S. Substance P and norepinephrine modulate murine chondrocyte proliferation and apoptosis. Arthritis Rheum. 2012;64(3):729–739. doi: 10.1002/art.33449. [DOI] [PubMed] [Google Scholar]
- 68.Bullock C.M., Wookey P., Bennett A., Mobasheri A., Dickerson I., Kelly S. Peripheral calcitonin gene-related peptide receptor activation and mechanical sensitization of the joint in rat models of osteoarthritis pain. Arthritis Rheum. 2014;66(8):2188–2200. doi: 10.1002/art.38656. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Benschop R.J., Collins E.C., Darling R.J., Allan B.W., Leung D., Conner E.M., Nelson J., Gaynor B., Xu J., Wang X.F., Lynch R.A., Li B., McCarty D., Nisenbaum E.S., Oskins J.L., Lin C., Johnson K.W., Chambers M.G. Development of a novel antibody to calcitonin gene-related peptide for the treatment of osteoarthritis-related pain. Osteoarthritis Cartilage. 2014;22(4):578–585. doi: 10.1016/j.joca.2014.01.009. [DOI] [PubMed] [Google Scholar]
- 70.Uematsu T., Sakai A., Ito H., Suzuki H. Intra-articular administration of tachykinin NK(1) receptor antagonists reduces hyperalgesia and cartilage destruction in the inflammatory joint in rats with adjuvant-induced arthritis. Eur. J. Pharmacol. 2011;668(1–2):163–168. doi: 10.1016/j.ejphar.2011.06.037. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.