Abstract
Background
Combined immune checkpoint inhibitor (ICI) treatment targeting PD-1 and CTLA-4 was suggested to yield clinical benefit over chemotherapy in malignant pleural mesothelioma (MPM), whereas aPD-1 monotherapy failed to provide benefit in phase-III trials. Success of ICI depends on the presence and activation of tumor-specific T cells. Therefore, we investigated whether T-cell characteristics are underlying clinical efficacy of ICI treatment in MPM.
Methods
Comprehensive immune cell profiling was performed on screening and on treatment peripheral blood samples of mesothelioma patients treated with nivolumab (aPD-1) monotherapy (NCT02497508), or a combination of nivolumab and ipilimumab (aCTLA-4) (NCT03048474).
Findings
aPD-1/aCTLA-4 combination treatment induced a profound increase in proliferation and activation of T cells, which was not observed upon aPD-1 monotherapy. Moreover, patients that responded to combination treatment had low frequencies of naive CD8 T cells and high frequencies of effector memory CD8 T cells that re-expressed RA (TEMRA) at screening. The frequency of Granzyme-B and Interferon-γ producing TEMRAs was also higher in responding patients.
Interpretation
High proportions of TEMRAs and cytokine production by TEMRAs before treatment, was associated with a better clinical outcome. TEMRAs, which likely comprise tumor-specific T cells, tend to require blockage of both aPD-1 and aCTLA-4 to be reactivated. In conclusion, peripheral blood TEMRAs can play a key role in explaining and predicting clinical benefit upon aPD-1/aCTLA-4 combination treatment.
Funding
Bristol-Myers Squibb sponsored NivoMes and INITIATE clinical trials and provided study drugs. No external funding was applicable for the flow cytometric analyses of peripheral blood samples described in this manuscript.
Keywords: Malignant pleural mesothelioma, Immune checkpoint inhibitors, Immunotherapy, Immune monitoring, Nivolumab, Ipilimumab
Research in context.
Evidence before this study
Immune monitoring, the assessment of peripheral blood immune cell subsets, yielded valuable insight into peripheral blood T-cell responses to immune checkpoint inhibitors (ICI) in non-small cell lung cancer (NSCLC) and melanoma patients. We searched Pubmed for scientific literature published between Jan 1st 2010 and June 15th 2020 with the following terms: “mesothelioma” AND (“PD-1” OR “PD-L1” OR “CTLA-4” OR “checkpoint”) AND (“peripheral blood” OR “immune monitoring”). No previous studies have assessed the peripheral blood immune cell compartment upon ICI treatment in malignant pleural mesothelioma (MPM).
Added value of this study
To our knowledge, we are the first to perform extensive immune monitoring in MPM patients treated with both aPD-1 monotherapy and aPD-1/aCTLA4 combination therapy. Recently, promising results of Checkmate-743 (NCT02899299) demonstrated that treatment of MPM patients with nivolumab and ipilimumab yielded a statistically significant and clinically meaningful improvement in overall survival, compared to platinum-based chemotherapy plus pemetrexed. These results are in contrast to the lack of benefit seen earlier in the PROMISE-meso trial (NCT02991482) that investigated nivolumab monotherapy as compared to chemotherapy in MPM. We here provide a rationale for the benefit observed upon aPD-1/aCTLA-4 combination treatment in MPM by indicating differences in the peripheral blood T-cell compartment in two phase II clinical trials that assessed aPD-1 monotherapy and aPD-1/aCTLA4 combination therapy.
Implications of all the available evidence
Combination checkpoint inhibition appears to be more effective than their use alone in MPM, which was already shown in the MAPS2 phase II randomized trial. Preliminary results of the Checkmate-743 support this statement. These findings, combined with our peripheral blood analyses, warrant further research into aPD-1/aCTLA-4 combination in MPM with in-depth peripheral blood and intratumoral T-cell characterization.
Alt-text: Unlabelled box
1. Introduction
Malignant pleural mesothelioma (MPM) is a malignancy arising from the mesothelial cells in the pleural cavity, primarily caused by asbestos exposure. Treatment options for MPM are very limited, as platinum-based chemotherapy combined with an antifolate and the optional addition of bevacizumab, are the only approved first-line treatment for MPM. This treatment leads to a median overall survival (OS) of 12–16 months [1,2]. Currently, no registered second-line treatments are available, illustrating the urgent need for new treatment options.
Immunotherapies aim for activation of the immune system, leading to efficient tumor-specific immune responses. In current clinical practice, these therapies include monoclonal antibodies that block inhibitory checkpoint receptors, i.e. programmed death 1 (PD-1), programmed death ligand 1 (PD-L1) and cytotoxic T lymphocyte associated antigen 4 (CTLA-4), thereby reinvigorating anti-tumor immune responses [3]. So-called immune checkpoint inhibitor (ICI) treatments have transformed the treatment landscape for various malignancies, such as non-small cell lung cancer (NSCLC) and melanoma [4,5].
Unfortunately, ICI treatments are less effective in MPM as compared to other malignancies. The DETERMINE trial showed no survival benefit of ipilimumab (anti-CTLA-4, aCTLA-4) monotherapy over placebo [6] and pembrolizumab and nivolumab, both anti-PD-1 (aPD-1) monotherapies, demonstrated objective response rates (ORR) of 21% and 26% in the KEYNOTE-028 and NivoMes trials respectively [7,8]. Recently, the PROMISE-meso phase III randomized trial (NCT02991482) failed to show improvement in PFS (progression-free survival) and OS upon second line aPD-1 treatment (pembrolizumab), as compared to single agent chemotherapy (institutional choice of gemcitabine or vinorelbine) [9]. The lack of effective ICI treatment in MPM is thought to be dependent on the small number of tumor-infiltrating lymphocytes (TILs) in MPM [10,11] and the immunosuppressive tumor microenvironment [12,13].
Combining aPD-1 and aCTLA-4 therapy has been shown to induce synergistic effects in both preclinical and clinical studies [14,15]. Phase II trials in MPM also suggest improved clinical responses upon combination ICI treatment, as the MAPS2 trial (nivolumab plus ipilimumab), the NIBIT-MESO trial (durvalumab (aPD-L1) plus tremelimumab (aCTLA-4)) and the INITIATE trial (nivolumab plus ipilimumab) reported better clinical responses upon combination ICI treatment than reported by trials that investigated monotherapy (nivolumab or pembrolizumab) [16], [17], [18]. Recently, the first positive results were announced for the Checkmate-743 [19], a phase III trial that combined aPD-1 (nivolumab) with aCTLA-4 (ipilimumab) treatment in previously untreated MPM patients. These results are very promising, although the magnitude of the benefit is still awaited.
Success of aPD-1 treatment in NSCLC and melanoma is thought to depend on pre-existing T-cell infiltration of the tumor [20], proliferation of peripheral PD-1-expressing CD8 T cells [21] and the ratio between T-cell reinvigoration and tumor burden [22]. It remains unclear whether the enhanced efficacy observed in ICI combination treatment trials is due to an additive effect of the respective therapies or truly depends on a novel immunological mechanism that is engaged by targeting both PD-1 and CTLA-4 [23].
In order to dissect the immunological mechanisms responsible for the clinical benefit from aPD-1 and aCTLA-4 therapy in MPM, we aimed to investigate the characteristics of lymphocytes present in peripheral blood of MPM patients treated with aPD-1 monotherapy (nivolumab) in the NivoMes trial [8] and aPD-1 and aCTLA-4 combination therapy (nivolumab/ipilimumab) in the INITIATE trial [16]. We specifically aimed to evaluate the T- and NK-cell compartment of the peripheral blood, since prior studies established the value of this compartment in the context of aPD-1 and aCTLA-4 treatment [21,22,24].
2. Methods
2.1. Study population
Patients in this study were enrolled in either the NivoMes study (NCT02497508) or the INITIATE study (NCT03048474). Both studies were approved by the institutional review board of the Netherlands Cancer Institute and in accordance with the Declaration of Helsinki. All patients provided written informed consent before enrolment. Collection and analysis of immune cell subsets in peripheral blood were planned a priori as part of the two trials. Clinical results of the NivoMes and INITIATE were previously published [8,16]. In summary, in the NivoMes trial, 34 MPM patients progressing after at least one cycle of platinum based chemotherapy, were treated with nivolumab 3 mg/kg every 2 weeks. In the INITIATE trial, 35 MPM patients progressing after at least one cycle of platinum based chemotherapy were treated with nivolumab (240 mg flat dose every 2 weeks) plus ipilimumab (1 mg/kg every 6 weeks up to four times). Peripheral blood was collected from patients on the day of the first ICI treatment and after six weeks of treatment. These samples correspond to the ‘screening’ and ‘on treatment’ time points. Response to treatment was assessed according to modified RECIST criteria for mesothelioma [25]. For comparison purposes, we decided to define responding patients as having a complete response (CR), partial response (PR) or stable disease (SD) at six months of follow up and non-responding patients as having progressive disease (PD) at six months of follow up. All patients in the ‘responder’ group experienced a PFS of six months or longer and all patients in in the ‘non-responder’ group progressed within six months.
2.2. Processing of peripheral blood
Fifty milliliters of blood was drawn at screening and on treatment time points in EDTA tubes and processed. Peripheral blood mononuclear cells (PBMC) were isolated via standard density-gradient centrifugation using Ficoll-Hypaque (GE Healthcare, Chicago, IL, USA). Cells were cryopreserved in 10% dimethylsulfoxide (Sigma-Aldrich, Saint Louis, MO, USA), 40% FCS (Gibco, ThermoFisher, Waltham, MA, USA) and RPMI (Invitrogen, ThermoFisher, Waltham, MA, USA) until further use.
2.3. Flow cytometry
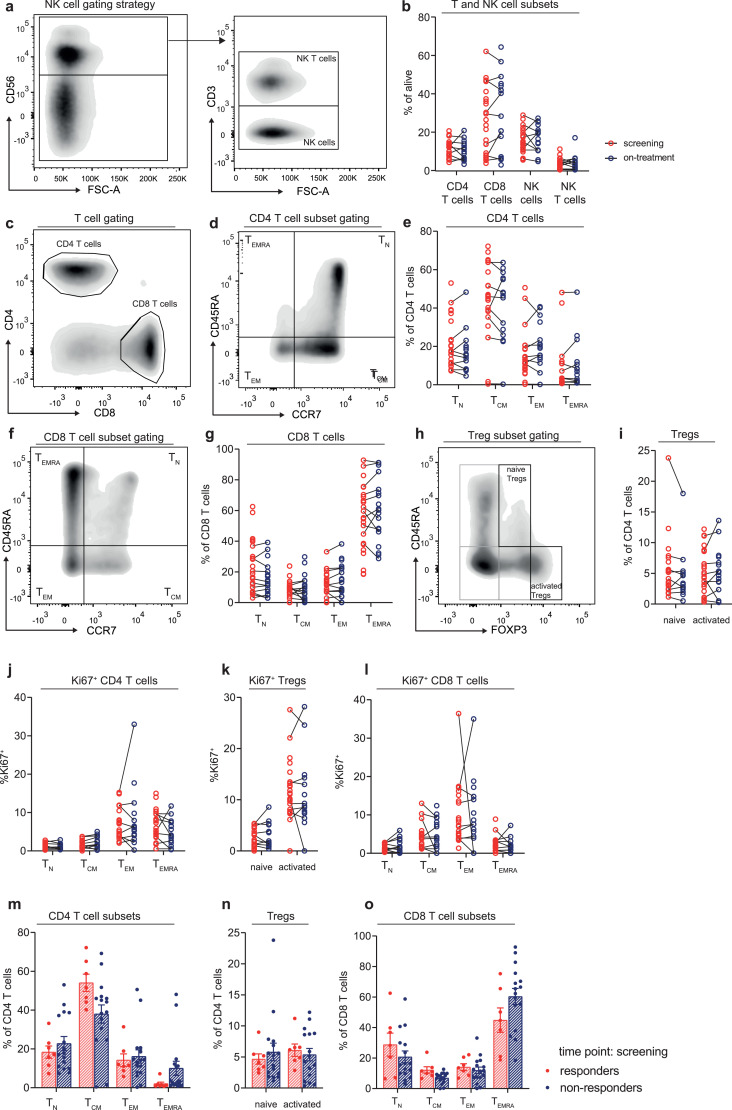
Flow cytometry staining was performed on the cryopreserved PBMC samples. After thawing of the PBMCs, cells were stimulated for 4 hours with phorbol 12-myristate 13-acetate and ionomycin (both from Sigma-Aldrich, Saint Louis, MO, USA) and GolgiStop (BD Biosciences, Franklin Lakes, NJ, USA), prior to continuation of the cytokine staining. Supplementary table 1 lists the antibodies used for the different stainings. First, extracellular markers were stained for 30 min at 4 °C. Secondly, the cells were stained with LIVE/DEAD Fixable Aqua Dead Cell Stain Kit (Invitrogen, ThermoFisher, Waltham, MA, USA) for 10 min at 4 °C in order to identify dead cells. Next, FoxP3 transcription factor fixation/permeabilisation mix (eBioscience, ThermoFisher, Waltham, MA, USA) was used to fixate the cells. Subsequently, intracellular markers were stained for 60 min at 4 °C. Data were acquired using an LSR II flow cytometer equipped with three lasers. We used FlowJo v10 (BD Biosciences, Franklin Lakes, NJ, USA) to analyze the data. Fig. 1A, C, D, F and H show the gating strategy. Specific maturation subsets of T cells were identified by the cell surface markers CD45RA and CCR7. Fractions of CD45RA+CCR7+ naive (N) T cells, CD45RA−CCR7+ central memory (CM) T cells, CD45RA−CCR7− effector memory (EM) T cells and CD45RA+CCR7− effector memory re-expressing RA (EMRA) T cells were identified in both the CD4 and CD8 T-cell compartments.
Fig. 1.
T- and NK-cell characteristics before and during aPD-1 monotherapy
(a, c, d, f, h) Gating strategy for NK-cells (a), T-cells (c), CD4 T-cells subsets (d), CD8 T-cells subsets (f) and Treg subsets (h) respectively. (b, e, g, i) Percentage of T-and NK-cell subsets (b), CD4 T-cell subsets (e), CD8 T-cells subsets (g) and Treg subsets (I) respectively, at screening and on-treatment time points. (j, k, l) Percentage of Ki67+ CD 4 T-cell subsets (j), Tregs subsets (k) and CD8 T-cell subsets (l) respectively, at screening and on-treatment time points. (m, n, o). Paired samples are shown connected by black lines. Percentage of CD4 T-cell subsets (m), Treg subsets (n) and CD8 T-cell subsets (o) respectively, at the screening time point in responding and non-responding patients. Bars depict mean values with standard error of the mean.
2.4. Statistical analysis
Statistical analyses were performed in R version 4.0.2 and GraphPad V8.0 (GraphPad, San Diego, CA, USA). P < 0.05 was considered statistically significant. Significant differences between the groups were determined with Mann–Whitney U tests (non-parametric, non-paired data) and Wilcoxon signed rank tests (non-parametric, paired data). P values were corrected for multiple testing, using the Benjamini and Hochberg False Discovery Rate [26]. Log rank test was used to compare Kaplan-Meier curves for PFS and OS. To stratify PFS and OS for proportions of T-cell subsets, the median was used as a cut off for high vs low proportions.
2.5. Role of funding sources
Bristol-Myers Squibb sponsored the clinical studies and provided the study drugs in both the NivoMes and INITIATE clinical trials. The analyses of peripheral blood mononuclear cells (PBMCs) by flow cytometry, described in this manuscript, were not sponsored by any external funding.
3. Results
3.1. Patient characteristics
Table 1 demonstrates the numbers of peripheral blood samples available from the two clinical trials. Baseline characteristics are shown for the patients of whom PBMCs were collected at screening and at least 1 CT-scan for response evaluation was available.
Table 1.
Characteristics of patients included in translational analysis.
| NivoMes | INITIATE | ||
|---|---|---|---|
| Patients screened | 38 | 38 | |
| Included, received at least 1 cycle of treatment | 34 | 35 | |
| At least 1 CT for response evaluation available | 33 | 34 | |
| At least 1 PBMC sample for FCM available at screening or on-treatment time point | 31 | 38 | |
| PBMC sample at screening time point available | 24 | 38 | |
| PBMC sample at screening time point and response evaluation available | 23 | 32 | |
| Baseline characteristics | |||
| n | 23 | 32 | |
| Age (years) (range) | 67 (62–73) | 65 (62–72) | |
| Gender (%) | Male | 19 (17.4%) | 24 (75%) |
| Female | 4 (82.6%) | 8 (25%) | |
| Histological subtype (%) | Epithelioid | 21 (91.3%) | 28 (87.5%) |
| Sarcomatoid | 2 (8.7%) | 2 (6.2%) | |
| Mixed | 0 (0%) | 2 (6.2%) | |
| WHO (%) | 0 | 10 (43.5%) | 11 (34.4%) |
| 1-2 | 13 (56.5%) | 21 (65.6%) | |
| 6 months response (%) | CR | 0 (0%) | 0 (0%) |
| PR | 6 (26.1%) | 12 (37.5%) | |
| Epithelioid | 6 (100%) | 11 (91.7%) | |
| Sarcomatoid | 0 (0%) | 1 (8.3%) | |
| Mixed | 0 (0%) | 0 (0%) | |
| SD | 1 (4.3%) | 4 (12.5%) | |
| Epithelioid | 1 (100%) | 2 (50%) | |
| Sarcomatoid | 0 (0%) | 1 (25%) | |
| Mixed | 0 (0%) | 1 (25%) | |
| PD | 16 (69.6%) | 16 (50%) | |
| Epithelioid | 14 (87.5%) | 15 (93.8%) | |
| Sarcomatoid | 2 (12.5%) | 0 (0%) | |
| Mixed | 0 (0%) | 1 (6.2%) | |
| PFS (months) (95% CI) | 2.44 (1.3–10.0) | 6.25 (4.1–11.0) | |
| OS (months) (95% CI) | 11.5 (5.1–21.6) | 23.0 (12.5-not reached) | |
3.2. Monotherapy with aPD-1 treatment does not induce T-cell proliferation
In both NSCLC and melanoma, it was shown that aPD-1 treatment increased proliferation of CD8 T cells in peripheral blood, and the majority of these proliferating CD8 T cells were PD-1 positive [21,22]. We therefore analyzed whether aPD-1 monotherapy induced similar changes in T- or NK cell subsets of MPM patients. No significant differences were observed in the frequencies of T cells (Fig. 1B), T-cell subsets (Fig. 1E, G, I), NK cells and NK T cells (Fig. 1B) between screening and 6 weeks after start of treatment. Surprisingly, aPD-1 monotherapy also induced no increase in proliferation of T-cell subsets, as assessed by Ki-67 expression, a cell cycle marker expressed by cycling or recently divided cells (Fig. 1J–L).
Next, we examined whether differences in the frequencies and phenotype of T cells prior to treatment, could help identify patients that responded to aPD-1 monotherapy. We found that MPM patients with a response upon aPD-1 had slightly higher frequency of CM CD4 T cells, whereas all other T-cell frequencies were similar between responding and non-responding MPM patients (Fig. 1M–O). No changes were found in the proportions of proliferating T- and NK cells, assessed by Ki67 expression (data not shown).
Furthermore, no changes in the frequencies of PD-1, CD28, 4-1BB, HLA-DR, inducible T-cell costimulator (ICOS), CD39, lymphocyte-activation gene 3 (LAG-3), T-cell immunoglobulin and mucin-domain containing-3 (TIM-3) and CTLA-4 expressing T-cell subsets induced by aPD-1 treatment or between responding and non-responding patients were observed (data not shown).
In conclusion, aPD-1 treatment did not induce changes in the proportion and proliferation of T-cell and NK cell subsets in MPM patients. No major differences were found between responding and non-responding patients prior to treatment.
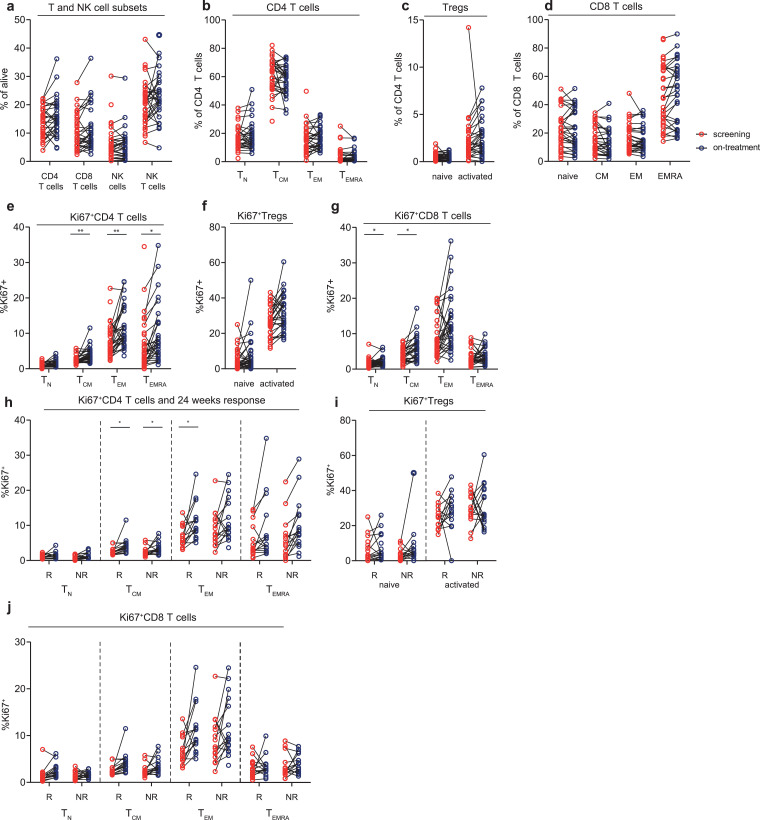
3.3. aPD-1 and aCTLA-4 combination therapy promotes proliferation of memory T-cell subsets
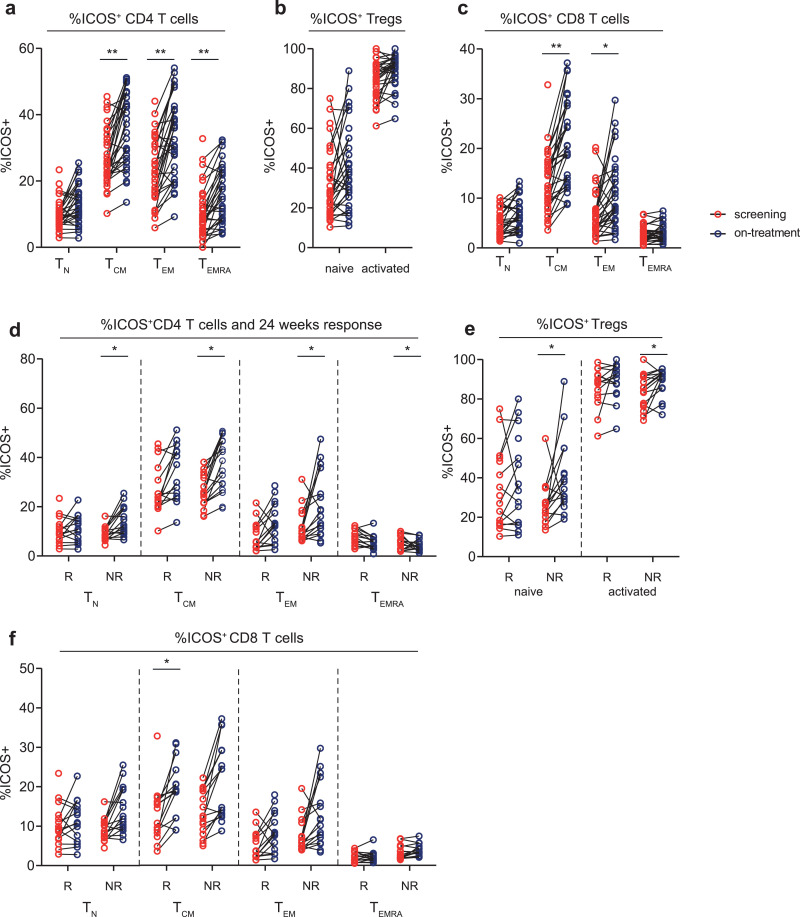
Secondly, we examined whether aPD-1 and aCTLA-4 combination treatment induced proliferation and activation of T cells. We found that combination treatment increased the proliferation of CM, EM and EMRA CD4 T-cells and in naive and CM CD8 T-cells (Fig. 2E–G). This increase in proliferation was independent of clinical response (Fig. 2 H–J). Furthermore, the frequency of CM, EM and EMRA CD4 T-cell subsets, and CM and EM CD8 T cells that expressed ICOS increased upon combination therapy, indicating that combination therapy induced T-cell activation (Fig. 3A–C). In the CD4 T-cell compartment, this activation was most prominent in non-responding patients (Fig. 3D). Combination treatment did not induce differences in the frequency of the activation and inhibitory markers CD28, 4-1BB, HLA-DR, PD-1, LAG-3, TIM-3, CD39 and CTLA-4 in both CD4 and CD8 T-cell subsets (data not shown).
Fig. 2.
T- and NK-cell characteristics before and during aPD-1/CTLA-4 combination therapy
(a, b, c, d) Percentage of T-and NK-cell subsets (a), CD4 T-cell subsets (b), Treg subsets (c) and CD8 T-cells subsets (d) respectively, at screening and on-treatment time points. (e, f, g) Percentage of Ki67+ CD 4 T-cell subsets, (TCM p = 0.003, TEM p = 0.007, TEMRA p = 0.028) (e), Tregs subsets (f) and CD8 T-cell subsets (TN p = 0.036, TCM p = 0.03,) (g) respectively, at screening and on-treatment time points. (h, i, j) Comparison between responding (R) and non-responding (NR) patients for the percentage of Ki67+ CD 4 T-cell subsets (TCM R p = 0.01, TCM NR p = 0.04, TEM R p = 0.01) (h), Tregs subsets (i) and CD8 T-cell subsets (j) respectively, at screening and on-treatment time points. Paired samples are shown connected by black lines in each graph. Significance (Wilcoxon signed-rank test for paired analysis of screening and on-treatment samples and Mann–Whitney U test for comparison of response groups) is shown in each graph, with * p < 0.05 and ** p < 0.01. P values were corrected for multiple testing, using the Benjamini and Hochberg False Discovery Rate.
Fig. 3.
Percentage of ICOS+ T cell subsets before and during aPD-1/CTLA-4 combination therapy
(a, b, c) Percentage of ICOS+ CD 4 T-cell subsets (TCM p = 0.002, TEM p = 0.003, TEMRA p = 0.004) (a), Tregs subsets (b) and CD8 T-cell subsets (TCM p = 0.003, TEM p = 0.012) (c) respectively, at screening and on-treatment time points. (d, e, f) Comparison between responding (R) and non-responding (NR) patients for the percentage of ICOS+ CD 4 T-cell subsets (TN NR p = 0.01, TCM NR p = 0.02, TEM NR p = 0.03, TEMRA NR p = 0.01 (d), Tregs subsets (nTreg NR p = 0.01) (e) and CD8 T-cell subsets (TCM R p = 0.03) (f) respectively, at screening and on-treatment time points. Paired samples are shown connected by black lines in each graph. Significance (Wilcoxon signed-rank test) is shown in each graph, with * p < 0.05 and ** p < 0.01. P values were corrected for multiple testing, using the Benjamini and Hochberg False Discovery Rate
In conclusion, combining aPD-1 and aCTLA-4 treatment induced proliferation and activation of memory T-cell subsets, however, this proliferation was independent of clinical response.
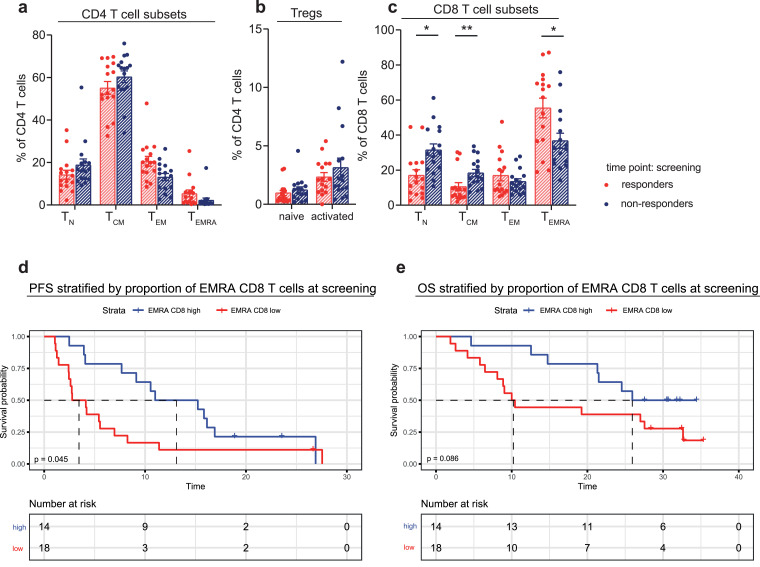
3.4. MPM patients responding to combined aPD-1 and aCTLA-4 treatment showed an altered distribution of CD8 T-cell subsets prior to treatment
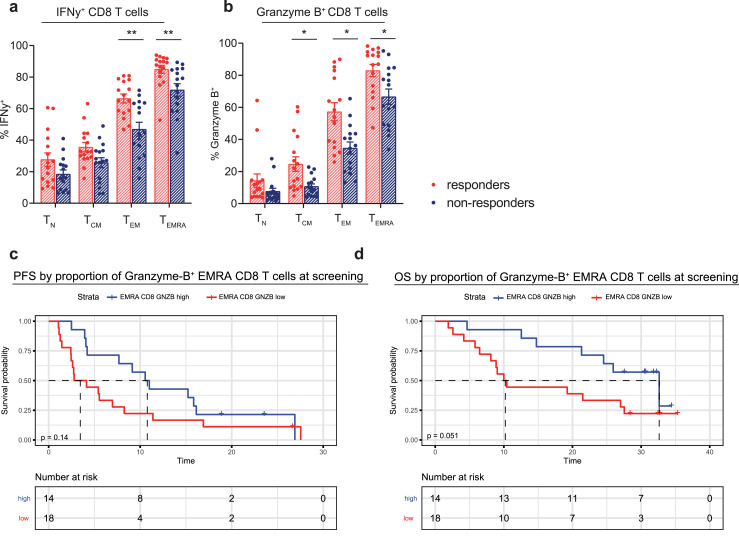
We investigated whether the frequency or phenotype of T-cell subsets was different prior to treatment in patients that responded, compared to patients that did not respond to aPD-1 and aCTLA-4 combination treatment. MPM patients that responded had a different distribution of their T-cell compartment prior to treatment, with significantly lower frequencies of naive and CM CD8 T cells and a higher frequency of EMRA CD8 T cells (Fig. 4A–C). Log rank test revealed that patients with a high EMRA CD8 T-cell proportion (cut-off based on the median proportion) at screening, had a significantly longer PFS upon combination treatment (median PFS of 13.1 vs 3.5 months, p = 0.045). Although the OS curves also appeared to differ (median OS of 25.9 vs 10.2 months), this difference was not statistically significant (Fig. 4D and E). Upon further characterization of these EMRA CD8 T cells, we found that the frequency of Granzyme-B and IFNγ-expressing EMRA CD8 T cells was increased in responding patients (Fig. 5A and B). Increased cytokine expression was also observed in CM CD8 T cells and EM CD8 T cells (Fig. 5A and B). High or low proportion of Granzyme-B positive EMRA CD8 T cells (cut-off based on the median proportion) prior to treatment was used to stratify PFS and OS. Median PFS was 10.8 months vs 3.5 months for the high vs low groups and median OS was 32.6 vs 10.2 months. Log rank test did not reveal any significant differences between the two curves for both PFS and OS, although a clear trend was seen in the OS curves.
Fig. 4.
Comparison of T-cell characteristics before aPD-1/CTLA-4 combination therapy in responding and non-responding patients
(a, b, c) Percentage of CD4 T-cell subsets (a), Treg subsets (b) and CD8 T-cell subsets (TN p = 0.017, TCM p = 0.008, TEMRA p = 0.028) (c) respectively, at the screening time point in responding and non-responding patients. Bars depict mean values with standard error of the mean. Significance (Mann–Whitney U test) is shown in each graph, with * p < 0.05 and ** p < 0.01. P values were corrected for multiple testing, using the Benjamini and Hochberg False Discovery Rate. (d, e) EMRA CD8 T-cells proportions prior to treatment were used to stratify progression-free survival (PFS) (d) and overall survival (OS) (e). Median proportion of EMRA CD8 T cells was used as a cut off between the ‘high’ vs ‘low’ group. Statistical significance of the difference between the two Kaplan–Meier curves was tested by log rank test with p = 0.045 for PFS (median PFS of 3.5 vs 13.1 months) and p = 0.086 for OS (median OS of 10.2 vs 25.9 months).
Fig. 5.
Comparison of cytokine frequencies in CD8 T-cell subsets before aPD-1/CTLA-4 combination therapy in responding and non-responding patients
(a, b) Percentage of IFNγ+ CD8 T-cell subsets (TEM p = 0.008, TEMRA p = 0.006) (a) and Granzyme-B+ CD8 T-cell subsets (TN p = 0.02, TCM p = 0.032, TEMRA p = 0.02) (b) respectively, at the screening time point in responding and non-responding patients. Bars depict mean values with standard error of the mean. Significance (Mann–Whitney U test) is shown in each graph, with * p < 0.05 and ** p < 0.01. P values were corrected for multiple testing, using the Benjamini and Hochberg False Discovery Rate. (c, d) Proportions of Granzyme-B+ EMRA CD8 T-cells prior to treatment were used to stratify progression-free survival (PFS) (d) and overall survival (OS) (e). Median proportion of Granzyme-B+ EMRA CD 8 T cells was used as a cut off between the ‘high’ vs ‘low’ group. Statistical significance of the difference between the two Kaplan–Meier curves was tested by log rank test with p = 0.14 for PFS (not significant, median PFS of 3.5 vs 10.8 months) and p = 0.051 for OS (not significant, median OS of 10.2 vs 32.6 months).
In conclusion, patients that responded to combined treatment with aPD-1 and aCTLA-4 had a different T-cell distribution, in particular more EMRA CD8 T cells and less naive CD8 T cells, prior to treatment. The frequency of cytokine-expressing memory CD8 T cells was increased in responding patients, indicating that these memory CD8 T cells are more functionally active.
4. Discussion
Recently, the first positive results were announced for the Checkmate-743 trial, demonstrating that combining aPD-1 and aCTLA-4 therapy led to improved OS in MPM, as compared to chemotherapy [19]. In contrast, aPD-1 monotherapy failed to improve PFS and OS [9]. Understanding the immunological mechanisms explaining why combination therapy of aPD-1 and aCTLA-4 is effective and monotherapy is not, is thus vital to select effective treatment options for MPM. To the best of our knowledge, we are the first to investigate T-cell characteristics of MPM patients treated with either aPD-1 monotherapy or aPD-1/aCTLA4 combination therapy, treated during two ICI trials [8,16].
Using comprehensive immune monitoring, we demonstrate that combining aPD-1 with aCTLA-4 treatment strongly induces memory T-cell proliferation and activation of both CD4 and CD8 T cells. Higher frequencies of ICOS-expressing CD4 T cells were only observed in the combination therapy. Since this proliferation and activation was irrespective of clinical response, these results could indicate that aPD1/aCTLA-4 treatment induces proliferation and activation of bystander, non-tumor specific T cells, which lack the ability to respond to tumor antigens and do not result in a successful anti-tumor immune response. However, the distribution of T-cell subsets prior to treatment was different in MPM patients with a clinical response to combined aPD-1 and aCTLA-4 treatment. Herein, we found increased frequencies of EMRA CD8 T cells (TEMRAs) at the cost of naive CD8 T cells. Survival analysis also showed that PFS was significantly longer in patients with high frequencies of TEMRAs prior to treatment. Furthermore, in responding patients, we found higher frequencies of TEMRAs expressing Granzyme-B and IFNγ. Thus, combined aPD-1/aCTLA-4 treatment was associated with the activation and proliferation of memory T cells, but only MPM patients with high frequencies of TEMRAs prior to start of treatment, did benefit. The beneficial presence of TEMRAs could indicate that TEMRAs in particular comprise tumor-specific memory T cells that can be reinvigorated by combination treatment, but not by aPD-1 monotherapy, as these associations were not found in the aPD-1 monotherapy study.
Our results are supported by several studies investigating memory CD8 T-cell biology, both in general and in relation to ICI treatment. Characterization of TILs in melanoma patients treated with combined aPD1/aCTLA-4 therapy revealed that tumors of responding patients harbored an effector memory T-cell population (CD8+ EOMES+CD69+CD45RO+) that was less abundant in non-responding patients [27]. Wei et al. revealed that dual blockade of PD-1 and CTLA-4 engages biological pathways partly different from aPD-1 monotherapy [28]. Combined aCTLA-1/aPD-1 treatment increased the frequencies of a terminally differentiated TBET+EOMES+ CD8 T-cell subset in peripheral blood of melanoma patients, whereas aPD-1 monotherapy did not. Therefore, the authors speculated that combination therapy may be sufficient to attenuate or even reverse T-cell exhaustion. Both studies demonstrated that the combination of aPD1/aCTLA4 has a distinct effect on borderline terminally differentiated memory T-cells, which was not observed upon aPD-1 monotherapy.
Our findings indicate that combination ICI treatment, in contrast to aPD-1 monotherapy, is able to reactivate these crucial TEMRA cells. Further research should provide mechanistic insight in how combined aPD-1 and aCTLA-4 treatment reactivates TEMRAs and should indicate their specificity.
In contrast to the observations of others in NSCLC and melanoma patients, we did not observe increases in T-cell proliferation upon aPD-1 monotherapy in MPM patients. These studies reported that the increase in proliferation peaked 3 weeks after start of treatment, and declined afterwards [21,22]. As we evaluated immunological differences 6 weeks after start of treatment, we were most likely too late to assess the effects of aPD-1 monotherapy. However, these differences could also be dependent on tumor type, as aPD-1 therapy depends on pre-existing tumor-specific PD-1-expressing cells, which could be more frequent in NSCLC and melanoma as compared to MPM. Moreover, it has been described earlier that aPD-1 and aCTLA-4 therapy induced longer lasting transcriptional alterations as compared to aPD-1 monotherapy [29], potentially enabling us to detect changes in T-cell characteristics in combination ICI treatment in peripheral blood at a later point in time.
It is important to highlight that the immunological differences found in the two treatment modalities, although they clearly seem to fit response observations, could still be of a phenomenological nature. Thus, our results do not warrant any general conclusions on differences in ICI monotherapy and combination therapy in tumor types other than MPM. Given the limited number of patients analyzed in these studies and the limited number of responding patients, especially in the aPD-1 monotherapy study, our findings need to be validated in a larger and independent MPM patient cohort. Investigating the immunological changes induced by ICI treatment on multiple time points after start of treatment will also provide insight into the duration of these immunological changes upon different ICI treatments, and enable the comparison between MPM and other malignancies. Furthermore, it is not known whether changes in peripheral T-cell subsets reflect changes in the tumor microenvironment (TME) in MPM, and whether tumor specific T cells migrated from the peripheral blood into the TME or vice versa. We are also aware of the fact that nivolumab was administered in a weight dependent dose of 3 mg/kg every 3 weeks in NivoMes, thus modestly differing from the fixed dose of 240 mg/kg every 3 weeks that was administered in INITIATE. However, since Selby et al. [15] demonstrated that no significant alterations in lymphocyte subsets were seen upon different dosing regimens of nivolumab in macaques, we believe that the immune cell alterations described in this manuscript are most likely not caused by dosing differences. At last, it is important to keep in mind that the presumed similarity between pembrolizumab and nivolumab is subject to an ongoing debate in MPM, especially since several studies in non-Caucasian populations demonstrated ORRs to nivolumab that appear to be higher than what was seen in studies performed in Europe and the United States [30,31].
In conclusion, the combined treatment of aPD-1 and aCTLA-4 induced a robust T-cell proliferation and activation in MPM patients, whereas aPD-1 monotherapy did not. The absence of a correlation to clinical response could indicate that these are bystander T-cells, unable to react to tumor-antigens. High proportions of TEMRAs that expressed cytokines, prior to treatment, were associated with a better clinical outcome to combination therapy, likely because TEMRAs comprise tumor-specific T cells. This also suggests that TEMRAs can only be reactivated upon combined blockade of both aPD-1 and aCTLA-4. These findings have important implications for future clinical trial design. First, it provides an explanation for the discouraging results of aPD-1 monotherapy in MPM, since aPD-1 monotherapy appears unable to reinvigorate tumor-specific terminally differentiated memory CD8 T cells in MPM. Second, it grants directions for future research, since aPD-1/aCTLA-4 appears to be a promising treatment modality for MPM, especially now that we are able to select patients up front that are likely to respond. And, finally, it provides a rationale for studying the efficacy of combining these treatments with vaccination strategies like dendritic cell vaccines in non-responding patients, since these vaccines have been shown to induce tumor specific T cells [32].
Contributors
J.M. Mankor: Investigation, Formal analysis, Visualization, Writing - Original Draft, M. Disselhorst: Investigation, Formal analysis, Writing - Original Draft, M. Poncin: Investigation P. Baas: Writing - Review & Editing, Resources. J.G.J.V. Aerts: Conceptualization, Writing - Review & Editing, Supervision, Resources. H. Vroman: Conceptualization, Methodology, Formal analysis, Writing - Review & Editing, Supervision. All authors read and approved the final version of the manuscript.
Data sharing
The data that support the findings described in this manuscript are available from the corresponding author upon request.
Declaration of Competing Interest
Prof. dr. Aerts reports personal fees from Eli Lilly, Roche, Boehringer Ingelheim, BMS, MSD, Amphera and AstraZeneca. Furthermore, prof. dr. Aerts has a patent pending on tumour lysate antigen (EP2938354A1) and is stock owner at Amphera B.V. Immunotherapy. Prof. dr. Baas reports grants from BMS, during the conduct of this study. Furthermore, he receives grants from MSD, outside the submitted work. All other authors do not have any financial relationships to disclose.
Acknowledgments
Bristol-Myers Squibb sponsored the clinical studies and provided the study drugs in both the NivoMes and INITIATE clinical trials. The analyses of peripheral blood mononuclear cells (PBMCs) by flow cytometry, described in this manuscript, were not sponsored by any external funding.
Footnotes
Supplementary material associated with this article can be found in the online version at doi:10.1016/j.ebiom.2020.103040.
Appendix. Supplementary materials
References
- 1.Vogelzang NJ, Rusthoven JJ, Symanowski J, Denham C, Kaukel E, Ruffie P. Phase III study of pemetrexed in combination with cisplatin versus cisplatin alone in patients with malignant pleural mesothelioma. J Clin Oncol. 2003;21:2636–2644. doi: 10.1200/JCO.2003.11.136. [DOI] [PubMed] [Google Scholar]
- 2.Zalcman G, Mazieres J, Margery J, Greillier L, Audigier-Valette C, Moro-Sibilot D. Bevacizumab for newly diagnosed pleural mesothelioma in the Mesothelioma Avastin Cisplatin Pemetrexed Study (MAPS): a randomised, controlled, open-label, phase 3 trial. Lancet. 2016;387:1405–1414. doi: 10.1016/S0140-6736(15)01238-6. [DOI] [PubMed] [Google Scholar]
- 3.Ribas A, Wolchok JD. Cancer immunotherapy using checkpoint blockade. Science. 2018 doi: 10.1126/science.aar4060. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Herbst RS, Morgensztern D, Boshoff C. The biology and management of non-small cell lung cancer. Nature. 2018;553:7689. doi: 10.1038/nature25183. 446. [DOI] [PubMed] [Google Scholar]
- 5.Michielin O, Van Akkooi ACJ, Ascierto PA, Dummer R, Keilholz U. Cutaneous melanoma: ESMO Clinical Practice Guidelines for diagnosis, treatment and follow-up. Ann Oncol. 2019;30:1884–1901. doi: 10.1093/annonc/mdz411. [DOI] [PubMed] [Google Scholar]
- 6.Maio M, Scherpereel A, Calabrò L, Aerts J, Perez SC, Bearz A. Tremelimumab as second-line or third-line treatment in relapsed malignant mesothelioma (DETERMINE): a multicentre, international, randomised, double-blind, placebo-controlled phase 2b trial. Lancet Oncol. 2017;18:1261–1273. doi: 10.1016/S1470-2045(17)30446-1. [DOI] [PubMed] [Google Scholar]
- 7.Alley EW, Lopez J, Santoro A, Morosky A, Saraf S, Piperdi B. Clinical safety and activity of pembrolizumab in patients with malignant pleural mesothelioma (KEYNOTE-028): preliminary results from a non-randomised, open-label, phase 1b trial. Lancet Oncol. 2017;18:623–630. doi: 10.1016/S1470-2045(17)30169-9. [DOI] [PubMed] [Google Scholar]
- 8.Quispel-Janssen J, van der Noort V, de Vries JF, Zimmerman M, Lalezari F, Thunnissen E. Programmed death 1 blockade with nivolumab in patients with recurrent malignant pleural mesothelioma. J Thorac Oncol. 2018;13:1569–1576. doi: 10.1016/j.jtho.2018.05.038. [DOI] [PubMed] [Google Scholar]
- 9.Popat S, Curioni-Fontecedro A, Polydoropoulou V, Shah R, O’Brien M, Pope A. A multicentre randomized phase III trial comparing pembrolizumab (P) vs single agent chemotherapy (CT) for advanced pre-treated malignant pleural mesothelioma (MPM): results from the European Thoracic Oncology Platform (ETOP 9-15) PROMISE-meso trial. Ann Oncol. 2019;30:v931. doi: 10.1016/j.annonc.2020.09.009. [DOI] [PubMed] [Google Scholar]
- 10.Marcq E, Siozopoulou V, de Waele J, Van Audenaerde J, Zwaenepoel K, Santermans E. Prognostic and predictive aspects of the tumor immune microenvironment and immune checkpoints in malignant pleural mesothelioma. OncoImmunology. 2017;6 doi: 10.1080/2162402X.2016.1261241. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Coussens LM, Zitvogel L, Palucka AK. Neutralizing tumor-promoting chronic inflammation: a magic bullet? Science. 2013;339:286–291. doi: 10.1126/science.1232227. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Minnema-Luiting J, Vroman H, Aerts J, Cornelissen R. Heterogeneity in immune cell content in malignant pleural mesothelioma. Int J Mol Sci. 2018;19 doi: 10.3390/ijms19041041. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Yap TA, Aerts JG, Popat S, Fennell DA. Novel insights into mesothelioma biology and implications for therapy. Nat Rev Cancer. 2017;17:475–488. doi: 10.1038/nrc.2017.42. [DOI] [PubMed] [Google Scholar]
- 14.Das R, Verma R, Sznol M, Boddupalli CS, Gettinger SN, Kluger H. Combination therapy with anti-CTLA-4 and anti-PD-1 leads to distinct immunologic changes in vivo. J Immunol. 2015;194:950–959. doi: 10.4049/jimmunol.1401686. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Selby MJ, Engelhardt JJ, Johnston RJ, Lu LS, Han M, Thudium K. Preclinical development of ipilimumab and nivolumab combination immunotherapy: mouse tumor models, In vitro functional studies, and cynomolgus macaque toxicology. PLoS One. 2016;11 doi: 10.1371/journal.pone.0161779. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Disselhorst MJ, Quispel-Janssen J, Lalezari F, Monkhorst K, de Vries JF, van der Noort V. Ipilimumab and nivolumab in the treatment of recurrent malignant pleural mesothelioma (INITIATE): results of a prospective, single-arm, phase 2 trial. Lancet Respir Med. 2019;7:260–270. doi: 10.1016/S2213-2600(18)30420-X. [DOI] [PubMed] [Google Scholar]
- 17.Calabrò L, Morra A, Giannarelli D, Amato G, D’Incecco A, Covre A. Tremelimumab combined with durvalumab in patients with mesothelioma (NIBIT-MESO-1): an open-label, non-randomised, phase 2 study. Lancet Respir Med. 2018;6:451–460. doi: 10.1016/S2213-2600(18)30151-6. [DOI] [PubMed] [Google Scholar]
- 18.Scherpereel A, Mazieres J, Greillier L, Lantuejoul S, Dô P, Bylicki O. Nivolumab or nivolumab plus ipilimumab in patients with relapsed malignant pleural mesothelioma (IFCT-1501 MAPS2): a multicentre, open-label, randomised, non-comparative, phase 2 trial. Lancet Oncol. 2019;20:239–253. doi: 10.1016/S1470-2045(18)30765-4. [DOI] [PubMed] [Google Scholar]
- 19.Bratman SV, Yang SYC, Iafolla MAJ, Liu Z, Hansen AR, Bedard PL. Personalized circulating tumor DNA analysis as a predictive biomarker in solid tumor patients treated with pembrolizumab. Nat Cancer. 2020 doi: 10.1038/s43018-020-0096-5. [DOI] [PubMed] [Google Scholar]
- 20.Tumeh PC, Harview CL, Yearley JH, Shintaku IP, Taylor EJ, Robert L. PD-1 blockade induces responses by inhibiting adaptive immune resistance. Nature. 2014;515(7528):568–571. doi: 10.1038/nature13954. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Kamphorst AO, Pillai RN, Yang S, Nasti TH, Akondy RS, Wieland A. Proliferation of PD-1+ CD8 T cells in peripheral blood after PD-1-targeted therapy in lung cancer patients. Proc Natl Acad Sci. 2017 doi: 10.1073/pnas.1705327114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Huang AC, Postow MA, Orlowski RJ, Mick R, Bengsch B, Manne S. T-cell invigoration to tumour burden ratio associated with anti-PD-1 response. Nature. 2017 doi: 10.1038/nature22079. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Fundamental mechanisms of immune checkpoint blockade therapy, (2018). [DOI] [PubMed]
- 24.Wei SC, Levine JH, Cogdill AP, Zhao Y, Anang N-AAS, Andrews MC. Distinct cellular mechanisms underlie anti-CTLA-4 and anti-PD-1 checkpoint blockade. Cell. 2017;170:1120–1133. doi: 10.1016/j.cell.2017.07.024. e17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Byrne MJ, Nowak AK. Modified RECIST criteria for assessment of response in malignant pleural mesothelioma. Ann Oncol. 2004;15:257–260. doi: 10.1093/annonc/mdh059. [DOI] [PubMed] [Google Scholar]
- 26.Benjamini Y, Hochberg Y. Controlling the false discovery rate: a practical and powerful approach to multiple testing on JSTOR. J R Stat Soc Ser B. 1995;57:289–300. [Google Scholar]
- 27.Gide TN, Quek C, Menzies AM, Tasker AT, Shang P, Holst J. Distinct immune cell populations define response to anti-PD-1 monotherapy and anti-PD-1/anti-CTLA-4 combined therapy. Cancer Cell. 2019;35:238–255. doi: 10.1016/j.ccell.2019.01.003. e6. [DOI] [PubMed] [Google Scholar]
- 28.Wei SC, Levine JH, Cogdill AP, Zhao Y, Anang N-AAS, Andrews MC. Distinct cellular mechanisms underlie anti-CTLA-4 and anti-PD-1 checkpoint blockade. Cell. 2017;170:1120–1133. doi: 10.1016/j.cell.2017.07.024. e17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Fairfax BP, Taylor CA, Watson RA, Nassiri I, Danielli S, Fang H. Peripheral CD8(+) T cell characteristics associated with durable responses to immune checkpoint blockade in patients with metastatic melanoma. Nat Med. 2020;26(2):193–199. doi: 10.1038/s41591-019-0734-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Nakamura A, Kondo N, Nakamichi T, Kuroda A, Hashimoto M, Matsumoto S. Initial evaluation of nivolumab in patients with post-operative recurrence of malignant pleural mesothelioma. Jpn J Clin Oncol. 2020;50(8):920–925. doi: 10.1093/jjco/hyaa069. [DOI] [PubMed] [Google Scholar]
- 31.Okada M, Kijima T, Aoe K, Kato T, Fujimoto N, Nakagawa K. Clinical efficacy and safety of nivolumab: results of a multicenter, open-label, single-arm, Japanese phase II study in malignant pleural mesothelioma (MERIT) Clin Cancer Res. 2019;25(18):5485–5492. doi: 10.1158/1078-0432.CCR-19-0103. [DOI] [PubMed] [Google Scholar]
- 32.Garg AD, Coulie PG, Van den Eynde BJ, Agostinis P. Integrating next-generation dendritic cell vaccines into the current cancer immunotherapy landscape. Trends Immunol. 2017;38(8):577–593. doi: 10.1016/j.it.2017.05.006. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.