Abstract
Incorporating chicken feather fibre (CFF) into natural based-nanocomposite comprising of glycerine plasticized-cassava starch binder with bentonite (BNT) as nanofiller, a thermal insulator (TIN) was synthesized. Central Composite Design (CCD) Response Surface Methodology was employed to carry out the experimental design using two factors (CFF and BNT) along with one response (thermal conductivity) to produce nine materials as insulators, comprising of 0%, 5%, and 10% BNT based on 8 g initial weight of CFF. A sample without CFF was used as the control. Developed thermal insulators were subjected to thermal conductivity tests using Lee's disc method at a steady state. The best insulator is TIN-4 with the lowest thermal conductivity of 0.0313 W/(mK) and the highest insulation property of 114.63 m2k/W, while TIN-10 with no CFF has the highest thermal conductivity of 0.0549 W/(mk) and lowest insulation property of 48.1603 m2k/W. Proportionate use of chicken feather fibre in starch-clay nanocomposite will help synthesize an effective thermal insulator with minimum cost.
Keywords: Chemical engineering, Materials science, Nanotechnology, Nanomaterials, Materials application, Materials processing, Materials synthesis, Thermal insulator, Nanofiller, Chicken feather, Nanocomposite, Crystallographic
Chemical engineering; Materials science; Nanotechnology; Nanomaterials; Materials application; Materials processing; Materials synthesis; Thermal insulator; Nanofiller; Chicken feather; Nanocomposite; Crystallographic.
1. Introduction
The improper management or disposal of by-products of materials processed from industrial, domestic, social, and commercial activities has posed many problems to the ecosystem. Effective waste management is expensive [1]. Global production of the chicken feather as waste is estimated to be about 15 million tons per year [1]. The burning of this waste brings about the release of harmful substances into the air [1, 2].
Production of thermal insulators using chicken feathers will address the challenge of waste disposal and create wealth from waste. Materials of low thermal conductivity are known as thermal insulators; they are used to reduce heat transfer between material in contact. The thermal conductivities of some materials, as reported somewhere [1, 3, 4] confirmed that chicken feather fibre (CFF) has very low thermal conductivity with unique structures and properties compare to any other natural or synthetic fibre [5, 6]. This justifies its use for thermal insulator and ease of wide industrial application. For instance, low-density value (0.8 g/cm3 compared to about 1.5 g/cm3 and 1.3 g/cm3 for cellulose fiber and wool, respectively), excellent compressibility and resiliency, ability to dampen sound, and distinctive morphological structures [6, 7, 8] make them unique. Chicken feathers contain about 91% keratin protein, 1% lipids, and 8% water [9] comprising of three different parts, the rachis, the barbs, and the barbules, which are attached one to the other (the barbules to the barbs and the barbs to the rachis) [10].
Feathers are in two microcrystalline forms, the fiber, and the quill. The thermal energy required to perturb the quill is less than that required by fibers; hence, the fibres can withstand thermal and mechanical stress [5]. The elasticity moduli of feather keratin range from 0.045 GPa to 10 GPa, while the tensile strength of oven-dried CFF, in the range of 41–130 MP (5). Thermogravimetric analysis of CFF, as reported elsewhere [11] suggests that the drying temperature for this fiber should be above 328.15K, with the processing temperature of a reinforced composite being controlled below 493.15K. Differential scanning calorimetry of CFF indicated that temperatures below 383.15K may not allow for moisture progression [12]. The utilization of chicken feather barbs for use as a thermal insulator relates to its intriguing property of honeycomb structure and thermal resistivity ability due to air pockets in the feather [13, 14]. The binding of chicken feathers in various composites has been achieved by using various polymers such as chitosan-starch blend, high-density polyethylene (HDPE), elastic polyurethane, polylactic acid, polybutyrate adipate terephthalate, and polyvinyl alcohol [4, 15, 16, 17, 18, 19].
This study considered the use of glycerine-plasticized cassava starch (CST) as a binder. Starch has been used for composites production in various works [20, 21, 22, 23]. According to Van Heemst et al. [24], cassava starch occurs naturally as a granular structure and cannot be used directly as a thermoplastic material except by destructuring through mechanical works at elevated temperatures in the presence of water and other possible processing agents e.g. plasticisers like polyols and fatty acids, forming thermoplastic starch (TPS) [25]. The end products of starch have poor dimensional stability and mechanical properties [26, 27, 28]. Still, its limitations can be resolved by introducing plasticizers (such as glycerine, glycol, and sorbitol) to enhance flexibility and processing ability [29, 30, 31]. TPS and nanocomposite mixture possess improved thermal and mechanical properties [32, 33, 34, 35].
Nanomaterials'shape and size have widespread applications in industries and research fields such as their introduction into polymer matrix [36]. Their usage as doped material has improved the properties of the polymer [37, 38, 39]. Nanofillers are introduced in the polymer at rates from 1-10% by mass. The composites' properties are impacted by organic matrix and nanofiller mixture ratio [40] to yield improved mechanical, thermal, and fire retardation [41, 42]. The most widely used clay-based nanocomposites are the smectites (such as montmorillonite, bentonite and hectorite) [43]. Montmorillonites have a high cation exchange capacity, which is little affected by particle size. Therefore, the cation exchange capacity allows both inorganic and organic cations to bind [44], maximizing its utilization as a nanofiller to improve the composition's properties.
In this study, novel thermal insulators (TIN) were produced by incorporating CFF into glycerine plasticized-cassava starch (CST) and bentonite (BNT) nanocomposite. This study seeks the thermal insulators' production such that naturally available materials (BNT was used as doped nanofiller and strength impacts while CST was used as a binder) to convert waste chicken feathers to thermal insulators. Furthermore, this study was to develop insulators which are not dependent on synthetic polymers, thereby minimizing some health and ecosystem related issues instigated by synthetic polymers and the after use of chicken feather waste. Performance evaluation was carried out using the Lee's disc method of thermal conductivity test. The proposed CFF in starch-clay nanocomposites synthesis of TIN is convenient, economical, and environmentally friendly, thereby encouraging the conversion of waste to wealth.
2. Materials and method
2.1. Materials, reagent, and equipment
The chicken feathers were sourced in form of wastes from a poultry farm in Eket Village, Eket Local Government Area, Akwa Ibom State, Nigeria. Cassava tubers were obtained from the farm in Etinan Local Government Area, Akwa Ibom State, Nigeria. Industrial bentonite, glycerine, and 98% ethanol were obtained from Ekpo Scientific Enterprises, Eket Local Government Area, Akwa Ibom State, Nigeria. Equipment and apparatus used include mechanical sieving machine with 0.2 mm mesh size sieve, ceramic mortar and pestle, Soxhlet extractor, heating mantle, hot plate, electronic weighing balance, 500 ml beakers, 50 ml measuring cylinder, thermometer, inextensible string, steam can, micrometer screw guage, vernier caliper, Lee's disc apparatus, stainless steel batch reactor, manual compression pressing machine, electronic oven and universal testing machine.
2.2. Preparation of chicken feather fibre powder
The first step of the preparation process was cleaning chicken feathers by washing with soap and water. The feathers were utterly cleaned and then dried in a convection oven at 318.15 K for seven days to ensure complete evaporation. Fibre was then separated from the quill through scissors, and the feathers were grounded using mortar and pestle. Lipids present in the ground feathers were removed by contacting 20 ml of ethanol with 8 g of the feather powder, using Soxhlet extractor for 4 h. The lipid-free feather particles obtained were then dried and stored at room temperature.
2.3. Production of starch from cassava
The cassava roots obtained from the farm were peeled. The peeled roots were washed and grated. Small volumes of water were added to the grated cassava and stirred vigorously. The slurry obtained was filtered in a sieve of mesh size 140 μm. The filtrate was allowed to settle for 2 h and the supernatant decanted. The starch mash was dried in an oven until a moisture content of 10%–15% was obtained. The starch obtained was milled and packed at room temperature.
2.4. Production of feather/starch-clay nanocomposite
The design of experiments was achieved using the response surface methodology technique (central composite design (CCD)) with the aid of Design Expert 11 software (Table 1). Two factors (CFF and BNT) were used as the independent variables to obtain the dependent variable or the response, thermal conductivity. The effect of 0 g CFF content (i.e. TIN-10) was considered as the control. The starch was kept constant while varying the weight of BNT and CFF. The composites were produced considering 0%, 5%, and 10% BNT (nanofillers were introduced in the polymer at rates from 1–10% by mass) [40] to 8 g initial weight of CFF. At 0% concentration of BNT based on 8 g initial CFF, the CFF concentration is 8 g (100%); 5% BNT based on 8 g initial weight of CFF produced 0.40 g BNT with 7.6 g CFF; and 10% BNT based on 8 g initial weight of CFF produced 0.80 g BNT with 7.2 g CFF.
Table 1.
The experimental design generated using central composite design.
| Run | 1 | 2 | 3 | 4 | 5 | 6 | 7 | 8 | 9 | 10 |
|---|---|---|---|---|---|---|---|---|---|---|
| Factor1 (A: CFF) | 8.16 | 8 | 7.6 | 7.6 | 8 | 7.04 | 7.6 | 7.2 | 7.2 | 0 |
| Factor 2 (B: BNT) | 0.4 | 0.8 | 0.97 | 0.4 | 0 | 0.4 | 0 | 0 | 0.8 | 0.4 |
The experimental runs (Table 1) were generated with the CCD response surface methodology with the two factors (A = CFF and B = BNT) considered using their lowest weight content (A = 7.2 g and B = 0 g) and the highest weight content (A = 8 g and B = 0.80 g) selected based on the percentages considered above. The ten experimental runs were carried out with a constant composition of 20 g of starch, 35 ml of water and 6 ml of glycerol. The raw materials were feed into the reactor and mixed in this order; CST, water, glycerol, and CFF.
The reaction proceeded with continuous stirring for about 30 min, at 373.15K. The rigid material obtained was transferred to a cylindrical mold, compressed in a cylindrical mold, and cooled to 298.15k. The resulting composites were oven-dried at 100 °C and kept for further studies.
2.5. Analytical method
2.5.1. Dimensional analysis
The average thickness, d (mm), of the samples was measured with a micrometer screw gauge by measuring five different points on the material [45]. The diameter, D (mm) of the samples obtained as well (using the same approach as in thickness) through the use of an electronic digital caliper (ISO9001).
2.5.2. X-ray diffraction (XRD) analysis
XRD analysis was used to check for the nanofiller properties utilized to ascertain whether the material is BNT as desired. The XRD analysis was operated with the continuous scan type from 5° 2θ to 75° 2θ diffractometer with step time of 32.90 s, the temperature of 298.15K, and generator settings kept at 40 mA and 45 kV. Copper anode (λ = 1.54060 Å) material was used to carry out this analysis.
2.5.3. Thermal conductivity test
The thermal conductivity test was achieved using Lee's disc experiment (Figure 1). Through a steam can, steam was generated and allowed to pass over the nano-sized composite material produced with its outlet connected to the steam chamber's inlet using a rubber tube. In the meantime, the weight of the Lee's disc, M, was obtained with a weighing balance. The specific heat capacity of the metal slab, C was recorded from a constant table. The composite was placed on metal slab (the disc) and then the steam chamber on the composite and suspended from the clamp stand. A thermometer was inserted into the Lee's disc and the steam chamber, allowed to read until a constant temperature was obtained for these thermometers. Steady-state temperatures were attained and recorded as T1 and T2 for the steam chamber and disc, respectively. Constant temperature reading by the thermometers was recorded after 10 min. The thermometers were interchanged, and the new temperatures at steady state were recorded, then the average of the readings was recorded (and for disc and chamber, respectively). The steam chamber was raised, and the composite was removed. The steam chamber directly heated Lee's disc until its temperature was about + 283.15K in steady-state (Figure 1). The steam chamber was removed with the heat allowed to distribute uniformly for 2–3 min over the disc. The composite material was then placed on the disc while observing the temperature reading of the thermometer. With the disc allowed to cool to , the temperature was read and recorded immediately for every 30 s (using a stopwatch) until the temperature falls to about 283.15K below the temperature, .. Using Microsoft Excel 2007, the rate of cooling, for each composite was deduced. Conductive heat flow occurs in the decreasing temperature (from the steam chamber through the sample to the disc). Fourier's Law expresses conductive heat transfer, as shown in Eq. (1).
| (1) |
Figure 1.
Schematic diagram of the Lee's disc experiment.
At steady state, the mean temperatures, are constant, and the rate of heat transfer (H) through the disc equal heat loss (Equation 2), using the cooling rate of the top brass disc covered with the chicken feather insulation, the cooling rate () was determined.
| (2) |
Combining Eqs. (1) and (2), the thermal conductivity, , was obtained as given in Eq. (3). Where M is the mass of the lower disc, and C is the specific heat.
| (3) |
2.5.4. Comparison of the experimental with the predicted values
The experimental response values obtained were tested for accuracy by comparing the experimental values with the predicted values of the CCD generated model. Design Expert 11.1.2.0 was used for the design and interpretation of results. The significance of the quadratic model was also tested with analysis of variance (ANOVA).
3. Results and discussion
3.1. Sample diameter measurement
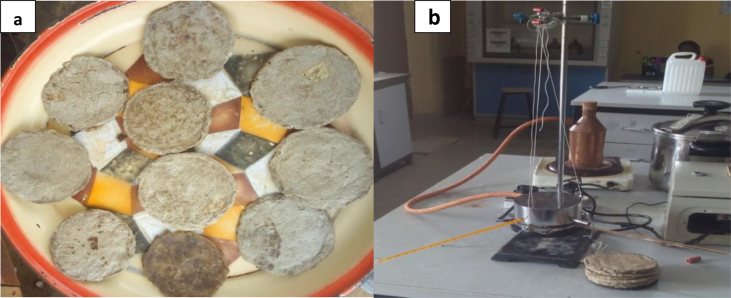
The average diameter, D, and thickness, d of the thermal insulator (TIN) samples (TIN-1 to TIN-10) were measured and recorded as given in Table 2. The insulators (Figure 3(a)) with CFF were seen to have more thickness than the sample without the CFF (TIN-10) in it.
Table 2.
Average thickness, d, and diameter, D, of the insulators.
| Sample | d (mm) | D (mm) |
|---|---|---|
| TIN-1 | 3.300 | 100.322 |
| TIN-2 | 3.516 | 97.032 |
| TIN-3 | 2.888 | 101.480 |
| TIN-4 | 2.848 | 101.346 |
| TIN-5 | 3.246 | 108.312 |
| TIN-6 | 3.588 | 106.686 |
| TIN-7 | 3.520 | 106.532 |
| TIN-8 | 3.430 | 99.094 |
| TIN-9 | 3.378 | 102.194 |
| TIN-10 | 2.644 | 95.524 |
Figure 3.
(a) The developed insulator and (b) The experimental set up of Lee's method for the thermal conductivity test.
3.2. XRD analysis
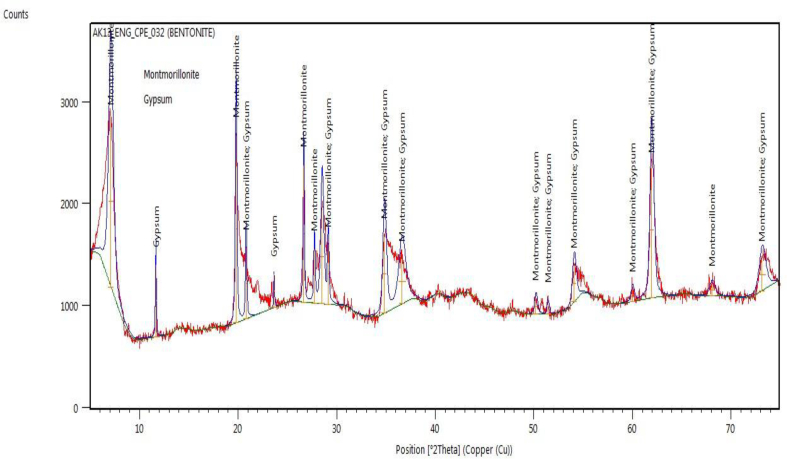
The XRD analysis of BNT nanofiller is as provided in Figure 2. The diffractogram showed that the substance contains two minerals: montmorillonite and gypsum, and both minerals have a monoclinic crystal system. The chemical formula for the montmorillonite (MMT) is Si7.80Al1.72Cs0.16Fe0.20Mg0.28O20.00, while for gypsum is Ca4.00S4.00O24.00H4.00. The crystallographic parameters are given in Table 3. Bentonite is an aluminuimphyllosillicate clay generated frequently from the alteration of volcanic ash consisting mostly of smectite minerals, usually montmorillonite [46].
Figure 2.
X-Ray diffractogram for the BNT nanofiller.
Table 3.
Crystallographic parameters of the minerals in the BNT.
| Crystallographic |
Mineral |
|
|---|---|---|
| Parameters | Montmorillonite | Gypsum |
| a (Å)a | 5.181 | 6.2742 |
| b (Å) | 8.945 | 5.67 |
| c (Å) | 12.34 | 5.67 |
| Alpha (Å) | 90 | 90 |
| Beta (Å) | 99.62 | 113.908 |
| Gamma (Å) | 90 | 90 |
| Calculated density | 1.84 | 2.27 |
| (g/cm3) | ||
Å (Angstrom unit) = 10−10m.
In addition to montmorillonite, it contains a small portion of other mineral matter like quartz, zeolites, gypsum, pyrite, or feldspar [47]. From Figure 2, the pattern shows that the substance contains MMT which is the major mineral formed at various peaks which can be seen at 7.10 2θ with the d-spacing of 12.4369Å, 26.512 2θ (d = 3.3459Å), 29.12 2θ (d = 3.0665Å), 36.63 2θ (d = 2.4531Å), 61.93 2θ (d = 1.44984Å) and further reflection at 68.06 2θ (d = 1.3777Å). Also, gypsum was noticed at about 11.66 2θ with a d-spacing of 7.5869Å and further confirmed at 23.63 2θ with a d-spacing of 3.7653Å. The diffractogram generated is similar to works carried out by Carlson, 2004 [48] and El Miz et al., 2017 [49].
3.3. Thermal conductivity analysis
The mass M of the Lee's disc was measured and recorded as 0.7794 kg, while the specific heat capacity of brass, C, was obtained as 0.38 kJ/kg K [50]. The mean temperature at steady state (Table 4) was obtained from the experimental setup, Figure 3(b).
Table 4.
Mean steady-state temperature for the insulators.
| Sample | TIN-1 | TIN-2 | TIN-3 | TIN-4 | TIN-5 | TIN-6 | TIN-7 | TIN-8 | TIN-9 | TIN-10 | |
|---|---|---|---|---|---|---|---|---|---|---|---|
| TM (K) | TMA | 369.75 | 369.75 | 367.15 | 369.45 | 367.65 | 369.65 | 364.45 | 368.45 | 369.65 | 370.45 |
| TMC | 320.15 | 317.15 | 317.05 | 315.45 | 322.45 | 316.65 | 327.65 | 319.65 | 324.05 | 341.15 |
The influence of the CFF can be noticed (Table 4) on the temperature readings. Sample TIN-10 having no CFF allowed more heat to pass through it. For the mean temperature of 370.45K recorded by the thermometer inserted into A (the steam chamber), the sample allowed more heat to pass through it with 341.15K temperature of the disc. The higher the ability of a sample to retain heat, the lesser the heat released to C. This means, the higher the conductivity of heat by the insulator, the higher the temperature value read by the thermometer. Better retention of heat was observed by the insulators, TIN-1 to TIN-9 (with CFF) instead of TIN-10 with no CFF. The temperature recorded by TIN-1 to TIN-9 was much lesser than that of TIN-10 (Table 4). This result shows the tendency of CFF to retard heat flow.
The synthesized TIN's insulation efficiency was determined by measuring the cooling rate of the brass disc at steady-state temperature with the brass disc top covered with synthesized TIN. The disc's temperature in each run was recorded per minute and represented in Figure 4, and their respective coefficient of determination R2 was recorded in Table 5. The linearity of temperature with time was observed from the temperature per time plot that temperature reduces with increasing time (Figure 4 and Table 5).
Figure 4.
Variation in temperature of the synthesized TIN with time.
Table 5.
Parameters and their values for the thermal insulators.
| Sample | BNT (g) | (K/s) | R2 | '' W/(mK) | RT (mK/W) | RV (m2K/W) |
|---|---|---|---|---|---|---|
| TIN-1 | 0.4 | 0.017 | 0.982 | 0.038 | 26.385 | 87.071 |
| TIN-2 | 0.8 | 0.013 | 0.999 | 0.035 | 28.736 | 101.035 |
| TIN-3 | 0.97 | 0.02 | 0.970 | 0.042 | 23.697 | 68.436 |
| TIN-4 | 0.4 | 0.014 | 0.977 | 0.031 | 31.949 | 90.990 |
| TIN-5 | 0.0 | 0.023 | 0.981 | 0.052 | 19.231 | 62.423 |
| TIN-6 | 0.4 | 0.014 | 0.977 | 0.032 | 30.960 | 111.084 |
| TIN-7 | 0.0 | 0.018 | 0.998 | 0.057 | 17.513 | 61.646 |
| TIN-8 | 0.0 | 0.022 | 0.986 | 0.059 | 16.863 | 57.842 |
| TIN-9 | 0.8 | 0.021 | 0.991 | 0.056 | 17.825 | 60.214 |
| TIN-10 | 0.4 | 0.014 | 0.990 | 0.055 | 18.215 | 48.160 |
At steady-state, the rate of cooling is the same as the rate of heating. Hence, the rate of cooling was obtained (Figure 4), where the cooling temperature for each of the experimental run was measured per 30 s after the steady-state temperature was attained and recorded in Table 4. The plot for each of the samples has a negative slope (Figure 4), indicating the drop in temperature due to cooling experienced by the disc through heat loss to the environment and the covering insulator. The cooling rate () for sample TIN-1 to TIN-10 are given in Table 5. The cooling rate in each case is almost similar, with the highest value observed to be 0.023 K/s in TIN-5 and the lowest being 0.013 K/s in TIN-2, accounting for only a difference of 0.10 K/s between the highest and lowest rate. The coefficient of determination R2 was used to assess the experimental data's accuracy or fitness (Table 5). From Table 5, the obtained R squared values were above 97%, indicating accuracy since they are close to 100%. The values obtained for the parameters needed to achieve the thermal conductivity () for each of the samples were substituted into Eq. (3). Table 5 shows relevant parameters for the different insulators.
3.3.1. Comparison of the thermal conductivity of the samples based on the weight of BNT
The thermal conductivity across the thermal insulators as given in Table 5, was further evaluated categorically based on their BNT weight concentrations. Four groups, namely; 0 g, 0.4 g, 0.8 g, and 0.97 g, were considered. TIN-5, TIN-7, and TIN-8 fall under 0 g BNT; TIN-1, TIN-6, and TIN-10 fall under 0.4g BNT; TIN-2 and TIN-9 fall under 0.8g BNT; and finally, TIN-3 (being the only sample) falls under 0.97g BNT.
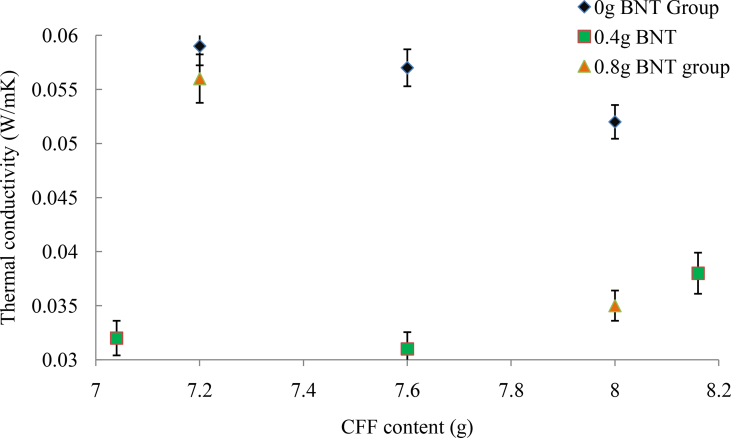
Considering the 0 g BNT group, the samples' thermal conductivity increased with a reduction in CFF content from samples TIN-5, TIN-7 to TIN-8. The thermal conductivity values across each sample were deduced as 0.0520 W/(mK), 0.0571 W/(mK), and 0.0593 W/(mK), respectively (Figure 5). Thermal conductivities for the 0.4g BNT category were 0.0379 W/(mk), 0.0313 W/(mK), 0.0323 W/(mK), and 0.0549 W/(mK) for TIN-1, TIN-4, TIN-6, and TIN-10 respectively. The 0.4 g BNT followed the similar trend as that of 0 g BNT with the only exception for TIN-1 with a greater value than TIN-4 which could be due to the environmental condition at which the experiment was conducted. For the 0.8 g BNT group, TIN-2 and TIN-9 made up of 8 g, and 7.2 g of CFF have value 0.0348 W/(mK) and 0.0561 W/(mK) respectively. The thermal conductivity for TIN-2 is lesser than that of TIN- 9 (Figure 5). Finally, the 0.97 g BNT with TIN-3 the thermal conductivity value of 0.0422 W/(mK).
Figure 5.
Categorical comparison of samples based on BNT content.
The effect of CFF on the thermal conductivity (Figure 6) is that increase in CFF content resulted in decreased thermal conductivity, indicating that CFF has suitable insulation property. Since the property of a thermal insulator is also obtained as thermal resistivity, RT (the inverse of thermal conductivity), the higher the resistivity, the better the insulation property of the material.
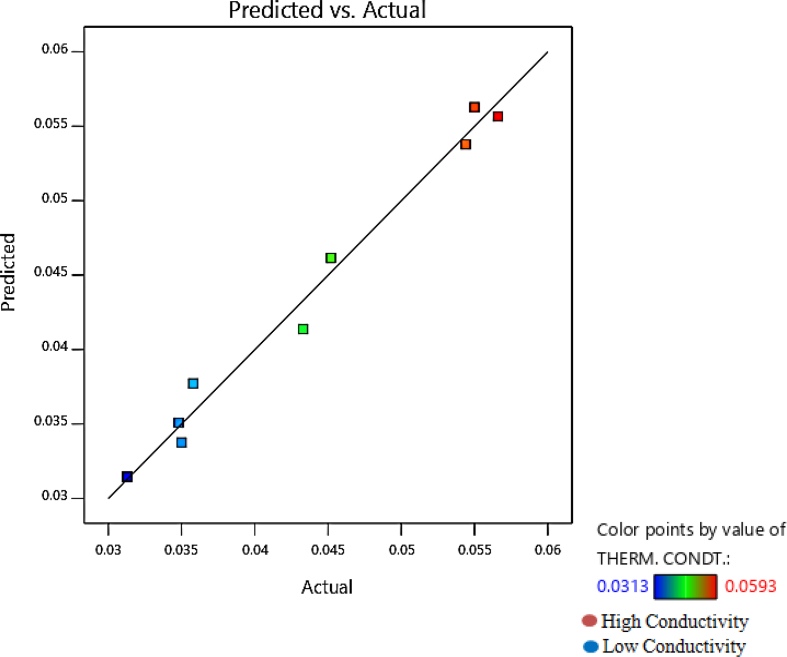
Figure 6.
The plot of predicted (W/mK) against actual value (W/mK) for the response.
Comparing best insulators in these groups, for the group 0 g BNT, TIN-8 is the best insulator with a 0.0593 W/mK thermal conductivity. The 0.4g BNT group has TIN-4 with 0.0313 W/(mK) as the best. TIN-2 with thermal conductivity of 0.0348 W/(mK) for 0.8 g BNT group. Finally, TIN-3 of 0.97 g BNT (the only sample if this group) has 0.0422 W/(mK). Considering these insulators, TIN-4 with 0.0313 W/(mK) has the lowest heat flow allowance, accounting for the best property as an insulator. TIN-10 with 0 g CFF has a thermal conductivity of 0.0549 W/(mK), which is the highest in the 0.4 g BNT group. TIN-10 can be termed as having the most deficient insulation property with no CFF compared to the rest materials in this group. The results are similar to that reported elsewhere [4, 52].
3.3.2. The resistance value (RV)
The resistance value (Table 5) is a property for checking how well a material resists conductive flow [51, 52]. The higher the RV, the more excellent its resistance and so its thermal insulating property. The 0% containing CFF nanocomposite was the least of this property (48.1603 m2K/W) than the other insulators. The highest value was noticed for TIN-6 (111.0836 m2K/W), followed by TIN-2 with 101.0345 m2K/W, and TIN-4 with 90.9904 m2K/W. Others are TIN-1, TIN-3, TIN-5, TIN-7, TIN-8, and TIN-9 following sequentially. However, it is often preferred for economic reasons that a thermal insulator should offer excellent resistance to heat flow at a lower thickness. Considering this fact, TIN-4 with a lesser thickness of 2.84 m is preferable, compared to TIN-6 (3.588 m) and TIN-2 (3.516 m). If we assume that TIN-4, TIN-6, and TIN-2 were all made up of 3.588 m thickness (as possessed by TIN-6 with the highest R-value), then TIN-4 will maintain even a higher RV of 114.6326 m2K/W TIN-6 and TIN-2 with RV of 111.0836 m2K/W and 103.1034 m2K/W.
3.4. Comparison of experimental and the predicted values from CCD
The experimental results obtained were compared to the predicted values by the CCD method, where the R2 was obtained as 0.8773. To get an R2 above 0.8999, the response (thermal conductivity) attained were adjusted (Tables 6 and 7) to fit with the response predicted. The fit statistics is presented in Table 7. The predicted R2 of 0.8773 is in reasonable agreement with the adjusted R2 of 0.9683 because of the difference of which is less than 0.2 (Design Expert 11). Also, adequate precision, which measures the signal to noise ratio (where a ratio greater than 4 is desirable) was found to be a ratio of 17.854. This indicated that the signal was adequate. Therefore, this model (Equation 4) can be used to navigate the design space. The lack of fit and degrees of freedom analysis was obtained as 3, which agrees with the recommended value of at least three and pure error of one. These conditions ensured a valid lack of fit test.
| (4) |
Table 6.
Diagnostics reports showing Experimental, Actual, Predicted and Residual Values for adjustment.
| Sample | TIN-1 | TIN-2 | TIN-3 | TIN-4 | TIN-5 | TIN-6 | TIN-7 | TIN-8 | TIN-9 | TIN-10 | |
|---|---|---|---|---|---|---|---|---|---|---|---|
| Response (Therm. Cond.) | Experimental | 0.0379 | 0.0348 | 0.0422 | 0.0313 | 0.052 | 0.0323 | 0.0571 | 0.0593 | 0.0561 | 0.0549 |
| Value | |||||||||||
| Actual Value | 0.035 | 0.0348 | 0.0452 | 0.0313 | 0.055 | 0.0358 | 0.0544 | 0.0566 | 0.0433 | - | |
| Predicted Value | 0.0338 | 0.0351 | 0.0461 | 0.0315 | 0.0563 | 0.0377 | 0.0538 | 0.0557 | 0.0414 | - | |
| Residual (experimental – predicted value) | 0.0012 | -0.0003 | -0.0009 | -0.0002 | -0.0013 | -0.0019 | 0.0006 | 0.0009 | 0.0019 | - | |
| Leverage | 0.64 | 0.626 | 0.664 | 0.462 | 0.734 | 0.64 | 0.413 | 0.734 | 0.626 | - | |
| Internally Studentized Residuals | 1.16 | -0.262 | -0.913 | -0.118 | -1.376 | -1.781 | 0.449 | 1.019 | 1.756 | - | |
| Externally Studentized Residuals | 1.234 | -0.229 | -0.888 | -0.102 | -1.641 | -3.392 | 0.399 | 1.025 | 3.177 | - | |
| Cook's Distance | 0.399 | 0.019 | 0.274 | 0.002 | 0.87 | 0.941 | 0.024 | 0.477 | 0.858 | ||
| Influence on Fitted Value DFFITS | 1.646 | -0.296 | -1.249 | -0.095 | -2.726 | -4.524 | 0.335 | 1.702 | 4.107 | ||
| Standard Order | 6 | 4 | 8 | 9 | 2 | 5 | 7 | 1 | 3 | ||
Table 7.
Fit statistics of the R2.
| Std. Dev. | 0.0018 | R2 | 0.9859 |
|---|---|---|---|
| Mean | 0.0423 | Adjusted R2 | 0.9683 |
| C.V. % | 4.25 | Predicted R2 | 0.8773 |
| Adequate Precision | 17.8542 |
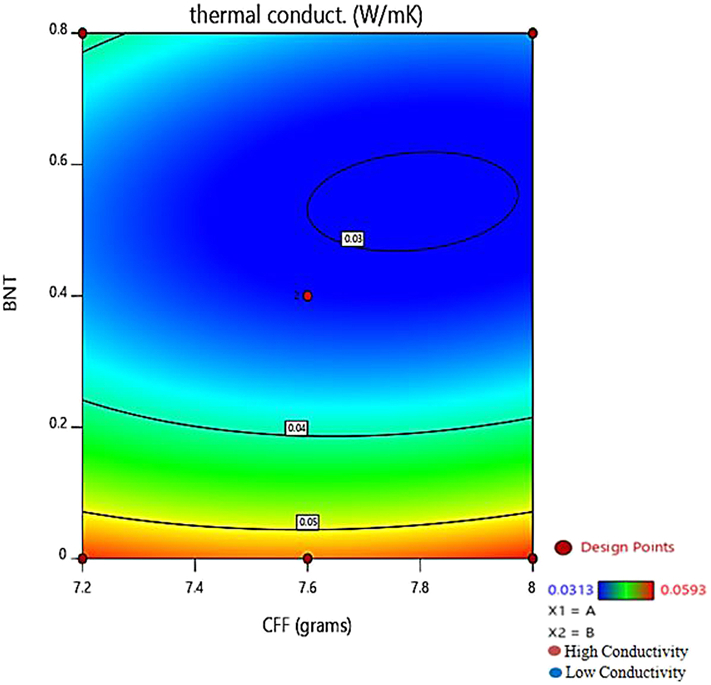
The plot of predicted against the actual value of the response (thermal conductivity) is given in Figure 6 shows a close agreement of the actual values with the predicted value. The contour (Figure 7) shows a value of 0.03W/(mK), which is almost similar to the experimental value of 0.0313W/(mK) (sample 4). The interaction between BNT and CFF on the thermal conductivity response is presented in Figure 8. The contour plot and the 3D response curves help analyze the interactions between factors on the responses, determining optimum levels for factors and responses [53, 54]. In the diagnostic report (Table 7), the leverage for each run is reported with values greater than 0 and less than 1. The leverage of a point ranges from 0 to 1 and indicates how much an individual design point influences the model's predicted values. The more the leverage is closer to 1, the more the residual value will be closer to 0. Hence, the closeness of the observed values of the experiment to the predicted values can be noticed from the table.
Figure 7.
The contour plot of predicted (thermal conductivity) against BNT and CFF.
Figure 8.
3D Surface response of thermal conductivity versus CFF and BNT.
3.5. Analysis of variance
Analysis of variance (ANOVA), as provided in Table 8, was applied to the results to determine the statistical significance and fitting of the quadratic model to this study. From the fit summary where different process orders were examined like the linear model, quadratic, cubic, mean, linear, and the interactions were tested, the quadratic was seen to have the highest F-value and lowest P-value. Hence, the quadratic model was selected for the analysis. The quadratic model was further tested to obtain more statistical results. The model fitness test is achieved by checking for F-test. When a P-value for a test is less than 5%, it implies that a model term is significant. The F-test returned an F-value of 56.05 and p-value (<0.05), which implies that the model adopted for the study is accurate and highly significant. The amount which the model prediction missed the observation known as lack of fit under the degrees of freedom (DOF) analysis column (Table 8) was obtained as 3, which is in agreement with the recommended value of at least three (3). This conditioned, along with a pure error of 1 ensured a valid lack of fit test. The terms expressed in model for B (linear value), A2 and B2 (squared values) are significant model terms. Meaning that the linear effect of BNT (B) and the quadratic effects of A (CFF) and B (BNT) are significant in the production of the insulator. P-value > 0.10 indicates the model terms are not significant, and this is the case with term AB (interaction of CFF and BNT on production of insulator is not significant, having no pronounced effect).
Table 8.
ANOVA (analysis of variance) of quadratic model.
| Source | Sum of Squares | DOF | Mean Square (Variance) | F-value | p-value | Interpretation |
|---|---|---|---|---|---|---|
| Model | 0.0009 | 5 | 0.0002 | 56.05 | 0.0009 | Highly significant |
| A-CFF | 0.0000 | 1 | 0.0000 | 4.94 | 0.0904 | Marginally significant |
| B-BNT | 0.0005 | 1 | 0.0005 | 156.03 | 0.0002 | Highly significant |
| AB | 0.0000 | 1 | 0.0000 | 3.70 | 0.1269 | Not significant |
| A2 | 0.0000 | 1 | 0.0000 | 7.12 | 0.0559 | Significant |
| B2 | 0.0006 | 1 | 0.0006 | 191.55 | 0.0002 | Highly significant |
| Residual | 0.0000 | 4 | 3.22E-06 | |||
| Lack of Fit | 0.0000 | 3 | 4.29E-06 | |||
| Pure Error | 0.0000 | 1 | 0.0000 | |||
| Cor Total | 0.0009 | 9 |
4. Conclusion
In this study, thermal insulators were developed using CFF in composite with BNT as nanofiller and starch binder. Nanocomposites of 0%, 5% and 10% bentonite clay to 8 g of feather were formed. The quadratic process model was analyzed using ANOVA. The thermal conductivity () of the composites materials is affected by the BNT content of the samples and was noticed by the various categories. Better insulation property was observed by group 0.4 g BNT in TIN-4 with 0.0313 W/mk against 0 g BNT in TIN-8 with 0.0593 W/mk, 0.8 g BNT in TIN-2 with 0.0348 W/mk and 0.9 g BNT in TIN-3 with of 0.0422 W/mk. However, the control sample TIN-10 with 0 g of CFF has the highest value of 0.0549 W/mk and can be termed the poorest in terms of thermal conductivity and with the least insulation property of 48.1603 m2k/W. In contrast, sample TIN-4 has the highest value of 114.63 m2k/W. The experimental value thus agreed with the predicted values obtained from the central composite design through leverage analysis.
5. Recommendation
This study seeks the development and encouragement of synthetic-independent thermal insulators where waste chicken feathers are used as the primary source by curtailing some health and ecosystem related issues instigated by synthetic polymers and the after use of chicken feather waste. Hence, it is recommended that:
-
i.
More investigations should be carried out on the application of chicken feather wastes to develop synthetic-independent thermal insulators, which will be an inexpensive raw material for the industry.
-
ii.
Thermogravimetry and thermal diffusivity studies should be carried out to better diversify and harness the application of the CFF as a thermal insulator.
-
iii.
Utilization of this material as an alternative thermal insulation source whose application will be useful in construction, domestic and industrial purposes due to its light weight and low cost.
Declarations
Author contribution statement
Rasheed Babalola: Conceived and designed the experiments; Performed the experiments; Analyzed and interpreted the data; Wrote the paper.
Augustine O. Ayeni: Analyzed and interpreted the data; Contributed reagents, materials, analysis tools or data; Wrote the paper.
Peter S. Joshua: Performed the experiments; Analyzed and interpreted the data.
Ayodeji A. Ayoola & Vincent E. Efeovbokhan: Analyzed and interpreted the data; Contributed reagents, materials, analysis tools or data.
Ukeme O. Isaac: Analyzed and interpreted the data.
Umo Aniediong: Performed the experiments; Analyzed and interpreted the data; Wrote the paper.
James A. Omoleye: Contributed reagents, materials, analysis tools or data.
Funding statement
This research did not receive any specific grant from funding agencies in the public, commercial, or not-for-profit sectors.
Competing interest statement
The authors declare no conflict of interest.
Additional information
No additional information is available for this paper.
Acknowledgements
The authors appreciate the Management of Akwa Ibom State University for providing the enabling environment for this study and Covenant University, Canaan land, Ota, Nigeria, for sponsoring the publication of this study.
References
- 1.Worldbank . April, 2019. Solid Waste Management. Understanding Poverty Urban Development.http://www.worldbank.org/en/topic/urbandevelopment/brief/solid-waste-management [Google Scholar]
- 2.Van Heemst J.J., Zant E., Schennink G.G.J., Rodenburg J., Rodenburg T. 4th April, 2019. Starch-based Biodegradable Polymer; Method of Manufacture and Articles Thereof, Patent EP2493975B1(2010)https://patents.google.com/patent/EP249397B1#similarDocuments [Google Scholar]
- 3.Ye W., Broughton M.R. Chicken feather as a fibre source for nonwoven insulation. Int. Nonwovens J. 2019;8:1–9. [Google Scholar]
- 4.Qin X. The University of Waikato; 2015. Chicken Feather Fibre Mat/PLA Composites for thermal Insulation.https://researchcommons.waikato.ac.nz/bitstream/handle/10289/9371/thesis.pdf?sequence=3&isAllowed=y Master of Engineering Thesis. [Google Scholar]
- 5.Chinta S.K., Landage S.M., Yadav K. Application of chicken feathers in technical textiles. Int. J. Innov. Res. Sci. Eng. Technol. 2013;2:5493–5498. http://www.ijirset.com/upload/april/42_Application.pdf [Google Scholar]
- 6.Reddy N., Yang Y. Structure and properties of chicken feather barbs as natural protein fibers. J. Polym. Environ. 2007;15:81–87. [Google Scholar]
- 7.Jones L.N., E Riven D., Tucker D.J. Marcel Dekker Inc.; New York: 1998. Handbook of Fiber Chemistry. [Google Scholar]
- 8.Barone J.R., Schmidt W.F. Polyethylene reinforced with keratin fibres obtained from chicken feathers. Compos. Sci. Technol. 2005;65:173–181. [Google Scholar]
- 9.Lederer R. 2005. Integument, feathers, and molt, ornithology: the science of birds. [Google Scholar]
- 10.Pahua-Ramos M.E., Hernández-Melchor D.J., Camacho-Pérez B., Quezada-Cruz M. Degradation of chicken feathers: a review, BioTechnol.: Indian J. For. 2017;13:153. [Google Scholar]
- 11.Dullaart R., Mousquès J. Vol. 164. Nova Science Publishers; Hauppauge, New York: 2012. Keratin: Structure, Properties, and Application. [Google Scholar]
- 12.Kock J.W. Georgia Institute of Technology; May 2006. Physical and Mechanical Properties Chicken Feather Materials. Master of Science Thesis. [Google Scholar]
- 13.Bansal G., Singh V.K. Review on chicken feather fiber (CFF) a livestock waste in composite material development. Int. J. Waste Resour. 2016;6:1–4. [Google Scholar]
- 14.Griffith B. U.S. Patent application No. 101008. 2001. https://patents.justia.com/inventor/barry-allen-griffith
- 15.Flores-Hernandez C.G., Colin-Cruz A., Velasco-Santos C., Castaño V.M., Rivera-Armenta J.L., Almendarez-Camarillo A., Garcia-Cassilas P.E., Martinez-Hernandez A.L. All green composites from fully renewable biopolymers: chitosan-starch reinforced with keratin from feathers. Polym. 2014;6:686–705. [Google Scholar]
- 16.Oladele I.O., Omotoyimbo J.A., Ayemidejor S.H. Mechanical properties of chicken feather and cow hair fibre reinforced high density polyethylene composites. Int. J. Sci. Technol. 2014;3:66–72. [Google Scholar]
- 17.Wrześniewska-Tosik K., Zajchowski S., Bryśkiewicz A., Ryszkowska J. Feathers as a flame-retardant in elastic polyurethane foam. Fibres Text. East. Eur. 2014;103:119–128. [Google Scholar]
- 18.Aranberri I. S.Montes, I. Azcune, A. Rekonde, H-J Grande, Fully biodegradable biocomposites with high chicken feather content. Polym. 2017;9:593. doi: 10.3390/polym9110593. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Chen X., Wu S., Yi M., Ge J. Preparation and physicochemical properties of blend films of feather keratin and poly(vinyl alcohol) Compatibilized by tris(hydroxymethyl)aminomethane. Polym. 2018;10:1054. doi: 10.3390/polym10101054. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Cao X., Chen Y., R P., Chang Muir A.D., Falk G. Starch-based nanocomposites reinforced with flax cellulose nanocrystals. Express Polym. Lett. 2008;2(2008):502–510. [Google Scholar]
- 21.Zainuddin S.Y.Z., Ahmed I., Kargarzadeh H. Preparation and characterization of cassava starch nanocomposite reinforced kenaf. Adv. Mater. Res. 2012;545(2012):348–352. [Google Scholar]
- 22.Karimi S., Dufresne A., Tahir M., Karimi A., Abdulkhani A. Biodegradable starch-based composites: effect of micro and Nano reinforcements on composite properties. J. Mater. Sci. 2014;49:4513–4521. [Google Scholar]
- 23.Issa A.T., Schimmel K.A., Worku M., Shahbazi A., Ibrahim S.A., Tahergorabi R. Sweet potato starch-based nanocomposites: development, characterization and biodegradability. Starch. 2018;70(2018):1700273. [Google Scholar]
- 24.Van Heemst J.J., Zant E., Schennink G.J., Rodenburg J., Rodenburg T. Starch-based biodegradable polymer; method of manufacture and articles thereof. PCT, WO 2011/053131. 2010. https://patentimages.storage.googleapis.com/45/fc/2a/13fd5a2c254d24/WO2011053131A1.pdf
- 25.Bastioli C. Global status of the production of biobased packaging materials. Starch. 2001;53:351–355. [Google Scholar]
- 26.Tapia-Blácido D.R. P.J. do Amaral Sobral, F.C. Menegalli, Optimization of amaranth flour films plasticized with glycerol and sorbitol by multi-response analysis. LWT - Food Sci. Technol. (Lebensmittel-Wissenschaft -Technol.) 2011;44:1731–1738. [Google Scholar]
- 27.Muscat D., Adhikari B. R.Adhikari, D.S. Chaudhary, Comparative study of film forming behaviour of low and high amylose starches using glycerol and xylitol as plasticizers. J. Food Eng. 2012;109:189–201. [Google Scholar]
- 28.Dang K.M., Yoksan R. Development of thermoplastic starch blown film by incorporating plasticized chitosan. Carbohydr. Polym. 2015;115:575–581. doi: 10.1016/j.carbpol.2014.09.005. [DOI] [PubMed] [Google Scholar]
- 29.Sanyang M.L., Sapuan S.M., Jawaid M., Ishak M.R., Sahari J. Effect of plasticizer type and concentration on tensile, thermal and barrier properties of biodegradable films based on sugar palm (Arenga pinnata) Starch. Polym. 2015;7:1106–1124. doi: 10.1007/s13197-015-2009-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Aguirre A., Borneo R., León A.E. Properties of triticale protein films and their relation to plasticizing–antiplasticizing effects of glycerol and sorbitol. Ind. Crop. Prod. 2013;50:297–303. [Google Scholar]
- 31.Gutiérrez T.J., Morales N.J., Pérez E., Tapia M.S., Famá L. Physico-chemical properties of edible films derived from native and phosphated cush-cush yam and cassava starches, Food Packag. Shelf Life. 2015;3:1–8. [Google Scholar]
- 32.Bilck A.P., Müller C.M.O., Olivato J.B., Mali S., Grossmann M.V.E., Yamashita F. Using glycerol produced from biodiesel as a plasticizer in extruded in extruded biodegradable films. Polímeros. 2015;25:33–335. [Google Scholar]
- 33.Cyras V.P., Manfredi L.B., Minh-Tan T.T., Vazquez A. Physical and mechanical properties of thermoplastic starch/montmorillonitenanocomposite films. Carbohydr. Polym. 2008;73:55–63. [Google Scholar]
- 34.Majdzadeh-Ardakani K., Nazari B. Improving the mechanical properties of thermoplastic starch/poly(vinyl alcohol)/clay nanocomposites. Compos. Sci. Technol. 2010;70:1557–1563. [Google Scholar]
- 35.Thakur V.K., Vennerberg D., Kessler M.R. Green aqueous surface modification of polypropylene for novel polymer nanocomposites. ACS Appl. Mater. Interfaces. 2014;6:9349–9356. doi: 10.1021/am501726d. [DOI] [PubMed] [Google Scholar]
- 36.Delhom C.D. Development and thermal characterization of cellulose/clay nanocomposites. Eng. Sci. Univer. New Orleans. 2009;62 [Google Scholar]
- 37.Lee J.W., Khan S.B., Akhtar K., Kim K.I., Yoo T.W., Seo K.W., Han H., Asiri A.M. Fabrication of composite membrane based on silicotungstic heteropolyacid doped polybenzimidazole for high temperature PEMFC. Int. J. Electrochem. Sci. 2012;7:6276–6288. [Google Scholar]
- 38.Kim D., Jang M., Seo J., Namb K.H., Han H., Khan S.B. UV-cured poly (urethane acrylate) composite films containing surface-modified tetrapod ZnO whiskers. Compos. Sci. Technol. 2013;7:84–92. [Google Scholar]
- 39.Khan S.B., Lee J.W., Marwani H.M., Akhtar K., Asiri A.M., Seo J., Khan A.A.P., Han H. Polybenzimidazole hybrid membranes as a selective adsorbent of mercury. Compos: Part B.56. 2014:392–396. [Google Scholar]
- 40.Damien M.M., Eric G., Carine C. Properties of nanofillers in polymer, nanocomposites and polymers with analytical methods, John Cuppoletti. IntechOpen. 2011 [Google Scholar]
- 41.Azmi A.I., Lin R.J.T., Bhattacharyya D. Experimental study of machinability of GFRP composites by end milling. Mater. Manuf. Process. 2012;27:1045–1050. [Google Scholar]
- 42.Panamoottil S.M., Poetschke P., Lin R.J.T. Conductivity of microfibrillar polymer polymer composites with CNT-loaded microfibrils or compatibilizer: a comparative study, Express Polym. Lett. 2013;7:607–620. [Google Scholar]
- 43.Onnainty R., Granero G. Chitosan-clays based nanocomposites: promising materials for drug delivery applications. Nanomed. Nanotechnol. J. 2017;1:114. [Google Scholar]
- 44.Weber J.B., Perry P.W., Upchurch R.P. The influence of temperature and time on the adsorption of paraquat, diquat, 2,4-D, and prometone by clays, charcoal, and an anion-exchange resin, Soil Sci. Soc. Am. Proc. 1965;29:678–688. [Google Scholar]
- 45.Monteiroa M.K.S., Oliveirab V.R.L., Santosb F.K.G., Barros Netoa E.L., Leiteb R.H.L., Arouchab E.M.M., Silvaa R.R., Silvaa K.N.O. Incorporation of bentonite clay in cassava starch films for the reduction of water vapor permeability. Food Res. Int. 2018;105:637–644. doi: 10.1016/j.foodres.2017.11.030. [DOI] [PubMed] [Google Scholar]
- 46.Abdullahi S.L., Audu A.A. Comparative analysis on chemical composition of bentonite clays obtained from Ashaka and Tango deposits in Gombe State, Nigeria. Chemsearch J. 2017;8:35–40. [Google Scholar]
- 47.Jinsong L., Ivars N. Physical and Chemical stability of the bentonite buffer. Chem. Eng. Technol. 2008:1–43. [Google Scholar]
- 48.Carlson L. Bentonite mineralogy, geological survey of Finland. Jan. 2004. http://www.posiva.fi/files/2257/POSIVA-2004-02_Working-report_web.pdf Finland.
- 49.El Miz M., Akichoh H., Berraaouan D., Salhi S., Tahani A. Chemical and physical characterization of Moroccan bentonite taken from Nador (North of Morocco) Am. J. Chem. 2017;7:105–112. [Google Scholar]
- 50.Engineering ToolBox Specific heat of common substances. https://www.engineeringtoolbox.com/specifec-heat-capacity-d_391.html
- 51.Rabl A., Curtiss P. second ed. 2005. Principles of Load Calculations, CRC Handbook of Mechanical Engineering.http://www.cementechnology.ir/Library/CRC%20Mechanical%20Engineering%20Handbook.pdf [Google Scholar]
- 52.Kosny J., Yarbrough D.W. second ed. 2017. Thermal Bridges in Building Structures, CRC Handbook of Thermal Engineering. [Google Scholar]
- 53.Ayeni A.O., Daramola M.O., Sekoai P.T., Adeeyo O., Garba M.J., Awosusi A.A. Statistical modelling and optimization of alkaline peroxide oxidation pretreatment process on rice husk cellulosic biomass to enhance enzymatic convertibility and fermentation to ethanol. Cellulose. 2018;25:2487–2504. [Google Scholar]
- 54.Ayeni A.O., Daramola M.O., Taiwo O., Olanrewaju O.I., Oyekunle D.T., Sekoai P.T., Elehinafe F.B. Production of citric acid from the fermentation of pineapple waste by Aspergillus Niger. Open Chem. Eng. J. 2019;13:88–96. [Google Scholar]