Abstract
Pulmonary lymphatic epithelioma-like carcinoma (LELC) is a rare and unique subtype, accounting for 0.9% of all lung cancers. To date, just over 200 cases have been reported worldwide. The Epstein–Barr virus plays a role in the pathogenesis of LELC. Most patients are from East Asia, especially southeastern China. Chest computed tomography mainly shows a single lump or nodule around the lung. In this article, we report a 49-year-old male patient from a non-epidemic area who was hospitalized for “intermittent blood in his phlegm for more than 4 months”. Imaging revealed two nodules in the left lower lobe of his lung. Transbronchial lung biopsy was performed on one of the nodules, and he was diagnosed with primary LELC. Single-photon emission computed tomography revealed that he had hypertrophic pulmonary osteoarthropathy, which is a rare symptom of paraneoplastic syndrome. Because the preoperative evaluation considered early-stage disease, video-assisted thoracoscopic surgery for the left lower lobe and mediastinal lymph node dissection were performed. Both lesions were eventually diagnosed as LELC. Fortunately, lymph node metastasis did not occur, and he did not receive other postoperative treatments. He was followed up for 1 year, and no recurrence was found.
Keywords: Pulmonary lymphoepithelioma-like carcinoma, lung cancer, Epstein–Bar virus, hypertrophic pulmonary osteoarthropathy, paraneoplastic syndrome, non-endemic region
Introduction
Primary lymphatic epithelioma-like carcinoma (LELC) is a rare clinical malignant tumor, which Begin et al.1 first reported in 1987. The prognosis of patients with LELC is better than that of patients with other types of non-small cell lung cancer (NSCLC).2 Currently, there is no standard treatment method for LELC. The disease is primarily found in young non-smokers and has clear geographical distribution characteristics. Globally, LELC patients are mainly from East Asia, especially the southern regions of China, such as Guangdong and Hong Kong.3 Few cases have been reported in other regions. Solitary lumps or nodules around the lung are the main imaging manifestations of the disease.4 Hypertrophic pulmonary osteoarthropathy (HPOA) is closely related to tumors and is a manifestation of paraneoplastic syndrome. Two domestic and international reports found that the incidence of lung cancer combined with HPOA was 1% to 1.87%.5,6 Active treatment of the primary lesions, including surgical resection, radiotherapy and chemotherapy, can significantly improve the symptoms of most HPOA patients and even completely relieve their symptoms.
Here, we report a rare case of two primary LELCs accompanied by HPOA in a patient from a non-epidemic area. No recurrence was found at follow-up within 1 year after the operation.
Case report
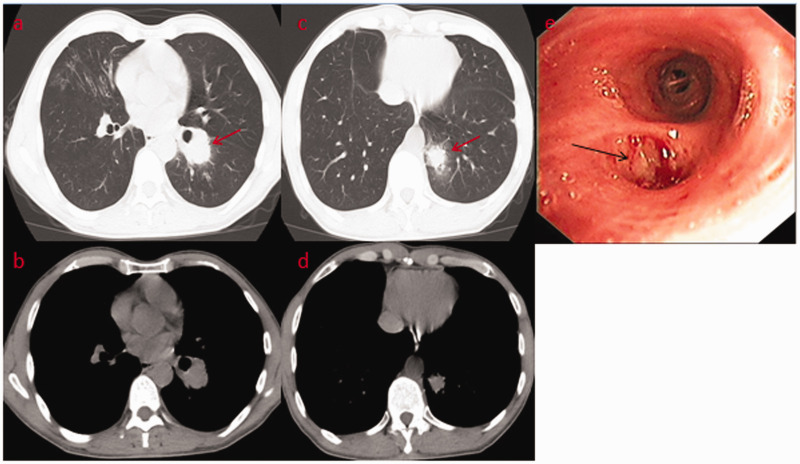
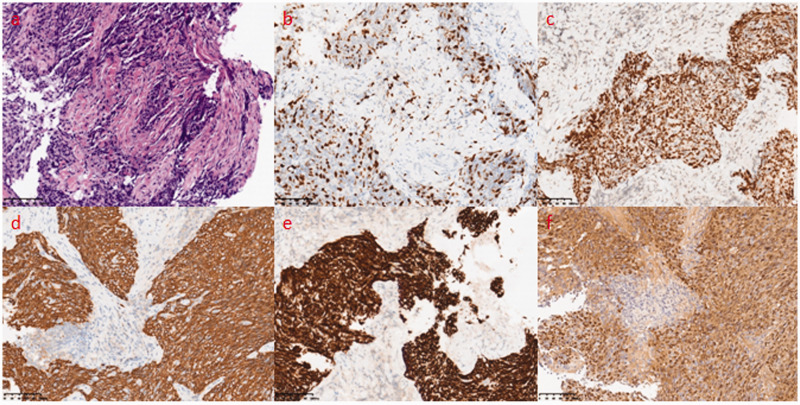
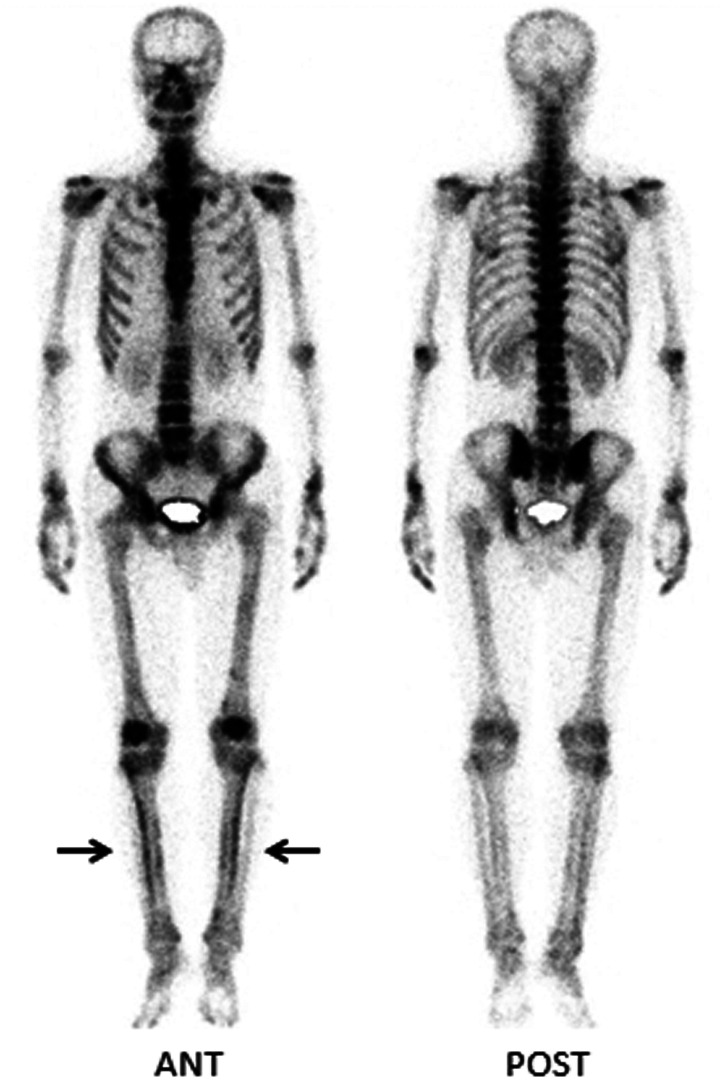
A 49-year-old male garment factory worker who was formerly in good health and denied smoking was admitted to our hospital on 30 December 2018 because of “intermittent blood in his phlegm for more than 4 months”. The patient suffered from intermittent bright red blood streaks in his phlegm after working long hours before April, but he had no formal diagnosis or treatment. One week before the admission of the patient to our hospital, a chest computed tomography (CT) scan revealed two masses in the left lower lobe of the lung located in the dorsal segment near the hilum (size, 31.3 × 35.6 mm) and the posterior basal segment (size, 18.4 × 20.2 mm) with superficial foliation, peripheral burr and a halo sign (Figure 1a–d). In addition, the image showed that the middle lobe of the right lung and the lower lobe of the left lung were slightly infected (Figure 1a, c). His vital signs were stable after physical examination, the lung had slightly moist rale sounds, and he had no swelling or clubbed fingers. In addition, his tumor markers were negative. Under bronchoscopy, the opening of the left dorsal segment was narrowed, and the lens body (Olympus BF-P260F) could not reach the distal end, but no new organisms were found. Lavage, brushing and transbronchial lung biopsy were performed (Figure 1e). The pathology of the dorsal segment showed poorly differentiated squamous cell carcinoma (Figure 2a). The immunohistochemistry results were as follows: leukocyte common antigen (+) lymphocytes, synaptophysin (−), chromogranin A (−), Ki-67 (+, 30%), pan-cytokeratin (+++), CD56 (−), thyroid transcription factor 1 (−), p40 (+++), cytokeratin 5/6 (++) and napsin A (−) (Figure 2b–e). In situ hybridization of Epstein–Barr virus (EBV)-encoded small RNA 1 (EBER-1) was diffusely positive (Figure 2f). Based on these results, primary LELC was considered. No sign of bone metastasis was found with whole-body bone imaging by emission computed tomography (ECT), but the findings were consistent with HPOA. Technetium 99m-methyl diphosphonate (99mTc-MDP) bone scintigraphy showed diffusely increased uptake along the cortical margins of long bones, and a parallel track pattern in the tibiae was observed (Figure 3). Because of medical insurance policies and other reasons, the patient refused further systemic evaluations and requested to return to his local hospital for further diagnosis and treatment.
Figure 1.
(a–d) Chest computed tomography revealed two masses in the left lower lobe of the lung located in the dorsal segment near the hilum (size, 31.3 × 35.6 mm) and the posterior basal segment (size, 18.4 × 20.2 mm) with superficial foliation, peripheral burr and a halo sign (arrows). (e) Under bronchoscopy, the opening of the left dorsal segment was narrowed, but no new organisms were found (arrow).
Figure 2.
(a) Hematoxylin and eosin staining showed nests of neoplastic cells with distinct pleomorphism, irregular nuclei and an epithelioid appearance and increased lymphocytic infiltration in the stroma. Immunohistochemistry demonstrated (b) Ki-67 (+, index of approximately 30%), (c) p40 (+), (d) pan-cytokeratin (+) and (e) cytokeratin 5/6 (+). (f) In situ hybridization for Epstein–Barr virus-encoded small RNA 1 was diffusely positive. Original magnification, ×200.
Figure 3.
Technetium 99m-methyl diphosphonate bone scintigraphy showed diffusely increased uptake along the cortical margins of the long bones, and a parallel track pattern in tibias was observed (arrows).
ANT, anterior; POST, posterior.
Positron emission tomography/CT in the local hospital indicated no tumor metastasis or primary nasopharyngeal carcinoma. Video-assisted thoracoscopic surgery was performed on the left lower lobe for resection. Intraoperative frozen pathology indicated NSCLC, and mediastinal lymph node dissection was performed. The postoperative pathology indicated that both lesions of the left lower lung were LELC. The tumor cells did not involve the visceral pleura, and no metastasis was found in the lymph nodes in the examination area (0/25), which included group 4 (0/1), group 5 (0/2), group 6 (0/3), group 7 (0/6), group 8 (0/1), group 9 (0/4), group 10 (0/3), group 11 (0/3) and group 12 (0/2). Immunohistochemistry of the nodule lesions near the hilum showed cytokeratin CAM 5.2 (+), CD56 (−), cytokeratin 5/6 (+), cytokeratin 7 (−), Ki-67 (+, 40%), napsin A (−), p40 (+), p63 (+), synaptophysin (−) and thyroid transcription factor 1 (−). Two surgical specimens were EBER (++) by molecular pathology, and gene detection showed epidermal growth factor receptor (EGFR) (−), anaplastic lymphoma kinase (−), ROS proto-oncogene 1(−) and B-Raf proto-oncogene (−) status. The serological test for EBER was positive after the operation. No obvious abnormality was found through nasopharyngeal magnetic resonance imaging or nasopharyngeal endoscopy. No other comprehensive treatments were given to the patient after the operation, and no recurrence has been found at present.
Discussion
LELC is histologically similar to undifferentiated carcinoma of the nasopharynx but can also be found in other organs of a foregut origin, such as the lung, thymus, salivary gland, liver, stomach, cervix and bladder. Primary LELC is a clinically rare malignant tumor with ∼200 cases reported worldwide.7 Begin et al. were the first to report and define this tumor as a large-cell lung carcinoma in 1987. Later, it was found that most pulmonary LELCs are poorly differentiated squamous cell carcinoma that may originate from epithelial tissues.3 In our case, cytokeratin 5/6 and p40 were both positive, which was consistent with previous reports.3 In 2015, the World Health Organization’s histological classification of lung tumors classified LELC as an untyped tumor derived from epithelial tissue.8
A previous study1 showed that EBER-1 was isolated from malignant tumor cells with obvious nucleoli. Tumors often express B-cell lymphoma 2 apoptosis regulators and latent membrane protein 1 and are enriched with CD8-positive T lymphocytes, suggesting a role of the EBV in its pathogenesis. The study also showed that EGFR mutations are often associated with the high expression of programmed death ligand-1, and the occurrence rate of these mutations in pulmonary LELC is relatively low. The negative results of gene detection for postoperative specimens in this patient are in accordance with previous literature reports. Recently, it was reported that plasma EBV DNA could be used as a tumor marker for pulmonary LELC and to predict the therapeutic effect and prognosis of this type of tumor.9 Unfortunately, we contacted the patient, but the patient refused to return to the hospital.
In addition, pulmonary LELCs are mainly found in the southern regions of China, such as Guangdong and Hong Kong.3 However, this patient lived in a city in the western countryside for a long time since birth. One year prior to presenting at our hospital, he came to work in an eastern city, which is not a high-incidence area for nasopharyngeal carcinoma. LELC cases in non-epidemic areas in China are rare. Pulmonary LELC primarily affects young non-smokers. The clinical manifestations include dry cough, hemoptysis, chest pain, weight loss and fever. The patient was admitted to the hospital with blood in his sputum. However, as many as one-third of patients may be diagnosed by accident.10 The patient’s age, smoking status and clinical manifestations are consistent with the literature reports.10
Previous reports indicate that the CT images of primary pulmonary LELC mainly show a single tumor or nodule around the lung, but sometimes the mass may involve the bronchus.4 Most masses are located in the medial zone inside the lower lobe of the lung, close to the mediastinal pleura, and are primarily round or quasi-round with clear boundaries. Tracheal endoscopy of the patient suggested the possibility of early involvement of the mucosa in the dorsal segment of the left lower lobe. However, the boundary around the two masses was blurred, and the appearance of the halo sign is relatively rare, which may be the cause of the coinfection. In addition, there have been no reports of two nodule lesions in the left lung pathologically diagnosed as LELC. This suggests the polymorphism of LELC images, and the diagnosis depends on pathological examination.
HPOA is closely related to malignant tumors and is a symptom of paraneoplastic syndrome. The pathogenesis is still unclear, but several theories have been proposed. First, one theory11 states that HPOA is an inflammatory reaction with surrounding cell infiltration, vascular hyperplasia and osteoid and calcareous changes in the tissues, leading to the formation of heteromorphic bones and periosteum hyperplasia. 99mTc-MDP bone scintigraphy shows diffusely radioactive concentrations along the cortical margins of long bones. Second, in the neurogenic pathway, afferent fibers of the vagus nerve are stimulated by tumor cells, inducing a neural reflex and leading to vasodilatation and blood flow to the extremities, which may account for some signs and symptoms of HPOA.12 Third, in the humoral pathway, one hypothesis suggests that the malignancy produces some biochemical factors, including growth factors, growth hormone-releasing hormone, gonadotropins and vascular endothelial growth factor, which are released into the circulation and promote features of hypertrophic osteoarthropathy, such as vascular hyperplasia, new bone formation and edema.13 An additional humoral hypothesis suggests that platelets and megakaryocytes that are normally destroyed in the lung gain access to the periphery when the pulmonary vasculature is destroyed. Once in the periphery, they release platelet-derived growth factor, which is a growth promoter, leading to fibroblast proliferation and new bone growth.14
The incidence of HPOA in lung cancer patients has been inconsistently reported. Ito et al.6 reported HPOA in 19 of 2625 lung cancer patients, with an incidence of less than 1%. China reported 6.151 cases of lung cancer, of which 1.87% had HPOA, 91% were male, 76% had a history of smoking, and the average patient age was 62 years.5 The patient in this case report had a relatively rare low-grade malignant primary LELC accompanied by HPOA. HPOA can appear several months earlier than respiratory symptoms, and the patient did not complain of accompanying symptoms, such as limb pain or swelling, before HPOA was found, which lead to difficulties in the early diagnosis of the disease to some extent. The patient’s age and smoking history do not appear to be consistent with the above literature, and there may be differences between LELC, a rare type of lung cancer, and other lung cancers.
ECT bone imaging is a highly sensitive examination method for diagnosing HPOA. Bone changes in HPOA are characterized by regular and symmetrical increases in radiation uptake of the long bone cortex, commonly in the lower limbs and the distal end of long bones, and radioactive concentrations around joints may be found. Bone imaging is particularly sensitive to abnormal changes in bone metabolism and can reflect the involvement of bones in the entire body earlier and more comprehensively than X-ray. These signs are different from bone lesions caused by bone metastasis of lung cancer. The latter mainly involves the medial axis and proximal ends of bone, especially the spine, ribs and ilium, and the radioactive concentration is irregular. When the long bone is involved, the uptake also presents asymmetry. The lesions are mainly located in the bone marrow cavity. Therefore, it is of great significance to distinguish between the two types of disease. HPOA treatment is mainly used to treat primary lesions, whereas bone metastases require corresponding symptomatic treatment. At present, the accepted treatment plan is actively treating the primary lesions, including with surgical resection, radiotherapy and chemotherapy, and the symptoms of most HPOA patients can be significantly improved or even completely relieved. As the patient has felt no obvious discomfort, he has not yet agreed to be reexamined by ECT.
At present, there is no unified treatment strategy for primary LELC. According to previous studies, the prognosis of primary LELC patients is better than that of patients with other types of NSCLC.2 Early-stage patients should be treated with surgery, and mid- to late-stage patients can be treated with a surgery-based comprehensive treatment approach.15
Because of the rarity of the disease and the lack of long-term follow-up reports domestically and internationally, there are limited data on this type of tumor. However, two small retrospective analyses indicated that compared with other NSCLCs, LELC usually has a better prognosis. The 5-year progression-free survival rate and overall survival rate in a previous study were 53% and 80%, respectively.16 In another study, the 5-year overall survival rate was 62%, and the overall survival rate for all lung cancers was 17.7%.17 There are indications that these tumors are more sensitive to radiotherapy and chemotherapy than other NSCLCs. Chemotherapy drugs effective against LELC include platinum, gemcitabine, pemetrexed, vinorelbine, taxane and 5-fluorouracil.18 Recently, it was reported that immunotherapy also achieved promising results.19 We believe that more cases and further studies are needed to optimize the treatment of this rare type of lung cancer. For this rare histological type, experienced pathologists suggest that a correct diagnosis, avoidance of overtreatment and consultation on the prognosis of patients are necessary. The patient was considered to have early-stage lung cancer after the operation. The patient was followed up for 1 year after treatment during which no recurrence of the lung lesions was found, further follow-up is needed.
Footnotes
Ethics statement: Written informed consent for the publication of all clinical details and images was obtained from the patient.
Declaration of conflicting interest: The authors declare that there is no conflict of interest.
Funding: This study was supported by the Science and Technology Innovation 2025 Major Project of Ningbo (2019B10037).
ORCID iD: Ning Zhu https://orcid.org/0000-0001-5902-0602
References
- 1.Bégin L, Eskandari J, Joncas J, et al. Epstein-Barr virus related lymphoepithelioma-like carcinoma of lung. J Surg Oncol 1987; 36: 280–283. [DOI] [PubMed] [Google Scholar]
- 2.Huang C, Feng A, Fang Y, et al. Multimodality treatment and long-term follow-up of the primary pulmonary lymphoepithelioma-like carcinoma. Clin Lung Cancer 2012; 13: 359–362. [DOI] [PubMed] [Google Scholar]
- 3.Han A, Xiong M, Gu Y, et al. Lymphoepithelioma-like carcinoma of the lung with a better prognosis. A clinicopathologic study of 32 cases. Am J Clin Pathol 2001; 115: 841–850. [DOI] [PubMed] [Google Scholar]
- 4.Ma H, Wu Y, Lin Y, et al. Computed tomography characteristics of primary pulmonary lymphoepithelioma-like carcinoma in 41 patients. Eur J Radiol 2013; 82: 1343–1346. [DOI] [PubMed] [Google Scholar]
- 5.Qian X, Qin J. Hypertrophic pulmonary osteoarthropathy with primary lung cancer. Oncol Lett 2014; 7: 2079–2082. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Ito T, Goto K, Yoh K, et al. Hypertrophic pulmonary osteoarthropathy as a paraneoplastic manifestation of lung cancer. J Thorac Oncol 2010; 5: 976–980. [DOI] [PubMed] [Google Scholar]
- 7.Yener N, Balikçi A, Çubuk R, et al. Primary lymphoepithelioma-like carcinoma of the lung: report of a rare case and review of the literature. Turk Patoloji Derg 2012; 28: 286–289. [DOI] [PubMed] [Google Scholar]
- 8.Travis W, Brambilla E, Nicholson A, et al. The 2015 World Health Organization Classification of Lung Tumors: Impact of Genetic, Clinical and Radiologic Advances Since the 2004 Classification. J Thorac Oncol 2015; 10: 1243–1260. [DOI] [PubMed] [Google Scholar]
- 9.Chen B, Chen X, Zhou P, et al. Primary pulmonary lymphoepithelioma-like carcinoma: a rare type of lung cancer with a favorable outcome in comparison to squamous carcinoma. Respir Res 2019; 20: 262. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Castro C, Ostrowski M, Barrios R, et al. Relationship between Epstein-Barr virus and lymphoepithelioma-like carcinoma of the lung: a clinicopathologic study of 6 cases and review of the literature. Hum Pathol 2001; 32: 863–872. [DOI] [PubMed] [Google Scholar]
- 11.Vaquer R, Dunn E, Bhat S, et al. Reversible pulmonary uptake and hypertrophic pulmonary osteoarthropathic distribution of technetium-99m methylene diphosphonate in a case of Pneumocystis carinii pneumonia. J Nucl Med 1989; 30: 1563–1567. [PubMed] [Google Scholar]
- 12.Treasure T. Hypertrophic pulmonary osteoarthropathy and the vagus nerve: an historical note. J R Soc Med 2006; 99: 388–390. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Olán F, Portela M, Navarro C, et al. Circulating vascular endothelial growth factor concentrations in a case of pulmonary hypertrophic osteoarthropathy. Correlation with disease activity. J Rheumatol Suppl 2004; 31: 614–616. [PubMed] [Google Scholar]
- 14.Dickinson C. The aetiology of clubbing and hypertrophic osteoarthropathy. Eur J Clin Invest 1993; 23: 330–338. [DOI] [PubMed] [Google Scholar]
- 15.Lin C, Chen Y, Hsieh M, et al. Advanced primary pulmonary lymphoepithelioma-like carcinoma: clinical manifestations, treatment, and outcome. J Thorac Dis 2017; 9: 123–128. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Mok F, Chan O, Chang A, et al. Treatment outcomes of primary pulmonary lymphoepithelioma-like carcinoma: a series of 22 patients and treatment strategy review. Hong Kong J. Radiol 2013: 270–277. [Google Scholar]
- 17.Liang Y, Wang L, Zhu Y, et al. Primary pulmonary lymphoepithelioma-like carcinoma: fifty-two patients with long-term follow-up. Cancer 2012; 118: 4748–4758. [DOI] [PubMed] [Google Scholar]
- 18.Chan A, Teo P, Lam K, et al. Multimodality treatment of primary lymphoepithelioma-like carcinoma of the lung. Cancer 1998; 83: 925–929. [DOI] [PubMed] [Google Scholar]
- 19.Qiu Z, Zhou P, Wang K. Primary pulmonary lymphoepithelioma-like carcinoma response favorably to nivolumab: a case report. Onco Targets Ther 2019; 12: 8595–8600. [DOI] [PMC free article] [PubMed] [Google Scholar]