Objective
The purpose of the study was to investigate the effect of dexmedetomidine hydrochloride (Dex) on the recovery of cognitive function, hemodynamics, and postoperative analgesia in patients undergoing intracranial aneurysm craniotomy. Methods: general anesthesia was performed on patients undergoing intracranial aneurysm craniotomy in neurosurgery. Patients were randomly divided into three groups: Dex 1 group (Dex dose: 1 μg/kg), Dex 2 group (Dex dose: 0.5 μg/kg), and blank control group (normal saline). The changes of heart rate, arterial pressure, intraoperative brain function index, and postoperative pain score were recorded and compared. Results: in Dex 1 group and Dex 2 group, the heart rate of T1 and T2 phase was significantly lower than that of T3-T7 phases (P < 0.05); compared with the control group, the heart rate of Dex 1 group and Dex 2 group was significantly lower (P < 0.05). The average arterial pressure of the control group and Dex groups was significantly different (P < 0.05). Compared with the control group, there were significant differences between Dex 1 group and Dex 2 group: S100 β protein in T7-T10, NSE (neuron specific enolase) in T9 and T10, pain score in T8, T9 and T10 after operation. Conclusion: the application of Dex in the resection of intracranial aneurysms can protect the brain of patients, minimize the influence of operation on hemodynamics, and relieve postoperative pain, which is worthy of clinical application.
Keywords: Dexmedetomidine hydrochloride, Intracranial aneurysm, Cerebral protection, Stress response, Hemodynamics
1. Introduction
Intracranial aneurysm refers to the cystic bulging of the intracranial arterial vessel wall. If the intracranial aneurysm is not treated in time, it will result in subarachnoid hemorrhage (Luo et al., 2016). Once the aneurysm ruptures, the risk of re-bleeding and vasospasm is extremely high in patients. Therefore, the treatment of intracranial aneurysm is based on the principle of preventing aneurysm rupture. If the aneurysm ruptures, the aneurysm should be removed by craniotomy after the condition of patient is stable at the early stage (Nonaka et al., 2016). In the process of surgical removal of an aneurysm, it is first necessary to clamp or ligature the tumor artery, block the blood supply of the aneurysm, and avoid the occurrence of re-bleeding. In such a process, due to the exposure of the surgical field and the pulling and oppression of the brain tissue during hemostasis, different degrees of hypoxia and ischemic damages may occur (Seddighi et al., 2016). If the brain is ischemic and hypoxic, the small molecule S100β protein enters the cerebrospinal fluid from the damaged glial cells and then enters the peripheral blood circulation through the blood–brain barrier. Once the concentration of S100β protein is too high, it induces neuronal apoptosis.
Dexmedetomidine hydrochloride (Dex) is a novel and highly selective α2 adrenergic receptor agonist in recent years. Due to its characteristics, such as analgesia, sedation, hypnosis, anti-anxiety, inhibition of sympathetic excitation, and maintenance of stable hemodynamics, it has been widely used in clinical practices (Jiang et al., 2017). Compared to metopyrimidine, Dex is more selective; it has a shorter half-life and can be used clinically for sedation of patients who start intubation and use a ventilator during intensive care treatment. Meanwhile, Dex can also reduce the amount of anesthetic, improve the hemodynamic stability during surgery, and decrease the incidence of myocardial ischemia. Some scholars have studied patients with intracranial aneurysm undergoing craniotomy. It has been confirmed that Dex can reduce various external irritations and increase the stability of the hemodynamic system during operations. The auxiliary medication of Dex for surgical anesthesia helps the patient to smoothly pass the recovery period of anesthesia after craniotomy. In recent years, there have been increasingly more studies on brain protection mechanism of Dex. Clinical studies have shown that Dex can alleviate brain damages caused by cerebral ischemia and hypoxia through anti-oxidative stress and anti-apoptosis and can contribute to the recovery of perioperative brain functions in patients with intracranial aneurysms (Bilgi et al., 2016).
An important objective of surgical anesthesia for intracranial aneurysms is to ensure stable hemodynamics during operations. The intraoperative intracranial hypertension or insufficient perfusion may lead to a series of related complications and even affect postoperative recovery (Kumar et al., 2017). The application of Dex in patients during perioperative anesthesia can reduce the dosage of intraoperative anesthetics. In terms of general anesthesia, it can reduce the dosage of postoperative analgesic drugs and has a positive effect on the recovery of postoperative cognitive functions. In this study, the drug constituents and brain protection mechanism of Dex was analyzed. Then, based on the composition analysis, the effects of Dex on perioperative hemodynamics, postoperative analgesia, and cognitive function recovery of patients with intracranial aneurysm craniotomy were investigated. It was expected to provide a clinical reference for the application of drugs to protect the brains and reduce the postoperative pain of patients during intracranial aneurysm resection.
2. Composition analysis of Dex and its mechanism of cerebral protection
Dex is a potent α2-adrenoreceptor agonist, which is clinically used for sedation of patients during intubation or as a ventilator during intensive care (Hernández et al., 2016). The molecular formula of Dex is C13H16N2-HCl; its molecular weight is 236.76. In addition, Dex is white crystalline powder and very soluble in water. Also, it is soluble in methanol, ethanol, and chloroform, but almost insoluble in ether. Its density is 1.17 g/cm3, its specific optical rotation is + 52.4°, its optical rotation is + 48° to + 58°, and its melting point is 153 °C to 158 °C (Pan et al., 2016). 10 mg of Dex was taken and added with water to formulate 1 mL of Dex solution, which was clear and colorless. The measured pH value was 3.5–4.5. The chemical structure of Dex is shown in Fig. 1.
Fig. 1.
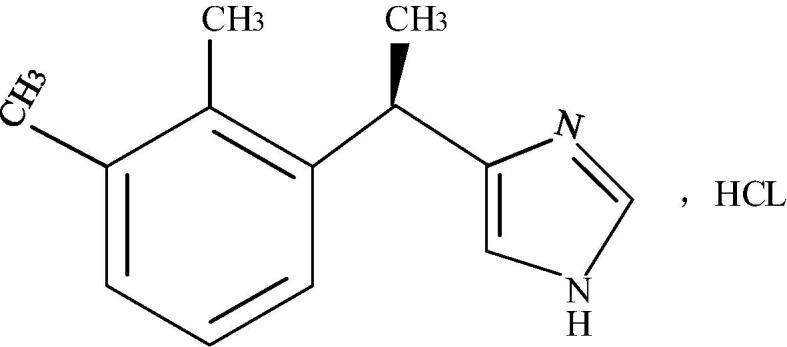
Chemical structure of Dex.
The α2 adrenergic receptor is a G protein-coupled receptor, and its 3 subtypes are widely distributed in the central, peripheral, and autonomic nervous system, as well as organs and blood vessels throughout the body. Dex is a relatively selective α2-adrenoreceptor agonist with central hypo-tensive, anti-sympathetic, analgesic, sedative, and anti-anxiety effects (Martin et al., 2016). In addition, Dex can also reduce the release of catecholamines in the adrenergic nerve endings, or directly functions in the brain to reduce the release of catecholamines in the brain tissue, thereby inhibiting the sympathetic nerve activity and reducing the stimulating reaction of brain tissue. The binding site of Dex also includes imidazoline 1 and imidazoline 2 receptors. Among the receptors, the imidazoline 1 receptor is involved in the regulation of blood pressure, while the imidazoline 2 receptor is related to the neuroprotection after cerebral ischemic injury (Willey et al., 2016).
The regulation of the cardiovascular system by Dex is bidirectional. The dosage and rate of administration directly affect the hemodynamic changes. If the concentration is too high or the rate of administration is too fast, the sympathetic nervous system will be activated under stress actions; thus, the blood pressure will increase transiently and will then drop, while the heart rate will gradually slow down (Debabrata et al., 2016). A transient increase in blood pressure in a short period of time is associated with Dex directly activating α2-adrenergic receptors on vascular smooth muscle cells leading to vasoconstriction, after which blood pressure drops and drugs act on the cardiovascular regulatory center, inhibiting the sympathetic nervous system and also increasing the vagal excitability. After intravenous infusion, the pharmacokinetic parameters of Dex are as follows: the distribution half-life (t1/2) of the rapidly distributed phase is about 6 min; the terminal elimination half-life (t1/2) is about 2 h; the distribution volume (Vss) is about 118 L, and the clearance rate is about 39 L/h (Khafagy et al., 2017).
3. Materials and methods
3.1. Research objects and grouping
A total of 66 patients with intracranial aneurysms admitted to our hospital from April 2017 to April 2019 were enrolled. All patients underwent general anesthesia for intracranial aneurysm craniotomy. The enrolled patients were aged 27–59 years old, with 35 males and 31 females. The experiment had been submitted to the approval of the ethics committee of the hospital. All the patients and their families were aware of the experimental contents had signed the informed consent forms of anesthesia and operations.
Inclusion criteria: (1) patients who were diagnosed as intracranial aneurysms by magnetic resonance imaging (MRI) or digital subtraction angiography (DSA) and underwent craniotomy; (2) according to the grading standards of American Society of Anesthesiologists (ASA) (Panchgar et al., 2017), all patients should be Grade I to II; also, patients should have excellent tolerance toward anesthesia and operations with stable anesthesia processes; (3) patients whose Mini Mental State Examination (MMSE) scores were higher than 24 points; (4) patients who had no history of anesthesia allergies. Exclusion criteria: (1) patients whose operation duration was greater than 3 h or accidents occurred during operation; (2) patients who had history of traumatic brain injuries, epilepsy, and mental diseases; (3) patients with severe heart, lung, liver, kidney, or metabolic diseases; (4) patients who had bradycardia or chronic arrhythmia.
All patients were divided into 3 groups according to the random number table method, i.e., the Dex 1 group (dosage of Dex was 1 μg/kg), the Dex 2 group (dosage of Dex was 0.5 μg/kg), and the blank control group (saline). Each group consisted of 22 patients. The comparisons of general references between patients in groups showed no statistically significant differences (P < 0.05), which indicated the comparability of patients, as shown in Table 1. Patients in the Dex 1 group were injected with Dex intravenously at a dosage of 1 μg/kg, and the injection time was controlled at 10 min. Patients in the Dex 2 group were injected with Dex intravenously at a dosage of 0.5 μg/kg, and the injection time was controlled at 10 min. Patients in the blank control group were injected with saline intravenously.
Table 1.
Comparisons of general references between patients in groups.
| Groups | Gender ratio (male/female) | Age | Height (cm) | Weight (kg) |
|---|---|---|---|---|
| Dex 1 | 12/10 | 47.3 ± 9.7 | 169.3 ± 15.9 | 62.2 ± 6.3 |
| Dex 2 | 13/9 | 45.5 ± 11.2 | 167.4 ± 14.3 | 60.8 ± 5.9 |
| The control group | 11/11 | 47.9 ± 9.1 | 166.9 ± 17.1 | 59.3 ± 7.3 |
3.2. Research methods
(1) Preparation before anesthesia: after the patients entered the operating room, the venous accesses were established. The routine dynamic electrocardiogram (ECG) monitoring (mainly led by II lead) and blood oxygen saturation monitoring were performed. The unilateral radial artery was taken for a puncture, and the direct arterial pressure and the dynamic monitoring of the average arterial pressure were performed once every 3 min. The internal jugular vein puncture was performed, and the central venous catheter was indwelled to monitor the central venous pressure.
(2) Anesthesia induction and anesthesia maintenance: anesthesia induction was started after mask oxygen inhalation, Patients were intravenously injected with 0.05 mg/kg of midazolam, 0.4 ug/kg of sufentanil, 0.2 mg/kg of atracurium cis-sulfonate, and 0.2 mg/kg of etomidate. After induction, the tracheal intubation was performed. The position of the catheter was confirmed correct, the anesthesia machine was connected for mechanical ventilation, the oxygen flow was controlled at 2 L/min, the tidal volume was controlled at 9 mL/kg, the respiratory rate was controlled at 14 times/min, the ratio of suction to breath was controlled at 1:2, and the partial pressure of carbon dioxide in the end-of-life was maintained between 30 and 40 mmHg. Once the anesthesia induction was finished, patients were grouped for anesthesia maintenance. Patients in the Dex 1 group were injected with Dex intravenously at a dosage of 1 μg/kg, and the injection time was controlled at 10 min. Patients in the Dex 2 group were injected with Dex intravenously at a dosage of 0.5 μg/kg, and the injection time was controlled at 10 min. Patients in the blank control group were injected with saline intravenously. Intraoperative maintenance of anesthesia ensured that the bi-spectral index (BIS) was between 40 and 60. During the intraoperative clamping of the tumor-bearing artery, drugs could be used for controlled hypotension.
3.3. Detection indicators and evaluation standards
-
(1)
Comparison of heart rates and average arterial pressure: The heart rates and average arterial pressure at the following time nodes were recorded, including entering the operating room, anesthesia, starting the operation, clamping the artery, clamping the tumor, restoring the movement, and ending the operation, respectively. These time nodes were represented by T1 to T7 successively.
-
(2)
Comparison of S100β protein and NSE levels: The internal jugular vein blood was taken at the following time points, including entering the operating room (T1), end of the operation (T7), postoperative 6 h (T8), postoperative 12 h (T8), and postoperative 24 h (T10). The serum central nervous-specific protein (S100β protein) and neuron-specific enolase (NSE) levels were determined by enzyme-linked immunosorbent assay (ELISA).
-
(3)
Comparison of postoperative pain scales: The visual analog scale (VAS) was used to evaluate the pain of patients at preoperative (T1), postoperative 6 h (T8), postoperative 12 h (T9), and postoperative 24 h (T10). The scales at both ends of the 10-cm-dial were “0′ and “10”, respectively, indicating “painless” and “painfulness”, and patients were independently evaluated according to the specific situations (Sheikh et al., 2018).
3.4. Statistical method
In this study, the SPSS 21.0 statistics software was used for statistical analysis. The measurement data were expressed as mean number ± standard deviation. The independent sample t-test was used to compare the variance analysis of the repeated measurement design. The comparison between groups was performed by student T-test. P < 0.05 indicated the statistical significance of the difference.
4. Results
4.1. Comparison of heart rate and average arterial pressure between patients in groups at different time nodes
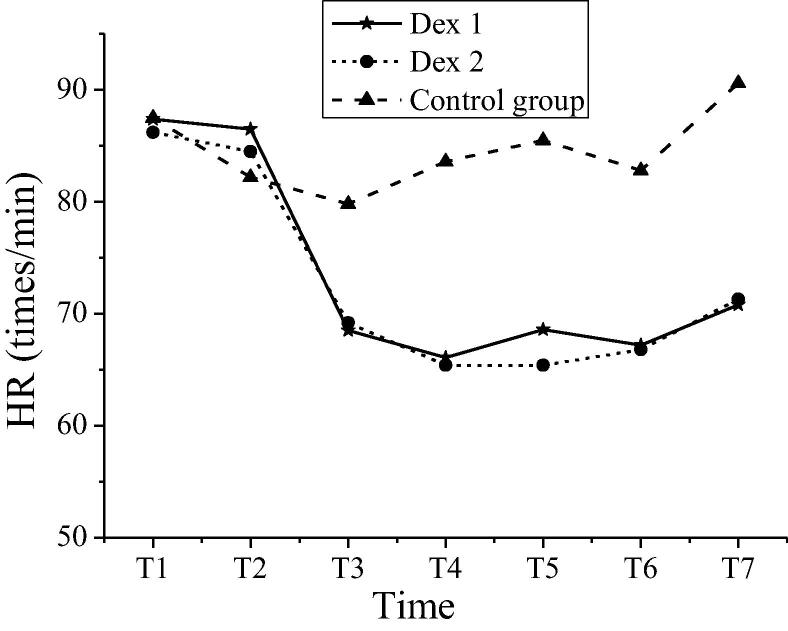
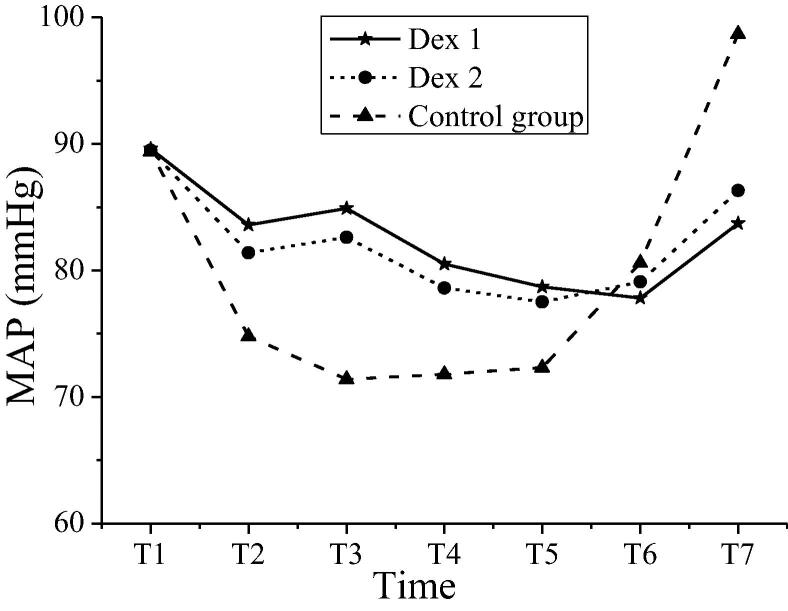
The comparison of heart rate between patients in groups at different time nodes was shown in Table 2. The intra-group comparisons were carried out within the Dex 1 group and Dex 2 group. Compared with the heart rates at T3 ~ T7 time nodes, the heart rates at T1 and T2 time nodes decreased significantly, and the differences were statistically significant (P < 0.05). Compared with the control group, the heart rates of patients in both Dex groups decreased significantly (P < 0.05). The comparison of average arterial pressure between patients in groups at a different time was shown in Table 3. The intra-group comparisons were carried out within the Dex 1 group and Dex 2 group. Compared with the average arterial pressure at different time nodes, the variations were not apparent, and the differences were not statistically significant (P > 0.05). Compared with the average arterial pressure of the control group at preoperative period (T1), intraoperative period (T2 ~ T6), and postoperative period (T7), the average arterial pressure of patients in both Dex groups had statistical significance (P < 0.05). Variations in heart rates of patients in groups at different time nodes were shown in Fig. 2. Variations in average arterial pressure of patients in groups at different time nodes were shown in Fig. 3. The heart rate and mean arterial pressure of patients in the Dex 1 and Dex 2 groups shared the same variation tendency. The heart rate of the control group changed less at each time node, which was more stable than that in the Dex 1 and Dex 2 groups. The variation amplitude of arterial pressure was large; especially, the levels of T2-T5 were low, and the mean arterial pressure value rebounded significantly at T6 and T7.
Table 2.
The heart rates of patients in groups at T1–T7 (times/min).
| Groups | T1 | T2 | T3 | T4 | T5 | T6 | T7 |
|---|---|---|---|---|---|---|---|
| Dex 1 | 87.4 ± 6.9 | 86.5 ± 6.0 | 68.5 ± 7.2*# | 66.1 ± 4.7*# | 68.6 ± 6.8*# | 67.2 ± 6.5*# | 70.8 ± 6.3*# |
| Dex 2 | 86.2 ± 7.2 | 84.5 ± 5.5 | 69.2 ± 6.7*# | 65.4 ± 3.8*# | 65.4 ± 3.8*# | 66.8 ± 5.4*# | 71.3 ± 6.7*# |
| The control group | 87.5 ± 6.6 | 82.2 ± 4.3 | 79.8 ± 5.4 | 83.6 ± 5.7 | 85.5 ± 6.2 | 82.8 ± 4.7 | 90.6 ± 5.3 |
Note: * indicated comparison with the same group at T1 and T2, P < 0.05; # indicated comparison with the control group at the same time nodes, P < 0.05.
Table 3.
The average arterial pressure of patients in groups at T1–T7 (mmHg).
| Groups | T1 | T2 | T3 | T4 | T5 | T6 | T7 |
|---|---|---|---|---|---|---|---|
| Dex 1 | 89.6 ± 6.4 | 83.6 ± 6.8*# | 84.9 ± 5.3*# | 80.5 ± 4.3*# | 78.7 ± 5.6*# | 77.8 ± 6.6*# | 83.7 ± 7.5*# |
| Dex 2 | 89.5 ± 6.9 | 81.4 ± 5.7*# | 82.6 ± 5.4*# | 78.6 ± 6.7*# | 77.5 ± 6.3*# | 79.1 ± 5.8*# | 86.3 ± 6.7*# |
| The control group | 89.4 ± 5.7 | 74.8 ± 6.7 | 71.4 ± 5.6 | 71.8 ± 7.1 | 72.3 ± 4.5 | 80.6 ± 5.5 | 98.7 ± 4.9 |
Note: * indicated comparison between the Dex 1 group and Dex 2 group at the same time nodes, P < 0.05; # indicated comparison with the control group at the same time nodes, P < 0.05.
Fig. 2.
Variations in heart rates of patients in groups at different time nodes.
Fig. 3.
Variations in average arterial pressure of patients in groups at different time nodes.
4.2. Comparison of S100β protein and NSE levels between patients in groups at different time nodes
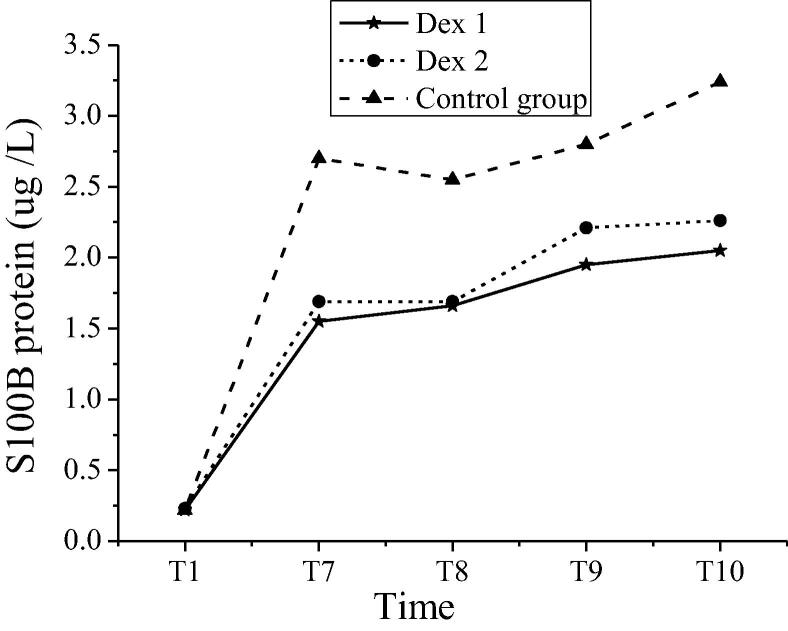
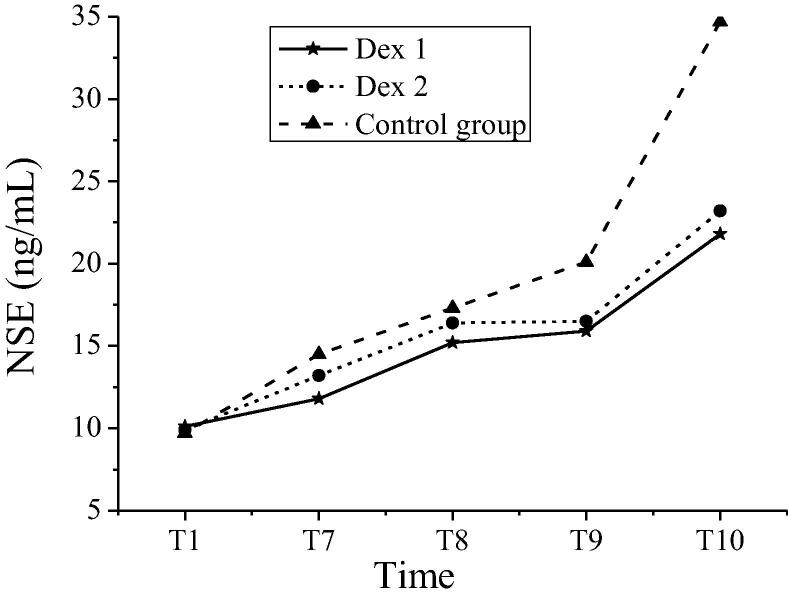
The S100β protein and NSE levels of patients in groups at different time nodes were shown in Table 4. In the Dex 1 group and the Dex 2 group, the S100β protein at T7 ~ T10 time nodes and the NSE levels at T9 and T10 time nodes of patients were significantly different from those in the control group (P < 0.05). In the Dex 1 group and the Dex 2 group, the comparisons of S100β protein and NSE levels had no statistical significances (P > 0.05). Variations in S100β protein of patients in groups at different time nodes were shown in Fig. 4. Variations in NSE levels of patients in groups at different time nodes were shown in Fig. 5. The S100β protein contents and NSE levels of patients in the Dex 1 and Dex 2 groups shared the same variation tendency at each time node. The S100β protein contents of T7 to T10 in the control group were higher than those in the Dex 1 and Dex 2 groups. Compared with the other two groups, the levels of NSE levels in T9-T10 patients of the control group increased more significantly. Table 5.
Table 4.
Comparison of S100β protein and NSE levels between patients in groups at different time nodes.
| Groups | T1 | T7 | T8 | T9 | T10 | |
|---|---|---|---|---|---|---|
| S100β protein (μg /L) |
Dex 1 | 0.22 ± 0.10 | 1.55 ± 0.68# | 1.66 ± 0.75# | 1.95 ± 0.65# | 2.05 ± 0.34# |
| Dex 2 | 0.23 ± 0.12 | 1.69 ± 0.92# | 1.69 ± 0.83# | 2.21 ± 0.66# | 2.26 ± 0.31 | |
| The control group | 0.22 ± 0.11 | 2.70 ± 1.02 | 2.55 ± 0.98 | 2.80 ± 0.74 | 3.24 ± 0.25 | |
| NSE (ng/mL) | Dex 1 | 10.1 ± 1.2 | 11.8 ± 1.5 | 15.2 ± 4.1 | 15.9 ± 3.5# | 21.8 ± 4.9# |
| Dex 2 | 9.9 ± 0.8 | 13.2 ± 0.9 | 16.4 ± 3.4 | 16.5 ± 3.7# | 23.2 ± 4.6# | |
| The control group | 9.7 ± 0.9 | 14.5 ± 0.8 | 17.3 ± 4.3 | 20.1 ± 4.2 | 34.7 ± 4.6 | |
Note: # indicated comparison with the control group at the same time nodes, P < 0.05.
Fig. 4.
Variations in S100β protein of patients in groups at different time nodes.
Fig. 5.
Variations in NSE levels of patients in groups at different time nodes.
Table 5.
Comparison of VAS scales between patients in groups at different time nodes.
| Groups | TI | T8 | T9 | T10 |
|---|---|---|---|---|
| Dex 1 | 3.57 ± 0.87 | 0.87 ± 0.67 | 0.60 ± 0.43 | 0.57 ± 0.49 |
| Dex 2 | 3.66 ± 0.76 | 0.95 ± 0.73 | 0.74 ± 0.51 | 0.66 ± 0.48 |
| The control group | 3.62 ± 0.80 | 1.76 ± 0.63 | 1.72 ± 0.60 | 1.17 ± 0.61 |
Note: # indicated comparison with the control group at the same time nodes, P < 0.05.
4.3. Comparison of postoperative VAS pain scales between patients in groups
The preoperative pain degrees of patients in groups were evaluated. Only several patients had the pain of 5–6 points. The comparisons between all 3 groups had no significant differences (P > 0.05). After the operations, at T8, T9, and T10 time nodes, compared with the control group, the pain scales of patients in both Dex groups were significantly reduced, and the differences were statistically significant (P < 0.05). The comparisons of pain scales between patients in the Dex 1 group and the Dex 2 group at different time nodes were not statistically significant (P > 0.05).
5. Discussion
Intracranial aneurysm is a highly damaging arterial disease caused by thinning and dilatation of the cerebral vessel wall. The mortality after aneurysm rupture is extremely high. Therefore, the timely removal of aneurysm clipping is very important. Before the aneurysm clipping operation, the blood flow inside the whole tumor is weak, resulting in excessive expression of secretory nitric oxide in endothelial cells. Also, the red blood cells, white blood cells, and platelets tend to adhere to the wall of the aneurysm, resulting in damages of endothelial cells (Bhattacharjee et al., 2016). Therefore, the aneurysm clipping process should fully consider the impacts of operations on the hemodynamics of patients to reduce the impacts of operations on the heart rates and average arterial pressure of patients as much as possible. In addition, since the aneurysm removal operation is often craniotomy, it is necessary to expose the brain tissue. When the brain tissue is damaged by ischemia or hypoxia, the neurons are degenerated and necrotic, and the glial cells are damaged. The NSE and S100β proteins enter the cerebrospinal fluid, causing damages to the brain functions of the patients and affecting the postoperative recovery.
As a new type of highly selective α2-receptor agonist, Dex can inhibit the up-regulation of pain signals by activating the posterior horn of the spinal cord and prominating the posterior membrane α2 of the inter-neurons, which has excellent analgesic and sedative effect and is used as a central analgesic drug (Abdelhamid et al., 2016). Studies have shown that Dex and opioids have synergistic effects, which can reduce the number of opioid analgesics used in patients during the perioperative period. It has a positive effect on maintaining hemodynamic stability. In recent years, several studies have confirmed that Dex also has a certain effect on improving heart damages and can also reduce the incidence of cardiac operation complications. Dex, by exciting the vagus nerve, not only slows heart rate but also effectively suppresses the inflammatory reactions.
Based on the pharmacological action and mechanism of Dex, in this study, the effects of Dex on hemodynamics and brain function protection during operations in patients treated with intracranial aneurysm clipping were further explored. The results showed that in both Dex groups, compared with the control group, the heart rates and the average arterial pressure of patients during the operations were stable; in addition, and the 1 μg/kg dosage group was better than the 0.5 μg/kg dosage group (P < 0.05). In both Dex groups, the postoperative S100β protein and NSE levels of patients were significantly lower than those in the control group, and the pain scores were also superior to the control group. The difference was statistically significant (P < 0.05). Therefore, the application of Dex in the of intracranial aneurysms could reduce the impacts of the operations on hemodynamics, ensure the stability of heart rate and arterial pressure, and reduce the effects of intraoperative hypoxia and brain ischemia on postoperative brain functions of patients, thereby relieving the postoperative pains of patients, which had significant values in clinical applications. This study mainly analyzed the effects of different Dex dosages on hemodynamics but did not compare it with other nerve block analgesics to confirm the superiority of Dex. In the subsequent study, other drugs should be introduced into clinical research to comprehensively analyze the pharmacological effects and safety of the drugs.
Acknowledgement:
The study was supported by Medical Science Research Key Subject of Health Commission of Hebei Province (No. 20160693).
Footnotes
Peer review under responsibility of King Saud University.
References
- Luo X., Zheng X., Huang H. Protective effects of dexmedetomidine on brain function of glioma patients undergoing craniotomy resection and its underlying mechanism. Clin. Neurol. Neurosurg. 2016;146:105–108. doi: 10.1016/j.clineuro.2016.05.004. [DOI] [PubMed] [Google Scholar]
- Nonaka T., Inamori M., Miyashita T. Feasibility of deep sedation with the combination of propofol and dexmedetomidine hydrochloride for esophageal endoscopic submucosal dissection. Digest. Endosc. Official J. Japan Gastroenterol. Endosc. Soc. 2016;28(2):145–151. doi: 10.1111/den.12559. [DOI] [PubMed] [Google Scholar]
- Seddighi R., Odoi A., Doherty T.J. Effect of dexmedetomidine hydrochloride on tiletamine hydrochloride-zolazepam hydrochloride anesthesia in alpacas. Am. J. Vet. Res. 2016;77(10):1057–1063. doi: 10.2460/ajvr.77.10.1057. [DOI] [PubMed] [Google Scholar]
- Jiang W., Wang Q., Xu M. Assessment of different loading doses of dexmedetomidine hydrochloride in preventing adverse reaction after combined spinal-epidural anesthesia. Experim. Therapeut. Med. 2017;13(6):2946–2950. doi: 10.3892/etm.2017.4335. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bilgi K.V., Vasudevan A., Bidkar P.U. Comparison of dexmedetomidine with fentanyl for maintenance of intraoperative hemodynamics in hypertensive patients undergoing major surgery: A randomized controlled trial. Anesth Essays Res. 2016;10(2):332–337. doi: 10.4103/0259-1162.176408. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kumar A., Sinha C., Kumar A. The effect of intravenous dexmedetomidine compared to propofol on patients hemodynamics as a sedative in brachial plexus block: a comparative study. Anesthesia Essays Res. 2017;11(1):201–205. doi: 10.4103/0259-1162.200236. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hernández G., Tapia P., Alegría L. Effects of dexmedetomidine and esmolol on systemic hemodynamics and exogenous lactate clearance in early experimental septic shock. Crit. Care. 2016;20(1):234. doi: 10.1186/s13054-016-1419-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pan W., Wang Y., Lin L. Outcomes of dexmedetomidine treatment in pediatric patients undergoing congenital heart disease surgery: a meta-analysis. Paediatr Anaesth. 2016;26(3):239–248. doi: 10.1111/pan.12820. [DOI] [PubMed] [Google Scholar]
- Martin F., Bannardsmith J., Blackburn T. Dexmedetomidine: a valuable sedative currently not widely available in the UK. Bja British J. Anaesthesia. 2016;117(2):263–264. doi: 10.1093/bja/aew202. [DOI] [PubMed] [Google Scholar]
- Willey J.L., Julius T.M., Claypool S.P. Evaluation and comparison of xylazine hydrochloride and dexmedetomidine hydrochloride for the induction of emesis in cats: 47 cases (2007–2013) J. Am. Vet. Med. Assoc. 2016;248(8):923. doi: 10.2460/javma.248.8.923. [DOI] [PubMed] [Google Scholar]
- Debabrata M., Anjan D., Subinay C. The effect of dexmedetomidine added to preemptive (2% lignocaine with adrenaline) infiltration on intraoperative hemodynamics and postoperative pain after ambulatory maxillofacial surgeries under general anesthesia. Anesthesia Essays Res. 2016;10(2):324–331. doi: 10.4103/0259-1162.167837. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Khafagy H.F., Ebied R.S., Mohamed A.H. Effect of dexmedetomidine infusion on desflurane consumption and hemodynamics during BIS guided laparoscopic cholecystectomy: a randomized controlled pilot study. Egyptian J. Anaesthesia. 2017;33(3):227–231. [Google Scholar]
- Panchgar V., Shetti A.N., Sunitha H.B. The effectiveness of intravenous dexmedetomidine on perioperative hemodynamics, analgesic requirement, and side effects profile in patients undergoing laparoscopic surgery under general anesthesia. Anesthesia Essays Res. 2017;11(1):72–77. doi: 10.4103/0259-1162.200232. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sheikh T.A., Dar B.A., Akhter N. A comparative study evaluating effects of intravenous sedation by dexmedetomidine and propofol on patient hemodynamics and postoperative outcomes in cardiac surgery. Anesth Essays Res. 2018;12(2):555–560. doi: 10.4103/aer.AER_46_18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bhattacharjee D.P., Saha S., Paul S. A comparative study of esmolol and dexmedetomidine on hemodynamic responses to carbon dioxide pneumoperitoneum during laparoscopic surgery. Anesthesia Essays Res. 2016;10(3):580–584. doi: 10.4103/0259-1162.183564. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Abdelhamid A.M., Aaa M., Abdelhaq M.M. Dexmedetomidine as an additive to local anesthetics compared with intravenous dexmedetomidine in peribulbar block for cataract surgery] Saudi J. Anaesthesia. 2016;10(1):50–54. doi: 10.4103/1658-354X.169475. [DOI] [PMC free article] [PubMed] [Google Scholar]