We read with great interest the article by Nicholson et al1 that described 4 patients with coronavirus disease 2019 (COVID-19) presenting with parenchymal hemorrhages during their critical care admission. We agree with the authors’ proposal that a thrombotic microangiopathy may cause hemorrhagic neurologic manifestations in COVID-19. This is supported by imaging features of coagulopathy and endothelial dysfunction in multiple organ systems observed in such patients. However, there remains a degree of uncertainty with regard to potentially shared underlying pathophysiologic mechanisms in the critically ill COVID-19 cohort.
Herein, we present 2 male patients with COVID-19 (48 and 65 years of age) with CT findings of isolated intraventricular hemorrhage (IIVH) during their prolonged critical care admission. Both patients were treated for COVID-19-related respiratory failure, requiring mechanical ventilation, and were placed on Continuous Veno-Venous Hemofiltration (CVVH; https://www.massgeneral.org/medicine/nephrology/treatments-and-services/cvvh). Neither patient required extracorporeal membrane oxygenation. The platelet count, prothrombin level, and activated partial thromboplastin times were within the normal referenced ranges. At least 2 weeks into the admission, CT of the head was performed due to reduced consciousness levels despite a sedation hold. CT revealed a dependent hyperdense fluid level in the occipital horn, consistent with IIVH. Noninvasive angiography (CT/MR imaging) did not reveal any underlying vascular abnormality. Findings of MR imaging of the head (48-year-old patient) showed cerebral microbleeds (CMB) in the splenium of the corpus callosum and subcortical white matter (Figure). Transient T2-weighted hyperintensity (resolved on subsequent MR imaging 2 weeks later) was evident in the splenium of the corpus callosum without diffusion restriction or pathologic enhancement. A transthoracic echocardiogram excluded features of infective endocarditis. Both patients recovered from their acute respiratory illness sufficiently for hospital discharge.
FIGURE.
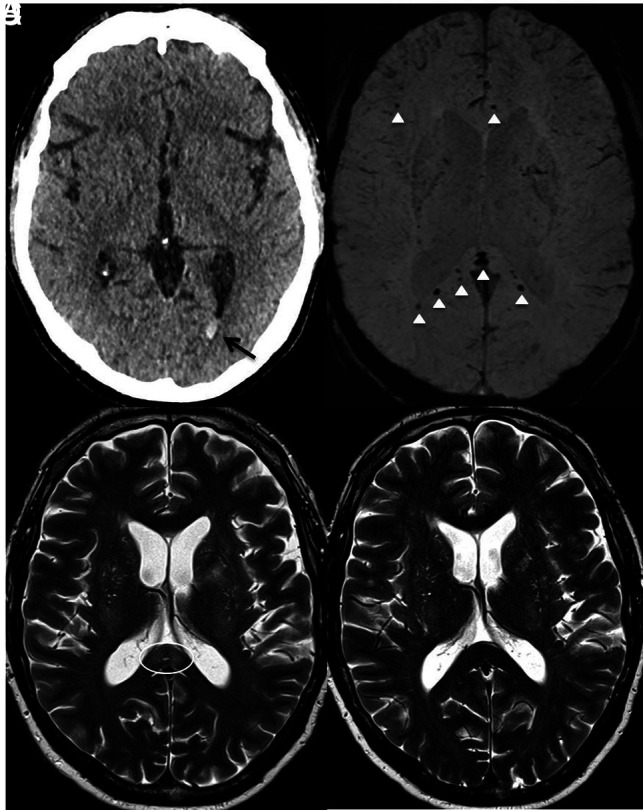
Axial images of the 48-year-old male patient. A, Un-enhanced CT demonstrates isolated intraventricular hemorrhage (black arrow). B, Susceptibility-weighted imaging shows microbleeds in the subcortical white matter, splenium, and genu of the corpus callosum (white arrowhead). C, T2-weighted image shows the splenial hyperintense lesion (white circle). D, T2-weighted imaging shows resolution after 2 weeks.
IIVH as a presenting feature on CT imaging is a comparatively rare entity in adults, accounting for about 3% of all intracranial hemorrhages, with coagulopathy as a known risk factor. No underlying cause may be identified in up to 25%–46% of patients.2 Most interestingly, both patients with IIVH had normal coagulation parameters. The hyperinflammatory syndrome or “cytokine storm” strongly associated with severe COVID-19 infection could explain the transient splenial lesion demonstrated.3 The observed CMBs are atypical for hypertensive or amyloid angiopathy causes. Instead, thrombotic microangiopathy and endotheliitis, also described in relation to COVID-19, may be contributory factors in sepsis/critical illness-related CMBs. However, corpus callosal CMBs have been reported in patients with acute respiratory distress syndrome with a resemblance seen in CMB-related high-altitude exposure, sharing a common underlying etiology of hypoxemia.4 This resemblance could similarly explain the findings in our case. Other variables that may influence the presence and/or extent of microhemorrhage in patients with COVID-19 include therapeutic anticoagulation (for pulmonary embolus or renal support) and raised cerebral venous pressure due to ventilator measures to optimize alveolar recruitment and patient oxygenation.
The exact etiology of the presented cryptogenic IIVH and CMB demonstrated in patients with COVID-19 remains unclear. Indeed, it is likely that shared pathophysiologic mechanisms are responsible for the various neurologic manifestations of COVID-19, particularly in the critically unwell.
Footnotes
Disclosures: Robert Lenthall—UNRELATED: Payment for Development of Educational Presentations: The European Course in Minimally Invasive Neurological Therapy (ECMINT) faculty, Comments: lecturer in the ECMINT course, multiple talks not on coronavirus disease 2019 (COVID-19) as yet; Other: equipment manufacturers, Comments: I have received support to attend educational meetings from multiple device manufacturers on multiple occasions for 20 years.
References
- 1.Nicholson P, Alshafai L, Krings T. Neuroimaging findings in patients with COVID-19. AJNR Am J Neuroradiol 2020;41:1380–83 10.3174/ajnr.A6630 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Weinstein R, Ess K, Sirdar B, et al. . Primary intraventricular hemorrhage: clinical characteristics and outcomes. J Stroke Cerebrovasc Dis 2017;26:995–99 10.1016/j.jstrokecerebrovasdis.2016.11.114 [DOI] [PubMed] [Google Scholar]
- 3.Mehta P, McAuley DF, Brown M, et al. ; HLH Across Speciality Collaboration, UK. COVID-19: consider cytokine storm syndromes and immunosuppression. Lancet 2020;395:1033–34 10.1016/S0140-6736(20)30628-0 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Fanou EM, Coutinho JM, Shannon P, et al. . Critical illness-associated cerebral microbleeds. Stroke 2017;48:1085–87 10.1161/STROKEAHA.116.016289 [DOI] [PubMed] [Google Scholar]


