Abstract
Among all cancer types, colorectal cancer is the third most common in men and the second most common in women globally. Generally, the risk of colorectal cancer increases with age, and colorectal cancer is modulated by various genetic alterations. Alterations in the immune response serve a significant role in the development of colorectal cancer. In primary cancer types, immune cells express a variety of inhibitory molecules that dampen the immune response against tumor cells. Additionally, few reports have demonstrated that classical chemotherapy promotes the immunosuppressive microenvironment in both tissues and immune cells. This study assessed the expression levels of genes using RT-qPCR associated with the immune system, including interferon-γ, programmed death-1, β2-microglobulin, human leukocyte antigen-A, CD3e, CD28 and intracellular adhesion molecule 1, in patients with colorectal cancer, as these genes are known to serve important roles in immune regulation during cancer incidence. Gene expression analysis was performed with the whole blood cells of patients with colorectal cancer and healthy volunteers. Compared with the normal controls, programmed death-1was highly expressed in patients with advanced-stage colorectal cancer. Furthermore, the expression of programmed death-1 was higher in patients receiving adjuvant therapy, which suggests the therapy dampened the immune response against tumor cells. The results of the present study indicate that classical adjuvant therapies, which are currently used for patients with colorectal cancer, should be modulated, and a combination of classical therapy with anti-programmed death-1 antibody should be conducted for improved management of patients with colorectal cancer.
Keywords: colorectal cancer, adjuvant therapy, immune suppression, circulating cells, programmed death-1
Introduction
Colorectal cancer (CRC) is the third most common cause of cancer and cancer-associated mortality worldwide.1 The CRC incidence rate is rapidly increasing in countries with a medium to high human development index (HDI), such as countries in Asia, Eastern Europe, and South America. While the mortality rate is declining in very high HDI countries, such as Australia, and Northern America and Western European countries.2 CRC affects both men and women with 746, 000, and 614,000 cases reported per year, respectively.3 Data collected from the Saudi Cancer Registry revealed that CRC has been the most commonly diagnosed malignancy among men and the third most common among women in Saudi Arabia since 2002.4
The survival rate for patients with CRC varies depending on the stage of the tumor growth; the rate is 90% for a localized tumor and <15% for an advanced metastatic tumor.5,6 The incidence of CRC increases with age; it can be detected in young individuals but increases dramatically in individuals >50 years old. Lifestyle habits, including smoking, poor diet, obesity, and heavy alcohol consumption, have all been reported to increase the risk of CRC.7
It is well understood that the host’s immune cells can detect and eliminate tumors, which relies on the recognition and elimination of newly formed cancer cells in a process termed immunosurveillance.8 The understanding of the association between the immune system and cancer cells has encouraged researchers worldwide to develop novel vaccines that boost the patients’ own immune cells to specifically kill cancer cells.9 Immunotherapeutic strategies include administering monoclonal antibodies (mAbs), which stimulate CD8+ T cells or block immune checkpoint inhibitors, injecting cytokines, peptide-based vaccines, and adoptive cell therapy of ex vivo activated T and natural killer cells.10-12 Although conventional cancer therapies, such as chemotherapy and radiotherapy, are commonly used to treat CRC, immunotherapy is emerging as an alternative powerful approach for treating advanced CRC. Some of these approaches are now in stage I-III clinical trials.13-15 The present study aimed to systemically examine RNA expression levels of selected immune system-associated genes in the whole blood, evaluate the associations with CRC progression, and find a biomarker for clinical use.
Materials and Methods
Patients and Blood Samples
The present study involved 84 male and female volunteers aged between 40 and 70 years. The patients with CRC (n = 50) consisted of 39 males and 11 females. The healthy control individuals (n = 34) included 23 males and 11 females. Samples were collected from King Abdulaziz University Hospital, and other hospitals from Jeddah, Saudi Arabia. For RNA extraction, 2 ml whole blood was collected in EDTA vacutainer tubes. The purpose of the research was explained and written informed consent was obtained from all individuals involved in the study. The General Directorate of Health Affairs in Jeddah approved this study, as did the unit of Biomedical Ethics at the Faculty of Medicine, King Abdulaziz University.
RNA Extraction and Complementary DNA (cDNA) Synthesis
RNA was extracted from the whole blood sample using the QIAamp RNA Blood Mini kit (Qiagen GmbH, Hilden, Germany; cat. no. 52304). Then, cDNA was synthesized from highly purified extracted RNA samples (300 ng, after normalizing with nuclease-free H2O) using a high capacity cDNA reverse transcription kit (Thermo Fisher Scientific, Inc., Waltham, MA, USA; cat. no. 4368814). RNAse inhibitor (Thermo Fisher Scientific, Inc.; cat. no. N8080119) was used with the cDNA reagent to prevent the degradation of RNA.
Primers Design
Primers for the qPCR reaction were designed to measure the expression levels of genes associated with the immune system. The primer sequences of the genes were obtained using the USCS browser (https://genome.ucsc.edu/index.html). mRNA sequences (only exons) were selected and primers were designed (Table 1) using the Primer3web tool (http://primer3.ut.ee/) after confirming all primer characteristics. Moreover, the sequences of the primers were confirmed in (in silico PCR) using the USCS browser.
Table 1.
Primers Used for the Analysis of Immune System-Associated Genes by Reverse Transcription-Quantitative PCR.
| Gene | Forward Sequence (5’-3’) | Reverse Sequence (5’-3’) |
|---|---|---|
| IFNγ | GAGTGTGGAGACCATCAAGGA | CAGTTCAGCCATCACTTGGA |
| PD-1 | AAACCCTGGTGGTTGGTGT | TTCTCTCGCCACTGGAAATC |
| β2 M | GTGCTCGCGCTACTCTCTCT | GTCAACTTCAATGTCGGATGG |
| CD3e | GGAGCAAGAATAGAAAGGCCA | TCAGGCCAGAATACAGGTCC |
| CD28 | CGGACCTTCTAAGCCCTTTT | ATAGGGCTGGTAATGCTTGC |
| HLA-A | GAGTGGCTCCGCAGATACC | CCAAAGAGAACTAGGCCAGC |
| ICAM1 | AACCTCAGCCTCGCTATGG | GATGACTTTTGAGGGGGACA |
IFNγ, interferon-γ; PD-1, programmed death-1; β2 M, β2-microglobulin; HLA-A, human leukocyte antigen-A; ICAM1, intracellular adhesion molecule 1.
Optimization of Immune Genes
The optimization experiments were performed using cDNAs of 30 subjects (20 patients with CRC and 10 controls) to check the quality, the design of primers, and qPCR conditions. After checking the melting curves for the 10 primers, 3 primers were excluded (CD273, CD274, and CTLA4) as they exhibited a primer-dimer or >1 PCR product. Subsequently, the qPCR experiments were performed with the 7 genes (n = 84), as they demonstrated a high quality during the optimization experiments. RPL11 was used as a housekeeping gene to calculate and normalize the relative expression fold change for the target genes using DDCT method.
qPCR
For qPCR, 2(-Delta Delta C(T) method was followed.16 Universal 2X PowerUpTM SYBRTM Green Master mix (cat. no. A25741; Thermo Fisher Scientific, Inc.) was used for the qPCR experiments, according to the manufacturer’s protocol. To perform qPCR, 10 µL SYBR Green master mix, 8µl nuclease-free water, 1µL specific (both the forward and reverse) primer and 1µL cDNA were mixed together to create a final volume of 20 μL/well. Bio-Rad CFX Connect™ Real-Time PCR Detection system was used to perform the reaction. The appropriate volume of reaction was transferred to the optical plate and sealed using an adhesive cover. The applied thermal cycler condition settings were dependent on the primer melting temperature, which was <60°C for all primers used in the study. REST 2009 software (QIAGEN, version 1) was used for the analysis of RT-qPCR gene expression data.
Statistical Analysis
A 2-tailed P < 0.05 was considered to indicate a statistically significant difference. Statistical analysis of the data was performed using GraphPad Prism (version 6; GraphPad Software, Inc., La Jolla, CA, USA) and graphically represented. Data are presented as the mean ± standard error of the mean. Mann-Whitney U test was used to compare the differences between 2 non-parametric groups.
Results
Expression of Immune System-Associated Genes in Patients With Different Stages of CRC
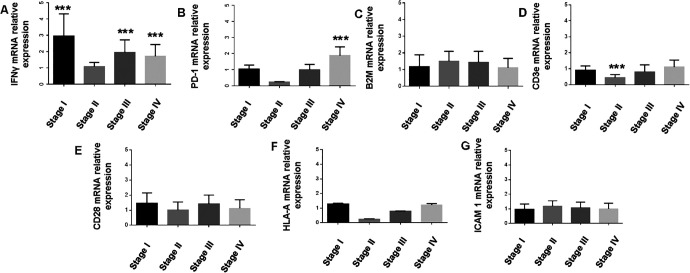
The present study selected 7 genes (Table SI) that are known to serve a key role in immune regulation in cancer, including interferon-γ (IFNγ), programmed death-1 (PD-1), β2-microglobulin (β2 M), human leukocyte antigen-A (HLA-A), CD3e, CD28 and intracellular adhesion molecule 1 (ICAM1). A whole blood sample was collected from patients with different stages of CRC and analyzed for gene expression. There was no significant difference in CD28, HLA-A, ICAM-1, and β2 M gene expression (Figure 1). CD3e expression was similar in tumor stages I, III, and IV (Figure 1). However, CD3e gene expression was markedly lower in patients with stage II CRC compared with the other stages (Figure 1). IFNγ gene expression was variable between the groups, with remarkable higher expression in patients with stage I, III, and IV compared with the control group (Figure 1). A lower level of IFNγ gene expression was detected in the samples obtained from patients with stage II CRC compared with other stages (Figure 1). Notably, the level of PD-1 expression was associated with tumor stage. PD-1 gene expression was significantly increased in stage IV compared with stages II and III (Figure 1).
Figure 1.
Relative expression levels of 7 immune system-associated genes in CRC patients compared with healthy controls, as analyzed by RT-qPCR and values are expressed as fold change. (A) IFNγ was significantly upregulated in patients with stage I (P = 0.004), III (P = 0.009) and IV (P = 0.01) CRC. (B) PD-1 was significantly upregulated in patients with stage IV CRC. (C) No significant difference was identified in the expression of β2 M. (D) CD3e was downregulated in patients with stage II CRC. No significant differences were identified in the expression levels of (E) CD28, (F) HLA-A and (G) ICAM1. ***P≤0.01; CRC, colorectal cancer; RT-qPCR, reverse transcription-quantitative PCR; IFNγ, interferon-γ; PD-1, programmed death-1; β2 M, β2-microglobulin; HLA-A, human leukocyte antigen-A; ICAM1, intracellular adhesion molecule 1.
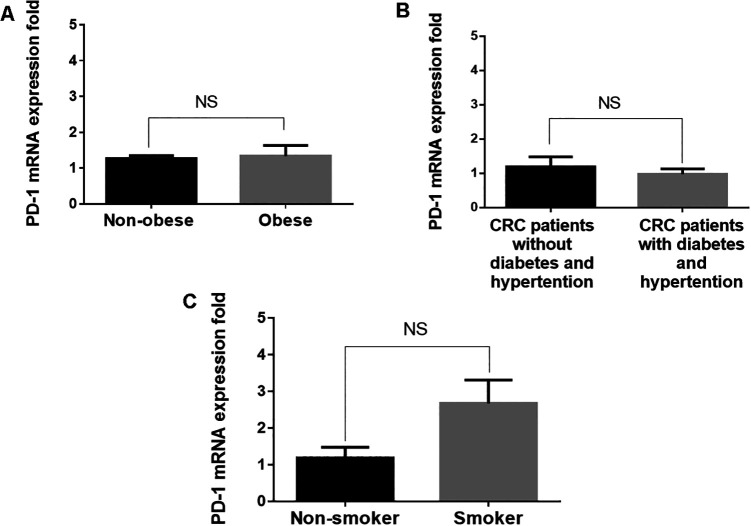
PD-1 Expression Is Not Associated With Obesity, Hypertension or Diabetes
PD-1/programmed death ligand-1 (PD-L1) interaction serves a major role in immune evasion17; therefore, the present study further investigated the correlation of PD-1 gene expression level with obesity, hypertension, and diabetes in patients with CRC. The results demonstrated that obesity was not associated with PD-1 expression. Both obese and non-obese patients expressed similar levels of PD-1 gene expression (Figure 2). Hypertension and diabetes were also not associated with PD-1 expression. Patients with CRC with hypertension and diabetes exhibited a similar pattern of PD-1 gene expression to patients without hypertension and diabetes (Figure 2). By contrast, PD-1 expression was higher, although insignificantly, in smokers compared with non-smokers (Figure 2).
Figure 2.
Comparison of PD-1 expression in patients with CRC with different characteristics by RT-qPCR. Values are expressed as fold change. No significant differences were identified in the relative expression of PD-1 in (A) patients with and without obesity, (B) patients with and without diabetes or hypertension, and (C) non-smokers and smokers. ***P≤0.01; NS, not significant. PD-1, programmed death-1; CRC, colorectal cancer; RT-qPCR, reverse transcription-quantitative PCR.
PD-1 Expression Increases During Therapy
Next, the present study assessed PD-1 gene expression in CRC patients receiving anticancer drugs (Table 2) and compared it with untreated patients. The results revealed that PD-1 expressed was significantly higher in patients receiving treatment compared with untreated patients (P≤0.01; Figure 3).
Table 2.
Patient Pathophysiological Characteristics.
| Characteristic | Number of patients (n = 50) |
|---|---|
| Sex, male: female (%) | 39 (78):11 (22) |
| Age, mean (range) | 56.94 (36-85) |
| Primary location, colon: rectum (%) | 44 (88):6 (12) |
| Smoking, yes: no (%) | 18 (36):32 (64) |
| Colorectal cancer stage | |
| 0 | 0 |
| I | 6 |
| IIA | 1 |
| IIB | 3 |
| IIC | 2 |
| IIIA | 5 |
| IIIB | 8 |
| IIIC | 0 |
| IVA | 8 |
| IVB | 14 |
| IVC | 3 |
| Medical history | |
| Diabetes | 5 |
| Hypertension | 4 |
| Hypertension-diabetes | 5 |
| Allergy | 1 |
| Hypertension-allergy | 1 |
| Thyroid dysfunction | 1 |
| Osteoporosis | 3 |
| Other | 4 |
| None | 26 |
| Anticancer therapy | |
| Xelox | 4 |
| Folfox | 2 |
| Folfiri | 1 |
| Xeloda | 2 |
| Xeloda, Xelox | 15 |
| Xeloda, Xeliri | 1 |
| Xeloda, Xelox, Xeliri | 1 |
| Xeloda, Xelox, Bevacizumab | 1 |
| Xeloda, Xelox, Xeliri, Bevacizumab | 3 |
| Xeloda, Xelox, Xeliri, Bevacizumab, Folfox | 1 |
| Xeloda, Xelox, Xeliri, Bevacizumab, Folfiri | 1 |
| Xelox, Regorafenib, Folfiri | 1 |
| CRT, Xelox | 2 |
| CRT, Xeloda | 1 |
| Other | 8 |
| None | 6 |
Figure 3.
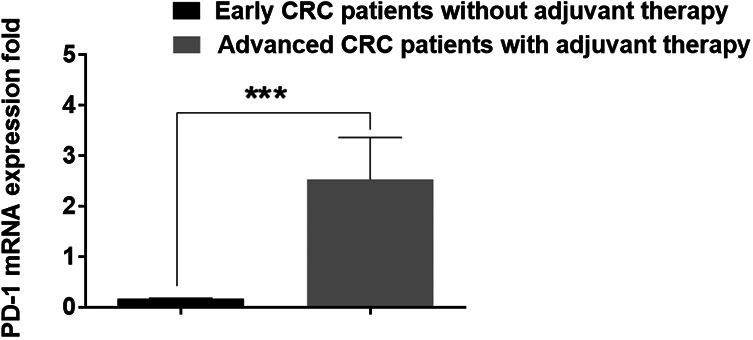
Comparison of PD-1 expression in patients with advanced CRC with and without adjuvant therapy by RT-qPCR. Values are expressed as fold change. PD-1 expression was significantly higher in patients with advanced CRC during adjuvant therapy compared with patients without adjuvant therapy. ***P≤0.01. PD-1, programmed death-1; CRC, colorectal cancer; RT-qPCR, reverse transcription-quantitative PCR.
Correlation of Clinical Parameters With PD-1 Expression in Patients With CRC
The current study selected a total of 50 patients with CRC according to the inclusion criteria (Table 2). Among all selected patients, 78% were male and 22% were female. In addition, 36% of patients had a history of smoking and 64% did not. In 88% of the patients, the primary location of cancer was the colon, and in the remaining 12%, the primary location was the rectum. Furthermore, 52% of the patients had no history of other diseases and 48% of the patients had a history of at least one other disease, such as diabetes, hypertension, allergies, thyroid dysfunction, and osteoporosis. Comparisons between physical characteristics of the patients with CRC and the controls were performed, and the results analyzed by a t-test revealed that there was a significant difference in age between the patients and controls (P = 0.022). In addition, weight (P = 0.002) and BMI (P = 0.001) demonstrated a significant difference between the 2 groups. However, there was no significant difference in height and waist/hip ratio (Table 3).
Table 3.
Comparison of Patient and Control Groups Regarding Physical Characteristics (***p ≤ 0.01; **p ≤ 0.02; *p ≤ 0.03).
| Characteristic | Controls (n = 34) | Patients (n = 50) | P-value |
|---|---|---|---|
| Age, years | 51.85 ± 1.57 | 56.94 ± 1.76 | 0.02** |
| Height, cm | 165.55 ± 1.41 | 166.1 ± 1.32 | 0.40 |
| Weight, kg | 85.26 ± 2.51 | 75.78 ± 2.08 | 0.00*** |
| BMI, kg/m2 | 31.3 ± 1.06 | 27.54 ± 0.74 | 0.00*** |
| Waist/Hip ratio | 0.97 ± 0.02 | 0.91 ± 0.02 | 0.03 |
Discussion
Colorectal cancer is one of the leading causes of cancer-associated mortality worldwide. Despite advances in medical technologies, the survival rate for patients with advanced CRC remains low. The interaction between PD-1 on activated T cells with its ligands PD-L1/L2 leads to T cell exhaustion. Different tumor cells, including CRC cells, have been reported to express PD-L1 as a major mechanism to escape immune destruction.18-20 To overcome this obstacle, mAbs have been developed to interfere with the PD-1/PD-L1 signaling pathway and restore antigen-specific CD8+ T cell proliferation and effector functions.17 Certain mAb-based drugs, including nivolumab and pembrolizumab, were approved by the Food and Drug Administration in 2014.21 However, the outcome following treatment with these drugs is variable among patients. A recent study by Kallergi et al 22 demonstrated that high levels of PD-L1+ circulating tumor cells in patients with advanced non-small cell lung cancer before treatment resulted in poor clinical outcomes. Similar results were observed by Guibert et al. 23 Several studies have reported that sensitive detection of PD-L1 expression in circulating or epithelial tumor cells could be a potential strategy to select patients eligible for treatment with anti-PD-1/L1 antibodies in early and late tumor stages.23-25
Previous studies have confirmed the expression of PD-L1 on circulating tumor cells, including head and neck, non-small cell lung cancer, and breast cancer cells26-28; however, to the best of our knowledge, PD-1 expression on circulating CRC cells remains to be investigated.
The present study is the first clinicopathological study to investigate the expression of PD-1 in patients with solid CRC tumors using whole blood samples and RT-qPCR. It was revealed that PD-1 expression was associated with tumor stage. A significantly higher mRNA expression level was detected in patients with stage IV CRC compared with earlier stages (Figure 3). This could be due to a higher number of activated lymphocytes expressing PD-1 receptor in the tumor and circulation. The correlation between a high lymphocyte number and the stage of the tumor was reported by Matkowski et al 29 in a breast cancer model; their data showed that a higher number of tumor-infiltrating CD4+ and CD8+ T cells in breast cancer resulted in a worse cancer-specific overall survival compared with patients with a lower number of tumor-infiltrating lymphocytes. The ligation of PD-L1 on tumor cells with PD-1 co-inhibitory receptor on tumor antigen-specific T cells inhibits effector functions, which allows tumor cells to grow more rapidly. This may explain the correlation between higher PD-1 expression in patients with late-stage CRC and the tumor size from this study and opens a window to potential biomarkers.
Notably, factors, including obesity, smoking, and hypertension, were not associated with PD-1 expression in the blood of patients with CRC (Figure 2). The expression level of PD-1 was almost identical in obese and non-obese CRC patients and similar results were shown for patients with and without diabetes and hypertension (Figure 2). These results indicated that obesity and hypertension may not influence the expression level of PD-1. However, examining PD-1 expression in tumor-infiltrating lymphocytes in these populations, having obesity and hypertension may be required before any conclusions can be made. By contrast, higher PD-1 expression was detected in smokers compared with non-smokers (Figure 2). Further studies may be required to verify the effect of smoking on the expression level of PD-1.
This is a pilot study. Even though the finding of this study is significant, there are still some limitations. Firstly, this is an investigation on CRC patients, and due to the unavailability of MSI/MSS status, their correlation with the relative expression profile of these immune genes is absent. Furthermore, the inadequate sample size may affect the accuracy of the results to some extent and make some confounding bias. Future studies will require to separate the cells from whole blood and look at the expression level of this biomarker in different cell types using other methods (e.g., high dimensional flow cytometry, deep RNA profiling by RNAseq, and mass cytometry). Hence, the outcomes of this study need further validation in a larger cohort.
Supplemental Material
Supplemental Material, TCRT-20-0339.R1_Supplimentary_file_blinded for High Expression of Pd-1 in Circulating Cells of Patients With Advanced Colorectal Cancer Receiving Adjuvant Therapy by Muhammed A. Bakhrebah, Mohammad Nasrullah, Wesam H. Abdulaal, Mohammed A. Hassan, Halima Siddiqui, Huda A. Al Doghaither, Ulfat M. Omar, Nawal Helmi, Mohannad M. Fallatah, Ayat B. Al-Ghafari, Mohammad Imran Khan and Hani Choudhry in Technology in Cancer Research & Treatment
Acknowledgments
The authors acknowledge the laboratory facility of Cancer and Mutagenesis Unit, King Fahd Medical Research Center, and support from the Deanship of Scientific Research, King Abdulaziz University. Also, the authors thank King Abdulaziz City for Science and Technology (KACST) for supporting this research.
Abbreviations
- β2 M
beta2-microglobulin
- CD4+
Helper T cell
- CD8+
Cytotoxic T cell
- HDI
Human development index
- HLA-A
Human leukocyte antigen-A
- cDNA
Complementary DNA
- CRC
Colorectal cancer
- ICAM1
Intracellular adhesion molecule 1
- IFNγ
including interferon-γ
- mAbs
monoclonal antibodies
- MSI
Microsatellite Instable
- MSS
Microsatellite Stable
- PD-1
Programmed death-1
- PDL-1
Programmed death ligand-1
- RPL11
Ribosomal Protein L11.
Footnotes
Author Contributions: Muhammed A. Bakhrebah and Mohammad Nasrullah contributed equally to this work. MAB, MN and HC: concept and design of the work; MN, MAH, HD, AAG, and: data collection; MN, MAH, HC, and MIK: data analysis and interpretation; MN, HC, and MIK: drafting the article; WHA, HAA, UMO, NH, MMF, MIK, and HC: critical revision of the article; MN, MIK, and HC: final approval.
Declaration of Conflicting Interests: The author(s) declared the following potential conflicts of interest with respect to the research, authorship, and/or publication of this article: The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Ethics Statement: This study was approved by the General Directorate of Health Affairs in Jeddah, Saudi Arabia (Approval Number A00221). The aim of the present study was explained to all participants, following which a written informed consent was obtained.
Funding: The author(s) disclosed receipt of the following financial support for the research, authorship, and/or publication of this article: This project was funded by the Deanship of Scientific Research (DSR) at King Abdulaziz University (KAU), Jeddah, Saudi Arabia under Grant Number (G: 638-130-38).
ORCID iD: Mohammad Nasrullah  https://orcid.org/0000-0003-1346-355X
https://orcid.org/0000-0003-1346-355X
Supplemental Material: Supplemental material for this article is available online.
References
- 1. Siegel RL, Miller KD, Jemal A. Cancer statistics, 2016. CA Cancer J Clin. 2016;66(1):7–30. doi:10.3322/caac.21332 [DOI] [PubMed] [Google Scholar]
- 2. Center MM, Jemal A, Smith RA, Ward E. Worldwide variations in colorectal cancer. CA Cancer J Clin. 2009;59(6):366–378. doi:10.3322/caac.20038 [DOI] [PubMed] [Google Scholar]
- 3. Tariq K, Ghias K. Colorectal cancer carcinogenesis: a review of mechanisms. Cancer Biol Med. 2016;13(1):120–135. doi:10.28092/j.issn.2095-3941.2015.0103 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Alsanea N, Abduljabbar AS, Alhomoud S, Ashari LH, Hibbert D, Bazarbashi S. Colorectal cancer in Saudi Arabia: incidence, survival, demographics and implications for national policies. Ann Saudi Med. 2015;35(3):196–202. doi:10.5144/0256-4947.2015.196 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Herrmann R. Systemic treatment of colorectal cancer. Eur J Cancer. 1993;29(4):583–586. doi:10.1016/S0959-8049(05)80156-X [DOI] [PubMed] [Google Scholar]
- 6. Kalyan A, Kircher S, Shah H, Mulcahy M, Benson A. Updates on immunotherapy for colorectal cancer. J Gastrointest Oncol. 2018;9(1):160–169. doi:10.21037/jgo.2018.01.17 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Akhtar R, Chandel S, Sarotra P, Medhi B. Current status of pharmacological treatment of colorectal cancer. World J Gastrointest Oncol. 2014;6(6):177–183. doi:10.4251/wjgo.v6.i6.177 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Farkona S, Diamandis EP, Blasutig IM. Cancer immunotherapy: the beginning of the end of cancer? BMC Med. 2016;14(1):73 doi:10.1186/s12916-016-0623-5 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Rosenberg SA, Restifo NP, Yang JC, Morgan RA, Dudley ME. Adoptive cell transfer: a clinical path to effective cancer immunotherapy. Nat Rev Cancer. 2008;8(4):299–308. doi:10.1038/nrc2355 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Dine J, Gordon R, Shames Y, Kasler M, Barton-Burke M. Immune checkpoint inhibitors: an innovation in immunotherapy for the treatment and management of patients with cancer. Asia-Pacific J Oncol Nurs. 2017;4(2):127 doi:10.4103/apjon.apjon_4_17 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Lee S, Margolin K. Cytokines in cancer immunotherapy. Cancers (Basel). 2011;3(4):3856–3893. doi:10.3390/cancers3043856 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Pernot S, Terme M, Voron T, et al. Colorectal cancer and immunity: what we know and perspectives. World J Gastroenterol. 2014;20(14):3738 doi:10.3748/wjg.v20.i14.3738 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Das S, Allen A, Berlin J. Immunotherapy after immunotherapy: response rescue in a patient with microsatellite instability-high colorectal cancer post-pembrolizumab. Clin Colorectal Cancer. 2020;19(2). doi:10.1016/j.clcc.2020.02.006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Payandeh Z, Khalili S, Somi MH, et al. PD-1/PD-L1-dependent immune response in colorectal cancer. J Cell Physiol. 2020;235(7-8):5461–5475. doi:10.1002/jcp.29494 [DOI] [PubMed] [Google Scholar]
- 15. Lee JJ, Chu E. Recent advances in the clinical development of immune checkpoint blockade therapy for mismatch repair proficient (pMMR)/non-MSI-H metastatic colorectal cancer. Clin Colorectal Cancer. 2018;17(4):258–273. doi:10.1016/j.clcc.2018.06.004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Livak KJ, Schmittgen TD. Analysis of relative gene expression data using real-time quantitative PCR and the 2−ΔΔCT method. Methods. 2001;25(4):402–408. doi:10.1006/meth.2001.1262 [DOI] [PubMed] [Google Scholar]
- 17. Alsaab HO, Sau S, Alzhrani R, et al. PD-1 and PD-L1 Checkpoint signaling inhibition for cancer immunotherapy: mechanism, combinations, and clinical outcome. Front Pharmacol. 2017;8:561 doi:10.3389/fphar.2017.00561 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Masugi Y, Nishihara R, Yang J, et al. Tumour CD274 (PD-L1) expression and T cells in colorectal cancer. Gut. 2017;66(8):1463–1473. doi:10.1136/gutjnl-2016-311421 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Davick JJ, Frierson HF, Smolkin M, Gru AA. PD-L1 expression in tumor cells and the immunologic milieu of bladder carcinomas: a pathologic review of 165 cases. Hum Pathol. 2018;81:184–191. doi:10.1016/j.humpath.2018.06.028 [DOI] [PubMed] [Google Scholar]
- 20. Dix Junqueira Pinto G, de Souza Viana L, Scapulatempo Neto C, Vicente Serrano S. Evaluation of PD-L1 expression in tumor tissue of patients with lung carcinoma and correlation with clinical and demographic data. J Immunol Res. 2016;2016:1–12. doi:10.1155/2016/9839685 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Gong J, Chehrazi-Raffle A, Reddi S, Salgia R. Development of PD-1 and PD-L1 inhibitors as a form of cancer immunotherapy: a comprehensive review of registration trials and future considerations. J Immunother Cancer. 2018;6(1):8 doi:10.1186/s40425-018-0316-z [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Kallergi G, Vetsika E-K, Aggouraki D, et al. Evaluation of PD-L1/PD-1 on circulating tumor cells in patients with advanced non-small cell lung cancer. Ther Adv Med Oncol. 2018;10:175883401775012 doi:10.1177/1758834017750121 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Guibert N, Delaunay M, Lusque A, et al. PD-L1 expression in circulating tumor cells of advanced non-small cell lung cancer patients treated with nivolumab. Lung Cancer. 2018;120:108–112. doi:10.1016/j.lungcan.2018.04.001 [DOI] [PubMed] [Google Scholar]
- 24. Schott DS, Pizon M, Pachmann U, Pachmann K. Sensitive detection of PD-L1 expression on circulating epithelial tumor cells (CETCs) could be a potential biomarker to select patients for treatment with PD-1/PD-L1 inhibitors in early and metastatic solid tumors. Oncotarget. 2017;8(42):72755–72772. doi:10.18632/oncotarget.20346 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Yue C, Jiang Y, Li P, et al. Dynamic change of PD-L1 expression on circulating tumor cells in advanced solid tumor patients undergoing PD-1 blockade therapy. Oncoimmunology. 2018;7(7):e1438111 doi:10.1080/2162402X.2018.1438111 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Nicolazzo C, Raimondi C, Mancini M, et al. Monitoring PD-L1 positive circulating tumor cells in non-small cell lung cancer patients treated with the PD-1 inhibitor Nivolumab. Sci Rep. 2016;6(1):31726 doi:10.1038/srep31726 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Kulasinghe A, Perry C, Kenny L, Warkiani ME, Nelson C, Punyadeera C. PD-L1 expressing circulating tumour cells in head and neck cancers. BMC Cancer. 2017;17(1):333 doi:10.1186/s12885-017-3316-3 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Mazel M, Jacot W, Pantel K, et al. Frequent expression of PD-L1 on circulating breast cancer cells. Mol Oncol. 2015;9(9):1773–1782. doi:10.1016/j.molonc.2015.05.009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Matkowski R, Gisterek I, Halon A, et al. The prognostic role of tumor-infiltrating CD4 and CD8 T lymphocytes in breast cancer. Anticancer Res. 2009;29(7):2445–2451. Accessed March 2, 2019 http://www.ncbi.nlm.nih.gov/pubmed/19596912 [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplemental Material, TCRT-20-0339.R1_Supplimentary_file_blinded for High Expression of Pd-1 in Circulating Cells of Patients With Advanced Colorectal Cancer Receiving Adjuvant Therapy by Muhammed A. Bakhrebah, Mohammad Nasrullah, Wesam H. Abdulaal, Mohammed A. Hassan, Halima Siddiqui, Huda A. Al Doghaither, Ulfat M. Omar, Nawal Helmi, Mohannad M. Fallatah, Ayat B. Al-Ghafari, Mohammad Imran Khan and Hani Choudhry in Technology in Cancer Research & Treatment