Abstract
Background
The reported high mortality of COVID-19 patients in intensive care has given rise to a debate over whether patients with this disease are being intubated too soon and might instead benefit from more non-invasive ventilation.
Methods
This review is based on articles published up to 12 June 2020 that were retrieved by a selective literature search on the topic of invasive and non-invasive ventilation for respiratory failure in COVID-19. Guideline recommendations and study data on patients with respiratory failure in settings other than COVID-19 are also considered, as are the current figures of the intensive care registry of the German Interdisciplinary Association for Intensive Care and Emergency Medicine (Deutsche Interdisziplinäre Vereinigung für Intensiv- und Notfallmedizin).
Results
The high mortality figures among patients receiving invasive ventilation that have been reported in studies from abroad cannot be uncritically applied to the current situation in Germany. Study data on ventilation specifically in COVID-19 patients would be needed to do justice to the special pathophysiology of this disease, but such data are lacking. Being intubated too early is evidently associated with risks for the patient, but being intubated too late is as well. A particularly important consideration is the potential harm associated with prolonged spontaneous breathing, with or without non-invasive assistance, as any increase in respiratory work can seriously worsen respiratory failure. On the other hand, it is clearly unacceptable to intubate patients too early merely out of concern that the medical staff might become infected with COVID-19 if they were ventilated non-invasively.
Conclusion
Nasal high flow, non-invasive ventilation, and invasive ventilation with intubation should be carried out in a stepwise treatment strategy, under appropriate intensive-care monitoring and with the observance of all relevant anti-infectious precautions. Germany is better prepared that other countries to provide COVID-19 patients with appropriate respiratory care, in view of the high per capita density of intensive-care beds and the availability of a nationwide, interdisciplinary intensive care registry for the guidance and coordination of intensive care in patients who need it.
In Germany a debate has arisen on the optimal medical care of patients with COVID-19 who require mechanical ventilation (1– 3). This has led to considerable uncertainty among physicians of various specialties and also beyond the medical profession. As a direct reaction to the discussion, the German Respiratory Society (DGP, Deutsche Gesellschaft für Pneumologie und Beatmungsmedizin) has compiled detailed recommendations on ventilation treatment in COVID-19, focusing especially on the use of non-invasive ventilation (NIV) (1).
However, there are still no randomized controlled trials of ventilation treatment in COVID-19. For this reason, the prevailing recommendations regarding ventilation are based primarily on physicians’ experience and on studies in other categories of patients (1, 4, 5). Nevertheless, in writing this review we sought to cast light on scientific considerations and findings that may provide assistance, against the background of the ongoing debate, to clinicians involved in decisions regarding ventilation in the explicit context of COVID-19 pneumonia.
NIV and invasive ventilation: competing or complementary treatment options?
In the current discussion on “excessively early” intubation, NIV and invasive ventilation are regarded as competing approaches. However, this assumption by no means reflects the scientific evidence or the reality of clinical treatment. For acute NIV, there are a large number of randomized controlled trials on conditions other than COVID-19 in which NIV and invasive ventilation are not compared with each other (4). Rather, NIV in addition to a standard treatment (oxygen, medication) is compared with the standard treatment alone, usually in an early phase of illness. The crucial outcome parameters are avoidance of intubation, length of hospital stay, and mortality. NIV is hence no better or worse than invasive ventilation; rather, it should always be viewed primarily as an additional measure early in the disease process as part of a stepwise approach, at a time when the criteria for intubation are not yet fulfilled. NIV thus has the potential to delay or even prevent the need for intubation, making it a fixed component of the intensive care repertoire.
Intubation of patients with COVID-19: are findings from other countries valid for Germany?
A study from China reported mortality of 97% among intubated patients; the median duration of ventilation was 4 days (6). In another analysis from China, however, only 3.2% of a total of 80 409 patients with COVID-19 of all degrees of severity were actually intubated (7). The data from an Italian intensive care cohort of 1591 patients show that 88% were intubated, while the death rate among those who completed intensive care treatment was 64% (8). Furthermore, the incidence of intubation in a French study was just under 69% (9). Data from a collective made up of residents of England, Wales, and Northern Ireland demonstrated mortality of 67% among 1795 invasively ventilated patients (10). Finally, recent data from the area in and around New York City show that 3% of invasively ventilated patients survived and 25% died (11); 72% were still undergoing inpatient treatment, so this was only a snapshot.
The circumstances in the above-mentioned countries are in no way comparable to the situation in Germany. Early data from Germany show a long duration of intensive care treatment in general, with a median of 10 days, although intensive therapy was ongoing (12). Moreover, Germany has the highest density of intensive care beds in the world (13). Because the pandemic affected Germany later than Italy, for example, this country had more time to make preparations. Decisive in this regard was the enhancement of intensive care capacity, e.g., by means of internal restructuring with suspension of elective interventions and subsequent creation of intensive care beds in the field of surgical intensive therapy. These measures ensured that, in contrast to other countries, long stays in intensive care units have been possible without, to date, any shortfall in intensive care in Germany. Very recently published data from the USA as well show much lower mortality among invasively ventilated patients compared to other countries (36%), which is attributed to longer preparation times and higher numbers of intensive care beds (14). Finally, the authors of a recent systematic review likewise come to the conclusion that the high mortality figures in the early publications were most likely due to the limited intensive care resources (15).
What role can be played by the nationwide DIVI intensive care registry?
Another crucial factor was the extremely rapid establishment of a nationwide intensive care registry by the German Interdisciplinary Association for Intensive Care and Emergency Medicine (DIVI, Deutsche Interdisziplinäre Vereinigung für Intensiv- und Notfallmedizin) (16). The DIVI registry provides daily updates of available capacity, enabling regional and supraregional coordination of intensive care bed allocation and facilitating scientific evaluation of the pertinent data.
In some other countries, overcrowding of emergency rooms and intensive care facilities has led to scenes of chaos. It may well be that the options of NIV or nasal high-flow (NHF) treatment were not exploited to the full, with lack of coordination meaning that intubation was followed by restricted intensive care monitoring. A recent report summarized the essential limitations of intensive care services in China: shortages of beds and staff, the variable level of intensive care, isolated high patient volumes, and the high rate of infection-related absence among medical personnel (17). The daily report from the German intensive care registry shows mortality of only 25 to 30% for completed intensive care treatments (18). These are not study data, however, so they have to be interpreted cautiously. Nevertheless, early, not yet fully analyzed data from Germany will show mortality of somewhat more than 50% in ventilated, hospitalized patients.
What are the limits of NIV in patients with acute hypoxemia?
NIV must be viewed critically against the backdrop of the two following considerations and may possibly have to be converted to invasive ventilation after analgosedation/intubation:
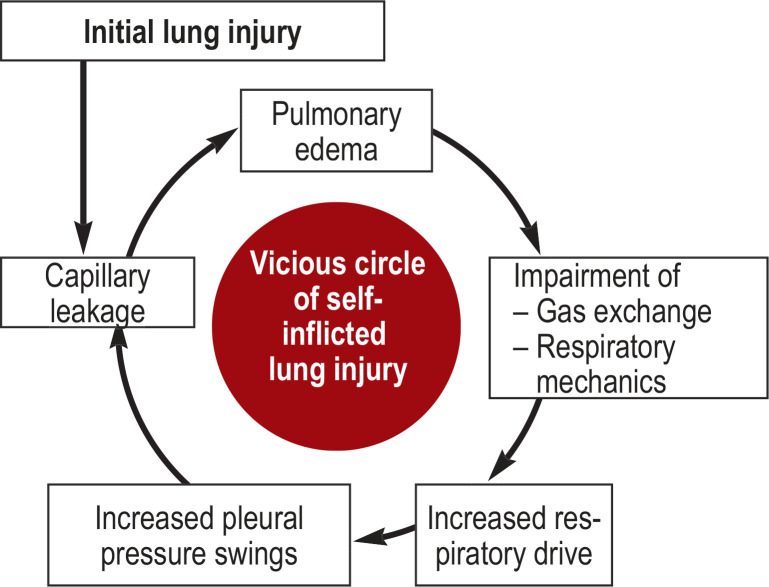
One crucial hypothesis is that elevated respiratory drive with increased fluctuation of pleural pressure may cause patient self-inflicted lung injury (P-SILI) (19). Essentially, this involves enlargement of a pre-existing capillary leak and consequently worsening of pulmonary edema (Figures 1 and 2). Recently published research shows that measurement of esophageal pressure, as surrogate for pleural pressure, can predict NIV failure accurately at an early stage (20). The tidal changes in esophageal pressure after 2 h of NIV were significantly lower in patients with NIV success than in those with NIV failure.
The Lung Safe Study, a large epidemiological study with data from 50 countries, showed that NIV was associated with elevated mortality when the Horovitz index (PaO2/FIO2) was <150 mm Hg (21). This agrees with the conclusion reached by earlier studies, i.e., that delayed intubation is prognostically unfavorable (4).
Figure 1.
Self-inflicted lung injury
A central role is played by the high respiratory drive of the spontaneously breathing and conscious patient, with or without NIV, with increasingly impaired gas exchange and restricted respiratory mechanics (19). The consequent high respiratory work may then lead to high, regionally variable, fluctuations in transpulmonary pressure. It is crucial to realize that struggling to breathe in results in a lowering of pleural pressure that exceeds the intravascular pressure decrease. With an additional elevation of intrathoracic blood volume on inspiration, this causes an increase in transmural pulmonary vascular pressure. The result is a higher risk of pulmonary edema, especially in an already damaged lung (capillary leakage). Completing the vicious circle, this then leads to further impairment of breathing mechanics with decreased compliance and restriction of gas exchange, which in turn favors shortness of breath and thus a further increase in respiratory work.
NIV, Non-invasive ventilation
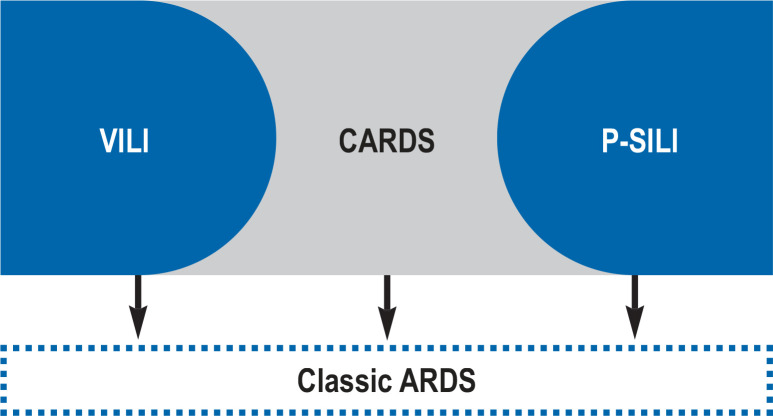
Figure 2.
The possible causes of classic ARDS in patients with COVID-19 pneumonia
COVID-19: a special form of ARDS?
Evidence is accumulating that the course of hypoxemic lung injury in COVID-19 pneumonia may very well differ in various ways from that in other entities, including a greater degree of heterogeneity (1, 22, 23). In that it also goes along with damage to the vascular epithelium, and therefore the danger of multiple organ failure, COVID-19 pneumonia can be viewed as a systemic disease (23).
In the initial phase, radiological imaging shows ground-glass infiltrates subpleurally and along the fissures, although the elasticity of the lung may be preserved. Hypoxemia, sometimes severe, may nevertheless already be present. This may be explained in part by loss of the capacity for hypoxia-driven vasoconstriction and by disordered regulation of perfusion, which subsequently goes along with a distinct lowering of the ventilation–perfusion ratio (low VA/Q). This then corresponds to the hypothesized L type (low elastance, low ventilation to perfusion ratio, low lung weight, low recruitability) (22, 23).
Some patients go on to develop severe lung injury with extensive consolidations seen on imaging, whereby classic acute respiratory distress syndrome (ARDS) can have various causes. There is a fall in lung compliance as a result of edema formation with a decrease in the number of lung segments aerated, which is associated with a subsequent increase in right–left shunt and aggravation of hypoxemia. This corresponds to the hypothesized H type (high elastance, high right to left shunt, high lung weight, high recruitability) (22, 23). Relevant bacterial and fungal superinfections may occur.
It must be noted, however, that the division into L and H types is theoretical and cannot always be applied equally to all cases of COVID-19 pneumonia. Furthermore, a recent autopsy study found that fulminant and also peripheral pulmonary embolisms, and not least deep vein thromboses, are frequent occurrences in COVID-19 (24). Moreover, a separate autopsy study with small case numbers demonstrated the presence of typical histopathological patterns differing from those in influenza, in particular severe endothelial damage, more thromboses in microangiopathy, and angiogenesis (25). In summary, the respiratory physiology, clinical, radiological, and histopathological findings provide strong evidence that ARDS in COVID-19 is subject to disease-specific mechanisms, on which further research is needed. Even though the physiological criteria for ARDS may be fulfilled in the initial phase of lung injury, diffuse alveolar injury, documented more regularly later at autopsy, is not automatically present at this early stage (24, 25). In the first phase (L type), therefore, non-invasive treatment strategies (e.g., NIV or NHF) may be feasible. If this option is pursued, it is important first to increase the inspiratory oxygen concentration, with defined limits.
If non-invasive treatment strategies prove ineffective or are not accepted, intubation may thus become necessary even at this early stage (L type). In this phase the tidal volumes should be low (6 mL/kg predicted body weight) (26). In some cases moderate positive end-expiratory pressures (PEEP) may suffice because the lung injury is not yet severe (26). In particular, excessively high PEEP in the presence of unrestricted or only slightly restricted compliance may have negative hemodynamic consequences, while a benefit from recruitment cannot always be expected (22, 23). It should be noted, however, that no published studies have evaluated the PEEP settings in the early phase of COVID-19. For this reason, individualized decisions on the best form of treatment are indispensable. If, nonetheless, the disease develops to the advanced phase (H type), this is the last opportunity, alongside PEEP adjustment according to the ARDS Network table, to introduce further elements of lung protective ventilation, accompanied if necessary by prone positioning and/or extracorporeal procedures (5, 26, 27). New observations in small series of cases have also shown that especially prone positioning may be helpful even at an earlier stage of respiratory failure in COVID-19, to improve oxygenation and ameliorate tachypnea, both in spontaneously breathing patients (28) and in those receiving NIV (29). However, further research is needed.
At what point should patients with COVID-19 pneumonia be intubated?
Deciding when intubation is indicated requires careful consideration of the classic—yet never clearly defined—intubation triggers. One important parameter is persistent tachypnea despite NIV (breathing rate ≥ 30/min). However, an increase in breathing rate does not simply reflect greater respiratory effort. Altered respiratory mechanics, the effect of inflammation on the respiratory drive, and, not least, respiratory drive–modulating medications can all have an impact on the actual respiratory effort (30, 31).
The most reliable way of assessing the elevated respiratory work is to measure the esophageal pressure, but this is often difficult in the clinical setting and is usually reserved for research. An easier method for clinicians is palpation of the phasically increased contraction of the muscles used in respiration, particularly the sternocleidomastoid muscle (31).
Since hypoxemia does not necessarily lead to end organ damage, it cannot serve as sole intubation trigger (30, 31). One must recall that tissue oxygen supply depends not only on the oxygen saturation, but also on the hemoglobin concentration and the cardiac output. Moreover, the reason for the patient’s shortness of breath is frequently not restricted oxygenation alone; rather, limitations of respiratory mechanics often also play a role (30, 31). Correspondingly, clinical observations show that severely disordered oxygenation in COVID-19 is not necessarily accompanied by severe dyspnea, especially in cases where the compliance is not yet badly affected. The significance of restricted oxygenation should not be underestimated, however, as suggested by increased rates of cardiovascular arrest owing to COVID-19 (32).
An essential factor is whether the restricted oxygenation arises from a depressed ventilation–perfusion ratio or the actual presence of an intrapulmonary right–left shunt. In the case of the former, a substantial improvement in oxygenation can be expected from increasing the the oxygen supply, so intubation can indeed sometimes be avoided at first. The situation is different in the event of non-response to elevation of the oxygen supply, which is most likely to rest on an increase in right–left shunt. In this case, the gas volume is reduced, necessitating lung protective ventilation with correspondingly adjusted PEEP, above all prone positioning, and extracorporeal membrane oxygenation (ECMO) if required (26).
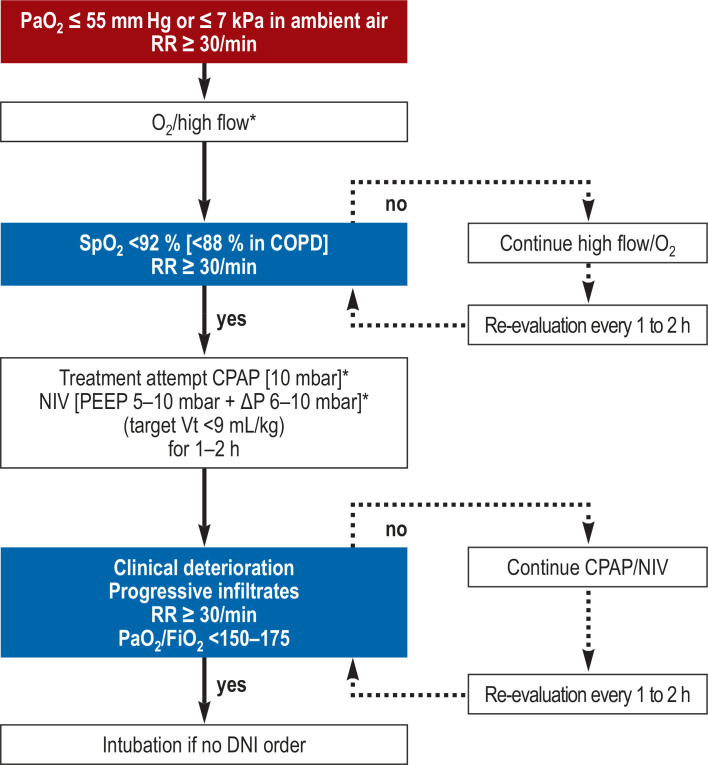
The decision whether to intubate an individual patient should therefore be based primarily on the sum of various parameters (26, 31, 33). At this juncture, it is important to recall that elevated respiratory work and severe refractory hypoxemia point to the presence of a right–left shunt despite non-invasive treatment, and also to remember that there may be subjective lack of acceptance of such therapeutic strategies, particularly based on the assumption of extended treatment periods, sometimes over 2 weeks. In this context, Figures 3 shows an algorithm for the use of instrument-based treatment that was formulated by the DGP (1) and has been adopted by the professional associations for intensive care medicine in Germany in their revised expert consensus guideline (26).
Figure 3.
Possible instrument-based treatment escalation in the case of acute respiratory insufficiency as a result of COVID-19, as recommended in the position paper of the German Respiratory Society (DGP) (1).
* Wearing personal protective equipment as recommended by the Robert Koch Institute. COPD, chronic obstructive pulmonary disease; CPAP, continuous positive airway pressure; DNI, do not intubate; FiO2, inspiratory oxygen fraction; kPa, kilopascal; NIV, non-invasive ventilation; PaO2, oxygen partial pressure; PEEP, positive end-expiratory pressure; RR, respiratory rate; SpO2, peripheral oxygen saturation; Vt, tidal volume (Reproduced by kind permission of Georg Thieme Verlag)
What circumstances may speak against intubation?
In certain circumstances the advisability of intubation may have to be considered particularly carefully despite positive physiological intubation triggers:
The patient must be in agreement with intubation and invasive ventilation. For patients with a DNI (do not intubate) order, primarily non-invasive treatment methods can be used and palliative strategies can be pursued if applicable (4).
The fear of transmission to medical personnel should never be considered an intubation trigger (1). For this reason, staff protection has top priority. The professional associations have published clear recommendations on the use of NIV including advise on hygiene requirements (1, 26).
The problem concerning the intubation criteria for COVID-19 is that while in conventional ARDS the severe oxygenation disorder due to the prevailing high intrapulmonary shunt represents the primary intubation criterion, in COVID-19, in contrast, there is often a good treatment response to administration of oxygen/NIV due to the low VA/Q (L type). In this event NHF therapy may also represent a promising treatment option, although neither in NIV nor NHF therapy is any significant escape of infectious aerosols likely as long as leakage is minimized by a close-fitting interface (1, 34).
Owing to the potential pathophysiological instability, with the danger of rapid clinical deterioration, all non-invasive treatments must be closely monitored by intensive care personnel. Intubation must be possible at all times (1, 4).
Finally, intubation with invasive ventilation must always be viewed as one component in the overall medical treatment plan. On the one hand, it is often the only way of ensuring that the patient receives enough oxygen to continue living. In those of advanced age with comorbidities and other organ dysfunctions, however, especially in the event of prolonged ventilation as is the case in COVID-19, problems in weaning the patient off ventilation must be anticipated (35). Weaning failure can be associated with worsening, sometimes dramatic, of the prognosis and of the patient’s quality of life (36– 38). Recent research in Germany shows that prolonged weaning is unsuccessful in 36% of cases (around 15% of patients die and circa 21% are transferred to out-of-hospital weaning facilities), despite treatment in specialized weaning centers (38). Although no data have yet been published on prolonged weaning after COVID-19 pneumonia, the experience to date means that the indications for intubation of patients with risk factors for weaning failure must be considered with great care.
| ARDS | Acute respiratory distress syndrome |
| CARDS | Special form of ARDS in the initial phase of COVID-19 |
| VILI | Ventilator-induced lung injury |
| P-SILI | Patient self-inflicted lung injury |
Acknowledgments
Translated from the original German by David Roseveare
Acknowledgment
The authors are extremely grateful to Prof. Uwe Janssens for his critical scrutiny of the manuscript.
Footnotes
Confict of interest statement
Prof. Windisch has received lecture honoraria from Heinen and Löwenstein, Res Med, and Philips International. He has received financial support for research of his own initiation from Weinmann, Vivisol, Heinen and Löwenstein, and VitalAire/Germany.
Prof. Weber-Carstens has received financial support for research of his own initiation from Dräger.
Prof. Kluge has received research support from the companies Ambu, E.T.View Ltd, Fisher & Paykel, Pfizer, and Xenios. He has received lecture honoraria from ArjoHuntleigh, Astellas, Astra, Basilea, Bard, Baxter, Biotest, CSL Behring, Cytosorbents, Fresenius, Gilead, MSD, Orion, Pfizer, Philips, Sedana, Sorin, Xenios, and Zoll. He has received payments for consultation from AMOMED, Astellas, Baxter, Bayer, Fresenius, Gilead, MSD, Pfizer, and Xenios.
Prof. Karagiannidis has received payments for consultation from Bayer and Xenios. He has received reimbursement of travel and accommodation expenses from Bayer and lecture honoraria from Bayer and Xenios.
The remaining authors declare that no conflict of interest exists.
References
- 1.Pfeifer M, Ewig S, Voshaar T, et al. Positionspapier zur praktischen Umsetzung der apparativen Differenzialtherapie der akuten respiratorischen Insuffizienz bei COVID-19 Deutsche Gesellschaft für Pneumologie und Beatmungsmedizin e. V. (DGP) Pneumologie. 2020;74:337–357. doi: 10.1055/a-1157-9976. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Soldt R. Es wird zu häufig intubiert Frankfurter Allgemeine Zeitung (published on 7 April 2020) www.faz.net/aktuell/gesellschaft/gesundheit/coronavirus/beatmung-beim-coronavirus-lungenfacharzt-im-gespraech-16714565.html?premium=0xad2896d4cdb0c5a8f3c9f83fef8d12fd (last accessed on 29 June 2020) [Google Scholar]
- 3.Voshaar T, Dellweg D, Hetzel M. published by Verband Pneumologischer Kliniken: Empfehlung zur Behandlung respiratorischer Komplikationen bei akuter Virusinfektion außerhalb der Intensivstation. www.vpneumo.de/fileadmin/pdf/VPK_Empfehlung_neu_21.03.2020.pdf (last accessed on 29 June 2020) [Google Scholar]
- 4.Westhoff M, Schönhofer B, Neumann P, et al. S3 Leitlinien: Nicht-invasive Beatmung als Therapie der akuten respiratorischen Insuffizienz. Pneumologie. 2015;69:719–756. doi: 10.1055/s-0034-1393309. [DOI] [PubMed] [Google Scholar]
- 5.Fichtner F, Moerer O, Laudi S, Weber-Carstens S, Nothacker M, Kaisers U. Clinical practice guideline: mechanical ventilation and extracorporeal membrane oxygenation in acute respiratory insufficiency. Dtsch Arztebl Int. 2018;115:840–847. doi: 10.3238/arztebl.2018.0840. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Wang Y, Lu X, Li Y, et al. Clinical course and outcomes of 344 intensive care patients with COVID-19. J Am J Respir Crit Care Med. 2020;201;:1430–1434. doi: 10.1164/rccm.202003-0736LE. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Meng L, Qiu H, Wan L, et al. Intubation and ventilation amid the COVID-19 outbreak Wuhan’s experience. Anesthesiology. 2020;132:1317–1332. doi: 10.1097/ALN.0000000000003296. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Grasselli G, Zangrillo A, Zanella A, et al. COVID-19 lombardy ICU network Baseline characteristics and outcomes of 1591 patients infected with SARS-CoV-2 admitted to ICUs of the Lombardy region, Italy. JAMA. 2020;323:1574–1581. doi: 10.1001/jama.2020.5394. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Simonnet A, Chetboun M, Poissy J, et al. Lille intensive care COVID-19 and obesity study group High prevalence of obesity in severe acute respiratory syndrome coronavirus-2 (SARS-CoV-2) requiring invasive mechanical ventilation. Obesity. 2020;28:1195–1199. doi: 10.1002/oby.22831. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.ICNARC. ICNARC report on COVID-19 in critical care 17 April 2020. www.icnarc.org (last accessed on 29 June 2020) [Google Scholar]
- 11.Richardson S, Hirsch JS, Narasimhan M, et al. Presenting characteristics, comorbidities, and outcomes among 5700 patients hospitalized with COVID-19 in the New York City Area. JAMA. 2020;323:2052–2059. doi: 10.1001/jama.2020.6775. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Dreher M, Kersten A, Bickenbach J, et al. The characteristics of 50 hospitalized COVID-19 patients with and without ARDS. Dtsch Arztebl Int. 2020;117:271–278. doi: 10.3238/arztebl.2020.0271. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Rhodes A, Ferdinande P, Flaatten H, Guidet B, Metnitz PG, Moreno RP. The variability of critical care bed numbers in Europe. Intensive Care Med. 2012;38:1647–1653. doi: 10.1007/s00134-012-2627-8. [DOI] [PubMed] [Google Scholar]
- 14.Auld SC, Caridi-Scheible M, Blum JM, et al. Emory COVID-19 quality and clinical research collaborative ICU and ventilator mortality among critically ill adults with coronavirus disease 2019. Crit Care Med. 2020 doi: 10.1097/CCM.0000000000004457. DOI:10.1097/CCM.0000000000004457. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Quah P, Li A, Phua J. Mortality rates of patients with COVID-19 in the intensive care unit: a systematic review of the emerging literature. Crit Care. 2020;24 doi: 10.1186/s13054-020-03006-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.DIVI. DIVI Intensivregister. www.intensivregister.de (last accessed on 29 June 2020) [Google Scholar]
- 17.Li L, Gong S, Yan J. Covid-19 in China: ten critical issues for intensive care medicine. Crit Care. 2020;24 doi: 10.1186/s13054-020-02848-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.DIVI. tagesreport. www.divi.de/register/tagesreport(last accessed on 29 June 2020) [Google Scholar]
- 19.Brochard L, Slutsky A, Pesenti A. Mechanical ventilation to minimize progression of lung injury in acute respiratory failure. Am J Respir Crit Care Med. 2017;195:438–442. doi: 10.1164/rccm.201605-1081CP. [DOI] [PubMed] [Google Scholar]
- 20.Tonelli R, Fantini R, TabbiÌ L, et al. Inspiratory effort assessment by esophageal manometry early predicts noninvasive ventilation outcome in de novo respiratory failure: a pilot study. Am J Respir Crit Care Med. 2020 doi: 10.1164/rccm.201912-2512OC. 10.1164/rccm.201912-2512OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Bellani G, Laffey JG, Pham T, et al. Noninvasive ventilation of patients with acute respiratory distress syndrome Insights from the LUNG SAFE Study. Am J Respir Crit Care Med. 2017;195:67–77. doi: 10.1164/rccm.201606-1306OC. [DOI] [PubMed] [Google Scholar]
- 22.Gattinoni L, Chiumello D, Caironi P, et al. COVID-19 pneumonia: different respiratory treatments for different phenotypes? Intensive Care Med. 2020;46:1099–1102. doi: 10.1007/s00134-020-06033-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Marini JJ, Gattinoni L. Management of COVID-19 respiratory distress. JAMA. 2020;323:2329–2330. doi: 10.1001/jama.2020.6825. [DOI] [PubMed] [Google Scholar]
- 24.Edler C, Schröder AS, Aepfelbacher M, et al. Dying with SARS-CoV-2 infection—an autopsy study of the first consecutive 80 cases in Hamburg, Germany. Int J Legal Med. 2020;134:1275–1284. doi: 10.1007/s00414-020-02317-w. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Ackermann M, Verleden SE, Kuehnel M, et al. Pulmonary vascular endothelialitis, thrombosis, and angiogenesis in Covid-19. N Engl J Med. 2020 doi: 10.1056/NEJMoa2015432. DOI: 10.1056/NEJMoa2015432. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Kluge S, Janssens U, Welte T, et al. S1-Leitlinie Empfehlungen zur intensivmedizinischen Therapie von Patienten mit COVID- www.awmf.org/uploads/tx_szleitlinien/113-001l_S1_Intensivmedizinische-Therapie-von-Patienten-mit-COVID-19_2020-06.pdf (last accessed on 19 June 2020) doi: 10.1007/s10405-020-00359-w. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Karagiannidis C, Bein T, Windisch W. Was hat sich seit Publikation der S3-Leitlinie „Invasive Beatmung und Einsatz extrakorporaler Verfahren“ getan? Pneumologie. 2020;74:46–49. doi: 10.1055/a-1065-6230. [DOI] [PubMed] [Google Scholar]
- 28.Elharrar X, Trigui Y, Dols AM, et al. Use of prone positioning in nonintubated patients with COVID-19 and hypoxemic acute respiratory failure. JAMA. 2020;323:2336–2338. doi: 10.1001/jama.2020.8255. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Sartini C, Tresoldi M, Scarpellini P, et al. Respiratory parameters in patients with COVID-19 after using noninvasive ventilation in the prone position outside the intensive care unit. JAMA. 2020;323:2338–2340. doi: 10.1001/jama.2020.7861. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Tobin MJ, Gardner WN. Tobin MJ, editor. Monitoring of the control of ventilation Principles and practice of intensive care monitoring. McGraw-Hill, Inc. New York, 1998:415–464. [Google Scholar]
- 31.Tobin M. Basing respiratory management of coronavirus on physiological principles. Am J Respir Crit Care Med. 2020;201 doi: 10.1164/rccm.202004-1076ED. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Baldi E, Sechi GM, Mare C, et al. Lombardia CARe Researchers Out-of-hospital cardiac arrest during the Covid-19 outbreak in Italy. N Engl J Med. 2020;382:1708–1720. doi: 10.1056/NEJMc2010418. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Laghi F, Tobin MJ. Indications for mechanical ventilation principles and practice of mechanical ventilation. In: Tobin MJ, editor. McGraw-Hill Inc. 3. New York: 2012. pp. 129–162. [Google Scholar]
- 34.Hui DS, Chow BK, Lo T. Exhaled air dispersion during high-flow nasal cannula therapy versus CPAP via different masks. Eur Respir J. 2019;53 doi: 10.1183/13993003.02339-2018. pii: 1802339. [DOI] [PubMed] [Google Scholar]
- 35.Schönhofer B, Geiseler J, Dellweg D, et al. Prolongiertes Weaning - S2k-Leitlinie herausgegeben von der Deutschen Gesellschaft für Pneumologie und Beatmungsmedizin e. V. Pneumologie. 2019;73:723–814. doi: 10.1055/s-0033-1359038. [DOI] [PubMed] [Google Scholar]
- 36.Huttmann SE, Windisch W, Storre JH. Invasive home mechanical ventilation: living conditions and health-related quality of life. Respiration. 2015;89:312–321. doi: 10.1159/000375169. [DOI] [PubMed] [Google Scholar]
- 37.Huttmann SE, Magnet FS, Karagiannidis C, Storre JH, Windisch W. Quality of life and life satisfaction are severely impaired in patients with long-term invasive ventilation following ICU treatment and unsuccessful weaning. Ann Intensive Care. 2018;8 doi: 10.1186/s13613-018-0384-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Windisch W, Dellweg D, Geiseler G, et al. Prolonged weaning from mechanical ventilation Results from specialized weaning centers—a registery-based study from the WeanNet Initiative. Dtsch Arztebl Int. 2020;117:197–204. doi: 10.3238/arztebl.2020.0197. [DOI] [PMC free article] [PubMed] [Google Scholar]