Highlights
-
•
There are no commercially available effective antiviral medications or vaccines to deal with novel coronavirus disease (COVID-19).
-
•
Exosomes carry complex cargo including bioactive which are transmitted to adjacent and distant cells.
-
•
Due to their ability to deliver and exchange intracellular messages and interact with receiver cells, exosomes can applied in diseases treatment.
-
•
Due to the specific structure of the exosomes various drugs can be inserted into them so they can be used in a variety of diseases as drug delivery.
Keywords: MSC exsosomes, Exosome based therapy, covid-19
Abstract
There are no commercially available effective antiviral medications or vaccines to deal with novel coronavirus disease (COVID-19). Hence there is a substantial unmet medical need for new and efficacious treatment options for COVID-19. Most COVID-19 deaths result from acute respiratory distress syndrome (ARDS). This virus induces excessive and aberrant inflammation so it is important to control the inflammation as soon as possible. To date, results of numerous studies have been shown that mesenchymal stem cells and their derivatives can suppress inflammation. Exosomes function as intercellular communication vehicles to transfer bioactive molecules (based on their origins), between cells. In this review, the recent exosome-based clinical trials for the treatment of COVID-19 are presented. Potential therapy may include the following items: First, using mesenchymal stem cells secretome. Second, incorporating specific miRNAs and mRNAs into exosomes and last, using exosomes as carriers to deliver drugs.
1. Covid19
The novel coronavirus disease 2019 (COVID-19) is an enveloped RNA virus of the Coronaviridae family that was first found in Wuhan, China, at the end of 2019 and declared a global public health emergency. The World Health Organization (WHO) has named the virus-associated disease SARS-CoV-2 (Zhao, 2020). Transmission from human to human occurs through the respiratory droplets or contact with contaminated surfaces. The incubation period is on average 5 days varies from 1 to 14 days (Zhu et al., 2020). The virus is transmitted through direct or indirect contact. In direct contact, the virus is transmitted from person to person like a handshake. Indirectly, transmission occurs through fomite, such as paper tissue or contaminated surfaces. Airborne can also transmit the virus without physical contact. Through sneeze and cough, droplet sprays can directly contaminate people, or inhaling airborne microscopic aerosol can also infect people with this virus (Zhu et al., 2020; Asadi et al., 2020).
Most patients have mild respiratory tract infection, with fever and cough. However, in some people, infection with Covid-19 may be asymptomatic, which may be involved in the spread of the virus. More than half of the patients are asymptomatic with CT and laboratory findings disorders (Kronbichler et al., 2020).
Elderly patients were stated to have a more serious symptom with the ICU admission ratio compare to younger patients (Sheahan et al., 2020). Obesity and underlying diseases such as diabetes can lead to young patients being admitted to the ICU (Kass et al., 2020; Lu et al., 2020).
However, effective antiviral medications nor vaccines are not commercially available to deal with this emergency. Once infected, the patient mainly relies on his immune system to resist SARS-CoV-2, with the supportive treatment given if complications occur (Lu et al., 2020; Xu et al., 2020; Zhou et al., 2020). SARS-CoV-2 can enter the cell through binding to angiotensin I converting enzyme 2 receptor (ACE2) by its spike protein receptor-binding domain (RBD). This virus induces excessive and aberrant host immune responses which are often accompanied by overproduction of pro-inflammatory cytokines including IL-2, IL-6, IL-7, GSCF, IP10, MCP1, MIP1A, and TNF (Wan et al., 2020). By targeting lung tissue, these cytokines induce pulmonary edema and subsequent acute lung injury (ALI) or acute respiratory distress syndrome (ARDS) (Metcalfe, 2020). Therefore, introducing appropriate therapeutic approaches for both cytokine storm suppression and lung repair functions are urgently needed.
Mesenchymal stem cells (MSCs) have several features that make them a potential candidate for COVID-19 treatment: these cells can modulate immune responses, reduce inflammation, and thus protecting alveolar epithelial cells during ALI and ARDS. Also, due to the presence of specific cytokines, MSCs can suppress viral infection (Ji et al., 2020). To date, several studies have proposed MSCs for COVID-19 pneumonia treatment (Metcalfe, 2020; Leng et al., 2020; Gentile and Sterodimas, 2020; Golchin et al., 2020). In a research by Shu and his colleges, it has been shown that intravenous transplantation of hUC-MSCs is an effective and safe method for the treatment of severe COVID-19. Their results showed that, the time to clinical improvement in the hUCMSC treatment group, was shorter than that of the control group. In addition, the 28-day mortality rate in the hUC-MSC treatment group was 0, compared with 10.34 % in the control group (Shu et al., 2020). Few studies have paid attention to stem cell secretome like extracellular vesicles (EVs) (Kumar et al., 2020). In a study by Sengupta et al. exosomes derived from allogeneic bone marrow mesenchymal stem cells (ExoFlo) were used for treatment of SARS-CoV-2 PCR positive patients. They received a single 15 mL intravenous dose of ExoFlo, and safety and efficacy were evaluated 1–14 day post-treatment. The results showed a survival rate of 83 %. 71 % of the patients recovered, 13 % remained seriously ill though stable and, 16 % expired for reasons not related to the treatment. Furthermore laboratory results showed significant improvements in neutrophil and CD3+, CD4+ and CD8+ lymphocyte numbers, with a decline in C-reactive protein (CRP) and Ferritin (Sengupta et al., 2020).
2. MSCs derived exosomes
Exosomes are one of the extracellular vesicles enclosed by a lipid bilayer. Based on their primary sources, exosomes carry complex cargo including bioactive molecules such as nucleic acids, microRNA, lipids, and proteins, which are transmitted to adjacent and distant cells and thus alter the fate of the recipient cell (Andaloussi et al., 2013).Various methods for the extraction of exosomes have been developed, such as centrifugation series followed by high-speed ultracentrifugation. In this process, the separation of exosomes is based on the mass, shape and size of the sequential separations. One of the advantages of this approach is reduced costs and lower chances of contamination, but it cannot differentiate between exosomes and other small vesicles or large protein aggregates (Théry et al., 2002). Another method is gradient ultracentrifugation which provides a high yield of isolated exosomes that retain their functions, but this approach has a low specificity compared to size exclusion chromatography (Farooqi et al., 2018). Size and protein markers are the most important characteristics for exosome characterization. Exosomes can be detected using scanning electron microscopy (SEM), transmission electron microscopy (TEM), and nanoparticle tracking analysis (NTA) (Sokolova et al., 2011). Furthermore biochemical characterization can reveal common characteristic of exosomal proteins (Kesimer et al., 2009). The proteins which are most abundant are CD9, CD63, CD81, and CD82), MHC molecules, 14-3-3 proteins, heat shock proteins (HSPs), and Tsg101 (Witwer et al., 2013).
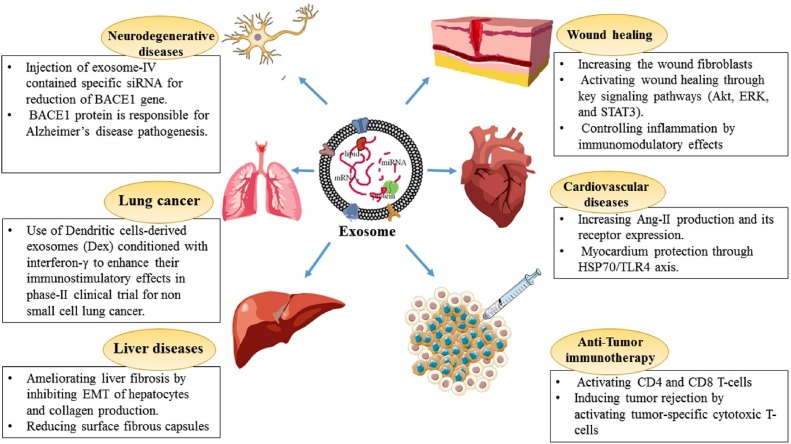
As we reviewed recently (Rahmati et al., 2020; Rezakhani MA et al., 2020), due to their unique ability to deliver and exchange intracellular chemical messages and interact with receiver cells, exosomes have been increasingly applied in the treatment of several diseases (Fig. 1 ).
Fig. 1.
Several exosome therapeutic application in various disease(25).
Up to now, (9/03/2020) 7 clinical trials using exosome to treat COVID 19 are underway (Table 1 ). Researches have shown that the potential of exosomes for COVID 19 treatment can be divided into three general categories. First, using exosomal particles secreted by different sources of mesenchymal stem cells instead of cell therapy. Second, incorporating specific miRNAs and mRNAs into exosomes a last, using exosomes as carriers to deliver drugs to treat COVID-19.
Table 1.
List of registered exosome based clinical trials for treating COVID-19.
| location | Study identifier | Main goal | Study design | Primary Outcome Measures |
|---|---|---|---|---|
| China, Shanghai Public Health Clinical Center | NCT04276987 | explore the safety and efficiency of aerosol inhalation of the exosomes derived from allogenic adipose mesenchymal stem cells (MSCs-Exo) in treatment of severe patients with novel coronavirus pneumonia (NCP) | Clinical Trial. N/A, 30 participants 5 times aerosol inhalation of MSCs-Exo (2 × 10 (Xu et al., 2020) nano vesicles/3 mL at Day 1to Day 5). | 1.Safety evaluation within 28 days after the first treatment, including frequency of adverse reaction (AE) and severe adverse reaction (SAE) |
| 2.Efficiency evaluation within 28 days, including the time to clinical improvement (TTIC) | ||||
| Kayseri, Melikgazi, Turkey | NCT04389385 | Treatment of COVID-19 patients -who are at early stages of pulmonary disease- with COVID-19 Specific T Cell-derived exosomes (CSTC-Exo) to control disease progression. | Clinical Trial. N/A, 60 participants. COVID-19 Specific T Cell-derived exosomes (CSTC-Exo). Inhaler CSTC-Exo treatment will be applied daily x 5 times (2.0 × 108 nano vesicle / 3 mL; on day 1 to day 5). | 1. Safety Assessment: Adverse reaction (AE) and severe AE (SAE) within 28 days |
| 2. Efficacy Assessment .28 Days | ||||
| Time to Clinical Recovery (TTCR) | ||||
| 3. The Rate of Recovery Without Mechanical Ventilator, within 28 days | ||||
| United States, Florida. Landmark Hospital | NCT04384445 | Investigate safety and potential efficacy of human amniotic fluid (HAF) derived acellular product in subjects suffering from COVID-19 infection with the severe acute respiratory syndrome (SARS). | Phase I/II Randomized, 20 participants, Parallel Assignment, two groups of treatment and placebo, each with 10 subjects (n = 20). Randomized and double-blinded. Organicell Flow will be administered intravenously with 1 mL, containing 2−5 × 10^11 particles/mL in addition to the Standard Care | 1. Safety will be defined by the incidence of infusion associated with adverse events as assessed by treating physician within 60 Days. |
| 2. Safety will be defined by the incidence of severe adverse events as assessed by treating physician within 60 Days. | ||||
| China | ChiCTR2000030261 | Investigate the effect of stem cell exosomes to inhibiting inflammatory factors and enhancing the immunity of the body, and by atomizing into the lung to contact the focus directly in COVID-19 patients | Phase 0 clinical, Exocrine group:13; Control group:13; Aerosol inhalation of exosomes | Lung CT, Nucleic acid detection of the pharyngeal test, Leukocytes and lymphocytes in blood routine |
| China | ChiCTR2000030484 | HUMSCs and Exosomes Treating Patients with Lung Injury following Novel Coronavirus Pneumonia (COVID-19) | Phase N/A clinical. Treatment group 30 and control group 30. HUMSCs intravenous infusion, 5 × 10 (Lu et al., 2020) cells / time, once / week, twice / course; Exosomes: intravenous administration, 180 mg/time, 1 time/day, 7 days | PaO2/FiO2 or respiratory rate (without oxygen), The number and range of lesions indicated by CT and X-ray of the lung, Time for the cough to become mild or absent. Inflammatory cytokines (CRP / PCT / SAA, etc.) |
| Samara, Russian Federation, 443,095 | NCT04491240 | Explore the safety and efficiency of aerosol inhalation of the exosomes in the treatment of severe patients hospitalized with novel coronavirus pneumonia. | Clinical Trial, three groups, each with 30 subjects (n = 90). All eligible study subjects are randomized, double-blinded, to either the two treatment groups or placebo group | 1. Safety assessment such as adverse events will be registered. Adverse events will be monitored during all trial |
| 2. Safety assessments such as adverse events during the inhalation procedures will be registered. | ||||
| No Contacts or Locations Provided | NCT04493242 | Evaluation of the safety and efficacy of intravenous administration of bone marrow-derived extracellular vesicles, ExoFlo, versus placebo as a treatment for moderate-to-severe Acute Respiratory Distress Syndrome (ARDS) in patients with severe COVID-19. | Multi-center, placebo-controlled, randomized clinical trial. 60 participants, | 1. All-cause mortality |
| 2. Median days to recovery |
3. Using mesenchymal stem cells secretome
However, it is now clear that MSCs act via a paracrine mechanism (Rahmati et al., 2020). MSC-secretome consists of both extracellular vesicles and several soluble proteins, cytokines, chemokines, and growth factors (Crivelli et al., 2017). This secretome can interact with the target cells and modify the recipient cell's fate by endogenous stem cells activation, apoptosis suppression, regulating the inflammatory response, fibrosis reduction, and mediating the chemoattraction (Di Rocco et al., 2016).
Several studies (Bari et al., 2019a, 2020; Ferreira et al., 2018) have shown that the therapeutic effects of MSCs secretome such as anti-inflammatory, regenerative, immunomodulatory pro-angiogenic, and anti-protease properties (Abraham and Krasnodembskaya, 2020), are similar to the MSCs of origin. Therefore, this conditioned medium harvested from MSCs, can be considered as a promising cell-free therapeutic tool in the treatment of lung diseases. The results of a research (Leng et al., 2020) showed that the intravenous infusion of MSCs resulted in increased peripheral lymphocytes, overactivation of some types of T cells, and the reduction of the C-reactive protein.
In preclinical ARDS, the efficacy of MSCs secretome is obvious in both in vivo and ex vivo, for instance, secretome distributed through both lungs after intravenous injection, with the high stability in the blood flow (Bari et al., 2020). Notably, the use of exosomes in therapy has more benefits than MSCs. Exosomes are smaller and less complex than MSCs, thus the production and their storage are more feasible. In addition, they do not have the ability to self-replicate, so the risk of endogenous tumor formation is reduced. Besides, they show low immunogenicity effect and low emboli formation after intravenous injection as a result they are safer than routine MSCs, finally the cost of using MSCs secretome will be much lower than monoclonal antibody therapy (Rahmati et al., 2020), which is so important in a pandemic treatment. For the first time, Bari and colleagues suggested that MSC-secretome can be formulated as inhaling aerosolized drugs/EVs (Bari et al., 2019b). Administering MSC-secretome by inhalation leads to improved clinical outcomes. Essentially, this administration route is a non-invasive means with fewer side effects that enable lower doses to have the same effect as an injection or oral therapy.
Based on the available evidence, MSC-secretome, which is made as a freeze-dried powder for intravenously or inhaled use, appears to be a suitable candidate for treating patients with COVID-19 pneumonia. In this regard, two recent clinical trials at http://www.clinicaltrials.gov are in progress. one research group (NCT04276987) is currently studying the inhalation of the exosomes derived from allogenic adipose mesenchymal stem cells for the treatment of COVID-19 pneumonia and (NCT04313647) that evaluate their safety and tolerance in healthy-looking volunteers. Please refer to Table 1 for more details.
4. Incorporating specific miRNAs and mRNAs into exosomes
The mechanism of viral replication in the infected cell is the use of a nucleic acid and protein synthesis system (Stern-Ginossar et al., 2019). Protein synthesis in animal cells is regulated by microRNA or miRNA (mRNA- inhibiting RNA). miRNAs are a class of the small non-coding RNAs that regulate the mRNA translation of target genes by interaction with 3’ untranslated region of relating mRNA (Abdollahi et al., 2019). Since viral protein synthesis occurs in the host cell, this regulatory function of miRNAs can be used against viral diseases. Using miRNAs in this purpose requires perfect or near-perfect matches with the mRNA of the viral genome (gRNA) or parts of its genome that is protein-coding. This binding, cause cleavage and degradation of the target gene (Yekta et al., 2004). Therefore, in order to minimize the side effects, it is important to select a sequence of miRNAs that specifically bind to the virus genome. Complementary sequences to the human genome can including non-specific gene silencing (Singh et al., 2011). There are several powerful programs to identify and predict gene targets of miRNAs, such as MirTarget, MiRanda, TargetScan and, PicTar II (Sethupathy et al., 2006). Ivashchenko and colleagues (Ivashchenko et al., 2020), decided to identify human miRNAs that affect the expression of SARS-CoV-2 coronavirus genomes. Their goal was to design a set of miRNAs that would specifically bind to the coronavirus genome to destroy it, without any side effects on human gene expression which may have the potential to be used as a new approach in coronavirus treatment. Using MirTarget, they identified miR-5197-3p out of the 2565 miRNAs that had the greatest potential to interaction with the gRNA of SARS-CoV-2, without having target genes among the 17,508 human coding genes.
A possible explanation for the fact that SARS-CoV-2 infection is less frequently observed in children aged less than 15 years compared to adults over 60 years is that the expression of piwi-interacting RNA (pi-RNA) and miRNA is Age-Dependent phenomena, in fact pi-RNA expression is decreased with age, while miRNA expression is increased. It seems that during ontogenesis the expression of one or more pi-RNAs that can bind to the viral gRNA decreases. Likewise, the concentration of miRNAs capable of binding to the gRNA and expressed in the early stages of ontogenesis may decrease, and the virus begins to multiply (Zhou et al., 2018; Lee et al., 2020).,
5. Exosomes for drug delivery
In long-distance cell to cell communication, exosomes are of great importance because they can enter the circulation and pass through additional biological barriers. More recent evidence indicates that they can carry rich cargo between cells. Due to the specific structure of the exosomes various drugs can be inserted into them so they can be used in a variety of diseases as drug delivery systems (Vader et al., 2016). In addition, in personalized medicine, exosomes can be separated from the patient's plasma, then filled with the exogenous drug of interest, and finally given back to the same patient (Lamichhane et al., 2015). The immunomodulatory cargo of MSCs exosomes combined with the anti-viral drugs makes them a novel intervention tool for the treatment of the disease (Gupta et al., 2020). Remdesivir antiviral drug, which is prescribed for the treatment of patients with Covid-19 (Zhou et al., 2020; Grein et al., 2020), can be load in to the exosome. However, in the case of COVID-19, more scrutiny is essential to have a full understanding of their safety, specificity, proficiency, and delivery mechanisms of antiretroviral drugs to target tissues.
6. Conclusion
To overcome the pandemic of COVID-19, we need to develop a multi-directional strategy to minimize its prevalence. In this infection, severe cytokine storm is the main contributor to organ damage, MSCs not only suppress the cytokine storm but also facilitate endogenous regenerative mechanisms due to their strong immunomodulatory capability (Shetty, 2020).In addition to MSCs, their derivatives including exosomes have earned considerable interest as a therapeutic technique. Exosome-based therapy is an attractive strategy for achieving the therapeutic benefits of MSCs without the complications and difficulties of the cells to the patients. In particular, the possible use of exosomes in preclinical models to alleviate ARDS is well known (Ghorbani et al., 2017).
This technique can be used as an effective therapy for the delivery of different synthetic and biological molecules in cellular therapy of various diseases. Indeed, these cells can be considered as potent drug stores releasing biologically active substances. Furthermore in comparison with other treatments such as monoclonal antibody therapy, the costs of MSC-secretome seem probably lower which is important in treating a pandemic (Caplan, 2017). Despite the potential of MSC-derived exosomes for treatment of SARS-CoV-2, the use of exosomes for any purpose in SARS-CoV-2, including but not limited to reducing cytokine storm, exerting regenerative effects or delivering drugs are currently pending the generation of appropriate manufacturing and quality control provisions (Borger et al., 2020).Current clinical trials highlight the potential benefits of stem cell secretome therapies for COVID-19 patients. However, further studies are therefore important to clarify the safety and effectiveness of these therapies and their long-term outcomes.
Declaration of Competing Interest
The authors report no declarations of interest.
Acknowledgments
The authors acknowledge the Cellular and Molecular Research Center of Kurdistan University of Medical Sciences for supporting our team. This research did not receive any specific grant from funding agencies in the public, commercial, or not-for-profit sectors.
References
- Abdollahi A., Rahmati S., Ghaderi B., Sigari N., Nikkhoo B., Sharifi K. A combined panel of circulating microRNA as a diagnostic tool for detection of the non-small cell lung cancer. QJM Int. J. Med. 2019;112(10):779–785. doi: 10.1093/qjmed/hcz158. [DOI] [PubMed] [Google Scholar]
- Abraham A., Krasnodembskaya A. Mesenchymal stem cell‐derived extracellular vesicles for the treatment of acute respiratory distress syndrome. Stem Cells Transl. Med. 2020;9(1):28–38. doi: 10.1002/sctm.19-0205. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Andaloussi S.E., Mäger I., Breakefield X.O., Wood M.J. Extracellular vesicles: biology and emerging therapeutic opportunities. Nat. Rev. Drug Discov. 2013;12(5):347–357. doi: 10.1038/nrd3978. [DOI] [PubMed] [Google Scholar]
- Asadi S., Bouvier N., Wexler A.S., Ristenpart W.D. Taylor & Francis; 2020. The Coronavirus Pandemic and Aerosols: Does COVID-19 Transmit Via Expiratory Particles? [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bari E., Ferrarotti I., Torre M.L., Corsico A.G., Perteghella S. Mesenchymal stem/stromal cell secretome for lung regeneration: the long way through “pharmaceuticalization” for the best formulation. J. Control. Release. 2019;309:11–24. doi: 10.1016/j.jconrel.2019.07.022. [DOI] [PubMed] [Google Scholar]
- Bari E., Ferrarotti I., Torre M.L., Corsico A.G., Perteghella S. Mesenchymal stem/stromal cell secretome for lung regeneration: the long way through “pharmaceuticalization” for the best formulation. J. Control. Release. 2019 doi: 10.1016/j.jconrel.2019.07.022. [DOI] [PubMed] [Google Scholar]
- Bari E., Ferrarotti I., Saracino L., Perteghella S., Torre M.L., Corsico A.G. Mesenchymal stromal cell secretome for severe COVID-19 infections: premises for the therapeutic use. Cells. 2020;9(4):924. doi: 10.3390/cells9040924. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Borger V., Weiss D.J., Anderson J.D., Borràs F.E., Bussolati B., Carter D.R. 2020. International Society for Extracellular Vesicles and International Society for Cell and Gene Therapy Statement on Extracellular Vesicles From Mesenchymal Stromal Cells and Other Cells: Considerations for Potential Therapeutic Agents to Suppress Coronavirus disease-19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Caplan A.I. Mesenchymal stem cells: time to change the name! Stem Cells Transl. Med. 2017;6(6):1445–1451. doi: 10.1002/sctm.17-0051. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Crivelli B., Chlapanidas T., Perteghella S., Lucarelli E., Pascucci L., Brini A.T. Mesenchymal stem/stromal cell extracellular vesicles: from active principle to next generation drug delivery system. J. Control. Release. 2017;262:104–117. doi: 10.1016/j.jconrel.2017.07.023. [DOI] [PubMed] [Google Scholar]
- Di Rocco G., Baldari S., Toietta G. Towards therapeutic delivery of extracellular vesicles: strategies for in vivo tracking and biodistribution analysis. Stem Cells Int. 2016;2016 doi: 10.1155/2016/5029619. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Farooqi A.A., Desai N.N., Qureshi M.Z., Librelotto D.R.N., Gasparri M.L., Bishayee A. Exosome biogenesis, bioactivities and functions as new delivery systems of natural compounds. Biotechnol. Adv. 2018;36(1):328–334. doi: 10.1016/j.biotechadv.2017.12.010. [DOI] [PubMed] [Google Scholar]
- Ferreira J.R., Teixeira G.Q., Santos S.G., Barbosa M.A., Almeida-Porada G., Gonçalves R.M. Mesenchymal stromal cell secretome: influencing therapeutic potential by cellular pre-conditioning. Front. Immunol. 2018;9:2837. doi: 10.3389/fimmu.2018.02837. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gentile P., Sterodimas A. Taylor & Francis; 2020. Adipose-derived Stromal Stem Cells (ASCs) As a New Regenerative Immediate Therapy Combating Coronavirus (COVID-19)-induced Pneumonia. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ghorbani F., Feizabadi M., Farzanegan R., Vaziri E., Samani S., Lajevardi S. An investigation of topics and trends of tracheal replacement studies using co-occurrence analysis. Tissue Eng. Part B Rev. 2017;23(2):118–127. doi: 10.1089/ten.TEB.2016.0254. [DOI] [PubMed] [Google Scholar]
- Golchin A., Seyedjafari E., Ardeshirylajimi A. Mesenchymal stem cell therapy for COVID-19: present or future. Stem Cell Rev. Rep. 2020:1–7. doi: 10.1007/s12015-020-09973-w. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grein J., Ohmagari N., Shin D., Diaz G., Asperges E., Castagna A. Compassionate use of remdesivir for patients with severe Covid-19. N. Engl. J. Med. 2020;382(24):2327–2336. doi: 10.1056/NEJMoa2007016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gupta S., Krishnakumar V., Sharma Y., Dinda A.K., Mohanty S. Mesenchymal stem cell derived exosomes: a nano platform for therapeutics and drug delivery in combating COVID-19. Stem Cell Rev. Rep. 2020:1–11. doi: 10.1007/s12015-020-10002-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ivashchenko A., Rakhmetullina A., Aisina D. 2020. How miRNAs Can Protect Humans From Coronaviruses COVID-19, SARS-CoV, and MERS-CoV. [Google Scholar]
- Ji F., Li L., Li Z., Jin Y., Liu W. Mesenchymal stem cells as a potential treatment for critically ill patients with coronavirus disease 2019. Stem Cells Transl. Med. 2020;9:813–814. doi: 10.1002/sctm.20-0083. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kass D.A., Duggal P., Cingolani O. Obesity could shift severe COVID-19 disease to younger ages. Lancet (London, England). 2020 doi: 10.1016/S0140-6736(20)31024-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kesimer M., Scull M., Brighton B., DeMaria G., Burns K., O’Neal W. Characterization of exosome‐like vesicles released from human tracheobronchial ciliated epithelium: a possible role in innate defense. Faseb J. 2009;23(6):1858–1868. doi: 10.1096/fj.08-119131. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kronbichler A., Kresse D., Yoon S., Lee K.H., Effenberger M., Shin J.I. Asymptomatic patients as a source of COVID-19 infections: a systematic review and meta-analysis. Int. J. Infect. Dis. 2020;98:180–186. doi: 10.1016/j.ijid.2020.06.052. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kumar S., Zhi K., Mukherji A., Gerth K. Repurposing antiviral protease inhibitors using extracellular vesicles for potential therapy of COVID-19. Viruses. 2020;12(5):486. doi: 10.3390/v12050486. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lamichhane T.N., Sokic S., Schardt J.S., Raiker R.S., Lin J.W., Jay S.M. Emerging roles for extracellular vesicles in tissue engineering and regenerative medicine. Tissue Eng. Part B Rev. 2015;21(1):45–54. doi: 10.1089/ten.teb.2014.0300. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee P.-I., Hu Y.-L., Chen P.-Y., Huang Y.-C., Hsueh P.-R. Are children less susceptible to COVID-19? J. Microbiol. Immunol. Infect. 2020;53(3) doi: 10.1016/j.jmii.2020.02.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Leng Z., Zhu R., Hou W., Feng Y., Yang Y., Han Q. Transplantation of ACE2-mesenchymal stem cells improves the outcome of patients with COVID-19 pneumonia. Aging Dis. 2020;11(2):216. doi: 10.14336/AD.2020.0228. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lu R., Zhao X., Li J., Niu P., Yang B., Wu H. Genomic characterisation and epidemiology of 2019 novel coronavirus: implications for virus origins and receptor binding. Lancet. 2020;395(10224):565–574. doi: 10.1016/S0140-6736(20)30251-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Metcalfe S.M. Mesenchymal stem cells and management of COVID-19 pneumonia. Med. Drug Discovery. 2020 doi: 10.1016/j.medidd.2020.100019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rahmati S., Shojaei F., Shojaeian A., Rezakhani L., Dehkordi M.B. An overview of current knowledge in biological functions and potential theragnostic applications of exosomes. Chem. Phys. Lipids. 2020;226 doi: 10.1016/j.chemphyslip.2019.104836. [DOI] [PubMed] [Google Scholar]
- L Rezakhani MA, E Sharifi, M Soleimannejad, A Alizadeh. Isolation and characterization of crab hemolymph exosomes and its effects on breast cancer cells Cell journal (Yakhteh). 2022;4T1 (inpress). [DOI] [PMC free article] [PubMed]
- Sengupta V., Sengupta S., Lazo A., Woods P., Nolan A., Bremer N. Exosomes derived from bone marrow mesenchymal stem cells as treatment for severe COVID-19. Stem Cells Dev. 2020:747–754. doi: 10.1089/scd.2020.0080. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sethupathy P., Megraw M., Hatzigeorgiou A.G. A guide through present computational approaches for the identification of mammalian microRNA targets. Nat. Methods. 2006;3(11):881–886. doi: 10.1038/nmeth954. [DOI] [PubMed] [Google Scholar]
- Sheahan T.P., Sims A.C., Leist S.R., Schäfer A., Won J., Brown A.J. Comparative therapeutic efficacy of remdesivir and combination lopinavir, ritonavir, and interferon beta against MERS-CoV. Nat. Commun. 2020;11(1) doi: 10.1038/s41467-019-13940-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shetty A.K. Mesenchymal stem cell infusion shows promise for combating coronavirus (COVID-19)-induced pneumonia. Aging Dis. 2020;11(2):462. doi: 10.14336/AD.2020.0301. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shu L., Niu C., Li R., Huang T., Wang Y., Huang M. Treatment of severe COVID-19 with human umbilical cord mesenchymal stem cells. Stem Cell Res. Ther. 2020;11(1):1–11. doi: 10.1186/s13287-020-01875-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Singh S., Narang A.S., Mahato R.I. Subcellular fate and off-target effects of siRNA, shRNA, and miRNA. Pharm. Res. 2011;28(12):2996–3015. doi: 10.1007/s11095-011-0608-1. [DOI] [PubMed] [Google Scholar]
- Sokolova V., Ludwig A.-K., Hornung S., Rotan O., Horn P.A., Epple M. Characterisation of exosomes derived from human cells by nanoparticle tracking analysis and scanning electron microscopy. Colloids Surf. B Biointerfaces. 2011;87(1):146–150. doi: 10.1016/j.colsurfb.2011.05.013. [DOI] [PubMed] [Google Scholar]
- Stern-Ginossar N., Thompson S.R., Mathews M.B., Mohr I. Translational control in virus-infected cells. Cold Spring Harb. Perspect. Biol. 2019;11(3) doi: 10.1101/cshperspect.a033001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Théry C., Zitvogel L., Amigorena S. Exosomes: composition, biogenesis and function. Nat. Rev. Immunol. 2002;2(8):569–579. doi: 10.1038/nri855. [DOI] [PubMed] [Google Scholar]
- Vader P., Mol E.A., Pasterkamp G., Schiffelers R.M. Extracellular vesicles for drug delivery. Adv. Drug Deliv. Rev. 2016;106:148–156. doi: 10.1016/j.addr.2016.02.006. [DOI] [PubMed] [Google Scholar]
- Wan Y., Shang J., Graham R., Baric R.S., Li F. Receptor recognition by the novel coronavirus from Wuhan: an analysis based on decade-long structural studies of SARS coronavirus. J. Virol. 2020;94(7) doi: 10.1128/JVI.00127-20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Witwer K.W., Buzás E.I., Bemis L.T., Bora A., Lässer C., Lötvall J. Standardization of sample collection, isolation and analysis methods in extracellular vesicle research. J. Extracell. Vesicles. 2013;2(1):20360. doi: 10.3402/jev.v2i0.20360. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xu X., Chen P., Wang J., Feng J., Zhou H., Li X. Evolution of the novel coronavirus from the ongoing Wuhan outbreak and modeling of its spike protein for risk of human transmission. Sci. China Life Sci. 2020;63(3):457–460. doi: 10.1007/s11427-020-1637-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yekta S., Shih I.-h, Bartel D.P. MicroRNA-directed cleavage of HOXB8 mRNA. Science. 2004;304(5670):594–596. doi: 10.1126/science.1097434. [DOI] [PubMed] [Google Scholar]
- Zhao R.C. Stem cell-based therapy for coronavirus disease 2019. Stem Cells Dev. 2020;(ja) doi: 10.1089/scd.2020.0071. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhou L., Lim M.Y.T., Kaur P., Saj A., Bortolamiol-Becet D., Gopal V. Importance of miRNA stability and alternative primary miRNA isoforms in gene regulation during Drosophila development. Elife. 2018;7 doi: 10.7554/eLife.38389. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhou P., Yang X.-L., Wang X.-G., Hu B., Zhang L., Zhang W. A pneumonia outbreak associated with a new coronavirus of probable bat origin. Nature. 2020;579(7798):270–273. doi: 10.1038/s41586-020-2012-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhu N., Zhang D., Wang W., Li X., Yang B., Song J. A novel coronavirus from patients with pneumonia in China, 2019. N. Engl. J. Med. 2020;395(10236):1544–1545. doi: 10.1056/NEJMoa2001017. [DOI] [PMC free article] [PubMed] [Google Scholar]