Despite a large cohort of 103 patients with COVID-19, the authors found a large number of symptomatic patients with negative neuroimaging findings, and no conclusions can be drawn concerning concrete associations between neuroimaging and COVID-19.
SUMMARY:
The rapid spread of the coronavirus disease 2019 (COVID-19) pandemic has shaken hospitals worldwide. Some authors suggest that neurologic involvement could further complicate the disease. This descriptive study is a cross-sectional review of 103 patients diagnosed with COVID-19 who underwent neuroimaging (of a total of 2249 patients with COVID-19 in our center). Analyzed variables were neurologic symptoms and acute imaging findings. The most frequent symptoms that motivated neuroimaging examinations were mild nonfocal neurologic symptoms, code stroke (refers to patients presenting with signs and symptoms of stroke whose hyperacute assessment and care is prioritized), focal neurologic symptoms, postsedation encephalopathy, and seizures. No cases of encephalitis or direct central nervous system involvement were detected. Thirteen patients presented with acute ischemic events, and 7, with hemorrhagic events; however, most reported multiple vascular risk factors. Despite the large cohort of patients with COVID-19, we found a large number of symptomatic patients with negative neuroimaging findings, and no conclusions can be drawn concerning concrete associations between neuroimaging and COVID-19.
The coronavirus disease 2019 (COVID-19) pandemic caused by the Severe Acute Respiratory Syndrome coronavirus 2 (SARS-CoV-2) started in Wuhan, China, in December 2019 and spread rapidly. The current focus is in North America and Europe, including Spain, and particularly Catalonia, where the virus has overwhelmed the hospitals and made it one of the most hard-hit regions of Europe.
The clinical hallmark of the disease is viral pneumonia with fever and dry cough. Patients can suddenly progress to acute respiratory distress syndrome and, in severe cases, to death due to respiratory or multiorgan failure. Early publications were centered on these most salient and emergent aspects of the disease, mainly respiratory, but later articles suggested different sorts of neurologic complications.1-5
Proposed mechanisms for neurologic implications include the following:
Direct central nervous system spread, based on the known neurotropism of previous SARS-COV strains, which could access the CNS via olfactory pathways or the bloodstream, causing meningitis and encephalitis.4,6 The involvement of the respiratory center in the brain stem may hypothetically justify the well-documented rapid respiratory deterioration with marked hypoxia despite a lack of symptomatic dyspnea.2,7
Indirect neurologic involvement due to an excessive systemic proinflammatory response, which may cause widespread dysregulation of homeostasis with coagulopathy and may also increase the risk of acute cerebrovascular diseases.8,9
Parainfectious autoimmune-based neurologic complications such as acute disseminated encephalomyelitis and Guillain-Barré syndromes, which are recognized complications of microbial infections.10-12
Several studies have described neurologic symptoms in patients with COVID-19. These symptoms mainly include dizziness, headache, ataxia, and confusion.5,7,13 One case report suggested viral meningoencephalitis and ventriculitis with reverse transcription polymerase chain reaction (RT-PCR) positive for SARS-CoV-2 in the CSF of a young patient with consciousness disturbance and seizures.6 Anosmia and dysgeusia, which are highly prevalent in early infection,14 have been proposed in support of the hypothesis of CNS spread via the olfactory tract.3 Cerebrovascular events in patients with COVID-19 have also been documented; Klok et al15 described 3 cases of acute ischemic stroke in a cohort of 184 patients (1.6%) in the intensive care unit, while another preprint article described acute cerebrovascular accidents (ischemic and hemorrhagic) in 13 patients of 221 (5.9%).16 Finally, some cases of parainfectious autoimmune-based neurologic manifestations concurrent with active COVID-19 have been described, including hemorrhagic necrotizing encephalopathy12 and Guillain-Barré syndrome.10,11
To the best of our knowledge, neuroimaging of the disease has not itself been evaluated to date.
Our objective is to present a large series of patients with COVID-19 with neurologic symptoms requiring neuroimaging.
MATERIALS AND METHODS
Case Series
This article was revised for publication by the research ethics committee of our tertiary hospital. The data of the patients were anonymized for this analysis. The confidential information of patients was protected in accordance with national and European Union norms. Nonspecific informed consent to participate in research projects was obtained from all patients. A waiver of a specific informed consent was provided by the ethics committee for this retrospective study.
We performed a retrospective cross-sectional review of patients admitted to our tertiary care center between March 1 and April 18, 2020, with RT-PCR positive for SARS-CoV-2 in whom brain neuroimaging was performed.
Eligibility criteria were the following: a positive record of RT-PCR for SARS-CoV-2; neuroimaging performed, including either head CT or MR imaging; and 16 years of age or older. Exclusion criteria were the following: neuroimaging performed >5 days before diagnosis (based on a median incubation period of 5.1 days17), or low-quality imaging on visual assessment.
Regarding the protocol of our center, the RT-PCR for SARS-CoV-2 testing was performed if the patient presented with severe respiratory symptoms (respiratory rate of >30 breaths per minute, blood oxygen saturation of <95%, with oxygen administered at 35%) or pulmonary infiltrates on x-ray suspicious for viral pneumonia. Furthermore, PCR testing was also performed on all inpatients, on patients who fulfilled the criteria for in-hospital admission, on candidates for invasive surgical or interventional procedures, on all hospital personnel with any respiratory or suspicious symptoms, and finally, on vulnerable populations such as immunocompromised patients.
The minimum required imaging protocol consisted of head CT with or without contrast from the cranial base to the apex or MR imaging, including T1WI, T2WI, T2*WI, DWI, and FLAIR. Available CTA was also reviewed but not included as an eligibility criterion.
Variables reviewed included basic demographic and clinical characteristics, symptoms motivating neuroimaging, and acute neuroimaging findings.
Reasons for neuroimaging were grouped into 7 categories: 1) mild nonfocal neurologic symptoms and including symptoms such as headache, transient mild ataxia, dysarthria, or mild confusion not fulfilling code stroke criteria; 2) activated code stroke/transient ischemic attack; 3) other focal neurologic symptoms; 4) traumatic brain injury; 5) postsedation encephalopathy; 6) seizures; and 7) miscellany.
All imaging studies were independently reviewed by 2 certified neuroradiologists (P.N.-B. and A.P.-E.). Demographics, clinical characteristics, and neuroimaging indications were extracted from patients’ clinical histories and neuroimaging. Quality assessment of the images was subjectively performed by both certified neuroradiologists (P.N.-B. and A.P.-E.). Disagreements were solved by consensus.
RESULTS
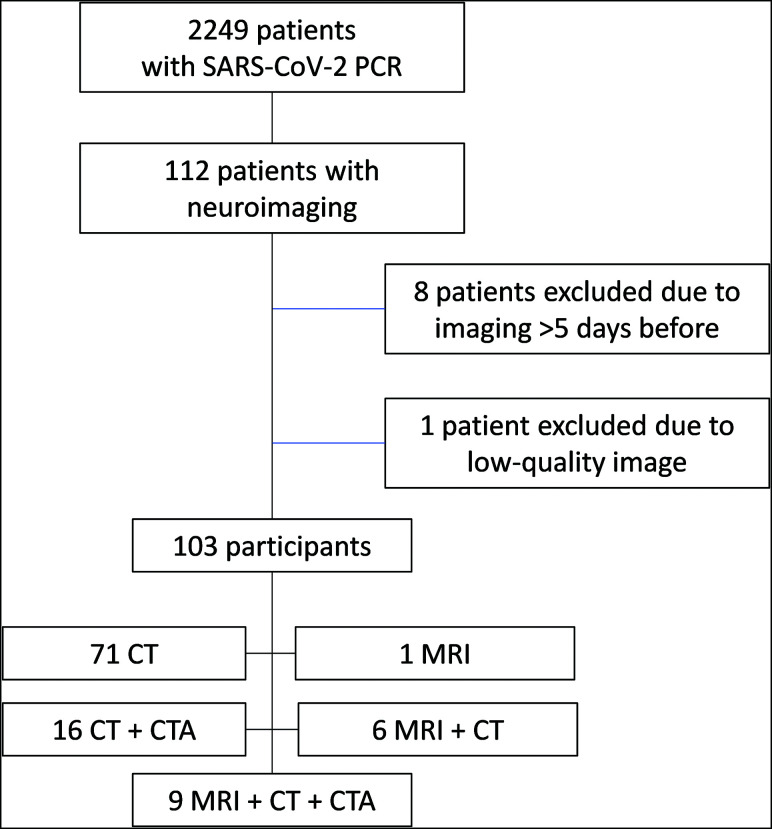
From March 1 to April 1, 2020, a total of 2249 patients with RT-PCR positive for SARS-CoV-2 were admitted in our center. During the hospitalization period, 112 of these patients underwent head neuroimaging (17 head MR imaging, 111 head CT, and 27 CTA). Of these patients, 9 were excluded (1 with MR imaging + CT; 2 with CT + CTA; 6 with CT), 8 of them because imaging was performed >5 days before SARS-CoV-2 diagnosis and 1 because of low-quality imaging (Fig 1). Accordingly, the final number of participants was 103 (Table 1).
Fig 1.
Recruitment flow chart.
Table 1:
Demographic and imaging technique characteristicsa
| All Patients | Inpatients | Emergency Department | |
|---|---|---|---|
| No. | 103 | 64 | 39 |
| Sex (No.) (%) | |||
| Male | 63 (61%) | 37 (58%) | 26 (67%) |
| Female | 40 (39%) | 27 (42%) | 13 (33%) |
| Age (yr) | 74 (50.2–90) | 71.5 (48–90) | 75 (30.3–89) |
| Imaging technique | |||
| CT | 102 (99%) | 63 (98%) | 39 (100%) |
| CTA | 25 (24%) | 14 (22%) | 11 (28%) |
| MR imaging | 16 (16%) | 13 (20%) | 3 (8%) |
Categoric variables are (No.) (%); age is (median) (fifth to 95th percentile).
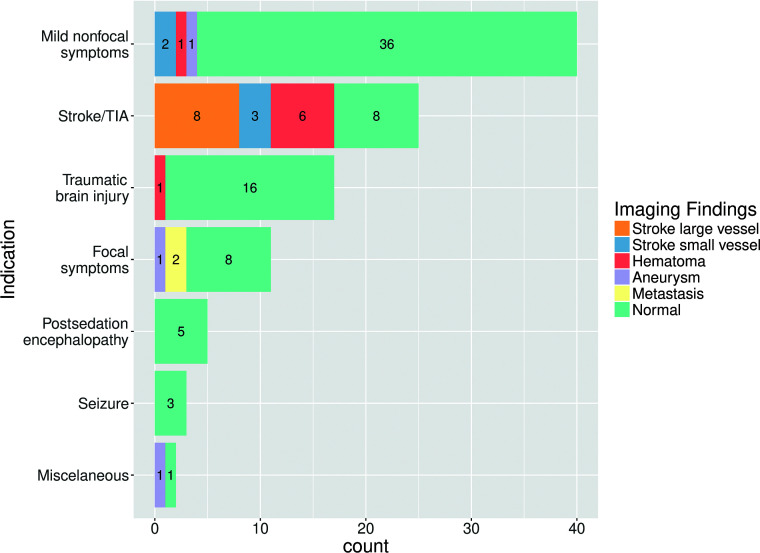
Reasons for neuroimaging matched with neuroimaging findings are summarized in Fig 2 and are presented below by categories. Specific results in patients with MR imaging only are presented in Table 2.
Fig 2.
Summary of results, including all patients undergoing neuroimaging (MR imaging/CT). Reasons for neuroimaging are on the y-axis. Neuroimaging findings are color-coded in the legend. Note that “Mild nonfocal neurologic symptoms” refer to any mild state of altered consciousness, mild transient dysarthria, mild transient gait abnormality, or headache. Lacunar or small distal cortical infarctions not susceptible to thrombectomy were considered “small-vessel.”
Table 2:
Patients undergoing MR imaging
|
Reason for MR Imaging |
No. | Findings of MR Imaging | No. |
|---|---|---|---|
| Code stroke | 8 | Acute ischemic (small-vessel) | 3 |
| Acute ischemic (large-vessel) | 2 | ||
| Parenchymal hemorrhage | 3 | ||
| Other focal neurologic symptoms | 4 | Metastasis | 2 |
| Aneurysm | 1 | ||
| Normal | 1 | ||
| Mild nonfocal neurologic symptoms | 4 | Acute ischemic | 1 |
| Parenchymal hemorrhage | 1 | ||
| Normal | 2 |
Mild Nonfocal Neurologic Symptoms
The most common reason for neuroimaging was a nonspecific state of headache, mild alteration of consciousness, transitory dysarthria, or gait abnormality, in 40 patients (4 CT + MRI, 2 CT + CTA, and 34 CT scans). Neuroimaging showed no acute findings in 36 patients. Two patients had distal small-vessel acute infarctions (1 cerebellar, 2 left prefrontal), a single patient had a left parietal lobar acute hematoma, and another had a basilar tip aneurysm.
Stroke/TIA
The second most common reason for neuroimaging was an activated code stroke or transient ischemic attack in 25 patients (7 CT + CTA + MRI, 1 CT + MRI, 11 CT + CTA, and 6 CT scans). We found 6 acute parenchymal hematomas: 3 deep basal ganglia and 3 lobar. Large-vessel occlusion was observed in 8 patients. Included were 3 patients categorized as having small-vessel occlusion, 2 acute lacunar infarctions, and 1 patient with multiple, multi-territory small distal acute parenchymal infarctions. Finally, 8 patients had no acute neuroimaging findings.
Focal Neurologic Symptoms
Eleven patients underwent neuroimaging for focal neural symptoms that did not fulfill criteria for code stroke (2 CT + CTA + MRI, 1 CT + MRI, 1 MRI, 1 CT + CTA, and 6 CT scans). Two patients with known malignancy had an increase in the size of previously known brain metastases: One of them presented with visual field disturbance; the other, with mild acral paresis. Another patient with abducens nerve palsy had a large aneurysm at the origin of the right posterior-inferior cerebellar artery. The other 8 patients had no acute neuroimaging findings: One of them presented with diplopia, and the other 7, with mild acral paresis.
Traumatic Brain Injury
Seventeen patients underwent CT for trauma involving the craniofacial region. Sixteen had no relevant acute intracranial findings. One had a focal left-parietal parenchymal hemorrhagic concussion.
Postsedation Encephalopathy
Five patients underwent CT (1 of them also with CTA) because of a Glasgow Coma Scale score below 7. Four of them were patients with delayed recovery of consciousness after prolonged sedation in the intensive care unit. One was a patient with severe respiratory failure. None had any acute findings on CT or CTA.
Seizures
Three patients had CT performed due to seizures. None of them had acute findings. Two of them were known to have had epileptogenic lesions: One had chronic calcified neurocysticercosis lesions, and the other had extensive areas of encephalomalacia due to a prior cerebrovascular accident. For the 1 patient with no history of seizures or epileptogenic lesions, neuroimaging findings were normal, and seizures were considered to be related to carbapenem neurotoxicity, which was administered due to concurrent extended-spectrum β-lactamase Klebsiella pneumoniae infection.
Miscellaneous Cases
Two isolated miscellaneous cases included a case of COVID-19 initial presentation with Guillain-Barré syndrome and normal neuroimaging (CT) findings, and a case of Staphylococcus aureus endocarditis with mycotic aneurysms on CTA.
The above cases include 20 cases of nontraumatic cerebrovascular accidents, with 3 not presenting as code stroke. Details of cardiovascular risk factors in these patients are provided in Table 3. Most notably, 75% of all patients with a cerebrovascular accident had at least 1 vascular risk factor, and 61% had at least 2, without considering age. However, in the case of the 7 patients with parenchymal hematomas, 3 had no vascular risk factors and were younger than 70 years of age. Moreover, of the 4 patients with lobar hematomas, none had imaging characteristics or clinical history of cerebral amyloid angiopathy or any other predisposing factor.
Table 3:
Demographic and vascular risk factors in cerebrovascular accidents and all patients with code stroke
| All Code Stroke and CVA | Ischemic | Hematoma | Normal | |
|---|---|---|---|---|
| No. | 28 | 13 | 7 | 8 |
| Sex (No.) (%) | ||||
| Male | 16 (57%) | 7 (54%) | 6 (86%) | 3 (38%) |
| Female | 12 (43%) | 6 (46%) | 1 (14%) | 5 (63%) |
| Age (median) (range) (yr) | 71 (45–89) | 74 (45–89) | 68 (49–78) | 73.5 (67–77) |
| Vascular risk factors (No.) (%) | ||||
| Hypertension | 20 (71%) | 9 (69%) | 4 (57%) | 7 (88%) |
| Hypercholesterolemia | 14 (50%) | 7 (54%) | 2 (29%) | 5 (63%) |
| Diabetes mellitus | 9 (32%) | 3 (23%) | 2 (29%) | 4 (50%) |
| Smoker | 2 (7%) | 0 | 1 (14%) | 1 (13%) |
| Atrial fibrillation | 2 (7%) | 2 (15%) | 0 | 0 |
| At least 1 CV risk factor | 21 (75%) | 10 (77%) | 4 (57%) | 7 (89%) |
| At least 2 CV risk factors | 17 (61%) | 7 (54%) | 3 (43%) | 7 (89%) |
Note:—CV indicates cardiovascular; CVA, cerebrovascular accident.
DISCUSSION
We have analyzed one of the largest series of patients with COVID-19 published to date and focused on those patients with neurologic symptoms requiring neuroimaging. The patients included in our analysis presented with a varied spectrum of neuroimaging indications and findings. Nevertheless, a large number of symptomatic patients appeared to have negative neuroimaging findings.
A causal relationship with COVID-19 infection may be reasonably ruled out in some patients, such as the ones with neuroimaging performed because of traumatic brain injury or the case of bacterial endocarditis. Cases with vague symptoms such as a mild transitory altered level of consciousness or mild nonspecific focal neurologic symptoms had mostly normal neuroimaging results or alternative diagnoses independent of COVID-19, such as brain metastases and unruptured aneurysms. Furthermore, 4 patients with encephalopathy after prolonged sedation had normal neuroimaging findings. A nonspecific delay in conscious-level recovery is not uncommon in patients with deep and prolonged sedation, which many patients with COVID-19 require. Neuro-imaging is performed in these patients to rule out other occult complications, which, in these cases, were indeed ruled out. The remaining patients, in our opinion, warranting consideration as possibly related to COVID-19 included 13 patients with acute ischemic lesions, 7 patients with acute hemorrhagic lesions (4 lobar and 3 deep basal ganglia), 3 patients with seizures, and 1 patient with Guillain-Barré syndrome and normal neuroimaging findings.
There was a high prevalence of vascular risk factors among acute ischemic cerebrovascular events. Nevertheless, in the case of acute hemorrhagic lesions, several cases had no previous risk factors. Moreover, as illustrative data, during the same period, the number of code stroke protocols activated in our center dropped 30% from the previous year. Of 97 patients with activated code stroke in this time period, 18 were positive for SARS-CoV-2 (19%) versus 79 who tested negative. To date, no reliable data are available on the prevalence of the infection in the local population.
The neurologic symptoms in patients with COVID-19 described in several articles are nonspecific, and inconclusive for any underlying organic neurologic damage. These symptoms in-cluded dizziness, headache, ataxia, and confusion, which are frequent transient symptoms of diverse scenarios such as infections, prolonged hospitalization periods, and posttreatment or postprocedural states, among others.5,13 A case report suggested viral meningoencephalitis and ventriculitis in a patient with RT-PCR positive determination on CSF and negative on the nasopharyngeal swab for SARS-CoV-2. This patient presented with nonspecific neurologic symptoms such as consciousness disturbance and seizures, and imaging findings were not specific.6 Regarding anosmia and dysgeusia, a pre-peer review study suggested that non-neural support cells but not sensory neural cells express the angiotensin-converting enzyme 2 receptor, which is targeted by the virus. This finding would support the hypothesis that anosmia and dysgeusia are merely a peripheral phenomenon.18 As for acute cerebrovascular events in COVID-19, some considerations prevent establishing causality based on published studies.15,16 Prior common patient underlying conditions/risk factors that may cause cerebrovascular events seem overlooked; and risk-stratified control datasets are not used to robustly confirm a higher incidence of cerebrovascular events or the real increase of risk in patients with COVID-19. Finally, parainfectious processes are thought to be triggered by an immune response, and about two-thirds of patients have a recent history of viral or bacterial respiratory or gastrointestinal tract infection,19 so it seems perfectly plausible that SARS-COV-2 may also trigger these kinds of diseases, as is suggested in the literature.10-12
There are several important limitations to this study, mainly due to the rapid expansion of the disease and the critical situation of many patients, which requires a reorganization of hospital resources centered on providing the best possible assistance. First, despite the relatively large sample of patients with COVID-19 (2249), only 103 who underwent neuroimaging could be included in this study. This could be partially explained by the following: Severely ill patients may not have neurologic symptoms or may not be able to undergo imaging; or the concern for transporting infected patients and contaminating radiology equipment may prompt a higher threshold for imaging indications. Second, not all presumably infected patients were tested, so the number of patients with COVID-19 may be underestimated. Third, the availability full clinical and follow-up information was limited, and a complete neurologic examination was not always performed by an experienced neurologist, meaning that our results do not represent all the clinical neurologic syndromes affecting these patients. Nevertheless, despite these limitations, we believe this local review is relevant mainly because COVID-19 is a global phenomenon and many other centers probably experience the same hindrance of robust data analysis.
CONCLUSIONS
We have analyzed one of the largest series of patients with COVID-19 published to the date and focused on those with neurologic symptoms requiring neuroimaging. We have not found specific neuroimaging presentations of the virus, and a large number of symptomatic patients appear to have negative neuroimaging findings. The well-demonstrated virus-associated coagulopathy may logically increase the risk of cerebrovascular events (in our experience possibly more hemorrhagic), but further studies with risk-stratified control cohorts are required to determine the real impact. Finally, autoimmune parainfectious entities seem plausible, as they are in the context of other infectious processes.
ABBREVIATIONS:
- COVID-19
coronavirus disease 2019
- RT-PCR
reverse transcription polymerase chain reaction
- SARS-COV
Severe Acute Respiratory Syndrome coronavirus
References
- 1.Pérez CA. Looking ahead: the risk of neurologic complications due to COVID-19. Neurology Clinical Practice April 9, 2020; https://cp.neurology.org/content/early/2020/04/08/CPJ.0000000000000836. Accessed April 15, 2020 [DOI] [PMC free article] [PubMed]
- 2.Li Y, Bai W, Hashikawa T. The neuroinvasive potential of SARS‐CoV2 may be at least partially responsible for the respiratory failure of COVID‐19 patients. J Med Virol 2020. February 27. [Epub ahead of print] 10.1002/jmv.25728 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Wu Y, Xu X, Chen Z, et al. . Nervous system involvement after infection with COVID-19 and other coronaviruses. Brain Behav Immun 2020. March 30. [Epub ahead of print] 10.1016/j.bbi.2020.03.031 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Baig AM, Khaleeq A, Ali U, et al. . Evidence of the COVID-19 virus targeting the CNS: tissue distribution, host–virus interaction, and proposed neurotropic mechanisms. ACS Chem Neurosci 2020;11:995–98 10.1021/acschemneuro.0c00122 [DOI] [PubMed] [Google Scholar]
- 5.Mao L, Jin H, Wang M, et al. . Neurologic manifestations of hospitalized patients with coronavirus disease in Wuhan, China. JAMA Neurol 2020. April 10. [Epub ahead of print] 10.1001/jamaneurol.2020.1127 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Moriguchi T, Harii N, Goto J, et al. . A first case of meningitis/encephalitis associated with SARS-coronavirus-2. Int J Infect Dis 2020;94:55–58 10.1016/j.ijid.2020.03.062 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Asadi-Pooya AA, Simani L. Central nervous system manifestations of COVID-19: a systematic review. J Neurol Sci 2020;413:116832 10.1016/j.jns.2020.116832 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Zhou F, Yu T, Du R, et al. . Clinical course and risk factors for mortality of adult inpatients with COVID-19 in Wuhan, China: a retrospective cohort study. Lancet 2020;395:1054–62 10.1016/S0140-6736(20)30566-3 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Tang N, Bai H, Chen X, et al. . Anticoagulant treatment is associated with decreased mortality in severe coronavirus disease 2019 patients with coagulopathy. J Thromb Haemost 2020;18:1094–99 10.1111/jth.14817 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Zhao H, Shen D, Zhou H, et al. . Guillain-Barré syndrome associated with SARS-CoV-2 infection: causality or coincidence? Lancet Neurol 2020;19:383–84 10.1016/S1474-4422(20)30109-5 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Toscano G, Palmerini F, Ravaglia S, et al. . Guillain–Barré syndrome associated with SARS-CoV-2. N Engl J Med 2020. April 17. [Epub ahead of print] 10.1056/NEJMc2009191 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Poyiadji N, Shahin G, Noujaim D, et al. . COVID-19–associated acute hemorrhagic necrotizing encephalopathy: CT and MRI features. Radiology 2020. March 31. [Epub ahead of print] 10.1148/radiol.2020201187 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Helms J, Kremer S, Merdji H, et al. . Neurologic features in severe SARS-CoV-2 infection. N Engl J Med 2020. Apr 15. [Epub ahead of print] 10.1056/NEJMc2008597 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Giacomelli A, Pezzati L, Conti F, et al. . Self-reported olfactory and taste disorders in patients with severe acute respiratory coronavirus 2 infection: a cross-sectional study. Clin Infect Dis 2020. March 26. [Epub ahead of print] 10.1093/cid/ciaa330 [DOI] [PMC free article] [PubMed]
- 15.Klok FA, Kruip M, van der Meer NJ, et al. . Incidence of thrombotic complications in critically ill ICU patients with COVID-19. Thromb Res 2020. April 10. [Epub ahead of print] 10.1016/j.thromres.2020.04.013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Li Y, Wang M, Zhou Y, et al. . Acute cerebrovascular disease following COVID-19: a single center: retrospective, observational study. SSRN Electron Journal January 2020. researchgate.net/publication/340154622_Acute_Cerebrovascular_Disease_Following_COVID-19_A_Single_Center_Retrospective_Observational_Study. Accessed April 15, 2020 [DOI] [PMC free article] [PubMed]
- 17.Lauer SA, Grantz KH, Bi Q, et al. . The incubation period of coronavirus disease 2019 (COVID-19) from publicly reported confirmed cases: estimation and application. Ann Intern Med 2020;172:577–82 10.7326/M20-0504 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Brann DH, Tsukahara T, Weinreb C, et al. . Non-neural expression of SARS-CoV-2 entry genes in the olfactory epithelium suggests mechanisms underlying anosmia in COVID-19 patients. bioRxiv March 28, 2020 https://www.biorxiv.org/content/10.1101/2020.03.25.009084v1. Accessed April 16, 2020 [DOI] [PMC free article] [PubMed]
- 19.Hahn AF. Guillain-Barré syndrome. Lancet 1998;352:635–41 10.1016/S0140-6736(97)12308-X [DOI] [PubMed] [Google Scholar]