Abstract
A 65-year-old woman had a ground glass nodule, which was suspicious for lung cancer, in her right lung S6 by chest computed tomography. For diagnosis, video-assisted thoracoscopic surgery was performed, and the specimen showed a pathological pattern of lymphocytic interstitial pneumonia (LIP). Four years after surgery, new localized ground glass shadows gradually increased on the base of the lung. However, because she had no respiratory symptoms and had normal respiratory function, she was observed with no medication. Subsequently, no other underlying diseases associated with LIP developed. The ground glass nodule was the initial lesion of LIP.
Keywords: Lymphocytic interstitial pneumonia, Idiopathic interstitial pneumonias, Ground glass nodule, Video-assisted thoracoscopic surgery (VATS)
1. Introduction
Lymphocytic interstitial pneumonia (LIP) is a type of idiopathic interstitial pneumonia (IIP) originally classified by Liebow et al. [1]. LIP is classified as a rare IIP by the current international classification and is defined morphologically as diffuse high density lymphocyte infiltration in the lung alveolar septum [2]. Characteristic image findings of LIP including multiple nodules, ground glass shadows, and cyst formation have been reported [3,4]. Here, we report a case who had a ground glass nodular lesion by chest computed tomography (CT) that was similar to lung cancer. The final pathological diagnosis was LIP pattern.
2. Case report
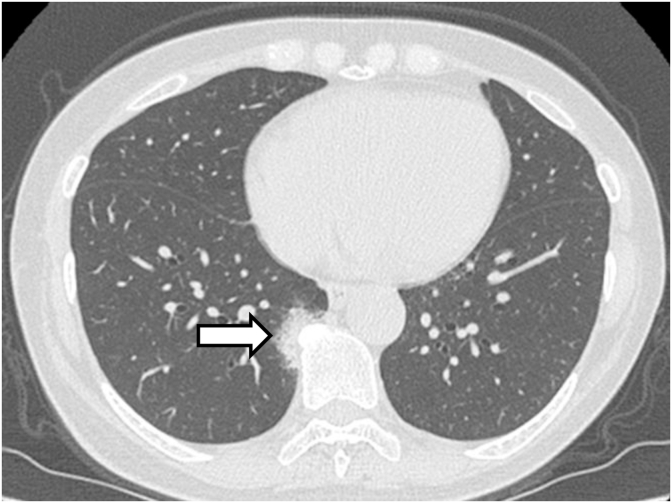
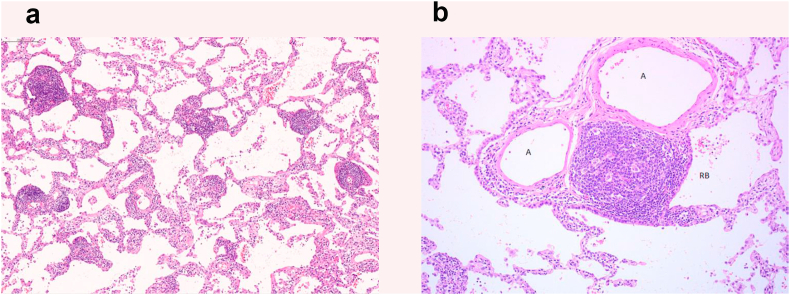
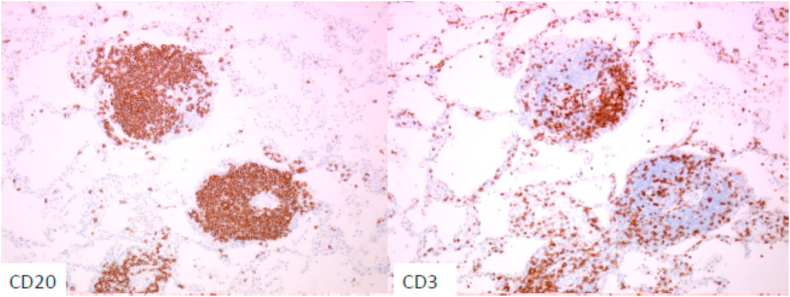
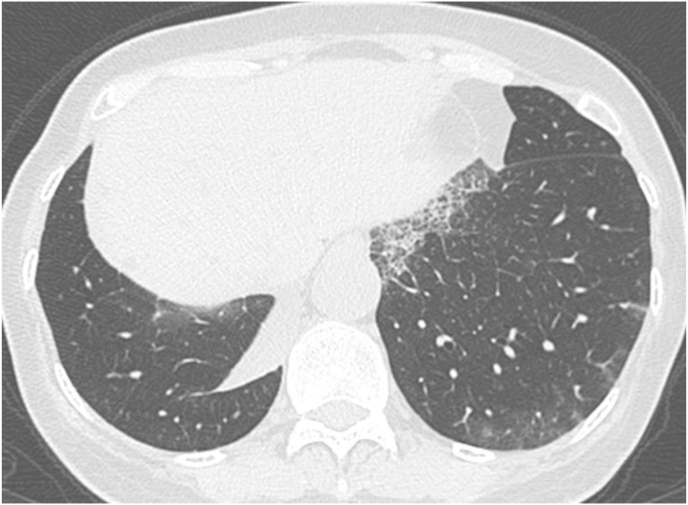
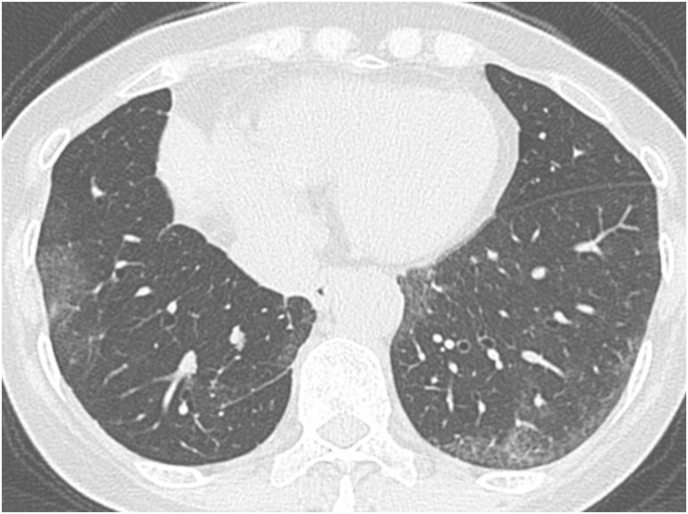
A 65-year-old woman with no medical history visited our hospital with an abnormal chest shadow. Her physical findings were normal including peripheral edema, digital clubbing, cyanosis, or sicca syndrome, and her blood test findings including tumor markers and a respiratory function test were also normal. Her chest CT showed a ground glass nodule with a diameter of 1.5cm in the lower lobe (S6) of the right lung (Fig. 1). Because we suspected lung cancer, a transbronchial lung biopsy (TBLB) was performed: the specimen showed atypical cells with no obvious malignant findings. Three months later, the size of the nodule had increased slightly. For a definitive diagnosis, video-assisted thoracoscopic surgery was performed, and the pathological findings showed diffuse lymphoid follicular hyperplasia, infiltration of the alveolar septum by small lymphocytes and plasma cells, and follicular bronchiolitis (Fig. 2A). There were many lymphoid follicles in the tissue and lymphocyte exudation was present in the alveolar space (Fig. 2B). Immunohistochemically, the follicular area was CD20 positive, and CD3 positive T cells had infiltrated into the alveolar walls (Fig. 3). There was no monoclonal growth of κ or λ positive cells. There were a few lymphoepithelial lesions, and lymphocyte infiltration was observed between the bronchiole epithelium. Based on these pathological findings, a LIP pattern was considered. There were no physical or serological findings associated with collagen disease. Seven months after surgery, new localized ground glass shadows appeared (Fig. 4), and they gradually increased on the basal lesion of the lung for four years (Fig. 5). However, because she had no respiratory symptoms and normal respiratory function, she was observed with no medication. In addition, no other underlying diseases associated with LIP developed.
Fig. 1.
High resolution CT shows a diffuse a ground-glass nodule in the lower lobe of the right lung near the seventh to ninth thoracic vertebrae (arrow).
Fig. 2.
Histological findings of the resected lung.
(A) The lung tissue shows diffuse lymphoid follicular hyperplasia, lymphocyte infiltration in the alveolar septum, and macrophage and lymphocyte exudation in the alveolar space (HE stain, original magnification, × 10). (B) The lung tissue shows small lymphocytes, plasma cell infiltration, and follicular bronchiolitis in the alveolar septum (HE stain, original magnification, × 40). A: Artery, pulmonary RB: Respiratory bronchiole.
Fig. 3.
Photomicrographs of immunostained lung tissue.
The follicular area was CD20 positive and alveolar septal lymphocytes were CD3 positive (original magnification, × 40).
Fig. 4.
After 7 months, high resolution CT showed a ground glass shadow on the dorsal side of the left lower lung field.
Fig. 5.
After 2 years, high resolution CT showed a ground glass shadow on the dorsal side of both lower lung fields.
3. Discussion
When a ground glass nodule on chest CT is observed, neoplastic lesions such as atypical adenomatous hyperplasia (AAH), adenocarcinoma in situ (AIS), minimally invasive adenocarcinoma (MIA), lung adenocarcinoma and lymphoproliferative disorders must be considered as differential diseases. In addition, non-neoplastic diseases such as localized fibrotic lesions, focal pneumonia, pulmonary mycosis, eosinophilic pneumonia, and organized pneumonia should also be considered as differential diseases [[5], [6], [7], [8]]. Large and persistent findings of ground glass nodule may be strongly suggestive of a neoplastic condition [9]. In a retrospective report, 93% of lesions with a ground glass opacity (GGO) diameter of 20 mm or more, 75% of lesions with a diameter of 10–20 mm, and 43% of lesions with a GGO diameter of 10 mm or less were malignant lesions [10]. In our case, the chest CT showed a ground glass nodule with a diameter of 15 mm and the size of the nodule had increased slightly. Therefore, video-assisted thoracoscopic surgery (VATS) was performed, and the pathological findings showed a LIP pattern.
The histological features of the LIP pattern determined by pathological diagnosis according to the 2002 international classification include: a) lesions containing diffuse interstitial (inflammatory cell) infiltration; b) mainly alveolar wall distribution; c) infiltrating cells are mainly T lymphocytes, plasma cells, and macrophages; and d) numerous lymphoid follicles present in the tissues [9]. In the 2013 statement, LIP was described as rare [2]. Lymphocytes and plasma cells infiltrate into the alveolar septum, and then are widely dispersed throughout the bronchi and blood vessels. These findings may develop to remodeling and fibrosis of the lung structure. In our case, the pathological findings showed almost no architectural distortion. Therefore, we considered this case to be at an early stage of LIP.
LIP is classified as a rare IIP in the current international classification [2]. For IIPs, it is often difficult to differentiate between LIP and cellular nonspecific interstitial pneumonia (NSIP) with high lymphocyte infiltration. In cases of LIP, there is a high degree of lymphocyte and plasma cell infiltration mainly in the alveolar septum. In particular, the findings of lymphoid follicular hyperplasia strongly suggest LIP [11]. In our case, the pathological findings showed diffuse lymphoid follicular hyperplasia, and infiltration of the alveolar septum by small lymphocytes and plasma cells. Therefore, the pathological analysis suggested the LIP pattern rather than the cellular NSIP pattern.
Chest CT findings of LIP were reported to be mainly bilateral diffuse interstitial shadows, with cyst formation (82%), ground glass opacity (GGO) (76%), multiple nodules (71%), and airspace integration (18%) [4]. In a report of 22 cases, GGO was observed in all cases, and bilateral, diffuse and mottled shadows were observed in 95%, 64% and 23% of cases, respectively [12]. In our case, we initially observed a single small GGO nodule in the lower lobe of the lung, and after surgical resection, additional multiple GGO gradually increased in the lower lobe of the lung. An initial single GGO nodule similar to lung cancer is an early stage finding of LIP. Regarding a potential association between LIP and lung cancer, a resected lung lesion around an adenocarcinoma showed the infiltration of numerous lymphocytes in the alveolar septum, consistent with the pathological findings of LIP [13]. This was considered to reflect the local immune response to antigenic stimulation caused by the lung cancer. However, there have been no reports of LIP with CT findings similar to lung cancer. Therefore, our case is rare and provides valuable information regarding the early stage of LIP, which appears as a nodular lesion by CT.
Idiopathic LIP is rarely reported. Many cases of LIP are recognized as lung injuries caused by immune abnormalities such as abnormal proteinemia, viral infection, and collagen disease [14]. The most common cause of secondary LIP is Sjögren's syndrome (SS), which occurs in about 25%–50% of cases [14,15]. SS is an autoimmune disorder, diagnosis of which is frequently delayed due to nonspecific clinical presentation. Pulmonary manifestations in SS include airway involvement, interstitial lung disease, and lymphoproliferative disorders such as pulmonary lymphoma [16]. Other rare causes include autoimmune diseases such as systemic lupus erythematosus and rheumatoid arthritis, as well as malignant lymphoma [15]. LIP secondary to SS is associated with infection by Epstein-Barr virus (EBV) [17], human T cell lymphotropic virus type-1 (HTLV-1) [18], and human immunodeficiency virus (HIV) [19], and human herpes virus-6 (HHV-6) [20]. Therefore, if LIP is proven by VATS, SS should initially be considered suspicious for secondary LIP. Additional tests, such as serological tests including rheumatoid factor, antinuclear antibodies, anti SS-A/SS-B antibodies, and virologic tests including EBV, HHV-6, HTLV-1, and HIV, as well as saliva volume by a gum test and tear volume by Schirmer's test are needed. Systemic examination will be also performed in consideration of malignant lymphoma. In our case, there were no physical and serological findings suspicious of collagen disease and the above-mentioned virologic tests were all negative. Furthermore, there was no malignant finding including malignant lymphoma in the resected lung specimen and positron emission tomography with 18-fluorodeoxyglucose (FDG-PET). Four years after VATS, there has been no finding suspicious of secondary LIP.
4. Conclusion
We report a case of LIP presenting with a ground glass nodule, which was similar to lung cancer, as an initial finding.
Declaration of competing interest
The authors declare no conflicts of interest associated with this manuscript.
References
- 1.Carrington B.C., Liebow A.A. Lymphocytic interstitial pneumonia. Am. J. Pathol. 1966;48:36a. [Google Scholar]
- 2.Travis W.D., Costabel U., Hansell D.M. An official American Thoracic Society/European Respiratory Society statement: update of the international multidisciplinary classification of the idiopathic interstitial pneumonias. Am. J. Respir. Crit. Care Med. 2013;188:733–748. doi: 10.1164/rccm.201308-1483ST. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Hare S.S., Souza C.A., Bain G. The radiological spectrum of pulmonary lymphoproliferative disease. Br. J. Radiol. 2012;85:848–864. doi: 10.1259/bjr/16420165. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Honda O., Johkoh T., Ichikado K. Differential diagnosis of lymphocytic interstitial pneumonia and malignant lymphoma on high-resolution CT. AJR Am. J. Roentgenol. 1999;173:71–74. doi: 10.2214/ajr.173.1.10397102. [DOI] [PubMed] [Google Scholar]
- 5.Tsutui S., Ashizawa K., Kido Y. Differential diagnosis of focal GGO on HRCT. Nihon Kyobu Rinsho. 2009;68:102–109. [Google Scholar]
- 6.Park C.M., Goo J.M., Lee H.J. Nodular ground-glass opacity at thin-section CT: histologic correlation and evaluation of change at follow-up. Radiographics. 2007;27:391–408. doi: 10.1148/rg.272065061. [DOI] [PubMed] [Google Scholar]
- 7.Park C.M., Goo J.M., Lee H.J. Focal interstitial fibrosis manifesting as nodular ground-glass opacity: thin-section CT findings. Eur. Radiol. 2007;17:2325–2331. doi: 10.1007/s00330-007-0596-z. [DOI] [PubMed] [Google Scholar]
- 8.Yang Z.G., Sone S., Takashima S. High-resolution CT analysis of small peripheral lung adenocarcinomas revealed on screening helical CT. Am. J. Roentgenol. 2001;176:1399–1407. doi: 10.2214/ajr.176.6.1761399. [DOI] [PubMed] [Google Scholar]
- 9.Infante M., Lutman R.F., Imparato S. Differential diagnosis and management of focal ground-glass opacities. Eur. Respir. J. 2009;33:821–827. doi: 10.1183/09031936.00047908. [DOI] [PubMed] [Google Scholar]
- 10.Yoon H.E., Fukuhara K., Michiura T. Pulmonary nodules 10 mm or less in diameter with groundglass opacity component detected by highresolution computed tomography have a high possibility of malignancy. Jpn. J. Thorac. Cardiovasc. Surg. 2005;53:8–22. doi: 10.1007/s11748-005-1004-8. [DOI] [PubMed] [Google Scholar]
- 11.American Thoracic Society European Respiratory Society International Multidisciplinary Consensus Classification of the Idiopathic Interstitial Pneumonias This joint statement of the American thoracic society (ATS), and the European respiratory society (ERS) was adopted by the ATS board of directors, june 2001 and by the ERS executive committee, june 2001. Am. J. Respir. Crit. Care Med. 2002;165:277–304. doi: 10.1164/ajrccm.165.2.ats01. [DOI] [PubMed] [Google Scholar]
- 12.Johkoh T.1, Müller N.L., Pickford H.A. Lymphocytic interstitial pneumonia: thin-section CT findings in 22 patients. Radiology. 1999;212:567–572. doi: 10.1148/radiology.212.2.r99au05567. [DOI] [PubMed] [Google Scholar]
- 13.Bandoh S., Fujita J., Haba R. Lung cancer with focal lymphocytic interstitial pneumonia. Intern. Med. 2002;41:997–1001. doi: 10.2169/internalmedicine.41.997. [DOI] [PubMed] [Google Scholar]
- 14.Cha S.I., Fessler M.B., Cool C.D. Lymphoid interstitial pneumonia: clinical features, associations and prognosis. Eur. Respir. J. 2006;28 doi: 10.1183/09031936.06.00076705. 364 - 349. [DOI] [PubMed] [Google Scholar]
- 15.Jeffrey J.S., Gerald J.B., Thomas A.R. Lymphoid interstitial pneumonia. Chest. 2002;122:2150–2164. doi: 10.1378/chest.122.6.2150. [DOI] [PubMed] [Google Scholar]
- 16.Kreider M., Highland K. Pulmonary involvement in Sjögren syndrome. Semin. Respir. Crit. Care Med. 2014;35:255–264. doi: 10.1055/s-0034-1371529. [DOI] [PubMed] [Google Scholar]
- 17.Kaan P.M., Hegele R.G., Hayashi S. Expression of bcl-2 and Epstein-Barr virus LMP 1 in Lymphocytic interstitial pneumonia. Thorax. 1997;52:12–16. doi: 10.1136/thx.52.1.12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Terada K., Katamine S., Eguchi K. Prevalence of serum and salivary antibodies to HTLV-1 in Sjögren’s syndrome. Lancet. 1994;344:1116–1119. doi: 10.1016/s0140-6736(94)90630-0. [DOI] [PubMed] [Google Scholar]
- 19.Travis W.D., Fox C.H., Devaney K.O. Lymphoid pneumonitis in 50 adult patients infected with the human immunodeficiency virus ; Lymphocytic interstitial pneumonitis versus nonspecific interstitial pneumonitis. Hum. Pathol. 1992;23:529–541. doi: 10.1016/0046-8177(92)90130-u. [DOI] [PubMed] [Google Scholar]
- 20.Totani Y., Demura Y., Ameshima S. A case of lymphocytic interstitial pneumonia with sjogren's syndrome and systemic lupus erythematisus in which human herpes virus-6 infection was the suspected pathogen. Nihon Kokyuki Gakkaishi (Journal of Japanese Respiratory Society) 2001;39:763–769. [PubMed] [Google Scholar]