Abstract
Elite controllers are HIV-1 positive subjects who control viral replication without antiretroviral therapy. Many of these subjects have replication-competent virus and thus represent a model of a functional cure. Peripheral CD4+ T cells in these subjects have small reservoirs with a low frequency of intact proviruses. Furthermore, recent studies suggest that many of these intact proviruses are disproportionally integrated at sites that have limited transcriptional activity raising the possibility that replication-competent viruses do not replicate because they are in a “blocked and locked” state. However, this feature is probably a consequence rather than a cause of elite control. Additionally, evolution of plasma virus has been detected in many elites suggesting that there continues to be ongoing viral replication in other compartments. While exceptional elite controllers with very limited viral reservoirs have recently been described, more work is needed to determine whether these patients have achieved a sterilizing cure.
Keywords: HIV, Elite controllers, HIV controllers, Viremic controllers, Reservoirs
1. Introduction
Latently infected CD4+ T cells are quiescent memory CD4+ T cells that have the viral genome integrated into the host genome. While viral replication does not occur in these resting CD4+ T cells, activation can lead to viral transcription and new rounds of viral replication. Thus, these cells represent the major barrier to HIV eradication [1]. There are two possible ways of curing HIV; a sterilizing cure involves the eradication of all replication-competent HIV proviruses whereas a functional cure involves controlling HIV replication without antiretroviral therapy. HIV controllers are subjects who are models of a functional cure [2]; many are infected with replication-competent virus, but they control viral replication without antiretroviral therapy. A large percentage of these patients have protective HLA alleles [3,4] and potent T cell responses [5] that are thought to play a role in controlling viral replication. However, HIV controllers are a heterogeneous group of patients and some do not have protective HLA alleles or HIV-specific immune responses. Multiple factors that have not yet been fully characterized, including viral fitness, may contribute to elite control in these subjects. HIV controllers are divided into 2 categories based on the degree of control: elite controllers (ECs) maintain viral loads below the limit of detection of commercial viral load assays and viremic controllers (VCs) have viral loads that are detectable but less than 2000 copies/ml [6]. A better understanding of the viral reservoir in these subjects is needed if a functional cure is to be achieved in patients with progressive disease who are on suppressive antiretroviral (ART) regimens (chronic progressors, CPs). New technology has led to improved characterization of the viral reservoir in CPs, and several recent studies have applied these methods to ECs. Here we review recent advances in our understanding of the viral reservoir in ECs and discuss the lessons we can apply to CPs.
2. Evidence of replication-competent virus in ECs
The Sydney Blood Bank cohort is made up of individuals who were infected by blood transfusions from a patient who was infected with an HIV-1 isolate that contained large deletions in the nef gene and the LTR. All of the recipients became slow progressors with low levels of viremia or ECs [7]. More recently, a patient who was infected with a vpr-defective HIV-1 molecular clone and became an EC has been described [8] and members of a transmission cluster of ECs have been found to be infected with HIV-1 isolates that contain related attenuated env genes [9]. Furthermore, the high percentage of people living with HIV-2 who become controllers suggests that viral factors may play a role in clinical outcomes [10]. Taken together, such studies suggest that infection with attenuated or defective viruses can lead to elite control, but they do not indicate that all ECs are infected with defective virus. Although it has been challenging to isolate replication-competent virus from most ECs, several labs have successfully isolated virus from a subset of these patients using some version of the quantitative viral outgrowth assay (QVOA, [11], [12], [13], [14], [15]). In one study, full genome sequence analyses of replication-competent isolates from 4 ECs showed no evidence of large deletions, hypermutations, or known attenuating mutations and phenotypic analyses also proved that these viruses were as fit as laboratory HIV-1 isolates [11]. Isolates from some EC have also been shown to replicate vigorously and cause CD4+ T cell depletion in humanized mice [16]. Furthermore, replication-competent virus has been isolated from both members of four HIV controller/CP transmission pairs [17], [18], [19] and comparable viral fitness from both members was seen in 3 out of 4 of these pairs [18,19]. Interestingly, in one of these studies, the pattern of escape mutations present in HLA-B*57 restricted Gag epitopes suggested that the elite controller had transmitted virus to the patient who became a CP [18]. Finally, there have been reports of ECs transiently [20] or permanently [19,21,22] losing control of viral replication and becoming viremic. Thus, it has become clear that some ECs are capable of controlling viruses that are fully replication-competent.
2.1. Characteristics of EC proviruses and integration sites
While many of the studies described above activated CD4+ T cells in QVOAs to isolate replication-competent virus, other studies have performed molecular analyses on unmanipulated CD4+ T cells. A low frequency of total [13] and integrated [23] HIV DNA has been reported in studies using standard PCR assays, however PCR does not distinguish between defective and infectious proviruses. A study based on more than 400 near full-length sequences of provirus from 28 CPs demonstrated that only 2.4% of proviruses in CPs did not contain large deletions or hypermutations and thus were said to be “intact” [24]. It is important to note that not all intact proviruses are replication-competent, as mutations and small insertions and deletions in critical areas of the viral genome can compromise viral fitness. However, some intact proviruses have been shown to replicate in vitro and thus intact proviruses represent a good surrogate for replication-competent virus [24]. Recently Bruner et al developed a high throughput intact proviral DNA assay (IPDA) that approximates the frequency of intact proviruses as determined by full genome sequencing [25]. Kwaa et al used the IPDA to compare the size of the viral reservoir in patients on ART and ECs and found that the frequency of both total and intact DNA in EC was approximately 20-fold lower than the frequency seen in patients on ART [26]. Jiang and colleagues sequenced 1,385 and 2,388 full length proviral genomes from 64 ECs and 41 CPs respectively and also found significantly lower levels of intact and total DNA in ECs [27]. Interestingly, the ratio of intact to total DNA was the same or higher in ECs compared to subjects on ART in the 2 cohorts of patients suggesting that ECs do not have a higher portion of defective virus.
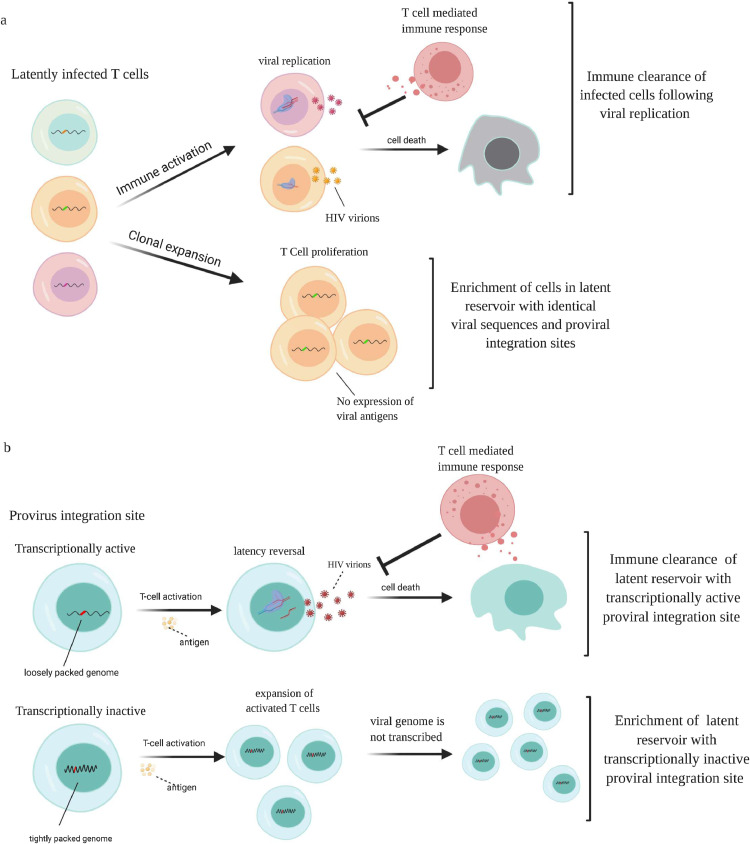
Sequence analysis revealed no evidence of enrichment of escape mutations [28] or gross genetic defects [29] in proviral DNA from ECs compared to CPs. Digital PCR studies enabled the amplification and sequence analysis of clonal proviruses isolated from peripheral CD4+ T cells. Interestingly, proviral Gag [30] and Env proviruses [31] showed evidence of identical sequences which was unexpected given the low fidelity of HIV polymerase. It is now clear that this phenomenon is due to clonal expansion of infected CD4+ T cells where integrated viral genomes are copied by host DNA polymerase during cell proliferation [32]. Boritz et al analyzed 99 sequences from env in a cohort that was largely made up of viremic controllers and showed that the frequency of expanded clones ranged from 32.7% to 96.8% of sequences from peripheral CD4+ T cells [33]. Veenhuis et al found a comparable frequency of expanded env clones in ECs and showed that the frequency is higher in EC than in subjects on ART [34]. While one cannot determine whether clonally expanded virus is defective or replication-competent by sequencing individual genes, full genome sequence analysis of multiple viral isolates obtained from a culture assay from an EC with a large reservoir proved that there was clonal expansion of replication-competent virus. Jiang and colleagues also showed that there was clonal expansion of genome-intact viruses in EC and the frequency of these presumed replication-competent clonally expanded viruses was higher in EC than in subjects on ART [27]. It is possible that this enrichment of clonally expanded proviruses in ECs may be due to the fact that while viral replication results in the synthesis of viral proteins that can be identified by the immune system, clonal expansion can take place without antigen expression or virus production [35,36]. Thus, HIV-specific immune responses are more likely to eradicate CD4+ T cells containing actively replicating virus than infected CD4+ T cells that have undergone clonal expansion (Fig. 1A). Interestingly, Antar and colleagues did not find an enrichment of clonally expanded proviruses in 3 ECs on ART[37]. This could be due to the fact the initiation of ART in ECs leads to a reduction in the frequency of HIV-specific CD8+ effector T cells [14,38] which would lead to less selective pressure in these individuals.
Fig. 1.
Effect of clonal expansion and integration sites on the viral reservoir: A) Activation of latently infected T cells will lead to viral transcription and replication. The synthesis of viral protein will lead to immune recognition and elimination of these cells by the immune system. In contrast, clonal expansion of latently infected cells is unlikely to lead to viral transcription and replication, and will not trigger an immune response, leading to enrichment of cells with identical viral sequences and proviral integration sites. B) Proviruses can be integrated into transcriptionally active or inactive sites. Upon activation, cells with provirus integrated into transcriptionally active sites will make viral proteins and will be eliminated by the immune system. In contrast, cells with provirus integrated into transcriptionally inactive sites will not make viral proteins and can evade the immune system. This will lead to an accumulation of cells with proviruses that are integrated into transcriptionally inactive sites in ECs.
Integration site analysis in HIV controllers was first performed by Boritz et al [33]. Approximately 90% of all env clones amplified from peripheral CD4+ T cells were integrated into NSD1 gene in one subject and in the POLM gene in another. Veenhuis et al showed that in an elite controller with a large reservoir, 15% of proviral clones amplified from 60 peripheral CD4+ T cells were integrated at the same site in WNK1, and 10% were integrated in the same site in ZNF470 [34]. Neither study distinguished between replication-competent and defective proviruses, but Jiang et al. used matched integration site and proviral sequencing [39] to analyze both integration sites and the corresponding proviral sequence [27]. They studied 92 integration sites from intact proviruses from 11 ECs and showed that 40% of proviral clones from ECs were integrated into non-genic or pseudogenic regions compared to 13% of intact proviral clones from subjects on ART. In addition, there was a significant enrichment of integration sites that occurred either in satellite DNA or in genes in the zinc-finger protein family, compared to integration sites in patients on ART. This is noteworthy as HIV integration rarely occurs in satellite DNA and this would be expected to lead to a higher level of transcriptional repression in ECs. Indeed, the authors found a lower ratio of viral transcripts to viral DNA in EC subjects compared to subjects on ART which confirms prior studies showing low levels of cell associated HIV RNA in ECs [40,41].
The authors show that superinfecting CD4+ T cells from elite controllers with a lab strain leads to a normal integration pattern [27] so it is unlikely that the majority of these individuals are infected with virus that happened to integrate at sites with heterochromatin features. It is much more likely that strong immune responses in ECs selectively eliminate CD4+ T cells that harbor transcriptionally competent virus over time due to expression of viral proteins in these cells. This is consistent with a study that found that latency reversal in a primary cell model was rare and depended on integration in an open chromatin context [42]. Selective immune mediated elimination of these transcriptionally-competent cells would lead to an enrichment of CD4+ T cells that harbor proviruses that are in a state of deep latency and thus do not express any viral proteins (Fig. 1B). This state of deep latency probably contributes to control of viral replication and may also provide an explanation for why it has been so challenging to culture virus from many ECs since immune activation with T cell mitogens may not lead to viral transcription and latency reversal in many infected cells.
3. Evidence of ongoing viral replication in ECs
The question then arises, if the majority of remaining proviruses in elite controllers exist in a ‘locked and blocked state’, is there any viral replication occurring in these subjects? The answer appears to be yes. Many laboratories have detected and quantified virus in the plasma of these subjects [43], [44], [45], [46] and sequence analysis shows a major discordance between plasma virus and virus in resting CD4+ T cells [30,47]. While virus from CD4+ T cells rarely have escape mutations, virtually every plasma virus amplified in Gag of HLA-B*57 ECs has at least one escape mutation. The presence of escape mutations in Gag in plasma virus has been confirmed [48] as has the discordance between proviral and plasma sequences in ECs [33]. This discordance suggests two things: 1) strong selective pressure is being exerted on viruses in these subjects and 2) virus replication is taking place in some compartment. Further evidence of ongoing viral replication in HLA-B*57 elite controllers comes from studies by O'Connell and colleagues. While no evolution was detected in provirus in peripheral CD4+ T cells in elite controllers, there was evolution of plasma virus in the same subjects over the same time frame [49,50]. Additionally, the proviral sequences were ancestral to the plasma sequences. This evolution of plasma virus was seen in a separate study in a different cohort of elite controllers where protective HLA alleles were not as overrepresented [51]. Studies in the monkey model of elite control suggest that the ongoing viral replication may be taking place in B cell follicles in lymphoid tissue [52] where CD8+ T cells are generally excluded [53]. Taken together these results suggest that the presence of deep latency seen in peripheral CD4+ T cells does not mean that viral replication is completely inhibited in these subjects. And while some studies have suggested that plasma virus from ECs are less fit than plasma virus from patients on ART [48,[54], [55], [56], [57]], the fact that viral evolution occurs proves that the virus is capable of ongoing replication [49], [50], [51].
4. Exceptional elite controllers
“Exceptional elite controllers” (EECs), are a heterogeneous group of patients who may have achieved a higher degree of viral control, and possibly sterilizing immunity in some cases. Mendoza et al described 4 ECs who had weakly positive HIV western blots and proviral DNA levels that were 50-fold lower than that seen in traditional ECs [58]. Canouii et al found a subset of ECs who always had undetectable viral loads and had significantly lower proviral DNA levels and T cell activation than EC with occasional blips [59]. Zaunders et al recently described a member of the original Sydney Blood Bank Cohort who was infected with attenuated virus and currently has weak antibody responses and undetectable levels of proviral DNA in peripheral blood [60] and Casado and colleagues described 3 subjects who had been infected for more than 20 years who had extremely low levels of cell associated HIV DNA and RNA, and significantly lower levels of proviral diversity than traditional ECs [61]. Kwaa et al described a 200-fold variation in the level of intact proviral DNA that could be detected in the IPDA in ECs. One subject who has routinely had negative culture assays over greater than 10 years also had undetectable proviral DNA levels [26]. Jiang et al identified 2 EC who have extremely low levels of intact proviral DNA. A single intact proviral clone was detected from 1.02 billion CD4+ T cells in one subject and no detectable intact proviral clones were seen in 1.5 billion PBMCs from the other. Furthermore, they were unable to culture virus from more than 340 million CD4+ T cells and the IPDA did not detect intact DNA in a sample of 14 million CD4+ T cells [27]. These cases raise the question of whether a sterilizing cure has been achieved in some ECs. While transplantation with stem cells from donors with the delta 32 CCR5 mutation has resulted in possible sterilizing cures in 2 cases [62], [63], we have learned that other interventions that dramatically reduce the number of latently infected CD4+ T cells in patients on ART do not lead to a cure [64], [65], [66]. A sterilizing cure is almost impossible to prove in elite controllers since the most stringent test of cure, a failure of viral rebound during a prolonged treatment interruption, cannot be applied to these subjects as the very definition of elite control is based on patients being aviremic without ART.
5. Implications for cure strategies
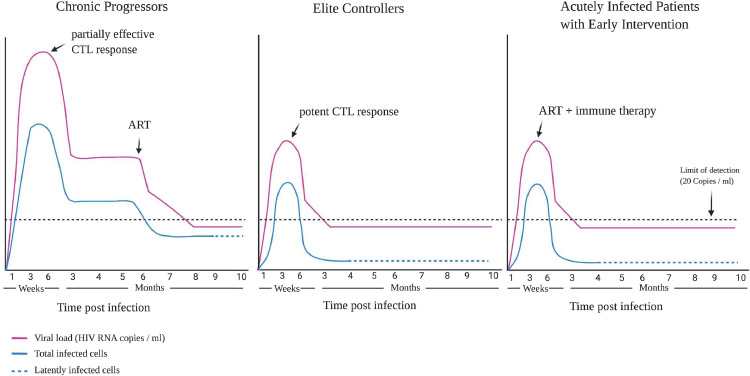
Overall it appears that most ECs have limited viral reservoirs that are controlled by the host immune response. The high percentage of CD4+ T cells with clonally expanded provirus and/or with provirus integrated in intergenic sites or sites with heterochromation features is probably a consequence of elite control rather than a cause. There have been very few studies of ECs during primary infection, but it appears that many of these patients never develop the high levels of viremia that is often seen in CPs and control viral replication shortly after infection [19,[67], [68], [69]]. The mechanism of control is unclear but may be due to rapid expansion of HIV-specific CD8+ T cells. Natural killer cells were also found to inhibit viral replication effectively during primary infection in one HIV controller [19], but more studies are needed to confirm that this is a common mechanism of early control. In one study where control of viral replication was regained following chemotherapy, the rate of viral decay was similar to that seen in subjects started on ART [20]. Treatment with ART in primary infection may be a way to mimic this rapid control and should dramatically limit the size of the viral reservoir [66,70,71] and in some cases lead to post-treatment control [72], [73], [74]. The induction of a potent T cell response or some other form of immunotherapy such as immunotoxin therapy [75] could lead to the selective elimination of cells harboring transcriptionally competent provirus. This response will probably be most effective very early in the course of infection when infected CD4+ T cells are still expressing HIV antigens and have not reached true latency (Fig. 2). Complete eradication of SIV appears to have been accomplished in macaques who were treated with a CMV vector-based vaccine that triggered very potent atypical SIV-specific CD8+ T cells prior to infection and this serves as an important proof of concept of the effect of early immune therapy on viral reservoirs [76]. A long-lasting immune response may be needed to eliminate any residual latently infected cell that reactivates after immune activation. This may require strategies such as repeated therapeutic vaccination or adoptive transfer of chimeric antigen receptor T cells [77] or infusions with broadly neutralizing antibodies [78]. Immune responses that are capable of penetrating sanctuaries such as B cell follicles will probably be needed to eliminate the source of ongoing viral replication seen in ECs. This will lead to enrichment of cells that harbor proviruses that are in a state of deep latency and are unlikely to produce infectious virus even when they become activated. It will probably be more challenging to block and lock proviruses that are already integrated at sites that favor transcription although a recent study that used the Tat inhibitor didehydro-Cortistatin A to mediate epigenetic silencing of the HIV promoter in infected humanized mice on ART suggests this approach may be promising [79]. Exceptional elite controllers who have very limited viral reservoirs in peripheral blood have been described, but before this is accepted as a sign of viral eradication, one must prove that there is no evidence of ongoing replication and viral evolution at other sites.
Fig. 2.
HIV viremia and viral reservoirs in different groups of patients. Primary infection in chronic progressors is defined by very high levels of viremia leading to widespread seeding of viral reservoirs. A partially effective immune response leads to the lowering of the viral load to a set point. The introduction of ART inhibits viral replication to undetectable viral loads and reduces the number of infected CD4+ T cells. Elite controllers have much lower viral loads during primary infection and rapid control of viral replication leading to markedly lower number of infected cells. Treatment of patients with ART during primary infection also leads to lower levels of viremia and a reduction in the number of infected cells. Immunotherapy started at this time should lead to a further reduction in the size of the viral reservoir and potentially to a functional cure.
6. Outstanding questions
Most studies of viral reservoirs in elite controllers have focused on peripheral CD4+ T cells and it will be important to analyze myeloid cells, lymph nodes and other compartments. Very few studies have analyzed the reservoir during primary infection and it is not known whether the rate of decay of infected cells in this phase is similar to that seen after the initiation of ART in CPs. The mechanism of control of the viral reservoir in ECs who have weak HIV-specific CD8+ T cell responses is still unknown. This may inform strategies for CPs who do not have protective HLA alleles.
Search Strategy and Selection Criteria. Data for this Review were identified by searches of PubMed with search terms including combinations of HIV, controllers, elite, reservoirs. Search terms reservoir, cure and remission were also used to access the most recent articles on HIV cure strategies for CPs
Author contributions
BAW, AKK and JNB all searched the literature and wrote the paper. BAW made the Fig.s.
Fig.s/Tables: Both Fig.s are original and have not been published previously. Figures were created with BioRender.Com
Acknowledgements
This work was supported by the Johns Hopkins University Center for AIDS Research (P30AI094189) and the National Institute of Allergies and Infectious Diseases (R01AI120024, JNB). The funders had no role in the writing of the paper.
References
- 1.Sengupta S, Siliciano RF. Targeting the latent reservoir for HIV-1. Immunity. 2018;48(5):872–895. doi: 10.1016/j.immuni.2018.04.030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Deeks SG, Walker BD. Human immunodeficiency virus controllers: mechanisms of durable virus control in the absence of antiretroviral therapy. Immunity. 2007;27(3):406–416. doi: 10.1016/j.immuni.2007.08.010. [DOI] [PubMed] [Google Scholar]
- 3.Migueles SA, Sabbaghian MS, Shupert WL, Bettinotti MP, Marincola FM, Martino L. HLA B*5701 is highly associated with restriction of virus replication in a subgroup of HIV-infected long term nonprogressors. Proc Natl Acad Sci U S A. 2000;97(6):2709–2714. doi: 10.1073/pnas.050567397. PMID: 10694578PMCID: PMC15994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.International HIV Controllers Study. Pereyra F, Jia X, McLaren PJ, Telenti A, de Bakker PI, Walker BD. The major genetic determinants of HIV-1 control affect HLA class I peptide presentation. Science. 2010;330(6010):1551–1557. doi: 10.1126/science.1195271. Epub 2010 Nov 4. PMID: 21051598PMCID: PMC3235490. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Migueles SA, Connors M. Success and failure of the cellular immune response against HIV-1. Nat Immunol. 2015;16(6):563–570. doi: 10.1038/ni.3161. [DOI] [PubMed] [Google Scholar]
- 6.Pereyra F, Addo MM, Kaufmann DE, Liu Y, Miura T, Rathod A. Genetic and immunologic heterogeneity among persons who control HIV infection in the absence of therapy. J Infect Dis. 2008;197(4):563–571. doi: 10.1086/526786. PMID: 18275276. [DOI] [PubMed] [Google Scholar]
- 7.Zaunders J, Dyer WB, Churchill M. The sydney blood bank cohort: implications for viral fitness as a cause of elite control. Curr Opin HIV AIDS. 2011;6(3):151–156. doi: 10.1097/COH.0b013e3283454d5b. [DOI] [PubMed] [Google Scholar]
- 8.Ali A, Ng HL, Blankson JN, Burton DR, 3rd Buckheit RW, Moldt B. Highly attenuated infection with a VPR-deleted molecular clone of human immunodeficiency virus-1. J Infect Dis. 2018;218(9):1447–1452. doi: 10.1093/infdis/jiy346. PMID: 29878133PMCID: PMC6151090. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Casado C, Marrero-Hernández S, Márquez-Arce D. Viral characteristics associated with the clinical nonprogressor phenotype are inherited by viruses from a cluster of HIV-1 elite controllers. mBio. 2018;9(2) doi: 10.1128/mBio.02338-17. e02338-17. Published 2018 Apr 10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Esbjörnsson J, Jansson M, Jespersen S, Månsson F, Hønge BL, Lindman J, Medina C, da Silva ZJ, Norrgren H, Medstrand P, Rowland-Jones SL, Wejse C. HIV-2 as a model to identify a functional HIV cure. AIDS Res Ther. 2019;16(1):24. doi: 10.1186/s12981-019-0239-x. PMID: 31484562PMCID: PMC6727498. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Blankson JN, Bailey JR, Thayil S, Yang HC, Lassen K, Lai J. Isolation and characterization of replication-competent human immunodeficiency virus type 1 from a subset of elite suppressors. J Virol. 2007;81(5):2508–2518. doi: 10.1128/JVI.02165-06. Epub 2006 Dec 6. PMID: 17151109PMCID: PMC1865922. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Lamine A, Caumont-Sarcos A, Chaix ML, Saez-Cirion A, Rouzioux C, Delfraissy JF. Replication-competent HIV strains infect HIV controllers despite undetectable viremia (ANRS EP36 study) AIDS. 2007;21(8):1043–1045. doi: 10.1097/QAD.0b013e3280d5a7ac. PMID: 17457100. [DOI] [PubMed] [Google Scholar]
- 13.Julg B, Pereyra F, Buzón MJ, Piechocka-Trocha A, Clark MJ, Baker BM. Infrequent recovery of HIV from but robust exogenous infection of activated CD4(+) T cells in HIV elite controllers. Clin Infect Dis. 2010;51(2):233–238. doi: 10.1086/653677. PMID: 20550452PMCID: PMC3749734. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Chun TW, Shawn Justement J, Murray D, Kim CJ, Blazkova J, Hallahan CW. Effect of antiretroviral therapy on HIV reservoirs in elite controllers. J Infect Dis. 2013;208(9):1443–1447. doi: 10.1093/infdis/jit306. Epub 2013 Jul 11. PMID: 23847057PMCID: PMC3789563. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Noel N, Peña R, David A, Avettand-Fenoel V, Erkizia I, Jimenez E. Long-term spontaneous control of HIV-1 is related to low frequency of infected cells and inefficient viral reactivation. J Virol. 2016;90(13):6148–6158. doi: 10.1128/JVI.00419-16. PMID: 27122576PMCID: PMC4907242. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Salgado M, Swanson MD, Pohlmeyer CW, Buckheit RW, 3rd, Wu J, Archin NM. HLA-B*57 elite suppressor and chronic progressor HIV-1 isolates replicate vigorously and cause CD4+ T cell depletion in humanized BLT mice. J Virol. 2014;88(6):3340–3352. doi: 10.1128/JVI.03380-13. Epub 2014 Jan 3. PMID: 24390323PMCID: PMC3957943. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Bailey JR, O'Connell K, Yang HC, Han Y, Xu J, Jilek B. Transmission of human immunodeficiency virus type 1 from a patient who developed AIDS to an elite suppressor. J Virol. 2008;82(15):7395–7410. doi: 10.1128/JVI.00800-08. Epub 2008 May 21. PMID: 18495769PMCID: PMC2493308. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Buckheit RW, 3rd, Allen TG, Alme A, Salgado M, O'Connell KA, Huculak S. Host factors dictate control of viral replication in two HIV-1 controller/chronic progressor transmission pairs. Nat Commun. 2012;3:716. doi: 10.1038/ncomms1697. PMID: 22395607PMCID: PMC3549550. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Walker-Sperling VE, Pohlmeyer CW, Veenhuis RT, May M, Luna KA, Kirkpatrick AR. Factors associated with the control of viral replication and virologic breakthrough in a recently infected HIV-1 controller. EBioMedicine. 2017;16:141–149. doi: 10.1016/j.ebiom.2017.01.034. Epub 2017 Jan 26. PMID: 28159573PMCID: PMC5474502. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Smith NM, Mlcochova P, Watters SA, Aasa-Chapman MM, Rabin N, Moore S. Proof-of-principle for immune control of global HIV-1 reactivation in vivo. Clin Infect Dis. 2015;61(1):120–128. doi: 10.1093/cid/civ219. Epub 2015 Mar 16. PMID: 25778749PMCID: PMC4463006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Bailey JR, Zhang H, Wegweiser BW, Yang HC, Herrera L, Ahonkhai A. Evolution of HIV-1 in an HLA-B*57-positive patient during virologic escape. J Infect Dis. 2007;196(1):50–55. doi: 10.1086/518515. Epub 2007 May 17. PMID: 17538883. [DOI] [PubMed] [Google Scholar]
- 22.Rosás-Umbert M, Llano A, Bellido R, Olvera A, Ruiz-Riol M, Rocafort M, Fernández MA, Cobarsi P, Crespo M, Dorrell L. Mechanisms of abrupt loss of virus control in a cohort of previous HIV controllers. J Virol. 2019;93(4) doi: 10.1128/JVI.01436-18. e01436-18PMID: 30487276PMCID: PMC6363998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Graf EH, Mexas AM, Yu JJ, Shaheen F, Liszewski MK, Di Mascio M. Elite suppressors harbor low levels of integrated HIV DNA and high levels of 2-LTR circular HIV DNA compared to HIV+ patients on and off HAART. PLoS Pathog. 2011;7(2) doi: 10.1371/journal.ppat.1001300. Epub 2011 Feb 24. Erratum in: PLoS Pathog. 2011 Mar;7(3). doi: 10.1371/annotation/0d21de23-d44c-49c0-9a9f-53d421648cbf. PMID: 21383972PMCID: PMC3044690. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Ho YC, Shan L, Hosmane NN, Wang J, Laskey SB, Rosenbloom DI. Replication-competent noninduced proviruses in the latent reservoir increase barrier to HIV-1 cure. Cell. 2013;155(3):540–551. doi: 10.1016/j.cell.2013.09.020. Epub 2013 Oct 24. PMID: 24243014PMCID: PMC3896327. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Bruner KM, Wang Z, Simonetti FR, Bender AM, Kwon KJ, Sengupta S. A quantitative approach for measuring the reservoir of latent HIV-1 proviruses. Nature. 2019;566(7742):120–125. doi: 10.1038/s41586-019-0898-8. Epub 2019 Jan 30. PMID: 30700913PMCID: PMC6447073. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Kwaa AK, Garliss CC, Ritter KD, Laird GM, Blankson JN. Elite suppressors have low frequencies of intact HIV-1 proviral DNA. AIDS. 2020;34(4):641–643. doi: 10.1097/QAD.0000000000002474. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Jiang C, Lian X, Gao C, Sun X, Einkauf KB, Chevalier JM. Distinct viral reservoirs in individuals with spontaneous control of HIV-1. Nature. 2020;585(7824):261–267. doi: 10.1038/s41586-020-2651-8. Epub 2020 Aug 26. PMID: 32848246. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Migueles SA, Laborico AC, Imamichi H, Shupert WL, Royce C, McLaughlin M. The differential ability of HLA B*5701+ long-term nonprogressors and progressors to restrict human immunodeficiency virus replication is not caused by loss of recognition of autologous viral gag sequences. J Virol. 2003;77(12):6889–6898. doi: 10.1128/jvi.77.12.6889-6898.2003. PMID: 12768008PMCID: PMC156173. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Miura T, Brockman MA, Brumme CJ, Brumme ZL, Carlson JM, Pereyra F. Genetic characterization of human immunodeficiency virus type 1 in elite controllers: lack of gross genetic defects or common amino acid changes. J Virol. 2008;82(17):8422–8430. doi: 10.1128/JVI.00535-08. Epub 2008 Jun 18. PMID: 18562530PMCID: PMC2519665. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Bailey JR, Williams TM, Siliciano RF, Blankson JN. Maintenance of viral suppression in HIV-1-infected HLA-B*57+ elite suppressors despite CTL escape mutations. J Exp Med. 2006;203(5):1357–1369. doi: 10.1084/jem.20052319. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Bailey JR, Lassen KG, Yang HC, Quinn TC, Ray SC, Blankson JN. Neutralizing antibodies do not mediate suppression of human immunodeficiency virus type 1 in elite suppressors or selection of plasma virus variants in patients on highly active antiretroviral therapy. J Virol. 2006;80(10):4758–4770. doi: 10.1128/JVI.80.10.4758-4770.2006. PMID: 16641269PMCID: PMC1472047. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Liu R, Simonetti FR, Ho YC. The forces driving clonal expansion of the HIV-1 latent reservoir. Virol J. 2020;17(1):4. doi: 10.1186/s12985-019-1276-8. Published 2020 Jan 7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Boritz EA, Darko S, Swaszek L, Wolf G, Wells D, Wu X. Multiple origins of virus persistence during natural control of HIV Infection. Cell. 2016;166(4):1004–1015. doi: 10.1016/j.cell.2016.06.039. Epub 2016 Jul 21. PMID: 27453467PMCID: PMC4983216. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Veenhuis RT, Kwaa AK, Garliss CC, Latanich R, Salgado M, Pohlmeyer CW. Long-term remission despite clonal expansion of replication-competent HIV-1 isolates. JCI Insight. 2018;3(18) doi: 10.1172/jci.insight.122795. PMID: 30232278PMCID: PMC6237238. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Hosmane NN, Kwon KJ, Bruner KM, Capoferri AA, Beg S, Rosenbloom DI. Proliferation of latently infected CD4+ T cells carrying replication-competent HIV-1: potential role in latent reservoir dynamics. J Exp Med. 2017;214(4):959–972. doi: 10.1084/jem.20170193. Epub 2017 Mar 24. PMID: 28341641PMCID: PMC5379987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Musick A, Spindler J, Boritz E, Pérez L, Crespo-Vélez D, Patro SC. HIV infected T cells can proliferate in vivo without inducing expression of the integrated provirus. Front Microbiol. 2019;10:2204. doi: 10.3389/fmicb.2019.02204. PMID: 31632364PMCID: PMC6781911. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Antar AA, Jenike KM, Jang S, Rigau DN, Reeves DB, Hoh R. Longitudinal study reveals HIV-1-infected CD4+ T cell dynamics during long-term antiretroviral therapy. J Clin Invest. 2020;130(7):3543–3559. doi: 10.1172/JCI135953. PMID: 32191639PMCID: PMC7324206. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Jilg N, Garcia-Broncano P, Peluso M, Segal FP, Bosch RJ, Roberts-Toler C. Hiv controllers maintain viral suppression despite waning T-cell responses on art. J Infect Dis. 2020 doi: 10.1093/infdis/jiaa294. jiaa294Epub ahead of print. PMID: 32496516. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Einkauf KB, Lee GQ, Gao C, Sharaf R, Sun X, Hua S. Intact HIV-1 proviruses accumulate at distinct chromosomal positions during prolonged antiretroviral therapy. J Clin Invest. 2019;129(3):988–998. doi: 10.1172/JCI124291. Epub 2019 Jan 28. PMID: 30688658PMCID: PMC6391088. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Hatano H, Somsouk M, Sinclair E, Harvill K, Gilman L, Cohen M. Comparison of HIV DNA and RNA in gut-associated lymphoid tissue of HIV-infected controllers and noncontrollers. AIDS. 2013;27(14):2255–2260. doi: 10.1097/QAD.0b013e328362692f. PMID: 24157906PMCID: PMC4143147. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Pohlmyer CW, Bullen CK, Martin AR, Laird GM, Chioma SU, Walker-Sperling VEK. Characterization of elite suppressors cell-associated HIV-1 mRNA at baseline and with T cell activation. Yale J Biol Med. 2017;90(2):331–336. PMID: 28656019PMCID: PMC5482309. [PMC free article] [PubMed] [Google Scholar]
- 42.Battivelli E, Dahabieh MS, Abdel-Mohsen M, Svensson JP, Tojal Da Silva I, Cohn LB, Gramatica A, Deeks S, Greene WC, Pillai SK, Verdin E. Distinct chromatin functional states correlate with HIV latency reactivation in infected primary CD4+ T cells. Elife. 2018;7:e34655. doi: 10.7554/eLife.34655. PMID: 29714165PMCID: PMC5973828. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Dinoso JB, Kim SY, Siliciano RF, Blankson JN. A comparison of viral loads between HIV-1-infected elite suppressors and individuals who receive suppressive highly active antiretroviral therapy. Clin Infect Dis. 2008;47(1):102–104. doi: 10.1086/588791. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Pereyra F, Palmer S, Miura T, Block BL, Wiegand A, Rothchild AC. Persistent low-level viremia in HIV-1 elite controllers and relationship to immunologic parameters. J Infect Dis. 2009;200(6):984–990. doi: 10.1086/605446. PMID: 19656066PMCID: PMC3725728. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Migueles SA, Osborne CM, Royce C, Compton AA, Joshi RP, Weeks KA. Lytic granule loading of CD8+ T cells is required for HIV-infected cell elimination associated with immune control. Immunity. 2008;29(6):1009–1021. doi: 10.1016/j.immuni.2008.10.010. Epub 2008 Dec 8. PMID: 19062316PMCID: PMC2622434. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Hatano H, Delwart EL, Norris PJ, Lee TH, Dunn-Williams J, Hunt PW. Evidence for persistent low-level viremia in individuals who control human immunodeficiency virus in the absence of antiretroviral therapy. J Virol. 2009;83(1):329–335. doi: 10.1128/JVI.01763-08. Epub 2008 Oct 22. PMID: 18945778PMCID: PMC2612329. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Bailey JR, Brennan TP, O'Connell KA, Siliciano RF, Blankson JN. Evidence of CD8+ T-cell-mediated selective pressure on human immunodeficiency virus type 1 nef in HLA-B*57+ elite suppressors. J Virol. 2009;83(1):88–97. doi: 10.1128/JVI.01958-08. Epub 2008 Oct 22. PMID: 18945771PMCID: PMC2612327. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Miura T, Brockman MA, Schneidewind A, Lobritz M, Pereyra F, Rathod A. HLA-B57/B*5801 human immunodeficiency virus type 1 elite controllers select for rare gag variants associated with reduced viral replication capacity and strong cytotoxic T-lymphocyte [corrected] recognition. J Virol. 2009;83(6):2743–2755. doi: 10.1128/JVI.02265-08. Epub 2008 Dec 30. Erratum in: J Virol. 2009 Jun;83(11):5961. PMID: 19116253PMCID: PMC2648254. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.O'Connell KA, Brennan TP, Bailey JR, Ray SC, Siliciano RF, Blankson JN. Control of HIV-1 in elite suppressors despite ongoing replication and evolution in plasma virus. J Virol. 2010;84(14):7018–7028. doi: 10.1128/JVI.00548-10. Epub 2010 May 5. PMID: 20444904PMCID: PMC2898225. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Salgado M, Brennan TP, O'Connell KA, Bailey JR, Ray SC, Siliciano RF. Evolution of the HIV-1 nef gene in HLA-B*57 positive elite suppressors. Retrovirology. 2010;7:94. doi: 10.1186/1742-4690-7-94. PMID: 21059238PMCID: PMC2993647. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Mens H, Kearney M, Wiegand A, Shao W, Schønning K, Gerstoft J. HIV-1 continues to replicate and evolve in patients with natural control of HIV infection. J Virol. 2010;84(24):12971–12981. doi: 10.1128/JVI.00387-10. Epub 2010 Oct 6. PMID: 20926564PMCID: PMC3004307. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Fukazawa Y, Lum R, Okoye AA, Park H, Matsuda K, Bae JY. B cell follicle sanctuary permits persistent productive simian immunodeficiency virus infection in elite controllers. Nat Med. 2015;21(2):132–139. doi: 10.1038/nm.3781. Epub 2015 Jan 19. PMID: 25599132PMCID: PMC4320022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Bronnimann MP, Skinner PJ, Connick E. The B-Cell follicle in HIV infection: barrier to a cure. Front Immunol. 2018;9:20. doi: 10.3389/fimmu.2018.00020. Published 2018 Jan 25. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Chen J, Tibroni N, Sauter D, Galaski J, Miura T, Alter G. Modest attenuation of HIV-1 Vpu alleles derived from elite controller plasma. PLoS One. 2015;10(3) doi: 10.1371/journal.pone.0120434. PMID: 25793728PMCID: PMC4368696. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Brumme ZL, Li C, Miura T, Sela J, Rosato PC, Brumme CJ. Reduced replication capacity of NL4-3 recombinant viruses encoding reverse transcriptase-integrase sequences from HIV-1 elite controllers. J Acquir Immune Defic Syndr. 2011;56(2):100–108. doi: 10.1097/QAI.0b013e3181fe9450. PMID: 21124229PMCID: PMC3078702. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Lassen KG, Lobritz MA, Bailey JR, Johnston S, Nguyen S, Lee B. Elite suppressor-derived HIV-1 envelope glycoproteins exhibit reduced entry efficiency and kinetics. PLoS Pathog. 2009;5(4) doi: 10.1371/journal.ppat.1000377. Epub 2009 Apr 10. PMID: 19360131PMCID: PMC2661022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Toyoda M, Ogata Y, Mahiti M, Maeda Y, Kuang XT, Miura T. Differential ability of primary HIV-1 Nef isolates to downregulate HIV-1 entry receptors. J Virol. 2015;89(18):9639–9652. doi: 10.1128/JVI.01548-15. Epub 2015 Jul 15. PMID: 26178998PMCID: PMC4542390. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Mendoza D, Johnson SA, Peterson BA, Natarajan V, Salgado M, Dewar RL. Comprehensive analysis of unique cases with extraordinary control over HIV replication. Blood. 2012;119(20):4645–4655. doi: 10.1182/blood-2011-10-381996. Epub 2012 Apr 5. PMID: 22490332PMCID: PMC3367872. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Canouï E, Lécuroux C, Avettand-Fenoël V, Gousset M, Rouzioux C, Saez-Cirion A. A subset of extreme human immunodeficiency virus (HIV) controllers is characterized by a small HIV blood reservoir and a weak T-cell activation level. Open Forum Infect Dis. 2017;4(2) doi: 10.1093/ofid/ofx064. ofx064PMID: 28584850PMCID: PMC5450900. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Zaunders J, Dyer WB, Churchill M, Munier CML, Cunningham PH, Suzuki K. Possible clearance of transfusion-acquired nef/LTR-deleted attenuated HIV-1 infection by an elite controller with CCR5 Δ32 heterozygous and HLA-B57 genotype. J Virus Erad. 2019;5(2):73–83. doi: 10.1016/S2055-6640(20)30696-11. PMID: 31191910PMCID: PMC6543488. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Casado C, Galvez C, Pernas M, Tarancon-Diez L, Rodriguez C, Sanchez-Merino V. Permanent control of HIV-1 pathogenesis in exceptional elite controllers: a model of spontaneous cure. Sci Rep. 2020;10(1):1902. doi: 10.1038/s41598-020-58696-y. PMID: 32024974PMCID: PMC7002478. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Hütter G, Nowak D, Mossner M, Ganepola S, Müssig A, Allers K. Long-term control of HIV by CCR5 Delta32/Delta32 stem-cell transplantation. N Engl J Med. 2009;360(7):692–698. doi: 10.1056/NEJMoa0802905. PMID: 19213682. [DOI] [PubMed] [Google Scholar]
- 63.Gupta RK, Abdul-Jawad S, McCoy LE, Mok HP, Peppa D, Salgado M. HIV-1 remission following CCR5Δ32/Δ32 haematopoietic stem-cell transplantation. Nature. 2019;568(7751):244–248. doi: 10.1038/s41586-019-1027-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Luzuriaga K, Gay H, Ziemniak C, Sanborn KB, Somasundaran M, Rainwater-Lovett K. Viremic relapse after HIV-1 remission in a perinatally infected child. N Engl J Med. 2015;372(8):786–788. doi: 10.1056/NEJMc1413931. PMID: 25693029PMCID: PMC4440331. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Henrich TJ, Hanhauser E, Marty FM, Sirignano MN, Keating S, Lee TH. Antiretroviral-free HIV-1 remission and viral rebound after allogeneic stem cell transplantation: report of 2 cases. Ann Intern Med. 2014;161(5):319–327. doi: 10.7326/M14-1027. PMID: 25047577PMCID: PMC4236912. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Henrich TJ, Hatano H, Bacon O, Hogan LE, Rutishauser R, Hill A. HIV-1 persistence following extremely early initiation of antiretroviral therapy (ART) during acute HIV-1 infection: An observational study. PLoS Med. 2017;14(11) doi: 10.1371/journal.pmed.1002417. PMID: 29112956PMCID: PMC5675377. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Goujard C, Chaix ML, Lambotte O, Deveau C, Sinet M, Guergnon J. Spontaneous control of viral replication during primary HIV infection: when is "HIV controller" status established? Clin Infect Dis. 2009;49(6):982–986. doi: 10.1086/605504. PMID: 19681706. [DOI] [PubMed] [Google Scholar]
- 68.Moosa Y, Tanko RF, Ramsuran V, Singh R, Madzivhandila M, Yende-Zuma N. Case report: mechanisms of HIV elite control in two African women. BMC Infect Dis. 2018;18(1):54. doi: 10.1186/s12879-018-2961-8. PMID: 29370775PMCID: PMC5785875. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Morley D, Lambert JS, Hogan LE, De Gascun C, Redmond N, Rutishauser RL. Rapid development of HIV elite control in a patient with acute infection. BMC Infect Dis. 2019;19(1):815. doi: 10.1186/s12879-019-4374-8. Erratum in: BMC Infect Dis. 2019 Oct 22;19(1):877. PMID: 31533639PMCID: PMC6749690. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Persaud D, Gay H, Ziemniak C, Chen YH, Piatak M, Jr, Chun TW. Absence of detectable HIV-1 viremia after treatment cessation in an infant. N Engl J Med. 2013;369(19):1828–1835. doi: 10.1056/NEJMoa1302976. Epub 2013 Oct 23. PMID: 24152233PMCID: PMC3954754. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Leyre L, Kroon E, Vandergeeten C, Sacdalan C, Colby DJ, Buranapraditkun S. Abundant HIV-infected cells in blood and tissues are rapidly cleared upon ART initiation during acute HIV infection. Sci Transl Med. 2020;12(533) doi: 10.1126/scitranslmed.aav3491. eaav3491PMID: 32132218PMCID: PMC7293182. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Salgado M, Rabi SA, O'Connell KA, Buckheit RW, 3rd, Bailey JR, Chaudhry AA. Prolonged control of replication-competent dual- tropic human immunodeficiency virus-1 following cessation of highly active antiretroviral therapy. Retrovirology. 2011;8:97. doi: 10.1186/1742-4690-8-97. PMID: 22141397PMCID: PMC3293762. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Sáez-Cirión A, Bacchus C, Hocqueloux L, Avettand-Fenoel V, Girault I, Lecuroux C. Post-treatment HIV-1 controllers with a long-term virological remission after the interruption of early initiated antiretroviral therapy ANRS VISCONTI Study. PLoS Pathog. 2013;9(3) doi: 10.1371/journal.ppat.1003211. Epub 2013 Mar 14. PMID: 23516360PMCID: PMC3597518. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Namazi G, Fajnzylber JM, Aga E, Bosch RJ, Acosta EP, Sharaf R. The control of HIV after antiretroviral medication pause (CHAMP) study: posttreatment controllers identified from 14 clinical studies. J Infect Dis. 2018;218(12):1954–1963. doi: 10.1093/infdis/jiy479. PMID: 30085241PMCID: PMC6217727. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Denton PW, Long JM, Wietgrefe SW, Sykes C, Spagnuolo RA, Snyder OD, Perkey K, Archin NM, Choudhary SK, Yang K, Hudgens MG, Pastan I, Haase AT, Kashuba AD, Berger EA, Margolis DM, Garcia JV. Targeted cytotoxic therapy kills persisting HIV infected cells during ART. PLoS Pathog. 2014;10(1) doi: 10.1371/journal.ppat.1003872. Epub 2014 Jan 9. PMID: 24415939PMCID: PMC3887103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Hansen SG, Piatak M, Jr, Ventura AB, Hughes CM, Gilbride RM, Ford JC. Immune clearance of highly pathogenic SIV infection. Nature. 2013;502(7469):100–104. doi: 10.1038/nature12519. Epub 2013 Sep 11. Erratum in: Nature. 2014 Oct 30;514(7524):654. Erratum in: Nature. 2017 Jul 6;547(7661):123-124. PMID: 24025770PMCID: PMC3849456. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Riley JL, Montaner LJ. Cell-mediated immunity to target the persistent human immunodeficiency virus reservoir. J Infect Dis. 2017;215(suppl_3):S160–S171. doi: 10.1093/infdis/jix002. PMID: 28520969PMCID: PMC5853458. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Grobben M, Stuart RA, van Gils MJ. The potential of engineered antibodies for HIV-1 therapy and cure. Curr Opin Virol. 2019;38:70–80. doi: 10.1016/j.coviro.2019.07.007. Epub 2019 Aug 15. PMID: 31421319. [DOI] [PubMed] [Google Scholar]
- 79.Kessing CF, Nixon CC, Li C, Tsai P, Takata H, Mousseau G, Ho PT, Honeycutt JB, Fallahi M, Trautmann L, Garcia JV, Valente ST. In vivo suppression of HIV rebound by didehydro-cortistatin A, a "block-and-lock" strategy for HIV-1 treatment. Cell Rep. 2017;21(3):600–611. doi: 10.1016/j.celrep.2017.09.080. PMID: 29045830PMCID: PMC5653276. [DOI] [PMC free article] [PubMed] [Google Scholar]