Abstract
Study Objectives
The differentiation of isolated rapid eye movement (REM) sleep behavior disorder (iRBD) or its prodromal phase (prodromal RBD) from other disorders with motor activity during sleep is critical for identifying α-synucleinopathy in an early stage. Currently, definite RBD diagnosis requires video polysomnography (vPSG). The aim of this study was to evaluate automated 3D video analysis of leg movements during REM sleep as objective diagnostic tool for iRBD.
Methods
A total of 122 participants (40 iRBD, 18 prodromal RBD, 64 participants with other disorders with motor activity during sleep) were recruited among patients undergoing vPSG at the Sleep Disorders Unit, Department of Neurology, Medical University of Innsbruck. 3D videos synchronous to vPSG were recorded. Lower limb movements rate, duration, extent, and intensity were computed using a newly developed software.
Results
The analyzed 3D movement features were significantly increased in subjects with iRBD compared to prodromal RBD and other disorders with motor activity during sleep. Minor leg jerks with a duration < 2 seconds discriminated with the highest accuracy (90.4%) iRBD from other motor activity during sleep. Automatic 3D analysis did not differentiate between prodromal RBD and other disorders with motor activity during sleep.
Conclusions
Automated 3D video analysis of leg movements during REM sleep is a promising diagnostic tool for identifying subjects with iRBD in a sleep laboratory population and is able to distinguish iRBD from subjects with other motor activities during sleep. For future application as a screening, further studies should investigate usefulness of this tool when no information about sleep stages from vPSG is available and in the home environment.
Keywords: RBD, iRBD, synucleinopathy, prodromal RBD, PSG, legs, SINBAR, software, screening
Statement of Significance
Isolated rapid eye movement (REM) sleep behavior disorder (iRBD) is an early phase α-synucleinopathy. Its recognition is therefore of utmost importance, particularly considering that neuroprotective drugs might become available in the next few years. The gold standard for iRBD diagnosis, video polysomnography, is costly and time consuming. Therefore, we evaluated automated 3D video analysis of leg movements during REM sleep for identifying iRBD and its prodromal phase and for distinguishing them from mimics. Minor leg jerks with a duration < 2 seconds discriminated with high accuracy (90.4%) iRBD from other motor activity during sleep. Further studies should investigate usefulness of this tool when no information about sleep stages is available and in home settings.
Introduction
Rapid eye movement (REM) sleep behavior disorder (RBD) is characterized by abnormal behaviors during REM sleep and the loss of physiological muscle atonia during REM sleep (REM sleep without atonia [RWA]) [1]. The resulting REM sleep behaviors range from brief twitches to complex movements, in which affected subjects may inflict injuries to themselves and/or their bedpartners [2]. Isolated RBD (iRBD)—i.e. RBD in the absence of associated comorbidities—is now widely recognized as an early-stage α-synucleinopathy [3].
A precursory state of RBD (prodromal RBD), where symptoms and signs are presents but do not yet fulfill diagnostic criteria for RBD, has been proposed [3–5]. Originally, this state has been called “subclinical RBD” by Schenck et al. and defined as “either PSG abnormalities alone” (i.e. isolated RWA) “or with non-clinical behaviors in REM sleep, such as limb twitching and jerking, and simple behaviors.” [5] For long time prodromal RBD has not been a main focus of research, and its evolution over time is still not completely clarified. Hypothetically, there is a complex set of possibilities, ranging from partial or complete resolution to progression to iRBD or overt α-synucleinopathy (passing or not through the stage of iRBD). More recently, a continuous evolution from initially normal REM sleep with preserved atonia to prodromal RBD, gradually further progressing into iRBD, and eventually RBD with clinically overt α-synucleinopathy has been suggested [3, 4]. Although more evidence is needed, several recent studies increasingly support this concept [6–9].
An accurate diagnosis of iRBD or even prodromal RBD and their distinction from other disorders with motor manifestations during sleep is critical for identifying α-synucleinopathy in an early stage. This is of utmost relevance in the light of possible future availability of neuroprotective drugs, which would probably have a higher effect in earlier disease stages.
Currently, definite diagnosis of RBD requires video polysomnography (vPSG), demonstrating the presence of RWA [1]. This is a costly examination not widely available. Moreover, RBD might be overseen in sleep centers not experienced in this condition or focused on breathing disorders during sleep, but not on motor disorders or neurological aspects of sleep. To overcome these limitations, automated systems for RBD detection have been developed during the last years. These focused mainly on computerized electromyography (EMG) analysis for quantification of EMG activity during REM sleep [10–13]. However, these require EMG recording and are therefore not suitable as large-scale screening methods. Moreover, the presence of RWA might represent prodromal RBD or iRBD (if associated with RBD behaviors in the video), and quantification of motor activity using EMG alone cannot distinguish between these two conditions. An automated system able to detect RBD behaviors based on video recordings would overcome these limitations.
Automated 3D video analysis allows a contactless and thus noninvasive assessment of a person’s movements during sleep. Moreover, in contrast to EMG, video analysis is not limited to specific muscles or body parts. The usefulness of 3D video analysis has already been demonstrated in detecting sleep apnea [14–16], periodic leg movements during sleep [17], and sleeping pose recognition [18]. Aim of this study was to examine the usefulness of automated 3D video analysis of leg movements during REM sleep as a diagnostic tool for prodromal RBD and iRBD. We hypothesized that subjects with iRBD and possibly prodromal RBD can be distinguished from patients with other sleep disorders with motor manifestations during sleep based on 3D video analysis of lower limbs movements during REM sleep. To this aim, 3D video and vPSG data from a sleep laboratory sample of patients with different sleep disorders with motor manifestations during sleep were recorded and analyzed.
Materials and Methods
Participants
Participants were prospectively recruited among patients undergoing 8-hour vPSG at the Sleep Disorders Unit of the Department of Neurology, Medical University of Innsbruck, diagnosed with a sleep disorder known to go along with motor manifestations during sleep. Accordingly, a total of 122 participants were included: 40 patients with iRBD, 18 patients with prodromal RBD, and 64 patients with other sleep disorders. iRBD was diagnosed according to the ICSD-3 criteria [1]. Prodromal RBD was defined as the presence of RWA in the SINBAR montage [19] exceeding the SINBAR cutoff [20], and/or the presence of a number of movements during REM sleep exceeding 34.7/h. As prodromal RBD is a new concept and values for movements during REM sleep differentiating prodromal RBD from normal REM sleep are not defined, this cutoff was selected because it represents the 90th percentile of published normative values of movements per hour during REM sleep in healthy subject [21]. The group of patients with other sleep disorders included sleep apnea (n = 11), periodic limb movements during sleep (PLMS; n = 4), sleep apnea with PLMS (n = 44), and restless legs syndrome (n = 5). These sleep disorders were diagnosed according to standard criteria [22, 23]. Exclusion criteria were the presence of secondary RBD (i.e. RBD associated with a neurological condition such as Parkinson’s disease, dementia with Lewy bodies, multiple system atrophy or narcolepsy), a total duration of REM sleep below 5 min, and faulty recordings due to technical issues. Since both PLMS and sleep apnea are common comorbidities in patients with RBD, their presence in the iRBD and prodromal RBD groups did not represent an exclusion criteria [19, 24].
The study was approved by the ethics committee of the Medical University of Innsbruck, Austria. All participants gave their written informed consent prior to inclusion in the study.
Video polysomnography
vPSG was performed according to the American Academy of Sleep Medicine (AASM) guidelines [23] and consisted of electrooculography, electroencephalography (F3, F4, C3, C4, O1, O2, M1, and M2 electrodes), cardiorespiratory recording (single channel electrocardiography, recording of nasal air flow [thermocouple], nasal pressure cannula, tracheal microphone, thoracic and abdominal respiratory movements [piezo], transcutaneous oxygen saturation), and EMG including the mental, submental, both anterior tibialis muscles, and both flexor digitorum superficialis muscles. Time-synchronized digital videography was recorded with an infrared camera (Sony IPELA SNC-VM600B).
Leg movements were recorded according to the AASM criteria, using surface electrodes placed longitudinally and symmetrically around the middle of the tibialis anterior muscle, 2–3 cm apart [23]. PLMS were scored according to the AASM criteria using a validated automated algorithm integrated in the PSG system [25].
RWA was quantified using a validated software [10], and subsequently a trained scorer performed manual artifact correction, consisting in the exclusion of miniepochs containing snoring/respiratory artifacts, electrocardiographic artifacts, or EMG activity in the context of arousals.
In a subgroup of subjects (n = 33), video during REM sleep was carefully analyzed and all visible movements were counted. Leg movement-like movements, movements related to arousals, respiratory movements, comfort movements, and voluntary movements were excluded from the count. Two movements were considered separate when they were clearly seen as separate in the video and there was an interval of 1 second between the two movements [21].
3D video
Recording
The 3D videos were recorded using a Microsoft Kinect v2 sensor (Microsoft Corporation) mounted to the ceiling above the bed. The distance between the surface of the bed and the sensor was 190 cm. The device is a so called time-of-flight sensor and emitted (invisible) infrared light, captured its reflections, and computed the distance between the sensor and the reflecting surface (e.g. the sleeping person or the bed) based on the time the emitted light needed to be detected by the sensor as reflections.
All recordings were taken under usual sleep laboratory conditions. No additional illumination in the room was required. The patients were sleeping under bed sheets and blankets.
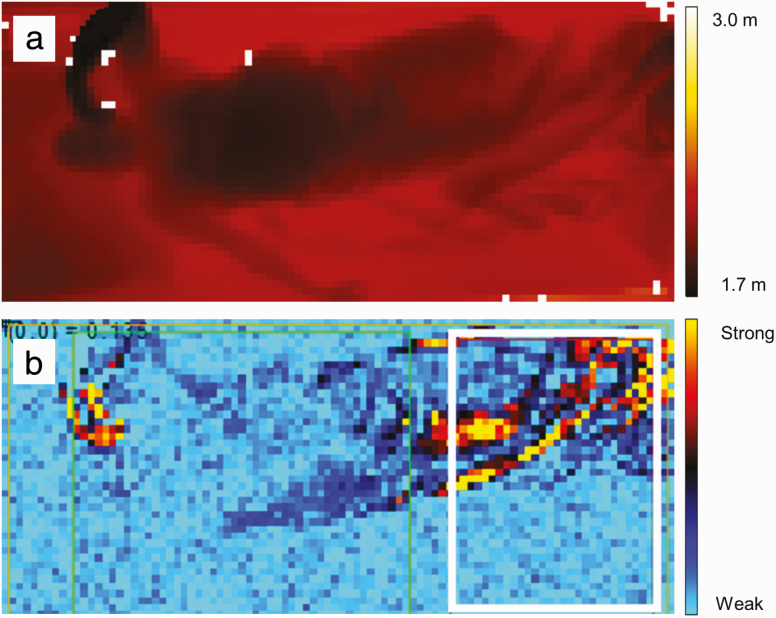
The measured distance was visualized as a depth image of the color-coded distance values at a specific point in time (see Figure 1a). Each depth image had a resolution of 512 × 424 pixels and represented one frame of the 3D video, which was recorded with 30 frames per second. The 3D video recordings were transferred via a mini-PC from the patient room to an encrypted external hard-drive and then processed offline using software developed in Python 3.4 (Python Software Foundation) consisting of a generic movement detection module (AIT, Austrian Institute of Technology GmbH) and a specific RBD evaluation module (Medical University of Innsbruck, and AIT Austrian Institute of Technology GmbH).
Figure 1.
(a) Depth image of the distance between the sleeping subject and the 3D sensor. (b) Motion image that shows the pixel-wise variation of the 3D video frames over time with movements visible in “hot” colors (red/orange/yellow). The white rectangular image section represents the area in which movements were analyzed to identify leg movements.
Movement detection
Movements in the 3D videos were detected by a series of automated processing steps including resampling, exclusion of faulty pixel values, and computing of the motion signal, i.e. the pixel-wise variation of the 3D video frames over time (see Figure 1b). Large motion signal values in multiple neighboring pixels indicated thereby a movement, which was identified by sequential thresholding. Sudden increases of the motion signal corresponded to rapid movements and a simultaneous increase in many pixels corresponded to movements of larger parts of the body. To assign the detected movements to a body region, we deployed the movement detection in manually preselected rectangular image sections in the 3D videos (see white rectangle in Figure 1b). In this pilot study, we focused only on the image section corresponding to the lower part of the bed to analyze leg movements.
3D leg movement features
Based on the detected leg movements, a set of features was developed to quantify the RBD-specific movement properties during REM sleep. Movements related to respiratory events were hereby excluded. The movement index (3D index) was defined as the average number of movements per hour of REM sleep. The movement ratio (3D ratio) was defined as the total movement duration during REM sleep divided by total REM sleep duration. The movement extent (3D extent) was defined as the mean number of active pixels per movement during REM sleep. The movement intensity (3D intensity) was defined as the average area under the motion signal per movement and pixel during REM sleep. Although the 3D extent and 3D intensity might overlap, the 3D extent is an index of the extent of the body area involved in the movement, whereas the 3D intensity takes into account the pixel-wise variation of the 3D video frame over time and is therefore a measure of how fast the movement is. These features were computed separately for leg movements with a duration between 0.1 and 15 seconds and for short jerks with a duration between 0.1 and 2 seconds. The rationale of selecting these leg movements’ durations was based on previous studies showing that the majority of RBD-associated REM events are minor distal jerks [26–29]. As the more common movements in iRBD are short jerks, the duration of 2 seconds was chosen to identify those brief movements typical of iRBD. On the other side, PLMS are usually longer (up to 15 seconds in case of bilateral leg movements), as are also movements related to respiratory events.
We analyzed these features both for all and for only the nonperiodic leg movements during REM sleep. Periodicity was defined applying the EMG-based WASM criteria for bilateral leg movements to leg movements detected with 3D video [30].
Statistical analysis
Statistical analyses were conducted using MATLAB 2018b (The MathWorks, Inc.). Data were tested for normal distribution using the Shapiro-Wilk test. Descriptive statistics of the three patient groups were provided as numbers (percentages) or, since not all group data had a normal distribution, as median values (interquartile range [IQR]). Group differences of demographic, clinical, and 3D features were assessed using Pearson’s χ 2 statistic as well as Kruskal–Wallis tests with subsequent multiple comparison testing. The 3D movement index was compared with the number of REM events per hour from video analysis and the SINBAR RWA score by using a linear regression approach and Spearman’s rank correlation coefficient (Spearman’s ρ). We assumed significance for p < 0.05. In case of multiple testing, p-values were corrected by using the Bonferroni–Holm method [31]. Finally, optimal discriminative cutoff values of the individual 3D movement features were determined by maximizing the area under the receiver operating characteristic (ROC-AUC) curve and by calculating the according accuracies as well as sensitivity and specificity.
Results
Demographic and clinical data
A total of 122 patients (28 female, median age 60 years) undergoing 8-hours vPSG at the Sleep Laboratory of the Department of Neurology at the Medical University of Innsbruck, Austria, were included in this study. Of them, 40 subjects were diagnosed with iRBD, 18 with prodromal RBD, and 64 with other sleep disorders known to go along with motor manifestations during sleep. Table 1 summarizes the demographic and clinical sample characteristics for the three patient groups, as well as the p-values of group difference testing. Subjects with iRBD were older than those with prodromal RBD (p = 0.0247) and those with other disorders (p = 0.0022). The iRBD group also had a lower median apnea–hypopnea index (p = 0.0497) and a higher positive airways pressure (PAP) therapy rate (p = 0.0395) than subjects with prodromal RBD, as well as a higher antidepressant therapy rate than subjects with other sleep disorders (p = 0.0035).
Table 1.
Demographic and group statistics of participants with isolated RBD, prodromal RBD, and other disorders with motor activity during sleep
| (1) iRBD | (2) Prodromal RBD | (3) Other* | p** | ||||
|---|---|---|---|---|---|---|---|
| N = 40 | N = 18 | N = 64 | Group | 1 vs. 2 | 1 vs. 3 | 2 vs. 3 | |
| Age, y | 67.0 (14.0) | 55.5 (10.0) | 58.5 (16.0) | 0.0014 | 0.0247 | 0.0022 | 1.0000 |
| Sex, female | 6 (15.0%) | 3 (16.7%) | 19 (29.7%) | 0.1785 | 1.0000 | 0.2532 | 0.7433 |
| REM duration, minutes | 72.3 (44.0) | 72.0 (25.5) | 75.0 (52.5) | 0.8033 | 1.0000 | 1.0000 | 1.0000 |
| PLMS index | 25.4 (33.9) | 22.3 (34.6) | 15.1 (27.1) | 0.0771 | 0.8329 | 0.0712 | 1.0000 |
| Apnea-hypopnea index | 6.9 (10.0) | 19.9 (17.8) | 11.4 (23.0) | 0.0247 | 0.0497 | 0.0820 | 1.0000 |
| PAP therapy | 17 (42.5%) | 2 (11.1%) | 14 (21.9%) | 0.0187 | 0.0395 | 0.0653 | 1.0000 |
| Antidepressant therapy | 21 (52.5%) | 4 (22.2%) | 14 (21.9%) | 0.0033 | 0.0682 | 0.0035 | 1.0000 |
Age, REM duration, PLMS index, and apnea–hypopnea index are summarized by median values and interquartile ranges. Sex, PAP therapy, and antidepressant therapy are provided as absolute numbers and percentages. Group differences were assessed, as appropriate, by Kruskal–Wallis and Pearson’s χ 2 tests. Statistically significant differences between groups after Bonferroni-Holm correction are shown as bold values. iRBD, isolated REM sleep behavior disorder; REM, rapid eye movement; RWA, REM sleep without atonia; PLMS, periodic limb movements during sleep; PAP, positive airways pressure.
*Sleep apnea (n = 11), PLMS (n = 4), sleep apnea with PLMS (n = 44), and other (n = 5).
**Bonferroni–Holm corrected.
Video polysomnography
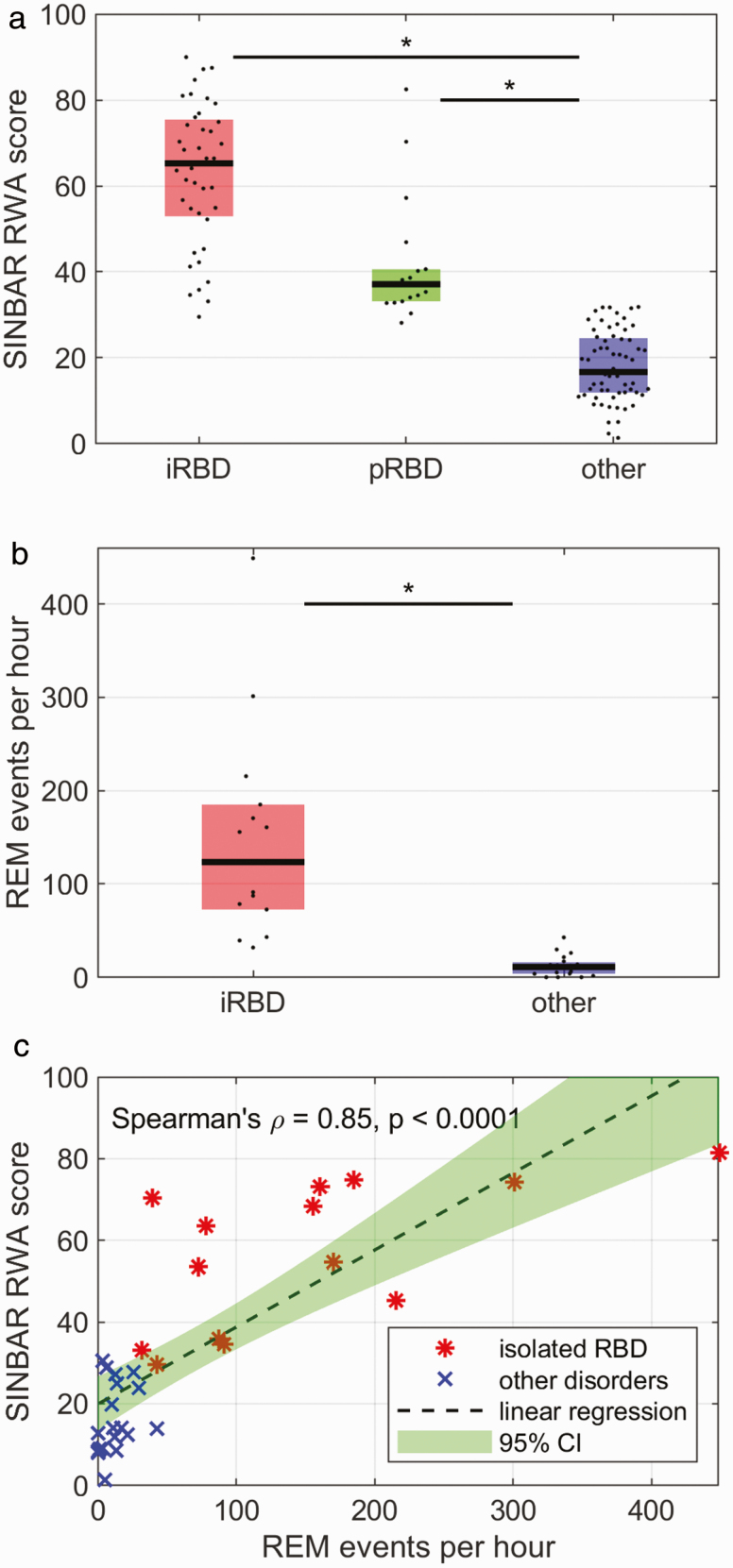
Subjects with iRBD had a median RWA score of 65.3 (IQR 22.6), which was significantly higher (p < 0.0001) than the RWA score of subjects with other sleep disorders (median 16.7, IQR 12.7). The median RWA score of subjects with prodromal RBD was 37.1 (IQR 7.5) and significantly higher (p < 0.0001) than the score of subjects with other sleep disorders as well. There was no significant group difference in RWA between iRBD and prodromal RBD in our sample (p = 0.1492), see Figure 2a.
Figure 2.
(a) RWA scores as determined by the SINBAR method for subjects with iRBD, prodromal RBD, and other disorders with motor activity during sleep (other). Significant differences were observed between iRBD and other as well as between prodromal RBD and other. (b) The number of REM events as determined from visual video analysis in a subsample of 14 subjects with iRBD and 19 with other sleep disorders were significantly higher in the iRBD group. (c) Regression subsample analysis of the number of REM events per hour and RWA scores showed a significantly positive correlation between these scores. iRBD, isolated RBD; RBD, REM sleep behavior disorder; REM, rapid eye movement; RWA, REM sleep without atonia; SINBAR, Sleep Innsbruck Barcelona.
Visual video analysis of motor events during REM sleep was conducted in a subsample of 14 subjects with iRBD and 19 subjects with other sleep disorders. In the iRBD group, the median number of motor events per hour of REM sleep was 123.4 (IQR 112.5), which was significantly higher (p < 0.0001) than in the group with other disorders with motor activity during sleep (median 10.9, IQR 12.4), see Figure 2b. The REM event index exceeded a value of 200 REM events/h in three and a value of 400/h in one subject with iRBD. Within the same subsample, we analyzed the relationship between the number of REM events per hour and SINBAR RWA scores, see Figure 2c. The linear regression (dashed line with green 95% confidence interval) indicates a positive relation between REM event index and RWA score (Spearman’s ρ = 0.85, p < 0.0001).
3D leg movement index compared with video polysomnography
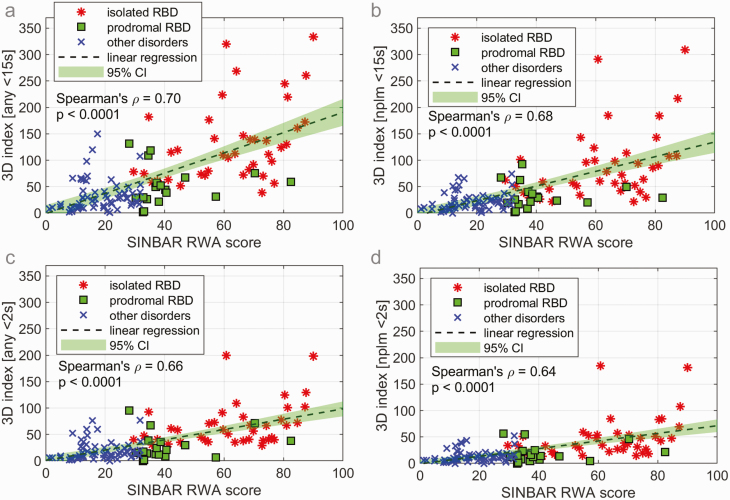
The RWA score and the number of leg movements per hour of REM sleep as assessed from 3D video (3D index) were positive related with a Spearman’s ρ of 0.70 and p < 0.0001, see Figure 3a. For the subsample of only nonperiodic leg movements shorter than 15 seconds, we observed a slightly lower Spearman’s ρ of 0.68 (p < 0.0001), see Figure 3b. The number of leg jerks with duration less than 2 seconds per hour of REM sleep was lower (approximately half) in all three groups. Figure 3c illustrates the positive relation of the number of leg jerks per hour of REM sleep with the RWA score (Spearman’s ρ = 0.66, p < 0.0001). Figure 3d compares the nonperiodic leg jerks with the RWA score (Spearman’s ρ = 0.64, p < 0.0001).
Figure 3.
Regression analysis of rapid eye movement (REM) sleep without atonia (RWA) scores and the 3D leg movements per hour of REM sleep for (a) any leg movements with duration < 15 s, (b) nonperiodic leg movements with duration < 15 s, (c) any leg jerks with duration < 2 s, and (d) nonperiodic leg jerks with duration < 2 s. RBD, REM sleep behavior disorder; REM, rapid eye movement; RWA, REM sleep without atonia; SINBAR, Sleep Innsbruck Barcelona.
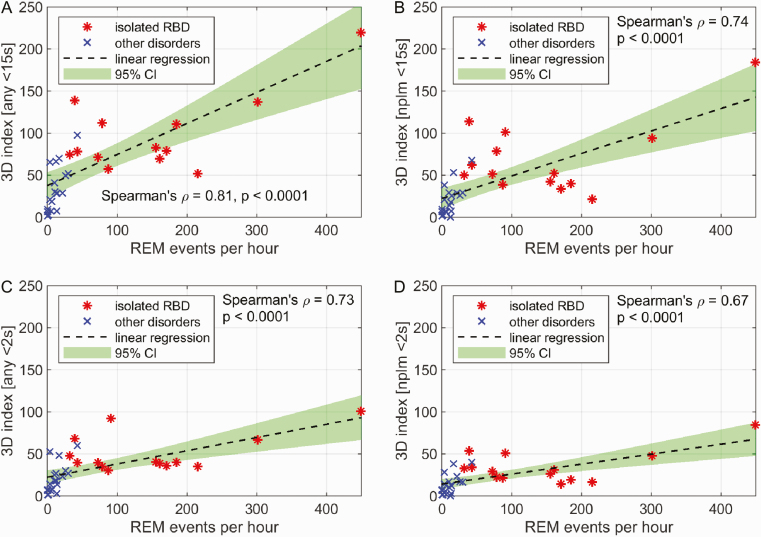
The results of the correlation analysis of visually identified REM events and 3D index in a subsample of 14 subjects with iRBD and 19 with other disorders with motor activity during sleep are shown in Figure 4a. There was a significant positive relationship between the number of leg movements found in 3D and the number of REM events per hour of REM sleep with a Spearman’s ρ of 0.81 (p > 0.0001). The REM event index correlated with the 3D index of nonperiodic movements only (Spearman’s ρ = 0.74, p < 0.0001), see Figure 4b. Figure 4c and d shows the results of regression analysis for REM event index versus any jerks and nonperiodic jerks, respectively. In both cases, the relation was significant and positive (Spearman’s ρ = 0.73 and 0.67 with p < 0.0001 in both cases).
Figure 4.
Regression subsample analysis of the number of REM events and the 3D leg movements per hour of REM sleep for (a) any leg movements with duration < 15 s, (b) nonperiodic leg movements with duration < 15 s, (c) any leg jerks with duration < 2 s, and (d) nonperiodic leg jerks with duration < 2 s. RBD, REM sleep behavior disorder; REM, rapid eye movement; RWA, REM sleep without atonia; SINBAR, Sleep Innsbruck Barcelona.
3D video analysis for RBD diagnosis
Table 2 presents the group differences between iRBD, prodromal RBD, and other disorders with motor activity during sleep, when looking into the 3D leg movement features. The comparison of the median 3D index of any leg movements and any jerks with values of 113.60 versus 58.74 in iRBD, 46.67 versus 19.08 in prodromal RBD, and 24.91 versus 12.84 in other disorders with motor activity during sleep indicates that, on average, 49% of the detected leg movements had a duration less than 2 seconds. For both jerks and any leg movements, the nonperiodic movements accounted for 63% of all movements. There was a significant difference in all 3D features between iRBD and prodromal RBD as well as between iRBD and other disorders with motor activity during sleep. The most significant results (i.e. with lowest p-values) were observed for the 3D features of any leg jerks with duration less than 2 seconds. No significant differences were observed between the prodromal RBD and other disorders with motor activity during sleep.
Table 2.
3D leg movement features of participants with isolated RBD, prodromal RBD, and other disorders with motor activity during sleep: The number of leg movements per hour of REM sleep (3D index), the total movement duration during REM sleep divided by total REM sleep duration (3D ratio), the mean number of active pixels per movement during REM sleep (3D extent), and the average area under the motion signal per movement and pixel during REM sleep (3D intensity) were computed separately for any leg movements shorter than 15 s and shorter than 2 s, as well as nonperiodic leg movements shorter than 15 s and shorter than 2 s
| (1) iRBD | (2) Prodromal RBD | (3) Other* | p ** | ||||
|---|---|---|---|---|---|---|---|
| N = 40 | N = 18 | N = 64 | Group | 1 vs 2 | 1 vs 3 | 2 vs 3 | |
| Any LMs 0.1–15 s | |||||||
| 3D index (any <15 s) | 113.60 (92.51) | 46.67 (38.52) | 24.91 (28.61) | <0.0001 | 0.0004 | <0.0001 | 0.1467 |
| 3D ratio (any <15 s) | 0.09 (0.07) | 0.03 (0.03) | 0.02 (0.02) | <0.0001 | 0.0005 | <0.0001 | 0.1278 |
| 3D extent (any <15 s) | 9.67 (9.44) | 3.24 (2.97) | 2.82 (3.14) | <0.0001 | 0.0002 | <0.0001 | 1.0000 |
| 3D intensity (any <15 s) | 0.30 (0.33) | 0.08 (0.15) | 0.09 (0.11) | <0.0001 | 0.0012 | <0.0001 | 0.8106 |
| Any jerks 0.1–2 s | |||||||
| 3D index (any <2 s) | 58.74 (40.30) | 19.08 (25.83) | 12.84 (18.39) | <0.0001 | 0.0001 | <0.0001 | 0.4906 |
| 3D ratio (any <2 s) | 0.02 (0.01) | 0.01 (0.01) | 0.01 (0.01) | <0.0001 | 0.0001 | <0.0001 | 0.4139 |
| 3D extent (any <2 s) | 0.82 (0.53) | 0.25 (0.16) | 0.12 (0.16) | <0.0001 | <0.0001 | <0.0001 | 0.3916 |
| 3D intensity (any <2 s) | 0.04 (0.02) | 0.01 (0.01) | 0.01 (0.01) | <0.0001 | 0.0001 | <0.0001 | 0.4049 |
| Nonperiodic LMs 0.1–15 s | |||||||
| 3D index (nplm <15 s) | 67.85 (60.11) | 27.29 (20.59) | 18.03 (18.40) | <0.0001 | 0.0003 | <0.0001 | 0.2939 |
| 3D ratio (nplm <15 s) | 0.06 (0.04) | 0.02 (0.01) | 0.01 (0.01) | <0.0001 | 0.0001 | <0.0001 | 0.4783 |
| 3D extent (nplm <15 s) | 5.61 (4.64) | 2.30 (1.73) | 1.92 (2.56) | <0.0001 | <0.0001 | <0.0001 | 1.0000 |
| 3D intensity (nplm <15 s) | 0.18 (0.15) | 0.06 (0.05) | 0.06 (0.08) | <0.0001 | 0.0001 | <0.0001 | 1.0000 |
| Nonperiodic jerks 0.1–2 s | |||||||
| 3D index (nplm <2 s) | 35.67 (29.53) | 13.90 (16.25) | 9.97 (11.24) | <0.0001 | 0.0003 | <0.0001 | 0.6375 |
| 3D ratio (nplm <2 s) | 0.01 (0.01) | 0.01 (0.01) | 0.01 (0.01) | <0.0001 | 0.0001 | <0.0001 | 0.5405 |
| 3D extent (nplm <2 s) | 0.48 (0.42) | 0.16 (0.14) | 0.10 (0.10) | <0.0001 | <0.0001 | <0.0001 | 0.6907 |
| 3D intensity (nplm <2 s) | 0.02 (0.02) | 0.01 (0.01) | 0.01 (0.01) | <0.0001 | 0.0002 | <0.0001 | 0.6436 |
The data are summarized by median values and interquartile ranges with Bonferroni–Holm-corrected p-values from Kruskal–Wallis testing. Statistically significant differences between groups after Bonferroni-Holm correction are shown as bold values. iRBD, isolated REM sleep behavior disorder; REM, rapid eye movement; RWA, REM sleep without atonia; LM, leg movement.
*Sleep apnea (n = 11), PLMS (n = 4), sleep apnea with PLMS (n = 44), and other (n = 5).
**Bonferroni–Holm corrected.
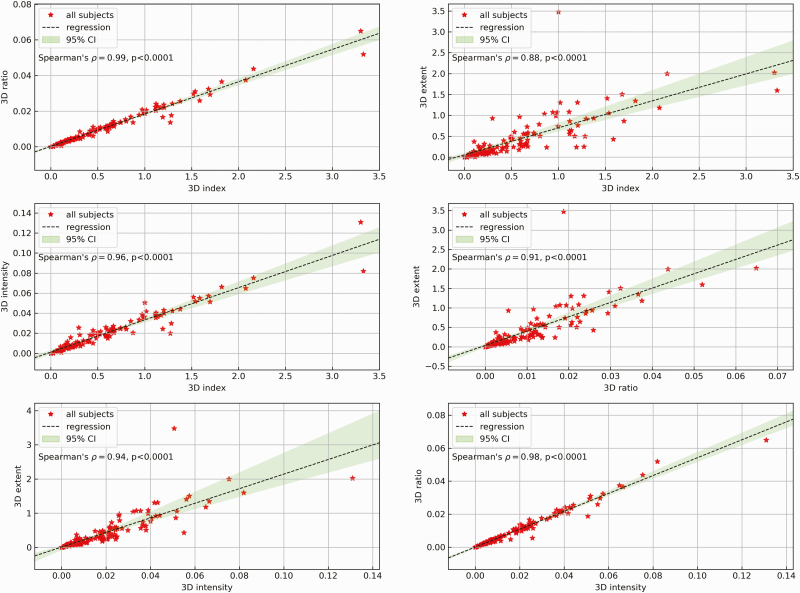
To evaluate dependencies between the different analyzed 3D features (index, ratio, extent, and intensity), we calculated Spearman correlations for all combinations, using any jerks (0.1–2 seconds) of all subjects as input data (Figure 5). The rho values are all above 0.88 and all p-values are <0.0001, indicating that all the analyzed features have a strong correlation with each other.
Figure 5.
Regression analysis of 3D features with Spearman’s ρ and p-values for (a) 3D ratio over 3D index, (b) 3D extent over 3D index, (c) 3D intensity over 3D index, (d) 3D extent over 3D ratio, (e) 3D extent over 3D intensity, and (f) 3D ratio over 3D intensity.
To determine optimal discriminative cutoff values for the individual 3D features, ROC curve analysis was used. Values are provided for discrimination between the three patient groups, as well as for discrimination of iRBD and prodromal RBD from other disorders with motor activity during sleep, and for iRBD versus prodromal RBD and other disorders with motor activity during sleep (Table 3). The highest accuracies were observed in discriminating iRBD from prodromal RBD, iRBD from other disorders with motor activity during sleep, and iRBD from the combination of prodromal RBD and other disorders with motor activity during sleep. The highest accuracy of 0.904 (sensitivity 0.975, specificity rate 0.859) for the 3D ratio of leg jerks allowed distinguishing between subjects with iRBD and other disorders with motor activity during sleep. Overall, leg jerks with duration less than 2 seconds better identified subjects with iRBD, and any leg movements were more accurate than only nonperiodic ones. Accordingly, the optimal cutoff values for identifying subjects with iRBD based on features of any jerks with duration less than 2 seconds are as follows: 33.56 jerks per hour of REM sleep (3D index), 0.01 or 1% of REM sleep spent moving (3D ratio), 0.48 active pixels per jerk during REM sleep (3D extent), and finally, an area under the motion signal of 0.017 per jerk and pixel during REM sleep (3D intensity).
Table 3.
Classification accuracy, sensitivity, and specificity of the individual 3D features as derived from ROC-AUC curve analysis
| Accuracy | Sensitivity | Specificity | |||||
|---|---|---|---|---|---|
| iRBD versus other | iRBD versus prodromal RBD | Prodromal RBD versus other | iRBD+prodromal RBD versus other | iRBD versus prodromal RBD+other | |
| Any LMs 0.1–15 s | |||||
| 3D index (any <15 s) | 0.894 | 0.825 | 0.938 | 0.845 | 0.900 | 0.722 | 0.793 | 0.167 | 0.969 | 0.811 | 0.845 | 0.781 | 0.877 | 0.825 | 0.902 |
| 3D ratio (any <15 s) | 0.894 | 0.900 | 0.891 | 0.828 | 0.900 | 0.667 | 0.805 | 0.167 | 0.984 | 0.811 | 0.724 | 0.891 | 0.869 | 0.700 | 0.951 |
| 3D extent (any <15 s) | 0.837 | 0.675 | 0.938 | 0.862 | 0.900 | 0.778 | 0.805 | 0.222 | 0.969 | 0.746 | 0.534 | 0.938 | 0.828 | 0.600 | 0.939 |
| 3D intensity (any <15 s) | 0.827 | 0.850 | 0.813 | 0.828 | 0.950 | 0.556 | 0.793 | 0.167 | 0.969 | 0.754 | 0.690 | 0.813 | 0.803 | 0.575 | 0.915 |
| Any jerks 0.1–2 s | |||||
| 3D index (any <2 s) | 0.885 | 0.925 | 0.859 | 0.879 | 0.975 | 0.667 | 0.793 | 0.056 | 1.000 | 0.811 | 0.810 | 0.813 | 0.861 | 0.850 | 0.866 |
| 3D ratio (any <2 s) | 0.904 | 0.975 | 0.859 | 0.897 | 0.950 | 0.778 | 0.805 | 0.111 | 1.000 | 0.820 | 0.776 | 0.859 | 0.877 | 0.950 | 0.841 |
| 3D extent (any <2 s) | 0.894 | 0.800 | 0.953 | 0.879 | 0.850 | 0.944 | 0.780 | 0.000 | 1.000 | 0.820 | 0.845 | 0.797 | 0.902 | 0.800 | 0.951 |
| 3D intensity (any <2 s) | 0.885 | 0.900 | 0.875 | 0.897 | 0.975 | 0.722 | 0.793 | 0.056 | 1.000 | 0.795 | 0.707 | 0.875 | 0.861 | 0.875 | 0.854 |
| Nonperiodic LMs 0.1–15 s | |||||
| 3D index (nplm <15 s) | 0.865 | 0.850 | 0.875 | 0.828 | 0.925 | 0.611 | 0.793 | 0.056 | 1.000 | 0.787 | 0.776 | 0.797 | 0.844 | 0.675 | 0.927 |
| 3D ratio (nplm <15 s) | 0.875 | 0.825 | 0.906 | 0.845 | 0.875 | 0.778 | 0.793 | 0.056 | 1.000 | 0.779 | 0.638 | 0.906 | 0.861 | 0.825 | 0.878 |
| 3D extent (nplm <15 s) | 0.827 | 0.750 | 0.875 | 0.862 | 0.875 | 0.833 | 0.780 | 0.000 | 1.000 | 0.721 | 0.552 | 0.875 | 0.836 | 0.750 | 0.878 |
| 3D intensity (nplm <15 s) | 0.827 | 0.750 | 0.875 | 0.828 | 0.850 | 0.778 | 0.793 | 0.111 | 0.984 | 0.730 | 0.569 | 0.875 | 0.828 | 0.750 | 0.866 |
| Nonperiodic jerks 00.1–2 s | |||||
| 3D index (nplm <2 s) | 0.846 | 0.825 | 0.859 | 0.845 | 0.925 | 0.667 | 0.805 | 0.111 | 1.000 | 0.770 | 0.672 | 0.859 | 0.836 | 0.750 | 0.878 |
| 3D ratio (nplm <2 s) | 0.856 | 0.800 | 0.891 | 0.845 | 0.925 | 0.667 | 0.805 | 0.111 | 1.000 | 0.770 | 0.828 | 0.719 | 0.844 | 0.700 | 0.915 |
| 3D extent (nplm <2 s) | 0.885 | 0.900 | 0.875 | 0.862 | 0.825 | 0.944 | 0.780 | 0.000 | 1.000 | 0.803 | 0.741 | 0.859 | 0.877 | 0.800 | 0.915 |
| 3D intensity (nplm <2 s) | 0.837 | 0.775 | 0.875 | 0.845 | 0.875 | 0.778 | 0.805 | 0.111 | 1.000 | 0.762 | 0.845 | 0.688 | 0.828 | 0.600 | 0.939 |
iRBD, isolated REM sleep behavior disorder; REM, rapid eye movement; RWA, REM sleep without atonia; LM, leg movement; other, other disorders with motor activity during sleep.
Discussion
IRBD is widely recognized as an early-stage α-synucleinopathy [3], and a precursory state of RBD (prodromal RBD) has been described, where symptoms and signs are presents but do not yet fulfill diagnostic criteria for RBD [3–5]. An accurate diagnosis of iRBD or even prodromal RBD and their distinction from other disorders with motor manifestations during sleep is critical for identifying α-synucleinopathy in a very early stage [3]. Diagnosis of RBD requires vPSG, which is time consuming and not feasible as screening method. Moreover, minor jerks are often difficult to spot in visual video analysis, as they might be barely visible for the human eye or e.g. hidden by blanket. Therefore, we evaluated the usefulness of automated 3D video analysis of leg movements during REM sleep for identifying iRBD. Rate, duration, extent, and intensity of leg movements (particularly of those with a duration lower than 2 seconds) during REM sleep showed high accuracy rates.
These results are consistent with previous studies showing that the majority of RBD-associated REM events are minor distal jerks [26–29]. We observed significantly higher accuracies when using any (i.e. both periodic and nonperiodic) leg movements than when using only nonperiodic leg movements. This is particularly interesting considering that 48 of 64 (75%) subjects with other disorders with motor activity during sleep exhibited PLMS. However, PLMS are also common in RBD, particularly during REM sleep [19, 32]. The fact that PLMS during REM sleep are seldom if physiological REM sleep atonia control is preserved can explain why both periodic and nonperiodic automatic detected leg movements are more frequent in RBD than in subjects with other disorders with motor activity during sleep.
Our data did not show any significant differences in the leg movement activity of subjects with prodromal RBD when compared with those with other disorders with motor activity during sleep. Most of these subjects were classified as prodromal RBD due to increased EMG activity, and mainly few visible movements were present. As the automatic 3D analysis is based on detectable movements, this might explain while prodromal RBD was not identified with the studied automatic detection software. To better investigate this aspect, the potential application of 3D analysis in prodromal RBD should be evaluated in subjects with motor events in video exceeding proposed cutoffs, independently from RWA values. Another possible explanation is that the majority of visible RBD events might be limited to the upper body region (not taken into account in the present study), as it is known that in RBD movements often involve the upper extremities [3, 19, 33–35].
Automatically detected leg movements using 3D video analysis correlated with both RWA and the number of REM events per hour as manually determined through video analysis, confirming the potential and reliability of this method. In some subjects, however, we observed a low number of REM events or low RWA score and high 3D movement index, or vice versa. The former could be attributed to movements that are hardly visible to human eyes in video or occurring in other muscle groups than the ones recorded by EMG, while the latter may be due to motor activity in body regions other than the legs, e.g. in the upper extremities.
With the aim of having a representative sample of subjects with motor activity during sleep, we included subjects with iRBD, prodromal RBD, and other sleep disorders known to go along with motor activities during sleep. The three groups were recruited consecutively without age-matching, and participants with iRBD were significantly older than the other two groups. Since we compared different sleep disorders with different age prevalence, we do not consider this as a major limitation. The iRBD group also had a lower median apnea–hypopnea index, probably due to higher PAP therapy rate, than subjects with prodromal RBD. However, a bias due to respiration is unlikely as a recent study of iRBD with and without obstructive sleep apnea showed no effect of respiration on the diagnostic value of REM sleep motor activity [36]. Moreover, subjects with iRBD had a higher antidepressant therapy rate than subjects with other sleep disorders, probably due to the well-known frequent comorbidity of iRBD and depression and to the fact that depression represents a premotor symptom of Parkinson’s disease. A causal relationship between antidepressant therapy and RBD onset was excluded.
A limitation of the presented 3D method is the restriction of the analysis to the stage of REM sleep and the consequential necessity of EEG for sleep scoring. We analyzed the 3D leg movement features also for the remaining sleep stages, both individually and altogether as non-REM sleep. Only few significant differences with low classification accuracies were found between the groups (data not shown). Incorporating the upper body and respiration may add additional information for REM and non-REM identification without vPSG support. This should be investigated in future studies, as well as applicability of this technology in the home environment.
In conclusion, our study has shown that automated 3D video analysis of leg movements during REM sleep is a promising diagnostic tool to identify subjects with iRBD in a sleep laboratory population and to distinguish them from subjects with other types of motor activity during sleep. The movement rate, duration, extent, and intensity during REM sleep were all significantly increased in subjects with iRBD when compared with prodromal RBD and other sleep disorders. Especially automatically detected leg jerks with a duration lower than 2 seconds emerged as reliable indicator of iRBD with high accuracy. The fact that no significant differences were found between subjects with prodromal RBD and those with other sleep disorders suggests that either most motor activity in this prodromal stage is limited to increased EMG activity without visible movements or that movements are present but limited to other body regions than the legs (e.g. the upper extremities). Further research needs to include upper body and head regions to examine this aspect more closely and to confirm the potential of 3D video analysis as diagnostic tool for iRBD.
Funding
This study was funded by the Austrian Science Fund (FWF), Project KLI 677-B31.
Conflict of interest statement. None declared.
References
- 1. American Academy of Sleep Medicine. The International Classification of Sleep Disorders: Diagnostic and Coding Manual. 3rd ed. rev. Darien, IL: American Academy of Sleep Medicine; 2014. [Google Scholar]
- 2. Schenck CH, et al. REM sleep behavior disorder: clinical, developmental, and neuroscience perspectives 16 years after its formal identification in SLEEP. Sleep. 2002;25(2):120–138. [DOI] [PubMed] [Google Scholar]
- 3. Högl B, et al. Idiopathic REM sleep behaviour disorder and neurodegeneration – an update. Nat Rev Neurol. 2018;14(1):40–55. [DOI] [PubMed] [Google Scholar]
- 4. Sixel-Döring F, et al. The evolution of REM sleep behavior disorder in early Parkinson disease. Sleep. 2016;39(9):1737–1742. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Schenck CH, et al. Subclinical REM sleep behavior disorder and its clinical and research implications. Sleep. 2008;31(12):1627. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Stefani A, et al. Long-term follow-up investigation of isolated rapid eye movement sleep without atonia without rapid eye movement sleep behavior disorder: a pilot study. J Clin Sleep Med. 2015;11(11):1273–1279. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Ferri R, et al. REM sleep without atonia with REM sleep-related motor events: broadening the spectrum of REM sleep behavior disorder. Sleep. 2018;41(12). doi: 10.1093/sleep/zsy187 [DOI] [PubMed] [Google Scholar]
- 8. Dijkstra F, et al. REM sleep without atonia and the relation with Lewy body disease. Parkinsonism Relat Disord. 2019;67:90–98. [DOI] [PubMed] [Google Scholar]
- 9. Dede HÖ, et al. Rapid eye movement sleep without atonia constitutes increased risk for neurodegenerative disorders. Acta Neurol Scand. 2019;140(6):399–404. [DOI] [PubMed] [Google Scholar]
- 10. Frauscher B, et al. Validation of an integrated software for the detection of rapid eye movement sleep behavior disorder. Sleep. 2014;37(10):1663–1671. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Cesari M, et al. Comparison of computerized methods for rapid eye movement sleep without atonia detection. Sleep. 2018;41(10). doi: 10.1093/sleep/zsy133 [DOI] [PubMed] [Google Scholar]
- 12. Frandsen R, et al. Analysis of automated quantification of motor activity in REM sleep behaviour disorder. J Sleep Res. 2015;24(5):583–590. [DOI] [PubMed] [Google Scholar]
- 13. Ferri R, et al. A quantitative statistical analysis of the submentalis muscle EMG amplitude during sleep in normal controls and patients with REM sleep behavior disorder. J Sleep Res. 2008;17(1):89–100. [DOI] [PubMed] [Google Scholar]
- 14. Garn H, et al. 3D detection of the central sleep apnoea syndrome. Curr Dir Biomed Eng. 2017;3:829–833. [Google Scholar]
- 15. Falie D. Sleep monitoring and sleep apnea event detection using a 3D camera. Int Conf Commun. 2010;177–180. [Google Scholar]
- 16. Falie D, et al. Statistical algorithm for detection and screening sleep apnea. Int Symp Signals, Circuits Syst. 2009;1–4. [Google Scholar]
- 17. Garn H, et al. 3D detection of periodic limb movements in sleep. Conf Proc IEEE Eng Med Biol Soc. 2016;2016:427–430. [DOI] [PubMed] [Google Scholar]
- 18. Kohli P, et al. Developments in Human Pose Estimation for Kinect. Consumer Depth Cameras for Computer Vision. Springer, London, UK; 2013. [Google Scholar]
- 19. Frauscher B, et al. ; SINBAR (Sleep Innsbruck Barcelona) Group Quantification of electromyographic activity during REM sleep in multiple muscles in REM sleep behavior disorder. Sleep. 2008;31(5):724–731. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Frauscher B, et al. ; SINBAR (Sleep Innsbruck Barcelona) Group Normative EMG values during REM sleep for the diagnosis of REM sleep behavior disorder. Sleep. 2012;35(6):835–847. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Stefani A, et al. A prospective video-polysomnographic analysis of movements during physiological sleep in 100 healthy sleepers. Sleep. 2015;38(9):1479–1487. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Allen RP, et al. ; International Restless Legs Syndrome Study Group Restless legs syndrome/Willis-Ekbom disease diagnostic criteria: updated International Restless Legs Syndrome Study Group (IRLSSG) consensus criteria–history, rationale, description, and significance. Sleep Med. 2014;15(8):860–873. [DOI] [PubMed] [Google Scholar]
- 23. Berry RB, et al. The AASM Manual for the Scoring of Sleep and Associated Events: Rules, Terminology and Technical Specifications. Darien, IL: American Academy of Sleep Medicine; 2018. [Google Scholar]
- 24. Gabryelska A, et al. Prevalence of obstructive sleep apnoea in REM behaviour disorder: response to continuous positive airway pressure therapy. Sleep Breath. 2018;22(3):825–830. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Stefani A, et al. Validation of a leg movements count and periodic leg movements analysis in a custom polysomnography system. BMC Neurol. 2017;17(1):42. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Frauscher B, et al. Video analysis of motor events in REM sleep behavior disorder. Mov Disord. 2007;22(10):1464–1470. [DOI] [PubMed] [Google Scholar]
- 27. Frauscher B, et al. The relation between abnormal behaviors and REM sleep microstructure in patients with REM sleep behavior disorder. Sleep Med. 2009;10(2):174–181. [DOI] [PubMed] [Google Scholar]
- 28. Manni R, et al. Motor-behavioral episodes in REM sleep behavior disorder and phasic events during REM sleep. Sleep. 2009;32(2):241–245. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Frauscher B, et al. Quality control for diagnosis of REM sleep behavior disorder: criteria, questionnaires, video, and polysomnography. In Videnovic A, Högl B, eds. Disorders of Sleep and Circadian Rhythms in Parkinson’s Disease. Wien, Austria: Springer; 2015: 145–157. [Google Scholar]
- 30. Ferri R, et al. ; International and European Restless Legs Syndrome Study Groups (IRLSSG and EURLSSG) World Association of Sleep Medicine (WASM) 2016 standards for recording and scoring leg movements in polysomnograms developed by a joint task force from the International and the European Restless Legs Syndrome Study Groups (IRLSSG and EURLSSG). Sleep Med. 2016;26:86–95. [DOI] [PubMed] [Google Scholar]
- 31. Holm S, A simple sequentially rejective multiple test procedure. Scand J Stat. 1979;6:65–70. [Google Scholar]
- 32. Fantini ML, et al. Periodic leg movements in REM sleep behavior disorder and related autonomic and EEG activation. Neurology. 2002;59(12):1889–1894. [DOI] [PubMed] [Google Scholar]
- 33. Frauscher B, et al. Defining muscle activities for assessment of rapid eye movement sleep behavior disorder: from a qualitative to a quantitative diagnostic level. Sleep Med. 2013;14(8):729–733. [DOI] [PubMed] [Google Scholar]
- 34. Iranzo A, et al. ; SINBAR (Sleep Innsbruck Barcelona) Group Usefulness of the SINBAR electromyographic montage to detect the motor and vocal manifestations occurring in REM sleep behavior disorder. Sleep Med. 2011;12(3):284–288. [DOI] [PubMed] [Google Scholar]
- 35. Fernández-Arcos A, et al. Diagnostic value of isolated mentalis versus mentalis plus upper limb electromyography in idiopathic REM sleep behavior disorder patients eventually developing a neurodegenerative syndrome. Sleep. 2017;40(4). doi: 10.1093/sleep/zsx025 [DOI] [PubMed] [Google Scholar]
- 36. McCarter SJ, et al. Diagnostic REM sleep muscle activity thresholds in patients with idiopathic REM sleep behavior disorder with and without obstructive sleep apnea. Sleep Med. 2017;33:23–29. [DOI] [PMC free article] [PubMed] [Google Scholar]