Abstract
Background:
Cervical lymph node (LN) metastasis of papillary thyroid carcinoma (PTC) is critical for treatment and prognosis. To examine the feasibility of MRI radiomics to preoperatively predict cervical LN metastasis in patients with PTC.
Methods:
Between January 2015 and March 2018, a total of 61 patients with pathologically confirmed PTC were analyzed retrospectively. The patients were divided into cervical LN metastasis group (n = 37) and no cervical LN metastasis (n = 24). T2WI and T2WI-fat-suppression (T2WI-FS) images were collected. A number of radiomic features were automatically extracted from the largest section of tumor. Three types of classifier (the random forests, the support vector machine classifier and the generalized linear model) based on T2WI and T2WI-FS images of cervical LN metastasis and no cervical LN metastasis were constructed and evaluated with a nested cross-validation scheme.
Results:
Radiomic features extracted from T2WI images were more discriminative than T2WI-FS images. The random forests model showed the best discriminate performance with the highest area under the curve (0.85, CI:0.76 -1), accuracy (0.87), sensitivity (0.83), specificity (1.00), positive predictive value (PPV = 1.00) and negative predictive value (NPV = 0.88).
Conclusion:
MRI radiomics analysis based on conventional T2WI and T2WI-FS can predict cervical LN metastasis in patients with PTC, and the radiomics is shown to be an assistant diagnosis tool for radiologists.
Keywords: thyroid cancer, lymphatic metastasis, magnetic resonance imaging, forecasting, feasibility studies
Introduction
Thyroid cancer is one of the most common malignancy and its incidence has increased in recent years.1,2 Papillary thyroid carcinoma (PTC), the most common histologic type of thyroid cancer, accounts for approximately 90% of all thyroid malignancies.3-5 Most PTCs show a slow growth rate and excellent outcomes after surgery, however, some PTCs show aggressive behavior and recurrence.6-8 The risk factors include male sex, extrathyroidal extension, cervical lymph node(LN) and tumor size.9 Recent studies showed that PTC has a high incidence of metastasis to the cervical LNs, accounting for approximately 30%-80%.10,11 Cervical LN metastasis is one of the most important risk factors for locoregional recurrence,7 and probably means a second or more time surgical resection, which will seriously impact the life quality of patients. Therefore, cervical LN metastasis is a major issue that concerns clinicians and accurate identification of LN metastasis before resection is the key to prevent recurrence.
According to the American Thyroid Association (ATA), when patients with known or suspected thyroid nodules, cervical LN exploration should be performed before thyroid operation.4 Imaging modalities for thyroid nodules evaluation include ultrasound (US), computed tomography (CT), magnetic resonance imaging (MRI), positron emission tomography (PET) and scintigraphy.12 US examination is a popular technique for assessment of thyroid nodules, owing to being in real time, convenience, radiation-free, non-invasive and inexpensive, but limited to instrument resolution, operator’s experience, and degree of detail operation, moreover, assessing parapharyngeal and level VII LN is inadequate,13 which seriously affects the accuracy of the assessment. Compared with US, MRI and CT are more objective in the diagnosis of cervical LN metastasis. However, prior studies have shown that the diagnostic accuracy of CT and MRI have failed to prove its benefit over US for predicting LN metastasis.14,15 Biopsy is the best way to confirm whether a LN is involved or not, however, it is invasive, risk of infection expensive and time-consuming.16 Therefore, looking for more objective assessment methods rather than biopsy is a key point for clinical work.
The diagnosis of pathological diseases such as PTC based on radiological images by doctors is subjective, many relevant information can not to be recognized by the human visual system. During the past decades, medical imaging and its post processing methods were innovated with the development of computer science and the establishment of large databases, radiomics emerged and quantitative imaging.17 Being different from the traditional megascopic analysis based on experiences, radiomics is a machine learning based technique that detects pathological changes that cannot be perceived by the human eye, using an automated high-throughput extraction of large amounts of quantitative features of medical images and to draw statistical information18 . Previous studies have proved the value of radiomics in predicting LN metastasis, gene expression, treatment responses and survival of cancer.19-21 However, to our knowledge, MRI radiomics approach has not been used in predicting LN metastasis of PTC.
The current study aimed to explore whether radiomics could be a potential method in predicting LN metastasis of PTC with quantitative features extracted from MRI images and compare the performances of different machine learning approaches and imaging modalities.
Materials and Methods
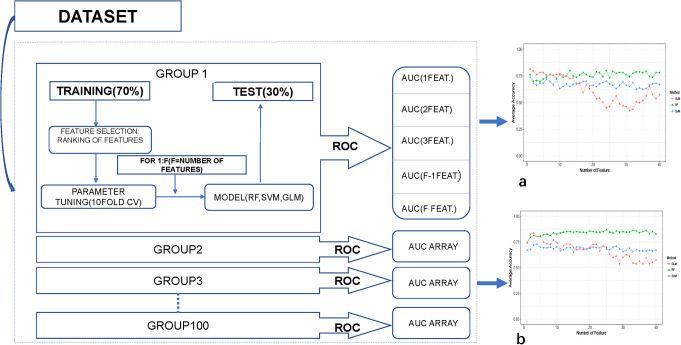
A summary of the workflow is shown in Figure 1.
Figure 1.
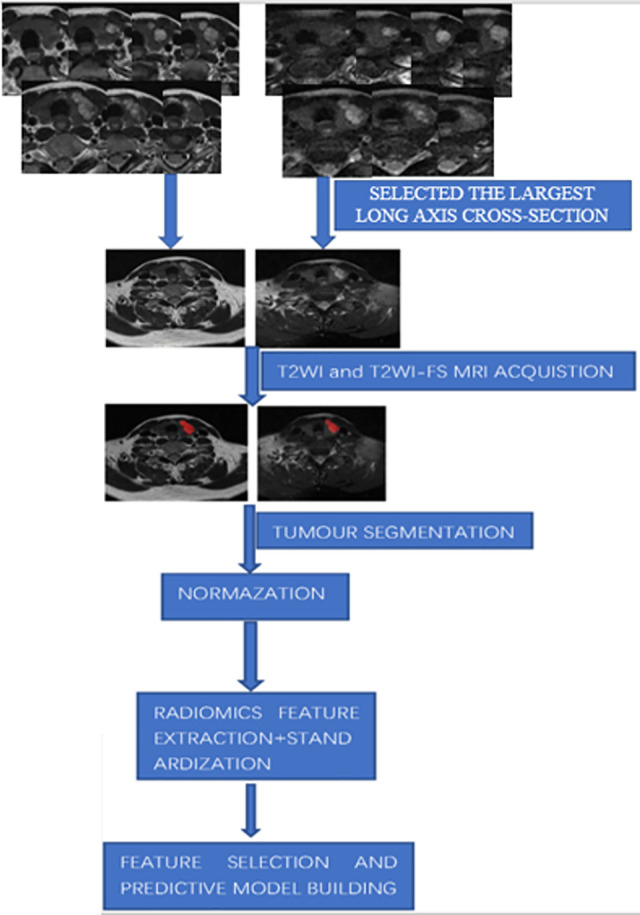
Overall workflow of the study.
Patients
Ethics committee in our hospital approved this retrospective study, and the requirement for informed consent was waived. A total of 87 consecutive patients in our institution with pathologically confirmed PTC who had undergone MR imaging in the immediate preoperative period from January 2015 to March 2018 were retrospectively evaluated.
Inclusion criteria were: (1) Each patient underwent neck LNs dissection with ipsilateral lobectomy or total thyroidectomy; (2) Patients should be pathologically confirmed as PTC; (3) No previous treatment, biopsy or surgical resection on thyroid. Exclusion criteria were: (1) PTC diameter ≤5 mm, the region of interest (ROI) method has lower accuracy; (2) The MRI images were too indistinct to draw a ROI on the tumor. Ultimately, 61 patients (15 men and 46 women, mean age 46.63 years, age range 24-74 years) were included in the present study. Patients were divided into 2 groups according to the pathological results of the operation: 37 (60.66%) patients had cervical LN metastasis, 24(39.34%) patients did not metastasis.
MR Image Acquisition
All examinations were performed on 1.5 Tesla MR scanner (GE Signa HD 1.5 T MR scanner; GE Healthcare Systems, Milwaukee, WI, USA) with an eight-channel high-resolution receiver synergy-head/neck phased-array coil. The applied MRI protocols consisted of T1WI, T2WI, DWI, and contrast-enhanced MRI. The spin echo based T1WI sequence (repetition time (TR)/ echo time (TE) = 520/14 ms) in axial view, the fast spin echo based T2WI sequence (TR/TE = 3500/95 ms) with and without fat suppression in axial and coronal views were employed for imaging acquisitions. DWI was acquired at 2 different b values (0 and 500 s/mm2) using the STIR fat-suppressed single-shot echo planar imaging spin echo sequences in 3 orthogonal directions. The shimming adjustment has also been properly applied in the thyroid region prior to image acquisition, so that the neck area which is sensitive to local field in homogeneities can be visualized with accepted image quality. Of all patients, contrast enhanced T1WI (TR/TE = 520/14 ms) were obtained with or without fat-suppression immediately after the intravenous injection of 0.1mmol/kg Gd-DTPA at an injection speed of 1.5 mL/s (Magnevist, Schering AG, Germany). The parameters were as follows: section thickness 3 mm, with a 1-mm intersection gap, field of view (FOV) = 40 × 28 cm2, matrix = 256 × 256, NEX = 4. The whole examination was completed within 30 minutes.
Regions of Interest Segmentation
The slices of T2WI and T2WI-FS including the largest long axis cross-section were exported into ITK-SNAP software (version 3.6.0, www.itksnap.org), and PTC was manually segmented by drawing a ROI along the tumor edge (Figure 1). Before we segmented the ROI, we reviewed previously published papers, and found different researcher using different ROI segmentation methods. Some used 2D ROI and the others used 3D ROI.22,23 Also, some researchers compared the performance between the 2D ROI features and 3D ROI features, and found they had the similar performance. Two radiologists (with 5 years and 10 years experiences in MRI diagnosis, respectively), jointly selected the largest tumor area, and when they were in different opinions, the negotiation should be made to reach agreement.
Image Preprocessing
Before feature extraction, some preprocess techniques were applied to improve texture resolving ability. Firstly, intensities in the ROI were normalized to μ ± 3 σ method (μ is the ROI’s mean gray level value and σ is the standard deviation) to enhance the differences between classes.24 Gray-level discretization (reduction of the levels of gray used to represent the image) was conducted to reduce the computational time and to improve the signal-to-noise ratio of the texture outcome.25
Feature Extraction and Selection
396 radiomic features were extracted using the A.K. software (AnalysisKit, version3.2.0, GE Healthcare), which included 5 main types: 42 intensity histogram features, 9 shape features, 154 gray-level cooccurrence matrix (GLCM) features, 180 gray-level run-length matrix (GLRLM) features and 11 gray-level size-zone matrix (GLSZM) features. Histogram features describe how voxel intensities within the ROI are distributed. Shape features describe the geometric properties of the tumor. GLCM features represent the intensity relationship of discrete gray levels of adjacent pixels, or voxels in a 3D volume. GLRLM features assesses the distribution of intensities run lengths. GLSZM features quantify the gray level regions in the image. Testing all possible combinations from all techniques, modalities and datasets would give a very large number of features. If all features were evaluated together, it is very likely that our classification model would be over-fitted and poorly generalized. Therefore, the redundant features were eliminated at first. The absolute values of pair-wise correlations are considered. If 2 variables had a high correlation (spearman correlation coefficient larger than 0.9), we looked at the mean absolute correlation of each variable and removes the variable with the largest mean absolute correlation.
Model Training and Evaluation
Considering the small sample size of our study, the nested cross-validation (CV) scheme was chosen to evaluate the performance of classifier without holding out some of the samples as an independent test set (Figure 2). Good estimates of the model performance can be achieved using the validation data when the number of samples is not large. The outer resampling loop of the nested CV scheme was used to optimize the number of features and the inner resampling loop was used to tune the model parameter.26
Figure 2.
Scheme of the nested cross validation method used to evaluate the different datasets of features. Texture data extracted from the PTC images were randomly divided into training and test sets N = 100 times to evaluate the 3 model using different of samples to obtain averaged results (a) T2WI-FS images with the average accuracy, (b) T2WI images with the average accuracy. Changes of average accuracy with the increase in feature number during feature selection.
The outer resampling loop used a Leave-group-out CV (LGOCV) scheme. This method randomly divided each dataset into training and test sets N times, forming N groups (N = 100). A total of N = 100 groups were used to reduce the variance of the CV results. In each group, 70% of the samples were randomly selected as training set and the remaining 30% were used as test set. The samples of the training set were used to construct the model and then using the samples of the test set to evaluate the model performance. Each group is constructed and evaluated independently. In the end, the classification results provided by the estimates of all groups are averaged. Three different predictive models: random forests (RF), Generalized Linear Model (GLM)and support vector machine (SVM) were studied to evaluate the discrimination power of different model of the radiomic features extracted from T2WI and T2WI-FS images.
Statistical Method
Continuous variables of clinical data were tested using the unpaired student’s t-test or the Mann-Whitney U test while categorical variables were analyzed by Pearson’s χ2 test or the Fisher’s exact test. A filter feature selection method based on the p-value provided by the Mann-Whitney U test was employed to generate a ranking of the features with the most discriminative power. All statistical analyses were conducted with R software (version 3.5.1; http://www.Rproject.org) to build and evaluate the prediction models. P < 0.05 was considered statistically significant. The classification results were estimated using the area under the receiver operating characteristic curve (AUC) averaged over groups estimates (mean standard deviation).
Results
Patient Characteristics
Basic information of the PCTs in our study is shown in Table 1. There were no significant differences in terms of patient sex, age, location and largest diameter in the PTC cases with and without cervical LN metastasis. An important clinical feature of our research is patients with cervical LN metastasis 37 (60.66%) more than without cervical LN metastasis 24(39.34%), because US examination is the first choice for PTC in our hospital, MRI is only selected when LN metastasis is suspected. This may be the cause of more PTC LN metastasis than non-metastatic patients in this study.
Table 1.
Comparison of Clinical Features According to Lymph Node Metastasis.
| Variables | LN metastasis (n = 37) | non- LN metastasis (n = 24) | P value |
|---|---|---|---|
| Age(y), Mean ± SD | 44.65 ± 11.29 | 49.71 ± 11.82 | 0.099 |
| Sex | 0.586 | ||
| Male Female |
10 (27.02%) 27 (72.98%) |
5 (20.83%) 19 (79.17%) |
|
| Largest diameter (mm) Mean ± SD |
13.41 ± 6.74 |
11.29 ± 5.34 |
0.201 |
| Location | 0.520 | ||
| Left lobe | 16 | 10 | |
| Right lobe | 21 | 13 | |
| Isthmus | 0 | 1 |
Inter-Observer and Intra-Observer Reproducibility of Feature Extraction
Before training the model, we tested the inter- and intra-observer agreement using ICCs and remained the features meeting the inter- and intra- ICCs larger than 0.75. Finally, we found all the feature met the acquirement. The intra-ICC’s range was 0.78-0.98, and inter-ICC’s range was 0.76-0.98.
Prediction Models Based on Radiomics
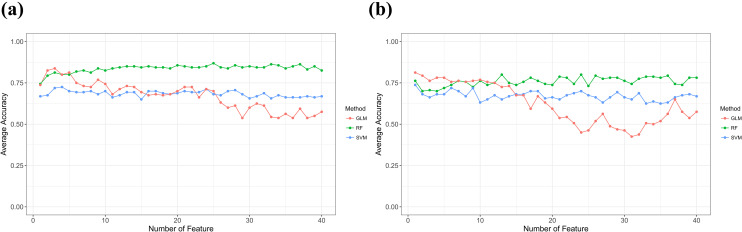
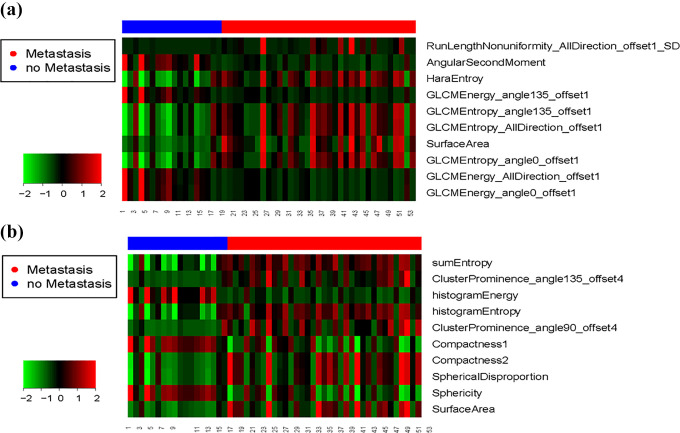
In Figure 3, the predictive capabilities of RF classifier were consistent when the number of features increasing, while the performance of SVM and GLM classifiers would decrease. Table 2 shows the top 10 radiomic features used to evaluate the RF model. Among the top 10 most predictive features in T2WI, 9 were texture features and 1 was shape feature. For T2WI-FS, 5 were shape features, 3 first-order feature and only 2 texture features. Heat map of top 10 radiomic features, each row corresponds to 1 radiomic feature, and each 1 column corresponds to 1 patient (Figure 4).
Figure 3.
The predictive capabilities of the 3 classifier varies the number of features increasing. (a) T2WI images with the average accuracy, (b) T2WI-FS images with the average accuracy.
Table 2.
Top 10 Features of the Best Dataset Ranked According to Their Average P-Value Computed With the Welch’s t-Test in the One-Versus-One Analysis.
| Feature | Average Rank | P value |
|---|---|---|
| T2WI | ||
| RunLengthNonuniformity_ AllDirection_offset1_SD | 1.17 | 0.0000044 |
| AngularSecondMoment | 2.09 | 0.00000537 |
| HaraEntroy | 3.36 | 0.00000593 |
| GLCMEnergy_angle135_offset1 | 4.13 | 0.00000876 |
| GLCMEntropy_angle135_offset1 | 5.42 | 0.00000876 |
| GLCMEntropy_AllDirection_offset1 | 5.96 | 0.00000964 |
| SurfaceArea | 6.46 | 0.00000964 |
| GLCMEntropy_angle0_offset1 | 7.93 | 0.0000106 |
| GLCMEnergy_AllDirection_offset1 | 9.04 | 0.0000116 |
| GLCMEnergy_angle0_offset1 | 9.79 | 0.0000116 |
| T2WI-FS | ||
| sumEntropy | 1.21 | 0.00000238 |
| ClusterProminence_angle135_offset4 | 2.03 | 0.00000369 |
| histogramEnergy | 3.75 | 0.000014 |
| histogramEntropy | 4.01 | 0.000014 |
| ClusterProminence_angle90_offset4 | 5.45 | 0.0000159 |
| Compactness1 | 6.47 | 0.000029 |
| Compactness2 | 6.76 | 0.000029 |
| Spherical Disproportion | 7.04 | 0.000029 |
| Sphericity | 7.21 | 0.000029 |
| SurfaceArea | 9.59 | 0.0000317 |
Figure 4.
Heat map of top 10 radiomic features.
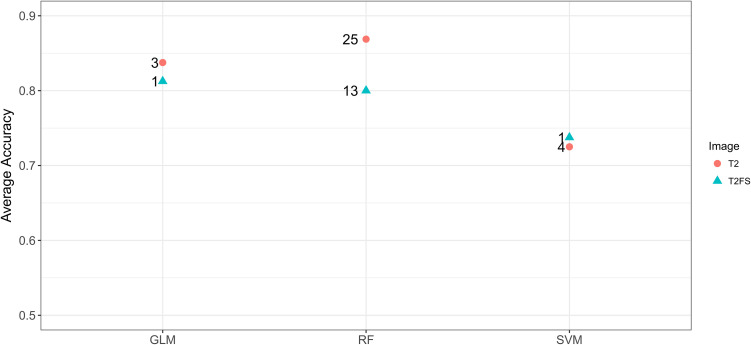
As shown in Figure 5, we comparison 3 models’ performance when the number of features used to achieve the maximum average accuracy. The RF model based on T2WI images reaches the optimal value of 0.85 when the first 25 features were used in classification. Likewise, the RF model based on T2WI-FS images reaches the optimal value of 0.80 when the first 13 features were used in classification.
Figure 5.
Comparison 3 model performance (RF, SVM, GLM) using radiomic features in this study. The numbers on the figure indicate the number of features used to achieve the maximum average accuracy.
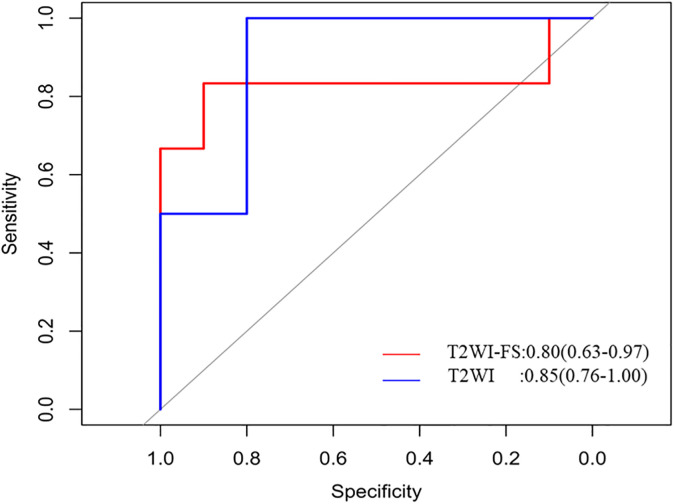
As showed in Figure 5 and Figure 6, all the prediction models constructed using the radiomic features extracted from T2WI had better performance than those using T2WI-FS. Especially, RF classifier combining with T2WI had the highest AUC value (0.85, CI:0.76 -1), accuracy (0.87), sensitivity (0.83), specificity (1.00), PPV (1.00) and NPV (0.88), as shown in Table 3. The main advantage of RF is that each tree in the forest is randomly different, and each tree has a high degree of irrelevance. In theory, the more trees that make up a RF, the less likely it is to overfit. RF is suitable for the study of small samples of 2 classifications, and is also robust to outliers.27 These advantages of the RF model may be the reason for its best performance.
Figure 6.
The ROC curve of RF model based on T2WI images (blue line) and T2WI-FS images (red line).
Table 3.
Additional Metrics Obtained Using the RF Model on the Best Dataset.
| AUC | Accuracy | Sensitivity | Specificity | PPV | NPV | |
|---|---|---|---|---|---|---|
| T2WI | 0.85 (0.76 -1.00) | 0.87 (0.75-0.92) | 0.83 | 1.00 | 1.00 | 0.88 |
| T2WI-FS | 0.80 (0.63-0.97) | 0.82 (0.71-0.89) | 0.83 | 0.90 | 0.89 | 0.74 |
Totally, both T2WI and T2WI-FS achieved high predictive performance, as shown in Figure 6, the sensitivity and specificity of the best model based on T2WI and T2WI-FS (0.83 and 1.00, 0.83 and 0.90, respectively).
Discussion
The objective of this research was to provide a tool that preoperative prediction of the cervical LN metastasis, based on the PTCs MRI radiomics. To the best of our knowledge, there are no reported studies in this field. In this study, we evaluated 3 models to predict the cervical LN metastasis of PTC in which RF model produce the best classification accuracy (achieving an average AUC > 0.8 in both cases), which suggests that RF model could be employed to discriminate whether the cervical LNs of PTC metastasize. The high AUC signified that the radiomics feature was a potential biomarker to discriminate cervical LN metastasis. Our study finding indicates that MRI radiomics could be a promising tool to predict the cervical LN metastasis of PTC. This would be helpful for preoperative decision making. According to the ATA guidelines4 for patients with positive lymph node determined clinically, the therapeutic level VI dissections was recommended.
Predicting cervical LN metastasis of PTC patients is significant for guiding clinical treatment. LN metastasis has been used in several staging systems, including TNM (tumor, node, and metastasis), AMES (age, metastasis, ETE, and size), and MACIS (metastasis, age, completeness of resection, invasion, and size). If a high of LN metastasis is detected in the patients by preoperative examination, the patient may undergo surgery for the resection of LNs. But owing to the complexity of neck dissection, LN resection is prone to accidental injuries and complications such as hemorrhage, lymphatic leak and nerve injury.28 This suggests that complications of cervical LN dissection may affect the quality of patients’ life. Therefore, it is necessary in order to reduce the incidence of complications while ensuring the curative effect. The reduction in the incidence of complications must be based on reduction of unnecessary cervical LN dissection. Therefore, how to determine whether LN of PTC needs to be dissected and the range of LN dissection in clinical practice is an important issue.
Unfortunately, a high proportion of patients with PTC had clinically negative for nodal involvement in the preoperative evaluation, but were found to have cervical LN metastasis at the time of surgery and in the pathology specimens. No matter what kind of image inspection method is an empirical judgment of experts based on image features, lack of objectivity, and showed extremely unbalanced specificity and sensitivity in predicting LN metastasis status.13,28
Compared to the conventional image analysis, radiomic features independent of the subjective factors and level of expertise, and provides objective information on the lesion image radiomic features in cancer studies has proved to be a successful source of information to increase the precision in diagnosis, to evaluate the prognosis and to predict treatment response.29 Previous studies on radiomics in thyroid nodes have mostly focused on differentiating benign and malignant nodules by US images or detecting LN metastasis by using relatively simple histogram, GLCM and texture analysis techniques.19,30,31 In our study, the A.K. software was used to extract radiomic features of each PTC’s MRI images. Which allows the high-throughput extraction of informative imaging features to quantify differences in intratumor heterogeneity and phenotype. This new radiomics concept is referred to the analysis of medical images by an exhaustive extraction of features from regions of these images and the corresponding data mining to create predictive models to help in the decision support.32
Due to our study was a preliminary study on prediction of cervical LN metastasis in PTC, we only extracted the radiomic features from T2WI and T2WI-FS images. The members of our team reviewed all patients’ multiparametric MRI images, including T2WI, T2WI-FS, ADC and CE-T1 images. T2WI and T2WI-FS images showed the clearest evidence of PTC. The research conducted by Brown et al33 confirmed that radiomic features from a single MRI sequence can obtain good value and prospect of application in the diagnostic and prognosis. In addition, another finding of our study is that the texture parameters extracted from T2WI images are more meaningful than T2WI-FS images. The main reason is that the T2WI sequence has a relatively long echo time, which increases the contrast between tissues, and makes the image contain more texture features with differential diagnosis.34 LGOCV (100 times) was applied to validate our study. Some previous studies26,23 indicated that validation of this approach was suitable for the modeling and verification of small samples, can demonstrated the reliability and accuracy of the model. Concerning the features with the most discriminative power, the nested CV scheme does not allow determining the exact ranking of features because the feature selection step is recomputed for every group. After that, an average ranking was obtained.26 Our samples were all from the same batch. So that, we used leave group out cross validation method to validate the model. Cross validation was the best method to avoid overfit and decrease the false positive rate. Table 2 shows the top 10 T2WI texture features used to evaluate the models: features derived from GLCM occupy the majority. This finding has been corroborated by recent studies that identified GLCM features as superior to all other texture classes in distinguishing benign and malignant thyroid nodes.33 Table 2 also shows the average p-value computed for these features: significant p-value (p < 10-5) was obtained for these 10 features, which give an idea of their discriminative power. The results indicated that the process of LN metastatic spread of PTCs is linked to profound changes in the tissue microarchitecture, related to proliferation of distinct tumor cell clusters and subsequent migration via the lymphatic system.
Seo et al.35 indicated that male gender, young age, conventional variant, tumor size > 0.5 cm, multiplicity, bilaterally and extrathyroidal extension were independent predictors for central LN metastasis. However, this study suggested that there was no significant differences in age, gender, and lesion diameter between patients with LN metastasis of PTC and those with no LN metastasis. The reason may be the sampling error caused by the small sample size. Thus, no clinical characteristics have been involved in the construction of our predictive model. The next step would be to collect data with a larger sample size to further validate and improve the current models.
Our study has several limitations. Firstly, our study is retrospective, inevitably, some bias may exist or may have affected the analysis. Secondly, the sample size of the study is small, more samples would be needed to build and test a final predictive model. Thirdly, we only included MR images acquired with the same scanner and imaging parameters since texture features can be affected by differences in scan parameters, a multicenter study on this specific application should be performed to evaluate this limitation.
In summary, radiomics provided a method to predict whether or not the LN metastasis occurred in the patients of PTCs. Moreover, the study was aimed to reduce the unnecessary LN resection, and the patients with LN metastasis may early undergo reasonable treatment. In conclusion, radiomics provided a way to predict scientifically, quantitatively, and accurately whether LN metastasis occurred in patients with PTC. It was highly promising in determining reasonable surgical range, reducing unnecessary LN dissection, and further improving the quality of life of patients.
Acknowledgments
The authors wish to thank Dr. Danjun Yuan, Weixia Li and Yerong Chen for their technical support in editing the manuscript.
Footnotes
Author Contributions: Heng Zhang, Wang Xian and Junlin He are contributed equally to this work.
Declaration of Conflicting Interests: The author(s) declared the following potential conflicts of interest with respect to the research, authorship, and/or publication of this article: All authors have read and approved the submitted manuscript. There are no conflicts of interest. The manuscript has not been submitted elsewhere nor published elsewhere in whole or in part. All relevant ethical safeguards had been met.
Funding: The author(s) disclosed receipt of the following financial support for the research, authorship, and/or publication of this article: This study was supported in part by a grant-in-aid for scientific research from the Technology Plan of Jiangsu (Project No. H2019087), the Technology Plan of Wuxi (Project No. MS201901) and the Science and Technology Development Plan of Wuxi (Project No. N20192027).
ORCID iD: Shudong Hu  https://orcid.org/0000-0002-4454-3432
https://orcid.org/0000-0002-4454-3432
References
- 1. Siegel RL, Miller KD, Jemal A. Cancer statistics, 2019. CA Cancer J Clin. 2019;69(1):7–34. doi:10.3322/caac.21551 [DOI] [PubMed] [Google Scholar]
- 2. Miller KD, Nogueira L, Mariotto AB, et al. Cancer treatment and survivorship statistics, 2019. CA Cancer J Clin. 2019;69(5):363–385. doi:10.3322/caac.21565 [DOI] [PubMed] [Google Scholar]
- 3. Vaccarella S, Franceschi S, Bray F, Wild CP, Plummer M, Dal Maso L. Worldwide thyroid-cancer epidemic? The increasing impact of overdiagnosis. N Engl J Med. 2016;375(7):614–617. doi:10.1056/NEJMp1604412 [DOI] [PubMed] [Google Scholar]
- 4. Haugen BR, Alexander EK, Bible KC, et al. 2015 American thyroid association management guidelines for adult patients with thyroid nodules and differentiated thyroid cancer: the American thyroid association guidelines task force on thyroid nodules and differentiated thyroid cancer. Thyroid. 2016;26(1):1–133. doi:10.1089/thy.2015.0020 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Davies L, Welch HG. Current thyroid cancer trends in the United States. JAMA Otolaryngol Head Neck Surg. 2014;140(4):317–322. doi:10.1001/jamaoto.2014.1 [DOI] [PubMed] [Google Scholar]
- 6. Kim SY, Kwak JY, Kim EK, Yoon JH, Moon HJ. Association of preoperative us features and recurrence in patients with classic papillary thyroid carcinoma. Radiology. 2015;277(2):142470 doi:10.1148/radiol.2015142470 [doi] [DOI] [PubMed] [Google Scholar]
- 7. Lee YK, Kim D, Shin DY, et al. The prognosis of papillary thyroid cancer with initial distant metastasis is strongly associated with extensive extrathyroidal extension: a retrospective cohort study. Ann Surg Oncol. 2019;26(7):2200–2209. doi:10.1245/s10434-019-07314-x [DOI] [PubMed] [Google Scholar]
- 8. Chen L, Zhu Y, Zheng K, et al. The presence of cancerous nodules in lymph nodes is a novel indicator of distant metastasis and poor survival in patients with papillary thyroid carcinoma. J Cancer Res Clin Oncol. 2017;143(6):1035–1042. doi:10.1007/s00432-017-2345-2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Hei H, Song Y, Qin J. Individual prediction of lateral neck metastasis risk in patients with unifocal papillary thyroid carcinoma. Eur J Surg Oncol. 2019;45(6):1039–1045. doi:10.1016/j.ejso.2019.02.016 [DOI] [PubMed] [Google Scholar]
- 10. Lim YC, Liu L, Chang JW, Koo BS. Lateral lymph node recurrence after total thyroidectomy and central neck dissection in patients with papillary thyroid cancer without clinical evidence of lateral neck metastasis. Oral Oncol. 2016;62:109–113. doi:10.1016/j.oraloncology.2016.10.010 [DOI] [PubMed] [Google Scholar]
- 11. Mulla M, Schulte KM. Central cervical lymph node metastases in papillary thyroid cancer: a systematic review of imaging-guided and prophylactic removal of the central compartment. Clin Endocrinol. 2012;76(1):131–136. doi:10.1111/j.1365-2265.2011.04162.x [DOI] [PubMed] [Google Scholar]
- 12. Intenzo CM, Dam HQ, Manzone TA, Kim SM. Imaging of the thyroid in benign and malignant disease. Sem Nucl Med. 2012;42(1):49–61. doi:10.1053/j.semnuclmed.2011.07.004 [DOI] [PubMed] [Google Scholar]
- 13. Jin S, Bao W, Yang YT, Bai T, Bai Y. Establishing a prediction model for lateral neck lymph node metastasis in patients with papillary thyroid carcinoma. Sci Rep. 2018;8(1):17355 doi:10.1038/s41598-018-35551-9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Park JE, Lee JH, Ryu KH, et al. Improved diagnostic accuracy using arterial phase CT for lateral cervical lymph node metastasis from papillary thyroid cancer. AJNR Am J Neuroradiol. 2017;38(4):782–788. doi:10.3174/ajnr.A5054 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Liu X, Ouyang D, Li H, Zhang R, Lv Y, Yang A, Xie C. Papillary thyroid cancer: dual-energy spectral CT quantitative parameters for preoperative diagnosis of metastasis to the cervical lymph nodes. Radiology. 2015;275(1):167–176. doi:10.1148/radiol.14140481 [DOI] [PubMed] [Google Scholar]
- 16. Suh CH, Choi YJ, Lee JJ, et al. Comparison of core-needle biopsy and fine-needle aspiration for evaluating thyroid incidentalomas detected by (18)F-fluorodeoxyglucose positron emission tomography/computed tomography: a propensity score analysis. Thyroid. 2017;27(10):1258–1266. doi:10.1089/thy.2017.0192 [DOI] [PubMed] [Google Scholar]
- 17. Lambin P, Rios-Velazquez E, Leijenaar R, et al. Radiomics: extracting more information from medical images using advanced feature analysis. Eur J Cancer. 2012;48(4):441–446. doi:10.1016/j.ejca.2011.11.036 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Lubner MG, Smith AD, Sandrasegaran K, Sahani DV, Pickhardt PJ. CT texture analysis: definitions, applications, biologic correlates, and challenges. radiographics: a review publication of the radiological society of North America, Inc. 2017;37(5):1483–1503. doi:10.1148/rg.2017170056 [DOI] [PubMed] [Google Scholar]
- 19. Schob S, Meyer HJ, Dieckow J, et al. Histogram analysis of diffusion weighted imaging at 3 T is useful for prediction of lymphatic metastatic spread, proliferative activity, and cellularity in thyroid cancer. Int J Mol Sci. 2017;18(4). doi:10.3390/ijms18040821 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Cui Y, Yang X, Shi Z, et al. Radiomics analysis of multiparametric MRI for prediction of pathological complete response to neoadjuvant chemoradiotherapy in locally advanced rectal cancer. Eur Radiol. 2019;29(3):1211–1220. doi:10.1007/s00330-018-5683-9 [DOI] [PubMed] [Google Scholar]
- 21. Wu J, Cao G, Sun X, et al. Intratumoral spatial heterogeneity at perfusion MR imaging predicts recurrence-free survival in locally advanced breast cancer treated with neoadjuvant chemotherapy. Radiology. 2018;288(1):26–35. doi:10.1148/radiol.2018172462 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Mao N, Yin P, Wang Q, et al. Added value of radiomics on mammography for breast cancer diagnosis: a feasibility study. J Am Coll Radiol 2019;16(4 Pt A):485–491. doi:10.1016/j.jacr.2018.09.041 [DOI] [PubMed] [Google Scholar]
- 23. Ortiz-Ramon R, Larroza A, Arana E, Moratal D. A radiomics evaluation of 2D and 3D MRI texture features to classify brain metastases from lung cancer and melanoma. Annu Int Conf IEEE Eng Med Biol Soc. 2017(undefined):493–496. [DOI] [PubMed] [Google Scholar]
- 24. Collewet G, Strzelecki M, Mariette F. Influence of MRI acquisition protocols and image intensity normalization methods on texture classification. Magn Reson Imaging. 2004;22(1):81–91. doi:10.1016/j.mri.2003.09.001 [DOI] [PubMed] [Google Scholar]
- 25. Gibbs P, Turnbull LW. Textural analysis of contrast-enhanced MR images of the breast. Magn Reson Med. 2003;50(1):92–98. doi:10.1002/mrm.10496 [DOI] [PubMed] [Google Scholar]
- 26. Ortiz-Ramon R, Larroza A, Ruiz-Espana S, Arana E, Moratal D. Classifying brain metastases by their primary site of origin using a radiomics approach based on texture analysis: a feasibility study. Eur Radiol. 2018;28(11):4514–4523. doi:10.1007/s00330-018-5463-6 [DOI] [PubMed] [Google Scholar]
- 27. Goldstein BA, Navar AM, Carter RE. Moving beyond regression techniques in cardiovascular risk prediction: applying machine learning to address analytic challenges. Eur Heart J. 2017;38(23):1805–1814. doi:10.1093/eurheartj/ehw302 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. McMullen C, Rocke D, Freeman J. Complications of bilateral neck dissection in thyroid cancer from a single high-volume center. JAMA Otolaryngol Head Neck Surg. 2017;143(4):376–381. doi:10.1001/jamaoto.2016.3670 [DOI] [PubMed] [Google Scholar]
- 29. Liu Z, Wang S, Dong D, et al. The applications of radiomics in precision diagnosis and treatment of oncology: opportunities and challenges. Theranostics. 2019;9(5):1303–1322. doi:10.7150/thno.30309 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Zhang LX, Xiang JJ, Wei PY, et al. Diagnostic value of computed tomography (CT) histogram analysis in thyroid benign solitary coarse calcification nodules. J Zhejiang Univ Sci B. 2018;19(3):211–217. doi:10.1631/jzus.B1700119 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Kim SY, Lee E, Nam SJ, et al. Ultrasound texture analysis: association with lymph node metastasis of papillary thyroid microcarcinoma. PloS One. 2017;12(4):e01761 03. doi:10.1371/journal.pone.0176103 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Gillies RJ, Kinahan PE, Hricak H. Radiomics: images are more than pictures, they are data. Radiology. 2016;278(2):563–577. doi:10.1148/radiol.2015151169 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Brown A, Nagala S, McLean M, et al. Multi-institutional validation of a novel textural analysis tool for preoperative stratification of suspected thyroid tumors on diffusion-weighted MRI. Magn Reson Med. 2016;75(4):1708–1716. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Horvat N, Veeraraghavan H, Khan M, et al. MR imaging of rectal cancer: radiomics analysis to assess treatment response after neoadjuvant therapy. Radiology. 2018;287(3):833–843. doi:10.1148/radiol.2018172300 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Kim SK, Park I, Woo JW, et al. Predictive factors for lymph node metastasis in papillary thyroid microcarcinoma. Ann Surg Oncol. 2016;23(9):2866–2873. doi:10.1245/s10434-016-5225-0 [DOI] [PubMed] [Google Scholar]