Abstract
Background
Patients from ethnic minority groups are disproportionately affected by Coronavirus disease (COVID-19). We performed a systematic review and meta-analysis to explore the relationship between ethnicity and clinical outcomes in COVID-19.
Methods
Databases (MEDLINE, EMBASE, PROSPERO, Cochrane library and MedRxiv) were searched up to 31st August 2020, for studies reporting COVID-19 data disaggregated by ethnicity. Outcomes were: risk of infection; intensive therapy unit (ITU) admission and death. PROSPERO ID: 180654.
Findings
18,728,893 patients from 50 studies were included; 26 were peer-reviewed; 42 were from the United States of America and 8 from the United Kingdom. Individuals from Black and Asian ethnicities had a higher risk of COVID-19 infection compared to White individuals. This was consistent in both the main analysis (pooled adjusted RR for Black: 2.02, 95% CI 1.67–2.44; pooled adjusted RR for Asian: 1.50, 95% CI 1.24–1.83) and sensitivity analyses examining peer-reviewed studies only (pooled adjusted RR for Black: 1.85, 95%CI: 1.46–2.35; pooled adjusted RR for Asian: 1.51, 95% CI 1.22–1.88). Individuals of Asian ethnicity may also be at higher risk of ITU admission (pooled adjusted RR 1.97 95% CI 1.34–2.89) (but no studies had yet been peer-reviewed) and death (pooled adjusted RR/HR 1.22 [0.99–1.50]).
Interpretation
Individuals of Black and Asian ethnicity are at increased risk of COVID-19 infection compared to White individuals; Asians may be at higher risk of ITU admission and death. These findings are of critical public health importance in informing interventions to reduce morbidity and mortality amongst ethnic minority groups.
Keywords: SARS-CoV-2, COVID-19 Black, Asian, Hispanic, ethnic, ethnicity, race, disporportionate, outcome, infection, transmission, ITU admission, death
Research in context.
Evidence before this study
Increasing evidence suggests that individuals from certain ethnic groups may have worse clinical outcomes from Coronavirus disease (COVID-19). However, whether the associations are related to increased vulnerability to infection, or more severe disease (intensive therapy unit (ITU) admission or mortality) is unknown. There is no published meta-analysis of existing data on this topic.
Added value of this study
In this systematic review and meta-analysis, we searched multiple databases (within MEDLINE, EMBASE, PROSPERO and the Cochrane library) and preprint data on MedRxiv from 1st December 2019 to 31st August 2020. 18,728,893 patients from 50 studies were included in the meta-analyses; by 31st August, 26 were peer-reviewed. In pooled adjusted analyses, Black and Asian individuals had an increased risk of infection with Severe Acute Respiratory Syndrome Coronavirus-2 (SARS-CoV-2) compared to White individuals, consistent in both the main analysis and sensitivity analysis examining only peer-reviewed studies. Asian patients may also have a higher risk of ITU admission (although all studies examining ITU admission in Asians were not yet peer-reviewed); and death (pooled adjusted risk ratios approached significance).
Implications of all the available evidence
This is the first meta-analysis to report on the effect of ethnicity on clinical outcomes in patients with COVID-19. We found increased risk of infection amongst those of Black and Asian ethnicities compared to White individuals. Asian individuals may also be at higher risk of ITU admission and death, even when confounders such as age, sex and comorbidities are adjusted for. Future studies must explore the reasons for this suggested association, adjusting for the risk of infection. Our findings are of critical public health importance and should inform policy on minimising SARS-CoV-2 exposure in ethnic minority groups.
Alt-text: Unlabelled box
1. Introduction
Over 39 million people have been infected with Severe Acute Respiratory Syndrome Coronavirus-2 (SARS-CoV-2) and over 1 million have died from Coronavirus Disease (COVID-19) since December 2019 [1]. Ethnicity has come under scrutiny as an important risk factor for infection, severe disease and death, with evidence that ethnic minorities may be at increased risk of COVID-19 morbidity and mortality [2,3].
Understanding the relationship between ethnicity and COVID-19 is an urgent research priority, in order to reduce the disproportionate burden of disease in Black, Asian and other minority ethnic groups [4,5]. Recently there has been an increase in the volume of literature, both published and in preprint servers, on the association of ethnicity with vulnerability to COVID-19 infection and clinical outcomes [6]. A comprehensive synthesis of existing evidence examining the relationship between ethnicity and COVID-19 is urgently needed in order to inform clinical care and public health policy. In particular, it is important to disentangle whether worse reported outcomes in ethnic minority groups are attributable to an increased risk of becoming infected, developing severe COVID-19 pneumonia or death. We therefore conducted a systematic review and meta-analysis of both published and preprint research to study the association of ethnicity with COVID-19 infection and outcomes. Specifically, we aimed to identify ethnic differences in the risk of becoming infected with SARS-CoV-2 as well as subsequent intensive therapy unit (ITU) admission (a surrogate marker for severe COVID-19 pneumonia) and death.
2. Methods
We conducted the research according to the Preferred Reporting Items for Systematic Reviews and Meta-Analyses (PRISMA) guidelines, and registered our review on PROSPERO (PROSPERO ID: 180654) on 21st April 2020 [7].
2.1. Data sources and searches
An academic librarian (PD) developed the search strategies, and carried out a search of the databases MEDLINE, EMBASE, PROSPERO and the Cochrane Library, as shown in the Supplementary materials 1. We searched for any articles published in English from 1st December 2019 to 31st August 2020. We also reviewed any peer reviewed publications on MedRxiv during the same period.
We included studies with original clinical data on COVID-19 infection, ITU admission, or mortality disaggregated by ethnicity. We excluded correspondence pieces, area level studies (reporting aggregated data rather than individual risk), and predictive modelling (mathematical modelling, machine learning or computational) studies, or those that only included basic science or animal data or did not report individual data (e.g. studies of infection breakouts). Retracted papers were also excluded. If studies assessed race and ethnicity separately, data were only extracted for mutually exclusive groups. For example, if two separate variables were presented: for ‘race’ and ‘ethnicity’, the variable which included ‘Black, Asian and White’ was chosen to represent ethnicity. We predicted that this would most commonly occur in some American studies, where ethnicity may be used to refer to ‘Hispanic’ or ‘Non-Hispanic’, and race to refer to ‘Black, Asian and White’. This was a pragmatic way of ensuring that we assessed ethnicity in a standardised way, across multiple studies which assessed ethnicity or race differently.
We attempted to minimise the possibility of including patients from the same population twice when exploring one outcome. Where multiple studies of what is likely to be the same population were identified (for example, multiple studies using the UK Biobank database or data from national GP records), the most recent version up to 31st August 2020 was used, with published peer-reviewed studies favoured over those in the preprint database (up to 31st August 2020). Papers which covered a larger number of patients over a longer period of time were favoured over smaller studies, should it be likely that they both investigated the same patients. However, studies which assessed different cohorts of patients (for example, from different countries) in the same paper, or studies which were based on the same population but explored different outcomes were included in the analysis.
2.2. Study selection and data collection
DP and SS independently screened titles, abstracts and full-text articles reporting potentially eligible studies. Disagreements were resolved by discussion or consultation with an adjudicator (MP) when necessary.
2.3. Data extraction
One reviewer (DP, SS, CAM, JN, JSM, LBN) independently extracted data from each potentially eligible article. Data extraction was duplicated for all papers by an independent researcher (CN). We stratified patients into the following ethnic groups based on the categorisations used in the included papers: White (including White British, Caucasian, White European); Asian (including South Asian, Asian/Pacific-Islander and Chinese); Black (including Black Caribbean and Black African); Hispanic (including Hispanic and Latino); Native American; Mixed and Other. Individuals who had ethnicity data missing were excluded. When the proportion of patients of each ethnicity was not presented in the text, we calculated the proportion from data presented in tables, or supplementary material from the manuscript. The outcomes studied in relation to different ethnic groups were:
-
-
Infection with SARS-CoV-2
-
-
ITU admission
-
-
Death
Patients with COVID-19 were defined as those who tested positive for SARS-CoV-2 by nasopharyngeal swab or had clinical evidence of COVID-19, as indicated by clinical signs and symptoms, along with radiology and laboratory tests. We excluded studies which defined patients with COVID-19 as positive by serology, since serological tests are not always initially positive during acute infection and were not widely available or validated when we started our meta-analysis in April 2020.
2.4. Quality assessment
Quality assessment was carried out by DP, SS, CAM, JN, JSM, and LBN. The Joanna Briggs Institute Critical Appraisal Tools were used to assess the quality of evidence for all studies relevant to each study design [8]. Each primary study was assigned two points if they satisfied the criteria used in the relevant tool; one if only partially satisfied, and zero if not satisfied. Any disagreements were resolved through group consensus. A quality appraisal score was calculated by using the numerator and denominator relevant for each study. Publication bias was assessed visually using funnel plots and formally with Egger's test for primary analyses including at least 10 studies [9].
2.5. Statistical analysis
We first synthesised data on the prevalence of each outcome and unadjusted data by ethnicity. We excluded studies which did not record all clinical outcomes. Raw counts were used for the unadjusted data to calculate odds ratios (OR) and 95% confidence intervals (CI).
We also synthesised data adjusted for key confounders. For risk of infection and ITU admission, we extracted adjusted risk ratios (RR). Adjusted OR were converted to adjusted RR using the conversion method as recommended by the Cochrane Handbook [10]. For mortality, we extracted adjusted hazard ratios (HR) (95% CI) where possible, and assumed adjusted RR to approximate an adjusted HR.
Some studies presented multiple models with different sets of confounders. We included the model which most closely matched our a priori chosen confounders of age, sex, deprivation, obesity, and comorbidities. We recorded other confounders that a study had adjusted for, including the way comorbidities were considered. For both the adjusted and unadjusted comparisons, data were extracted for analyses which used White ethnicity as the reference group.
For ITU admission and mortality, we included studies which reported suspected or confirmed COVID-19 patients in their analyses. For mortality, we conducted a further analysis to include studies which looked at the risk of death from COVID-19 in the general population (i.e., those with and without COVID-19). Sensitivity analyses were also conducted excluding:
-
-
For the outcome of death, studies which did not include data for those still hospitalised at the end of the follow-up, since these studies may underestimate death;
-
-
Studies which were of mixed populations (hospitalised and non-hospitalised patients), since these studies may also underestimate ITU admission or death;
-
-
Studies which were not peer reviewed (on MedRxiv)
For all outcomes and data types, we synthesised data (prevalence, unadjusted OR and adjusted RR/HR) using the DerSimonian and Laird random effects model [11]. I2 was used to assess heterogeneity. All meta-analyses were conducted using Stata version 16.1 (StataCorp, United States). P values <0.05 were considered to be statistically significant.
2.6. Role of funding
The funders had no role in study design, data collection and analysis, decision to publish, or preparation of the manuscript.
3. Results
3.1. Study selection
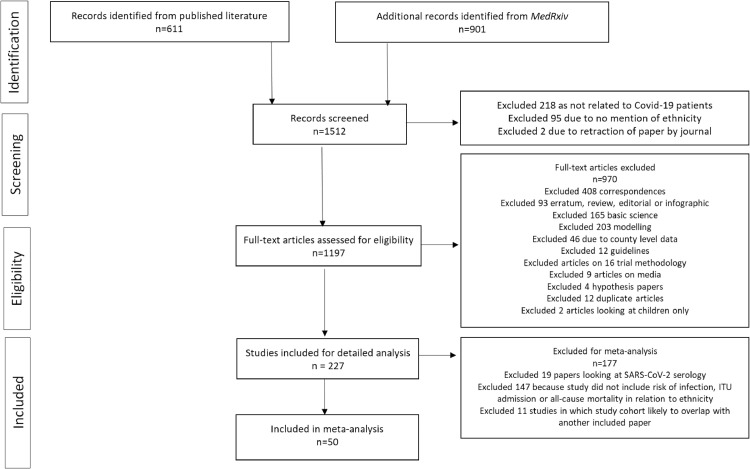
611 articles were identified in the published literature between 1st December 2019 and 31st August 2020 as shown in Fig. 1 . An additional 901 articles were identified from MedRxiv within the same period, giving a total of 1512 articles. 218 articles were excluded because they did not report on individuals with COVID-19; 95 were excluded as they did not mention ethnicity. Two papers were excluded due to retraction during the study period. Of the remaining 1197 articles that were assessed for eligibility in full-text screening, 970 did not meet inclusion criteria (reasons for exclusion, Fig. 1). A further 177 were excluded from the meta-analysis, including 19 studies which defined patients with COVID-19 as those with positive SARS-CoV-2 serology. 147 studies which provided no data on our predefined outcomes, and 11 studies in which the cohort was likely to have overlapped with another paper included in our data synthesis.
Fig. 1.
PRISMA flowchart of study.
3.2. Study characteristics
A total of 18,728,893 patients from 50 papers were included in the meta-analyses after excluding any missing data within the studies; 14,506,023 (77%) were White; 1,267,802 (7%) were Asian; 527,944 (3%) were Black, 1,578,192 (8%) were Hispanic, 1,113 were Native American, 229,822 (2%) were Mixed, and 617,997(3%) were of Other ethnic group [12], [13], [14], [15], [16], [17], [18], [19], [20], [21], [22], [23], [24], [25], [26], [27], [28], [29], [30], [31], [32], [33], [34], [35], [36], [37], [38], [39], [40], [41], [42], [43], [44], [45], [46], [47], [48], [49], [50], [51], [52], [53], [54], [55], [56], [57], [58], [59], [60], [61], 81].
Detailed descriptions of the included studies are shown in Table 1 . All studies included patients who had a positive SARS-CoV-2 polymerase-chain reaction (PCR) test by nasopharyngeal swab; two published studies also included patients who were diagnosed with COVID-19 based on clinical suspicion (suggestive clinical presentation, radiology and other blood tests/observations) [29,36]. Most studies (n = 42, 84%) were from the USA; the remaining eight (16%) were from the UK (Table 1). One study described two separate cohorts from the USA and the UK [21]. One study was a case series; one was a cohort and a case control; three were cross-sectional and the remaining were cohort studies. 28 (56%) reported on patients in hospital; nine (18%) reported on patients in the community; 13 (26%) reported on both. As of 31st August 2020, over half (n = 29, 58%) of papers included were published.
Table 1.
Characteristics of included studies. Peer reviewed studies are highlighted in bold.
| Study first author | Country | Study design | Setting | Published period | Population | White | Asian | Black | Hispanic | Mixed | Native American | Other |
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Ahmed | USA | Cohort | Community and Hospital | May 2020 | All patients within Uhealth, Utah Health system | 13151 | 446 | 361 | 2804 | 0 | 223 | 542 |
| Argenziano | USA | Case series | Hospital | May 2020 | All patients who received care at a New York Hospital | 144 | 19 | 181 | 248 | 0 | 0 | 408 |
| Auld | USA | Cohort | Hospital | May 2020 | Critically ill adults across one academic health care system | 39 | 7 | 153 | 0 | 0 | 0 | 18 |
| Azar | USA | Cohort | Hospital/Community | May 2020 | All patients at Sutter Health healthcare system in Northern California | 6779 | 1432 | 940 | 2681 | 0 | 98 | 2105 |
| Bril | UK | Cohort | Hospital | June 2020 | Retrospective cohort study of first 450 patients admitted to one hospital with confirmed COVID-19 | 265 | 51 | 33 | 0 | 0 | 0 | 77 |
| Caraballo | USA | Cohort | Hospital | May 2020 (subsequently published in September 2020) | Yale Heart Failure Registry (NCT04237701) includes 26,703 patients with heart failure across a 6-hospital integrated health care system in Connecticut was queried on April 16th, 2020 for all patients. | 119 | 0 | 62 | 0 | 0 | 0 | 0 |
| Chamie | USA | Cohort | Community | August 2020 | Patients from the United in Health Study – looking at participants in San-Francisco | 1442 | 326 | 0 | 1427 | 0 | 327 | 327 |
| Crouse | USA | Cohort | Community | July 2020 | Retrospective observational study of subjects at the University of Alabama in Birmingham Hospital | 220 | 0 | 311 | 0 | 0 | 0 | 73 |
| Ebinger | USA | Cohort | Hospital | July 2020 | All patients who presented to a Healthcare system in LA | 283 | 35 | 58 | 0 | 0 | 0 | 66 |
| Ellington | USA | Cohort | Community | June 2020 | CDC reports of women of reproductive age | 18,817 | 2123 | 16,381 | 25,442 | 0 | 0 | 2620 |
| Garibaldi | USA | Cohort | Hospital | May 2020 (subsequently published in September 2020) | Five hospitals in the Johns Hopkins Medicine system | 266 | 48 | 333 | 134 | 0 | 0 | 43 |
| Gold | USA | Cohort | Hospital | May 2020 | Hospitalised adults in Georgia (primarily metropolitan Atlanta) | 32 | 8 | 247 | 10 | 0 | 0 | 0 |
| Gu | USA | Cohort | Community | June 2020 | Participants tested in Michigan | 3374 | 0 | 981 | 0 | 0 | 0 | 486 |
| Jun | USA | Cohort | Hospital | August 2020 | Participants presenting to five acute care hospitals in New York City, within the Mount Sinai Health system | 689 | 144 | 825 | 892 | 0 | 0 | 458 |
| Khan | USA | Cohort | Community and Hospital | May 2020 | Patients presenting to TriNetX, which has access to healthcare records from 34 healthcare organisations | 3435 | 0 | 2621 | 0 | 0 | 0 | 0 |
| Kim | USA | Cohort | Hospital | May 2020 | 154 acute care hospitals in 74 counties in 13 states, hospitalised patients | 1178 | 0 | 755 | 306 | 0 | 0 | 251 |
| Levy | USA | Cohort | Hospital | June 2020 | Thirteen acute care hospitals in the New York City Area, within the Northwell Health System | 4250 | 952 | 2336 | 0 | 0 | 0 | 3048 |
| Lo | UK | Cohort | Community | June 2020 | Participants in the USA and UK who used the Covid symptom study smartphone application | 2104829 | 64662 | 13057 | 2379 | 48908 | 0 | 8893 |
| Lo | USA | Cohort | Community | June 2020 | Participants in the USA and UK who used the Covid symptom study smartphone questrionnaire | 147325 | 6828 | 4977 | 9251 | 4774 | 00 | 2044 |
| Lusignan | UK | Cross-section | Community and Hospital | May 2020 | Patients from primary care medical records in the oxford RCGP research and surveillance centre network | 2497 | 152 | 58 | 0 | 81 | 0 | 0 |
| Marcello | USA | Cohort | Hospital | June 2020 | Patients presenting to New York City Health and Hospitals | 2316 | 1739 | 5790 | 6249 | 0 | 0 | 6013 |
| Marie Del Vale | USA | Cohort | Hospital | August 2020 | Hospitalised patients at the Mount Sinai Health System in New York | 277 | 73 | 278 | 577 | 0 | 0 | 63 |
| Martin | UK | Cohort | Community and Hospital | July 2020 | Patients presenting to University Hospitals of Leicester NHS Trust, UK | 3067 | 710 | 122 | 0 | 0 | 0 | 152 |
| McCarty | USA | Cohort | Hospital | August 2020 | Patients presenting to 9 Massachusetts hospitals | 189 | 52 | 14 | 113 | 0 | 0 | 11 |
| Mendy | USA | Cohort | Community and Hospital | June 2020 | Patients diagnosed at the University of Cincinnati health system | 201 | 0 | 176 | 224 | 0 | 0 | 88 |
| Miles | UK | Cohort | Hospital | July 2020 | Patients admitted to one hospital in London, aged 70+ | 138 | 16 | 16 | 0 | 19 | 0 | 28 |
| Monteiro | USA | Cohort | Hospital | August 2020 | Patients admitted to the UCLA hospital System | 49 | 9 | 7 | 33 | 0 | 0 | 14 |
| Narain | USA | Cohort | Hospital | June 2020 | Hospitalised patients in the Northwell health system | 1042 | 410 | 576 | 0 | 177 | 0 | 893 |
| Niedzwiedz | UK | Cohort | Community and Hospital | May 2020 | UK Biobank analysis | 371,460 | 8456 | 6395 | 0 | 2356 | 0 | 3429 |
| Williamson | UK | Cohort | Community | July 2020 | NHS primary healthcare data; CPNS inpatient hospital death notifications; ONS death data | 10,962,999 | 1,030,980 | 343,437 | 0 | 171,929 | 0 | 323,813 |
| Patel N | USA | Cohort | Hospital | August 2020 | Clinical outcomes of adults hospitalised with COVID-19 affiliated with Northwestern Medicine | 611 | 0 | 288 | 662 | 0 | 0 | 152 |
| Patel M | USA | Cohort | Hospital | August 2020 | Patients admitted to Temple University Hospital in Philadelphia | 9 | 0 | 53 | 23 | 0 | 0 | 19 |
| Petrak | USA | Cohort | Hospital | June 2020 (subsequently published in September 2020) | To evaluate the clinical outcomes of patients treated with Tocilizumab | 56 | 5 | 43 | 32 | 0 | 0 | 9 |
| Perez-Guzman | UK | Cohort | Hospital | August 2020 | Consecutive patients admitted for COVID-19 in 3 large London hospitals | 235 | 94 | 133 | 0 | 0 | 0 | 17 |
| Price-haywood | USA | Cohort | Community and Hospital | May 2020 | Patients seen within Ochsner Health in Louisiana | 1030 | 0 | 2451 | 0 | 0 | 0 | 0 |
| Rajiter | USA | Cohort | Hospital | June 2020 | Hospitalised patients from 4 hospitals in Florida | 76 | 0 | 153 | 33 | 0 | 0 | 13 |
| Ramaswamy | USA | Cohort | Hospital | May 2020 | Patients part of Cone Health's enterprise analytics data | 38 | 0 | 40 | 0 | 0 | 0 | 8 |
| Rentsch | USA | Cohort | Community and Hospital | April 2020 | Data from the veterans’ association (VA) national cooporate data warehouse on members from the VA birth cohort | 2135 | 0 | 1126 | 294 | 0 | 0 | 234 |
| Rosenberg | USA | Cohort | Hospital | May 2020 | A random sample of all admitted patients in 25 hospitals representing 88% of patients with COVID-19 in the New York region | 321 | 0 | 371 | 364 | 0 | 0 | 243 |
| Rozenfeld | USA | Cross sectional | Community | July 2020 | Patients tested for COVID-19 as part of Providence Health System | 24,799 | 1713 | 1649 | 0 | 0 | 465 | 0 |
| Sakowicz | USA | Cohort | Community | August 2020 | Pregnant women presenting to NorthWestern Memorial Hospital | 795 | 107 | 169 | 0 | 0 | 0 | 346 |
| Sapey | UK | Cohort | Hospital | August 2020 (subsequently published in September 2020) | Hospitalised patients admitted to University Hospitals Birmingham | 1540 | 410 | 134 | 0 | 18 | 0 | 67 |
| Somers | USA | Cohort | Hospital | July 2020 | Patients admitted to Michigan Medicine for COVID-19 pneumonia | 41 | 0 | 81 | 0 | 0 | 0 | 32 |
| Tartof | USA | Cohort | Hospital | August 2020 | All Kaiser Permanente Southern California members diagnosed with COVID-19 | 1210 | 1036 | 584 | 3751 | 0 | 0 | 335 |
| Vahidy | USA | Cross-sectional | Community and Hospital | July 2020 | COVID-19 surveillance and outcomes registry (CURATOR) at the Houston Methodist Hospital system | 12602 | 1860 | 4396 | 263 | 1578 | 0 | 0 |
| Velu | USA | Cohort | Community and Hospital | June 2020 | Clinical validation and implementation of lab-developed real time RT-PCR | 488 | 101 | 171 | 0 | 0 | 0 | 210 |
| Wang | USA | Cohort | Community and Hospital | June 2020 | Patients with COVID-19 in New York City | 2044 | 385 | 1787 | 0 | 0 | 0 | 2860 |
| Wang | USA | Cohort | Hospital | May 2020 | Patient-level data were extracted from electronic medical records for 28,336 patients tested for SARS-CoV-2 at the Mount Sinai Health System | 802848 | 142080 | 107255 | 0 | 0 | 0 | 254682 |
| Yehia | USA | Cohort | Hospital | August 2020 | Cohort of patients presenting to 92 hospitals across 12 states in the USA | 4606 | 0 | 4180 | 0 | 0 | 0 | 2424 |
| Zakeri | UK | Case-control and Cohort | Community and Hospital | July 2020 | Patients admitted to King's College Hospital Foundation Trust | 2313 | 322 | 1363 | 0 | 0 | 0 | 362 |
| Zimmerman | USA | Cohort | Hospital | August 2020 | Patients admitted to the Nuvance Health System | 177 | 11 | 36 | 0 | 0 | 0 | 29 |
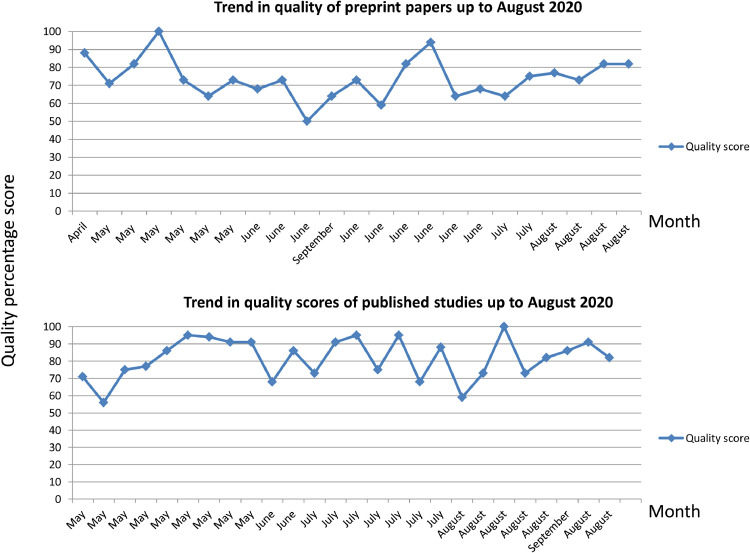
The overall quality of published articles was higher than those in preprint (median published quality score: 84%, interquartile range 73%−91%; median preprint article score: 73%, interquartile range 66%−82%); although both published articles and those presented on preprint servers maintained relatively high quality scores throughout the study period, as shown in Fig. 2 .
Fig. 2.
Temporal trends in quality assessment scores of published studies and those on MedRxiv.
Table 2 shows the quality assessment score of all 50 articles, the outcomes each study investigated, comorbidities included in the paper and confounders that were adjusted for. 14 (28%) studies investigated the risk of infection; 15 (30%) investigated the risk of ITU admission and 33 (66%) investigated the risk of death. Only one paper investigating the risk of infection did not consider comorbidities. 15 (30%) studies did not adjust for any confounders when assessing outcomes related to ethnicity.
Table 2.
Quality assessment scores of the 50 included studies; description of studies by outcome, comorbidities considered and confounders adjusted for. References for the studies are those from Table 1.
| Study first author | Quality assessment | Outcomes investigated for | Comorbidities | Confounders adjusted for (excluding ethnicity) |
|---|---|---|---|---|
| Ahmed | 71% | Infection | Fluid/electrolyte disorder, renal Failure, alcohol abuse, anaemia, chronic pulmonary disease, depression, hypertensive renal disease without renal failure, hypertension (uncomplicated), obesity | Cough, fever, breathlessness, known contact with SARS-CoV-2 |
| Argenziano | 55% | ITU admission | Hypertension, diabetes, coronary artery disease, congestive heart failure, pulmonary disease, asthma, COPD, obstructive sleep apnoea, interstitial lung disease, renal disease, stroke, active cancer, transplant, rheumatological disease, HIV, viral hepatitis, cirrhosis, obesity (BMI >30) | No adjustment for confounders |
| Auld | 56% | Death | Hypertension, diabetes, obesity, heart failure, coronary artery disease, chronic kidney disease, asthma, COPD | No adjustment for confounders |
| Azar | 75% | ITU admission and death | Hypertension, diabetes, cardiovascular disease, cancer, depression, heart failure, COPD, Asthma | No adjustment for confounders |
| Bril | 68% | Death | Hypertension, cardiac condition, diabetes, respiratory condition, immunosuppression | No adjustment for confounders |
| Caraballo | 73% | Death | Heart failure, hypertension, COPD, coronary artery disease, renal disease | No adjustment for confounders |
| Chamie | 73% | Infection | Chronic lung disease, heart disease, hypertension, diabetes, smoking | Sex, frontline worker status, household income, COVID-19 contact; referent ethnic group is: ‘Non-Latino’ rather than ‘White’ |
| Crouse | 75% | Death | Obesity, hypertension, diabetes | Age, sex and comorbidities |
| Ebinger | 75% | ITU admission | Obesity, hypertension, diabetes, previous myocardial infarction, COPD or asthma | Age, sex, hypertension, use of ACE inhibitors |
| Ellington | 86% | Death | Diabetes, chronic lung disease, cardiovascular disease, chronic renal disease, chronic liver disease, immunocompromised, neurological disorder | No adjustment for confounders with regards to ethnicity |
| Garibaldi | 100% | Death | Hypertension, coronary artery disease, heart failure, chronic kidney disease, diabetes mellitus, asthma, COPD, cancer, liver disease, immunosuppression, AIDS/ HIV, transplant | Age, sex, deprivation, comorbidities |
| Gold | 77% | ITU admission and death | Diabetes, cardiovascular diseases, coronary artery disease, congestive heart failure, arrhythmia, chronic lung disease, asthma, COPD, severe obesity, immunocompromising conditions or therapies, end stage renal disease, Liver disease, hypertension, neurological disorder, chronic kidney disease | No adjustment for confounders |
| Gu | 73% | Infection, ITU admission and death | Respiratory, cardiovascular disease, cancer, diabetes mellitus, kidney disease, liver disease, autoimmune disease | Age, sex, deprivation, comorbidities, BMI, smoking, alcohol for all three outcomes |
| Jun | 77% | Death | Hypertension, diabetes, coronary artery disease, heart failure, atrial fibrillation, chronic kidney disease, COPD/asthma, obesity, cancer | Age, sex, comorbidities, initial oxygen saturations. However, only odds ratios presented; no absolute numbers |
| Khan | 82% | ITU admission and death | Essential hypertension, diabetes mellitus, chronic lower respiratory diseases, chronic kidney disease, heart failure, ischaemic heart diseases, cerebrovascular diseases, nicotine dependence, alcohol related disorders | Propensity scoring rather than adjustment for confounders |
| Kim | 86% | ITU admission and death | Hypertension, obesity, diabetes mellitus, asthma, COPD, coronary artery disease, heart failure, neurological disease, renal disease, immunosuppressive conditions, gastrointestinal or liver disease, haematological conditions, autoimmune/ rheumatological, pregnancy | Age, sex, comorbidities, smoking, medications |
| Levy | 50% | Death | Coronary artery disease, diabetes, hypertension, heart failure, lung disease, kidney disease | No adjustment for confounders with regards to ethnicity |
| Lo | 73% | Infection | Diabetes, lung disease, heart disease, kidney disease, cancer | Age, sex, BMI, smoking. To account for likelihood of receiving testing, authors used separate inverse probability weighting as a function of race/ethnicity and other factors such as age, symptom burden, COVID-19 exposure risk factors and socioeconomic status |
| Lusignan | 95% | Infection | Obesity, hypertension, chronic kidney disease, diabetes, chronic heart disease, chronic respiratory disease, cancer, immunocompromised | Age, sex, deprivation, household size, population density, comorbidity |
| Marcello | 59% | Infection and death | Diabetes, hypertension, arrhythmia, cardiovascular disease, chronic heart disease, asthma, COPD, liver disease, chronic kidney disease, cancer, HIV | No adjustment for confounders |
| Marie Del Vale | 100% | Death | Hypertension, obesity, diabetes, chronic kidney disease, cancer, atrial fibrillation, heart failure, asthma, COPD, sleep apnoea, smoking | Age, sex, comorbidity, smoking, blood tests, cytokine markers of inflammation |
| Martin | 95% | Infection | Hypertension, diabetes mellitus, cardiovascular disease, respiratory disease, kidney disease | Age, sex, deprivation, household size, population density, comorbidity, NEWS-2 score on admission |
| McCarty | 73% | ITU admission and death | Hypertension, hyperlipidaemia, diabetes mellitus, arrhythmia, coronary artery disease, thyroid disorder, renal disorder, heart failure, stroke, pulmonary disorder, smoking | Age, sex and comorbidity |
| Mendy | 82% | ITU admission and death | obesity, diabetes, hypercholesterolaemia, asthma, COPD, chronic kidney disease, cardiovascular disease, cancer, osteoarthritis | Age, sex and smoking |
| Miles | 68% | Death | No comorbidities | Age, sex, clinical frailty scale, testing positive for SARS-CoV-2 on nasopharyngeal swab, deprivation |
| Monteiro | 82% | ITU admission | Obesity, hypertension, diabetes, COPD, coronary artery disease, cancer, asthma, atrial fibrillation, chronic kidney disease, transplant recipient | Age, sex, comorbidity, smoking |
| Narain | 68% | Death | Asthma COPD, hypertension, diabetes mellitus, chronic kidney disease, cardiovascular disease, haemodialysis, cancer, autoimmune, liver disease, interstitial lung disease | Age, sex, comorbidities, laboratory parameters, disease severity score, treatment, insurance |
| Niedzwiedz | 94% | Infection | Unknown – variables mentioned as ‘number of long term conditions’ | Age, sex, deprivation, comorbidities, healthcare worker and behaviour risk factors for infection |
| Williamson | 95% | Death | Smoking, obesity (BMI), blood pressure, respiratory disease, asthma, chronic heart disease, diabetes, cancer, haematological malignancy, liver disease, stroke/dementia, other neurological, kidney disease, organ transplant, spleen diseases, rheumatoid/lupus/psoriasis, other immunosuppressive condition | Age, sex, deprivation, comorbidities, |
| Patel N | 73% | ITU admission and death | Charlson comorbidity score | Age, sex, body mass index, glucose on admission, use of antiplatelet agents |
| Patel M | 59% | ITU admission | Hypertension, diabetes mellitus, lung disease, heart disease, chronic kidney disease | No adjustment for comorbidities |
| Petrak | 64% | Death | Diabetes, COPD bronchospastic illness, chronic cardiac or renal disease, immunodeficiency, neoplastic disease | Age, sex, comorbidity, timing of Tocilizumab; referent ethnic group was ‘Non-white’ rather than ‘white’ |
| Perez-Guzman | 86% | Death | Hypertension, diabetes, ischaemic heart disease, chronic heart failure, stroke, chronic kidney disease, dementia, previous DVT/PE, atrial fibrillation, COPD, liver disease, malignancy, HIV/AIDS | Age, sex, comorbidity, deprivation, admission NEWS-2 score |
| Price-haywood | 91% | Death | Obesity, asthma, COPD, diabetes mellitus, hypertension, heart failure, coronary artery disease, chronic kidney disease, solid organ transplant, liver disease, cancer, HIV | Age, sex, comorbidity, deprivation, obesity, admission observations and laboratory findings |
| Rajter | 68% | Death | Diabetes, cardiovascular disease, pulmonary disease, BMI, renal disease, cancer, hypertension, neurological disease, HIV, thyroid disease | Age, sex, comorbidity, smoking, severe presentation, white cell count, lymphocyte count, ivermectin use |
| Ramaswamy | 73% | Death | Smoker, diabetes mellitus, COPD, hypertension, heart disease, cancer, atrial fibrillation, stroke, anaemia, peripheral vascular disease | Age, sex, comorbidity, average modified early warning score (MEWS) during the inpatient stay, use of high-flow nasal cannula, maximum flow rate used on nasal cannula, highest level of care (ICU, Progressive, etc.), if mechanical ventilation was required, number of opioid doses, number of anti-diabetic drug doses, number of anticoagulant doses and number of antibiotic doses |
| Rentsch | 88% | Infection and ITU admission | Asthma, cancer, CKD, COPD, DM, HTN, Liver disease, vascular disease, | Age, sex, comorbidity, vital signs on admission, laboratory findings on admission, medications |
| Rosenberg | 91% | Death | Obesity, cancer, kidney disease, lung disease, diabetes, hypertension, coronary heart disease, congestive heart failure, dementia | No adjustment for confounders with regards to ethnicity |
| Rozenfeld | 73% | Infection | BMI, diabetes mellitus chronic kidney disease, HIV/AIDS, dementia, serious persistent mental illness, substance use disorder | Age, sex, deprivation, population density, comorbidity |
| Sakowicz | 82% | Infection | Obesity, hypertension, diabetes mellitus, pulmonary disease, gestational diabetes mellitus | No adjustment for confounders |
| Sapey | 90% | ITU admission and death | Count of morbidities used (multi-morbidity); hypertension, cerebrovascular disease, atrial fibrillation, ischaemic heart disease, angina, myocardial infarction, diabetes (type 1 and 2), asthma, COPD, interstitial lung disease, chronic kidney disease, any active malignancy, dementia (all types), obesity | Age, sex, deprivation, comorbidities |
| Somers | 91% | Death | Hypertension, congestive heart failure, COPD, asthma, sleep apnoea, diabetes, chronic kidney disease, chronic liver disease, solid organ transplant | Age, sex and treatment with Tocilizumab |
| Tartof | 91% | Death | Myocardial infarction, chronic heart failure, peripheral vascular disease, cerebrovascular disease, COPD, renal disease, cancer, immune disease, hyperlipidaemia, hypertension, asthma, organ transplant, diabetes | Age, sex, deprivation, comorbidity |
| Vahidy | 88% | Infection | Hypertension, diabetes, obesity | Age, sex, deprivation, comorbidities, household size, population density |
| Velu | 94% | Infection | No comorbidities listed | No adjustment for confounders |
| Wang A | 64% | Death | Asthma, chronic kidney disease, cancer, COPD, HIV, obesity, hypertension, diabetes | No adjustment for confounders |
| Wang Z | 64% | Infection and death | Diabetes mellitus, hypertension, asthma, chronic obstructive pulmonary COPD, HIV, obesity and cancer. | No adjustment for confounders with regards to ethnicity |
| Yehia | 82% | Death | Asthma, cancer, chronic kidney disease, chronic liver disease, COPD, chronic heart failure, coronary artery disease, diabetes, hypertension, obesity, organ transplant | Age, sex, insurance, comorbidities, deprivation, site of care |
| Zakeri | 64% | ITU admission and death | Asthma, COPD, coronary heart disease, hypertension, diabetes, chronic kidney disease, obesity | Age, sex, deprivation, comorbidity |
| Zimmerman | 82% | Death | No comorbidities listed | Age, gender, body mass index |
3.3. Risk of SARS-CoV-2 infection
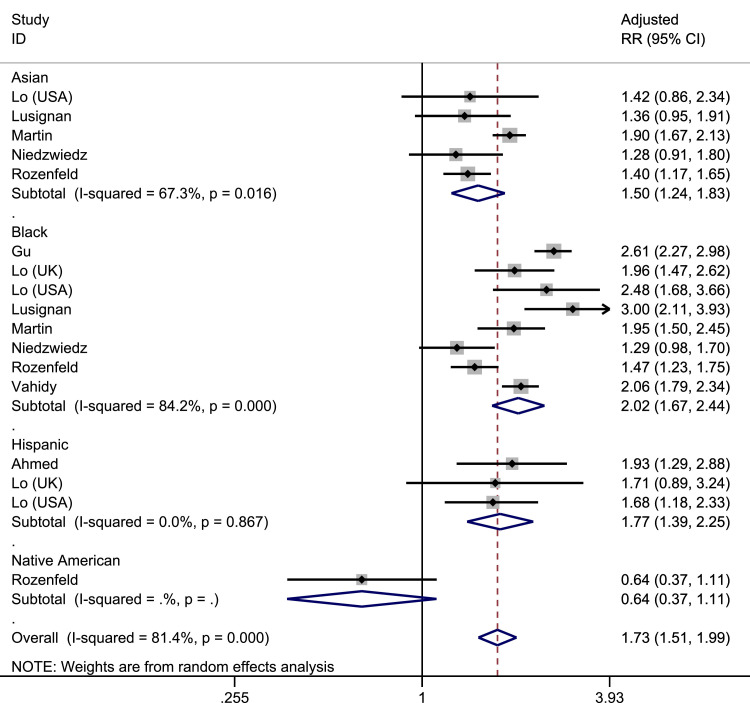
Pooled prevalence, unadjusted odds ratios (OR) and adjusted risk ratios (RR) for the risk of SARS-CoV-2 infection, stratified by ethnicity is shown in Table 3 . Pooled prevalence of infected patients was highest in those of Black ethnicity. In adjusted analyses, those of Black ethnicity were twice as likely to become infected with SARS-CoV-2 compared to White individuals (pooled adjusted RR 2.02, 95% CI 1.67–2.44, I 2=84.2%, amongst 8 studies); Asian and Hispanic individuals were also more likely to become infected compared to White individuals (Asian: pooled adjusted: RR 1.50, 95% CI 1.24–1.83, I 2=67.3% across 5 studies; Hispanic: pooled adjusted RR 1.77, 95% CI 1.39–2.25 across 3 studies, I 2=0.0%), as shown in Fig. 3 . In sensitivity analyses examining only peer-reviewed publications, increased risk of infection amongst Black and Asian groups were maintained amongst adjusted analyses (Black pooled adjusted RR:1.85, 95%CI: 1.46–2.35, I 2=84.2% across 5 studies; Asian pooled adjusted RR: 1.51, 95% CI:: 1.22–1.88, I 2=74.8% across 4 studies), but no studies investigating Hispanic individuals had yet been peer-reviewed. Data for Mixed and Native American ethnicities were limited by small numbers of patients. Across all pooled analyses, there were mixed levels of heterogeneity.
Table 3.
Data syntheses for risk of SARS-CoV-2 infection by ethnic group.
| Studies | Pooled prevalence (95% CI) |
I2 | Studies | Pooled unadjusted OR (95% CI) |
I2 | Studies | Pooled RR (95% CI) |
I2 | |
|---|---|---|---|---|---|---|---|---|---|
| (1) Studies from pre-prints and peer-reviewed publications | |||||||||
| White | 18 | 0.11 (0.11, 0.12) | 99.9 | Reference | Reference | ||||
| Asian | 14 | 0.16 (0.14, 0.19) | 99.6 | 14 | 1.61 (1.23, 2.10) | 95.3 | 5 | 1.50 (1.24, 1.83) | 67.3 |
| Black | 17 | 0.26 (0.20, 0.32) | 99.9 | 17 | 2.43 (1.93, 3.07) | 95.6 | 8 | 2.02 (1.67, 2.44) | 84.2 |
| Hispanic | 8 | 0.19 (0.08, 0.30) | 99.9 | 8 | 2.58 (1.99, 3.35) | 94.5 | 3 | 1.77 (1.39, 2.25) | 0 |
| Mixed | 1 | 0.33 (0.16, 0.56) | – | 1 | 0.96 (0.36, 2.58) | – | 0 | – | – |
| Native American | 2 | 0.03 (0.01, 0.04) | – | 2 | 0.44 (0.26, 0.74) | 0 | 1 | 0.64 (0.37, 1.11) | – |
| (2) Studies only from peer-reviews publications | |||||||||
| White | 9 | 0.15 (0.11, 0.18) | 99.8 | Reference | Reference | ||||
| Asian | 8 | 0.21 (0.14, 0.29) | 99.0 | 8 | 1.87 (1.42, 2.45) | 80.9 | 4 | 1.51 (1.22, 1.88) | 74.8 |
| Black | 8 | 0.22 (0.17, 0.28) | 96.5 | 8 | 2.29 (1.60, 3.29) | 91.1 | 5 | 1.85 (1.46, 2.35) | 84.2 |
| Hispanic | 2 | 0.08 (0.07, 0.09) | – | 2 | 10.74 (0.14, 833.68) | 94.8 | 0 | – | – |
| Mixed | 1 | 0.33 (0.16, 0.56) | – | 1 | 0.96 (0.36, 2.58) | – | 0 | – | – |
| Native American | 2 | 0.03 (0.01, 0.04) | – | 2 | 0.44 (0.26, 0.74) | 0 | 1 | 0.64 (0.37, 1.11) | – |
Fig. 3.
Forrest plot of pooled adjusted risk of SARS-CoV-2 infection by ethnicity (Reference group: White).
3.4. ITU admission
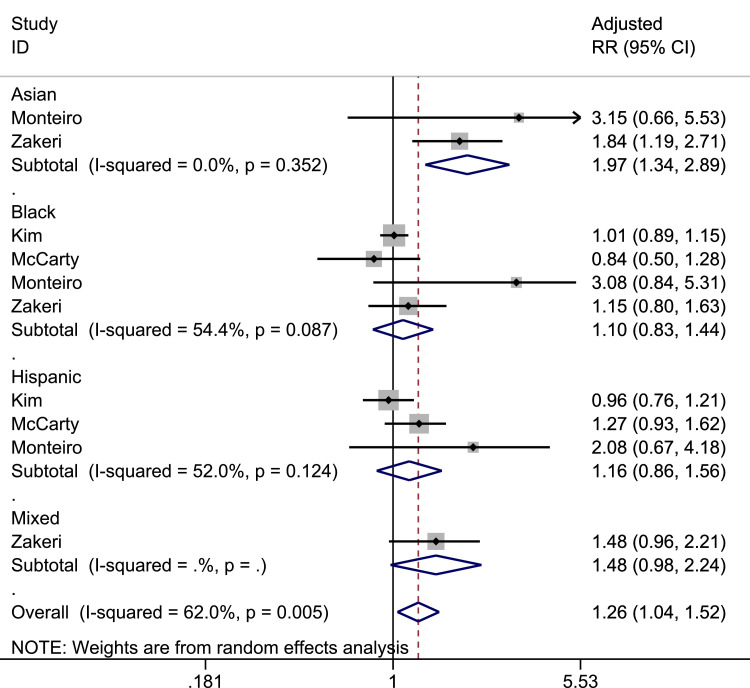
Pooled prevalence, unadjusted OR and adjusted RR for the risk of ITU admission stratified by ethnicity is shown in Table 4 . Pooled prevalence was highest among those of Black ethnicity. In adjusted analyses examining only hospitalised patients, Asians were more likely to be admitted to ITU compared to White individuals (pooled adjusted RR: 1.97, 95% CI: 1.34–2.89. I 2=0.0% amongst 2 studies) as shown in Fig. 4 . However, no published studies by 31st August have assessed the risk of ITU admission amongst Asian cohorts. Black (pooled adjusted RR: 1.10, 95% CI:0.83–1.44, I 2=54.5% amongst four studies) and Hispanic (pooled adjusted RR: 1.16, 95% CI: 0.86–1.56, I 2=52% across 3 studies) patients were not at increased risk of ITU admission compared to White individuals. When considering studies in which the denominator were a combination of hospitalised patients and those with COVID-19 in the community, the risk of ITU admission was only higher in those of Black ethnicity compared to White ethnicity (pooled adjusted RR: 1.90, 95% CI: 1.38–2.61, I 2=52.7% across 3 studies). Data for all ethnicities were limited by small numbers of studies. Across all pooled analyses, there were lower levels of heterogeneity compared to risk of infection..
Table 4.
Data syntheses for risk of ITU admission amongst different ethnic groups.
| Studies | Pooled prevalence (95% CI) |
I2 | Studies | Pooled unadjusted OR (95% CI) |
I2 | Studies | Pooled adjusted RR (95% CI) |
I2 | |
|---|---|---|---|---|---|---|---|---|---|
| (1) Studies considering hospitalised populations only or reporting a subgroup analysis for hospitalised patients only | |||||||||
| White | 14 | 0.28 (0.21, 0.34) | 97.5 | Reference | Reference | ||||
| Asian | 7 | 0.27 (0.19, 0.35) | 75.0 | 7 | 1.97 (1.25, 3.09) | 70.1 | 2 | 1.97 (1.34, 2.89) | 0 |
| Black | 15 | 0.33 (0.26, 0.41) | 97.0 | 14 | 1.28 (1.06, 1.56) | 62.8 | 4 | 1.10 (0.83, 1.44) | 54.4 |
| Hispanic | 9 | 0.33 (0.22, 0.44) | 95.9 | 9 | 1.06 (0.77, 1.44) | 68.7 | 3 | 1.16 (0.86, 1.56) | 52.0 |
| Mixed | 1 | 0.19 (0.14, 0.26) | – | 1 | 1.99 (1.26, 3.16) | – | 1 | 1.48 (0.98, 2.24) | – |
| (2) Studies considering inpatient/outpatient populations | |||||||||
| White | 7 | 0.10 (0.07, 0.14) | 98.2 | Reference | Reference | ||||
| Asian | 3 | 0.05 (0.00, 0.11) | 0.0 | 3 | 0.96 (0.41, 2.21) | 75.8 | 0 | – | – |
| Black | 7 | 0.19 (0.10, 0.28) | 99.1 | 7 | 2.08 (1.39, 3.13) | 89.3 | 3 | 1.90 (1.38, 2.61) | 52.7 |
| Hispanic | 4 | 0.09 (0.02, 0.17) | 95.0 | 4 | 1.10 (0.84, 1.45) | 29.3 | 1 | 3.04 (1.47, 6.28) | – |
| Native American | 0 | – | – | 1 | 1.65 (0.08, 34.79) | – | 0 | – | – |
| (3) Excluding studies from (1) which were not from peer-reviewed publications | |||||||||
| White | 7 | 0.33 (0.22, 0.43) | 96.9 | Reference | Reference | ||||
| Asian | 4 | 0.34 (0.12, 0.56) | 84.1 | 4 | 1.31 (0.84, 2.05) | 23.6 | 0 | – | – |
| Black | 8 | 0.33 (0.23, 0.43) | 96.4 | 7 | 1.04 (0.90, 1.20) | 7.0 | 2 | 1.00 (0.88, 1.13) | 0 |
| Hispanic | 6 | 0.30 (0.18, 0.43) | 96.8 | 6 | 0.89 (0.69, 1.14) | 53.6 | 2 | 1.09 (0.83, 1.43) | 55.7 |
Fig. 4.
Forrest plot of pooled adjusted risk of ITU admission by ethnicity (Reference group: White).
3.5. Mortality
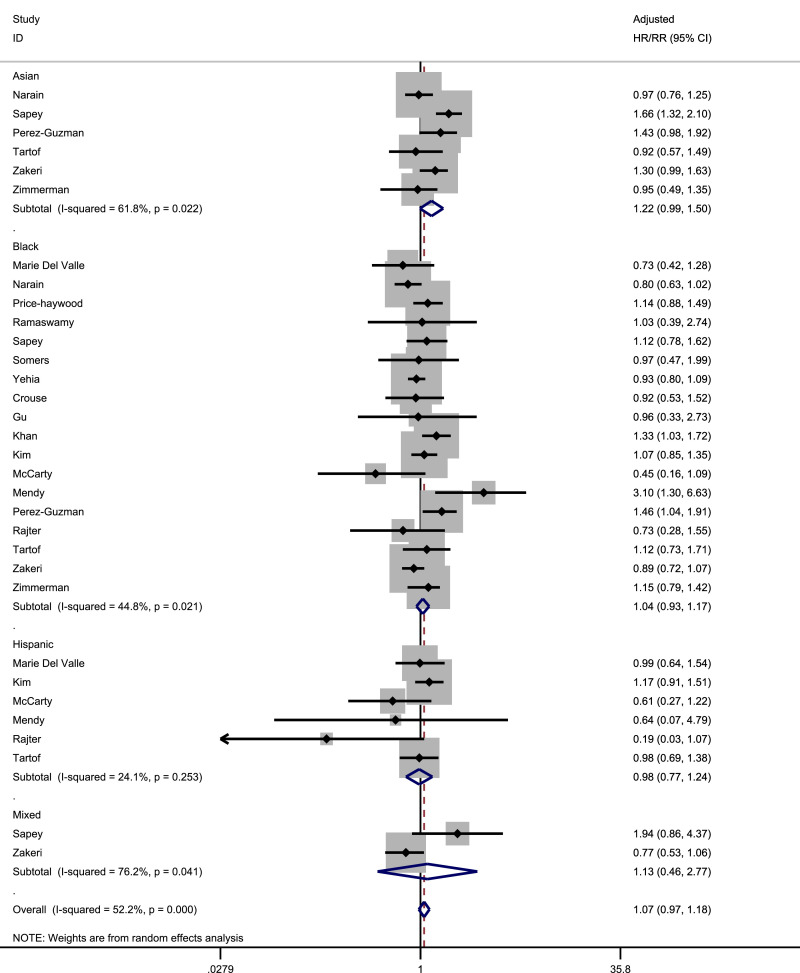
Pooled prevalence, unadjusted OR and adjusted hazard ratio (HR)/RR for the risk of death stratified by ethnicity is shown in Table 5 . Pooled prevalence was highest amongst White and Asian ethnicities. In adjusted analyses of patients with COVID-19 only, there was a signal towards increased risk of death in Asian individuals compared to White (pooled adjusted HR/RR: 1.22, 95% CI: 0.99–1.63, I 2=61.8% amongst 6 studies) as shown in Fig. 5 . This signal was stronger in sensitivity analyses looking only at studies including patients with COVID-19 as well as those without COVID-19 in the general population (pooled adjusted HR/RR: 1.33, 95% CI: 1.11–1.60, I 2=69.0% amongst 8 studies) as well as studies where the denominator was hospitalised patients only (pooled adjusted HR/RR: 1.27, 95% CI: 1.01–1.57 across 5 studies, I 2=64.7%) but was weaker when we examined studies that only included those who had a documented outcome of discharge, or death (pooled adjusted HR/RR: 1.18, 95% CI:0.92–1.51 across 5 studies, I2=67.9%) and studies which had been peer reviewed (pooled adjusted HR/RR:1.19, 95% CI:0.77–1.83 across 2 studies, I 2=54.3%). In adjusted analyses, those of Black and Hispanic ethnicities were not at increased risk of death compared to White individuals, across all sensitivity analyses. Data for Native American, Mixed and Other ethnicities were limited by small numbers of studies. Across all pooled analyses, there were lower levels of heterogeneity compared to risk of infection.
Table 5.
Data syntheses for risk of mortality by different ethnic groups.
| Studies | Pooled prevalence (95% CI) |
I2 | Studies | Pooled unadjusted OR (95% CI) |
I2 | Studies | Pooled adjusted HR/RR (95% CI) |
I2 | |
|---|---|---|---|---|---|---|---|---|---|
| (1) Studies including suspected or confirmed COVID-19 patients only | |||||||||
| White | 25 | 0.22 (0.18, 0.27) | 99.2 | Reference | Reference | ||||
| Asian | 14 | 0.23 (0.15, 0.30) | 97.5 | 14 | 0.81 (0.69, 0.95) | 36.1 | 6 | 1.22 (0.99, 1.50) | 61.8 |
| Black | 26 | 0.18 (0.14, 0.21) | 97.3 | 25 | 0.88 (0.74, 1.04) | 84.6 | 18 | 1.04 (0.93, 1.17) | 44.8 |
| Hispanic | 11 | 0.10 (0.06, 0.14) | 98.5 | 11 | 0.48 (0.36, 0.63) | 65.1 | 6 | 0.98 (0.77, 1.24) | 24.1 |
| Mixed | 2 | 0.18 (0.12, 0.23) | – | 2 | 0.63 (0.21, 1.89) | 76.3 | 2 | 1.13 (0.46, 2.77) | 76.2 |
| (2) Studies including suspected or confirmed COVID-19 patients plus studies of general populations | |||||||||
| White | – | – | – | Reference | Reference | ||||
| Asian | – | – | – | 15 | 0.86 (0.73, 1.01) | 60.9 | 8 | 1.33 (1.11, 1.60) | 69.0 |
| Black | – | – | – | 26 | 0.90 (0.75, 1.07) | 87.3 | 20 | 1.09 (0.95, 1.26) | 68.8 |
| Hispanic | – | – | – | 11 | 0.48 (0.36, 0.63) | 65.1 | 6 | 0.98 (0.77, 1.24) | 24.1 |
| Mixed | – | – | – | 3 | 0.62 (0.37, 1.04) | 67.8 | 4 | 1.19 (0.74, 1.91) | 74.6 |
| (3) Excluding studies from (1) which did not include hospitalised patients in outcome (i.e. only considered those who were discharged or died) | |||||||||
| White | 21 | 0.22 (0.17, 0.26) | 99.0 | Reference | Reference | ||||
| Asian | 12 | 0.21 (0.14, 0.28) | 96.5 | 12 | 0.77 (0.63, 0.93) | 32.8 | 5 | 1.18 (0.92, 1.51) | 67.9 |
| Black | 21 | 0.17 (0.14, 0.21) | 97.5 | 21 | 0.91 (0.74, 1.12) | 84.9 | 13 | 1.04 (0.90, 1.20) | 42.9 |
| Hispanic | 9 | 0.10 (0.06, 0.15) | 98.8 | 9 | 0.52 (0.38, 0.71) | 67.3 | 4 | 0.93 (0.69, 1.26) | 8.1 |
| Mixed | 2 | 0.18 (0.12, 0.23) | – | 2 | 0.63 (0.21, 1.89) | 76.3 | 2 | 1.13 (0.46, 2.77) | 76.2 |
| (4) Excluding studies from (1) where the denominator was not of hospitalised patients | |||||||||
| White | 18 | 0.28 (0.25, 0.31) | 93.7 | Reference | Reference | ||||
| Asian | 11 | 0.27 (0.25, 0.30) | 22.6 | 11 | 0.84 (0.73, 0.97) | 17.5 | 5 | 1.27 (1.01, 1.58) | 64.7 |
| Black | 18 | 0.21 (0.19, 0.24) | 86.2 | 18 | 0.74 (0.64, 0.85) | 63.4 | 13 | 1.00 (0.89, 1.11) | 34.8 |
| Hispanic | 9 | 0.12 (0.05, 0.20) | 96.6 | 9 | 0.47 (0.33, 0.66) | 65.1 | 4 | 0.90 (0.60, 1.35) | 52.4 |
| Mixed | 2 | 0.18 (0.12, 0.23) | – | 2 | 0.63 (0.21, 1.89) | 76.3 | 2 | 1.13 (0.46, 2.77) | 76.2 |
| (5) Excluding studies from (1) which were not from peer-reviewed publications | |||||||||
| White | 11 | 0.21 (0.15, 0.28) | 98.9 | Reference | Reference | ||||
| Asian | 6 | 0.16 (0.09, 0.23) | 92.4 | 6 | 0.87 (0.51, 1.48) | 69.0 | 2 | 1.19 (0.77, 1.83) | 54.3 |
| Black | 12 | 0.17 (0.12, 0.23) | 97.6 | 11 | 0.91 (0.74, 1.13) | 69.3 | 8 | 1.05 (0.90, 1.22) | 41.7 |
| Hispanic | 6 | 0.07 (0.04, 0.09) | 94.4 | 6 | 0.49 (0.35, 0.68) | 64.0 | 4 | 1.04 (0.87, 1.25) | 0 |
Fig. 5.
Forrest plot of pooled adjusted risk of death by ethnicity (Reference group: White).
3.6. Risk of bias across studies
For prevalence estimates of infection, there was clear asymmetry in funnel plots for Asian and White ethnic groups (Egger's test p = 0.002 and p<0.001 respectively, Supplementary materials 5). Studies with very low estimates of infection had very high precision, whereas studies with higher infection estimates had lower precision. For unadjusted OR estimate of infection in Asian vs White ethnic groups, Egger's test indicates potential bias (p = 0.002). However the funnel plot indicates that this result is being driven by an outlier study (one with low precision but a high estimate of OR).
For prevalence estimates of ITU admission, Egger's test for White ethnic group indicates potential bias (p = 0.002). Again, the funnel plot indicates an outlier study possibly driving this result.
For prevalence estimates of mortality, there was asymmetry in the funnel plots for the Asian and Black ethnic groups (Egger's test p = 0.0028 and p<0.001, respectively).
4. Discussion
In a previous systematic review, we highlighted the need for more studies to investigate the relation between COVID-19, ethnicity and specific clinical outcomes [2]. Some months later, a large body of literature is available. We now present the first meta-analysis investigating this issue. We found that individuals from Asian and Black ethnic groups are more likely to be infected by SARS-CoV-2compared to those of White ethnicity. Those of Asian ethnicity may be at increased risk of death compared to White patients; this may be related to a higher likelihood of developing severe COVID-19 pneumonia and being admitted to ITU although this finding is limited by the relatively small number of studies which investigated ITU admission as an outcome.
Our findings suggest that the disproportionate impact of COVID-19 on Black and Asian communities is mainly attributable to increased infection amongst these communities. Many explanations exist as to why there may be an elevated level of SARS-CoV-2 infection in ethnic minority groups. Contact tracing studies provide strong evidence that sustained close contact with someone who is infected with SARS-CoV-2 drives the majority of new infections. SARS-CoV-2 is much more efficiently spread in enclosed and crowded environments [62]. Individuals from ethnic minority backgrounds are more likely to live in larger household sizes comprised of multiple generations [26,63]. They are also more likely to have lower socioeconomic status, which may increase the likelihood of living in overcrowded households, or accommodation with shared facilities or communal areas [64,65]. Furthermore, individuals from ethnic minority backgrounds are more likely to be employed as essential workers, or less able to work from home, and as a result have continued to have contact with others through work or commuting [63]. In the Bureau of Labour Statistics Current Population Survey for the year 2019, Hawkins found that Black and Asian workers in the USA were more likely to be employed in occupations with both frequent exposures to infections and proximity to others [66]. There is also evidence that ethnic minority groups experience disproportionate rates of COVID-19 in some of these occupational groups, for example among healthcare workers [67].
We found some evidence that Asian individuals had a higher risk of severe infection, as marked by an increased risk of ITU admission and possibly death from COVID-19, even when common key confounders, such as a higher prevalence of diabetes and cardiovascular disease are taken into account. In this respect, our findings are consistent with national census data from the Office for National Statistics, and the most recent Intensive Care National Audit and Research Centre report which found over-representation of Asian ethnic groups to hospital and intensive care units in the UK [68,69]. However, we do note that few studies included in our meta-analysis directly considered multi-generational living, overcrowding or known recent contact with a confirmed positive case as confounders to risk our predefined outcomes. Only two studies considered the role of occupation in the context of infection;none considered it as a confounder for severe infection or death [32,57]. Future population studies investigating COVID-19 mortality must also try to ascertain what proportion of patients tested positive for SARS-CoV-2. Given the clustering nature of SARS-CoV-2 transmission, adjusting for testing positive would help to explain whether increased transmission between certain communities would be the reason for higher death. Throughout the course of this analysis, we found three large studies of the UK population (one included and another two excluded due to likelihood of overlapping populations) which found a higher risk of death amongst Asian individuals compared to White individuals, but did not adjust for testing positive for SARS-CoV-2 [29,70,71].
Racism and structural discrimination may also contribute to an increased risk of worse clinical outcomes within ethnic minority communities [72,73]. These processes are complex and systemic, underpinned by unequal power relations and beliefs, and operating at individual, community, and organisational levels, resulting in stigmatisation, discrimination, and marginalisation of ethnic minorities [74]. Within a healthcare context, this contributes to inequities in the delivery of care, barriers to accessing care, loss of trust, and psychosocial stressors [75,76]. There is evidence to suggest that ethnic minorities and migrant groups have been less likely to implement public health measures, be tested, or seek care when experiencing symptoms due to such barriers and inequities in the availability and accessibility of care [77], underscoring critical healthcare disparities [3,5]. Large scale political-economic forces that have played out over generations have resulted in deep-seated social, economic and power inequities, which shape the distribution of risks and resources for health, resulting in social and spatial clustering of infectious diseases amongst certain ethnic groups which have long been underserved [78]. Within the USA and the UK,COVID-19 has evolved from a pandemic to a syndemic - until policies and programmes are devised to address such disparities, we must be careful not to prematurely attribute worse clinical outcomes in ethnic minority groups to genetics [79].
We found that minority ethnic groups continue to be under-represented in research [80], which is likely to be exacerbated by the same barriers that contribute to disparities in access to care and health outcomes [73]. White individuals may be more likely to access testing for SARS-CoV-2 than those from ethnic minority backgrounds; or a larger proportion of White individuals who were asymptomatic were tested compared to those from ethnic minority backgrounds. This in turn may have had influence on the relation between ethnicity and rates of infection, or severity of disease. We examined for temporal patterns in the data, but did not identify any differences. Amongst our pooled cohort, only 16% of patients were from non-White ethnic backgrounds, and a particularly small proportion were attributable to Hispanic and Native American groups. This impacted on our pooled adjusted risk with regards to ITU admission based on a small number of studies, thereby limiting generalisability in these outcomes from our data. This was avoidable; we excluded 147 studies because although they had raw data on ethnicity, none investigated this variable in relation to infection, ITU admission or death and another third of included studies did not adjust for any confounding variables when investigating outcomes relating to ethnicity. As the COVID-19 pandemic continues to evolve, it is critically important that studies aim to present outcomes (infection, ITU admission and death) of patients with COVID-19 disaggregated by ethnicity, with data adjusted by key confounders.
Our study had several limitations. Half of our pooled analyses involved studies which had not been peer reviewed. However, inclusion of studies awaiting peer review helped to provide a broader view of the emerging literature, in a rapidly evolving field. In September 2020, four of the preprint publications had been published. We also adjusted for whether a paper was peer-reviewed or not in subsequent sensitivity analyses. Variations across papers in relation to populations, setting, treatment context, and reporting of ethnicity and outcomes, resulted in high heterogeneity. However, this does not preclude pooling of data and is consistent with other meta-analyses on infection in diverse populations [82,83]. Instead, we explored heterogeneity through sensitivity analyses. The analyses provide an important visualisation of the data available, and highlight the heterogeneity across the research and the need to improve data collection and analysis, including greater standardisation in adjusted analyses.
Several studies may have overlapping populations [84]. For example, we found several studies from Mount Sinai investigating mortality from COVID-19; quite possibly from the same population [53,[85], [86], [87], [88]]. We have minimised this error by excluding studies which were clearly done on the same database, though we urge greater transparency in reporting for future research.
We used broad categories of ethnicity. We did this in order to maximise inclusion within our pooled analyses – however, this will have affected precise estimates of risk for any further subgroup categorisations of ethnicity. For example, ‘Asian’ could be separated into ‘Bangladeshi’, ‘South Asian’ and ‘Chinese’, each of which may have a differing prognostic implication. Furthermore the terms race and ethnicity can be considered to be different; patients who are of ‘Black’ race may be of ‘Hispanic’ ethnicity. However, many studies did not define what they meant by ‘Asian’, race or ethnicity. Until a more standardised approach to the definition of ethnicity exists across studies, we believe our method to be a pragmatic approach for data synthesis.
Finally, all studies included were from the UK and USA. Whilst both these countries have ethnically diverse populations, generalisability of our findings to other countries should be cautioned, where management of patients (such as criteria for ITU admission) may be different. Further research should be undertaken in other country contexts and diverse income-level settings. In particular, a robust investigation of clinical outcomes in countries where those of White ethnicity do not make up the majority of the population would help to ascertain the role of any biological disposition to infection, severe disease or death.
In conclusion, we found clear evidence that patients of Black, Asian and Hispanic ethnicity are more likely to be infected with SARS-CoV-2, compared to those of White ethnicity, and a possible association of higher risk of ITU admission and death from COVID-19 in Asians, even when most confounders are adjusted for.. Our findings should inform public health strategies to minimise exposure risk of SARS-CoV-2 in ethnic minority groups, by facilitating timely access to healthcare resources, and targeting the social determinants, structural racism, and occupational risk underlying inequities.
4.1. Data sharing statement
All data used in this manuscript can be found in the online versions of the studies that were accessed. Our own data synthesis of these manuscripts are available upon reasonable request.
Funding
NIHR and UKRI.
Author contribution
SS and DP wrote the first draft of the manuscript. SS, DP, CBN, CAM, JN, JSM and LBN performed the data collection. PD performed the database search. LJG and CRN performed the statistical analyses. KK, CAM, CRN, JN. KRA, LJG and LBN and MP reviewed and revised the manuscript. SS, DP and CRN have verified the underlying data.
Declaration of Competing Interest
KRA has served as a paid consultant, providing unrelated methodological advice, to; Abbvie, Amaris, Allergan, Astellas, AstraZeneca, Boehringer Ingelheim, Bristol-Meyers Squibb, Creativ-Ceutical, GSK, ICON/Oxford Outcomes, Ipsen, Janssen, Eli Lilly, Merck, NICE, Novartis, NovoNordisk, Pfizer, PRMA, Roche and Takeda, and has received research funding from Association of the British Pharmaceutical Industry (ABPI), European Federation of Pharmaceutical Industries & Associations (EFPIA), Pfizer, Sanofi and Swiss Precision Diagnostics. He is a Partner and Director of Visible Analytics Limited, a healthcare consultancy company. KK has received honoraria from AstraZeneca, Boehringer Ingelheim, Eli Lilly, Janssen, Merck Sharp & Dohme, Novartis, Novo Nordisk, Sanofi, Takeda, Servier and Pfizer, and research support from AstraZeneca, Boehringer Ingelheim, Eli Lilly, Merck Sharp & Dohme, Novartis, Novo Nordisk, Sanofi and Pfizer. KK is Director for the University of Leicester Centre for BME Health, Trustee of the South Asian Health Foundation, national NIHR ARC lead for Ethnicity and Diversity, Chair of the SAGE subgroup on Ethnicity and COVD and a member of Independent SAGE. MP reports grants and personal fees from Gilead Sciences and personal fees from QIAGEN, outside the submitted work. All other authors report no conflict of interest.
Funding
DP, SS and CAM are supported by NIHR Academic Clinical Fellowships. JSM is funded by an NIHR Clinical Lectureship in Older People and Complex Health Needs. CRN works for the Complex Reviews Support Unit which is funded by the NIHR (project number 14/178/29). KRA is supported by Health Data Research (HDR) UK, the UK National Institute for Health Research (NIHR) Applied Research Collaboration East Midlands (ARC EM), and as a NIHR Senior Investigator Emeritus (NF-SI-0512-10159). LBN receives funding from the Academy of Medical Sciences (SBF005\1047), the Medical Research Council/Economic and Social Research Council/Arts and Humanities Research Council (MR/T046732/1), and UKRI/MRC (MR/V027549/1). LJG receives funding from UK National Institute for Health Research (NIHR) and UKRI. KK, KM, MP and LG are supported by the National Institute for Health Research (NIHR) Applied Research Collaboration East Midlands (ARC EM). MP and KRA are members of the Health Data Research (HDR) UK COVID-19 Taskforce. MP is supported by a NIHR Development and Skills Enhancement Award and UKRI/MRC/NIHR (MR/V027549/1). KK and MP are supported by NIHR Leicester Biomedical Research Centre (BRC). The views expressed are those of the authors and not necessarily those of the NIHR, NHS or the Department of Health and Social Care.
Footnotes
Supplementary material associated with this article can be found in the online version at doi:10.1016/j.eclinm.2020.100630.
Appendix. Supplementary materials
References
- 1.Johns Hopkins University of Medicie Coronavirus Resource Center. COVID-19 dashboard by the Center for Systems Science and Engineering (CSSE) at Johns Hopkins University (JHU). https://coronavirus.jhu.edu/map.html (accessed Sept 4, 2020).
- 2.Pan D., Sze S., Minhas J.S., et al. The impact of ethnicity on clinical outcomes in COVID-19: a systematic review. EClinicalMedicine. 2020;23 doi: 10.1016/j.eclinm.2020.100404. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Williams D., Cooper L. COVID-19 and health equity – a new kind of ‘herd immunity’. JAMA. 2020;323:2478–2480. doi: 10.1001/jama.2020.8051. [DOI] [PubMed] [Google Scholar]
- 4.Pareek M., Bangash M.N., Pareek N., et al. Ethnicity and COVID-19: an urgent public health research priority. Lancet. 2020;395:1421–1422. doi: 10.1016/S0140-6736(20)30922-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Yancy C.W. COVID-19 and African Americans. JAMA – J Am Med Assoc. 2020;323:1891–1892. doi: 10.1001/jama.2020.6548. [DOI] [PubMed] [Google Scholar]
- 6.LITCOVID. LitCovid – ethnicity search. https://www.ncbi.nlm.nih.gov/research/coronavirus/docsum?text=Ethnicity (accessed Sept 4, 2020).
- 7.PROSPERO. The impact of ethnicity on clinical outcomes in COVID-19: a systematic review. https://www.crd.york.ac.uk/prospero/display_record.php?RecordID=180654 (accessed Sept 4, 2020). [DOI] [PMC free article] [PubMed]
- 8.Aromataris E., Munn Z. JBI manual for evidence synthesis. DOI: 10.46658/JBIMES-20-01.
- 9.Egger M., Smith G.D., Schneider M., Minder C. Bias in meta-analysis detected by a simple, graphical test. Br Med J. 1997;315:629. doi: 10.1136/bmj.315.7109.629. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Higgins J., Thomas J. 6th ed. 2019. The Cochrane handbook for systematic reviews of interventions. [Google Scholar]
- 11.Jackson D., Bowden J., Baker R. How does the DerSimonian and Laird procedure for random effects meta-analysis compare with its more efficient but harder to compute counterparts? J Stat Plan Inference. 2010;140:961–970. [Google Scholar]
- 12.Ahmed S.M., Shah R.U., Bale M., et al. Comprehensive testing highlights racial, ethnic, and age disparities in the COVID-19 outbreak. medRxiv. 2020 2020.05.05.20092031. [Google Scholar]
- 13.Argenzian M.G., Bruc S.L., Slate C.L., et al. Characterization and clinical course of 1000 patients with coronavirus disease 2019 in New York: retrospective case series. BMJ. 2020;369 doi: 10.1136/bmj.m1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Caraballo C., McCullough M., Fuery M.A., et al. COVID-19 infections and outcomes in a live registry of heart failure patients across an integrated health care system. PLoS ONE. 2020;15 doi: 10.1371/journal.pone.0238829. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Gold J.A.W., Wong K.K., Szablewski C.M., et al. Characteristics and clinical outcomes of adult patients hospitalized with COVID-19 — Georgia, March 2020. Morb Mortal Wkly Rpt. 2020;69:1–6. doi: 10.15585/mmwr.mm6918e1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Gu T., Mack J., Salvatore M., et al. COVID-19 outcomes, risk factors and associations by race: a comprehensive analysis using electronic health records data in Michigan Medicine. medRxiv. 2020 doi: 10.1101/2020.06.16.20133140. [DOI] [Google Scholar]
- 17.Jun T., Nirenberg S., Kovatch P., Huang K. Sex-specificity of mortality risk factors among hospitalized COVID-19 patients in New York City: prospective cohort study. medRxiv. 2020 2020.07.29.20164640. [Google Scholar]
- 18.Khan A., Chatterjee A., Singh S. Comorbidities and disparities in outcomes of COVID-19 among black and white patients. medRxiv. 2020 doi: 10.1101/2020.05.10.20090167. [DOI] [Google Scholar]
- 19.Kim L., Garg S., O'Halloran A., et al. Risk factors for intensive care unit admission and in-hospital mortality among hospitalized adults identified through the U.S. coronavirus disease 2019 (COVID-19)-associated hospitalization surveillance network (COVID-NET) Clin Infect Dis. 2020 doi: 10.1093/cid/ciaa1012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Levy T.J., Richardson S., Coppa K., et al. Development and validation of a survival calculator for hospitalized patients with COVID-19. medRxiv Prepr Serv Heal Sci. 2020 doi: 10.1101/2020.04.22.20075416. [DOI] [Google Scholar]
- 21.Lo C.-.H., Nguyen L.H., Drew D.A., et al. Racial and ethnic determinants of Covid-19 risk. medRxiv. 2020 2020.06.18.20134742. [Google Scholar]
- 22.de Lusignan S., Dorward J., Correa A., et al. Risk factors for SARS-CoV-2 among patients in the Oxford Royal College of general practitioners research and surveillance centre primary care network: a cross-sectional study. Lancet Infect Dis. 2020;20:1034–1042. doi: 10.1016/S1473-3099(20)30371-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Marcello R., Dolle J., Grami S., et al. Characteristics and outcomes of COVID-19 patients in New York City's Public hospital system. medRxiv. 2020 doi: 10.1101/2020.05.29.20086645. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Auld S.C., Caridi-Scheible M., Blum J.M., et al. ICU and ventilator mortality among critically ill adults with coronavirus disease 2019. Crit Care Med. 2020:1–6. doi: 10.1097/CCM.0000000000004457. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Del Valle D.M., Kim-Schulze S., Huang H.-.H., et al. An inflammatory cytokine signature predicts COVID-19 severity and survival. Nat Med. 2020:1–8. doi: 10.1038/s41591-020-1051-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Martin C.A., Jenkins D.R., Minhas J.S., et al. Socio-demographic heterogeneity in the prevalence of COVID-19 during lockdown is associated with ethnicity and household size: results from an observational cohort study. EClinicalMedicine. 2020;25 doi: 10.1016/j.eclinm.2020.100466. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.McCarty T., Hathorn K., Redd W., et al. How do presenting symptoms and outcomes differ by race/ethnicity among hospitalised patients with COVID-19 infection? Experience in Massachusetts. Clin Infect Dis. 2020 doi: 10.1093/cid/ciaa1245. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Mendy A., Apewokin S., Wells A.A., Morrow A.L. Factors associated with hospitalisation and disease severity in a racially and ethnically diverse population of COVID-19 patients. MedRxiv. 2020 [Google Scholar]
- 29.Miles A., Webb T.E., Mcloughlin B.C., et al. Outcomes from COVID-19 across the range of frailty: excess mortality in fitter older people. Eur Geriatr Med. 2020:851–855. doi: 10.1007/s41999-020-00354-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Costa Monteiro A.C., Suri R., Emeruwa I.O., et al. Obesity and smoking as risk factors for invasive mechanical ventilation in COVID-19: a retrospective, observational cohort study. medRxiv Prepr Serv Heal Sci. 2020 doi: 10.1101/2020.08.12.20173849. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Narain S., Stefanov D., Chau A., et al. Comparative survival analysis of immunomodulatory therapy for COVID-19 ‘Cytokine Storm’: a retrospective observational cohort study. medRxiv. 2020 doi: 10.1101/2020.06.16.20126714. [DOI] [Google Scholar]
- 32.Niedzwiedz C.L., O'Donnell C.A., Jani B.D., et al. Ethnic and socioeconomic differences in SARS-CoV-2 infection: prospective cohort study using UK Biobank. BMC Med. 2020;18:1–14. doi: 10.1186/s12916-020-01640-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Williamson E.J., Walker A.J., Bhaskaran K., et al. Factors associated with COVID-19-related death using OpenSAFELY. Nature. 2020;584:430–436. doi: 10.1038/s41586-020-2521-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Patel N.G., Bhasin A., Feinglass J.M., et al. Clinical outcomes of hospitalized patients with COVID-19 on therapeutic anticoagulants. medRxiv. 2020:1–30. [Google Scholar]
- 35.Azar K., Shen Z., Romanelli R., et al. Dispairities in Outcomes among COVID-19 patients in a large health care system in California. Health Aff. 2020;39 doi: 10.1377/hlthaff.2020.00598. [DOI] [PubMed] [Google Scholar]
- 36.Patel M., Gangemi A., Marron R., et al. Retrospective analysis of high flow nasal therapy in COVID-19-related moderate-to-severe hypoxaemic respiratory failure. BMJ Open Respir Res. 2020;7:1–11. doi: 10.1136/bmjresp-2020-000650. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Petrak R.M., Skorodin N.C., Van Hise N.W., et al. Tocilizumab as a therapeutic agent for critically ill patients infected with sARS‐CoV‐2. Clin Transl Sci. 2020 doi: 10.1111/cts.12894. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Perez-Guzman P.N., Daunt A., Mukherjee S., et al. Clinical characteristics and predictors of outcomes of hospitalized patients with coronavirus disease 2019 in a multiethnic London national health service trust: a retrospective cohort study. Clin Infect Dis. 2020:1–11. doi: 10.1093/cid/ciaa1091. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Price-Haywood E.G., Price-Haywood E.G., Burton J., Fort D., Seoane L. Hospitalization and mortality among black patients and white patients with Covid-19. N Engl J Med. 2020;382:2534–2543. doi: 10.1056/NEJMsa2011686. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Rajter J.C., Sherman M.S., Fatteh N., Vogel F., Sacks J., Rajter J.-.J. ICON (Ivermectin in COvid Nineteen) study: use of ivermectin is associated with lower mortality in hospitalised patients with COVID-19. medRxiv. 2020 doi: 10.1016/j.chest.2020.10.009. 2020.06.06.20124461. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Ramaswamy M., Mannam P., Comer R., Sinclair E., McQuaid D.B., Schmidt M.L. Off-label real world experience using tocilizumab for patients hospitalized with COVID-19 disease in a regional community health system: a case-control study. medRxiv. 2020 2020.05.14.20099234. [Google Scholar]
- 42.Rentsch C.T., Kidwai-khan F., Tate J.P., et al. COVID-19 testing, hospital admission and intensive care among 2,026,277 United States veterans aged 54-75 years. 2020;: 1–32.
- 43.Rosenberg E.S., Dufort E.M., Udo T., et al. Association of treatment with hydroxychloroquine or azithromycin with in-hospital mortality in patients with COVID-19 in New York state. JAMA – J Am Med Assoc. 2020;323:2493–2502. doi: 10.1001/jama.2020.8630. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Rozenfeld Y., Beam J., Maier H., et al. A model of disparities: risk factors associated with COVID-19 infection. Int J Equity Health. 2020;19 doi: 10.1186/s12939-020-01242-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Sakowicz A., Ayala A., Ukeje C., Witting C., William A., Miller E. Risk factors for severe acute respiratory syndrome. Am J Obstet Gynaecol MFM. 2020 doi: 10.1016/j.ajogmf.2020.100198. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Brill S.E., Jarvis H.C., Ozcan E., et al. COVID-19: a retrospective cohort study with focus on the over-80s and hospital-onset disease. BMC Med. 2020;18:1–9. doi: 10.1186/s12916-020-01665-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Sapey E., Gallier S., Mainey C., et al. Ethnicity and risk of death in patients hospitalised for COVID-19 infection in the UK: an observational cohort study in an urban catchment area. BMJ Open Respir Res. 2020;7:1–11. doi: 10.1136/bmjresp-2020-000644. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Somers E., Eschenauer G., Troost J., et al. Tocilizumab for treatment of mechanically ventilated patients with COVID-19. Clin Infect Dis DOI:10.1093/cid/ciaa839. [DOI] [PMC free article] [PubMed]
- 49.Tartof S., Qian L., Wei R., et al. Obesity and mortality among patients diagnosed with COVID-19: results from an integrated healthcare organisation. Ann Intern Med. 2020 doi: 10.7326/M20-3742. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Vahidy F.S., Nicolas J.C., Meeks J.R., et al. Racial and ethnic disparities in SARS-CoV-2 pandemic: analysis of a COVID-19 observational registry for a diverse US metropolitan population. BMJ Open. 2020;10 doi: 10.1136/bmjopen-2020-039849. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Velu P., Craney A., Ruggiero P., et al. Rapid implementation of SARS-CoV-2 emergency use authorization RT-PCR 2 testing and experience at an academic medical institution. medRxiv. 2020 doi: 10.1101/2020.06.05.20109637. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Wang A.-.L., Zhong X., Hurd Y. Comorbidity and sociodemographic determinants in COVID-19 mortality in an US Urban healthcare system. medRxiv. 2020 2020.06.11.20128926. [Google Scholar]
- 53.Wang Z., Zheutlin A.B., Kao Y.-.H., et al. Analysis of hospitalized COVID-19 patients in the Mount Sinai Health system using electronic medical records (EMR) reveals important prognostic factors for improved clinical outcomes. medRxiv. 2020 2020.04.28.20075788. [Google Scholar]
- 54.Yehia B.R., Winegar A., Fogel R., et al. Association of race with mortality among patients hospitalized with coronavirus disease 2019 (COVID-19) at 92 US hospitals. JAMA Netw open. 2020;3 doi: 10.1001/jamanetworkopen.2020.18039. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Zakeri R., Bendayan R., Ashworth M., et al. A case-control and cohort study to determine the relationship between ethnic background and severe COVID-19. 2020;: 0–2. [DOI] [PMC free article] [PubMed]
- 56.Zimmerman P., Stroever S., T B., et al. Mortality associated with intubation and mechanical ventilation in patients with COVID-19. MedRxiv. 2020 [Google Scholar]
- 57.Chamie G., Marquez C., Crawford E., et al. Community transmission of severe acute respiratory syndrome coronavirus 2 disproportionately affects the Latinx population during shelter-in-place in San Francisco. Clin Infect Dis. 2020:1–9. doi: 10.1093/cid/ciaa1234. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Crouse A., Grimes T., Li P., Might M., Ovalle F., Shalev A. Metformin use is associated with reduced mortality in a diverse population with Covid-19 and diabetes. medRxiv Prepr Serv Heal Sci. 2020 doi: 10.1101/2020.07.29.20164020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Ebinge J.E., Achamallah N., Ji H., et al. Pre-existing traits associated with Covid-19 illness severity. PLoS ONE. 2020;15:1–16. doi: 10.1371/journal.pone.0236240. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Ellington S., Strid P., Tong V., et al. Characteristics of women of reproductive age with laboratory confirmed SARS-CoV-2 infection by pregnancy status – United States, January 22-June 7, 2020. Centers Dis Control Prev Morb Mortal Wkly Rep. 2020:69. doi: 10.15585/mmwr.mm6925a1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Garibaldi B., Fiksel J., Muschelli J., et al. Patient trajectories among persons hospitalized for COVID-19 –a cohort study. Ann Intern Med. 2020 doi: 10.7326/M20-3905. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Cevik M., Marcus J., Buckee C., Smith T. SARS-CoV-2 transmission dynamics should inform policy. Clin Infect Dis. 2020 doi: 10.1093/cid/ciaa1442. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Office for National Statistics United Kingdom Government. Coronavirus (COVID-19) related deaths by occupation, England and Wales: deaths registered up to and including 20 April 2020. 2020. https://www.ons.gov.uk/peoplepopulationandcommunity/healthandsocialcare/causesofdeath/bulletins/coronaviruscovid19relateddeathsbyoccupationenglandandwales/deathsregistereduptoandincluding20april2020 (accessed Sept 4, 2020).
- 64.Jing Q.-.L., Liu M.-.J., Zhang Z.-.B., et al. Household secondary attack rate of COVID-19 and associated determinants in Guangzhou, China: a retrospective cohort study. Lancet Infect Dis. 2020;3099:1–10. doi: 10.1016/S1473-3099(20)30471-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Iacobucci G. Covid-19: deprived areas have the highest death rates in England and Wales. BMJ. 2020;369:m1810. doi: 10.1136/bmj.m1810. [DOI] [PubMed] [Google Scholar]
- 66.Hawkins D. Differential occupational risk for COVID-19 and other infection exposure according to race and ethnicity. Am J Ind Med. 2020;63:817–820. doi: 10.1002/ajim.23145. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Nguyen L.H., Drew D.A., Graham M.S., et al. Risk of COVID-19 among front-line health-care workers and the general community: a prospective cohort study. Lancet Public Heal. 2020 doi: 10.1016/s2468-2667(20)30164-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Intensive Care National Audit and Research. ICNARC report on COVID-19 in critical care : England, Wales and Northern Ireland. 2020;: 1–19.
- 69.Office for National Statistics United Kingdom Government. Coronavirus (COVID-19) related deaths by ethnic group, England and Wales: 2 March 2020 to 15 May 2020. https://www.ons.gov.uk/peoplepopulationandcommunity/birthsdeathsandmarriages/deaths/articles/coronaviruscovid19relateddeathsbyethnicgroupenglandandwales/2march2020to15may2020.
- 70.Holman N., Knighton P., Kar P., et al. Risk factors for COVID-19-related mortality in people with type 1 and type 2 diabetes in England: a population-based cohort study. Lancet Diabetes Endocrinol. 2020;8:823–833. doi: 10.1016/S2213-8587(20)30271-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Barron E., Bakhai C., Kar P., et al. Associations of type 1 and type 2 diabetes with COVID-19-related mortality in England: a whole-population study. Lancet Diab Endocrinol. 2020;8:813–822. doi: 10.1016/S2213-8587(20)30272-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Egede L.E., Walker R.J. Structural racism, social risk factors and COVID-19 – a dangerous convergence for black Americans. N Engl J Med. 2020 doi: 10.1056/NEJMp2023616. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Hooper M., Napoles A., Perez-Stable E. COVID-19 and racial/ethnic disparities. JAMA. 2020;323:2466–2467. doi: 10.1001/jama.2020.8598. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Szczepura A. Access to health care for ethnic minority populations. Postgrad Med J. 2005;81:141–147. doi: 10.1136/pgmj.2004.026237. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Doctors of the World. A rapid needs assessment of excluded people in England during the 2020 COVID-19 pandemic. 2020.
- 76.Caldwell J.G., Price E.V., Schroeter AL, Fletcher GF. Aortic regurgitation in the Tuskegee study of untreated syphilis. J Chronic Dis. 1973;26:187–194. doi: 10.1016/0021-9681(73)90089-1. [DOI] [PubMed] [Google Scholar]
- 77.Devakumar D., Shannon G., Bhopal S.S., Abubakar I. Racism and discrimination in COVID-19 responses. Lancet. 2020;395:1194. doi: 10.1016/S0140-6736(20)30792-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Gravlee C.C. Systemic racism, chronic health inequities, and COVID-19: a syndemic in the making? Am J Hum Biol. 2020;32:1–8. doi: 10.1002/ajhb.23482. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Horton R. Offline: COVID-19 is not a pandemic. Lancet. 2020;396:874. doi: 10.1016/S0140-6736(20)32000-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Chastain D., Osae S., Henao-Martinez A., Franco-Paredes C., Chastain J., Young H. Racial disproportionality in COVID clinical trials. N Engl J Med. 2020;383 doi: 10.1056/NEJMp2021971. [DOI] [PubMed] [Google Scholar]
- 81.Caraballo C., McCullough M., Fuery M.A., et al. COVID-19 infections and outcomes in a live registry of heart failure patients across an integrated healthcare system. medRxiv. 2020 doi: 10.1371/journal.pone.0238829. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Nellums L.B., Thompson H., Holmes A., et al. Antimicrobial resistance among migrants in Europe: a systematic review and meta-analysis. Lancet Infect Dis. 2018;18:796–811. doi: 10.1016/S1473-3099(18)30219-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Aldridge R.W., Nellums L.B., Bartlett S., et al. Global patterns of mortality in international migrants: a systematic review and meta-analysis. Lancet. 2018;392:2553–2566. doi: 10.1016/S0140-6736(18)32781-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Senn S.J. Overstating the evidence - Double counting in meta-analysis and related problems. BMC Med Res Methodol. 2009;9:1–7. doi: 10.1186/1471-2288-9-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Sigel K., Swartz T., Golden E., et al. Coronavirus 2019 and people living with human immunodeficiency virus: outcomes for hospitalized patients in New York City. Clin Infect Dis. 2020;10029:1–6. doi: 10.1093/cid/ciaa880. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Malle L., Gao C., Bouvier N., Percha B., Bogunovic D. COVID-19 hospitalization is more frequent and severe in down syndrome and affects patients a decade younger. medRxiv. 2020 doi: 10.1101/2020.05.26.20112748. [DOI] [Google Scholar]
- 87.Wang B., Van Oekelen O., Mouhieddine T.H., et al. A tertiary center experience of multiple myeloma patients with COVID-19: lessons learned and the path forward. J Hematol Oncol. 2020;13:1–12. doi: 10.1186/s13045-020-00934-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Paranjpe I., Russak A.J., De Freitas J.K., et al. Clinical characteristics of hospitalized Covid-19 patients in New York City. medRxiv. 2020:1–20. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.