Abstract
Outbreaks and the rapid transmission of viruses, such as coronaviruses and influenza viruses, are serious threats to human health. A major challenge in combating infectious diseases caused by viruses is the lack of effective methods for prevention and treatment. Nanotechnology has provided a basis for the development of novel antiviral strategies. Owing to their large modifiable surfaces that can be functionalized with multiple molecules to realize sophisticated designs, nanomaterials have been developed as nanodrugs, nanocarriers, and nano-based vaccines to effectively induce sufficient immunologic memory. From this perspective, we introduce various nanomaterials with diverse antiviral mechanisms and summarize how nano-based antiviral agents protect against viral infection at the molecular, cellular, and organismal levels. We summarize the applications of nanomaterials for defense against emerging viruses by trapping and inactivating viruses and inhibiting viral entry and replication. We also discuss recent progress in nano-based vaccines with a focus on the mechanisms by which nanomaterials contribute to immunogenicity. We further describe how nanotechnology may improve vaccine efficacy by delivering large amounts of antigens to target immune cells and enhancing the immune response by mimicking viral structures and activating dendritic cells. Finally, we provide an overview of future prospects for nano-based antiviral agents and vaccines.
Keywords: Virus, Infection diseases, Nanomaterials, Antiviral agents and mechanisms, Nanovaccines
Graphical abstract
1. Introduction
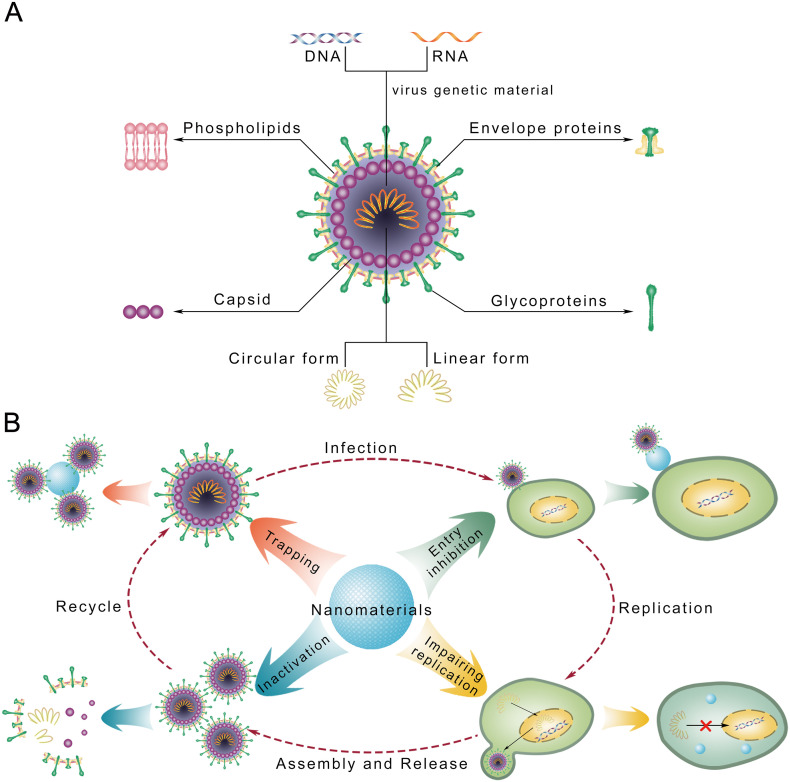
A virus particle is made up of genetic material and a capsid (Fig. 1A). Housed inside the protein-based capsid, the viral genome consists of single-stranded or double-stranded DNA or RNA in linear or circular form. Some viruses, such as human immunodeficiency virus and coronaviruses, have viral envelopes covering capsids. Typically derived from host cell membranes, the envelopes are composed of phospholipids and proteins and may include viral glycoproteins. Infectious diseases caused by viruses have long been serious threats to global public health [1,2]; for example, smallpox and yellow fever have resulted in millions of deaths. In recent years, public health crises have emerged due to epidemics and pandemics of new viruses, including SARS-CoV, MERS-CoV, and H7N9 [3,4]. The recent pandemic of COVID-19 caused by SARS-CoV-2 has become a global health crisis [5,6]. The lack of effective treatments remains a primary challenge in the fight against emerging viral threats [7,8].
Fig. 1.
Schematic representation of how nanomaterials inhibit virus infections. (A) The composition of a virus with envelop. (B) Nano particles could play antiviral effects by mechanisms including: inactivating virus; trapping and detention of virus; inhibiting cellular entry of virus; blocking the replication of virus.
The majority of available antiviral agents are synthetic agents, such as nucleoside analogues that prevent viral genome replication and protease inhibitors that selectively bind to viral proteases and block proteolytic cleavage of viral protein precursors [[9], [10], [11]]. Recently, antibodies targeting specific viral proteins have been developed [12,13]. However, novel antiviral agents are urgently needed for newly emerging virus strains. Operating at the nanoscale (1–100 nm), nanotechnology paves a new path for the development of antiviral agents. The unique properties of nanomaterials, such as their small sizes, high surface-to-volume ratios, and modifiable surfaces, are beneficial for contact with viruses and contribute to multiple antiviral effects, such as the inactivation of viruses and blocking viruses from entering host cells [14,15].
Historically, vaccines have been vital against smallpox, polio, hepatitis A, and papilloma [[16], [17], [18]]. However, conventional vaccines are not applicable to some viral infections for two key reasons. First, some viruses are difficult to produce in vitro, which is required for the development of vaccines composed of inactive or attenuated viruses. Second, although vaccines carrying peptide antigens or mRNAs encoding antigens are an alternative, they are limited by low stability and degradation in vivo. Nanomaterials can as act carriers to protect antigens from degradation and improve immune responses, which in turn improves the effectiveness of nanovaccines. Since the first nanovaccine against hepatitis B virus (HBV) was licensed in 1986, nanotechnology has been applied to develop vaccines against human papillomaviruses (HPV) and hepatitis E virus (HEV), and positive preclinical outcomes have been obtained for HIV and respiratory viruses [[19], [20], [21]]. In light of emerging viruses, such as SARS-CoV-2, nano-based vaccines have received substantial attention [22].
In this review, we focus on nano-based antiviral agents and vaccines, which are among the most promising approaches for countering outbreaks of emerging viral infections. To provide insight into the use of nanotechnology to manage viral threats, the antiviral mechanisms of nanomaterials as well as recent progress in the development of nano-based vaccines are summarized.
2. Antiviral effects of nanomaterials
Viruses invade cells in three steps: (i) contact with the cell membrane and entry into the intracellular space; (ii) amplification of the viral genome and expression of the viral proteome; (iii) assembly of the new virus and release to the extracellular space, inducing infection [[23], [24], [25]]. Nanomaterials have been reported to suppress cell entry and viral replication; moreover, their numerous surface binding sites facilitate interactions with target molecules, consequently trapping and inactivating viruses (Fig. 1B; Table 1 ) [26,27].
Table 1.
Nano-based antiviral agents.
| Antiviral effect | Nanomaterial | Virus | Mechanism |
|---|---|---|---|
| Trapping virus | CD4+ T-cell-derived vesicles | HIV-1 | Attaching effectively to HIV-1, preventing it from binding to and entering healthy CD4+ T cells [29] |
| Membrane vesicle coated with human sodium taurocholate co-transporting polypeptide | HBV | Trapping HBV, protecting host cells from viral infection [30] | |
| Plasma membranes of CD4+ T cells coated on polymeric cores | HIV | Neutralizing HIV and diverting the viruses away from peripheral mononuclear blood cells and human-monocyte-derived macrophages by selectively binding with viral gp120 [32] | |
| Polymeric core covered by membrane | Zika virus | Trapping Zika virus (ZIKV) and divert it away from its healthy cellular targets [34] | |
| Polymeric cores coated by the plasma membranes derived from human lung epithelial type II cells or human macrophages | SARS-CoV-2 | Neutralizing SARS-CoV-2 and protecting healthy cell from viral infection, as a result of displaying the same protein receptors required by SARS-CoV-2 for cellular entry [36] | |
| Inhibiting viral entry | Polyethylene glycol encapsulated AuNPs | HIV-1 | Blocking gp120 attachment with CD4+ cells [38] |
| SiNPs with –OH and –NH2 groups | HIV-1 | Blocking gp120 attachment with CD4+ cells [39] | |
| AgNPs | HIV-1 | Interacting with viral gp120 in both cell-free and cell-associated virus [40] | |
| AgNPs | H7N3 | Blocking the function of viral hemagglutinin, leading to the hindered viral entry [41] | |
| AgNPs | HSV-2 | Interacting with sulfhydryl group of membrane glycoproteins, thus preventing viral internalization [42] | |
| Tannic acid functionalized AgNPs | HSV-2 | Tannic-acid modification increases biological affinity of AgNPs for viral glycoproteins [44] | |
| Glycosaminoglycan modified SiNPs | HSV-1, 2 | Glycosaminoglycans could bind to the viral glycoproteins [45] | |
| Nanogels based on dendritic polyglycerol sulfate to mimic cellular membrane heparan sulfate | HSV-1 | Multivalently interacting with viral glycoproteins, shielding virus surfaces, and efficiently blocking virus infection [15] | |
| Polyquaternary phosphonium oligochitosans decorated AgNPs (PQPOCs-AgNPs) | Hepatitis A virus (HAV), norovirus (NoV) and Coxsackievirus B4 (CoxB4) | Binding of AgNPs to the viral active sites and electrostatic interaction between the positive brushes of PQPOCs and negative targets of viruses [47] | |
| Carbon-based fullerenes | Pseudotyped viral particles (Ebola virus) | Blocking membrane DC-SIGN mediated viral entry [[48], [49], [50]] | |
| Carbon dots | Human Norovirus virus-like-particles | Blocking membrane HBGAs mediated viral entry [53] | |
| Inhibiting viral replication | AuNPs | Foot- and mouth- disease virus (FMDV) | Binding to FMDV RNA, sub-genomic RNA, or viral replicative proteins [54] |
| Fullerene derivatives | Wild-type HIV-1, resistant HIV-1 | Affecting viral maturation of wild-type HIV-1 by inhibiting Gag processing, and maturation of resistant HIV-1 viruses by impairing viral polyprotein processing through a protease-independent mechanism [56] | |
| AgNPs | Peste des petits ruminants' virus (PPRV) | Interacting with virion surface and core protein, impairing viral replication and entry [57] | |
| AgNPs | dsRNA viruses | Interacting with viral genome [58] | |
| Carbon dots | PRV, porcine reproductive and respiratory syndrome virus | Inducing antiviral response of interferon-α (IFN-α) production and the expression of IFN-stimulating genes (ISGs) in host cells [59] | |
| Poly(aniline-co-pyrrole) polymerized nanoregulators (PASomes) | H1N1, H3N2, and H9N2 | Controlling intracellular ROS levels, resulting in downregulating MEK/ERK pathway-based viral replication [60] | |
| Viral inactivation | Protoporphyrin IX attached acid-functionalized multi-walled carbon nanotubes | H3N2 | Binding and destroying viral envelope by photoactivated protoporphyrin IX [62] |
| Negatively charged GO | Pseudorabies virus (PRV) and porcine epidemic diarrhea virus | Viral envelop damage due to its single-layer structure and sharp edge [63] | |
| TiO2 NPs | Influenza virus (H3N2) | Interacting with and destroying the viral envelop [64] | |
| Graphene oxide (GO) and GO conjugated Ag nanoparticles | Feline Coronavirus (FCoV) | Binding to viral lipid tails, leading to rupture of the envelop [65] | |
| AgNPs | HSV-1, 2 and HPIV-3 | Interacting with and destroying the viral envelop [43] | |
| TiO2 NPs | MS2 | Binding and destroying viral capsid protein through a photocatalytic effect [14,66] | |
| AgNP-MHCs | Murine norovirus, Adenovirus serotype 2 and Bacteriophage ɸX174 | Binding and damaging thiol group-containing biomolecules embedded in the capsid proteins [68] | |
| AgNPs | Poliovirus | Interacting with and destroy the viral proteins, leading to damaging the structure of virus particles [69] | |
| Au/CuS core/shell NPs | Norovirus-Like Particles | Capsid protein degradation and capsid damage [70] |
2.1. Trapping effects of nanomaterials
To invade, a virus first attaches itself to the membrane of host cells. This behavior is the basis for broad-spectrum anti-infection nanomaterials based on cell membrane properties, known as nanodecoys. The surface of nanodecoys may be derived from cell membranes, providing cell-surface receptors that are recognized by and trap the virus [28]. Indeed, vesicles derived from CD4+ T cell membranes show effective attachment by HIV-1, thereby preventing HIV-1 from binding to and entering healthy CD4+ T cells [29]. Another artificially engineered membrane-derived vesicle in which the surface is coated with a human sodium taurocholate co-transporting polypeptide effectively blocks HBV infection, spread, and replication in a mouse model [30].
However, cell membrane-derived vesicles are prone to membrane fusion due to the fluid-filled vesicles and cell membranes, thus generating a risk that vesicles bring detained viruses to healthy cells [31]. Therefore, cell membrane-coated nanostructures, in which the membrane is fixed and stabilized by a nano-core, have been developed to avoid unwanted membrane fusion [28,32,33]. Cell membrane-coated nanodecoys effectively trap Zika virus (ZIKV), divert it from healthy cells, and successfully mitigate ZIKV-induced inflammatory responses and fetal microcephaly [34]. In addition to the decreased membrane fusion property, the core-coated structure in which a coated lipid membrane is stabilized by a nanoparticle core also stabilizes cell membrane-coated nanomaterials, which is beneficial for in vivo applications with an extended half-life [35]. Recently, cell membrane-coated nanodecoys for SARS-CoV-2 have been established by using plasma membranes derived from human lung epithelial type II cells or human macrophages to coat polymeric cores [36]. Displaying receptors similar to those required by SARS-CoV-2 for cellular entry, these nanodecoys neutralize SARS-CoV-2 and protect mice from infection [36].
2.2. Inhibition of viral entry by nanomaterials
The inhibitory effect of nanomaterials on cell entry by viruses and related mechanisms have been studied extensively. Viral entry usually requires the interaction between viral surface protein(s) and receptor(s) on host cell membranes; thus, nanomaterials that interfere with such interactions are promising antiviral agents.
Some nanomaterials interrupt virus–cell interactions by blocking viral surface proteins. The HIV envelope protein gp120, a glycoprotein exposed on the surface of the HIV envelope that targets CD4+ cells, is critical for the binding of HIV to CD4+ T cells [37]. Two anti-HIV-1 nanomaterials, gold nanoparticles (AuNPs) encapsulated by polyethylene glycol and silicon nanoparticles (SiNPs) modified with -OH and -NH2 groups, function by blocking gp120 on the HIV-1 surface [38,39]. Silver nanoparticles (AgNPs) are able to bind to multiple molecules and have broad-spectrum antiviral effects by inhibiting viral entry. For example, commercially available AgNPs show a strong effect against HIV-1 by interacting with viral gp120 in both cell-free and cell-attached viruses, thus impeding viral binding and entry [40]. AgNPs also hinder the attachment of Avian influenza A virus H7N3 to host cells by blocking influenza hemagglutinin, which plays a critical role in cell entry [41]. Moreover, AgNPs block the entry of herpes simplex virus (HSV)-1, HSV-2, and human parainfluenza virus (HPIV)-3 by interacting with the sulfhydryl group on viral glycoproteins [42,43]. In addition to nanoparticles (NPs), dendritic polyglycerol sulfate-based nanogels that mimic the cellular membrane heparan sulfate can actively interact with viral glycoproteins, shield virus surfaces, and efficiently block HSV-1 infection [15].
Modifications to nanomaterials promote their ability to block viral surface proteins. AgNPs modified with TA have an increased affinity for viral glycoproteins and reduce HSV-2 infectivity by blocking viral attachment, penetration, and spread [44]. Similar inhibitory effects against HSV-1 and HSV-2 entry have been observed using SiNPs modified with glycosaminoglycans [45], which bind to the “repeat structure” of viral glycoproteins and amyloids [46]. Polyquaternary phosphonium oligochitosan (PQPOC)-decorated AgNPs can inhibit multiple human enteric viruses, including hepatitis A virus (HAV), norovirus (NoV), and Coxsackievirus B4 (CoxB4), via electrostatic interactions between the positively charged PQPOC brushes and negatively charged viral surface targets [47].
Additionally, nanomaterials may inhibit viral entry by blocking cell membrane receptors. For example, several carbon-based fullerenes inhibit the entry of pseudotyped viral particles by blocking the dendritic cell (DC)-specific intercellular adhesion molecule-3-grabbing non-integrin (DC-SIGN) on the cell membrane [[48], [49], [50]], which plays a critical role in the internalization of Ebola virus [51]. Carbon dots established from 2,2′-(ethylenedioxy)bis(ethylamine) and 3-ethoxypropylamine suppress the entry of human norovirus-like particles by interacting with the histoblood group antigens (HBGAs) of host cells, a norovirus receptor [52,53].
2.3. Inhibition of viral replication by nanomaterials
Nanomaterials can inhibit viral replication by interacting with the viral protein/genome or inducing a suppressive environment for intracellular viral replication. AuNPs arrest foot-and-mouth disease virus (FMDV) replication at the post-entry stage and block viral transcription in the host cell by binding to FMDV RNA, sub-genomic RNA, or viral replicative proteins via electrostatic interactions [54,55]. Moreover, several fullerene derivatives can affect the maturation of both wild-type HIV-1 and drug-resistant HIV-1 by inhibiting Gag polyprotein processing, which generates the matrix, capsid, nucleocapsid, and p6 gag proteins required for a functional virus, thereby hindering viral replication [56]. AgNPs also inhibit the replication of dsRNA viruses and Peste des petits ruminants virus (PPRV) by interacting with the viral genome or core proteins related to RNA synthesis [57,58].
In addition to direct contact with the viral protein/genome, nanomaterials can also inhibit viral replication by producing an unfavorable intracellular environment for viral replication. Carbon dots established from PEG-diamine and ascorbic acid induce interferon-α (IFN-α) production and the expression of IFN-stimulating genes in host cells, resulting in powerful antiviral responses and the inhibition of the replication of pseudorabies virus (PRV) and porcine reproductive and respiratory syndrome virus (PRRS) [59]. As an organic nanomaterial, poly(aniline-co-pyrrole) polymerized nanoregulators (PASomes) control intracellular reactive oxygen species (ROS) levels in vitro to inhibit viral replication and cell death [60]. Since certain viruses can hijack host cell pathways to cause the biphasic activation of the MEK/ERK cascade, which improves viral replication [61], investigations of the precise regulation of the intracellular response to viral infection are still warranted when designing antiviral agents.
2.4. Viral inactivation effects of nanomaterials
Nanomaterials, such as Ag, Ti, and carbon-based nanomaterials, directly contact viruses and induce viral inactivation by different mechanisms depending on the nanomaterial and virus. The destruction of the viral envelope by photocatalytic oxidation or physical damage is a common mechanism by which nanomaterials induce the inactivation of viruses with envelopes. A multi-walled carbon nanotube attached to photoactivating protoporphyrin IX interacts with and destabilizes the envelope of influenza virus H3N2 by ROS [62]. Another interesting viral inactivation effect was observed using negatively charged graphene oxide (GO), which interacts with the viral envelope by electrostatic interactions and damages the viral envelope via its sharp-edged single-layer structure [63]. There are also envelope-binding NPs that cause the destruction and disintegration of viruses by unknown mechanisms, including TiO2 NPs on H3N2 [64]. Additionally, GO-conjugated AgNPs bind to the lipid tails of feline coronavirus (FCoV) and lead to envelop rupture [65].
Similar to the envelope-rupture effect, some nanomaterials inactivate viruses by destroying the viral capsid via photocatalytic oxidation and protein degradation. TiO2 NPs interact with viral capsid proteins and inactivate viruses, such as influenza virus and MS2 bacteriophage, via photocatalytic oxidation in which catalytic inactivation is mediated by hydroxide radicals [14,66,67]. AgNP-MHCs (aminopropyl-functionalized Fe3O4-SiO2 core-shell magnetic hybrid colloid-decorated AgNPs) bind to and degrade thiol group-containing viral capsid proteins, thus inactivating murine norovirus, adenovirus serotype 2, bacteriophage ɸX174, and poliovirus [68,69]. Moreover, by interacting with capsid proteins and inducing degradation via copper ions in NPs, an Au/CuS core-shell NP causes the inactivation of human norovirus-like particles [70].
3. Nano-based vaccines
Nano-based vaccines, or nanovaccines, are vaccines that use NPs as carriers. A variety of NPs could be used for nanovaccine construction, such as protein/peptide self-assembled virus-like particles (VLPs), inorganic-based NP-like AuNPs, and organic-based NPs, such as lipid nanoparticles (LNPs) (Table 2 ). Nanovaccines improve antigen bioavailability in vivo and influence the innate and adaptive immune responses, including the generation of memory cells, which are essential for the efficacy of a vaccine (Fig. 2 ) [[71], [72], [73], [74], [75]].
Table 2.
Nano-based vaccines.
| Virus | Antigen | Nanovaccine | Outcome |
|---|---|---|---|
| HBV | HBsAg | VLPs | In clinical use [109] |
| HPV | Capsid L1 proteins | VLPs | In clinical use [81] |
| HEV | HEV p239 (aa 368–606) | VLPs | In clinical use [82] |
| SARS-CoV | Spike proteins | VLPs formed by SARS-CoV spike protein and influenza M1 protein | Induced strong immune response and protectd mice from death [110] |
| SARS-CoV | Spike proteins | AuNPs | Increased IgG response [111] |
| MERS-CoV | Spike proteins | Spike protein NPs | Stimulated significant titers of neutralizing antibody and Th2 immune response [112] |
| MERS-CoV | Spike proteins | Ferritin assembled VLPs | Stimulated CD4+ T-cells and IFN−/ TNF- responses [113] |
| MERS-CoV | Spike proteins | Hollow polymeric NPs | Stimulated remarkable levels of humoral responses and IgG2a antibodies [114] |
| MERS-CoV | Spike proteins | Spike protein NPs with aluminum or Matrix M1 as adjuvant | Produced high titer anti-spike neutralizing antibody and protected mice from MERS-CoV infection in vivo [148,149] |
| SARS-CoV-2 | mRNA encoding a full-length spike protein | Lipid nanoparticles (LNPs) | Passed phase I trial, induced high titer of neutralizing antibodies (NCT04283461); currently under phase III trial (NCT04470427) |
| SARS-CoV-2 | mRNA encoding spike protein or its different fragments | LNPs | In clinical trials (NCT04368728; NCT04449276, ISRCTN1707269 and NCT04480957) |
| HIV | Envelope protein (Env) trimers | Env trimer of various HIV-1 strains self-assembled VLPs | Stimulated broadly neutralizing antibodies (bNAs) against diverse virus strains in rabbits [108] |
| HIV | Env gp120/gp41 | Env trimer self-assembled VLPs | Induced high titers of bNAs against diverse virus strains in rabbits [125,126] |
| HIV | HIV Env antigens | Polystyrene NPs | An increase in both bNAs and antibody-secreting cells in mice [127] |
| Influenza virus | Influenza whole virus | Mucoadhesive carrier chitosan | Successful nasal mucosa immunization in rabbits, significant levels of anti-hemagglutinin antibody, local anti-influenza-virus IgA, systemic IL-2, and IFN-γ were detected in the serum [131] |
| Influenza virus | Killed swine influenza virus antigens | Cationic alpha-D-glucan nanoparticles with TLR3 agonist poly(I:C) as adjuvant | Induced high levels of virus neutralizing antibodies in bronchoalveolar lavage fluid and cross-reactive virus-specific secretory IgA antibodies in the nasal passage and lungs [132] |
| H9N2 | Formalin-inactivated H9N2 virus | Poly(lactic-co-glycolic acid) (PLGA) | Generating a significantly stronger antibody response in chickens, as indicated by the HI titer, than non-encapsulated forms [133] |
| Influenza virus | Influenza split vaccine | VLPs | Shaped cellular immune responses toward T helper type 1 responses increasing IgG2a isotype antibodies as well as IFN-γ producing cells in mucosal and systemic sites of mice [150] |
| Foot-and-mouth virus (FMDV) | FMDV VP1 protein | Calcium mineralized FMDV VP1 VLPs | Improved thermal stability and extend the storage time of the vaccine, accompanied with effectively activating DCs to express high levels of surface MHC-II, costimulatory molecules, and proinflammatory cytokines, leading to enhanced immune response [95] |
| FMDV | VP1 protein | Polyelectrolyte complexation of chitosan and heparin with tumor necrosis factor α (TNF) or CpG as adjuvants | Induced strong immune activation toward antibody production, elicited strong IgA titers, and conferred effective protection against lethal virus challenge in mice comparable to the traditional vaccine [135] |
| FMDV | FMDV capsid proteins VP0, VP1, and VP3 | Hollow mesoporous silica nanoparticles (HMSNs) loaded the (FMDV) VLPs | Induced persistent humoral immunity with high-level antibody titer for more than three months, accompanied with improved T-lymphocyte proliferation and IFN-γ, and the ideal protection against FMDV challenge in guinea pigs [136] |
| Dengue virus (DENV) | Dengue EDIII antigens | Bacterial membrane vesicles | Evoking dengue-specific humoral immune responses and confering effective protection against DENV-2 infection in mice [137] |
| DENV | DENV-2 E protein | PLGA NP | Increased the anti-E IgG titer and improved the neutralizing capacity of the antibodies in mice, compared to free E protein [138] |
| DENV | UV-inactivated DENV-2 | Chitosan with Mycobacterium bovis Bacillus Calmette-Guerin cell wall components as an adjuvant | Vaccinated mice exhibited upregulated expression of IFN-γ, IL-2, IL-5, IL-12p40, IL-12p70, and IL-17, an increased frequency of CD4+ and IFN+ T cells, and higher levels of IgG antibodies [139,140] |
| DENV | DENV-3 E protein | Chitosan NP | Taken up more efficiently by nasal epithelial cells than free E protein and resulted in increased secretion of IL-1β, IL-6, and TNF-α [141] |
| West Nile virus (WNV) | Domain III of the envelope glycoprotein | VLPs | Inducing high titers of virus-neutralizing antibodies, and completely protecting mice from WNV infection [142] |
| Hepatitis C virus (HCV) | E2 subunit of the envelope glycoprotein | Lipid-based nanovaccines | Eliciting 6- to 20-fold higher E2-specific serum IgG titers in mice, compared to soluble antigens [143] |
| Respiratory syncytial virus (RSV) | RSV F protein | VLPs | Passed phase III clinical trials (NCT02624947) |
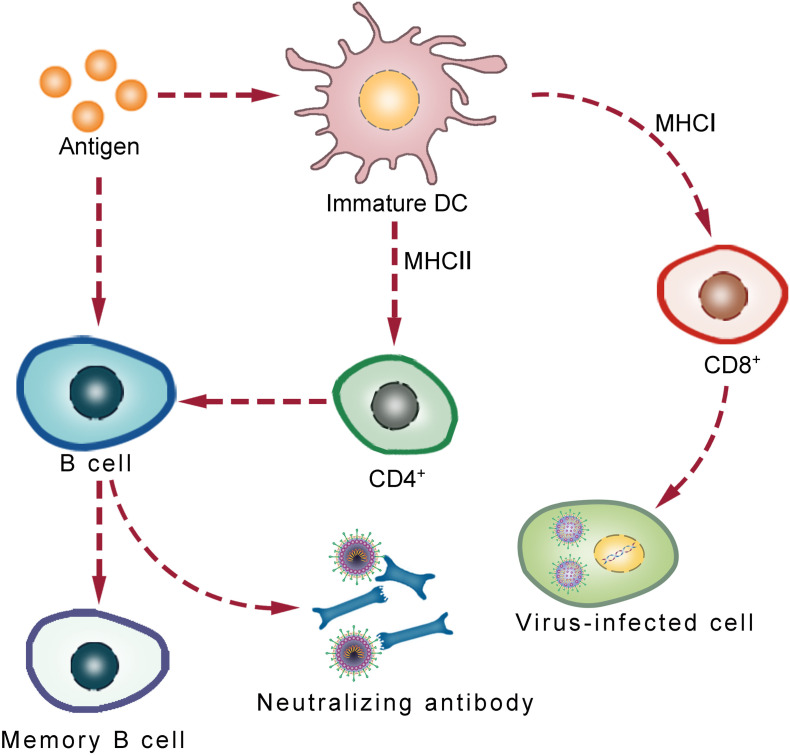
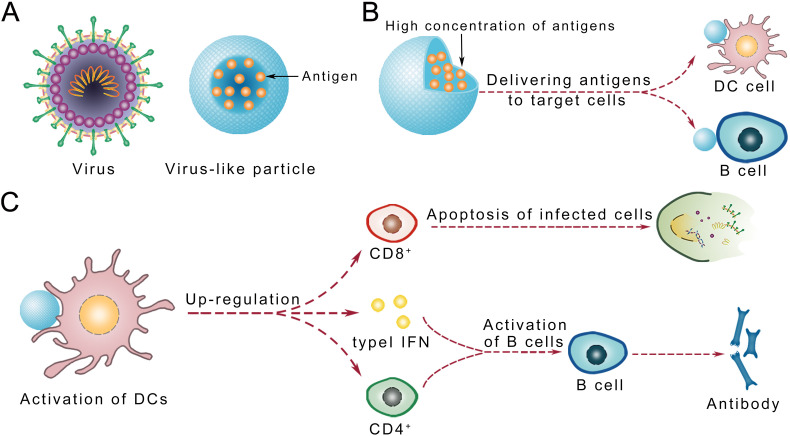
Fig. 2.
Schematic representation of the key steps of immune responses induced by nanovaccines. B cell activation is facilitated by directly attached antigens or CD4+ cell-passed antigens that come from DC cells. B cell activation leads to the production of antibodies that neutralize antigens. Moreover, DC cells pass the antigen to CD8+ cells, which activates of CD8+ cells to kill infected cells.
3.1. Nanoparticle properties related to the induction of the immunological response
Immunological benefits of nano-based vaccines result from a combination of the following properties: the ability to mimic viruses in terms of size and structure (pathogen-mimicking VLPs), the ability to deliver viral antigens to target immune cells, the ability to activate DCs, and the ability to efficiently activate B cells and induce humoral immune responses (Fig. 3 ).
Fig. 3.
Schematic representation of the advantages of nano-base vaccines. (A) Model of virus-like particle (VLPs). (B) Nano-based vaccines load a high concentration of antigens and deliver them to targeted immune cells; (C) Nano-based vaccines activate dendritic cells and B cells.
3.1.1. Pathogen mimicry and virus-like particles
VLPs are self-assembled noninfectious NPs that lack genetic materials [76,77]. They could be formed by antigen peptides or self-assembled proteins, such as ferritin. Antigens on VLPs optimize the activation of specific immune responses, including humoral and cellular immunity [78]. With innate viral structures, VLP vaccines benefit from their ability to interact with the immune system without causing infections and inducing an immune response without an adjuvant, which improves the immune response of vaccines [79,80].
VLPs are the most widely applied nanosystem for antiviral vaccines. Currently available HPV vaccines, such as Gardasil, Gardasil 9, and Cervarix, which effectively protect women from HPV infection, are all nano-based VLP vaccines [81]. A VLP vaccine against HEV, Hecolin, is also commercially available [82].
3.1.2. Loading and delivery of viral molecules
The targeted delivery of antigen peptides or proteins to specific tissues is a major advantage of nanovaccines [19,20,83]. Moreover, since unprotected peptide antigens are unstable in vivo, nanomaterials provide protection against enzymatic degradation [[84], [85], [86]]. Furthermore, NPs may improve antigen transport to the lymphatic system [[87], [88], [89]], since lymphatic gaps preferentially allow the passage of molecules between 20 and 200 nm [90]. For example, in a mouse intradermal injection study, ultra-small nanoparticles (25 nm) are efficiently transported into the lymphatic system and target a subset of lymph DCs [91].
3.1.3. Activation of dendritic cells
The presentation of exogenous antigens on MHC class I molecules by DCs, known as cross-presentation, is essential for the initiation of the CD8+ T cell response [92]. Conventional antigen peptide-based vaccines are insufficient for cross-presentation, while nanovaccines can activate DCs by adequate cross-presentation [93,94]. In addition, nanovaccines have also been reported to enhance the MHC II pathway, which passes antigens to CD4+ T cells, leading to improved B cell activation [95].
Pattern-recognition receptors (PRRs), such as Toll-like receptors, NOD-like receptors, and RIG-I-like receptors, are expressed on DCs and associated with the recognition of pathogen-associated molecular patterns, DC maturation, and the expression of type I IFN, an antiviral inflammatory cytokine [[96], [97], [98], [99]]. Thus, PRR agonists have been used in nanovaccines as adjuvants, which bind to PRRs and upregulate related pathways, thus activating DCs and enhancing immune responses [100,101].
3.1.4. B cell activation and humoral response
Antibody titers are closely correlated with vaccine effectiveness. Nanovaccines have been shown to induce high antibody titers [[102], [103], [104], [105]], probably due to their superiority in target delivery and DC activation as well as the capacity to carry large amounts of antigens [106]. Nanovaccines can be constructed to carry large quantities of peptides or protein antigens; this provides a high concentration of antigens for efficient B cell receptor (BCR) signaling, consequently improving the B cell response and antibody production (Fig. 3) [107]. For example, despite the relatively low immunogenicity of HIV-1 envelope (Env) trimer antigens, the enhanced induction of neutralizing antibodies against HIV-1 has been observed in animals immunized with nanovaccines loaded with these antigens [108].
3.2. Nanovaccine candidates
Nanotechnology is increasingly used for the development of antiviral vaccines. Since a nanovaccine against hepatitis B virus (HBV) was first licensed in 1986, this approach has been used to develop vaccines against HPV and HEV, which have achieved positive preclinical outcomes against viruses, such as HIV and respiratory viruses [[19], [20], [21],109].
3.2.1. Coronaviruses
Vaccines for coronaviruses usually use viral surface spike proteins as antigens, which play a critical role in viral attachment to host cells. A VLP vaccine was produced by the co-recombination of SARS-CoV spike protein and the influenza M1 protein; the VLPs induced a strong immune response and had a protective effect in mice [110]. Another AuNP-based vaccine increased the IgG response from B cells by stimulating macrophages, DCs, and lymphocytes, resulting in the induction of proinflammatory cytokine production [111].
Positive results have also been achieved in the development of NP-based vaccines for MERS-CoV. A ferritin fusion protein with a viral antigen receptor-binding domain can self-assemble into VLPs, which stimulate CD4+ T cells and IFN/TNF responses. Loaded with a STING (stimulator of interferon genes) agonist as an adjuvant to increase DC activation, hollow polymeric NPs were able to stimulate remarkable humoral responses and IgG2a production. Another nanovaccine formed by the full-length spike protein of MERS-CoV also stimulated significant titers of neutralizing antibodies [[112], [113], [114]].
In terms of SARS-CoV-2, mRNA-based nanovaccines have been developed using LNPs as carriers to protect mRNAs from degradation by extracellular RNases and to efficiently deliver mRNAs to immune cells and thereby trigger the translation of antigen proteins [[115], [116], [117], [118]]. Moreover, the sustained release of mRNAs from LNPs leads to continuous protein translation, stimulating the production of high antibody titers and enhanced B cell and T cell immune responses [119]. There are currently five LNP-encapsulated mRNA vaccines under clinical trials (NCT04470427, NCT04368728, NCT04449276, ISRCTN1707269, and NCT04480957) using mRNAs encoding the full-length spike protein of SARS-CoV-2 or spike protein fragments as antigens. One of these mRNA-based LNP vaccines was reported to induce a strong immune response in participants, resulting in the production of high titers of neutralizing antibodies against SARS-CoV-2 [22], and is now under a phase III trial (NCT04470427) [120].
3.2.2. HIV
HIV is an RNA virus; owing to its high mutation frequency, it is challenging to develop a successful vaccine capable of inducing antibodies against diverse strains [[121], [122], [123], [124]]. A vaccine assembled from HIV Env trimers from various HIV-1 strains stimulated a broad spectrum of neutralizing antibodies (bNAs) against diverse virus strains [108]. Another Env gp120/gp41 trimer-assembled VLP had similar effects [125,126]. In mice immunized with HIV Env-based polystyrene NPs, which enhance the activation of CD4+ cells and lead to improved B cell responses, increases in both bNAs and antibody-secreting cells were found [127].
3.2.3. Influenza virus
A conventional method to protect against influenza virus is vaccination with a trivalent inactivated influenza vaccine (TIV), including two influenza A viruses and one influenza B virus [128]. However, the available TIV formulation is insufficient for mucosal protection, since TIV is not able to induce sufficient IgA, which is distributed on and protects the mucous membranes of the lungs, sinuses, stomach, and intestines [129,130]. Nanovaccines have been developed to induce mucosal protection from influenza virus. Successful nasal immunization was achieved in rabbits immunized with whole influenza virus incorporated into a mucoadhesive carrier chitosan; significant levels of anti-hemagglutinin antibody, local anti-influenza virus IgA, systemic IL-2, and IFN-γ were detected in the serum [131]. Another successful nanovaccine was established by adsorbing both inactivated swine influenza virus antigens and TLR3 agonist poly(I:C) onto cationic alpha-d-glucan NPs [132]. The poly(I:C) adjuvant promoted cytokine production; thus, the nanovaccine induced high levels of virus-neutralizing antibodies in bronchoalveolar lavage fluid and IgA antibodies in the nasal passage and lungs of immunized pigs [132]. Beneficial effects of mucosal immunization with NPs were also observed in chickens immunized via the aerosol route. In this study, poly(lactic-co-glycolic acid) (PLGA) loaded with formalin-inactivated H9N2 virus and CpG generated a significantly stronger antibody response than that generated by non-encapsulated antigens [133].
3.2.4. FMDV
Foot-and-mouth disease (FMD) caused by FMDV, a picornavirus, and the prototypic member of the genus Aphthovirus, is a serious health threat, especially in children [134]. Traditional vaccines against FMDV require multiple injections to confer sufficient immunity. The use of calcium mineralization to fabricate FMDV VLPs is a simple improvement. Such bio-mineralization improves the thermal stability and storage time of the vaccine and effectively activates DCs to express high levels of surface MHC-II, costimulatory molecules, and proinflammatory cytokines, leading to enhanced immune responses [95]. Another vaccine combines the polyelectrolyte complexation of chitosan and heparin to co-encapsulate the VP1 protein antigen from enterovirus 71, which causes FMD, with TNFα or CpG as adjuvants [135]. This nanovaccine induced strong immune activation toward antibody production and conferred effective protection against a lethal virus challenge in mice. Moreover, it elicited strong IgA titers, which may provide unique advantages for mucosal protection [135]. Another FMDV VLP loaded in a hollow mesoporous silica nanoparticle (HMSN) induced persistent humoral immunity with high antibody titers for longer than 3 months in guinea pigs, accompanied by improved T cell proliferation and IFN-γ production [136].
3.2.5. Dengue virus
The applications of a major Dengue virus (DENV) immunogen, the E protein, in NPs has produced promising results in mouse models. Engineered bacterial membrane vesicles carrying dengue EDIII antigens effectively evoke humoral immune responses and confer effective protection against DENV-2 infection in mice [137]. Compared with antigens for free DENV-2 E protein, the combination of PLGA NP with E protein increased the anti-E IgG titer and improved neutralizing antibodies in mice [138]. Another NP vaccine composed of chitosan with UV-inactivated DENV-2 induced high IgG levels in mice [139,140]. An NP nasal vaccine based on chitosan has been tested for the delivery of DENV-3 E protein; it was taken up efficiently by nasal epithelial cells, resulting in increased IL-1β, IL-6, and TNF-α secretion [141].
3.2.6. Hepatitis C virus, respiratory syncytial virus, and West Nile virus
Nano-based vaccines have also been developed against other viruses, such as hepatitis C virus (HCV), respiratory syncytial virus (RSV), and West Nile virus (WNV). HCV and WNV nanovaccines have successfully stimulated the immune system to produce antibodies and protect animal models from viral infection in preclinical tests [142,143]. Vaccines against RSV have been successful in clinical trials (NCT02624947).
4. Conclusions and outlook
Pandemics and epidemics caused by newly emerged viruses, such as SARS-CoV-2, have revealed the urgent need to develop antiviral agents that function by novel mechanisms and various routes to combat viruses. Nanotechnology has a tremendous opportunity to improve the prevention and treatment of viral diseases for several reasons. First, nanomaterials can be flexibly functionalized with multiple molecules to realize sophisticated antiviral designs. Second, nanomaterials inhibit virus infection from multiple directions based on various antiviral effects (Fig. 1B). Third, nanomaterials have broad-spectrum antiviral properties due to common underlying mechanisms. For example, nanodecoys trap diverse viruses prone to attachment to cell membrane-derived or -coated structures [28].
Nanomaterials have many advantages for the development of antiviral drugs. Compared with antibodies, nanomaterials are relatively easy to produce and thus more affordable. Moreover, rational design could be introduced to improve clinical effects [144,145]. Nanomaterials could also be designed to target viral factors, such as antibodies. For example, AuNPs and AgNPs could be designed to target gp120 in HIV and hemagglutinin in influenza virus, respectively [38,41]. Moreover, the combined use of nanomaterials with other antiviral drugs may resulted in enhanced antiviral effects. For example, carbon dots can be used to induce IFN-α production and IFN-stimulating gene expression in host cells, which may increase the inhibitory effects of synthetic agents, such as nucleosides, on viral replication [59].
Vaccination is an efficient intervention to protect against viral infection. Nano-based vaccines have many clinical advantages over conventional vaccines, including their ability to transport high concentrations of antigens to B cells and to induce abundant immune responses (Fig. 3). Although many nano-based vaccines remain in pre-clinical stages, progress in the application of nanotechnology to vaccines against viruses, such as coronaviruses, HIV, and FMDV, suggests that this approach is promising for the development of vaccines.
Finally, although they have various advantages over other approaches, the safety of antiviral nanomaterials needs to be carefully evaluated before the clinical translation of novel nanotechnologies. Given the lack of knowledge about the risks of exposure to nanomaterials in humans, in-depth studies of nanotoxicity, especially under long-term observation, are needed [146]. Further investigations of the distribution, accumulation, and clearance of nanomaterials in the human body are also warranted. Fortunately, nano-based anti-cancer candidates are already undergoing clinical trials (www.cancer.gov/nano/cancer-nanotechnology) [147], which will provide insight into their safety in humans and extend their use as antiviral agents and vaccines.
List of chemical compounds
polyethylene glycol; polyglycerol sulfate; heparan sulfate; tannic acid; glycosaminoglycans; poly(aniline-co-pyrrole); protoporphyrin IX; ascorbic acid; 2,2′-(ethylenedioxy)bis(ethylamine); 3-ethoxypropylamine.
Declaration of competing interest
There are no conflicts to declare.
Acknowledgements
We sincerely appreciate the investigators and authors who have contributed to this field and apologize that we could not discuss and cite all of them in this review due to space limitations. This work was supported by the Natural Science Foundation of China (31671195 and 31971066), the China Postdoctoral Science Foundation (2019M652658), the Front Youth Program of HUST, and Integrated Innovative Team for Major Human Diseases Program of Tongji Medical College, HUST.
References
- 1.Cohen M.L. Changing patterns of infectious disease. Nature. 2000;406(6797):762–767. doi: 10.1038/35021206. [DOI] [PubMed] [Google Scholar]
- 2.Braden C.R., Dowell S.F., Jernigan D.B., Hughes J.M. Progress in global surveillance and response capacity 10 years after severe acute respiratory syndrome. Emerg. Infect. Dis. 2013;19(6):864–869. doi: 10.3201/eid1906.130192. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Pati R., Shevtsov M., Sonawane A. Nanoparticle vaccines against infectious diseases. Front. Immunol. 2018;9:2224. doi: 10.3389/fimmu.2018.02224. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Li Y., Yang C., Wang S., Yang D., Zhang Y., Xu L. Copper and iron ions accelerate the prion-like propagation of alpha-synuclein: a vicious cycle in Parkinson’s disease. Int. J. Biol. Macromol. 2020;163:562–573. doi: 10.1016/j.ijbiomac.2020.06.274. [DOI] [PubMed] [Google Scholar]
- 5.Wu J.T., Leung K., Leung G.M. Nowcasting and forecasting the potential domestic and international spread of the 2019-nCoV outbreak originating in Wuhan, China: a modelling study. Lancet. 2020;395(10225):689–697. doi: 10.1016/S0140-6736(20)30260-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Yang D., Xiao Y., Chen J., Chen Y., Luo P., Liu Q. COVID-19 & chronic renal disease: clinical characteristics & prognosis. QJM. 2020 doi: 10.1093/qjmed/hcaa258. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Sullivan N.J., Sanchez A., Rollin P.E., Yang Z.Y., Nabel G.J. Development of a preventive vaccine for Ebola virus infection in primates. Nature. 2000;408(6812):605–609. doi: 10.1038/35046108. [DOI] [PubMed] [Google Scholar]
- 8.Abbink P., Larocca R.A., De La Barrera R.A., Bricault C.A., Moseley E.T., Boyd M. Protective efficacy of multiple vaccine platforms against Zika virus challenge in rhesus monkeys. Science. 2016;353(6304):1129–1132. doi: 10.1126/science.aah6157. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Patick A.K., Potts K.E. Protease inhibitors as antiviral agents. Clin. Microbiol. Rev. 1998;11(4):614–627. doi: 10.1128/cmr.11.4.614. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Hoenen T., Groseth A., Feldmann H. Therapeutic strategies to target the Ebola virus life cycle. Nat Rev Microbiol. 2019;17(10):593–606. doi: 10.1038/s41579-019-0233-2. [DOI] [PubMed] [Google Scholar]
- 11.Miao M., De Clercq E., Li G. Clinical significance of chemokine receptor antagonists. Expert Opin. Drug Metab. Toxicol. 2020;16(1):11–30. doi: 10.1080/17425255.2020.1711884. [DOI] [PubMed] [Google Scholar]
- 12.Gao R., Sheng Z., Sreenivasan C.C., Wang D., Li F. Influenza a virus antibodies with antibody-dependent cellular cytotoxicity function. Viruses. 2020;12(3):276. doi: 10.3390/v12030276. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Huo J., Le Bas A., Ruza R.R., Duyvesteyn H.M.E., Mikolajek H., Malinauskas T. Neutralizing nanobodies bind SARS-CoV-2 spike RBD and block interaction with ACE2. Nat. Struct. Mol. Biol. 2020 doi: 10.1038/s41594-020-0469-6. [DOI] [PubMed] [Google Scholar]
- 14.Nakano R., Ishiguro H., Yao Y., Kajioka J., Fujishima A., Sunada K. Photocatalytic inactivation of influenza virus by titanium dioxide thin film. Photochem Photobiol Sci. 2012;11(8):1293–1298. doi: 10.1039/c2pp05414k. [DOI] [PubMed] [Google Scholar]
- 15.Dey P., Bergmann T., Cuellar-Camacho J.L., Ehrmann S., Chowdhury M.S., Zhang M. Multivalent flexible nanogels exhibit broad-spectrum antiviral activity by blocking virus entry. ACS Nano. 2018;12(7):6429–6442. doi: 10.1021/acsnano.8b01616. [DOI] [PubMed] [Google Scholar]
- 16.Lien G., Heymann D.L. The problems with polio: toward eradication. Infect. Dis. Ther. 2013;2(2):167–174. doi: 10.1007/s40121-013-0014-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Chappuis F., Farinelli T., Deckx H., Sarnecki M., Go O., Salzgeber Y. Immunogenicity and estimation of antibody persistence following vaccination with an inactivated virosomal hepatitis a vaccine in adults: a 20-year follow-up study. Vaccine. 2017;35(10):1448–1454. doi: 10.1016/j.vaccine.2017.01.031. [DOI] [PubMed] [Google Scholar]
- 18.Pezzotti P., Bellino S., Prestinaci F., Iacchini S., Lucaroni F., Camoni L. The impact of immunization programs on 10 vaccine preventable diseases in Italy: 1900-2015. Vaccine. 2018;36(11):1435–1443. doi: 10.1016/j.vaccine.2018.01.065. [DOI] [PubMed] [Google Scholar]
- 19.Nandedkar T.D. Nanovaccines: recent developments in vaccination. J. Biosci. 2009;34(6):995–1003. doi: 10.1007/s12038-009-0114-3. [DOI] [PubMed] [Google Scholar]
- 20.Peek L.J., Middaugh C.R., Berkland C. Nanotechnology in vaccine delivery. Adv. Drug Deliv. Rev. 2008;60(8):915–928. doi: 10.1016/j.addr.2007.05.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Jorquera P.A., Tripp R.A. Synthetic biodegradable microparticle and nanoparticle vaccines against the respiratory syncytial virus. Vaccines (Basel) 2016;4(4):45. doi: 10.3390/vaccines4040045. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Jackson L.A., Anderson E.J., Rouphael N.G., Roberts P.C., Makhene M., Coler R.N. An mRNA vaccine against SARS-CoV-2 — preliminary report. N. Engl. J. Med. 2020 doi: 10.1056/NEJMoa2022483. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Mao J., Lin E., He L., Yu J., Tan P., Zhou Y. Autophagy and viral infection. Adv. Exp. Med. Biol. 2019;1209:55–78. doi: 10.1007/978-981-15-0606-2_5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Azarm K.D., Lee B. Differential features of fusion activation within the Paramyxoviridae. Viruses. 2020;12(2):161. doi: 10.3390/v12020161. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Tabata K., Neufeldt C.J., Bartenschlager R. Hepatitis C virus replication. Cold Spring Harb Perspect Med. 2020;10(3):a037093. doi: 10.1101/cshperspect.a037093. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Jiao M., Zhang P., Meng J., Li Y., Liu C., Luo X. Recent advancements in biocompatible inorganic nanoparticles towards biomedical applications. Biomaterials Science. 2018;6(4):726–745. doi: 10.1039/c7bm01020f. [DOI] [PubMed] [Google Scholar]
- 27.Li L., Lu Y., Jiang C., Zhu Y., Yang X., Hu X. Actively targeted deep tissue imaging and Photothermal-chemo therapy of breast Cancer by antibody-functionalized drug-loaded X-ray-responsive bismuth sulfide@Mesoporous silica Core-Shell nanoparticles. Adv. Funct. Mater. 2018;28(5) doi: 10.1002/adfm.201704623. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Rao L., Tian R., Chen X. Cell-membrane-mimicking Nanodecoys against infectious diseases. ACS Nano. 2020;14(3):2569–2574. doi: 10.1021/acsnano.0c01665. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.de Carvalho J.V., de Castro R.O., Silva E.Z. da, Silveira P.P., Silva-Januario M.E. da, Arruda E. Nef neutralizes the ability of exosomes from CD4+ T cells to act as decoys during HIV-1 infection. PLoS One. 2014;9(11) doi: 10.1371/journal.pone.0113691. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Liu X., Yuan L., Zhang L., Mu Y., Li X., Liu C. Bioinspired artificial nanodecoys for hepatitis B virus. Angew Chem Int Ed Engl. 2018;57(38):12499–12503. doi: 10.1002/anie.201807212. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Prada I., Meldolesi J. Binding and fusion of extracellular vesicles to the plasma membrane of their cell targets. Int. J. Mol. Sci. 2016;17(8):1296. doi: 10.3390/ijms17081296. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Wei X., Zhang G., Ran D., Krishnan N., Fang R.H., Gao W. T-cell-mimicking nanoparticles can neutralize HIV infectivity. Adv. Mater. 2018;30(45) doi: 10.1002/adma.201802233. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Zhang Y., Chen Y., Lo C., Zhuang J., Angsantikul P., Zhang Q. Inhibition of pathogen adhesion by bacterial outer membrane-coated nanoparticles. Angew Chem Int Ed Engl. 2019;58(33):11404–11408. doi: 10.1002/anie.201906280. [DOI] [PubMed] [Google Scholar]
- 34.Rao L., Wang W., Meng Q.F., Tian M., Cai B., Wang Y. A biomimetic Nanodecoy traps Zika virus to prevent viral infection and fetal microcephaly development. Nano Lett. 2019;19(4):2215–2222. doi: 10.1021/acs.nanolett.8b03913. [DOI] [PubMed] [Google Scholar]
- 35.Hu C.M., Zhang L., Aryal S., Cheung C., Fang R.H., Zhang L. Erythrocyte membrane-camouflaged polymeric nanoparticles as a biomimetic delivery platform. Proc. Natl. Acad. Sci. U. S. A. 2011;108(27):10980–10985. doi: 10.1073/pnas.1106634108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Zhang Q., Honko A., Zhou J., Gong H., Downs S.N., Vasquez J.H. Cellular Nanosponges inhibit SARS-CoV-2 infectivity. Nano Lett. 2020;20(7):5570–5574. doi: 10.1021/acs.nanolett.0c02278. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Wilen C.B., Tilton J.C., Doms R.W. HIV: cell binding and entry. Cold Spring Harb Perspect Med. 2012;2(8) doi: 10.1101/cshperspect.a006866. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Vijayakumar S., Ganesan S. Gold nanoparticles as an HIV entry inhibitor. Curr. HIV Res. 2012;10(8):643–646. doi: 10.2174/157016212803901383. [DOI] [PubMed] [Google Scholar]
- 39.de Souza E.S.J.M., Hanchuk T.D., Santos M.I., Kobarg J., Bajgelman M.C., Cardoso M.B. Viral inhibition mechanism mediated by surface-modified silica nanoparticles. ACS Appl. Mater. Interfaces. 2016;8(26):16564–16572. doi: 10.1021/acsami.6b03342. [DOI] [PubMed] [Google Scholar]
- 40.Lara H.H., Ayala-Nunez N.V., Ixtepan-Turrent L., Rodriguez-Padilla C. Mode of antiviral action of silver nanoparticles against HIV-1. J Nanobiotechnology. 2010;8(1) doi: 10.1186/1477-3155-8-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Fatima M., Zaidi N.U., Amraiz D., Afzal F. In vitro antiviral activity of Cinnamomum cassia and its nanoparticles against H7N3 influenza a virus. J. Microbiol. Biotechnol. 2016;26(1):151–159. doi: 10.4014/jmb.1508.08024. [DOI] [PubMed] [Google Scholar]
- 42.Hu R.L., Li S.R., Kong F.J., Hou R.J., Guan X.L., Guo F. Inhibition effect of silver nanoparticles on herpes simplex virus 2. Genet. Mol. Res. 2014;13(3):7022–7028. doi: 10.4238/2014.March.19.2. [DOI] [PubMed] [Google Scholar]
- 43.Gaikwad S., Ingle A., Gade A., Rai M., Falanga A., Incoronato N. Antiviral activity of mycosynthesized silver nanoparticles against herpes simplex virus and human parainfluenza virus type 3. Int. J. Nanomedicine. 2013;8:4303–4314. doi: 10.2147/IJN.S50070. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Orlowski P., Tomaszewska E., Gniadek M., Baska P., Nowakowska J., Sokolowska J. Tannic acid modified silver nanoparticles show antiviral activity in herpes simplex virus type 2 infection. PLoS One. 2014;9(8) doi: 10.1371/journal.pone.0104113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Lee E.C., Davis-Poynter N., Nguyen C.T., Peters A.A., Monteith G.R., Strounina E. GAG mimetic functionalised solid and mesoporous silica nanoparticles as viral entry inhibitors of herpes simplex type 1 and type 2 viruses. Nanoscale. 2016;8(36):16192–16196. doi: 10.1039/c6nr03878f. [DOI] [PubMed] [Google Scholar]
- 46.Chen Y., Yang D., Cheng B., Chen J., Peng A., Yang C. Clinical characteristics and outcomes of patients with diabetes and COVID-19 in association with glucose-lowering medication. Diabetes Care. 2020;43(7):1399–1407. doi: 10.2337/dc20-0660. [DOI] [PubMed] [Google Scholar]
- 47.Sofy A.R., Hmed A.A., Abd El Haliem N.F., Zein M.A., Elshaarawy R.F.M. Polyphosphonium-oligochitosans decorated with nanosilver as new prospective inhibitors for common human enteric viruses. Carbohydr. Polym. 2019;226 doi: 10.1016/j.carbpol.2019.115261. [DOI] [PubMed] [Google Scholar]
- 48.Luczkowiak J., Munoz A., Sanchez-Navarro M., Ribeiro-Viana R., Ginieis A., Illescas B.M. Glycofullerenes inhibit viral infection. Biomacromolecules. 2013;14(2):431–437. doi: 10.1021/bm3016658. [DOI] [PubMed] [Google Scholar]
- 49.Munoz A., Sigwalt D., Illescas B.M., Luczkowiak J., Rodriguez-Perez L., Nierengarten I. Synthesis of giant globular multivalent glycofullerenes as potent inhibitors in a model of Ebola virus infection. Nat. Chem. 2016;8(1):50–57. doi: 10.1038/nchem.2387. [DOI] [PubMed] [Google Scholar]
- 50.Munoz A., Illescas B.M., Luczkowiak J., Lasala F., Ribeiro-Viana R., Rojo J. Antiviral activity of self-assembled glycodendro[60]fullerene monoadducts. J. Mater. Chem. B. 2017;5(32):6566–6571. doi: 10.1039/c7tb01379e. [DOI] [PubMed] [Google Scholar]
- 51.X. Ji, G.G. Olinger, S. Aris, Y. Chen, H. Gewurz, and G.T. Spear, Mannose-binding lectin binds to Ebola and Marburg envelope glycoproteins, resulting in blocking of virus interaction with DC-SIGN and complement-mediated virus neutralization. J Gen Virol, 86 (Pt 9) (2005) 2535–2542. [DOI] [PubMed]
- 52.Huang P., Farkas T., Zhong W., Tan M., Thornton S., Morrow A.L. Norovirus and histo-blood group antigens: demonstration of a wide spectrum of strain specificities and classification of two major binding groups among multiple binding patterns. J. Virol. 2005;79(11):6714–6722. doi: 10.1128/JVI.79.11.6714-6722.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Dong X., Moyer M.M., Yang F., Sun Y.P., Yang L. Carbon Dots’ antiviral functions against Noroviruses. Sci. Rep. 2017;7(1):519. doi: 10.1038/s41598-017-00675-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Rafiei S., Rezatofighi S.E., Roayaei Ardakani M., Rastegarzadeh S. Gold nanoparticles impair foot-and-mouth disease virus replication. IEEE Trans Nanobioscience. 2016;15(1):34–40. doi: 10.1109/TNB.2015.2508718. [DOI] [PubMed] [Google Scholar]
- 55.Luther D.C., Huang R., Jeon T., Zhang X., Lee Y.W., Nagaraj H. Delivery of drugs, proteins, and nucleic acids using inorganic nanoparticles. Adv. Drug Deliv. Rev. 2020 doi: 10.1016/j.addr.2020.06.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Martinez Z.S., Castro E., Seong C.S., Ceron M.R., Echegoyen L., Llano M. Fullerene derivatives strongly inhibit HIV-1 replication by affecting virus maturation without impairing protease activity. Antimicrob. Agents Chemother. 2016;60(10):5731–5741. doi: 10.1128/AAC.00341-16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Khandelwal N., Kaur G., Chaubey K.K., Singh P., Sharma S., Tiwari A. Silver nanoparticles impair Peste des petits ruminants virus replication. Virus Res. 2014;190:1–7. doi: 10.1016/j.virusres.2014.06.011. [DOI] [PubMed] [Google Scholar]
- 58.Ronavari A., Kovacs D., Igaz N., Vagvolgyi C., Boros I.M., Konya Z. Biological activity of green-synthesized silver nanoparticles depends on the applied natural extracts: a comprehensive study. Int. J. Nanomedicine. 2017;12:871–883. doi: 10.2147/IJN.S122842. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Du T., Liang J., Dong N., Liu L., Fang L., Xiao S. Carbon dots as inhibitors of virus by activation of type I interferon response. Carbon. 2016;110:278–285. [Google Scholar]
- 60.Kim H.O., Yeom M., Kim J., Kukreja A., Na W., Choi J. Reactive oxygen species-regulating Polymersome as an antiviral agent against influenza virus. Small. 2017;13(32) doi: 10.1002/smll.201700818. [DOI] [PubMed] [Google Scholar]
- 61.Bonjardim C.A. Viral exploitation of the MEK/ERK pathway - a tale of vaccinia virus and other viruses. Virology. 2017;507:267–275. doi: 10.1016/j.virol.2016.12.011. [DOI] [PubMed] [Google Scholar]
- 62.Banerjee I., Douaisi M.P., Mondal D., Kane R.S. Light-activated nanotube-porphyrin conjugates as effective antiviral agents. Nanotechnology. 2012;23(10) doi: 10.1088/0957-4484/23/10/105101. [DOI] [PubMed] [Google Scholar]
- 63.Ye S., Shao K., Li Z., Guo N., Zuo Y., Li Q. Antiviral activity of Graphene oxide: how sharp edged structure and charge matter. ACS Appl. Mater. Interfaces. 2015;7(38):21571–21579. doi: 10.1021/acsami.5b06876. [DOI] [PubMed] [Google Scholar]
- 64.Mazurkova N.A., Spitsyna Y.E., Shikina N.V., Ismagilov Z.R., Zagrebel’nyi S.N., Ryabchikova E.I. Interaction of titanium dioxide nanoparticles with influenza virus. Nanotechnologies in Russia. 2010;5(5):417–420. [Google Scholar]
- 65.Chen Y.N., Hsueh Y.H., Hsieh C.T., Tzou D.Y., Chang P.L. Antiviral activity of graphene-silver nanocomposites against non-enveloped and enveloped viruses. Int. J. Environ. Res. Public Health. 2016;13(4):430. doi: 10.3390/ijerph13040430. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Syngouna V.I., Chrysikopoulos C.V. Inactivation of MS2 bacteriophage by titanium dioxide nanoparticles in the presence of quartz sand with and without ambient light. J. Colloid Interface Sci. 2017;497:117–125. doi: 10.1016/j.jcis.2017.02.059. [DOI] [PubMed] [Google Scholar]
- 67.Jafry H.R., Liga M.V., Li Q., Barron A.R. Simple route to enhanced photocatalytic activity of p25 titanium dioxide nanoparticles by silica addition. Environ Sci Technol. 2011;45(4):1563–1568. doi: 10.1021/es102749e. [DOI] [PubMed] [Google Scholar]
- 68.Park S., Park H.H., Kim S.Y., Kim S.J., Woo K., Ko G. Antiviral properties of silver nanoparticles on a magnetic hybrid colloid. Appl. Environ. Microbiol. 2014;80(8):2343–2350. doi: 10.1128/AEM.03427-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Huy T.Q., Hien Thanh N.T., Thuy N.T., Chung P.V., Hung P.N., Le A.T. Cytotoxicity and antiviral activity of electrochemical - synthesized silver nanoparticles against poliovirus. J. Virol. Methods. 2017;241:52–57. doi: 10.1016/j.jviromet.2016.12.015. [DOI] [PubMed] [Google Scholar]
- 70.Broglie J.J., Alston B., Yang C., Ma L., Adcock A.F., Chen W. Antiviral activity of gold/copper sulfide Core/Shell nanoparticles against human Norovirus virus-like particles. PLoS One. 2015;10(10) doi: 10.1371/journal.pone.0141050. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Lambotin M., Raghuraman S., Stoll-Keller F., Baumert T.F., Barth H. A look behind closed doors: interaction of persistent viruses with dendritic cells. Nat Rev Microbiol. 2010;8(5):350–360. doi: 10.1038/nrmicro2332. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Bachmann M.F., Jennings G.T. Vaccine delivery: a matter of size, geometry, kinetics and molecular patterns. Nat Rev Immunol. 2010;10(11):787–796. doi: 10.1038/nri2868. [DOI] [PubMed] [Google Scholar]
- 73.Barry A.E., Arnott A. Strategies for designing and monitoring malaria vaccines targeting diverse antigens. Front. Immunol. 2014;5:359. doi: 10.3389/fimmu.2014.00359. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Wadhwa S., Jain A., Woodward J.G., Mumper R.J. Lipid nanocapsule as vaccine carriers for his-tagged proteins: evaluation of antigen-specific immune responses to HIV I his-gag p41 and systemic inflammatory responses. Eur. J. Pharm. Biopharm. 2012;80(2):315–322. doi: 10.1016/j.ejpb.2011.10.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Zaric M., Lyubomska O., Touzelet O., Poux C., Al-Zahrani S., Fay F. Skin dendritic cell targeting via microneedle arrays laden with antigen-encapsulated poly-D,L-lactide-co-glycolide nanoparticles induces efficient antitumor and antiviral immune responses. ACS Nano. 2013;7(3):2042–2055. doi: 10.1021/nn304235j. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Kushnir N., Streatfield S.J., Yusibov V. Virus-like particles as a highly efficient vaccine platform: diversity of targets and production systems and advances in clinical development. Vaccine. 2012;31(1):58–83. doi: 10.1016/j.vaccine.2012.10.083. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Yang C., Huang K. Clinical applications of virus-like particles: opportunities and challenges. Curr. Protein Pept. Sci. 2019;20(5):488–489. doi: 10.2174/138920372005190327120752. [DOI] [PubMed] [Google Scholar]
- 78.Jeong H., Seong B.L. Exploiting virus-like particles as innovative vaccines against emerging viral infections. J. Microbiol. 2017;55(3):220–230. doi: 10.1007/s12275-017-7058-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Noad R., Roy P. Virus-like particles as immunogens. Trends Microbiol. 2003;11(9):438–444. doi: 10.1016/s0966-842x(03)00208-7. [DOI] [PubMed] [Google Scholar]
- 80.Zhang L.F., Zhou J., Chen S., Cai L.L., Bao Q.Y., Zheng F.Y. HPV6b virus like particles are potent immunogens without adjuvant in man. Vaccine. 2000;18(11−12):1051–1058. doi: 10.1016/s0264-410x(99)00351-5. [DOI] [PubMed] [Google Scholar]
- 81.Jakimovski D., Weinstock-Guttman B., Ramanathan M., Dwyer M.G., Zivadinov R. Infections, vaccines and autoimmunity: a multiple sclerosis perspective. Vaccines (Basel) 2020;8(1) doi: 10.3390/vaccines8010050. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Wu T., Li S.W., Zhang J., Ng M.H., Xia N.S., Zhao Q. Hepatitis E vaccine development: a 14 year odyssey. Hum Vaccin Immunother. 2012;8(6):823–827. doi: 10.4161/hv.20042. [DOI] [PubMed] [Google Scholar]
- 83.Zhao L., Seth A., Wibowo N., Zhao C.X., Mitter N., Yu C. Nanoparticle vaccines. Vaccine. 2014;32(3):327–337. doi: 10.1016/j.vaccine.2013.11.069. [DOI] [PubMed] [Google Scholar]
- 84.Mahapatro A., Singh D.K. Biodegradable nanoparticles are excellent vehicle for site directed in-vivo delivery of drugs and vaccines. J Nanobiotechnology. 2011;9:55. doi: 10.1186/1477-3155-9-55. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Dhakal S., Hiremath J., Bondra K., Lakshmanappa Y.S., Shyu D.L., Ouyang K. Biodegradable nanoparticle delivery of inactivated swine influenza virus vaccine provides heterologous cell-mediated immune response in pigs. J. Control. Release. 2017;247:194–205. doi: 10.1016/j.jconrel.2016.12.039. [DOI] [PubMed] [Google Scholar]
- 86.Amirnasr M., Fallah Tafti T., Sankian M., Rezaei A., Tafaghodi M. Immunization against HTLV-I with chitosan and tri-methylchitosan nanoparticles loaded with recombinant env23 and env13 antigens of envelope protein gp46. Microb. Pathog. 2016;97:38–44. doi: 10.1016/j.micpath.2016.05.012. [DOI] [PubMed] [Google Scholar]
- 87.Kim S.Y., Noh Y.W., Kang T.H., Kim J.E., Kim S., Um S.H. Synthetic vaccine nanoparticles target to lymph node triggering enhanced innate and adaptive antitumor immunity. Biomaterials. 2017;130:56–66. doi: 10.1016/j.biomaterials.2017.03.034. [DOI] [PubMed] [Google Scholar]
- 88.Gutjahr A., Phelip C., Coolen A.L., Monge C., Boisgard A.S., Paul S. Biodegradable polymeric nanoparticles-based vaccine adjuvants for lymph nodes targeting. Vaccines (Basel) 2016;4(4):34. doi: 10.3390/vaccines4040034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Xiang J., Xu L., Gong H., Zhu W., Wang C., Xu J. Antigen-loaded Upconversion nanoparticles for dendritic cell stimulation, tracking, and vaccination in dendritic cell-based immunotherapy. ACS Nano. 2015;9(6):6401–6411. doi: 10.1021/acsnano.5b02014. [DOI] [PubMed] [Google Scholar]
- 90.Reddy S.T., Rehor A., Schmoekel H.G., Hubbell J.A., Swartz M.A. In vivo targeting of dendritic cells in lymph nodes with poly(propylene sulfide) nanoparticles. J. Control. Release. 2006;112(1):26–34. doi: 10.1016/j.jconrel.2006.01.006. [DOI] [PubMed] [Google Scholar]
- 91.Reddy S.T., van der Vlies A.J., Simeoni E., Angeli V., Randolph G.J., O’Neil C.P. Exploiting lymphatic transport and complement activation in nanoparticle vaccines. Nat. Biotechnol. 2007;25(10):1159–1164. doi: 10.1038/nbt1332. [DOI] [PubMed] [Google Scholar]
- 92.Joffre O.P., Segura E., Savina A., Amigorena S. Cross-presentation by dendritic cells. Nat Rev Immunol. 2012;12(8):557–569. doi: 10.1038/nri3254. [DOI] [PubMed] [Google Scholar]
- 93.Molino N.M., Anderson A.K., Nelson E.L., Wang S.W. Biomimetic protein nanoparticles facilitate enhanced dendritic cell activation and cross-presentation. ACS Nano. 2013;7(11):9743–9752. doi: 10.1021/nn403085w. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Mukai Y., Yoshinaga T., Yoshikawa M., Matsuo K., Yoshikawa T., Matsuo K. Induction of endoplasmic reticulum-endosome fusion for antigen cross-presentation induced by poly (gamma-glutamic acid) nanoparticles. J. Immunol. 2011;187(12):6249–6255. doi: 10.4049/jimmunol.1001093. [DOI] [PubMed] [Google Scholar]
- 95.Du P., Liu R., Sun S., Dong H., Zhao R., Tang R. Biomineralization improves the thermostability of foot-and-mouth disease virus-like particles and the protective immune response induced. Nanoscale. 2019;11(47):22748–22761. doi: 10.1039/c9nr05549e. [DOI] [PubMed] [Google Scholar]
- 96.Kawai T., Akira S. The role of pattern-recognition receptors in innate immunity: update on toll-like receptors. Nat. Immunol. 2010;11(5):373–384. doi: 10.1038/ni.1863. [DOI] [PubMed] [Google Scholar]
- 97.Suresh R., Mosser D.M. Pattern recognition receptors in innate immunity, host defense, and immunopathology. Adv. Physiol. Educ. 2013;37(4):284–291. doi: 10.1152/advan.00058.2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Zhang Y., Guo X., Yan W., Chen Y., Ke M., Cheng C. ANGPTL8 negatively regulates NF-kappaB activation by facilitating selective autophagic degradation of IKKgamma. Nat. Commun. 2017;8(1):2164. doi: 10.1038/s41467-017-02355-w. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Zhou Q., Cheng C., Wei Y., Yang J., Zhou W., Song Q. USP15 potentiates NF-kappaB activation by differentially stabilizing TAB2 and TAB3. FEBS J. 2020;287(15):3165–3183. doi: 10.1111/febs.15202. [DOI] [PubMed] [Google Scholar]
- 100.Lee Y.R., Lee Y.H., Kim K.H., Im S.A., Lee C.K. Induction of potent antigen-specific cytotoxic T cell response by PLGA-nanoparticles containing antigen and TLR agonist. Immune Netw. 2013;13(1):30–33. doi: 10.4110/in.2013.13.1.30. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Li A.V., Moon J.J., Abraham W., Suh H., Elkhader J., Seidman M.A. Generation of effector memory T cell-based mucosal and systemic immunity with pulmonary nanoparticle vaccination. Sci. Transl. Med. 2013;5(204):204ra130. doi: 10.1126/scitranslmed.3006516. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Turner L.H., Kinder J.M., Wilburn A., D’Mello R.J., Braunlin M.R., Jiang T.T. Preconceptual Zika virus asymptomatic infection protects against secondary prenatal infection. PLoS Pathog. 2017;13(11) doi: 10.1371/journal.ppat.1006684. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Pileggi C., Papadopoli R., Bianco A., Pavia M. Hepatitis B vaccine and the need for a booster dose after primary vaccination. Vaccine. 2017;35(46):6302–6307. doi: 10.1016/j.vaccine.2017.09.076. [DOI] [PubMed] [Google Scholar]
- 104.Ng S., Saborio S., Kuan G., Gresh L., Sanchez N., Ojeda S. Association between Haemagglutination inhibiting antibodies and protection against clade 6B viruses in 2013 and 2015. Vaccine. 2017;35(45):6202–6207. doi: 10.1016/j.vaccine.2017.09.036. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Reikie B.A., Naidoo S., Ruck C.E., Slogrove A.L., de Beer C., la Grange H. Antibody responses to vaccination among south African HIV-exposed and unexposed uninfected infants during the first 2 years of life. Clin. Vaccine Immunol. 2013;20(1):33–38. doi: 10.1128/CVI.00557-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.Veneziano R., Moyer T.J., Stone M.B., Wamhoff E.C., Read B.J., Mukherjee S. Role of nanoscale antigen organization on B-cell activation probed using DNA origami. Nat. Nanotechnol. 2020;15(8):716–723. doi: 10.1038/s41565-020-0719-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.Avalos A.M., Ploegh H.L. Early BCR events and antigen capture, processing, and loading on MHC class II on B cells. Front. Immunol. 2014;5:92. doi: 10.3389/fimmu.2014.00092. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108.Brouwer P.J.M., Antanasijevic A., Berndsen Z., Yasmeen A., Fiala B., Bijl T.P.L. Enhancing and shaping the immunogenicity of native-like HIV-1 envelope trimers with a two-component protein nanoparticle. Nat. Commun. 2019;10(1):4272. doi: 10.1038/s41467-019-12080-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109.Roldao A., Mellado M.C., Castilho L.R., Carrondo M.J., Alves P.M. Virus-like particles in vaccine development. Expert Rev Vaccines. 2010;9(10):1149–1176. doi: 10.1586/erv.10.115. [DOI] [PubMed] [Google Scholar]
- 110.Liu Y.V., Massare M.J., Barnard D.L., Kort T., Nathan M., Wang L. Chimeric severe acute respiratory syndrome coronavirus (SARS-CoV) S glycoprotein and influenza matrix 1 efficiently form virus-like particles (VLPs) that protect mice against challenge with SARS-CoV. Vaccine. 2011;29(38):6606–6613. doi: 10.1016/j.vaccine.2011.06.111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111.Sekimukai H., Iwata-Yoshikawa N., Fukushi S., Tani H., Kataoka M., Suzuki T. Gold nanoparticle-adjuvanted S protein induces a strong antigen-specific IgG response against severe acute respiratory syndrome-related coronavirus infection, but fails to induce protective antibodies and limit eosinophilic infiltration in lungs. Microbiol. Immunol. 2020;64(1):33–51. doi: 10.1111/1348-0421.12754. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112.Jung S.Y., Kang K.W., Lee E.Y., Seo D.W., Kim H.L., Kim H. Heterologous prime-boost vaccination with adenoviral vector and protein nanoparticles induces both Th1 and Th2 responses against Middle East respiratory syndrome coronavirus. Vaccine. 2018;36(24):3468–3476. doi: 10.1016/j.vaccine.2018.04.082. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113.Kim Y.S., Son A., Kim J., Kwon S.B., Kim M.H., Kim P. Chaperna-mediated assembly of ferritin-based Middle East respiratory syndrome-coronavirus nanoparticles. Front. Immunol. 2018;9:1093. doi: 10.3389/fimmu.2018.01093. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114.Lin L.C., Huang C.Y., Yao B.Y., Lin J.C., Agrawal A., Algaissi A. Viromimetic STING agonist-loaded hollow polymeric nanoparticles for safe and effective vaccination against Middle East respiratory syndrome coronavirus. Adv. Funct. Mater. 2019;29(28) doi: 10.1002/adfm.201807616. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115.Chauhan G., Madou M.J., Kalra S., Chopra V., Ghosh D., Martinez-Chapa S.O. Nanotechnology for COVID-19: therapeutics and vaccine research. ACS Nano. 2020;28;14(7):7760–7782. doi: 10.1021/acsnano.0c04006. [DOI] [PubMed] [Google Scholar]
- 116.Thanh Le T., Andreadakis Z., Kumar A., Gomez Roman R., Tollefsen S., Saville M. The COVID-19 vaccine development landscape. Nat. Rev. Drug Discov. 2020;19(5):305–306. doi: 10.1038/d41573-020-00073-5. [DOI] [PubMed] [Google Scholar]
- 117.Lung P., Yang J., Li Q. Nanoparticle formulated vaccines: opportunities and challenges. Nanoscale. 2020;12(10):5746–5763. doi: 10.1039/c9nr08958f. [DOI] [PubMed] [Google Scholar]
- 118.Reichmuth A.M., Oberli M.A., Jaklenec A., Langer R., Blankschtein D. mRNA vaccine delivery using lipid nanoparticles. Ther. Deliv. 2016;7(5):319–334. doi: 10.4155/tde-2016-0006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 119.Kanasty R., Dorkin J.R., Vegas A., Anderson D. Delivery materials for siRNA therapeutics. Nat. Mater. 2013;12(11):967–977. doi: 10.1038/nmat3765. [DOI] [PubMed] [Google Scholar]
- 120.Le T.T., Cramer J.P., Chen R., Mayhew S. Evolution of the COVID-19 vaccine development landscape. Nat. Rev. Drug Discov. 2020;19(10):667–668. doi: 10.1038/d41573-020-00151-8. [DOI] [PubMed] [Google Scholar]
- 121.Ahmed Y., Tian M., Gao Y. Development of an anti-HIV vaccine eliciting broadly neutralizing antibodies. AIDS Res. Ther. 2017;14(1):50. doi: 10.1186/s12981-017-0178-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 122.Caskey M., Klein F., Nussenzweig M.C. Broadly neutralizing antibodies for HIV-1 prevention or immunotherapy. N. Engl. J. Med. 2016;375(21):2019–2021. doi: 10.1056/NEJMp1613362. [DOI] [PubMed] [Google Scholar]
- 123.Julg B., Tartaglia L.J., Keele B.F., Wagh K., Pegu A., Sok D. Broadly neutralizing antibodies targeting the HIV-1 envelope V2 apex confer protection against a clade C SHIV challenge. Sci. Transl. Med. 2017;9(406) doi: 10.1126/scitranslmed.aal1321. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 124.Korber B., Hraber P., Wagh K., Hahn B.H. Polyvalent vaccine approaches to combat HIV-1 diversity. Immunol. Rev. 2017;275(1):230–244. doi: 10.1111/imr.12516. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 125.Sanders R.W., van Gils M.J., Derking R., Sok D., Ketas T.J., Burger J.A. HIV-1 VACCINES. HIV-1 neutralizing antibodies induced by native-like envelope trimers. Science. 2015;349(6244) doi: 10.1126/science.aac4223. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 126.Sliepen K., Ozorowski G., Burger J.A., van Montfort T., Stunnenberg M., LaBranche C. Presenting native-like HIV-1 envelope trimers on ferritin nanoparticles improves their immunogenicity. Retrovirology. 2015;12:82. doi: 10.1186/s12977-015-0210-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 127.Brewer M.G., DiPiazza A., Acklin J., Feng C., Sant A.J., Dewhurst S. Nanoparticles decorated with viral antigens are more immunogenic at low surface density. Vaccine. 2017;35(5):774–781. doi: 10.1016/j.vaccine.2016.12.049. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 128.Schotsaert M., Garcia-Sastre A. Inactivated influenza virus vaccines: the future of TIV and QIV. Curr Opin Virol. 2017;23:102–106. doi: 10.1016/j.coviro.2017.04.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 129.Muszkat M., Greenbaum E., Ben-Yehuda A., Oster M., Yeu’l E., Heimann S. Local and systemic immune response in nursing-home elderly following intranasal or intramuscular immunization with inactivated influenza vaccine. Vaccine. 2003;21(11–12):1180–1186. doi: 10.1016/s0264-410x(02)00481-4. [DOI] [PubMed] [Google Scholar]
- 130.Su F., Patel G.B., Hu S., Chen W. Induction of mucosal immunity through systemic immunization: phantom or reality? Hum Vaccin Immunother. 2016;12(4):1070–1079. doi: 10.1080/21645515.2015.1114195. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 131.Dehghan S., Tafaghodi M., Bolourieh T., Mazaheri V., Torabi A., Abnous K. Rabbit nasal immunization against influenza by dry-powder form of chitosan nanospheres encapsulated with influenza whole virus and adjuvants. Int. J. Pharm. 2014;475(1–2):1–8. doi: 10.1016/j.ijpharm.2014.08.032. [DOI] [PubMed] [Google Scholar]
- 132.Renu S., Feliciano-Ruiz N., Lu F., Ghimire S., Han Y., Schrock J. A nanoparticle-poly(I:C) combination adjuvant enhances the breadth of the immune response to inactivated influenza virus vaccine in pigs. Vaccines (Basel) 2020;8(2):229. doi: 10.3390/vaccines8020229. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 133.Singh S.M., Alkie T.N., Abdelaziz K.T., Hodgins D.C., Novy A., Nagy E. Characterization of immune responses to an inactivated avian influenza virus vaccine Adjuvanted with nanoparticles containing CpG ODN. Viral Immunol. 2016;29(5):269–275. doi: 10.1089/vim.2015.0144. [DOI] [PubMed] [Google Scholar]
- 134.Belsham G.J., Kristensen T., Jackson T. Foot-and-mouth disease virus: prospects for using knowledge of virus biology to improve control of this continuing global threat. Virus Res. 2020;281 doi: 10.1016/j.virusres.2020.197909. [DOI] [PubMed] [Google Scholar]
- 135.Qiao D., Liu L., Chen Y., Xue C., Gao Q., Mao H.Q. Potency of a scalable Nanoparticulate subunit vaccine. Nano Lett. 2018;18(5):3007–3016. doi: 10.1021/acs.nanolett.8b00478. [DOI] [PubMed] [Google Scholar]
- 136.Bai M., Dong H., Su X., Jin Y., Sun S., Zhang Y. Hollow mesoporous silica nanoparticles as delivery vehicle of foot-and-mouth disease virus-like particles induce persistent immune responses in Guinea pigs. J. Med. Virol. 2019;91(6):941–948. doi: 10.1002/jmv.25417. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 137.Yuan J., Yang J., Hu Z., Yang Y., Shang W., Hu Q. Safe staphylococcal platform for the development of multivalent Nanoscale vesicles against viral infections. Nano Lett. 2018;18(2):725–733. doi: 10.1021/acs.nanolett.7b03893. [DOI] [PubMed] [Google Scholar]
- 138.Metz S.W., Tian S., Hoekstra G., Yi X., Stone M., Horvath K. Precisely molded nanoparticle displaying DENV-E proteins induces robust serotype-specific neutralizing antibody responses. PLoS Negl. Trop. Dis. 2016;10(10) doi: 10.1371/journal.pntd.0005071. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 139.Hunsawong T., Sunintaboon P., Warit S., Thaisomboonsuk B., Jarman R.G., Yoon I.K. A novel dengue virus serotype-2 nanovaccine induces robust humoral and cell-mediated immunity in mice. Vaccine. 2015;33(14):1702–1710. doi: 10.1016/j.vaccine.2015.02.016. [DOI] [PubMed] [Google Scholar]
- 140.Hunsawong T., Sunintaboon P., Warit S., Thaisomboonsuk B., Jarman R.G., Yoon I.K. Immunogenic properties of a BCG Adjuvanted chitosan nanoparticle-based dengue vaccine in human dendritic cells. PLoS Negl. Trop. Dis. 2015;9(9) doi: 10.1371/journal.pntd.0003958. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 141.Nantachit N., Sunintaboon P., Ubol S. Responses of primary human nasal epithelial cells to EDIII-DENV stimulation: the first step to intranasal dengue vaccination. Virol. J. 2016;13:142. doi: 10.1186/s12985-016-0598-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 142.Niikura K., Matsunaga T., Suzuki T., Kobayashi S., Yamaguchi H., Orba Y. Gold nanoparticles as a vaccine platform: influence of size and shape on immunological responses in vitro and in vivo. ACS Nano. 2013;7(5):3926–3938. doi: 10.1021/nn3057005. [DOI] [PubMed] [Google Scholar]
- 143.Bazzill J.D., Ochyl L.J., Giang E., Castillo S., Law M., Moon J.J. Interrogation of antigen display on individual vaccine nanoparticles for achieving neutralizing antibody responses against hepatitis C virus. Nano Lett. 2018;18(12):7832–7838. doi: 10.1021/acs.nanolett.8b03601. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 144.Wang C., Yang C., Chen Y.C., Ma L., Huang K. Rational Design of Hybrid Peptides: a novel drug design approach. Curr Med Sci. 2019;39(3):349–355. doi: 10.1007/s11596-019-2042-2. [DOI] [PubMed] [Google Scholar]
- 145.Li Y., Wang Z., Chen Y., Petersen R.B., Zheng L., Huang K. Salvation of the fallen angel: reactivating mutant p53. Br. J. Pharmacol. 2019;176(7):817–831. doi: 10.1111/bph.14572. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 146.Accomasso L., Cristallini C., Giachino C. Risk assessment and risk minimization in Nanomedicine: a need for predictive, alternative, and 3Rs strategies. Front. Pharmacol. 2018;9:228. doi: 10.3389/fphar.2018.00228. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 147.von Roemeling C., Jiang W., Chan C.K., Weissman I.L., Kim B.Y.S. Breaking down the barriers to precision Cancer Nanomedicine. Trends Biotechnol. 2017;35(2):159–171. doi: 10.1016/j.tibtech.2016.07.006. [DOI] [PubMed] [Google Scholar]
- 148.Coleman C.M., Liu Y.V., Mu H., Taylor J.K., Massare M., Flyer D.C. Purified coronavirus spike protein nanoparticles induce coronavirus neutralizing antibodies in mice. Vaccine. 2014;32(26):3169–3174. doi: 10.1016/j.vaccine.2014.04.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 149.Coleman C.M., Venkataraman T., Liu Y.V., Glenn G.M., Smith G.E., Flyer D.C. MERS-CoV spike nanoparticles protect mice from MERS-CoV infection. Vaccine. 2017;35(12):1586–1589. doi: 10.1016/j.vaccine.2017.02.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 150.Kim M.C., Kim K.H., Lee J.W., Lee Y.N., Choi H.J., Jung Y.J. Co-delivery of M2e virus-like particles with influenza Split vaccine to the skin using microneedles enhances the efficacy of cross protection. Pharmaceutics. 2019;11(4):188. doi: 10.3390/pharmaceutics11040188. [DOI] [PMC free article] [PubMed] [Google Scholar]