Abstract
The COVID-19 pandemic is a worldwide threat, and information on physiopathological aspects of the disease is limited. Despite efforts in searching treatment options, a better understanding of the SARS-CoV-2 pathways can contribute to managing severe cases. In this study, we aim to describe pathological and immunopathogenic findings of two different cases, both in the high-risk group. Post-mortem lung biopsies were analyzed by traditional and immunohistochemical methods. Tissue expression of innate and adaptive immune response biomarkers was tested. We observed a higher innate response in case 1 with an abundance of mast cells, scarce CD8+ lymphocytes, high expression of TNF-alpha, and almost absent adaptative immune response. In case 2, the adaptative immune response was present, with numerous CD8+ lymphocytes and higher levels of IL-4 and TGF-beta. Both cases converged to a prothrombotic state expressing high IL-6, followed by ICAM-1 expression and endotheliites leading to systemic inflammatory response syndrome. In conclusion, differences in age and comorbidities and immune response described here may be related to the SARS-CoV-2 delay in the adaptative immune response, evolution stage of diffuse alveolar damage, and progression for systemic inflammatory response syndrome.
Keywords: COVID-19, Biopsy, Interleukin-6, Cell adhesion molecules, Systemic inflammatory response syndrome
1. Introduction
The world is currently going through the COVID-19 pandemics, and the death toll is still on the rise worldwide. Amidst this troubling time, researchers and health care providers strive to find the best therapeutic strategies, and despite all efforts, there is still no robust evidence towards a definitive treatment.
Patients infected with the SARS-CoV-2 tend to develop acute respiratory distress syndrome (ARDS) presenting ground-glass opacities on chest computed tomography exams [1], and studies show about 3.2% of patients in China required the onset of assisted breathing therapies such as intubation [2].
The SARS-CoV-2 pandemic is unique due to a few notable features in the virus: first, we observe its high affinity to the angiotensin-convertase-enzyme-2 (ACE-2) receptors [3]; secondly, it is high glycosylated spike proteins that have low immunogenicity, thirdly its recent leap from animal to human meaning that there is a high susceptibility rate. The high viability in the external environment and, finally, delayed adaptive immune response to the virus means patients are vectors for this virus for long periods. All these factors may contribute to the quick and sustained spreading of the virus [[4], [5], [6]].
Once infection reaches the lungs, pneumocytes and macrophages will initiate an innate immune response favoring a disproportionate immunologic response and endotheliites with microthrombi formation [4], pathological injuries also recently called endotheliopathy and immunothrombosis [7,8]. The complement cascade activation is then followed by the phenomenon referred to as a cytokine storm that will culminate on endothelial activation and consequently may cause a Systemic Inflammatory Response Syndrome (SIRS) with multiple organ failure and death [9].
In this article, we described findings of two post-mortem biopsies, the first being an 87-year-old woman with various comorbidities and the second a 53-year-old, obese, man without any other previous illness known. We assessed the immune response to the SARS-CoV-2 infection of these two cases and possible immunopathogenic mechanisms of the therapeutic modalities being tested during this outbreak.
2. Methods
2.1. Ethics approval
The National Research Ethics Committee approves this study (Conselho Nacional de Ética em Pesquisa - CONEP - 3.944.734/2020), and families consented to the post-mortem biopsy.
2.2. Medical records
Clinical data of both cases were obtained from medical records during hospitalization in the Intensive Care Unit (ICU) at Hospital Marcelino Champagnat in Curitiba-Brazil. Testing for COVID-19 was performed on nasopharyngeal swabs taken during ICU hospitalization, and real-time reverse transcriptase-polymerase chain reaction (rRT-PCR) performed was positive for SARS-CoV2 in both cases.
2.3. Biopsy technique and analysis
2.3.1. Autopsy procedure
Initially, the Imaging exams such as X-rays and CT's were analyzed to identify the pulmonary segments that have been lesioned, especially the lingular lobe, since it is removed in a more agile and practical technique of minimally invasive biopsy. Once we confirmed a radiographically evident lesion on the lingular segment in the upper left pulmonary lobe, we proceeded to collect the sample through a left anterior mini-thoracotomy on the fourth or fifth intercostal space.
2.3.2. Sample collection
In cases 1 and 2, the samples were similarly sized (3 × 3 cm), and the samples were delicately handled and resected using surgical scissors. Both samples presented normal pulmonary consistency. The samples were then inserted into a 10% concentration formalin solution and kept in it for at least 24 hours until blocking and slicing for microscopic analysis.
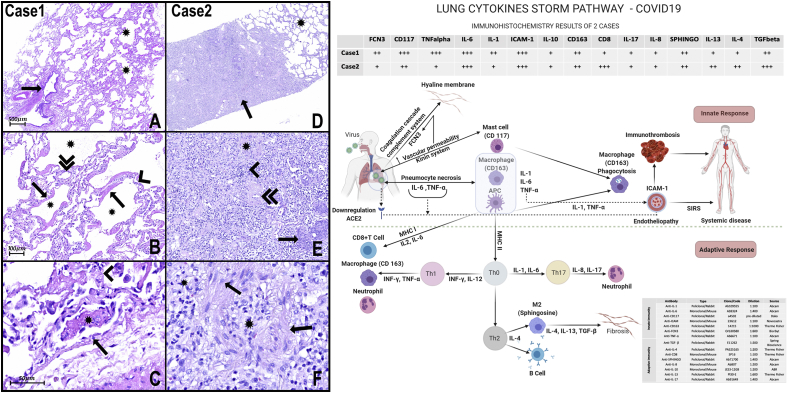
The lung formalin-fixed paraffin-embedded (FFPE) samples were stained with hematoxylin and eosin (H&E). The immunohistochemistry technique was used to identify innate and adaptive immunity biomarkers (Fig. 1 - scheme).
Fig. 1.
H&E Photomicrograph:
A- The panoramic view of the lung sample of case 1 shows one bronchiole (arrow) without histopathological alterations. There are alveoli (asterisks) with multifocal thick hyaline membranes that characterize diffuse alveolar damage (DAD).
B- Numerous alveoli (asterisks) with thick hyaline membranes (arrows) covering delicate alveolar septa (arrowhead). The septal inflammatory infiltrates very mild or almost absent, composed mainly of lymphocytes and some neutrophils, and the septal capillaries are congested (double arrowhead). There is very subtle and focal intra-alveolar edema with slight red cell overflow in some foci. Type II pneumocyte hyperplasia can also be observed.
C- There are some alveolar capillaries and small vessels with signs of endothelial activation (arrow), microthrombi (asterisk), and endotheliitis. Hyaline membranes are recovering delicate septa with very rare lymphocytes and neutrophils (arrowhead).
D- Panoramic view of lung samples of case 2 shows scattered areas of pulmonary fibrosis (arrow) interspersed with areas of histologically preserved lung tissue (asterisk).
E− Lung fibrosis area with collagen and fibroblasts (asterisk) and inflammatory infiltrate (arrowhead). The pulmonary vessels also show signs of endothelial activation and endotheliitis. There is also a thickening of the wall of medium and small arteries (double arrowhead), in addition to foci of squamous metaplasia (arrow).
F- The pulmonary fibrosis areas presented with dense inflammatory infiltrate composed mainly of lymphocytes, macrophages (arrows), and fibroblasts interspersed with areas of intense collagenization (asterisks).
Scheme of Innate and adaptive responses present in cases of COVID-19: In these cases, in response to necrosis of pneumocytes injured by SARS-COV2, we note the massive presence of mast cells (CD117) and macrophages (CD163), capable of secreting cytokines such as TNF-α and interleukins (IL) type 1 and 6, respectively. IL-6 and TNF-α could elevate vascular permeability, promoting exudation of plasma proteins, including ficolin 3 (FCN3). FCN3 and other plasmatic proteins inside the alveoli lumen are responsible for promoting opsonization, activating the complement and the coagulation cascade, and, as observed in the present work, promoting hyaline membranes. The presence of IL-1 and TNF-α, inducing massive expression of ICAM-1 in both cases, can promote the activation of endothelial cells and endotheliitis and may be involved in the progression for systemic inflammatory response syndrome (SIRS). APCs can present the antigens for T lymphocytes, via MHC (main histocompatibility complex), and secreting cytokines that play critical roles in the differentiation of T cells into effector cells. APCs express MHC I molecules and present TCD8+ lymphocytes.
On the other hand, macrophages and APC activate TCD4+ lymphocytes via MHC II, organizing responses classified into subgroups, such as Th1, Th2, Th17, depending on cell differentiation. In case 1 of the present study, a high immunoexpression of CD117 (mast cells) FCN3, TNF-α, IL-1, and Il-6 is observed, suggesting that the inflammatory process is sustained, mainly, through innate immunity. Besides, there is a low number of TCD8+ lymphocytes, suggesting that adaptive immunity is not still adequately stimulated. In contrast, in case 2, in addition to the presence of TCD8+ lymphocytes, there is a marked expression of Sphingosine (M2 macrophages), IL-4, IL1-3, and TGFβ, suggesting the presence of adequately stimulated of adaptive immunity with a tendency to resolve with fibrogenesis. A slight immunoexpression IL-8 and IL-17 is also observed, suggesting low stimulation of the Th17 response and explaining the almost absence of alveolar neutrophil migration. . (For interpretation of the references to colour in this figure legend, the reader is referred to the Web version of this article.)
3. Case DESCRIPTION
3.1. Case 1
Female, 87 years old, presenting systemic arterial hypertension (SAH), dyslipidemia, hypothyroidism, and senile dementia with two previous hospitalizations in less than 15 days. The first one, two weeks before the present hospitalization when she had a diagnosis of femur fracture, in which she had contact with a COVID-19 person (assistant physician). The second hospitalization was due to gastrointestinal bleeding (endoscopic diagnosis of Mallory Weiss laceration of the esophageal mucosa). Two days before going to the emergency department, she had flu-like symptoms without a fever. In the first hours of hospitalization, her breathing pattern worsened, requiring mechanical ventilation and vasoactive drugs. The patient's illness progressed with SIRS.
Pronation maneuver was necessary to maintain PaO2/FiO2, but the patient developed multiple organ dysfunction and died on the 8th day of hospitalization (see Table 1).
Table 1.
Summary of ventilatory parameters, therapeutic interventions, tomographic, and laboratory results.
| CASE 1 - 87-year-old woman | CASE 2 - 53-year-old man | |
|---|---|---|
| Comorbidities | Systemic Arterial Hypertension Dyslipidemia Hypothyroidism Senile dementia |
Class II obesity |
| Chronic use drugs | Losartan 50mg/day | No |
| Ventilatory parameters | Decreased positive end-expiratory pressure (PEEP), with ideal PEEP = 10 Compliance = 52 cmH2O Plateau pressure of 24 cmH2O Driving pressure of 14 cmH2O Inspiratory fraction of oxygen (FiO2) of 60% Pressure partial Oxygen (PaO2): PaO2/FiO2 = 126 |
Decreased positive end-expiratory pressure (PEEP), with ideal PEEP = 08 Compliance = 65 cmH2O Plateau pressure = 19 cmH2O Driving pressure = 11 cmH2O Inspiratory fraction of oxygen (FiO2) = 50% Pressure partial Oxygen (PaO2): PaO2/FiO2 = 132 |
| Pronation maneuver | Partial response - PaO2/FiO2 ratio of 191 | Adequate response - PaO2/FiO2 ratio = 270 |
| Admission Computed tomography | Diffuse and bilateral “opacities with ground-glass attenuation” Thickening of the pulmonary septum, suggestive of viral pulmonary infection |
Diffuse and bilateral “opacities with ground-glass attenuation”, suggestive of viral pulmonary infection |
| Admission laboratory tests | C Reactive Protein = 313 mg/L; D-dimer = 2014 μg/dL; Total leukocytes = 15,100/neutrophils = 1208 (8%) and lymphocytes = 604 (4%); Creatinine = 1.1 mg/dL |
C-Reactive Protein = 146 mg/L; D-dimer = 425 μg/mL (normal); Total leukocytes = 11,000/neutrophils = 550 (5%) and lymphocytes = 990 (9%). |
| Laboratory tests (24 hours before death) | C-Reactive Protein = 201 mg/dL; Leukocytes = 15,000/neutrophils = 2400 (16%) and lymphocytes = 1200 (8%); Creatinine = 2.65 mg/dL. |
C-Reactive Protein = 133 mg/dL; Leukocytes = 50,200/neutrophils = 3012 (6%) and lymphocytes = 3514 (7%). |
| Therapeutic drugs | Hydroxychloroquine 800 mg/day on the 1st day and 400 mg/day on the other days + Azithromycin 500 mg/day for 5 days; Oseltamivir 150 mg/day for 5 days Piperacillin + Tazobactam 18 mg/day |
Hydroxychloroquine 800 mg/day on the 1st day and 400 mg/day on the other days + Azithromycin 500 mg/day for 5 days; Oseltamivir 150 mg/day for 5 days; Ceftriaxone 2g/day |
| Invasive procedure | No | ECMO (Extracorporeal Membrane Oxygenation) |
3.2. Case 2
Male, 53 years old, class II obesity, reported traveling to the COVID-19 endemic area ten days before going to the emergency department. He had flu-like symptoms, fever, headache, and remained on spontaneous ventilation with supplemental oxygen therapy, progressing to respiratory failure and mechanical ventilation on the third day of hospitalization. During his hospitalization, his clinical condition evolved to SIRS.
Daily protonation measures were performed with good response, however, on the 10th day of hospitalization, he had a significant worsening of tissue oxygenation, requiring ECMO (Extracorporeal Membrane Oxygenation) and full anticoagulation, with diffuse bleeding as a complication, resulting in death at the 13th day of hospitalization and eighth day of mechanical ventilation (see Table 1).
4. Results
4.1. Pathological findings
Case 1 H&E analysis presented severe diffuse alveolar damage (DAD) with type II pneumocyte hyperplasia and a large number of hyaline membranes. Almost absent septal inflammatory infiltrate (neutrophils and lymphocytes) and very mild and focal intra-alveolar edema and red blood cells. There is hyperplasia of type II pneumocytes and marked endothelial activation with micro thrombosis and neutrophils on small vessel walls (endotheliitis) (Fig. 1).
In case 2, areas with preserved alveolar structure interspersed with foci of extensive alveolar fibrosis were observed. In fibrotic areas, we observed extensive alveolar collagenization associated with inflammatory infiltrate (lymphocytes and macrophages) and the presence of thickening of the vascular walls and squamous metaplasia. An intense endothelial activation with signs of endotheliitis was also observed (Fig. 1).
4.2. Immunohistochemical analysis
Case 1 revealed a predominance of innate response biomarkers tissue expression, such as ficolin 3 (FCN3) in hyaline membranes, IL-1 and TNF-alpha in pneumocytes and alveolar macrophages and abundant mast cells (CD117). Also, a slight expression of adaptive response tissue biomarkers such as CD8+ lymphocytes and IL-4, IL-13, and TGF-beta in type II pneumocytes and alveolar macrophages (Fig. 1, Fig. 2).
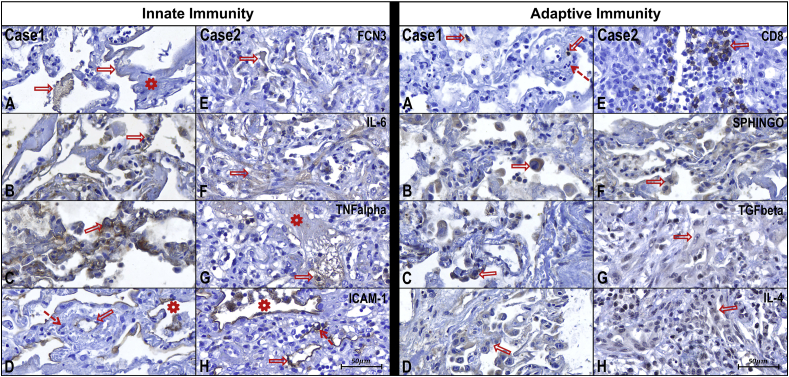
Fig. 2.
Case 1 – Innate Immunity - Photomicrographs from A to D
In photomicrograph A it can be observed FCN3 (arrows) expressed along the thick hyaline membranes (asterisk).
In B, it observed the expression of IL-6 in the alveolar septum (arrow) by lymphocytes, type II pneumocytes, and alveolar macrophages.
In C, it can observe the expression of TNF alpha in the alveolar septum (arrow) by type II pneumocytes and alveolar macrophages.
In D, it observed endothelium (arrow) and pneumocytes (asterisk) expressing ICAM-1. Presence of polymorphonuclear cells permeating the vascular wall (dashed arrow) configuring endotheliitis.
Case 2 – Innate Immunity - Photomicrographs from E to H
In photomicrograph A, there is a scarce expression of FCN3 in a few alveoli still with remaining hyaline membranes (arrow).
In B, it can observe a marked expression of IL-6 in the areas of fibrosis (arrow).
In C, it can observe a discrete expression of TNF alpha (arrow) only in a few alveoli with remaining hyaline membranes (asterisk).
In D, it observed a marked expression of ICAM-1 in endothelial cells (arrow) and pneumocytes (asterisk). Vessels show activation of endothelial cells and endotheliitis (dashed arrow).
Case 1 – Adaptive Immunity - Photomicrographs from A to D
In photomicrograph A, it can be observed scattered (about 2 per medium power field) CD8+T lymphocytes (arrows). Vessel with polymorphonuclear cells on its wall (dashed arrow) configuring endotheliitis.
In image B, it observed numerous alveolar macrophages (arrow) expressing SPHINGOSINE, which characterizes the M2 type macrophage.
In C, it can be observed a slight expression of TGF-beta in type II pneumocytes (arrow) and alveolar macrophages.
The photomicrograph D can observe moderate expression of IL-4 in type II pneumocytes (arrow) and alveolar macrophages.
Case 2 – Adaptive Immunity - Photomicrographs from E to H
In photomicrograph A, it can observe numerous (about 20–30 per medium-magnification field) CD8+ T lymphocytes (arrow).
In image B, it observed numerous alveolar macrophages (arrow) expressing SPHINGOSINE, which characterizes the M2 type macrophage.
In C, it observed a moderate expression of TGF-beta in macrophages and fibroblasts (arrow).
It can observe moderate to a marked expression of IL-4 (arrow) in macrophages and lymphocytes in photomicrography D.
In case 2, the expression of innate and adaptive response was the opposite of case 1. There was a scarce innate response, such as lower FCN3, IL-1, TNF-alpha, CD117 tissue expression. Also, a predominance of adaptive response was observed, such as higher CD8, IL-4, IL-13, and TGF-beta tissue expression (Fig. 1, Fig. 2).
Markers highly expressed in both cases were CD163 (macrophages), SPHINGO (M2 macrophages), IL6 (pneumocytes and alveolar macrophages), and ICAM-1 (activated endothelium and pneumocytes) (Fig. 1, Fig. 2).
In both cases, markers that were equally under-expressed were IL-10, IL-8, and IL-17 in type 2 pneumocytes and macrophages (Fig. 1, Fig. 2).
5. Discussion
Based on two lung biopsies, our immunohistochemical findings may be related to the SARS-CoV-2 ability to induce an earlier and sustained Th2 response along with a marked release of innate immune cytokines.
Acute Respiratory Distress Syndrome (ARDS) is a clinical condition that affects patients with a multitude of diseases ranging from cases of pneumonia to exposition to toxic fumes [10]. This process is characterized by the typical histological finding of Diffuse Alveolar Damage (DAD) in its acute phase, as well as type II pneumocyte hyperplasia and hyaline membranes, and the condition evolves to tissue organization and fibrosis [11]. In this context, both patients showed evident signs of ARDS in different stages, while patient 1 showed more acute signs of ARDS such as type II pneumocyte hyperplasia and hyaline membranes; patient 2 was already in the organization phase with evident fibrosis and scarce hyaline membranes.
Although laboratory findings evidenced SIRS in both cases, there were differences in serum lymphocyte levels at admission and lymphopenia in case 1. There is evidence that lymphopenia is a useful indicator of the severity and hospitalization in COVID-19 patients [12]. Besides, in case 1, histopathological analysis revealed few TCD8 + lymphocytes in the alveolar septa, which may have been caused by the marked lymphopenia.
The D-dimer level was a fourfold increase in case 1, average in case 2, and high levels on admission could effectively predict mortality in patients with COVID-19 [13]. Accordingly, case 1 presented the foci of micro thrombosis in capillaries and small-caliber pulmonary vessels.
The radiological findings, ventilatory parameters applied, therapeutic administered, clinical and laboratory evidence of impaired tissue oxygenation, and finally, the protonation protocol was comparable in both cases. We may hypothesize that the virus infection per se may cause all pathologic changes into lung-morphology since the ventilation of the patients during the intensive care unit (ICU) hospitalization was gentle and controlled.
SIRS caused by Cytokine Release Syndrome (CRS) or Cytokine Storm may have an essential role in the outcome of these two patients [4,9]. Diffuse Alveolar Damage (DAD) is a sign in critically ill COVID-19 infected patients [14,15], and, during its activation, the inflammatory response must be strictly regulated to prevent systemic damage (SIRS).
The lung injury caused by SARS-CoV-2 appears to begin in the upper airways where the virus replicates in the ACE-2 receptor rich respiratory mucosa [5,6]. After reaching the alveoli, the virus begins to replicate in the pneumocytes, causing cell death and alveolar septum collagen exposure. The pneumocyte necrosis causes IL-6 and TNF-alpha to be released and mast cell degranulation [4]. This scenario would lead to the triggering of the coagulation and complement cascades; it would also increase vascular permeability [9]. A sequent formation of hyaline membranes characteristic of the acute phase of DAD (case 1) is seen. In approximately two weeks of evolution, DAD initiates its resolution phase, and the hyaline membranes are replaced by type II pneumocyte hyperplasia, a chronic inflammatory process followed by pulmonary alveolar fibrosis, as shown in case 2 [14,15]. Notably, in case 1 (87-year-old woman), there is no significant alveolar edema, commonly seen in acute cases of DAD. Neutrophils and lymphocytes in the alveolar septa are also not seen, another characteristic commonly seen in viral interstitial pneumonitis. Neither case showed signs of secondary bacterial infections, a common cause of death for patients with viral pneumonitis and DAD [14].
Our combined results showed an increased number of mast cells (CD177) with TNF-alpha, IL-6, and IL-1 release (Th2 response) in case 1 and lower levels of these parameters in case 2. We also observed a high number of macrophages (CD163) in both cases. Mast cells and macrophages releasing TNF-alpha in airways with a Th2 response predisposition, as observed in case 1, have high potential to contribute to DAD [16,17]. However, in case 2, the Th2 response was still found. In our observation, case 1 showed few Th1 immune responses since the number of TCD8+ lymphocytes was discrete, contrary to case 2. Different stages of lung injury among cases could explain this discrepancy. Case 1 might have died before the fibrotic response was activated, and the immune senescence might play a role as well. While there is a four-day gap between both patients from hospitalization date to the death date, the lack of fibrotic response and inflammatory infiltrate might not be only to the time of disease progression but also to the patient's ability to respond to the infection and the effective immune response. Thus, the age gap and inherent predisposition to a Th1 or Th2 response may play significant roles in the final lung fibrosis and tissue remodeling.
The spectrum of SARS-CoV-2 injuries and their symptoms, from asymptomatic to the most severe forms, could also be related to the individual genetic background that could drive the initial prevalent Th2 response without an effective antivirus Th1 immunity since this Th1 response could be activated later. Another explanation could be the high spike proteins glycosylation present in SARS-CoV-2, which would hinder the antigenic presentation and the recollection of the virus by T cells. However, the impact of individual immune responses on the severity of SARS-CoV-2 infection is not clear [6,18].
An unusual lack of alveolar neutrophils was found and could be explained by the low expression of IL-8 and IL-17 in both cases. As the IL-17A/IL-8 are pro-inflammatory cytokines mainly dependent on activated T cells, the poor (case 1) and later (case 2) Th1 response of cases could explain this phenomenon [19].
Deposition of Ficolin-3 (FCN3) within lung septal microvasculature has been described, promoting complement system activation in COVID-19. We observed the expression of FCN3 in both cases, more evidently in case 1 (acute phase). The complement cascade precedes endothelial injury and leads to the clotting pathway activation culminating in systemic micro thrombosis and multiple organ failure [20].
Moreover, endotheliopathy and immunothrombosis may have a crucial role in SIRS observed in patients with COVID-19, as some studies described the endothelium as a potential target of the SARS-CoV-2 since they are described to express ACE-2 type receptors [21]. We also observed high expression of ICAM-1, suggesting endothelial activation as a common finding in both cases.
IL-6 levels have been associated with mortality and could be an indicator of its protagonism in the cytokine storm in COVID-19 [22], as observed in both our cases.
Regarding multiple organ failure, the patient's advanced age in case 1 could compromise the immune system activation “per se,” as shown by the inadequate adaptive immune response observed with scarce of TCD8+ lymphocytes, however, even with higher expression of CD8+ lymphocytes in case 2, the fibrosis promoted by M2 macrophage (SPHINGO) induced by a higher expression of TGF-beta, IL-13 and IL-4 that could explain the severity of lung damage observed in this case [23]. It is also important to consider that case 2 underwent 24 hours of extra corporeal membrane oxygenation and received vasoactive drugs and blood transfusion before death as these procedures may have interfered in this case's final outcome.
Clinical trials are looking at whether blocking one or more of these pro-inflammatory mediators would affect clinical outcomes. TNF-alpha blockers, IL-1 inhibitors, IL-6 antagonists, as well as complement inhibitors can reduce pro-inflammatory cytokines [3]and activation of M1/M2 macrophages leading to decreased expression of ICAM-1 and less endothelial activation and endotheliitis. The use of corticosteroids in COVID-19 could attenuate mast cells' stimulation contributing to triggering the cytokine storm [9,24,25].
This study analyzed two post mortem samples through immunohistochemistry and therefore has a limited number of patients. Besides, autopsies samples only allow for end-stage and static observation. This analysis does not necessarily reflect the evolving changes in the disease course. The patients were from different risk groups that might have influenced their immune response, thus influencing the interpretation of our results.
In summary, different immune responses presented here may be related to the SARS-CoV-2 intrinsic characteristics delaying Th1 and exacerbating Th2 response, to the stage of DAD, as well as the clinical characteristics of patients, such as age and presence or absence of comorbidities. Therefore, managing the cytokine storm, the direct virus-induced tissue damage, or the synergistic effects of both phenomena warrant further clarification.
Author contribution
Study conception (LN, CPB), study design (LN, CPB, AFRSM, JSMJ), data collection (AFRSM, JSMJ, CBVP, SN, MASM, LBC) interpretation (LN, CBVP, SN, MASM, LBC, AMN), manuscript writing (AFSM, JSMJ, LBC, AMN, LN), critical review (CPB, LBC, LN).
Funding
This research did not receive any specific grant from funding agencies in the public, commercial, or not-for-profit sectors.
Data and code availability
This study did not generate/analyze datasets or codes.
Declaration of competing interest
Authors have no conflict of interest to disclose.
Acknowledgments
The authors would like to thank the families that kindly authorized the post-mortem biopsies to be performed on their loved ones. We also thank the caregivers and staff at the Hospital Marcelino Champagnat for their bravery in these dire times. The authors thank the Postgraduate Program of Health Sciences at the school of medicine in Pontifica Universidade Catolica do Parana for funding this research.
References
- 1.Zhou S., Wang Y., Zhu T., Xia L. CT features of coronavirus disease 2019 (COVID-19) pneumonia in 62 patients in wuhan, China. AJR Am. J. Roentgenol. 2020 doi: 10.2214/AJR.20.22975. [DOI] [PubMed] [Google Scholar]
- 2.Meng L., Qiu H., Wan L., Ai Y., Xue Z., Guo Q., Deshpande R., Zhang L., Meng J., Tong C., Liu H., Xiong L. Anesthesiology; 2020. Intubation and Ventilation amid the COVID-19 Outbreak. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Hirano T., Murakami M. COVID-19: a new virus, but a familiar receptor and cytokine release syndrome. Immunity. 2020 doi: 10.1016/j.immuni.2020.04.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.McGonagle D., Sharif K., O'Regan A., Bridgewood C. The role of cytokines including interleukin-6 in COVID-19 induced pneumonia and macrophage activation syndrome-like disease. Autoimmun. Rev. 2020 doi: 10.1016/J.AUTREV.2020.102537. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Li W., Moore M.J., Vasllieva N., Sui J., Wong S.K., Berne M.A., Somasundaran M., Sullivan J.L., Luzuriaga K., Greeneugh T.C., Choe H., Farzan M. Angiotensin-converting enzyme 2 is a functional receptor for the SARS coronavirus. Nature. 2003:450–454. doi: 10.1038/nature02145. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Mercurio I., Tragni V., Busco F., De Grassi A., Pierri C.L. Protein structure analysis of the interactions between SARS-CoV-2 spike protein and the human ACE2 receptor: from conformational changes to novel neutralizing antibodies. BioRxiv. 2020:2020. doi: 10.1101/2020.04.17.046185. 04.17.046185. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Goshua G., Pine A.B., Meizlish M.L., Chang C.-H., Zhang H., Bahel P., Baluha A., Bar N., Bona R.D., Burns A.J., Dela Cruz C.S., Dumont A., Halene S., Hwa J., Koff J., Menninger H., Neparidze N., Price C., Siner J.M., Tormey C., Rinder H.M., Chun H.J., Lee A.I. Endotheliopathy in COVID-19-associated coagulopathy: evidence from a single-centre, cross-sectional study. Lancet. Haematol. 2020 doi: 10.1016/S2352-3026(20)30216-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Ackermann M., Verleden S.E., Kuehnel M., Haverich A., Welte T., Laenger F., Vanstapel A., Werlein C., Stark H., Tzankov A., Li W.W., Li V.W., Mentzer S.J., Jonigk D. Pulmonary vascular endothelialitis, thrombosis, and angiogenesis in covid-19. N. Engl. J. Med. 2020 doi: 10.1056/nejmoa2015432. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Mehta P., McAuley D.F., Brown M., Sanchez E., Tattersall R.S., Manson J.J. Lancet; London, England): 2020. HLH across Speciality Collaboration, UK, COVID-19: Consider Cytokine Storm Syndromes and Immunosuppression. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Ferguson N.D., Fan E., Camporota L., Antonelli M., Anzueto A., Beale R., Brochard L., Brower R., Esteban A., Gattinoni L., Rhodes A., Slutsky A.S., Vincent J.L., Rubenfeld G.D., Taylor Thompson B., Marco Ranieri V. The Berlin definition of ARDS: an expanded rationale, justification, and supplementary material. Intensive Care Med. 2012 doi: 10.1007/s00134-012-2682-1. [DOI] [PubMed] [Google Scholar]
- 11.Cardinal-Fernandez P., Lorente J.A., Ballen-Barragan A., Matute-Bello G. Acute respiratory distress syndrome and diffuse alveolar damage new insights on a complex relationship. Ann. Am. Thorac. Soc. 2017 doi: 10.1513/AnnalsATS.201609-728PS. [DOI] [PubMed] [Google Scholar]
- 12.Tan L., Wang Q., Zhang D., Ding J., Huang Q., Tang Y.Q., Wang Q., Miao H. Lymphopenia predicts disease severity of COVID-19: a descriptive and predictive study. Signal Transduct. Target. Ther. 2020 doi: 10.1038/s41392-020-0148-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Zhang L., Yan X., Fan Q., Liu H., Liu X., Liu Z., Zhang Z. D-dimer levels on admission to predict in-hospital mortality in patients with Covid-19. J. Thromb. Haemostasis. 2020 doi: 10.1111/jth.14859. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Fox S.E., Akmatbekov A., Harbert J.L., Li G., Brown J.Q., Vander Heide R.S. Pulmonary and cardiac pathology in covid-19: the first autopsy series from new orleans. MedRxiv. 2020 doi: 10.1101/2020.04.06.20050575. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Barton L.M., Duval E.J., Stroberg E., Ghosh S., Mukhopadhyay S. COVID-19 autopsies, Oklahoma, USA. AJCP/Orig. Artic. Am J Clin Pathol. 2020 doi: 10.1093/ajcp/aqaa062. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Marcet C.W., Laurent C.D. St, Moon T.C., Singh N., Befus A.D. Limited replication of influenza A virus in human mast cells. Immunol. Res. 2013 doi: 10.1007/s12026-012-8377-4. [DOI] [PubMed] [Google Scholar]
- 17.Soy M., Atagündüz P., Atagündüz I., Sucak G.T. Hemophagocytic lymphohistiocytosis: a review inspired by the COVID-19 pandemic. Rheumatol. Int. 2020 doi: 10.1007/s00296-020-04636-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Severance E.G., Bossis I., Dickerson F.B., Stallings C.R., Origoni A.E., Sullens A., Yolken R.H., Viscidi R.P. Development of a nucleocapsid-based human coronavirus immunoassay and estimates of individuals exposed to coronavirus in a U.S. metropolitan population. Clin. Vaccine Immunol. 2008 doi: 10.1128/CVI.00124-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Wong C.K., Cao J., Yin Y.B., Lam C.W.K. Interleukin-17A activation on bronchial epithelium and basophils: a novel inflammatory mechanism. Eur. Respir. J. 2010 doi: 10.1183/09031936.00088309. [DOI] [PubMed] [Google Scholar]
- 20.Magro C., Mulvey J.J., Berlin D., Nuovo G., Salvatore S., Harp J., Baxter-Stoltzfus A., Laurence J. Complement associated microvascular injury and thrombosis in the pathogenesis of severe COVID-19 infection: a report of five cases. Transl. Res. 2020 doi: 10.1016/j.trsl.2020.04.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Varga Z., Flammer A.J., Steiger P., Haberecker M., Andermatt R., Zinkernagel A.S., Mehra M.R., Schuepbach R.A., Ruschitzka F., Moch H. Endothelial cell infection and endotheliitis in COVID-19. Lancet. 2020 doi: 10.1016/S0140-6736(20)30937-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Wang W., He J., Lie P., Huang L., Wu S., Lin Y., Liu X. The definition and risks of cytokine release syndrome-like in 11 COVID-19-infected pneumonia critically ill patients: disease characteristics and retrospective analysis. MedRxiv. Feb. 2020;27 doi: 10.1101/2020.02.26.20026989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Uhl E.W., Castleman W.L., Sorkness R.L., Busse W.W., Lemanske J., McAllister P.K. Parainfluenza virus-induced persistence of airway inflammation, fibrosis, and dysfunction associated with TGF-β1 expression in brown Norway rats. Am. J. Respir. Crit. Care Med. 1996 doi: 10.1164/ajrccm.154.6.8970378. [DOI] [PubMed] [Google Scholar]
- 24.Zhao M. Cytokine storm and immunomodulatory therapy in COVID-19: role of chloroquine and anti-IL-6 monoclonal antibodies. Int. J. Antimicrob. Agents. 2020 doi: 10.1016/j.ijantimicag.2020.105982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Feldmann M., Maini R.N., Woody J.N., Holgate S.T., Winter G., Rowland M., Richards D., Hussell T. Trials of anti-tumour necrosis factor therapy for COVID-19 are urgently needed. Lancet. 2020 doi: 10.1016/S0140-6736(20)30858-8. (London, England) [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
This study did not generate/analyze datasets or codes.