Abstract
Hydrogels are three-dimensional platforms that serve as substitutes for native extracellular matrix. These materials are starting to play important roles in regenerative medicine because of their similarities to native matrix in water content and flexibility. It would be very advantagoues for researchers to be able to regulate cell behavior and fate with specific hydrogels that have tunable mechanical properties as biophysical cues. Recent developments in dynamic chemistry have yielded designs of adaptable hydrogels that mimic dynamic nature of extracellular matrix. The current review provides a comprehensive overview for adaptable hydrogel in regenerative medicine as follows. First, we outline strategies to design adaptable hydrogel network with reversible linkages according to previous findings in supramolecular chemistry and dynamic covalent chemistry. Next, we describe the mechanism of dynamic mechanical microenvironment influence cell behaviors and fate, including how stress relaxation influences on cell behavior and how mechanosignals regulate matrix remodeling. Finally, we highlight techniques such as bioprinting which utilize adaptable hydrogel in regenerative medicine. We conclude by discussing the limitations and challenges for adaptable hydrogel, and we present perspectives for future studies.
Keywords: Adaptable hydrogel, Dynamic mechanical microenvironment, Supramolecular chemistry, Dynamic covalent chemistry, Yes-associated protein
Graphical abstract
Highlights
-
•
Introduction of adaptable hydrogels with dynamic mechanical properties as 3D extracellular matrix.
-
•
Summary of reversible linkages based on supramolecular interactions and dynamic covalent bonds.
-
•
Discussion of how adaptable hydrogels provide dynamic mechanical microenvironment and influence cell behaviors and fate.
-
•
Overview of applications of adaptable hydrogel in regenerative medicine.
1. Introduction
The limited innate capacity for self-regeneration of human beings caused wide attention to tissue regeneration, and propelled the advances of regenerative medicine which could replace or repair diseased tissue or organs. Despite the inherent challenges, in recent decates, successes in cell and biomateirals for regenerative medicine has led to practical applications like artificial skin [1], ear-shaped cartilage [2], bone reconstruction [3,4], nerve regeneration [5], etc. Furthermore, some commercialized products for stem cell therapy [[6], [7], [8]] and numerous candidates in clinical trials [[9], [10], [11]] have been developed. However, further advances in tissue regeneration will require extracellular matrix (ECM) that goes beyond immutable physical support. We need ECM that provides chemical and physical dynamsism, in order to control cell behavior, cell fate, and cell growth [12].
Hydrogel is an efficient three-dimensional material for tissue engineering because of its high water content and flexibility, which allows it to mimic native ECM. Benefiting from the aqueous state close to physiological condition, hydrogel could provide biomimetic microenvironment [13]. The similar stiffness of hydrogel and soft tissue is also beneficial to tissue regeneration [14]. At the early-stage studies of regenerative medicine, covalent hydrogels were dominant; these included glutaraldehyde (GA) crosslinked poly (vinyl alcohol) (PVA) and chitosan [15], photo-crosslinked methacrylated gelatin (GelMA) [16], and transglutaminases crosslinked fibrinogen [17], etc. Such covalent crosslinked hydrogels are stable but lack of the necessary dynamic for cell expansion, ECM remodeling and other cell behaviors. Thus, researchers turned their attention to degradable polymers which could impart dynamic changes for cell proliferation and tissue regeneration [18,19]. Such hydrogels based on degradable polymers can adapt to cell culture in terms of ECM chemistry, mechanical properties, cell type, crosslinking density, and adhesion ligand density [20]. However, the tradeoff between mechanical loss due to degradation and required physical support decides the limitation of irreversible change of degradable covalently crosslinked hydrogel.
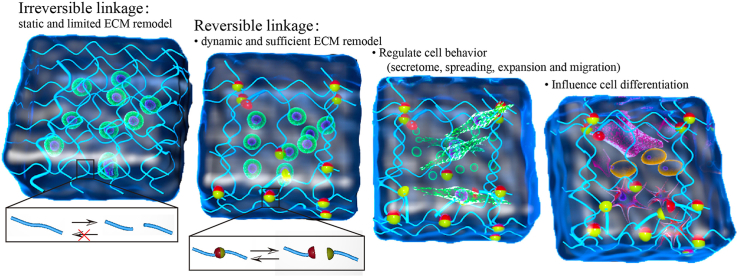
In recent decades, adaptable hydrogels with reversible linkages have become able to mimic the dynamic changes of native ECM. Native ECM is dynamic with stress relaxation and be remodeled and to provide dynamic mechanics to cells [21,22]. However, conventional hydrogels with irreversible linkages are static which own finite stress relaxation and could hardly be remodeled without degradation. Advances in supramolecular chemistry and dynamic covalent chemistry provide opportunities to develop reversible linkages for hydrogels with self-healing, shear thinning, and fast stress relaxation properties to supply dynamic physical cues [23,24]. Further studies focus on spatial dynamics (plasticity that enables remodeling) and mechanical dynamics (stress relaxation that provides biophysical signals) of matrices with reversible linkages [25]. For the purposes of our review, we define adaptable hydrogel for tissue engineering as polymer networks with adaptable linkages that can be broken and re-formed in a reversible manner without external triggers [26].
Previous reviews have outlined the types of reversible linkages which could be utilized to fabricate adaptable hydrogels. Previous reviews have also discussed the applications of adaptable hydrogels in bio-printing and tissue engineering [26,27]. However, the literature has lacked a systematic summary of how to utilize those reversible linkages to build dynamic networks to accomplish specific influences on cell behavior. In this review, we summarizes recent advances in (1) reversible linkages which could be utilized to construct adaptable hydrogels; (2) the influences of hydrogel stress relaxation and spatial plasticity on cell behavior and fate; (3) practical applications of adaptable hydrogels in the field of tissue regeneration. We discuss the rational design of adaptable networks that provide biophysical cues to achieve hydrogels for regeneration of different tissues/organs. Finally, we conclude the limitation of adaptable hydrogels and perspective developments.
2. Reversible linkages for adaptable hydrogels
Adaptable hydrogels are designed to provide mechanical and spatial dynamic, which plays an important role during cell proliferation, differentiation, and immigration as well as tissue regeneration. Thus adaptable hydrogels must provide a physical scaffold to maintain the structural integrity of multicellular organisms, and also serve as a reservoir for biochemical and biophysical signals to support cell survival, organization, and differentiation. Three characteristics of adaptable hydrogels are required for these hydrogels: they must be easy to remodel, instantaneously reversible, and they must feature fast stress relaxation. . The remodeling of the matrix is important for cell expansion, and migration, which cannot be achieved by traditional hydrogel networks. The instantaneous reversibility could allow for injectability, and it could maintain hydrogel stability as physical support even with multiple deformations. Lastly, fast stress relaxation is a newly discovered regulator to cell behavior that could reflect specific mechanosignals to cells, and this makes adaptable hydrogels different from conventional irreversible hydrogels.
In the design of adaptable materials, in addition to the common molecular weight, stoichiometry of reactive functional groups and crosslink density, reversible linkage between molecular chains is essential. The degree of equilibrium reversibility is the key to balance stability and mechanical dynamic. In the past, supramolecular chemistry has provided plenty of non-covalent interactions to obtain reversible linkages, including macrocyclic host–guest interactions, hydrogen bonds, electrostatic interactions, and hydrophobic interactions. Also, dynamic covalent chemistry provides several options, including reversible Diels–Alder reactions, hydrazone bonds, thioester exchanges, boronate bonds, etc.
2.1. Non-covalent interactions
2.1.1. Macrocyclic host–guest interactions
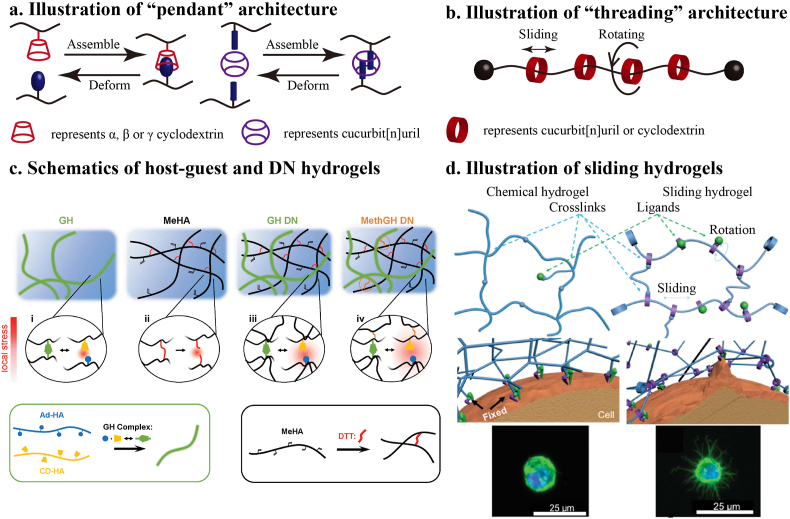
Macrocyclic host-guest complexation is a kind of non-covalent interaction which consists of macrocyclic host such as cyclodextrin (CD) or cucurbit[n]uril (CB[n]s), and paired guests including specific small molecules and linear polymers. The combination form of macrocyclic host-guest complex could be “pendant” or “threading” architecture (Fig. 1a and b).
Fig. 1.
Macrocyclic host–guest design for adaptable hydrogel. a) Illustration of “pendant” architecture; b) Illustration of “threading” architecture; c) Network architectures examined included a host-guest (GH) hydrogel, covalently crosslinked (MeHA) hydrogel, GH double network (DN), and MethGH DN. Local stress under loading (red) dissipated through reversible GH complex rupture [34]. Copyright (2016) Wiley. d) Schematic illustrations of sliding hydrogel (right) and covalent crosslinked chemical hydrogel (left). And the confocal microscopy images of cells cultured in these two kinds of hydrogel [44]. Copyright (2016) Wiley.
The “pendant” means the host or guest molecule was decorated with functional groups on the polymer chains. The host and guest could be paired, according to specific combinations, and typical equilibrium binding constants of specific pairs have been summarized in some reviews [28,29]. Because of the relatively weak interaction and rapid formation, hydrogels with “pendant” host-guest interactions are prone to shear thinning and can sometimes be injected. Specific pairs present various stimulus responses (thermal, pH, light, ion and redox) because of the mutative formation and deformation [30].
Cyclodextrin is one kind of macrocyclic saccharide with a cavity, and it could be divided into three kinds (α, β and γ) according to the number of units. Because of the poor solubility, β-cyclodextrin can be more easily purified after modification compared to α-, and γ-cyclodextrin. β-cyclodextrin is widely studied because it has good equilibrium association constants and widely-available derivatives [31]. Rodell and Burdick et al. have reported their systematic research on hyaluronic acid (HA) hydrogel based on host-guest interactions modified with host (β-cyclodextrin) and guest (adamantine or azobenzene) [32,33]. Such host-guest hydrogels have properties such as shear-thinning, self-healing and injectability. Furthermore, to make the hydrogels more tunable, a secondary network with covalent crosslink was developed (Fig. 1c) [34,35]. Additionally, limited chemical cross-linking was adopted to address the reduced stability of adaptable hydrogels with reversible linkages. Feng et al. reported preassembled host-guest complexation based on gelation and acrylate β-cyclodextrin to fabricate stable adaptable hydrogels [36,37]. The tailored degradation and molecule release respond to proteases, and they are ensured by dithiol peptide crosslinker [38]. Such peptide was able to cleave the matrix metalloproteinase and accomplish cell invasion in the process of tissue regeneration. Practical applications including gene therapy, stem cell therapy and 3D print were also attempted [39,40].
Cucurbit[n]uril (CB[n], n = 5–8, 10) has a similar structure to cyclodextrin, but it has been limited by several factors: it is insoluble, it lacks a homologous series of different-sized hosts, and it is unable to access CB[n] derivatives [41]. So far, only a few hydrogels consisting of CB [6] and CB [8] have been reported for stem cell therapy or drug delivery, even though CB[n] has high affinity to specific guest molecules, high selectivity, and strong binding interactions [42,43]. As shown in Fig. 1a, host-guest interactions based on cucurbit[n]uril could include functional group as cyclodextrin, but also linkers for more than one guest moiety.
The “threading” architecture, also called rotaxane, means the linear polymer, such as poly (ethylene oxide) (PEO), poly (ε-caprolactone) (PCL), or poly (vinyl alcohol) (PVA) serves as a guest molecule that passes through the cavity of host molecule. When the linear polymer passes through the cavity of macrocyclic host molecules, the latter could slide along the guest polymer chain causing flexibility. Such a structure exhibit flexible dynamism without breaking, which is essentially different from most reversible hydrogels.
Highlighted in dynamic hydrogels, sliding hydrogels with rotaxane architecture represent both stability and mobility. Even though sliding hydrogels are ideal strengthen material with complex dynamic, most reported ones were not biocompatible. Yang et al. [44] made sliding hydrogels for constructing synthetic stem cell niches (Fig. 1d). Molecular mobilities of sliding hydrogel render the hydrogel network more adaptable, giving stem cells the freedom to respond to soluble factors and to adjust their behavior accordingly.
2.1.2. Hydrogen bonds
Hydrogen bonds are ubiquitous secondary interactions which could be destroyed easily due to weak bond energy. Multiple hydrogen bonds could form dynamic reversible hydrogels. The large overall association constant ensures such hydrogels remain reliable and adaptable for tissue engineering. In the proceeding of regenerative materials, substrates with multiple hydrogen bonds, including polyphenols, nucleotides, and β-sheets of peptides, were adopted to fabricate adaptable hydrogels.
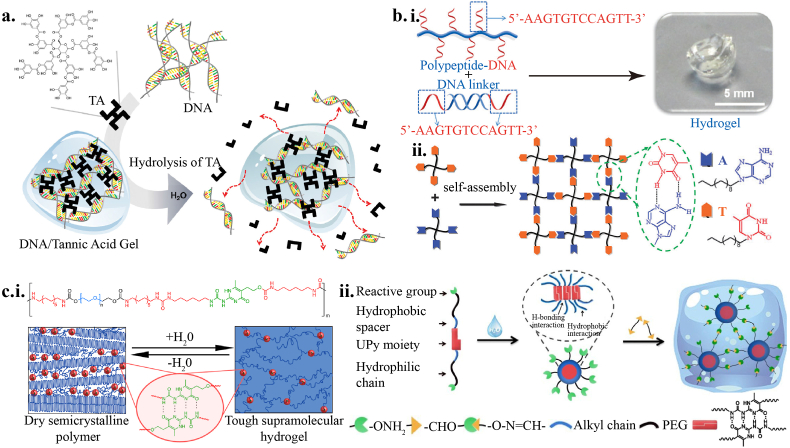
Polyphenols, also called vegetable tannins, have more than one hydroxyl group on a benzene ring. They can form hydrogen bonds with themselves, protein, or DNA [45]. Plenty of works have reported bio-adhesive polyphenols in tissue engineering [46]. With reversible yet strong interaction between DNA and tannic acid (TA), hydrogel was formed without chemical modification [47]. As shown in Fig. 2a, TA plays a role as a “molecular glue”, a new mode of reversible action in phosphodiester bonds, which is different from the crosslinking that uses complementary sequences. Shin et al. [48] reported gallol-derived hydrogel; such reversible hydrogen bonds would transform to covalent bonds after oxidation, in a process that may last several hours. As a result, such hydrogels could only be adaptable in a short time for cell encapsulation and 3D bio-printing. The self-healing of supramolecular hydrogels also provides opportunities to print multiple materials and complex structures at high resolutions [49].
Fig. 2.
a) Schematic illustrations of the formation and hydrolysis of DNA/TA hybrid hydrogel [47]. Copyright (2015) Wiley. b) (i) Polypeptide–DNA conjugate forms hydrogel based on hydrogen bonds between double-stranded DNA [52]. Copyright (2015) Wiley. (ii) Schematic illustrations of four-arm PEG hydrogel network based on hydrogen bonds between thymine and adenine functionalities [51]. Copyright (2012) The Royal Society of Chemistry. c) (i) Schematic illustrations of PEG-UPy chain-extended (co)polymers with segmented multiblock architectures [55]. Copyright (2014) American Chemical Society. (ii) Schematic illustrations of non-swellable hydrogel based on quadruple hydrogen bonds. Copyright (2019) American Chemical Society.
Nucleotides, famous for Watson Crick base pairing, are the basic components of genetic substrates. DNA hydrogels could be crosslinked under physiological conditions via efficient ligase-mediated reactions [50]. As shown in Fig. 2b, the functional group (single-stranded DNA or specific base pairing) modified on the polymer chains could be available to design adaptable hydrogels [51,52].
Ureido pyrimidinone (UPy) could form self-complementary dimers via quadruple hydrogen bonds. However, the strong affinity of UPy dimers for this kind of bonding can only be retained in hydrophobic microenvironments, which are not biocompatible; thus, the application of UPy based hydrogel in tissue engineering was a challenge [50,53]. Block copolymerization or graft with hydrophilic polymer (Fig. 2c i) may overcome the limited application of UPy in aqueous condition [54,55]. Another strategy is to crosslink the micelle of amphiphilic copolymer with UPy units (Fig. 2c ii) [56].
β-Sheets are common motifs of protein secondary structure. They consist of β-strands with more than two backbone hydrogen bonds. Among biomaterials in tissue engineering, several natural proteins and programmable peptides could form hydrogels via β-sheets. Mechanical properties and gelation of natural materials (silk and collagen I) are largely dictated by domains of β-sheet peptide assemblies. Also, synthetic peptides, including MAX1 (β-hairpin peptide), RADA16 and (FEFEFKFK)2-OH, etc., have been designed and programmed to fabricate hydrogels for tissue engineering [[57], [58], [59]]. Due to the weak interaction of β-sheets, composition of such synthetic peptide hydrogels based on β-sheets and supporting matrix are reliable in practical application [60]. Researchers describe general rheological analysis [61] and tutorials [62] which highlights the impact of the conditions used for peptide assembly on the formation and mechanical properties of the corresponding hydrogels.
2.1.3. Electrostatic interaction
Electrostatic interaction is a molecular interaction between cationic and anionic electric charges. Based on electrostatic interaction, hydrogels could be formed between polyelectrolytes, or between multivalent cationic and anionic polymers. Most natural macromolecules (e.g. alginate, hyaluronic acid, chondroitin sulphate, etc.) are anionic, and they could assemble with cationic polyelectrolyte (e.g. chitin, chitosan, polylysine or synthetic polymers) or crosslinks with multivalent cations (Ca2+, Mg2+, Fe3+, Zn2+, etc.) [63,64].
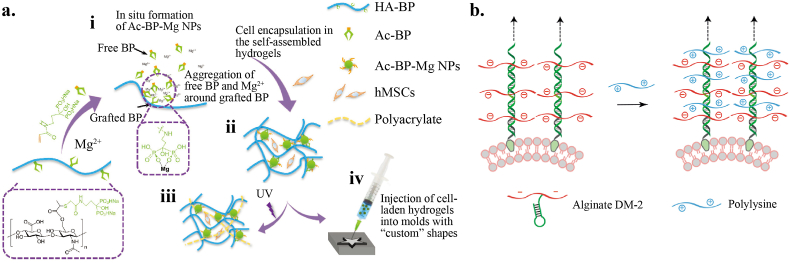
Alginate-Ca2+ is widely applied in tissue engineering because it is easy to form and control (Fig. 3a) [65,66]. The mechanical properties of alginate hydrogels have been studied according to the influences of mechanical signals on cell behavior and fate (see 3.1 for detail). In the progress of additive manufacturing, 3D bioprinting can combine stem cells, and other materials to create complex bio-hybrid structures for various applications [67,68]. The easy and rapid access to digital design and fabrication propels practical application in tissue engineering such as bone/cartilage regeneration, and cardiovascular diseases. Adaptable hydrogels were also developed as bio-inks, because of their dynamic and shear-thinning features. In recent decades, adaptable hydrogels have been printed by extrusion technique. Such technique allows high cell density with low cost, but it is hindered by slow speed and moderate resolution compared to other techniques (inkjet, laser-assisted, and stereolithography) [69]. Alginate expansion lattices for neural progenitor cells were extrusion-printed via stepwise crosslinking. The first-stage crosslink gave pre-printed hydrogels proper stiffness that could support cells and allow for injection, and second-stage crosslink formed a robust and stable lattice [70]. Another more common strategy for bio-printing is photo-crosslink extruded bio-ink with UV light. Stable and precise structure was achieved by covalent cross-linking or supramolecular interactions [71]. Additionally, as shown in Fig. 3b, Mg2+ could stabilize hydrogels by modified with bisphosphonate, which could adapt cell culture and promote a physiological response [72].
Fig. 3.
a) Schematic illustration of the fabrication of the self-assembled HA-BP-Mg nanocomposite hydrogel. i) The in situ self-assembly of Ac-BP-Mg NPs around the grafted BP groups of the HA-BP macromer via BP-Mg2+ coordination. ii) The self-assembled cell-laden hydrogel are stabilized by the Ac-BP-Mg NPs. iii) UV-initiated radical polymerization of the acrylate groups at the surface of Ac-BP-Mg NPs leads to the secondary crosslinking and a further increase in the mechanical property of the self-assembled HA-BP-Mg nanocomposite hydrogel. iv) The cell-laden hydrogel can be injected and rapidly remolded to conform to the geometry of the injection sites [72]. Copyright (2017) Wiley b) DNA-templated crosslinking of alginate and polylysine via polyelectrolyte complexation [76]. Copyright (2019) Springer Nature.
Polyelectrolyte hydrogels have been formed via electrostatic interactions, which have advantages including solubility, quick gelation, protection for bioactive moluecles, and response stimulation [[73], [74], [75]]. The quick gelation needs to be utilized skillfully such as through layer by layer self-assembly; random mixture would cause unexpected precipitation. As shown in Fig. 3c, polyelectrolyte complexes consist of alginate and polylysine were built stepwise with a DNA template [76].
2.1.4. Hydrophobic interaction
Derived by the entropic increase, amphiphilic molecules could assemble by burying the hydrophobic faces away from aqueous environment. Amphiphilic block polymers and coiled-coil peptides are common candidates for adaptable hydrogels assembled via hydrophobic interaction.
Amphiphilic block polymers could consist of synthetic polymers, polypeptides, or grafted derivatives of former two compounds. As shown in Fig. 4a, amphiphilic polypeptide KmLn could be synthesized by a block of hydrophilic polylysine (K) and hydrophobic polyleucine (L), in which m and n represent unit number of polylysine and polyleucine [77]. Synthetic polymers like hydrophilic polyethylene glycol (PEG) are often blocked with hydrophobic polymers like polyphenylene sulfide (PPS) to build amphiphilic block polymers [78]. Besides, amphiphilic polymers poly (N-isopropylacrylamide) are popular for fabricating adaptable hydrogels for cell culture, release, and separation [79,80].
Fig. 4.
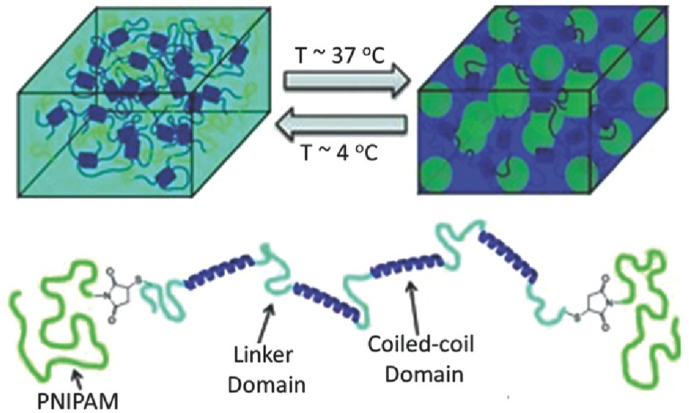
Schematic of a shear-thinning hydrogel formed by coiled-coil self-assembly and reinforced through temperature-responsive aggregation of poly (N-isopropylacrylamide) [84]. Copyright (2013) Wiley.
Coiled-coil peptides are structural sequences common in fibrous proteins, which consists of more than two α-helices [81]. Typically, the primary structure of the α-helices consists of (a-b-c-d-e-f-g) n, where “a” and “d” are usually occupied by hydrophobic amino acids, such as leucine and valine, “e” and “g” are occupied by charged residues [82]. Similar to functional groups, adaptable hydrogels could be formed by self-assembly of polymers grafted with coiled-coil peptides, or by crosslink of micelles that consist of peptide-grafted polymers with reactive groups [83]. As shown in Fig. 4b. elastin-like peptide/protein is another kind of peptide that could form adaptable hydrogels by self-assemble via hydrophobic interaction [84].
2.2. Dynamic covalent linkages
Dynamic covalent bonds are a class of reversible chemical bonds that can be broken and reformed under equilibrium conditions. From the safety consideration, toxic catalysts or relatively extreme conditions such as temperature and pH reversible reactions are not available for adaptive tissue regenerative materials. Table 1 shows the dynamic covalent bonds that have been studied, including Diels-Alder reactions, hydrazine bonds, Schiff bases, hydrazine bonds, thioesters, borate esters, and disulfide bonds.
Table 1.
Dynamic covalent bonds applied for adaptable hydrogel.
2.2.1. Reversible Diels–Alder reaction
The Diels-Alder (DA) reaction is a click chemistry with high selectivity and good yield, without any by-products. DA reactions can occur in a liquid physiological environment, and the equilibrium of the reaction can be controlled by temperature. In past studies, reversible DA reactions required high temperatures (above 100 °C) which are unsuitable for the physiological environment [85]. However, recent studies have found that furan and maleimide can undergo reversible DA reaction at 37 °C, which can be used for tissue engineering [86]. With disulfide bonds that contain norborene and methylphenyltetrazine, Delplace et al. designed inverse electron-demand DA reactions which could happen under physiological conditions (pH and temperature) [87]. However the gelation based on inverse electron-demand DA reactions is still slow (over 30 min) with pH 5.5 which is not sutiable for most cells. Two alternative strategies could be adpoted: (1) replacing the furan diene with an even more electron-rich cyclic diene, such as a fulvene, would further enhance the reaction rate of the DA reaction [88,89], or (2) adopting additional weak linkages like thermosensitive furyl-modified hydroxypropyl chitin (FGE-HPCH) to stablize the hydrogels before DA reaction is accomplished [90].
2.2.2. Hydrazone bonds
The hydrazone bonds produced from the reaction between aldehyde group and hydrazine could be formed rapidly under physiological conditions. Under neutral pH-, the formation and hydrolysis rate of aliphatic aldehyde-derived hydrazones are much faster than that of aromatic aldehyde-derived hydrazones. Hdrogels crosslinked completely with aliphatic aldehyde-derived hydrazones have rapid mechanical relaxation, which is more conducive to cell spreading and migration [91]. Hydrogels based on hydrazone bonds feature rapid geletion and pH-responsive degradation. They have been widely studied in different applications including cell delivery and drug release [[92], [93], [94], [95], [96]].
2.2.3. Schiff base bonds
The Schiff base is produced from the reaction between aldehyde groups or ketone and amines. Due to the harsh reaction conditions of ketone and amine, only aldehyde groups could be applied for biological material. The self-healing property of Schiff base is based on rapid dissociation and recombination without external stimuli. Aliphatic Schiff bases are typically more suitable for cell growth because of the easier reversible breakage [97,98]. Because of the wide range of substances which have aldehyde groups or ketone and amines, hydrogels based on Schiff base are common. Furthermore, with ε-polylysine and aldehyde groups modified Pluronic F127, Wang et al. reported a method to fabricate adapatble hydrogels based on micelles and ε-polylysine assembled nanoparticles [99]. Similarly, Zhou et al. reported polypeptide-based hybrid nanosystems with mutifunctions based on Schiff base reaction between -polylysine and aldehyde groups modified Pluronic F127 [100].
2.2.4. Thioester
Thioesters (e.g. coenzyme A) are widely used in biosynthesis, and their exchange rate in aqueous solution depends on the nucleophilicity and protonation state of sulfide. The formation of alkyl thioesters exceeds the competitive hydrolysis reaction at physiological pH. Changes in pH and reactant metering can modulate thioester exchange to yield thioester-based adaptable hydrogel [101,102].
2.2.5. Boronate bonds
The reaction between boric acid and diol compounds could produce dynamic boronate bonds, which are common in biofilms of bacteria that combine carbohydrates with lectin. Phenylboronic acid (PBA), also called “synthetic lectin”, was applied to mimic chemical structure of lectins in bacteria and obtain self-healing materials [103]. However, its primary/secondary amines are toxic, and PBA needs to be modified to a polymer backbone at a pH lower than the physiological environment. By synthesizing monomers with PBA, adaptable hydrogels could be formed under physiological conditions [104]. Additionally, numerous attempts have been made to improve the arylboronic acid/diol binding affinity by lowering the pKa of the boronic acid. Wu et al. reported nopoldiol, a rigid unnatural cisdiol that provides fast, stable, and bioorthogonal binding with arylboronic acids to build novel type of network with acid/polyol resistance, fast gelation [105]. The cis-diol moiety on furanose ring of guanosine can also react with borates or boronates via boronic acid-diol complexation, and this property is usually used to improve the stability of guanosine-based hydrogels [106].
2.2.6. Disulfide bonds
In living organisms, disulfide bonds formed by the thiol side chains of cysteine residues play a key role in protein folding and assembly. Although the thiol is not active in its reduced state and the formation of the disulfide requires oxidant, the mild reaction condition is biocompatible. Potential limitations of this cross-linking mechanism involve an off-target reaction with a protein that may interfere with its biological activity. In addition, it may exhibit poor stability in the presence of a reductant like glutathione [107].
3. Adaptable hydrogel's effect on cells
Protection is the major aim of hydrogel for cell encapsulation, mostly in terms of immunoisolation. Immuno-privilege is an essential difficulty that cell therapy must face, which requires a protective barrier to ensure high cell viability in vivo. Additional multiple functions have also been proposed for cell encapsulation systems, such as exchange bioactive substances [108,109]. Hydrogels are common matrix for 3D cell encapsulation because of their hydrophilicity, injectability, etc (Fig. 5c). In comparison to conventional hydrogels, most adaptable hydrogels are injectable and self-healing through reversible linkages, which provide better protection of encapsulated cells through dissipating shear/compress force. As shown in Fig. 5a and b, adaptable hydrogel could dissipate force when sheared or pressed [110,111]. Additionally, the successful and easy access to cell encapsulation was the basement of further bio-printing, tissue regeneration and other applications. In terms of bio-printing, the shear-thinning hydrogel with reversile linkages could prevent cell sediment, which is common in conventional covlant hydrogel such as GelMA, PEGDA, etc. [112].
Fig. 5.
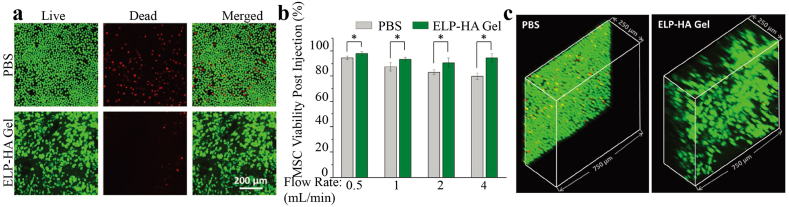
Shear-thinning and self-healing ELP–HA hydrogel can protect cells during syringe needle flow. a) Live/dead (green/red) confocal projection images of MSCs exposed to syringe needle flow (28 gauge, 0.5 in., 4 mL min−1) within PBS or ELP–HA hydrogel. b) Quantification of cell viability immediately postinjection for multiple flow rates. Error bars represent mean ± SD. *p < 0.05, n = 5. c) Confocal 3D reconstructions of live/dead MSCs (green/red) postinjection (28 gauge, 0.5 in., 4 mL min−1) within PBS and ELP–HA hydrogel [111]. Copyright (2017) Wiley.
In past decades, strategies including adding bioactive factors and designing materials surface and interface, have been developed to regulate or guide cell behavior and fate for tissue engineering. However, in recent years, dynamic physical cues were discovered to have essential effects in maintenance of stemness, cell secretome regulation, and cell spread, expansion, migration, and differentiation.
3.1. Maintenance of stemness
Maintenance of stemness and further differentiation potential of stem cells is critical for clinical application [113,114]. Substrates with high stiffness were proven to be helpful for directional differentiation of stem cells, which would cause loss of stemness [[115], [116], [117]]. Previous studies focus on matrix stiffness, topological morphology, and other interface structure [118,119]. However, how to maintain stemness in 3D culture is still difficult.
Besides of stiffness and degradability, stress relaxation of adaptable hydrogels with reversible linkages has inspired researchers to study the influence of dynamic mechanical environments on stem cells [111,120]. Different from the constant elastic modulus of hydrogels with irreversible linkages, the dynamic elastic modulus of adaptable hydrogels with reversible linkages is enabled by fast stress relaxation. Max et al. utilized alginate hydrogels crosslinked with electrostatic interaction to set a series of hydrogels with different stress relaxation rates to verify their influences on the maintenance of stemness [121]. As a result, gene expression of human cortical neuron progenitors (hNPC) and MSCs showed sensitvity to the stress relaxation of alginate hydrogel. Furthermore, fast stress relaxation of adaptable hydrogels is consistant with native ECM, which caused hydrogels with reversible linkages are more similar to native ECM than those hydrogels with irreversible linkages. The reversible and dynamic network of adaptable hydrogels was also proven to be helpful for ECM remodeling like native ECM, and would permit cell spreading and cell-cell contact [122,123].
3.2. Cell secretome regulation
In the progress of cell proliferation and tissue regeneration, secretion plays an important role in cell behavior and cell fate [124,125]. Stem cells could influence tissue regeneration not only by cell proliferation but also by cell secretome regulation [126,127]. Secretome from mesenchymal stem cells (MSCs) includes a great variety of cytokines and chemokines, which have been shown to play important roles in tissue repair and regeneration. Therapies with the application of secretome have a superior safety profile compared to live cell administration [128,129].
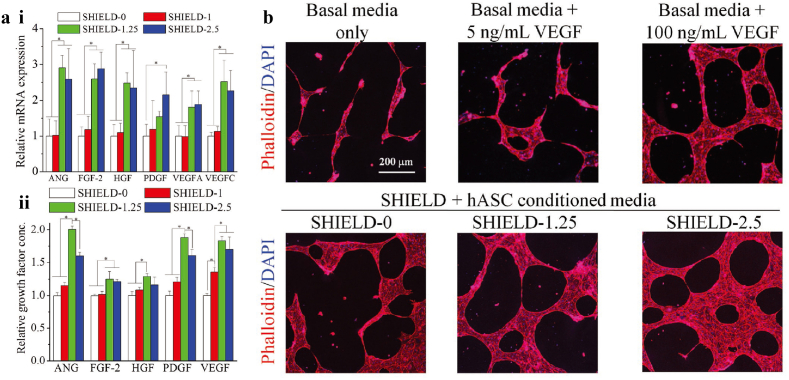
The constant stiffness of materials was hypothesized to facilitate the regulation of stem cell secretome [130]. With engineered recombinant proteins, which crosslinked with tunable stiffness, the stem cell secretome was regulated in situ [124]. Such an adaptable design of “shear-thinning hydrogels for injectable encapsulation and long-term delivery” was named “SHIELD”, and applied to culture human adipose-derived stem cells (hASCs). Two stages of gelation were adopted to form SHIELD: (1) weak gelation of peptide assemble, ands (2) strong gelation of a thermoresponsive PNIPAM. The series of hydrogels were named as “SHIELD-n”, in which n represnets content of PNIPAM. As shown in Fig. 6a, gene expression and secreted paracrine factors of cells cultured SHIELD- 1.25 and SHIELD-2.5 hydrogels were proven in higher level. As a result, enhanced secretome of hASCs cultured in SHIELD hydrogels could lead to better angiogenic function of hMVECs. As shown in Fig. 6b. Significantly more network junctions and loops were observed in hASCs cultured in SHIELD than those cultured in basal media.
Fig. 6.
a) i. Confocal 3D projection images of hASCs within Matrigel sandwiches stained with DAPI (blue) for cell nuclei and rhodamine phalloidin (red) for F-actin cytoskeleton after 2 days exposure to various media treatments. ii. Relative mRNA expression of hASCs within SHIELD at day 14 post injection. b). Relative growth factor concentration of collected media conditioned by hASCs within SHIELD at day 14 post injection. *p < 0.05, n = 4 [124]. Copyright (2016) Wiley.
3.3. Cell spreading, expansion and migration
As a basic physical quality, stress relaxation is a matrix biophysical signal related to cell behaviors such as cell spreading, fiber remodeling, focal adhesion formation, volume expansion, and migration [131,132]. Both physical and dynamic chemical crosslinked adaptable networks were studied as regulators of cell spreading. Compared with constant elastic modulus and static spatial architecture of hydrogels with irreversible linkages, hydrogels with reversible linkages show stress relaxation and plasticity [132]. The stress relaxation of adaptable hydrogels contributes to biophysical signals to integrin that guides cell behavior including spreading and expansion. On the contrary, the static mechanical environment is responsible for inhibited focal adhesion formation and further cell spreading and expansion [122]. As a result, restriction of expansion by hydrogels with slow stress relaxation with diminishes in osteogenesis. Beyond the knowledge of protease-dependent and pores or channels required migration, the adaptable network allows cells to migrate through confining matrix without protease [133].
3.4. Cell differentiation
Tunable properties of ECM could signal cells to influence their differentiation, with which hydrogels could be designed to induce cells differentiation [134]. For instance, with viscoelastic alginate-Ca2+ hydrogels that present stress relaxation and creep, chondrogenesis differentiation was found to be related to faster relaxation [135]. The slow stress relaxation restricts cell volume expansion, which further increases the secretion of IL-1 (cell factor associated with cartilage degradation and cell death), and finally triggers chondrogenesis differentiation. Also, when encapsulated in amphiphilic PNIPAM based hydrogel, hMSC differentiated into nucleus pulposus like cells with undesirable bone formation under hypoxic conditions [136]. Benefitting from the conductivity of polyelectrolytes, self-assembly hydrogels consist of cationic PEG-peptide and anionic poly(3,4-ethylenedioxythiophene): polystyrene sulfonate (PEDOT: PSS) could cause MSCs to differentiate into myocardiocyte-like cells [137].
As above mentioned, matrix cues have been demonstrated as potent regulators of epigenetics and stem cells function. In the past, most effects of adaptable hydrogels were ascribed to the regulator response to mechanical and spatial dynamic [138]. Additionally, pathways activated by these cues including matrix stiffness, degradability and stress relaxation; this illustrates how cells functions are regulated were discovered [139]. Commonly, biophysical signals of extracellular matrix are received by integrins, then affect cell by pathways e.g. Rho/ROCK (Rho protein/Rho-associated kinase), which is necessary for the activity of the YAP (Yes-associated protein) and TAZ (WW domain containing transcription regulator) transcription factors [140].
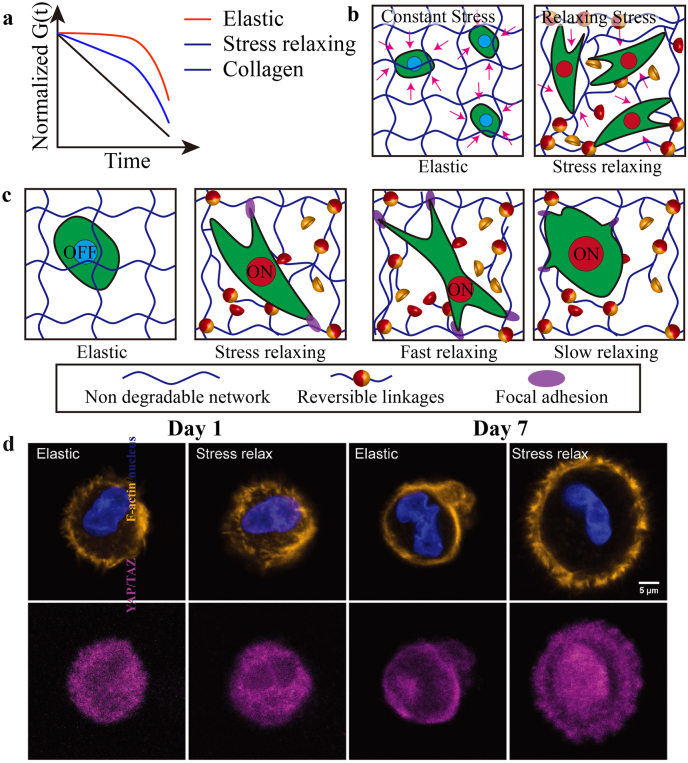
In recent years, YAP/TAZ were studied as two highly related mechanosensitive transcriptional regulators for cells [141]. Matrices with varied mechanical behaviors affect cells via different YAP/TAZ responses (activation/inhibition). Such responses result from ECM remodeling and biophysical signals, and further decide cell fates. Specifically speaking, native ECM mainly consists on collagen which shows fast stress relaxing and could be easily remodeled (Fig. 7a). Adaptable hydrogels with reversible linakges present similar stress relaxing with collegan which could provide dynamic mechanical environment for cells. However, non-degradable covalent networks are elasctic and provide constant mechnical environment which is not adaptable for cells. Also, spatial ECM remodeling is important for cells. As shown in Fig. 7b and c,.the activation of YAP/TAZ, maturation of FA and F-actin remodeling are impacted by the mechnical environment. Compared to elastic hydrogels, cells cultured in stress relax hydrogels present activated YAP/TAZ, and further cell volume expansion and spread (Fig. 7d) [104].
Fig. 7.
a. Stress relaxation of elastic hydrogel based on irreversible covalent bonds, stress relaxing hydrogels based on reversible linkages and native ECM collagen. b. Illustration of static mechanical environment and fixed architecture of elastic hydrogels, and dynamic mechanical environment and remodelable architecture of stress relaxing hydrogels. c. Inhibited YAP/TAZ of cell incubated in elastic hydrogels, and activated YAP/TAZ in stress relaxing hydrogels. Also, the stress relaxing rate impacts maturation of FA and F-actin remodeling. d. Representative immunofluorescence staining for F-actin (orange), nucleus (blue), and YAP/TAZ (magenta) in hMSCs cultured in elastic and stress-relaxing gels for 1 and 7 d. Images are shown as one cross-sectional slice of a z-stack with a step size of 1.5 μm. Scale bar = 5 μm [104]. Copyright (2018) The Authors.
Additionally, more pathways have been studied. For instance, volume expansion controls nuclear localization of RUNX2 (Runt-related transcription factor 2), but not YAP, was demonstrated to promote osteogenesis in 3D culture [132].
4. Application of adaptable hydrogel
4.1. Cardiac regeneration after myocardial infarction
Myocardial infarctions are the leading cause of mortality in heart diseases. These infarctions result in permanent loss of contractile cardiomyocytes (native muscle cells in the heart). The major challenge to heart regeneration is the limited capacity for renewal of cardiomyocytes in the adult heart [142]. In the past, various hydrogels have been developed for treating myocardial infarction, which still could not meet the complex demands of heart tissue repair. Most covalent crosslinked hydrogel could not cover the entire surface area of tissues with irregular surfaces or those that are heavily folded. The gelation of conventional hydrogels with irreversible linkages is uncontrollable, which means fast gelation brings difficulty to operation and slow gelation would cause failed retention of cells or drugs. Furthermore, the brittle and/or unable to accommodate the dynamic movement of tissues in the body. Stapleton et al. reported supramolecular polymeric hydrogels composed of dodecyl (C12)-modified hydroxypropylmethylcellulose and poly(ethylene glycol)-b-poly(lactic acid) nanoparticles as an effective post-operative pericardial adhesion barrier [143]. Such supramolecular polymeric hydrogels could be injected and self-heal rapidly to reversibly transition from viscous flow back to a solid-like barrier, allowing the hydrogels to quickly adhere and settle on the target tissue. Compared to irreversible cardiac adhesions, supramolecular polymeric hydrogels showed better retention and remodeling that suit soft tissue. Additionally, adaptable hydrogels might be helpful to accomplish heart regeneration by cellular therapy or gene delivery. Burdick and his colleagues [38] developed an adaptable hydrogel with shear-thinning and self-healing properties based on macrocyclic host-guest complexation. Such an adaptable hydrogel is composed of adamantine modified hyaluronic acid (HA) microgel and cyclodextrin modified HA with granular structure. Besides of injectablity, stress relaxation, and plasticity, the granular structure could be designed to control porosity of hydrogels for cell invasion.
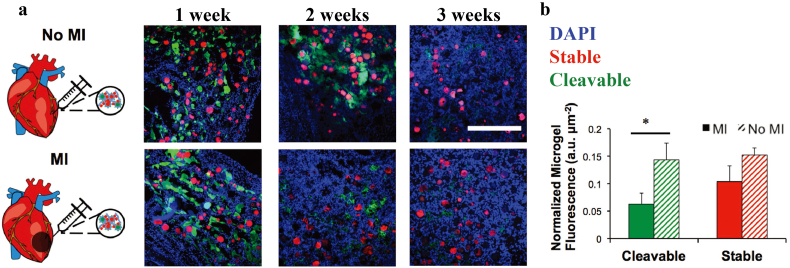
Furthermore, they applied such guest–host HA hydrogels to sustained miRNA delivery to promote cardiomyocyte proliferation and functional regeneration after ischemic injury [39]. MicroRNA mimic (miR-302) modified with cholesterol was assembled into hydrogel via host-guest interaction, and slowly released in vivo. After several weeks, obvious degradation could be seen in microgel morphology. As shown in Fig. 8a, the cleavable microgel was cleared in the MI condition, while large aggregates of partially degraded microgels remained in the healthy condition. Across all conditions, stable microgels remained present with spherical, nondegraded morphologies throughout the duration of the three weeks study. As shown in Fig. 8b, quantified microgel presence at the two weeks time point by measuring the fluorescence intensity and area of both microgel types in order to provide a metric of material mass and volume in the injection site. Cleavable material normalized fluorescence intensity in the MI condition was ~40% in the no MI condition and cleavable material normalized area was ~20% of the normalized area in the no MI condition. This indicated preferential degradation of cleavable microgels in the MI condition. There were no significant differences in stable microgel fluorescence intensity between conditions. Relative to the no MI condition, there was a small decrease in the amount of stable normalized microgel area in the MI group, possibly indicating increased dispersion of the stable microgels in the tissue after MI, likely due to increased cellular invasion as the cleavable microgels were degraded.
Fig. 8.
a) Two-component granular hydrogels labeled with FITC for cleavable microgels and rhodamine for stable microgels were injected into rat hearts either without myocardial infarction (no MI) or with MI. Representative images of microgels and cell nuclei (blue) at one, two, and three weeks after injection (scale bar = 500 μm). b) Quantification of remaining microgels at two weeks, as fluorescence intensity normalized to the injection site area (n = 3). Statistical significance was assessed relative to healthy controls with p < 0.05 [38]. Copyright (2018) Wiley.
4.2. Bone regeneration
Bone defect is a common clinical disease which raised numerous strategies including implantation, transplantation and cellular therapy, etc. Bian and his colleagues [144,145] have reported several works that applied adaptable hydrogel to bone regeneration. Bone regeneration is an important kind of tissue regeneration, but the bones must be very hard. One way to meet this strength requirement through hydrogels is by dual crosslinking [145].
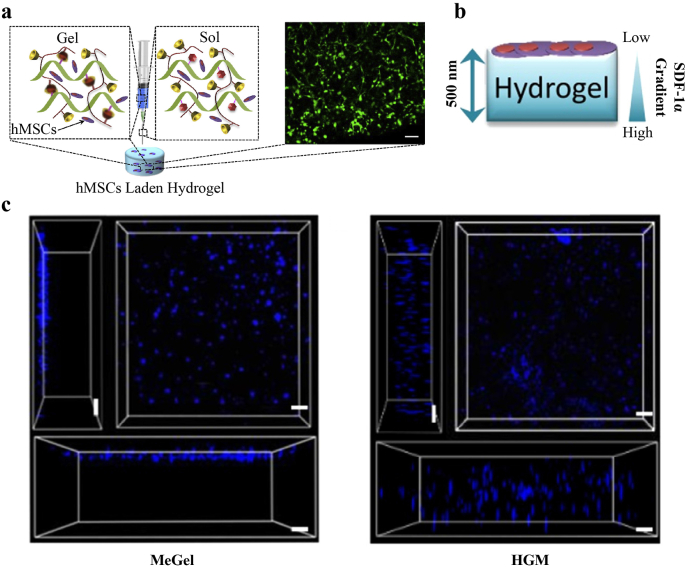
Feng et al. reported their work on gelatin hydrogels based on host-guest interactions to assist bone regeneration [36]. As shown in Fig. 9a, the host-guest macromer (HGM) hydrogels are crosslinked by supramolecular interaction between cyclodextrin and aromatic residues of gelatin. Injetablility and plasticity could be attributed to the reversible linkages, which enable minimally invasive therapy and cell infiltration. To compare the difference of cell infiltration in reversible and irreversible linkages. HGM hydrogels and methacrylated gelatin (MeGel) hydrogels were adpoted to culture hMSCs (Fig. 9b) with SDF-1α gradient. Fig. 9c revealed that HGM hydrogels could enable cell infiltration and migration because of the reversible nature while MeGel inhibited cell infiltration.
Fig. 9.
a) The schematic illustration of the injection of hMSC-laden HGM hydrogels: the shear stress of the injection coverts the solid HGM hydrogel into the “sol” state. Cell viability staining of the hMSC-laden HGM hydrogels after 3 days of in vitro culture following the injection of the HGM hydrogels. b) Schematic illustration of cell migration experiment. The hMSCs seeded on the surface of the HGM hydrogels infiltrate into the hydrogels, but few seeded cells infiltrate into the MeGel hydrogels. c) The 3D distribution of DAPI-stained hMSC nuclei clusters within the MeGel and HGM hydrogels. The confocal micrographs show the 3D distribution of DAPI-stained hMSC nuclei clusters within the MeGel hydrogelsand HGM hydrogels after 2 h of exposure to a chemoattractant gradient. Scale bar: 100 mm (a) and 100 μm (c) [36]. Copyright (2016) Elsevier.
Bisphosphonate-magnesium (Mg2+) coordination was also utilized to obtain adaptable hydrogels which could be infiltrated by cells or regulate differentiation [72]. Dexamethasone phosphate-laden hydrogels based on pamidronate-grafted hyaluronic acid crosslinked with magnesium ion (HA-Pam-Mg-DexP) were formed by bisphosphonate-magnesium coordination. As a result, self-healing and improved mechanical properties could be found in their adaptable hydrogels. Further design for osteogenic differentiation regulation was related to release of magnesium ions and dexamethasone, which was catalyzed by activation of alkaline phosphatase (ALP). Such injectable hydrogel combined with drugs and cells could effectively promote in situ bone regeneration via minimally invasive procedures.
4.3. Cartilage regeneration
Cartilage defect caused by osteoarthritis, trauma and other reasons is an important health issue but difficult to be repaired [[146], [147]]. Various strategies including structures [[149], [150]] (fibrous, porous, etc), mechanical stimulation, and degradability [151] have been attempted and achieved only limited success [125]. The differentiation and proliferation of chondrogenic cells are key points limiting clinical effect of cartilage regeneration [152]. Richardson et al. [132] reported an adaptable hydrazone covalent network for cartilage regeneration with tunable viscoelastic properties and time-dependent properties, which impacted chondrocyte proliferation and matrix deposition.
4.4. Osteochondral regeneration
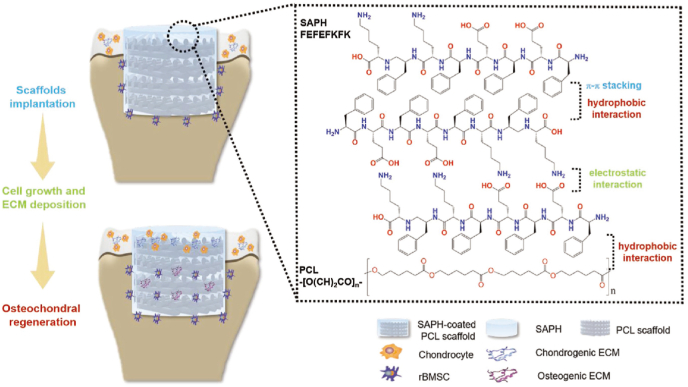
Clinically, osteochondral regeneration remains a great challenge because of the concurrence of subchondral bone injuries and cartilage damage. The integrative repair of osteochondral regeneration requires mechanical support which should be provided by subchondral bone or its substitute and nutrient exchange between cartilage and bone marrow cavities. As shown in Fig. 10, the combination of strong scaffolds and adaptable peptide hydrogel was adopted to meet mechanical support and 3D microenvironment for cell culture [60]. The hydrophobic interaction between β-sheet of peptide and repeated unit -[O(CH2)5CO]n-of polycaprolactone ensured the stable adhesive for a long time. Prolonged therapy was achieved by the separate deposition of chondrogenic and osteogenic ECM proteins.
Fig. 10.
Schematic illustration of self-assembling peptide hydrogel-coated PCL scaffolds in osteochondral defect model and the interactions between the peptide chains of SAPH and the possible interactions between SAPH and PCL [60]. Copyright (2018) Wiley.
4.5. Other applications
The applications of adaptable hydrogels have been expanded into wider fields including wound healing, adhesion, and drug delivery. Li et al. [153] attempted to build double crosslinked networks with dynamic covalent and physical linkages to achieve rapid gelation as well as shear thinning properties. Such hydrogel was constituted with hydrazide modified hyaluronic acid (HAAD) and benzaldehyde terminated F127 triblock copolymers (BAF127). The rapid gelation satisfied the injectability of hydrogel to fit irregular burn wounds. The transplantation of endothelial cells with adaptable hydrogel can overcome the limitation of transplanted cell death, which was confirmed as a promising therapeutic strategy for peripheral arterial disease [154].
Additionally, the adaptable network could include post-operative adhesions which should satisfy the injection to organs, viscoelasticity that enables organs and tissues to move freely, and effectively prevention of adhesions of tissues. Stapleton et al. [143] reported a dynamically-crosslinked supramolecular polymer–nanoparticle (PNP) hydrogel for post-operative adhesions. These PNP hydrogels are formed by simple mixing of aqueous solutions of dodecyl (C12)-modified hydroxypropylmethylcellulose (HPMC-C12) with biodegradable polymeric nanoparticles comprising poly(ethylene glycol)-b-poly(lactic acid) (PEG–PLA). The simplicity of their preparation through a self-assembly process enables PNP hydrogel manufacturing to be easily scaled up, producing materials with identical mechanical properties on any scale.
In terms of brain disease, conventional drug delivery strategies are typically limited by the blood-brain barrier. Local delivery could be an impossible way to ideal therapy which required vehicles should be mouldable, injectable, and mechanically softer than surrounding brain tissue, and readily tailorable cargo loading and delivery properties. Rowland et al. [43] developed physical hydrogel cross-linked via the host-guest interactions of cucurbit [8]uril (CB8) as drug-delivery vehicle for brain. Such hyaluronic acid cysteine-phenylalanine/cucurbit [8]uril hydrogel can be tuned to appropriately complement the surrounding environment, ensuring continuous structural and shape remodeling that would be coherent with tissue healing. Such hydrogels provide an effective alternative to carmustine loaded wafers in order to bypass the blood-brain barrier and provide synergistic, personalized therapies locally for patients with glioblastoma.
5. Conclusions and perspectives
In summary, numerous adaptable networks based on chemical or physical linkages have been developed by researchers, which has provided tools for further study. The effects of adaptable hydrogel with spatiotemporal and mechanical dynamic on cell behavior has been a rewarding object of inquiry. Two especially important factors for desigining these adaptable hydrogels are fast stress relaxation and easy remodeling capacity. Ease of remodeling is ensstional for maintenance of stemness and cell migrations, applications that require networks to break and reform rapidly. Various reversible linkages could be adopted with consideration of gelation rate, and physiological conditions (like pH, redox, etc). Additionally, fast stress relaxation should be intentionally controlled for specific tissue engineering to induce targeted cell differentiation. Plenty of practical applications have been proposed, and evidence has been provided to support the effectiveness of some.
However, some problems continue to exist, including the insufficient dynamic due to limited reversible linkage and balance between stability and dynamic, and the lack of reliable quantitative evaluation and necessary biological knowledge. Even though the reversible linkages could benefit cell behavior as a better dynamic ECM network and soft stiffness adapt cells, reversible linkages that can break and form even more quickly should be developed. Besides, the poor stability and mechanical strength of single reversible linkages limit practical applications. Most single reversible linkages could not satisfy practical mechanical support or long term gelation. More strategies, including double network or composition with strong scaffold should be developed. Additionally, with ideal injectable property, adaptable hydrogels would be important bio-ink for 3D bio-printing. Beyond the present polymer/monomer solutions ink, more styles like granular hydrogels, nanoparticle based hydrogels, microgels etc, could be developed to satisfy more functions like complex cell coculture or cargo delivery.
Several papers have reported how material matrices affect cell behavior, but conclusive knowledge requires more evidence and discoveries. For instance, the capability of modulating the behaviors of the immune cells (such as macrophage polarization, cell infiltration) with hydrogels which could be helpful to tissue regeneration was reported [155,156]. Macrophage polarization could be induced by adaptable hydrogels which could be verified by more M1 and M2 macrophage biomarkers expression in the regeneration process compared to conventional hydrogels [157,158]. Further study needs to be carried to figure out what factor (stiffness, dynamic mechanical, or spatial cues) should be responsible for the difference. Furthermore, quantitative analysis and precise model should be set up for universal design of adaptable matrices for tissue engineering. At present, quantitative analysis has been considered from chemical equilibrium view or combined view of viscoelasticity and corresponding cell behavior. On the one hand, we can understand how to obtain a fast gelation according to chemical equilibrium even though the stability and mechanical properties could not be predicted. On the other hand, by adjusting the materials, we can obtain materials with different stress relaxation properties and corresponding cell behaviors. But to meet the practical application, both views need to be considered and carried out for follow-up research. The former can provide researchers with a basis for material design, while the latter provides a good way to verify and evaluate materials.
Declaration of competing interest
The authors declare no conflict of interest.
Acknowledgments
We thank the financial support of the National Key Research and Development Program of China (2016YFE0132700), National Natural Science Foundation of China (51822306, 51673171), Science Technology Department of Zhejiang Province (2020C03042), and the Fundamental Research Funds for the Central Universities of China.
Footnotes
Peer review under responsibility of KeAi Communications Co., Ltd.
Supplementary data related to this article can be found at https://doi.org/10.1016/j.bioactmat.2020.10.029.
Contributor Information
Zhengwei Mao, Email: zwmao@zju.edu.cn.
Changyou Gao, Email: cygao@zju.edu.cn.
Appendix A. Supplementary data
The following is the supplementary data related to this article:
References
- 1.Burke J.F., Yannas I.V., Quinby W.C., Bondoc C.C., Jung W.K. Successful use of a physiologically acceptable Artificial skin in the treatment of extensive burn injury. Ann. Surg. 1981;194(4):413–428. doi: 10.1097/00000658-198110000-00005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Xue J., Feng B., Zheng R., Lu Y., Zhou G., Liu W. Engineering ear-shaped cartilage using electrospun fibrous membranes of gelatin/polycaprolactone. Biomaterials. 2013;34(11):2624–2631. doi: 10.1016/j.biomaterials.2012.12.011. [DOI] [PubMed] [Google Scholar]
- 3.Huebsch N., Lippens E., Lee K., Mehta M., Koshy S.T., Darnell M.C. Matrix elasticity of void-forming hydrogels controls transplanted-stem-cell-mediated bone formation. Nat. Mater. 2015;14(12):1269–1277. doi: 10.1038/nmat4407. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Bai X., Gao M., Syed S., Zhuang J., Xu X., Zhang X. Bioactive hydrogels for bone regeneration. Bioactive Materials. 2018;3(4):401–417. doi: 10.1016/j.bioactmat.2018.05.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Jekhmane S., Prachar M., Pugliese R., Fontana F., Medeiros Silva J., Gelain F. Design parameters of tissue‐engineering scaffolds at the atomic scale. Angew. Chem. 2019;131(47):17099–17107. doi: 10.1002/anie.201907880. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Pellegrini G., Ardigò D., Milazzo G., Iotti G., Guatelli P., Pelosi D. Navigating market authorization: the path holoclar took to become the first stem cell product approved in the European union. Stem Cell. Transl. Med. 2018;7(1):146–154. doi: 10.1002/sctm.17-0003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Allison M. Hemacord approval may foreshadow regulatory creep for HSC therapies. Nat. Biotechnol. 2012;30(4):304. doi: 10.1038/nbt0412-304. [DOI] [PubMed] [Google Scholar]
- 8.Allison M. Hemacord approval may foreshadow regulatory creep for HSC therapies. Nat. Biotechnol. 2012;30(4):304. doi: 10.1038/nbt0412-304. [DOI] [PubMed] [Google Scholar]
- 9.Timeline C. Willyard. Regrowing the body. Nature. 2016;540(7632):S50–S51. doi: 10.1038/540S50a. [DOI] [PubMed] [Google Scholar]
- 10.Syed B.A., Evans J.B. Stem cell therapy market. Nat. Rev. Drug Discov. 2013;12(3):185–186. doi: 10.1038/nrd3953. [DOI] [PubMed] [Google Scholar]
- 11.Langer R., Vacanti J. Tissue engineering. Science. 1993;260(5110):920–926. doi: 10.1126/science.8493529. [DOI] [PubMed] [Google Scholar]
- 12.Rosales A.M., Anseth K.S. The design of reversible hydrogels to capture extracellular matrix dynamics. Nat. Rev. Mater. 2016;1(2) doi: 10.1038/natrevmats.2015.12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Seliktar D. Designing cell-compatible hydrogels for biomedical applications. Science. 2012;336(6085):1124–1128. doi: 10.1126/science.1214804. [DOI] [PubMed] [Google Scholar]
- 14.Hussey G.S., Dziki J.L., Badylak S.F. Extracellular matrix-based materials for regenerative medicine. Nat. Rev. Mater. 2018;3(7):159–173. [Google Scholar]
- 15.Hennink W.E., van Nostrum C.F. Novel crosslinking methods to design hydrogels. Adv. Drug Deliv. Rev. 2012;64:223–236. doi: 10.1016/s0169-409x(01)00240-x. [DOI] [PubMed] [Google Scholar]
- 16.Nichol J.W., Koshy S.T., Bae H., Hwang C.M., Yamanlar S., Khademhosseini A. Cell-laden microengineered gelatin methacrylate hydrogels. Biomaterials. 2010;31(21):5536–5544. doi: 10.1016/j.biomaterials.2010.03.064. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Annabi N., Mithieux S.M., Zorlutuna P., Camci-Unal G., Weiss A.S., Khademhosseini A. Engineered cell-laden human protein-based elastomer. Biomaterials. 2013;34(22):5496–5505. doi: 10.1016/j.biomaterials.2013.03.076. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Hollister S.J. Porous scaffold design for tissue engineering. Nat. Mater. 2005;4(7):518–524. doi: 10.1038/nmat1421. [DOI] [PubMed] [Google Scholar]
- 19.Tayalia P., Mooney D.J., Controlled Growth factor delivery for tissue engineering. Adv. Mater. 2009;21(32–33):3269–3285. doi: 10.1002/adma.200900241. [DOI] [PubMed] [Google Scholar]
- 20.Jongpaiboonkit L., King W.J., Lyons G.E., Paguirigan A.L., Warrick J.W., Beebe D.J. An adaptable hydrogel array format for 3-dimensional cell culture and analysis. Biomaterials. 2008;29(23):3346–3356. doi: 10.1016/j.biomaterials.2008.04.040. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Lecuit T., Lenne P. Cell surface mechanics and the control of cell shape, tissue patterns and morphogenesis. Nat. Rev. Mol. Cell Biol. 2007;8(8):633–644. doi: 10.1038/nrm2222. [DOI] [PubMed] [Google Scholar]
- 22.Burla F., Mulla Y., Vos B.E., Aufderhorst-Roberts A., Koenderink G.H. From mechanical resilience to active material properties in biopolymer networks. Nature Reviews Physics. 2019;1(4):249–263. [Google Scholar]
- 23.Ding X., Wang Y. Weak bond-based injectable and stimuli responsive hydrogels for biomedical applications. J. Mater. Chem. B. 2017;5(5):887–906. doi: 10.1039/C6TB03052A. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Tong X., Yang F. Recent progress in developing injectable matrices for enhancing cell delivery and tissue regeneration. Adv. Healthc. Mater. 2018;7(7):1701065. doi: 10.1002/adhm.201701065. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Madl C.M., Heilshorn S.C., Blau H.M. Bioengineering strategies to accelerate stem cell therapeutics. Nature. 2018;557(7705):335–342. doi: 10.1038/s41586-018-0089-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Wang H., Heilshorn S.C. Adaptable hydrogel networks with reversible linkages for tissue engineering. Adv. Mater. 2015;27(25):3717–3736. doi: 10.1002/adma.201501558. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Heidarian P., Kouzani A.Z., Kaynak A., Paulino M., Nasri-Nasrabadi B. Dynamic hydrogels and polymers as inks for three-dimensional printing. ACS Biomater. Sci. Eng. 2019;5(6):2688–2707. doi: 10.1021/acsbiomaterials.9b00047. [DOI] [PubMed] [Google Scholar]
- 28.Appel E.A., Del Barrio J., Loh X.J., Scherman O.A. Supramolecular polymeric hydrogels. Chem. Soc. Rev. 2012;41(18):6195–6214. doi: 10.1039/c2cs35264h. [DOI] [PubMed] [Google Scholar]
- 29.Harada A., Takashima Y., Nakahata M. Supramolecular polymeric materials via cyclodextrin–guest interactions. Accounts Chem. Res. 2014;47(7):2128–2140. doi: 10.1021/ar500109h. [DOI] [PubMed] [Google Scholar]
- 30.Liu G., Yuan Q., Hollett G., Zhao W., Kang Y., Wu J. Cyclodextrin-based host–guest supramolecular hydrogel and its application in biomedical fields. Polym. Chem.-Uk. 2018;9(25):3436–3449. [Google Scholar]
- 31.Chen G., Jiang M. Cyclodextrin-based inclusion complexation bridging supramolecular chemistry and macromolecular self-assembly. Chem. Soc. Rev. 2011;40(5):2254–2266. doi: 10.1039/c0cs00153h. [DOI] [PubMed] [Google Scholar]
- 32.Rodell C.B., Kaminski A.L., Burdick J.A. Rational design of network properties in guest–host assembled and shear-thinning hyaluronic acid hydrogels. Biomacromolecules. 2013;14(11):4125–4134. doi: 10.1021/bm401280z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Rosales A.M., Rodell C.B., Chen M.H., Morrow M.G., Anseth K.S., Burdick J.A. Reversible control of network properties in azobenzene-containing hyaluronic acid-based hydrogels. Bioconjugate Chem. 2018;29(4):905–913. doi: 10.1021/acs.bioconjchem.7b00802. [DOI] [PubMed] [Google Scholar]
- 34.Rodell C.B., Dusaj N.N., Highley C.B., Burdick J.A. Injectable and cytocompatible tough double-network hydrogels through tandem supramolecular and covalent crosslinking. Adv. Mater. 2016;28(38):8419–8424. doi: 10.1002/adma.201602268. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Loebel C., Ayoub A., Galarraga J.H., Kossover O., Simaan-Yameen H., Seliktar D. Tailoring supramolecular guest–host hydrogel viscoelasticity with covalent fibrinogen double networks. J. Mater. Chem. B. 2019;7(10):1753–1760. doi: 10.1039/c8tb02593b. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Feng Q., Wei K., Lin S., Xu Z., Sun Y., Shi P. Mechanically resilient, injectable, and bioadhesive supramolecular gelatin hydrogels crosslinked by weak host-guest interactions assist cell infiltration and in situ tissue regeneration. Biomaterials. 2016;101:217–228. doi: 10.1016/j.biomaterials.2016.05.043. [DOI] [PubMed] [Google Scholar]
- 37.Feng Q., Xu J., Zhang K., Yao H., Zheng N., Zheng L. Dynamic and cell-infiltratable hydrogels as injectable carrier of therapeutic cells and drugs for treating challenging bone defects. Acs Central Sci. 2019;5(3):440–450. doi: 10.1021/acscentsci.8b00764. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Mealy J.E., Chung J.J., Jeong H., Issadore D., Lee D., Atluri P. Injectable granular hydrogels with multifunctional properties for biomedical applications. Adv. Mater. 2018;30(20):1705912. doi: 10.1002/adma.201705912. [DOI] [PubMed] [Google Scholar]
- 39.Wang L.L., Liu Y., Chung J.J., Wang T., Gaffey A.C., Lu M. Sustained miRNA delivery from an injectable hydrogel promotes cardiomyocyte proliferation and functional regeneration after ischaemic injury. Nat. Biomed. Eng. 2017;1(12):983–992. doi: 10.1038/s41551-017-0157-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Loebel C., Rodell C.B., Chen M.H., Burdick J.A. Shear-thinning and self-healing hydrogels as injectable therapeutics and for 3D-printing. Nat. Protoc. 2017;12(8):1521–1541. doi: 10.1038/nprot.2017.053. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Lagona J., Mukhopadhyay P., Chakrabarti S., Isaacs L. The Cucurbit[n]uril Family. Angew. Chem. Int. Ed. 2005;44(31):4844–4870. doi: 10.1002/anie.200460675. [DOI] [PubMed] [Google Scholar]
- 42.Yeom J., Kim S.J., Jung H., Namkoong H., Yang J., Hwang B.W. Supramolecular hydrogels for long-term bioengineered stem cell therapy. Adv. Healthc. Mater. 2015;4(2):237–244. doi: 10.1002/adhm.201400304. [DOI] [PubMed] [Google Scholar]
- 43.Rowland M.J., Parkins C.C., McAbee J.H., Kolb A.K., Hein R., Loh X.J. An adherent tissue-inspired hydrogel delivery vehicle utilised in primary human glioma models. Biomaterials. 2018;179:199–208. doi: 10.1016/j.biomaterials.2018.05.054. [DOI] [PubMed] [Google Scholar]
- 44.Tong X., Yang F. Sliding hydrogels with mobile molecular ligands and crosslinks as 3D stem cell niche. Adv. Mater. 2016;28(33):7257–7263. doi: 10.1002/adma.201601484. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Quideau S., Deffieux D., Douat-Casassus C., Pouységu L. Plant polyphenols: chemical properties, biological activities, and synthesis. Angew. Chem. Int. Ed. 2011;50(3):586–621. doi: 10.1002/anie.201000044. [DOI] [PubMed] [Google Scholar]
- 46.Madhurakkat Perikamana S.K., Lee J., Lee Y.B., Shin Y.M., Lee E.J., Mikos A.G. Materials from mussel-inspired chemistry for cell and tissue engineering applications. Biomacromolecules. 2015;16(9):2541–2555. doi: 10.1021/acs.biomac.5b00852. [DOI] [PubMed] [Google Scholar]
- 47.Shin M., Ryu J.H., Park J.P., Kim K., Yang J.W., Lee H. DNA/Tannic acid hybrid gel exhibiting biodegradability, extensibility, tissue adhesiveness, and hemostatic ability. Adv. Funct. Mater. 2015;25(8):1270–1278. [Google Scholar]
- 48.Shin M., Galarraga J.H., Kwon M.Y., Lee H., Burdick J.A. Gallol-derived ECM-mimetic adhesive bioinks exhibiting temporal shear-thinning and stabilization behavior. Acta Biomater. 2019;95:165–175. doi: 10.1016/j.actbio.2018.10.028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Highley C.B., Rodell C.B., Burdick J.A. Direct 3D printing of shear-thinning hydrogels into self-healing hydrogels. Adv. Mater. 2015;27(34):5075–5079. doi: 10.1002/adma.201501234. [DOI] [PubMed] [Google Scholar]
- 50.Um S.H., Lee J.B., Park N., Kwon S.Y., Umbach C.C., Luo D. Enzyme-catalysed assembly of DNA hydrogel. Nat. Mater. 2006;5(10):797–801. doi: 10.1038/nmat1741. [DOI] [PubMed] [Google Scholar]
- 51.Tan H., Xiao C., Sun J., Xiong D., Hu X. Biological self-assembly of injectable hydrogel as cell scaffold via specific nucleobase pairing. Chem. Commun. 2012;48(83):10289. doi: 10.1039/c2cc35449g. [DOI] [PubMed] [Google Scholar]
- 52.Li C., Faulkner-Jones A., Dun A.R., Jin J., Chen P., Xing Y. rapid formation of a supramolecular polypeptide-DNA hydrogel for In Situ three-dimensional multilayer bioprinting. Angew. Chem. 2015;127(13):4029–4033. doi: 10.1002/anie.201411383. [DOI] [PubMed] [Google Scholar]
- 53.Dankers P.Y.W., Hermans T.M., Baughman T.W., Kamikawa Y., Kieltyka R.E., Bastings M.M.C. Hierarchical formation of supramolecular transient networks in water: a modular injectable delivery system. Adv. Mater. 2012;24(20):2703–2709. doi: 10.1002/adma.201104072. [DOI] [PubMed] [Google Scholar]
- 54.Zhang G., Chen Y., Deng Y., Ngai T., Wang C. Dynamic supramolecular hydrogels: regulating hydrogel properties through self-complementary quadruple hydrogen bonds and thermo-switch. ACS Macro Lett. 2017;6(7):641–646. doi: 10.1021/acsmacrolett.7b00275. [DOI] [PubMed] [Google Scholar]
- 55.Guo M., Pitet L.M., Wyss H.M., Vos M., Dankers P.Y.W., Meijer E.W. Tough stimuli-responsive supramolecular hydrogels with hydrogen-bonding network junctions. J. Am. Chem. Soc. 2014;136(19):6969–6977. doi: 10.1021/ja500205v. [DOI] [PubMed] [Google Scholar]
- 56.Qin Z., Yu X., Wu H., Li J., Lv H., Yang X. Nonswellable and tough supramolecular hydrogel based on strong micelle cross-linkings. Biomacromolecules. 2019;20(9):3399–3407. doi: 10.1021/acs.biomac.9b00666. [DOI] [PubMed] [Google Scholar]
- 57.De Leon Rodriguez L.M., Hemar Y., Cornish J., Brimble M.A. Structure–mechanical property correlations of hydrogel forming β-sheet peptides. Chem. Soc. Rev. 2016;45(17):4797–4824. doi: 10.1039/c5cs00941c. [DOI] [PubMed] [Google Scholar]
- 58.Castillo Diaz L.A., Elsawy M., Saiani A., Gough J.E., Miller A.F. Osteogenic differentiation of human mesenchymal stem cells promotes mineralization within a biodegradable peptide hydrogel. J. Tissue Eng. 2016;7:1013652270. doi: 10.1177/2041731416649789. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Tang J.D., Mura C., Lampe K.J. Stimuli-responsive, pentapeptide, nanofiber hydrogel for tissue engineering. J. Am. Chem. Soc. 2019;141(12):4886–4899. doi: 10.1021/jacs.8b13363. [DOI] [PubMed] [Google Scholar]
- 60.Li L., Li J., Guo J., Zhang H., Zhang X., Yin C. 3D molecularly functionalized cell‐free biomimetic scaffolds for osteochondral regeneration. Adv. Funct. Mater. 2019;29(6):1807356. [Google Scholar]
- 61.Yan C., Pochan D.J. Rheological properties of peptide-based hydrogels for biomedical and other applications. Chem. Soc. Rev. 2010;39(9):3528. doi: 10.1039/b919449p. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Raeburn J., Zamith Cardoso A., Adams D.J. The importance of the self-assembly process to control mechanical properties of low molecular weight hydrogels. Chem. Soc. Rev. 2013;42(12):5143. doi: 10.1039/c3cs60030k. [DOI] [PubMed] [Google Scholar]
- 63.Yan X., Tong Z., Chen Y., Mo Y., Feng H., Li P. Bioresponsive materials for drug delivery based on carboxymethyl chitosan/poly(γ-glutamic acid) composite microparticles. Mar. Drugs. 2017;15(5):127. doi: 10.3390/md15050127. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Tong Z., Yang J., Lin L., Wang R., Cheng B., Chen Y. In situ synthesis of poly (γ- glutamic acid)/alginate/AgNP composite microspheres with antibacterial and hemostatic properties. Carbohydr. Polym. 2019;221:21–28. doi: 10.1016/j.carbpol.2019.05.035. [DOI] [PubMed] [Google Scholar]
- 65.Sun J., Zhao X., Illeperuma W.R.K., Chaudhuri O., Oh K.H., Mooney D.J. Highly stretchable and tough hydrogels. Nature. 2012;489(7414):133–136. doi: 10.1038/nature11409. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Tong Z., Chen Y., Liu Y., Tong L., Chu J., Xiao K. Preparation, characterization and properties of alginate/poly(γ-glutamic acid) composite microparticles. Mar. Drugs. 2017;15(4):91. doi: 10.3390/md15040091. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Truby R.L., Lewis J.A. Printing soft matter in three dimensions. Nature. 2016;540(7633):371–378. doi: 10.1038/nature21003. [DOI] [PubMed] [Google Scholar]
- 68.Lee A., Hudson A.R., Shiwarski D.J., Tashman J.W., Hinton T.J., Yerneni S. 3D bioprinting of collagen to rebuild components of the human heart. Science. 2019;365(6452):482–487. doi: 10.1126/science.aav9051. [DOI] [PubMed] [Google Scholar]
- 69.Donderwinkel I., van Hest J.C.M., Cameron N.R. Bio-inks for 3D bioprinting: recent advances and future prospects. Polym. Chem.-Uk. 2017;8(31):4451–4471. [Google Scholar]
- 70.Lindsay C.D., Roth J.G., LeSavage B.L., Heilshorn S.C. Bioprinting of stem cell expansion lattices. Acta Biomater. 2019;95:225–235. doi: 10.1016/j.actbio.2019.05.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Ouyang L., Highley C.B., Rodell C.B., Sun W., Burdick J.A. 3D printing of shear-thinning hyaluronic acid hydrogels with secondary cross-linking. ACS Biomater. Sci. Eng. 2016;2(10):1743–1751. doi: 10.1021/acsbiomaterials.6b00158. [DOI] [PubMed] [Google Scholar]
- 72.Zhang K., Feng Q., Xu J., Xu X., Tian F., Yeung K.W.K. Self-assembled injectable nanocomposite hydrogels stabilized by bisphosphonate-magnesium (Mg2+) coordination regulates the differentiation of encapsulated stem cells via dual crosslinking. Adv. Funct. Mater. 2017;27(34):1701642. [Google Scholar]
- 73.Cross D., Jiang X., Ji W., Han W., Wang C. Injectable hybrid hydrogels of hyaluronic acid crosslinked by well-defined synthetic polycations: preparation and characterization in vitro and in vivo. Macromol. Biosci. 2015;15(5):668–681. doi: 10.1002/mabi.201400491. [DOI] [PubMed] [Google Scholar]
- 74.Li H., Zhang W., Tong W., Gao C. Enhanced cellular uptake of bowl-like microcapsules. Acs Appl. Mater. Inter. 2016;8(18):11210–11214. doi: 10.1021/acsami.6b02965. [DOI] [PubMed] [Google Scholar]
- 75.Chen Y., Wang F., Zhang N., Li Y., Cheng B., Zheng Y. Preparation of a 6-OH quaternized chitosan derivative through click reaction and its application to novel thermally induced/polyelectrolyte complex hydrogels. Colloids Surf. B Biointerfaces. 2017;158:431–440. doi: 10.1016/j.colsurfb.2017.07.028. [DOI] [PubMed] [Google Scholar]
- 76.Shi P., Zhao N., Coyne J., Wang Y. DNA-templated synthesis of biomimetic cell wall for nanoencapsulation and protection of mammalian cells. Nat. Commun. 2019;10(1) doi: 10.1038/s41467-019-10231-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Nowak A.P., Breedveld V., Pakstis L., Ozbas B., Pine D.J., Pochan D. Rapidly recovering hydrogel scaffolds from self-assembling diblock copolypeptide amphiphiles. Nature. 2002;417(6887):424–428. doi: 10.1038/417424a. [DOI] [PubMed] [Google Scholar]
- 78.Zhang J., Tokatlian T., Zhong J., Ng Q.K.T., Patterson M., Lowry W.E. Physically associated synthetic hydrogels with long-term covalent stabilization for cell culture and stem cell transplantation. Adv. Mater. 2011;23(43):5098–5103. doi: 10.1002/adma.201103349. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Cai L., Dewi R.E., Heilshorn S.C. Injectable hydrogels with in situ double network formation enhance retention of transplanted stem cells. Adv. Funct. Mater. 2015;25(9):1344–1351. doi: 10.1002/adfm.201403631. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Ekerdt B.L., Fuentes C.M., Lei Y., Adil M.M., Ramasubramanian A., Segalman R.A. Thermoreversible hyaluronic acid-PNIPAAm hydrogel systems for 3D stem cell culture. Adv. Healthc. Mater. 2018;7(12):1800225. doi: 10.1002/adhm.201800225. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Rapp P.B., Omar A.K., Shen J.J., Buck M.E., Wang Z., Tirrell D.A. Analysis and control of chain mobility in protein hydrogels. J. Am. Chem. Soc. 2017;139(10):3796–3804. doi: 10.1021/jacs.6b13146. [DOI] [PubMed] [Google Scholar]
- 82.Conticello V., Hughes S., Modlin C. Biomaterials made from coiled-coil peptides. Sub-cellular biochemistry. 2017;82:575. doi: 10.1007/978-3-319-49674-0_17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Liu Y., Liu B., Riesberg J.J., Shen W. In situ forming physical hydrogels for three-dimensional tissue morphogenesis. Macromol. Biosci. 2011;11(10):1325–1330. doi: 10.1002/mabi.201100119. [DOI] [PubMed] [Google Scholar]
- 84.Glassman M.J., Chan J., Olsen B.D. Reinforcement of shear thinning protein hydrogels by responsive block copolymer self-assembly. Adv. Funct. Mater. 2013;23(9):1182–1193. doi: 10.1002/adfm.201202034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Kirchhof S., Brandl F.P., Hammer N., Goepferich A.M. Investigation of the Diels–Alder reaction as a cross-linking mechanism for degradable poly(ethylene glycol) based hydrogels. Journal of materials chemistry. B, Materials for biology and medicine. 2013;1(37):4855. doi: 10.1039/c3tb20831a. [DOI] [PubMed] [Google Scholar]
- 86.Ghanian M.H., Mirzadeh H., Baharvand H. In situ forming, cytocompatible, and self-recoverable tough hydrogels based on dual ionic and click cross-linked alginate. Biomacromolecules. 2017;19(5):1646–1662. doi: 10.1021/acs.biomac.8b00140. [DOI] [PubMed] [Google Scholar]
- 87.Delplace V., Pickering A.J., Hettiaratchi M.H., Zhao S., Kivijärvi T., Shoichet M.S. Inverse electron-demand diels–alder methylcellulose hydrogels enable the Co-delivery of chondroitinase ABC and neural progenitor cells. Biomacromolecules. 2020;21(6):2421–2431. doi: 10.1021/acs.biomac.0c00357. [DOI] [PubMed] [Google Scholar]
- 88.Madl C.M., Heilshorn S.C. Rapid diels–alder cross-linking of cell encapsulating hydrogels. Chem. Mater. 2019;31(19):8035–8043. doi: 10.1021/acs.chemmater.9b02485. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Smith L.J., Taimoory S.M., Tam R.Y., Baker A.E.G., Binth Mohammad N., Trant J.F. Diels–alder click-cross-linked hydrogels with increased reactivity enable 3D cell encapsulation. Biomacromolecules. 2018;19(3):926–935. doi: 10.1021/acs.biomac.7b01715. [DOI] [PubMed] [Google Scholar]
- 90.Bi B., Ma M., Lv S., Zhuo R., Jiang X. In-situ forming thermosensitive hydroxypropyl chitin-based hydrogel crosslinked by Diels-Alder reaction for three dimensional cell culture. Carbohydr. Polym. 2019;212:368–377. doi: 10.1016/j.carbpol.2019.02.058. [DOI] [PubMed] [Google Scholar]
- 91.Richardson B.M., Wilcox D.G., Randolph M.A., Anseth K.S. Hydrazone covalent adaptable networks modulate extracellular matrix deposition for cartilage tissue engineering. Acta Biomater. 2019;83:71–82. doi: 10.1016/j.actbio.2018.11.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Koivusalo L., Kauppila M., Samanta S., Parihar V.S., Ilmarinen T., Miettinen S. Tissue adhesive hyaluronic acid hydrogels for sutureless stem cell delivery and regeneration of corneal epithelium and stroma. Biomaterials. 2019;225:119516. doi: 10.1016/j.biomaterials.2019.119516. [DOI] [PubMed] [Google Scholar]
- 93.Koivisto J.T., Gering C., Karvinen J., Maria Cherian R., Belay B., Hyttinen J. Mechanically biomimetic gelatin–gellan gum hydrogels for 3D culture of beating human cardiomyocytes. Acs Appl. Mater. Inter. 2019;11(23):20589–20602. doi: 10.1021/acsami.8b22343. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Suo A., Xu W., Wang Y., Sun T., Ji L., Qian J. Dual-degradable and injectable hyaluronic acid hydrogel mimicking extracellular matrix for 3D culture of breast cancer MCF-7 cells. Carbohydr. Polym. 2019;211:336–348. doi: 10.1016/j.carbpol.2019.01.115. [DOI] [PubMed] [Google Scholar]
- 95.Xu F., Gough I., Dorogin J., Sheardown H., Hoare T. Nanostructured degradable macroporous hydrogel scaffolds with controllable internal morphologies via reactive electrospinning. Acta Biomater. 2020;104:135–146. doi: 10.1016/j.actbio.2019.12.038. [DOI] [PubMed] [Google Scholar]
- 96.Sharma P.K., Taneja S., Singh Y. Hydrazone-linkage-based self-healing and injectable xanthan–poly(ethylene glycol) hydrogels for controlled drug release and 3D cell culture. Acs Appl. Mater. Inter. 2018;10(37):30936–30945. doi: 10.1021/acsami.8b07310. [DOI] [PubMed] [Google Scholar]
- 97.Tan H., Rubin J.P., Marra K.G. Injectable in situ forming biodegradable chitosan-hyaluronic acid based hydrogels for adipose tissue regeneration. Organogenesis. 2014;6(3):173–180. doi: 10.4161/org.6.3.12037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Yang B., Zhang Y., Zhang X., Tao L., Li S., Wei Y. Facilely prepared inexpensive and biocompatible self-healing hydrogel: a new injectable cell therapy carrier. Polym. Chem.-Uk. 2012;3(12):3235. [Google Scholar]
- 99.Wang S., Zheng H., Zhou L., Cheng F., Liu Z., Zhang H. Nanoenzyme-reinforced injectable hydrogel for healing diabetic wounds infected with multidrug resistant bacteria. Nano Lett. 2020;20(7):5149–5158. doi: 10.1021/acs.nanolett.0c01371. [DOI] [PubMed] [Google Scholar]
- 100.Zhou L., Xi Y., Xue Y., Wang M., Liu Y., Guo Y. Injectable self‐healing antibacterial bioactive polypeptide‐based hybrid nanosystems for efficiently treating multidrug resistant infection, skin‐tumor therapy, and enhancing wound healing. Adv. Funct. Mater. 2019;29(22):1806883. [Google Scholar]
- 101.Shih H., Greene T., Korc M., Lin C. Modular and adaptable tumor niche prepared from visible light initiated thiol-norbornene photopolymerization. Biomacromolecules. 2016;17(12):3872–3882. doi: 10.1021/acs.biomac.6b00931. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Brown T.E., Carberry B.J., Worrell B.T., Dudaryeva O.Y., McBride M.K., Bowman C.N. Photopolymerized dynamic hydrogels with tunable viscoelastic properties through thioester exchange. Biomaterials. 2018;178:496–503. doi: 10.1016/j.biomaterials.2018.03.060. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Hong S.H., Shin M., Park E., Ryu J.H., Burdick J.A., Lee H. Alginate‐boronic acid: pH‐triggered bioinspired glue for hydrogel assembly. Adv. Funct. Mater. 2020;30(26):1908497. [Google Scholar]
- 104.Tang S., Ma H., Tu H., Wang H., Lin P., Anseth K.S. Adaptable fast relaxing boronate-based hydrogels for probing cell-matrix interactions. Adv. Sci. 2018;5(9):1800638. doi: 10.1002/advs.201800638. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Wu D., Wang W., Diaz-Dussan D., Peng Y., Chen Y., Narain R. In situ forming, dual-crosslink network, self-healing hydrogel enabled by a bioorthogonal nopoldiol–benzoxaborolate click reaction with a wide pH range. Chem. Mater. 2019;31(11):4092–4102. [Google Scholar]
- 106.Hu J., Hu Q., He X., Liu C., Kong Y., Cheng Y. Stimuli‐responsive hydrogels with antibacterial activity assembled from guanosine, aminoglycoside, and a bifunctional anchor. Adv. Healthc. Mater. 2020;9(2):1901329. doi: 10.1002/adhm.201901329. [DOI] [PubMed] [Google Scholar]
- 107.Xu Q., He C., Zhang Z., Ren K., Injectable X. Chen. Biomolecule-responsive polypeptide hydrogels for cell encapsulation and facile cell recovery through triggered degradation. Acs Appl. Mater. Inter. 2016;8(45):30692–30702. doi: 10.1021/acsami.6b08292. [DOI] [PubMed] [Google Scholar]
- 108.Correia C.R., Reis R.L., Mano J.F. Design principles and multifunctionality in cell encapsulation systems for tissue regeneration. Adv. Healthc. Mater. 2018;7(19):1701444. doi: 10.1002/adhm.201701444. [DOI] [PubMed] [Google Scholar]
- 109.Hasturk O., Kaplan D.L. Cell armor for protection against environmental stress: advances, challenges and applications in micro- and nanoencapsulation of mammalian cells. Acta Biomater. 2019;95:3–31. doi: 10.1016/j.actbio.2018.11.040. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110.Chen X., Dong C., Wei K., Yao Y., Feng Q., Zhang K. Supramolecular hydrogels cross-linked by preassembled host–guest PEG cross-linkers resist excessive, ultrafast, and non-resting cyclic compression. NPG Asia Mater. 2018;10(8):788–799. [Google Scholar]
- 111.Wang H., Zhu D., Paul A., Cai L., Enejder A., Yang F. Covalently adaptable elastin-like protein-hyaluronic acid (ELP-HA) hybrid hydrogels with secondary thermoresponsive crosslinking for injectable stem cell delivery. Adv. Funct. Mater. 2017;27(28):1605609. doi: 10.1002/adfm.201605609. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112.Dubbin K., Tabet A., Heilshorn S.C. Quantitative criteria to benchmark new and existing bio-inks for cell compatibility. Biofabrication. 2017;9(4):44102. doi: 10.1088/1758-5090/aa869f. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113.Xu Q., A S., Gao Y., Guo L., Creagh-Flynn J., Zhou D. A hybrid injectable hydrogel from hyperbranched PEG macromer as a stem cell delivery and retention platform for diabetic wound healing. Acta Biomater. 2018;75:63–74. doi: 10.1016/j.actbio.2018.05.039. [DOI] [PubMed] [Google Scholar]
- 114.Dong D., Hao T., Wang C., Zhang Y., Qin Z., Yang B. Zwitterionic starch-based hydrogel for the expansion and “stemness” maintenance of brown adipose derived stem cells. Biomaterials. 2018;157:149–160. doi: 10.1016/j.biomaterials.2017.12.011. [DOI] [PubMed] [Google Scholar]
- 115.Chowdhury F., Li Y., Poh Y., Yokohama-Tamaki T., Wang N., Tanaka T.S. Soft substrates promote homogeneous self-renewal of embryonic stem cells via downregulating cell-matrix tractions. PloS One. 2010;5(12) doi: 10.1371/journal.pone.0015655. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 116.Gilbert P.M., Havenstrite K.L., Magnusson K.E.G., Sacco A., Leonardi N.A., Kraft P. Substrate elasticity regulates skeletal muscle stem cell self-renewal in culture. Science. 2010;329(5995):1078–1081. doi: 10.1126/science.1191035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 117.Lü D., Luo C., Zhang C., Li Z., Long M. Differential regulation of morphology and stemness of mouse embryonic stem cells by substrate stiffness and topography. Biomaterials. 2014;35(13):3945–3955. doi: 10.1016/j.biomaterials.2014.01.066. [DOI] [PubMed] [Google Scholar]
- 118.Randriantsilefisoa R., Hou Y., Pan Y., Camacho J.L.C., Kulka M.W., Zhang J. Interaction of human mesenchymal stem cells with soft nanocomposite hydrogels based on polyethylene glycol and dendritic polyglycerol. Adv. Funct. Mater. 2020;30(1):1905200. [Google Scholar]
- 119.Madl C.M., LeSavage B.L., Dewi R.E., Dinh C.B., Stowers R.S., Khariton M. Maintenance of neural progenitor cell stemness in 3D hydrogels requires matrix remodelling. Nat. Mater. 2017;16(12):1233–1242. doi: 10.1038/nmat5020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 120.Madl C.M., LeSavage B.L., Dewi R.E., Dinh C.B., Stowers R.S., Khariton M. Maintenance of neural progenitor cell stemness in 3D hydrogels requires matrix remodelling. Nat. Mater. 2017;16(12):1233. doi: 10.1038/nmat5020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 121.Darnell M., O Neil A., Mao A., Gu L., Rubin L.L., Mooney D.J. Material microenvironmental properties couple to induce distinct transcriptional programs in mammalian stem cells. Proc. Natl. Acad. Sci. Unit. States Am. 2018;115(36):E8368–E8377. doi: 10.1073/pnas.1802568115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 122.Lou J., Stowers R., Nam S., Xia Y., Chaudhuri O. Stress relaxing hyaluronic acid-collagen hydrogels promote cell spreading, fiber remodeling, and focal adhesion formation in 3D cell culture. Biomaterials. 2018;154:213–222. doi: 10.1016/j.biomaterials.2017.11.004. [DOI] [PubMed] [Google Scholar]
- 123.Chaudhuri O., Gu L., Klumpers D., Darnell M., Bencherif S.A., Weaver J.C. Hydrogels with tunable stress relaxation regulate stem cell fate and activity. Nat. Mater. 2016;15(3):326–334. doi: 10.1038/nmat4489. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 124.Cai L., Dewi R.E., Goldstone A.B., Cohen J.E., Steele A.N., Woo Y.J. Regulating stem cell secretome using injectable hydrogels with in situ network formation. Adv. Healthc. Mater. 2016;5(21):2758–2764. doi: 10.1002/adhm.201600497. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 125.Oliveira E., Assunção-Silva R.C., Ziv-Polat O., Gomes E.D., Teixeira F.G., Silva N.A. Influence of different ECM-like hydrogels on neurite outgrowth induced by adipose tissue-derived stem cells. Stem Cell. Int. 2017;2017:1–10. doi: 10.1155/2017/6319129. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 126.Silva N.A., Moreira J., Ribeiro-Samy S., Gomes E.D., Tam R.Y., Shoichet M.S. Modulation of bone marrow mesenchymal stem cell secretome by ECM-like hydrogels. Biochimie. 2013;95(12):2314–2319. doi: 10.1016/j.biochi.2013.08.016. [DOI] [PubMed] [Google Scholar]
- 127.Schneider M.C., Barnes C.A., Bryant S.J. Characterization of the chondrocyte secretome in photoclickable poly(ethylene glycol) hydrogels. Biotechnol. Bioeng. 2017;114(9):2096–2108. doi: 10.1002/bit.26320. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 128.Rao V.V., Vu M.K., Ma H., Killaars A.R., Anseth K.S. Rescuing mesenchymal stem cell regenerative properties on hydrogel substrates post serial expansion. Bioeng Transl Med. 2019;4(1):51–60. doi: 10.1002/btm2.10104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 129.Liu F., Hu S., Yang H., Li Z., Huang K., Su T. Hyaluronic acid hydrogel integrated with mesenchymal stem cell-secretome to treat endometrial injury in a rat model of asherman's syndrome. Adv. Healthc. Mater. 2019;8(14) doi: 10.1002/adhm.201900411. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 130.Weber K.T., Sun Y., Bhattacharya S.K., Ahokas R.A., Gerling I.C. Myofibroblast-mediated mechanisms of pathological remodelling of the heart. Nat. Rev. Cardiol. 2013;10(1):15–26. doi: 10.1038/nrcardio.2012.158. [DOI] [PubMed] [Google Scholar]
- 131.Wisdom K.M., Adebowale K., Chang J., Lee J.Y., Nam S., Desai R. Matrix mechanical plasticity regulates cancer cell migration through confining microenvironments. Nat. Commun. 2018;9(1):4144. doi: 10.1038/s41467-018-06641-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 132.Lee H.P., Stowers R., Chaudhuri O. Volume expansion and TRPV4 activation regulate stem cell fate in three-dimensional microenvironments. Nat. Commun. 2019;10(1):529. doi: 10.1038/s41467-019-08465-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 133.Wisdom K.M., Adebowale K., Chang J., Lee J.Y., Nam S., Desai R. Matrix mechanical plasticity regulates cancer cell migration through confining microenvironments. Nat. Commun. 2018;9(1):4144. doi: 10.1038/s41467-018-06641-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 134.Murphy W.L., McDevitt T.C., Engler A.J. Materials as stem cell regulators. Nat. Mater. 2014;13(6):547–557. doi: 10.1038/nmat3937. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 135.Lee H., Gu L., Mooney D.J., Levenston M.E., Chaudhuri O. Mechanical confinement regulates cartilage matrix formation by chondrocytes. Nat. Mater. 2017;16(12):1243–1251. doi: 10.1038/nmat4993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 136.Thorpe A.A., Dougill G., Vickers L., Reeves N.D., Sammon C., Cooper G. Thermally triggered hydrogel injection into bovine intervertebral disc tissue explants induces differentiation of mesenchymal stem cells and restores mechanical function. Acta Biomater. 2017;54:212–226. doi: 10.1016/j.actbio.2017.03.010. [DOI] [PubMed] [Google Scholar]
- 137.Xu Y., Yang X., Thomas A.K., Patsis P.A., Kurth T., Kräter M. Noncovalently assembled electroconductive hydrogel. Acs Appl. Mater. Inter. 2018;10(17):14418–14425. doi: 10.1021/acsami.8b01029. [DOI] [PubMed] [Google Scholar]
- 138.Ma Y., Lin M., Huang G., Li Y., Wang S., Bai G. 3D spatiotemporal mechanical microenvironment: a hydrogel-based platform for guiding. Stem Cell Fate. 2018;30(49) doi: 10.1002/adma.201705911. [DOI] [PubMed] [Google Scholar]
- 139.Major L.G., Choi Y.S. Developing a high-throughput platform to direct adipogenic and osteogenic differentiation in adipose-derived stem cells. J. Tissue Eng. Regen. M. 2018;12:2021–2028. doi: 10.1002/term.2736. [DOI] [PubMed] [Google Scholar]
- 140.Crowder S.W., Leonardo V., Whittaker T., Papathanasiou P., Stevens M.M. Material cues as potent regulators of epigenetics and stem cell function. Cell Stem Cell. 2016;18(1):39–52. doi: 10.1016/j.stem.2015.12.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 141.Brusatin G., Panciera T., Gandin A., Citron A., Piccolo S. Biomaterials and engineered microenvironments to control YAP/TAZ-dependent cell behaviour. Nat. Mater. 2018;17(12):1063–1075. doi: 10.1038/s41563-018-0180-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 142.Laflamme M.A., Murry C.E. Heart regeneration. Nature. 2011;473(7347):326–335. doi: 10.1038/nature10147. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 143.Stapleton L.M., Steele A.N., Wang H., Hernandez H.L., Yu A.C., Paulsen M.J. Use of a supramolecular polymeric hydrogel as an effective post-operative pericardial adhesion barrier. Nat. Biomed. Eng. 2019;3(8):611–620. doi: 10.1038/s41551-019-0442-z. [DOI] [PubMed] [Google Scholar]
- 144.Shi L., Wang F., Zhu W., Xu Z., Fuchs S., Hilborn J. Self-healing silk fibroin-based hydrogel for bone regeneration: dynamic metal-ligand self-assembly approach. Adv. Funct. Mater. 2017;27(37):1700591. [Google Scholar]
- 145.Zhang K., Jia Z., Yang B., Feng Q., Xu X., Yuan W. Adaptable hydrogels mediate cofactor-assisted activation of biomarker-responsive drug delivery via positive feedback for enhanced tissue regeneration. Adv. Sci. 2018;5(12):1800875. doi: 10.1002/advs.201800875. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 146.Brittberg M., Lindahl A., Nilsson A., Ohlsson C., Isaksson O., Peterson L. Treatment of deep cartilage defects in the knee with autologous chondrocyte transplantation. N. Engl. J. Med. 1994;331(14):889–895. doi: 10.1056/NEJM199410063311401. [DOI] [PubMed] [Google Scholar]
- 147.Runhaar J. 2013. Development and Prevention of Knee Osteoarthritis: the Load of Obesity. [Google Scholar]
- 149.Zhang Y., Liu X., Zeng L., Zhang J., Zuo J., Zou J. Polymer fiber scaffolds for bone and cartilage tissue engineering. Adv. Funct. Mater. 2019;29(36):1903279. [Google Scholar]
- 150.Kim I.L., Khetan S., Baker B.M., Chen C.S., Burdick J.A. Fibrous hyaluronic acid hydrogels that direct MSC chondrogenesis through mechanical and adhesive cues. Biomaterials. 2013;34(22):5571–5580. doi: 10.1016/j.biomaterials.2013.04.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 151.Kloxin A.M., Kasko A.M., Salinas C.N., Anseth K.S. Photodegradable hydrogels for dynamic tuning of physical and chemical properties. Science (American Association for the Advancement of Science) 2009;324(5923):59–63. doi: 10.1126/science.1169494. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 152.Huey D.J., Hu J.C., Athanasiou K.A. Unlike bone, cartilage regeneration remains elusive. Science. 2012;338(6109):917–921. doi: 10.1126/science.1222454. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 153.Li Z., Zhou F., Li Z., Lin S., Chen L., Liu L. Hydrogel cross-linked with dynamic covalent bonding and micellization for promoting burn wound healing. Acs Appl. Mater. Inter. 2018;10(30):25194–25202. doi: 10.1021/acsami.8b08165. [DOI] [PubMed] [Google Scholar]
- 154.Foster A.A., Dewi R.E., Cai L., Hou L., Strassberg Z., Alcazar C.A. Protein-engineered hydrogels enhance the survival of induced pluripotent stem cell-derived endothelial cells for treatment of peripheral arterial disease. Biomater. Sci.-Uk. 2018;6(3):614–622. doi: 10.1039/c7bm00883j. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 155.Loebel C., Rodell C.B., Chen M.H., Burdick J.A. Shear-thinning and self-healing hydrogels as injectable therapeutics and for 3D-printing. Nat. Protoc. 2017;12(8):1521–1541. doi: 10.1038/nprot.2017.053. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 156.Tong X., Yang F. Sliding hydrogels with mobile molecular ligands and crosslinks as 3D stem cell niche. Adv. Mater. 2016;28(33):7257–7263. doi: 10.1002/adma.201601484. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 157.Shin M., Ryu J.H., Park J.P., Kim K., Yang J.W., Lee H. DNA/Tannic acid hybrid gel exhibiting biodegradability, extensibility, tissue adhesiveness, and hemostatic ability. Adv. Funct. Mater. 2015;25(8):1270–1278. [Google Scholar]
- 158.Li C., Faulkner-Jones A., Dun A.R., Jin J., Chen P., Xing Y. rapid formation of a supramolecular polypeptide-DNA hydrogel for in situ three-dimensional multilayer bioprinting. Angew. Chem. Int. Ed. 2015;54(13):3957–3961. doi: 10.1002/anie.201411383. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.