Abstract
Oral microbiome mediated nitrate reductase (NR) activity regulates nitric oxide (NO) bioavailability and signaling. While deficits in NO-bioavailability impact several morbidities of extreme prematurity including bronchopulmonary dysplasia (BPD), whether oral NR activity is associated with morbidities of prematurity is not known. We characterized NR activity in extremely preterm infants from birth until 34 weeks' post menstrual age (PMA), determined whether changes in the oral microbiome contribute to changes in NR activity, and determined whether changes in NR activity correlated with disease. In this single center prospective cohort study (n = 28), we observed two surprising findings: (1) NR activity unexpectedly peaked at 29 weeks' PMA (p < 0.05) and (2) when infants were stratified for BPD status, infants who developed BPD had significantly less NR activity at 29 weeks’ PMA compared to infants who did not develop BPD. Oral microbiota and NR activity may play a role in BPD development in extremely preterm infants, indicating potential for disease prediction and therapeutic targeting.
Keywords: Bronchopulmonary dysplasia, Nitrate reductase, Nitric oxide, Extreme prematurity, Nitrite
Abbreviations: BPD, bronchopulmonary dysplasia; PMA, postmenstrual age; NO, nitric oxide; NR, nitrate reductase
1. Introduction
Many distinct microbial ecosystems have been described throughout the human body. Associations between these microbiota and human disease have focused efforts into understanding mechanisms by which these organisms may modulate disease susceptibility, progression, and severity. The microbiota over the tongue dorsum are particularly unique in that several facultative anaerobes residing in its crypts express an enzyme, nitrate reductase (NR) [1]. NR containing bacteria catalyze the reduction of nitrate to nitrite; the latter is a substrate for nitric oxide (NO) via protonation within the acidic gastric environment or further 1-electron reduction through a number of hypoxia sensitive enzymes and proteins in blood and tissues [2,3]. Accumulating evidence suggests that reduction of nitrate to nitrite and NO is an important and parallel pathway, with nitric oxide synthase, to control systemic NO-bioavailability [4]. Several studies have demonstrated that nitrate administration lowers systolic and diastolic blood pressures by levels comparable to a single antihypertensive agent [[5], [6], [7]]. Conversely, decreased oral and plasma nitrite and subsequent NO bioavailability following the use of antibacterial mouthwash is associated with increased blood pressure and loss of NO-dependent signaling [[8], [9], [10]].
In preterm infants, disruption of pulmonary angiogenesis contributes to the development of bronchopulmonary dysplasia (BPD), a common disease of extreme prematurity that significantly contributes to infant mortality and long-term morbidity [11]. Multiple studies show NO therapy improves gas exchange, lung angiogenesis and regulates alveolar epithelial development in the newborn animal models [12]. Furthermore, a growing body of evidence demonstrate that nitrate and/or nitrite can protect against acute and chronic airway and pulmonary vascular diseases [2,4,[13], [14], [15], [16]].
However, the role of oral NR activity in infants is thought to be minimal. This perspective is based on data demonstrating that, relative to adults, NR activity in newborns is significantly lower with the activity slowly increasing over the first year of life [17,18]. Albeit lower, these data do not preclude a potential role for oral NR activity in newborn pathophysiology, and no studies have assessed potential temporal changes in oral NR activity in extremely preterm infants. Here, we conducted a longitudinal study measuring NR activity in extremely preterm infants from birth through 34 weeks' postmenstrual age (PMA) and evaluated associations with the oral microbiome, co-morbidities, exposures, and nutrition. We observed oral NR varies as a function of PMA, peaking at 29 weeks’ PMA, and surprisingly occurrence of this peak associates with lower incidence of BPD. These data support a novel hypothesis that oral microbiome mediated differences in NR activity provide a noninvasive biomarker for BPD development with the potential for therapeutic targeting.
2. Methods
Institutional Review Board approval at the University of Alabama at Birmingham was obtained prior to study initiation and informed consent from parent(s) obtained prior to patient enrollment. Infants were recruited from the University of Alabama at Birmingham Regional Neonatal Intensive Care Unit between August 2016 and January 2017 and sample collection completed in April 2017. Preterm infants enrolled were <29 weeks’ gestation at birth. Infants with major congenital anomalies were excluded.
2.1. Sample collection
Initial samples for oral microbiome and NR activity analyses were taken within 72 h after birth. Thereafter, weekly samples were collected 2 h post feeding until infants reached 34 weeks' PMA. For NR activity analysis, samples were taken from each infant's right posterior tongue dorsum as previously described [19] using sterile wood Fisherbrand cotton-tipped applicators (Cat. No. 23-400-115) by rotating the applicator 360°. All samples were collected by the same researcher to minimize inter-sampling variability. Samples were then inserted immediately in 1.5 ml of Brain Heart Infusion Broth (Anaerobe Systems, Morgan Hill, CA), placed on ice and NR activity measured within 2 h of collection. Fisherfinest Dry Transport swabs (Fisher HealthCare, Houston, Texas) were used for microbiome samples. At the same time points used for NR activity sampling, the applicator was rotated 360° over the left posterior tongue dorsum, immersed in 1 ml sterile normal saline, and placed in a −80 °C freezer until further analysis.
2.2. Nitrate reductase activity
NR activity was measured twice after sample collection: within 2 h of collection and after 18-h incubation under aerobic conditions in BHI at 37 °C. Two 90 μL aliquots (one for control and one to which nitrate was later added) were incubated in a water bath at 37 °C for 10 min prior to analysis and baseline nitrite levels measured. 10 μL of 10 mM nitrate was then added (1 mM final nitrate concentration), samples vortexed (~3 s), and nitrite formation measured over 20 min using triiodide chemiluminescence as previously described [19,20]. The nitrite levels from sample controls (no nitrate added) were then subtracted from the nitrate-enriched samples. Standard curves generated on the day of sampling from a nitrite solution of known concentration was used to calculate nitrite concentrations.
Differences in mean NR activity were compared over time. Associations between NR activity and oral microbiota were conducted via methods later described. Additional analyses were conducted between NR activity and the covariates of BPD (using the traditional definition defined at 36 weeks’ PMA as treatment with supplemental oxygen or respiratory support [21]), mode of delivery (caesarean section vs vaginal), time of sample collection, nutrition source (breast milk vs formula), and antibiotic exposure.
2.3. Oral microbiome analysis
DNA was extracted using a QIAGEN QIAamp DNA Stool Mini Kit (QIAGEN, Germany). Microbiome analysis using 16S rRNA sequencing was performed via methods previously described [22]. To determine bacterial numbers, colony forming unit assays were performed on each sample used to measure NR activity. Ten μL of sample were serially diluted in normal saline and plated in triplicate on Tryptic Soy Blood Agar plates (Anaerobe Systems), incubated for 24 h, and colony forming units averaged between the three plated samples.
To determine the oral microbiota associated with NR production, the top 100 abundant operational taxonomic units (OTU) represented across all samples were compared between four time points: birth, 27 weeks' PMA, 29 weeks' PMA, and 34 weeks’ PMA. The following analyses were conducted: (a) longitudinal comparisons of specific oral microbiota by PMA, (b) comparisons of oral microbiota by BPD status at each PMA of collection, and (c) comparisons of inter and intra-sample diversity by PMA and BPD status via alpha diversity and beta diversity respectively. The Shannon index was used to report alpha diversity. For the beta diversity analysis, we first calculated the beta diversities and then used the commonly used method, PERMANOVA, to test the difference of beta diversity between different PMAs and between infants with and without BPD [23]. We calculated three beta diversity measures: Bray-Curtis, Unweighted-Unifrac, and Weighted-Unifrac. Comparisons of the relative abundance of microbiota by PMA and BPD status were represented in stacked plots and heat map analyses. Comparisons with a p-value of <0.10 were considered significant.
2.4. Statistical analyses
Continuous demographic variables were reported as median and interquartile ranges with comparison between BPD and no-BPD infants made by Mann-Whitney unpaired t-test or chi-squared analysis. One-way ANOVA mixed model with Tukey post-test was performed to compare NR activity between each postnatal week. Paired t-tests were used to compare NR activity between two post-natal ages and unpaired t-test was used when assessing how clinical co-variates affecting NR activity. Unpaired t-tests were used to compare relative abundance of oral microbiota at each postnatal age. Spearman correlation metrics determined which microbiota were positively or negatively correlated with NR activity. All data were analyzed for normality using D'Agostino and Pearson test and appropriate post-test selected.
3. Results
3.1. Infant characteristics
Twenty-eight extremely preterm infants were enrolled in the study. The median gestational age of the preterm cohort was 25.8 weeks (interquartile range: 25, 27) with a median birth weight of 688 g (550, 891). All infants were exposed to at least 48 h of antibiotics (ampicillin and gentamicin) immediately after birth. Three infants died and one infant was transferred prior to eligibility for BPD assessment; a total of six samples were obtained for these infants for time points ranging from birth to 28 weeks’ PMA. Of the remaining 24 infants, fifteen developed BPD (60%). Other demographic data are displayed in Table 1.
Table 1.
Infant characteristics and Co-morbidities by BPD status.
| Characteristic | ALL Infants | No BPDa (N = 9) | BPDa (N = 15) | P |
|---|---|---|---|---|
| Gestational age (weeks), median (Q1,Q3) | 25.8 (25,27) | 26 (25,27) | 25 (23,27) | 0.30 |
| Birth weight (g), median (Q1,Q3) | 688 (550,891) | 710 (690,790) | 650 (543,913) | 0.18 |
| Male Sex, n (%) | 17 (60.7) | 4 (44) | 11 (73) | 0.16 |
| Race | ||||
| White, n (%) | 17 (61) | 6 (67) | 9 (60) | |
| Non-white, n (%) | 12 (43) | 3 (33.3) | 7 (47) | 0.74 |
| Multiple gestation | 6 (21) | 1 (11) | 4 (27) | 0.75 |
| Maternal antibiotic prophylaxis, n (%) | 12 (43) | 4 (44) | 8 (53) | 0.67 |
| Infants receiving 48 h antibiotics, n (%) | 28 (100) | 9 (100) | 15 (100) | >0.99 |
| Infants receiving >48 h antibiotics, n (%) | 19 (68) | 5 (56) | 11 (73) | 0.37 |
| Histologic chorioamnionitis, n (%) | 10 (36) | 3 (33.3) | 6 (40) | 0.74 |
| Antenatal corticosteroids, n (%) | 27 (96) | 8 (89) | 15 (100) | 0.19 |
| Caesarean section, n (%) | 18 (64) | 5 (56) | 10 (67) | 0.59 |
| Indication for delivery | ||||
| Preterm labor, n (%) | 14 (50) | 6 (67) | 7 (47) | 0.34 |
| Non reassuring fetal monitoring, n (%) | 8 (29) | 2 (22) | 4 (27) | 0.81 |
| Hypertensive disorder of pregnancy, n (%) | 4 (14) | 1 (11) | 2 (13) | 0.87 |
| Other | 2 (7) | 0 (0) | 2 (13) | – |
| 5 min Apgar, median (Q1,Q3) | 6 (3, 7) | 7 (6,8) | 6 (2,7) | 0.24 |
| Intubation at delivery, n (%) | 14 (50) | 3 (33.3) | 10 (67) | 0.59 |
| Surfactant. n (%) | 23 (82) | 6 (67) | 14 (93) | 0.09 |
| Exclusively breast milk fed, n (%) | 9 (32) | 2 (22) | 6 (40) | 0.47 |
| Pneumothorax, n (%) | 3 (11) | 0 (0) | 2 (13) | 0.25 |
| Bronchopulmonary dysplasia, n (%) | 16 (57.0) | – | – | – |
| Patent ductus arteriosus, n (%) | 15 60) | 3 (33) | 8 (53) | 0.34 |
| Pharmacologic treatment of PDA, n (%) | 4 (14) | 0 (0) | 3 (20) | 0.15 |
| Intracranial hemorrhage, n (%) | 8 (29) | 2 (22) | 4 (27) | 0.81 |
| Grade 3–4 intracranial hemorrhage, n (%) | 5 (18) | 0 (0) | 3 (20) | 0.15 |
| Early onset sepsis, n (%) | 1 (4) | 0 (0) | 1 (7) | >0.99 |
| Late onset sepsis, n (%) | 3 (11) | 0 (0) | 3 (20) | 0.26 |
| Necrotizing enterocolitis, n (%) | 1 (4) | 0 (0) | 1 (7) | 0.43 |
| Retinopathy of prematurity, n (%) | 14 (50) | 5 (56) | 9 (60) | 0.83 |
| Severe retinopathy of prematurity, n (%) | 5 (18) | 1 (11) | 4 (27) | 0.36 |
| Death, n (%) | 6 (21 | 0 (0) | 3 (20) | 0.15 |
Data represent median (Q1,Q3) for continuous variables and N (%) for categorical variables.
Excluding 3 infants that died and 1 patient transferred prior to BPD assessment.
3.2. Measuring nitrate reductase activity
NR activity was measured in 190 samples taken from 28 preterm infants between birth and 34 weeks’ PMA; median number of samples per patient was 8 (quartile ranges 7,10). For samples taken at birth, the median time of sample collection was 48.5 h after birth. In the initial 23 samples collected, from the first seven preterm infants enrolled (birth to 21 days postnatal age), NR activity was undetectable when measured within 2 h of swab collection; Supplementary Fig 1 shows representative traces for NO formation mediated by triiodide-dependent reduction of nitrite. However, when tongue swabs were cultured for 18 h first and then nitrate-dependent formation of nitrite assessed, a significant NR activity was observed (Supplementary Fig 1, 18h). Nitrate reductase activity was undetectable in samples collected from swabs exposed to air and cultured in parallel with patient-derived samples (Supplementary Fig 1, air swab) confirming that NR activity was oral cavity (tongue swab) derived. Subsequent measurements of NR activity were made after 18 h culturing of tongue swabs.
3.3. Longitudinal changes in nitrate reductase activity
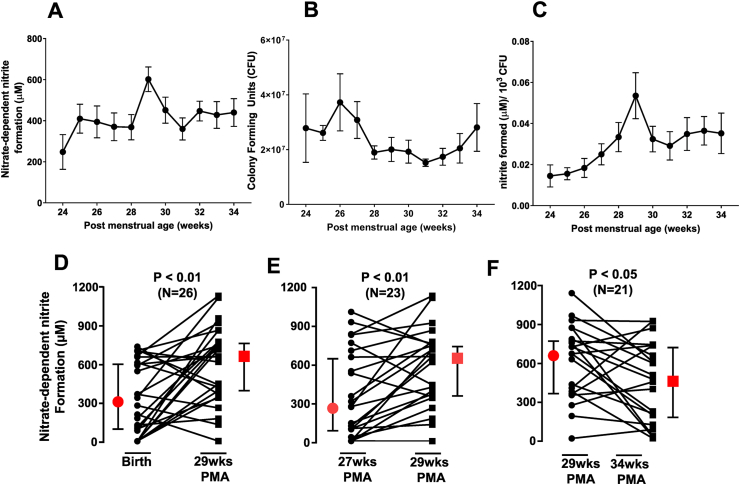
Fig. 1A–C plots the changes in NR activity, bacterial number (CFU) and NR activity normalized to bacterial number, as a function of PMA. NR activity increased ~1.7-fold between 24- and 25- weeks' PMA. Thereafter, NR activity remained steady at all times except at 29 weeks' PMA, where a further ~1.6-fold peak increase was observed (Fig. 1A). No changes in CFU were observed (p = 0.13 by one-way RM-mixed models ANOVA) (Fig. 1B). Since differences in sampling and bacterial number may modulate NR activity, the latter was normalized to CFU (Fig. 1C). After normalization to CFU, NR activity was observed to increase with PMA, peaking at 29 weeks followed by rapid 40% decrease at 30 weeks. NR activity per CFU remained steady thereafter. Fig. 1D–F presents paired analyses and show higher NR activity at 29 weeks' PMA compared to birth (Fig. 1D; p < 0.01), 27 weeks' PMA (Fig. 1E; p < 0.01), and 34 week's PMA (Fig. 1F; p < 0.05).
Fig. 1.
Nitrate reductase activity was measured in samples collected longitudinally from preterm infants (n = 28). Panel A shows nitrate-reductase activity (measured by nitrite formation after addition of nitrate to swab-derived cultures), p = 0.055 by mixed model 1way RM-ANOVA. Panel B shows CFU following 18 h culture of swabs (collected at indicated times). No significant changes over time were observed (p = 0.13 by mixed model one-way RM-ANOVA). Panel C: Nitrate reductase activity normalized to CFU. p < 0.04 by mixed model one-way RM-ANOVA. All data are mean ± SEM, with number of replicates ranging between 9 and 26 across PMAs. Panels D–F show changes in NR activity between birth and 29 weeks'PMA; 27–29 weeks' PMA and 29–34 weeks' PMA respectively. Each line indicates an individual patient. Red symbols indicate median with 95% CI. Indicated p-values determined by parametric paired t-test for panels E–F, and non-parametric paired t-test for panel D. N denotes number of patients per analysis. (For interpretation of the references to colour in this figure legend, the reader is referred to the Web version of this article)
3.4. Nitrate reductase activity and clinical covariates
Subgroup analyses for associations between NR activity before and after normalization to CFU, and clinical covariates were conducted (Supplementary Figure 2). Preterm infants born via spontaneous vaginal delivery had higher NR activity at birth compared to those born via cesarean section, however differences were not significant after normalizing to CFU (Supplementary Figure 2A). Longitudinal differences in NR activity beyond birth were not observed between infants born by cesarean compared to vaginal delivery (not shown). Samples collected >48 h after delivery had higher NR activity compared to samples taken within 48 h after delivery, however these differences were lost when normalized to CFU (Supplementary Figure 2B). Nutrition data were available for 18 infants; NR activity was significantly higher in infants fed exclusively maternal breast milk compared to infants fed a mixture of formula and/or breast milk but only when normalized to CFU (Supplementary Figure 2C). Exposure to antenatal antibiotics tended to increase NR activity (p < 0.07). However, when normalized to CFU, no difference in NR activity was observed between preterm infants exposed to maternal antibiotics compared to those that were not (Supplementary Figure 2D).
3.5. Changes in nitrate reductase activity predict BPD risk
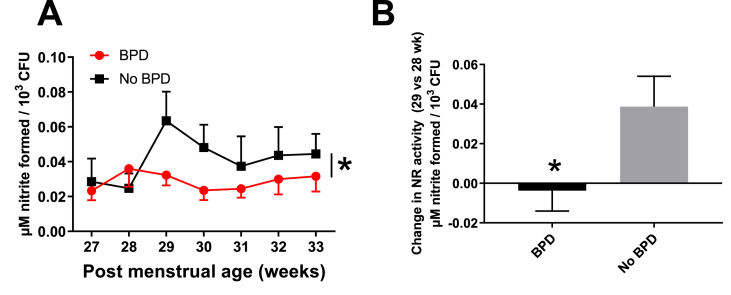
Temporal changes in NR activity between 27 and 33 weeks' PMA were replotted after separating patients who were diagnosed with BPD at 36 weeks' PMA. Infants who later developed BPD had no increase in NR activity at 29 weeks' PMA (Fig. 2A). In contrast, infants in whom NR activity increased at 29 weeks' PMA, did not develop BPD at 36 weeks' PMA; Fig. 2B shows the change in NR activity between 29- and 28- weeks’ PMA in BPD versus non-BPD patients.
Fig. 2.
Panel A. Nitrate reductase activity as a function of PMA and as a function of BPD diagnosis determined at 36 weeks' PMA. *p < 0.03 by two-way ANOVA. Data are mean ± SEM (n = 16 for BPD and n = 9 for no BPD group). Panel B: Change in NR activity between 28 and 29 weeks' PMA in BPD vs no-BPD infants. Data are mean ± SEM, p < 0.05 by Mann-Whitney t-test. n = 16 for BPD and n = 9 for no BPD group.
3.6. Longitudinal differences in oral microbiota
From the 28 infants in the cohort, the microbiome was analyzed from 101 isolates at the time points: birth, 27, 29, and 34 weeks’ PMA. Supplementary Figure 3 shows the top 100 identified OTUs based on relative abundance within each time point. Alpha diversity of oral microbiota was higher at birth (4.41) compared to all other time points of 27 weeks' PMA (2.35), 29 weeks' PMA (2.59), and ≥34 weeks' PMA (2.60) (p < 0.05).
Bacteria whose relative abundance significantly differed between 29 weeks' PMA and either birth, 27, or 34 weeks' PMA are depicted in a heat map (Fig. 3A); 29 weeks was chosen as a reference for these analyses as peak NR activity was observed at this time. There were 51 OTUs whose relative abundance differed at birth compared to at 29 weeks' PMA, while only 3 OTUs differed in relative abundance between 27 and 29 weeks' PMA; compared to infants at 27 weeks' PMA, Staphylococcus was less abundant and Streptococcus I and Stenotrophomonas were more abundant in infants at 29 weeks' PMA (p < 0.10). Compared to infants at 34 weeks' PMA, Veillonella, Streptococcus 2, Rothia mucilaginosa, and Corynebacterium 3 were less abundant and Staphlococcus and Bacillus were more abundant in infants at 29 weeks’ PMA (p < 0.10).
Fig. 3.
Panel A: Heat map showing relative abundance of bacteria for which relative abundance differed between 29 weeks' PMA and either birth, 27 weeks' PMA or 34 weeks' PMA. a p < 0.1 between birth and 29 weeks' PMA; b p < 0.1 between 27 and 29 weeks' PMA; c p < 0.1 relative abundance between 29 and 34 weeks' PMA by unpaired t-test. Panel B: Heat map showing relative abundance of bacteria for which relative abundance differed between BPD and no-BPD patients at the indicated times. a p < 0.1 at birth; b p < 0.1 at 29 weeks' PMA c p < 0.1 at 34 weeks' PMA.
3.7. Relative abundance of NR producing bacteria by BPD status
A heat map plotting the relative abundance of the 30 bacteria that differed by BPD status at any of indicated PMAs is represented in Fig. 3B. Nineteen of these differed at birth in BPD versus non-BPD infants, 15 at 29 weeks' PMA, and 3 at 34 weeks' PMA, and 9 bacteria showed differences at both birth and 29 weeks’ PMA. No differences were observed at 27 weeks' PMA. There were no differences in beta diversity between infants with and without BPD at each of the PMAs analyzed with p values of 0.409, 0.419, 0.482 (Bray-Curtis, Unweighted-Unifrac, and Weighted-Unifrac).
3.8. Relative abundance of microbiota and nitrate reductase activity
Previous studies have identified several candidate bacteria (Actinomyces, Rothia, Veillonella, Lactobacillus, Hemophilus, and Prevotella) as potential mediators of NR activity including in preterm infants [17,18,[24], [25], [26], [27], [28]]. To provide further insight into which bacteria may modulate NR activity in preterm infants, correlations between relative abundance and NR activity, pre and post normalization to CFU, were assessed using all samples, independent of PMA and clinical covariates (Table 2). Of these bacteria, relative abundance of Akkermansia muciniphila 2, Burkholderia, Chryseobacterium, f_Enterobacteriaceae, and f_Gemellaceae was significantly different at birth versus 29 weeks' PMA, and for Stenotrophomonas and Streptococcus 1 at 34 weeks' PMA versus 29 weeks’ PMA (Fig. 3A). Furthermore, relative abundance of Akkermansia muciniphila 2, Burkholderia, Chryseobacterium, Neisseria subflava, Prevotella 4, Prevotella pallens and f_Gemellaceae differed between BPD and no-BPD infants at one or more PMAs (Fig. 3B).
Table 2.
Bacteria whose relative abundance significantly (p < 0.1) correlated with NR activity or NR activity/CFU.
| NR activity without CFU normalization | Spearman Correlation Coefficient | P-value |
|---|---|---|
| Akkermansia muciniphilia 2 | 0.212 | 0.035 |
| Burkholderia | 0.207 | 0.040 |
| Chryseobacterium | 0.177 | 0.080 |
| Enterococcus | −0.195 | 0.053 |
| f__Enterobacteriaceae | −0.254 | 0.011 |
| Mycobacterium | −0.194 | 0.054 |
| Neisseria subflava | −0.183 | 0.070 |
| Prevotella 4 | 0.208 | 0.039 |
| Prevotella pallens | −0.169 | 0.094 |
| Tissierella Soehngenia | −0.177 | 0.080 |
|
| ||
| NR activity with CFU normalization |
Spearman Correlation Coefficient |
P-value |
| Enterococcus | −0.265 | 0.009 |
| f__Enterobacteriaceae | −0.179 | 0.083 |
| f__Gemellaceae | 0.191 | 0.064 |
| Mycobacterium | −0.233 | 0.023 |
| Staphylococcus | −0.232 | 0.024 |
| Stenotrophomonas | 0.172 | 0.095 |
| Streptococcus 1 | 0.317 | 0.002 |
4. Discussion
Formation of NO in mammals is mediated by nitric oxide synthase dependent and independent mechanisms. The latter involves nitrate-reduction to nitrite by commensal oral nitrate-reducing bacteria. The nitrite formed provides substrate for various nitrite-reduction pathways that mediate NO-signaling by hypoxia and pH-dependent mechanisms [9]. Using this rationale, we conducted a prospective cohort study to evaluate longitudinal changes in oral NR activity in extremely preterm infants. We observed two surprising and striking findings. First, there was a statistically significant increase in NR activity at 29 weeks' PMA compared to other time points. The second was the association that infants who did not show an increase in NR activity at 29 weeks' PMA subsequently developed BPD thereby supporting the prognostic potential for NR activity measurements in predicting BPD development. Currently, there are few predictive biomarkers for BPD and our observations suggest that measurement of oral NR activity at 29 weeks' PMA may predict BPD risk. However, longitudinal assessment allowing for calculation of the change in NR activity between 29 weeks’ PMA and comparing periods before and after this time point (see Fig. 3E) may be a more sensitive index in predicting BPD. Importantly, since assessment of oral NR activity can occur by non-invasive sampling, multiple time points for assessment is feasible. While encouraging, these findings need to be validated in larger clinical studies capable of adjusting for other covariates known to influence BPD risk, and if validated could result in new clinical management of these at-risk patients.
In addition to a predictive function, our data suggest a potential mechanistic link between deficits in NR-activity and associated NO-signaling at 29 weeks' PMA, and BPD development. Further studies are required to assess first whether NR-dependent nitrite and NO-formation is indeed altered at 29 weeks’ PMA and in turn, how this may affect lung development. Notably, targeting NO-signaling has been explored as a therapeutic to treat BPD. Inhaled nitric oxide (iNO) improves gas exchange and angiogenesis in animal models [29,30]. While multiple large, randomized controlled trials of iNO in preterm infants have not shown a reduction in death or BPD [31] recent analyses suggest that iNO may reduce BPD in certain patient populations [32]. Furthermore, inhibition of endogenous NO production and/or genotypes wherein basal NO production is decreased have been shown to increase the risk for BPD development [33,34]. Our data suggest that changes in oral NR activity, and ensuing changes in nitrite-derived NO, should be considered as a possible NO-dependent pathway affecting lung development and function, and potentially represents a novel target to modulate BPD risk. Furthermore, given that inhaled NO is routinely used in infants with persistent pulmonary hypertension [31,35], and that up to 39% of infants with severe BPD have associated pulmonary hypertension [36], we speculate that oral NR activity may also play a role in pulmonary vascular function/dysfunction.
Interestingly, the 29 weeks' PMA time point coincides with a critical window in diseases associated with prematurity, specifically the time of peak necrotizing enterocolitis (NEC) occurrence [37]. Since disruptions in the balance of active intestinal vasoconstriction and vasodilation, mediated in part by lower NO bioavailability, have been implicated in NEC pathogenesis [38], the increased oral NR activity observed at 29 weeks' PMA may have physiological significance in providing an additional pathway for NO generation at this crucial time in development. Our study was not powered to observe associations between NR activity and NEC as only one patient in this cohort developed NEC. Further, while we cannot explain the peculiar time course for changes in NR activity, the coincidence of NR peaking at 29 weeks’ PMA with the window when NEC is observed warrants additional studies to assess how NR activity may be linked to changes in NOS-independent NO production, and NEC pathogenesis.
Our results are consistent with previous conclusions in older preterm infants with an average gestational age of 30 weeks at birth wherein NR activity remained undetectable until two weeks of age, and then when activity became detectable, it remained low, ~10% that of adults [17,18]. Oral NR activity in the data presented here was low and only detectable after bacterial amplification; no NR activity was evident in swabs exposed to air only, underscoring the low abundance of NR bacteria in the oral cavity from preterm infants. This observation is similar to our prior studies investigating oral NR activity in adult mice [19]. Importantly, nitrate- and oral microbiome dependent NO-signaling has been demonstrated in mice [19] suggesting that despite bacterial abundance and NR activity being relatively low, this may still be biologically functional. Moreover, it is possible that compared to adults, the amount of nitrite needed to elicit NO-dependent effects in the newborn is lower. Thus, while the oral NR activity is low, our data suggests that it is present and increases from birth in premature babies, though further studies are required to assess functional importance by correlating oral NR activity with plasma based metrics of NO availability.
Our recent studies have identified associations between lung microbiome in premature infants and BPD [22] but little is known on the potential role for oral microbiome in this patient group. Variables previously noted to influence initial lung or intestinal microbiota include delivery mode [39], the diversity and progression of breast milk flora [40] vs formula [41], and maternal intrapartum antibiotic exposure [42]. The oral cavity is colonized within hours [43] and, while initially similar, divergence between oral and intestinal microbiota later occurs at around 15 days of life [44]. We note that oral NR activity was higher in infants receiving maternal breast milk compared to formula fed infants, which of particular interest as NEC and other diseases of prematurity occur significantly more frequently in formula fed infants [[45], [46], [47]]. Human milk contains nitrate which is considered to be a key substrate for NO generation in newborns [48], and dietary nitrate promotes colonization of the oral cavity with NR-expressing bacteria in adults [49]. Our data showing that an increased NR activity in preterm infants fed breast milk was only observed when normalized to CFU is consistent with these concepts and support hypotheses that breast milk positively selects for bacteria that mediate nitrate-reduction. We also observed that delivery mode influenced the NR activity as did the time of first isolate collection. In both of these instances, differences were lost when NR activity was normalized to CFU suggesting that vaginal delivery and time post birth positively influence bacterial colonization rather than select for bacteria that promote nitrate reduction. Finally, we note that maternal antibiotic administration did not inhibit NR activity and in fact tended to increase NR activity. Further studies are needed to determine how antibiotics may modulate oral microbiome and specifically the bacteria that modulate NR activity.
To gain insights into how oral microbiota may influence observed NR activity, we characterized bacteria based on relative abundance at four different time points and as a function of whether infants developed BPD. In addition to understanding relationships between specific bacteria and observed changes in NR activity, these insights could also lead to additional bacterial markers that could be developed to assess BPD risk. Changes in NR activity are unlikely to result from isolated changes in relative abundance of a single bacterium and more likely to reflect changes in the bacterial community. However, there were no differences in beta diversity between infants with and without BPD. Furthermore, both bacteria that positively and negatively correlated with NR activity were identified. Amongst the bacteria that positively correlated with NR activity were Streptococcus, Stenotrophomonas, and Gemellaceae. These microbiota have been reported to modulate the oral microbiome in infants [50,51] and known to associate with NR activity [52]. Recent studies show the gut and lung microbiomes modulate disease risk [53,54] with potential interrelationships between bacteria across these distinct sites noted. Further studies assessing how the oral microbiome axis relates to the lung and gut microbiome axis may yield new insights into our understanding of how commensal organisms are colonized from birth and how they modulate disease susceptibility.
There are other limitations to this study in addition to those noted above. Its observational nature necessarily constraints all conclusions to associations. We cannot exclude the potential that the oral NR activity measured here may not directly relate to any nitrate-dependent activation of NO-signaling cascades in conferring BPD risk. In addition, we recognize the limitations associated with culturing tongue swabs for 18 h and that NR activity measured after this protocol may not reflect activity mediated by native microbiome. Future studies with appropriate power to adjust for other covariates influential to BPD risk are needed to both validate these findings and determine the predictive utility of oral NR measurement in estimating BPD risk. Lastly, in the absence of gastrointestinal pathology within this cohort, the role of NR in NEC pathogenesis remains speculative.
5. Summary
In this cohort of extremely preterm infants, NR activity significantly peaked at 29 weeks’ PMA and was associated with subsequent development of BPD. This increase in NR activity during a critical period of gastrointestinal maturity may be of physiologic necessity to limit diseases associated with prematurity. Furthermore, we demonstrate that delivery mode and nutrition potentially influence NR activity via postnatal changes in the oral microbiota of extremely preterm infants. Further studies testing the power of NR activity measurements to predict BPD and to establish cause-effect relationships between NR activity, oral microbiome and BPD are warranted.
Funding source
American Heart Association 17SDG32720009 (CVL), National Institute of Health National Heart, Lung and Blood Institute (NHLBI) Grants K08HL141652 (CVL), R01HL129907 (NA), U01HL133536 (NA), and National Institute of Health National Institute of Child Health and Human Development (NICHD) R21HD100917 (RP, SG).
Financial disclosure
The authors have indicated they have no financial relationships relevant to this article to disclose.
Declaration of competing interest
RPP is a coinventor on the use of nitrite salts for the treatment of cardiovascular conditions and chronic ischemia. SJG, KA, CVL and RPP are co-inventors on a provisional patent for methods to diagnose and predict chronic lung and bowel disease in pre-term infants.
Acknowledgments
SG and RPP acknowledge support from NICHD R21 HD100917.
Footnotes
Supplementary data to this article can be found online at https://doi.org/10.1016/j.redox.2020.101782.
Appendix A. Supplementary data
The following is the Supplementary data to this article:
References
- 1.Duncan C. Chemical generation of nitric oxide in the mouth from the enterosalivary circulation of dietary nitrate. Nat. Med. 1995;1(6):546–551. doi: 10.1038/nm0695-546. [DOI] [PubMed] [Google Scholar]
- 2.Kapil V. The noncanonical pathway for in vivo nitric oxide generation: the nitrate-nitrite-nitric oxide pathway. Pharmacol. Rev. 2020;72(3):692–766. doi: 10.1124/pr.120.019240. [DOI] [PubMed] [Google Scholar]
- 3.Lundberg J.O., Gladwin M.T., Weitzberg E. Strategies to increase nitric oxide signalling in cardiovascular disease. Nat. Rev. Drug Discov. 2015;14(9):623–641. doi: 10.1038/nrd4623. [DOI] [PubMed] [Google Scholar]
- 4.Koch C.D. Enterosalivary nitrate metabolism and the microbiome: intersection of microbial metabolism, nitric oxide and diet in cardiac and pulmonary vascular health. Free Radic. Biol. Med. 2017;105:48–67. doi: 10.1016/j.freeradbiomed.2016.12.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Larsen F.J. Effects of dietary nitrate on blood pressure in healthy volunteers. N. Engl. J. Med. 2006;355(26):2792–2793. doi: 10.1056/NEJMc062800. [DOI] [PubMed] [Google Scholar]
- 6.Kapil V. Inorganic nitrate supplementation lowers blood pressure in humans: role for nitrite-derived NO. Hypertension. 2010;56(2):274–281. doi: 10.1161/HYPERTENSIONAHA.110.153536. [DOI] [PubMed] [Google Scholar]
- 7.Webb A.J. Acute blood pressure lowering, vasoprotective, and antiplatelet properties of dietary nitrate via bioconversion to nitrite. Hypertension. 2008;51(3):784–790. doi: 10.1161/HYPERTENSIONAHA.107.103523. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Kapil V. Physiological role for nitrate-reducing oral bacteria in blood pressure control. Free Radic. Biol. Med. 2013;55:93–100. doi: 10.1016/j.freeradbiomed.2012.11.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Govoni M. The increase in plasma nitrite after a dietary nitrate load is markedly attenuated by an antibacterial mouthwash. Nitric Oxide. 2008;19(4):333–337. doi: 10.1016/j.niox.2008.08.003. [DOI] [PubMed] [Google Scholar]
- 10.Joshipura K. Vol. 29. Blood Press; 2020. pp. 103–112. (Over-the-counter Mouthwash Use, Nitric Oxide and Hypertension Risk). 2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Stoll B.J. Trends in Care practices, morbidity, and mortality of extremely preterm neonates, 1993-2012. J. Am. Med. Assoc. 2015;314(10):1039–1051. doi: 10.1001/jama.2015.10244. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Savani R.C. Modulators of inflammation in bronchopulmonary dysplasia. Semin. Perinatol. 2018;42(7):459–470. doi: 10.1053/j.semperi.2018.09.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Baliga R.S. Dietary nitrate ameliorates pulmonary hypertension: cytoprotective role for endothelial nitric oxide synthase and xanthine oxidoreductase. Circulation. 2012;125(23):2922–2932. doi: 10.1161/CIRCULATIONAHA.112.100586. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Zuckerbraun B.S. Nitrite potently inhibits hypoxic and inflammatory pulmonary arterial hypertension and smooth muscle proliferation via xanthine oxidoreductase-dependent nitric oxide generation. Circulation. 2010;121(1):98–109. doi: 10.1161/CIRCULATIONAHA.109.891077. [DOI] [PubMed] [Google Scholar]
- 15.Honavar J. Nitrite therapy improves survival postexposure to chlorine gas. Am. J. Physiol. Lung Cell Mol. Physiol. 2014;307(11):L888–L894. doi: 10.1152/ajplung.00079.2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Hunter C.J. Inhaled nebulized nitrite is a hypoxia-sensitive NO-dependent selective pulmonary vasodilator. Nat. Med. 2004;10(10):1122–1127. doi: 10.1038/nm1109. [DOI] [PubMed] [Google Scholar]
- 17.Kanady J.A. Nitrate reductase activity of bacteria in saliva of term and preterm infants. Nitric Oxide. 2012;27(4):193–200. doi: 10.1016/j.niox.2012.07.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Timby N. Effects of age, sex and diet on salivary nitrate and nitrite in infants. Nitric Oxide. 2020;94:73–78. doi: 10.1016/j.niox.2019.10.012. [DOI] [PubMed] [Google Scholar]
- 19.Ahmed K.A. Measuring nitrate reductase activity from human and rodent tongues. Nitric Oxide. 2017;66:62–70. doi: 10.1016/j.niox.2017.04.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Pelletier M.M. The measurement of blood and plasma nitrite by chemiluminescence: pitfalls and solutions. Free Radic. Biol. Med. 2006;41(4):541–548. doi: 10.1016/j.freeradbiomed.2006.05.001. [DOI] [PubMed] [Google Scholar]
- 21.Jobe A.H., Bancalari E. Bronchopulmonary dysplasia. Am. J. Respir. Crit. Care Med. 2001;163(7):1723–1729. doi: 10.1164/ajrccm.163.7.2011060. [DOI] [PubMed] [Google Scholar]
- 22.Lal C.V. The airway microbiome at birth. Sci. Rep. 2016;6:31023. doi: 10.1038/srep31023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.McMurdie P.J., Holmes S. phyloseq: an R package for reproducible interactive analysis and graphics of microbiome census data. PLoS One. 2013;8(4) doi: 10.1371/journal.pone.0061217. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Doel J.J. Evaluation of bacterial nitrate reduction in the human oral cavity. Eur. J. Oral Sci. 2005;113(1):14–19. doi: 10.1111/j.1600-0722.2004.00184.x. [DOI] [PubMed] [Google Scholar]
- 25.Burleigh M. Dietary nitrate supplementation alters the oral microbiome but does not improve the vascular responses to an acute nitrate dose. Nitric Oxide. 2019;89:54–63. doi: 10.1016/j.niox.2019.04.010. [DOI] [PubMed] [Google Scholar]
- 26.Kapil V. Sex differences in the nitrate-nitrite-NO(*) pathway: role of oral nitrate-reducing bacteria. Free Radic. Biol. Med. 2018;126:113–121. doi: 10.1016/j.freeradbiomed.2018.07.010. [DOI] [PubMed] [Google Scholar]
- 27.Tribble G.D. Frequency of tongue cleaning impacts the human tongue microbiome composition and enterosalivary circulation of nitrate. Front Cell Infect Microbiol. 2019;9:39. doi: 10.3389/fcimb.2019.00039. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Vanhatalo A. Nitrate-responsive oral microbiome modulates nitric oxide homeostasis and blood pressure in humans. Free Radic. Biol. Med. 2018;124:21–30. doi: 10.1016/j.freeradbiomed.2018.05.078. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Kinsella J.P., Ivy D.D., Abman S.H. Inhaled nitric oxide improves gas exchange and lowers pulmonary vascular resistance in severe experimental hyaline membrane disease. Pediatr. Res. 1994;36(3):402–408. doi: 10.1203/00006450-199409000-00022. [DOI] [PubMed] [Google Scholar]
- 30.McCurnin D.C. Inhaled NO improves early pulmonary function and modifies lung growth and elastin deposition in a baboon model of neonatal chronic lung disease. Am. J. Physiol. Lung Cell Mol. Physiol. 2005;288(3):L450–L459. doi: 10.1152/ajplung.00347.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Barrington K.J., Finer N., Pennaforte T. Inhaled nitric oxide for respiratory failure in preterm infants. Cochrane Database Syst. Rev. 2017;1:CD000509. doi: 10.1002/14651858.CD000509.pub5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Askie L.M. Race effects of inhaled nitric oxide in preterm infants: an individual participant data meta-analysis. J. Pediatr. 2018;193:34–39 e2. doi: 10.1016/j.jpeds.2017.10.004. [DOI] [PubMed] [Google Scholar]
- 33.Davis C.W. Expression of nitric oxide synthases and endogenous NO metabolism in bronchopulmonary dysplasia. Pediatr. Pulmonol. 2008;43(7):703–709. doi: 10.1002/ppul.20848. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Trittmann J.K. An arginase-1 SNP that protects against the development of pulmonary hypertension in bronchopulmonary dysplasia enhances NO-mediated apoptosis in lymphocytes. Phys. Rep. 2016;4(22) doi: 10.14814/phy2.13041. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Barrington K.J. Nitric oxide for respiratory failure in infants born at or near term. Cochrane Database Syst. Rev. 2017;1:CD000399. doi: 10.1002/14651858.CD000399. [DOI] [PubMed] [Google Scholar]
- 36.Arjaans S. Identification of gaps in the current knowledge on pulmonary hypertension in extremely preterm infants: a systematic review and meta-analysis. Paediatr. Perinat. Epidemiol. 2018;32(3):258–267. doi: 10.1111/ppe.12444. [DOI] [PubMed] [Google Scholar]
- 37.Yee W.H. Incidence and timing of presentation of necrotizing enterocolitis in preterm infants. Pediatrics. 2012;129(2):e298–304. doi: 10.1542/peds.2011-2022. [DOI] [PubMed] [Google Scholar]
- 38.Nankervis C.A., Giannone P.J., Reber K.M. The neonatal intestinal vasculature: contributing factors to necrotizing enterocolitis. Semin. Perinatol. 2008;32(2):83–91. doi: 10.1053/j.semperi.2008.01.003. [DOI] [PubMed] [Google Scholar]
- 39.Dominguez-Bello M.G. Delivery mode shapes the acquisition and structure of the initial microbiota across multiple body habitats in newborns. Proc. Natl. Acad. Sci. U. S. A. 2010;107(26):11971–11975. doi: 10.1073/pnas.1002601107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Cabrera-Rubio R. The human milk microbiome changes over lactation and is shaped by maternal weight and mode of delivery. Am. J. Clin. Nutr. 2012;96(3):544–551. doi: 10.3945/ajcn.112.037382. [DOI] [PubMed] [Google Scholar]
- 41.Patel K. Trends and determinants of gastric bacterial colonization of preterm neonates in a NICU setting. PLoS One. 2015;10(7) doi: 10.1371/journal.pone.0114664. e0114664. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Keski-Nisula L. Maternal intrapartum antibiotics and decreased vertical transmission of Lactobacillus to neonates during birth. Acta Paediatr. 2013;102(5):480–485. doi: 10.1111/apa.12186. [DOI] [PubMed] [Google Scholar]
- 43.Nelson-Filho P. Dynamics of microbial colonization of the oral cavity in newborns. Braz. Dent. J. 2013;24(4):415–419. doi: 10.1590/0103-6440201302266. [DOI] [PubMed] [Google Scholar]
- 44.Costello E.K. Microbiome assembly across multiple body sites in low-birthweight infants. mBio. 2013;4(6) doi: 10.1128/mBio.00782-13. e00782-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Sisk P.M. Early human milk feeding is associated with a lower risk of necrotizing enterocolitis in very low birth weight infants. J. Perinatol. 2007;27(7):428–433. doi: 10.1038/sj.jp.7211758. [DOI] [PubMed] [Google Scholar]
- 46.Meinzen-Derr J. Role of human milk in extremely low birth weight infants' risk of necrotizing enterocolitis or death. J. Perinatol. 2009;29(1):57–62. doi: 10.1038/jp.2008.117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Lucas A., Cole T.J. Breast milk and neonatal necrotising enterocolitis. Lancet. 1990;336(8730):1519–1523. doi: 10.1016/0140-6736(90)93304-8. [DOI] [PubMed] [Google Scholar]
- 48.Hord N.G. Nitrate and nitrite content of human, formula, bovine, and soy milks: implications for dietary nitrite and nitrate recommendations. Breastfeed. Med. 2011;6(6):393–399. doi: 10.1089/bfm.2010.0070. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Kapil V. Dietary nitrate provides sustained blood pressure lowering in hypertensive patients: a randomized, phase 2, double-blind, placebo-controlled study. Hypertension. 2015;65(2):320–327. doi: 10.1161/HYPERTENSIONAHA.114.04675. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Ruiz L. Microbiota of human precolostrum and its potential role as a source of bacteria to the infant mouth. Sci. Rep. 2019;9(1):8435. doi: 10.1038/s41598-019-42514-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Gomez-Arango L.F. Antibiotic treatment at delivery shapes the initial oral microbiome in neonates. Sci. Rep. 2017;7:43481. doi: 10.1038/srep43481. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Hyde E.R. Metagenomic analysis of nitrate-reducing bacteria in the oral cavity: implications for nitric oxide homeostasis. PLoS One. 2014;9(3) doi: 10.1371/journal.pone.0088645. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Gentle S.J., Lal C.V. Predicting BPD: lessons learned from the airway microbiome of preterm infants. Front Pediatr. 2019;7:564. doi: 10.3389/fped.2019.00564. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Enaud R. The gut-lung Axis in health and respiratory diseases: a place for inter-organ and inter-kingdom crosstalks. Front Cell Infect Microbiol. 2020;10:9. doi: 10.3389/fcimb.2020.00009. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.